Abstract
We report on a detailed study on the molecular diversity and evolutionary relationships of Tn1546-like elements in vancomycin-resistant enterococci (VRE) from humans and animals. Restriction fragment length polymorphism (RFLP) analysis of the VanA transposon of 97 VRE revealed seven different Tn1546 types. Subsequent sequencing of the complete VanA transposons of 13 VRE isolates representing the seven RFLP types followed by sequencing of the identified polymorphic regions in 84 other VanA transposons resulted in the identification of 22 different Tn1546 derivatives. Differences between the Tn1546 types included point mutations in orf1, vanS, vanA, vanX, and vanY. Moreover, insertions of an IS1216V-IS3-like element in orf1, of IS1251 in the vanS-vanH intergenic region, and of IS1216V in the vanX-vanY intergenic region were found. The presence of insertion sequence elements was often associated with deletions in Tn1546. Identical Tn1546 types were found among isolates from humans and farm animals in The Netherlands, suggesting the sharing of a common vancomycin resistance gene pool. Application of the genetic analysis of Tn1546 to VRE isolates causing infections in hospitals in Oxford, United Kingdom, and Chicago, Ill., suggested the possibility of the horizontal transmission of the vancomycin resistance transposon. The genetic diversity in Tn1546 combined with epidemiological data suggest that the DNA polymorphism among Tn1546 variants can successfully be exploited for the tracing of the routes of transmission of vancomycin resistance genes.
In recent years, the nosocomial prevalence of infections caused by vancomycin-resistant enterococci (VRE) has increased significantly in the United States (13, 36), while virtually no VRE have been found in the gut flora of healthy people (16). The epidemiology of VRE in Europe differs from that in the United States. The prevalence of VRE in Europe is low among strains causing hospital-associated infections (20, 22, 44), while VanA-positive enterococci can easily be detected outside the hospital in several European countries (20, 43, 46, 47, 48, 49, 51). A possible source of VRE is the food chain since VRE have been isolated from farm animals and animal products in several European countries (2, 7, 8, 14, 17, 33, 48, 49, 50, 53). It has been suggested that the use of the antibiotic avoparcin as a feed additive in animal husbandry in numerous European countries has resulted in the selection of vancomycin resistance in strains from farm animals (1, 7, 32). This is consistent with the lack of non-hospital-associated VRE in the United States, where the use of avoparcin has not been permitted (16).
Although resistance to glycopeptides has spread primarily in enterococci, vanA- and vanB-related genes were recently isolated from various other gram-positive bacteria like Arcanobacterium haemolyticum (41), Oerskovia turbata (41), Streptococcus bovis (42), and Bacillus circulans (21). Vancomycin resistance may disseminate to other pathogens, such as methicillin-resistant Staphylococcus aureus strains, which would result in a highly dangerous pathogen that could cause an infection that would be difficult to treat with currently available antibiotics. Indeed, conjugative transfer of glycopeptide resistance from Enterococcus faecalis to S. aureus has been reported under laboratory conditions (39). The possibility that such a transfer will eventually occur in nature stresses the need to limit the spread of VRE and to gain insight into the factors that contribute to the selection of VRE and the routes of dissemination.
The genes encoding the VanA and VanB types of vancomycin resistance are located on mobile DNA elements. Therefore, the horizontal transfer of resistance genes among enterococci may have a more significant impact on the dissemination of vancomycin resistance than the clonal spread of resistant enterococci. The isolation of genetically unrelated VREs during well-documented nosocomial outbreaks suggests such a mechanism (10, 15, 24, 35, 38). Thus, direct comparison of the vancomycin resistance determinants may provide additional insight into the epidemiology of vancomycin resistance. The vanA gene is the most frequently encountered gene among isolates causing VRE infections in humans (9, 19, 20, 22, 31). This gene is part of the transposable element Tn1546, which was first characterized in 1993 by Arthur et al. (5). Genetic heterogeneity in Tn1546-related elements has been documented previously (3, 5, 23, 27, 29, 34, 37, 48, 50, 54, 55). The polymorphisms described so far have included insertion of the insertion sequence (IS) elements IS1216V, IS1251, IS1476, and IS1542 and deletions at both the left (orf1 side) and right (vanZ side) ends of the transposon that includes the orf1 and vanZ genes. Recently, a point mutation in the vanX gene has been described (29, 48).
The aim of the present study was to perform a detailed molecular characterization of the DNA polymorphisms in the VanA gene cluster originating from human and animal sources. By means of restriction fragment length polymorphism (RFLP) analysis and DNA sequencing, 22 different VanA transposon types among 97 VRE strains were identified. Differences included point mutations in the orf1, vanA, vanX, and vanY genes, the presence of the IS elements IS1251 and IS1216V, and deletions associated with IS insertions. Indistinguishable Tn1546-like elements were found among enterococci isolated from human and animal sources, suggesting the existence of a common vancomycin resistance gene pool.
MATERIALS AND METHODS
Bacterial strains.
The VRE used in this study are listed in Table 1. Stool samples from nonhospitalized individuals were collected and cultured in kanamycin-esculin azide enrichment broth (Oxoid Ltd., Basingstoke, United Kingdom) supplemented with 6 μg of vancomycin per ml. Bacteria from tubes whose contents turned black after 1 or 2 days of incubation at 37°C were subcultured onto Slanetz and Bartley agar (Oxoid Ltd.) supplemented with 6 μg of vancomycin per ml. VRE were identified to the species level and were tested for the presence of the vanA gene by means of a PCR described by Dutka-Malen et al. (18). Fecal samples from veal calves were examined as described above. Dutch clinical isolates (isolates 11 to 21), pig isolates (isolates 27 to 37), and chicken isolates (isolates 38 to 45) have been described previously (20, 49, 50), as have the isolates from the United Kingdom (isolates 46 to 87) (8, 31) and the United States (isolates 88 to 97) (11, 12).
TABLE 1.
Enterococcal isolates used in this study
| Strain no. | Strain | Enterococcal species | Source | Countrya | Tn1546-types | Reference |
|---|---|---|---|---|---|---|
| 1 | 9600188 | E. faecium | Human stool | NL | A2 | This study |
| 2 | 9600205 | E. faecalis | Human stool | NL | A1 | This study |
| 3 | 9600220 | E. faecium | Human stool | NL | A1b | This study |
| 4 | 9600224 | E. faecium | Human stool | NL | A2 | This study |
| 5 | 9600253 | E. faecium | Human stool | NL | A2 | This study |
| 6 | 9600266 | E. faecium | Human stool | NL | A2 | This study |
| 7 | 9600276 | E. faecium | Human stool | NL | A1 | This study |
| 8 | 9600291 | E. faecium | Human stool | NL | A1 | This study |
| 9 | 9700196 | E. faecium | Human stool | NL | A1 | This study |
| 10 | 9700228 | E. faecium | Human stool | NL | A2 | This study |
| 11 | 22-R | E. faecium | Human stool | NL | A2 | 20 |
| 12 | 10-A | E. faecium | Human wound | NL | A1 | 20 |
| 13 | 10-B | E. faecium | Human ascites | NL | A2 | 20 |
| 14 | 10-C | E. faecium | Human blood | NL | A2 | 20 |
| 15 | 10-D | E. faecium | Human urine | NL | A2 | 20 |
| 16 | 10-G | E. faecium | Human bile | NL | A2 | 20 |
| 17 | 10-H | E. faecium | Human blood | NL | A2b | 20 |
| 18 | 10-J | E. faecalis | Human ascites | NL | A1 | 20 |
| 19 | 1245964 | E. faecium | Human urine | NL | A2 | This study |
| 20 | 2074639 | E. faecium | Human ascites | NL | A2 | This study |
| 21 | 4252948 | E. faecium | Human ascites | NL | E6 | This study |
| 22 | 1-A2 | E. gallinarum | Veal calf | NL | A1b | This study |
| 23 | 1-A6 | E. flavescens | Veal calf | NL | A3b | This study |
| 24 | 1-A8 | E. faecalis | Veal calf | NL | B1b | This study |
| 25 | 1-A10 | E. avium | Veal calf | NL | A1 | This study |
| 26 | 1-A11 | E. faecium | Veal calf | NL | A4b | This study |
| 27 | A2 | E. faecium | Pig | NL | A2 | 49 |
| 28 | A4 | E. faecium | Pig | NL | A2 | 49 |
| 29 | A16 | E. faecium | Pig | NL | A2 | 49 |
| 30 | B9 | E. faecium | Pig | NL | A2 | 49 |
| 31 | B37 | E. faecium | Pig | NL | A2 | 49 |
| 32 | M4 | E. faecium | Pig | NL | A2 | 49 |
| 33 | M7 | E. faecium | Pig | NL | A2 | 49 |
| 34 | M11 | E. faecium | Pig | NL | A2 | 49 |
| 35 | O12 | E. faecium | Pig | NL | A2 | 49 |
| 36 | O118 | E. faecium | Pig | NL | A2 | 49 |
| 37 | O122 | E. faecium | Pig | NL | A2 | 49 |
| 38 | chicken 2 | E. faecium | Chicken | NL | E3 | 50 |
| 39 | chicken 3 | E. faecium | Chicken | NL | A1 | 50 |
| 40 | chicken 43 | E. faecium | Chicken | NL | A2 | 50 |
| 41 | chicken 48 | E. faecium | Chicken | NL | B2 | 50 |
| 42 | chicken 57 | E. faecium | Chicken | NL | E2b | 50 |
| 43 | chicken 59 | E. faecium | Chicken | NL | A1 | 50 |
| 44 | chicken 69 | E. faecium | Chicken | NL | E5 | 50 |
| 45 | chicken 72 | E. faecium | Chicken | NL | A1 | 50 |
| 46 | 58538 (GP) | E. faecium | Human stool | UK | E2 | 31 |
| 47 | 61741 (GP3) | E. faecium | Human stool | UK | A1 | 8 |
| 48 | 55859 (patient 12) | E. faecium | Human stool | UK | D1b | 31 |
| 49 | 59479 | E. faecium | Human stool | UK | D1 | 31 |
| 50 | 60761 | E. faecium | Human stool | UK | D1 | 31 |
| 51 | 63910 | E. faecium | Human stool | UK | Cb | 31 |
| 52 | 67668 | E. faecium | Human stool | UK | A1 | 31 |
| 53 | 53864 (patient 3) | E. faecium | Human stool | UK | D1 | 31 |
| 54 | 77364 (patient 10) | E. faecium | Human stool | UK | D1 | 31 |
| 55 | 58155 (patient 9) | E. faecium | Human urine | UK | D1 | 31 |
| 56 | 62899 (patient 11) | E. faecium | Human urine | UK | D2 | 31 |
| 57 | 68521 (patient 15) | E. faecium | Human urine | UK | D1 | 31 |
| 58 | 72801 (patient 12) | E. faecium | Human wound | UK | D1 | 31 |
| 59 | 80103 (BC20) | E. faecium | Human blood | UK | D1 | 8 |
| 60 | 89407 (U22) | E. faecium | Human urine | UK | D1 | 8 |
| 61 | 26712 (patient 1) | E. faecium | Human urine | UK | D1 | 31 |
| 62 | 38658 (patient 2) | E. faecium | Human blood | UK | D1 | 31 |
| 63 | 42757 (patient 3) | E. faecium | Human urine | UK | D1 | 31 |
| 64 | 43088 (patient 4) | E. faecium | Human urine | UK | D1 | 31 |
| 65 | 68140 (patient 10) | E. faecium | Human urine | UK | D1 | 31 |
| 66 | 66925 (patient 13) | E. faecium | Human urine | UK | D1 | 31 |
| 67 | 74198 (patient 14) | E. faecium | Human pus | UK | D4 | 31 |
| 68 | 70040 (patient 16) | E. faecium | Human urine | UK | D4 | 31 |
| 69 | 75436 (patient 18) | E. faecium | Human pus | UK | D1 | 31 |
| 70 | S1 (C2) | E. faecium | Sewage inlet A | UK | E1b | 8 |
| 71 | S5 (L#3) | E. faecium | Sewage inlet B | UK | D3 | 8 |
| 72 | S10 (C1) | E. faecium | Sewage inlet A | UK | E7 | 8 |
| 73 | S17 (M7) | E. faecium | Sewage inlet B | UK | E3 | 8 |
| 74 | S25 (M2) | E. faecium | Sewage inlet C | UK | Gb | 8 |
| 75 | S26 (M3) | E. faecium | Sewage inlet C | UK | A2 | 8 |
| 76 | S27 (Mixed 0.1#1) | E. faecium | Sewage inlet A | UK | A1 | 8 |
| 77 | A1 (VF1) | E. faecium | Pig | UK | A2 | 8 |
| 78 | A6 (Pig 22) | E. faecium | Pig | UK | A2 | 8 |
| 79 | A10 (Pig 2,19) | E. faecium | Pig | UK | A2b | 8 |
| 80 | C2 (Sim Chick) | E. faecium | Uncooked chicken | UK | B3 | 8 |
| 81 | C3 (T2) | E. faecium | Uncooked chicken | UK | A1 | 8 |
| 82 | C4 (Chicken 1) | E. faecium | Uncooked chicken | UK | B1 | 8 |
| 83 | C5 (Grade A) | E. faecium | Uncooked chicken | UK | E4 | 8 |
| 84 | C12 (VF4) | E. faecium | Turkey | UK | A1 | 8 |
| 85 | C13 (VF7 alfa) | E. faecium | Duck | UK | A1 | 8 |
| 86 | C14 (VF8) | E. faecium | Chicken | UK | A1 | 8 |
| 87 | C15 (VF9) | E. faecium | Pony | UK | A1 | 8 |
| 88 | VS1 | E. faecium | Human | USA | F2b | 12 |
| 89 | VS2 | E. faecium | Human | USA | F2 | 12 |
| 90 | VS3 | E. faecium | Human | USA | F2 | 12 |
| 91 | VS4 | E. faecium | Human | USA | F1 | 12 |
| 92 | VS5 | E. faecium | Human | USA | F2 | 12 |
| 93 | VS6 | E. faecium | Human | USA | F2 | 12 |
| 94 | VS7 | E. faecium | Human | USA | F2 | 12 |
| 95 | VS8 | E. faecium | Human | USA | F2 | 12 |
| 96 | VS9 | E. faecium | Human | USA | F2 | 12 |
| 97 | VS10 | E. faecium | Human | USA | F2 | 12 |
NL, The Netherlands; UK, United Kingdom; USA, United States.
Tn1546 types which were sequenced entirely.
Susceptibility testing.
MICs were determined by the agar dilution method on Mueller-Hinton II agar plates (BBL, Becton Dickinson, Cockeysville, Md.). Inocula (approximately 108 CFU/ml) were prepared from overnight cultures on Columbia agar plates supplemented with defribrinated horse blood (Oxoid Ltd.). The antimicrobial agents tested were vancomycin (Eli Lilly, Indianapolis, Ind.), teicoplanin (Hoechst Marion Roussel Inc., Frankfurt, Germany), and avoparcin (Roche Pharmaceuticals, Basel, Switzerland).
PFGE.
Pulsed-field gel electrophoresis (PFGE) analysis was performed as described previously (50). The banding patterns were interpreted as described by Tenover et al. (45), and the different types were identified by capital-letter codes.
RFLP analysis.
Genomic DNAs from all VRE were isolated by a modification of the initial steps of the method described by Ausubel et al. (6). The bacterial pellets were suspended in 557 μl of 10 mM Tris–1 mM EDTA, and 10 μl of a 50-mg/ml solution of egg white lysozyme (Boehringer Mannheim, Mannheim, Germany) was added. After incubation for 15 min at 37°C, the bacteria were lysed by the addition of 30 μl of 10% sodium dodecyl sulfate and 3 μl of a 20-mg/ml proteinase K (Merck, Darmstadt, Germany) solution. Subsequently, the protocol described by Ausubel et al. (6) was used. Chromosomal DNA preparations were digested with HaeIII and XbaI (Boehringer Mannheim), respectively, separated by agarose gel electrophoresis (1.5% agarose gels), transferred onto a Hybond N+ nylon membrane (Nycomed Amersham plc, Buckinghamshire, United Kingdom) with a vacuum blotting system (Millipore, Bedford, Mass.), and subsequently hybridized with internal Tn1546 PCR fragments (probes 1, 2, 3, and 4 generated with primers 22.F-1913.R, 3514.F-5374.R, 5235.F-7035.R, and 8544.F-10716.R, respectively; see Table 2 and Fig. 1). Labeling of the PCR fragments and subsequent detection of hybrids were performed as described in the instructions for the ECL direct nucleic acid labeling and detection kit (Nycomed Amersham plc.).
TABLE 2.
PCR and sequence primers used in this study
| Primera | Sequence | Positionsb |
|---|---|---|
| Tn1546 primers | ||
| 22.F | 5′-GGATTTACAACGCTAAGCC | 22–40 |
| 184.R | 5′-ACCATATGTCGCCCTTAG | 184–167 |
| 934.F | 5′-TGTGGATTTGCATCTGC | 934–950 |
| 1009.R | 5′-ACGGTACAACATCTTCGTC | 1009–991 |
| 1292.R | 5′-TTACTCATGGATGTGGCC | 1292–1275 |
| 1723.F | 5′-ACAGGTGAGTCATCAGGC | 1723–1740 |
| 1890.F | 5′-TAAATAATCATAGTCGGCAGG | 1890–1910 |
| 1913.R | 5′-CGTCCTGCCGACTATG | 1913–1898 |
| 1924.R | 5′-TAGGAACTTGCACGTCCT | 1924–1908 |
| 2768.F | 5′-AGGATGGACTAACACCAATC | 2768–2787 |
| 2880.R | 5′-TGCTGTTCAATTAGCTGTTC | 2880–2861 |
| 3514.F | 5′-ACTGTAATGGCTGGTGTTAAC | 3514–3534 |
| 3560.R | 5′-TATCCGAATAAGATCTCGCT | 3560–3542 |
| 3940.R | 5′-ATTTATCAGATTATAGGGCCG | 3940–3920 |
| 3992.F | 5′-TTATTGTGGATGATGAACATG | 3992–4012 |
| 4426.F | 5′-AACGAGAAGCAGTTATCCC | 4426–4444 |
| 4511.R | 5′-TCGGAGCTAACCACATTC | 4511–4494 |
| 5235.F | 5′-ATATCACGTTGGACAAAGC | 5235–5253 |
| 5374.R | 5′-TTCATCGGTCATCTGCAC | 5374–5357 |
| 5747.F | 5′-ACGTTTAGGGTAGAGCTTCC | 5747–5766 |
| 6039.F | 5′-GTTTATGGATGTGAGCAGG | 6039–6057 |
| 6113.R | 5′-TATCGTTGCCATAACGC | 6113–6097 |
| 6964.F | 5′-AAAGGAGACAGGAGCATG | 6964–6981 |
| 7035.R | 5′-TTACGTCATGCTCCTCTGAG | 7035–7017 |
| 7486.R | 5′-CAAAAACAGGATAGGTAAACG | 7486–7466 |
| 7875.F | 5′-CCGCATTGTACTGAACG | 7875–7891 |
| 7986.R | 5′-CAAGCGGTCAATCAGTTC | 7986–7969 |
| 8544.F | 5′-GCATATAGCCTCGAATGG | 8544–8561 |
| 8691.R | 5′-TTACATACGTCGGGTTTCC | 8691–8673 |
| 8969.R | 5′-GATTGTGCCGTTTTGC | 8969–8954 |
| 9519.F | 5′-ACCAGCAGGTTATAGTGAGC | 9519–9538 |
| 9580.R | 5′-TCGTCAAGCTTGATCCTAC | 9580–9562 |
| 9970.R | 5′-GCCATCCTTACCTCCTTG | 9970–9953 |
| 10716.R | 5′-TTTTCCCCTCACTTCACAC | 10716–10698 |
| 10778.F | 5′-TTTAGTGCTGAGGAATTGG | 10778–19796 |
| IS1216V primers | ||
| IS1216V.A | 5′-GGAAAGCAATTTCAGCAG | 254–271 |
| IS1216V.B | 5′-TCGATGCAGATGGTTTAAC | 516–534 |
| IS1216V.C | 5′-CACTTGTAATAGAGGGGGC | 659–641 |
| IS1216V.D | 5′-TGGGATTCCCAATAATACC | 895–913 |
| IS1216V.E | 5′-AGCTTAAATCATAGATACCGTAAGG | 913–935 |
| IS1216V.F | 5′-TTCATCGTCATTCCTCCTCCTG | 243–225 |
The names of the Tn1546 primers indicate the position of the first nucleotide and the orientation of the primer (F, forward; R, reverse).
FIG. 1.
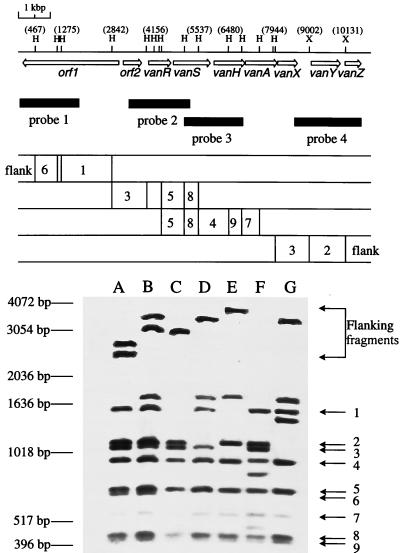
RFLP analysis and physical and genetic maps of Tn1546. The position and direction of transcription of genes and open reading frames (orf’s) are indicated with open arrows. Black horizontal bars indicate the position of internal Tn1546 fragments used as probes (probes 1 to 4). The numbers 1 to 9 represent the restriction fragments visualized after hybridization with the Tn1546-specific probes 1 to 4 and are indicated on the right side of the blot. The positions of the molecular size markers are indicated on the left side of the blot. Letters above the lanes represent the Tn1546 RFLP types. Only the restriction enzyme recognition sites relevant for this study are shown. H, HaeIII; X, XbaI. The positions of some restriction sites are indicated in parentheses.
DNA sequence analysis.
The PCR products described below were purified with a Qiagen PCR purification kit (Qiagen Inc., Hilden, Germany) according to the manufacturer’s instructions. Subsequently, the purified PCR products were sequenced directly with the ABI PRISM Big Dye cycle sequencing ready reaction kit (Perkin-Elmer, Applied Biosystems, Foster City, Calif.) on an ABI PRISM 377 DNA Sequencer (Perkin-Elmer). All VRE isolates were analyzed for the point mutation in the orf1, vanS, and vanX genes. To determine the DNA sequence of the left end of the truncated VanA transposon derivatives, type A2, B3, C, D1 to D4, E1 to E7, F1, F2, and G DNA fragments were amplified with Tn1546 primer 184.R, 1009.R, 1292.R, or 4511R in combination with IS1216 primer IS1216V.B. The exact integration site and orientation of IS1216V in the vanX-vanY intergenic region were determined by amplifying a DNA fragment with primers 7875.F and 10716.R, and the sequence was determined with the IS1216V primers IS1216.E and IS1216.F. Finally, all VRE isolates carrying Tn1546 types F1 and F2 were analyzed for the mutation in the vanA and vanY genes, as determined with isolate VS1, by sequencing the corresponding region of the PCR fragments generated with primers 6964.F, 8691.R, 7875.F, and 10716.R.
RESULTS
RFLP analysis of Tn1546-like elements.
In order to identify polymorphic regions in the vancomycin-resistant transposon Tn1546, 97 different vanA gene-carrying VRE (Table 1) isolated from different sources were analyzed by means of RFLP analysis.
Seven different RFLP patterns, types A to G, were detected (Fig. 1). The banding pattern of type A was identical to the predicted pattern for the published sequence of Tn1546 (5). For types B, D, E, and G, an additional fragment of approximately 1,800 bp was present, suggesting an insertion. The lack of fragment 1 or 6 in types C to G suggests that these transposons had deletions from the left end. Furthermore, the lack of fragment 2 in types D and G suggests polymorphism at the right end of the transposon. No polymorphism was found among the restriction fragments from the central regions of Tn1546, vanR, vanS, vanH, and vanA. The high-molecular-mass bands present in types A to E and G represent DNA fragments flanking the VanA transposon. The absence of flanking fragments in type F is partially explained by deletions from the left end of the transposon (see above). In addition, the flanking fragment at the right end appeared to migrate at the position of fragment 4, while the original fragment 4 in lane F was absent, probably due to a rearrangement in this region.
Sequence analysis of the VanA transposons of representatives of the seven RFLP types.
Thirteen representatives of the seven different Tn1546 RFLP types (strains 3, 17, 22, 23, 24, 26, 42, 48, 51, 70, 74, 79, and 88 [Table 1]) were analyzed in more detail by determining the nucleotide sequence of the entire transposon. Overlapping internal fragments of Tn1546 were amplified and were subsequently sequenced by using combinations of 35 Tn1546-specific primers (Table 2). The sequences that were obtained were compared with the published sequence of Tn1546. Consistent with the RFLP analysis, RFLP types C, D, E, F, and G lacked sequences at the left end of the transposon. In order to determine the exact left ends of the truncated Tn1546-related elements, DNA fragments were amplified with a combination of Tn1546-derived primers and primers based on the insertion element IS1216V. IS1216V was found to be located upstream from Tn1546 in strains of RFLP types D, E, F, and G. In strains of RFLP type C, no IS1216V was present upstream of the transposon, so that the exact left end of the transposon could not be determined and was estimated from the RFLP data to be between 1,275 and 2,842 bp.
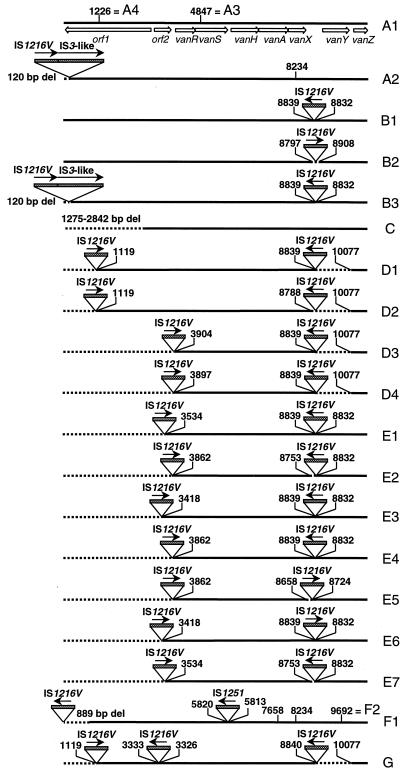
The major rearrangements among the 13 strains investigated were the insertion of a IS1216V-IS3-like element at the left end of the transposon (types A2 and B3), the insertion of one or two copies of IS1216V (types B and D to G), the insertion of one copy of IS1251 (type F), deletions associated with IS insertions downstream of vanX (types D to G), and at the left end of the transposon, deletions that affect the transposase or the resolvase gene (types C to G) (Fig. 2). Insertion of the IS1216V-IS3-like element at the left end of the transposon and insertion of IS1216V in the vanXY intergenic region have been described previously (3, 26). It is interesting that copies of IS1216V inserted in the vanXY intergenic region in strains 24, 42, 48, 70, and 74, which were completely sequenced, contained a synonymous T-to-C point mutation at position 826 relative to the published sequence of IS1216V (GenBank accession no. L40841). In all strains with IS1216V insertions except strains in which the IS insertions were accompanied by small adjacent deletions, an 8-bp duplication of the target sequence (CCCATTGT) was found. Insertion of IS1216V in the vanXY intergenic region also explained the presence of the additional 1.8-kbp fragment in types B, D, E, and G (Fig. 1). Insertion of IS1251 in the vanSH intergenic region resulted in an 8-bp duplication of the target sequence, ATAATTTT. Furthermore, insertion of IS1251 in this region explained the absence of fragment 4 in lane F (Fig. 1). Insertion of IS1251 at this site has also been described previously (27). Furthermore, DNA polymorphism due to point mutations in orf1 (1226), vanS (4847), vanA (7658), vanX (8234), and vanY (9692) were found (Fig. 2). Altogether 11 different Tn1546 types were distinguished among the 13 strains whose transposons were sequenced: type A1 (which is Tn1546), A2, A3, A4, B1, C, D1, E1, E2, F2, and G (Fig. 2).
FIG. 2.
Genetic maps of 22 Tn1546 types. The thick horizontal lines represent the Tn1546 types A1 to A4, B1 to B3, C, D1 to D4, E1 to E7, F1, F2, and G. The positions of genes and open reading frames (orf’s) and the direction of transcription are depicted with open arrows. Dotted boxes represent IS elements. The positions of the first nucleotide upstream and the first nucleotide downstream from the IS insertion sites are depicted. Filled arrows indicate the transcriptional orientations of the inserted IS elements. Deletions (del) are indicated by dotted lines. The positions of base pair mutations are indicated above the different Tn1546 types: 1226, T→A (K→stop); 4847, T→C; 7658, T→C (V→A); 8234, G→T (K→N); 9692, C→T (P→L).
Analysis of the polymorphic regions in Tn1546 in other isolates of VRE.
We analyzed the polymorphic regions of Tn1546 of 87 additional VRE which were initially examined by RFLP analysis. The presence of the point mutations in the vanX, vanS, and orf1 genes, the exact integration sites and the orientations of IS1216V and IS1251, the deletions surrounding the IS1216V insertion site, and the size of the left-end deletion were assessed by means of DNA sequencing. Furthermore, isolates of VRE carrying the type F transposon were analyzed for the point mutation in the vanA and the vanY genes.
DNA sequencing finally distinguished 22 different transposon types. RFLP type A could be subdivided into four subtypes (subtypes A1 to A4), type B could be subdivided into three subtypes (subtypes B1 to B3), type D could be subdivided into four subtypes (subtypes D1 to D4), type E could be subdivided into seven subtypes (subtypes E1 to E7), and type F could be subdivided into two subtypes (subtypes F1 and F2). Types C and G could not be subdivided. On the basis of RFLP analysis, types D3 and D4 were initially designated E subtypes since they both lacked fragments 6, 1, and 3 at the left ends of their transposons. However, since these two types also lacked the vanY gene, which is indicative of type D, they were renamed D3 and D4. The identification of IS1216V in the vanXY intergenic region in types D1, D2, and D4 in strains 46 to 69 contradicts the results published previously by Jensen et al. (29) since in that study the same strains were partly analyzed, but no sequence or size variation was observed in the amplicons of the vanXY intergenic region.
Glycopeptide susceptibility patterns of isolates.
The MICs of vancomycin, teicoplanin, and avoparcin for the 97 different isolates were determined by the agar dilution method. Generally, no association was found between the resistant phenotype and the transposon genotype. All isolates were resistant to vancomycin (MICs at which 50% [MIC50] and 90% [MIC90] of isolates are inhibited, 512 and 1,024 μg/ml, respectively) and avoparcin (MIC50 and MIC90, 256 and 1,024 μg/ml, respectively). Exceptions were strains with deletions of the vanY gene (types D1, D2, D3, D4, and G). These strains were less resistant to teicoplanin (MIC50 and MIC90, 16 and 64 μg/ml, respectively) than strains belonging to the other types (MIC50 and MIC90, 128 and 256 μg/ml, respectively). It is conceivable that the deletion of vanY affects the transcription of vanZ, resulting in a lower MIC of teicoplanin, because vanZ has been shown to be involved in teicoplanin resistance (4, 48).
Tn1546 types among VRE isolated from hospitalized patients.
Our collection of VRE comprised two sets of strains isolated from hospitalized patients. One set of 22 VRE originated from an outbreak at the John Radcliffe Hospital in Oxford, United Kingdom (31). These 22 isolates represented eight different ribotypes and 13 different PFGE types (Table 3), which suggests that at least 13 different enterococcal strains were involved in this outbreak. In contrast, 17 of the 22 isolates contained the same D1 type of Tn1546 (Table 3). Furthermore, an additional three strains harbored either Tn1546 type D2 or Tn1546 type D4, which could be derived from D1 by a single DNA rearrangement (Fig. 3). Tn1546 type D1 was found among nine different strain types.
TABLE 3.
Ribotypes, PFGE types, and Tn1546 types of VRE isolated from the John Radcliffe Hospital, Oxford, and the Cook County Hospital, Chicago
| Strain no. | Source | City | Ribotypea | PFGE typeb | Tn1546 type |
|---|---|---|---|---|---|
| 48 | Human stool | Oxford | 2 | G′ | D1 |
| 49 | Human stool | Oxford | 4 | U | D1 |
| 50 | Human stool | Oxford | 11 | Q | D1 |
| 53 | Human stool | Oxford | 1 | H′ | D1 |
| 54 | Human stool | Oxford | 1 | H | D1 |
| 55 | Human urine | Oxford | 1 | I | D1 |
| 56 | Human urine | Oxford | 4 | G | D2 |
| 57 | Human urine | Oxford | 5 | P | D1 |
| 58 | Human wound | Oxford | 2 | F | D1 |
| 59 | Human blood | Oxford | 6 | P′ | D1 |
| 60 | Human urine | Oxford | 6 | H | D1 |
| 61 | Human urine | Oxford | 1 | H | D1 |
| 62 | Human blood | Oxford | 1 | H | D1 |
| 63 | Human urine | Oxford | 1 | H | D1 |
| 64 | Human urine | Oxford | 1 | H | D1 |
| 65 | Human urine | Oxford | 1 | H | D1 |
| 66 | Human urine | Oxford | 1 | H | D1 |
| 67 | Human pus | Oxford | 1 | H" | D4 |
| 68 | Human urine | Oxford | 1 | H | D4 |
| 69 | Human pus | Oxford | 1 | H | D1 |
| 51 | Human stool | Oxford | 8 | A | C |
| 52 | Human stool | Oxford | 9 | R | A1 |
| 88 | Humanc | Chicago | NDd | UU | F2 |
| 89 | Humanc | Chicago | ND | VV | F2 |
| 90 | Humanc | Chicago | ND | WW | F2 |
| 91 | Humanc | Chicago | ND | XX | F1 |
| 92 | Humanc | Chicago | ND | YY | F2 |
| 93 | Humanc | Chicago | ND | ZZ | F2 |
| 94 | Humanc | Chicago | ND | AAA | F2 |
| 95 | Humanc | Chicago | ND | BBB | F2 |
| 96 | Humanc | Chicago | ND | CCC | F2 |
| 97 | Humanc | Chicago | ND | DDD | F2 |
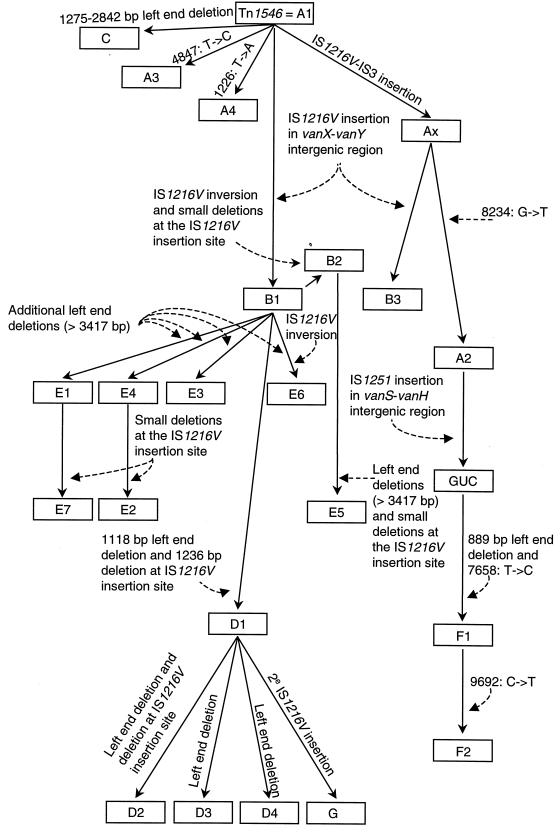
FIG. 3.
Hypothetical evolutionary scheme for the various Tn1546 derivatives characterized in this study from the archetypal transposon Tn1546 (type A1) as described by Arthur et al. in 1993 (5). Boxes represent the different Tn1546 types. Filled arrows indicate the transition of Tn1546 type A1 to the other Tn1546 types. The different DNA rearrangements, insertions, deletions, and point mutations are indicated. Strain GUC has been described by Handwerger et al. (27). 2e, secondary.
A second set of 10 strains originated from a 7-week survey for VRE contamination at Cook County Hospital, Chicago, Ill. (11, 12). All 10 E. faecium strains had different PFGE types (Table 3). Interestingly, all isolates except one contained the same Tn1546 derivative, Tn1546 type F2. One isolate, isolate VS4, contained the type F1 transposon, which differed from type F2 by a single base pair.
The data on the prevalence of transposon types in the Oxford and Chicago hospitals suggest the possibility of horizontal transmission of vancomycin resistance transposon types D1 and F, respectively, among different enterococcal hosts.
DISCUSSION
To facilitate understanding of the molecular epidemiology of vancomycin resistance, we undertook a detailed study of the molecular diversity and the evolutionary relationships of Tn1546-like elements in enterococci from humans and animals. Knowledge of the diversity of Tn1546 is important for distinguishing between the dissemination of a single VRE clone and the transmission of a particular Tn1546 type through a genetically divergent population of enterococci. Typing of VRE by methods such as PFGE and ribotyping has shown the clonal dissemination of VRE in hospitals (9, 25, 35, 40). However, transmission of particular Tn1546 types has not been documented before. Nevertheless, various studies suggest that this occurs since genetic divergence in VRE genomes was found among strains isolated from epidemics caused by VRE (10, 15, 24, 35, 38).
In this study we have identified and characterized polymorphic regions in Tn1546-like elements from 97 VRE originating from animal and human sources. By means of a combination of RFLP analysis and DNA sequencing, 22 different Tn1546-like elements were distinguished. Three types of polymorphisms were found: point mutations, insertions of IS elements, and deletions generally associated with the insertion of IS elements. The point mutations were located in the orf1, vanS, vanA, vanX, and vanY genes. The only point mutation described previously is in the vanX gene at position 8234 (29, 48). Jensen et al. (29) also found this mutation in the vanX gene in three strains which we have also analyzed, strains 77 to 79.
The vast majority (74 of 97) of strains contained one to three copies of the insertion sequence IS1216V inserted in the vancomycin resistance transposon. Insertion of this IS element in the vanXY intergenic region and its presence on either side of Tn1546 have been described previously (3, 26, 28). The presence of IS element insertions was often associated with deletions, a phenomenon which has been described previously (30, 52). Thirty isolates containing the type A2 and B3 VanA transposons had similar genetic organizations at the left end of the VanA transposon, as in strain GUC described by Handwerger and Skoble (26). In these types as well as in strain GUC, a copy of an IS1216V-IS3 like element is present at the left end of the VanA transposon, resulting in a deletion of the first 120 bp. In strain GUC the Tn1546-like element is located on a large chromosomal mobile element designated Tn5482. Preliminary analysis of two representative isolates carrying type A2 transposons indicated a chromosomal location of the VanA element (data not shown), which is similar to the case for strain GUC, which may suggest that type A2 and B3 VanA transposons are part of a larger chromosomal mobile element. In strains 77 to 79 the presence of the IS1216V-IS3 element at the left end of the Tn1546-like element is consistent with the finding of Jensen et al. (29). In addition to IS1216V, insertions of IS1251 in the vanSH intergenic region were found. Although the insertion of IS1251 at this site was published previously, the transposon in E. faecium GUC described by Handwerger and colleagues (26, 27) was clearly distinct from the Tn1546 type F transposon, since no insertion of an IS1216V-IS3 like element was present directly upstream from Tn1546 in the type F transposons.
Remarkable was the finding that 72 (74%) of the analyzed strains (types A2, B3, C, D1 to D4, E1 to E7, F1, F2, and G) carried small or large deletions in the transposase and resolvase regions of the Tn1546-like transposon. A similar finding has recently been reported by others (55). Although it is expected that deletions in the transposase and resolvase regions which abolish transposition may affect the dissemination of truncated Tn1546-like elements, other studies have shown that Tn1546-like elements are often part of chromosomal mobile elements (26) or plasmids that can be mobilized (28).
In this study we investigated in detail the polymorphism in Tn1546 with the aim of exploiting differences in this genetic element for future studies on the epidemiology of vancomycin resistance. Because we examined a large number of strains from a variety of sources, some preliminary conclusions may be drawn. Tn1546 types A1 and A2 were the most prevalent in The Netherlands both among isolates from humans and among isolates from farm animals (Table 4), suggesting an epidemiological link between animal and human reservoirs. The presence of identical VanA transposons in VRE isolated from humans and animals has also been described recently in Denmark and the United Kingdom (29, 55). In VRE from hospitalized patients in the United States we found transposons which contain insertions of IS1251. So far this IS element was been found only by Handwerger et al. (27), Jensen et al. (29), and MacKinnon et al. (34) in isolates from U.S. patients.
TABLE 4.
Distribution of 22 different Tn1546 derivatives among 97 isolates of VRE from human and animal sources
| Tn1546 type | No. of isolates from the following sourcesa:
|
|||||
|---|---|---|---|---|---|---|
| Human (NL) (n = 21) | Animal (NL) (n = 24) | Human (UK) (n = 24) | Animal (UK) (n = 18) | Human (USA) (n = 10) | Total | |
| A1 | 7 | 5 | 2 | 6 | 20 | |
| A2 | 13 | 12 | 4 | 29 | ||
| A3 | 1 | 1 | ||||
| A4 | 1 | 1 | ||||
| B1 | 1 | 1 | 2 | |||
| B2 | 1 | 1 | ||||
| B3 | 1 | 1 | ||||
| C | 1 | 1 | ||||
| D1 | 17 | 17 | ||||
| D2 | 1 | 1 | ||||
| D3 | 1 | 1 | ||||
| D4 | 2 | 2 | ||||
| E1 | 1 | 1 | ||||
| E2 | 1 | 1 | 2 | |||
| E3 | 1 | 1 | 2 | |||
| E4 | 1 | 1 | ||||
| E5 | 1 | 1 | ||||
| E6 | 1 | 1 | ||||
| E7 | 1 | 1 | ||||
| F1 | 1 | 1 | ||||
| F2 | 9 | 9 | ||||
| G | 1 | 1 | ||||
| Total | 21 | 24 | 24 | 18 | 10 | 97 |
NL, The Netherlands; UK, United Kingdom; USA, United States; n, total number of isolates from that source.
It is intriguing that the majority of the transposon types found in hospitals in the United Kingdom and the United States (types D1 and F2) have no counterpart in animals. For the U.S. isolates, this is explained by the fact that so far no VRE have been isolated from animals in the United States. The fact that no D types were found among the isolates from animals in the United Kingdom may suggest that once it was introduced in the Oxford hospital the VanA transposon has evolved independently from the transposons from counterpart strains from animals. This is consistent with the scheme presented in Fig. 3. Figure 3 depicts a hypothetical evolutionary scheme in an attempt to explain the relationships between the 22 transposon types. In Fig. 3 transposon types D (types D1, D2, and D4) and F (types F1 and F2) are located separately from the majority of the subtypes found outside hospitals. In the scheme presented in Fig. 3 we assume that the various Tn1546 variants evolved by base pair substitutions, transpositions, and deletions. We did not include homologous recombination events, although they could lead to a more parsimonious phylogeny. The preliminary data on region specificity suggest that geographic isolation contributed to differences in the prevalence of particular Tn1546 subtypes at different geographic sites.
The combination of the polymorphism in Tn1546 and the epidemiological data indicate that the DNA polymorphism among Tn1546 variants can be exploited successfully for the tracing of the routes of transmission of vancomycin resistance genes. Indicative of this is the finding of identical or closely related VanA transposon types among genetically different enterococci in the Oxford hospital as well as in the hospital in Chicago. Studies are in progress to use the tools developed in this study to investigate in detail the prevalence of subtypes of Tn1546 among humans and animals. This may resolve the controversial issue of the spillover of vancomycin resistance to humans from the animal reservoir due to the use in animal husbandry of glycopeptide antibiotics, such as avoparcin, for growth promotion. Avoparcin has been used in Europe for more than 20 years, but it is anticipated that the current ban on the veterinary use of this antibiotic will also lead to an overall decrease in the frequency of vancomycin resistance among enterococci colonizing the human digestive tract.
ACKNOWLEDGMENTS
We are grateful to Marc Bonten, Cook County Hospital, Chicago, Ill., for providing the strains from the United States; Hubert Endtz, Erasmus Medical Center Rotterdam, Rotterdam, The Netherlands, for providing the Dutch clinical isolates; J. Zoe Jordens, John Radcliffe Hospital, Oxford, United Kingdom, for providing the English strains; and Ellen Stobberingh and Ton van den Bogaard, University Hospital Maastricht, Maastricht, The Netherlands, for providing the isolates from Dutch pigs. We thank Remco van den Hoek and Ans van Veen from the National Institute of Public Health, Bilthoven, The Netherlands, and Kees Veldman from the DLO-Institute for Animal Science and Health, Lelystad, The Netherlands, for technical support. Finally, we thank Han de Neeling and Tjeerd Kimman from the National Institute of Public Health, Bilthoven, The Netherlands, for helpful discussions and critical reading of the manuscript.
REFERENCES
- 1.Aarestrup F M. Occurrence of glycopeptide resistance among Enterococcus faecium isolated from conventional and ecological poultry farms. Microb Drug Resist. 1995;1:255–257. doi: 10.1089/mdr.1995.1.255. [DOI] [PubMed] [Google Scholar]
- 2.Aarestrup F M, Ahrens P, Madsen M, Pallesen L V, Poulsen R L, Westh H. Glycopeptide susceptibility among Danish Enterococcus faecium and Enterococcus faecalis isolates of animal and human origin and PCR identification of genes within the VanA cluster. Antimicrob Agents Chemother. 1996;40:1938–1940. doi: 10.1128/aac.40.8.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arthur M, Depardieu F, Gerbaud G, Galimand M, Leclercq R, Courvalin P. The VanS sensor negatively controls VanR-mediated transcriptional activation of glycopeptide resistance genes of Tn1546 and related elements in the absence of induction. J Bacteriol. 1997;179:97–106. doi: 10.1128/jb.179.1.97-106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arthur M, Depardieu F, Molinas C, Reynolds P, Courvalin P. The vanZ gene of Tn1546 from Enterococcus faecium BM4147 confers resistance to teicoplanin. Gene. 1995;154:87–92. doi: 10.1016/0378-1119(94)00851-i. [DOI] [PubMed] [Google Scholar]
- 5.Arthur M, Molinas C, Depardieu F, Courvalin P. Characterization of Tn1546, a Tn3-related transposon conferring glycopeptide resistance by synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J Bacteriol. 1993;175:117–127. doi: 10.1128/jb.175.1.117-127.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl L. Current protocols in molecular biology, section 2.4. Brooklyn, N.Y: Greene Publishing Associates; 1989. [Google Scholar]
- 7.Bager F, Madsen M, Christensen J, Aarestrup F M. Avoparcin used as a growth promoter is associated with the occurrence of vancomycin-resistant Enterococcus faecium on Danish poultry and pig farms. Prev Vet Med. 1997;31:95–112. doi: 10.1016/s0167-5877(96)01119-1. [DOI] [PubMed] [Google Scholar]
- 8.Bates J, Jordens J Z, Griffiths D T. Farm animals as a putative reservoir for vancomycin-resistant enterococcal infection in man. J Antimicrob Chemother. 1994;34:507–514. doi: 10.1093/jac/34.4.507. [DOI] [PubMed] [Google Scholar]
- 9.Biavasco F, Miele A, Vignaroli C, Manso E, Lupidi R, Varaldo P E. Genotypic characterization of a nosocomial outbreak of VanA Enterococcus faecalis. Microb Drug Resist. 1996;2:231–237. doi: 10.1089/mdr.1996.2.231. [DOI] [PubMed] [Google Scholar]
- 10.Bingen E H, Denamur E, Lambert-Zechovsky N Y, Elion J. Evidence for the genetic unrelatedness of nosocomial vancomycin-resistant Enterococcus faecium strains in a pediatric hospital. J Clin Microbiol. 1991;29:1888–1892. doi: 10.1128/jcm.29.9.1888-1892.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonten M J M, Hayden M K, Nathan C, Rice T W, Weinstein R A. Stability of vancomycin-resistant enterococcal genotypes isolated from long-term-colonized patients. J Infect Dis. 1998;177:378–382. doi: 10.1086/514196. [DOI] [PubMed] [Google Scholar]
- 12.Bonten M J M, Hayden M K, Nathan C, van Voorhis J, Matushek M, Slaughter S, Rice T, Weinstein R A. Epidemiology of colonisation of patients and environment with vancomycin-resistant enterococci. Lancet. 1996;348:1615–1619. doi: 10.1016/S0140-6736(96)02331-8. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Nosocomial enterococci resistant to vancomycin—United States, 1989–1993. Morbid Mortal Weekly Rep. 1993;42:597–599. [PubMed] [Google Scholar]
- 14.Chadwick P R, Woodford N, Kaczmarski E B, Gray S, Barrell R A, Oppenheim B A. Glycopeptide-resistant enterococci isolated from uncooked meat. J Antimicrob Chemother. 1996;38:908–909. doi: 10.1093/jac/38.5.908. [DOI] [PubMed] [Google Scholar]
- 15.Clark N C, Cooksey R C, Hill B C, Swenson J M, Tenover F C. Characterization of glycopeptide-resistant enterococci from U.S. hospitals. Antimicrob Agents Chemother. 1993;37:2311–2317. doi: 10.1128/aac.37.11.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coque T M, Tomayko J F, Ricke S C, Okhyusen P C, Murray B E. Vancomycin-resistant enterococci from nosocomial, community, and animal sources in the United States. Antimicrob Agents Chemother. 1996;40:2605–2609. doi: 10.1128/aac.40.11.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devriese L A, Ieven M, Goossens H, Vandamme P, Pot B, Hommez J, Haesebrouck F. Presence of vancomycin-resistant enterococci in farm and pet animals. Antimicrob Agents Chemother. 1996;40:2285–2287. doi: 10.1128/aac.40.10.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dutka-Malen S, Evers S, Courvalin P. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J Clin Microbiol. 1995;33:24–27. doi: 10.1128/jcm.33.1.24-27.1995. . (Erratum, 33:1434.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eliopoulos G M, Wennersten C B, Gold H S, Schulin T, Souli M, Farris M G, Cerwinka S, Nadler H L, Dowzicky M, Talbot G H, Moellering R C. Characterization of vancomycin-resistant Enterococcus faecium isolates from the United States and their susceptibility in vitro to dalfopristin-quinupristin. Antimicrob Agents Chemother. 1998;42:1088–1092. doi: 10.1128/aac.42.5.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Endtz H, van den Braak N, van Belkum A, Kluytmans J A J W, Koeleman J G M, Spanjaard L, Voss A, Weersink A J L, Vandenbroucke-Grauls C M J E, Buiting A G M, van Duin A, Verbrugh H A. Fecal carriage of vancomycin-resistant enterococci in hospitalized patients and those living in the community in The Netherlands. J Clin Microbiol. 1997;35:3026–3031. doi: 10.1128/jcm.35.12.3026-3031.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fontana R, Ligozzi M, Pedrotti C, Padovani E M, Cornaglia G. Vancomycin-resistant Bacillus circulans carrying the vanA gene responsible for vancomycin resistance in enterococci. Eur J Clin Microbiol Infect Dis. 1997;16:473–474. doi: 10.1007/BF02471915. [DOI] [PubMed] [Google Scholar]
- 22.Gordts B, Van Landuyt H, Ieven M, Vandamme P, Goossens H. Vancomycin-resistant enterococci colonizing the intestinal tracts of hospitalized patients. J Clin Microbiol. 1995;33:2842–2846. doi: 10.1128/jcm.33.11.2842-2846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haaheim H, Dahl K H, Simonsen G S, Olsvik O, Sundsfjord A. Long PCRs of transposons in the structural analysis of genes encoding acquired glycopeptide resistance in enterococci. BioTechniques. 1998;24:432–437. doi: 10.2144/98243st02. [DOI] [PubMed] [Google Scholar]
- 24.Hall L M, Duke B, Guiney M, Williams R. Typing of Enterococcus species by DNA restriction fragment analysis. J Clin Microbiol. 1992;30:915–919. doi: 10.1128/jcm.30.4.915-919.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Handwerger S, Raucher B, Altarac D, Monka J, Marchione S, Singh K V, Murray B E, Wolff J, Walters B. Nosocomial outbreak due to Enterococcus faecium highly resistant to vancomycin, penicillin, and gentamicin. Clin Infect Dis. 1993;16:750–755. doi: 10.1093/clind/16.6.750. [DOI] [PubMed] [Google Scholar]
- 26.Handwerger S, Skoble J. Identification of chromosomal mobile element conferring high-level vancomycin resistance in Enterococcus faecium. Antimicrob Agents Chemother. 1995;39:2446–2453. doi: 10.1128/aac.39.11.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Handwerger S, Skoble J, Discotto L F, Pucci M J. Heterogeneity of the vanA gene cluster in clinical isolates of enterococci from the northeastern United States. Antimicrob Agents Chemother. 1995;39:362–368. doi: 10.1128/aac.39.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heaton M P, Discotto L F, Pucci M J, Handwerger S. Mobilization of vancomycin resistance by transposon-mediated fusion of a VanA plasmid with an Enterococcus faecium sex pheromone-response plasmid. Gene. 1996;171:9–17. doi: 10.1016/0378-1119(96)00022-4. [DOI] [PubMed] [Google Scholar]
- 29.Jensen L B, Ahrens P, Dons L, Jones R N, Hammerum A M, Aarestrup F M. Molecular analysis of Tn1546 in Enterococcus faecium isolated from animals and humans. J Clin Microbiol. 1998;36:437–442. doi: 10.1128/jcm.36.2.437-442.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jilk R A, Makris J C, Borchardt L, Reznikoff W S. Implications of Tn5-associated adjacent deletions. J Bacteriol. 1993;175:1264–1271. doi: 10.1128/jb.175.5.1264-1271.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jordens J Z, Bates J, Griffiths D T. Faecal carriage and nosocomial spread of vancomycin-resistant Enterococcus faecium. J Antimicrob Chemother. 1994;34:515–528. doi: 10.1093/jac/34.4.515. [DOI] [PubMed] [Google Scholar]
- 32.Klare I, Heier H, Claus H, Bohme G, Marin S, Seltmann G, Hakenbeck R, Antanassova V, Witte W. Enterococcus faecium strains with vanA-mediated high level glycopeptide resistance isolated from animal food-stuffs and faecal samples of humans in the community. Microb Drug Resist. 1995;1:265–272. doi: 10.1089/mdr.1995.1.265. [DOI] [PubMed] [Google Scholar]
- 33.Klare I, Heier H, Claus H, Reissbrodt R, Witte W. vanA-mediated high-level glycopeptide resistance in Enterococcus faecium from animal husbandry. FEMS Microbiol Lett. 1995;125:165–171. doi: 10.1111/j.1574-6968.1995.tb07353.x. [DOI] [PubMed] [Google Scholar]
- 34.MacKinnon M G, Drebot M A, Tyrrell G J. Identification and characterization of IS1476, an insertion sequence-like element that disrupts VanY function in a vancomycin-resistant Enterococcus faecium strain. Antimicrob Agents Chemother. 1997;41:1805–1807. doi: 10.1128/aac.41.8.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mato R, Delencastre H, Roberts R B, Tomasz A. Multiplicity of genetic backgrounds among vancomycin-resistant Enterococcus faecium isolates recovered from an outbreak in a New York City Hospital. Microb Drug Resist. 1996;2:309–317. doi: 10.1089/mdr.1996.2.309. [DOI] [PubMed] [Google Scholar]
- 36.McDonald L C, Kuehnert M J, Tenover F C, Jarvis W R. Vancomycin-resistant enterococci outside the health-care setting: prevalence, sources, and public health implications. Emerg Infect Dis. 1997;3:311–317. doi: 10.3201/eid0303.970307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miele A, Bandera M, Goldstein B P. Use of primers selective for vancomycin resistance genes to determine van genotype in enterococci and to study gene organization in VanA isolates. Antimicrob Agents Chemother. 1995;39:1772–1778. doi: 10.1128/aac.39.8.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morris J G, Jr, Shay D K, Hebden J N, McCarter R J, Jr, Perdue B E, Jarvis W, Johnson J A, Dowling T C, Polish L B, Schwalbe R S. Enterococci resistant to multiple antimicrobial agents, including vancomycin. Establishment of endemicity in a university medical center. Ann Intern Med. 1995;123:250–259. doi: 10.7326/0003-4819-123-4-199508150-00002. [DOI] [PubMed] [Google Scholar]
- 39.Noble W C, Virani Z, Cree R G. Co-transfer of vancomycin and other resistance genes from Enterococcus faecalis NCTC 12201 to Staphylococcus aureus. FEMS Microbiol Lett. 1992;72:195–198. doi: 10.1016/0378-1097(92)90528-v. [DOI] [PubMed] [Google Scholar]
- 40.Pegues D A, Pegues C F, Hibberd P L, Ford D S, Hooper D C. Emergence and dissemination of a highly vancomycin-resistant vanA strain of Enterococcus faecium at a large teaching hospital. J Clin Microbiol. 1997;35:1565–1570. doi: 10.1128/jcm.35.6.1565-1570.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Power E G, Abdulla Y H, Talsania H G, Spice W, Aathithan S, French G L. vanA genes in vancomycin-resistant clinical isolates of Oerskovia turbata and Arcanobacterium (Corynebacterium) haemolyticum. J Antimicrob Chemother. 1995;36:595–606. doi: 10.1093/jac/36.4.595. [DOI] [PubMed] [Google Scholar]
- 42.Poyart C, Pierre C, Quesne G, Pron B, Berche P, Trieu-Cuot P. Emergence of vancomycin resistance in the genus Streptococcus: characterization of a vanB transferable determinant in Streptococcus bovis. Antimicrob Agents Chemother. 1997;41:24–29. doi: 10.1128/aac.41.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schouten M A, Hoogkamp-Korstanje J A A, Voss A. Controlling glycopeptide-resistant enterococci. Clin Microbiol Infect. 1997;3:592–593. doi: 10.1111/j.1469-0691.1997.tb00321.x. [DOI] [PubMed] [Google Scholar]
- 44.Suppola J P, Volin L, Valtonen V V, Vaara M. Overgrowth of Enterococcus faecium in the feces of patients with hematologic malignancies. Clin Infect Dis. 1996;23:694–697. doi: 10.1093/clinids/23.4.694. [DOI] [PubMed] [Google Scholar]
- 45.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torres C, Reguera J A, Sanmartin M J, Perez-Diaz J C, Baquero F. vanA-mediated vancomycin-resistant Enterococcus spp. in sewage. J Antimicrob Chemother. 1994;33:553–561. doi: 10.1093/jac/33.3.553. [DOI] [PubMed] [Google Scholar]
- 47.Van Belkum A, van den Braak N, Thomassen R, Verbrugh H, Endtz H. Vancomycin-resistant enterococci in cats and dogs. Lancet. 1996;348:1038–1039. doi: 10.1016/s0140-6736(05)64973-2. [DOI] [PubMed] [Google Scholar]
- 48.Van den Bogaard A E, Jensen L B, Stobberingh E E. Vancomycin-resistant enterococci in turkeys and farmers. N Engl J Med. 1997;337:1558–1559. doi: 10.1056/NEJM199711203372117. [DOI] [PubMed] [Google Scholar]
- 49.Van den Bogaard A E, Mertens P, London N H, Stobberingh E E. High prevalence of colonization with vancomycin- and pristinamycin-resistant enterococci in healthy humans and pigs in The Netherlands: is the addition of antibiotics to animal feeds to blame? J Antimicrob Chemother. 1997;40:454–456. doi: 10.1093/jac/40.3.454. [DOI] [PubMed] [Google Scholar]
- 50.Van den Braak N, van Belkum A, van Keulen M, Vliegenthart J, Verbrugh H A, Endtz H P. Molecular characterization of vancomycin-resistant enterococci from hospitalized patients and poultry products in The Netherlands. J Clin Microbiol. 1998;36:1927–1932. doi: 10.1128/jcm.36.7.1927-1932.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van der Auwera P, Pensart N, Korten V, Murray B E, Leclercq R. Influence of oral glycopeptides on the fecal flora of human volunteers: selection of highly glycopeptide-resistant enterococci. J Infect Dis. 1996;173:1129–1136. doi: 10.1093/infdis/173.5.1129. [DOI] [PubMed] [Google Scholar]
- 52.Wang G, Xu X, Chen J M, Berg D E, Berg C M. Inversions and deletions generated by a mini-gamma delta (Tn1000) transposon. J Bacteriol. 1994;176:1332–1338. doi: 10.1128/jb.176.5.1332-1338.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wegener H C, Madsen M, Nielsen N, Aarestrup F M. Isolation of vancomycin resistant Enterococcus faecium from food. Int J Food Microbiol. 1997;35:57–66. doi: 10.1016/s0168-1605(96)01221-4. [DOI] [PubMed] [Google Scholar]
- 54.Werner G, Klare I, Witte W. Arrangement of the vanA gene cluster in enterococci of different ecological origin. FEMS Microbiol Lett. 1997;155:55–61. doi: 10.1111/j.1574-6968.1997.tb12685.x. [DOI] [PubMed] [Google Scholar]
- 55.Woodford N, Adebiyl A-M A, Palepou M-F I, Cookson B D. Diversity of VanA glycopeptide resistance elements in enterococci from humans and nonhuman sources. Antimicrob Agents Chemother. 1998;42:502–508. doi: 10.1128/aac.42.3.502. [DOI] [PMC free article] [PubMed] [Google Scholar]