Abstract
Mitochondrial DNA (mtDNA) has been identified as a significant genetic biomarker in disease, cancer and evolution. Mitochondria function as modulators for regulating cellular metabolism. In the clinic, mtDNA variations (mutations/single nucleotide polymorphisms) and dysregulation of mitochondria-encoded genes are associated with survival outcomes among cancer patients. On the other hand, nuclear-encoded genes have been found to regulate mitochondria-encoded gene expression, in turn regulating mitochondrial homeostasis. These observations suggest that the crosstalk between the nuclear genome and mitochondrial genome is important for cellular function. Therefore, this review summarizes the significant mechanisms and functional roles of mtDNA variations (DNA level) and mtDNA-encoded genes (RNA and protein levels) in cancers and discusses new mechanisms of crosstalk between mtDNA and the nuclear genome.
Keywords: mitochondria, SNP, mutation, ncRNA, prognostic marker, cancer
1. Introduction
The sequence of the mitochondrial genome was first identified in 1981 [1]. Mitochondria contain distinct cell membranes and their own genome (circular DNA), which encode 2 rRNAs, 22 tRNAs and 13 mitochondrial protein subunits [2]. Mitochondria function as modulators for regulating cellular metabolism, including the tricarboxylic acid (TCA) cycle, oxidative phosphorylation (OXPHOS), fatty acid metabolism, amino acid metabolism and nucleotide metabolism [3,4]. Adenosine triphosphate (ATP) is the major energy source for maintaining cellular function. ATP is produced by glycolysis and OXPHOS. ATP is quickly generated by glycolysis and supports cancer cell survival, drug resistance and tumor metastasis [5]. In contrast, OXPHOS can produce 36 ATPs per glucose. Due to high glycolysis utilization in cancer cells, OXPHOS is downregulated in several cancer types [6], and several studies have found that OXPHOS is also upregulated in some cancers [6]. These findings suggest that OXPHOS plays dual roles in cancer progression. An imbalance in mitochondrial homeostasis causes excessive reactive oxygen species (ROS) production, which leads to DNA damage, apoptosis, aging and cancer progression [7,8]. To prevent excessive ROS, cells maintain homeostasis through the activation of the antioxidant reaction. Manganese superoxide dismutase (MnSOD) acts as the scavenger to eliminate ROS produced from mitochondria [9]. In the current central dogma of biology, it is well-known that DNA contains the important genetic information for making RNA (transcription), and then, RNA makes proteins (translation). Subsequently, proteins are responsible for regulating multiple cellular functions. In addition, a kind of gene, referred to as a noncoding gene, can be transcribed to RNA, but it cannot translate to a protein. Increasing evidence supports that noncoding RNA (ncRNA) also play an important role in modulating cell growth, metastasis, cell metabolism and mitochondria homeostasis [10]. Functional and clinical studies have reported that mitochondrial DNA (mtDNA) mutations, mtDNA single-nucleotide polymorphisms (SNPs), mtDNA-encoded microRNAs (mitomiRs), mitochondria-derived long noncoding RNAs (lncRNAs) and mitochondrial proteins are involved in cancer progression (Figure 1). Furthermore, nuclear-encoded genes, including transcription factors and ncRNAs, regulate mitochondria-related gene expression and homeostasis. Alterations in nuclear DNA-encoded genes lead to an imbalance in mitochondrial homeostasis, suggesting that crosstalk between the nuclear and mitochondrial genomes is important in cancer biology. Taken together, these findings suggest that mitochondria-related molecule (DNA, RNA and protein) is crucial for several important physiological homeostasis and cancer progression. Therefore, this review summarizes the functions and clinical relevance of the mitochondrial DNA (DNA level), mitochondria-encoded ncRNA (RNA level) and proteins (protein level) in cancer progression and discusses new mechanisms of crosstalk between mtDNA and the nuclear genome.
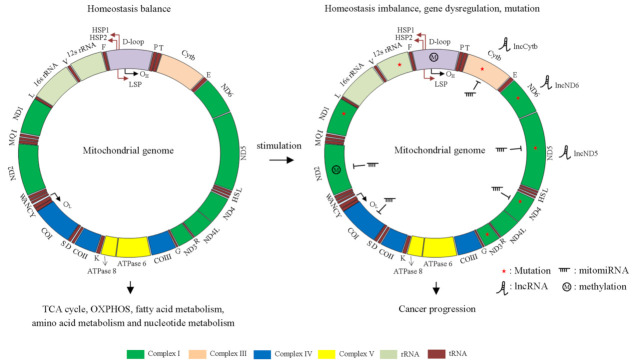
Figure 1.
Overview of the human mitochondrial genome, including protein-coding genes, noncoding RNAs and control regions. The mtDNA is double-stranded and circular, with approximately 16,569 base pairs, which encode 2 rRNAs, 22 tRNAs and 13 mitochondrial protein subunits. The rRNA genes are in teal. Complex I genes are in green. Complex III genes are in peach buff. Complex IV genes are in blue. Complex V genes are in yellow. The regulatory region, D-loop, is shown in amethyst. Two promoters in mtDNA, heavy strand promoters (HSP) and light strand promoters (LSP), are shown. Left panel: Normal mitochondria functions as regulators for maintaining cellular homeostasis, such as the TCA cycle, OXPHOS and fatty acid metabolism. Right panel: Mitochondrial DNA mutations (red star), mitochondrial genes dysfunction and methylation lead to homeostasis imbalance and cancer progression. F: tRNA Phe, V: tRNA Val, L: tRNA Leu, I: tRNA Ile, Q: tRNA Gln, M: tRNA Met, W: tRNA Trp, A: tRNA Ala, N: tRNA Asn, C: tRNA Cys, Y: tRNA Tyr, S: tRNA Ser, D: tRNA Asp, K: tRNA Lys, G: tRNA Gly, R: tRNA Arg, H: tRNA His, S: tRNA Ser, L: tRNA Leu, E: tRNA Glu, T: tRNA Thr and P: tRNA Pro.
2. The Genetic Information of mtDNA and Nuclear DNA on Cancer Progression
Increasing evidence indicates that DNA mutation, SNP or gene expression act as a useful prognostic marker in cancer formation [11,12,13]. Here, we summarize the mtDNA/nuclear DNA variations and mitochondrial/nuclear gene expression on cancer progression.
2.1. The Role of mtDNA (SNP/Mutation) in Cancer
In fact, tumor cells can reprogram bioenergetic signal transduction, such as glucose metabolism and ROS production, to support survival [14,15]. On the other hand, mtDNA variations have been confirmed to be correlated with cancer progression. Jin et al. [16] investigated the association between mitochondrial ND3 SNPs and gastric cancer development. Three SNPs (rs28358278, rs2853826 and rs41467651) of ND3 were significantly correlated with the risk of gastric cancer. mtDNA mutations in the ND1 gene (G3842A), ND4 gene (A11708G) and ND5 gene (12418insA) were found to be correlated with hepatocellular carcinoma (HCC) progression [17]. In addition, Akouchekian and co-workers demonstrated that MT-ND1 gene mutation (T4216C) was associated with colorectal cancer (CRC) progression [18]. Previously, MT-ND2 mutation (G4776A) enhanced the cell growth of head and neck cancer cell via the induction of HIF1α [19]. Furthermore, this mutation enhanced pyruvate dehydrogenase kinase 2 expression through the induction of ROS production. Another report demonstrated that the impairment of mitochondrial complex I activity caused by mtDNA mutation was associated with the cisplatin-resistant phenotype. Based on a structural analysis, MT-ND2 mutation (T4587C) may play an important effector for promoting drug resistance [20]. Ishikawa and co-workers demonstrated that the MT-ND6 insertion mutation (13885insC) suppressed complex I activity and induced ROS production, resulting in the induction of cell metastasis in lung and breast cancer cells [21,22]. Another mutation in MT-ND6 (C12084T) was identified and correlated with metastatic capacity in a breast cancer cell line [23]. A previous study indicated that SNPs in MT-ND1 (T3394C and C3497T) were correlated with distant metastasis [24]. Accordingly, OXPHOS-related genes are important in regulating cancer cell metastasis. Kim et al. demonstrated that SNPs in uncoupling protein 2 (UCP2) and UCP3 genes are associated with mitochondria-related metabolism and healthy aging in females [25]. These findings suggest that significant variations in the corresponding genes may interfere with their own gene expression, subsequently disrupting cellular homeostasis and contributing to cancer formation. Shidara et al. demonstrated that ectopic expression of ATP6 mutation (T8993G or T9176C) induced tumor growth through inhibition of apoptosis [26]. A previous meta-analysis found that mutations in mitochondrial tRNA genes have also been linked to cancer progression. The study indicated that five tRNA mutations (mt-tRNAAla, mt-tRNAArg, mt-tRNALeu, mt-tRNASer and mt-tRNAThr) were rarely found in healthy specimens [27]. These mutations may affect tRNA metabolism in lung cancer. In addition, Meng et al. [28] reported that tRNA mutations (mt-tRNAASP) caused their tertiary structure and led to impairment of mitochondrial protein synthesis in breast cancer. Two noncoding genes (MT-RNR1 and MT-RNR2) are encoded by mitochondrial DNA and responsible for mitochondrial protein synthesis. Notably, SNPs in those noncoding regions were found to act as prognostic markers. Our group demonstrated that MT-RNR1 was significantly correlated with the survival outcomes of patients with HCC [29]. In addition, the expression levels of the glycolytic regulator hexokinase 2 (HK2) were higher in the MT-RNR1 709A group than in the MT-RNR1 709G group. Notably, the combined effects of MT-RNR1 709A and HK2 on survival outcome in HCC patients were poor. Taken together, we found that MT-RNR1 709A acted as an independent risk factor for overall survival and metastasis-free survival. Another study indicated that MT-RNR1 mutations (652G insertion and 716G) were identified only in gastric cancerous tissues, especially in intestinal type gastric cancer [30], suggesting MT-RNR1 mutations were correlated with incidence of intestinal type gastric cancer. Therefore, the mtDNA SNP/mutation may cause nonsense, missense or frameshift mutation, leading to loss of protein function or alteration of RNA structure and then modulated cancer progression.
2.2. The Association between Mitochondrial-Related Noncoding RNA and Cancer Progression
Recently, ncRNAs have been identified by next-generation sequencing (NGS) and act as crucial regulators of cancer progression. Depending on the length of ncRNAs, ncRNAs can be divided into two categories. One is small ncRNAs. The other is long ncRNAs. In fact, several different small ncRNAs have been identified. Of these, microRNAs (miRNAs) are involved in regulating cellular function via modulation of their target gene expression [31]. Notably, mtDNA-encoded miRNAs have been described. The term mitomiRs indicates that these miRNAs are mainly expressed in mitochondria [3]. Previous evidence reported that mitomiRs targeted nuclear- or mitochondrial-encoded mRNA and suppressed target gene expression, leading to the regulation of mitochondrial homeostasis (Figure 2A). The mRNAs encoded by the mitochondrial genome without or with short 3′UTRs may cause miRNA-RISC to not directly interact with target genes. Accordingly, the regulatory mechanism of mitomiR and its target gene remain unclear. Overexpression of mitomiR-2392 has been shown to induce cisplatin resistance through modulation of mitochondrial complex activity [32]. The results obtained in vitro were similar to those in vivo. Furthermore, several mitochondrial genes, such as ND2, ND4, ND5, CYTB and COX1, were regulated by mitomiR-2392 in an AGO2-dependent manner. In the clinic, mitomiR-2392 was negatively correlated with chemosensitivity and overall survival. The same group provided additional evidence to support that mitomiR is involved in cellular functions [33]. They found that mitomiR-5787 expression levels were increased in cisplatin-resistant tongue squamous cell carcinoma (TSCC) cells. Knockdown of mitomiR-5787 repressed MT-CO3 translation. Functionally, mitomiR-5787 regulates cisplatin resistance, glucose consumption and OXPHOS in TSCC cells. Giuliani et al. [34] demonstrated that mitomiR-34a, -181a and 146a were highly expressed in replicative senescent human umbilical vein endothelial cells (HUVECs) compared to younger HUVECs. In senescent HUVECs, these mitomiRs regulated mitochondrial function and autophagy through modulation of Bcl-2. Taken together, these mitomiRs are mainly expressed in mitochondria and responsible for regulating cell growth, drug resistance and apoptosis.
Figure 2.
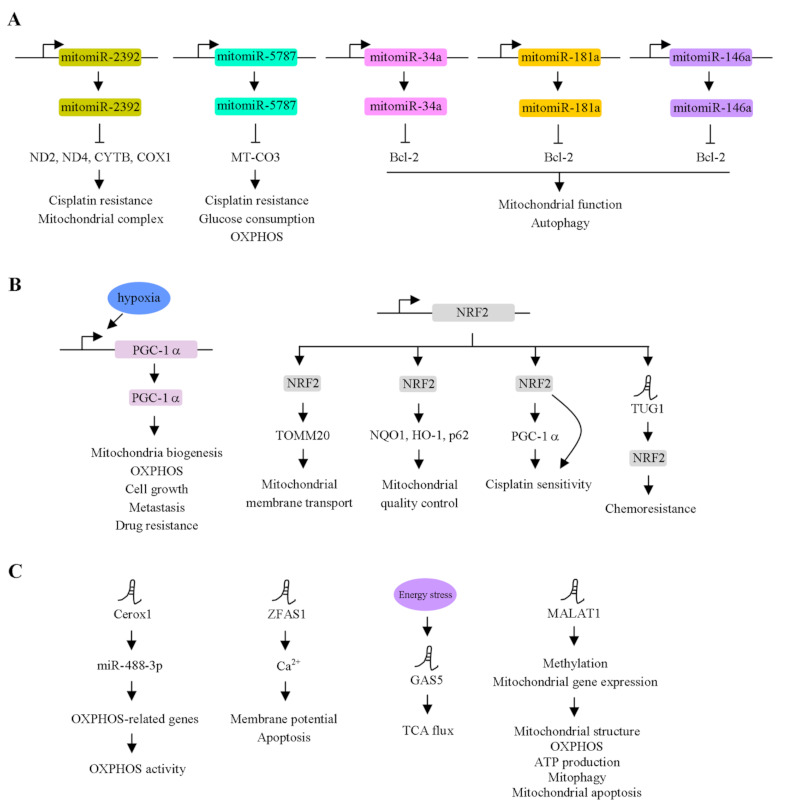
Functional roles of mtDNA-encoded miRNAs and nuclear DNA-encoded genes in cancer progression. (A) mtDNA-encoded miRNAs, including mitomiR-2392, mitomiR-5787, mitomiR-34a, mitomiR-181a and mitomiR-146a, inhibited the corresponding target genes and contributed to modulate drug resistance, mitochondrial functions, OXPHOS and autophagy. (B) Nuclear DNA-encoded protein coding genes, PGC-1α and NRF2, regulated the mitochondrial functions, cell growth, metastasis and drug resistance via modulation different downstream effectors. (C) Nuclear DNA-encoded lncRNAs regulate the mitochondria-related functions via modulation of downstream gene expression.
LncRNAs can associate with proteins and form a complex to regulate target gene expression by different mechanisms, such as miRNA sponges, recruitment of chromatin modifiers and regulation of mRNA splicing, stability and translation [35]. Emerging evidence has shown that lncRNAs can be encoded from the mitochondrial genome. Rackham and colleagues demonstrated that mitochondrial long ncRNAs, including lncND5, lncND6 and lncCyt b RNA, were identified by deep sequencing [36]. Specifically, lncND5 RNA levels were highly abundant compared to lncND6 and lncCyt b RNA levels. The three lncRNAs were regulated by nuclear-encoded proteins [37]. Another report indicated that the lncND5 transcript was positively regulated by pentatricopeptide repeat domain (PTCD) 1 [38]. On the other hand, depletion of PTCD2 repressed lncND5 and lncND6 expression [38]. In addition, a nuclear DNA-encoded protein, mitochondrial RNase P protein 3, localized in mitochondria regulated lncND5, lncND6 and lncCyt b RNA abundance [37]. A lncRNA named long intergenic noncoding RNA predicting cardiac remodeling (LIPCAR) is a chimeric fusion transcript between the 5′-end of COX2 and the 3′ end of CYTB [39,40]. Furthermore, LIPCAR served as a novel biomarker for predicting survival outcomes in patients with heart failure. Therefore, lncRNAs encoded from mitochondria genome is involved in regulating cellular homeostasis and cancer progression.
2.3. Dysregulation of Mitochondrial-Encoded Protein-Coding Genes in Cancer Progression
As mentioned above, several genes are encoded by mitochondria genome. In this section, we summarize the association between mitochondrial-related proteins and cancer progression. A previous study showed that MT-ND2 expression was increased in colorectal cancer samples compared to corresponding noncancerous tissues [41]. MT-ND2 expression in Caco-2 cells was upregulated by treatment with a demethylation drug, 5-aza-2′deoxycytidine (5-Aza). Notably, methylation status in the D-loop region was found to be associated with MT-ND2 expression. Liu et al. demonstrated that highly methylated D-loop region of mtDNA was observed in bone metastatic tumor cells from renal cell carcinoma (RCC) compared to those in the primary RCC tissues [42]. In addition, mRNA levels of MT-ND2, MT-ND3, MT-ND4L, MT-ND6, ATP6, ATP8, COI and COII were significantly downregulated in bone metastatic tumor cells derived from RCC compared to those in parental RCC cells. Notably, 5-Aza treatment contributed to repression of tumor metastasis. These observations suggested that mitochondrial gene expression was controlled by epigenetic regulation. Li and co-workers demonstrated that MT-ND5 and MT-ND6 expression was inversely associated with tumor stage in lung squamous cell carcinoma (LUSC) and lung adenocarcinoma (LUAD) [43]. Furthermore, in the Cox regression analysis, a lower expression of these two genes was significantly associated with shorter survival outcomes. A group demonstrated that the overexpression of cytochrome B (Cytb) mutation (mtCytb) in MB49 bladder cancer cell lines promoted ROS production, oxygen utilization and lactate production, which led to tumor growth, metastasis and angiogenesis induction in vitro and in vivo [44]. Mechanistically, mtCytb stimulated the nuclear factor-κB2 (NF-κB2) signaling pathway. These findings supported that mitochondrial-encoded protein mutations (i.e., Cytb mutation) played an oncogenic role in a bladder cancer cell line. Notably, OXPHOS was regulated by mitochondrial ATP synthase [45]. Direct or indirect inhibition of ATP synthase by inhibitors resulted in the repression of cell growth in multiple cancer cell lines [46]. Compared with adjacent normal tissues, the expression levels of MT-COI, MT-CYB, MT-ND1 and MT-RNR1 in CRC adenomas and adenocarcinomas were progressively increased using a quantitative real-time PCR analysis [47]. Therefore, the dysregulation of mitochondria-encoded genes contributes to cancer progression.
3. Nuclear DNA-Encoded Genes Function as Modulators to Coordinate Mitochondrial Gene Expression and Mitochondrial Functions
Accumulating evidence indicates that nuclear DNA-encoded genes serve as a component of OXPHOS or leads to regulating mitochondria homeostasis via the modulation of mitochondria-related gene expression [48]. These findings suggest that both of them are a key effector for maintaining cellular functions. Accordingly, we summarize how nuclear gene-encoded transcription factors crosstalk with mitochondrial gene expression (Figure 2B,C).
3.1. Action of Peroxisome Proliferator-Activated Receptor-γ Coactivatior-1α (PGC-1α) in Mitochondria
PGC-1α functions as a transcription factor for modulating mitochondrial biogenesis, gluconeogenesis and OXPHOS [49,50,51]. LeBleu et al. demonstrated that the transcription coactivator PGC-1α is involved in mitochondrial biogenesis and OXPHOS in an invasive cell line [52]. The expression level of the α subunit of ATP synthase was upregulated by PGC-1α in 4T1 cells. In contrast, the α subunit of ATP synthase was downregulated in prostate cancer and correlated with earlier-onset prostate cancer [53]. On the other hand, the β subunit of ATP synthase was expressed at lower levels in hepatoma, colon cancer and LUAD [54,55]. Another report showed that hypermethylation in the promoter of the ATP5B gene was found, thereby silencing its gene expression [56]. A previous study indicated that hypoxia induced cell growth, metastasis and mitochondrial function by the promotion of PGC-1α expression in CRC cell lines [57]. Moreover, the overexpression of PGC-1α in hypoxia abolished the 5-fluorouracil-induced CRC cell line by apoptosis. These findings suggest that nuclear gene-encoded PGC-1α is responsible for regulating mitochondrial gene expression and cell migration.
3.2. Action of NF-E2-Related Factor 2 (NRF2) in Mitochondria
Similar to PGC-1α, the nuclear DNA-encoded gene NF-E2-related factor 2 (NRF2) is an important transcription factor for regulating nuclear and mitochondrial gene expression, which are involved in the electron transfer chain (ETC) reaction [58,59,60]. Under normal physiological conditions, NRF2 is regulated by the proteasome degradation pathway via the Kelch-like ECH-associated protein 1 (Keap1)-Cul3 E3 ligase. Keap1 is oxidized by ROS, resulting in the promotion of NRF2 expression [61]. Subsequently, NRF2 can translocate into the nucleus and associate with the Maf protein to bind to the antioxidant response element (ARE; 5′-TGACNNNGC-3′) of the target genes, in turn promoting their expression [62]. Heiss et al. demonstrated that the activation of NRF2 induced glucose utilization in fibroblasts. Moreover, alteration of the glucose concentration led to the repression of NRF2-mediated detoxication. NRF2 modulates the pentose phosphate pathway through the regulation of glucose 6-phosphate dehydrogenase expression, resulting in the regulation of nucleotide and NADPH production [63]. A previous study [64] indicated that NAD(P)H quinone oxidoreductase (NQO1), heme oxygenase-1 (HO-1) and p62 genes involved in mitochondrial quality control were regulated by NRF2. Previously, the overexpression of NRF2 contributed to induction of the oxygen consumption rate, higher basal ATP levels and higher basal mitochondrial membrane potential [65]. Transcription factor A, mitochondria (TFAM), transcription factor B1, mitochondria (TFB1M) and TFB2M are regulated by NRF1 [66]. Additionally, translocase outer mitochondrial membrane (TOMM) 20, responsible for modulating mitochondrial membrane transport, was also regulated by NRF2 [67]. Increasing evidence indicates that mitochondrial biogenesis and gene expression are tightly regulated by the PGC-1α/NRF1/NRF2 axis [68]. Deng et al. demonstrated that PGC-1α modulated NRF2-mediated functions via the regulation of GSK3β in the ovarian cancer cell line SKOV3, which is resistant to platinum [69]. Moreover, PGC-1α is directly regulated by NRF2 at the transcriptional level [70]. Taken together, reciprocal regulation between PGC-1α and NRF2 was a pivotal effector for regulating mitochondrial functions.
3.3. Action of lncRNAs in Mitochondria
In this subsection, we summarize the association between nuclear-encoded lncRNAs and cancer progression. Two lncRNAs, cardiomyocyte-enriched noncoding transcript (Caren) and cardiac apoptosis-related lncRNA (CARL), are encoded by the nuclear genome and function as modulators for the regulation of mitochondrial biogenesis and fission [71,72]. An oncogenic lncRNA, taurine upregulated gene 1 (TUG1), had no effect on the NRF2 mRNA levels. Alternatively, NRF2 protein expression was dramatically regulated by TUG1 by interacting with NRF2, in turn conferring chemoresistance in esophageal carcinoma [73]. P32 functions as a master regulator to ordinate OXPHOS and glycolysis [74]. In melanoma, lncRNA SAMMSON is associated with p32 and results in the regulation of prooncogenic function [75]. Sirey and co-workers [76] demonstrated that Cerox1 was a cytoplasmic lncRNA and regulated OXPHOS-related gene expression, leading to modulating OXPHOS enzymatic activity. Furthermore, the effect of Cerox1 on mitochondria was mediated by miR-488-3p. Previous findings showed that the overexpression of ZFAS1 lncRNA reduced the mitochondria membrane potential and triggered the mitochondria apoptosis pathway through the alteration of Ca2+ homeostasis in cardiomyocyte [77]. Zhao et al. demonstrated that metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) was highly expressed in the mitochondria of HCC cell lines [78]. Notably, MALAT1 associated with mitochondrial genes such as D-loop, ND3, COX2 and CYTB. The repression of MALAT1 modulated mtDNA methylation and mitochondrial gene expression, leading to alteration of the mitochondrial structure, OXPHOS, ATP production, mitophagy and mitochondrial apoptosis. Recently, growth arrest-specific 5 (GAS5) lncRNA has been found to be a tumor suppressor for modulating energy homeostasis in cells [79]. GAS5 was regulated upon energy stress. GAS5 modulated TCA flux by altering the association of components of the TCA cycle, including citrate synthase, fumarate hydratase and malate dehydrogenase. In the clinic, GAS5 expression was negatively correlated with those genes in breast cancer tissues. This finding suggests that nuclear DNA-encoded genes (lncRNAs and proteins) and mtDNA-encoded genes crosstalk together and then maintain cellular homeostasis.
4. Strategy for Investigating Mitochondrial Function in Cell Lines
The effects of mtDNA-mediated cellular functions have been well-documented, playing an important role in metabolism and energy production. More than a decade ago, long-term and low-dose EtBr treatment intercalated into circular DNA and repressed mtDNA replication and transcription without interfering with the corresponding nuclear DNA. Thus, to explore the importance of mitochondria in vitro, mitochondrial DNA-depleted cell lines (ρ0) were generated by ethidium bromide (EtBr) treatment in specific cell lines. Then, human subject mitochondria could be transferred into the ρ0 cell line, and the transferred cell line was called the cytoplasmic hybrid (cybrid) cell line. Notably, this model was used to investigate mitochondria-related cancers. However, the ρ0 cell lines were only generated in specific cell lines, such as cervical cancer (HeLa) and hepatoma (SK-Hep1) cell lines, because long-term EtBr treatment may cause DNA mutation and cell death. Alternatively, Correia-Melo et al. [80] designed another methodology to deplete mtDNA: that is, enforced mitophagy. Overexpression of the ubiquitin E3 ligase Parkin, combined with mitochondrial inhibitors carbonyl cyanide 3-chlorophenylhydeazone (CCCP), antimycin A and oligomycin A, induced mitophagy, thereby effectively depleting mitochondria in cell lines. This methodology maintained ρ0 cell lines for approximately one month with minimal cell cytotoxicity and long-term investigations. It is difficult to investigate the functional role of a single SNP or mutation in the mtDNA sequence, because mtDNA has a high copy number in the cell. A previous study [81] demonstrated that mtDNA levels and transcription were repressed by clustered regularly interspaced short palindromic repeats (CRISPR)-Cas9-mediated mtDNA cleavage. To avoid Cas9 ubiquitously expressed in the nucleus or mitochondria, the authors generated a Cas9—namely, mitoCas9—that was only localized in mitochondria. Then, this modification successfully reduced the mtDNA levels and transcription in the HEK293 cell line but not in nuclear DNA. These findings may raise an important question: Is it possible to study point mutations or SNPs of mtDNA by CRISPR-Cas9 that are involved in cancer progression? Enforced mitophagy and CRISPR-Cas9 are both novel methodologies for investigating mitochondrial importance in cancer biology. More successful mtDNA-depleted cell lines and studies to support those two methods are needed to observe the mitochondrial function.
5. Mitochondria Target Therapies and Their Application
Energy production and cellular functions are tightly controlled by mitochondria. The dysregulation of mitochondrial homeostasis may cause cancer progression. This is why mitochondria act as a target for cancer therapy. The different consequences of mitochondria-mediated cellular functions may be caused by different mtDNA SNPs, mutation sites and dysregulated gene-mediated mitochondrial function. Several reagents/inhibitors have been designed and tested to regulate mitochondrial homeostasis for cancer treatment. These agents are divided into different actions of mitochondrial therapy, including mitochondrial protection, biogenesis, quality control and signaling pathways. One type of molecule, delocalized lipophilic cations (DLCs), was designed and successfully accumulated in the mitochondrial matrix. Subsequently, one DLC, tetraphenylphosphonium (TPP), has been used to assist drug delivery to target mitochondria. Antioxidants such as lipoic acid and vitamin E conjugated with TPP preferentially accumulate in mitochondria [82,83]. Notably, the anticancer drug doxorubicin was also conjugated with TPP and applied for treating drug resistance in breast cancer [84]. They found that TPP-doxorubicin treatment caused more cell death than doxorubicin treatment. Although these findings indicated that positive and lipophilic molecules were useful for cancer treatment, the uptake of these positive molecules by cells was less specific. The consequences of these side effects may cause unexpected results.
Intracellular antioxidant coenzyme Q10 (CoQ10) is well-known for protecting against peroxidation-induced plasma membrane damage and has been applied for treating metabolic syndrome and type 2 diabetes patients [85,86]. A study demonstrated that a liposomal-based nanocarrier MITO-Porter was successfully designed and applied for cancer treatment [87,88]. This carrier delivers the drug into the mitochondria through membrane fusion. MITO-Porter associates with CoQ10, in turn inducing its accumulation in mitochondria. 5-Aminoimidazole-4-carboxamide ribotide (AICAR) regulated the ATP levels and suppressed ROS production. Additionally, AICAR promoted mitochondrial biogenesis but did not modulate the mitochondrial membrane potential. Many compounds have been designed and applied for targeting energy homeostasis via the modulation of AMP-activated protein kinase (AMPK), mammalian target of rapamycin (mTOR), NRF1 and TFAM [89].
6. Conclusions
Recently, mitochondrial homeostasis has become an important event for cancer progression. An increasing number of studies have found that mtDNA SNPs and mutations act as predictors or prognostic biomarkers in cancers. Notably, mtDNA-encoded genes and nuclear DNA-encoded genes tightly crosstalk and maintain cellular functions. The mtDNA SNPs (DNA level), mutations (DNA level), mtDNA-encoded genes (RNA and protein levels) and nuclear DNA-encoded gene (RNA and protein levels) networks modulating mitochondrial functions in cancers are comprehensively listed in Table 1 and Table 2. As mentioned previously, these studies focused on the functional roles of one specific target gene encoded by mtDNA or nuclear DNA. Now, the promising omics technology (from genomics to transcriptomics to proteomics to metabolomics) is crucial to investigate the mitochondria-associated cancer progression step by step. Eventually, integration with those results will be important for accurately identifying specific signaling pathways associated with mitochondrial dysfunction and developing the therapeutic interventions. In this review paper, we highlighted the functional roles of these phenomena associated with mitochondrial homeostasis and summarized their potential mechanisms.
Table 1.
The association between mtDNA SNP/mutations (DNA level) and cancer progression.
| Gene Name | SNP/Mutation | Mediated-Cellular Functions | Cancer | Reference |
|---|---|---|---|---|
| ND1 | G3842A | - | HCC | [17] |
| ND1 | T4216C | - | CRC | [18] |
| ND1 | T3394C | Metastasis | - | [24] |
| ND1 | C3497T | Metastasis | - | [24] |
| ND2 | G4776A | Cell growth and ROS production | Head and neck cancer | [19] |
| ND2 | T4587C | Drug resistance and Mitochondrial complex I activity | RCC | [20] |
| ND3 | rs28358278, rs2853826, and rs41467651 | - | Gastric Cancer | [16] |
| ND4 | A11708G | - | HCC | [17] |
| ND5 | 12418insA | - | HCC | [17] |
| ND6 | 13885insC | Complex I activity and ROS production | Lung and Breast Cancer | [21,22] |
| ND6 | C12084T | Metastasis | Breast Cancer | [23] |
| UCP2 | rs591758 and rs675547 | - | - | [25] |
| UCP3 | rs1626521 | - | - | [25] |
| MT-ATP6 | T8993G and T9176C | Cell growth and Apoptosis | Head and neck squamous cell carcinoma | [26] |
| MT-RNR1 | G709A | Metastasis | HCC | [29] |
| MT-RNR1 | 652G insertion and 716G | - | Gastric cancer | [30] |
Table 2.
The actions of mitochondria-encoded and nuclear-encoded genes (RNA and protein level) in cancer progression.
| Gene Name | Principal Functions | Molecules and Signaling Pathways Involved | Study Model | Prognostic Markers in Cancer | Cancer Development | Reference |
|---|---|---|---|---|---|---|
| mitomiR-2392 | Cisplatin resistance, mitochondrial complex activity | ND2, ND4, ND5, CYTB, COX1 | TSCC | ✓ | Progression | [32] |
| mitomiR-5787 | Glucose metabolism, chemoresistance | MT-CO3 | TSCC | ✓ | Repression | [33] |
| mitomiR-34a | Autophagy | Bcl-2 | HUVEC | - | - | [34] |
| mitomiR-181a | Autophagy | Bcl-2 | HUVEC | - | - | [34] |
| mitomiR-146a | Autophagy | Bcl-2 | HUVEC | - | - | [34] |
| lncND5 | Mitochondrial gene expression | PTCD1, mitochondria RNase P protein 3 | - | - | - | [36,37] |
| lncND6 | Mitochondrial gene expression | PTCD1, PTCD2, mitochondria RNase P protein 3 | - | - | - | [36,37] |
| lncCytb | Mitochondrial gene expression | Mitochondria RNase P protein 3 | - | - | - | [36,37] |
| LIPCAR | - | - | Heart failure | ✓ | [39] | |
| ND2 | Epigenetic regulation, mtDNA copy number | Methylation in D-loop | Colorectal cancer | - | Progression | [41] |
| ND5 | - | - | LUSC, LUAD |
✓ | Repression | [43] |
| ND6 | - | - | LUSC, LUAD |
✓ | Repression | [43] |
| CYTB | ROS production, oxygen utilization, lactate production, metastasis, angiogenesis | NFκB2 signaling pathway | Bladder cancer | - | Repression | [44] |
| PGC-1α | Mitochondria biogenesis, OXPHOS, metastasis | ATP synthase | Breast cancerColorectal cancer | ✓ | Progression | [52] |
| ATP5F1A | OXPHOS | - | Prostate cancer | ✓ | Progression | [53] |
| ATPase | HSP60 | - | LUAD | ✓ | Repression | [56] |
| ATP5B | Hypermethylation, drug resistance | - | Chronic myeloid leukemia | - | - | [56] |
| NRF1 | Metabolic homeostasis | TFAM, TFB1M, TFB2M | - | ✓ | - | [66] |
| NRF2 | Oxygen consumption, ATP level, mitochondrial membrane potential, mitochondria membrane transport | TOMM20 | Glial cells, mouse embryonic fibroblast | ✓ | Progression/Repression | [69] |
| lncRNA Caren | Mitochondrial biogenesis and fission | ATM/DDR pathway, Hint1 expression | Cardiomyocyte | - | - | [71] |
| lncRNA CARL | Mitochondrial fission and apoptosis | miR-539, PHB2 | Cardiomyocyte | - | - | [72] |
| TUG1 | Chemoresistance | NRF2 interaction | Esophageal carcinoma | - | - | [73] |
| SAMMSON | OXPHOS, glycolysis, survival | P32 | Melanoma | ✓ | - | [75] |
| Cerox1 | OXPHOS, enzymatic activity | OXPHOS-related genes expressions, miR-488-3p | Mouse Neuro-2a neuroblastoma cells, HEK293 cells | - | - | [76] |
| ZFAS1 | Mitochondria membrane potential, mitochondria apoptosis | Ca2+ homeostasis | cardiomyocyte | - | - | [77] |
| GAS5 | Energy stress, TCA flux | Citrate synthase, fumarate hydratase and malate dehydrogenase | breast cancer | ✓ | Repression | [79] |
| MALAT1 | Metabolic reprogramming | D-loop, ND3, COX2, CYTB | HCC | ✓ | Progression | [78] |
Author Contributions
Writing—Original Draft Preparation: Y.-H.L., C.-Y.C. and H.-C.C.; Writing—Review and Editing: S.-N.L. and Supervision: C.-T.Y. and W.-R.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from Chang Gung Memorial Hospital, Taiwan (CMRPG3K2292, CMRPG3J0693 and CMRPG3J1681 to W.-R.L. and CMRPG3L1211 and NRRPG3L6011 to Y.-H.L.) and from the Ministry of Science and Technology of the Republic of China (MOST 109-2314-B-182A-068- and MOST 110-2314-B-182A-095- to W.-R.L and MOST 110-2311-B-182A-001-MY3 to Y.-H.L.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors have no conflict of interest to disclose.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Anderson S., Bankier A.T., Barrell B.G., De Bruijn M.H.L., Coulson A.R., Drouin J., Eperon I.C., Nierlich D.P., Roe B.A., Sanger F., et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 2.Liu X., Shan G. Mitochondria Encoded Non-coding RNAs in Cell Physiology. Front. Cell Dev. Biol. 2021;9:713729. doi: 10.3389/fcell.2021.713729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gusic M., Prokisch H. ncRNAs: New players in mitochondrial health and disease? Front. Genet. 2020;11:95. doi: 10.3389/fgene.2020.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez-Reyes I., Chandel N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020;11:102. doi: 10.1038/s41467-019-13668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu C., Jin Y., Fan Z. The mechanism of Warburg effect-induced chemoresistance in cancer. Front. Oncol. 2021;11:698023. doi: 10.3389/fonc.2021.698023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashton T.M., McKenna W.G., Kunz-Schughart L.A., Higgins G.S. Oxidative phosphorylation as an emerging target in cancer therapy. Clin. Cancer Res. 2018;24:2482–2490. doi: 10.1158/1078-0432.CCR-17-3070. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y., Qi H., Liu Y., Duan C., Liu X., Xia T., Chen D., Piao H.-L., Liu H.-X. The double-edged roles of ROS in cancer prevention and therapy. Theranostics. 2021;11:4839–4857. doi: 10.7150/thno.56747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Indo H.P., Davidson M., Yen H.-C., Suenaga S., Tomita K., Nishii T., Higuchi M., Koga Y., Ozawa T., Majima H.J. Evidence of ROS generation by mitochondria in cells with impaired electron transport chain and mitochondrial DNA damage. Mitochondrion. 2007;7:106–118. doi: 10.1016/j.mito.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 9.Indo H.P., Yen H.-C., Nakanishi I., Matsumoto K.-I., Tamura M., Nagano Y., Matsui H., Gusev O., Cornette R., Okuda T., et al. A mitochondrial superoxide theory for oxidative stress diseases and aging. J. Clin. Biochem. Nutr. 2015;56:1–7. doi: 10.3164/jcbn.14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anastasiadou E., Jacob L.S., Slack F.J. Non-coding RNA networks in cancer. Nat. Rev. Cancer. 2018;18:5–18. doi: 10.1038/nrc.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang B.-F., Tzeng H.-E., Chen P.-C., Wang C.-Q., Su C.-M., Wang Y., Hu G.-N., Zhao Y.-M., Wang Q., Tang C.-H. HMGB1 genetic polymorphisms are biomarkers for the development and progression of breast cancer. Int. J. Med. Sci. 2018;15:580–586. doi: 10.7150/ijms.23462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H., Guo J., Cheng G., Wei Y., Liu S., Qi Y., Wang G., Xiao R., Qi W., Qiu W. Identification and validation of SNP-containing genes with prognostic value in gastric cancer via integrated bioinformatics analysis. Front. Oncol. 2021;11:564296. doi: 10.3389/fonc.2021.564296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H., Zhao W., Gu D., Du M., Gong W., Tan Y., Wang M., Wen J., Zhai Y., Xu Z. Association of antioxidative enzymes polymorphisms with efficacy of platin and fluorouracil-based adjuvant therapy in gastric cancer. Cell. Physiol. Biochem. 2018;48:2247–2257. doi: 10.1159/000492642. [DOI] [PubMed] [Google Scholar]
- 14.DeBerardinis R.J., Chandel N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016;2:e1600200. doi: 10.1126/sciadv.1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Courtney K.D., Bezwada D., Mashimo T., Pichumani K., Vemireddy V., Funk A., Wimberly J., McNeil S.S., Kapur P., Lotan Y., et al. Isotope tracing of human clear cell renal cell carcinomas demonstrates suppressed glucose oxidation in vivo. Cell Metab. 2018;28:793–800.e2. doi: 10.1016/j.cmet.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin E.-H., Sung J., Lee S.-I., Hong J.-H. Mitochondrial NADH Dehydrogenase Subunit 3 (MTND3) polymorphisms are associated with gastric cancer susceptibility. Int. J. Med. Sci. 2018;15:1329–1333. doi: 10.7150/ijms.26881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin P.-H., Wu C.-C., Lin J.-C., Chi C.-W., Wei Y.-H., Lee H.-C. Somatic mutations of mitochondrial genome in hepatocellular carcinoma. Mitochondrion. 2010;10:174–182. doi: 10.1016/j.mito.2009.12.147. [DOI] [PubMed] [Google Scholar]
- 18.Akouchekian M., Houshmand M., Akbari M.H.H., Kamalidehghan B., Dehghan M. Analysis of mitochondrial ND1 gene in human colorectal cancer. J. Res. Med. Sci. 2011;16:50–55. [PMC free article] [PubMed] [Google Scholar]
- 19.Sun W., Zhou S., Chang S.S., McFate T., Verma A., Califano J.A. Mitochondrial mutations contribute to HIF1 accumulation via increased reactive oxygen species and up-regulated pyruvate dehydrogenease kinase 2 in head and neck squamous cell carcinoma. Clin. Cancer Res. 2009;15:476–484. doi: 10.1158/1078-0432.CCR-08-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horibe S., Ishikawa K., Nakada K., Wake M., Takeda N., Tanaka T., Kawauchi S., Sasaki N., Rikitake Y. Mitochondrial DNA mutations are involved in the acquisition of cisplatin resistance in human lung cancer A549 cells. Oncol. Rep. 2021;47:1–12. doi: 10.3892/or.2021.8243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishikawa K., Takenaga K., Akimoto M., Koshikawa N., Yamaguchi A., Imanishi H., Nakada K., Honma Y., Hayashi J.-I. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008;320:661–664. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- 22.Ishikawa K., Hayashi J.-I. A novel function of mtDNA: Its involvement in metastasis. Ann. N. Y. Acad. Sci. 2010;1201:40–43. doi: 10.1111/j.1749-6632.2010.05616.x. [DOI] [PubMed] [Google Scholar]
- 23.Imanishi H., Hattori K., Wada R., Ishikawa K., Fukuda S., Takenaga K., Nakada K., Hayashi J.-I. Mitochondrial DNA mutations regulate metastasis of human breast cancer cells. PLoS ONE. 2011;6:e23401. doi: 10.1371/journal.pone.0023401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beadnell T.C., Scheid A.D., Vivian C.J., Welch D.R. Roles of the mitochondrial genetics in cancer metastasis: Not to be ignored any longer. Cancer Metastasis Rev. 2018;37:615–632. doi: 10.1007/s10555-018-9772-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim S., Myers L., Ravussin E., Cherry K.E., Jazwinski S.M. Single nucleotide polymorphisms linked to mitochondrial uncoupling protein genes UCP2 and UCP3 affect mitochondrial metabolism and healthy aging in female nonagenarians. Biogerontology. 2016;17:725–736. doi: 10.1007/s10522-016-9643-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shidara Y., Yamagata K., Kanamori T., Nakano K., Kwong J.Q., Manfredi G., Oda H., Ohta S. Positive contribution of pathogenic mutations in the mitochondrial genome to the promotion of cancer by prevention from apoptosis. Cancer Res. 2005;65:1655–1663. doi: 10.1158/0008-5472.CAN-04-2012. [DOI] [PubMed] [Google Scholar]
- 27.He Z., Zheng L., Xie D., Yu S., Zhao J. Mutational analysis of mitochondrial tRNA genes in patients with lung cancer. Balk. J. Med. Genet. 2016;19:45–50. doi: 10.1515/bjmg-2016-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meng X.-L., Meng H., Zhang W., Qin Y.-H., Zhao N.-M. The role of mitochondrial tRNA variants in female breast cancer. Mitochondrial DNA Part A. 2015;27:3199–3201. doi: 10.3109/19401736.2015.1007332. [DOI] [PubMed] [Google Scholar]
- 29.Lin Y.-H., Chu Y.-D., Lim S.-N., Chen C.-W., Yeh C.-T., Lin W.-R. Impact of an MT-RNR1 gene polymorphism on hepatocellular carcinoma progression and clinical characteristics. Int. J. Mol. Sci. 2021;22:1119. doi: 10.3390/ijms22031119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han C.-B., Ma J.-M., Xin Y., Mao X.-Y., Zhao Y.-J., Wu D.-Y., Zhang S.-M., Zhang Y.-K. Mutations of mitochondrial 12S rRNA in gastric carcinoma and their significance. World J. Gastroenterol. 2005;11:31–35. doi: 10.3748/wjg.v11.i1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watson C.N., Belli A., Di Pietro V. Small Non-coding RNAs: New class of biomarkers and potential therapeutic targets in neurodegenerative disease. Front. Genet. 2019;10:364. doi: 10.3389/fgene.2019.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan S., Tian T., Chen W., Lv X., Lei X., Zhang H., Sun S., Cai L., Pan G., He L., et al. Mitochondrial miRNA determines chemoresistance by reprogramming metabolism and regulating mitochondrial transcription. Cancer Res. 2019;79:1069–1084. doi: 10.1158/0008-5472.CAN-18-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen W., Wang P., Lu Y., Jin T., Lei X., Liu M., Zhuang P., Liao J., Lin Z., Li B., et al. Decreased expression of mitochondrial miR-5787 contributes to chemoresistance by reprogramming glucose metabolism and inhibiting MT-CO3 translation. Theranostics. 2019;9:5739–5754. doi: 10.7150/thno.37556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giuliani A., Cirilli I., Prattichizzo F., Mensà E., Fulgenzi G., Sabbatinelli J., Graciotti L., Olivieri F., Procopio A.D., Tiano L., et al. The mitomiR/Bcl-2 axis affects mitochondrial function and autophagic vacuole formation in senescent endothelial cells. Aging. 2018;10:2855–2873. doi: 10.18632/aging.101591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pang Y., Mao C., Liu S. Encoding activities of non-coding RNAs. Theranostics. 2018;8:2496–2507. doi: 10.7150/thno.24677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mercer T.R., Neph S., Dinger M.E., Crawford J., Smith M.A., Shearwood A.-M.J., Haugen E., Bracken C.P., Rackham O., Stamatoyannopoulos J.A., et al. The human mitochondrial transcriptome. Cell. 2011;146:645–658. doi: 10.1016/j.cell.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rackham O., Shearwood A.-M.J., Mercer T.R., Davies S.M., Mattick J.S., Filipovska A. Long noncoding RNAs are generated from the mitochondrial genome and regulated by nuclear-encoded proteins. RNA. 2011;17:2085–2093. doi: 10.1261/rna.029405.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lightowlers R.N., Chrzanowska-Lightowlers Z.M.A. PPR (pentatricopeptide repeat) proteins in mammals: Important aids to mitochondrial gene expression. Biochem. J. 2008;416:e5–e6. doi: 10.1042/BJ20081942. [DOI] [PubMed] [Google Scholar]
- 39.Jusic A., Devaux Y. Mitochondrial noncoding RNA-regulatory network in cardiovascular disease. Basic Res. Cardiol. 2020;115:23. doi: 10.1007/s00395-020-0783-5. [DOI] [PubMed] [Google Scholar]
- 40.Kumarswamy R., Bauters C., Volkmann I., Maury F., Fetisch J., Holzmann A., Lemesle G., de Groote P., Pinet F., Thum T. Circulating long noncoding RNA, LIPCAR, predicts survival in patients with heart failure. Circ. Res. 2014;114:1569–1575. doi: 10.1161/CIRCRESAHA.114.303915. [DOI] [PubMed] [Google Scholar]
- 41.Gao J., Wen S., Zhou H., Feng S. De-methylation of displacement loop of mitochondrial DNA is associated with increased mitochondrial copy number and nicotinamide adenine dinucleotide subunit 2 expression in colorectal cancer. Mol. Med. Rep. 2015;12:7033–7038. doi: 10.3892/mmr.2015.4256. [DOI] [PubMed] [Google Scholar]
- 42.Liu Z., Tian J., Peng F., Wang J. Hypermethylation of mitochondrial DNA facilitates bone metastasis of renal cell carcinoma. J. Cancer. 2022;13:304–312. doi: 10.7150/jca.62278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li N., Zhao J., Ma Y., Roy B., Liu R., Kristiansen K., Gao Q. Dissecting the expression landscape of mitochondrial genes in lung squamous cell carcinoma and lung adenocarcinoma. Oncol. Lett. 2018;16:3992–4000. doi: 10.3892/ol.2018.9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dasgupta S., Hoque M.O., Upadhyay S., Sidransky D. Mitochondrial Cytochrome B gene mutation promotes tumor growth in bladder cancer. Cancer Res. 2008;68:700–706. doi: 10.1158/0008-5472.CAN-07-5532. [DOI] [PubMed] [Google Scholar]
- 45.Boyer P.D. The ATP synthase—A splendid molecular machine. Annu. Rev. Biochem. 1997;66:717–749. doi: 10.1146/annurev.biochem.66.1.717. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan L.B., Gui D.Y., Hosios A.M., Bush L.N., Freinkman E., Vander Heiden M.G. Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell. 2015;162:552–563. doi: 10.1016/j.cell.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wallace L., Mehrabi S., Bacanamwo M., Yao X., Aikhionbare F.O. Expression of mitochondrial genes MT-ND1, MT-ND6, MT-CYB, MT-COI, MT-ATP6, and 12S/MT-RNR1 in colorectal adenopolyps. Tumor Biol. 2016;37:12465–12475. doi: 10.1007/s13277-016-5101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boland M.L., Chourasia A.H., MacLeod K.F. Mitochondrial dysfunction in cancer. Front. Oncol. 2013;3:292. doi: 10.3389/fonc.2013.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Austin S., St-Pierre J. PGC1α and mitochondrial metabolism–emerging concepts and relevance in ageing and neurodegenerative disorders. J. Cell Sci. 2012;125:4963–4971. doi: 10.1242/jcs.113662. [DOI] [PubMed] [Google Scholar]
- 50.Uguccioni G., Hood D.A. The importance of PGC-1alpha in contractile activity-induced mitochondrial adaptations. Am. J. Physiol. Endocrinol. Metab. 2011;300:E361–E371. doi: 10.1152/ajpendo.00292.2010. [DOI] [PubMed] [Google Scholar]
- 51.Miller K.N., Clark J.P., Anderson R.M. Mitochondrial regulator PGC-1a—Modulating the modulator. Curr. Opin. Endocr. Metab. Res. 2019;5:37–44. doi: 10.1016/j.coemr.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.LeBleu V.S., O’Connell J.T., Gonzalez Herrera K.N., Wikman H., Pantel K., Haigis M.C., de Carvalho F.M., Damascena A., Chinen L.T.D., Rocha R.M., et al. PGC-1alpha mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat. Cell Biol. 2014;16:992–1003. doi: 10.1038/ncb3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feichtinger R.G., Schäfer G., Seifarth C., Mayr J.A., Kofler B., Klocker H. Reduced levels of ATP synthase subunit ATP5F1A correlate with earlier-onset prostate cancer. Oxidative Med. Cell. Longev. 2018;2018:1347174. doi: 10.1155/2018/1347174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cuezva J.M., Krajewska M., De Heredia M.L., Krajewski S., Santamaría G., Kim H., Zapata J.M., Marusawa H., Chamorro M., Reed J.C. The bioenergetic signature of cancer: A marker of tumor progression. Cancer Res. 2002;62:6674–6681. [PubMed] [Google Scholar]
- 55.Cuezva J.M., Chen G., Alonso A.M., Isidoro A., Misek D.E., Hanash S.M., Beer D.G. The bioenergetic signature of lung adenocarcinomas is a molecular marker of cancer diagnosis and prognosis. Carcinogenesis. 2004;25:1157–1163. doi: 10.1093/carcin/bgh113. [DOI] [PubMed] [Google Scholar]
- 56.Li R.J., Zhang G.S., Chen Y.H., Zhu J.F., Lu Q.J., Gong F.J., Kuang W.Y. Down-regulation of mitochondrial ATPase by hypermethylation mechanism in chronic myeloid leukemia is associated with multidrug resistance. Ann. Oncol. 2009;21:1506–1514. doi: 10.1093/annonc/mdp569. [DOI] [PubMed] [Google Scholar]
- 57.Yun C.W., Lee J.H., Lee S.H. Hypoxia-induced PGC-1α regulates mitochondrial function and tumorigenesis of colorectal cancer cells. Anticancer Res. 2019;39:4865–4876. doi: 10.21873/anticanres.13672. [DOI] [PubMed] [Google Scholar]
- 58.Evans M.J., Scarpulla R.C. NRF-1: A trans-activator of nuclear-encoded respiratory genes in animal cells. Genes Dev. 1990;4:1023–1034. doi: 10.1101/gad.4.6.1023. [DOI] [PubMed] [Google Scholar]
- 59.He F., Antonucci L., Karin M. NRF2 as a regulator of cell metabolism and inflammation in cancer. Carcinogenesis. 2020;41:405–416. doi: 10.1093/carcin/bgaa039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zimta A.-A., Cenariu D., Irimie A., Magdo L., Nabavi S.M., Atanasov A.G., Berindan-Neagoe I. The role of Nrf2 activity in cancer development and progression. Cancers. 2019;11:1755. doi: 10.3390/cancers11111755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dinkova-Kostova A.T., Abramov A.Y. The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol. Med. 2015;88:179–188. doi: 10.1016/j.freeradbiomed.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Itoh K., Mimura J., Yamamoto M. Discovery of the negative regulator of Nrf2, Keap1: A historical overview. Antioxidants Redox Signal. 2010;13:1665–1678. doi: 10.1089/ars.2010.3222. [DOI] [PubMed] [Google Scholar]
- 63.Singh A., Happel C., Manna S.K., Acquaah-Mensah G., Carrerero J., Kumar S., Nasipuri P., Krausz K.W., Wakabayashi N., Dewi R., et al. Transcription factor NRF2 regulates miR-1 and miR-206 to drive tumorigenesis. J. Clin. Investig. 2013;123:2921–2934. doi: 10.1172/JCI66353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kasai S., Shimizu S., Tatara Y., Mimura J., Itoh K. Regulation of Nrf2 by mitochondrial reactive oxygen species in physiology and pathology. Biomolecules. 2020;10:320. doi: 10.3390/biom10020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Holmstrom K.M., Baird L., Zhang Y., Hargreaves I., Chalasani A., Land J.M., Stanyer L., Yamamoto M., Dinkova-Kostova A.T., Abramov A.Y. Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol. Open. 2013;2:761–770. doi: 10.1242/bio.20134853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Piantadosi C.A., Carraway M.S., Babiker A., Suliman H.B. Heme Oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory Factor-1. Circ. Res. 2008;103:1232–1240. doi: 10.1161/01.RES.0000338597.71702.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blesa J.R., Prieto-Ruiz J.A., Hernandez J.M., Hernandez-Yago J. NRF-2 transcription factor is required for human TOMM20 gene expression. Gene. 2007;391:198–208. doi: 10.1016/j.gene.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 68.Ventura-Clapier R., Garnier A., Veksler V. Transcriptional control of mitochondrial biogenesis: The central role of PGC-1 alpha. Cardiovasc. Res. 2008;79:208–217. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- 69.Deng X., Lin N., Fu J., Xu L., Luo H., Jin Y., Liu Y., Sun L., Su J. The Nrf2/PGC1alpha pathway regulates antioxidant and proteasomal activity to alter cisplatin sensitivity in ovarian cancer. Oxid. Med. Cell. Longev. 2020;2020:4830418. doi: 10.1155/2020/4830418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gureev A.P., Shaforostova E.A., Popov V.N. Regulation of mitochondrial biogenesis as a way for active longevity: Interaction between the Nrf2 and PGC-1α signaling pathways. Front. Genet. 2019;10:435. doi: 10.3389/fgene.2019.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sato M., Kadomatsu T., Miyata K., Warren J.S., Tian Z., Zhu S., Horiguchi H., Makaju A., Bakhtina A., Morinaga J., et al. The lncRNA Caren antagonizes heart failure by inactivating DNA damage response and activating mitochondrial biogenesis. Nat. Commun. 2021;12:2529. doi: 10.1038/s41467-021-22735-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang K., Long B., Zhou L.-Y., Liu F., Zhou Q.-Y., Liu C.-Y., Fan Y.-Y., Li P.-F. CARL lncRNA inhibits anoxia-induced mitochondrial fission and apoptosis in cardiomyocytes by impairing miR-539-dependent PHB2 downregulation. Nat. Commun. 2014;5:3596. doi: 10.1038/ncomms4596. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Z., Xiong R., Li C., Xu M., Guo M. LncRNA TUG1 promotes cisplatin resistance in esophageal squamous cell carcinoma cells by regulating Nrf2. Acta Biochim. Biophys. Sin. 2019;51:826–833. doi: 10.1093/abbs/gmz069. [DOI] [PubMed] [Google Scholar]
- 74.Fogal V., Richardson A., Karmali P.P., Scheffler I.E., Smith J.W., Ruoslahti E. Mitochondrial p32 protein is a critical regulator of tumor metabolism via maintenance of oxidative phosphorylation. Mol. Cell. Biol. 2010;30:1303–1318. doi: 10.1128/MCB.01101-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leucci E., Vendramin R., Spinazzi M., Laurette P., Fiers M., Wouters J., Radaelli E., Eyckerman S., Leonelli C., Vanderheyden K., et al. Melanoma addiction to the long non-coding RNA SAMMSON. Nature. 2016;531:518–522. doi: 10.1038/nature17161. [DOI] [PubMed] [Google Scholar]
- 76.Sirey T.M., Roberts K., Haerty W., Bedoya-Reina O., Rogatti-Granados S., Tan J.Y., Li N., Heather L.C., Carter R.N., Cooper S., et al. The long non-coding RNA Cerox1 is a post transcriptional regulator of mitochondrial complex I catalytic activity. eLife. 2019;8:e45051. doi: 10.7554/eLife.45051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang Y., Jiao L., Sun L.-H., Li Y., Gao Y., Xu C., Shao Y., Li M., Li C., Lu Y., et al. LncRNA ZFAS1 as a SERCA2a Inhibitor to cause intracellular Ca2+ overload and contractile dysfunction in a mouse model of myocardial infarction. Circ. Res. 2018;122:1354–1368. doi: 10.1161/CIRCRESAHA.117.312117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao Y., Zhou L., Li H., Sun T., Wen X., Li X., Meng Y., Li Y., Liu M., Liu S., et al. Nuclear-encoded lncRNA MALAT1 epigenetically controls metabolic reprogramming in HCC cells through the mitophagy pathway. Mol. Ther.-Nucleic Acids. 2020;23:264–276. doi: 10.1016/j.omtn.2020.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sang L., Ju H.-Q., Yang Z., Ge Q., Zhang Z., Liu F., Yang L., Gong H., Shi C., Qu L., et al. Mitochondrial long non-coding RNA GAS5 tunes TCA metabolism in response to nutrient stress. Nat. Metab. 2021;3:90–106. doi: 10.1038/s42255-020-00325-z. [DOI] [PubMed] [Google Scholar]
- 80.Correia-Melo C., Ichim G., Tait S.W.G., Passos J. Depletion of mitochondria in mammalian cells through enforced mitophagy. Nat. Protoc. 2016;12:183–194. doi: 10.1038/nprot.2016.159. [DOI] [PubMed] [Google Scholar]
- 81.Hussain S.-R.A., Yalvac M.E., Khoo B., Eckardt S., McLaughlin K.J. Adapting CRISPR/Cas9 System for Targeting Mitochondrial Genome. Front. Genet. 2021;12:627050. doi: 10.3389/fgene.2021.627050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smith R.A.J., Porteous C.M., Coulter C.V., Murphy M.P. Selective targeting of an antioxidant to mitochondria. Eur. J. Biochem. 1999;263:709–716. doi: 10.1046/j.1432-1327.1999.00543.x. [DOI] [PubMed] [Google Scholar]
- 83.Smith R.A.J., Porteous C.M., Gane A.M., Murphy M.P. Delivery of bioactive molecules to mitochondria in vivo. Proc. Natl. Acad. Sci. USA. 2003;100:5407–5412. doi: 10.1073/pnas.0931245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Han M., Vakili M.R., Soleymani Abyaneh H., Molavi O., Lai R., Lavasanifar A. Mitochondrial delivery of doxorubicin via triphenylphosphine modification for overcoming drug resistance in MDA-MB-435/DOX cells. Mol. Pharm. 2014;11:2640–2649. doi: 10.1021/mp500038g. [DOI] [PubMed] [Google Scholar]
- 85.Zozina V.I., Covantev S., Goroshko O.A., Krasnykh L.M., Kukes V.G. Coenzyme Q10 in cardiovascular and metabolic diseases: Current state of the problem. Curr. Cardiol. Rev. 2018;14:164–174. doi: 10.2174/1573403X14666180416115428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gutierrez-Mariscal F.M., Larriva A.P.A.-D., Limia-Perez L., Romero-Cabrera J.L., Yubero-Serrano E.M., López-Miranda J. Coenzyme Q10 Supplementation for the reduction of oxidative stress: Clinical implications in the treatment of chronic diseases. Int. J. Mol. Sci. 2020;21:7870. doi: 10.3390/ijms21217870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sato Y., Nakamura T., Yamada Y., Harashima H. Development of a multifunctional envelope-type nano device and its application to nanomedicine. Pt BJ. Control. Release. 2016;244:194–204. doi: 10.1016/j.jconrel.2016.06.042. [DOI] [PubMed] [Google Scholar]
- 88.Abe J., Yamada Y., Harashima H. Validation of a strategy for cancer therapy: Delivering aminoglycoside drugs to mitochondria in HeLa cells. J. Pharm. Sci. 2016;105:734–740. doi: 10.1002/jps.24686. [DOI] [PubMed] [Google Scholar]
- 89.Andreux P.A., Houtkooper R.H., Auwerx J. Pharmacological approaches to restore mitochondrial function. Nat. Rev. Drug Discov. 2013;12:465–483. doi: 10.1038/nrd4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.