Abstract
Aims
The national incidence, risk factors, and associated mortality of atrial fibrillation (AF) in breast cancer patients are unknown.
Methods and results
Using the Surveillance, Epidemiology, and End Results-Medicare-linked database, we identified females, ≥66 years old, with a new primary diagnosis of breast cancer from 2007 through 2014. These patients were individually matched 1:1 to Medicare enrolees without cancer, and each pair was followed for 1 year to identify a primary outcome of AF. Cumulative incidence was calculated using competing risk survival statistics. Following this, identifying risk factors of AF among breast cancer patients was conducted using the adjusted Cox proportional hazards model. Finally, Kaplan–Meier methods and adjusted Cox proportional hazards modelling were performed to estimate mortality in breast cancer patients with incident and prevalent AF. This study included 85 423 breast cancer patients. Among these 9425 (11.0%) had AF diagnosis prior to the breast cancer diagnosis. New-onset AF was diagnosed in 2993 (3.9%) patients in a 1-year period after the breast cancer diagnosis [incidence 3.3%, 95% confidence interval (CI) 3.0–3.5%, at 1 year; higher rate in the first 60 days (0.6%/month)]. Comparatively, the incidence of new-onset AF in matched non-cancer controls was 1.8% (95% CI 1.6–2.0%). Apart from traditional demographic and cardiovascular risk factors, breast cancer stage was strongly associated with the development of AF [American Joint Committee on Cancer (AJCC) Stage II/III/IV vs. I: adjusted hazard ratio (aHR) 1.51/2.63/4.21, respectively]. New-onset AF after breast cancer diagnosis (aHR 3.00) is associated with increased 1-year cardiovascular mortality.
Conclusion
AF incidence is significantly higher in women after a breast cancer diagnosis. Higher breast cancer stages at diagnos are significantly associated with a higher risk of AF. New-onset AF in the new breast cancer diagnosis setting increases 1-year cardiovascular mortality but not breast cancer-related mortality.
Key Question
What are the incidence, prevalence, risk factors and mortality outcomes of atrial fibrillation (AF) in a multi-ethnic representative United States cohort of breast cancer patients?
Key Finding
Annual incidence for AF is 3.9% with highest rate in the first 60 days after cancer diagnosis. Cancer stage and grade are the strongest risk factors for AF. New onset AF after breast cancer increases all-cause and cardiovascular mortality.
Take Home Message
AF incidence is higher in breast cancer patients and is associated with later stage and grade at diagnosis of breast cancer. Involving cardio-oncology in those who develop AF after cancer diagnosis should be encouraged to improve their cardiovascular and overall prognosis.
Keywords: Atrial fibrillation, Breast cancer, Incidence, Risk factors, Mortality, SEER-Medicare
Graphical Abstract
See the editorial comment for this article ‘Atrial fibrillation and breast cancer: casual or causal relationship?’, by Jose L. Merino, https://doi.org/10.1093/eurheartj/ehab807.
Introduction
Atrial fibrillation (AF) is a leading cause of significant thrombotic morbidity and overall cardiovascular mortality.1–4 Over the last two decades, AF has become an increasingly common concern, particularly among patients diagnosed with cancer. In the USA, breast cancer is the leading cause of malignancy among women. Over the last two decades, there has been an increase in the treatment efficacy with resulting increase in survival following a breast cancer diagnosis.
However, increased cardiovascular disease has become a limitation of optimal outcomes among breast cancer patients.5,6 While AF in the non-cancer population is associated with an overall poor prognosis after diagnosis,7–9 little is known regarding the outcomes of AF after cancer diagnosis or how AF affects breast cancer prognosis.6,10–16
Within this study, we leveraged a multi-ethnic representative cohort of early- and late-stage breast cancer patients from the Surveillance, Epidemiology, and End Results (SEER)-Medicare registry to determine the incidence, prevalence, cancer-specific risk factors, and mortality outcomes of AF development among contemporary breast cancer populations.
Methods
Data source
This study used linked SEER-Medicare databases from 2007 to 2014.17 The SEER programme, supported by the National Cancer Institute (NCI), collects data from various state registries and covers 35% of the US population. The Medicare programme insures over 95% of Americans above the age of 65 years. The SEER-Medicare linkage started in 1991 and has been updated every 3–4 years, with the final relevant linkage done in 2014. For each linkage, 95% of persons aged 65 years and older in SEER files were matched to the Medicare enrolment files. SEER provides data from a 5% random sample of Medicare beneficiaries without cancer residing in SEER geographic regions, which enabled us to compare the risk of AF in patients with breast cancer vs. matched patients without cancer.18,19 The Ohio State University's institutional review board approved this study under exempt status due to the de-identified nature of the registry.
Study population
Patients included in this study were females 66 years of age or older diagnosed with any stage of breast cancer between 2007 and 2013. The date of the last follow-up was 31 December 2014.
A breast cancer diagnosis was established using the ICD-O-3 site recode classification C500 to C509. Patients were required to have Medicare Parts A and B and not be members of a health maintenance organization (HMO) for 1 year before and after their breast cancer diagnosis. The identification of comorbidities and AF is not complete for HMO members. In addition, patients should have qualified for Medicare based on age only. Patients were excluded if their cancer was diagnosed at autopsy, their month of cancer diagnosis was missing, if they had a pre-cancerous or in situ lesion only (e.g., ductal carcinoma in situ), or had previously been diagnosed with any cancer.20
The non-cancer control population was matched to breast cancer patients by year of birth, race [White or non-white (Black, Asian, Pacific Islander, others)], SEER registry (a surrogate for geographic region categorized into Northeast, South, Midwest, and West regions), and Charlson comorbidity index in the year before study entry (dichotomized into 0 or ≥1).18,19 Non-cancer patients were ineligible for matching if they lacked Medicare Part A or B coverage, belonged to an HMO, or had a Medicare claim for AF before the index date.
The index date for matching was also referred to as pseudo-diagnosis date in non-cancer controls. Non-cancer patients were first matched using incidence-density sampling, where one breast cancer patient was matched to multiple non-cancer patients. Then, the control group was narrowed to a 1:1 match using a propensity-matched sample using calliper matching where calliper width was set at 10%.
Data extraction and definitions
We used two different methods to determine a new and prior diagnosis of AF. The cohort of breast cancer patients and matched non-cancer patients were merged to their Medicare inpatient, outpatient, and provider claims. These claims were coded using ICD-9-CM codes. Those who had at least one inpatient, one provider, or two outpatient claims for AF (427.31) after a breast cancer diagnosis were considered to have new-onset AF.21 The chronic condition flag file that accompanies the claims file and is a part of the Chronic Condition segment of the Master Beneficiary Summary File was also utilized to identify AF since it is one of the 27 tracked chronic conditions. If the AF diagnosis date appeared in more than one source, then the earliest date of diagnosis was used. Those who were determined to have AF before cancer diagnosis were considered to have a prior diagnosis of AF.
Covariates were divided into four broad groups, namely, demographic, cancer-specific, non-cancer comorbidities, and medications. Further discussion regarding covariates is presented in Supplementary material online, Methods with the definition of each covariate, and the source of data is listed in Supplementary material online, Table S1. A comorbidity score was calculated using the cancer-specific SEER-Medicare comorbidity index and Klabunde's adaptation of the Charlson comorbidity index.22
Outcomes
This study quantifies the incidence of AF in those with a new diagnosis of breast cancer compared to those without cancer, identifies the cancer-specific risk factors that are associated with the incidence of AF, and assesses if incident AF or prior AF is associated with increased mortality after a breast cancer diagnosis. Secondary outcomes include the quantification of cause-specific mortality.
Statistics
Descriptive statistics were used to evaluate baseline characteristics of breast cancer patients stratified by the development of AF. As death is a frequent competing risk in patients with cancer that can preclude AF from developing, competing risk survival statistics accounting for death were used to calculate the cumulative incidence of AF.23 The follow-up was limited to 1 year. The incidence of AF between breast cancer and non-cancer patients was compared by performing the Gray-K test.24 Follow-up was calculated from the case patient's date of cancer diagnosis until AF (event), death (competing risk), or end of study (end of follow-up). We further presented the standardized incidence at 1 year after breast cancer diagnosis.2
To evaluate the association of cancer-specific variables with the development of new-onset AF, all covariates were checked for proportional hazards assumption. Schoenfeld's residual P-values and univariable hazard ratios (HRs) from Cox models for all variables are presented in Supplementary material online, Table S1. If cancer-specific variables did not meet the proportional hazards assumption, then extended Cox models were used. The non-cancer variables that met proportional hazards assumption were used for adjusting in a Fine–Gray competing risk model where cause-specific HRs were presented for cancer-specific risk factors. The non-cancer variables that did not meet the proportional hazards assumption were added as stratifying variables. The final multivariable model was adjusted or stratified for age, race, Hispanic ethnicity, SEER registry, marital status, urban location of the patient, poverty level, marital status, history of obesity, history of smoking, history of hypertension, history of stroke, and SEER-Medicare comorbidity index. The decision to not include variables in the final model was based on the univariable HR results or if the variable was accounted for by the SEER-Medicare index, thus avoiding multicollinearity.
Subgroups of breast cancer grade, American Joint Committee on Cancer (AJCC) stage, first treatment as surgery, and first treatment as radiation therapy were performed. The same cancer-specific variables included in the primary risk factor analysis were considered in each subgroup analysis (Supplementary material online, Figure S1). An interaction term was introduced in the model to study one significant cancer-specific variable's effect in relation to another significant cancer-specific variable. This is also known as a joint test.25 For example, if radiation therapy was significant in the above analysis, effect modification of breast cancer surgery and cancer stage would be evaluated in the subgroup of patients who did and did not undergo radiation therapy. Missing data were not imputed due to sufficient statistical power obtained from patients where data were available. However, none of the cancer variables analysed had >10% missing data.
After appropriate proportional hazards testing, proportions, 1-year overall survival, and HRs were calculated for all-cause mortality, stratified by incident AF and AF prior to breast cancer diagnosis. Kaplan–Meier survival curves were generated to determine median-time-to-event for mortality in those who developed AF. Multivariable Cox proportional hazards models were used to estimate the association between AF and all-cause mortality in the form of crude and adjusted HRs. The adjustment scheme included variables in the following order: demographics, followed by breast cancer-specific variables, followed by cardiovascular comorbidities, anticancer medications, and finally, cardiovascular medications (Supplementary material online, Table S2). This analysis was repeated for cancer-specific mortality, determined by breast cancer cause of death code of 26000, and cardiovascular-specific mortality, determined by death code for ‘disease of heart’ (50060) or ‘cerebrovascular diseases’ (50080). Appropriate ICD-9 codes were utilized to re-classify the cause of death in breast cancer patients with incident AF related to heart failure, ischaemic stroke, systemic embolism, or arrhythmic. Those breast cancer patients who died of other aetiologies contributed person-time to the analysis until the end of follow-up due to mortality from a different cause than being assessed. Survival curves were generated using the unadjusted (Model 1, Supplementary material online, Table S2) and fully adjusted model (Model 6, Supplementary material online, Table S2).
An exploratory analysis to study the role of anticoagulation use and direct-current cardioversion for the outcome of all-cause mortality was performed. Given the limited sample size for those who developed new AF, the Cox proportional hazards model was adjusted for age, race, Hispanic ethnicity, SEER registry, history of hypertension, history of stroke, SEER-Medicare comorbidity index, and AJCC stage.
SAS version 9.4 (Cary, NC, USA) was used for analysis. All statistical tests were two-sided, and a P-value of <0.05 was considered statistically significant.
Results
Demographics
A total of 85 423 patients aged 66 years or older with early and advanced breast cancer were identified (Supplementary material online, Figure S1). Among these, 9425 (11.0%) had an AF diagnosis prior to a breast cancer diagnosis, with a median age of 81 [interquartile range (IQR) 75–85) years. In the 1 year after a breast cancer diagnosis, 2993 (3.9%) patients were diagnosed with new-onset AF, with a median age of 78 (IQR 72–84) years. The demographics of breast cancer patients with a new diagnosis of AF, AF prior to cancer diagnosis, and no AF diagnosis at 1-year follow-up are presented in Table 1. Patients with new-onset or prior AF were older (78 and 81 years, respectively; P < 0.001) than those who did not develop AF (74 years) during the 1-year follow-up. In addition, the incidence of AF after a breast cancer diagnosis was higher in those who had not received surgery (23.5% vs. 10.4%; P < 0.001) or radiation (66.5% vs. 52.3%; P < 0.001) as their first course of therapy and had advanced- vs. early-stage disease at diagnosis (Stage IV 14.8% vs. 6.3%; P < 0.001).
Table 1.
Characteristics of breast cancer patients included in the study from 2007 to 2014
| Variable | New-onset AF after cancer diagnosis (n = 2993) | Prior AF before cancer diagnosis (n = 9425) | No AF at 1-year follow-up (n = (73 005) | P-value* |
|---|---|---|---|---|
| Age at cancer diagnosis, years, median (IQR) | 78 (72–84) | 81 (75–85) | 74 (69–80) | <0.001 |
| Race, n (%) | <0.001 | |||
| White | 2585 (86.4) | 8590 (91.1) | 61 879 (84.8) | |
| Black | 280 (9.4) | 571 (6.1) | 6686 (9.2) | |
| Other | 128 (4.3) | 264 (2.8) | 4440 (6.1) | |
| Hispanic, n (%) | 144 (4.8) | 377 (4.0) | 4721 (6.5) | <0.001 |
| Registry, n (%)a | <0.001 | |||
| West | 1227 (41.0) | 3579 (38.0) | 31 642 (43.3) | |
| Northeast | 649 (21.7) | 2074 (22.0) | 13 423 (18.4) | |
| Midwest | 544 (18.2) | 1837 (19.5) | 12 495 (17.1) | |
| South | 573 (19.1) | 1935 (20.5) | 15 445 (21.2) | |
| Marital status, n (%) | <0.001 | |||
| Unmarried, single | 271 (9.1) | 651 (6.9) | 6440 (8.8) | |
| Married | 1089 (36.4) | 3192 (33.9) | 32 237 (44.2) | |
| Previously married | 1499 (50.1) | 5205 (55.2) | 31 226 (42.8) | |
| Unmarried partnered | 134 (4.5) | 377 (4.0) | 3102 (4.3) | |
| Urban, n (%) | 0.0002 | |||
| Large metro | 1783 (59.6) | 5178 (54.9) | 40 684 (55.8) | |
| Small metro | 805 (26.9) | 2788 (29.6) | 21 768 (29.8) | |
| Other urban areas | 362 (12.1) | 1285 (13.6) | 9285 (12.7) | |
| Rural | 43 (1.4) | 174 (1.9) | 1268 (1.7) | |
| Poverty, n (%) | 0.47 | |||
| 0–<5% | 678 (23.0) | 2228 (24.0) | 17 478 (24.2) | |
| 5–<10% | 837 (28.4) | 2594 (27.9) | 19 793 (27.4) | |
| 10–<20% | 861 (29.2) | 2705 (29.1) | 20 758 (28.8) | |
| 20–100% | 572 (19.4) | 1762 (19.0) | 14 135 (19.6) | |
| Breast cancer characteristicsb | ||||
| Laterality—left, n (%) | 1482 (50.2) | 4742 (50.8) | 37 219 (51.3) | 0.32 |
| Grade, n (%) | <0.001 | |||
| 1 | 481 (18.4) | 2169 (25.6) | 17 595 (26.1) | |
| 2 | 1161 (44.4) | 3839 (45.2) | 31 217 (46.4) | |
| 3 | 958 (36.6) | 2418 (28.5) | 18 188 (27.0) | |
| 4 | 15 (0.6) | 59 (0.7) | 343 (0.5) | |
| AJCC stage, n (%)c | <0.001 | |||
| I | 987 (35.5) | 4205 (48.5) | 37 182 (53.3) | |
| II | 908 (32.6) | 2947 (34.0) | 21 630 (31.0) | |
| III | 476 (17.1) | 945 (10.9) | 6509 (9.3) | |
| IV | 411 (14.8) | 568 (6.6) | 4384 (6.3) | |
| SEER stage, n (%) | <0.001 | |||
| I (localized) | 1540 (52.5) | 6125 (66.7) | 49 692 (69.0) | |
| II (regional direct extension) | 157 (5.4) | 438 (4.8) | 2062 (2.9) | |
| III (regional lymph node extension only) | 599 (20.4) | 1627 (17.7) | 13 206 (18.3) | |
| IV (regional direct and lymph node extension) | 218 (7.4) | 410 (4.5) | 2624 (3.6) | |
| VII (distant) | 417 (14.2) | 587 (6.4) | 4478 (6.2) | |
| Surgical therapy, n (%) | <0.001 | |||
| No surgery | 682 (23.5) | 1573 (17.0) | 7277 (10.4) | |
| Localized therapy such as lumpectomy | 1217 (41.9) | 4618 (50.0) | 40 754 (58.0) | |
| Total simple mastectomy | 452 (15.6) | 1495 (16.2) | 11 173 (15.9) | |
| Modified radical mastectomy | 556 (19.1) | 1557 (16.9) | 11 038 (15.7) | |
| Lymph node surgery, n (%) | <0.001 | |||
| <4 lymph nodes removed | 938 (48.1) | 3460 (55.7) | 34 829 (59.1) | |
| ≥4 lymph nodes removed | 1013 (51.9) | 2756 (44.3) | 24 080 (40.9) | |
| Radiation therapy, n (%) | <0.001 | |||
| No radiotherapy | 1869 (66.5) | 5908 (66.7) | 35 885 (52.3) | |
| Beam radiation | 902 (32.1) | 2731 (30.8) | 30 155 (43.9) | |
| Implanted radiation | 40 (1.4) | 220 (2.5) | 2631 (3.8) | |
| Tumour oestrogen receptor status, n (%) | 2191 (80.3) | 7434 (85.0) | 59 082 (85.4) | <0.001 |
| Tumour progesterone receptor status, n (%) | 1816 (67.0) | 6324 (72.8) | 50 440 (73.4) | <0.001 |
| Tumour HER2 status, n (%)d | 237 (14.0) | 563 (11.3) | 4323 (10.9) | 0.0003 |
| Breast tumour subtype based on combination receptor status, n (%)d | <0.001 | |||
| HER2+/hormone receptor (HR)+ | 158 (9.4) | 400 (8.1) | 2985 (7.5) | |
| HER2+/HR− | 77 (4.6) | 161 (3.2) | 1325 (3.4) | |
| HER2−/HR+ | 1233 (73.4) | 3905 (78.7) | 31 451 (79.5) | |
| HER2−/HR− | 213 (12.7) | 496 (10.0) | 3809 (9.6) | |
| Comorbidities before breast cancer diagnosis, n (%) | ||||
| Hypertension | 22 234 (74.6) | 8874 (94.2) | 48 725 (66.7) | <0.001 |
| Diabetes | 1115 (37.3) | 4295 (45.6) | 20 155 (27.6) | <0.001 |
| Obesitye | 233 (7.8) | 981 (10.4) | 2281 (3.1) | <0.001 |
| History of ischaemic stroke/transient ischaemic attack | 429 (14.3) | 2509 (26.6) | 6552 (9.0) | <0.001 |
| Hyperlipidaemia | 1933 (64.6) | 7944 (84.3) | 46 117 (63.2) | <0.001 |
| History of congestive heart failure | 935 (31.2) | 5824 (61.8) | 11 487 (15.7) | <0.001 |
| History of myocardial infarction | 121 (4.0) | 726 (7.7) | 1310 (1.8) | <0.001 |
| History of ischaemic heart disease | 1340 (44.8) | 7158 (76.0) | 23 205 (31.8) | <0.001 |
| History of lung disease | 714 (23.9) | 3451 (36.6) | 11 852 (16.2) | <0.001 |
| Smokinge | 377 (12.6) | 1388 (14.7) | 4616 (6.3) | <0.001 |
| Peripheral vascular disease | 208 (7.0) | 1090 (11.6) | 2732 (3.7) | <0.001 |
| Rheumatological diseases | 1506 (50.3) | 6526 (69.2) | 32 984 (45.2) | <0.001 |
| Alzheimer’s dementia | 393 (13.1) | 1842 (19.5) | 5982 (8.2) | <0.001 |
| History of depression | 749 (25.0) | 3356 (35.6) | 15 818 (21.7) | <0.001 |
| Chronic kidney disease | 545 (18.2) | 2722 (28.9) | 7909 (10.8) | <0.001 |
| History of anaemia | 1492 (49.9) | 6629 (70.3) | 29 557 (40.5) | <0.001 |
| History of hypothyroidism | 780 (26.1) | 3888 (41.3) | 17 764 (24.3) | <0.001 |
| Charlson comorbidity indexf (mean ± SD) | 0.71 ± 1.60 | 1.18 ± 2.05 | 0.36 ± 1.03 | <0.001 |
| National Cancer Institute comorbidity indexf (mean ± SD) | 0.76 ± 1.51 | 1.28 ± 1.90 | 0.43 ± 1.04 | <0.001 |
Demographics, cancer-specific data, and comorbidities are presented.
AF, atrial fibrillation; HER, human epidermal growth factor receptor; IQR, interquartile range; SD, standard deviation; SEER, Surveillance, Epidemiology, and End Results.
West = San Francisco, Hawaii, New Mexico, Seattle, Utah, San Jose, Los Angeles; Northeast = Connecticut, New Jersey; Midwest = Detroit, Iowa, Kentucky; and South = Atlanta, rural Georgia, Louisiana, greater Georgia.
Present proportions exclude missing data.
II (includes II, II not otherwise specified, IIA, IIB, IIC); III (includes III, III not otherwise specified, IIIA, IIIB, IIIC); IV (includes IV, IV not otherwise specified, IVA, IVB, IVC).
Only available after 2010.
Underreported and overall proportion reported and not just prior to breast cancer diagnosis.
These indices were calculated using macro provided by SEER-Medicare (Klabundke’s modification of Charlson comorbidity index and NCI comorbidity index). Charlson comorbidity index is calculated using 7 years prior to breast cancer diagnosis utilized. NCI comorbidity index is calculated using 1 year prior to breast cancer diagnosis utilized.
P-value is Pearson’s Chi-square test for categorical variables and analysis of variance (ANOVA) for continuous variables.
Incidence
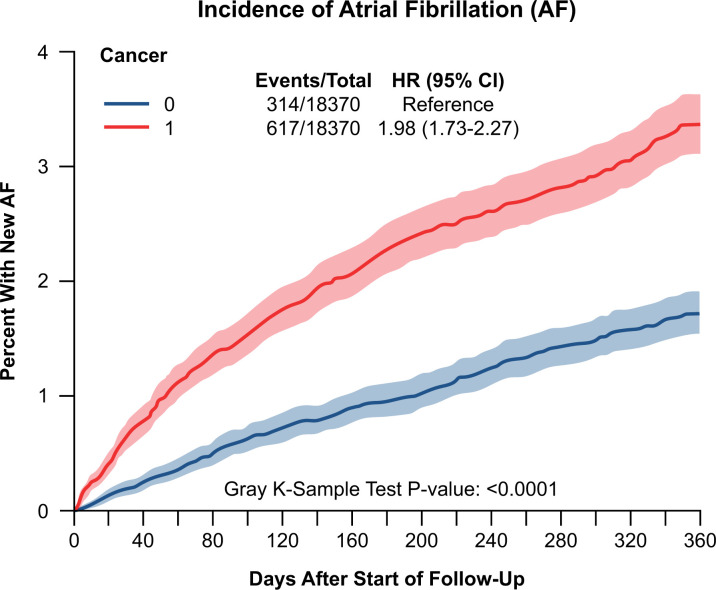
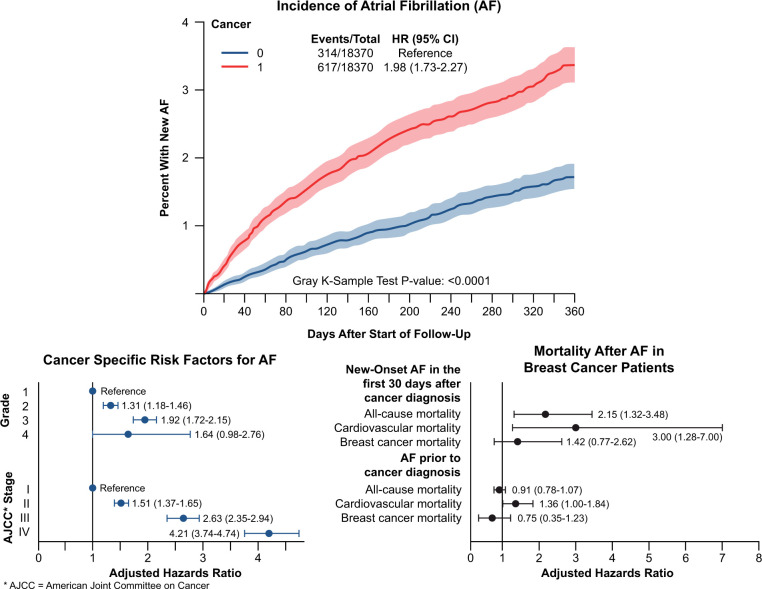
The incidence of new-onset AF after breast cancer diagnosis was 0.6% [95% confidence interval (CI) 0.5–0.7%] at 30 days, 2.1% (95% CI 1.9–2.4%) at 6 months and 3.3% (95% CI 3.0–3.5%) at 1 year. This remained higher than the non-cancer matched control through the 1-year follow-up (Figure 1). Among the breast cancer population (n = 75 998), the rate of a new diagnosis of AF was highest in the first 60 days, increasing at 0.6%/30 days followed by 0.3%/30 days over the period of 1-year follow-up (Supplementary material online, Figure S2A). The 1-year incidence across the entire cohort was 40.4 per 1000 person-years (Supplementary material online, Figure S2B). The race-standardized AF incidence was 31.9 per 1000 person-years in females aged 66–70 years, with an increase in AF incidence with age. From 2007 to 2014, there was an annual increase in AF incidence by 3.4% (Supplementary material online, Table S3). The age-standardized AF incidence was 49.9 per 1000 person-years in Whites vs. 58.8 per 1000 person-years in Black females in 2014.
Figure 1.
Cumulative incidence function plot for atrial fibrillation in breast cancer patients compared to 1:1 incidence density sampling and propensity-matched non-cancer patients. The breast cancer and non-cancer patients were obtained from Surveillance, Epidemiology, and End Results-Medicare 2007–2014 with matched 5% non-cancer control Medicare sample. Death was a competing risk. Matched for the year of birth, race, US state, and Charlson score; follow-up period of 1 year after cancer diagnosis.
Risk factors for AF
Age, race, and several other socioeconomic features were strongly associated with the development of new-onset AF in this breast cancer population (Supplementary material online, Table S1). Multiple cardiovascular risk factors such as hypertension [HR 1.46 (95% CI 1.34–1.58)], diabetes [HR 1.55 (95% CI 1.44–1.67)], prior history of stroke [HR 1.70 (95% CI 1.53–1.88)], and the NCI comorbidity index >0 [HR 1.84 (95% CI 1.70–2.00)] were associated with a new diagnosis of AF. Notably, history of depression [HR 1.21 (95% CI 1.12–1.32)] and anaemia [HR 1.46 (95% CI 1.36–1.57)] were also associated with the development of AF. Among the cancer-specific covariates, cancer stage was strongly associated with the development of AF [AJCC Stage II vs. I: adjusted HR (aHR) 1.51 (95% CI 1.37–1.65); AJCC Stage III vs. I: aHR 2.63 (95% CI 2.35–2.94); AJCC Stage IV vs. I: aHR 4.21 (95% CI 3.74–4.74)]. Those who did not undergo surgery or radiation as their first-line treatment were at a higher risk of developing AF [Table 2; aHR for no surgery vs. simple mastectomy as first treatment option in the first 90 days after cancer diagnosis: 4.39 (95% CI 3.80–5.07); aHR for no radiation therapy vs. external beam radiation as first option: 1.46 (95% CI 1.34–1.59)].
Table 2.
Multivariable cause-specific hazards ratio of cancer-specific variables modelling for new-onset atrial fibrillation in breast cancer patients
| Variable | HR 0–90 days after cancer diagnosis (95% CI) | HR 91–180 days after cancer diagnosis (95% CI) | HR >180 days after cancer diagnosis (95% CI) |
|---|---|---|---|
| SEER stage | |||
| I (localized) | Ref | Ref | Ref |
| II (regional direct extension) | 2.52 (1.98–3.21) | 1.55 (1.05–2.27) | 1.69 (1.24–2.30) |
| III (regional lymph node extension only) | 1.50 (1.29–1.75) | 1.55 (1.28–1.88) | 1.48 (1.26–1.73) |
| IV (regional direct and lymph node extension) | 2.91 (2.35–3.61) | 2.65 (1.98–3.53) | 1.93 (1.47–2.53) |
| VII (distant) | 4.70 (4.04–5.48) | 3.48 (2.75–4.40) | 2.20 (1.72–2.83) |
| Surgical therapy | |||
| No surgery | 4.39 (3.80–5.07) | 2.83 (2.31–3.48) | 1.70 (1.39–2.07) |
| Localized therapy such as lumpectomy | ref | ref | Ref |
| Total simple mastectomy | 1.55 (1.31–1.85) | 1.20 (0.95–1.51) | 1.16 (0.97–1.40) |
| Modified radical mastectomy | 2.07 (1.75–2.44) | 1.63 (1.32–2.01) | 1.35 (1.13–1.62) |
| Variable | HRa |
|---|---|
| Laterality—left vs. right | 0.95 (0.89–1.02) |
| Grade | |
| 1 | Ref |
| 2 | 1.31 (1.18–1.46) |
| 3 | 1.92 (1.72–2.15) |
| 4 | 1.64 (0.98–2.76) |
| AJCC stage | |
| I | Ref |
| II | 1.51 (1.37–1.65) |
| III | 2.63 (2.35–2.94) |
| IV | 4.21 (3.74–4.74) |
| Radiation therapy | |
| No radiotherapy | 1.46 (1.34–1.59) |
| Beam radiation | Ref |
| Implanted radiation | 0.51 (0.37–0.70) |
| Tumour oestrogen receptor status vs. not | 0.66 (0.60–0.73) |
| Tumour progesterone receptor status vs. not | 0.72 (0.66–0.78) |
| Tumour HER2 status vs. not | 1.37 (1.19–1.58) |
| Breast tumour subtype based on combination receptor status | |
| HER2+/hormone receptor (HR)+ | Ref |
| HER2+/HR− | 1.26 (0.95–1.66) |
| HER2−/HR+ | 0.75 (0.63–0.89) |
| HER2−/HR− | 1.10 (0.89–1.36) |
Univariable analysis =presented in Supplementary material online, Table S1 was utilized in model building. All variables are adjusted for age, race, ethnicity, marital status, poverty level, urban location status, geographic SEER location, NCI comorbidity index, obesity, smoking, history of hypertension, history of depression, history of anaemia, and history of stroke. Lymph node biopsy status had >10% missing data and hence not modelled for. HER2 status and type of breast cancer included breast cancer patients after 2010.
CI, confidence interval; HER, human epidermal growth factor receptor; HR, hazard ratio; NCI, National Cancer Institute; SEER, Surveillance, Epidemiology, and End Results.
Variables at the latter part of the table meet proportional hazards assumption and presented hazard ratios are for the entire year.
Cancer grade and stage (subgroup analysis)
Among the individual breast cancer grades (1–3, Supplementary material online, Table S4) and individual AJCC stages (I–IV, Supplementary material online, Table S5), associations noted between covariates and incident AF were similar to those noted in Table 2. However, there was greater likelihood of AF in those not undergoing hormonal therapy with lower-grade breast cancer compared with higher-grade breast cancer [comparison of no hormonal therapy to treatment with hormonal therapy: grade 1, aHR 11.18 (95% CI 8.90–14.04); grade 2, aHR 8.32 (95% CI 5.97–11.60); grade 3, aHR 6.28 (95% CI 4.81–8.21); joint test P-value = 0.01; Supplementary material online, Table S4]. Similar differences were noted across the different grades among those receiving HER2-targeted therapy as first-line therapy for breast cancer (joint test P-value across all grades = 0.008; Supplementary material online, Table S4). Cardiac medications (beta-blockers, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, and spironolactone/eplerenone) lowered the likelihood of incident AF in those with breast cancer across all grades with no significant difference across the grades (joint test P-values >0.05 for all three classes of medications). For example, in grade 3 breast cancer, those on beta-blockers had a lower likelihood of developing AF than those not on beta-blockade (aHR 0.27; 95% CI 0.18–0.37).
A difference was noted across the various breast cancer subtypes based on receptor status across the four AJCC cancer stages (joint test P-value = 0.02; Supplementary material online, Table S5). HER−/HR+ receptor status was associated with a lower risk of AF in those with Stage I (aHR 0.71; 95% CI 0.52–0.97) and Stage II (aHR 0.75; 95% CI 0.57–0.99) diseases as compared to those with HER+/HR+ receptor status. Beta-blockers and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers lowered the likelihood of incident AF in those with breast cancer across all grades with no significant difference across the grades (joint test P-values >0.05 for both medications; Supplementary material online, Table S5).
Cancer treatment: surgery and radiation (subgroup analysis)
Although the highest risk of AF was associated with those who did not undergo surgery, the risk of AF was higher in more complex surgeries compared to those undergoing simple breast cancer surgeries such as lumpectomy (total simple mastectomy vs. lumpectomy, aHR in the first 90 days after cancer diagnosis: 1.55; 95% CI 1.31–1.85; modified radical mastectomy vs. lumpectomy: aHR 2.07; 95% CI 1.75–2.44; Table 2). There was no difference across the other cancer-specific covariates and cardiac medications among the four categories of surgery (all joint test P-values >0.05; Supplementary material online, Table S6).
The risk of AF was noted to be lower in those who received implanted radiation vs. beam radiation as first-choice treatment (aHR 0.51; 95% CI 0.37–0.70; Table 2). In all categories of surgical therapy, and in non-hormonal therapy patients, the likelihood of AF was noted to be higher in those who received beam radiation as the first treatment of choice vs. no radiation therapy (joint test P-values <0.05; e.g. aHR for those receiving modified radical mastectomy vs. lumpectomy in breast cancer patients who have received beam radiation therapy as first treatment of choice: 2.28; 95% CI 1.67–3.12 vs. who have not received any radiation therapy: 1.62; 95% CI 1.30–2.02; joint test P-values <0.0001; Supplementary material online, Table S7). There was no difference across the other cancer-specific covariates and cardiac medications among the four categories of surgery (all joint test P-values >0.05; Supplementary material online, Table S7).
Mortality outcomes
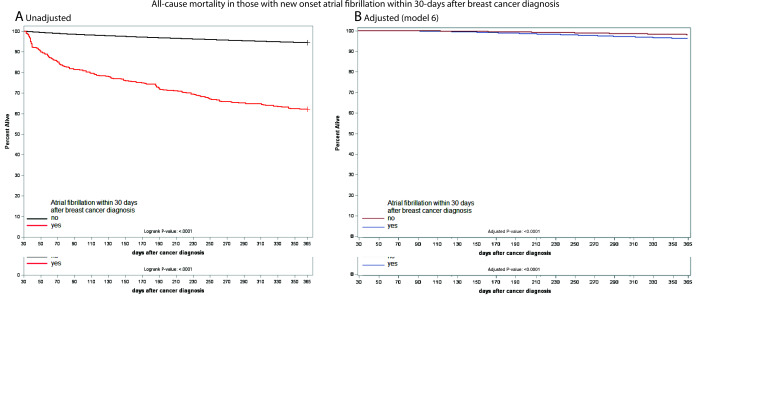
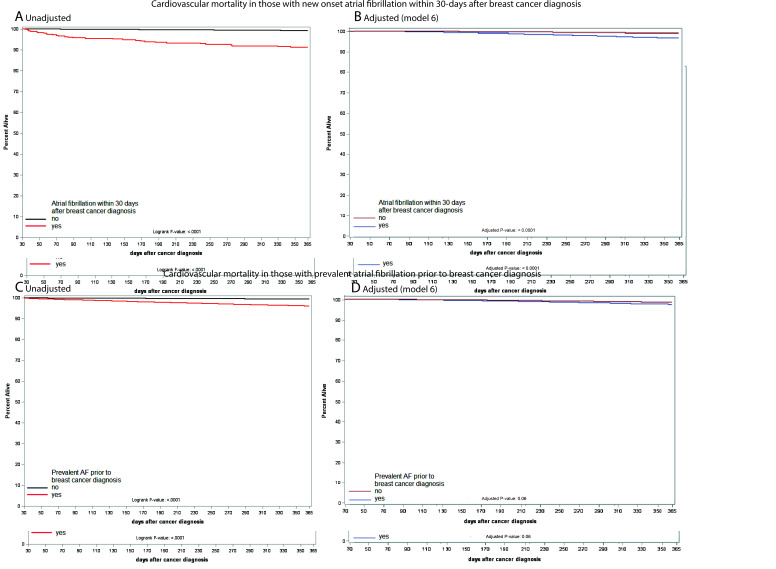
Mortality differed among breast cancer patients depending on the time of AF onset. We studied two groups: those who developed AF within 30 days after breast cancer diagnosis (Group 1), and those who had AF prior to breast cancer diagnosis (Group 2). Among those who developed AF within the first 30 days after breast cancer diagnosis (Group 1), the 1-year survival was 62.2% (95% CI 57.6–67.1%, Supplementary material online, Figure S3A). However, 1-year survival among those in Group 2 was ∼85% (Supplementary material online, Figure S3B). In the adjusted Cox proportional hazards model, after full adjustment (Model 7, Table 3), there was a significant increase in all-cause mortality at 1 year with incident AF within the first 30 days after breast cancer diagnosis [Group 1; aHR 2.15 (95% CI = 1.32–3.48); Figure 2] but no difference in those with AF prior to cancer diagnosis [Group 2; aHR 0.91 (95% CI 0.78–1.07); Supplementary material online, Figure S4]. Cardiovascular mortality was increased in breast cancer patients with incident AF within the first 30 days of breast cancer diagnosis [Table 3, Model 7, Group 1: aHR 3.00 (95% CI 1.28–7.00); Figure 3A and B] but not in those with AF prior to breast cancer diagnosis [Table 3, Model 7, Group 2: aHR 1.36 (95% CI 1.00–1.84); Figure 3C and D]. The cause of cardiovascular death in breast cancer patients with incident AF was heart failure (63.3%), systemic embolism (16.7%), ischaemic stroke (16.7%), and arrhythmic (13.3%). In contrast, there was no difference in breast cancer-specific mortality at 1 year, either in females with breast cancer and new-onset AF within 30 days of breast cancer diagnosis [Table 3, Model 7, Group 1: aHR 1.42 (95% CI 0.77–2.62); Supplementary material online, Figure S5], or in those with AF prior to breast cancer diagnosis [Table 3, Model 7, Group 2: aHR 0.75 (95% CI 0.35–1.23); Supplementary material online, Figure S6].
Table 3.
Association of atrial fibrillation with all-cause mortality, cardiovascular mortality, and cancer-specific mortality at 1 year after cancer diagnosis: results from Cox proportional hazards model
| AF group and adjustment model | HR (95% CI) |
||
|---|---|---|---|
| All-cause mortality | Cardiovascular mortality | Cancer-specific mortality | |
| Breast cancer patients with new-onset AF in first 30 days after breast cancer diagnosis (Group 1) | |||
| Model 1—unadjusted | 7.63 (6.50–8.96) | 8.99 (6.25–12.94) | 7.65 (6.20–9.43) |
| Model 2 | 7.68 (6.55–9.01) | 8.95 (6.22–12.89) | 7.71 (6.25–9.52) |
| Model 3 | 3.52 (2.34–5.28) | 6.43 (3.17–13.01) | 2.86 (1.57–5.22) |
| Model 4 | 3.94 (2.30–6.76) | 9.16 (3.71–22.63) | 2.44 (1.07–5.58) |
| Model 5 | 3.11 (2.06–4.68) | 5.46 (2.69–11.10) | 2.65 (1.44–4.87) |
| Model 6 | 2.20 (1.46–3.31) | 3.85 (1.89–7.84) | 1.85 (1.00–3.40) |
| Model 7 | 2.15 (1.32–3.48) | 3.00 (1.28–7.00) | 1.42 (0.77–2.62) |
| Breast cancer patients with AF prior to breast cancer diagnosis (Group 2) | |||
| Model 1—unadjusted | 2.26 (2.12–2.40) | 4.74 (4.17–5.40) | 1.63 (1.49–1.79) |
| Model 2 | 2.23 (2.10–2.38) | 4.76 (4.17–5.43) | 1.61 (1.47–1.76) |
| Model 3 | 2.28 (2.00–2.60) | 3.62 (2.85–4.60) | 1.58 (1.27–1.97) |
| Model 4 | 2.20 (1.82–2.66) | 3.72 (2.63–5.26) | 1.39 (1.01–1.92) |
| Model 5 | 1.54 (1.34–1.78) | 1.90 (1.46–2.48) | 1.29 (1.02–1.65) |
| Model 6 | 1.02 (0.88–1.18) | 1.27 (0.97–1.67) | 0.83 (0.65–1.06) |
| Model 7 | 0.91 (0.78–1.07) | 1.36 (1.00–1.84) | 0.75 (0.35–1.23) |
Model 2: Model 1 + demographic features; Model 3: Model 2 + breast cancer-related features; Model 4: Model 3 + breast cancer tumour receptor subtype; Model 5: Model 3 + cardiovascular risk factors for AF; Model 6: Model 5 + breast cancer medication; Model 7: Model 6 + cardiac medications (beta-blocker, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, spironolactone/eplerenone). Detailed model description is presented in Supplementary material online, Table S2.
AF, atrial fibrillation; CI, confidence interval; HR, hazard ratio.
Figure 2.
Survival curves showing all-cause mortality plot of breast cancer patients who developed atrial fibrillation within 30 days of breast cancer diagnosis compared to those who did not at 1-year follow-up (A, unadjusted—Kaplan–Meier; B, adjusted). The plot is adjusted for standard demographic features, breast cancer-related features, cardiovascular risk factors for atrial fibrillation, and breast cancer medication. Detailed model description is presented in Supplementary material online, Table S2.
Figure 3.
(A, B) Survival curves showing cardiovascular mortality plot in breast cancer patients who developed atrial fibrillation within 30 days of breast cancer diagnosis compared to those who did not at 1-year follow-up (A, unadjusted—Kaplan–Meier; B, adjusted). (C, D) Survival curves showing cardiovascular mortality plot in breast cancer patients who had atrial fibrillation prior to breast cancer diagnosis compared to those who did not at 1-year follow-up (C, unadjusted—Kaplan–Meier; D, adjusted). The plot is adjusted for standard demographic features, breast cancer-related features, cardiovascular risk factors for atrial fibrillation, and breast cancer medication. Detailed model description presented in Supplementary material online, Table S2.
An exploratory analysis showed a decrease in all-cause mortality (aHR 0.43; 95% CI 0.21–0.89) in breast cancer patients with incident AF who were anticoagulated vs. not. Only two direct-current cardioversions were performed in breast cancer patients with incident AF, and the exploratory mortality analysis did not converge.
Discussion
In this contemporary evaluation of older female breast cancer patients, the incidence, risk factors, and mortality associated with AF were quantified using the SEER-Medicare registry. The incidence for AF was highest in the first 60 days, and higher among older and Black females. Breast cancer severity, i.e. stage and grade, is strongly associated with the risk of AF development, with over 300% higher likelihood of AF in those with advanced- (Stage IV) vs. early-stage (Stage I) breast cancer (Graphical Abstract). In fact, patients who did not receive surgery, radiation therapy, or hormonal therapy as first-line treatment for breast cancer were at the higher risk of developing AF than those who received these treatments. Furthermore, more complex surgeries such as modified radical mastectomy have a higher risk of AF development than simple surgeries such as lumpectomy. Also, implanted radiation therapy is associated with a lower risk than beam radiation in the development of AF. Notably, cardiovascular medications, namely, beta-blockers, angiotensin-converting enzyme inhibitors/angiotensin receptor blocker, and spironolactone/eplerenone use lowered the risk of developing AF. In fully adjusted models, mortality in breast cancer patients is higher at 1 year among those who have new-onset AF after a breast cancer diagnosis, and this mortality risk is predominantly cardiovascular and not related to breast cancer. This risk was not mitigated despite the use of the above cardiovascular medications. Involving cardiovascular specialists or cardio-oncology programmes in the care of breast cancer patients who develop AF after cancer diagnosis should be encouraged.
Structured Graphical Abstract.
Cumulative incidence function plot for atrial fibrillation in breast cancer patients compared with matched non-cancer patients. The start of follow-up is at breast cancer diagnosis or pseudo-diagnosis date for the non-cancer cohort. Partial forest plot showing risk factor analysis of atrial fibrillation after breast cancer diagnosis. Partial forest plot showing fully adjusted model for all-cause, cardiovascular, and breast cancer-specific mortality in those after new-onset atrial fibrillation after breast cancer diagnosis as well as those with atrial fibrillation prior to breast cancer diagnosis. AF, atrial fibrillation; HR, hazard ratios; ref, reference; AJCC, American Joint Committee on Cancer.
This study found that the incidence of AF is higher in the first 60 days after cancer diagnosis. This finding is in line with two prior studies,11,16 which observed a higher incidence of AF in the first year after a breast cancer diagnosis, and contradictory to a study from Denmark where AF incidence was lower in the first 6 months after a breast cancer diagnosis compared with matched controls in those above the age of 60.15 It is important to note that the Danish study does not reflect our study multi-ethnic composition. Nevertheless, they observed that the risk of AF increased after the first 6 months in patients with breast cancer compared to non-cancer controls, similar to our study. This study found a higher incidence of AF in Black females. This finding is contrary to what is known from the general population, where the incidence of AF is known to be higher in Whites compared to Black females.2,3,26 Although not wholly explained by our data, Black females have a higher likelihood of ER/PR− and later stage of a breast cancer diagnosis, which may be contributing factors.27,28 Our finding that patients who take hormonal therapy are at a relatively lower risk of AF may also help to explain the higher incidence of AF in Black females.
Although no direct evidence was provided in our study, it is speculated that the increased incidence of AF subsequent to cancer diagnosis might be biologically explained by pro-inflammatory state, electrolyte and fluid imbalance, as well as a direct effect of cancer therapy.29,30 Preclinical and clinical studies demonstrating cardiac fibrosis in late-stage cancer may also support our finding of AF in late-stage breast cancer.30,31 We speculate that the epidemiologically higher burden of AF in breast cancer patients may be explained by lead-time bias due to a higher level of comprehensive screening for various cardiovascular comorbidities that may affect cancer therapy.32 Finally, Navi et al. noted an increase in stroke risk in the first year after cancer diagnosis in another SEER-Medicare analysis.19 This increase in stroke risk may now be partially explained by the increase in AF burden after cancer diagnosis, as noted in our study.
After adjustment for traditional risk factors, higher breast cancer stage and grade stood out as significant risk factors associated with an increased incidence of AF. Remarkably, left-sided breast cancer and breast cancer subtype based on receptor status (HER2Neu/HR) were not associated with AF risk. Moreover, patients who did not receive initial treatment with surgery, radiation, or hormonal therapy were noted to be at a higher risk of AF. This finding is potentially related to the fact that those with higher-stage cancer, which is related to a higher risk of AF, are likely to receive systemic chemotherapy as first-line treatment.33,34 This is further supported by the joint test results shown in Supplementary material online, Table S7, where aHR for surgical therapy and hormonal therapy is higher in those who did not receive radiation therapy vs. those who did. In addition, complex surgical treatments such as modified radical mastectomy increased the risk of AF compared to simple surgeries like lumpectomy. Although not described before, blood loss or electrolyte imbalance associated with longer cancer surgeries35, the impact of cardiac fibrosis in late-stage cancer as described above, may explain this finding.30 This is also the first study to report a difference in AF risk based on beam radiation vs. implanted radiation. The lower risk with implanted radiation, also known as brachytherapy, is likely due to lower cardiac dose from radiation implants into the breast.36 Furthermore, neither anthracycline nor HER2-targeted therapy was found to increase the risk of AF, consistent with prior findings.11 Although this differs from a recent study of the World Health Organization dataset VigiBase which reported that anthracyclines were disproportionally used among those who developed AF [reporting odds ratio 2.32 (95% CI 1.36 − 3.97)] compared to other medications, taken together with data from this study,37 AF development after a breast cancer diagnosis may also be linked to poor systemic health related to the cancer state rather than cardiotoxic therapies alone. Finally, cardiovascular medications such as angiotensin-converting enzyme inhibitors appeared to lower the risk of developing AF, suggesting the potential for cardio-protection against AF in breast cancer patients.38
This is the first study to evaluate the morbidity and mortality of new-onset AF after a breast cancer diagnosis. It is noted that new-onset AF worsens all-cause mortality, which is mainly driven by cardiovascular mortality. The common causes of mortality were heart failure followed by systemic embolism and ischaemic stroke. There is no change in breast cancer-related mortality in those with new-onset AF or AF prior to cancer diagnosis. The increased mortality is similar to that observed in patients with left ventricular dysfunction after cancer diagnosis, whether due to cancer therapy or other causes.39,40 Given that variably used cardiovascular medications did not reduce the risk of cardiovascular mortality at 1-year follow-up, it may be important to screen and proactively mitigate AF in the first month after cancer diagnosis to reduce cardiovascular morbidity and mortality. Since AF was not associated with any specific cancer therapy, this study is reassuring from the standpoint of continuing cancer therapy even after AF diagnosis. Notably, anticoagulant use lowered all-cause mortality; however, the study was not powered to study cardiovascular mortality. We speculate that this reduction in mortality was due to reducing the burden of systemic embolism, but more studies are needed to explore this further.
Several limitations should be addressed in this study. First, patients below the age of 66 years were excluded since we used the Medicare database. Second, given that this study is based on medical claims, the findings are potentially less reliable than clinically collected data. Nevertheless, prior studies have shown good sensitivity and specificity of AF diagnosis in Medicare claims.41 In addition, the increased likelihood of cardiovascular events proximal to cancer diagnosis has been noted in other SEER-Medicare studies.19 Third, even though we performed two-step matching for cancer and non-cancer patients using incidence density sampling followed by propensity matching, hidden confounders may not have all been accounted for. This issue may be resolvable by future studies on this topic. Fourth, SEER-Medicare covers ∼30% of the USA17 and represents the population at large; nevertheless, if compared with data gathered from the entire USA, minor discrepancies could be observed. Fifth, even though Medicare claims may identify major conditions like AF, the prevalence of obesity was noted to be around 3–10% in our study. This finding likely represents obesity ICD-9 code under-reporting, given that at least one-third of Americans above the age of 65 are obese.42 Along the lines of such, essential risk factors for AF, such as performance status, are not available in SEER. Sixth, the analysis that included part D data should inherently be considered limited since 30–50% of patients who are enrolled in Medicare at the age of 65 do not utilize part D for oral medications.43 Seventh, we limited our study to 1-year follow-up. Prior studies have shown that following cancer patients for extended durations have led to proportional hazards assumption violation, perhaps due to differential mortality related to disparate cancer strata; hence, we limited the incidence and HR quantification to 1 year.44 In addition, the role of rhythm control measures on mortality could not be studied efficiently due to sample size limitation. Also, the identification of specific cardiovascular causes of death is relatively uncertain due to the coding system used by SEER. Finally, this study was conducted during the period of 2007 to 2014 to avoiding crossing over to ICD-10, which appeared in claims in 2015.
Conclusion
AF incidence is significantly higher in women after a breast cancer diagnosis. Apart from traditional risk factors, higher breast cancer stages and grades at diagnosis are associated with increased risk of AF, suggesting a systemic effect of advanced breast cancer itself on the heart. All-cause mortality was found to be increased in those with breast cancer who have new-onset AF, which is mainly driven by cardiovascular mortality. Early involvement of cardiology and targeted application of cardioprotective medications after a breast cancer diagnosis may mitigate this excess mortality; however, further prospective studies are required.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported in part by NIH grants P30 CA016058, K12-CA133250, and K23-HL155890 (D.A.) and by American Heart Association‐Robert Wood Johnson Foundation (Harold Amos) grant (D.A.). N.L.W. is supported by NIH grants HL124097, HL126949, HL134354, AR070029, and AG064895. A.G. and N.L.W. are supported by American Heart Association-Strategically Focused Research Network Grant in Disparities in Cardio-Oncology (# 847740, # 863620). A.A. is supported by NIH grant K24HL148521. M.G.F. receives unrelated research funding from Medtronic and consulting fee from Abbott and AstraZeneca.
Conflict of interest: none declared.
Data availability
The data underlying this article were provided by SEER-Medicare under licence to the Ohio State University. Data may be shared on request to the corresponding author with permission of SEER-Medicare.
Supplementary Material
References
- 1.Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X.. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol 2013;112:1142–1147. [DOI] [PubMed] [Google Scholar]
- 2.Piccini JP, Hammill BG, Sinner MF. et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993-2007. Circ Cardiovasc Qual Outcomes 2012;5:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen AY, Contreras R, Sobnosky S, Shah AI. et al. Racial/ethnic differences in the prevalence of atrial fibrillation among older adults—a cross-sectional study. J Natl Med Assoc 2010;102:906–913. [DOI] [PubMed] [Google Scholar]
- 4.Virani SS, Alonso A, Aparicio HJ. et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2021 update. Circulation 2021;143:e254–e743. [DOI] [PubMed] [Google Scholar]
- 5.Guha A, Dey AK, Jneid H, Addison D.. Acute coronary syndromes in cancer patients. Eur Heart J 2019;40:1487–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guha A, Dey AK, Jneid H, Ibarz JP, Addison D, Fradley M.. Atrial fibrillation in the era of emerging cancer therapies. Eur Heart J 2019;40:3007–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piccini JP, Hammill BG, Sinner MF. et al. Clinical course of atrial fibrillation in older adults: the importance of cardiovascular events beyond stroke. Eur Heart J 2014;35:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D.. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98:946–952. [DOI] [PubMed] [Google Scholar]
- 9.Emdin CA, Wong CX, Hsiao AJ. et al. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: systematic review and meta-analysis of cohort studies. BMJ 2016;532:h7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jakobsen CB, Lamberts M, Carlson N. et al. Incidence of atrial fibrillation in different major cancer subtypes: a Nationwide population-based 12 year follow up study. BMC Cancer 2019;19:1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdel-Qadir H, Thavendiranathan P, Fung K. et al. Association of early-stage breast cancer and subsequent chemotherapy with risk of atrial fibrillation. JAMA Netw Open 2019;2:e1911838–e1911838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guzzetti S, Costantino G, Vernocchi A, Sada S, Fundarò C.. First diagnosis of colorectal or breast cancer and prevalence of atrial fibrillation. Internal Emerg Med 2008;3:227–231. [DOI] [PubMed] [Google Scholar]
- 13.Nouraie M, Kansal V, Belfonte C. et al. Atrial fibrillation and colonic neoplasia in African Americans. PLoS One 2015;10:e0135609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conen D, Wong JA, Sandhu RK. et al. Risk of malignant cancer among women with new-onset atrial fibrillation. JAMA Cardiol 2016;1:389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Souza M, Smedegaard L, Madelaire C. et al. Incidence of atrial fibrillation in conjunction with breast cancer. Heart Rhythm 2019;16:343–348. [DOI] [PubMed] [Google Scholar]
- 16.Matthews AA, Peacock Hinton S, Stanway S. et al. Risk of cardiovascular diseases among older breast cancer survivors in the United States: a matched cohort study. J Natl Compr Canc Netw 2021. Jan 5;doi: 10.6004/jnccn.2020.7629. [DOI] [PubMed] [Google Scholar]
- 17.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF.. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 2002;40:IV– IV18. [DOI] [PubMed] [Google Scholar]
- 18.Navi BB, Reiner AS, Kamel H. et al. Arterial thromboembolic events preceding the diagnosis of cancer in older persons. Blood 2019;133:781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Navi BB, Reiner AS, Kamel H. et al. Association between incident cancer and subsequent stroke. Ann Neurol 2015;77:291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nattinger AB, Laud PW, Bajorunaite R, Sparapani RA, Freeman JL.. An algorithm for the use of Medicare claims data to identify women with incident breast cancer. Health Serv Res 2004;39:1733–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S.. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf 2012;21:141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klabunde CN, Potosky AL, Legler JM, Warren JL.. Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000;53:1258–1267. [DOI] [PubMed] [Google Scholar]
- 23.Cheng L, Swartz MD, Zhao H. et al. Hazard of recurrence among women after primary breast cancer treatment—a 10-year follow-up using data from SEER-Medicare. Cancer Epidemiol Biomarkers Prev 2012;21:800–809. [DOI] [PubMed] [Google Scholar]
- 24.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. The Annals of Statistics 1988;16:1141–1154. [Google Scholar]
- 25.Baltagi BH, Li Q.. A joint test for serial correlation and random individual effects. Statistics & Probability Letters 1991;11:277–280. [Google Scholar]
- 26.Mou L, Norby FL, Chen LY. et al. Lifetime risk of atrial fibrillation by race and socioeconomic status: ARIC study (Atherosclerosis Risk in Communities). Circ Arrhythm Electrophysiol 2018;11:e006350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stead LA, Lash TL, Sobieraj JE. et al. Triple-negative breast cancers are increased in black women regardless of age or body mass index. Breast Cancer Res 2009;11:R18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chatterjee NA, He Y, Keating NL.. Racial differences in breast cancer stage at diagnosis in the mammography era. Am J Public Health 2013;103:170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farmakis D, Parissis J, Filippatos G.. Insights into onco-cardiology: atrial fibrillation in cancer. J Am Coll Cardiol 2014;63:945–953. [DOI] [PubMed] [Google Scholar]
- 30.Anker MS, Sanz AP, Zamorano JL. et al. Advanced cancer is also a heart failure syndrome: a hypothesis. J Cachexia Sarcopenia Muscle 2021;12:533–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mühlfeld C, Das SK, Heinzel FR. et al. Cancer induces cardiomyocyte remodeling and hypoinnervation in the left ventricle of the mouse heart. PLoS One 2011;6:e20424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andersson TM-L, Rutherford MJ, Humphreys K.. Assessment of lead-time bias in estimates of relative survival for breast cancer. Cancer Epidemiol 2017;46:50–56. [DOI] [PubMed] [Google Scholar]
- 33.Cardoso F, Paluch-Shimon S, Senkus E. et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol 2020;31:1623–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korde LA, Somerfield MR, Carey LA. et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol 2021;39:1485–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Higuchi S, Kabeya Y, Matsushita K. et al. Incidence and complications of perioperative atrial fibrillation after non-cardiac surgery for malignancy. PLoS One 2019;14:e0216239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beck RE, Kim L, Yue NJ, Haffty BG, Khan AJ, Goyal S.. Treatment techniques to reduce cardiac irradiation for breast cancer patients treated with breast-conserving surgery and radiation therapy: a review. Front Oncol 2014;4:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alexandre J, Salem JE, Moslehi J. et al. Identification of anticancer drugs associated with atrial fibrillation: analysis of the WHO pharmacovigilance database. Eur Heart J Cardiovasc Pharmacother 2021;7:312–320. [DOI] [PubMed] [Google Scholar]
- 38.Schaer BA, Schneider C, Jick SS, Conen D, Osswald S, Meier CR.. Risk for incident atrial fibrillation in patients who receive antihypertensive drugs: a nested case-control study. Ann Intern Med 2010;152:78–84. [DOI] [PubMed] [Google Scholar]
- 39.Felker GM, Thompson RE, Hare JM. et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med 2000;342:1077–1084. [DOI] [PubMed] [Google Scholar]
- 40.Ryberg M, Nielsen D, Skovsgaard T, Hansen J, Jensen BV, Dombernowsky P.. Epirubicin cardiotoxicity: an analysis of 469 patients with metastatic breast cancer. J Clin Oncol 1998;16:3502–3508. [DOI] [PubMed] [Google Scholar]
- 41.Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF.. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care 2005;43:480–485. [DOI] [PubMed] [Google Scholar]
- 42.Flegal KM, Carroll MD, Ogden CL, Curtin LR.. Prevalence and trends in obesity among US adults, 1999-2008. JAMA 2010;303:235–241. [DOI] [PubMed] [Google Scholar]
- 43.Lauffenburger JC, Robinson JG, Oramasionwu C, Fang G.. Racial/ethnic and gender gaps in the use of and adherence to evidence-based preventive therapies among elderly Medicare Part D beneficiaries after acute myocardial infarction. Circulation 2014;129:754–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bellera CA, MacGrogan G, Debled M, de Lara CT, Brouste V, Mathoulin-Pélissier S.. Variables with time-varying effects and the Cox model: some statistical concepts illustrated with a prognostic factor study in breast cancer. BMC Med Res Methodol 2010;10:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were provided by SEER-Medicare under licence to the Ohio State University. Data may be shared on request to the corresponding author with permission of SEER-Medicare.