Abstract
Nonsteroidal anti-inflammatory drug (NSAID)-exacerbated respiratory disease (NERD) is a unique condition characterized by aspirin/NSAID hypersensitivity, adult-onset asthma, and/or chronic rhinosinusitis with nasal polyps. Arachidonic acid metabolism dysregulation and intense eosinophilic/type 2 inflammation are central mechanisms in NERD. Studies have been conducted on various biomarkers, and urinary leukotriene E4 is considered the most available biomarker of NERD. However, the pathophysiology of NERD is heterogeneous and complex. Epithelial cells and platelets can interact with immune cells in NERD, and novel biomarkers related to these interactions have recently been investigated. We summarize emerging novel biomarkers of NERD and discuss their roles in the management of NERD.
Keywords: Aspirin, hypersensitivity, asthma, biomarkers, leukotrienes, eosinophils, epithelial cells, platelets
INTRODUCTION
Nonsteroidal anti-inflammatory drug (NSAID)-exacerbated respiratory disease (NERD) is characterized by chronic eosinophilic inflammation of the respiratory tract in patients with asthma and/or chronic rhinosinusitis (CRS) accompanied by nasal polyps (NPs) as well as hypersensitivity to NSAIDs, including aspirin.1 The prevalence of NERD has been reported at 7.1% in adult asthmatic populations and ranges from 5.5% to 12.4% in general populations.2 The prevalence of NERD has been estimated to be higher in severe asthmatic cohorts at 14.9%,2 which is approximately twice that in asthmatic cohorts. The majority of patients with NERD suffer from more severe and persistent asthma symptoms than NSAID-tolerant asthmatics, and more severe sinus mucosal inflammation than CRS patients without NERD.1 Therefore, there is a considerable socioeconomic burden associated with the management of NERD.
NERD as an endotype of asthma has a unique pathophysiology due to the dysregulation of arachidonic acid (AA) metabolism as well as chronic and extensive type 2/eosinophilic inflammation.3 Dysregulation in the cyclooxygenase (COX) and lipoxygenase (LO) pathway causes overproduction of cysteinyl leukotrienes (cysLTs) and prostaglandin (PG) D2 and decreases the level of PGE2, resulting in persistent eosinophilic inflammation in the upper and lower airway mucosae.1,3 Therefore, most of the previous studies exploring the biomarkers of NERD have focused on lipid mediators, cells, cytokines, or genetic factors associated with AA metabolism and/or type 2/eosinophilic inflammation.4 However, the pathophysiologic mechanism underlying NERD is more complex and thought to involve interactions among immune cells, epithelial cells and platelets. Novel biomarkers related to these interactions have recently been investigated. In this review, we summarize emerging biomarkers beyond leukotrienes (LTs) that can be potentially applied in the management of NERD.
BIOMARKERS RELATED TO AA METABOLISM
LTs
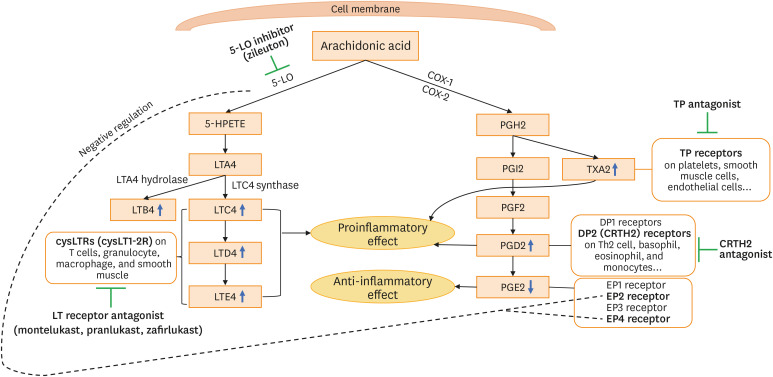
LTs are synthesized from AA by various inflammatory cells, including eosinophils, mast cells, basophils, neutrophils, and macrophages.5 Upon oxidation by 5-LO, AA undergoes conversion in the order of LTC4, LTD4, and LTE4 (called cysLTs) where LTE4 is a stable end product (Fig. 1).3,6 Therefore, LTE4 levels had been detected in various samples, including the urine, nasal mucosa, sputum, saliva, and blood.7,8,9,10,11,12,13,14,15 LTE4 levels were significantly higher in the induced sputum, blood, and saliva from NERD patients than from aspirin/NSAID-tolerant asthmatics (ATAs) (Table).7,8 Urinary levels of LTE4 at baseline were higher in NERD patients than in ATA patients and further increased after aspirin challenges.7,11,12,13 Urine collection is relatively easy to perform than other samples, and both random urine and urine collected at 24 hours after aspirin challenges have been reported to be useful for discriminating NERD from ATA.12,14 A recent meta-analysis has demonstrated similar diagnostic accuracy with better sensitivity between them.15 In addition, baseline urinary LTE4 levels were associated with falls in forced expiratory volume in 1 second (FEV1%) as well as upper and lower airway symptoms during aspirin challenges, and could predict the severity of airflow obstruction.16,17,18 Taken together, the urinary LTE4 level is considered a well-validated biomarker for the diagnosis of NERD and predicts the disease severity. In addition, it may be useful for pediatric patients due to its noninvasiveness and ease for collection.
Fig. 1. Arachidonic acid metabolism and therapeutic approach for the management of NERD. Arachidonic acid metabolism is initiated by 2 major enzymes, COX and 5-LO. 5-LO enzyme generates LTA4, which is then converted to LTC4 by LTC4 synthase. LTC4 is metabolized in the order of LTD4 and LTE4. LTC4, LTD4, and LTE4 are designated as cysLT. LTA4 can also be converted to LTB4 by LTA4 hydrolase. COX (COX-1 and COX-2) catalyzes arachidonic acid to PGG2 and subsequently PGH2, which can be further metabolized into PGI2, PGF2, PGD2, PGE2, and TXA2 by corresponding specific synthases. NERD is characterized with systemic elevations in PGD2 along with reduction in PGE2 and overproduction of cysLTs. The inhibition of the COX pathway results in the reduction in PGE2 production and excessive production of cysLTs in NERD.
COX, cyclooxygenase; LO, lipoxygenase; LT, leukotriene; cysLT, cysteinyl leukotriene; PG, prostaglandin; TX, thromboxane; NERD, nonsteroidal anti-inflammatory drug-exacerbated respiratory disease; TP, thromboxane prostanoid; DP, D-prostanoid; CRTH2, chemoattractant receptor-homologous molecule expressed on TH2 cells; HPETE, hydroperoxyeicosatetraenoic acid.
Table. Summary of biomarkers of NERD vs. ATA.
| Variables | Biomarker | Sample | Methodology | Baseline vs. ATA | Ref. | Response after ASA challenges vs. ATA | Ref. |
|---|---|---|---|---|---|---|---|
| Arachidonic acid metabolism | LTE4 | Urine | Immunoassay | ↑ | 7,11,14 | ↑ | 7,11 |
| Metabolomic analysis | ↑ | 10 | |||||
| Sputum | Immunoassay | ↑ | 7 | ||||
| Mass spectrometry | ↑ | 8 | ↑ | 8 | |||
| Saliva | Immunoassay | ↑ | 7 | ||||
| Blood | Immunoassay | ↑ | 7 | ↑ | 7 | ||
| Metabolomic analysis | ↑ | 10 | ↑ | 10 | |||
| LTE4/PGF2α | Urine | Metabolomic analysis | ↑ | 10 | ↑ | 10 | |
| Mass spectrometry | ↑ | 27 | |||||
| Blood | Metabolomic analysis | ↑ | 10 | ↑ | 10 | ||
| Mass spectrometry | ↑ | 27 | |||||
| LTB4 glucuronide | Urine | Immunoassay | ↔ | 22 | ↑ | 22 | |
| PGD2 | Sputum | Mass spectrometry | ↑ | 8 | ↑ | 8 | |
| 9α,11β-PGF2 | Blood | Mass spectrometry | ↑ | 19 | |||
| PGD-M | Urine | Mass spectrometry | ↑ | 24 | |||
| Eosinophilic inflammation | Eosinophils | Bronchial biopsy | Immunohistochemical staining | ↑ | 40 | ||
| Nasal fluid | Morphological count of stained slide | ↑ | 41,42 | ↑ | 41 | ||
| EDN | Blood | Immunoassay | ↑ | 46 | |||
| Mast cells | Bronchial biopsy | Morphological count | ↑ | 49 | |||
| Basophils | Nasal fluid | Morphological count | ↑ | 41 | |||
| Epithelial cells | Periostin | Blood | Immunoassay | ↑ | 62 | ||
| TGF-β | Blood | Immunoassay | ↑ | 64,65 | |||
| DPP10 | Blood | Immunoassay | ↑ | 65 | |||
| SPD | Blood | Immunoassay | ↓ | 68 | |||
| FCN | Blood | Immunoassay | ↑ | 72 | |||
| Platelets | Platelet-associated leukocytes | Blood | Flow cytometry | ↑ | 74,75 | ||
| Nasal polyp tissue | Immunohistochemistry | ↑ | 74 | ||||
| sP-selectin and sCD40L | Blood | Immunoassay | ↑ | 75 |
ASA, aspirin; ATA, aspirin-tolerant asthma; NERD, nonsteroidal anti-inflammatory drug-exacerbated respiratory disease; LTE4, leukotriene E4; PGF2α, prostaglandin F2α; LTB4, leukotriene B4; PGD2, prostaglandin D2; 9α,11β-PGF2: 9 alpha, 11 beta-prostaglandin F2; PGD-M, prostaglandin D2 metabolite; EDN, eosinophil-derived neurotoxin; TGF-β, transforming growth factor-beta; DPP10, dipeptidyl-peptidase 10; SPD, surfactant protein D; FCN, folliculin; sP-selectin, soluble P-selectin; sCD40L, soluble CD40 ligand.
LTA4 can also be converted to LTB4 as well as cysLTs by LTA4 hydrolase in eosinophils, neutrophils, macrophages, and mast cells.6,19 LTB4 is a proinflammatory mediator with potent chemoattractant properties mediated through the G-protein-coupled surface receptor (originally termed) BLT1 expressed on target cells.20 LTB4 levels were reported to be elevated in the NP tissue of NERD patients21 and increased in urine and peripheral blood monocytes after aspirin challenges (Table).22,23 However, more consistent results are warranted to apply LTB4 as a biomarker of NERD.
Prostanoids
AA is catalyzed to PGH2 by COX-1 and COX-2, which produce 5 preliminary downstream metabolites, including PGD2, PGE2, PGF2, thromboxane (TX) A2, and prostacyclin (PGI2), depending on the specific synthases.13 PGD2, PGE2, and TXA2 have been involved in the pathogenesis of NERD. PGD2 is the main product of COX-derived intermediates in mast cells and is also produced by eosinophils. PGD2 plays various roles through its receptors, thromboxane prostanoid (TP) receptors and D-prostanoid (DP) receptors, DP1 and DP2. PGD2 can play the role in bronchoconstriction and chemoattraction for eosinophils and basophils through chemoattractant receptor-homologous molecule expressed on TH2 cells (CRTH2), also known as the DP2 receptor.6,13 Baseline levels of PGD2 and its metabolite 9α,11β-PGF2 in the blood, urine, and sputum were higher in patients with NERD than with ATA, and further increased after aspirin challenges (Table).8,19,24 In addition, the stability of urinary 9α,11β-PGF2 was previously proven.25,26 Aspirin-induced PGD2 overproduction was inversely correlated with the decrease in FEV1%.13 These findings suggest that increased PGD2 level serves as a potential biomarker for the diagnosis of NERD. Serum and urine levels of LTE4/PGF2α were significantly higher in NERD patients than in ATA patients and healthy controls in a recent study.27 Moreover, the discriminative value of NERD from ATA was slightly better in urinary LTE4/PGF2α than in urinary LTE4. Thus, cysLT/PGF2α may be a new diagnostic biomarker for NERD.
PGE2 has 4 different receptors (EP 1-4) and its action may vary, depending on these receptors.28 It exerts anti-inflammatory and bronchoprotective effects through EP2. The protective effect of PGE2 is compromised in patients with NERD.29,30 PGE2 is known to be relatively stable in vitro.31,32 The baseline level of PGE2 was lower in the nasal tissue and blood of patients with NERD than in those of healthy controls.33,34 Patients with NERD had lower COX-2 expression in the airway tissue, resulting in decreased production of PGE2 in the NP tissue.35,36 Thus, PGE2 may be a potential therapeutic target of NERD and its applicability should be tested in further studies.
TXA2, a proinflammatory prostanoid induced by COX, is involved in platelet activation and aggregation as well as leukocyte chemoattraction. TXA2 serves as a potent bronchoconstrictor and induces airway hyperresponsiveness by interacting with the TP receptor.6,37 The TP receptor can interact with both PGD2 metabolite and TXA2. This interaction plays a role in the development of lower respiratory symptoms and FEV1% fall during aspirin challenges in patients with NERD.13 TXA2 and TP receptor are considered novel therapeutic targets of NERD. A clinical trial of TP receptor antagonist in patients with NERD is ongoing.38
BIOMARKERS RELATED TO INFLAMMATORY CELLS AND CYTOKINES
Eosinophil activation
Intense eosinophilic airway inflammation plays a major role in the pathogenesis of NERD, and numerous studies have described enhanced eosinophilic inflammation in NERD. Several eosinophil-related factors, such as eosinophil counts and eosinophil-related mediators, were suggested to play an important role (Fig. 2).4,39 Patients with NERD showed an increase in the number of eosinophils in bronchial biopsies as compared to patients with ATA and healthy controls (Table).40 The influx of eosinophils in the nasal lavage fluid after nasal aspirin challenges was significantly higher in patients with NERD than with ATA.41 The number of eosinophils in the NP tissue was higher in patients with NERD than with allergic fungal sinusitis and chronic hyperplastic eosinophilic sinusitis.42,43 Increased eosinophil counts in the sputum and the blood could predict the degree of eosinophilic airway inflammation, symptom severity, and treatment response in NERD patients,44,45 but it may not be a consistent biomarker for representing the phenotype of NERD because clinical features of NERD patients are heterogeneous.18 In addition, the plasma eosinophil-derived neurotoxin level was significantly higher in patients with NERD than with ATA and was associated with the decrease in lung functions after aspirin challenges (Table), highlighting its potential role as a novel biomarker for distinguishing the phenotype between NERD and ATA.46
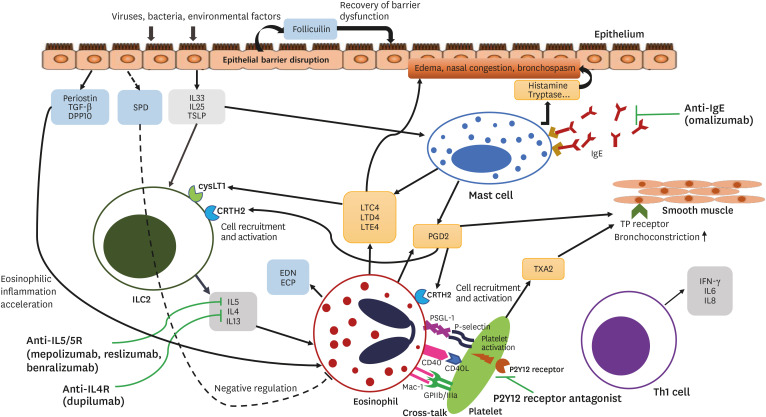
Fig. 2. Inflammatory pathway and therapeutic approach for the management of NERD. Various exogenous factors, such as viruses, bacteria, and environmental factors, can initiate epithelial injury and release of alarmins, IL33, IL25, and TSLP. These elevated levels of alarmins and cytokines from epithelial cells induce type 2 inflammatory responses by activating mast cells and ILC2 which are capable of activating eosinophils. Activated mast cells and eosinophils release various cytokines and chemokines that can induce mucosal inflammation and bronchoconstriction. The increased burden of platelet-associated leukocytes in NERD can further enhance the production of cysLTs, PGD2, and TXA2.
NERD, nonsteroidal anti-inflammatory drug-exacerbated respiratory disease; IL, interleukin; TSLP, thymic stromal lymphopoietin; ILC2, innate lymphoid type-2 cell; cysLT, cysteinyl leukotriene; PGD2, prostaglandin D2; TXA2, thromboxane A2; TGF, transforming growth factor; DPP10, dipeptidyl peptidase 10; SPD, surfactant protein D; LT, leukotriene; PG, prostaglandin; TX, thromboxane; INF, interferon.
Mast cells/basophils
Mast cells are known to release large amounts of key mediators, including cysLTs and PGD2, and have been demonstrated to play a central role in the pathogenesis of NERD, although there are some inconsistent reports (Fig. 2).40,43,47,48 The number of mast cells was reported to be higher in bronchial biopsies of NERD patients than of ATA patients (Table),49 and elevated levels of serum tryptase during aspirin challenges in NERD patients reflected mast cell activation.50 Mast cell activation is mediated by both IgE-dependent and -independent mechanisms.48 The IgE level was significantly higher in the NP tissue from NERD patients than from ATA patients with CRS with/without NP, and was associated with faster recurrence of NP.51 In addition, IgE antibodies to staphylococcal enterotoxins enhanced eosinophil activation of NPs in patients with NERD.52,53 However, a higher total IgE level is not a reliable biomarker of NERD, as it depends on the atopy status of patients with NERD or ATA. Considering that there are consistent reports demonstrating significantly higher levels of LTE4 and PGD2 after aspirin challenges in NERD patients, activated mast cells are thought to play a key role in the pathogenesis of NERD. However, further investigations are needed to identify reliable biomarkers in order to represent the status of mast cell activation in NERD patients.
Basophils, another potent granulocyte type, can release various proinflammatory mediators such as histamine, cysLTs, interleukin (IL) 4, and IL5.54 Basophils play crucial roles in type 2 inflammation, but only a few studies have investigated their potential as a biomarker of respiratory disease and NERD. The percentage of basophils in the nasal lavage fluid after nasal aspirin challenges was significantly higher in patients with NERD than with ATA (Table).40 In a recent study, the number of basophils in the NP tissue was found to be significantly higher in NERD patients than in CRS patients with NP (CRSwNP). The blood basophil count was also higher in NERD patients than in CRSwNP patients and correlated with eosinophil counts in NERD patients.54 Basophils may, thus, act as an important biomarker for predicting upper respiratory disease severity of NERD, although further studies are imperative.
Cytokines
Numerous studies have investigated the roles of cytokines and chemokines in NERD, which was found to exhibit both type 2- and type 1-like immune profiles.4,39 Eosinophilic asthma and sinus disease are characterized by type 2 cytokine profiles, including IL4, IL5, IL13, granulocyte–macrophage colony-stimulating factor, and eotaxin.55,56 Increased expression of type 2 cytokines, including IL5 (which is related to eosinophil activation and prolonged survival), was observed in the NP tissue of patients with NERD. Eosinophil cationic protein levels in the nasal tissue were higher (about 5-fold) in patients with NERD than with ATA with CRSwNP.57 More evidence is warranted to support type 2 cytokines as biomarkers of NERD beyond eosinophilic inflammation.
NERD also appears as a type 1 inflammatory milieu with prominent expression of interferon (IFN)-γ.4,39 The elevated expression of IFN-γ in the nasal tissue could distinguish NERD from chronic hyperplastic eosinophilic sinusitis.58 Other type 1 cytokines, including IL6 and IL8, were also associated with NERD. Baseline serum levels of IL8 were significantly higher in NERD patients than in ATA patients, and serum levels of IL6 decreased after oral aspirin challenges in NERD patients.59 The clinical utility of these type 1 inflammatory milieu as biomarkers of NERD needs to be further evaluated and validated.
Epithelial cells
The airway epithelium is a first-line barrier involved in the innate immunity through secretion of alarming cytokines such as IL33, IL25, and thymic stromal lymphopoietin, which results in the activation of type 2 innate lymphoid cells, eosinophils, and basophils in asthmatic airways. The interaction between the epithelium and immune cells is thought to be a key phenomenon in airway inflammation and airway remodeling in asthma patients including those with NERD.60
Several studies have been conducted on the roles of different molecules released into the airway epithelium as biomarkers of NERD. Periostin (a secreted protein mainly induced by IL4 and IL13 from airway epithelial cell) is found to be stable in serum.61 Periostin accelerates eosinophilic inflammation and airway remodeling in asthma and appears to be a useful biomarker of type 2 immunity. The serum level of periostin was reported to be increased in patients with NERD as compared to those with ATA, and positively correlated with the eosinophil count in the blood and sputum (Table).62 Therefore, periostin is a potential biomarker for identifying a subgroup of NERD patients with eosinophilic inflammation.
Transforming growth factor (TGF)-β1 plays a central role in epithelial cell apoptosis, subepithelial fibrosis, and mucus hypersecretion, and is involved in airway inflammation and remodeling. Previous studies have suggested a crosstalk between periostin and TGF-β1, wherein periostin is thought to induce TGF-β1 production. Serum levels of TGF-β1 and periostin were higher in eosinophilic asthma patients than in non-eosinophilic asthma patients, and positively correlated with each other.63 Furthermore, serum levels of TGF-β1 were significantly higher in NERD patients than in ATA patients or healthy controls, and positively correlated with urinary LTE4 level (Table).64 TGF-β1 is considered an ancillary biomarker for the diagnosis of NERD, which warrants further studies.
Dipeptidyl peptidase 10 (DPP10) expressed in the airway epithelium is associated with asthma and lung function decline.65,66 Higher serum DPP10 levels negatively correlated with FEV1% in patients with NERD than in those with ATA (Table). Furthermore, a positive correlation was observed between serum levels of DPP10 and TGF-β1 in patients with NERD.65 A genome-wide association study (GWAS) demonstrated the significant association of a single nucleotide polymorphism in DPP10 with NERD.66,67 These findings have suggested that DPP10 could contribute to the decline in the lung function in NERD. DPP10 may be able to accelerate type 2 inflammation through orchestration with TGF-β1 and periostin, and affect the subphenotypes of NERD. DPP10 may be a potential biomarker to distinguish NERD from ATA and predict disease severity.
Surfactant protein D (SPD), a member of the collectin family of proteins, is mainly produced by airway epithelial cells. SPD induces phagocytosis of pathogens by interacting with phagocytic cells and may exert protective functions against eosinophilia.68,69 SPD levels were elevated in the sera of asthmatic, and were associated with the severity of airway dysfunction.69 However, serum SPD levels were lower and negatively correlated with FEV1% decrease after aspirin challenges in patients with NERD than with ATA (Table). SPD treatment may attenuate airway inflammation and remodeling in mice,68 suggesting its role as a novel therapeutic target as well as a biomarker for the diagnosis of NERD.
Folliculin is an intracellular protein expressed in various types of cells, including airway epithelial cells, and has been thought to regulate cell-cell adhesion and affect the integrity of the epithelial barrier.70 Epithelial barrier disruption and cellular attachment to the basement membrane were noted in NERD patients with CRSwNP.71 Higher serum levels of folliculin were reported in patients with NERD than in those with ATA (Table),72 suggesting that folliculin may be a biomarker of epithelial activation and a therapeutic target for epithelial barrier dysfunction in NERD. These epithelium-derived biomarkers are expected to better differentiate between NERD and ATA than other type 2 biomarkers, therefore, they would be novel biomarkers for NERD.
Platelets
The main function of platelets is to repair damaged vessels, but these cells also have the capacity to interact with other immune cells and facilitate granulocyte recruitment. Platelets are known to be involved in various inflammatory diseases, including asthma,73 and are thought to be associated with NERD.73,74 Higher serum levels of soluble P-selectin and soluble CD40 ligand were observed in patients with NERD than with ATA (Table).75 Platelet-associate leukocyte counts were elevated in the NP tissue from NERD patients than from ATA patients and positively correlated with urinary LTE4 levels, but negatively correlated with the lung function.74,75 Thus, activated platelets may serve as additional therapeutic targets for NERD.
GENETIC BIOMARKERS
Most studies on the genetic mechanism of NERD have focused on genes related to CysLT synthesis and eosinophil activation.3,4 LTC4S, ALOX5, CYSLTR1, and CYSLTR2 are the most significant genetic biomarkers involved in AA metabolism in NERD.67 Several GWASs have been conducted to explore potential genetic biomarkers that distinguish NERD from ATA.4,67 HLA DPB1*0301 is known to be a significant allele that could potentially represent the phenotype of NERD. Further studies are imperative to determine a valid genetic marker.
MANAGEMENT OF NERD AND APPLICATION OF BIOMARKERS
The standard management of NERD is based on the management of asthma, CRS, and NSAID hypersensitivity. Patients with NERD should avoid aspirin and NSAIDs, and alternative NSAIDs should be confirmed by drug provocation tests. The majority of patients with NERD could be managed according to asthma treatment guidelines, including inhaled corticosteroids and long-acting beta-2 agonists with/without LT modifiers.1,3 The management of NERD with CRS is similar to that of patients without NSAID hypersensitivity, and involves intranasal corticosteroids as a first-line treatment. Nasal irrigation, systemic steroids, and antibiotics can be used to alleviate severe nasal symptoms. In addition, aspirin desensitization (AD) is an option for patients with severe NERD as it can improve nasal and asthmatic symptoms, olfactory function, and quality of life.1,3,76 However, AD could not alleviate the increased levels of LTE4, PGD2, and TXA2, contrary to improved symptoms,13,16,77,78 indicating that there are no available biomarkers for predicting AD status.
Some patients with NERD need more specific strategies to control asthma and/or CRS symptoms. Several specific treatment options according to pathophysiological mechanisms have been considered (Figs. 1 and 2). The efficacy of leukotriene-modifying drugs (LTMDs) has been proven in patients with NERD in terms of improved lung function, decreased rescue inhaler usage, and improved quality-of-life.1,3,79,80 LTMD treatment could reduce aspirin-induced bronchospasm81 and has been widely prescribed in adults and children due to relatively few side-effects. NERD is mainly adult-onset disease, but 3.5% of patients with NERD reported early onset of the disease (before 18 years of age).82 LTMD can be helpful to pediatric patients with clinical features of NERD. Serum and urinary levels of LTE4 and LTE4/PGF2α remained high in NERD patients treated with LTRA and were related to eosinophilic inflammation and disease severity.27 An additional treatment is warranted for NERD patients with sustained high levels of cysLTs despite LTRA treatment.
For patients with severe NERD, some biologics are recommended to control both asthma and CRS symptoms. Omalizumab, a monoclonal antibody against human IgE, is efficacious in patients with severe allergic asthma and other allergic diseases.83 It is beneficial to control asthma and CRS/NP in patients with severe NERD. Omalizumab treatment reduced urinary LTE4 and PGD2 and 9a,11b-PGF2 levels, indicating that it could suppress mast cells.83 In a recent randomized controlled study, omalizumab treatment decreased the concentration of urinary LTE4 and respiratory symptoms during oral aspirin challenges,84 which suggests its potential therapeutic application for severe NERD.1,3
Anti-IL5 monoclonal antibodies such as mepolizumab, reslizumab, and benralizumab are approved for severe eosinophilic asthma, and have been effective in treating CRS and NPs.85,86,87,88 Blood eosinophil counts and eosinophil-derived neurotoxin levels significantly decreased after 1 month of anti-IL5 antibody treatment, but no change in periostin and TGF-β1 levels was observed.85 Dupilumab, anti-IL4α receptor antibody blocking both IL4 and IL13 signaling, is approved for moderate-severe type 2 asthma and is the first biologic agents approved for the treatment of CRSwNP.86 These biologics have been considered to be more effective in NERD; however, a few studies have investigated their therapeutic efficacy for NERD.3 A recent study in patients with NERD showed improved CRSwNP outcomes, including clinical symptoms and abnormal radiographic findings, and type 2 inflammatory biomarkers such as FeNO, total serum IgE, and 24 hour urine LTE4 levels after 6 months of dupilumab treatment.87
Recent clinical trials for NERD have investigated the efficacy of platelet-targeted therapy including a P2Y12-receptor antagonist and a TP receptor antagonist. Prasugrel, a P2Y12 inhibitor used in acute coronary syndromes for its antiplatelet activity, is known to attenuate the platelet-leukocyte aggregation.3,89 Prasugrel alleviated aspirin-induced reactions only in a small subset of NERD patients with higher baseline platelet activation and milder upper respiratory symptoms, but did not reduce aspirin-induced symptoms in most patients with NERD.90 Either urinary levels of LTE4, PGD2 metabolite, and TXB2 or serum levels of tryptase were not increased after aspirin challenges in this subset of NERD patients. These results are thought to be associated with the low release of lipid mediators by mast cells. Ifetroban, an oral TP receptor antagonist, is suggested to have a preventive effect on aspirin-induced reactions. A clinical study is ongoing in patients with NERD.38 The investigation of biomarkers of platelet activation in NERD is necessary for NERD management and phenotyping.
PGD2 activates and recruits effector cells through CRTH2 in NERD. Two recent clinical trials have investigated CRTH2 antagonists for blocking PGD2 signaling in asthma.91 The clinical utility of CRTH2 as a biomarker of NERD is yet to be determined.
CONCLUSION
Studies on biomarkers of NERD have mainly been conducted in relation to cysLT overproduction and eosinophilic inflammation. Urinary LTE4 is still the most significant biomarker for the diagnosis of NERD. Epithelial cell markers, such as periostin, folliculin, and DPP10, have recently emerged as novel biomarkers of NERD, although further studies are essential to confirm their stability, reproducibility, as well as discriminative and predictive values. NERD is a heterogeneous disease with diverse clinical manifestations and complex mechanisms. It is, therefore, necessary to develop various biomarkers that can be individually applied according to the subtype along with the commonly used biomarkers for the diagnosis and management of NERD.
ACKNOWLEDGMENTS
This study was supported by a grant of the Korea Health Technology R&D Project (HR16C0001) through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea.
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
References
- 1.Kowalski ML, Agache I, Bavbek S, Bakirtas A, Blanca M, Bochenek G, et al. Diagnosis and management of NSAID-exacerbated respiratory disease (N-ERD)-a EAACI position paper. Allergy. 2019;74:28–39. doi: 10.1111/all.13599. [DOI] [PubMed] [Google Scholar]
- 2.Rajan JP, Wineinger NE, Stevenson DD, White AA. Prevalence of aspirin-exacerbated respiratory disease among asthmatic patients: a meta-analysis of the literature. J Allergy Clin Immunol. 2015;135:676–681.e1. doi: 10.1016/j.jaci.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 3.Woo SD, Luu QQ, Park HS. NSAID-exacerbated respiratory disease (NERD): from pathogenesis to improved care. Front Pharmacol. 2020;11:1147. doi: 10.3389/fphar.2020.01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park H, Choi Y, Jung CG, Park HS. Potential biomarkers for NSAID-exacerbated respiratory disease. Mediators Inflamm. 2017;2017:8160148. doi: 10.1155/2017/8160148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters-Golden M, Gleason MM, Togias A. Cysteinyl leukotrienes: multi-functional mediators in allergic rhinitis. Clin Exp Allergy. 2006;36:689–703. doi: 10.1111/j.1365-2222.2006.02498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sehanobish E, Asad M, Barbi M, Porcelli SA, Jerschow E. Aspirin actions in treatment of NSAID-exacerbated respiratory disease. Front Immunol. 2021;12:695815. doi: 10.3389/fimmu.2021.695815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaber F, Daham K, Higashi A, Higashi N, Gülich A, Delin I, et al. Increased levels of cysteinyl-leukotrienes in saliva, induced sputum, urine and blood from patients with aspirin-intolerant asthma. Thorax. 2008;63:1076–1082. doi: 10.1136/thx.2008.101196. [DOI] [PubMed] [Google Scholar]
- 8.Mastalerz L, Celejewska-Wójcik N, Wójcik K, Gielicz A, Januszek R, Cholewa A, et al. Induced sputum eicosanoids during aspirin bronchial challenge of asthmatic patients with aspirin hypersensitivity. Allergy. 2014;69:1550–1559. doi: 10.1111/all.12512. [DOI] [PubMed] [Google Scholar]
- 9.Fischer AR, Rosenberg MA, Lilly CM, Callery JC, Rubin P, Cohn J, et al. Direct evidence for a role of the mast cell in the nasal response to aspirin in aspirin-sensitive asthma. J Allergy Clin Immunol. 1994;94:1046–1056. doi: 10.1016/0091-6749(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 10.Ban GY, Cho K, Kim SH, Yoon MK, Kim JH, Lee HY, et al. Metabolomic analysis identifies potential diagnostic biomarkers for aspirin-exacerbated respiratory disease. Clin Exp Allergy. 2017;47:37–47. doi: 10.1111/cea.12797. [DOI] [PubMed] [Google Scholar]
- 11.Christie PE, Tagari P, Ford-Hutchinson AW, Charlesson S, Chee P, Arm JP, et al. Urinary leukotriene E4 concentrations increase after aspirin challenge in aspirin-sensitive asthmatic subjects. Am Rev Respir Dis. 1991;143:1025–1029. doi: 10.1164/ajrccm/143.5_Pt_1.1025. [DOI] [PubMed] [Google Scholar]
- 12.Divekar R, Hagan J, Rank M, Park M, Volcheck G, O’Brien E, et al. Diagnostic utility of urinary LTE4 in asthma, allergic rhinitis, chronic rhinosinusitis, nasal polyps, and aspirin sensitivity. J Allergy Clin Immunol Pract. 2016;4:665–670. doi: 10.1016/j.jaip.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cahill KN, Bensko JC, Boyce JA, Laidlaw TM. Prostaglandin D2: a dominant mediator of aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2015;135:245–252. doi: 10.1016/j.jaci.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bochenek G, Stachura T, Szafraniec K, Plutecka H, Sanak M, Nizankowska-Mogilnicka E, et al. Diagnostic accuracy of urinary LTE4 measurement to predict aspirin-exacerbated respiratory disease in patients with asthma. J Allergy Clin Immunol Pract. 2018;6:528–535. doi: 10.1016/j.jaip.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Hagan JB, Laidlaw TM, Divekar R, O’Brien EK, Kita H, Volcheck GW, et al. Urinary leukotriene E4 to determine aspirin intolerance in asthma: a systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2017;5:990–997.e1. doi: 10.1016/j.jaip.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Daffern PJ, Muilenburg D, Hugli TE, Stevenson DD. Association of urinary leukotriene E4 excretion during aspirin challenges with severity of respiratory responses. J Allergy Clin Immunol. 1999;104:559–564. doi: 10.1016/s0091-6749(99)70324-6. [DOI] [PubMed] [Google Scholar]
- 17.Nizankowska E, Bestyńska-Krypel A, Ćmiel A, Szczeklik A. Oral and bronchial provocation tests with aspirin for diagnosis of aspirin-induced asthma. Eur Respir J. 2000;15:863–869. doi: 10.1034/j.1399-3003.2000.15e09.x. [DOI] [PubMed] [Google Scholar]
- 18.Bochenek G, Kuschill-Dziurda J, Szafraniec K, Plutecka H, Szczeklik A, Nizankowska-Mogilnicka E. Certain subphenotypes of aspirin-exacerbated respiratory disease distinguished by latent class analysis. J Allergy Clin Immunol. 2014;133:98–103.e1-6. doi: 10.1016/j.jaci.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Bochenek G, Nagraba K, Nizankowska E, Szczeklik A. A controlled study of 9α,11β-PGF2 (a prostaglandin D2 metabolite) in plasma and urine of patients with bronchial asthma and healthy controls after aspirin challenge. J Allergy Clin Immunol. 2003;111:743–749. doi: 10.1067/mai.2003.1387. [DOI] [PubMed] [Google Scholar]
- 20.Huang WW, Garcia-Zepeda EA, Sauty A, Oettgen HC, Rothenberg ME, Luster AD. Molecular and biological characterization of the murine leukotriene B4 receptor expressed on eosinophils. J Exp Med. 1998;188:1063–1074. doi: 10.1084/jem.188.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinto S, Gallo O, Polli G, Boccuzzi S, Paniccia R, Brunelli T, et al. Cyclooxygenase and lipoxygenase metabolite generation in nasal polyps. Prostaglandins Leukot Essent Fatty Acids. 1997;57:533–537. doi: 10.1016/s0952-3278(97)90556-1. [DOI] [PubMed] [Google Scholar]
- 22.Mita H, Higashi N, Taniguchi M, Higashi A, Akiyama K. Increase in urinary leukotriene B4 glucuronide concentration in patients with aspirin-intolerant asthma after intravenous aspirin challenge. Clin Exp Allergy. 2004;34:1262–1269. doi: 10.1111/j.1365-2222.2004.02034.x. [DOI] [PubMed] [Google Scholar]
- 23.Juergens UR, Christiansen SC, Stevenson DD, Zuraw BL. Inhibition of monocyte leukotriene B4 production after aspirin desensitization. J Allergy Clin Immunol. 1995;96:148–156. doi: 10.1016/s0091-6749(95)70002-1. [DOI] [PubMed] [Google Scholar]
- 24.Buchheit KM, Cahill KN, Katz HR, Murphy KC, Feng C, Lee-Sarwar K, et al. Thymic stromal lymphopoietin controls prostaglandin D2 generation in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2016;137:1566–1576.e5. doi: 10.1016/j.jaci.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu X, Cai H, Xiang YB, Cai Q, Yang G, Liu D, et al. Intra-person variation of urinary biomarkers of oxidative stress and inflammation. Cancer Epidemiol Biomarkers Prev. 2010;19:947–952. doi: 10.1158/1055-9965.EPI-10-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berenguer PH, Camacho IC, Câmara R, Oliveira S, Câmara JS. Determination of potential childhood asthma biomarkers using a powerful methodology based on microextraction by packed sorbent combined with ultra-high pressure liquid chromatography. Eicosanoids as case study. J Chromatogr A. 2019;1584:42–56. doi: 10.1016/j.chroma.2018.11.041. [DOI] [PubMed] [Google Scholar]
- 27.Ban GY, Kim SH, Park HS. Persistent eosinophilic inflammation in adult asthmatics with high serum and urine levels of leukotriene E4. J Asthma Allergy. 2021;14:1219–1230. doi: 10.2147/JAA.S325499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanak M. Eicosanoid mediators in the airway inflammation of asthmatic patients: what is new? Allergy Asthma Immunol Res. 2016;8:481–490. doi: 10.4168/aair.2016.8.6.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rusznak M, Peebles RS., Jr Prostaglandin E2 in NSAID-exacerbated respiratory disease: protection against cysteinyl leukotrienes and group 2 innate lymphoid cells. Curr Opin Allergy Clin Immunol. 2019;19:38–45. doi: 10.1097/ACI.0000000000000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White AA, Stevenson DD. Aspirin-exacerbated respiratory disease. N Engl J Med. 2018;379:1060–1070. doi: 10.1056/NEJMra1712125. [DOI] [PubMed] [Google Scholar]
- 31.Lee MG. A study on the stability of prostaglandin E2 in methylhydroxyethylcellulose gel by gas chromatography. J Clin Hosp Pharm. 1982;7:67–70. doi: 10.1111/j.1365-2710.1982.tb00910.x. [DOI] [PubMed] [Google Scholar]
- 32.Fitzpatrick FA, Aguirre R, Pike JE, Lincoln FH. The stability of 13,14-dihydro-15 keto-PGE2. Prostaglandins. 1980;19:917–931. doi: 10.1016/0090-6980(80)90126-4. [DOI] [PubMed] [Google Scholar]
- 33.Pérez-Novo CA, Watelet JB, Claeys C, Van Cauwenberge P, Bachert C. Prostaglandin, leukotriene, and lipoxin balance in chronic rhinosinusitis with and without nasal polyposis. J Allergy Clin Immunol. 2005;115:1189–1196. doi: 10.1016/j.jaci.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 34.Schmid M, Göde U, Schäfer D, Wigand ME. Arachidonic acid metabolism in nasal tissue and peripheral blood cells in aspirin intolerant asthmatics. Acta Otolaryngol. 1999;119:277–280. doi: 10.1080/00016489950181819. [DOI] [PubMed] [Google Scholar]
- 35.Roca-Ferrer J, Garcia-Garcia FJ, Pereda J, Perez-Gonzalez M, Pujols L, Alobid I, et al. Reduced expression of COXs and production of prostaglandin E2 in patients with nasal polyps with or without aspirin-intolerant asthma. J Allergy Clin Immunol. 2011;128:66–72.e1. doi: 10.1016/j.jaci.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 36.Picado C, Fernandez-Morata JC, Juan M, Roca-Ferrer J, Fuentes M, Xaubet A, et al. Cyclooxygenase-2 mRNA is downexpressed in nasal polyps from aspirin-sensitive asthmatics. Am J Respir Crit Care Med. 1999;160:291–296. doi: 10.1164/ajrccm.160.1.9808048. [DOI] [PubMed] [Google Scholar]
- 37.Kohyama K, Hashimoto M, Abe S, Kodaira K, Yukawa T, Hozawa S, et al. Thromboxane A2 receptor +795T>C and chemoattractant receptor-homologous molecule expressed on Th2 cells -466T>C gene polymorphisms in patients with aspirin-exacerbated respiratory disease. Mol Med Rep. 2012;5:477–482. doi: 10.3892/mmr.2011.680. [DOI] [PubMed] [Google Scholar]
- 38.Cumberland Pharmaceuticals. Oral ifetroban to treat aspirin exacerbated respiratory disease (AERD) Bethesda (MD): National Library of Medicine; 2021. [cited 2021 Nov 6]. Available from: https://clinicaltrials.gov/ct2/show/NCT03028350. [Google Scholar]
- 39.Steinke JW, Payne SC, Borish L. Eosinophils and mast cells in aspirin-exacerbated respiratory disease. Immunol Allergy Clin North Am. 2016;36:719–734. doi: 10.1016/j.iac.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cowburn AS, Sladek K, Soja J, Adamek L, Nizankowska E, Szczeklik A, et al. Overexpression of leukotriene C4 synthase in bronchial biopsies from patients with aspirin-intolerant asthma. J Clin Invest. 1998;101:834–846. doi: 10.1172/JCI620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kupczyk M, Kurmanowska Z, Kupryś-Lipińska I, Bocheńska-Marciniak M, Kuna P. Mediators of inflammation in nasal lavage from aspirin intolerant patients after aspirin challenge. Respir Med. 2010;104:1404–1409. doi: 10.1016/j.rmed.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 42.Adamjee J, Suh YJ, Park HS, Choi JH, Penrose JF, Lam BK, et al. Expression of 5-lipoxygenase and cyclooxygenase pathway enzymes in nasal polyps of patients with aspirin-intolerant asthma. J Pathol. 2006;209:392–399. doi: 10.1002/path.1979. [DOI] [PubMed] [Google Scholar]
- 43.Payne SC, Early SB, Huyett P, Han JK, Borish L, Steinke JW. Evidence for distinct histologic profile of nasal polyps with and without eosinophilia. Laryngoscope. 2011;121:2262–2267. doi: 10.1002/lary.21969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360:1715–1721. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- 45.Lee Y, Lee JH, Yang EM, Kwon E, Jung CG, Kim SC, et al. Serum levels of eosinophil-derived neurotoxin: a biomarker for asthma severity in adult asthmatics. Allergy Asthma Immunol Res. 2019;11:394–405. doi: 10.4168/aair.2019.11.3.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shin SW, Park JS, Park CS. Elevation of eosinophil-derived neurotoxin in plasma of the subjects with aspirin-exacerbated respiratory disease: a possible peripheral blood protein biomarker. PLoS One. 2013;8:e66644. doi: 10.1371/journal.pone.0066644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park HS, Nahm DH, Park K, Suh KS, Yim HE. Immunohistochemical characterization of cellular infiltrate in nasal polyp from aspirin-sensitive asthmatic patients. Ann Allergy Asthma Immunol. 1998;81:219–224. doi: 10.1016/s1081-1206(10)62815-3. [DOI] [PubMed] [Google Scholar]
- 48.Kuruvilla ME, Vanijcharoenkarn K, Levy JM. The role of mast cells in aspirin-exacerbated respiratory disease (AERD) pathogenesis: Implications for future therapeutics. J Asthma Allergy. 2020;13:463–470. doi: 10.2147/JAA.S237463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nasser SM, Pfister R, Christie PE, Sousa AR, Barker J, Schmitz-Schumann M, et al. Inflammatory cell populations in bronchial biopsies from aspirin-sensitive asthmatic subjects. Am J Respir Crit Care Med. 1996;153:90–96. doi: 10.1164/ajrccm.153.1.8542168. [DOI] [PubMed] [Google Scholar]
- 50.Cahill KN, Murphy K, Singer J, Israel E, Boyce JA, Laidlaw TM. Plasma tryptase elevation during aspirin-induced reactions in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2019;143:799–803.e2. doi: 10.1016/j.jaci.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buchheit KM, Dwyer DF, Ordovas-Montanes J, Katz HR, Lewis E, Vukovic M, et al. IL-5Rα marks nasal polyp IgG4- and IgE-expressing cells in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2020;145:1574–1584. doi: 10.1016/j.jaci.2020.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bachert C, Zhang N, Holtappels G, De Lobel L, van Cauwenberge P, Liu S, et al. Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. J Allergy Clin Immunol. 2010;126:962–968. 968.e1–966. doi: 10.1016/j.jaci.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 53.Gevaert P, Holtappels G, Johansson SG, Cuvelier C, Cauwenberge P, Bachert C. Organization of secondary lymphoid tissue and local IgE formation to Staphylococcus aureus enterotoxins in nasal polyp tissue. Allergy. 2005;60:71–79. doi: 10.1111/j.1398-9995.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 54.Stevens WW, Staudacher AG, Hulse KE, Poposki JA, Kato A, Carter RG, et al. Studies of the role of basophils in aspirin-exacerbated respiratory disease pathogenesis. J Allergy Clin Immunol. 2021;148:439–449.e5. doi: 10.1016/j.jaci.2021.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bachert C, Wagenmann M, Hauser U, Rudack C. IL-5 synthesis is upregulated in human nasal polyp tissue. J Allergy Clin Immunol. 1997;99:837–842. doi: 10.1016/s0091-6749(97)80019-x. [DOI] [PubMed] [Google Scholar]
- 56.Hamilos DL, Leung DY, Huston DP, Kamil A, Wood R, Hamid Q. GM-CSF, IL-5 and RANTES immunoreactivity and mRNA expression in chronic hyperplastic sinusitis with nasal polyposis (NP) Clin Exp Allergy. 1998;28:1145–1152. doi: 10.1046/j.1365-2222.1998.00380.x. [DOI] [PubMed] [Google Scholar]
- 57.Stevens WW, Ocampo CJ, Berdnikovs S, Sakashita M, Mahdavinia M, Suh L, et al. Cytokines in chronic rhinosinusitis. Role in eosinophilia and aspirin-exacerbated respiratory disease. Am J Respir Crit Care Med. 2015;192:682–694. doi: 10.1164/rccm.201412-2278OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steinke JW, Liu L, Huyett P, Negri J, Payne SC, Borish L. Prominent role of IFN-γ in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2013;132:856–865.e1-3. doi: 10.1016/j.jaci.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pezato R, Świerczyńska-Krępa M, Niżankowska-Mogilnicka E, Holtappels G, De Ruyck N, Sanak M, et al. Systemic expression of inflammatory mediators in patients with chronic rhinosinusitis and nasal polyps with and without Aspirin Exacerbated Respiratory Disease. Cytokine. 2016;77:157–167. doi: 10.1016/j.cyto.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 60.Choi Y, Lee Y, Park HS. Which factors associated with activated eosinophils contribute to the pathogenesis of aspirin-exacerbated respiratory disease? Allergy Asthma Immunol Res. 2019;11:320–329. doi: 10.4168/aair.2019.11.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palme S, Christenson RH, Jortani SA, Ostlund RE, Kolm R, Kopal G, et al. Multicenter evaluation of analytical characteristics of the Elecsys® Periostin immunoassay. Clin Biochem. 2017;50:139–144. doi: 10.1016/j.clinbiochem.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 62.Kim MA, Izuhara K, Ohta S, Ono J, Yoon MK, Ban GY, et al. Association of serum periostin with aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2014;113:314–320. doi: 10.1016/j.anai.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 63.Choi Y, Lee DH, Lee JH, Shin YS, Kim SH, Park HS. Immunomodulatory function of surfactant protein D in eosinophilic asthma. Allergy. 2019;74:192–195. doi: 10.1111/all.13588. [DOI] [PubMed] [Google Scholar]
- 64.Choi Y, Sim S, Lee DH, Lee HR, Ban GY, Shin YS, et al. Effect of TGF-β1 on eosinophils to induce cysteinyl leukotriene E4 production in aspirin-exacerbated respiratory disease. PLoS One. 2021;16:e0256237. doi: 10.1371/journal.pone.0256237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sim S, Choi Y, Lee DH, Lee HR, Shin YS, Park HS. Contribution of dipeptidyl peptidase 10 to airway dysfunction in patients with NSAID-exacerbated respiratory disease. Clin Exp Allergy. 2022;52:115–126. doi: 10.1111/cea.14003. [DOI] [PubMed] [Google Scholar]
- 66.Kim SH, Choi H, Yoon MG, Ye YM, Park HS. Dipeptidyl-peptidase 10 as a genetic biomarker for the aspirin-exacerbated respiratory disease phenotype. Ann Allergy Asthma Immunol. 2015;114:208–213. doi: 10.1016/j.anai.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 67.Dahlin A, Weiss ST. Genetic and epigenetic components of aspirin-exacerbated respiratory disease. Immunol Allergy Clin North Am. 2016;36:765–789. doi: 10.1016/j.iac.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choi Y, Lee DH, Trinh HK, Ban GY, Park HK, Shin YS, et al. Surfactant protein D alleviates eosinophil-mediated airway inflammation and remodeling in patients with aspirin-exacerbated respiratory disease. Allergy. 2019;74:78–88. doi: 10.1111/all.13458. [DOI] [PubMed] [Google Scholar]
- 69.Benfante A, Battaglia S, Principe S, Di Mitri C, Paternò A, Spatafora M, et al. Asthmatics with high levels of serum surfactant protein D have more severe disease. Eur Respir J. 2016;47:1864–1867. doi: 10.1183/13993003.02142-2015. [DOI] [PubMed] [Google Scholar]
- 70.Kim JH, Kim S, Park SY, Kim HJ, An J, Kwon HS, et al. Serum folliculin is related to lower pulmonary function in patients with asthma. Allergy Asthma Immunol Res. 2021;13:822–826. doi: 10.4168/aair.2021.13.5.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takahashi T, Kato A, Berdnikovs S, Stevens WW, Suh LA, Norton JE, et al. Microparticles in nasal lavage fluids in chronic rhinosinusitis: potential biomarkers for diagnosis of aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2017;140:720–729. doi: 10.1016/j.jaci.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trinh HK, Pham DL, Choi Y, Kim HM, Kim SH, Park HS. Epithelial folliculin enhances airway inflammation in aspirin-exacerbated respiratory disease. Clin Exp Allergy. 2018;48:1464–1473. doi: 10.1111/cea.13253. [DOI] [PubMed] [Google Scholar]
- 73.Laidlaw TM, Boyce JA. Platelets in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2015;135:1407–1414. doi: 10.1016/j.jaci.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Laidlaw TM, Kidder MS, Bhattacharyya N, Xing W, Shen S, Milne GL, et al. Cysteinyl leukotriene overproduction in aspirin-exacerbated respiratory disease is driven by platelet-adherent leukocytes. Blood. 2012;119:3790–3798. doi: 10.1182/blood-2011-10-384826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mitsui C, Kajiwara K, Hayashi H, Ito J, Mita H, Ono E, et al. Platelet activation markers overexpressed specifically in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2016;137:400–411. doi: 10.1016/j.jaci.2015.05.041. [DOI] [PubMed] [Google Scholar]
- 76.Kowalski ML, Wardzyńska A, Makowska JS. Clinical trials of aspirin treatment after desensitization in aspirin-exacerbated respiratory disease. Immunol Allergy Clin North Am. 2016;36:705–717. doi: 10.1016/j.iac.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 77.Tyrak KE, Kupryś-Lipińska I, Czarnobilska E, Jakieła B, Pajdzik K, Ćmiel A, et al. Sputum biomarkers during aspirin desensitization in nonsteroidal anti-inflammatory drugs exacerbated respiratory disease. Respir Med. 2019;152:51–59. doi: 10.1016/j.rmed.2019.04.021. [DOI] [PubMed] [Google Scholar]
- 78.Klaewsongkram J, Buranapraditkun S, Mongkolpathumrat P, Palapinyo S, Chantaphakul H. Clinical characteristics, urinary leukotriene E4 levels, and aspirin desensitization results in patients with NSAID-induced blended reactions. Allergy Asthma Immunol Res. 2021;13:229–244. doi: 10.4168/aair.2021.13.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dahlén SE, Malmström K, Nizankowska E, Dahlén B, Kuna P, Kowalski M, et al. Improvement of aspirin-intolerant asthma by montelukast, a leukotriene antagonist: a randomized, double-blind, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165:9–14. doi: 10.1164/ajrccm.165.1.2010080. [DOI] [PubMed] [Google Scholar]
- 80.Dahlén B, Nizankowska E, Szczeklik A, Zetterström O, Bochenek G, Kumlin M, et al. Benefits from adding the 5-lipoxygenase inhibitor zileuton to conventional therapy in aspirin-intolerant asthmatics. Am J Respir Crit Care Med. 1998;157:1187–1194. doi: 10.1164/ajrccm.157.4.9707089. [DOI] [PubMed] [Google Scholar]
- 81.Berges-Gimeno MP, Simon RA, Stevenson DD. The effect of leukotriene-modifier drugs on aspirin-induced asthma and rhinitis reactions. Clin Exp Allergy. 2002;32:1491–1496. doi: 10.1046/j.1365-2745.2002.01501.x. [DOI] [PubMed] [Google Scholar]
- 82.Tuttle KL, Schneider TR, Henrickson SE, Morris D, Abonia JP, Spergel JM, et al. Aspirin-exacerbated respiratory disease: not always “adult-onset”. J Allergy Clin Immunol Pract. 2016;4:756–758. doi: 10.1016/j.jaip.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hayashi H, Mitsui C, Nakatani E, Fukutomi Y, Kajiwara K, Watai K, et al. Omalizumab reduces cysteinyl leukotriene and 9α,11β-prostaglandin F2 overproduction in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2016;137:1585–1587.e4. doi: 10.1016/j.jaci.2015.09.034. [DOI] [PubMed] [Google Scholar]
- 84.Hayashi H, Fukutomi Y, Mitsui C, Kajiwara K, Watai K, Kamide Y, et al. Omalizumab for aspirin hypersensitivity and leukotriene overproduction in aspirin-exacerbated respiratory disease. A randomized controlled trial. Am J Respir Crit Care Med. 2020;201:1488–1498. doi: 10.1164/rccm.201906-1215OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jang JH, Woo SD, Lee Y, Kim CK, Shin YS, Ye YM, et al. Changes in type 2 biomarkers after anti-IL5 treatment in patients with severe eosinophilic asthma. Allergy Asthma Immunol Res. 2021;13:330–338. doi: 10.4168/aair.2021.13.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Agache I, Cojanu C, Laculiceanu A, Rogozea L. Critical points on the use of biologicals in allergic diseases and asthma. Allergy Asthma Immunol Res. 2020;12:24–41. doi: 10.4168/aair.2020.12.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mustafa SS, Vadamalai K, Scott B, Ramsey A. Dupilumab as add-on therapy for chronic rhinosinusitis with nasal polyposis in aspirin exacerbated respiratory disease. Am J Rhinol Allergy. 2021;35:399–407. doi: 10.1177/1945892420961969. [DOI] [PubMed] [Google Scholar]
- 88.Bochner BS, Stevens WW. Biology and function of eosinophils in chronic rhinosinusitis with or without nasal polyps. Allergy Asthma Immunol Res. 2021;13:8–22. doi: 10.4168/aair.2021.13.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rodríguez-Jiménez JC, Moreno-Paz FJ, Terán LM, Guaní-Guerra E. Aspirin exacerbated respiratory disease: current topics and trends. Respir Med. 2018;135:62–75. doi: 10.1016/j.rmed.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 90.Laidlaw TM, Cahill KN, Cardet JC, Murphy K, Cui J, Dioneda B, et al. A trial of P2Y12 receptor inhibition with prasugrel identifies a potentially distinct endotype of patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2019;143:316–324.e7. doi: 10.1016/j.jaci.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brightling CE, Gaga M, Inoue H, Li J, Maspero J, Wenzel S, et al. Effectiveness of fevipiprant in reducing exacerbations in patients with severe asthma (LUSTER-1 and LUSTER-2): two phase 3 randomised controlled trials. Lancet Respir Med. 2021;9:43–56. doi: 10.1016/S2213-2600(20)30412-4. [DOI] [PubMed] [Google Scholar]