This case series uses adaptive optics scanning light ophthalmoscopy to assess the short-term integrity of the cone mosaic following subretinal injections of adeno-associated virus-mediated hCHM gene augmentation in patients with choroideremia.
Key Points
Question
To what extent is cone photoreceptor mosaic integrity and density maintained following subretinal injection of adeno-associated virus vector designed to deliver a functional version of the CHM gene (AAV2-hCHM) augmentation for choroideremia?
Findings
In this case series of 9 patients, cone mosaic integrity and cone photoreceptor density were maintained following subretinal injection of AAV2-hCHM for choroideremia, although mild shortening of the photoreceptor outer segments was detected.
Meaning
These findings provide safety information at the cellular level for both the subretinal injection surgical technique and the AAV2-hCHM study agent.
Abstract
Importance
Subretinal injection for gene augmentation in retinal degenerations forcefully detaches the neural retina from the retinal pigment epithelium, potentially damaging photoreceptors and/or retinal pigment epithelium cells.
Objective
To use adaptive optics scanning light ophthalmoscopy (AOSLO) to assess the short-term integrity of the cone mosaic following subretinal injections of adeno-associated virus vector designed to deliver a functional version of the CHM gene (AAV2-hCHM) in patients with choroideremia.
Design, Setting, and Participants
This longitudinal case series study enrolled adult patients with choroideremia from February 2015 to January 2016 in the US. To be included in the study, study participants must have received uniocular subfoveal injections of low-dose (5 × 1010 vector genome per eye) or high-dose (1 × 1011 vector genome per eye) AAV2-hCHM. Analysis began February 2015.
Main Outcomes and Measures
The macular regions of both eyes were imaged before and 1 month after injection using a custom-built multimodal AOSLO. Postinjection cone inner segment mosaics were compared with preinjection mosaics at multiple regions of interest. Colocalized spectral-domain optical coherence tomography and dark-adapted cone sensitivity was also acquired at each time point.
Results
Nine study participants ranged in age from 26 to 50 years at the time of enrollment, and all were White men. Postinjection AOSLO images showed preservation of the cone mosaic in all 9 AAV2-hCHM–injected eyes. Mosaics appeared intact and contiguous 1 month postinjection, with the exception of foveal disruption in 1 patient. Optical coherence tomography showed foveal cone outer segment shortening postinjection. Cone-mediated sensitivities were unchanged in 8 of 9 injected and 9 of 9 uninjected eyes. One participant showed acute loss of foveal optical coherence tomography cone outer segment–related signals along with cone sensitivity loss that colocalized with disruption of the mosaic on AOSLO.
Conclusions and Relevance
Integrity of the cone mosaic is maintained following subretinal delivery of AAV2-hCHM, providing strong evidence in support of the safety of the injections. Minor foveal thinning observed following surgery corresponds with short-term cone outer segment shortening rather than cone cell loss. Foveal cone loss in 1 participant raises the possibility of individual vulnerability to the subretinal injection.
Introduction
The advent of gene therapy for inherited retinal degenerations has revolutionized the field of ophthalmic care.1 The US Food and Drug Administration’s recent approval of Luxturna (Spark Therapeutics) has provided the first clinically available treatment option for RPE65-associated retinal degeneration2 and given hope to numerous other patients who experience genetic blinding disease.3 Indeed, numerous clinical trials testing gene augmentation to treat other inherited retinal degenerations are being conceived or are in progress.4,5,6
Choroideremia is one such inherited retinal degeneration where gene augmentation is being tested in multi-institutional gene therapy clinical trials.7,8,9,10,11,12,13,14,15,16,17,18 Choroideremia is an X-linked degeneration caused by variants in the CHM gene, which encodes Rab escort protein 1 (REP1), a protein thought to be involved in membrane trafficking.19,20 Variants in CHM lead to progressive degeneration of the photoreceptors, retinal pigment epithelium (RPE), and choroid.21,22,23,24 Patients with choroideremia typically present in their youth with nyctalopia and visual field defects. The earliest clinically detectable abnormalities include RPE demelanization, disruption of the photoreceptor outer segments, and severe rod photoreceptor dysfunction starting in the near midperipheral retina.25,26,27 Cone dysfunction as well as centrifugal and centripetal movement of the degenerative process21,27 causes progressive constriction of the visual field and eventual involvement of the foveal center, which results in degraded visual acuity typically in the fifth decade of life.21,25,27,28,29
Retinal imaging in choroideremia has demonstrated retained central islands of neural retina, with sharp borders demarcating the transition to severely degenerated areas.29,30 Cross-sectional imaging with optical coherence tomography (OCT) has revealed that, independent of the cellular origin of the primary mechanism of disease, shortening or loss of the photoreceptor outer segments are the earliest clinically detectable abnormalities, preceding overt structural loss within the RPE and choroid.21,24,27,29,31
At the cellular level, imaging with adaptive optics scanning laser ophthalmoscopy (AOSLO) has enabled visualization of the photoreceptor mosaic both in health and disease.32 This technique involves measuring and compensating for the optical aberrations of the living eye to obtain diffraction-limited imaging through the pupil.33 In choroideremia, AOSLO imaging has revealed the photoreceptor mosaic remains contiguous up to the edge of sharp borders between relatively preserved and atrophic retina.29,34,35 Within the retained central islands, local regions of the photoreceptor mosaic can exhibit either normal or reduced cone densities with dim and mottled wave-guided reflectance profiles. AOSLO imaging combined with microperimetry has confirmed the existence of sharp transitions between functioning retina and severe sensitivity losses that collocate with the rapid transitions in retinal structure.30 The results suggest the RPE, in addition to rods, is an autonomous site of degeneration in choroideremia. Cones ultimately are lost as well, either by mechanisms that occur in parallel or as a consequence of severe RPE and/or choroidal changes.21,27,29,30,34,36,37,38,39
Current gene augmentation strategies for managing choroideremia use a subretinal injection of adeno-associated virus vector to deliver normal copies of the CHM gene (AAV2-hCHM) to the residual islands of viable retina hoping to restore REP1 function and thereby maintain or improve levels of central vision.7,17,21 The procedure is not without risk in that it involves forcefully delivering a fluid under the neural retina, thus separating the photoreceptors within the residual island from the underlying supportive RPE. This process of intentionally detaching the photoreceptors from the RPE has the potential to damage the structural integrity of the entire retina, particularly the RPE and photoreceptors. In the present study, we use AOSLO to gain insight into the short-term changes of the cone mosaic following macular subretinal injections of adeno-associated virus-mediated (AAV2) hCHM in choroideremia. We evaluate the cone mosaic structure in conjunction with foveal measures of cone outer segment length and vision prior to and 1 month after the subfoveal injections.
Methods
Study Participants and General Procedures
This research adhered to the Declaration of Helsinki40 and was approved by the institutional review boards at the University of Pennsylvania and the Children’s Hospital of Philadelphia. All participants provided written and verbal informed consent before voluntarily enrolling (February 2015 to January 2016) in the study and were compensated $10 per hour for participating. The study participants also provided informed consent and were enrolled in a dose escalation phase 1/2 clinical trial testing the safety of the subretinal delivery of AAV2-hCHM in choroideremia (NCT02341807).
Study participants underwent a complete ophthalmic examination before and 1 month after the subretinal delivery of AAV2-hCHM, including dark-adapted foveal sensitivity testing, OCT imaging, and AOSLO imaging. Cone sensitivity was measured at the fovea using a modified Humphrey Field Analyzer (HFA II-I; Carl Zeiss Meditec) using a 1.75° diameter, 650-nm stimuli presented at the fovea following 30 minutes of dark adaptation.21 Axial lengths were recorded using an IOL-Master (Carl Zeiss Meditec). AOSLO images were proportionally scaled by axial length. Within 87 days from the baseline imaging session (mean [SD], 27 [25] days; range, 4-87 days), study participants received unilateral subretinal injection of low-dose (up to 5 × 1010 vector genome per eye) or high-dose (up to 1 × 1011 vector genome per eye) AAV2-hCHM per the phase 1/2 clinical trial protocol. As previously reported, the injection blebs covered the entire extent of the residual central islands including the foveal center.16 For quantitative analyses of the OCT cross-sections, images from postoperative visits were coregistered to baseline and resampled at 10-fold the original resolution. Longitudinal reflectivity profiles from the foveal center (juxtafovea in jpatient 07 to avoid retinal tracks and ellipsoid zone [EZ] discontinuation) were generated using ImageJ.21,41 Longitudinal reflectivity profiles aligned by the main RPE/Bruch membrane (BrM) signal peak were used to determine the distance between the EZ signal peak and the peak at the base of the RPE/BrM. Finally, changes in dark-adapted cone sensitivity and foveal EZ-to-RPE/BrM distance were compared with cone mosaic morphology as determined by AOSLO imaging.
AOSLO Imaging
The custom-built, multimodal AOSLO system used in this study has been previously described.42,43 Three photomultiplier tubes (Hamamatsu Corporation) were configured to record confocal and nonconfocal split-detection near-infrared reflectance image sequences at 18 Hz simultaneously. AOSLO image sequences were acquired over the central 3° surrounding fixation and along each meridian until reaching the atrophic border or reaching approximately 15° eccentricity.
AOSLO image sequences from each imaging session were desinusioded, registered, and semiautomatically montaged as previously described.44,45,46 Montages from each time point were then manually aligned using Adobe Photoshop. Macroscopic image features, such as blood vessels and the contours of the central island, were used for an initial alignment; the longitudinal alignment was then refined for cone-by-cone accuracy over regions of interest (ROIs). When necessary, 1-month images were scaled to baseline images.
Cone Density Measurements
Four ROIs from each eye were selected for measurement of cone densities. ROIs were manually cropped from the baseline and 1 month postinjection split detection AOSLO montages for both injected and control eyes of all study participants. One grader (J.I.W.M.) manually identified cones in all ROIs using custom software.47 The grader was masked to injected vs control eye and time point for each study participant and was able to adjust the brightness and contrast of the image while selecting cones. Cone centers were used to determine Voronoi boundaries and bound cone density was calculated for each ROI.48 Using the generalized estimating equations, cone densities, cone densities change from baseline, and percent change in cone densities from baseline were compared between uninjected and injected eyes, while accounting for intereye correlation and repeated measure correlation for cone densities at 4 locations.49 Similar analysis were performed for comparing cone densities between baseline and 1-month time points in uninjected and injected eyes. Analysis began February 2015. Two sided P < .05 was considered to be statistically significant.
Results
Nine patients with molecularly confirmed choroideremia participated in the study. Study participant characteristics are shown in the eTable in the Supplement. All patients self-reported as White men and ranged in age from 26 to 50 years at the time of enrollment. As previously reported, surgeries were uneventful.16 Foveal cone sensitivity was unchanged at 1 month postinjection for 8 of 9 injected eyes and all 9 control eyes. One participant, patient 11, showed a loss in foveal cone sensitivity in the injected eye (eTable in the Supplement). As previously reported,16 this same participant demonstrated a loss in visual acuity (3 lines) in the injected eye while all other visual acuities remained unchanged.
Shortening of Cone Outer Segments After Subretinal Gene Therapy
Overall, at 1 month postinjection, OCTs show that compared with baseline images the laminar architecture of the retina is qualitatively unchanged in both the injected and uninjected eyes and that the subretinal bleb containing AAV2-hCHM resolved in injected eyes (eFigure 1 in the Supplement). Quantitative analyses showed a normal foveal EZ-to-RPE/BrM distance (mean [2SD] normal, 52 [15] μm) at baseline in all study participants except patients 05 and 07 with the most severe foveal abnormalities. At the 1 month postinjection time point, this distance was shorter in the AAV2-hCHM–injected eyes, suggestive of foveal cone outer segment shortening (eFigure 1C in the Supplement). The differences between the measures at 1 month and baseline exceeded the variability of the measurements (±4.42 μm) in 4 study participants, was borderline relative to the variability in another 4 participants and unchanged in 1 participant. The EZ-to-RPE/BrM distance in uninjected eyes remained unchanged at 1 month compared with baseline.
Photoreceptor Mosaic Integrity and Cone Density
Nonconfocal split detection AOSLO at baseline revealed the photoreceptor mosaic within the central island of remaining retina was intact and contiguous out to the border of atrophy, at which point, the photoreceptor mosaic exhibited a sharp transition to atrophic retinal regions. AOSLO at 1 month postinjection also revealed a contiguous mosaic in 8 of 9 injected eyes and all 9 uninjected eyes (Figure 1 and eFigures 2-18 in the Supplement). Global features within the montages could be aligned longitudinally. However, local distortions in adjacent images both within and between time points precluded cone-by-cone alignment across the full montage in both injected and uninjected eyes. Thus, ROIs within the montages were selected for cone-by-cone alignment across time points using rigid transforms (translation, rotation, scale) only (eFigures 19-27 in the Supplement). Cone-by-cone alignment was attained at multiple retinal locations within the montage in all eyes. Qualitatively, this manual alignment was easier to perform in uninjected eyes and ROIs in uninjected eyes showed accurate cellular alignments over a larger distance than ROIs in injected eyes.
Figure 1. Nonconfocal Split Detection Adaptive Optics Scanning Laser Ophthalmoscopy Montage of the Photoreceptor Inner Segment Mosaic at Baseline and 1 Month Postinjection in the Injected Eye of Patient 06.
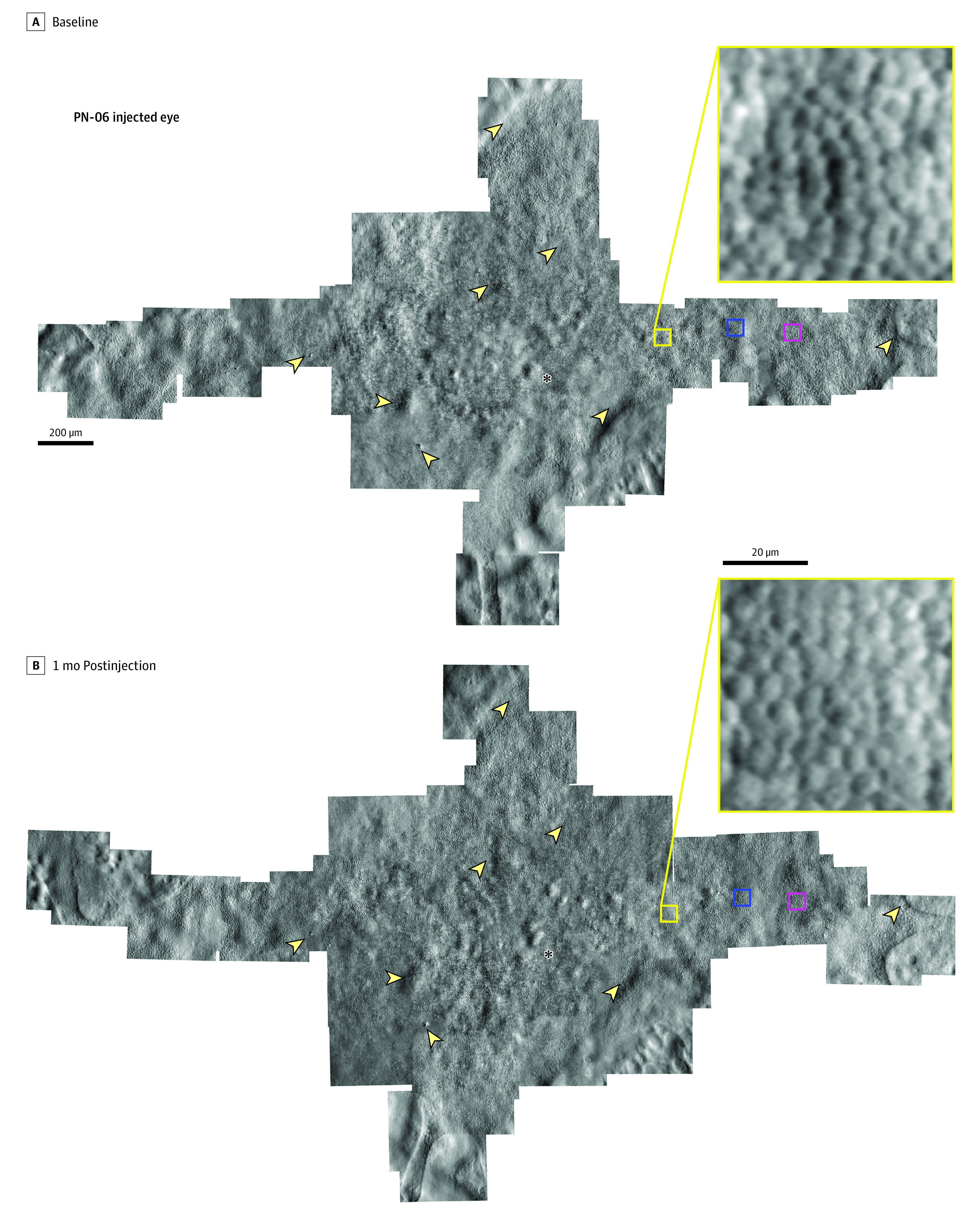
The same retinal features are observed longitudinally in both montages and the photoreceptor mosaic remains intact following the subretinal injection of adeno-associated virus-mediated hCHM. Asterisks denote the foveal location in the mosaic. Arrowheads highlight example features of the cone mosaic that are observed at both time points. Yellow boxes show an enlarged region of the cone mosaic within the montage; this location was one of the regions of interest used for cone density measurement. Blue and magenta boxes denote the locations of the regions of interest used for cone density measurements shown in Figure 2.
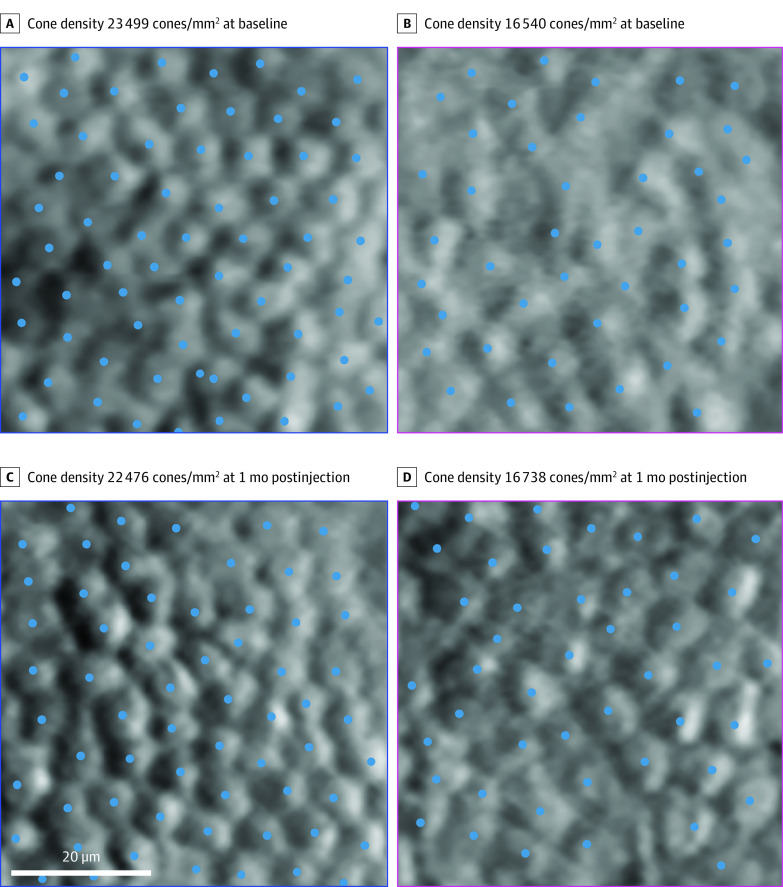
Cones could be manually identified and bound cone density determined for longitudinally aligned ROIs in all eyes (Figure 2). Cone densities were similar across time points for all ROIs in both injected and uninjected eyes (eFigure 28 in the Supplement). In injected eyes, cone density (mean [SE]) was 24 027 (1991) cones/mm2 at 1 month postinjection compared with 24 401 (2361) cones/mm2 at baseline (mean [SE] cones/mm2, 24 027 [1991] vs 24 401 [2361]; P = .43). Mean (SD) cone densities in uninjected eyes were 24 284 (3051) cones/mm2 at 1 month compared with 24 491 (3022) cones/mm2 at baseline (mean [SE] cones/mm2, 24 284 [3051] vs 24 491 [3022]; P = .41). Summarizing across all ROIs, there was no statistical difference observed between cone densities measured in injected and uninjected eyes at baseline (mean [SE] cones/mm2, 24 401 [2361] vs 24 491 [3022]; P = .97; Table) or at 1 month (mean [SE] cones/mm2, 24 027 [1991] vs 24 284 [3051]; P = .91; Table). There was no difference between injected and uninjected eyes in cone density change from baseline (mean [SE] cones/mm2, 374 [472] vs 207 [249]; P = .80; Table) nor in percent change from baseline in cone density at 1 month (mean [SE] %, −0.56 [2.56] vs 1.2 [1.05]; P = .60; Table).
Figure 2. Adaptive Optics Regions of Interest Aligned Between Time Points From the Injected Eye of Patient 06.
An intact cone mosaic is visible before and after the subretinal injection of adeno-associated virus-mediated hCHM. Cones were manually identified (blue dots) and bound cone density was calculated for each region of interest. The adaptive optics montages in Figure 1 show the retinal locations of the 2 regions of interest, based on the corresponding blue and magenta colored squares.
Table. Comparison of Cone Density (Cones/mm2) Between Injected and Uninjected Eyes .
| Variable | Eyes, mean (SE)a | Difference (95% CI)b | P valueb | |
|---|---|---|---|---|
| Uninjected | Injected | |||
| Baseline | 24 491 (3022) | 24 401 (2361) | 90 (−4505 to 4685) | .97 |
| 1 mo Postinjection | 24 284 (3051) | 24 027 (1991) | 257 (−4119 to 4632) | .91 |
| Difference (baseline – 1 mo postinjection) | 207 (249) | 374 (472) | −167 (−1447 to 1113) | .80 |
| % Difference | 1.20 (1.05) | −0.56 (2.56) | 1.76 (−4.79 to 8.32) | .60 |
Eighteen eyes were analyzed.
From the generalized estimating equations49 that account for the correlations from repeated measures at 4 locations and intereye correlation.
One-month postinjection AOSLO images of participant patient 09 revealed a local loss of cones at the fovea (Figure 3). This area of cone loss was colocated both with the retinal region that revealed a loss of foveal sensitivity and with a region showing disruption of the EZ band on OCT at the 1-month postinjection time point (eFigure 1 in the Supplement). Despite the foveal cone loss, cones were visible in the surrounding parafoveal regions at the same time point. Similar to the other participants, the photoreceptor mosaic was intact at regions outside of the fovea for patient 09, longitudinal alignments of the cone mosaic were possible, and cone densities were unchanged in ROIs selected for cone density quantifications.
Figure 3. Adaptive Optics Nonconfocal Split Detection Montage in the Injected Eye of Patient 09 at 1 mo Postinjection.
The black box shows an area of loss of cone inner segments surrounded by intact cone inner segments at the fovea; yellow boxes show the intact cone inner segment mosaic in parafoveal regions.
Discussion
Managing choroideremia by gene augmentation represents a substantial departure from the treatment scenario of the earlier experience in RPE65-inherited retinal degenerations that culminated with the first US Food and Drug Administration–approved gene therapy product for use in the clinic.3,18 In mid- to end-stage choroideremia, there is no alternative but to treat small fragile central islands of relatively preserved retina that sustain limited fields of vision that often support reading levels that are above the legal limit of blindness. The scenario is quite different from the treatment of functionally blind retinas that are less fragile in diseases within the spectrum of RPE65–Leber congenital amaurosis.3,18 In fact, the resulting shift in the benefit-to-risk ratio is to be expected for the larger group of non–Leber congenital amaurosis inherited retinal degenerations at the disease stages that are typically considered for initial clinical trials, making choroideremia a model for an entire group of genetic retinal diseases that await treatment solutions. Although the safety profile of the subretinal injections is now better understood, quantitative approaches, particularly at the cellular level, are needed to understand the mechanisms that lead to unwanted outcomes, particularly when the remaining vision is threatened by an invasive procedure, such as a therapeutic retinal detachment. In the current study, we used OCT and AOSLO imaging to document possible short-term changes of the photoreceptor mosaic following subfoveal injections of AAV2-hCHM. We demonstrate that at 1 month postinjection, the cone mosaic resettled on the RPE following resolution of the subretinal bleb. The cone mosaic remained intact in 8 of 9 study participants (except patient 09) and outside of the foveal center in all participants. Quantification of cone densities revealed no measurable difference between the injected and uninjected eyes and no measurable changes in cone densities between baseline and 1 month postinjection. These results show there was no widespread cone loss across the retained area of central retina targeted by the retinal detachment. Thus, we make 2 important conclusions regarding the safety of the AAV2-hCHM experimental therapy. First, cone photoreceptors did not drop out as a consequence of mechanical or acute inflammatory changes in response to the presence of AAV2-hCHM in the subretinal space. Second, the therapeutic retinal detachment, as performed by us, did not result in detectable short-term changes of the density of the photoreceptor mosaic, although mild shortening of the photoreceptor outer segments was detected. Altogether, our results provide safety information at the cellular level for both the surgical technique and the AAV2-hCHM study agent and confirm cellular-level safety signals that up to now were only available through histopathologic studies in normal nonhuman primates.50,51,52,53,54
However, the subretinal injection is not without risk. Foveal thinning and the occurrence of full thickness loss of retinal tissue (macular holes) are known complications of the procedure.3 The loss of the photoreceptors at the fovea in 1 study participant (patient 09) raises the possibility of individual vulnerability to the subfoveal injection, an issue reported in at least 1 participant in each of the CHM gene therapy clinical trial reports.18 Patient 09’s surgery was considered uneventful. However, it is unclear why the photoreceptors at the fovea of patient 09 did not withstand the subfoveal injection because the parafoveal cones did survive the intervention (Figure 3); we suggest that the cone loss may have resulted from mechanical factors of the surgery rather than toxicity to the study agent. All study participants were at a similar stage of their central retinal disease with remodeled foveas that were within normal limits of thickness (except patient 05) or even thicker than normal.21,27 In fact, 2 patients (patients 07 and 08) showed proximity of the transitional zones of structural disorganization to the foveal center, a factor known to predict the decline in visual acuity as part of the natural history of the disease,21 yet they did not have an unfavorable outcome.16 Inspection of patient 09’s OCT cross-sections reveals faint EZ and interdigitation zone signal that may be indicative of fragile or more abnormal photoreceptors, as has been described in certain forms of cone photoreceptor inherited degenerations.55,56 Perhaps these may be structural signs that dictate modified surgical approaches.57,58 Further studies are needed to address predisposing factors in patients with similar outcomes.
Our OCT results at 1 month postinjection showed a decrease in outer segment length in comparison with baseline. Preliminary results reported from an ongoing randomized clinical trial (NCT02341807) showed that the foveal thickness slowly recovers over 6 months postinjection.16 This, taken together with the AOSLO imaging results, leads to the conclusion that the measured decrease in thickness is caused by short-term outer segment shortening as opposed to cone loss. We hypothesize the forced detachment between the outer segment tips and the RPE from the surgical injection rather than the vector itself causes this short-term shortening of the outer segments. For unknown reasons, the recovery of the outer segment length after retinal detachments follows a time course that differs from the normal renewal rate of the outer segment and seems to be independent of the cause of the retinal detachment, whether short-lived and intentional, such as during the delivery of gene therapy products by subretinal injections, or after spontaneous primary macula-off detachments.59,60,61,62 Beyond purely mechanical factors affecting the outer retina, complex interactions are known to occur after retinal detachments, such as the response of the inner retina and the underlying RPE to the therapeutic detachment, topics in need of investigation.63,64,65,66
Although there have been reports documenting the safety of subretinal injections targeting the macula in choroideremia and other inherited retinal degenerations, this work is, to our knowledge, the first study to apply AOSLO to investigate gene therapy intervention for a blinding disease. Gene therapy aims to prevent cellular death and/or restore function to cells that are still surviving and AOSLO enables noninvasive visualization of individual cells. As a practical matter, the design and economics of clinical trials puts a high value on accurately measuring outcomes reasonably soon after the experimental interventions. The Spark Therapeutics–funded clinical trial for choroideremia did not include AOSLO imaging as an outcome measure, but the results from this ancillary study suggest that AOSLO might be suitable as a precise anatomic outcome measure in future trials involving subretinal injections. Further, techniques such as optoretinography67,68,69,70,71 and AOSLO microperimetry30,72,73,74 have complemented AOSLO imaging by allowing the direct or indirect evaluation of photoreceptor function at the cellular level. The emergence of these tools may prove impactful for assessing the short- and long-term safety and efficacy of gene therapies for blinding diseases.
Limitations
Longitudinal cone density measurements from AOSLO images are limited by manual image processing techniques including cell-by-cell alignment and cone identification over small regions of interest. Techniques such as automated longitudinal montaging44 and automatic quantification of cone density over the full montage75 will be beneficial, especially for studies that enroll large numbers of participants.
Conclusions
In conclusion, our data support the short-term safety of subretinal injections of AAV2-hCHM. Additional follow-up will be required to assess the long-term safety and efficacy of subretinal injection and delivery of AAV2-hCHM for preventing or restoring vision loss caused by choroideremia.
eTable. Study Participant Characteristics
eFigure 1. Cone outer segment shortening after subretinal gene therapy
eFigure 2. AOSLO montages, PN-01 Injected Eye
eFigure 3. AOSLO montages, PN-01 Uninjected Eye
eFigure 4. AOSLO montages, PN-03 Injected Eye
eFigure 5. AOSLO montages, PN-03 Uninjected Eye
eFigure 6. AOSLO montages, PN-04 Injected Eye
eFigure 7. AOSLO montages, PN-04 Uninjected Eye
eFigure 8. AOSLO montages, PN-05 Injected Eye
eFigure 9. AOSLO montages, PN-05 Uninjected Eye
eFigure 10. AOSLO montages, PN-06 Uninjected Eye
eFigure 11. AOSLO montages, PN-07 Injected Eye
eFigure 12. AOSLO montages, PN-07 Uninjected Eye
eFigure 13. AOSLO montages, PN-08 Injected Eye
eFigure 14. AOSLO montages, PN-08 Uninjected Eye
eFigure 15. AOSLO montages, PN-09 Injected Eye
eFigure 16. AOSLO montages, PN-09 Uninjected Eye
eFigure 17. AOSLO montages, PN-11 Injected Eye
eFigure 18. AOSLO montages, PN-11 Uninjected Eye
eFigure 19. Cone mosaic ROIs, PN-01
eFigure 20. Cone mosaic ROIs, PN-03
eFigure 21. Cone mosaic ROIs, PN-04
eFigure 22. Cone mosaic ROIs, PN-05
eFigure 23. Cone mosaic ROIs, PN-06
eFigure 24. Cone mosaic ROIs, PN-07
eFigure 25. Cone mosaic ROIs, PN-08
eFigure 26. Cone mosaic ROIs, PN-09
eFigure 27. Cone mosaic ROIs, PN-11
eFigure 28. Cone density at one-month post-injection versus baseline
eReferences
References
- 1.MacLaren RE, Bennett J, Schwartz SD. Gene Therapy and stem cell transplantation in retinal disease: the new frontier. Ophthalmology. 2016;123(10S):S98-S106. doi: 10.1016/j.ophtha.2016.06.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apte RS. Gene therapy for retinal degeneration. Cell. 2018;173(1):5. doi: 10.1016/j.cell.2018.03.021 [DOI] [PubMed] [Google Scholar]
- 3.Maguire AM, Bennett J, Aleman EM, Leroy BP, Aleman TS. Clinical perspective: treating RPE65-associated retinal dystrophy. Mol Ther. 2021;29(2):442-463. doi: 10.1016/j.ymthe.2020.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson DA, Iannaccone A, Ali RR, et al. ; Monaciano Consortium . Advancing clinical trials for inherited retinal diseases: recommendations from the second monaciano symposium. Transl Vis Sci Technol. 2020;9(7):2. doi: 10.1167/tvst.9.7.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garafalo AV, Cideciyan AV, Héon E, et al. Progress in treating inherited retinal diseases: early subretinal gene therapy clinical trials and candidates for future initiatives. Prog Retin Eye Res. 2020;77:100827. doi: 10.1016/j.preteyeres.2019.100827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiCarlo JE, Mahajan VB, Tsang SH. Gene therapy and genome surgery in the retina. J Clin Invest. 2018;128(6):2177-2188. doi: 10.1172/JCI120429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacLaren RE, Groppe M, Barnard AR, et al. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 2014;383(9923):1129-1137. doi: 10.1016/S0140-6736(13)62117-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards TL, Jolly JK, Groppe M, et al. Visual acuity after retinal gene therapy for choroideremia. N Engl J Med. 2016;374(20):1996-1998. doi: 10.1056/NEJMc1509501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimopoulos IS, Hoang SC, Radziwon A, et al. Two-year results after AAV2-mediated gene therapy for choroideremia: the Alberta experience. Am J Ophthalmol. 2018;193:130-142. doi: 10.1016/j.ajo.2018.06.011 [DOI] [PubMed] [Google Scholar]
- 10.Xue K, Jolly JK, Barnard AR, et al. Beneficial effects on vision in patients undergoing retinal gene therapy for choroideremia. Nat Med. 2018;24(10):1507-1512. doi: 10.1038/s41591-018-0185-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lam BL, Davis JL, Gregori NZ, et al. Choroideremia gene therapy phase 2 clinical trial: 24-month results. Am J Ophthalmol. 2019;197:65-73. doi: 10.1016/j.ajo.2018.09.012 [DOI] [PubMed] [Google Scholar]
- 12.Fischer MD, Ochakovski GA, Beier B, et al. Changes in retinal sensitivity after gene therapy in choroideremia. Retina. 2020;40(1):160-168. doi: 10.1097/IAE.0000000000002360 [DOI] [PubMed] [Google Scholar]
- 13.Cehajic Kapetanovic J, Barnard AR, MacLaren RE. Molecular therapies for choroideremia. Genes (Basel). 2019;10(10):E738. doi: 10.3390/genes10100738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cehajic Kapetanovic J, Patrício MI, MacLaren RE. Progress in the development of novel therapies for choroideremia. Expert Rev Ophthalmol. 2019;14(6):277-285. doi: 10.1080/17469899.2019.1699406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer MD, Ochakovski GA, Beier B, et al. Efficacy and safety of retinal gene therapy using adeno-associated virus vector for patients with choroideremia: a randomized clinical trial. JAMA Ophthalmol. 2019;137(11):1247-1254. doi: 10.1001/jamaophthalmol.2019.3278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aleman TS, Huckfeldt RM, Serrano L, et al. AAV2-hCHM subretinal delivery to the macula in choroideremia: 2 year results of an ongoing phase I/II gene therapy trial. Invest Ophthalmol Vis Sci. 2019;60:5173. [DOI] [PubMed] [Google Scholar]
- 17.Duncan JL. Gene therapy for choroideremia-progress and remaining questions. JAMA Ophthalmol. 2019;137(11):1254-1255. doi: 10.1001/jamaophthalmol.2019.3295 [DOI] [PubMed] [Google Scholar]
- 18.MacDonald IM, Moen C, Duncan JL, Tsang SH, Cehajic-Kapetanovic J, Aleman TS. Perspectives on gene therapy: choroideremia represents a challenging model for the treatment of other inherited retinal degenerations. Transl Vis Sci Technol. 2020;9(3):17. doi: 10.1167/tvst.9.3.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Preising M, Ayuso C. Rab escort protein 1 (REP1) in intracellular traffic: a functional and pathophysiological overview. Ophthalmic Genet. 2004;25(2):101-110. doi: 10.1080/13816810490514333 [DOI] [PubMed] [Google Scholar]
- 20.Fry LE, Patrício MI, Williams J, et al. Association of messenger RNA level with phenotype in patients with choroideremia: potential implications for gene therapy dose. JAMA Ophthalmol. 2020;138(2):128-135. doi: 10.1001/jamaophthalmol.2019.5071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aleman TS, Han G, Serrano LW, et al. Natural history of the central structural abnormalities in choroideremia: a prospective cross-sectional study. Ophthalmology. 2017;124(3):359-373. doi: 10.1016/j.ophtha.2016.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coussa RG, Traboulsi EI. Choroideremia: a review of general findings and pathogenesis. Ophthalmic Genet. 2012;33(2):57-65. doi: 10.3109/13816810.2011.620056 [DOI] [PubMed] [Google Scholar]
- 23.MacDonald IM, Russell L, Chan CC. Choroideremia: new findings from ocular pathology and review of recent literature. Surv Ophthalmol. 2009;54(3):401-407. doi: 10.1016/j.survophthal.2009.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Syed N, Smith JE, John SK, Seabra MC, Aguirre GD, Milam AH. Evaluation of retinal photoreceptors and pigment epithelium in a female carrier of choroideremia. Ophthalmology. 2001;108(4):711-720. doi: 10.1016/S0161-6420(00)00643-6 [DOI] [PubMed] [Google Scholar]
- 25.Duncan JL, Aleman TS, Gardner LM, et al. Macular pigment and lutein supplementation in choroideremia. Exp Eye Res. 2002;74(3):371-381. doi: 10.1006/exer.2001.1126 [DOI] [PubMed] [Google Scholar]
- 26.Heon E, Alabduljalil T, McGuigan DB III, et al. Visual function and central retinal structure in choroideremia. Invest Ophthalmol Vis Sci. 2016;57(9):OCT377-OCT387. doi: 10.1167/iovs.15-18421 [DOI] [PubMed] [Google Scholar]
- 27.Jacobson SG, Cideciyan AV, Sumaroka A, et al. Remodeling of the human retina in choroideremia: rab escort protein 1 (REP-1) mutations. Invest Ophthalmol Vis Sci. 2006;47(9):4113-4120. doi: 10.1167/iovs.06-0424 [DOI] [PubMed] [Google Scholar]
- 28.Coussa RG, Kim J, Traboulsi EI. Choroideremia: effect of age on visual acuity in patients and female carriers. Ophthalmic Genet. 2012;33(2):66-73. doi: 10.3109/13816810.2011.623261 [DOI] [PubMed] [Google Scholar]
- 29.Morgan JI, Han G, Klinman E, et al. High-resolution adaptive optics retinal imaging of cellular structure in choroideremia. Invest Ophthalmol Vis Sci. 2014;55(10):6381-6397. doi: 10.1167/iovs.13-13454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tuten WS, Vergilio GK, Young GJ, et al. Visual function at the atrophic border in choroideremia assessed with adaptive optics microperimetry. Ophthalmol Retina. 2019;3(10):888-899. doi: 10.1016/j.oret.2019.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meschede IP, Burgoyne T, Tolmachova T, Seabra MC, Futter CE. Chronically shortened rod outer segments accompany photoreceptor cell death in choroideremia. PLoS One. 2020;15(11):e0242284. doi: 10.1371/journal.pone.0242284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morgan JI. The fundus photo has met its match: optical coherence tomography and adaptive optics ophthalmoscopy are here to stay. Ophthalmic Physiol Opt. 2016;36(3):218-239. doi: 10.1111/opo.12289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang J, Williams DR, Miller DT. Supernormal vision and high-resolution retinal imaging through adaptive optics. J Opt Soc Am A Opt Image Sci Vis. 1997;14(11):2884-2892. doi: 10.1364/JOSAA.14.002884 [DOI] [PubMed] [Google Scholar]
- 34.Syed R, Sundquist SM, Ratnam K, et al. High-resolution images of retinal structure in patients with choroideremia. Invest Ophthalmol Vis Sci. 2013;54(2):950-961. doi: 10.1167/iovs.12-10707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun LW, Johnson RD, Williams V, et al. Multimodal imaging of photoreceptor structure in choroideremia. PLoS One. 2016;11(12):e0167526. doi: 10.1371/journal.pone.0167526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tolmachova T, Wavre-Shapton ST, Barnard AR, MacLaren RE, Futter CE, Seabra MC. Retinal pigment epithelium defects accelerate photoreceptor degeneration in cell type-specific knockout mouse models of choroideremia. Invest Ophthalmol Vis Sci. 2010;51(10):4913-4920. doi: 10.1167/iovs.09-4892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wavre-Shapton ST, Tolmachova T, Lopes da Silva M, Futter CE, Seabra MC. Conditional ablation of the choroideremia gene causes age-related changes in mouse retinal pigment epithelium. PLoS One. 2013;8(2):e57769. doi: 10.1371/journal.pone.0057769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foote KG, Roorda A, Duncan JL. Multimodal imaging in choroideremia. Adv Exp Med Biol. 2019;1185:139-143. doi: 10.1007/978-3-030-27378-1_23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Esteve-Rudd J, Hazim RA, Diemer T, et al. Defective phagosome motility and degradation in cell nonautonomous RPE pathogenesis of a dominant macular degeneration. Proc Natl Acad Sci U S A. 2018;115(21):5468-5473. doi: 10.1073/pnas.1709211115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 41.Nti AA, Serrano LW, Sandhu HS, et al. Frequent subclinical macular changes in combined BRAF/MEK inhibition with high-dose hydroxychloroquine as treatment for advanced metastatic BRAF mutant melanoma: preliminary results from a phase I/II clinical treatment trial. Retina. 2019;39(3):502-513. doi: 10.1097/IAE.0000000000002027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dubra A, Sulai Y. Reflective afocal broadband adaptive optics scanning ophthalmoscope. Biomed Opt Express. 2011;2(6):1757-1768. doi: 10.1364/BOE.2.001757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scoles D, Sulai YN, Langlo CS, et al. In vivo imaging of human cone photoreceptor inner segments. Invest Ophthalmol Vis Sci. 2014;55(7):4244-4251. doi: 10.1167/iovs.14-14542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen M, Cooper RF, Gee JC, Brainard DH, Morgan JIW. Automatic longitudinal montaging of adaptive optics retinal images using constellation matching. Biomed Opt Express. 2019;10(12):6476-6496. doi: 10.1364/BOE.10.006476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dubra A, Harvey Z. Registration of 2D images from fast scanning ophthalmic instruments. In: Lecture Notes in Computer Science. 2010;6204:60-71. [Google Scholar]
- 46.Chen M, Cooper RF, Han GK, Gee J, Brainard DH, Morgan JI. Multi-modal automatic montaging of adaptive optics retinal images. Biomed Opt Express. 2016;7(12):4899-4918. doi: 10.1364/BOE.7.004899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morgan JIW, Chen M, Huang AM, Jiang YY, Cooper RF. Cone identification in choroideremia: repeatability, reliability, and automation through use of a convolutional neural network. Transl Vis Sci Technol. 2020;9(2):40. doi: 10.1167/tvst.9.2.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cooper RF, Wilk MA, Tarima S, Carroll J. Evaluating descriptive metrics of the human cone mosaic. Invest Ophthalmol Vis Sci. 2016;57(7):2992-3001. doi: 10.1167/iovs.16-19072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang KY, Zeger, S.L.. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13-22. doi: 10.1093/biomet/73.1.13 [DOI] [Google Scholar]
- 50.Ochakovski GA, Bartz-Schmidt KU, Fischer MD. Retinal gene therapy: surgical vector delivery in the translation to clinical trials. Front Neurosci. 2017;11:174. doi: 10.3389/fnins.2017.00174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ochakovski GA, Peters T, Michalakis S, et al. ; RD-CURE Consortium . Subretinal injection for gene therapy does not cause clinically significant outer nuclear layer thinning in normal primate foveae. Invest Ophthalmol Vis Sci. 2017;58(10):4155-4160. doi: 10.1167/iovs.17-22402 [DOI] [PubMed] [Google Scholar]
- 52.Reichel FF, Dauletbekov DL, Klein R, et al. ; RD-CURE Consortium . AAV8 can induce innate and adaptive immune response in the primate eye. Mol Ther. 2017;25(12):2648-2660. Medline:28970046 doi: 10.1016/j.ymthe.2017.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weed L, Ammar MJ, Zhou S, et al. Safety of same-eye subretinal sequential readministration of AAV2-hRPE65v2 in non-human primates. Mol Ther Methods Clin Dev. 2019;15:133-148. doi: 10.1016/j.omtm.2019.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tobias P, Philipp SI, Stylianos M, et al. ; RD-Cure Consortium . Safety and toxicology of ocular gene therapy with recombinant AAV vector rAAV.hCNGA3 in nonhuman primates. Hum Gene Ther Clin Dev. 2019;30(2):50-56. doi: 10.1089/humc.2018.188 [DOI] [PubMed] [Google Scholar]
- 55.Gill JS, Georgiou M, Kalitzeos A, Moore AT, Michaelides M. Progressive cone and cone-rod dystrophies: clinical features, molecular genetics and prospects for therapy. Br J Ophthalmol. 2019;bjophthalmol-2018-313278. doi: 10.1136/bjophthalmol-2018-313278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song H, Rossi EA, Stone E, et al. Phenotypic diversity in autosomal-dominant cone-rod dystrophy elucidated by adaptive optics retinal imaging. Br J Ophthalmol. 2018;102(1):136-141. doi: 10.1136/bjophthalmol-2017-310498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xue K, Groppe M, Salvetti AP, MacLaren RE. Technique of retinal gene therapy: delivery of viral vector into the subretinal space. Eye (Lond). 2017;31(9):1308-1316. doi: 10.1038/eye.2017.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fischer MD, Hickey DG, Singh MS, MacLaren RE. Evaluation of an optimized injection system for retinal gene therapy in human patients. Hum Gene Ther Methods. 2016;27(4):150-158. doi: 10.1089/hgtb.2016.086 [DOI] [PubMed] [Google Scholar]
- 59.Smith AJ, Telander DG, Zawadzki RJ, et al. High-resolution Fourier-domain optical coherence tomography and microperimetric findings after macula-off retinal detachment repair. Ophthalmology. 2008;115(11):1923-1929. doi: 10.1016/j.ophtha.2008.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rashid S, Pilli S, Chin EK, Zawadzki RJ, Werner JS, Park SS. Five-year follow-up of macular morphologic changes after rhegmatogenous retinal detachment repair: fourier domain OCT findings. Retina. 2013;33(10):2049-2058. doi: 10.1097/IAE.0b013e3182891e81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matlach J, Pflüger B, Hain J, Göbel W. Inner and outer central retinal findings after surgery for rhegmatogenous retinal detachment using different spectral-domain optical coherence tomography devices. Graefes Arch Clin Exp Ophthalmol. 2015;253(3):369-380. doi: 10.1007/s00417-014-2713-4 [DOI] [PubMed] [Google Scholar]
- 62.dell’Omo R, Viggiano D, Giorgio D, et al. Restoration of foveal thickness and architecture after macula-off retinal detachment repair. Invest Ophthalmol Vis Sci. 2015;56(2):1040-1050. doi: 10.1167/iovs.14-15633 [DOI] [PubMed] [Google Scholar]
- 63.Chang CJ, Lai WW, Edward DP, Tso MO. Apoptotic photoreceptor cell death after traumatic retinal detachment in humans. Arch Ophthalmol. 1995;113(7):880-886. doi: 10.1001/archopht.1995.01100070054025 [DOI] [PubMed] [Google Scholar]
- 64.Lewis GP, Charteris DG, Sethi CS, Leitner WP, Linberg KA, Fisher SK. The ability of rapid retinal reattachment to stop or reverse the cellular and molecular events initiated by detachment. Invest Ophthalmol Vis Sci. 2002;43(7):2412-2420. [PubMed] [Google Scholar]
- 65.Luna G, Keeley PW, Reese BE, Linberg KA, Lewis GP, Fisher SK. Astrocyte structural reactivity and plasticity in models of retinal detachment. Exp Eye Res. 2016;150:4-21. doi: 10.1016/j.exer.2016.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cook B, Lewis GP, Fisher SK, Adler R. Apoptotic photoreceptor degeneration in experimental retinal detachment. Invest Ophthalmol Vis Sci. 1995;36(6):990-996. [PubMed] [Google Scholar]
- 67.Cooper RF, Tuten WS, Dubra A, Brainard DH, Morgan JIW. Non-invasive assessment of human cone photoreceptor function. Biomed Opt Express. 2017;8(11):5098-5112. doi: 10.1364/BOE.8.005098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cooper RF, Brainard DH, Morgan JIW. Optoretinography of individual human cone photoreceptors. Opt Express. 2020;28(26):39326-39339. doi: 10.1364/OE.409193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Azimipour M, Migacz JV, Zawadzki RJ, Werner JS, Jonnal RS. Functional retinal imaging using adaptive optics swept-source OCT at 1.6 MHz. Optica. 2019;6(3):300-303. doi: 10.1364/OPTICA.6.000300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Azimipour M, Valente D, Vienola KV, Werner JS, Zawadzki RJ, Jonnal RS. Optoretinogram: optical measurement of human cone and rod photoreceptor responses to light. Opt Lett. 2020;45(17):4658-4661. doi: 10.1364/OL.398868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pandiyan VP, Jiang X, Maloney-Bertelli A, Kuchenbecker JA, Sharma U, Sabesan R. High-speed adaptive optics line-scan OCT for cellular-resolution optoretinography. Biomed Opt Express. 2020;11(9):5274-5296. doi: 10.1364/BOE.399034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tuten WS, Tiruveedhula P, Roorda A. Adaptive optics scanning laser ophthalmoscope-based microperimetry. Optom Vis Sci. 2012;89(5):563-574. doi: 10.1097/OPX.0b013e3182512b98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harmening WM, Tuten WS, Roorda A, Sincich LC. Mapping the perceptual grain of the human retina. J Neurosci. 2014;34(16):5667-5677. doi: 10.1523/JNEUROSCI.5191-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Q, Tuten WS, Lujan BJ, et al. Adaptive optics microperimetry and OCT images show preserved function and recovery of cone visibility in macular telangiectasia type 2 retinal lesions. Invest Ophthalmol Vis Sci. 2015;56(2):778-786. doi: 10.1167/iovs.14-15576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cooper RF, Aguirre GK, Morgan JIW. Fully automated estimation of spacing and density for retinal mosaics. Transl Vis Sci Technol. 2019;8(5):26. doi: 10.1167/tvst.8.5.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Study Participant Characteristics
eFigure 1. Cone outer segment shortening after subretinal gene therapy
eFigure 2. AOSLO montages, PN-01 Injected Eye
eFigure 3. AOSLO montages, PN-01 Uninjected Eye
eFigure 4. AOSLO montages, PN-03 Injected Eye
eFigure 5. AOSLO montages, PN-03 Uninjected Eye
eFigure 6. AOSLO montages, PN-04 Injected Eye
eFigure 7. AOSLO montages, PN-04 Uninjected Eye
eFigure 8. AOSLO montages, PN-05 Injected Eye
eFigure 9. AOSLO montages, PN-05 Uninjected Eye
eFigure 10. AOSLO montages, PN-06 Uninjected Eye
eFigure 11. AOSLO montages, PN-07 Injected Eye
eFigure 12. AOSLO montages, PN-07 Uninjected Eye
eFigure 13. AOSLO montages, PN-08 Injected Eye
eFigure 14. AOSLO montages, PN-08 Uninjected Eye
eFigure 15. AOSLO montages, PN-09 Injected Eye
eFigure 16. AOSLO montages, PN-09 Uninjected Eye
eFigure 17. AOSLO montages, PN-11 Injected Eye
eFigure 18. AOSLO montages, PN-11 Uninjected Eye
eFigure 19. Cone mosaic ROIs, PN-01
eFigure 20. Cone mosaic ROIs, PN-03
eFigure 21. Cone mosaic ROIs, PN-04
eFigure 22. Cone mosaic ROIs, PN-05
eFigure 23. Cone mosaic ROIs, PN-06
eFigure 24. Cone mosaic ROIs, PN-07
eFigure 25. Cone mosaic ROIs, PN-08
eFigure 26. Cone mosaic ROIs, PN-09
eFigure 27. Cone mosaic ROIs, PN-11
eFigure 28. Cone density at one-month post-injection versus baseline
eReferences