Highlights
-
•
Adenosarcoma is a rare tumor of the uterus and cervix occuring mostly in post-menopausal women.
-
•
Our patient was a 37-year old nullipara presenting with lower abdominal pain and backache.
-
•
She was diagnosed as a case of multiple leiomyomata and proceeded for myomectomy.
-
•
Intraoperative findings raised suspicion of malignancy.
-
•
She underwent total abdominal hysterectomy and bilateral salpingo-oophorectomy. Histology confirmed the diagnosis.
Keywords: Uterus, Mullerian, Adenosarcoma, Adenomyoma, Sarcoma, Leiyomyoma
1. Introduction
Uterine sarcoma is a rare mixed Mullerian neoplasm which contains both epithelial and mesenchymal components (McCluggage, 2010). According to the World Health Organization’s 2014 classification of uterine malignancies, uterine sarcomas comprise of leiomyosarcoma, endometrial stromal sarcoma, adenosarcoma, undifferentiated endometrial sarcoma and their histologic sub-types (Tse et al., 2011). All the aforementioned types are rare but adenosarcomas are the rarest of them all, making up roughly 0.2% of all uterine malignancies (Nathenson et al., 2016).
A uterine sarcoma is labeled as an adenosarcoma if its epithelial component is benign or atypical and its mesenchymal component is low-grade malignant (McCluggage, 2016). Despite the limited malignant nature, it is capable of recurrence and metastasis. It usually arises from the female genital tract, specifically the uterine corpus where it is initially endometrial in origin and then undergoes myometrial invasion. It may also arise from adnexal sites such as the cervix, vagina and ovaries. Extra-genital origin has been observed in the gastrointestinal and urinary tract as well as pelvic ligaments and pelvic walls (Karateke et al., 2014).
Adenosarcomas arising in an adenomyoma is a rare entity, for it does not arise from the eutopic endometrium but from the adenomyoma itself (Elshafie et al., 2013). Managing adenosarcomas are a diagnostic and therapeutic challenge. The varied age-range, menopausal status, clinical features and scarcity of pre-operative investigations make it difficult to diagnose, hence most cases are either missed, misdiagnosed or the diagnosis is made retrospectively on the basis of post-operative histopathological features (Shi et al., 2008). The challenge does not end when a definitive diagnosis is made, since the FIGO classification for its staging does not take into account its non-eutopic origin, leading to a potential over-diagnosis (Clarke et al., 2011).
The outcome of a unique, high-grade Mullerian adenosarcoma originating from a subserosal adenomyoma with no evidence of eutopic endometrial, myometrial or serosal involvement are described in this case report. The report was written after obtaining Institutional Review Board (IRB) approval (approval number 452–21) and informed consent from the patient.
2. Case report
A 37-year old nulliparous woman came to the gynaecology outpatient department (OPD) in January 2021 with a history of lower abdominal pain and backache for the last six months. She also complained of urinary discomfort and off-and-on left-sided flank pain. She had been diagnosed as a case of multiple submucosal uterine leiomyomata three years previously and was being managed conservatively as she did not have any menstrual complaints or significant mass effects.
Over the course of three years, her symptoms had worsened and she felt her lesions had increased in size. She had undergone an open cystectomy for a right sided complex adnexal cyst three years ago but there was no documentation to confirm a histopathological diagnosis. She did not have any history of contraceptive, chemotherapy or radiotherapy use. There was no history of tobacco smoking, alcohol or drug abuse. She did not recall any weight loss. Her systemic reviews were unremarkable. General physical examination revealed a distended abdomen with a right-sided suprapubic mass. The patient’s Body Mass Index was normal. Per-vaginal examination was not performed due to the patient’s wish.
Laboratory testing revealed a raised CA-125 level of 326 IU/ml (Normal < 16 IU/ml). All other lab parameters including hematology, chemistry and coagulation profiles were normal. Random blood sugar and HbA1C was also normal.
Pelvic ultrasound showed a large, solid-looking, lobulated, heterogeneous mass measuring 141 × 103 mm, arising from the pelvis, inseparable from the posterior uterine wall, pushing the uterus anteriorly. Mild right-sided pelvicalyceal fullness was noted on renal ultrasound, the rest of ultrasonographic findings were insignificant. Magnetic resonance imaging using diffusion weighted images showed multiple exophytic subserosal degenerating leiyomyomata in the uterine fundus, body and cervix with evidence of haemorrhage and necrosis. The largest leiyomyoma measured 12 × 6 × 9 cm. These were exerting significant mass effects on the bladder. Right ovary was compressed along the lateral aspects of the mass. Mild right hydrosalpinx was also noted. Tiny nabothian cysts were noted but cervix was essentially unremarkable. Rectum was normal. No significant lymphadenopathy or ascites was seen (See Fig. 1, Fig. 2
Fig. 1.
Coronal T1, coronal post contrast T1, sagittal T2 and axial T2 weighted images of MRI pelvis of female patient showing atleast three exophytic altered signal mass lesions (red arrows) arising from right lateral and posterior uterine wall resulting in deformity of uterine contour pushing uterus anteriorly (orange arrow). These lesions appear hypointense on T1 and heterogeneously isointense to hypointense on T2 weighted images showing enhancement similar to myometrium with internal non enhancing areas of necrosis. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
Ultrasound pelvis of female patient showing a large heterogeneous solid looking mass lesion in pelvis inseparable from posterior wall of uterus pushing it anteriorly.
Patient was counselled and planned for myomectomy. Urology team was taken on board regarding the patients urinary symptoms and upon reviewing the chart and imaging, they advised concurrent bilateral Double J stenting for three months since patient had symptoms in the left flank and imaging revealed right sided pelvicalyceal fullness. The patient was proceeded for myomectomy with a diagnosis of leiyomyomata in mind and proceeded as such but per op findings raised the suspicion of a sarcoma. On table consent was obtained from authorised person and she underwent total abdominal hysterectomy and bilateral salpingo-oophorectomy without nodal dissection. Per-operative findings revealed extensive adhesions between the uterine mass and abdominal musculature, omentum, intestine and appendix. There were no visible endometriotic implants, ascites or any other significant pelvic pathology. Appendectomy, adhenolysis and bilateral Double J stenting was also done. The patient was transfused 6 FFPs and 1 PRBCs per operatively due to intra-operative bleeding. The decision regarding transfusion was made by the anesthesiologist. Post-operatively the patient remained vitally stable and was discharged on the second post-operative day.
Post-operative Gross examination revealed an enlarged uterus measuring 9 × 6 × 6 cm which contained two subserosal leiyomyomas having areas of necrosis and haemorrhage. The larger subserosal leyiomyoma measured 10 × 9 × 6 cm and the smaller measured 9 × 7 × 6 cm. A separately lying tan brown tissue was also identified which measured 10 × 6 × 6 cm. Endometrial thickness measured 0.3 cm whereas myometrial thickness was 1.5 cm. Six intramural leiyomyomas were also identified each measuring 0.5 × 0.4 × 0.4 cm. Polypoidal tissue was also identified in the endometrial cavity measuring 1.5 × 1 cm. The appendix was unremarkable grossly.
Microscopic examination showed a biphasic tumour comprising of glandular and stromal elements. The endometrium was secretory and unremarkable. The tumor also did not show any myometrial invasion but instead arose from a previous adenomyoma of subserosal nodule. The sections from the subserosal nodule showed adenomyoma composed of endometrial glands interspersed in the background of leiomyoma. However there were few areas of adenosarcoma having “phyllodes- like” architecture on low power microscopy. There were leaf- like, slit shaped glands with benign looking mullerian type lining. Subepithelial tissue showed proliferation of stromal cells with high cellularity, moderate to severe nuclear pleomorphism and few very large nuclei. Mitosis was up to 6/10HPFs. This sarcomatous overgrowth was limited to subepithelial areas. CD10 immunostaining highlighted the stromal areas. P53 was positive in high grade areas. The tumor cells were positive for Estrogen & progesterone receptors. There is no invasion in the myometrium. The tumor is limited to subserosal adenomyoma. Both ovaries and fallopian tubes were also normal looking.
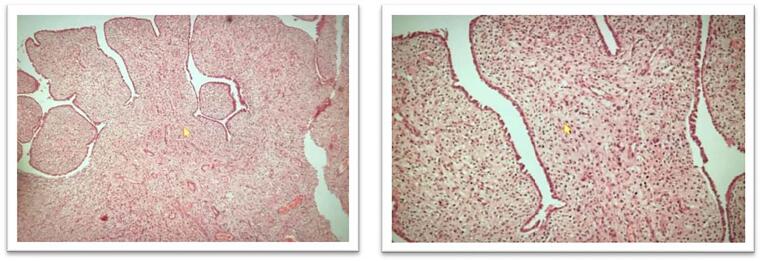
There was no evidence of any tissue, organ or lymphovascular invasion. The surgical margins were uninvolved by the sarcoma. Immunohistochemistry showed the stroma was positive for estrogen and progesterone receptors, as well as positive for p53 gene in high grade areas. CD10 and CyclinD1 was also positive. The histologic findings favoured a diagnosis of high-grade adenosarcoma arising from a subserosal adenomyoma (See Fig. 3, Fig. 4).
Fig. 3.
Subepithelial tissue showing proliferation of stromal cells with high cellularity, moderate to severe nuclear pleomorphism and few very large nuclei. Mitosis is up-to 6/10hpf.
Fig. 4.
The sections from a subserosal nodule show an adenomyoma composed of endometrial glands interspersed in the background of leiomyoma. There are few areas having phyllodes- like architechture. There are leaf- like, slit shaped glands with benign looking mullerian type lining. There is no invasion in the myometrium. The tumor is limited to subserosal adenomyoma.
The patient was followed up two weeks after surgery, oncology team was taken on board and patient was further evaluated with a full body PET-CT scan, which was unremarkable. The patient has been following up with regular physical examinations and imaging with no evidence of recurrence or metastasis. She is pain-free, asymptomatic and enjoying a good quality of life.
3. Discussion
The patient that presented to our center was a young 37 years old female, with no known co-morbids who was diagnosed as a case of uterine fibroids. Other studies have varied age ranges (from 20 years of age to 53 years of age) (WG; TKLNYMC) and parities. Ours is the only patient with adenosarcoma reported as nulliparous. Her presenting complaints included backache, lower abdominal pain, left flank pain and urinary discomfort. Other cases in literature cite menstrual irregularities such as menorrhagia and vaginal bleeding as a recurrent feature (Elshafie et al., 2013, WG; TKLNYMC). Our patient did not report any menstrual symptoms. Only one other study by Lee et al (Lee and Park, 2017) reports a suprapubic mass as in our case. The overall prognosis reported seems promising as none of the cases cited in literature have reported a recurrence, including our patient 12 months post-operatively (WG; TKLNYMC).
Laboratory testing does not reveal any significant findings; CA-125 may be raised as indicated by Talia et al (WG; TKLNYMC) and was also raised in our case. Imaging modalities are unreliable in its diagnosis as adenosarcomas arising from a background of adenomyosis can be tough to distinguish using cross-sectional imaging due to extensive adenomyomata concealing the plane of view.
Histologically speaking, the stromal portion, usually low grade, is made up of spindled or round cells with scanty cytoplasm, but it can be high grade rarely. The pathognomic feature of adenosarcoma is called ‘periglandular cuffing’ or ‘cambium layer’ which is characterised by intraglandular stromal protrusions which are located circumferentially across the glands. The 'cuffing' may be narrow (and hence missed), but it is the location where nucleoli most exhibit atypia. The cambium layer is also the site for the maximum mitoses (Elshafie et al., 2013).
The other cases in literature pertaining to adenosarcomas arise in an intramural adenomyoma with significant myoinvasion (WG; TKLNYMC). There is only one study in literature that investigates the presence of an adenosarcoma arising in a subserosal adenomyoma (Elshafie et al., 2013) reported by El Shafie et al. That case showed similar ultrasonographic findings of multiple subserosal myomata. The patient reported by them was also proceeded for myomectomy and had a suspicion of neoplasm raised intra-operatively in the manner similar to ours. They subsequently performed a total hysterectomy and bilateral salpingo-oopherectomy with pelvic lymphadenectomy. Our approach was the same but we did not perform nodal dissection. Unfortunately we could not take photographs of the intra-operative findings but we highly recommend all clinicians having suspicions of a malignancy to obtain consent for intra-operative photography.
Our experience with the case leads us to the conclusion that we must keep a high index of suspicion when operating upon leiyomyomata. Since there is no pathognomic clinical feature or sign on physical examination, the only time we can potentially pick the diagnosis before histopathology is during surgery. If it is missed by the surgeon, the onus is then on the histopathology team. These points make it difficult to diagnose, hence the scarcity in literature regarding this phenomenon. We need more reporting of this tumour so we can identify the missing link: is there a pattern to this malignancy that we are missing? The authors hope this case helps to shed light on this rare cancer and helps clinicians make the best decisions for their patients.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request
Potential reviewers
-
1.
Maliha Aziz, Shifa Clinical Research Center, Shifa International Hospitals Ltd, H-8/4, Islamabad, maliha.aziz@shifa.com.pk
-
2.
Sumaira Gulzar, Shifa Clinical Research Center, Shifa International Hospitals Ltd, H-8/4, Islamabad, sumairagulzar2050@gmail.com
CRediT authorship contribution statement
Shazia Fakhar: Conceptualization, Validation, Resources, Supervision. Tehreem Zahid: Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. Yamina Ishtiaq: Investigation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Tehreem Zahid, Email: dr.tehreemzahid@gmail.com.
Yamina Ishtiaq, Email: yamina.ishtiaq@icloud.com.
References
- Clarke B.A., Mulligan A.M., Irving J.A., McCluggage W.G., Oliva E. Müllerian adenosarcomas with unusual growth patterns. Int. J. Gynecol. Pathol. 2011;30(4):340–347. doi: 10.1097/PGP.0b013e31820b341e. [DOI] [PubMed] [Google Scholar]
- Elshafie M., Rahimi S., Ganesan R., Hirschowitz L. Müllerian adenosarcoma arising in a subserosal adenomyoma. Int. J. Surg. Pathol. 2013;21(2):186–189. doi: 10.1177/1066896912453852. [DOI] [PubMed] [Google Scholar]
- Karateke A., Kahramanoglu I., Bilgic R. Extragenital Müllerian Adenosarcoma in the Pouch of Douglas. Balkan Medical J. 2014;33(1):100–104. doi: 10.5152/balkanmedj.2013.9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.Y., Park J.Y. A rare case of intramural adenosarcoma arising from adenomyosis of the uterus. J. Pathol. Translational Med. 2017;51:433–440. doi: 10.4132/jptm.2017.06.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluggage W. Mullerian adenosarcoma of the female genital tract. Adv. Anatomic Pathol. 2010;17(2):122–129. doi: 10.1097/PAP.0b013e3181cfe732. [DOI] [PubMed] [Google Scholar]
- McCluggage W.G. A practical approach to the diagnosis of mixed epithelial and mesenchymal tumours of the uterus. Mod. Pathol. 2016;29(S1):S78–S91. doi: 10.1038/modpathol.2015.137. [DOI] [PubMed] [Google Scholar]
- Nathenson, M., Ravi, V., Fleming, N., Wang, W., Conley, A., 2016. Uterine adenosarcoma: a review. Curr. Oncol. Rep. 18(11). [DOI] [PubMed]
- Shi Y., Liu Z., Peng Z., Liu H., Yang K., Yao X. The diagnosis and treatment of Mullerian adenosarcoma of the uterus. Aust. N. Z. J. Obstet. Gynaecol. 2008;48(6):596–600. doi: 10.1111/j.1479-828X.2008.00914.x. [DOI] [PubMed] [Google Scholar]
- Tse K.Y., Crawford R., Ngan H.Y.S. Staging of uterine sarcomas. Best Practice & Res. Clin. Obstetrics & Gynaecol. 2011;25(6):733–749. doi: 10.1016/j.bpobgyn.2011.05.011. [DOI] [PubMed] [Google Scholar]
- WG; TKLNYMC. Uterine adenosarcoma originating in adenomyosis: Report of an extremely rare phenomenon and review of published literature [Internet]. Int. J. Gynecol. Pathol.: Official J. Int. Soc. Gynecol. Pathologists. U.S. National Library of Medicine; [cited 2022Jan31]. Available from: https://pubmed.ncbi.nlm.nih.gov/32947330/. [DOI] [PubMed]