Abstract
We evaluated the in vitro and in vivo potencies of a new lipid nanosphere that incorporates amphotericin B (AmB), NS-718, against Aspergillus fumigatus. The in vitro activity of NS-718 (the MIC at which 90% of strains are inhibited [MIC90], 0.25 μg/ml) against 18 isolates of A. fumigatus was similar to that of deoxycholate AmB (D-AmB; Fungizone; MIC90, 0.25 μg/ml), but NS-718 was more potent than liposomal AmB (L-AmB; AmBisome; MIC90, 1.0 μg/ml). The in vivo efficacy of NS-718 in a rat model of invasive pulmonary aspergillosis was compared with those of D-AmB and L-AmB. A low dose (1 mg/kg of body weight) of L-AmB was ineffective (survival rate, 0%), although equivalent doses of D-AmB and NS-718 were more effective (survival rate, 17%). However, a higher dose of NS-718 (3 mg/kg) was more effective (survival rate, 100%) than equivalent doses of D-AmB and L-AmB (survival rate, 0%). To explain these differences, pharmacokinetic studies showed higher concentrations of AmB in the plasma of rats treated with NS-718 than in the plasma of those treated with D-AmB. Our results suggest that NS-718, a new preparation of AmB, is a promising antifungal agent with activity against pulmonary aspergillosis.
The incidence of deep-seated mycosis has increased, probably due to an increase in the number of compromised hosts, e.g., patients with human immunodeficiency virus infection or patients who have undergone organ transplantation. Despite the introduction of new azole antifungal agents, such as fluconazole and itraconazole, amphotericin B (AmB) is still the first choice for the treatment of severe and refractory mycoses, particularly for invasive pulmonary aspergillosis (IPA). However, the clinical usefulness of AmB is often limited due to its adverse effects, including its nephrotoxicity. In order to improve the therapeutic activity and lower the toxicity of AmB, new drug delivery systems such as the lipid microsphere (LM) that incorporates AmB (LM-AmB) (9), liposomal AmB (L-AmB; AmBisome) (6, 10, 13), AmB colloidal dispersion (Amphocil) (3), and AmB lipid complex (Abelcet) (1) have been developed in the last 10 years. These new forms of AmB are less toxic than deoxycholate AmB (D-AmB; Fungizone).
The lipid nanosphere (LNS), a new drug career, is composed of purified soybean oil and purified egg yolk lecithin, and its structure and composition are similar to those of LM, which is used as a carrier of prostaglandins (15), steroids (16), and certain anti-inflammatory drugs (17). LNS is smaller (25 to 50 nm) than LM (200 nm). LNS was developed as a drug carrier by Nippon Shinyaku Co., Ltd., Kyoto, Japan (20).
In the present study, we investigated the efficacy of an LNS that incorporates AmB (NS-718) in vitro and in vivo in experimental IPA caused by Aspergillus fumigatus.
MATERIALS AND METHODS
Fungal strains, antifungal agents, and MIC measurement.
We used 18 clinical isolates of A. fumigatus isolated from the sputum of patients with pulmonary aspergillosis at Nagasaki University Hospital. The Aspergillus strains were identified by morphological methods and electrophoretic comparison of enzymes (12). D-AmB (Fungizone; Bristol-Myers Squibb K.K., Tokyo, Japan), L-AmB (AmBisome; NeXstar Pharmaceuticals, Cambridge, United Kingdom), and NS-718 (Nippon Shinyaku Co., Ltd.) were used in this study. NS-718 is a lyophilized preparation and is composed of 10 mg of AmB, 1 g of soybean oil, 1 g of purified egg yolk lecithin, and 2 g of maltose in a vial. After reconstitution with water, the average particle diameter was 25 to 50 nm, as determined by laser dynamic scattering analysis. D-AmB, L-AmB, and NS-718 were dissolved in sterile distilled water.
The fungal isolates were cultured on potato dextrose agar plates (Nissui Seiyaku, Tokyo, Japan) at 30°C for 2 weeks. The spores were harvested with 1 ml of sterile saline containing 0.05% Tween 80 (Wako Chemical, Tokyo, Japan). The final concentration of the spores in the inoculum was adjusted to 3 × 103 CFU/ml. The MICs of the antifungal agents were determined by a microdilution method modified from the method of the National Committee for Clinical Laboratory Standards (18) by using a round-bottom 96-well plate. The plates were incubated at 30°C for 72 h and the endpoint was defined as the point at which no growth was observed.
In vivo efficacy in rats with IPA.
Male Sprague-Dawley rats (age, 5 weeks) were purchased from Charles River Japan (Yokohama, Japan). Experimental IPA was induced in rats by the method described in our previous report (14). Briefly, the rats were immunosuppressed by subcutaneous injection of 150 mg of cortisone acetate (Wako Pure Chemical Industries, Osaka, Japan) per kg of body weight three times per week and were provided a low-protein diet (8% protein diet; Oriental Yeasts Industries, Chiba, Japan). Cortisone was continued for 1 week before and after inoculation (i.e., 1 week prior to antifungal therapy). The low-protein diet was continuously provided until the end of the study (28 days after inoculation). Tetracycline hydrochloride (250 mg/800 ml; Achromycin; Lederle Japan, Tokyo, Japan) was added to the drinking water throughout the experiment to prevent bacterial infection. A. fumigatus MF-13, which was isolated from the sputum of a patient with pulmonary aspergilloma, was used for infection. The experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of Nagasaki University School of Medicine.
Fungal isolates were cultured on potato dextrose agar plates at 30°C for 4 days, and the conidia were harvested with 0.02% Tween 80. After the conidia were washed, they were suspended in sterile saline and counted in a hemocytometer. Three days after the third cortisone acetate injection, the immunosuppressed rats were infected by intratracheal inoculation of 105 spores in 0.1 ml of saline by tracheostomy while they were under general anesthesia with enflurane (Ethrane; Abbott Laboratories, Chicago, Ill.). One hundred microliters of D-AmB, L-AmB, NS-718, or 5% dextrose (as control) was injected through the lateral tail vein once daily for 8 days starting at 2 h after inoculation. For mycological studies, the animals were killed after 1 or 3 days of inoculation, and the lungs were removed and homogenized and were then suspended in sterile saline. One hundred microliters of the suspension was inoculated onto Sabouraud dextrose agar (BBL, Cockeysville, Md.), and the plates were incubated at 30°C for 48 h, followed by counting of the colonies.
Concentration of AmB in plasma.
D-AmB, L-AmB, or NS-718 was administered (at 3.0 mg/kg) on the third day after infection, and a laparotomy was performed while the rats were under general anesthesia to exsanguinate and collect the whole blood through the inferior vena cava. Determination of the AmB concentration by high-pressure liquid chromatography (HPLC) was based on the method of Granich et al. (5), with some modifications. Plasma samples (0.1 ml) were combined with 1.0 ml of methanol containing 1.0 μg of the internal standard 1-amino-4-nitronaphthalene (Aldrich, Milwaukee, Wis.) per ml, and the components were mixed by vortexing. After centrifugation at 3,000 rpm for 10 min, the supernatant was dried under reduced pressure followed by redissolution with 0.2 ml of methanol for injection into the high-pressure liquid chromatograph. The high-pressure liquid chromatography system consisted of an SLC-10A system controller, an LC-10AD pump, an SIL-10A autosampler with a 20-μl sampler loop, an SCL-10A UV-visible detector set at 408 nm, a CTO-10AC column oven kept at 40°C, and a C-R5A chromatopac data station (Shimadzu, Kyoto, Japan). Analysis was performed with an L-column ODS (4.6 by 150 mm; Chemicals Inspection and Testing Institute, Tokyo, Japan) equipped with a LiChroCART guard cartridge (E. Merck, Darmstadt, Germany). The mobile phase was a mixture of acetonitrile and 10 mM sodium acetate buffer (pH 4.0) (11:17; vol/vol), and the flow rate was 1.0 ml/min. The concentration of AmB was determined from the ratio between the peak height of AmB to that of the internal standard.
Biochemical study with immunosuppressed rats.
The rats (Sprague-Dawley male rats; age, 5 weeks) in each group were immunosuppressed but not infected and were treated by the regimen (AmB at 3.0 mg/kg intravenously daily) for 8 days. Laparotomy was done 24 h after the last injection while the rats were under general anesthesia. Blood was collected from the inferior vena cava for measurement of serum glutamic oxalacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), blood urea nitrogen (BUN), and creatinine levels by standard procedures.
Statistical analysis.
Data are expressed as means ± standard deviations. The effect of treatment on the survival rate was tested by the generalized Wilcoxon test. Differences in the number of fungi between organs were tested by Scheffe’s multiple comparison test. Differences in the serum biochemical profiles were tested by Student’s t test or the Welch test. A P value of <0.05 was considered statistically significant.
RESULTS
In vitro activity of NS-718.
The MICs of D-AmB, L-AmB, and NS-718 for 18 isolates of A. fumigatus are presented in Table 1. The MIC of NS-718 (the MIC at which 90% of strains are inhibited [MIC90], 0.25 μg/ml) was similar to that of D-AmB (MIC90, 0.25 μg/ml) and was fourfold less than that of L-AmB (MIC90, 1.0 μg/ml).
TABLE 1.
In vitro antifungal activities of NS-718, D-AmB, and L-AmB against 18 clinical isolates of A. fumigatus
| Drug | MIC (μg/ml)a
|
||
|---|---|---|---|
| Range | 50% | 90% | |
| NS-718 | 0.0313–0.25 | 0.125 | 0.25 |
| D-AmB | 0.0625–0.50 | 0.25 | 0.25 |
| L-AmB | 0.0625–1.0 | 0.25 | 1.0 |
The MICs were determined by the microdilution method modified from the macrodilution method of the National Committee for Clinical Laboratory Standards. The MICs were measured three times.
In vivo activity of NS-718 against IPA.
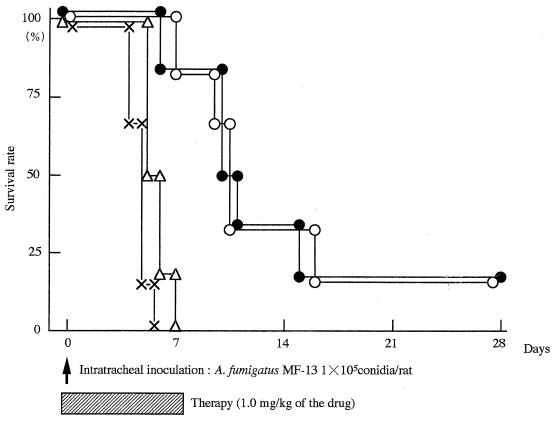
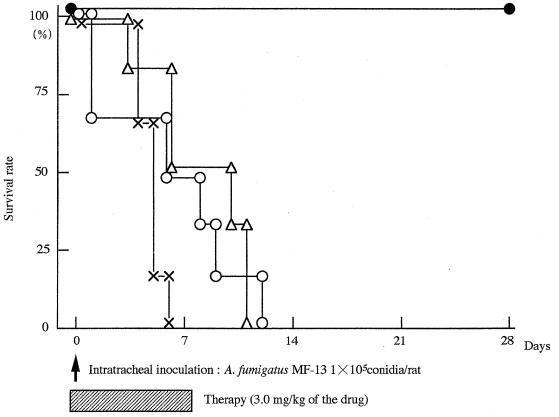
As shown in Fig. 1, all control rats died within 6 days after infection. The survival rate was less than 20% in all groups when a low dose of the drug (1.0 mg/kg) was used. NS-718 was significantly more effective than L-AmB (survival rate, 0%; P < 0.05). The efficacy of the high-dose therapy (3.0 mg/kg) is indicated in Fig. 2. NS-718 was significantly more effective (survival rate, 100%) than D-AmB (survival rate, 0%; P < 0.05) and L-AmB (survival rate, 0%; P < 0.05).
FIG. 1.
Survival rates for rats with IPA treated with an intravenous injection of 5% dextrose (×), NS-718 (●), D-AmB (○), or L-AmB (▵) (dose, 1.0 mg of AmB per kg of body weight). Six rats were used in each group. The study of the survival of rats with IPA was repeated twice.
FIG. 2.
Survival rates for rats with IPA treated with an intravenous injection of 5% dextrose (×), NS-718 (●), D-AmB (○), or L-AmB (▵) (dose, 3.0 mg of AmB per kg of body weight). Six rats were used in each group. The study of the survival of rats with IPA was repeated twice.
Table 2 indicates the number of aspergilli in the lungs of rats with IPA at 24 and 72 h after inoculation. At 24 h after inoculation, the numbers of aspergilli in the lungs of rats treated with L-AmB were similar to those in the lungs of control rats. The cell count in rats treated with NS-718 after the same time interval was significantly lower than that in control rats. The numbers of aspergilli in the lungs of rats 72 h after treatment with L-AmB were significantly lower than the numbers in control rats. The cell counts in NS-718- or D-AmB-treated rats after the same time period were significantly lower than the cell counts in those treated with L-AmB.
TABLE 2.
Number of A. fumigatus organisms in the lungs of rats with experimental IPA
| Treatment | No. of aspergilli (log10 CFU/g) at the following times postinoculationa:
|
|
|---|---|---|
| 24 h | 72 h | |
| Control | 3.95 ± 0.39 | 4.53 ± 0.31 |
| NS-718 | 3.11 ± 0.35b | 1.90 ± 0.24bc |
| D-AmB | 3.43 ± 0.54 | 1.87 ± 0.30bc |
| L-AmB | 3.91 ± 0.56 | 3.55 ± 0.31b |
Data are the mean ± standard deviation number of aspergilli in the lungs at 24 and 72 h after inoculation into rats treated with the indicated forms of AmB for 3 days. A dose of 3.0 mg of each form per kg was injected intravenously into each of six rats in each group.
P < 0.05 compared with the results for the respective control rats.
P < 0.05 compared with the results for the respective L-AmB-treated rats (Sheffe’s test).
Concentration of AmB in the plasma of rats with IPA.
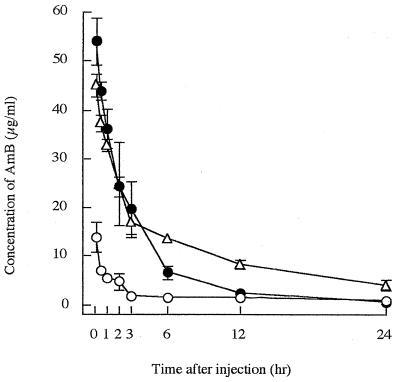
The concentration of AmB in plasma measured 3 h after a single administration of 3 mg of NS-718 per kg was higher than that measured 3 h after the administration of D-AmB, but the concentration decreased 12 h after injection and was similar to that in D-AmB-treated rats. However, the plasma AmB concentration in L-AmB-treated rats tended to be higher than those of the two other forms of AmB 6 h after injection (Fig. 3).
FIG. 3.
Concentrations of AmB in plasma after a single administration of one of three forms of AmB (3.0 mg/kg) to rats with IPA. ●, NS-718; ○, D-AmB; ▵, L-AmB.
Study of the serum biochemical profile for immunosuppressed rats.
The results of the study of the serum biochemical profile for immunosuppressed rats treated with NS-718, D-AmB, or L-AmB are summarized in Table 3. Renal insufficiency was evident in rats treated with D-AmB. In the rats in the NS-718-treated group, BUN and creatinine levels were increased compared with those in the rats in the control and L-AmB-treated groups. The highest elevations of SGOT and SGPT levels were observed in rats after the administration of L-AmB; however, the difference was not significant compared with the values for the control group.
TABLE 3.
Serum biochemical profiles for rats treated for 8 days by injection of NS-718, L-AmB, or D-AmBa
| Drug | No. of rats | SGOT level (mU/ml) | SGPT level (mU/ml) | BUN level (mg/ml) | Creatinine level (mg/ml) |
|---|---|---|---|---|---|
| Control | 6 | 437 ± 209 | 419 ± 220 | 11.3 ± 2.8 | 0.14 ± 0.04 |
| NS-718 | 6 | 581 ± 247 | 568 ± 231 | 34.9 ± 13.2b | 0.30 ± 0.10c |
| D-AmB | 5 | 388 ± 185 | 314 ± 149 | 63.3 ± 24.1b | 0.40 ± 0.07c |
| L-AmB | 5 | 660 ± 375 | 651 ± 287 | 16.7 ± 4.0c | 0.17 ± 0.06 |
Data are expressed as means ± standard deviations.
P < 0.05, compared with the results for the respective control rats (Welch test).
P < 0.05 compared with the results for the respective control rats (Student’s t test).
DISCUSSION
In the present study, we compared the in vitro and in vivo potencies of NS-718, D-AmB, and L-AmB against A. fumigatus. The A. fumigatus strains were more susceptible to NS-718 that to L-AmB; i.e., a smaller dose of NS-718 was required to inhibit fungal growth. In our previous study, among three AmB formulations NS-718 was found to be the most efficacious against Cryptococcus neoformans isolates in vitro (7). NS-718 was also found to be more effective than D-AmB or L-AmB against clinical isolates of Candida albicans in our previous study (8); thus, NS-718 has a broad spectrum of antifungal activity. Our results in this study indicate that NS-718 was efficacious in the treatment of rat IPA, which correlates with the results of the in vitro study. NS-718 at a dose of 3.0 mg/kg inhibited the growth of aspergilli in the lung, but the antifungal activity of L-AmB was weaker than that of NS-718.
It is necessary to study the toxicity of NS-718 in mammalian cells since at a high dosage AmB has harmful side effects. Lysis of erythrocytes has occasionally been observed with D-AmB but not with NS-718 (4). This phenomenon indicates that the effective therapeutic dose of NS-718 used for the treatment of deep-seated mycoses, including aspergillosis, has a low level of toxicity. According to the results obtained from study of the serum biochemical profile, the level of renal insufficiency was the highest in D-AmB-treated rats. We speculated that all rats died rapidly after therapy with a high dose of D-AmB (3.0 mg/kg) because of the acute toxicity of AmB, but equivalent doses of NS-718 were well tolerated, indicating a reduction in toxicity with the lipid formulation.
One of the reasons for the differences in the antifungal potencies and toxicities among AmB formulations was suggested by Espuelas et al. (2). Their formulation, which is an AmB-poly (ɛ-caprolacton) nanosphere, was less toxic than D-AmB and was more toxic than L-AmB. Their preliminary results indicated that their nanoparticles containing AmB were more potent in vitro against C. albicans than D-AmB. They mentioned that if the high degree of stability of L-AmB can explain its lower level of toxicity, it is also responsible for the decreased efficacy reported by several investigators (11, 19, 21).
In terms of the efficacy of NS-718 against fungal cells, we also speculated that the facilitation of AmB release and contact with the fungal cell surface might improve the MIC and potency of the lipid formulation. The release of AmB from L-AmB might be slow and slight because the transition temperatures of the gel-liquid crystal phases of the lipids used in L-AmB, hydrogenated soy lecithin and distearoylphosphatidylglycerol, are higher than the body temperature. This means that the liposomal particles of L-AmB are relatively rigid and tight at the body temperature and hold AmB molecules within the liposomal particles. In contrast, because NS-718 is composed of soybean oil and egg lecithin as liquid-like lipid particles, with the release of AmB from the lipid particles, it may be easy for the AmB to move to fungal cells, which have ergosterol as a membrane component, and it may be relatively hard for the AmB to move to mammalian cells, which do not contain ergosterol as a membrane component. Further investigations of physicochemical preparations should be done to confirm this hypothesis.
In the pharmacokinetic study, the concentrations of NS-718 in plasma were higher than those of D-AmB. The pharmacokinetic character of NS-718 suggested that a lower dose of AmB encapsulated in LNS could be as efficacious as D-AmB for the treatment of IPA. In our previous study, the concentration of AmB in pleural exudate after the intravenous injection of NS-718 was higher than that obtained after the injection of D-AmB (4, 20). The results indicated that NS-718 was retained in the blood circulation and easily permeated leaky blood vessels at the site of inflammation by a mechanism called the passive targeting effect. Although the injection of L-AmB resulted in high plasma AmB concentrations, a higher dose of L-AmB might be required for efficacy similar to that of NS-718 for the treatment of IPA in rats because of the low intrinsic potency of L-AmB, as discussed above.
NS-718 was less nephrotoxic than D-AmB but was more toxic than L-AmB in our study. However, SGOT and SGPT levels increased in rats injected with L-AmB. The reason for the liver insufficiency could not be explained clearly. In rats injected with NS-718, SGOT and SGPT levels increased less than those in rats injected with L-AmB, and there was no statistical difference compared with the results for the control rats.
In conclusion, the results obtained in the present study suggest that NS-718 may represent a more appropriate choice for patients with aspergillosis because of the good balance between efficacy and toxicity. Because NS-718 was more efficacious than L-AmB and had a lower level of toxicity than D-AmB, NS-718 may serve as an effective novel antifungal drug delivery system for the treatment of patients with IPA.
ACKNOWLEDGMENTS
We thank Yoshitsugu Miyazaki for academic support. We also thank F. G. Issa, Department of Medicine, University of Sydney, Sydney, Australia, for careful reading and editing of the manuscript.
REFERENCES
- 1.Clark J M, Whitney R R, Olsen S J, George R J, Swerdel M R, Kunselman L, Bonner D P. Amphotericin B lipid complex therapy of experimental fungal infections in mice. Antimicrob Agents Chemother. 1991;35:615–621. doi: 10.1128/aac.35.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Espuelas M S, Legrand P, Irache J M, Gamazo C, Orecchioni A M, Devissaguet J P, Ygartua P. Poly (ɛ-caprolacton) nanoparticles as an alternative way to reduce amphotericin B toxicity. Int J Pharm. 1997;158:19–27. [Google Scholar]
- 3.Fielding R M, Smith P C, Wang L H, Porter J, Guo L S. Comparative pharmacokinetics of amphotericin B after administration of a novel colloidal delivery system, ABCD, and a conventional formulation to rats. Antimicrob Agents Chemother. 1991;35:1208–1213. doi: 10.1128/aac.35.6.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukui H, Koike T, Saheki A, Sonoke S, Yoshikawa H, Sasaki H, Tomii Y, Seki J. Proceedings of the 23rd International Symposium on Controlled Release of Bioactive Materials. 1996. A novel antifungal drug delivery system: lipid nanosphere incorporating amphotericin B (LNS-AmB), abstr. 5026; p. 655. [Google Scholar]
- 5.Granich G G, Kobayashi G S, Krogstad D J. Sensitive high-pressure liquid chromatographic assay for amphotericin B which incorporates an internal standard. Antimicrob Agents Chemother. 1986;29:584–588. doi: 10.1128/aac.29.4.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hay R J. Liposomal amphotericin B, AmBisome. J Infect. 1994;28(Suppl. I):35–43. doi: 10.1016/s0163-4453(94)95956-0. [DOI] [PubMed] [Google Scholar]
- 7.Hossain M A, Maesaki S, Kakeya H, Noda T, Yanagihara K, Sasaki E, Hirakata Y, Tomono K, Tashiro T, Kohno S. Efficacy of NS-718, a novel lipid nanosphere-encapsulated amphotericin B, against Cryptococcus neoformans. Antimicrob Agents Chemother. 1998;42:1722–1725. doi: 10.1128/aac.42.7.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohno S, Otsubo T, Hara K, Tomii Y, Seki J. Program and abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. A new antifungal drug delivery system, lipid nanosphere encapsulating amphotericin B (LNS-AmB), its evaluation in the rat model of invasive pulmonary aspergillosis, abstr. 109; p. 131. [Google Scholar]
- 9.Kohno S, Hara K, Murahashi N, Watanabe T. Amphotericin B incorporated in lipid emulsion (lipid microsphere) In: Yamaguchi H, Kobayashi G S, Takahashi H, editors. Recent progress in antifungal chemotherapy. New York, N.Y: Marcel Dekker, Inc.; 1991. pp. 333–339. [Google Scholar]
- 10.Kohno S, Miyazaki T, Yamaguchi K, Tanaka H, Hayashi T, Hirota M, Saito A, Hara K, Sato T, Sunamoto J. Polysaccharide-coated liposomes with antimicrobial agents against intracytoplasmic pathogens and fungi. J Bioact Compat Polym. 1988;3:137–147. [Google Scholar]
- 11.Legrand P, Romero E A, Cohen B E, Bolard J. Effect of aggregation and solvent on the toxicity of amphotericin B to human erythrocytes. Antimicrob Agents Chemother. 1992;36:2518–2522. doi: 10.1128/aac.36.11.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuda H, Kohno S, Maesaki S, Yamada H, Koga H, Tamura M, Kuraishi H, Sugiyama J. Application of ubiquinone systems and electrophoretic comparison of enzymes to identification of clinical isolates of Aspergillus fumigatus and several other species of Aspergillus. J Clin Microbiol. 1992;30:1999–2005. doi: 10.1128/jcm.30.8.1999-2005.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitsutake K, Kohno S, Miyazaki Y, Noda T, Miyazaki H, Miyazaki T, Kaku M, Koga H, Hara K. In vitro and in vivo antifungal activities of liposomal amphotericin B, and amphotericin B lipid complex. Mycopathologia. 1994;128:13–17. doi: 10.1007/BF01104273. [DOI] [PubMed] [Google Scholar]
- 14.Miyazaki H, Kohno S, Miyazaki Y, Mitsutake K, Tomono K, Kaku M, Koga H, Hara K. Efficacy of intravenous itraconazole against experimental pulmonary aspergillosis. Antimicrob Agents Chemother. 1993;37:2762–2765. doi: 10.1128/aac.37.12.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mizushima Y. Lipo-prostaglandin preparations. Prostaglandins Leukot Essent Fatty Acids. 1991;42:1–6. doi: 10.1016/0952-3278(91)90058-d. [DOI] [PubMed] [Google Scholar]
- 16.Mizushima Y, Hamano T, Yokoyama K. Use of a lipid emulsion as a novel carrier for corticosteroids. J Pharm Pharmacol. 1982;34:49–50. doi: 10.1111/j.2042-7158.1982.tb04678.x. [DOI] [PubMed] [Google Scholar]
- 17.Mizushima Y, Wada Y, Etoh Y, Watanabe K. Anti-inflammatory effects of indomethacin ester incorporated in a lipid microsphere. J Pharm Pharmacol. 1983;35:398–399. doi: 10.1111/j.2042-7158.1983.tb02969.x. [DOI] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 19.Pahls S, Schaffner A. Comparison of the activity of free and liposomal amphotericin B in vitro and in a model of localized murine candidiasis. J Infect Dis. 1994;169:1057–1061. doi: 10.1093/infdis/169.5.1057. [DOI] [PubMed] [Google Scholar]
- 20.Seki J, Sasaki H, Doi M, Yoshikawa H, Takahashi Y, Yamane S, Fukui H, Sonoke S, Yamamoto H, Hirose M, Ezure Y, Ando T, Ushimaru K, Sugiyama M. Lipid nanosphere (LNS), a protein free analogue of lipoproteins, as a novel drug carrier for parenteral administration. J Controlled Release. 1994;28:352–353. [Google Scholar]
- 21.Van Etten E W M, Van den Heuvel-de Groot C, Bakker-Woudenberg I A J M. Efficacies of amphotericin B-deoxycholate (Fungizone), liposomal amphotericin B (AmBisome) and fluconazole in the treatment of systemic candidosis in immunocompetent and leukopenic mice. J Antimicrob Chemother. 1993;32:723–739. doi: 10.1093/jac/32.5.723. [DOI] [PubMed] [Google Scholar]