Abstract
Background
The prevalence of multiple sclerosis (MS) is increasing in Gulf Cooperation Council (GCC) countries. Multiple sclerosis contributes to significant burden on patients and caregivers. The pharmacological treatment in MS involves treating acute exacerbations and preventing relapses and disability progression using disease-modifying therapies. Clinical evidence suggests that teriflunomide is one of the therapeutic choices for patients with relapsing–remitting MS (RRMS). However, genetic and cultural differences across different regions may contribute to variations in drug use. Therefore, it is necessary to consider real-world evidence for teriflunomide usage in GCC countries.
Methods
An expert group for MS gathered from GCC countries in December 2020. The consensus highlighting role of teriflunomide in MS management has been developed using clinical experiences and evidence-based approach.
Results
The expert-recommended patient profile for teriflunomide usage includes individuals aged 18 years and above, both men and women (on effective contraceptives) with clinically isolated syndrome or RRMS. The factors considered were cost-effectiveness of the drug, patient preference, adherence, monitoring, established safety profile, and coronavirus disease 2019 status.
Conclusion
Expert recommendations based on their clinical experience will be more helpful to clinicians in clinical settings regarding the usage of teriflunomide and provide valuable insights applicable in day-to-day practice.
Keywords: multiple sclerosis, teriflunomide, real-world evidence, relapse, disease-modifying therapies
Introduction
Multiple sclerosis (MS) is a chronic central nervous system (CNS) disease that involves immune-mediated inflammation, demyelination, and subsequent axonal damage, leading to the loss of sensory and motor function. 1 The CNS plaques represent the hallmark of MS, which is composed of a mix of inflammatory cells, including macrophages, T and B lymphocytes, and variable degrees of immunoglobulin and complement deposition. 2 Further, the entry of inflammatory cells into the CNS causes subsequent lesions. The B lymphocytes are the significant contributor that acts either as antigen-presenting cells to T cells or by producing autoantibodies or cytokines that promote further autoreactive T-cell response. 3 Multiple sclerosis significantly affects the patients’ quality of life (QoL). However, patients with relapsing–remitting MS (RRMS) are known to have a better QoL than those with progressive disease.4,5
The prevalence of MS has increased globally from 2.3 million in 2013 to 2.8 million in 2020, making it one of the most common neurological disorders. 6 The Gulf countries, initially considered a low-risk area for MS, have now been classified under moderate-risk regions with a projected prevalence of 61.95/100,000 per Saudi nationals 7 and 39.31/100,000 population across individual Gulf states. 8 Hence, identifying an optimal approach to MS management is a significant priority.
The variety of treatments currently available and emerging therapies prompt that the management of MS cannot fit into the “one-size-fits-all” model. Hence, these treatments may be used as sequential monotherapies or for escalation or induction strategies based on the efficacy and safety measures. 9 However, the choice of treatment decision is essential and is highly specific to the individual undergoing treatment, considering the genetic and environmental factors that play a crucial role in the pathogenesis of MS and/or disease progression and response to drugs. 10
Currently, many disease-modifying therapies (DMTs) are approved for RRMS management. The oral DMTs have resulted in better compliance as compared with injectables. 11 Teriflunomide, an oral DMT, has shown promising clinical trial efficacy and safety outcomes in the management of RRMS. Most importantly, teriflunomide selectively and reversibly inhibits the key mitochondrial enzyme dihydroorotate dehydrogenase requisite for de novo pyrimidine synthesis pathway, leading to reduced proliferation of activated T and B lymphocytes without causing cell death. 12 This mechanism is effectively highlighted in phase III teriflunomide trials that state the therapeutic benefits of teriflunomide without causing clinical immune suppression. 12 Teriflunomide is known to be well tolerated with minimum safety concerns; the latter include its long half-life, potential teratogenicity, and monitoring for potential hepatotoxicity. 9
Therefore, in the current narrative review, we, a group of neurologists and experts in MS management from the Gulf Cooperation Council (GCC) countries, provide consensus recommendations to establish a broad patient profile recommendation for teriflunomide usage. This recommendation would support the treating physicians and patients to decide on the usage of teriflunomide as a treatment option in the management of MS patients.
Methods
The expert consensus provided in this review was developed based on a virtual meeting involving 13 experts from across the GCC countries.
The meeting objectives included:
Understanding the importance of real-world evidence (RWE) in MS management
Considering management of MS in view of teriflunomide usage
Studying the teriflunomide usage and RWE specifically in the GCC countries.
The experts shared and discussed their real-world experience in the light of the above objectives. Many cases, observational and cohort studies, and also cases with coronavirus disease 2019 (COVID-19) were discussed for the effective management of MS using teriflunomide. Thereafter, a consensus recommendation was achieved based on presentations at the meeting, expert discussion, and existing literature.
Results and discussion
The factors that accounted for recommending teriflunomide as an effective treatment option with high compliance were as follows:
Level of disease activity
Monitoring burden
Pregnancy status
COVID status
Cost-effectiveness of a drug
Patient preference
Established tolerability and safety profile
Physicians in the GCC countries may use international guidelines like the European and/or American Guidelines for MS management with teriflunomide usage. However, the practicing physicians may not always be constrained to them and decide to follow the local practice in the region.13,14 Therefore, the experts on the panel shared their real-world experiences in treating patients with MS. These experiences, along with published literature, highlight teriflunomide safety and effectiveness, along with patient-related outcomes, pregnancy-related data, and patients with COVID-19 infection.
Overview of clinical trials and case studies from GCC on teriflunomide
Kuwait MS cohort description
The expert from Kuwait presented a cohort of patients with MS from Kuwait who received teriflunomide (14 mg once daily) following its regulatory approval in 2014 (unpublished data). The study aimed at examining the efficacy and safety of teriflunomide treatment under clinical practice conditions in a contemporary cohort of real-world patients in Kuwait. A total of 93 patients were analyzed, and data of 52 patients with 1-year follow-up have been discussed herein.
An overview of real-world studies on teriflunomide and Kuwait cohort study is summarized in Table 1. Data for the Kuwait cohort were collected at baseline, that is, before teriflunomide and after teriflunomide (at 1 year of treatment with teriflunomide). In this study, a significant increase in the percentage of patients who were relapse-free (baseline vs. posttreatment: 53.8% vs. 92.3%, p < 0.001) and a significant decrease in annualized relapse rates (ARR) (baseline vs. posttreatment: 0.56 vs. 0.08, p < 0.0001) were reported (Table 1). The ARR reported by TEMSO (Teriflunomide Multiple Sclerosis Oral) and TOWER (an efficacy study of Teriflunomide in participants with relapsing multiple sclerosis) trials were 31% and 36% relative reduction, respectively, when compared with placebo 15 (Table 1).
Table 1.
Overview of real-world studies on teriflunomide and Kuwait cohort study.
| Parameters | TOPIC 16 | TESMO 17 | TOWER 18 | Teri-PRO 19 | Kuwait cohort study |
|---|---|---|---|---|---|
| Duration of the study | 108 weeks | 108 weeks | 48 weeks from the last randomized patienta | 48 weeks with dosage according to local labeling | 48 weeks |
| Study population | Total population, N = 618: Placebo (n = 197; females: 135/197 [68.5%]); teriflunomide 7-mg group (n = 205; females: 130/205 [63.4%]); teriflunomide 14-mg group (n = 216; females: 154/216 [71.2%]) | Total population, N = 1088: Placebo (n = 363; females: 275/363 [75.8%]); teriflunomide, 7-mg group (n = 366; females: 255/366 [69.7%]); teriflunomide 14-mg group (n = 359; females: 255/359 [71%]) | Total population, N = 1169: Placebo (n = 389; females: 273/ 389 [70.1%]); teriflunomide 7-mg group (n = 408; females: 300/408 [73.5%]); teriflunomide 14-mg group (n = 372; females: 258/372 [69.3%]) | Total population, N = 1000 (females: 756/1000 [75.6%]): teriflunomide 7-mg group (n = 72); teriflunomide 14-mg group (n = 928) | Patients analyzed: N = 93, and 52 patients were on 1-year follow-up (females: 57/93 [61.2%]) |
| Primary endpoint | Time to relapse confirming clinically definite MS b | ARR | ARR | Global satisfaction (TSQM) at 48 weeks or end of treatment | ARR |
| Key secondary endpoint | Time to relapse or new MRI lesions (gadolinium enhancement or new T2 lesion) | Time to 12-week confirmed disability worsening MRI total lesion volume | Time to 12-week confirmed disability worsening | 1. Change in TSQM in those switching from another DMT in the previous 6 months (baseline to 4 and 48 weeks or end of treatment) 2. Patient-reported disability (MSPS) and QoL (MusiQoL) from baseline to 48 weeks or end of treatment |
The mean change in EDSS at last follow-up and percentage of patients with MRI activity |
| Primary endpoint | ARR: Placebo: 0.284; teriflunomide 14-mg group: 0.194. Risk reduction vs. placebo: 31.9% (teriflunomide 14-mg group) | ARR: Placebo: 0.54, teriflunomide 14-mg group: 0.37 Risk reduction vs. placebo: 31.5% (teriflunomide 14-mg group) | ARR: Placebo: 0.50, teriflunomide 14-mg group: 0.32 Risk reduction vs. placebo: 36.3% (teriflunomide 14-mg group) | The mean TSQM Global Satisfaction score at 48 weeks was high (68.2) | ARR: Patients before teriflunomide treatment: 0.56; patients after teriflunomide treatment: 0.37, p < 0.0001 Risk reductions before and after teriflunomide treatment: Patients after teriflunomide treatment: 92.3%; patients before teriflunomide treatment: 53.8% |
| Adverse events | Placebo: 155/191 [81.1%], and teriflunomide 14-mg group: 183/216 [84.7%] | Placebo: 315/360 [87.5%], and teriflunomide 14-mg group: 325/358 [90.8%] | Placebo: 320/385 [83.1%], and teriflunomide 14-mg group: 320/371 [86.2%] | Teriflunomide group: 823/1000 [82.3%] | Teriflunomide group: 24/52 [46.2%] |
| Any adverse event leading to discontinuation of study medication | Placebo:19/191 [9.9%], and teriflunomide 14-mg group: 18/216 [8.3%] | Placebo: 29/360 [8.1%], and teriflunomide 14-mg group: 39/358 [10.9%] | Placebo: 24/385 [6.2%], and teriflunomide 14-mg group: 58/371 [15.6%] | Teriflunomide 14-mg group: 109/1000 [10.9%] | Teriflunomide 14-mg group: 14/52 [0.2%] |
Fixed end for all patients.
Defined as a new neurological abnormality separated by at least 30 days from the onset of a preceding clinical event, present for at least 24 h, and occurring in the absence of fever or known infection. At least one functional system score or the EDSS score must have increased compared with the previous EDSS assessment, consistent with the patient's symptoms. Screening of patients for entry into the TOPIC study was stopped due to 2010 revisions to the MS diagnostic criteria, which enabled an earlier diagnosis of MS (in some cases at the first clinical event). Patients who were still participating in the study at the stoppage could enter the extension study and receive active treatment with teriflunomide 14 mg or 7 mg.
ARR: Annualized relapse rate; MRI: Magnetic resonance imaging; EDSS: Expanded Disability Status Scale; DMT: Disease-modifying therapy; MRI: Magnetic resonance imaging; MS: Multiple sclerosis; MSPS: Multiple Sclerosis Performance Scale; MusiQoL: Multiple Sclerosis International Quality of Life score; QoL: Quality of life; TSQM: Treatment Satisfaction Questionnaire for Medication; TOPIC: Oral Teriflunomide for Patients with a First Clinical Episode Suggestive of Multiple Sclerosis; TEMSO: Teriflunomide Multiple Sclerosis Oral; TOWER: Teriflunomide Oral in People With Relapsing Multiple Sclerosis; Teri-PRO: Teriflunomide Patient-Reported Outcomes.
Another real-world study conducted in Canada reported no increase in the rate of relapses and well-tolerated teriflunomide treatment in RRMS patients at the early stages of the disease. 20 A case report of a 45-year-old female switchover patient also reports no clinical relapse and adverse effects except tolerable mild hair thinning during 12 months. 21
The Expanded Disability Status Scale (EDSS) score recorded in the Kuwait cohort showed no to minimum change, confirming no progression in disability. These study findings are in line with the Teri-PRO (study using patient-reported outcomes to evaluate teriflunomide treatment in relapsing MS patients) trial that confirmed no disease progression for the entire duration of the trial as reported by both the patient- and physician-reported measures. The probability of no evidence of magnetic resonance imaging (MRI) activity was found to be significantly greater (baseline vs. posttreatment: 48.1 vs. 92.3, p < 0.0003). The TEMSO core study also reported a greater probability of no evidence of MRI activity in recently diagnosed patients receiving teriflunomide 14 mg vs. placebo over 2 years.15,17
A study examining the rebound disease activity in patients who transitioned to teriflunomide reported no change in EDSS at 12 months as compared with baseline. 22 A complete nationwide population-based cohort study conducted in 3239 patients who were treated with teriflunomide depicted that there was a higher percentage of patients in the treatment-naive group experienced relapse when compared to the patients who had been treated with teriflunomide (p < 0.0001). 23
The adverse events reported (n/N [%]) in the Kuwait cohort were hair thinning (6/52 [11.5%]), diarrhea (5/52 [9.6%]), headache (4/52 [7.7%]), increase in alanine transaminase (3/52 [5.8%]), nausea (4/52 [7.7%]), and skin rashes (2/52 [3.8%]) (Table 1). These safety results were comparable to the major clinical trials, namely Teriflunomide Phase II, TEMSO, TOWER, and TOPIC (Table 1). 15 The real-world cases discussed by the experts (unpublished) reported hair thinning as a repeated side effect of teriflunomide. However, the experts agreed that this problem was transient and subsided after 3 months into the therapy, and patient education about hair thinning is important to prevent teriflunomide discontinuation. However, discontinuation of the drug is the only option in patients with severe hair loss even after 3 months.
A study with MS patients evaluated the effect of teriflunomide microstructural pathology in the gray matter (GM) and white matter, as measured by changes in brain volume and diffusion-tensor imaging (DTI). When compared to healthy controls, no significant deterioration was noted over the follow-up in brain volume or normal-appearing white matter (NAWM) global or tract-based spatial statistics DTI measures in MS patients. This study suggested that treatment with teriflunomide had shown potential effects by slowing down the accumulation of microstructural tissue damage in the GM and NAWM. 24 Additionally, no clinical progression or adverse events occurred during the follow-up. 24 The patient-reported outcome studies have reported increased preference, higher adherence, and greater patient satisfaction to oral DMTs.25,26
Oman MS cohort description
In Oman, 33 patients with MS were reported till November 2020 and were undergoing treatment with teriflunomide. Majority of patients on teriflunomide were females (22/33 [66.7%]). The age group of the patients with MS who switched to teriflunomide was 20–39 years. Majority of switchers to teriflunomide received treatment with interferons and 12 naïve patients received treatment with teriflunomide. One patient discontinued the drug due to side effects. Three patients stopped due to relapses. Unplanned pregnancy was reported in 2 patients while 3 other patients reported hair thinning. The common side effects reported in this cohort were headache, fatigue, hair fall, dizziness, numbness, abnormal liver function tests results, insomnia and diarrhea.
United Arab Emirates MS cohort description
In United Arab Emirates, a total of 14 patients (females: 11/14 [78.6%]; males: 3/14 [21.4%]) are under teriflunomide treatment. Relapse of the disease and safety issues were not observed in this group of the population, indicating greater efficacy and good tolerability of teriflunomide. Hair thinning is noted in six female patients and all returned to a normal pattern after few months. None of the patients reported lymphopenia/neutropenia and no patients stopped teriflunomide due to side effects/abnormal tests.
Pregnancy outcomes in patients with MS treated with teriflunomide
A 26-question survey conducted in women with MS reported oral treatment was significantly more common in women with no desire for children vs. short- and medium-term desire (p < 0.01 for both). This questionnaire-based study also stated issues surrounding pregnancy influence therapy choice in women of childbearing age with MS. 27 Another clinical study and post marketing data reported consistent pregnancy outcomes in the general population and patients with MS treated with teriflunomide monotherapy. This study even ruled out the teratogenic signal in teriflunomide-exposed pregnancies. 28 The Teriflunomide Pregnancy Registry Enrollment Update also reported similar findings and reported no structural or functional abnormalities in newborns of women or partners of men exposed to teriflunomide during pregnancy. 29
Based on the real-world clinical experience, experts from Oman seconded the study findings and they stated that, as per the standard protocol, women of reproductive age who are on effective contraception should limit the usage of teriflunomide.
Teriflunomide treatment in COVID-19 patients with MS
In the light of current pandemic situations, three cases of COVID-19-positive patients with MS (aged 19–36 years) were also discussed (unpublished data). These three patients were switchers to teriflunomide before contracting COVID-19, that is, two patients received interferon beta-1a and the third patient received dimethyl fumarate. They switched to teriflunomide due to side effects associated with these DMTs. Two cases were those of nurses (36-year-old female and 29-year-old male). The patients were cured of COVID-19 while on teriflunomide treatment without any event or side effects. In line with this observation, a case report and literature review of usage of teriflunomide in COVID-19 suggested that MS patients who develop the COVID-19 infection should not discontinue teriflunomide therapy. 30 Another study featuring five cases of COVID-19 in teriflunomide-treated MS patients stated that the patients continued the therapy with self-limiting infection and without relapse. 31 This review highlights synergy in the outcomes of the clinical trial and the real-world studies.
On the other hand, a multicenter, retrospective, observational cohort study in 347 patients depicted no association between DMT exposure and COVID-19 severity. Further, this French Covisep registry elucidated that age, EDSS, and obesity were independent risk factors for severe COVID-19. 32 The MS International Federation (MSIF) suggests that people with MS taking DMTs, including teriflunomide, do not have an increased risk of more severe COVID-19 symptoms. 33
Teriflunomide noncompetitively and reversibly inhibits dihydroorotate dehydrogenase (DHODH), a mitochondrial enzyme. Inhibition of DHODH interferes with pyrimidine synthesis and might affect the viral replication that might explain its potential antiviral effects, as reported by in vitro experiments. The in vitro experiment reported that inhibiting DHODH results in pyrimidine depletion, thereby delaying the coronavirus replication.34,35 In the case of COVID-19 infection, teriflunomide demonstrated antiviral activity and mild immunomodulatory potential. 35
Teriflunomide may be a relatively safe option to continue ongoing treatment or start with newly diagnosed MS patients with mild activity during the pandemic. Its safety reports in patients infected with COVID-19 are limited. 36
Precautions for reducing the risk of infection with COVID-19 in patients with MS 33
The general safety measures include social distancing; wearing a mask; washing hands frequently with soap and water or an alcohol-based hand rub; avoiding going to crowded places; avoiding touching eyes, nose, and mouth. Besides general safety measures, the following precautions have to be undertaken: Discuss with your healthcare provider about optimal care plans, through video consultations or in-person visits where needed. Do not avoid hospital visits if they are recommended based on your current health needs.
Stay active and try to take part in activities that will enhance your mental health and well-being. Physical exercise and social activities that can take place outside and with social distancing are encouraged.
Caregivers and family members who live with, or regularly visit, a person with MS in one of the higher-risk groups should also follow these recommendations to reduce the chance of bringing COVID-19 infection into the home.
COVID-19 vaccine guidance for MS patients on teriflunomide therapy
The effect of teriflunomide on immune responses to COVID-19 vaccine can be predicted based on prior reported investigations of existing vaccines. Generally, the response to the vaccine depends on three factors:
Type of vaccine (live attenuated vaccines are not recommended with teriflunomide). 36
Type of response that the vaccine generates, namely recall or response to novel antigen, and
Impact of the treatment on humoral and cellular immunity in response to the vaccine. 12
People with MS should avoid receiving live attenuated vaccines. As most COVID-19 vaccines, including the mRNA and adenovirus vector vaccines, do not contain live infectious agents, vaccination is safe for MS patients.33,37,38 Of note, there is no evidence that MS patients are at a higher risk of complications from the mRNA, nonreplicating viral vector, inactivated virus, or protein COVID-19 vaccines compared to the general population. 33
Although data on the safety and effectiveness of COVID-19 vaccines in those with MS is not yet available, science suggests that COVID-19 vaccines are likely to be safe and effective for those with MS. 37 Evidence suggests all patients with MS, including those on any class of DMT (none of which are associated with significant peripheral immunosuppression), can and should be vaccinated against COVID-19.33,37,38 The MSIF further suggested that the COVID-19 vaccine injection should not delay the initiation of DMTs including teriflunomide therapy in MS patients. No dose adjustments are needed if MS patients are already on DMTs including teriflunomide therapy.33,37
Patients with progressive MS, those who are older, those who have a higher level of physical disability, those with comorbidities (e.g. diabetes, high blood pressure, obesity, and heart and lung disease), and Black and Hispanic populations are among groups with the highest risk of hospitalization due to COVID-19. Individuals in these high-risk groups are especially encouraged to get vaccinated. 37
Safety and tolerability of teriflunomide
Data from four clinical trials conducted over 16 years show no new or unexpected safety signals for treatment with teriflunomide. 39 Hair thinning, diarrhea, and nausea are the AEs that are frequently associated with teriflunomide therapy. However, most of the AEs reported were at mild-to-moderate levels. 40
Long-term safety and tolerability data of teriflunomide as follows:
No increased risk of serious infections
No increased risk of malignancies
No significant impact on immunization
No significant impact on T-cell and B-cell viability
No confirmed cases of progressive multifocal leukoencephalopathy to date
Expert consensus and recommendations for teriflunomide usage
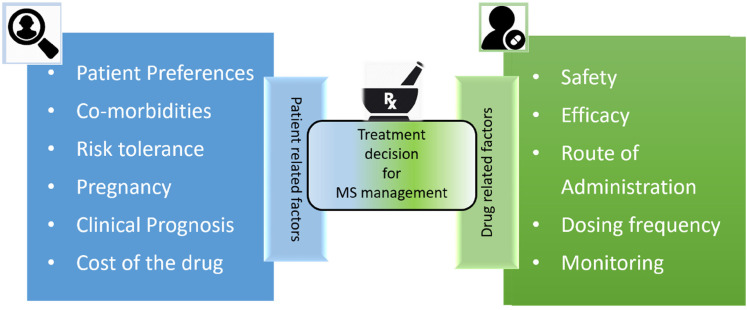
The experts discussed the need for considering patient-related factors and drug-related factors (Figure 1) to find an effective and safe treatment option for patients with MS.
Figure 1.
Factors affecting treatment decision for MS management. 41
MS: multiple sclerosis.
A discussion between the treating physician and the patient over the pointers as mentioned above will help in better treatment choices. This discussion may be considered a step forward toward personalized medicine.
The experts presented the following MS patient profile curated for the GCC countries that may result in the usage of teriflunomide treatment effectively and with minimum side effects or safety concerns.
Key recommendations.
Multiple sclerosis patient profile for teriflunomide usage include:
Patients aged 18 years and above
Women who are on contraceptive therapy
Patients with the clinically isolated syndrome
Patients with relapsing–remitting MS with mild-to-moderate activity
Patients who are treatment-naïve or switchers
No risk with COVID-19-positive patients
No risk on vaccination in patients with mild activity and absence of concurrent infection. 42
The reasons for opting for teriflunomide therapy in MS patients include cost-effectiveness 43 of the drug, patient preference, less patient monitoring, less frequent laboratory monitoring, adherence, and established safety profile.
Conclusion
The real-world experiences discussed by experts and the published literature helped establish a patient profile for effective MS management with teriflunomide. Incorporating essential factors, such as genetic and environmental factors, while choosing treatment options may lead to better control of MS in specific populations similar to those in the GCC countries.
Acknowledgements
All the authors have seen and approved the manuscript and contributed significantly to the work of this manuscript. The authors thank BioQuest Solutions Pvt. Ltd for providing medical writing support, funded by Sanofi Genzyme, in accordance with Good Publication Practice guidelines.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: Sanofi Genzyme offered editorial and logistical support for the development of this consensus statement.
ORCID iDs: Raed Alroughani https://orcid.org/0000-0001-5436-5804
Jihad Inshasi https://orcid.org/0000-0001-5892-751X
Contributor Information
Raed Alroughani, Division of Neurology, Department of Medicine, Al-Amiri Hospital, Kuwait City, Kuwait.
Jihad Inshasi, Neurology Department, Rashid Hospital and Dubai Medical College, Dubai Health Authority, Dubai, UAE.
Mona Al Khawajah, KFSH-R, Riyadh, Saudi Arabia.
Samar Farouk Ahmed, Ibn Sina Neurology Hospital, Kuwait City, Kuwait; Department of Neurology and Psychiatry, Minia University, Minia, Egypt.
Yaser Al Malik, College of Medicine, King Saud Bin Abdul Aziz University for Health Sciences, Riyadh, Saudi Arabia; King Abdullah International Medical Research Center, Riyadh, Saudi Arabia; Division of Neurology, King Abdul Aziz Medical City, National Guard Health Affairs, Riyadh, Saudi Arabia.
Jaber Alkhabouri, Khoula hospital, Muscat, Oman.
Ahmed Shatila, SSMC Hospital, Abu Dhabi, UAE.
Salman Aljarallah, College of Medicine, King Saud University, Riyadh, Saudi Arabia.
Edward J Cupler, King Faisal Specialist Hospital and Research Center, Jeddah, Saudi Arabia.
Shireen Al Qureshi, Johns Hopkins Aramco Healthcare, Dhahran, Saudi Arabia.
Mona Thakre, Al Zahraa Hospital, Dubai, UAE.
Heba Elhasin, Tawam Hospital, Abu Dhabi, UAE.
Aly Ezzat, Sanofi Genzyme, Dubai, UAE.
Sherif Roushdy, Sanofi Genzyme, Dubai, UAE.
References
- 1.Patejdl R, Penner IK, Noack TK, et al. Multiple sclerosis and fatigue: a review on the contribution of inflammation and immune-mediated neurodegeneration. Autoimmun Rev 2016; 15: 210–220. [DOI] [PubMed] [Google Scholar]
- 2.Popescu BF, Lucchinetti CF. Pathology of demyelinating diseases. Ann Rev Pathol 2012; 7: 185Y217. [DOI] [PubMed] [Google Scholar]
- 3.Korn T, Mitsdoerffer M, Kuchroo VK. Immunological basis for the development of tissue inflammation and organ-specific autoimmunity in animal models of multiple sclerosis. Results Probl Cell Differ 2010; 51: 43–74. [DOI] [PubMed] [Google Scholar]
- 4.Janardhan V, Bakshi R. Quality of life and its relationship to brain lesions and atrophy on magnetic resonance images in 60 patients with multiple sclerosis. Arch Neurol 2000; 57: 1485–1491. [DOI] [PubMed] [Google Scholar]
- 5.Patti F, Russo P, Pappalardo A, et al. Predictors of quality of life among patients with multiple sclerosis: an Italian cross-sectional study. J Neurol Sci 2007; 252: 121–129. [DOI] [PubMed] [Google Scholar]
- 6.Atlas of MS: Number of people with MS. Available at: https://www.atlasofms.org/map/global/epidemiology/number-of-people-with-ms#:~:text=2020%20worldwide%20study,accessing%20diagnosis%2C%20treatment%20and%20care (accessed 20 May 2021).
- 7.AlJumah M, Bunyan R, Al Otaibi H, et al. Rising prevalence of multiple sclerosis in Saudi Arabia, a descriptive study. BMC Neurol 2020; 20: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etemadifar M, Nikanpour Y, Neshatfar A, et al. Incidence and prevalence of multiple sclerosis in Persian gulf area: a systematic review and meta-analysis. Mult Scler Relat Disord 2020; 40: 101959. [DOI] [PubMed] [Google Scholar]
- 9.Wingerchuk DM, Carter JL. Multiple sclerosis: current and emerging disease-modifying therapies and treatment strategies. Mayo Clin Proc 2014; 89: 225–240. [DOI] [PubMed] [Google Scholar]
- 10.Waubant E, Lucas R, Mowry E, et al. Environmental and genetic risk factors for MS: an integrated review. Ann Clin Transl Neurol 2019; 6: 1905–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayas A, Mäurer M. Teriflunomide for the treatment of relapsing-remitting multiple sclerosis: patient preference and adherence. Patient Prefer Adherence 2015; 9: 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bar-Or A, Pachner A, Menguy-Vacheron F, et al. Teriflunomide and its mechanism of action in multiple sclerosis. Drugs 2014; 74: 659–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobson R, Dassan P, Roberts M, et al. UK Consensus on pregnancy in multiple sclerosis ‘association of British Neurologists’ guidelines. Pract Neurol 2019; 19: 106–114. [DOI] [PubMed] [Google Scholar]
- 14.Alroughani R, Inshasi J, Al-Asmi A, et al. Expert consensus from the Arabian Gulf on selecting disease-modifying treatment for people with multiple sclerosis according to disease activity. Postgrad Med 2020; 132: 368–376. [DOI] [PubMed] [Google Scholar]
- 15.Miller AE. Teriflunomide: a once-daily oral medication for the treatment of relapsing forms of multiple sclerosis. Clin Ther 2015; 37: 2366–2380. [DOI] [PubMed] [Google Scholar]
- 16.Miller AE, Wolinsky JS, Kappos L, et al. Oral teriflunomide for patients with a first clinical episode suggestive of multiple sclerosis (TOPIC): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13: 977–986. [DOI] [PubMed] [Google Scholar]
- 17.O’Connor P, Wolinsky JS, Confavreux C, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med 2011; 365: 1293–1303. [DOI] [PubMed] [Google Scholar]
- 18.Confavreux C, O’Connor P, Comi G, et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13: 247–256. [DOI] [PubMed] [Google Scholar]
- 19.Coyle PK, Khatri B, Edwards KR, et al. Patient-reported outcomes in relapsing forms of MS: real-world, global treatment experience with teriflunomide from the Teri-PRO study. Mult Scler Relat Disord 2017; 17: 107–115. [DOI] [PubMed] [Google Scholar]
- 20.Oh J, Vukusic S, Tiel-Wilck K, et al. Efficacy and safety of teriflunomide in multiple sclerosis across age groups: analysis from pooled pivotal and real-world studies. J Cent Nerv Syst Dis 2021; 13: 11795735211028781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin KS, Park JG, Park MS. Reduction of disease activity in patient with relapsing-remitting multiple sclerosis after switching to teriflunomide from interferon beta. J Korean Neurol Assoc 2016; 34: 77–79. [Google Scholar]
- 22.Cohan SL, Edwards K, Lucas L, et al. Reducing return of disease activity in patients with relapsing multiple sclerosis transitioned from natalizumab to teriflunomide: 12-month interim results of teriflunomide therapy. Mult Scler J Exp Transl Clin 2019; 5: 2055217318824618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papp V, Buron MD, Siersma V, et al. Real-world outcomes for a complete nationwide cohort of more than 3200 teriflunomide-treated multiple sclerosis patients in The Danish Multiple Sclerosis Registry. PLoS One 2021; 16: e0250820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zivadinov R, Bergsland N, Hagemeier J, et al. Effect of teriflunomide on gray and white matter brain pathology in multiple sclerosis using volumetric and diffusion-tensor imaging MRI measures. J Neurol Sci 2018; 388: 175–181. [DOI] [PubMed] [Google Scholar]
- 25.Labandeira-Gomez C, Gonzalez-Suarez I, Alvarez-Payero M, et al. Increased adherence and better satisfaction outcomes with oral treatments in relapsing-remitting multiple sclerosis patients. Mult Scler 2017; 23: 676–677. [Google Scholar]
- 26.Morawski J, Hass S, Cox F, et al. Treatment preferences related to route of administration in patients with MS: results from US and EU5. Mult Scler 2016; 22: 807–808. [Google Scholar]
- 27.Kamm CP, Muehl S, Mircsof D, et al. Role of family planning in women with multiple sclerosis in Switzerland: results of the women with multiple sclerosis patient survey. Front Neurol 2018; 9: 821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vukusic S, Coyle PK, Jurgensen S, et al. Pregnancy outcomes in patients with multiple sclerosis treated with teriflunomide: clinical study data and 5 years of post-marketing experience. Mult Scler 2020; 26: 829–836. [DOI] [PubMed] [Google Scholar]
- 29.Hellwig K, Lebrun-Frenay C, Rog D, et al. Teriflunomide (Aubagio [R]) pregnancy registry: design and enrolment procedures for pregnant women with multiple sclerosis exposed to teriflunomide. Eur J Neurol 2015; 22: 153–153. [Google Scholar]
- 30.Capone F, Motolese F, Luce T, et al. COVID-19 in teriflunomide-treated patients with multiple sclerosis: a case report and literature review. Mult Scler Relat Disord 2021; 48: 102734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maghzi AH, Houtchens MK, Preziosa P, et al. COVID-19 in teriflunomide-treated patients with multiple sclerosis. J Neurol 2020; 267: 2790–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Louapre C, Collongues N, Stankoff B, et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol 2020; 77: 1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MSIF: The Coronavirus and MS – updated global advice. Available at: The coronavirus and MS – updated global advice (msif.org) (accessed 10 March 2021).
- 34.Xiong R, Zhang L, Li S, et al. Novel and potent inhibitors targeting DHODH, a rate-limiting enzyme in de novo pyrimidine biosynthesis, are broad-spectrum antiviral against RNA viruses including newly emerged coronavirus SARS-CoV-2. BioRxiv. 2020. Available at: Novel and potent inhibitors targeting DHODH, a rate-limiting enzyme in de novo pyrimidine biosynthesis, are broad-spectrum antiviral against RNA viruses including newly emerged coronavirus SARS-CoV-2 | bioRxiv (accessed 15 March 2021). [DOI] [PMC free article] [PubMed]
- 35.Zrzavy T, Wimmer I, Rommer PSet al. et al. Immunology of COVID-19 and disease-modifying therapies: the good, the bad and the unknown. Eur J Neurol 2021; 28: 3503–3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng C, Kar I, Chen CK, et al. Multiple sclerosis disease-modifying therapy and the COVID-19 pandemic: implications on the risk of infection and future vaccination. CNS Drugs 2020; 34: 879–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.The MS Society consensus statement on MS treatments and COVID-19 vaccines. Available at: MS Society Medical Advisers consensus statement on MS treatments and COVID-19 vaccines. Multiple Sclerosis Society UK (accessed 20 March 2021).
- 38.Giovannoni G, Smets I, Reyes S. MS Minute: The COVID-19 Vaccine & Vaccine Readiness in MS. Available at: https://practicalneurology.com/articles/2021-jan/msminute-the-covid-19-vaccine-vaccine-readiness-in-ms (accessed 10 March 2021).
- 39.AUBAGIO. Summary of product characteristics. Available at: AUBAGIO, INN-teriflunomide (europa.eu) (accessed 15 March 2020).
- 40.Coyle PK, Khatri B, Edwards KR, et al. Teriflunomide real-world evidence: global differences in the phase 4 Teri-PRO study. Mult Scler Relat Disord 2019; 31: 157–164. [DOI] [PubMed] [Google Scholar]
- 41.Rotstein D, Montalban X. Reaching an evidence-based prognosis for personalized treatment of multiple sclerosis. Nat Rev Neurol 2019; 15: 287–300. [DOI] [PubMed] [Google Scholar]
- 42.Hamdy SM, Abdel-Naseer M, Shehata HS, et al. Managing disease-modifying therapies and breakthrough activity in multiple sclerosis patients during the COVID-19 pandemic: toward an optimized approach. Ther Clin Risk Manag 2020; 16: 651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu Y, Mao N, Chirikov V, et al. Cost-effectiveness of teriflunomide compared to interferon beta-1b for relapsing multiple sclerosis patients in China. Clin Drug Investig 2019; 39: 331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]