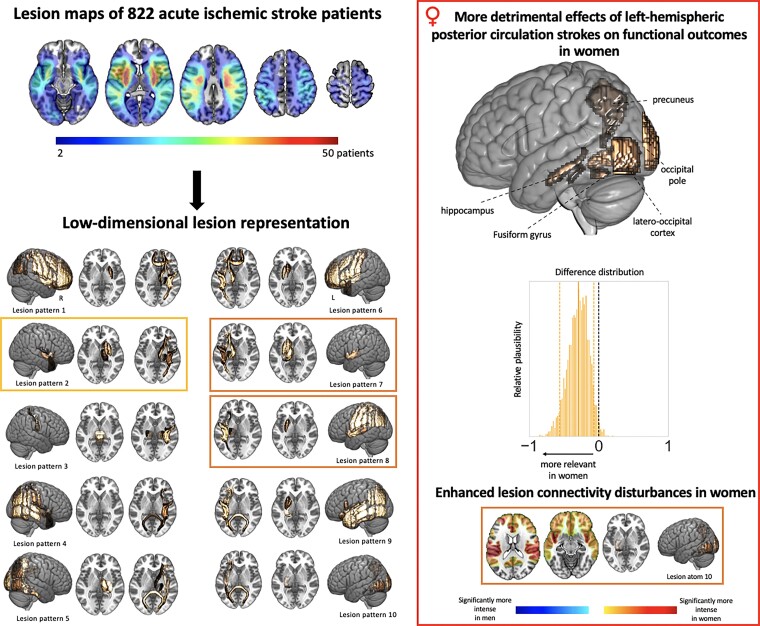
Abstract
Stroke represents a considerable burden of disease for both men and women. However, a growing body of literature suggests clinically relevant sex differences in the underlying causes, presentations and outcomes of acute ischaemic stroke. In a recent study, we reported sex divergences in lesion topographies: specific to women, acute stroke severity was linked to lesions in the left-hemispheric posterior circulation. We here determined whether these sex-specific brain manifestations also affect long-term outcomes. We relied on 822 acute ischaemic patients [age: 64.7 (15.0) years, 39% women] originating from the multi-centre MRI-GENIE study to model unfavourable outcomes (modified Rankin Scale >2) based on acute neuroimaging data in a Bayesian hierarchical framework. Lesions encompassing bilateral subcortical nuclei and left-lateralized regions in proximity to the insula explained outcomes across men and women (area under the curve = 0.81). A pattern of left-hemispheric posterior circulation brain regions, combining left hippocampus, precuneus, fusiform and lingual gyrus, occipital pole and latero-occipital cortex, showed a substantially higher relevance in explaining functional outcomes in women compared to men [mean difference of Bayesian posterior distributions (men – women) = −0.295 (90% highest posterior density interval = −0.556 to −0.068)]. Once validated in prospective studies, our findings may motivate a sex-specific approach to clinical stroke management and hold the promise of enhancing outcomes on a population level.
Keywords: acute ischaemic stroke, functional outcomes, sex differences, lesion patterns, Bayesian hierarchical modelling
Relying on neuroimaging and clinical data of 822 acute stroke patients, Bonkhoff et al. report substantially more detrimental effects of lesions in left-hemispheric posterior circulation regions on functional outcomes in women compared to men. These findings may motivate a sex-specific clinical stroke management to improve outcomes in the longer term.
Graphical Abstract
Graphical Abstract.
Introduction
Stroke results in a considerable burden of disease for both men and women. Converging evidence, however, underscores the relevant influence of biological sex on the idiosyncrasies of cause, presentation and outcome of acute ischaemic stroke (AIS).1 Of note, women appear to feature a higher AIS severity upon admission that cannot be satisfactorily explained by any sociodemographic or clinical factors, such as age and cardioembolic stroke subtype.2 In particular, this higher symptom load emerges despite comparable lesion characteristics,3,4 consistent with the possibility that male and female brains might react differently to ischaemia-induced lesions. More concretely, we recently noted distinct sex divergences in stroke lesion topographies: stroke severity in both men and women was explained by lesions affecting presumed bilateral motor regions and left-lateralized language regions. However, only in women, stroke severity was additionally explained by lesions extending to the posterior circulation of the left hemisphere, rendering female-specific lesion pattern more wide-spread. Whether these sex-specific effects have an impact on acute stroke symptoms only, or have a long-lasting character, is currently unknown. The aim of the present study was to address these gaps in our understanding of sex-specific lesion effects on long-term stroke outcomes.
Materials and methods
AIS patient sample
All MRI-Genetics Interface Exploration (MRI-GENIE)5 AIS patients with available high-quality diffusion-weighted imaging (DWI)-derived lesion segmentations6 and 3 months (60–190 days) modified Rankin Scale (mRS) data were included in this complete case study (c.f., Supplementary materials for a sample size calculation). MRI-GENIE is a large international collaboration, built upon the infrastructure of the Stroke Generics Network (SiGN).7 It assembled sociodemographic, clinical, neuroimaging and genetic data of ∼3300 AIS patients, with the primary aim to facilitate genetic analyses of neuroimaging phenotypes as derived from clinical scans.5 While MRI-GENIE merged data from 12 international sites overall, analyses here primarily relied on five individual studies that could additionally share functional outcomes (c.f., Supplementary Table 1 for study sizes and characteristics). Subjects gave written informed consent in accordance with the Declaration of Helsinki. The study protocol was approved by the Massachusetts General Hospital’s Institutional Review Board.
Stroke lesion representation derivation
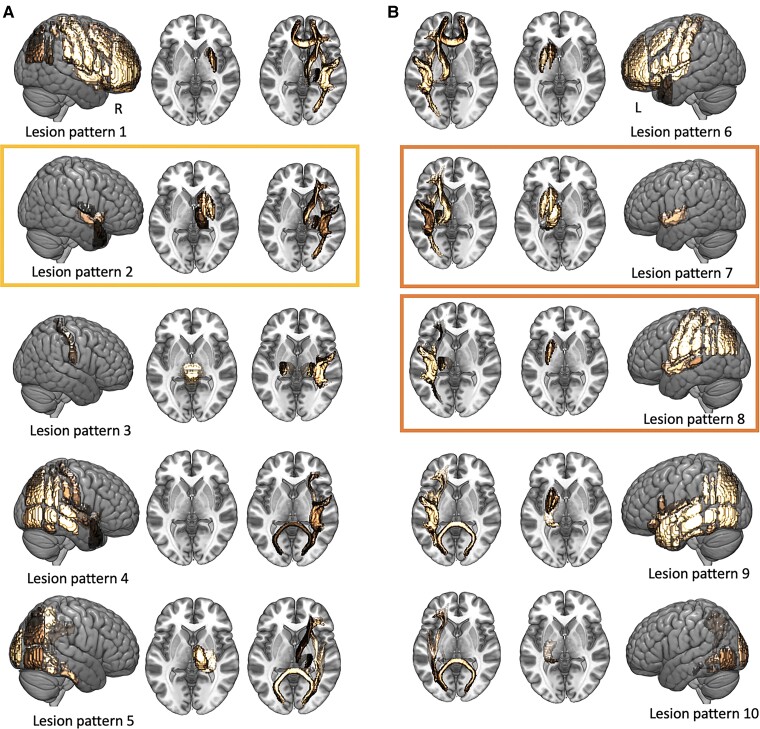
DWI data and respective automatically DWI-derived lesions were non-linearly spatially normalized (c.f., Wu et al.6 for details on lesion segmentation and spatial normalization and Supplementary materials for imaging parameters). Spatially normalized DWI data and lesion segmentations were carefully quality controlled by two experienced raters (A.K.B. and M.B.). Lesion data were parsed according to 94 cortical regions, 15 subcortical regions8 and 20 white matter tracts,9 i.e. the number of lesioned voxels per atlas-defined brain region was computed. Non-negative matrix factorization (NMF) was used to reduce the collection of 129 atlas-based region and tract lesion measures to 10 unique lesion patterns. The number 10 was chosen in line with previous work4,10–12 and further motivated by achieving a balance between faithful representation of the high-dimensional input space and mitigation of the risk of overfitting.
Statistical analyses: modelling unfavourable functional outcomes
Unfavourable functional outcome (mRS > 2) was modelled via the Bayesian hierarchical logistic regression. The 10 lesion patterns were incorporated as input variables with hierarchical priors capturing the biological sex of participants (as indicated in medical records), that is, one for female and one for male participants. The covariates age, age-squared, sex, cardiovascular risk factors (history of hypertension, atrial fibrillation, diabetes, ischaemic heart disease, prior stroke and smoking) and total DWI-derived lesion volume were included in the model (c.f., Supplementary materials for full model specifications).4,10,11 We did not incorporate admission stroke severity as an input variable, given that it likely reflects the extent and location of brain injury. Samples from the Bayesian posterior parameter distribution were drawn by employing the No U-Turn Sampler (NUTS), a type of Monte Carlo Markov Chain algorithm (setting: draws = 5000).13 The model classification performance of unfavourable outcomes was measured by the area under the receiver operating characteristic curve (AUC). We first assessed similarities between male- and female-specific lesion pattern effects and determined those lesion patterns with posterior distributions substantially differing from zero in both men and women. Differences between hierarchically estimated female and male lesion pattern effects were then evaluated via subtracting the corresponding posterior parameter distributions. As in previous work,4 we presumed substantial lesion pattern effects or sex differences if the resulting posterior (difference) distributions did not include zero in the 90% highest probability density interval (HPDI).
Sensitivity analyses
We evaluated whether there were any general sex differences in lesion anatomy, i.e. the total DWI-derived lesion volume, as well as parcel-wise lesion volumes and frequencies in how often parcels were affected.
There was a slight imbalance in the men:women ratio in our sample. Altogether, there were more male patients experiencing a favourable long-term outcome. To ensure that results were not skewed by varying frequencies of unfavourable functional outcomes in women and men, we repeated analyses after downsampling the majority class, i.e. matching the occurrence of favourable outcomes in the groups of men and women. More specifically, we downsampled the larger group of men with favourable outcomes (n = 386) to the size of the smaller group of women with favourable outcomes (n = 208). That is, we randomly chose 208 of the 386 men with favourable outcomes and repeated this process 20 times. Unfavourable long-term outcomes were present in the same number of male and female patients (n = 114 each). Afterwards, we repeatedly conducted the Bayesian logistic regression analyses.
Lesion network mapping analyses
Finally, we employed lesion network mapping14 (LNM) aiming to uncover links between lesion patterns and sex-specific lesion disconnection profiles. To achieve this goal, we implemented two main changes compared to classic LNM analyses15,16: first, instead of computing lesion network maps for lesions of individual patients, we utilized the 10 prototypical lesion patterns as regions of interest for the estimation of whole-brain lesion connectivity. We created 100 variations of each of the 10 lesion patterns by sampling a random number and collection of lesion pattern-affiliated brain regions (a brain region was considered affiliated, if the NMF-weight exceeded 0.05). Second, we relied on male- and female-specific normative connectomes, i.e. connectomes that were based on data of only male or female healthy participants (n = 346 each). Consequently, we obtained two LNM exemplars, a male- and a female-specific one, for each lesion pattern (conventionally: one LNM exemplar based on connectomes of n = 1000 healthy male and female participants).14 The preprocessing of sex-specific resting-state fMRI data itself was performed as previously described. Global signal regression was included. After estimating the whole-brain voxel-wise lesion connectivity and Fisher’s z-transformation, we summarized the positive and negative t-values, i.e. the ‘intensity’ of lesion connectivity, within Yeo/Schaefer-defined cortical networks17,18 (7 per hemisphere, therefore 14 in total; visual, somatomotor, dorsal attention, ventral attention, limbic, frontoparietal, default mode network). The Yeo–Schaefer atlas was here chosen to match the functional data at hand, as it was derived from functional data, in contrast to the Harvard–Oxford (HO)8 or JHU atlases,9 which are based on structural data. Eventually, we compared network-averaged intensities between the male- and female-connectome-based maps for each lesion pattern (two-sided t-tests, level of significance P < 0.05, false discovery rate-corrected for 14 hemisphere-specific network values for 10 lesion patterns).
Code and data availability
The authors agree to make data available to researchers for the explicit purposes of reproducing the here stated results, pending the permission for data sharing by the Massachusetts General Hospital’s institutional review board. The HO and JHU DTI-based white matter atlases are openly available at https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases. Analyses were implemented in the Python 3.7 (mainly employing the packages nilearn19 and pymc320). Exemplary code is available under: https://github.com/AnnaBonkhoff/BMH_functional_outcomes_sex_differences.
Results
Prediction of unfavourable functional outcomes across men and women
A total of 822 AIS patients were recruited to model unfavourable long-term outcomes [age: 64.7 (15.0) years, 39% women, 28% unfavourable functional outcome, c.f., Table 1 for further clinical characteristics and Supplementary Fig. 1 for a lesion overlap]. Individual atlas-parcellated lesions were represented in five anatomically plausible lesion patterns per hemisphere, with varying emphases on subcortical to cortical and anterior to posterior regions (Fig. 1). Three of these 10 unique patterns explained considerable variation in unfavourable outcomes concurrently in both male and female patients (AUC = 0.81). The relevance in explaining unfavourable outcomes across men and women of these three spatial lesion patterns were indicated by 90% posterior intervals of Bayesian parameter distributions excluding zero for both biological sexes (men: Lesion pattern #2: posterior mean: 0.20, 90% HPDI: 0.04–0.35; Lesion pattern #7: posterior mean: 0.57, 90% HPDI: 0.11–1.07; Lesion pattern #8: 0.37, 90% HPDI: 0.01–0.72; women: Lesion pattern #2: posterior mean: 0.21, 90% HPDI: 0.07–0.35; Lesion pattern #7: posterior mean: 0.76, 90% HPDI: 0.13–1.25; Lesion pattern #8: 0.39, 90% HPDI: 0.07–0.72; Supplementary Figs 2 and 3). Mainly implicated brain regions in these three patterns were bilateral subcortical grey matter nuclei and left-lateralized regions in proximity to the insula (Figs 1 and 2).
Table 1.
Patient characteristics
| All participants (n = 822) | Male participants (n = 500) | Female participants (n = 332) | Statistical comparison of male and female participants | |
|---|---|---|---|---|
| Age, years | 64.7 (15.0) | 63.9 (14.2) | 65.8 (16.2) | P = 0.07 |
| Sex | 39.2% | – | – | — |
| Unfavourable outcome (mRS > 2) | 27.7% | 22.8% | 35.4% | P = 0.0001 |
| Normalized DWI-derived stroke lesion volume (ml, median, interquartile range) | 3.3 (0–12.8) | 2.9 (0–11.3) | 3.8 (0–17.8) | P = 0.28 |
| Hypertension | 64.1% | 63.0% | 65.9% | P = 0.41 |
| Diabetes mellitus Type 2 | 21.8% | 23.0% | 19.9% | P = 0.30 |
| Atrial fibrillation | 16.8% | 14.6% | 20.2% | P = 0.04 |
| Coronary artery disease | 18.4% | 21.8% | 13.0% | P = 0.002 |
| Smoking | 55.0% | 61.0% | 45.7% | P < 0.0001 |
| Prior stroke | 9.7% | 9.4% | 10.2% | P = 0.72 |
Mean values and standard deviation, unless otherwise noted. Characteristics of men and women were compared either via two-sample t-tests or two-sided Fisher’s exact tests as appropriate. Significantly more women than men experienced unfavourable outcomes obtained ∼3 months post-stroke (women: 35.4% versus men: 22.8%). In view of this difference in our main outcome, we performed additional downsampling analyses, in which we repeatedly contrasted samples of male and female patients with the same ratios of favourable to unfavourable outcomes.
Figure 1.
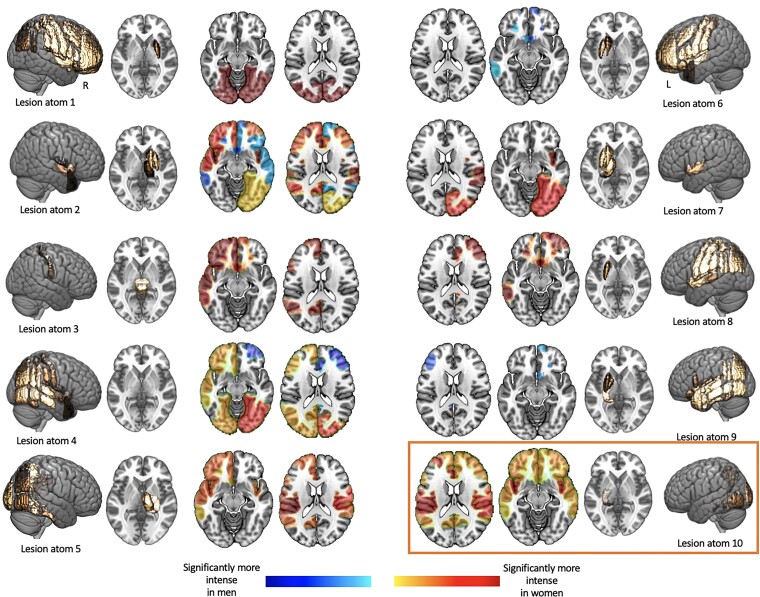
Anatomically plausible, parsimonious representation of stroke lesions. Ten unique, archetypical stroke lesion patterns were derived via an unsupervised pattern-discovery framework. Lesion pattern represented predominantly right-hemispheric stroke (A) and left-hemispheric stroke (B) with varying emphases on cortical–subcortical and anterior–medial–posterior regions. Three lesion patterns, framed in yellow for right-hemispheric stroke and in orange for left-hemispheric stroke, had a high relevance in explaining unfavourable functional outcome 3 months after stroke for both men and women. This relevance was discernible from their Bayesian posterior distributions that did not substantially overlap with zero (i.e. their 90% credibility intervals did not include zero).
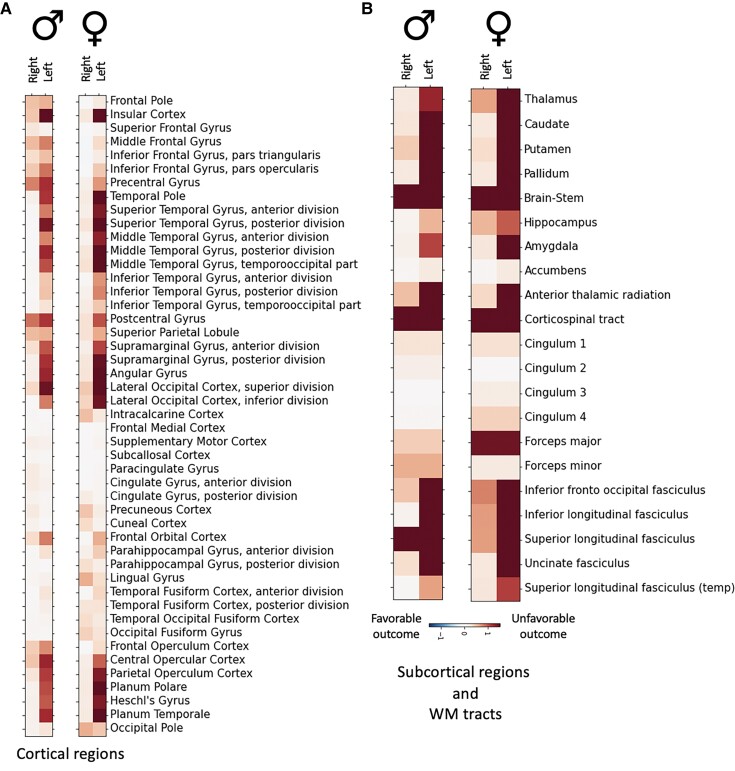
Figure 2.
Individual brain regions explaining unfavourable functional outcomes. Characteristic constellations of cortical (A) and subcortical brain regions, as well as white matter tracts (B) emerged that explained unfavourable outcomes ∼3 months after stroke in 500 male and 322 female stroke patients. Lesions in the left hemisphere were more strongly associated with unfavourable long-term outcomes than lesions in the right hemisphere for both men and women. Particularly relevant regions comprised left pre- and post-central, insular and opercular cortex, superior and middle temporal gyri, supramarginal and angular gyrus and lateral occipital cortex.
Sex-specific prediction of unfavourable functional outcomes
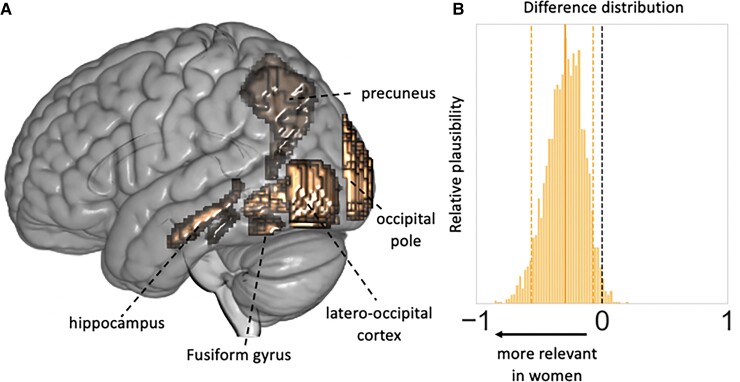
Next, we compared the relevance of lesion patterns in men versus in women via contrasting their respective sex-specific lesion pattern posterior distributions. We here observed one predominant sex difference: the left-hemispheric lesion pattern of posterior circulation brain regions was assigned a substantially higher relevance in explaining unfavourable functional outcome specifically in women [mean difference (men – women) of Bayesian marginal posterior distributions related to expressions of Lesion pattern #10: −0.30, 90% HPDI: −0.56 to −0.07, Fig. 3, c.f., Supplementary Figs 2 and 3 for an exhaustive display of difference distributions].
Figure 3.
Sex-specific effects relating to left-hemispheric posterior circulation lesion pattern. (A) Lesion pattern #10 represented left-hemispheric lesions in the presumed posterior circulation, combining the hippocampus, precuneus, fusiform and lingual gyrus, latero-occipital cortex and occipital pole. (B) The difference distribution of Bayesian posteriors for the male- and female-specific expression of Lesion pattern #10 indicated a substantially higher relevance in explaining unfavourable functional outcome specifically in women (mean of the difference posterior distribution = −0.295, HPDI of the posterior distribution covering 90% certainty = −0.556 to −0.068).
Sensitivity analyses indicate stability of results
Total lesion volume, parcel-wise lesion volumes, as well as parcel-wise frequencies of lesion status did not significantly differ between men and women (P > 0.05, family wise error-corrected, Supplementary Table 2). Results remained unchanged after reiterating the identical analysis workflow in balanced groups of men and women with favourable outcomes: In the 20 logistic regression analyses resulting after downsampling, we consistently observed female-specific Lesion pattern #10 effects, i.e. the difference distribution of Lesion pattern #10 posteriors did not overlap with zero in 16 cases (80% of cases). In the remaining four downsampling scenarios, the difference distributions had a minor overlap with zero. Importantly, only the female-specific Lesion pattern #10 posterior distributions indicated a substantial effect, while the male-specific Lesion pattern #10 distributions overlapped with zero. Altogether, these downsampling analyses thus reinforced the notion that detected sex differences were not due to imbalances in the men:women ratio or more favourable long-term outcomes in men.
Pronounced lesion connectivity disturbances in women in Lesion pattern #10
Lesion pattern #10 was characterized by the most pronounced differences in lesion connectivity as uncovered by LNM (Fig. 4). More specifically, Lesion pattern #10 featured nine significant male- and female-specific intensity differences, that were consistent with women expressing more intense lesion connectivity. Only one other lesion pattern (Lesion pattern #2) had the same number of significant sex-specific intensity differences. Here, higher intensity however occurred for male- and female-based connectivity. Altogether, these explorations thus suggest that sex differences, as ascertained for Lesion pattern #10, might be linked to differences in affected functional connectivity. In women, Lesion pattern #10 was associated with a more pronounced deterioration in functional connectivity than in men.
Figure 4.
Significantly altered male- and female-connectome-based lesion connectivity in 14 cortical networks (seven per hemisphere). Sex-specific lesion connectivity was computed for each of the 10 unique lesion patterns (c.f., Fig. 1) and subsequently statistically compared within each of the cortical networks. Networks with significantly different lesion connectivity are represented in colour (orange/red: significantly stronger in women; blue: significantly stronger in men). To allow for these statistical group comparisons in the first place, we inserted an additional simulation step: each lesion pattern was slightly varied 100 times by sampling a random number and collection of lesion pattern-affiliated brain regions. Exemplarily more in detail: Lesion pattern #1 primarily comprised the parcels pre- and post-central gyrus, superior, middle or inferior frontal gyrus, insular cortex, superior parietal and supramarginal cortex. We would here, for example, randomly choose pre- and post-central gyrus for a first Lesion pattern #1-like lesion, subsequently choose superior parietal, as well as supramarginal cortex for a second Lesion pattern #1-like lesion and so forth until we obtained 100 Lesion pattern #1-like lesions. Male- and female-specific lesion connectivity was then computed for each of these 100 simulated lesions per lesion pattern. Lesion patterns #2 and #10 comprised most connectivity differences (nine each). While Lesion pattern #2 was characterized by both higher connectivity in men and women, Lesion pattern #10 featured higher lesion connectivity exclusively in women.
Discussion
Our study provides evidence that left-lateralized posterior circulation strokes increase the likelihood of unfavourable 3-month outcomes specifically in women. This effect was not found in male AIS patients. Our present findings thereby support and extend previous inferences on sex-specific lesion topographies for acute stroke severity4: lesions affecting the left hippocampus, precuneus, lingual and fusiform gyrus, the occipital pole and latero-occipital cortex, do not only explain more severe strokes in women in the acute phase. Rather, they have an additional permanent female-specific effect and explain unfavourable outcomes in a later phase after stroke. Future studies are warranted to now test whether women with these brain regions at risk may benefit from more aggressive acute reperfusion treatments, special post-acute rehabilitative efforts and a sex-specific planning of care.
Sex divergences in acute symptoms and long-term outcomes post-stroke are frequently examined with respect to sociodemographic and clinical aspects: For example, previous studies have suggested that women experience a higher stroke severity partly due to their more advanced age and higher likelihood of pre-morbid disability, as well as higher frequency of atrial fibrillation and cardioembolic strokes.2,21 However, the importance of actual lesion characteristics has rarely been studied in a sex-specific manner, that is lesion effects in men and women have rarely been compared, neither in low dimensions (e.g. total lesion volume) nor in high-dimensional settings (e.g. spatially high-resolution lesion-symptom mapping studies).22 In most cases, if considered at all, the biological sex of patients is regressed out from lesion information,23 essentially rendering it impossible to subsequently deduce any linear sex-specific effects. In contrast, the here installed Bayesian hierarchical modelling framework allowed for the estimation and systematic comparison of male- and female-specific lesion effects. Our findings enabled by these sex-specific pattern analyses now (i) offer the potential to enhance stroke outcome predictions and (ii) motivate the focused investigation of sex-stratified stroke care decisions with the ultimate goal of improving long-term outcomes.
Neuroanatomically, it is particularly striking that observed sex differences pertained to the left hemisphere only. Rare examples of early sex-specific lesion studies already indicated that left-sided lesions may have comparatively more detrimental effects in women.24,25 These studies contrasted left- and right-hemispheric strokes and their effects on intelligence measures in men and women. They concluded that women experience deterioration of both verbal and performance scale IQ values after left-hemispheric lesions, while in men and in the case of right-hemispheric lesions just one of these qualities was affected.24,25 Additionally, a recent functional connectivity-based study on sex-stratified predictions of IQ scores reported a high predictive capacity of fusiform gyrus-related functional connectivity patterns specifically in women.26 Of note, the left fusiform gyrus comprises the ‘visual word form area’ that is thought to be involved in the identification of words from their visual shape and thus to contribute to high-level processing of word meaning.27 Collectively, these previous insights may motivate the hypothesis that the left occipital fusiform gyrus, as encompassed in our female-specific left posterior circulation lesion pattern, could be one of the driving forces for our observed sex divergences. Nonetheless, a direct comparison of these earlier findings and ours is hampered by the crudeness of our spatial lesion representation and choice of outcome score.
Therefore, the coarse-grained nature of our 10 lesion patterns, that each combined several individual brain regions, as well as of our global 3-month clinical endpoint, unfavourable outcome as measured on the mRS, can be considered limitations of this study. Nonetheless, the spatial granularity of our lesion patterns goes beyond one of the early intelligence-focused lesion studies that differentiated between left- and right-hemispheric strokes only.24,25 Anatomical configurations of stroke lesions are furthermore naturally constrained by the human vasculature and our data-driven dimensionality reduction approach addresses this circumstance overtly by combining brain regions that tend to be lesioned simultaneously. Furthermore, the here-derived low-dimensional lesion representations have been shown to be highly reproducible in independent datasets in previous work.4 The mRS captures a patient’s functional status on a very general level only, but is frequently used as a primary outcome measure in large clinical stroke trials.28 It may thus represent a natural first choice; especially, as ascertaining sex differences in lesion topographies for such a global outcome automatically suggests a meaningful impact on a broad stroke population basis. Future studies are, however, needed to pinpoint the exact brain correlates and brain functions of the detected female-specific effects and to furthermore disentangle the effects of pre-stroke functional states and acute recanalization therapies. For example, a concrete first step could be to conduct prospective stroke trials to systematically evaluate sex-specific treatment decisions, such as more aggressive endovascular reperfusion therapies of the posterior cerebral artery (PCA) occlusions in women. Especially since thrombectomy for primary distal PCA occlusion stroke was recently determined to be safe and potentially beneficial,29 we would hypothesize a resulting improvement in outcomes in view of our present findings. Importantly, posterior circulation strokes occur in ∼30% of all cases across both men and women30 and approximately 13% of all strokes more specifically relate to PCA occlusions,31 underscoring the potential benefit of enhanced sex-specific treatment outcomes. Finally, our performance estimates were computed based on the Bayesian posterior predictive distributions, as analyses were performed in a Bayesian hierarchical regression framework. Especially if future work shifted the focus from inference on sex differences, as applied here, to a focus on clinical outcome prediction,32,33 this future work could complement our work by running cross-validated out-of-sample predictions. We here aimed to mitigate the risk of overfitting by investigating a limited number of input variables in a large sample of ∼800 stroke patients. The Bayesian priors may moreover intrinsically exert some regularizing effect.
Our study brings into sharp focus the possibility that lesions in the left posterior circulation underlie a female-specific higher likelihood of unfavourable functional outcomes approximately 3 months after AIS. Of note, we did not observe any sex differences in lesion volumes per se and findings remained the same when controlling for varying rates in unfavourable outcomes between men and women. If confirmed in future research, these findings prompt a more sex-specific approach to the planning of acute stroke trials in the shorter and clinical stroke management in the longer term. Our findings suggest that women could, exemplarily, benefit from more aggressive acute treatments of distal PCA occlusions. These more sex-informed clinical practices may eventually augment AIS outcomes on a broad population basis.
Supplementary Material
Acknowledgements
We are grateful to our colleagues at the J. Philip Kistler Stroke Research Center for valuable support and discussions. Furthermore, we are grateful to our research participants without whom this work would not have been possible.
Abbreviations
- AIS =
acute ischaemic stroke
- AUC =
area under the receiver operating characteristic curve
- DWI =
diffusion-weighted imaging
- HO =
Harvard–Oxford
- HPDI =
highest probability density interval
- LNM =
lesion network mapping
- mRS =
modified Rankin Scale
- NMF =
non-negative matrix factorization
- PCA =
posterior cerebral artery
Appendix I
MRI-GENIE and GISCOME Investigators and the International Stroke Genetics Consortium
Anna K. Bonkhoff, Martin Bretzner, Sungmin Hong, Markus D. Schirmer, Alexander Cohen, Robert W. Regenhardt, Kathleen L. Donahue, Marco J. Nardin, Adrian V. Dalca, Anne-Katrin Giese, Mark R. Etherton, Brandon L. Hancock, Steven J. T. Mocking, Elissa C. McIntosh, John Attia, Oscar R. Benavente, Stephen Bevan, John W. Cole, Amanda Donatti, Christoph J. Griessenauer, Laura Heitsch, Lukas Holmegaard, Katarina Jood, Jordi Jimenez-Conde, Steven J. Kittner, Robin Lemmens, Christopher R. Levi, Caitrin W. McDonough, James F. Meschia, Chia-Ling Phuah, Arndt Rolfs, Stefan Ropele, Jonathan Rosand, Jaume Roquer, Tatjana Rundek, Ralph L. Sacco, Reinhold Schmidt, Pankaj Sharma, Agnieszka Slowik, Martin Söderholm, Alessandro Sousa, Tara M. Stanne, Daniel Strbian, Turgut Tatlisumak, Vincent Thijs, Achala Vagal, Johan Wasselius, Daniel Woo, Ramin Zand, Patrick F. McArdle, Bradford B. Worrall, Christina Jern, Arne G. Lindgren, Jane Maguire, Michael D. Fox, Danilo Bzdok, Ona Wu and Natalia S. Rost.
Contributor Information
MRI-GENIE and GISCOME Investigators and the International Stroke Genetics Consortium:
Anna K. Bonkhoff, Martin Bretzner, Sungmin Hong, Markus D. Schirmer, Alexander Cohen, Robert W. Regenhardt, Kathleen L. Donahue, Marco J. Nardin, Adrian V. Dalca, Anne-Katrin Giese, Mark R. Etherton, Brandon L. Hancock, Steven J. T. Mocking, Elissa C. McIntosh, John Attia, Oscar R. Benavente, Stephen Bevan, John W. Cole, Amanda Donatti, Christoph J. Griessenauer, Laura Heitsch, Lukas Holmegaard, Katarina Jood, Jordi Jimenez-Conde, Steven J. Kittner, Robin Lemmens, Christopher R. Levi, Caitrin W. McDonough, James F. Meschia, Chia-Ling Phuah, Arndt Rolfs, Stefan Ropele, Jonathan Rosand, Jaume Roquer, Tatjana Rundek, Ralph L. Sacco, Reinhold Schmidt, Pankaj Sharma, Agnieszka Slowik, Martin Söderholm, Alessandro Sousa, Tara M. Stanne, Daniel Strbian, Turgut Tatlisumak, Vincent Thijs, Achala Vagal, Johan Wasselius, Daniel Woo, Ramin Zand, Patrick F. McArdle, Bradford B. Worrall, Christina Jern, Arne G. Lindgren, Jane Maguire, Michael D. Fox, Danilo Bzdok, Ona Wu, and Natalia S. Rost
Funding
A.K.B. is supported by the Massachusetts General Hospital Executive Committee on Research (MGH ECOR) Fund for Medical Discovery (FMD) Clinical Research Fellowship Award. M.B. acknowledges support from the Société Française de Neuroradiologie, Société Française de Radiologie and Fondation ISITE-ULNE. A.V.D. is in part supported by the National Institutes of Health and the National Institute of Neurological Disorders and Stroke (NIH-NINDS, R01 NS103824, RF1 NS117643, R01 NS100417, U01NS100699 and U01NS110772). C.J.G. acknowledges support from the Swedish Research Council (2018-02543), the Swedish state under the agreement between the Swedish government and the county councils, the ‘Avtal om Läkarutbildning och Medicinsk Forskning’ (ALF) agreement (ALFGBG-720081); the Swedish Heart and Lung Foundation (20190203). A.G.L. acknowledges support from the Swedish Research Council (2019-01757), The Swedish Government (under the ‘Avtal om Läkarutbildning och Medicinsk Forskning, ALF’), The Swedish Heart and Lung Foundation, Region Skåne, Lund University, Skåne University Hospital, Sparbanksstiftelsen Färs och Frosta, Fremasons Lodge of Instruction Eos in Lund and the National Institutes of Health (NIH, 1R01NS114045-01). D.B. has been funded by the Brain Canada Foundation, through the Canada Brain Research Fund, with the financial support of the Health Canada, the National Institutes of Health (NIH R01 AG068563A), the Canadian Institute of Health Research (CIHR 438531), the Healthy Brains Healthy Lives initiative (Canada First Research Excellence fund), the Google (Research Award, Teaching Award) and by the Canada Institute for Advanced Research (CIFAR) Artificial Intelligence Chairs program. N.S.R. is in part supported by the National Institutes of Health and the National Institute of Neurological Disorders and Stroke (NIH-NINDS, R01NS082285, R01NS086905 and U19NS115388).
Competing interests
M.R.E. has received personal fees for consulting from Astra Zeneca and WorldCare Clinical Group. C.J.G. has received consulting honoraria from Microvention and Strykere, and research funding from Medtronic and Penumbra. A.V. has received research funding from Cerenovus. A.G.L. has received personal fees from Bayer, Astra Zeneca, BMS Pfizer and Portola. N.S.R. has received compensation as scientific advisory consultant from Omniox, Sanofi Genzyme and AbbVie Inc. All other authors declare no competing interests.
Supplementary material
Supplementary material is available at Brain Communications online.
References
- 1. Bushnell C, Howard VJ, Lisabeth L, et al. . Sex differences in the evaluation and treatment of acute ischaemic stroke. Lancet Neurol. 2018;17(7):641–650. [DOI] [PubMed] [Google Scholar]
- 2. Bonkhoff AK, Karch A, Weber R, Wellmann J, Berger K. Female stroke: Sex differences in acute treatment and early outcomes of acute ischemic stroke. Stroke. 2021;52(2):406–415. [DOI] [PubMed] [Google Scholar]
- 3. Silva GS, Lima FO, Camargo ECS, et al. . Gender differences in outcomes after ischemic stroke: Role of ischemic lesion volume and intracranial large-artery occlusion. Cerebrovasc Dis. 2010;30(5):470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonkhoff AK, Schirmer MD, Bretzner M, et al. . Outcome after acute ischemic stroke is linked to sex-specific lesion patterns. Nat Commun. 2021;12(1):3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Giese AK, Schirmer MD, Donahue KL, et al. . Design and rationale for examining neuroimaging genetics in ischemic stroke: The MRI-GENIE study. Neurol Genet. 2017;3(5):e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu O, Winzeck S, Giese AK, et al. . Big data approaches to phenotyping acute ischemic stroke using automated lesion segmentation of multi-center magnetic resonance imaging data. Stroke. 2019;50:1734–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meschia JF, Arnett DK, Ay H, et al. . Stroke Genetics Network (SiGN) study: Design and rationale for a genome-wide association study of ischemic stroke subtypes. Stroke. 2013;44(10):2694–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Desikan RS, Ségonne F, Fischl B, et al. . An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. [DOI] [PubMed] [Google Scholar]
- 9. Mori S, Wakana S, Van Zijl PC, Nagae-Poetscher LM. MRI atlas of human white matter. Elsevier; 2005. [DOI] [PubMed] [Google Scholar]
- 10. Bonkhoff AK, Lim J-S, Bae H-J, et al. . Generative lesion pattern decomposition of cognitive impairment after stroke. Brain Commun. 2021;3(2):fcab110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bonkhoff AK, Hong S, Bretzner M, et al. . Lesions in putative language and attention regions are linked to more severe strokes in patients with higher white matter hyperintensity burden. medRxiv. 2021. [Google Scholar]
- 12. Regenhardt RW, Bonkhoff AK, Bretzner M, et al. . Association of infarct topography and outcome after endovascular thrombectomy in patients with acute ischemic stroke. Neurology 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoffman MD, Gelman A. The No-U-Turn sampler: Adaptively setting path lengths in Hamiltonian Monte Carlo. J Mach Learn Res. 2014;15(1):1593–1623. [Google Scholar]
- 14. Fox MD. Mapping symptoms to brain networks with the human connectome. N Engl J Med. 2018;379(23):2237–2245. [DOI] [PubMed] [Google Scholar]
- 15. Ferguson MA, Lim C, Cooke D, et al. . A human memory circuit derived from brain lesions causing amnesia. Nat Commun. 2019;10(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cohen AL, Soussand L, Corrow SL, Martinaud O, Barton JJ, Fox MD. Looking beyond the face area: Lesion network mapping of prosopagnosia. Brain. 2019;142(12):3975–3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yeo BT, Krienen FM, Sepulcre J, et al. . The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schaefer A, Kong R, Gordon EM, et al. . Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb Cortex. 2018;28(9):3095–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pedregosa F, Varoquaux G, Gramfort A, et al. . Scikit-learn: Machine learning in Python. J Mach Learn Res. 2011;12:2825–2830. [Google Scholar]
- 20. Salvatier J, Wiecki TV, Fonnesbeck C. Probabilistic programming in Python using PyMC3. PeerJ Comput Sci. 2016;2:e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dehlendorff C, Andersen KK, Olsen TS. Sex disparities in stroke: Women have more severe strokes but better survival than men. J Am Heart Assoc. 2015;4(7):e001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bates E, Wilson SM, Saygin AP, et al. . Voxel-based lesion–symptom mapping. Nat Neurosci. 2003;6(5):448–450. [DOI] [PubMed] [Google Scholar]
- 23. Wu O, Cloonan L, Mocking SJT, et al. . Role of acute lesion topography in initial ischemic stroke severity and long-term functional outcomes. Stroke. 2015;46(9):2438–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Inglis J, Ruckman M, Lawson JS, MacLean AW, Monga TN. Sex differences in the cognitive effects of unilateral brain damage. Cortex. 1982;18(2):257–275. [DOI] [PubMed] [Google Scholar]
- 25. Inglis J, Ruckman M, Lawson JS, MacLean AW, Monga TN. Sex differences in the cognitive effects of unilateral brain damage: Comparison of stroke patients and normal control subjects. Cortex. 1983;19(4):551–555. [DOI] [PubMed] [Google Scholar]
- 26. Jiang R, Calhoun VD, Fan L, et al. . Gender differences in connectome-based predictions of individualized intelligence quotient and sub-domain scores. Cereb Cortex. 2020;30(3):888–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dehaene S, Cohen L. The unique role of the visual word form area in reading. Trends Cogn Sci. 2011;15(6):254–262. [DOI] [PubMed] [Google Scholar]
- 28. Thomalla G, Simonsen CZ, Boutitie F, et al. . MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379(7):611–622. [DOI] [PubMed] [Google Scholar]
- 29. Meyer L, Stracke CP, Jungi N, et al. . Thrombectomy for primary distal posterior cerebral artery occlusion stroke: The TOPMOST study. JAMA Neurol. 2021;78:434–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Frid P, Drake M, Giese AK, et al. . Detailed phenotyping of posterior vs. anterior circulation ischemic stroke: A multi-center MRI study. J Neurol. 2020;267(3):649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Drake M, Frid P, Hansen BM, et al. . Diffusion-weighted imaging, MR angiography, and baseline data in a systematic multicenter analysis of 3,301 MRI scans of ischemic stroke patients—neuroradiological review within the MRI-GENIE study. Front Neurol. 2020;11:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shmueli G. To explain or to predict? Stat Sci. 2010;25(3):289–310. [Google Scholar]
- 33. Bzdok D, Ioannidis JPA. Exploration, inference, and prediction in neuroscience and biomedicine. Trends Neurosci. 2019;42(4):251–262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors agree to make data available to researchers for the explicit purposes of reproducing the here stated results, pending the permission for data sharing by the Massachusetts General Hospital’s institutional review board. The HO and JHU DTI-based white matter atlases are openly available at https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases. Analyses were implemented in the Python 3.7 (mainly employing the packages nilearn19 and pymc320). Exemplary code is available under: https://github.com/AnnaBonkhoff/BMH_functional_outcomes_sex_differences.