Graphical abstract
Keywords: Ultrasound drying, Broccoli, Glucoraphanin, Sulforaphane, Melatonin, Pretreatment
Highlights
-
•
The components and factors in the metabolic pathways of glucoraphanin were analyzed.
-
•
Melatonin or vitamin C pretreatments protected broccoli sulforaphane during drying.
-
•
The exogenous pretreatments also protected broccoli myrosinase during drying.
-
•
Contact sonication during drying promoted the degradation of glucoraphanin.
Abstract
In this investigation, the combinations of exogenous pretreatment (melatonin or vitamin C) and contact ultrasound-assisted air drying were utilized to dry broccoli florets. To understand the influences of the studied dehydration methods on the conversion of glucoraphanin to bioactive sulforaphane in broccoli, various components (like glucoraphanin, sulforaphane, myrosinase, etc.) and factors (temperature and moisture) involved in the metabolism pathway were analyzed. The results showed that compared with direct air drying, the sequential exogenous pretreatment and contact ultrasound drying shortened the drying time by 19.0–22.7%. Meanwhile, contact sonication could promote the degradation of glucoraphanin. Both melatonin pretreatment and vitamin C pretreatment showed protective effects on the sulforaphane content and myrosinase activity during the subsequent drying process. At the end of drying, the sulforaphane content in samples dehydrated by the sequential melatonin (or vitamin C) pretreatment and ultrasound-intensified drying was 14.4% (or 26.5%) higher than only air-dried samples. The correlation analysis revealed that the exogenous pretreatment or ultrasound could affect the enzymatic degradation of glucoraphanin and the generation of sulforaphane through weakening the connections of sulforaphane-myrosinase, sulforaphane-VC, and VC-myrosinase. Overall, the reported results can enrich the biochemistry knowledge about the transformation of glucoraphanin to sulforaphane in cruciferous vegetables during drying, and the combined VC/melatonin pretreatment and ultrasound drying is conducive to protect bioactive sulforaphane in dehydrated broccoli.
1. Introduction
As an important cruciferous vegetable, broccoli (Brassica oleracea L. var. italica) is rich in nutrients and bioactive compounds, like vitamins, dietary fibers, phenolics, glucosinolates, and others [1], [2]. Epidemiological studies have exhibited that long-term consumption of broccoli can provide a protective effect on liver steatosis and cardiovascular disease and help reduce the risk of various cancers [3]. Many investigations confirm that the anti-cancer effect of broccoli is mainly attributed to products derived from the enzymatic hydrolysis of glucosinolates [4], [5]. Glucoraphanin is a major glucosinolate in broccoli, making up around 50% of glucosinolates [6]. Also, sulforaphane, a breakdown product of glucoraphanin, is the most closely linked with the health-beneficial properties of broccoli [7]. On the other hand, the high water content in fresh broccoli affects its quality, stability, and shelf life. Drying is beneficial to prolong the shelf life and is also an important intermediate method to manufacture vegetable powders. However, an important challenge for the drying of broccoli is to alleviate the loss of nutrients and bioactive substances and prevent deterioration of its quality.
Many studies manifest that ultrasound is an emerging technique to accelerate the convective drying rate of various fruits and vegetables and enhance the quality of dehydrated products [8], [9], [10], [11], [12]. Also, the metabolism of glucosinolates in broccoli during preservation and some thermal treatments have also been previously explored [13], [14], [15]. But only a limited studies focused on the metabolism of glucoraphanin and related metabolites under the simultaneous heating and water removeing treatments, liking drying [16], [17]. In one of our previous studies, the airborne-ultrasound assisted air drying of broccoli florets was studied and the quality of dehydrated products was evaluated [17]. It was found that although ultrasound treatment shortened the drying time and lowered the energy consumption, no significant difference in the retention of glucoraphanin and sulforaphane for the air-dried samples in the presence and absence of ultrasound were observed. To produce dried broccoli products with high nutritional value, more efforts are essential to improve the performance of the ultrasound-assisted drying technique to provide a better preservative effect on the broccoli bioactives. Besides, the scientific information about the effect of exogenous pretreatment before ultrasound drying on the accumulation of bioactive compounds in broccoli is scarce.
Melatonin is occasionally used in the preservation of different fruits and vegetables, including pear [18], tomato [19], Chinese flowering cabbage [20] and broccoli [1], [4], [21]. In the postharvest period, melatonin treatment can protect the cell structure, prevent DNA damage, and reduce peroxide levels by removing free radicals, thus enhancing the antioxidant capacity and inhibiting the lipid peroxidation of plant cells [22], [23], [24]. Wei et al. [21] observed that broccoli florets treated with melatonin exhibited higher contents of glucoraphanin and sulforaphane during the preservation period at 4 °C, owing to the up-regulated expression of genes synthesising glucoraphanin and myrosinase. Miao et al. [1] also reported that broccoli florets treated by melatonin sustained higher vitamin C (VC), phenolics, and glucosinolates than broccoli without melatonin treatment. On the other hand, VC is widely utilised in the postharvest of fruits and vegetables, preventing the oxidation of plant materials [25]. Thus, it is interesting to combine the appropriate pretreatments (such as melatonin, VC, etc.) with the ultrasound-assisted drying treatment to alleviate the loss of broccoli's bioactives during drying potentially.
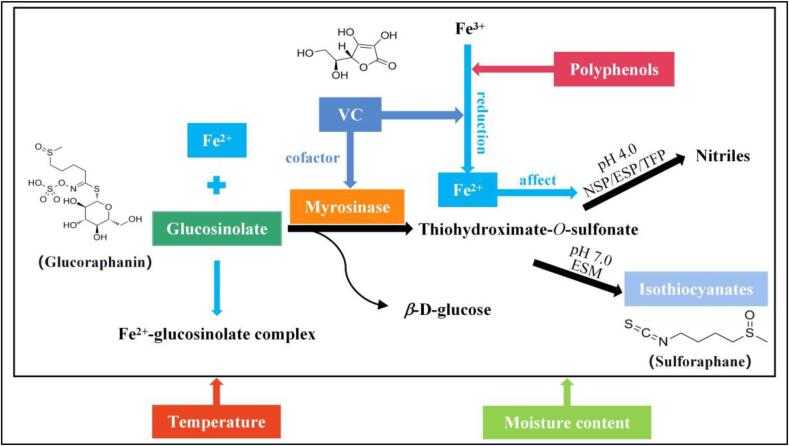
To better control the metabolism of glucosinolates and retain bioactive isothiocyanates, it is necessary to investigate the fates of glucosinolates, isothiocyanates and other influencing components in the metabolic pathway of glucosinolates. Based on the detailed literature review, the summarised metabolic pathway of glucosinolates under drying in several aspects is illustrated in Fig. 1, which includes the related factors. Specifically, glucosinolate can be hydrolysed by myrosinase if the cell structure is damaged [5]. Then, glucosinolate is broken down into β-D-glucose and the unstable thiohydroximate-O-sulfonate [15]. Thiohydroximate-O-sulfonate could decompose to nitriles through its interactions with the epithiospecifier protein (ESP), the nitrile-specifier proteins (NSP), or the thiocyanate-forming protein (TFP) at pH 4.0 [26]. Thiohydroximate-O-sulfonate can also be transformed to isothiocyanates, including sulforaphane by the epithiospecifier modifier protein (ESM) at pH 7.0 [27].
Fig. 1.
The summarised metabolic pathway of glucosinolates.
Meanwhile, Fe2+ can exert some adverse influence on the conversion of glucosinolate to sulforaphane. On the one hand, Fe2+ can combine with glucosinolate to form the Fe2+-glucosinolate complex [28]. Also, Fe2+ can affect the activities of ESP and NSP and induce forming nitriles by themselves [15]. Moreover, as a cofactor of myrosinase, VC at proper concentrations can promote the enzymatic degradation of glucosinolates by controlling the release of glucose molecules from the active sites of myrosinase [29]. The redox effects of VC and polyphenols can reduce Fe3+ to Fe2+, indirectly inhibiting the enzymatic degradation of glucosinolates to isothiocyanates [30]. Besides, the environmental factors, including temperature and water contents, can affect the mobility and chemical stability of molecules and the reaction rate stated above, thus exerting a certain influence on the metabolic pathways of glucosinolates. To study the metabolism of broccoli glucosinolates under drying, the components mentioned above and factors should be analysed.
This investigation aims to explore the metabolic pathway of glucoraphanin under the sequential melatonin or VC pretreatment and contact ultrasound-assisted air drying process. The fates of glucoraphanin, sulforaphane, VC, Fe2+, phenolics and myrosinase activity in broccoli florets throughout drying were analysed by chemical methods. Moreover, the correlations among the components and factors involved in the metabolic pathway of glucoraphanin were explored statistically. The obtained results can help to develop novel dehydration methods to produce quality and sulforaphane rich broccoli products, and provide some insights into glucoraphanin-related chemistry in the drying of Cruciferae vegetables.
2. Materials and methods
2.1. Plant materials
Fresh broccoli samples (Brassica oleracea L. var italica), cultivar “You Xiu” were purchased from Suguo Supermarket (Nanjing, China) and the origin was Nanjing Runhong Vegetable Co., Ltd. (Nanjing, China). All the broccoli samples were stored at 4 °C for maximally 24 h before experiments. Small broccoli florets weighing approximately 3.0 g per floret were prepared and immediately sent to the subsequent drying pretreatments. The initial moisture content of the broccoli florets was measured by the AOAC method [31], which was 9.44 kg/kg dry weight (DW).
2.2. Immersion pretreatments
Broccoli florets were immersed in 100 μmol/L each melatonin and VC solutions at a solid–liquid ratio of 1:20 (g:mL), separately. These immersion pretreatments were performed at room temperature (approximately 25 °C) in darkness. Following the literature [32], [33], [34], the immersion pretreatment in the melatonin solution lasted for 30 min. Meanwhile, some broccoli florets were kept in the VC solution for 60 min, based on our preliminary study. After these pretreatments, excessive liquid on broccoli florets was carefully removed using tissue papers. The samples immersed in distilled water for 60 min were taken as a control.
2.3. Contact ultrasound-assisted air drying
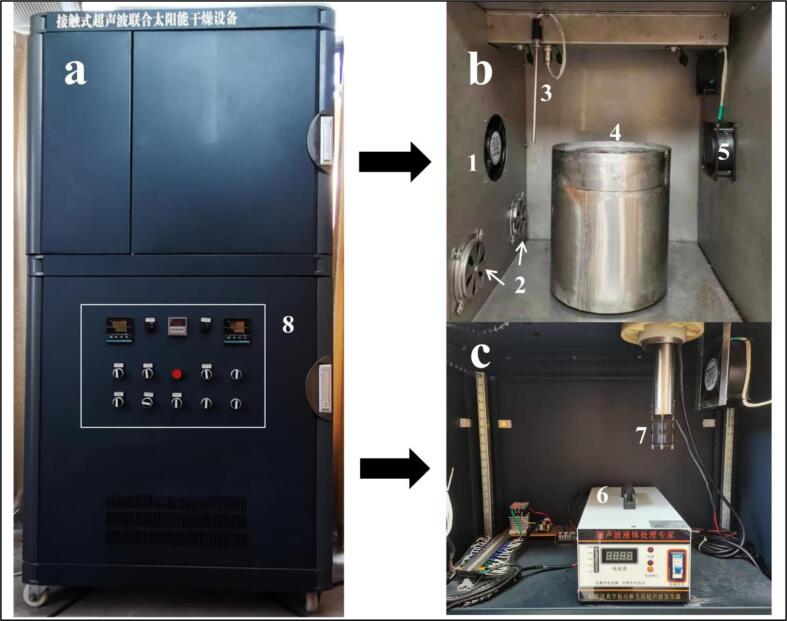
The broccoli florets, as prepared in Section 2.2, were dried in a self-assembly hot air dryer; into which an ultrasonic vibrating plate of 20 kHz with a diameter of 25 cm (Shangjia Biotechnology Co., Ltd., Wuxi, Jiangsu, China) was installed. The design of this system is different from the ultrasound dryer presented in our previous study [17], and this equipment was located in our laboratory in our university campus. The actual ultrasound power distributed on the surface of the vibrating plate was measured using the calorimetric method [35]. Exactly, distilled water of an already known weight was introduced onto the plate and then the plate was covered with plastic wrap to insulate the water from the environment. The temperature change was monitored by a K-type thermocouple connected with a data logger (RDXL 12SD, Omega, USA). The ultrasound intensity on the plate surface was determined to be 12.6 W/dm2. Broccoli samples weighing approximately 3.0 g per floret were spread directly onto the ultrasonic vibration plate during drying, and the sample weight was analysed periodically. Hot air at 65 °C and 4.5 m/s entered the dryer from the inlet and left the dryer from the outlet. Ultrasound was introduced in a pulse mode (5 s on and 5 s off). The image of the developed contact ultrasound-assisted dryer is shown in Fig. 2. The drying time is defined as the time needed to decrease the moisture content to 0.25 kg/kg DW, which was the equilibrium moisture content at the studied drying conditions. Air drying alone was performed as a control group. The applied drying treatments are summarised in Table 1. The process of the whole methodology is illustrated in Supplementary Fig. 1.
Fig. 2.
Schematic diagram of the contact ultrasound-assisted air dryer (a: the exterior of the dryer; b: the interior of the dryer; c: ultrasonic part). 1: air inlet, 2: electric fans to recycle hot air (not used in this investigation), 3: thermocouple, 4: vibrating plate driven by ultrasound transducers, 5: air outlet, 6: ultrasound generator; 7: electric fan to remove the heat generated by the ultrasound device, 8: control panel.
Table 1.
The utilised dehydration methods in this study.
| No. | Dehydration treatment | Abbreviation |
|---|---|---|
| 1 | Hot-air drying without pretreatment | HD |
| 2 | Sequential melatonin pretreatment and hot-air drying | MT & HD |
| 3 | Sequential vitamin C pretreatment and hot-air drying | VC & HD |
| 4 | Contact ultrasound-assisted hot-air drying without pretreatment | CUD |
| 5 | Sequential melatonin pretreatment and contact ultrasound-assisted hot-air drying | MT & CUD |
| 6 | Sequential vitamin C pretreatment and contact ultrasound-assisted hot-air drying | VC & CUD |
2.4. Physical and chemical analysis
2.4.1. Glucoraphanin content
Glucoraphanin in broccoli florets was extracted according to the method of Baenas et al. [36] with minor modifications. Broccoli samples (0.4 g for fresh samples and samples dried for 0.5 h, 0.3 g for samples dried for 2 h, and 0.1 g for samples at the end of drying) were mixed with 3 mL methanol (70% v/v) at 70 °C for 20 min to inactivate myrosinase. Then, the samples were fully milled for cell disruption and incubated in a shaker (DSHZ-300A, Qiangle, Taicang, China) at 70 °C under agitation at 100 rpm for another 20 min to extract glucoraphanin. The samples were centrifuged at 5000 rpm for 15 min, and the supernatant was collected. Glucoraphanin in the residual was then extracted for a second time using the same procedure. The two extracts were mixed and vacuum-concentrated in a rotary evaporater (RE-2000A, Xiande, Shanghai, China) at 45 °C till reaching a constant weight. The obtained samples were dissolved in 2 mL deionised water containing trifluoroacetic acid (0.05% v/v) and filtered through a 0.45 μm membrane filter.
Glucoraphanin in the broccoli extract was first identified using the ultrahigh performance liquid chromatography (Nexera X2, Shimadzu, Japan) equipped with a Triple-TOF-MS system (TripleTOF 4600, AB SCIEX, USA) with the DuoSprayTM ionisation source. The DuoSprayTM has an atmospheric pressure chemical ionisation inlet and an electrospray. The chromatographic separation was implemented through an Ultimate XB-C18 column (100 × 2.1 mm, 3 μm particle size). The mobile phases consisted of 0.1% formic acid (A) and acetonitrile (B). The employed chromatographic conditions were 0–2 min, 5% B; 2–19 min, 5–70% B; 19–21 min, 70–90% B; 21–25 min, 90–90% B; 25.1 min, 5% B; 25.1–30 min, 5% B. The other parameters followed were from our previous study [37].
After identification, glucoraphanin content was then measured in a Shimadzu HPLC system (LC-2010A, Shimadzu Corporation, Japan) equipped with an Eclipse XDB-C18 column (4.6 × 150 mm, 5 μm particle size; Agilent Technologies Co. Ltd) at 227 nm [17]. The injection volume was 20 μL, and the detection temperature was 30 °C. The mobile phases consisted of (A) deionised water containing trifluoroacetic acid (0.05% v/v) and (B) acetonitrile. The flow rate of the mobile phase was 1.0 mL/min. The gradient elution program was as follows: 0–15 min, 0–0% B; 15–25 min, 0–5% B; 25–40 min, 5–20% B; 40–50 min, 20–35% B; 50–55 min, 35–99% B; 55–60 min, 99–0% B. The glucoraphanin standard was used to build the calibration curve, and the glucoraphanin content is expressed as mg/g DW.
2.4.2. Sulforaphane content
Sulforaphane in broccoli florets was extracted according to the method described by Guo et al. [38] with slight modifications. Broccoli samples (0.4 g for fresh samples and samples dried for 0.5 h, 0.3 g for samples dried for 2 h, and 0.1 g for samples at the end of drying) were homogenised using 4 mL distilled water. The extraction was then performed in the same shaker under agitation at 37 °C and 100 rpm for 3 h. Sulforaphane in the mixture was then extracted using 10 mL ethyl acetate in triplicate at room temperature. The organic phase from each extraction was combined, and 1 g anhydrous sodium sulfate was then added for additional dehydration. The ethyl acetate fraction was dried at 35 °C in a rotary evaporator (RE-2000A, Xiande, China). Finally, the residue was dissolved in 2 mL acetonitrile and filtered through a 0.45 μm membrane filter. The identification of sulforaphane was performed following the same conditions as that for glucoraphanin.
The sulforaphane content in the extract was quantified using an Agilent 1200 HPLC system (Agilent Technologies Co. Ltd.) with an Eclipse XDB-C18 column (4.6 × 150 mm, 5 μm particle size; Agilent Technologies Co. Ltd) at 254 nm. The flow rate was 1.0 mL/min and the elution program was chosen as: 0–15 min, 20–60% B; 15–20 min, 60–100% B [17]. Sulforaphane was quantified through external calibration, and the results are expressed as mg/g DW.
2.4.3. Myrosinase activity
Myrosinase activity in broccoli florets was analysed according to the method described by Guo et al. [38] with slight modifications. First, broccoli samples (0.4 g for fresh samples and samples dried for 0.5 h, 0.3 g for samples dried for 2 h, and 0.1 g for samples at the end of drying) were mixed with 3 mL 0.1 mol/L sodium phosphate buffer and ground manually in an ice bath. The supernatant containing enzyme extract was collected after centrifugation at 5000 rpm and 4 °C for 15 min. The residual was mixed with another 3 mL fresh sodium phosphate buffer, and the extraction was repeated. The two supernatants were combined. Then, 500 μL each of crude enzyme extract and sinigrin (0.25 mmol/L) was mixed and incubated at 37 °C for 15 min, and the reaction was stopped by boiling for 5 min. D-Fructose/D-Glucose assay kit (Megazyme, Bray, Ireland) was then utilised to measure the amount of glucose formed by the reaction between myrosinase and the substrate. One myrosinase unit is defined as 1 μmol/L glucose released from sinigrin/min at 37 °C. Myrosinase activity is expressed as U/g DW.
2.4.4. VC content
VC content in broccoli florets was determined according to the method described by Cao et al. [17] with minor modifications. Broccoli samples (0.4 g for fresh samples and samples dried for 0.5 h, 0.3 g for samples dried for 2 h, and 0.1 g for samples at the end of drying) were homogenised with 4 mL distilled water containing oxalic acid (2.0% w/v), vortexed for 1 min and centrifuged at 5000 rpm for 15 min. The supernatant was collected, and the residue was mixed again with the fresh oxalic acid solution. The extraction procedures were repeated to extract the residual VC. The two supernatants were mixed and filtered through a 0.45 μm membrane filter. The VC content in the extract was quantified using a Shimadzu HPLC system (LC-2010A, Shimadzu Corporation, Japan) equipped with an Agilent ZOBAX-C18 column (4.6 × 250 mm, 5 μm particle size; Agilent Technologies Co. Ltd). Samples were eluted using the solvent containing 0.1% aqueous oxalic acid–methanol (95:5, v:v) at a flow rate of 0.8 mL/min. The detection wavelength was 254 nm, and the injection volume was 20 μL. The standard VC was used to make the calibration curve, and the result is expressed as mg/g DW.
2.4.5. Fe2+ content
Fe2+ content in broccoli florets was measured following the method of Bellostas et al. [28] with slight modifications. Broccoli samples (0.4 g for fresh samples and samples dried for 0.5 h, 0.3 g for samples dried for 2 h, and 0.1 g for samples at the end of drying) were ground manually with 3 mL distilled water, vortexed for 1 min and centrifuged at 5000 rpm for 15 min. The supernatant was collected, and the residue was extracted again using another 3 mL distilled water. The two supernatants were then mixed. 3 mL non-reducing protein precipitate solution (10 g trichloroacetic acid and 10 mL 37% HCl added to 100 mL distilled water) was then added to the obtained extract. The mixture containing non-reducing protein precipitate was left overnight at room temperature. The sample was then centrifuged at 5000 rpm for 15 min. Then, 100 µL supernatant was mixed with 200 µL HEPES (N-2-(hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid)) buffer (0.3 mol/L, pH 9.9) and 25 µL ferrozine chromogen solution (5 mg/mL in distilled water). The mixture was immediately moved to a 96-well plate and analysed in a microplate reader (Bio-Tek Instruments) at 570 nm. Fe2+ was quantified from a calibration curve using ferrous sulfate as a standard. The result is expressed as mg/100 g DW.
2.4.6. Total phenolic content
Phenolics in broccoli florets were extracted based on the method of Thomas et al. [2] with some modifications. Broccoli samples (0.4 g for fresh samples and samples dried for 0.5 h, 0.3 g for samples dried for 2 h, and 0.1 g for samples at the end of drying) were homogenised using 4 mL methanol (80% v/v). Phenolics in the mixture were then extracted under agitation at 37 °C and 100 rpm for 1 h. After centrifugation at 5000 rpm for 15 min, the supernatant was collected, and phenolics in the residuals were extracted again using fresh methanol (80% v/v). The two supernatants were then mixed, and the Folin-Ciocalteu method was employed to analyse the total phenolic content. The detailed procedure for the Folin-Ciocalteu method can be found in our previous work [39]. To be extract, 0.6 mL of the extract was mixed with 4.5 mL 10-fold-diluted Folin-Ciocalteu reagent and 4.5 mL of 7.5% (w/v) sodium carbonate. After standing at 25 °C in darkness for 2 h, the absorbance was read at 765 nm. The calibration curve was made using gallic acid as a standard. The result is expressed as mg gallic acid equivalents per gram of dry weight (mg GAE/g DW).
2.4.7. Low-field NMR analysis
Water status was analysed using the method of Xu et al. [40], with some modifications. The broccoli sample was placed in a Φ15 mm cylindrical glass tube. The relaxation time measurements were performed in an NMI20-analyst low-field (LF) NMR analyser (Niumag Co., Ltd, Suzhou, Jiangsu, China) with a magnetic field strength of 0.5 T at 21 MHz. The temperature of the LF-NMR instrument was maintained at 32 °C. A total of 10,000 echoes were acquired for analysis. T2 distributions were obtained using the Multi Exp Inv Analysis software (Niumag Co., Ltd., Suzhou, China).
2.5. Statistical analysis
The evolutions of the myrosinase activity and contents of Fe2+ and VC in broccoli florets during the drying process were modeled using the following Weibull model equation [41]:
| (1) |
where Ct is the value of each parameter (U/g DW for myrosinase, mg/g DW for VC and mg/100 g DW for Fe2+) at time t (h), a is the initial value of each parameter (U/g DW for myrosinase, mg/g DW for VC and mg/100 g DW for Fe2+), b is the descent rate constant during drying, and c (dimensionless) is the shape constant for Weibull model. Modelling was performed in Matlab, R2009a (The MathWorks, Inc., USA).
All the experiments and analyses were conducted in triplicate, and the data were expressed as the mean ± standard deviation. The data were evaluated by ANOVA and the Duncan’ s multiple-range test using SPSS 25 (SPSS Inc., Chicago, USA). Differences at p < 0.05 were considered significant. The Pearson correlation coefficient was also calculated using SPSS 25.
3. Results and discussion
3.1. Drying characteristics
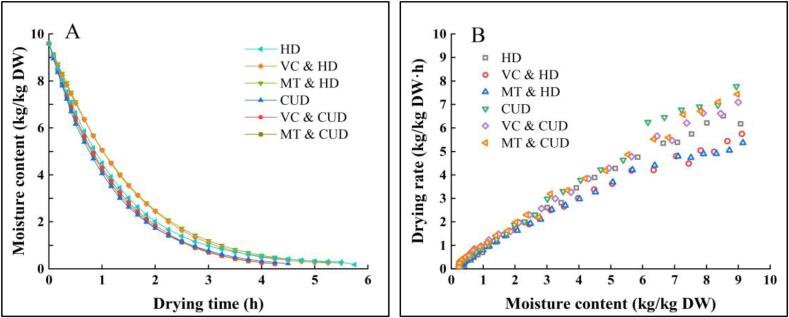
The experimental drying kinetic curves of broccoli florets by different dehydration methods are exhibited in Fig. 3A. As can be seen, the drying times required to achieve the moisture content of 0.25 kg/kg DW for HD, MT & HD and VC & HD are approximately 345, 330 and 315 min, respectively. Meanwhile, the drying times for CUD, MT & CUD and VC & CUD decrease to 270, 255 and 255 min, respectively. The mechanical, cavitation or even thermal effects of ultrasound can reduce drying time [42]. Tao et al. [11] reported a higher reduction of drying time in the case of contact ultrasound-assisted air drying of garlic slices. Apart from the applied air conditions, the properties of food matrices themselves can affect the drying efficiency under ultrasound treatment. Broccoli florets have an umbrella-like geometry with many air gaps, attenuating the ultrasound energy. Also, the exogenous pretreatments could benefit the drying process slightly. Both melatonin and VC pretreatments shortened the drying time by 4.3–8.7% compared with the drying treatment without pretreatments. It is speculated that the applied exogenous pretreatments could exert osmotic pressure and modify the cell structure, thus promoting the subsequent drying process. Moreover, based on Fig. 3B, the drying rate for all the samples decline with water content, implying that the falling rate period dominates throughout the air drying processes. Similar results were reported by Liu et al. [43], who found that the proper high-humidity hot air impingement blanching treatment can also enhance the drying rate of broccoli florets and the entire drying process occurred in the falling period. At the same water level, the drying rates for ultrasound-treated samples are higher than only air-dried samples, consistent with the drying kinetic results mentioned above.
Fig. 3.
Drying kinetic curves (A) and drying rate curves (B) of broccoli florets under the studied dehydration methods.
The internal temperature curves of broccoli florets under drying are plotted in Supplementary Fig. 2. The temperature in all the samples did not exceed the air temperature, demonstrating the absence of overheating problems. The transverse relaxation time curves (T2) under drying obtained from low-field NMR are plotted in Supplementary Fig. 3. The largest peak (T23) refers to the water with higher mobility existing in cell xylem and vacuole, and the other peaks denote the less mobile water and bound water [40]. Contact sonication made a more apparent reduction of the peak amplitude of T23. It further confirms that contact sonication could promote the removal of free water and speed up the drying process.
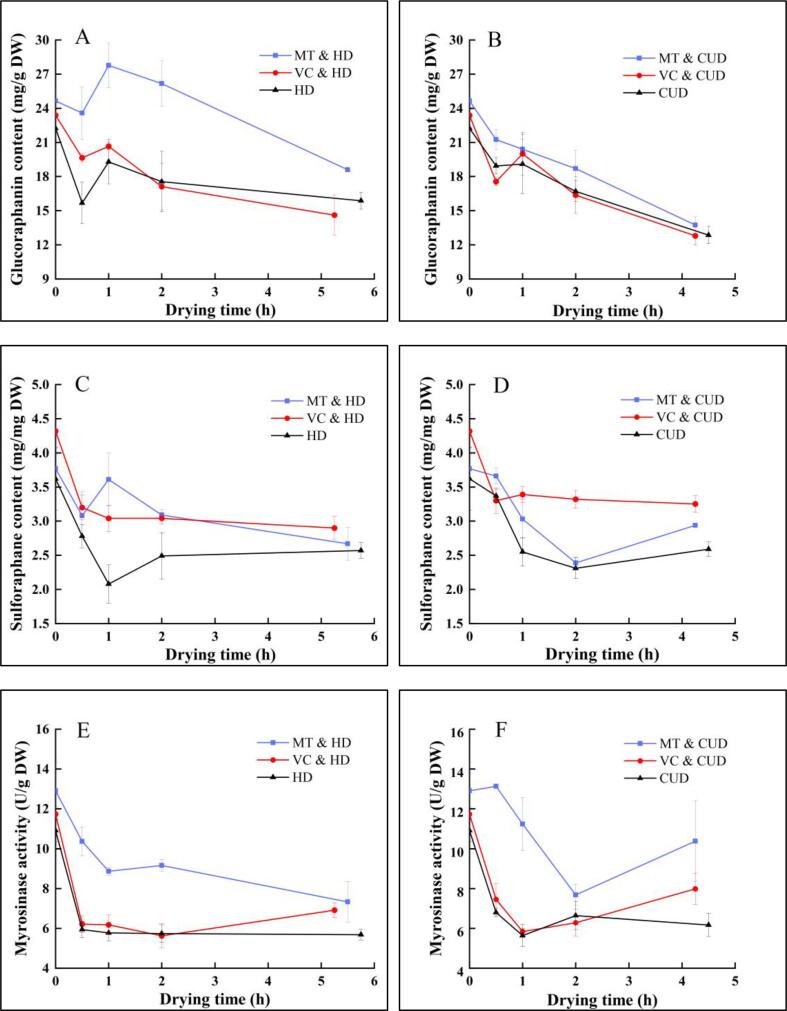
3.2. Effect of sequential exogenous pretreatments and contact ultrasound-assisted air drying on glucoraphanin content
Glucoraphanin is the major glucosinolate in broccoli and is also the precursor of sulforaphane [6]. The MS information about the identified glucoraphanin in the studied broccoli cultivar is listed in Supplementary Table 1. The variations of glucoraphanin content in broccoli florets throughout drying are illustrated in Fig. 4A and B. The amount of glucoraphanin in the fresh broccoli florets is 22.23 ± 1.01 mg/g DW. Similarly, the glucoraphanin content in the fresh broccoli (Brassica oleracea L. var. italica) reported by Wei et al. [21] was about 15.57 mg/g DW. Melatonin pretreatment led to a slight but significant (p < 0.05) increase of glucoraphanin content in fresh broccoli, whereas VC pretreatment had no significant effect on glucoraphanin in broccoli. Glucoraphanin content in broccoli immersed in 100 μmol/L melatonin solution was enhanced by 10.9%. Similar results were also found in the studies of Wei et al. [21] and Miao et al. [1], who found that melatonin spraying enriched glucoraphanin in broccoli. Wei et al. [21] reported that melatonin could induce the expression of biosynthetic genes (BoCYP79F1, BoCYP79B2) and the major transcription factors (BoMYB28, BoMYB34) that regulate glucosinolates synthesis in vivo. Melatonin could also reduce the expression level of AOP2 that converts glucoraphanin to alkenyl glucosinolates.
Fig. 4.
Variations of glucoraphanin content (A and B), sulforaphane content (C and D) and myrosinase activity (E and F) in broccoli florets during drying.
Under air drying both in the presence and absence of ultrasound, the glucoraphanin content exhibited a decreasing trend, denoting the degradation of glucoraphanin. Meanwhile, the glucoraphanin content in broccoli florets pretreated with exogenous melatonin is always higher than in the counterpart without any pretreatments throughout drying. For example, the glucoraphanin amount in samples dried by MT & CUD was 13.74 ± 0.73 mg/g DW at the end of drying and is 6.8% higher compared to samples dried by CUD. Theoretically, the presence of melatonin could inhibit the activity of cell wall degrading enzyme [44] and the contact between myrosinase and glucoraphanin, thus alleviating the hydrolysis of glucoraphanin. Besides, VC pretreatment did not affect the glucoraphanin content in the subsequent drying process.
The reduction of exposure time to hot air owing to contact ultrasound did not lead to the accumulation of glucoraphanin. Instead, the decomposition of glucoraphanin was intensified by contact ultrasound. After drying, the contents of glucoraphanin in samples dried by HD, MT & HD and VC & HD were 15.87 ± 0.75, 18.60 ± 0.13 and 14.62 ± 1.77 mg/g DW, respectively, which are 23.4%, 35.4% and 14.2% higher than the counterparts in the sonicated samples. It could be speculated that the sponge and cavitation effects of ultrasound might promote the breaking of covalent bonds between glucoraphanin and other organic substances in cells [45], thus facilitating the subsequent enzymatic and non-enzymatic degradation of glucoraphanin. Furthermore, sonication may disrupt the chemical bonds of glucoraphanin itself, like glycosidic bond [46], leading to the loss of glucoraphanin.
3.3. Effect of sequential exogenous pretreatments and contact ultrasound-assisted air drying on sulforaphane content
Sulforaphane is an important bioactive component in broccoli, which is responsible for the anti-cancer activity and can also affect the bitter taste [4]. The MS information about sulforaphane in the studied broccoli cultivar is listed in Supplementary Table 1. Based on Fig. 4C and D, the sulforaphane content in fresh broccoli florets is 3.62 ± 0.46 mg/g DW. After both melatonin and VC pretreatments, the sulforaphane contents increased to 3.77 ± 0.20 and 4.32 ± 0.25 mg/g DW, respectively. Throughout air drying in the presence and absence of ultrasound treatment, the content of sulforaphane in all the samples shows a pattern of similar change on the whole. Thus, the sulforaphane content decreases fast in the first 1 h of drying and then changes slowly with either a slow decline or a slight increase. Mahn et al. [47] also reported that the sulforaphane content in broccoli diminished at the first 15 min of tray drying at 60 °C and then increased until 1 h of drying. It is further deduced that the thermal treatment may result in a better inactivation of ESP than myrosinase, thus reducing the formation of sulforaphane nitrile and enhancing the sulforaphane production [48].
On the one hand, sulforaphane can be generated from the degradation of glucoraphanin under the catalytic action of myrosinase once the cell structure collapses [49]. On the other hand, sulforaphane itself is liable to degrade, especially when the temperature exceeds 40 °C [47]. A dozen factors affect the formation and degradation of sulforaphane, including water content, temperature, activities of myrosinase and ESP, contents of VC, Fe2+ and phenolics, and others [15], making the pattern of sulforaphane variation in broccoli under drying complicated.
During both air drying with and without ultrasound, broccoli samples with exogenous pretreatments exhibit higher sulforaphane than samples without pretreatments. For example, the sulforaphane content in the samples treated by VC & HD is 12.8% higher than in the HD-treated samples at the end of drying. Meanwhile, at the end of contact ultrasound-assisted air drying, the sulforaphane content in broccoli samples treated by VC & CUD is the highest (3.25 ± 0.12 mg/g DW), followed by samples dried by MT & CUD (2.94 ± 0.01 mg/g DW) and CUD (2.59 ± 0.11 mg/g DW). Moreover, in the study of Wei et al. [21], sulforaphane content in melatonin treated broccoli was also increased by about 50% after 25-day storage at 4 °C. However, the reason behind this phenomenon still needs to be clarified. Theoretically, VC can serve as a cofactor to activate myrosinase, resulting in the glucosinolate hydrolysis and accumulation of sulforaphane [50].
The above results also reveal that ultrasound-treated samples possess higher sulforaphane than non-sonicated samples at the end of drying. However, the differences are insignificant (p < 0.05). According to the study of Tian et al. [51], the electrophilic carbon atom of -N = C = S bond in sulforaphane can react with the hydroxyl group of water to form R-NH2, and the sulforaphane degradation rate increased with water content. It is believed that contact sonication could accelerate water loss, thus alleviating the sulforaphane degradation reaction in broccoli.
3.4. Effect of sequential exogenous pretreatments and contact ultrasound-assisted air drying on myrosinase activity
The changes of myrosinase activity in broccoli with different pretreatments under air drying are shown in Fig. 4E and F. First, the myrosinase activities in the fresh broccoli florets without pretreatments, melatonin-pretreated, and VC-pretreated samples are 10.91 ± 0.56, 12.91 ± 1.42 and 11.73 ± 0.35 U/g DW, respectively. Melatonin pretreatment seems to have a promotive but insignificant influence on broccoli myrosinase. Wei et al. [21] also found that the myrosinase activity of fresh-cut broccoli pretreated with 100 μmol/L melatonin was always higher than the control group during the 25-day storage. In the study of Miao et al. [1], the transcription level of BoTGG1, an important gene encoding myrosinase, was significantly up-regulated in broccoli samples pretreated with melatonin resulting in the intensification of myrosinase activity. Furthermore, VC immersion did not influence the myrosinase activity of broccoli.
Fig. 4E and F also reveal that dehydration weakens the myrosinase activity. In the groups of air drying alone, the myrosinase activity in broccoli declines by 41.1–47.9% at the end of drying compared to samples before drying. For the groups of contact ultrasound-intensified drying, the myrosinase activity decreases by 19.6–43.4% after drying. On the one hand, as the drying progresses, water removal diminishes the volume for myrosinase stretching, inhibiting the myrosinase activity [52]. The loss of water could also enhance the cell membrane permeability and make contact between myrosinase and proteases easier, indirectly weakening its ability to degrade glucosinolates [53]. On the other hand, myrosinase is highly susceptible to thermal processing [52].
Throughout the air-drying process, the myrosinase activities in broccoli samples pretreated with melatonin are always higher than VC-pretreated and control samples, demonstrating its ability to preserve myrosinase under exposure to hot air. Meanwhile, contact ultrasound also shows a protective effect on myrosinase in the melatonin-pretreated groups. For the drying of melatonin-pretreated broccoli, the myrosinase activities in ultrasound-treated samples are higher compared to samples without ultrasound, except for the samples dried for 2 h. At the end of drying under ultrasound, the myrosinase activities for samples dried by CUD, VC & CUD, and MT & CUD are 6.17 ± 0.58, 7.98 ± 0.79 and 10.38 ± 2.03 U/g DW, respectively.
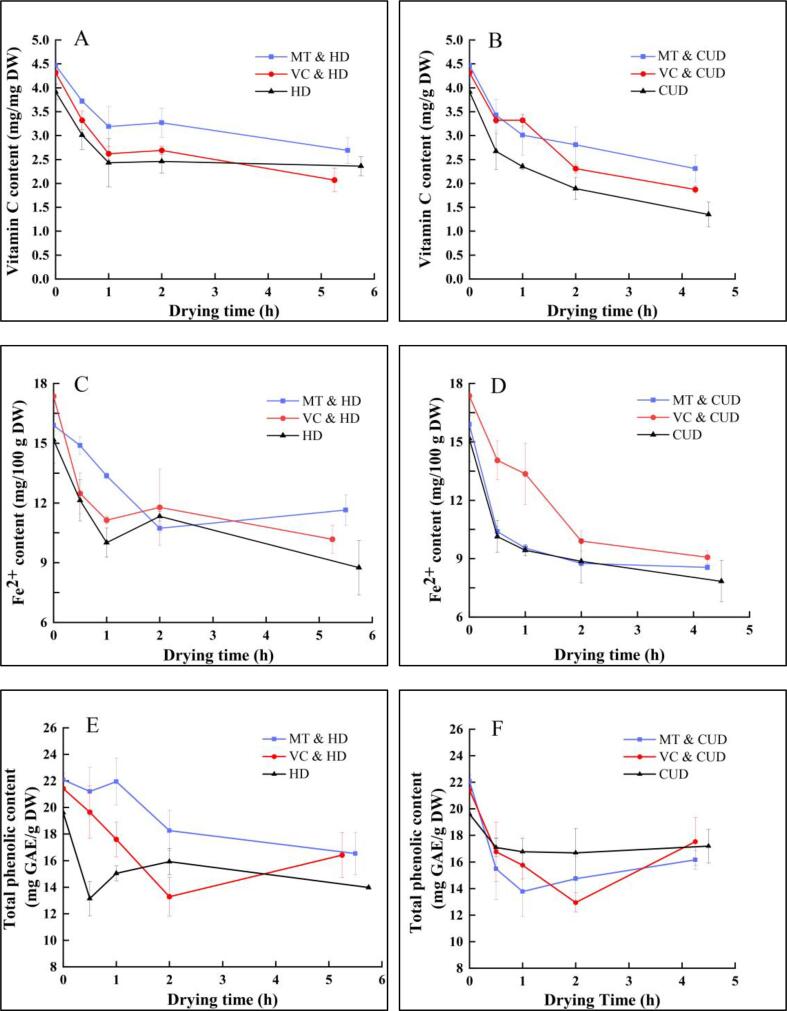
3.5. Effect of sequential exogenous pretreatments and contact ultrasound-assisted air drying on VC content
Based on Fig. 5A and B, the VC content in fresh broccoli samples without pretreatment is 3.92 ± 0.16 mg/g DW, which is close to the VC content (57.35–131.35 mg/100 g FW) in the commercial broccoli samples from grocery stores in the United States [54]. After melatonin and VC pretreatments, the VC contents slightly increased to 4.47 ± 0.52 mg/g DW and 4.32 ± 0.04 mg/g DW, respectively. However, these increments in VC content are insignificant.
Fig. 5.
Variations in the contents of VC (A and B), Fe2+ (C and D) and total phenolic (E and F) in broccoli florets during drying.
As expected, the VC content declined gradually with drying. The VC content in the presence of contact ultrasound decreased faster than in the absence of ultrasound. At the end of drying without any pretreatments, the VC content in the CUD-dried samples (1.35 ± 0.26 mg/g DW) is 42.8% lower than the HD-dried samples (2.36 ± 0.20 mg/g DW). The ultrasonic treatment can generate microagitation, microscopic channels, and cavitation [55]. As a result, VC is more likely to contact oxygen and oxidase, leading to the oxidation of VC to 2-furonic acid, 3-hydroxy-2-pyranone, etc. [56]. Gamboa-Santos et al. [57] also showed that VC retention in strawberries under ultrasound-assisted convective drying was significantly lower than convection drying without ultrasound.
At the end of ultrasound-assisted drying, the VC content in the samples dried by MT & CUD (2.31 ± 0.28 mg/g DW) is 71.1% higher than in the CUD-dried samples. Similarly, the samples dried by MT & HD contained a higher VC of 14.0% than those dried by HD at the end of air drying alone. Moreover, under both air drying with and without ultrasound, the VC contents in the dehydrated samples pretreated by melatonin were higher than in the dried samples without pretreatments. Melatonin can effectively remove excess reactive-oxygen species and improve the redox state of cells, protecting VC against oxidation [58]. During the storage of broccoli at 20 °C, the melatonin treatment also alleviated the degradation of VC [1].
3.6. Effect of sequential exogenous pretreatments and contact ultrasound-assisted air drying on Fe2+ content
Fe2+ can bind to glucosinolates, causing the non-enzymatic degradation of glucosinolates [28]. Consequently, the formation of isothiocyanates is inhibited. Fig. 5C and D show that the contents of Fe2+ in fresh broccoli samples pretreated with melatonin (15.90 ± 1.08 mg/100 g DW) and VC (17.36 ± 1.77 mg/100 g DW) are slightly and insignificantly (p > 0.05) higher compared to the control group (15.17 ± 1.19 mg/100 g DW). This is because melatonin possesses the antioxidant capacity, reducing Fe3+ in broccoli samples to Fe2+ [59]. Also, as an antioxidant, VC can reduce Fe3+ to Fe2+. It should also be pointed out that the stimulative effects of exogenous melatonin and VC pretreatments on Fe3+ reduction may affect the conversion of glucoraphanin to sulforaphane adversely. It is noted from Fig. 5C and D that the Fe2+ contents in all the samples decrease with air drying, and the declining rate of Fe2+ content is higher in the early stage of drying. At the end of air drying alone, Fe2+ contents in the HD-dried samples, MT & HD dried samples and VC & HD dried samples are 8.75 ± 1.37, 11.64 ± 0.76 and 10.18 ± 0.71 mg/100 g DW, respectively. Meanwhile, sonicated broccoli samples possess less Fe2+ at the end of drying than the non-sonicated samples. Fe2+ contents in broccoli dried by CUD, MT & CUD, and VC & CUD are 7.84 ± 1.06, 8.55 ± 0.13 and 9.07 ± 0.33 mg/100 g DW, respectively. On the one hand, the above-mentioned lower retention of VC in ultrasound-treated broccoli samples may contribute to the lower Fe2+ contents in these samples. On the other hand, ultrasound can damage the plant tissue structure [60], facilitating the contact between oxygen and Fe2+ and promoting the oxidation of Fe2+ to Fe3+.
3.7. Effect of sequential exogenous pretreatments and contact ultrasound-assisted air drying on total phenolic content
Phenolic compounds are not only another important group of bioactive substances in broccoli; their Fe-reducing power may influence the degradation rate of glucosinolates [30]. As shown in Fig. 5E and F, total phenolic contents in fresh broccoli samples without pretreatments, pretreated by melatonin, and pretreated by VC are 19.62 ± 0.39, 22.09 ± 1.94 and 21.43 ± 1.15 mg GAE/g DW, respectively. Similar to the changes of glucoraphanin, sulforaphane, and other chemical indices measured in this investigation, the phenolic content in broccoli also enhanced slightly after exogenous melatonin and VC pretreatments. About the postharvest handling of tomatoes, Sun et al. [61] found that 50 μmol melatonin treatment up-regulated the expression of genes coding chorismate mutase 2 in the phenylpropanoid pathway, contributing to the accumulation of phenolics. Also, VC might enhance the antioxidant network against active oxygen species in plant tissues [62], thereby increasing the total phenolic content.
Under air drying, total phenolic content in all the broccoli samples decreased fast in the early drying period. At the beginning of drying, the heating of the broccoli sample can promote the enzymatic oxidation and decomposition of polyphenols, resulting in the destruction of phenolic structure and the decrease of their content [63]. The loss of total phenolic content decreased with the continuous decline of water content. The total phenolic content even enhanced slightly in the latter drying stage of VC-pretreated samples. These phenomena may be because, with the increase of sample temperature during drying, polyphenol oxidase activity gradually decreases and even gets deactivated, thereby preventing further polyphenols oxidation [64]. Furthermore, the loss of water and long-term heating may weaken the binding between phenolics and proteins/saccharides in the cell wall, making some phenolics released from the cell wall [65], [66]. Que et al. [67] speculated that new phenolic substances might be generated during the heating process due to the non-enzymatic transformation of their precursors.
At the end of air drying only, total phenolic contents in the samples dried by HD, MT & HD, and VC & HD are 13.98 ± 0.09, 16.53 ± 1.59 and 16.41 ± 1.68 mg GAE/g DW, respectively. Also, after drying, samples processed by CUD, MT & CUD and VC & CUD possess total phenolic amounts of 17.19 ± 1.26, 16.16 ± 0.71 and 17.54 ± 1.80 mg GAE/g DW, respectively. Generally, contact ultrasound enhanced the retention of phenolics in the dried samples without any pretreatments. In contrast, no protective effect of ultrasound on phenolics could be observed in the samples pretreated by melatonin and VC. Tao et al. [10], Mello et al. [68] and Nascimento et al. [69] reported that simultaneous sonication with air drying could alleviate the loss of phenolics in blackberry, orange peel and passion fruit peel.
3.8. Kinetic modeling of the decline of myrosinase activity, VC and Fe2+ contents in broccoli
Since the myrosinase activity and Fe2+ and VC contents in the most studied drying treatments changed regularly, the obtained data well fit to the Weibull model. According to Table 2, the fitting qualities are acceptable except for the evolutions of myrosinase activity in melatonin-pretreated broccoli during ultrasound drying and Fe2+ content in melatonin-pretreated broccoli during air drying alone. The parameter b in the Weibull model reflects the rate constant for each attribute under dehydration. It can be observed that the b values for the decrease in the myrosinase activity under ultrasound-intensified drying are always lower compared to air drying alone. Also, the b values for the decrease in the myrosinase activity in melatonin-pretreated broccoli are lower than broccoli without pretreatments. These results further demonstrate that ultrasonication and melatonin immersion alleviate the loss of myrosinase activity during drying.
Table 2.
Kinetic parameters of the Weibull model for the selected attributes under different dehydration treatments.
| Attribute | Treatment | Regression coefficient | R2 | RMSEa | Fitting quality |
|---|---|---|---|---|---|
| Myrosinase | HD | a = 10.91b = 0.6275c = 0.0265 | 1.000 | 0.056 | Good |
| MT + HD | a = 12.91b = 0.3095c = 2.118 × 10-6 | 0.964 | 0.562 | Good | |
| VC + HD | a = 11.73b = 0.6426c = 3.099 × 10-7 | 0.966 | 0.648 | Good | |
| CUD | a = 10.91b = 0.5399c = 0.0348 | 0.956 | 0.624 | Good | |
| MT + CUD | a = 13.21b = 0.1769c = 0.5086 | 0.463 | 2.295 | Poor | |
| VC + CUD | a = 11.74b = 0.5717c = 3.099 × 10-7 | 0.850 | 1.277 | Good | |
| VC | HD | a = 3.925b = 0.3875c = 0.1953 | 0.937 | 0.235 | Good |
| MT + HD | a = 4.473b = 0.2739c = 0.3566 | 0.960 | 0.189 | Good | |
| VC + HD | a = 4.326b = 0.4036c = 0.3634 | 0.962 | 0.236 | Good | |
| CUD | a = 3.919b = 0.5228c = 0.4722 | 1.000 | 0.024 | Good | |
| MT + CUD | a = 4.471b = 0.3670c = 0.4012 | 0.995 | 0.080 | Good | |
| VC + CUD | a = 4.316b = 0.3484c = 0.6299 | 0.963 | 0.262 | Good | |
| Fe2+ | HD | a = 15.16b = 0.3108c = 0.2976 | 0.900 | 1.086 | Good |
| MT + HD | a = 16.05b = 0.1934c = 0.4044 | 0.761 | 1.493 | Poor | |
| VC + HD | a = 17.36b = 0.3894c = 0.1721 | 0.975 | 0.629 | Good | |
| CUD | a = 15.17b = 0.4695c = 0.2212 | 1.000 | 0.083 | Good | |
| MT + CUD | a = 15.90b = 0.5002c = 0.1749 | 0.996 | 0.283 | Good | |
| VC + CUD | a = 17.42b = 0.3166c = 0.5493 | 0.961 | 0.937 | Good |
: RMSE refers to the root mean square error. RMSE units for myrosinase, VC and Fe2+ are U/g DW, mg/g DW and mg/100 g DW, respectively.
On the other hand, considering the decrease of VC amount under all the air-drying treatments, excluding the VC pretreatments, the b values under sonication are higher than in the absence of contact sonication. Similarly, the b value for the decline of Fe2+ content in the CUD-dried broccolis (0.4695) is higher than HD-dried samples (0.3108). As indicated earlier, ultrasound itself may destabilise the tissue structure of broccoli, thus accelerating the decrease of VC and Fe2+ contents [70].
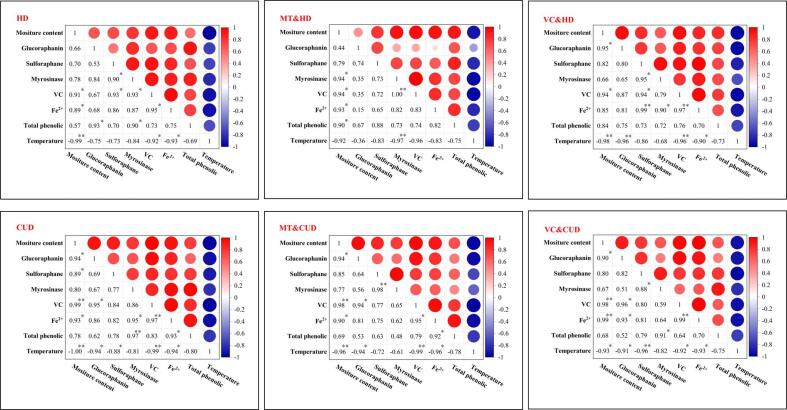
3.9. Correlation analysis
The Pearson correlation analysis explored the relationship between any two components (or factors) in glucoraphanin-related metabolic pathways under various drying treatments. According to Fig. 6, in all the drying treatments, temperature negatively influences the amounts of the indicated components, signifying that the increasing temperature is adverse to the preservation of glucoraphanin, sulforaphane, VC, Fe2+ and phenolics. In contrast, the correlation between any two components among glucoraphanin, sulforaphane, VC, Fe2+ and phenolics are always positive in all the treatments. However, these positive relationships differ with the drying treatments.
Fig. 6.
Pearson correlation coefficients among different components and factors involved in the metabolic pathway of glucoraphanin. * refers to the statistical significance at p ≤ 0.05, and ** refers to the statistical significance at p ≤ 0.01.
Under hot-air drying without pretreatment, significant positive correlations exist between sulforaphane-myrosinase (R = 0.90, p ≤ 0.05), sulforaphane-VC (R = 0.93, p ≤ 0.05), and VC-myrosinase (R = 0.93, p ≤ 0.05). These data confirm that the enhancement of myrosinase activity and VC content could facilitate the generation of sulforaphane resulting from the enzymatic degradation of glucoraphanin, and the existence of VC is beneficial to the myrosinase activity, which is in agreement with the illustrated metabolic pathway of glucosinolates shown in Fig. 1. Meanwhile, the pretreatments of VC immersion and melatonin immersion, contact ultrasound during drying, and their combinations weaken the connections of sulforaphane-myrosinase, sulforaphane-VC, and VC-myrosinase. For example, the correlations of sulforaphane-myrosinase and sulforaphane-VC for MT & HD treatment are insignificant. In CUD treatment, these correlations are all insignificant. It can thus be concluded that the role of VC and myrosinase to promote the formation of sulforaphane may be attenuated by the exerted drying pretreatment and ultrasound treatment.
In all the drying treatments excluding MT & HD, Fe2+ is significantly correlated with VC, owing to the ability of VC to reduce Fe3+ to Fe2+, as clarified in Fig. 1. However, none of the correlations about Fe2+-glucoraphanin and Fe2+-sulforaphane is negative, speculating that the existence of Fe2+ does not promote the formation of Fe2+-glucosinolate complex and nitriles. Besides, the correlation between Fe2+ and total phenolics is significantly positive in the treatments of CUD (R = 0.93, p ≤ 0.05) and MT & CUD (R = 0.92, p ≤ 0.05). In contrast, this relationship in all the other drying treatments is insignificant, deducing that contact sonication may intensify the redox effects of polyphenols to generate Fe2+ by reducing Fe3+.
Through the statistical exploration of the relationship among the metabolites and factors included in the metabolic pathway of glucosinolates, it can be tentatively concluded that the implemented drying pretreatments and contact ultrasound could affect the metabolism of glucoraphanin, particularly the enzymatic degradation of glucoraphanin and the generation of sulforaphane through weakening the connections between sulforaphane-myrosinase, sulforaphane-VC, and VC-myrosinase. However, there are still some deficiencies in this study. For example, the activities of specific proteins (i.e. ESP, TFP, ESM and NSP) and the contents of nitriles were not detected. The potential generation of glucoraphanin and degradation of sulforaphane in broccoli florets under drying were also not considered. To fully understand the metabolic pathways of glucosinolates under novel drying treatments, more chemical analysis is needed, which will be the focus of future studies.
4. Conclusion
This study applied some exogenous pretreatments before contact ultrasound-intensified air drying of broccoli florets. The results demonstrate that combining the exogenous pretreatment (melatonin immersion and VC immersion) and ultrasonication could complete the broccoli drying process faster than air drying alone and preserve sulforaphane and myrosinase in broccoli. Meanwhile, other components involved in the metabolic pathways of glucoraphanin, including phenolics, VC, and Fe2+, are also affected by the applied exogenous pretreatments and contact ultrasound. From the calculation of correlation coefficients among the metabolites in the metabolic pathways of glucoraphanin, it has been found that the correlations for sulforaphane-myrosinase, sulforaphane-VC, and VC-myrosinase are weakened by the VC and melatonin pretreatments and ultrasound, contributing to the formation of sulforaphane. Overall, this investigation guides the regulation of glucoraphanin metabolism and the preservation of bioactive sulforaphane under novel dehydration of cruciferous vegetables.
CRediT authorship contribution statement
Beini Liu: Investigation, Methodology, Software, Visualization, Writing – original draft, Formal analysis. Yang Tao: Conceptualization, Investigation, Validation, Data curation, Project Administration, Writing – review & editing, Supervision, Funding acquisition. Sivakumar Manickam: Writing – review & editing. Dandan Li: Writing – review & editing. Yongbin Han: Resources, Supervision, Writing – review & editing. Ying Yu: Methodology. Dongfeng Liu: Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank the National Natural Science Foundation of China (No. 32072351) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) to support this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2022.105977.
Contributor Information
Yang Tao, Email: yang.tao@njau.edu.cn.
Yongbin Han, Email: hanyongbin@njau.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Miao H.Y., Zeng W., Zhao M., Wang J.S., Wang Q.M. Effect of melatonin treatment on visual quality and health-promoting properties of broccoli florets under room temperature. Food Chem. 2020;319:126498. doi: 10.1016/j.foodchem.2020.126498. [DOI] [PubMed] [Google Scholar]
- 2.Thomas M., Badr A., Desjardins Y., Gosselin A., Angers P. Characterization of industrial broccoli discards (Brassica oleracea var. italica) for their glucosinolate, polyphenol and flavonoid contents using UPLC MS/MS and spectrophotometric methods. Food Chem. 2018;245:1204–1211. doi: 10.1016/j.foodchem.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 3.Aranaz P., Navarro-Herrera D., Romo-Hualde A., Zabala M., López-Yoldi M., González-Ferrero C., Gil A.G., Alfredo Martinez J., Vizmanos J.L., Milagro F.I., González-Navarro C.J. Broccoli extract improves high fat diet-induced obesity, hepatic steatosis and glucose intolerance in Wistar rats. J. Funct. Foods. 2019;59:319–328. [Google Scholar]
- 4.Luo F., Cai J.H., Zhang X., Tao D.B., Zhou X., Zhou Q., Zhao Y.B., Wei B.D., Cheng S.C., Ji S.J. Effects of methyl jasmonate and melatonin treatments on the sensory quality and bioactive compounds of harvested broccoli. RSC Adv. 2018;8(72):41422–41431. doi: 10.1039/c8ra07982j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun J., Wang Y.F., Pang X.Y., Tian S.H., Hu Q.B., Li X.F., Liu J., Wang J., Lu Y.J. The effect of processing and cooking on glucoraphanin and sulforaphane in brassica vegetables. Food Chem. 2021;360:130007. doi: 10.1016/j.foodchem.2021.130007. [DOI] [PubMed] [Google Scholar]
- 6.Bello C., Maldini M., Baima S., Scaccini C., Natella F. Glucoraphanin and sulforaphane evolution during juice preparation from broccoli sprouts. Food Chem. 2018;268:249–256. doi: 10.1016/j.foodchem.2018.06.089. [DOI] [PubMed] [Google Scholar]
- 7.Zhang S., Ying D.Y., Cheng L.J., Bayrak M., Jegasothy H., Sanguansri L., Augustin M.A. Sulforaphane in broccoli-based matrices: Effects of heat treatment and addition of oil. LWT-Food Sci. Technol. 2020;128:109443. [Google Scholar]
- 8.Gong W.J., Li D.D., Wu Y., Manickam S., Sun X., Han Y.B., Tao Y., Liu X.L. Sequential phenolic acid co-pigmentation pretreatment and contact ultrasound-assisted air drying to intensify blackberry drying and enhance anthocyanin retention: A study on mass transfer and phenolic distribution. Ultrason. Sonochem. 2021;80:105788. doi: 10.1016/j.ultsonch.2021.105788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schössler K., Jäger H., Knorr D. Effect of continuous and intermittent ultrasound on drying time and effective diffusivity during convective drying of apple and red bell pepper. J. Food Eng. 2012;108(1):103–110. [Google Scholar]
- 10.Tao Y., Li D.D., Chai W.S., Show P.L., Yang X.H., Manickam S., Xie G.J., Han Y.B. Comparison between airborne ultrasound and contact ultrasound to intensify air drying of blackberry: Heat and mass transfer simulation, energy consumption and quality evaluation. Ultrason. Sonochem. 2021;72:105410. doi: 10.1016/j.ultsonch.2020.105410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tao Y., Zhang J.L., Jiang S.R., Xu Y.Q., Show P.L., Han Y.B., Ye X.S., Ye M.R. Contacting ultrasound enhanced hot-air convective drying of garlic slices: Mass transfer modeling and quality evaluation. J. Food Eng. 2018;235:79–88. [Google Scholar]
- 12.Zhang M., Chen H.Z., Mujumdar A.S., Tang J., Miao S., Wang Y.C. Recent developments in high-quality drying of vegetables, fruits, and aquatic products. Crit. Rev. Food Sci. Nutr. 2017;57(6):1239–1255. doi: 10.1080/10408398.2014.979280. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira S.S., Monteiro F., Passos C.P., Silva A.M.S., Wessel D.F., Coimbra M.A., Cardoso S.M. Blanching impact on pigments, glucosinolates, and phenolics of dehydrated broccoli by-products. Food Res. Int. 2020;132:109055. doi: 10.1016/j.foodres.2020.109055. [DOI] [PubMed] [Google Scholar]
- 14.Castillejo N., Martínez-Zamora L., Gómez P.A., Pennisi G., Crepaldi A., Fernández J.A., Orsini F., Artés-Hernández F. Postharvest yellow LED lighting affects phenolics and glucosinolates biosynthesis in broccoli sprouts. J. Food Compos. Anal. 2021;103:104101. doi: 10.1002/jsfa.10820. [DOI] [PubMed] [Google Scholar]
- 15.Hanschen F.S., Lamy E., Schreiner M., Rohn S. Reactivity and stability of glucosinolates and their breakdown products in foods. Angew. Chem. 2014;53(43):11430–11450. doi: 10.1002/anie.201402639. [DOI] [PubMed] [Google Scholar]
- 16.Lekcharoenkul P., Tanongkankit Y., Chiewchan N., Devahastin S. Enhancement of sulforaphane content in cabbage outer leaves using hybrid drying technique and stepwise change of drying temperature. J. Food Eng. 2014;122:56–61. [Google Scholar]
- 17.Cao Y., Tao Y., Zhu X.H., Han Y.B., Li D.D., Liu C.Q., Liao X.J., Show P.L. Effect of microwave and air-borne ultrasound-assisted air drying on drying kinetics and phytochemical properties of broccoli floret. Drying Technol. 2020;38(13):1733–1748. [Google Scholar]
- 18.Zheng H.H., Liu W., Liu S., Liu C.C., Zheng L. Effects of melatonin treatment on the enzymatic browning and nutritional quality of fresh-cut pear fruit. Food Chem. 2019;299:125116. doi: 10.1016/j.foodchem.2019.125116. [DOI] [PubMed] [Google Scholar]
- 19.Hasan M.K., Liu C.X., Pan Y.T., Ahammed G.J., Qi Z.Y., Zhou J. Melatonin alleviates low-sulfur stress by promoting sulfur homeostasis in tomato plants. Sci. Rep. 2018;8:10182. doi: 10.1038/s41598-018-28561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan X.L., Zhao Y.T., Shan W., Kuang J.F., Lu W.J., Su X.G., Tao N.G., Lakshmanan P., Chen J.Y. Melatonin delays leaf senescence of postharvest Chinese flowering cabbage through ROS homeostasis. Food Res. Int. 2020;138:109790. doi: 10.1016/j.foodres.2020.109790. [DOI] [PubMed] [Google Scholar]
- 21.Wei L.Y., Liu C.H., Zheng H.H., Zheng L. Melatonin treatment affects the glucoraphanin-sulforaphane system in postharvest fresh-cut broccoli (Brassica oleracea L.) Food Chem. 2020;307:125562. doi: 10.1016/j.foodchem.2019.125562. [DOI] [PubMed] [Google Scholar]
- 22.Ze Y., Gao H.J., Li T.T., Yang B., Jiang Y.M. Insights into the roles of melatonin in maintaining quality and extending shelf life of postharvest fruits. Trends Food Sci. Technol. 2021;109:569–578. [Google Scholar]
- 23.Zhao C.X., Nawaz G., Cao Q.H., Xu T. Melatonin is a potential target for improving horticultural crop resistance to abiotic stress. Sci. Hortic. 2022;291:110560. [Google Scholar]
- 24.Gao S.W., Ma W.Y., Lyu X.N., Cao X.L., Yao Y.X. Melatonin may increase disease resistance and flavonoid biosynthesis through effects on DNA methylation and gene expression in grape berries. BMC Plant Biol. 2020;20:231. doi: 10.1186/s12870-020-02445-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saleem M.S., Anjum M.A., Naz S., Ali S., Hussain S., Azam M., Sardar H., Khaliq G., Canan I., Ejaz S. Incorporation of ascorbic acid in chitosan-based edible coating improves postharvest quality and storability of strawberry fruits. Int. J. Biol. Macromol. 2021;189:160–169. doi: 10.1016/j.ijbiomac.2021.08.051. [DOI] [PubMed] [Google Scholar]
- 26.Burow M., Wittstock U. Regulation and function of specifier proteins in plants. Phytochem. Rev. 2009;8(1):87–99. [Google Scholar]
- 27.Angelino D., Jeffery E. Glucosinolate hydrolysis and bioavailability of resulting isothiocyanates: Focus on glucoraphanin. J. Funct. Foods. 2014;7:67–76. [Google Scholar]
- 28.Bellostas N., Sørensen A.D., Sørensen J.C., Sørensen H. Fe2+-Catalyzed Formation of Nitriles and Thionamides from Intact Glucosinolates. J. Nat. Prod. 2008;71(1):76–80. doi: 10.1021/np070438d. [DOI] [PubMed] [Google Scholar]
- 29.Hanschen F.S., Klopsch R., Oliviero T., Schreiner M., Verkerk R., Dekker M. Optimising isothiocyanate formation during enzymatic glucosinolate breakdown by adjusting pH value, temperature and dilution in Brassica vegetables and Arabidopsis thaliana. Sci. Rep. 2017;7:40807. doi: 10.1038/srep40807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hennig K., de Vos R.C., Maliepaard C., Dekker M., Verkerk R., Bonnema G. A metabolomics approach to identify factors influencing glucosinolate thermal degradation rates in Brassica vegetables. Food Chem. 2014;155:287–297. doi: 10.1016/j.foodchem.2014.01.062. [DOI] [PubMed] [Google Scholar]
- 31.MacLean DavidB. The association of official analytical chemists - its international activities. Trends Anal. Chem. 1982;1(11):V–VII. [Google Scholar]
- 32.Pang L.L., Wu Y., Pan Y.F., Ban Z.J., Li L., Li X.H. Insights into exogenous melatonin associated with phenylalanine metabolism in postharvest strawberry. Postharvest Biol. Technol. 2020;168:111244. [Google Scholar]
- 33.Kong X.M., Ge W.Y., Wei B.D., Zhou Q., Zhou X., Zhao Y.B., Ji S.J. Melatonin ameliorates chilling injury in green bell peppers during storage by regulating membrane lipid metabolism and antioxidant capacity. Postharvest Biol. Technol. 2020;170:111315. [Google Scholar]
- 34.Wu C.H., Cao S.F., Xie K.Q., Chi Z.Y., Wang J., Wang H.F., Wei Y.Y., Shao X.F., Zhang C.D., Xu F., Gao H.Y. Melatonin delays yellowing of broccoli during storage by regulating chlorophyll catabolism and maintaining chloroplast ultrastructure. Postharvest Biol. Technol. 2021;172:111378. [Google Scholar]
- 35.Mason T.J., Lorimer J.P., Bates D.M. Quantifying sonochemistry: Casting some light on a ‘black art’. Ultrasonics. 1992;30:40–42. [Google Scholar]
- 36.Baenas N., Villano D., Garcia-Viguera C., Moreno D.A. Optimising elicitation and seed priming to enrich broccoli and radish sprouts in glucosinolates. Food Chem. 2016;204:314–319. doi: 10.1016/j.foodchem.2016.02.144. [DOI] [PubMed] [Google Scholar]
- 37.Tao Y., Wang Y.L., Pan M.S., Zhong S.R., Wu Y., Yang R.Q., Han Y.B., Zhou J.Z. Combined ANFIS and numerical methods to simulate ultrasound-assisted extraction of phenolics from chokeberry cultivated in China and analysis of phenolic composition. Sep. Purif. Technol. 2017;178:178–188. [Google Scholar]
- 38.Guo L.P., Yang R.Q., Wang Z.Y., Guo Q.H., Gu Z.X. Glucoraphanin, sulforaphane and myrosinase activity in germinating broccoli sprouts as affected by growth temperature and plant organs. J. Funct. Foods. 2014;9:70–77. [Google Scholar]
- 39.Li S.J., Tao Y., Li D.D., Wen G.Z., Zhou J.Z., Manickam S., Han Y.B., Chai W.S. Fermentation of blueberry juices using autochthonous lactic acid bacteria isolated from fruit environment: Fermentation characteristics and evolution of phenolic profiles. Chemosphere. 2021;276:130090. doi: 10.1016/j.chemosphere.2021.130090. [DOI] [PubMed] [Google Scholar]
- 40.Xu F.F., Jin X., Zhang L., Chen X.D. Investigation on water status and distribution in broccoli and the effects of drying on water status using NMR and MRI methods. Food Res. Int. 2017;96:191–197. doi: 10.1016/j.foodres.2017.03.041. [DOI] [PubMed] [Google Scholar]
- 41.Tao Y., Sun D.W., Górecki A., Błaszczak W., Lamparski G., Amarowicz R., Fornal J., Jeliński T. Effects of high hydrostatic pressure processing on the physicochemical and sensorial properties of a red wine. Innov. Food Sci. Emerg. Technol. 2012;16:409–416. [Google Scholar]
- 42.Tao Y., Han M.F., Gao X.G., Han Y.B., Show P.L., Liu C.Q., Ye X.S., Xie G.J. Applications of water blanching, surface contacting ultrasound-assisted air drying, and their combination for dehydration of white cabbage: Drying mechanism, bioactive profile, color and rehydration property. Ultrason. Sonochem. 2019;53:192–201. doi: 10.1016/j.ultsonch.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Liu Z.L., Bai J.W., Yang W.X., Wang J., Deng L.Z., Yu X.L., Zheng Z.A., Gao Z.J., Xiao H.W. Effect of high-humidity hot air impingement blanching (HHAIB) and drying parameters on drying characteristics and quality of broccoli florets. Drying Technol. 2019;37(10):1251–1264. [Google Scholar]
- 44.Tang Q., Li C.Y., Ge Y.H., Li X., Cheng Y., Hou J.B., Li J.R. Exogenous application of melatonin maintains storage quality of jujubes by enhancing anti-oxidative ability and suppressing the activity of cell wall-degrading enzymes. LWT-Food Sci. Technol. 2020;127:109431. [Google Scholar]
- 45.Malik M.A., Sharma H.K., Saini C.S. High intensity ultrasound treatment of protein isolate extracted from dephenolized sunflower meal: Effect on physicochemical and functional properties. Ultrason. Sonochem. 2017;39:511–519. doi: 10.1016/j.ultsonch.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 46.Hou F.R., Fan L.H., Ma X.B., Wang D.L., Wang W.J., Ding T., Ye X.Q., Liu D.H. Degradation of carboxymethylcellulose using ultrasound and beta-glucanase: Pathways, kinetics and hydrolysates' properties. Carbohydr. Polym. 2018;201:514–521. doi: 10.1016/j.carbpol.2018.07.092. [DOI] [PubMed] [Google Scholar]
- 47.Mahn A., Martin C., Reyes A., Saavedra A. Evolution of sulforaphane content in sulforaphane-enriched broccoli during tray drying. J. Food Eng. 2016;186:27–33. [Google Scholar]
- 48.Cai Y.X., Augustin M.A., Jegasothy H., Wang J.H., Terefe N.S. Mild heat combined with lactic acid fermentation: a novel approach for enhancing sulforaphane yield in broccoli puree. Food Funct. 2020;11:779–786. doi: 10.1039/c9fo02089f. [DOI] [PubMed] [Google Scholar]
- 49.Cai Y.X., Wang J.H., McAuley C., Augustin M.A., Terefe N.S. Fermentation for enhancing the bioconversion of glucoraphanin into sulforaphane and improve the functional attributes of broccoli puree. J. Funct. Foods. 2019;61:103461. [Google Scholar]
- 50.Lee J.G., Lim S., Kim J., Lee E.J. The mechanism of deterioration of the glucosinolate-myrosynase system in radish roots during cold storage after harvest. Food Chem. 2017;233:60–68. doi: 10.1016/j.foodchem.2017.04.104. [DOI] [PubMed] [Google Scholar]
- 51.Tian G.F., Li Y., Cheng L., Yuan Q.P., Tang P., Kuang P.Q., Hu J. The mechanism of sulforaphene degradation to different water contents. Food Chem. 2016;194:1022–1027. doi: 10.1016/j.foodchem.2015.08.107. [DOI] [PubMed] [Google Scholar]
- 52.Ghawi S.K., Methven L., Rastall R.A., Niranjan K. Thermal and high hydrostatic pressure inactivation of myrosinase from green cabbage: A kinetic study. Food Chem. 2012;131:1240–1247. [Google Scholar]
- 53.Wang J., Barba F.J., Sørensen J.C., Frandsen H.B., Sørensen S., Olsen K., Orlien V. The role of water in the impact of high pressure on the myrosinase activity and glucosinolate content in seedlings from Brussels sprouts. Innov. Food Sci. Emerging Technol. 2019;58:102208. [Google Scholar]
- 54.Koh E., Wimalasiri K.M.S., Chassy A.W., Mitchell A.E. Content of ascorbic acid, quercetin, kaempferol and total phenolics in commercial broccoli. J. Food Compos. Anal. 2009;22(7-8):637–643. [Google Scholar]
- 55.Frias J., Peñas E., Ullate M., Vidal-Valverde C. Influence of drying by convective air dryer or power ultrasound on the vitamin C and β-carotene content of carrots. J. Agric. Food Chem. 2010;58(19):10539–10544. doi: 10.1021/jf102797y. [DOI] [PubMed] [Google Scholar]
- 56.Yuan J.P., Chen F. Degradation of Ascorbic Acid in Aqueous Solution. J. Agric. Food Chem. 1998;46:5078–5082. [Google Scholar]
- 57.Gamboa-Santos J., Montilla A., Soria A.C., Carcel J.A., Garcia-Perez J.V., Villamiel M. Impact of power ultrasound on chemical and physicochemical quality indicators of strawberries dried by convection. Food Chem. 2014;161:40–46. doi: 10.1016/j.foodchem.2014.03.106. [DOI] [PubMed] [Google Scholar]
- 58.Wang S.Y., Shi X.C., Wang R., Wang H.L., Liu F., Laborda P. Melatonin in fruit production and postharvest preservation: A review. Food Chem. 2020;320:126642. doi: 10.1016/j.foodchem.2020.126642. [DOI] [PubMed] [Google Scholar]
- 59.Rastegar S., Khankahdani H.H., Rahimzadeh M. Effects of melatonin treatment on the biochemical changes and antioxidant enzyme activity of mango fruit during storage. Sci. Hortic. 2020;259:108835. [Google Scholar]
- 60.Rajewska K., Mierzwa D. Influence of ultrasound on the microstructure of plant tissue. Innov. Food Sci. Emerg. Technol. 2017;43:117–129. [Google Scholar]
- 61.Sun Q.Q., Zhang N., Wang J.F., Cao Y.Y., Li X.S., Zhang H.J., Zhang L., Tan D.X., Guo Y.D. A label-free differential proteomics analysis reveals the effect of melatonin on promoting fruit ripening and anthocyanin accumulation upon postharvest in tomato. J. Pineal Res. 2016;61(2):138–153. doi: 10.1111/jpi.12315. [DOI] [PubMed] [Google Scholar]
- 62.Lo’ay A.A., EL-Khateeb A.Y. Antioxidant enzyme activities and exogenous ascorbic acid treatment of ‘Williams’ banana during long-term cold storage stress. Sci. Hortic. 2018;234:210–219. [Google Scholar]
- 63.Guo C.T., Bi J.F., Li X., Lyu J., Xu Y., Hu J.X. Investigation on the phenolic composition, related oxidation and antioxidant activity of thinned peach dried by different methods. LWT-Food Sci. Technol. 2021;147:111573. [Google Scholar]
- 64.Jaiswal V., DerMarderosian A., Porter J.R. Anthocyanins and polyphenol oxidase from dried arils of pomegranate (Punica granatum L.) Food Chem. 2010;118(1):11–16. [Google Scholar]
- 65.Zhou L.Y., Cao Z.Z., Bi J.F, Yi J.Y., Chen Q.Q., Wu X.Y., Zhou M. Degradation kinetics of total phenolic compounds, capsaicinoids and antioxidant activity in red pepper during hot air and infrared drying process. Int. J. Food Sci. Technol. 2016;51(4):842–853. [Google Scholar]
- 66.Vidinamo F., Fawzia S., Karim M.A. Effect of drying methods and storage with agro-ecological conditions on phytochemicals and antioxidant activity of fruits: a review. Crit. Rev. Food Sci. Nutr. 2022;62(2):353–361. doi: 10.1080/10408398.2020.1816891. [DOI] [PubMed] [Google Scholar]
- 67.Que F., Mao L.C., Fang X.H., Wu T. Comparison of hot air-drying and freeze-drying on the physicochemical properties and antioxidant activities of pumpkin (Cucurbita moschata Duch.) flours. Int. J. Food Sci. Technol. 2008;43(7):1195–1201. [Google Scholar]
- 68.Mello R.E., Fontana A., Mulet A., Corrêa J.L.G., Cárcel J.A. PEF as pretreatment to ultrasound-assisted convective drying: Influence on quality parameters of orange peel. Innov. Food Sci. Emerg. Technol. 2021;72:102753. [Google Scholar]
- 69.do Nascimento E.M.G.C., Mulet A., Ascheri J.L.R., de Carvalho C.W.P., Cárcel J.A. Effects of high-intensity ultrasound on drying kinetics and antioxidant properties of passion fruit peel. J. Food Eng. 2016;170:108–118. [Google Scholar]
- 70.Schössler K., Thomas T., Knorr D. Modification of cell structure and mass transfer in potato tissue by contact ultrasound. Food Res. Int. 2012;49(1):425–431. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.