Abstract
8 - Difluoromethoxy - 1 - ethyl - 6 - fluoro - 1,4 - dihydro - 7 - [4 - (2 - methoxyphenyl) - 1 - piperazinyl] - 4 - oxoquinoline - 3 - carboxylic acid (K-12) has recently been identified as a potent and selective inhibitor of human immunodeficiency virus type 1 (HIV-1) transcription. In this study, we examined several combinations of K-12 and other antiretroviral agents for their inhibitory effects on HIV-1 replication in acutely and chronically infected cell cultures. Combinations of K-12 and a reverse transcriptase (RT) inhibitor, either zidovudine, lamivudine, or nevirapine, synergistically inhibited HIV-1 replication in acutely infected MT-4 cells. The combination of K-12 and the protease inhibitor nelfinavir (NFV) also synergistically inhibited HIV-1, whereas the synergism of this combination was weaker than that of the combinations with the RT inhibitors. K-12 did not enhance the cytotoxicities of RT and protease inhibitors. Synergism of the combinations was also observed in acutely infected peripheral blood mononuclear cells. The combination of K-12 and cepharanthine, a nuclear factor κB inhibitor, synergistically inhibited HIV-1 production in tumor necrosis factor alpha-stimulated U1 cells, a promonocytic cell line chronically infected with the virus. In contrast, additive inhibition was observed for the combination of K-12 and NFV. These results indicate that the combinations of K-12 and clinically available antiretroviral agents may have potential as chemotherapeutic modalities for the treatment of HIV-1 infection.
Continuous efforts are being made to find chemotherapeutic agents that are effective against human immunodeficiency virus type 1 (HIV-1). At present, six nucleoside reverse transcriptase (RT) inhibitors, three nonnucleoside RT inhibitors, and four protease inhibitors are available for the treatment of AIDS patients. However, clinical studies with these antiretroviral agents have revealed that monotherapy is insufficient for the long-term suppression of HIV-1 replication in HIV-1-infected patients. Therefore, combination chemotherapies with two or more drugs are required for effective treatment of the patients. In fact, recent development of combination chemotherapies with RT inhibitors and protease inhibitors has achieved a more than 2 log10 reduction in the viral RNA level in plasma for a considerable period of time (10, 14). In general, a combination chemotherapy has increased efficacy through additive or synergistic antiviral effects, has reduced levels of the toxic effects associated with the use of each compound at higher doses, and delays the emergence of drug-resistant viruses (17).
Transcription of the viral genome (integrated proviral DNA) into its mRNA is an essential step in the replicative cycle of HIV-1 and is considered to be a potential target for chemotherapeutic intervention for the restriction of HIV-1 replication (8, 18). Although a number of attempts have been made to discover an effective inhibitor of HIV-1 transcription, most of the compounds reported to date had marginal antiviral activities or considerable toxicities. For instance, the Tat antagonists Ro 5-3335 and Ro 24-7429 displayed significant anti-HIV-1 activities in cell cultures (15, 16). However, clinical trials of Ro 24-7429 were halted due to some side effects in patients before its antiviral activities could be demonstrated (9). In the meantime, we have recently identified 8-difluoromethoxy - 1 - ethyl - 6 - fluoro - 1,4 - dihydro - 7 - [4 - (2 - methoxyphenyl)-1-piperazinyl]-4-oxoquinoline-3-carboxylic acid (K-12) as a potent and selective inhibitor of HIV-1 transcription (1). K-12 inhibited viral replication in various cell culture systems acutely infected with HIV-1, including peripheral blood mononuclear cells (PBMCs). In addition, the compound could suppress HIV-1 production in chronically infected cells. Studies of its mechanism of action suggest that K-12 is a selective inhibitor of Tat-induced HIV-1 gene expression (4).
Recent studies have revealed that replication-competent virus can be recovered from resting CD4+ T cells even in patients with prolonged (more than 100 weeks) suppression of plasma viremia as a result of combination chemotherapy (11, 26). Therefore, it is clear that the current chemotherapy cannot be stopped unless such reservoir cells have been eradicated or viral release from these cells can be completely suppressed. In this regard, HIV-1 transcription inhibitors have the potential to inhibit the recovery of latent virus from resting CD4+ cells as well as infected macrophages, which are also considered to be a chronically infected cell population in HIV-1-infected patients with a long survival time (24). In this study, we evaluated several combinations of K-12 and clinically available antiretroviral agents for their inhibitory effects on HIV-1 replication not only in acutely infected cells but also in chronically infected cells. We found that combinations of K-12 and either zidovudine (ZDV), lamivudine (3TC), nevirapine (NVP), or nelfinavir (NFV) synergistically inhibited HIV-1 replication in acutely infected MT-4 cells and PBMCs. Furthermore, synergistic inhibition was also observed with the combination of K-12 and cepharanthine (CEP), a nuclear factor κB (NF-κB) inhibitor (23), in tumor necrosis factor alpha (TNF-α)-stimulated U1 cells.
MATERIALS AND METHODS
Compounds.
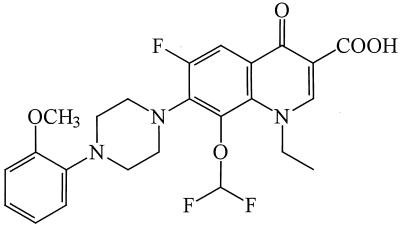
K-12 (Fig. 1) was synthesized by Daiichi Pharmaceutical Co. (Tokyo, Japan). 3TC and NVP were kindly provided by S. Yuasa (Mitsubishi Chemical Corporation, Yokohama, Japan), while NFV and CEP were supplied by Japan Tobacco Co. (Takatsuki, Japan) and Kaken Shoyaku Co. (Mitaka, Japan), respectively. ZDV was purchased from Sigma Chemical Co. (St. Louis, Mo.). All compounds were dissolved in dimethyl sulfoxide at a concentration of 20 mM or higher to exclude any antiviral or cytotoxic effect of dimethyl sulfoxide.
FIG. 1.
Structure of K-12.
Cells and virus.
MT-4 cells (22), PBMCs, and U1 cells (12) were used in the antiviral assays. PBMCs were obtained from healthy donors and were stimulated with phytohemagglutinin. Except for the PBMCs, the cells were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 100 U of penicillin G per ml, and 100 μg of streptomycin per ml. PBMCs were cultured in RPMI 1640 medium containing 20% fetal calf serum, antibiotics, and 20 U of interleukin-2 (Boehringer Mannheim, Mannheim, Germany) per ml. HIV-1IIIB was used for acute infection of MT-4 cells and PBMCs. The virus was propagated and titrated in MT-4 cells and was stored at −80°C until use.
Antiviral assays.
The activities of the compounds against acute HIV-1 infection were based on the inhibition of virus-induced cytopathogenicity in MT-4 cells as described previously (2). Briefly, the cells (105 cells/ml) were infected with HIV-1IIIB at a multiplicity of infection of 0.02 and were cultured in the presence of various concentrations of the test compounds. After a 4-day incubation at 37°C, the number of viable cells was measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method. Determination of anti-HIV-1 activities in PBMCs was based on the quantitative detection of HIV-1 p24 antigen in the culture supernatants with a sandwich enzyme-linked immunosorbent assay kit (Cellular Products, Buffalo, N.Y.). PBMCs stimulated with phytohemagglutinin for 3 days were infected with HIV-1IIIB at a multiplicity of infection of 0.02. After viral absorption for 2 h, the cells were extensively washed with culture medium and incubated in the presence of various concentrations of the test compounds for 6 days. The cytotoxicities of the compounds were evaluated in parallel with their antiviral activities. The mock-infected cells were cultured in the presence of various concentrations of the test compounds. The numbers of viable MT-4 cells and PBMCs were measured by the MTT method on days 4 and 6, respectively.
The activities of the compounds against chronic HIV-1 infection were based on the inhibition of p24 antigen production in U1 cells. U1 cells (105 cells/ml) were incubated in the presence or absence of the compounds for 2 h, stimulated with 1 ng of TNF-α (Boehringer Mannheim) per ml, and further incubated. After a 3-day incubation at 37°C, the culture supernatants were collected and examined for their p24 antigen levels. At the same time, the number of viable U1 cells was also determined by the MTT method.
Synergy calculations.
The multiple-drug effect was evaluated by the median-effect principle and the isobologram method (5, 6). This method involves the conversion of dose-effect curves for each compound and for multiple diluted fixed-ratio combinations of compounds into the median-effect plot. The slope and x intercept of the plot were used to calculate the combination index (CI). CIs of <1, 1, and >1 indicate a synergistic effect, an additive effect, and an antagonistic effect, respectively. All experiments were carried out in duplicate or triplicate, and each experiment was repeated two or three times for determination of anti-HIV-1 activities and CIs.
RESULTS
Before the start of the experiments, the combination ratio of two compounds had to be chosen in order to establish their optimal molar ratio. To this end, we referred to our database on the anti-HIV-1 activities of the tested compounds, and on the basis of their 50% effective concentrations (EC50s) in MT-4 cells, three different combination ratios were selected for each combination. When K-12 and the other test compounds were examined for their inhibitory effects on HIV-1 replication in MT-4 cells, a dose-dependent inhibition of virus-induced cytopathogenicity was observed with all compounds on day 4 after viral infection (data not shown). The EC50s of K-12 alone, ZDV alone, 3TC alone, NVP alone, and NFV alone were 0.50, 0.0043, 1.0, 0.12, and 0.068 μM, respectively (Table 1). Their EC90s) were also obtained, and they were 5- to 11-fold higher than the corresponding EC50s. Table 1 also presents the EC50s and EC90s of the drug combinations at different molar ratios. For instance, the EC50 of K-12 plus 3TC at a ratio of 1:1 was 0.36 μM (Table 1), which consisted of 0.18 μM K-12 and 0.18 μM 3TC. Thus, concentrations lower than those expected from the EC50s of each compound alone (0.25 μM K-12 and 0.5 μM 3TC) were required to achieve 50% inhibition of HIV-1 replication, indicating that the combination was synergistic. In fact, the CI at a level of 50% inhibition was 0.61 (Table 1), which indicated synergy.
TABLE 1.
Anti-HIV-1 activities of K-12, ZDV, 3TC, NVP, and NFV and their combinations in MT-4 cellsa
| Combination (molar ratio) | EC50b (μM) | EC90 (μM) | CI at the following % inhibitionc:
|
rd | ||
|---|---|---|---|---|---|---|
| 50 | 70 | 90 | ||||
| K-12 alone | 0.50 ± 0.22 | 3.0 ± 1.4 | 0.98 | |||
| ZDV alone | 0.0043 ± 0.0018 | 0.041 ± 0.026 | 0.98 | |||
| 3TC alone | 1.0 ± 0.36 | 11.4 ± 7.8 | 0.96 | |||
| NVP alone | 0.12 ± 0.07 | 0.81 ± 0.44 | 0.99 | |||
| NFV alone | 0.068 ± 0.014 | 0.39 ± 0.17 | 0.99 | |||
| K-12 + ZDV (400:1) | 0.25 ± 0.11 | 1.8 ± 1.2 | 0.66 ± 0.13 | 0.57 ± 0.13 | 0.45 ± 0.16 | 0.98 |
| K-12 + ZDV (200:1) | 0.18 ± 0.07 | 0.80 ± 0.61 | 0.60 ± 0.16 | 0.41 ± 0.07 | 0.23 ± 0.04 | 0.95 |
| K-12 + ZDV (100:1) | 0.14 ± 0.04 | 0.47 ± 0.26 | 0.63 ± 0.21 | 0.41 ± 0.11 | 0.22 ± 0.07 | 0.99 |
| K-12 + 3TC (4:1) | 0.41 ± 0.12 | 3.3 ± 2.0 | 0.86 ± 0.21 | 1.0 ± 0.4 | 1.3 ± 0.7 | 0.96 |
| K-12 + 3TC (2:1) | 0.32 ± 0.12 | 1.7 ± 1.3 | 0.61 ± 0.23 | 0.57 ± 0.32 | 0.54 ± 0.44 | 0.95 |
| K-12 + 3TC (1:1) | 0.36 ± 0.10 | 1.6 ± 1.1 | 0.61 ± 0.12 | 0.52 ± 0.20 | 0.44 ± 0.30 | 0.96 |
| K-12 + NVP (8:1) | 0.27 ± 0.13 | 1.3 ± 0.4 | 0.68 ± 0.10 | 0.60 ± 0.09 | 0.48 ± 0.08 | 0.99 |
| K-12 + NVP (4:1) | 0.21 ± 0.11 | 1.3 ± 0.5 | 0.63 ± 0.01 | 0.63 ± 0.08 | 0.64 ± 0.22 | 0.99 |
| K-12 + NVP (2:1) | 0.18 ± 0.10 | 1.3 ± 1.1 | 0.71 ± 0.06 | 0.69 ± 0.13 | 0.67 ± 0.28 | 0.97 |
| K-12 + NFV (20:1) | 0.27 ± 0.11 | 1.6 ± 0.1 | 0.82 ± 0.16 | 0.78 ± 0.10 | 0.74 ± 0.05 | 0.99 |
| K-12 + NFV (10:1) | 0.22 ± 0.09 | 0.82 ± 0.48 | 0.77 ± 0.14 | 0.63 ± 0.13 | 0.46 ± 0.12 | 0.99 |
| K-12 + NFV (5:1) | 0.19 ± 0.07 | 0.97 ± 0.47 | 0.83 ± 0.05 | 0.78 ± 0.14 | 0.72 ± 0.26 | 0.97 |
All data represent means ± standard deviations for at least three separate experiments.
Based on the inhibition of HIV-1-induced cytopathogenicity in MT-4 cells.
CIs giving 50, 70, or 90% inhibition of HIV-1 cytopathogenicity under the mutually exclusive assumption.
Average linear correlation coefficient of the median-effect plot.
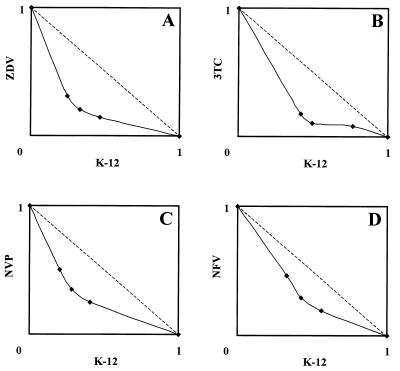
Except for one combination (K-12 plus 3TC at a ratio of 4:1), the CIs of all combinations were less than 1.0, irrespective of the inhibition levels (Table 1). The combination of K-12 and 3TC at a ratio of 4:1 exhibited synergism (CI = 0.86) at a level of 50% inhibition, whereas it displayed an additive effect (CI = 1.0) at 70% inhibition and antagonism (CI = 1.3) at 90% inhibition. Among the combinations, K-12 plus ZDV appeared to be the most synergistic, and K-12 plus 3TC and K-12 plus NVP followed. K-12 plus NFV was the least synergistic, although CIs of 0.77 to 0.83 were recorded at a level of 50% inhibition (Table 1). The isobologram presentation clearly demonstrated the synergies of all combinations, where the resulting isobolograms always fell below the broken line that indicated an additive effect (Fig. 2).
FIG. 2.
Isobolograms of the inhibitory effects of K-12 plus ZDV (A), K-12 plus 3TC (B), K-12 plus NVP (C), and K-12 plus NFV (D) on HIV-1 replication in MT-4 cells. MT-4 cells were infected with HIV-1IIIB and were cultured in the presence of various concentrations of the test compounds. After a 4-day incubation, the number of viable cells was determined by the MTT method. The data were analyzed by the median-effect principle, as described in Materials and Methods. Both x and y axes indicate the fractional inhibitory concentrations of each compound. The fractional inhibitory concentrations of 1.0 on the x and y axes represent the EC50s of K-12 and a compound with which it is combined, respectively. Broken lines represent the lines for CIs equal to 1.0 (additive effect). All data represent mean values for three separate experiments.
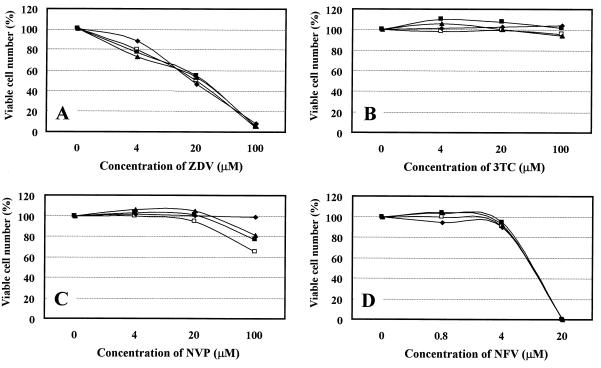
To exclude the possibility that K-12 enhanced the anti-HIV-1 activities of ZDV, 3TC, NVP, and NFV by enhancing their cytotoxicities for the host cells, the effect of K-12 on the cytotoxicities of these compounds was examined. In our previous studies, the 50% cytotoxic concentration (CC50) of K-12 was 3.2 μM for mock-infected MT-4 cells (3), yet at concentrations up to 1 μM it did not affect the proliferation and viability of the cells (data not shown). As shown in Fig. 3, K-12 did not affect the cytotoxicity of the RT and protease inhibitors to the host cells even when K-12 was used at a concentration of 1 μM. Interestingly, the cytotoxicity of NVP seemed to be reversed with increasing concentrations of K-12 (Fig. 3C). These results indicate that the synergistic anti-HIV-1 activities of the combinations are not due to the cytotoxicity of K-12.
FIG. 3.
Effects of K-12 on the cytotoxicities of ZDV (A), 3TC (B), NVP (C), and NFV (D) in mock-infected MT-4 cells. The cells were incubated in the presence of various concentrations of the test compounds in combination with K-12 at 0 μM (□), 0.04 μM (■), 0.8 μM (▴), or 1 μM (⧫). After a 4-day incubation, the number of viable cells was determined by the MTT method and was expressed as a percentage of the number of control viable cells (not treated with compound). All data represent mean values for three separate experiments.
The synergistic inhibition of HIV-1 replication by K-12 and either ZDV, 3TC, NVP, and NFV was confirmed in PBMCs. We chose for the assays the combination ratio that resulted in the highest degree of synergism with MT-4 cells, such as 100:1 for K-12 plus ZDV, 1:1 for K-12 plus 3TC, 8:1 for K-12 plus NVP, and 10:1 for K-12 plus NFV. Different from the results obtained in MT-4 cells, K-12 plus 3TC displayed the highest degree of synergism in PBMCs (Table 2). The CIs of this combination were almost constant (0.56 to 0.60), irrespective of the inhibition levels. Again, the weakest synergism was observed with K-12 plus NFV, and the CIs of this combination of close to 1.0 (0.89 to 0.96) indicated the additive effect of this combination in PBMCs.
TABLE 2.
Anti-HIV-1 activities of K-12, ZDV, 3TC, NVP, and NFV and their combinations in PBMCsa
| Combination (molar ratio) | EC50b (μM) | EC90 (μM) | CI at the following % inhibitionc:
|
rd | ||
|---|---|---|---|---|---|---|
| 50 | 70 | 90 | ||||
| K-12 alone | 0.18 ± 0.01 | 0.37 ± 0.10 | 0.96 | |||
| ZDV alone | 0.0013 ± 0.0001 | 0.0045 ± 0.0011 | 0.95 | |||
| 3TC alone | 0.14 ± 0.03 | 0.22 ± 0.03 | 0.95 | |||
| NVP alone | 0.034 ± 0.003 | 0.047 ± 0.08 | 0.96 | |||
| NFV alone | 0.0086 ± 0.0015 | 0.015 ± 0.002 | 0.95 | |||
| K-12 + ZDV (100:1) | 0.065 ± 0.005 | 0.14 ± 0.01 | 0.87 ± 0.13 | 0.80 ± 0.13 | 0.73 ± 0.14 | 0.99 |
| K-12 + 3TC (1:1) | 0.089 ± 0.002 | 0.15 ± 0.02 | 0.60 ± 0.10 | 0.58 ± 0.09 | 0.56 ± 0.07 | 0.97 |
| K-12 + NVP (8:1) | 0.10 ± 0.01 | 0.16 ± 0.02 | 0.86 ± 0.03 | 0.83 ± 0.01 | 0.79 ± 0.06 | 0.99 |
| K-12 + NFV (10:1) | 0.060 ± 0.005 | 0.11 ± 0.01 | 0.96 ± 0.03 | 0.93 ± 0.04 | 0.89 ± 0.04 | 0.99 |
All data represent means ± ranges for two separate experiments.
Based on the inhibition of p24 antigen production in PBMCs.
CIs giving 50, 70, or 90% inhibition of p24 antigen production under the mutually exclusive assumption.
Average linear correlation coefficient of the median-effect plot.
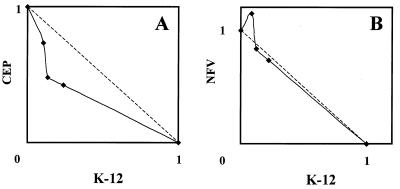
Since K-12 is an HIV-1 transcription inhibitor and is able to inhibit HIV-1 production in chronically infected cells, it was of particular interest to examine the effect of a combination of another transcription inhibitor or a protease inhibitor in these cells. Therefore, the combinations of K-12 and either the NF-κB inhibitor CEP or the protease inhibitor NFV were examined for their inhibitory effects on HIV-1 production in TNF-α-stimulated U1 cells. U1 cells are a promonocytic cell line chronically infected with HIV-1 (12). In the absence of any stimuli, U1 cells produce little or no HIV-1 particles and antigens. However, viral replication was markedly activated by the addition of a small amount of TNF-α into the culture medium. In our experiments, the p24 antigen levels in the culture supernatants were approximately 0.032 and 4.0 ng/ml in the absence or presence of 1 ng of TNF-α per ml, respectively (data not shown). Under such conditions, K-12, CEP, and NFV proved to be highly potent and selective inhibitors of HIV-1 replication, as determined by the reduction in the p24 antigen levels in the culture supernatants. The EC50s of K-12 alone, CEP alone, and NFV alone were 0.084, 0.047, and 0.13 μM, respectively (Table 3). The combination of K-12 and CEP at any ratio (1:4, 1:2, and 1:1) clearly resulted in synergistic inhibition of HIV-1 production, and the CIs were always less than 1.0. In particular, a CI value of 0.32 was achieved with the 1:1 combination at a level of 90% inhibition (Table 3), indicating strong synergism for inhibition of TNF-α-induced HIV-1 production in U1 cells. In contrast, the combination of K-12 and NFV in U1 cells resulted in an additive effect. The CIs of this combination were close to 1.0 or were even slightly greater than 1.0 at a level of 50% inhibition (Table 3). The isobologram presentation of these two combinations also contrasted the differences in the results obtained with K-12 plus CEP and those obtained with K-12 plus NFV (Fig. 4). The CC50 of K-12 was more than 5 μM for U1 cells, and we could not detect any cytotoxic effects on U1 cells at any of the concentrations used in the combination experiments (data not shown).
TABLE 3.
Anti-HIV-1 activities of K-12, CEP, and NFV and their combinations in TNF-α-stimulated U1 cellsa
| Combination (molar ratio) | EC50b (μM) | EC90 (μM) | CI at the following % inhibitionc:
|
rd | ||
|---|---|---|---|---|---|---|
| 50 | 70 | 90 | ||||
| K-12 alone | 0.084 ± 0.013 | 0.90 ± 0.49 | 0.99 | |||
| CEP alone | 0.047 ± 0.005 | 0.21 ± 0.03 | 0.99 | |||
| NFV alone | 0.13 ± 0.01 | 0.38 ± 0.16 | 0.95 | |||
| K-12 + CEP (1:4) | 0.043 ± 0.002 | 0.12 ± 0.03 | 0.85 ± 0.03 | 0.67 ± 0.04 | 0.47 ± 0.06 | 0.98 |
| K-12 + CEP (1:2) | 0.034 ± 0.002 | 0.11 ± 0.03 | 0.64 ± 0.07 | 0.53 ± 0.04 | 0.41 ± 0.02 | 0.96 |
| K-12 + CEP (1:1) | 0.040 ± 0.005 | 0.11 ± 0.03 | 0.69 ± 0.10 | 0.51 ± 0.04 | 0.32 ± 0.02 | 0.92 |
| K-12 + NFV (1:20) | 0.15 ± 0.02 | 0.27 ± 0.09 | 1.2 ± 0.1 | 1.0 ± 0.1 | 0.78 ± 0.20 | 0.96 |
| K-12 + NFV (1:10) | 0.12 ± 0.01 | 0.31 ± 0.10 | 0.96 ± 0.05 | 0.89 ± 0.09 | 0.83 ± 0.13 | 0.94 |
| K-12 + NFV (1:5) | 0.11 ± 0.00 | 0.26 ± 0.05 | 0.99 ± 0.08 | 0.85 ± 0.13 | 0.72 ± 0.20 | 0.95 |
All data represent means ± ranges for two separate experiments.
Based on the inhibition of p24 antigen production in TNF-α-stimulated U1 cells.
CIs giving 50, 70, or 90% inhibition of p24 antigen production under the mutually exclusive assumption.
Average linear correlation coefficient of the median-effect plot.
FIG. 4.
Isobolograms of the inhibitory effects of K-12 plus CEP (A) and K-12 plus NFV (B) on HIV-1 production in TNF-α-stimulated U1 cells. U1 cells were incubated in the presence of various concentrations of the test compounds for 2 h, stimulated with TNF-α, and further incubated. After a 3-day incubation, the culture supernatants were collected and examined for their p24 antigen levels. Both x and y axes indicate the fractional inhibitory concentrations of each compound, as described in the legend to Fig. 2. All data represent mean values for two separate experiments.
DISCUSSION
In this study, we demonstrated that combinations of K-12, a representative of the anti-HIV-1 fluoroquinolines, and the clinically available antiretroviral agents (ZDV, 3TC, NVP, and NFV) synergistically inhibited HIV-1 replication in acutely infected cell cultures. Synergistic inhibition of HIV-1 was also observed with the combination of K-12 and CEP in chronically infected cells. The anti-HIV-1 activities of fluoroquinoline derivatives were first described in a European patent, but their mechanism of action was not reported (19). Our recent studies of a series of novel fluoroquinoline derivatives revealed that the compounds, including K-12, selectively inhibited HIV-1 gene expression (3, 4). Reporter gene assays with HIV-1 long terminal repeat-driven chloramphenicol acetyltransferase or alkaline phosphatase revealed that the fluoroquinoline derivatives could suppress Tat-induced but not TNF-α-induced gene expression (4; data not shown). Interestingly, TNF-α-induced gene expression was moderately (20 to 30%) enhanced by the presence of 1 μM K-12 in CEM cells (data not shown). Thus, their anti-HIV-1 activities seem to be attributable to the inhibition of the HIV-1 Tat function, although we have recently found that the fluoroquinoline derivatives inhibit the Tat function in a transactivating response element-independent fashion (unpublished data). Further studies, such as a cell-free Tat transcription assay, are required to elucidate the pinpoint target of the compounds. Since Tat-mediated HIV-1 activation involves complex interactions with known and unknown cellular factors (7, 25, 27, 28), it is also possible that the fluoroquinoline derivatives target one of these factors. If K-12 is interacting with a cellular factor that plays a crucial role in gene expression, substantial toxicities may not be avoidable.
Current combination chemotherapies with HIV-1 RT inhibitors and protease inhibitors mainly focus on the suppression of the drug-resistant mutants that lead to the failure of drug efficacy in HIV-1-infected patients. On the other hand, the dose of each compound required to achieve the same degree of inhibition achieved with monotherapy could be reduced by use of a synergistic combination. This advantage of combination chemotherapy may also be quite important for such compounds that interact with cellular factors, because in vivo side effects seem to be a primary factor limiting their practical use. The concentrations of K-12 required for 50 and 90% inhibition of HIV-1 replication in MT-4 cells, could be reduced by more than one-third of the EC50 and EC90 of K-12 alone, respectively (Table 1). Similar results were also obtained with some combinations in PBMCs (Table 2).
Another interesting observation to be noted is that the combination of K-12 and CEP but not the combination of K-12 and NFV was found to inhibit synergistically HIV-1 production in chronically infected cells (Table 3 and Fig. 4). CEP is a plant alkaloid and has been shown to have anti-inflammatory, antiallergic, and immunomodulatory activities in vivo (13, 20, 21). A plant extract containing CEP as a major component has been used in Japan for the treatment of various chronic inflammatory diseases. We have recently found that CEP is a potent and selective inhibitor of HIV-1 expression in U1 cells (23). Its EC50 and CC50 for HIV-1 production were 0.026 and 3.6 μM, respectively, in phorbol 12-myristate 13-acetate-stimulated U1 cells. In the present study, the EC50 of CEP was 0.047 μM in TNF-α-stimulated U1 cells (Table 3), which confirmed the anti-HIV-1 activity of CEP in chronic HIV-1 infection. Although both K-12 and CEP are inhibitory to HIV-1 gene expression, the mechanism of HIV-1 inhibition by CEP clearly differs from that by K-12. CEP proved to interfere with the activation and translocation of NF-κB into the nucleus, whereas K-12 did not affect it, as determined by a gel mobility shift assay (3). This may be a reason for the synergy of K-12 plus CEP. In contrast, the combination of K-12 and the protease inhibitor NFV resulted in an additive effect in U1 cells (Table 3 and Fig. 4). Similarly, this combination (K-12 plus NFV) was the least synergistic in acutely infected MT-4 cells and PBMCs (Tables 1 and 2). Although it seems unlikely that K-12 affects the processing activity of HIV-1 protease, experiments with other protease inhibitors are needed to conclude that the combination of K-12 and a protease inhibitor generally exerts an additive inhibitory effect on HIV-1 replication.
In conclusion, the HIV-1 transcription inhibitor K-12 not only potently and selectively inhibits HIV-1 replication but also enhances the activities of clinically available antiretroviral agents in cell cultures. Thus, the combinations described here may have potential as effective chemotherapeutic modalities and should be further pursued in vivo unless K-12 (or its derivatives) is found to have serious toxicities or pharmacological problems in humans.
ACKNOWLEDGMENTS
U1 cells were kindly provided by Thomas Folks (Centers for Disease Control and Prevention, Atlanta, Ga.).
This work was supported in part by a grant-in aid for scientific research from the Ministry of Education, Science, Sports, and Culture of Japan and by a grant from the Japan Health Science Foundation.
REFERENCES
- 1.Baba M. Piperazinylfluoroquinolines as inhibitors of HIV-1 transcription. Int Antivir News. 1997;5:203–204. [Google Scholar]
- 2.Baba M, De Clercq E, Tanaka H, Ubasawa M, Takashima H, Sekiya K, Nitta I, Umezu K, Nakashima H, Mori S, Shigeta S, Walker R T, Miyasaka T. Potent and selective inhibition of human immunodeficiency virus type 1 (HIV-1) by 5-ethyl-6-phenylthiouracil derivatives through their interaction with the HIV-1 reverse transcriptase. Proc Natl Acad Sci USA. 1991;88:2356–2360. doi: 10.1073/pnas.88.6.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baba M, Okamoto M, Makino M, Kimura Y, Ikeuchi T, Sakaguchi T, Okamoto T. Potent and selective inhibition of human immunodeficiency virus type 1 transcription by piperazinyloxoquinoline derivatives. Antimicrob Agents Chemother. 1997;41:1250–1255. doi: 10.1128/aac.41.6.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baba M, Okamoto M, Kawamura M, Makino M, Higashida T, Takashi T, Kimura Y, Ikeuchi T, Tetsuka T, Okamoto T. Inhibition of human immunodeficiency virus type 1 replication and cytokine production by fluoroquinoline derivatives. Mol Pharmacol. 1998;53:1097–1103. [PubMed] [Google Scholar]
- 5.Belen’kii M S, Schinazi R F. Multiple drug effect analysis with confidence interval. Antivir Res. 1994;25:1–11. doi: 10.1016/0166-3542(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 6.Chou T C, Talalay P. Quantitative analysis of dose-effect relationship: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 7.Cujec T P, Okamoto H, Fujinaga K, Meyer J, Chamberlin H, Morgan D O, Peterlin B M. The HIV transactivator TAT binds to the CDK-activating kinase and activates the phosphorylation of the carboxy-terminal domain of RNA polymerase II. Genes Dev. 1997;11:2645–2657. doi: 10.1101/gad.11.20.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cullen B R. Regulation of human immunodeficiency virus replication. Annu Rev Microbiol. 1991;45:219–250. doi: 10.1146/annurev.mi.45.100191.001251. [DOI] [PubMed] [Google Scholar]
- 9.Cupelli L A, Hsu M-C. The human immunodeficiency virus type 1 Tat antagonist, Ro 5-3335, predominantly inhibits transcription initiation from the viral promoter. J Virol. 1995;69:2640–2643. doi: 10.1128/jvi.69.4.2640-2643.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deeks S G, Smith M, Holodniy M, Kahn J O. HIV-1 protease inhibitors. A review for clinicians. JAMA. 1997;277:145–153. [PubMed] [Google Scholar]
- 11.Finzi D, Hermankova M, Pierson T, Carruth L M, Back C, Chaisson R E, Quinn T C, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho D D, Richman D D, Siliciano R F. Identification of a reservoir for HIV-1 patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 12.Folks T M, Justement J, Kinter A, Dinarello C A, Fauci A S. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science. 1987;238:800–802. doi: 10.1126/science.3313729. [DOI] [PubMed] [Google Scholar]
- 13.Goto M, Zeller W P, Hurley P M. Cepharanthine (biscoclaurine alkaloid) treatment in endotoxic shock of suckling rats. J Pharm Pharmacol. 1991;43:589–591. doi: 10.1111/j.2042-7158.1991.tb03542.x. [DOI] [PubMed] [Google Scholar]
- 14.Havlir D V, Richman D D. Viral dynamics of HIV: implications for drug development and therapeutic strategies. Ann Intern Med. 1996;124:984–994. doi: 10.7326/0003-4819-124-11-199606010-00006. [DOI] [PubMed] [Google Scholar]
- 15.Hsu M-C, Schutt A D, Holly M, Slice L W, Sherman M I, Richman D D, Potash M J, Volsky D J. Inhibition of HIV replication in acute and chronic infections in vitro by a Tat antagonist. Science. 1991;254:1799–1802. doi: 10.1126/science.1763331. [DOI] [PubMed] [Google Scholar]
- 16.Hsu M-C, Dhingra U, Earley J V, Holley M, Keith D, Nalin C M, Richou A R, Schutt A D, Tam S Y, Potash M J, Volsky D J, Richman D D. Inhibition of type 1 human immunodeficiency virus replication by a Tat antagonist to which the virus remains sensitive after prolonged exposure in vitro. Proc Natl Acad Sci USA. 1993;90:6395–6399. doi: 10.1073/pnas.90.14.6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson V A. Combination therapy for HIV-1 infection. Overview: preclinical and clinical analysis of antiretroviral combinations. Antivir Res. 1996;29:35–39. doi: 10.1016/0166-3542(95)00912-4. [DOI] [PubMed] [Google Scholar]
- 18.Jones K A, Peterlin B M. Control of RNA initiation and elongation at the HIV promoter. Annu Rev Biochem. 1994;63:717–743. doi: 10.1146/annurev.bi.63.070194.003441. [DOI] [PubMed] [Google Scholar]
- 19.Kimura T, Katsube T. Aminoquinoline derivatives as anti-HIV agents. European patent 572259; December 1993. [Google Scholar]
- 20.Kondo Y, Imai Y, Hojo H, Hashimoto Y, Nozoe S. Selective inhibition of T-cell-dependent immune responses by bisbenzylisoquinoline alkaloids in vivo. Int J Immunopharmacol. 1992;14:1181–1186. doi: 10.1016/0192-0561(92)90053-n. [DOI] [PubMed] [Google Scholar]
- 21.Matsuno T, Orita K, Edashige K, Kobuchi H, Sato E F, Inouye B, Inoue M, Utsumi K. Inhibition of active oxygen generation in guinea-pig neutrophils by biscoclaurine alkaloids. Biochem Pharmacol. 1990;39:1255–1259. doi: 10.1016/0006-2952(90)90271-l. [DOI] [PubMed] [Google Scholar]
- 22.Miyosi I, Taguchi H, Kubonishi I, Yoshimoto S, Ohtsuki Y, Shiraishi Y, Akagi T. Type C virus-producing cell lines derived from adult T cell leukemia. Gann Monogr. 1982;28:219–228. [Google Scholar]
- 23.Okamoto M, Ono M, Baba M. Potent inhibition of HIV-1 replication by an anti-inflammatory alkaloid, cepharanthine, in chronically infected monocytic cells. AIDS Res Hum Retroviruses. 1998;14:1239–1245. doi: 10.1089/aid.1998.14.1239. [DOI] [PubMed] [Google Scholar]
- 24.Perelson A S, Neumann A U, Markowitz M, Leonard J M, Ho D D. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 25.Wei, P., M. E. Garber, S.-M. Fang, W. H. Fischer, and K. A. Johns. A novel CDK-9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell 92:451–462. [DOI] [PubMed]
- 26.Wong J K, Hezareh M, Günthard H F, Havlir D V, Ignacio C C, Spina C A, Richman D D. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1921–1925. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Q, Sharp P A. Tat-SF1: cofactor for stimulation of transcriptional elongation by HIV-1 Tat. Science. 1996;274:605–610. doi: 10.1126/science.274.5287.605. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Y, Pe’ery T, Peng J, Ramanathan Y, Marshall N, Marshall T, Amendt B, Mathews M B, Price D H. Transcription elongation factor P-TEFb is required for HIV-1 Tat transactivation in vitro. Genes Dev. 1997;11:2622–2632. doi: 10.1101/gad.11.20.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]