Abstract
Purpose
There are reports concerning mucus plugs detected on high-resolution computed tomography images and airflow obstruction in asthma and chronic obstructive pulmonary disease (COPD). However, little is known about the associations between mucus plugs and small airway dysfunction (SAD). We evaluated the relationship between mucus plugs and pulmonary function in patients with asthma, COPD, and asthma-COPD overlap (ACO), and investigated the relevance to SAD and type 2 inflammation in a retrospective study.
Methods
Subjects included 49 asthmatic, 40 ACO, and 41 COPD patients. ACO was diagnosed based on the Japanese Respiratory Society ACO guidelines. Clinical and laboratory parameters, including blood eosinophil count, serum total IgE levels, fractional exhaled nitric oxide (FeNO), spirometry, and forced oscillation technique (FOT), were compared between patients with and without mucus plugs.
Results
Mucus plugs were found in 29 (59%) asthmatic, 25 (65%) ACO, 17 (41%) COPD patients. Patients with mucus plugs had reduced spirometry and larger FOT parameters, especially in COPD patients. Mucus scores correlated positively with IgE in ACO and FeNO in asthmatic patients, but not in COPD patients. Multivariate logistic regression analysis revealed that SAD parameters, including forced vital capacity and resonant frequency, a respiratory reactance parameter, were significantly associated with the presence of mucus plugs in the whole studied population.
Conclusions
SAD, rather than large airway dysfunction, was associated with mucus plugs in asthma, ACO, and COPD patients.
Keywords: Asthma, chronic obstructive pulmonary disaese; diagnostic imaging; physiology
INTRODUCTION
An increasing number of reports show that mucus plugs are associated with airflow obstruction in asthma and chronic obstructive pulmonary disease (COPD).1,2,3,4 A previous study developed a method of quantifying mucus plugs based on the visual assessment of high-resolution computed tomography (HRCT) lung images in severe asthma and found that mucus score correlated negatively with forced expiratory volume in 1 second (FEV1) and positively with sputum eosinophils.1 Subsequently, the same researchers assessed the associations between mucus plugs and emphysema/airflow obstruction in smokers, including COPD patients, and found that the mucus score was associated with lower FEV1, independent of emphysema.4 Another recent study found an inverse relationship between CT scan-identified luminal plugging and FEV1 in COPD patients, but that luminal plugging was more prevalent in patients with emphysema than in those without.3
Although CT scan delineates relatively large airways from the trachea to the 6th generation bronchi, small airway dysfunction (SAD), a clinically relevant role in asthma and the primary site of airflow obstruction in COPD,5,6 can coexist in patients with mucus plugging. Among the physiological tests to explore the relevance and extent of SAD in a recent large cohort asthma study (ATLANTIS),5 we focused on the spirometric indices, including forced vital capacity (FVC) and forced expiratory flow at 25%–75% of FVC (FEF 25–75) and forced oscillation technique (FOT). Although the interpretation is still controversial,7,8 we adopted the forced oscillatory parameters to assess SAD in this study, including the difference between respiratory resistance at 5 and 20 Hz (R5–R20), respiratory reactance at 5 Hz (X5), low-frequency reactance area (ALX), and resonant frequency (Fres). In addition, patients with the clinical features of asthma and COPD, asthma-COPD overlap (ACO), have been a clinical issue in managing asthma and COPD.9 We hypothesized that there might be some differences in clinical features with mucus plugs among asthmatic, ACO, and COPD patients. In this retrospective study, we evaluated mucus plugs based on the visual assessment of CT lung images according to the previous studies1,3,4 and investigated the relevance to pulmonary function, especially SAD and type 2 biomarkers.
MATERIALS AND METHODS
Subjects
We retrospectively collected 130 consecutive patients who attended outpatient clinics at Shizuoka General Hospital for routine checkups and underwent HRCT scans between August 2018 and September 2020. The subjects were classified into 3 groups: asthma (n = 49), COPD (n = 41), and ACO (n = 40) (Fig. 1). Asthmatic and COPD patients fulfilled the definition of the Global Initiative for Asthma9 and the Global Initiative for Chronic Obstructive Lung Disease,10 respectively. ACO patients fulfilled the definition of the Japanese Respiratory Society (JRS) guidelines.11 Specifically, ACO was diagnosed if asthma patients were older than 40 years old, had post-bronchodilator FEV1/FVC < 0.7, and fulfilled at least one of the following criteria, including more than 10 pack-years smoking history, a presence of low attenuation area (LAA) on HRCT, or less than 80% of diffusing capacity of the lung for carbon monoxide (DLco)/alveolar volume (VA). In addition, ACO was diagnosed if COPD patients fulfilled at least 2 of the following criteria, including variable (diurnal, daily, or seasonal) or paroxysmal respiratory symptoms (cough, sputum, or dyspnea), an asthma history before 40 years, or fractional exhaled nitric oxide (FeNO) > 35 ppb, or fulfilled one of these criteria, and at least 2 of the following criteria, including perennial allergic rhinitis, airway reversibility (change in FEV1 > 12% and > 200 mL), blood eosinophil count > 5% or > 300 cells/μL, or elevated total IgE or positive specific IgE antibody (Fig. 1). Patients had been receiving medications, including inhaled or systemic corticosteroids, long-acting β2-agonist, long-acting muscarinic antagonist, leukotriene receptor antagonist, and biologics (benralizumab, omalizumab, or dupilumab). Patients were excluded from the study if they had an exacerbation or had an acute viral infection within at least 1 month at the time of HRCT scan.
Fig. 1. The flow of the subjects.
COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FeNO, fractional exhaled nitric oxide; LAA, low attenuation area; HRCT, high-resolution computed tomography; DLco, diffusing capacity of the lung for carbon monoxide; VA, alveolar volume; ACO, asthma-chronic obstructive pulmonary disease overlap.
Study design
This is a single-center, retrospective study. The Institutional Review Board of the Shizuoka General Hospital approved the study protocols (SGHIRB#20202057). The Board waived patient approval or informed consent because the study was a retrospective review of patient records and images.
The HRCT scans of the 130 patients were analyzed by a well-trained radiologist (S.K.) who was blinded to the clinical information. We also collected patients’ medical records and laboratory data, including blood and sputum eosinophil count, serum IgE levels, FeNO, patient-reported outcome measures, spirometry, and FOT.
HRCT analysis
Airway mucus plugs were identified and quantified using a scoring system as previously reported.1,3,4 Mucus plugs were defined as complete occlusion of an airway. The lung zone within 2 cm from the costal or diaphragmatic pleura was excluded because the airways in that zone are too small to ascertain a complete occlusion by luminal plugs. The mucus score, 0 or 1 based on the absence or presence of mucus plugs, was generated for each CT scan as an aggregation of the number of bronchopulmonary segments with luminal plugging, ranging from 1 to 18.
Tree-in-bud appearance was defined as the centrilobular nodules of 2–4 mm in diameter with peripheral location within 5 mm of the pleural surface. Bronchiectasis was defined as bronchial dilatation with an internal diameter of a bronchus > 150% the diameter of the accompanying pulmonary artery. Emphysema was evaluated automatically according to the Goddard classification reported previously,12 using SYNAPSE VINCENT, a 3D image analysis system (Fujifilm Medical Co., Tokyo, Japan). The lung field was divided into 6 sections: upper, middle, and lower lung fields on each side, and the total score was automatically calculated by classifying the degree of LAA in each region into 5 levels (0–4 points), thus yielding the Goddard score from 0 to 24.
Data collection
Severe asthma was defined according to the European Respiratory Society (ERS)/American Thoracic Society (ATS) severe asthma guidelines.13 Asthma control was assessed by Asthma Control Questionnaire (ACQ)14 and Asthma Control Test (ACT).15 Dyspnea and symptoms of COPD were assessed by the Modified British Medical Research Council (mMRC)16 and COPD Assessment Test (CAT),17 respectively. Sputum analysis was performed in 32 asthmatic, 20 ACO, and 11 COPD patients. For ACO and COPD patients, a cut-point of ≥ 2 for cough and phlegm items defined chronic mucus hypersecretion (CMH) in CAT.18 FeNO was measured by NIOX VERO (Chest M.I. Co. Ltd., Tokyo, Japan). Pulmonary function tests were performed while on daily medications, and measured data are expressed as post-bronchodilator values. We performed ACQ, ACT, mMRC, CAT, FeNO, FOT, and spirometry in that order for patients with asthma, ACO, and COPD as routine management. Spirometry and DLco/VA by the single-breath method were conducted using computerized equipment (model CHESTAC-8800; Chest M.I. Co. Ltd.) according to the ERS/ATS guidelines for spirometry.19 Predicted values for pulmonary function tests were obtained from the JRS guidelines.20 The FOT was measured by MostGraph-01 (Chest M.I. Co. Ltd.) to assess the airflow obstruction during the tidal breathing, which is sometimes different from that performed during forced expiration.21 Oscillatory indices were expressed as mean values during a respiratory cycle. FVC, FEF 25–75, R5–R20, and respiratory reactance parameters, including X5, Fres, and ALX, were interpreted as markers of SAD according to the previous report.5 In contrast, FEV1, FEV1/FVC, and R20 were interpreted as markers of large airways dysfunction. Sputum analysis was performed for patients who could expectorate sputum spontaneously.
Statistical analysis
Categorical variables are summarized as frequencies and proportions, and continuous variables are expressed as medians and interquartile ranges. The Kruskal-Wallis test was used to assess differences among 3 groups with continuous variables, and the Mann-Whitney U test was applied for 2-group comparisons. Fisher’s exact test was used to compare categorical variables between groups. Spearman's correlation coefficients were calculated to determine the associations between mucus score and spirometric and forced oscillatory parameters, blood eosinophil count, total IgE, and FeNO within each of the 3 groups. Univariate logistic regression analysis followed by multivariate logistic regression analysis was used to determine the associations between the presence of mucus plugging and demographic data, pulmonary function, and type 2 biomarkers. Heatmap clustering was drawn by Ward's method based on the Euclidean distance using the R “pheatmap” package. All analyses were performed with R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria). A 2-tailed P value of < 0.05 was considered statistically significant.
RESULTS
Characteristics of the subjects
The flow and clinical characteristics of the subjects are shown in Fig. 1 and Table 1. Forty ACO patients consisted of 22 patients derived from asthma and 18 derived from COPD. Asthma patients were younger, female-dominant, and had lower pack-years than those with ACO or COPD. Although some asthmatic patients had more than 10 pack-years, they did not fulfill FEV1/FVC < 0.7. Nine and 10 asthmatic patients had chronic rhinosinusitis and severe asthma, respectively. Asthma was less controlled in asthmatic patients than ACO patients, while there was no difference in dyspnea and symptoms between ACO and COPD patients. The prevalence of CMH was not high (approximately 20%) and similar in ACO and COPD patients. There was no difference in blood eosinophil count among asthmatic, ACO, and COPD patients. Serum total IgE was lower in COPD patients, while the number of positive specific IgE antibodies was higher in asthmatic patients than in ACO or COPD patients. Sputum eosinophil and FeNO levels were lower in COPD patients. In addition, %FEV1 and FEV1/FVC were higher in asthma patients than in ACO and COPD patients. %FVC was higher, and %FEF 25–75 was lower in COPD patients than in asthmatics or ACO patients. Respiratory resistance, including R5, R20, and R5–R20, was lower in COPD patients. Overall, respiratory reactance was larger in ACO patients than in asthmatics or COPD patients. Biologics were used in 4 asthmatics and 2 ACO patients.
Table 1. Characteristics of the subjects.
| Characteristics | Asthma (n = 49) | ACO (n = 40) | COPD (n = 41) | Overall P value* | Pairwise P value† | |||
|---|---|---|---|---|---|---|---|---|
| Asthma vs. ACO | Asthma vs. COPD | ACO vs. COPD | ||||||
| Mucus plugging | 29 (59) | 26 (65) | 17 (41) | 0.087 | 0.663 | 0.138 | 0.046 | |
| Mucus score | 1 (0, 6) | 2 (0, 5) | 0 (0, 1) | 0.006 | 0.749 | 0.012 | 0.002 | |
| Tree-in-bud appearance | 17 (35) | 21 (53) | 11 (27) | 0.056 | 0.131 | 0.496 | 0.024 | |
| Bronchiectasis | 4 (8) | 2 (1) | 4 (10) | 0.780 | 0.687 | 1.000 | 0.675 | |
| Goddard score | 0 (0, 0) | 12 (9, 12) | 12 (11, 12) | < 0.001 | < 0.001 | < 0.001 | 0.035 | |
| Age (yr) | 65 (54, 72) | 76 (66, 80) | 76 (69, 80) | < 0.001 | < 0.001 | < 0.001 | 0.974 | |
| Sex, male | 19 (39) | 35 (88) | 36 (88) | < 0.001 | < 0.001 | < 0.001 | 1.000 | |
| Body mass index (kg/m2) | 23.7 (20.6, 27.0) | 22.5 (19.8, 24.6) | 22.4 (20.3, 24.9) | 0.265 | 0.151 | 0.187 | 0.862 | |
| Childhood asthma | 9 (18) | 1 (3) | 0 (0) | 0.001 | 0.021 | 0.003 | 0.494 | |
| Allergic rhinitis | 17 (35) | 11 (28) | 6 (15) | 0.090 | 0.500 | 0.051 | 0.181 | |
| Chronic rhinosinusitis | 9 (19) | 3 (8) | 1 (2) | 0.039 | 0.212 | 0.019 | 0.359 | |
| Severe asthma | 10 (20) | 2 (15) | NA | 0.058 | NA | NA | NA | |
| Smoking history | < 0.001 | < 0.001 | < 0.001 | 0.712 | ||||
| Never smoker | 31 (63) | 0 (0) | 0 (0) | |||||
| Ex-smoker | 12 (24) | 37 (93) | 36 (85) | |||||
| Current smoker | 5 (10) | 3 (7) | 5 (12) | |||||
| Pack-years | 0 (0, 12) | 41 (31, 58) | 50 (36, 63) | < 0.001 | < 0.001 | < 0.001 | 0.180 | |
| ACQ | 1.8 (0.9, 3.0) | 0.4 (0.0, 1.0) | NA | NA | 0.002 | NA | NA | |
| ACT | 20 (13, 22) | 23 (21, 25) | NA | NA | < 0.001 | NA | NA | |
| mMRC | NA | 1.0 (1.0, 1.75) | 1.0 (0.0, 1.0) | NA | NA | NA | 0.086 | |
| COPD assessment test | NA | 7.0 (3.75, 14.0) | 5.0 (3.0, 11.0) | NA | NA | NA | 0.181 | |
| CMH | NA | 8 (22) | 7 (18) | NA | NA | NA | 0.776 | |
| Blood eosinophil count (cells/μL) | 151 (52, 391) | 209 (110, 465) | 168 (91, 227) | 0.219 | 0.250 | 0.827 | 0.064 | |
| Total IgE (I.U./mL) | 238 (55, 754) | 211 (88, 474) | 95 (26, 269) | 0.067 | 0.632 | 0.034 | 0.051 | |
| Positive specific IgE antibody | 2 (0, 13) | 1 (0, 7) | 0 (0, 11) | < 0.001 | 0.005 | < 0.001 | 0.330 | |
| Sputum eosinophils (%) | 1.8 (0, 9.9) | 2.5 (0, 5.3) | 0 (0, 0.8) | 0.053 | 0.992 | 0.023 | 0.030 | |
| Sputum neutrophils (%) | 95.3 (85.0, 97.0) | 93.0 (86.5, 98.1) | 99.0 (95.3, 99.8) | 0.099 | 0.967 | 0.034 | 0.076 | |
| FeNO (ppb) | 40.5 (18.0, 66.3) | 35.0 (21.8, 67.3) | 24.5 (17.0, 30.5) | 0.051 | 0.972 | 0.051 | 0.017 | |
| FEV1 (L) | 1.72 (1.39, 2.21) | 1.46 (1.03, 1.89) | 1.85 (1.22, 2.24) | 0.183 | 0.089 | 0.979 | 0.135 | |
| %FEV1 | 77.8 (65.3, 89.1) | 63.8 (44.6, 72.5) | 71.1 (51.0, 88.5) | 0.002 | < 0.001 | 0.147 | 0.075 | |
| FVC (L) | 2.38 (2.03, 3.08) | 2.76 (2.20, 3.67) | 3.35 (2.29, 3.82) | 0.009 | 0.112 | 0.002 | 0.147 | |
| %FVC | 91.3 (77.5, 101.7) | 88.9 (75.1, 104.5) | 101.6 (82.7, 117.6) | 0.011 | 0.962 | 0.005 | 0.015 | |
| FEV1/FVC | 71.6 (62.5, 78.3) | 55.3 (42.4, 62.0) | 53.9 (45.4, 63.9) | < 0.001 | < 0.001 | < 0.001 | 0.552 | |
| %FEF 25–75 | 37.6 (25.5, 51.6) | 21.4 (11.7, 28.9) | 20.8 (15.6, 30.0) | < 0.001 | < 0.001 | < 0.001 | 0.543 | |
| R5 (cmH2O/L/s) | 3.71 (2.91, 4.52) | 3.74 (2.67, 4.65) | 2.58 (2.11, 3.56) | 0.003 | 0.686 | 0.002 | 0.006 | |
| R20 (cmH2O/L/s) | 3.00 (2.44, 3.67) | 2.98 (2.14, 3.29) | 2.23 (1.84, 3.05) | 0.001 | 0.141 | < 0.001 | 0.021 | |
| R5–R20 (cmH2O/L/s) | 0.79 (0.45, 1.11) | 0.87 (0.60, 1.29) | 0.54 (0.26, 1.00) | 0.024 | 0.295 | 0.122 | 0.004 | |
| X5 (cmH2O/L/s) | −0.73 (−1.03, −0.41) | −0.93 (−2.72, −0.47) | −0.61 (−1.03, −0.26) | 0.079 | 0.076 | 0.489 | 0.041 | |
| Fres (Hz) | 9.28 (7.68, 12.52) | 12.90 (8.80, 18.72) | 10.30 (6.74, 14.25) | 0.045 | 0.015 | 0.839 | 0.066 | |
| ALX (cmH2O/L) | 3.26 (1.31, 5.29) | 5.02 (1.68, 23.24) | 2.49 (0.83, 5.84) | 0.065 | 0.043 | 0.724 | 0.043 | |
| Inhaled corticosteroid use | 37 (76) | 35 (88) | 6 (15) | < 0.001 | 0.183 | < 0.001 | < 0.001 | |
| LABA | 35 (71) | 36 (90) | 32 (78) | 0.087 | 0.036 | 0.628 | 0.226 | |
| LAMA | 0 (0) | 20 (50) | 35 (85) | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| Biologic use | 4 (8) | 2 (5) | 0 (0) | 0.160 | 0.687 | 0.123 | 0.241 | |
| Benralizumab | 3 (6) | 1 (3) | 0 (0) | |||||
| Omalizumab | 1 (2) | 0 (0) | 0 (0) | |||||
| Dupilumab | 0 (0) | 1 (3) | 0 (0) | |||||
| Systemic corticosteroid use | 1 (2) | 0 (0) | 0 (0) | 1.000 | 1.000 | 1.000 | 1.000 | |
Data are shown as median (interquartile range) or frequency (percentage).
ACO, asthma-chronic obstructive pulmonary disease overlap; COPD, chronic obstructive pulmonary disease; ACQ, Asthma Control Questionnaire; ACT, Asthma Control Test; NA, not applicable; mMRC, Modified British Medical Research Council; CMH, chronic mucus hypersecretion; FeNO, fractional exhaled nitric oxide; FEF 25–75, forced expiratory flow at 25%–75% of forced vital capacity; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; R5 and R20, respiratory resistance at 5 and 20 Hz; R5–R20, the difference between R5 and R20; X5, respiratory reactance at 5 Hz; ALX, low-frequency reactance area; Fres, resonant frequency; LABA, long-acting β2-agonists; LAMA, long-acting muscarinic antagonists.
*Kruskal-Wallis test, †Mann-Whitney U test, or Fisher's exact test. Sputum analysis was performed in 32 asthmatic, 20 ACO, and 11 COPD patients.
HRCT analysis
Mucus plugs were found in 29 (59%) asthma, 26 (65%) ACO, and 17 (41%) COPD patients, respectively, with a statistically significant difference between ACO and COPD patients (Fig. 2, Table 1). Mucus score was significantly lower in COPD patients than in asthmatic or ACO patients. The frequency distribution of mucus score is shown in Fig. 3. Asthmatic and ACO patients had more mucus plugs, and the scores were evenly distributed, whereas COPD patients had fewer mucus plugs overall. Tree-in-bud appearance was found in 17 asthmatic, 21 ACO, and 11 COPD patients, with a significant difference between ACO and COPD patients. There was no difference in the accompanying bronchiectasis among the 3 groups. The Goddard score was significantly higher in COPD patients.
Fig. 2. Representative high-resolution CT findings of mucus plugs in an asthmatic patient. (A) An axial plane showing 2 round opacities occluding the anterior and lateral basal bronchi of the right lower lobe. (B) A coronal plane showing a tubular opacity occluding the anterior basal bronchus, as visualized in the axial plane.
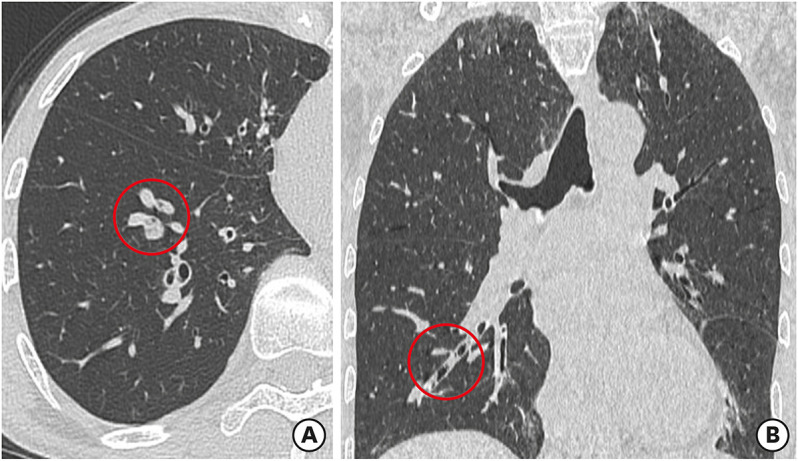
Fig. 3. The frequency distribution of mucus scores in patients with asthmatic, ACO, and COPD.
ACO, asthma-chronic obstructive pulmonary disease overlap; COPD, chronic obstructive pulmonary disease.
Comparison between patients with and without mucus plugging
The median mucus score was 5, 4, and 1 in asthmatic, ACO, and COPD patients with mucus plugs, respectively (Table 2). Tree-in-bud appearance was more frequently found in asthmatic and COPD patients with mucus plugs than those without mucus plugs. Bronchiectasis was more frequently found in COPD patients with mucus plugs alone. There was no difference in the Goddard score between patients with and without mucus plugs in all 3 diseases. There was no difference in age, sex, comorbidities, or smoking history between patients with and without mucus plugs. Body mass index in ACO patients with mucus plugs was lower than in those without mucus plugs. The prevalence of CMH was similar between ACO and COPD patients with and without mucus plugs. There was no difference in blood and sputum eosinophils and specific IgE antibodies between patients with and without mucus plugs. However, total IgE was significantly higher in asthmatics and ACO patients with mucus plugs than those without mucus plugs. FeNO was significantly higher in asthmatic patients with mucus plugs than in those without.
Table 2. Comparison between patients with and without mucus plugging.
| Characteristics | Asthma | ACO | COPD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mucus plugging | P value* | Mucus plugging | P value* | Mucus plugging | P value* | |||||
| Absent (n = 20) | Present (n = 29) | Absent (n = 14) | Present (n = 26) | Absent (n = 24) | Present (n = 17) | |||||
| Mucus score | 0 (0, 0) | 5 (2, 8) | < 0.001 | 0 (0, 0) | 4 (2, 6) | < 0.001 | 0 (0, 0) | 1 (1, 2) | < 0.001 | |
| Tree-in-bud appearance | 2 (10) | 15 (52) | 0.003 | 5 (36) | 16 (62) | 0.186 | 3 (13) | 8 (47) | 0.029 | |
| Bronchiectasis | 0 (0) | 4 (14) | 0.135 | 1 (7) | 1 (4) | 1.000 | 0 (0) | 4 (24) | 0.024 | |
| Goddard score | 0 (0, 0) | 0 (0, 0) | 1.000 | 11 (7, 12) | 12 (9, 12) | 0.294 | 12 (11, 12) | 12 (11, 13) | 0.534 | |
| Age (yr) | 57 (48, 69) | 68 (56, 76) | 0.056 | 71 (60, 79) | 78 (70, 82) | 0.095 | 76 (70, 79) | 76 (69, 80) | 0.758 | |
| Sex, male | 6 (30) | 13 (45) | 0.377 | 12 (86) | 23 (88) | 1.000 | 22 (92) | 14 (82) | 0.633 | |
| Body mass index (kg/m2) | 22.1 (20.8, 24.4) | 24.8 (20.4, 28.1) | 0.172 | 24.1 (23.7, 27.9) | 20.8 (18.9, 22.8) | < 0.001 | 23.6 (21.4, 25.2) | 21.3 (18.9, 23.7) | 0.192 | |
| Childhood asthma | 2 (10) | 7 (24) | 0.356 | 0 (0) | 1 (4) | 1.000 | NA | NA | NA | |
| Allergic rhinitis | 8 (40) | 9 (31) | 0.555 | 6 (43) | 5 (19) | 0.147 | 4 (17) | 2 (12) | 1.000 | |
| Chronic rhinosinusitis | 1 (5) | 8 (28) | 0.064 | 1 (7) | 2 (8) | 1.000 | 1 (4) | 0 (0) | 1.000 | |
| Severe asthma | 2 (10) | 8 (28) | 0.167 | 2 (14) | 0 (0) | 0.117 | NA | NA | NA | |
| Smoking history | 0.367 | 0.540 | 0.065 | |||||||
| Never smoker | 15 (75) | 16 (55) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||
| Ex-smoker | 3 (15) | 9 (31) | 14 (100) | 23 (88) | 19 (79) | 17 (100) | ||||
| Current smoker | 2 (10) | 3 (10) | 0 (0) | 3 (12) | 5 (21) | 0 (0) | ||||
| Pack-years | 0 (0, 4) | 0 (0, 12) | 0.387 | 38 (31, 44) | 42 (34, 70) | 0.326 | 48 (35, 62) | 50 (38, 63) | 0.768 | |
| CMH | NA | NA | NA | 2 (14) | 6 (27) | 0.441 | 4 (18) | 3 (19) | 1.000 | |
| Blood eosinophil count (cells/μL) | 127 (48, 195) | 201 (74, 592) | 0.171 | 266 (96, 457) | 176 (128, 447) | 0.927 | 170 (105, 243) | 133 (88, 198) | 0.525 | |
| Total IgE (I.U./mL) | 68 (48, 196) | 416 (100, 1,100) | 0.008 | 103 (5, 563) | 268 (184, 486) | 0.008 | 114 (54, 266) | 59 (13, 223) | 0.134 | |
| Positive specific IgE antibody | 2.0 (0, 7) | 2.0 (0, 13) | 0.290 | 0 (0, 6) | 1 (0, 7) | 0.930 | 1 (0, 11) | 0 (0, 3) | 0.050 | |
| Sputum eosinophils (%) | 0 (0, 2.5) | 2.0 (0.5, 12.5) | 0.170 | 2.0 (0.0, 31.0) | 2.5 (0.1, 4.8) | 0.956 | 0.3 (0.0, 0.9) | 0.0 (0.0, 0.0) | 0.621 | |
| Sputum neutrophils (%) | 96.0 (93.5, 97.0) | 95.0 (83.0, 97.5) | 0.797 | 79.0 (59.4, 97.5) | 93.0 (89.6, 98.1) | 0.456 | 98.0 (97.0, 99.4) | 99.0 (93.5, 100) | 0.959 | |
| FeNO (ppb) | 21 (11, 44) | 45 (25, 84) | 0.014 | 31 (27, 55) | 37 (19, 67) | 0.980 | 26 (23, 36) | 23 (16, 27) | 0.134 | |
| FEV1 (L) | 2.11 (0.36, 4.32) | 1.54 (0.57, 2.85) | 0.032 | 1.83 (0.56, 2.96) | 1.24 (0.47, 2.88) | 0.008 | 1.98 (1.17, 3.58) | 1.07 (0.53, 2.80) | < 0.001 | |
| %FEV1 | 84.6 (28.6, 112) | 69.4 (26.4, 109) | 0.018 | 70.7 (31.6, 92.1) | 59.2 (18.8, 84.6) | 0.019 | 75.9 (44.9, 127) | 50.9 (25.8, 97.5) | < 0.001 | |
| FVC (L) | 2.65 (0.66, 4.53) | 2.32 (0.78, 4.58) | 0.101 | 3.59 (1.46, 4.58) | 2.59 (1.30, 5.14) | 0.026 | 3.71 (1.79, 4.57) | 2.29 (1.49, 4.29) | < 0.001 | |
| %FVC | 98.0 (39.5, 115) | 84.1 (41.4, 117) | 0.040 | 102 (56.9, 122) | 84.8 (46.1, 124) | 0.033 | 107 (63.0, 129) | 81.7 (62.2, 121) | < 0.001 | |
| FEV1/FVC | 74.7 (66.8, 77.4) | 70.8 (60.9, 78.3) | 0.305 | 56.0 (49.9, 65.3) | 53.9 (38.7, 60.6) | 0.200 | 59.1 (53.4, 67.7) | 48.1 (43.6, 53.9) | 0.003 | |
| %FEF 25–75 | 42.3 (6.5, 117) | 30.6 (9.3, 90.8) | 0.041 | 23.8 (7.60, 54.9) | 20.2 (4.80, 32.2) | 0.115 | 25.3 (8.80, 99.3) | 15.6 (8.40, 37.7) | 0.005 | |
| R5 (cmH2O/L/s) | 3.52 (1.44, 6.95) | 3.86 (0.95, 6.34) | 0.259 | 3.55 (2.55, 4.81) | 4.46 (2.04, 6.22) | 0.470 | 2.26 (1.33, 3.56) | 4.39 (2.14, 5.99) | < 0.001 | |
| R20 (cmH2O/L/s) | 2.96 (1.55, 5.25) | 3.08 (0.95, 4.45) | 0.565 | 2.73 (1.93, 3.70) | 3.11 (1.48, 4.29) | 0.589 | 1.97 (1.19, 3.10) | 3.07 (1.84, 4.60) | 0.001 | |
| R5–R20 (cmH2O/L/s) | 0.74 (−0.27, 2.21) | 0.90 (−0.34, 2.12) | 0.305 | 0.66 (0.38, 1.57) | 1.18 (0.12, 1.95) | 0.197 | 0.41 (−0.24, 1.19) | 1.16 (−0.05, 1.96) | 0.001 | |
| X5 (cmH2O/L/s) | −0.56 (−5.40, 0.04) | −0.82 (−6.17, 0.14) | 0.049 | −0.69 (−4.91, 0.06) | −1.28 (−5.58, −0.02) | 0.068 | −0.45 (−2.04, 0.08) | −1.30 (−5.61, −0.07) | < 0.001 | |
| Fres (Hz) | 8.54 (4.71, 25.0) | 10.8 (4.0, 24.5) | 0.047 | 10.1 (4.57, 21.7) | 14.4 (5.14, 24.2) | 0.033 | 8.08 (4.64, 20.0) | 14.7 (5.37, 26.7) | < 0.001 | |
| ALX (cmH2O/L) | 2.06 (0.09, 52.5) | 3.62 (0, 56.8) | 0.044 | 2.95 (0.09, 40.2) | 7.37 (0.23, 48.7) | 0.051 | 1.55 (0.13, 16.1) | 8.07 (0.38, 55.7) | < 0.001 | |
| Inhaled corticosteroid use | 15 (75) | 22 (76) | 1.000 | 12 (86) | 23 (88) | 1.000 | 5 (21) | 1 (6) | 0.370 | |
| LABA | 14 (70) | 21 (72) | 1.000 | 12 (86) | 24 (92) | 0.602 | 19 (79) | 13 (76) | 1.000 | |
| LAMA | 0 (0) | 0 (0) | 1.000 | 8 (57) | 12 (46) | 0.741 | 20 (83) | 15 (88) | 1.000 | |
Data are shown as median (interquartile range) or frequency (percentage).
ACO, asthma-chronic obstructive pulmonary disease overlap; ALX, low-frequency reactance area; CMH, chronic mucus hypersecretion; COPD, chronic obstructive pulmonary disease; FeNO, fractional exhaled nitric oxide; FEF 25–75, forced expiratory flow at 25%–75% of forced vital capacity; FEV1, forced expiratory volume in 1 second; Fres, resonant frequency; FVC, forced vital capacity; LABA, long-acting β2-agonists; LAMA, long-acting muscarinic antagonists, NA, not applicable; R5 and R20, respiratory resistance at 5 and 20 Hz; R5–R20, the difference between R5 and R20; X5, respiratory reactance at 5 Hz.
*Mann-Whitney U test or Fisher’s exact test. Sputum analysis was performed in 32 asthmatic, 20 ACO, and 11 COPD patients.
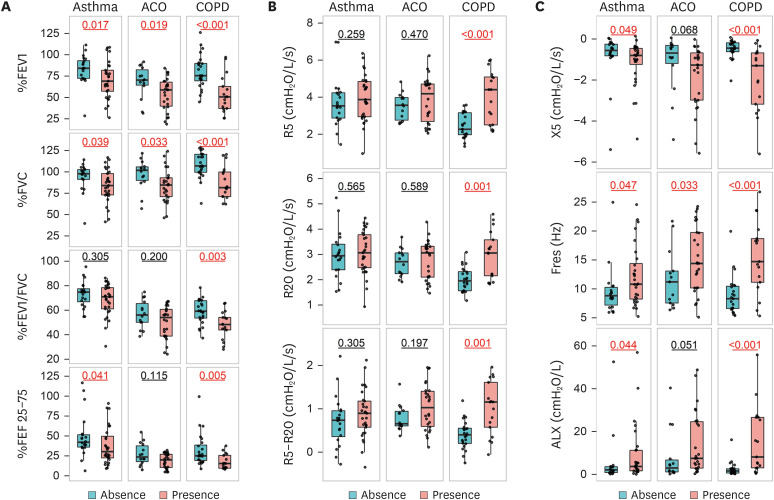
The %FEV1 and %FVC were significantly lower in patients with mucus plugs than those without (Fig. 4A). FEV1/FVC was significantly lower in COPD patients with mucus plugs. The %FEF 25–75 was lower in asthmatic and COPD patients with mucus plugs than those without. R5, R20, and R5–R20 were significantly higher in COPD patients with mucus plugs (Fig. 4B). Reactance parameters were larger in asthmatic and COPD patients with mucus plugs than those without (Fig. 4C). In ACO patients, Fres alone was larger in patients with mucus plugs than those without.
Fig. 4. Comparison of pulmonary function between patients with and without mucus plugs in asthmatic, ACO, and COPD patients. (A) Spirometry. (B) Respiratory resistance. (C) Respiratory reactance.
ACO, asthma-chronic obstructive pulmonary disease overlap; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FEF 25–75, forced expiratory flow at 25%–75% of forced vital capacity; R5 and R20, respiratory resistance at 5 and 20 Hz; R5–R20, the difference between R5 and R20; X5, respiratory reactance at 5 Hz; Fres, resonant frequency; ALX, low-frequency reactance area.
Correlation between mucus score and clinical parameters
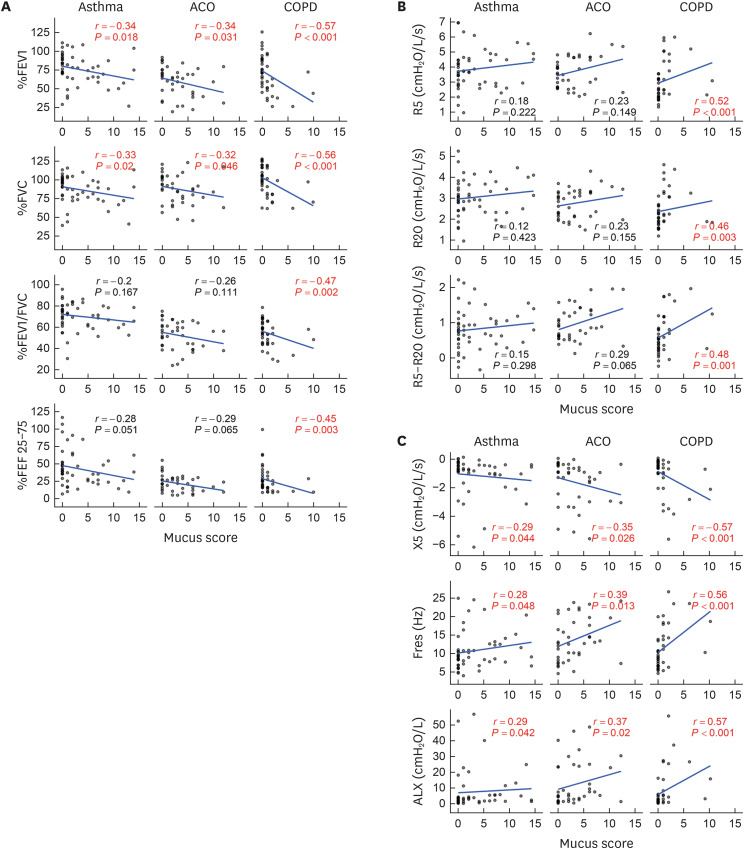
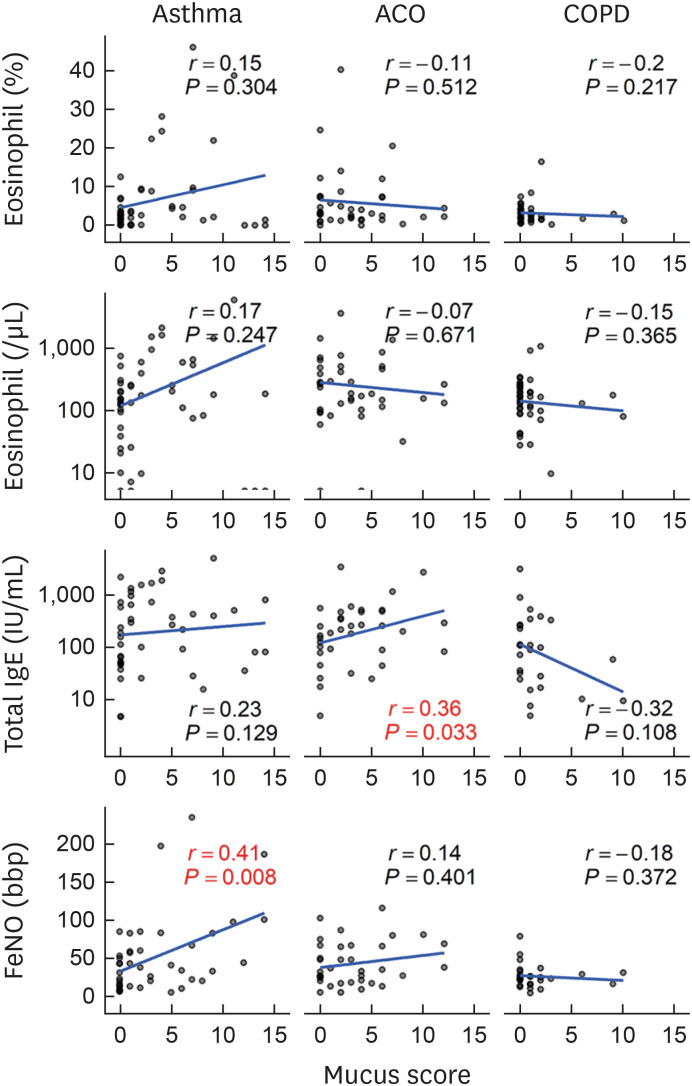
There was a negative correlation between mucus score and %FEV1 and %FVC in asthma, ACO, and COPD patients (Fig. 5A). There was also a negative correlation between mucus score and FEV1/FVC and %FEF 25–75 in COPD patients alone. There was a positive correlation between mucus score and R5, R20, and R5–R20 in COPD patients alone (Fig. 5B). There was a correlation between mucus score and X5, Fres, and ALX in asthma, ACO, and COPD patients (Fig. 5C). Overall, the correlations were stronger in COPD patients. Mucus score was positively correlated with total IgE in ACO patients and FeNO in asthma patients, respectively (Fig. 6). Although there was no significant (P < 0.05) correlation between mucus score and total IgE in asthma patients, the correlation coefficient was 0.23, indicating a weak correlation. However, the correlation coefficient was 0.23, indicating a weak correlation between mucus score and total IgE. However, there was no correlation between mucus score and eosinophil count in any diseases. There was no correlation between mucus score and Goddard score: asthma, r = −0.04, P = 0.781; ACO, r = 0.27, P = 0.097; and COPD, r = 0.11, P = 0.515.
Fig. 5. Correlation between mucus score and pulmonary function. (A) Spirometry. (B) Respiratory resistance. (C) Respiratory reactance.
ACO, asthma-chronic obstructive pulmonary disease overlap; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FEF 25–75, forced expiratory flow at 25%–75% of forced vital capacity; R5 and R20, respiratory resistance at 5 and 20 Hz; R5–R20, the difference between R5 and R20; X5, respiratory reactance at 5 Hz; Fres, resonant frequency; ALX, low-frequency reactance area.
Fig. 6. Correlation between mucus score and type 2 biomarkers. Mucus score positively correlated with FeNO in asthmatic patients and total IgE in ACO patients. However, there was no correlation between mucus score and type 2 biomarkers in COPD patients.
ACO, asthma-chronic obstructive pulmonary disease overlap; COPD, chronic obstructive pulmonary disease; FeNO, fractional exhaled nitric oxide.
Logistic regression analysis for variables associated with mucus plugs
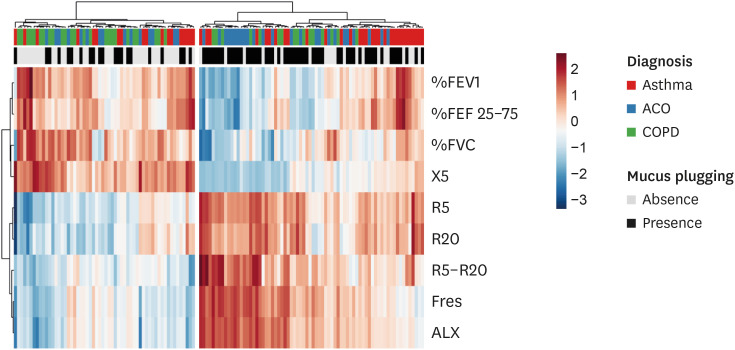
A logistic regression analysis was performed to analyze the relationships between mucus plugs and clinical and demographic variables in the whole study patients (Table 3). Variables with a P value < 0.15 in the univariate analysis and candidate variables are described. Stepwise multivariate logistic regression analysis revealed that the lower %FVC and higher Fres were significantly associated with the presence of mucus plugging in the whole studied population. The heatmap indicates such a relationship between the presence of mucus plugging and SAD parameters (Fig. 7).
Table 3. Univariate and multivariate logistic regression analysis of factors influencing the presence of mucus plugging.
| Variables | Univariate models | Multivariate model | ||||
|---|---|---|---|---|---|---|
| OR* | 95% CI | P value | OR* | 95% CI | P value | |
| Age (yr) | 1.37 | 0.96, 1.96 | 0.084 | |||
| Sex, male | 1.01 | 0.71, 1.43 | 0.953 | |||
| Body mass index (kg/m2) | 0.83 | 0.58, 1.18 | 0.305 | |||
| Pack-years | 1.04 | 0.73, 1.48 | 0.830 | |||
| %FEV1 | 0.40 | 0.26, 0.62 | < 0.001 | |||
| %FVC | 0.38 | 0.24, 0.60 | < 0.001 | 0.53 | 0.32, 0.91 | 0.020 |
| FEV1/FVC | 0.63 | 0.44, 0.92 | 0.016 | |||
| %FEF 25–75 | 0.57 | 0.38, 0.86 | 0.007 | |||
| R5 (cmH2O/L/s) | 2.03 | 1.36, 3.04 | 0.001 | |||
| R20 (cmH2O/L/s) | 1.73 | 1.18, 2.53 | 0.005 | |||
| R5–R20 (cmH2O/L/s) | 2.17 | 1.42, 3.29 | < 0.001 | |||
| X5 (cmH2O/L/s) | 0.43 | 0.26, 0.72 | 0.001 | |||
| Fres (Hz) | 2.62 | 1.65, 4.16 | < 0.001 | 1.80 | 1.05, 3.11 | 0.034 |
| ALX (cmH2O/L) | 2.23 | 1.32, 3.76 | 0.003 | |||
| Blood eosinophil count | 2.17 | 0.99, 4.74 | 0.052 | |||
| Total IgE | 1.61 | 0.88, 2.95 | 0.119 | |||
| FeNO | 1.61 | 0.95, 2.72 | 0.077 | |||
OR, odds ratio; CI, confidence interval; ALX, low-frequency reactance area; FeNO, fractional exhaled nitric oxide; FEF 25–75, forced expiratory flow at 25%–75% of forced vital capacity; FEV1, forced expiratory volume in 1 second; Fres, resonant frequency; FVC, forced vital capacity; R5 and R20, respiratory resistance at 5 and 20 Hz; R5–R20, the difference between R5 and R20; X5, respiratory reactance at 5 Hz.
*Per 1 standard deviation increase.
Fig. 7. Heatmap showing the relationship between the absence or presence of mucus plugs and pulmonary function in asthmatic, ACO, and COPD patients.
ACO, asthma-chronic obstructive pulmonary disease overlap; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FEF 25–75, forced expiratory flow at 25%–75% of forced vital capacity; R5 and R20, respiratory resistance at 5 and 20 Hz; R5–R20, the difference between R5 and R20; X5, respiratory reactance at 5 Hz; Fres, resonant frequency; ALX, low-frequency reactance area.
DISCUSSION
Mucus plugs were found in around 60% of patients with asthma and ACO, and less frequently, in 40% of COPD patients. Patients with mucus plugs had reduced spirometric indices and larger respiratory resistance and reactance parameters. The correlations between mucus score and pulmonary function were stronger in COPD patients than in asthmatic and ACO patients. There was a positive correlation between mucus score and type 2 biomarkers, including total IgE in ACO patients and FeNO in asthmatic patients, but not in COPD patients. In a multivariate logistic regression analysis, SAD parameters, including FVC and Fres, were significantly associated with the presence of mucus plugs in the whole patients. These results indicate that mucus plugs are associated with the pathophysiology of asthma, ACO, and COPD.
The prevalence of mucus plugging in asthmatic patients in this study was comparable with those in previous studies: 85 of 146 (58%)1 and 18 of 27 (67%),2 despite the low percentage of severe asthma (20%) compared with previous studies dealing with severe asthma alone. The prevalence of mucus plugging in COPD patients was in the middle of previous studies: 25% of 4003 and 67% of 299 patients,4 suggesting the differences in population studied or radiologists’ decision. Some COPD patients had coexisting asthma in previous studies: 8%3 and 23%,4 and the prevalence of mucus plugs was higher in patients with COPD and asthma than in those with COPD alone.3 A novel finding of this study was that mucus plugs were also assessed in ACO as a disease entity, which was diagnosed according to the JRS guidelines.11 We found that the prevalence of mucus plugs in ACO patients was comparable with that in asthmatic patients, but significantly higher than those with COPD. We evaluated emphysema automatically by using an image analysis system as Goddard scores and found no difference in the degree of emphysema between ACO and COPD patients with and without mucus plugs. This independent relationship between mucus plugs and emphysema was consistent with the previous study.4 The low prevalence of CMH (around 20%) in ACO and COPD patients in this study was also compatible with previous studies: 23% in severe asthma1 and 34% in COPD,4 confirming the asymptomatic nature of mucus plugging.
Like previous studies,1,2,3,4 we found that the mucus plugs were associated with low spirometric indices not only in asthmatic and COPD patients but also in ACO patients. The stronger correlations between mucus score and spirometry in COPD patients than in asthmatic and ACO patients suggest the significant effect of mucus plugging on the pathophysiology of COPD. A novel finding of this study was that the relationship between mucus plugs and airflow obstruction was also assessed by the FOT. The FOT is performed during tidal breathing, avoiding the need for any particular breathing maneuver or noticeable interference with respiration, and provides more information on the lung than obtained by forced expiration, spirometry.21 The correlation between spirometry and the FOT is generally modest, thus yielding some difference between the 2 modalities. This study found higher respiratory resistance values in COPD patients with mucus plugs than those without and a significant correlation in only COPD patients. As for respiratory reactance values, there were also significant differences between asthmatics and ACO patients with mucus plugs and those without mucus plugs, but the difference was more significant, and correlation coefficients were stronger in COPD patients. A previous asthma study found a positive correlation between the mucus score and the sputum eosinophil percentage and proposed that type 2 airway inflammation might promote mucus plug formation.1 In this study, we found a positive correlation between mucus score and total IgE or FeNO levels in ACO or asthma patients, but not in COPD patients, suggesting that non-type 2 mechanism might be involved in mucus plug formation in COPD patients. Further studies are needed to clarify the mechanism of mucus plug formation in different diseases.
Another novel finding of this study was that SAD rather than large airways dysfunction was associated with mucus plugging in obstructive airway diseases as a whole. Although previous studies have referred to the associations between mucus plugs and FVC1,3,4 or FEF 25–75,3 the relevance was not compared. A logistic regression analysis revealed that FVC and Fres were significantly associated with mucus plugs. Reduced FVC is interpreted as a marker of air trapping, and the site of airway closure is thought to be small airways.5,22 Previous systematic reviews positioned FVC as a marker of SAD.23,24 FVC is noninvasive, easy to perform, and correlates with residual volume/total lung capacity22 with high reproducibility and low variability. This suggests that FVC might have some utility in assessing SAD, mainly since FVC can be easily assessed by primary care physicians, so it could be undertaken as a serial assessment to monitor SAD.24 Fres is one of the commonly used forced oscillatory properties, where the respiratory reactance curve crosses zero and the elastic and inertial forces are equal in magnitude and opposite.21 We previously found the correlation between nitrogen phase III slope of single-breath washout (delta N2) and Fres in asthma,25 and that FVC, Fres, and the degree of emphysema were predictors of delta N2 in COPD,26 suggesting that Fres is a SAD parameter.
One of the limitations of this study is the retrospective study design, and there are some missing data, especially sputum analysis. However, because we performed ACQ, ACT, mMRC, CAT, FeNO, FOT, and spirometry for patients with asthma, ACO, and COPD as routine management, these data were reliable enough to analyze. Another limitation is that not all patients undergo chest CT in daily clinical practice, and there might be a selection bias in this retrospective study. Recently, however, chest CT has been done more often than ever for the management of patients with moderate to severe asthma, ACO, and COPD to detect emphysema and diagnose early lung cancer. Thus, the findings in this study are not far from clinical practice of asthma, ACO, and COPD in a real-life setting. These findings will help manage such patients.
In conclusion, mucus plugs were found in a subset of patients with asthma, ACO, and COPD by a visual and quantitative assessment of HRCT lung images. There was an inverse relationship between mucus score and pulmonary function, especially in COPD patients, and associations between type 2 inflammation and airflow obstruction were found in asthmatic and ACO patients. Overall, SAD, rather than large airways dysfunction, was associated with mucus plugs in the studied population.
Footnotes
Disclosure: T.S. reports personal fees from AstraZeneca Japan, GlaxoSmithKline Japan, Boehringer Ingelheim Japan, Novartis Pharma, and Sanofi outside of the submitted work. Other authors have nothing to disclose.
References
- 1.Dunican EM, Elicker BM, Gierada DS, Nagle SK, Schiebler ML, Newell JD, et al. Mucus plugs in patients with asthma linked to eosinophilia and airflow obstruction. J Clin Invest. 2018;128:997–1009. doi: 10.1172/JCI95693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Svenningsen S, Haider E, Boylan C, Mukherjee M, Eddy RL, Capaldi DP, et al. CT and functional MRI to evaluate airway mucus in severe asthma. Chest. 2019;155:1178–1189. doi: 10.1016/j.chest.2019.02.403. [DOI] [PubMed] [Google Scholar]
- 3.Okajima Y, Come CE, Nardelli P, Sonavane SK, Yen A, Nath HP, et al. Luminal plugging on chest CT scan: association with lung function, quality of life, and COPD clinical phenotypes. Chest. 2020;158:121–130. doi: 10.1016/j.chest.2019.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunican EM, Elicker BM, Henry T, Gierada DS, Schiebler ML, Anderson W, et al. Mucus plugs and emphysema in the pathophysiology of airflow obstruction and hypoxemia in smokers. Am J Respir Crit Care Med. 2021;203:957–968. doi: 10.1164/rccm.202006-2248OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Postma DS, Brightling C, Baldi S, Van den Berge M, Fabbri LM, Gagnatelli A, et al. Exploring the relevance and extent of small airways dysfunction in asthma (ATLANTIS): baseline data from a prospective cohort study. Lancet Respir Med. 2019;7:402–416. doi: 10.1016/S2213-2600(19)30049-9. [DOI] [PubMed] [Google Scholar]
- 6.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 7.Foy BH, Soares M, Bordas R, Richardson M, Bell A, Singapuri A, et al. Lung computational models and the role of the small airways in asthma. Am J Respir Crit Care Med. 2019;200:982–991. doi: 10.1164/rccm.201812-2322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zimmermann SC, Tonga KO, Thamrin C. Dismantling airway disease with the use of new pulmonary function indices. Eur Respir Rev. 2019;28:180122. doi: 10.1183/16000617.0122-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Global Initiative for Asthma. Global strategy for asthma management and prevention, 2020 [Internet] Fontana (WI): Global Initiative for Asthma; 2020. [cited 2021 Sep 4]. Available from: www.ginasthma.org. [Google Scholar]
- 10.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease [Internet] place unknown: Global Initiative for Chronic Obstructive Lung Disease; [cited 2021 Sep 4]. Available from: www.goldcopd.org. [Google Scholar]
- 11.The Japanese Respiratory Society. Asthma and COPD overlap (ACO). The JRS guidelines for the management of ACO. Tokyo: Medical Review Co., Ltd; 2018. [Google Scholar]
- 12.Goddard PR, Nicholson EM, Laszlo G, Watt I. Computed tomography in pulmonary emphysema. Clin Radiol. 1982;33:379–387. doi: 10.1016/s0009-9260(82)80301-2. [DOI] [PubMed] [Google Scholar]
- 13.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 14.Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14:902–907. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 15.Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher CM. Standardised questionnaire on respiratory symptoms: a statement prepared and approved by the MRC Committee on the Aetiology of Chronic Bronchitis (MRC breathlessness score) BMJ. 1960;2:1662. [Google Scholar]
- 17.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34:648–654. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 18.Stott-Miller M, Müllerová H, Miller B, Tabberer M, El Baou C, Keeley T, et al. Defining chronic mucus hypersecretion using the CAT in the SPIROMICS cohort. Int J Chron Obstruct Pulmon Dis. 2020;15:2467–2476. doi: 10.2147/COPD.S267002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 20.The Committee of Pulmonary Physiology, Japanese Respiratory Society. Guidelines for pulmonary function tests: spirometry, flow-volume curve, diffusing capacity of the lung. Tokyo: The Japanese Respiratory Society; 2004. [PubMed] [Google Scholar]
- 21.Shirai T, Kurosawa H. Clinical application of the forced oscillation technique. Intern Med. 2016;55:559–566. doi: 10.2169/internalmedicine.55.5876. [DOI] [PubMed] [Google Scholar]
- 22.Sorkness RL, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Chung KF, et al. Lung function in adults with stable but severe asthma: air trapping and incomplete reversal of obstruction with bronchodilation. J Appl Physiol (1985) 2008;104:394–403. doi: 10.1152/japplphysiol.00329.2007. [DOI] [PubMed] [Google Scholar]
- 23.Contoli M, Bousquet J, Fabbri LM, Magnussen H, Rabe KF, Siafakas NM, et al. The small airways and distal lung compartment in asthma and COPD: a time for reappraisal. Allergy. 2010;65:141–151. doi: 10.1111/j.1398-9995.2009.02242.x. [DOI] [PubMed] [Google Scholar]
- 24.Usmani OS, Singh D, Spinola M, Bizzi A, Barnes PJ. The prevalence of small airways disease in adult asthma: a systematic literature review. Respir Med. 2016;116:19–27. doi: 10.1016/j.rmed.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Shirai T, Mori K, Mikamo M, Shishido Y, Akita T, Morita S, et al. Respiratory mechanics and peripheral airway inflammation and dysfunction in asthma. Clin Exp Allergy. 2013;43:521–526. doi: 10.1111/cea.12083. [DOI] [PubMed] [Google Scholar]
- 26.Mikamo M, Shirai T, Mori K, Shishido Y, Akita T, Morita S, et al. Predictors of phase III slope of nitrogen single-breath washout in COPD. Respir Physiol Neurobiol. 2013;189:42–46. doi: 10.1016/j.resp.2013.06.018. [DOI] [PubMed] [Google Scholar]