Abstract
Haemophagocytic lymphohistiocytosis (HLH) is one of the rare haematological syndromes more commonly reported in infants/children than adults. This disease is known for its aggressive dysregulated immune response affecting the host rapidly, causing multiorgan dysfunction and thus carries a high mortality. The disease still remains cryptic in this current decade despite all the developments in the ever-evolving field of haematology. Due to its rare occurrence and being more frequent in infants and the paediatric population, the literature lacks enough data to standardise therapies. Such events in adults and the elderly are invariably related to an underlying insult such as infections, other autoimmune or rheumatological diseases or drugs. We describe an interesting case of a middle-aged Caucasian woman who presented with fever, pancytopenia and hepatitis, who was eventually diagnosed with HLH just in time to receive the life-saving specific treatment as per available guidelines.
Keywords: drugs and medicines, haematology (drugs and medicines), medical management
Background
Haemophagocytic lymphohistiocytosis (HLH) syndrome is a hyperinflammatory response evoked by secondary activation of macrophages and cytotoxic T cells leading to life-threatening organ damage. It can be hereditary or acquired caused by a broad spectrum of infections ‘primarily viral’, malignancies, autoimmune conditions and rarely drugs, ‘more so immunomodulatory medications’. To date, most of our understanding of HLH stems from the literature focusing on familial type, commonly observed in children. The growth in literature on adult HLH during the last two decades makes it more apparent that the incidence in adulthood could be almost comparable to the incidence in infancy/childhood despite the underlying cause. The primary and secondary forms of the disease share similar clinical features of multiorgan damage due to excessive inflammation. Primary or familial HLH is related to specific inherited genetic defects and thus is more prevalent in children. The secondary form is typically the result of other conditions like malignancy, rheumatological disorders or infections and is commonly seen in adults. HLH is often diagnosed late because of its rarity and non-specific presentation, which can delay effective treatment and thus prevent a good outcome.
The earliest case series with features of HLH was documented in 1939 by Dr Scott and Dr Robb Smith but were named as histiocytic medullary reticulosis with autopsy findings revealing similar features, including erythrophagocytosis.1 Later, Dr Anderson in 1944 and Dr Asher in 1946 reported identical cases.2 3 This established the very basis of our current knowledge of HLH. Dr Farquhar and Dr Claireaux first used the term ‘Hemophagocytosis’ to report a case involving two siblings/infants born at a 1-year interval succumbed to death within weeks of birth.4 With such a high mortality rate, especially when diagnosis gets delayed, HLH should be one of the active ongoing areas of interest in haematology, to our knowledge, with uptrending developments.
Case presentation
Early in the SARS CoV-2 pandemic (Spring 2020), a Caucasian woman in her late 30s presented with sudden-onset fever, debilitating myalgias and dark urine for the past 2–3 days. Her initial febrile episodes were relapsing despite conservative management, and worsening dark discoloured urination prompted inpatient care. She had a medical history of rheumatoid arthritis, which was in remission for more than a year, being off of disease-modifying antirheumatic drugs or steroids, and major depressive disorder with recent worsening requiring treatment modification with Lamotrigine—started 2 weeks before admission. She tested negative twice for COVID-19 using RT-PCR testing on the nasopharyngeal swab on the day of admission. There were no concerning systemic symptoms, with unrevealing social, family and travel history.
The initial set of vital signs was significant for high-grade fever with Tmax of 103°F with blood pressure and oxygenation on room air. Physical examination revealed shotty cervical and inguinal lymphadenopathy. Systemic examination was otherwise normal.
Investigations
Initial laboratory workup revealed leucopenia (2.07×106/L) with normal neutrophil count (1821 cells/µL) and lymphopenia (190 cells/µL), normochromic—normocytic erythrocytes (4.77×109/L) with haemoglobin of 13.1 g/L and thrombocytopenia (115×106/L). Renal function tests were within normal limits, but the hepatic function panel revealed direct hyperbilirubinaemia (2.6 mg/dL), elevated liver enzymes, including alkaline phosphatase (237 U/L), alanine transaminase (204 U/L) and aspartate transaminase (200 U/L). Urinalysis revealed large urine bilirubin with elevated urobilinogen of 12 mg/dL, suggesting the primary symptom of dark urine could be secondary to hyperbilirubinaemia in the setting of acute hepatitis or urobilinogen overload secondary to haemolytic anaemia.
The initial differential diagnosis included sepsis, cytokine storm secondary to COVID-19 or viral hepatitis. The patient was immediately started on broad-spectrum antibiotics and intravenous hydration.
During the hospital course, the patient progressed to develop a patchy, erythematous, non-itchy, blanching maculopapular rash in the abdomen and lower extremities. Additional infectious workups, including serology and cultures, were negative. CT revealed non-specific subpectoral and hilar lymphadenopathy with sparse ground-glass opacities in the lung and splenomegaly of size 15 cm with no other organ pathology. At that time, the differentials were broadened out to include macrophage activation syndrome and HLH. With her history of rheumatoid arthritis, she was immediately started on high-dose steroids. However, her rheumatoid factor and Anti–cyclic citrullinated peptide (anti-CCP) levels obtained prior to initiation of steroids returned to be within normal limits.
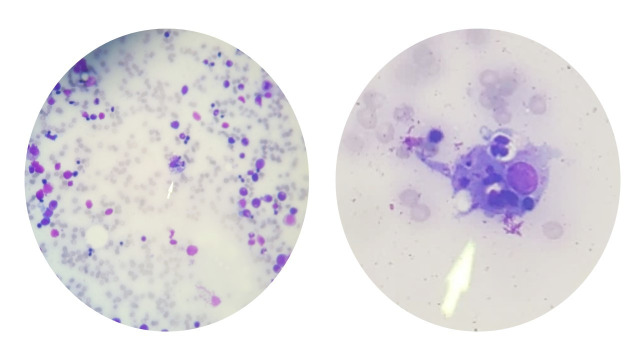
The workup for HLH was initiated with no further delay, including the Bone marrow biopsy. She had elevated lactate dehydrogenase of 1665 EnzU/L, ferritin of 24 090 ng/mL, triglycerides of 193 mg/dL and fibrinogen was low at 238 mg/dL. Soluble IL2 receptor level was sent to a higher centre laboratory, returning several days later, significantly elevated at 56 905 pg/mL. The bone marrow biopsy revealed trilineage haematopoiesis, megakaryocytic hyperplasia and haemophagocytosis, as in figure 1.
Figure 1.
Bone marrow biopsy smear highlighting haemophagocytosis of erythroid precursor cells.
The diagnostic and follow-up laboratory values trends are detailed in table 1. The reference range for each observed laboratory value is documented with the respective variable.
Table 1.
| Day 1 | Day 3 | Day 6 | Day 9 | Day 12 | Day 15 | |
| Temperature (36–38°C) | 39.3 | 39.4 | 37.2 | |||
| Haemoglobin (11.5–14.7 g/L) | 13.1 | 10.7 | 8.2 | 7.5 | ||
| RBCs (4.00–4.90×109/L) | 4.77 | 3.88 | 3.00 | 2.90 | ||
| WBCs (4.10–12.20×106/L) | 2.07 | 1.58 | 1.04 | 1.90 | ||
| Platelets (137–352×106/L) | 115 | 62 | 37 | 46 | ||
| Ferritin (13–50 ng/mL) | 24 094 | 22 513 | 1599 | 528 | 690 | |
| Fibrinogen (209–478 mg/dL) | 238 | 96 | 136 | 207 | 292 | |
| Triglycerides (<150 mg/dL) | 193 | |||||
| sIL-2R (175.3–858.2 U/mL) | 56 905 | |||||
| Pathology | Haemophagocytosis |
RBC, red blood cell; WBC, white blood cell.
Thus, as per histiocyte society HLH-2004 diagnostic guidelines, the patient fulfilled more than five out of eight criteria, reiterating HLH diagnosis. Genetic studies were not performed as the clinical picture was very much consistent with the secondary form of HLH.
Differential diagnosis
Looking back at the aetiology behind HLH in this patient, we considered the possibility of rheumatological-HLH (R-HLH) given the patient’s history of rheumatoid arthritis.
However, we eventually suspected iatrogenic (Rx) HLH secondary to lamotrigine given the fact that her rheumatoid arthritis was clinically (with absent polyarthritis) as well as biochemically inactive (RA factor and anti-CCP insignificant) for more than 1 year, and the short interval between the start of lamotrigine treatment and the onset of symptoms.
Treatment
The suspected inciting agent—lamotrigine, was discontinued since admission and recommended against its use at any point in the future. The steroids were adjusted to tapering Dexamethasone, which has better Central Nervous System (CNS) penetration. The patient was started on etoposide VP-16 150 mg/m2 two times weekly for 2 weeks, followed by 150 mg/m2 weekly for up to 8 weeks as initial therapy. Dexamethasone was started as 10 mg for 2 weeks, followed by 5 mg for 2 weeks, 2.5 mg for 2 weeks, 1.25 mg for 1 week and further tapering to end at 8 weeks. Her course was complicated by ischaemic optic neuropathy in the setting of anaemia needing blood transfusion during the initial phase of heightened inflammation, but the patient had a good outcome eventually. Her vision had improved over the past year but continues to have residual difficulties to date.
Outcome and follow-up
During the initial phases of treatment, the patient had a favourable response to the chosen therapy with a downtrending fever curve and improvement of blood counts and inflammatory markers. Additionally, the rash and other clinical symptoms improved rapidly with treatment and resolved. The patient was in remission before finishing the 8 weeks of initial treatment and remained in remission after her 1-year follow-up.
Discussion
HLH is a disease of obnoxious misactivation of the immune system with concurrent absence of downregulation responses, ultimately resulting in fatal multisystem failure. This disease often presents mimicking sepsis, delaying the diagnosis and timely action.5 Haemophagocytosis in peripheral smear can also happen following blood transfusion, haemolysis and other contexts, and it is not specific for HLH per se. Hence, a high level of suspicion with clinical expertise is needed for prompt diagnosis and intervention.
HLH has been previously classified as either primary or secondary based on the aetiology. The familial (primary) type is caused by autosomal recessive mutation of specific genes, including PRF1 (perforin), MUNC13-4 (UNC13D), STX11 (Syntaxin), STXBP2 and RAB27A,.6 Uncommonly they can even be X-linked in cases of SH2D1A, BIRC4 mutations. These familial or inherited types often manifest in early infancy to childhood. On the other hand, the secondary type is known to have multiple inciting factors such as infection, malignancy, inflammatory conditions or drugs.7 In 2019, North American Consortium for Histiocytosis society used the HLH syndrome definition to include all conditions which present with cytokine storm and meet at least five of the eight HLH 2004 diagnostic criteria. Additionally, a recent observational study in 2017 by Dr Hayden et al was found to have high sensitivity and specificity to rule in HLH with Ferritin levels>10 000 μg/mL and sIL-2R levels>10 000 U/mL.8 The differentiation between HLH disease and HLH disease mimics was also discussed in the HLH 2004 criteria. The former response to HLH specific immunosuppressive therapies exclusively encompasses the familial (primary) and secondary types, while the latter requires a different treatment approach.
With all these factors and features considered, the histiocyte society published their standardised recommendations on treating HLH disease in 2004, revised from the HLH-94 protocol. The initial therapy (weeks 1–8) includes the use of etoposide (VP-16), dexamethasone and cyclosporine (CSA) followed by continuation therapy.7 9 10 Dexamethasone has better blood-brain penetration, and as a corticosteroid, it suppresses the immune system overaction. Etoposide (derived from epipodophyllotoxins) inhibits DNA topoisomerase II alpha, resulting in cell division/proliferation blockage and, hence, anti-tumour activity. Ciclosporin, a calcineurin inhibitor that works by inhibiting the T cell activation, was used upfront rather than as a delayed consideration, a notable revision to the previous HLH-94 protocol. The intrathecal treatment with methotrexate (MTX) is recommended in cases of suspected CNS activity even during the initial phase of 8 weeks if required. MTX treatment must be monitored every 4 weeks with CSF analysis (pleocytosis and hyperproteinaemia). However, in cases of worsening or persistent symptoms or recurrent/reactivation of the disease, haematopoietic stem cell transplantation (HSCT) is considered the last treatment modality. Having discussed the chemotherapy regimen, the heterogeneity of adult HLH prohibits the use of standard protocol of all. Due to multiple comorbidities in adulthood, the patients are very quickly prone to various adverse effects, especially CSA limiting the use of treatment protocol. Hence, modified elements of HLH-94 protocol with pulsed corticosteroids±standard or modified dose of etoposide in recommended by recent therapeutic guidelines discussed by Dr Rosee et al in ASH 2019.9 Apart from those discussed, other chemotherapeutic agents were used in specific groups of patients. For example, in cases of infection associated with HLH (I-HLH) secondary to Epstein-Barr virus, the concurrent use of rituximab, aiming at the selective killing of B cells, is justified in some literature. There have also been cases supporting repeat stem cell transplantation aiming at a better prognosis.11 12 HLH is an evolving field, and despite the current advancement and treatment options, the 5-year survival rate is still grim.
In refractory disease, other than HSCT, salvage chemotherapy can be used.13 However, in 2016, Marsh et al conducted a detailed literature review on salvage therapies and found that most agents lacked superior efficacy. They included the use of anakinra (might be helpful in R-HLH), alemtuzumab (use in HLH could not be justified) and DEP regimen—doxorubicin, etoposide, methyl prednisone (lacks comparison data with the current etoposide and dexamethasone regimen).13 Currently, some studies have enlightened the use of more targeted therapies such as emapalumab (interferon-gamma neutraliser) and ruxolitinib (JAK1/2 inhibitor). A recent multicentric trial (published in 2020) showed that the use of emapalumab with dexamethasone achieved 63% overall response, especially in primary HLH cases that failed to respond to initial treatment.14 This has led the US food and drug administration (FDA) to approve the use of emapalumab with dexamethasone in refractory/relapse cases of HLH in May 2020. A couple more studies highlight the use of ruxolitinib, which showed successful control of pathological immune response with eventual transfusion independence and improved hospital discharge rates.15 16
This case presented a diagnostic dilemma between R-HLH and Rx-HLH. The patient was a young woman with the history of rheumatoid arthritis that was apparently in remission at the time of presentation. We initially considered R-HLH, but the worsening thrombocytopenia and hypofibrinogenaemia along with the absence of active rheumatological findings suggested otherwise. The history revealed recent initiation of lamotrigine treatment for depression within 2 weeks of presentation, suggesting medication-induced HLH. Reviewing the literature, it is evident that lamotrigine has been associated with iatrogenic/treatment associated HLH (Rx-HLH), although very rarely. To our knowledge, less than 10 cases were reported worldwide.17–23
Nevertheless, FDA released a safety announcement in April 2018 associating lamotrigine with HLH development. Apart from lamotrigine, there were also cases sparsely reported regarding HLH associated with other antiepileptic agents such as carbamazepine24 and oxcarbazepine.25 Although our patient had a good outcome, some cases were not as fortunate despite multiple therapies, including stem cell transplant. This highlights the need for more studies in the field of HLH to overcome therapeutic challenges.
Learning points.
Lamotrigine-associated haemophagocytic lymphohistiocytosis (HLH) is an important emerging cause of HLH and adults.
Obvious is not always that obvious. The possibility of HLH should always be considered in critical illnesses with rapid deterioration as early diagnosis and treatment are vital to improve survival.
Although the aetiologies of HLH disease might not make a difference in the treatment approach, it is vital to try identifying the inciting factor for prompt addressal.
HLH itself is a rare disease, and lamotrigine-associated HLH has been reported only in a handful of cases worldwide, including our case.
Acknowledgments
I thank Dr Hachem for mentoring us in this work and the patient who allowed us to use necessary information for educational purposes.
Footnotes
Contributors: DV was involved in the collection of the data and primary drafting. LK and SSB were involved in editing and supplemental data collection. AH was involved in mentoring, confirming the details and proofreading.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Bodley Scott R, Robb-Smith AHT. Histiocytic medullary reticulosis. The Lancet 1939;234:194–8. 10.1016/S0140-6736(00)61951-7 [DOI] [Google Scholar]
- 2.Anderson RG. Histiocytic medullary reticulosis with transient skin lesions. Br Med J 1944;1:220–1. 10.1136/bmj.1.4336.220-a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asher R. Histiocytic medullary reticulosis; a case without lymphadenopathy. Lancet 1946;1:650–1. 10.1016/S0140-6736(46)90495-3 [DOI] [PubMed] [Google Scholar]
- 4.FARQUHAR JW, CLAIREAUX AE. Familial haemophagocytic reticulosis. Arch Dis Child 1952;27:519–25. 10.1136/adc.27.136.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Fakih R, Mohamed SY, Alnounou R, et al. Secondary HLH case report highlighting clinical challenges. Case Rep Hematol 2018;2018:1–2. 10.1155/2018/1913938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henter J-I, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2007;48:124–31. 10.1002/pbc.21039 [DOI] [PubMed] [Google Scholar]
- 7.Jordan MB, Allen CE, Greenberg J, et al. Challenges in the diagnosis of hemophagocytic lymphohistiocytosis: recommendations from the North American Consortium for histiocytosis (NACHO). Pediatr Blood Cancer 2019;66:e27929. 10.1002/pbc.27929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayden A, Lin M, Park S, et al. Soluble interleukin-2 receptor is a sensitive diagnostic test in adult HLH. Blood Adv 2017;1:2529–34. 10.1182/bloodadvances.2017012310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.La Rosée P, Horne A, Hines M, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood 2019;133:2465–77. 10.1182/blood.2018894618 [DOI] [PubMed] [Google Scholar]
- 10.Naymagon L, Tremblay D, Mascarenhas J. The efficacy of Etoposide-Based therapy in adult secondary hemophagocytic lymphohistiocytosis. Acta Haematol 2021;144:560–8. 10.1159/000514920 [DOI] [PubMed] [Google Scholar]
- 11.Tong QJ, Godbole MM, Biniwale N, et al. An elusive diagnosis: case reports of secondary hemophagocytic lymphohistiocytosis and review of current literature. Cureus 2019;11:e4548. 10.7759/cureus.4548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McClain KL. Treatment of hemophagocytic lymphohistiocytosis in the era of new biologics. Pediatr Blood Cancer 2020;67:e28631. 10.1002/pbc.28631 [DOI] [PubMed] [Google Scholar]
- 13.Marsh RA, Jordan MB, Talano J-A, et al. Salvage therapy for refractory hemophagocytic lymphohistiocytosis: a review of the published experience. Pediatr Blood Cancer 2017;64. 10.1002/pbc.26308. [Epub ahead of print: 27 10 2016]. [DOI] [PubMed] [Google Scholar]
- 14.Locatelli F, Jordan MB, Allen C, et al. Emapalumab in children with primary hemophagocytic lymphohistiocytosis. N Engl J Med 2020;382:1811–22. 10.1056/NEJMoa1911326 [DOI] [PubMed] [Google Scholar]
- 15.Hansen S, Alduaij W, Biggs CM, et al. Ruxolitinib as adjunctive therapy for secondary hemophagocytic lymphohistiocytosis: a case series. Eur J Haematol 2021;106:654–61. 10.1111/ejh.13593 [DOI] [PubMed] [Google Scholar]
- 16.Ahmed A, Merrill SA, Alsawah F, et al. Ruxolitinib in adult patients with secondary haemophagocytic lymphohistiocytosis: an open-label, single-centre, pilot trial. Lancet Haematol 2019;6:e630–7. 10.1016/S2352-3026(19)30156-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suleman N, Ozdemirli M, Weisman D. Lamotrigine-associated hemophagocytic lymphohistiocytosis. BMJ Case Rep 2021;14:e238183. 10.1136/bcr-2020-238183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou JY, Martinez JA, Shen JP. Lamotrigine-induced hemophagocytic lymphohistiocytosis with Takotsubo cardiomyopathy: a case report. J Med Case Rep 2019;13:345. 10.1186/s13256-019-2295-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meador KJ, Gidal BE. Lamotrigine and hemophagocytic lymphohistiocytosis. Neurology 2019;92:979–80. 10.1212/WNL.0000000000007518 [DOI] [PubMed] [Google Scholar]
- 20.Kim T, Kulick CG, Kortepeter CM, et al. Hemophagocytic lymphohistiocytosis associated with the use of lamotrigine. Neurology 2019;92:e2401–5. 10.1212/WNL.0000000000007517 [DOI] [PubMed] [Google Scholar]
- 21.Hancock CL, Gálvez A. Lamotrigine-associated hemophagocytic lymphohistiocytosis. Blood 2019;133:1165. 10.1182/blood-2018-11-885509 [DOI] [PubMed] [Google Scholar]
- 22.Ignaszewski M, Ignaszewski MJ, Kohlitz P. Lamotrigine-Associated hemophagocytic lymphohistiocytosis. Am J Ther 2017;24:e493. 10.1097/MJT.0000000000000515 [DOI] [PubMed] [Google Scholar]
- 23.Gümüş H, Kumandaş S, Per H, et al. Hemophagocytic syndrome associated with high-dose lamotrigine. Pediatr Int 2007;49:672–3. 10.1111/j.1442-200X.2007.02433.x [DOI] [PubMed] [Google Scholar]
- 24.Oto M, Yoshitsugu K, Uneda S, et al. Prognostic factors and outcomes of adult-onset hemophagocytic lymphohistiocytosis: a retrospective analysis of 34 cases. Hematol Rep 2015;7:5841. 10.4081/hr.2015.5841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kırık S, Güneş H, Yurttutan S, et al. Hemophagocytic lymphohistiocytosis associated with oxcarbazepine. Turk J Pediatr 2019;61:297–300. 10.24953/turkjped.2019.02.025 [DOI] [PubMed] [Google Scholar]