Abstract
Peptic ulcer syndrome (PUD) has been acknowledged as one of the most frequent causes of morbidity and mortality worldwide throughout the 20th and 21st centuries. Several reports indicated the ability of plant derived dosages as antiulcer agents. Many prior investigations have implied some biological activities of Lawsonia inermis L. The aim of this current investigation was to estimate the antiulcer capability of Lawsonia inermis L. leaves and its nano formulation against hazardous biochemical and histological changes in aspirin-induced ulcer rats. Methods divided into 6 groups (6 rats/group), Normal control (negative), Group (1) receiving dose of (200 g/kg) Lawsonia inermis L. for 8 weeks, Group (2) receiving (200 g/kg) nano Lawsonia inermis L. leaves for 8 weeks, Group (3) ulcer control group receiving a single dose (500 mg aspirin/kg rat body weight),groups 4& 5 receiving aspirin and either Lawsonia inermis L. leaves or nano Lawsonia inermis L. leaves for 8 weeks. Results: improvements in all the tested parameters as well as hepatic enzymes activities and some blood biochemical parameters. Conclusion Lawsonia inermis L.at the tested dose could prevent ulcer formation in the tested animals that may offer safe and low cost effective treatment for gastric ulcer.
Keywords: Aspirin, Gastric ulcers, Herbal Medicines, Lawsonia inermis L., Pharmacological activities, Rat
Graphical Abstract
1. Introduction
Peptic ulcer syndrome (PUD) has been acknowledged as one of the leading reasons for morbidity and mortality throughout the 20th and 21st centuries and still represented a serious medical concern as a result of the increasingly extensive use of non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin [1].
Although the development of new drugs for the treatment of ulcer led to the decrease of the peptic ulcers deterioration worldwide, the excessive use of non-steroidal anti-inflammatory is still one of the leading causes for these pathologies development [2].
The public uses of non-steroidal anti-inflammatory (especially aspirin are still increasing even without medical advises (prescriptions), which in return is manifested as a factor associated with the high incidence of gastric ulcer worldwide.
A lot of research has been conducted to find natural means in the attempt of preventing ulcer formation /or deterioration. Several reports have indicated the possibility of using plant-derived formulations as antiulcer agents (Euphorbia umbellate, Allium sativum, Hibiscus cannabinus, Emblica officinalis, Curcuma longa and Lawsonia inermis [3].
Lawsonia inermis L. is generally recognized as ‘henna’. It is often planted in India, Middle East and on coastlines of the Mediterranean Sea. It is mostly used in cosmetics and in traditional weddings for staining hands and also for hair dyeing. Yet it possesses a marked place in traditional medicine as the leaves are usually applied as a prophylactic therapy for several dermatologic diseases [4]. Phytochemical studies of Henna had revealed the existence of phenolic substances that could be glycosylated, such as coumarins, flavonoids, naphthalene, and gallic acid derivatives [5]. Additional chemical components of this plant have been discovered, including triterpenoids, steroids, and aliphatic hydrocarbons [5], [6].
Nanotechnology has the capacity to enhance the herbal medicine science and that through helping in the detection, development, and delivery of many intervention strategies to enhance health insurance and limit the chance of risk and difficulties of many diseases. It is trusted that by highlighting these advances the potential advantage of nanomaterials is known. There is certainly little information about the potential health threats of nanoparticles.
Several previous studies have implied the antibacterial, antifungal and antitumor activities of Lawsonia inermis L. [7]. The gastro-protective potential of L. inermis was attributed to the bioactive ingredient existing in plant extract such as phenolic compounds, glycosides, tannins, gums, etc. [7]. Although the mechanism underlying the protective action of the extract against ethanol induced gastric lesions are unclear, further studies yet [8] claimed that flavonoids exhibit several biological effects such as anti-inflammatory, anti-hepatotoxic and antiulcer actions.
The aim of this study was to evaluate the ability of Lawsonia inermis L. leaves and its nano-formulation against aspirin-induced ulcer in rats.
2. Materials and methods
2.1. Plant collection
Lawsonia inermis L. leaves were obtained from trees growing at the Experimental Farm of The Medicinal and Aromatic Plants Research Department, El-Kanater El-Khaireya, Horticulture Research Institute, Agriculture Research Center.
2.2. Nano-Lawsonia inermis L. leaves preparation
The Nano-Lawsonia inermis L. leaves used in the experiment was prepared according to Solvent emulsification-diffusion method with novel modifications at the Nutrition and Food Science Department at National Research Center, according to [9], [10], [11], [12]. Nano-Lawsonia inermis L. leaves were assessed by Transmission Electron Microscopy and Zeta Sizer Nano-ZS. Where the nano size ranged from 1 There's patent for this work concluded the nano preparation method and all different measurements data specific for this work which registered by Egyptian Patent Office, Academy of Scientific Research & Technology with no. 1956/2020. to 100 nm. There's patent for this work concluded the nano preparation method and all different measurements data specific for this work which registered by Egyptian Patent Office, Academy of Scientific Research & Technology with no. 1956/2020.
2.3. Nanoparticles measurement techniques
2.3.1. Transmission electron microscopy
The samples were examined using a transmission electron microscope (TEM) (JEM - 1234), which has a 120 KV operating voltage, a magnification power of 600,000×, a resolving power of 0.3 nm, a CCD camera, and a programmed heating/cooling facility ranging from − 1900 to 10,000 °C. Samples were kept on a carbon-coated copper grid in the National Research Center's Central Lab.
2.3.2. Zeta sizer nano ZS
Photon correlation spectroscopy was used in National Research Centre Central Lab to estimate the Z-average hydrodynamic diameter of the analysed samples at 25 0.1 °C using a Zeta Sizer Nano ZS (MalvernInstruments Inc., Southborough, MA).
2.4. Antioxidant assays
The antioxidant activity of Lawsonia inermis L. Leaves were assessed using two methods; DPPH radical scavenging assay and ABTS radical scavenging assay as follows:
2.4.1. DPPH radical scavenging assay
1.5 mL of various dilutions of Lawsonia inermis L. were combined with 1.5 mL of a 0.2 mM methanolic DPPH solution to investigate antioxidant scavenging activity [13]. After a 30-minute incubation time at 25 °C, the absorbance at 520 nm, (the maximum absorption wavelength of DPPH), was measured using a spectrophotometer and recorded as A. (sample). The same approach was used to do a blank experiment in the absence of Lawsonia inermis L., and A. (blank) was assigned to the blank absorbance.
Lawsonia inermis L. Antioxidant activity was expressed as IC50, defined as the Lawsonia inermis L. concentration required to cause a 50% diminution in initial DPPH concentration. Vitamin C was employed as a standard. All of the measurements were repeated in triplicate.
2.4.2. ABTS radical scavenging assay
The radical scavenging capacity of ABTS samples (2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonate) radical cation was utilized by combining 7 mM ABTS at pH 7.4 (5 mM NaH2PO4, 5 mM Na2HPO4, and 154 mMNaCl) with 2.5 mM potassium persulfate (final concentration) [14]. The mixture was diluted with ethanol, and the absorbance at 734 nm was measured with a spectrophotometer. For each sample, a diluted Lawsonia inermis L. methanol solution (100 μL) was mixed with freshly ABTS solution (900 μL), and the absorbance was recorded 6 min after the initial mixing. The capacity of free radical scavenging was expressed as values determined using vitamin C as a reference, with IC50 (mg/L) denoting the concentration required to scavenge 50% of ABTS radicals. The following equation was used to calculate the IC50 of free radical scavenging capacity:
All measurements were performed in triplicate.
2.5. Experimental animals
Thirty-six male Sprague-Dawley rats between 1 and 2 months of age and weighing 150–200 g were obtained from the National Research Centre's Animal House Colony in Cairo, Egypt. For acclimatization and to guarantee normal growth and behavior, the animals were kept on a standard laboratory feed and water for one week prior to the experiment. The rats were acclimatized in individual solid bottom cages in a temperature regulated (23 °C), 40–60 g/100 g absolute moisture, and artificially illuminated (12 h dark/light cycle) room devoid of chemical contamination. All of rats were treated humanely and handled in accordance with the Animal Experiments Guidelines, which were approved by Egypt's National Research Centre's Ethical Committee of Medical Research. The national law on laboratory animal care and usage was observed.
2.6. Diet composition
Casein (150 g/1 kg diet), unsaturated fat (100 g/1 kg diet), sucrose (220 g/1 kg diet), maize starch (440 g/1 kg diet), cellulose (40 g/1 kg diet), salt mixture (40 g/1 kg diet), and vitamin mixture (10 g/1 kg diet) made up the basal synthetic diet [8], [9], [10], [11], [12], [15]. The salt and vitamin mixture were formed according to AIN-93 M diet [16].
2.7. Experimental design
It includes (36 rats) divided into 6 groups (6 for each) as follows:
-
•
Normal Control (Negative): Normal rats fed on the basal synthetic diet.
-
•
Group (1): Rats fed on the basal synthetic diet supplemented with Lawsonia inermis L. leaves with a dose (200 g/kg) for 8 weeks [8].
-
•
Group (2): Rats fed on the basal synthetic diet supplemented with nano Lawsonia inermis L. leaves with a dose (200 g/kg) for 8 weeks [8].
-
•
Group (3) (positive control): Peptic ulcer induced rats fed on the basal synthetic diet. Each rat was given a single dosage (500 mg aspirin/kg body weight) of dissolved aspirin in water via an oral gavage [17], [18].
-
•
Group (4): Peptic ulcer induced rats, through given a single dosage (500 mg aspirin/kg body weight) of dissolved aspirin in water via an oral gavage, fed on the basal synthetic diet supplemented with Lawsonia inermis L. leaves with a dose (200 g/kg).
-
•
Group (5): Peptic ulcer induced rats, through given a single dosage (500 mg aspirin/kg body weight) of dissolved aspirin in water via an oral gavage, fed on the basal synthetic diet supplemented with nano Lawsonia inermis L. leaves with a dose (200 g/kg).
2.8. Measurement of body weight change and food consumption
Weekly body weight of the rats for all groups were measured with the aid of a digital weighing balance to assess weekly weight gain or weight loss while the food intake was measured and calculated with the aid of the metabolic cages and digital weighing balance.
2.9. Blood samples collection
The animals were fasted for 12 h then anaesthetized with an intramuscular injection of ketamine hydrochloride (35 mg/kg, i.m.) before being euthanized by cervical dislocation at the end of the study period, which lasted two months for all groups. To assess the biochemical parameters, a blood sample per each animal had been collected from tail and the concentration of the blood sample taken was about 5 mL. Serum and plasma were separated by centrifuged (Sigma labor zentrifuge GMBH, West Germany, model 2–153360 osterode /Hertz) at 4000 rpm for 15 min and preserved at − 20 °C.
2.10. Gastric juice and pH measurement
The gastric juice was had been gathered and centrifuged at 3000 rpm for 10 min, after which it was measured in mL/100 g body weight. A digital pH meter was used to determine the pH of the supernatant [19].
2.11. Measurement of free and total acidity
Titrating with 0.01 N NaOH using Topfer's reagent and phenolphthalein as indicators, free and total acidity were calculated and represented as meq/L/100 g [20].
2.12. Determination of free acidity and total acidity
The gastric contents were centrifuged for 10 min at 1000 rpm 0.9 mL distilled water were used to dilute 1 mL of supernant. Titrated with 0.1 N sodium hydroxide from a microburette and 3–4 drops of Topfer's reagent as an indicator, a volume of 2 mL diluted gastric juice was titrated till canary yellow color was noticed. The amount of NaOH necessary was calculated based on the free acidity. A few more drops of phenolphthalein (3–4 drops) were added and titrated with NaOH until the pink color returned. This gives total acidity. Total acidity is the result of this. Total acidity and free acidity were measured in mL of 0.1 N HCl per 100 gm of gastric contents. This is equivalent to mEq/L. [21]. The formula for calculating acidity was used:
2.13. Biochemical parameters
Total cholesterol, HDL, LDL and triglycerides were evaluated as lipid profiles by enzymatic colorimetric method [22], [23], [24], [25], [26] Plasma alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP) activities were assessed as liver function indicators by colorimetric techniques [27], [28]. Plasma total protein and plasma albumin (A) were determined by colorimetric methods [29], [30] as extra indicators of liver function. Plasma globulin (G) and A/G ratio were calculated. Plasma malondialdehyde (MDA), as indicator of lipid peroxidation, plasma catalase activity, an antioxidant biomarker were estimated [31], [32]. Plasma total antioxidant was estimated by colorimetric method [33]. Creatinine, Urea and Uric acid were assessed as kidney function indicators by colorimetric methods [34], [35], [36].
2.14. Histopathological examination
All experimental rats' stomach and liver samples were gathered, preserved in 10% neutral buffered formalin, washed, dehydrated, clarified, and embedded in paraffin. For light microscopic analysis, the paraffin embedded blocks were sectioned at a thickness of 5 µm and stained with Haematoxylin & Eosin (Olympus BX50, Tokyo, Japan) [37]. An experienced pathologist blinded to treatments performed the histological analysis.
Histopathological alterations in the stomach and liver were graded from (0−3) through the determination of the percentage of the lesions in five randomly examined microscopic fields per animal as follows: (0) indicated no changes; [1], [2] and [3] indicated mild, moderate and severe changes, respectively. The grading was determined by percentage as follows: (<30%) showed mild changes, (<30–50%) indicated moderate changes and changes more than 50% (>50%) indicated severe changes [38].
2.15. Statistical analysis
Each value represents the mean ± standard error (n = 6); Statistical Means were compared by one-way analyses of variance (ANOVA) and post hoc multiple comparisons were performed using the Duncan test in the SPSS/PC software program (version 22.0; SPSS Inc., Chicago, IL, USA) to evaluate the differences in all biochemical parameters. Differences were considered significant if p < 0.05.
3. Results
3.1. Nanoparticles analysis techniques
3.1.1. Transmission electron microscopy (TEM) analysis
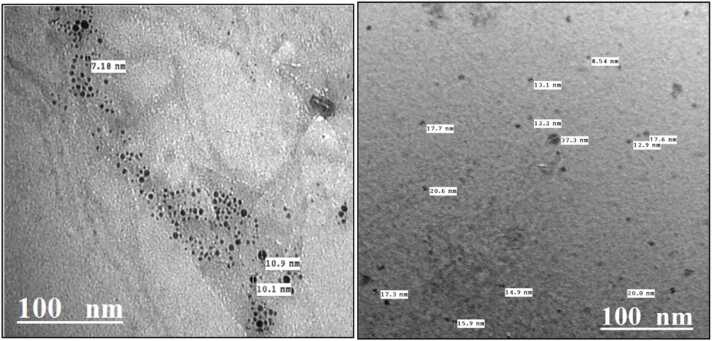
The shape and size of the resulting particles were demonstrated by the help of TEM, Fig. 1 shows the TEM micrographs revealing the presence of Lawsonia inermis L. with different sizes which are less than 100 nm, thus verifying that the tested particles are within the nano range. The TEM micrographs propose that the particles sizes were around 30 nm. The particles were of spherical shape. (Fig. 2).
Fig. (1).
- TEM micrographs of Lawsonia inermis L. nanoparticles.
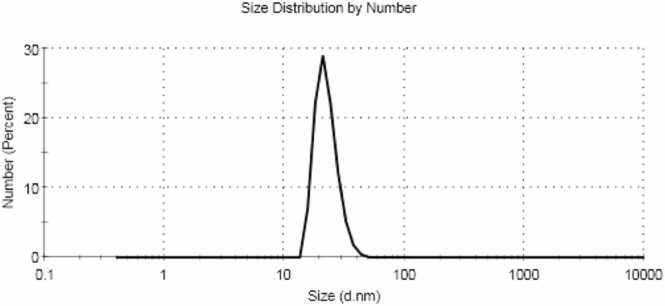
Fig. (2).
The Zeta Sizer ofLawsonia inermis L.
3.1.2. Refractive index zeta sizer
Where, Z-average (d.nm) = 45.43, Pdl= 0.227 and particle size (d.nm) = 22.67 with % Number= 99.8%.
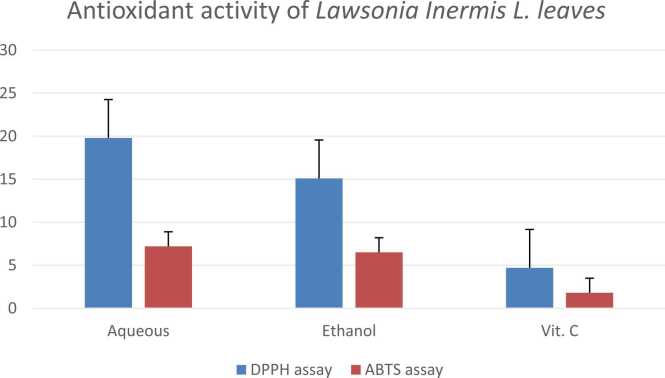
3.2. Antioxidant activity of Lawsonia inermis L. leaves
The antioxidant activity of Lawsonia inermis L. leaves in Fig. 3 was examined using two different methods which showed that the ethanolic extract had lower IC50 than the water extract in the tested methods.
Fig. (3).
Antioxidant activity of Lawsonia inermis L. leaves using DPPH and ABTS assays.
3.3. Anti-ulcer activity of Lawsonia inermis L. in normal and ulcer induced rats
Results in Table 1 revealed that there was an observed elevation in the aspirin treated group in all the tested parameters as the gastric juice volume was 3.5 mL in comparison to the normal untreated group that measures 2.25 while in the rats receiving Lawsonia inermis L. group and its nano formulation the gastric juice volume recorded 2.15 and 2.10 mL respectively. There was a significant reduction in the gastric juice volume toward the normal levels in both groups receiving Lawsonia inermis L.as an antiulcer agent with aspirin. The same pattern was observed for the free acidity marked elevation was observed in the group of rats receiving aspirin alone in comparison to the control group. This elevation was countered in the groups receiving Lawsonia inermis L. and higher activity was noticed for the nano formulation of Lawsonia inermis L., as the free acidity decreased towards normal recording 25.56 and 24.76 meq/L/100 g respectively the total acidity recorded noticeable increase in the aspirin treated group and this increase was reversed. The two groups receiving either Lawsonia inermis L. leaves or its nano formulation reported total acidity of 35.43 and 30.29 meq/L/100 g respectively. There was a noticeable decrease in the pH in the aspirin treated group that clearly indicate the increase in the gastric acidity while the Lawsonia inermis L. and its nano formulation treated groups showed records comparable to the control group the same observations was found in the aspirin and Lawsonia inermis L. treated groups.
Table (1).
Effect of Lawsonia inermis L. Leaves on Gastric juice, pH, free acidity and total acidity.
| Groups | Volume of gastric juice (mL) | pH | Free acidity meq/L/100 g | Total acidity meq/L/100 g |
|---|---|---|---|---|
| Normal Control (negative) | 2.25 ± 0.19 | 2.31 ± 0.18 | 25.42 ± 0.85 | 22.24 ± 3.31 |
| Group (1) | 2.15 ± 0.58a | 2.64 ± 0.05a | 23.78 ± 0.42a | 21.51 ± 6.27a |
| Group (2) | 2.10 ± 0.49a | 2.55 ± 0.03a | 22.23 ± 0.52a | 20.32 ± 4.33a |
| Group (3) (positive) | 3.5 ± 0.15b | 1.46 ± 0.37b | 58.9 ± 11.3b | 78.22 ± 1.29b |
| Group (4) | 1.93 ± 0.13c | 2.25 ± 0.12c | 25.56 ± 0.58c | 35.43 ± 1.15c |
| Group (5) | 1.53 ± 0.12d | 2.16 ± 0.15d | 24.76 ± 0.46d | 30.29 ± 1.19d |
** Values are expressed as Mean ± SE (n = 6) in which the same letters in each column imply a non-significant difference across varieties, whereas different letters imply a significant difference at P ≤ 0.05.
The pH levels were reduced significantly in the aspirin treated group yet the pH levels increased again in the groups treated with aspirin + Lawsonia inermis L. leaves and its nano formulation.
3.4. Nutritional parameters
The nutritional attributes were represented in Table 2 that indicates the absence of any significant differences in the initial body weights of all the tested groups and there have been no significant differences in the final body weights as well while we observed significant differences in the body gain between all the tested groups and also significant reduction in the total food intake in the aspirin treated group that was attenuated by adding the Lawsonia inermis L. leaves. (Table 3).
Table (2).
The effect of Lawsonia inermis L. Leaves in normal and nano scale on initial body weight, final body weight, body gain, total food intake and food efficiency.
| Group | Initial body weight (g) | Final body weight (g) | Body gain (g) | Total food intake (g) | Food efficiency |
|---|---|---|---|---|---|
| Normal Control (negative) | 187.8 ± 4.85 | 230.7 ± 5.48 | 42.8 ± 3.12 | 1190.5 ± 2.29 | 0.036 ± 0.003 |
| Group (1) | 185 ± 4.33a | 226.2 ± 7.87a | 41.2 ± 5.5a | 1191.2 ± 3.09a | 0.035 ± 0.005a |
| Group (2) | 183 ± 4.36a | 222.7 ± 4.77a | 39.7 ± 2.08b | 1194 ± 1.61a | 0.033 ± 0.002a |
| Group (3) (positive) | 182.8 ± 4.92a | 223.2 ± 5.35a | 40.3 ± 1.05c | 1142.2 ± 10.63b | 0.035 ± 0.001a |
| Group (4) | 182.2 ± 5.03a | 228.3 ± 3.67a | 46.2 ± 2.36d | 1183.8 ± 2.24c | 0.039 ± 0.002a |
| Group (5) | 182.2 ± 4.35a | 227.5 ± 4.5a | 45.3 ± 1.23e | 1183.5 ± 2.17d | 0.038 ± 0.001b |
** Values are expressed as Mean ± SE (n = 6) in which the same letters in each column imply a non-significant difference across varieties, whereas different letters imply a significant difference at P ≤ 0.05.
* * Food Efficiency= (Body Gain/ Total Food Intake).
Table (4).
The effect of Lawsonia inermis L. Leaves in normal and nano-scale onCatalase, lipid peroxide and total antioxidant capacity.
| Group | Catalase mg / dL | Lipid peroxide mg / dL | Total antioxidant mM/L |
|---|---|---|---|
| Normal Control (negative) | 52.2 ± 1.82 | 19 ± 1.78 | 1.13 ± 0.1 |
| Group (1) | 56.66 ± 1.93a | 15 ± 1.44a | 1.45 ± 0.11a |
| Group (2) | 56.80 ± 2.74a | 16 ± 1.2a | 1.71 ± 0.12b |
| Group (3)(positive) | 70.81 ± 1.01b | 26 ± 2.49b | 3.09 ± 0.09c |
| Group (4) | 36.14 ± 2.56c | 18 ± 0.43c | 1.85 ± 0.15d |
| Group (5) | 32.22 ± 2.58d | 17 ± 0.67d | 1.23 ± 0.11e |
** Values are expressed as Mean ± SE (n = 6) in which the same letters in each column imply a non-significant difference across varieties, whereas different letters imply a significant difference at P ≤ 0.05.
Table (3).
The effect of Lawsonia inermis L. Leaves in normal and nano-scale on Total cholesterol, HDL, LDL and Triglycerides.
| Group | Total cholesterol mg / dL | HDL mg / dL | LDL mg / dL | Triglycerides mg / dL |
|---|---|---|---|---|
| Normal Control (negative) | 34.5 ± 3 | 17.7 ± 1.56 | 10.8 ± 1.61 | 66 ± 6.30 |
| Group (1) | 36.1 ± 2.6a | 12.1 ± 1.7a | 12.7 ± 2.26a | 65.8 ± 3.31a |
| Group (2) | 29.2 ± 2.95a | 10.6 ± 1.35b | 10.3 ± 2.29a | 51.4 ± 5.64a |
| Group (3) (positive) | 78.2 ± 4.04b | 42.1 ± 3.22c | 25.3 ± 2.87b | 167.7 ± 6.42b |
| Group (4) | 29.2 ± 6.14c | 14.9 ± 0.97d | 13.2 ± 4.69c | 68.9 ± 7.05c |
| Group (5) | 23 ± 3.45d | 14.8 ± 0.75e | 8.2 ± 1.88d | 65.1 ± 4.31d |
* * Values are expressed as Mean ± SE (n = 6) in which the same letters in each column imply a non-significant difference across varieties, whereas different letters imply a significant difference at P ≤ 0.05.
3.5. Biochemical parameters
Assessment of the lipid profile of the tested groups revealed significant increment in all the lipid profile parameters in the aspirin treated group as the total cholesterol was 78.2 mg/dL accompanied by elevation of LDL to reach 25.3 mg/dL and triglyceride to reach 167.7 mg/dL. These increment as been improved in the groups receiving Lawsonia inermis L. Leaves. With the nano formulation the decrease was comparable to the normal or even better.
The assessment of catalase activity demonstrated a significant increase in catalase activity in blood in the aspirin treated group reaching 70 mg/dL. The catalase level decreased significantly in the Lawsonia inermis L. and its nano formulation treated groups to become 36 and 32 mg/dL respectively. The same pattern was found for lipid peroxidase and total antioxidant capacity in the aspirin group as they were 26 and 3.09 mM/L respectively while lipid peroxide recorded 18 and 17 mg/dL in the Lawsonia inermis L. and its nano formulation treated groups and the total antioxidant capacity decreased significantly to 1.85, 1.23 mM/L respectively. (Table 5).
Table (5).
The effect of Lawsonia inermis L. leaves in normal and nano-scale on creatinine, urea and uric acid.
| Group | Creatinine mg / dL | Urea mg / dL | Uric Acid mg / dL |
|---|---|---|---|
| Normal Control (negative) | 0.55 ± 0.07 | 38.4 ± 5.04 | 2.37 ± 0.14 |
| Group (1) | 0.54 ± 0.08a | 38.6 ± 4.33a | 2.52 ± 0.18a |
| Group (2) | 0.44 ± 0.02a | 35.7 ± 3.13a | 2.23 ± 0.28a |
| Group (3)(positive) | 0.94 ± 0.23a | 77.5 ± 3.12b | 3.62 ± 0.5b |
| Group (4) | 0.43 ± 0.02b | 37.5 ± 2.57c | 2.62 ± 0.19c |
| Group (5) | 0.4 ± 0.07c | 37.1 ± 3.37d | 2.20 ± 0.18d |
** Values are expressed as Mean ± SE (n = 6) in which the same letters in each column imply a non-significant difference across varieties, whereas different letters imply a significant difference at P ≤ 0.005.
The assessment of the kidney functions demonstrated severe alterations that was evident by the increase in the creatinine levels reaching 0.94 in the aspirin treated group and that was further supported by the results of urea and uric acid elevations reaching 77.5 mg/dL and 3.6 mg/dL respectively. That deterioration of kidney functions was attenuated by the use of Lawsonia inermis L. leaves and its nano formulation as all the parameters creatinine, urea and uric acid were significantly reduced to 2.6 mg/dL and 2.2 mg/dL respectively.
The results presented in Table 6 revealed significant elevation in the liver enzymes in the group of rats receiving aspirin alone. In fact, AST reached 57.7 mg/dL while the ALT and ALP were respectively of 36.3 µg / dL and 117.15 IU / L. In the groups receiving Lawsonia inermis L. leaves and its nano formulation there were significant improvement as all the liver enzymes significantly decreased towards normal levels.
Table (6).
The effect of Lawsonia inermis L. Leaves in normal and nano-scale on AST, ALT, and ALP.
| Group | AST mg / dL | ALT µg / dL | ALP IU / L |
|---|---|---|---|
| Normal Control (negative) | 32.8 ± 1.51 | 24.3 ± 3.07 | 65.07 ± 5.09 |
| Group (1) | 30.5 ± 1.96a | 21.3 ± 2.87a | 63.81 ± 4.78a |
| Group (2) | 27.5 ± 1.26b | 18.5 ± 2.92a | 62.41 ± 4.09a |
| Group (3)(positive) | 57.7 ± 1.05c | 36.3 ± 3.58b | 117.15 ± 9.78b |
| Group (4) | 37 ± 1.48d | 15.8 ± 1.83c | 53.15 ± 4.09c |
| Group (5) | 34.2 ± 2.17e | 18.7 ± 4.67d | 43.78 ± 5.48d |
* * Values are expressed as Mean ± SE (n = 6) in which the same letters in each column imply a non-significant difference across varieties, whereas different letters imply a significant difference at P ≤ 0.005.
Table 7 results revealed that group (3) revealed significant elevation in the blood levels of albumin, total proteins and globulins when compared with negative control animals. There’re a significant reduction in groups (4, 5) in albumin, total proteins and globulin levels when compared with positive control animals.
Table (7).
The effect of Lawsonia inermis L. leaves in normal and nano-scale on albumin, total protein and globulin.
| Group | Albumin g / dL | Total protein mmol/L | Globulin g / dL |
|---|---|---|---|
| Normal Control (negative) | 2.78 ± 0.02 | 5.17 ± 0.34 | 2.4 ± 0.33 |
| Group (1) | 2.77 ± 0.07a | 4.98 ± 0.23a | 2.21 ± 0.25a |
| Group (2) | 2.74 ± 0.09a | 4.62 ± 0.31a | 1.88 ± 0.31a |
| Group (3)(positive) | 3.29 ± 0.06b | 6.63 ± 0.19b | 3.33 ± 0.15b |
| Group (4) | 2.54 ± 0.03c | 3.57 ± 0.19c | 1.04 ± 0.21c |
| Group (5) | 2.39 ± 0.03d | 3.38 ± 0.18d | 0.99 ± 0.19d |
** Values are expressed as Mean ± SE (n = 6) in which the same letters in each column imply a non-significant difference across varieties, whereas different letters imply a significant difference at P ≤ 0.005.
3.6. Histopathological examination
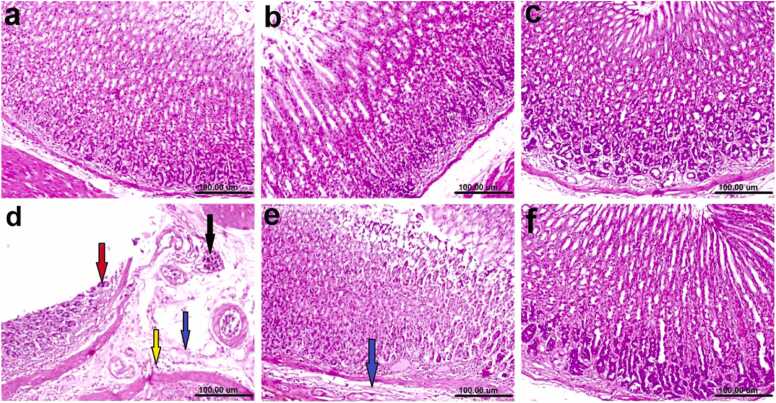
Histopathological alterations and lesion scores in the stomach and liver of different experimental groups were demonstrated in Figs. 4 & 5 as well as Table 8. Briefly light microscopic examination of stomach sections of negative control rats revealed the normal histological architecture of stomach layers (mucosa, submucosa, musculosa and serosa) (Fig. 4a). Moreover, rats' stomach treated with either Lawsonia inermis L. leaves or nano Lawsonia inermis L. leaves respectively exhibited no histopathological alterations (Fig. 4b & c). On contrary, sections of rats from ulcer group showed severe histopathologic damage which described by focal necrosis of gastric mucosa, sloughing and ulceration, submucosal edema, inflammatory cells infiltration and congested blood vessels (Fig. 4d). However, improved picture was noticed in stomach of ulcerated rats treated with Lawsonia inermis L. leaves, examined sections revealed submucosal edema (Fig. 4e). Furthermore, sections from ulcerated rats treated with nano Lawsonia inermis L. leaves showed marked regressed lesions and the stomach revealed normal gastric layers (Fig. 4f). Table 8 summarizes the histopathological lesion scores and ulcer severity in the gastric tissue.
Fig. (4).
Photomicrographs of H&E stained stomach sections of rats: (a) Normal control showing the normal histological architecture of gastric layers. (b& c) treated with Lawsonia inermis L. Leaves and nano Lawsonia inermis L. Leaves respectively showing no histopathological alterations. (d) Ulcer group (positive control), showing necrosis of gastric mucosa, ulceration (red arrow), submucosal edema (blue arrow), inflammatory cells infiltration (yellow arrow) and congested blood vessel (black arrow). (e) Ulcer treated with Lawsonia inermis L. Leaves, showing slight submucosal edema (blue arrow). (f) Ulcer + nano Lawsonia inermis L. Leaves showing normal gastric layers (scale bar, 100 µm).
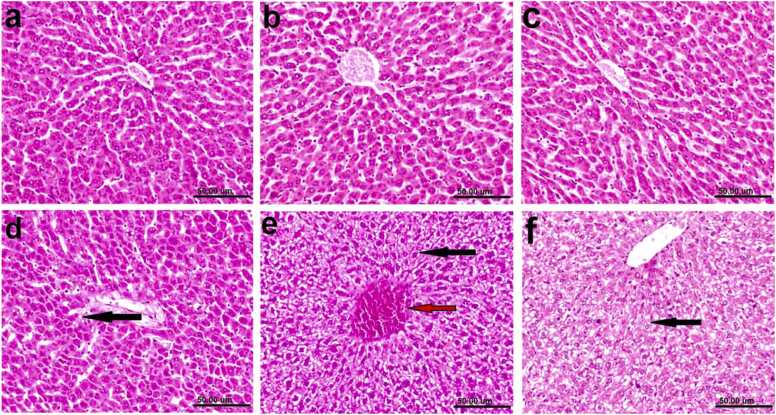
Fig. (5).
Photomicrographs of H&E stained liver sections of rats: (a) Normal control showing the normal histological architecture of hepatic parenchyma. (b& c) treated with Lawsonia inermis L. Leaves and nano Lawsonia inermis L. Leaves respectively showing no histopathological alterations. (d) Ulcer group (positive control), showing hepatocellular vacuolar degeneration (black arrow). (e) Ulcer treated with Lawsonia inermis L. Leaves, showing slight hydropic degeneration of some hepatocytes (black arrow) and congestion of central vein (red arrow). (f) Ulcer + nano Lawsonia inermis L. Leaves showing slight hydropic degeneration of hepatocytes (black arrow) (scale bar, 50 µm).
Microscopically, liver of normal control rats revealed the normal architecture of hepatic parenchyma which consists of central veins and hepatocytes arranged in hepatic cords (Fig. 5a). Moreover, liver of rats treated with either Lawsonia inermis L. leaves or nano Lawsonia inermis L. leaves respectively exhibited no histopathological alterations (Fig. 5b & c) meanwhile, liver of rats from ulcer group showed Kupffer cells activation and hepatocellular vacuolar degeneration (Fig. 5d). On the other hand, liver from ulcer treated with Lawsonia inermis L. leaves group revealed improved picture as examined sections showed slight hydropic degeneration of some hepatocytes and congestion of central vein (Fig. 5e). Likewise, slight hydropic degeneration of some hepatocytes was the only change observed in liver of rats from ulcer +nano Lawsonia inermis L. leaves group (Fig. 5f). Furthermore, the histopathological lesion scoring in the stomach and liver of rats in the different experimental groups was evaluated (Table 8), where the use of Lawsonia inermis L. leaves and its nano formulation markedly restored the induced lesion and ulcer scoring.
Table (8).
Histopathological lesions scores in different experimental animals.
| Organs | Lesions | Normal control (negative) | Group (1) | Group (2) | Group (3) (positive) | Group (4) | Group (5) |
|---|---|---|---|---|---|---|---|
| Stomach | -Focal necrosis of the mucosa - Congestion -Submucosal edema -Inflammatory cells Infiltration |
0 0 0 0 |
0 0 0 0 |
0 0 0 0 |
3 3 3 3 |
0 0 1 0 |
0 0 0 0 |
| Liver | - Kupffer cells activation - Congestion -Vacuolar degeneration ofhepatocytes -Hydropic degeneration of hepatocytes |
0 0 0 0 |
0 0 0 0 |
0 0 0 0 |
3 2 2 0 |
1 1 0 2 |
1 0 0 1 |
4. Discussion
Peptic ulcer syndrome (PUD) is a mucosal break of the upper gastro-intestinal tract related to acid peptic digestion causing in ulcer formation that spreads beyond then mucosae into the submucosa. Most commonly it happens in the stomach and first part of the duodenum yet it can occur also in the various other parts like the distal esophagus, distal duodenum, and/or jejunum and in the Meckel’s diverticulum with heterotrophic gastric mucosa [1].
Since earlier as, plants and plant derived-products has been the main source for medication in traditional healing all over the world as a cure for several disorders and diseases. Currently, herbal remedy regains its role and becomes an important unconventional medication over the present existing pharmaceutical applications for the control of PU. This is believed to have advantages on its lower cost, perceived effectiveness, and availability in addition to high safety profile, due to the limited or no side effects. A number of these herbal medications have proved gastro protective attributes [39], [40], [41].
Many reports [42], [43], [44], [45] mentioned the many different healing effects for Henna, also it was reported to possess antibacterial effects particularly for gram positive bacteria some studies indicated its antitumor activity in rat, antifungal activity against dermatophytes and wound healing [46], [47]. Incidence of Hyperacidity and ulcer nowadays are very frequent, resulting in sever human pain, Resulting from the imbalance between damaging factors within the lumen and the mechanisms that protect inside the mucosa of the alimentary canal. Despite the fact that prolonged anxiety, emotional stress, hemorrhagic surgical shock, burns and trauma are reported as a causative factor for severe gastric irritation (ulcer), yet the mechanism remains very poorly understood [48].
This study had addressed the anti-ulcerative ability of Henna leaves and the characters of its nano formulation as well as its bioactivity as anti-ulcer agent.
Here, when converted Lawsonia inermis L. leaves to nanoparticles, the resulted nanoparticles characterized with unique small size ranged from 10 to 100 nm with physicochemical and biological properties as compared to their larger counterparts that can greatly influence their interactions with biomolecules and cells and also with special property of high surface area to volume ratio and all of these make nanoparticles used as a promising tool to improve health and reduce the risk and complications of several diseases.
Estimation of the antioxidant capability of henna revealed that the bioactive compounds of henna possesses antioxidant activity comparable to vitamin C which may explain its medicinal activity in addition to its antibacterial ability against H. pylori which is a pronounced factor in peptic ulcer [49].
The obtained results demonstrated that there's a non-significant in groups (1, 2) in volume of gastric juice, pH, free acidity and total acidity when compared with negative normal control animals where group (3) result showed a significant elevation in volume of gastric juice, acidity (PH), free acidity and total acidity when compared with negative normal control animals. These results support previous report on aspirin to increase total acidity and gastric juice [50], [51], [52]. On the other hand groups (4, 5) showed a significant reduction in volume of gastric juice, pH, free acidity and total acidity when compared with positive ulcer control. This was in accordance with previous reports by [7] that Henna prohibited the mucosal lesions formation which led to the presumption that the bio-components present in henna had a suppressing activity on acid secretion leading to the retardation of gastric damage.
The groups of animals were divided randomly and there were no significant differences in the initial body weight and the same observation was found in the final body weight when compared with (negative & positive) animal controls these results were in harmony with the results of [53]. On the other hand a significant decrease in body weight gain in groups (3) were recorded in comparison with negative control animals while a significant elevation in body gain in groups (4, 5) when compared with positive control. In groups (1, 2), there was a significant increase in food intake where a significant reduction noticed in group (3) when compared with negative normal control animals and groups (4, 5) when compared with positive control. These results support previous reports by [52]. Moreover, there was a significant decrease in food efficiency in group (3) when compared with negative normal control animals where there’s a significant increase in groups (4, 5) when compared with positive control. These changes could be attributed to the deterioration in the stomach condition leading to refusal of food to decrease the stress on the alimentary canal and to decrease acid secretion.
The results showed a significant elevation in total cholesterol, HDL, LDL and triglyceride levels in group (3) when compared with negative normal control animals and a significant decrease occurred in groups (4, 5) when compared with positive control. These result although were surprising yet was in accordance with previous results obtained by [54], [55] who attributed this elevation to disruption of fatty acid metabolism, and indirectly to potential lipid peroxidation of the membrane and the release of free radicals affected by exposure to aspirin.
Our results in Table 4 showed a significant increase in catalase, total antioxidant and lipid peroxide levels in group (3) when compared with negative controls and that was a powerful marker indicating the excess oxidative stress in the tested animals as explained by [56] and a significant reduction noticed in groups (4, 5) when compared with positive controls. In total antioxidant results, which could be a result of the antioxidant properties of the henna leaves and nano-formulation provided to the tested animals, these results were further supported by the elevated levels of both Lipid Peroxide and Total Antioxidant In the aspirin treated group that was ameliorated in the henna treated groups.
The results point out a marked increment of Creatinine, urea and uric acid in group (3) receiving aspirin which concur with previous findings [54], [57] concluded positive control. A significant decrease observed in creatinine, urea and uric acid levels in groups (4, 5) when these groups compared with positive control that lead to the assumption that the antioxidant activity of the bioactive components of Lawsonia inermis L. leaves played an important role to decrease the oxidative stress leading to the improvement of kidney function. The kidney damage caused by aspirin could possibly be due to the prostaglandin synthesis reduction, where causes renal vascular constriction and impaired renal ischemia, resulting in acute renal abnormalities [58], [59], [60].
Treatment with aspirin revealed adverse effects on liver which was manifested by significant elevation of ALT and ALP activities. These results support those obtained by [57], who reported that ALT and AST serum activities elevation caused by aspirin administration for 8 weeks, pointing out the sub-chronic administration risks. There were negative impacts in normal rats were observed after aspirin induction [57].
The concerns of aspirin-induced liver toxicity are evident since the liver is indeed the main targeted organ for drug metabolism and hepatic biotransformation processes are thought to trigger hepatocyte death [61].While, there was a significant reduction in all liver enzymes activity in groups (4, 5) when compared with positive animal control which is reflecting the improvement of liver in the Lawsonia inermis L. leaves treated groups. These results are in harmony with previous reports [62].
Moreover, liver toxicity induced by aspirin administration might result from idiosyncratic metabolic-reaction caused by the drug metabolism that led to hazardous metabolites accumulation in the hepatocytes that directly links to cell proteins and lead to anomalies [60], [61]. This is supported by the total protein levels that have been measured. Protein synthesis in liver cells may be disrupted due to hepatic and renal dysfunction, which causes several range of enzymes and impairs protein synthesis [61]. The results suggested that Lawsonia inermis L. leaves or nano Lawsonia inermis L. are rich in phytochemicals and antioxidant bioactive components that have the ability to decrease the toxic oxidative stress on both liver and kidney and counteract the harmful effects of aspirin.
The use of nano formulation showed significant improvement in all the tested ulcer parameters represented in the significant decrease in the PH and total and free acidity the same improvement was observed in the biochemical parameters and the improvement surpassed even the normal Lawsonia inermis L. leaves as there was significant improvement over the normal Lawsonia inermis L. leaves treated group these results was supported by the histopathological findings and was also observed in the improvement in feed intake.
5. Conclusion
Results for the experiments conducted, suggested Lawsonia inermis L. leaves may possess some protective effect and healing benefits to peptic ulcer syndrome by counteracting the menaces caused in aspirin induced ulcer cases. This supports the use of Lawsonia inermis L. leaves in traditional medicine in the management of gastric ulcerative abnormalities. Using the nano technology provided additional improvement in all the tested groups which may provide additional benefits. However, more clinical trials will be needed to prove the Lawsonia inermis L. efficacy for patients suffering from stomach ulcers as this is considered a second phase trail. Third phase trail is encouraged to be conducted on humans with respect to ethical considerations and limitations.
Ethics approval and consent to participate
The animal experiment was performed in accordance with the United Kingdom's Animals (Scientific Procedures) Act, 1986 and associated guidelines, EU Directive 2010/63/EU for animal experiments (Publication No. 85–23, revised 1985), and The World Medical Association's Code of Ethics for animal experiments. The national law on the care and use of laboratory animals was followed.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
DM conceived of the research work, devised the work plan, performed supervision, prepared the samples, conducted the experiment in the open area, perform and prepared the nanoparticles and also perform the biochemical analysis, assisted with data analysis and visualization, wrote and reviewed the final manuscript; BS was involved in identifying the issue, and assisting with the writing of the manuscript and was present during the experiment in the open field; KA perform the histopathological examination, data analysis and reviewed the manuscript. MA prepared the samples, assisted with data analysis and visualization. The final manuscript was revised, read, and accepted by all authors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We'd really like to appreciate all of the authors of this article, as well as the National Research Centre.
Handling Editor: DR. Aristidis Tsatsakis
References
- 1.Malik T.F., Gnanapandithan K., Singh K. StatPearls Publishing; Treasure Island (FL): 2021. Peptic Ulcer Disease. [PubMed] [Google Scholar]
- 2.Falcão H.S., Mariath I.R., Diniz M.F., Batista L.M., Barbosa-Filho J.M. Plants of the American continent with antiulcer activity. Phytomedicine. 2008;15:132–146. doi: 10.1016/j.phymed.2007.07.057. [DOI] [PubMed] [Google Scholar]
- 3.Chaudhary B., Saxena M.S., Sharma S., Ansari B., Mohseen A review of some medicinal plants on their antiulcer and ulcer healing potential. Int. J. Pharm. Sci. Res. 2020;11(11):5308–5321. [Google Scholar]
- 4.Ahmed S., Rahman A., Alam A., Saleem M., Athar M., Sultana S. Evaluation of the efficacy of Lawsonia albain the alleviation of carbon tetrachloride induced oxidative stress. J. Ethnopharmacol. 2000;69(2):157–164. doi: 10.1016/s0378-8741(99)00091-4. [DOI] [PubMed] [Google Scholar]
- 5.Siddiqui B.S., Kardar M.N., Ali S.T., Khan S. Two new and a known compound from Lawsonia inermis. Helvetica Chimica. Acta. 2003;86(6):2164–2169. [Google Scholar]
- 6.Siddiqui B.S., Kardar M.N. Triterpenoids from Lawsonia alba. Phytochemistry. 2001;58(8):1195–1198. doi: 10.1016/s0031-9422(01)00329-6. [DOI] [PubMed] [Google Scholar]
- 7.Goswami M., Kulshreshtha M., Rao C.V., Yadav S. Anti-ulcer potential of Lawsonia inermis L. leaves against gastric ulcers in rats. J. Appl. Pharm. Sci. 2011;1:69–72. [Google Scholar]
- 8.Dhanasree, Basha S.Nizamuddin. Gastro protective activity of Lawsonia inermis (Henna). A well-known traditional medicinal plant. Int. J. Appl. Res. 2015;1(11):833–837. [Google Scholar]
- 9.Ezzat A., Abdelhamid A.O., El Awady M.K., Dawood Reham M., Mohammed Dina Mostafa. Biochemical differences between nano and normal formulation of tamoxifen and other natural bioactive materials ameliorate breast cancer in experimental rats. Bull. Natl. Res. Cent. 2021;45:78. [Google Scholar]
- 10.Ezzat A., Abdelhamid A.O., Mohammed D.M. The role of tamoxifen and some bioactive compounds in resistance to the development of toxicity causing breast cancer in experimental animals. SDRP J. Food Sci. Technol. 2018;3(5):440–449. [Google Scholar]
- 11.Ezzat A., Abdelhamid A.O., El Awady M.K., Abd El Azeem A.S., Mohammed D.M. The biochemical effects of nano tamoxifen and some bioactive components in experimental breast cancer. Biomed. Pharmacother. 2017;95:571–576. doi: 10.1016/j.biopha.2017.08.099. [DOI] [PubMed] [Google Scholar]
- 12.Ezzat A., Abdel hamid A.O., Amin A.I., Abdel-azeem A.S., Mohammed D.M. Biochemical studies on the effect of nano particles of some nutrients on apoptosis modulation of breast cancer cells in experimental animals. J. Appl. Sci. Res. 2013;9(1):658–665. [Google Scholar]
- 13.Babili F.E., Valentin A., Chatelain C. Lawsonia inermis: its anatomy and its antimalarial, antioxidant and human breast cancer cells MCF7 activities. Pharm. Anal. Acta. 2013;4:203. [Google Scholar]
- 14.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., et al. Antioxidant activity applying an improved ABTS radical cationdecolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 15.AOAC, Official methods of analysis of the association of official analytical chemists, 12th ed. Washington D.C (1990).
- 16.Reeves P.G., Nielsen F.H., Fahey G.C.J.R. AIN-93 purified diets for laboratory rodents: final report of the american institute of nutrition ad. hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 17.Zoobi J., Mohd A. Evaluation of antiulcer activity of the flowers of punicagranatum experimental animals. Int. J. Res. Ayurveda Pharm. 2011;2(4):1210–1213. [Google Scholar]
- 18.Amjad M., Tahir M. Effect of ethanolic extract of coconut (Cocosnucifera) on aspirin-induced gastric ulcer in albino rats. J. Gastrointest. Dig. Syst. 2017;7(3):1–6. [Google Scholar]
- 19.Patil K.S., Kumar S., Bahuguna Y.M., Shinkar A.S., Hugar D.S. Antiulcer actvity of leaves of Gossy piumar boreumin aspirin induced rats and pylorus ligated rats. Indian Drugs. 2008;45:325–331. [Google Scholar]
- 20.Rajkapoor, Anandan R., Jayakar B. Anti‐ulcer effect of Nigella sativa Linn against gastric ulcers in rats. Curr. Sci. 2002;83:177–179. [Google Scholar]
- 21.Gusdinar T., Herowati R., Kartasasmita R.E., Adnyana I.K. Anti-inflammatory and antioxidant activity of quercetin-3, 3′, 4′-triacetate. J. Pharmacol. Toxicol. 2011;6:182–188. [Google Scholar]
- 22.Richmond W. Preparation and properties of a cholesterol oxidase from nocardia sp. and its application to the enzymatic assay of total cholesterol in serum. Clin. Chem. 1973;19(12):1350–1356. [PubMed] [Google Scholar]
- 23.Allain C.C., Poon Lucy S., Chan Cicely S.G., Richmond W., Fu Paul C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974;20(4):470–475. [PubMed] [Google Scholar]
- 24.Wieland H., Seidel D. A simple specific method for precipitation of low density lipoproteins. J. Lipid Res. 1983;24(7):904–909. [PubMed] [Google Scholar]
- 25.Lopes-Virella M.F., Stone P., Ellis S., Colwell J.A. Cholesterol determination in high-density lipoproteins separated by three different methods. Clin. Chem. 1977;23:882–884. [PubMed] [Google Scholar]
- 26.Fossati P., Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin. Chem. 1982;28:2077–2080. [PubMed] [Google Scholar]
- 27.Reitman S., Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957;28(1):56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 28.Babson A.L., Greeley S.J., Coleman C.M., Phillips G.E. Phenolphthalein monophosphate as a substrate for serum alkaline phosphatas. Clin. Chem. 1966;12(8):482–490. [PubMed] [Google Scholar]
- 29.Rheinhold J.G. In: Seligron D., editor. vol. I. Academic press, Inc; New York: 1953. p. 88. (Total Protein, Albumin and Globulin in Standard Methods of Clinical Chemistry). [Google Scholar]
- 30.Doumas B.T., Watson W.A., Biggs H.G. Albumin standards and the measurement of serum albumin with bromcresol green. Clin. Chim. Acta. 1971;31(1):87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- 31.Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta. 1978;90(1):37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 32.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 33.Koracevic D., Koracevic G., Djordjevic V., Andrejevic S., Cosic V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001;54(5):356–361. doi: 10.1136/jcp.54.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schirmeister J. Determination of creatinine in serum. Dtsch. Med. Wschr. 1964;89:1940. [Google Scholar]
- 35.Fawcett J.K., Scott J.E. A rapid and precise method for the determination of urea. J. Clin. Pathol. 1960;13(2):156. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barham D., Trinder P. An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst. 1972;97:142–145. doi: 10.1039/an9729700142. [DOI] [PubMed] [Google Scholar]
- 37.Bancroft J.D., Gamble M. fifth ed. Churchill Livingstone Pub, London,; Edinburgh: 2002. Theory and Practice of Histological Techniques. [Google Scholar]
- 38.Ahmed K.A., Korany R.M.S., El Halawany H.A. Spirulinaplatensis alleviates arsenic-induced toxicity in male rats: biochemical, histopathological and immunohistochemical studies. Adv. Anim. Vet. Sci. 2019;7(8):701–710. [Google Scholar]
- 39.Hamedi S., Arian A.A., Farzaei M.H. Gastroprotective effect of aqueous stem bark extract of ziziphusjujuba l. Against hcl/ethanol-induced gastric mucosal injury in rats. J. Tradit. Chin. Med. 2015;35:666–670. doi: 10.1016/s0254-6272(15)30157-6. [DOI] [PubMed] [Google Scholar]
- 40.De Andrade S.F., Lemos M., Comunello E., Noldin V.F., CechinelFilho V., Niero R. Evaluation of the antiulcerogenic activity of Maytenusrobusta (Celastraceae) in different experimental ulcer models. J. Ethnopharmacol. 2007;113:252–257. doi: 10.1016/j.jep.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Boligon A.A., de Freitas R.B., de Brum T.F., Waczuk E.P., Klimaczewski C.V., de Ávila D.S., Athayde M.L., de Freitas Bauermann L. Antiulcerogenic activity of Scutiabuxifolia on gastric ulcers induced by ethanol in rats. Acta Pharm. Sin. B. 2014;4:358–367. doi: 10.1016/j.apsb.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khémiri I., Bitri L. Effectiveness of Opuntiaficusindica L. inermis seed oil in the protection and the healing of experimentally induced gastric mucosa ulcer. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/1568720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ardalani H., Hadipanah A., Sahebkar A. Medicinal plants in the treatment of peptic ulcer disease: a review. Mini Rev. Med. Chem. 2020;20(8):662–702. doi: 10.2174/1389557520666191227151939. [DOI] [PubMed] [Google Scholar]
- 44.Abd El-Ghffar E.A., Al-Sayed E., Shehata S.M., Eldahshan O.A., Efferth T. The protective role of Ocimumbasilicum L.(Basil) against aspirin-induced gastric ulcer in mice: impact on oxidative stress, inflammation, motor deficits and anxiety-like behavior. Food Funct. 2018;9(8):4457–4468. doi: 10.1039/c8fo00538a. [DOI] [PubMed] [Google Scholar]
- 45.Qadhi A., Elfky N.A. Study the protective effects of portulacaoleracea on peptic ulcer in male rats. Asian J. Med. Princ. Clin. Pract. 2019:1–8. [Google Scholar]
- 46.Ayatollahi Mousavi S.A., Abdolahi H., Kazemii N. Investigation of antifungal activity of 10 methanol extracts of medicinal herbs. J. Kerman Uni. Med. Sci. 1996;3:115–122. [Google Scholar]
- 47.Singh V.K., Pandey D.K. Fungitoxic studies on bark extract of L. inermis against ringworm fungi. Hindustan Atibitot. Bull. 1989;2:32–35. [PubMed] [Google Scholar]
- 48.Rao Ch.V., Sairam K., Goel R.K. Experimental evaluation of Bacopamonnieraon rat gastric ulceration and secretion. Indian J. Physiol. Pharmacol. 2000;44:35–41. [PubMed] [Google Scholar]
- 49.Manandhar S., Luitel S., Dahal R.K. In Vitro Antimicrobial Activity of Some Medicinal Plants against Human Pathogenic Bacteria. Journal of tropical medicine. 2019;2019 doi: 10.1155/2019/1895340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kannappan N.S., Jaikumar, Manavalan R., KottaiMuthu A. Anti-ulcer activity of methanolic extract of Jatrophacurcas on Aspirin-induced gastric lesions in Wistar rats. Pharmacologyonline. 2008;1:279–293. [Google Scholar]
- 51.Shah J.S., Patel J.R. Anti-ulcer activity of Lucer against experimentally induced gastric ulcers in rats. An International Quarterly Journal of Research in Ayurveda (Ay) 2012;33(2):314–316. doi: 10.4103/0974-8520.105260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adefisayo M.A., Akomolafe R.O., Akinsomisoye S.O., Alabi Q.K., Ogundipe O.L., Omole J.G., Olamilosoye K.P. Gastro-protective effect of methanol extract of Vernoniaamygdalina (del.) leaf on aspirin-induced gastric ulcer in Wistarrats. Toxicol. Rep. 2017;4:625–633. doi: 10.1016/j.toxrep.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hsien-Tsung Y., Shan-Ye H., Meng-Tsan C. Food Chem. Toxicol. 2008;46:1525–1534. [Google Scholar]
- 54.Amin H.M., Youssef M.A. Immunological, hematological and biochemical effects of aspirin in low and high doses in male albino rats. Eur. J. Mol. Clin. Med. 2021;7(11):6700–6713. [Google Scholar]
- 55.Ergul Y., Erkan T., Uzun H. Effect of vitamin C on oxidative liver injury due to isoniazid in rats. Pediatr. Int. J. 2010;52:69–74. doi: 10.1111/j.1442-200X.2009.02891.x. [DOI] [PubMed] [Google Scholar]
- 56.Al-Abrash A.S., Al-Quobaili F.A., Al-Akhras G.N. Catalase evaluation in different human diseases associated with oxidative stress. Saudi Med. J. 2000;21(9):826–830. [PubMed] [Google Scholar]
- 57.Vyas H., Ram A., Purohit, Jatwa R. Adverse effects of subchronic dose of aspirin on reproductive profile of male rats. J. Pharm. 2016;3:1–9. doi: 10.1155/2016/6585430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Purohit, Daradka H.M.M. Effect of mild hyperlipidaemia on testicular cell population dynamics in albino rats. Indian J. Exp. Biol. 1999;37(4):396–398. [PubMed] [Google Scholar]
- 59.Huerta J., Castellsague C., Varas-Lorenzo, Garcıa Rodr ´ıguez L.A. Nonsteroidal anti-inflammatory drugs and risk of ARF in the general population. Am. J. Kidney Dis. 2005;45(3):531–539. doi: 10.1053/j.ajkd.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 60.Matzke G.R. Nonrenal toxicities of acetaminophen, aspirin, and nonsteroidal anti-inflammatory agents. Am. J. Kidney Dis. 1996;28(1):S63–S70. doi: 10.1016/s0272-6386(96)90571-5. [DOI] [PubMed] [Google Scholar]
- 61.Aprioku J.S., Nwidu L.L., Amadi C.N. Evaluation of toxicological profile of ibuprofen in Wistar albino rats. Am. J. Biomed. Sci. 2014;6(1):32–40. [Google Scholar]
- 62.Kumar M., Kaur P., Chandel M., Singh A.P., Jain A., Kaur S. Antioxidant and hepatoprotective potential of Lawsonia inermis L. leaves against 2-acetylaminofluorene induced hepatic damage in male Wistar rats. BMC Complement. Altern. Med. 2017;17:56. doi: 10.1186/s12906-017-1567-9. [DOI] [PMC free article] [PubMed] [Google Scholar]