Abstract
Introduction
Pertussis is one of the top 10 diseases of children under 10 years of age, and the few vaccine-preventable diseases who is on a rise in China in recent years; however, the true burden of pertussis, including age-stratified incidence and risk factors of severe sequelae, are under-recognised. We aim to estimate the health burden of laboratory-confirmed pertussis by age groups, considering the setting of illness onset (ie, in community, outpatient and inpatient), in a Chinese population (~2.23 million in total) at two sites.
Methods and analysis
This paper describes the study design of a 1-year, prospective, age-stratified and population-based case–control study, including site selection, study population, case registry, ascertainment and enrolment, control recruitment, follow-up of case, microbiological methods, data collection, quality control activities and statistical methods used to generate incidence estimates. During June 2021 through May 2022, registry of suspected pertussis cases (namely chronic/persistent cough) will be conducted in several participating hospitals (SHs) at the two sites, which are selected based on Healthcare Utilisation and Attitudes Surveys (HUAS) carried out before study initiation. A case–control study will be conducted in the SHs and we aim to enrol a total of 1000 suspected pertussis cases (ie, all hospital admissions and the first 1–3 outpatient visits each week each hospital) and 2000 frequency matched healthy controls in community. Our primary study outcome, the laboratory-confirmed Bordetella pertussis infection, will be determined by a comprehensive laboratory methods and procedures (ie, culture, PCR and serological tests) in both cases and controls at enrolment and during 60-day’s follow-up visits. Finally, data from HUAS (ie, population size), case registry (ie, the total number of suspected pertussis cases) and case–control study (ie, the prevalence or population attributable fraction of Bordetella pertussis) will be combined to calculate incidence and its 95% CI through bootstrap method. Epidemiological analyses will be conducted to determine the risk factors associated with severe sequelae of pertussis.
Ethics and dissemination
This study has been approved by Chinese Centre for Disease Control and Prevention’s Institutional Review Board (no. ICDC-202110). Results will be disseminated via academic presentations and publication in peer-reviewed journals, and will provide valuable scientific data and some new insights into the incidence, aetiology and risk factors for severe sequelae of pertussis to academic societies and the public health authorities who is currently struggling and fighting against this burdensome disease worldwide.
Keywords: epidemiology, paediatric infectious disease & immunisation, epidemiology
Strengths and limitations of this study.
PertussisChina is a population-based study at two sites, covering approximately 2.23 million populations defined through conducting Healthcare Utilisation and Attitudes Surveys (HUAS) in community.
PertussisChina is a laboratory-based study, in which comprehensive laboratory methods (ie, culture, PCR and serological tests) and procedures (ie, 60-days follow-up) will be used to specifically measure pertussis disease burden.
PertussisChina is a case–control study in which the prevalence and population attributable fraction of Bordetella pertussis infection can be ready acquired.
All cases will be prospectively followed up to 60 days to collect interesting events (ie, adverse clinical outcomes of hospitalisation or death) at 2, 4 and 8 weeks after enrolment.
Limitations are that our incidence might be underestimated and cannot be extrapolated to represent the whole country due to the insensitive case definition used, short study period and relatively small population covered.
Background
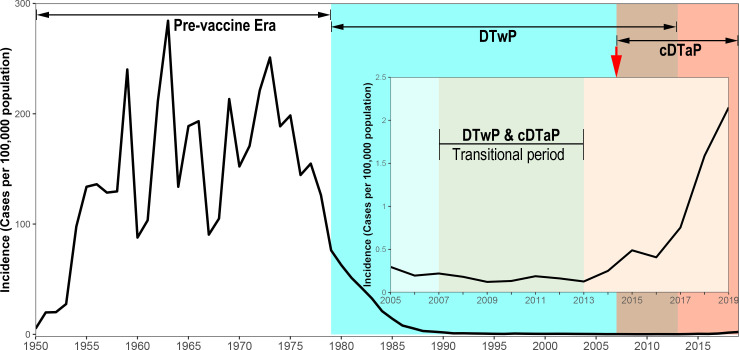
Whooping cough (pertussis) is a highly contagious respiratory disease caused by Bordetella pertussis.1 2 Despite a high vaccine coverage of third dose diphtheria–tetanus–pertussis vaccine (DTP3),3 the ‘resurgence of pertussis’ in recent years has posed a great threat to global public health,4–6 as well as to Chinese infants.7 8 In 2019, pertussis was one of the top 10 diseases with highest burden in children younger than 10 years,9 and the WHO estimates that pertussis kills about 160 700 children under 5 years old worldwide each year.10 In China, three types of pertussis vaccines are available till 31 Octorber 2021, that is, the copurified diphtheria and tetanus toxoids and acellular pertussis (cDTaP, used for routine immunisation), DTaP/Hib (Minhai Biotechnology Co., Beijing, China)11 and DTaP-IPV/Hib (Sanofi Pasteur, Lyon, France).12 13 The coverage of DTP3 remained high above 99% for children throughout the 2010 s,14 15 and the reported incidence of pertussis has been risen from 0.12 per 100 000 in 2013 to 2.14 per 100 000 in 2019 (figure 1). Unlike the other countries who had experience resurgence of pertussis, especially in adolescents/adults, primarily due to the waning of vaccine induced immunity,16–20 China observed no such changes of age distribution.21 The rise of pertussis in China was mainly concentrated in infants less than 1 year old, and less than 5% of reported pertussis were adolescents and adults.
Figure 1.
Incidence of reported pertussis from NNIDSS, China, 1952–2019. A cDTaP was introduced into national immunisation programme to replace DTwP in 2007 and the transition was fully completed in 2013. cDTaP, copurified diphtheria, tetanus toxoid and acellular pertussis vaccine; DTwP, combined diphtheria, tetanus toxoid and whole-cell pertussis vaccine; NNIDSS, National Notifiable Infectious Disease Surveillance System.
Since most epidemiological data on pertussis in China came from a passive reporting system, the National Notifiable Infectious Disease Surveillance System (NNIDSS),22 under-reporting was substantial in the system (≥90%) because of limited diagnosis and incompleteness of reporting.8 23 24 And the burden of pertussis remained under-recognised. It has been suggested that immunising schoolchildren is the key for curtailing transmission of pertussis in population.18 Due to a substantial knowledge gaps existed in age-specific burden of pertussis (ie, incidence and severity), no adolescent or adult immunisation are recommended in the country.25 Moreover, some important data such as clinical, laboratory and vaccine information are also not available, which is unfavourable for evaluating the effectiveness of vaccine and implementing of other disease control and prevention programmes (such as adult vaccination, diagnostic tests and postexposure prophylaxis of pertussis). Rigorously conducted, prospective, population-based studies can be used to strengthen the NNIDSS, by providing information on the burden of laboratory-confirmed pertussis, strains distribution, risk factors for severe sequelae and case fatality, and most importantly, to assist health authority in China to allocate health resources, prioritise health research investments, optimise interventions (ie, vaccination) and innovate vaccine development.
We designed the PertussisChina study, a 1-year, prospective, age-stratified, population-based longitudinal cohort and case–control study, which will enrol suspected pertussis patients (ie, chronic/persistent cough) seeking healthcare in several selected participating hospitals (SHs) at two sites of China, covering approximately 2.23 million censused population. This article describes the study design, including sites selection, study population, case registry, ascertainment and enrolment, control recruitment, follow-up of cases and controls, microbiological methods (ie, culture, PCR and serological tests), data collection, quality control activities and statistical methods used to generate incidence estimates of pertussis. We then further discuss the strengths and weaknesses of the study design.
Methods and analysis
Objectives of the study
The primary objective of the study is to measure the incidence of laboratory-confirmed pertussis by age groups (children, adolescents and adults), and by settings (community, outpatient and inpatient). The secondary objectives are as follows: (1) to describe the distribution of disease severity and outcomes across age groups; (2) to describe the patterns and factors of underdetection and under-reporting of pertussis; (3) to study the carrier (colonisation) status of the B. pertussis in the upper respiratory tract of healthy controls, and the serum levels of antipertussis toxin antibodies (anti-Ptx IgG) in both patients and healthy people and (4) to create a repository of well-characterised clinical specimens and B. pertussis isolates that can be used in future studies.
Study sites and population
Site selection criteria
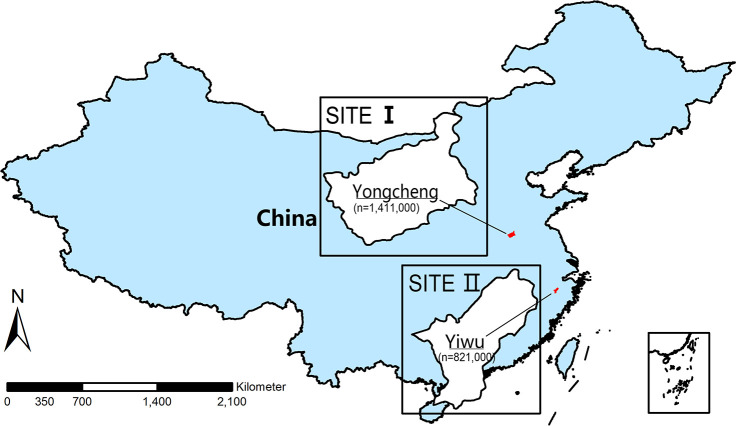
Sites are selected based on the following criteria: (1) have strong willingness to participate; (2) have capability and resources to conduct ongoing surveillance, namely staffs to facilitate specimen collection and case investigation, previous experience in disease surveillance, infrastructures to secure data collection and specimen storage or transportation and (3) provide a full list of healthcare facilities in the area and the information of built-in hospital information system (HIS) in the facilities. Currently, there are two sites in the study, including Yongcheng, Henan and Yiwu and Zhejiang (figure 2).
Figure 2.
Location and population size of study sites included in PertussisChina study.
Study population
In 2019, Yiwu had a permanent population of 821 000 (47 000 were children under 5 years of age) served by 24 healthcare facilities (ie, three tertiary care, four secondary care and 17 primary care hospitals). Most hospital admissions (≥80% of the total number) occurred in the three large tertiary hospitals, including a children’s hospital and two general hospitals; meanwhile, Yongcheng had a permanent population of 1 411 000 (94 000 were children under 5 years of age) served by 41 healthcare facilities (ie, five secondary care and 36 primary care hospitals). Most hospital admissions occurred in the five large secondary care hospitals, including four general hospitals and a maternal and paediatric hospital. In total, the two sites cover a total of 2.23 million permanent population in the study area.
Study overview and design
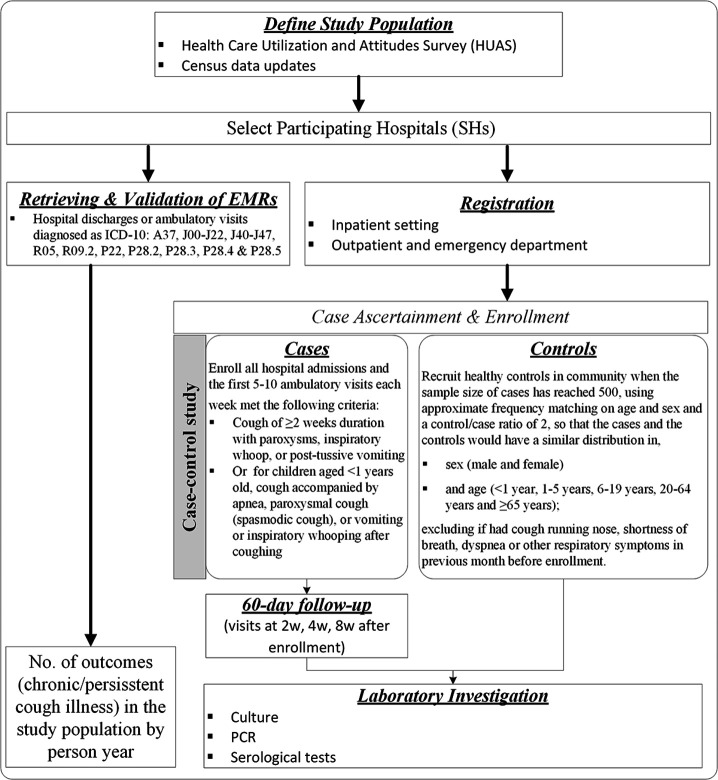
In order to achieve our study objectives, we will conduct the following study activities at the two sites from June 2021 through May 2022, including, (1) a Healthcare Utilisation and Attitudes Survey (HUAS) and a census data updating to define study population (ie, incidence denominator), so as to set up a sampling frame for the case–control study and selecting participating hospitals (ie, SH) for case registry and case recruitment; (2) the case–control study to acquire the prevalence of B. pertussis infection among suspected pertussis cases and healthy controls, as well as the calculation of population attributable fraction (AF) indicating the proportion of cases that can be prevented if B. pertussis was totally removed from the population and (3) case registry and the retrieval of electronic medical records (EMRs) from HIS to provide and validate the total number of suspected pertussis case patient (chronic/persistent cough) encountered in the SHs (ie, incidence numerator) (figure 3).
Figure 3.
Flow diagram of major study activities. EMRs, electronic medical records.
Defining and calibrating study population
Census data updating
Population census data at the two sites will be collected and updated during the study period. Population census is conducted every 10 years in China and the nearest one is in 2020. However, an intermittent survey of 1% sampling of the total population would be performed to update population census data every year between the two censuses. We will retain the up-to-date population data from the National Bureau of Statistics. Moreover, the population birth, mortality and population migration are recorded by the local government. We will also contact the local health bureau quarterly to access these data to give a precise estimation of population size in the two sites.
Healthcare utilisation and attitudes Surveys (HUAS)
HUAS will be conducted prior to recruiting cases and controls at the two sites, which will serve three purposes, (1) to set up a sampling frame for the case–control study; (2) to select SHs in which prospective enrolment of cases will be conducted and (3) to provide estimates of the population coverage for our SHs and healthcare seeking behaviour weights applied in estimating pertussis incidence in community.
In summary, a population-based cross-sectional study, with an age-stratified sample of 3000 children aged 0–59 months and 6000 adolescents/adults aged≥5 years, will be conducted in the community of the two sites. The sample size was calculated based on: (i) for children, a monthly prevalence of cough illness, π=1% (estimated from the reported incidence of lower respiratory tract infection of 0.15 per child year,26 allowable error (δ=0.5%), significant level (α=0.05) and design effect (deff=2); (ii) and for adolescents/adults, a monthly prevalence of cough illness, π=3.3%,27 allowable error (δ=0.66%), significant level (α=0.05) and design effect (deff=2).
A complex sampling method will be used to select survey respondents as follows. First, a probability proportionate to size sampling will be used to randomly select 50 clusters (eg, communities or villages) in the site’s administrative regions. At the second sampling stage in selected communities, quota sampling will be used to recruit interviewee. The quota required in each age stratum was calculated based on the age distribution of the population in the sites and the number of surveys allocated to each cluster. Trained work staff will go to the selected communities to conduct face-to-face surveys at several locations (residential areas, kindergartens and children’s vaccination clinics) Monday to Sunday during daytime in the study period. All residents living in the communities or villages for at least half a year prior to survey are eligible for and invited to participate in the interview. After the quota required in each age group is complete, the interviews will stop.
The following questions (online supplemental tables 1 and 2) are asked to respondents, (1) the occurrence and length of cough illness in the previous month prior to survey, (2) healthcare-seeking behaviour regarding the self-reported cough illness for the most recent episodes and the sources of healthcare facilities and (3) the willingness to seek healthcare and where would they choose to visit for an assumptive cough illness.
bmjopen-2021-053316supp001.pdf (246.9KB, pdf)
Based on the HUAS and census data, hospitals at which over 80% of respondents in each site choose to attend when hospital admission is required will be selected as our SHs. In case healthcare providers in the site change their practice or scope of service during our study period, for example the opening of new hospitals or the establishment of new branches of existing hospitals, an abbreviated HUAS with a smaller sample of 1000 will be administered at the middle or the end of the year during which cases are recruiting at SHs.
Case–control study
Case definition of suspected pertussis
Patients will be classified as suspected pertussis cases and offered to participate if they present chronic/persistent cough defined as cough of ≥2 weeks duration with one or more of the following symptoms, (1) paroxysmal cough; (2) inspiratory whoop or (3) post-tussive vomiting; or, for children aged <1 years old, cough (regardless of cough duration) accompanied by one or more of the following symptoms, (1) apnoea; (2) paroxysmal cough; (3) inspiratory whoop or (4) post-tussive vomiting.
We will exclude patients presenting with gastro-oesophageal reflux, spastic bronchitis and clearly diagnosed tuberculosis, mycoplasma/chlamydia infection or chronic sinusitis. Adults/adolescents with a measured body temperature of ≥38.5°C at enrolment will also be excluded.
Sample size considerations
We planned to enrol approximately 250 suspected cases and two matched controls for each case in each age stratum (ie, children under 5 years, and adolescents/adults aged≥5 years) for laboratory investigation at each site, which would add up to approximately 1000 suspected cases and 2000 controls at the two sites. We calculated the above sample size based on a prevalence of B. pertussis in chronic/persistent cough of 20% (range=12%–32%),28–30 an allowable error of 5% and a significant level of 0.05. This sample size would have a 90% power (two-sided α=0.05) to detect an OR of 2 between case and control for a site and age stratum-specific comparison, if the true prevalence of B. pertussis is 20% in case; or an OR of 3, if the true prevalence is 10%. Although the carrier state of B. pertussis is transient in family contacts,31 32 B. pertussis is rarely identified in healthy people,33 34 and we expected a larger OR of ≥2 in the study. This sample size means that the laboratory would process average 115 samples per week, which is feasible and acceptable for our laboratories.
Case registry, ascertainment and enrolment
Case registry, ascertainment and enrolment for suspected case will be conducted in SHs during the study period. Clinicians or trained nurses working in selected departments of the SHs (ie, respiratory, paediatric, infectious disease and emergency department) will carry out case registry of suspected pertussis cases every weekday (ie, Monday through Sunday) except national holidays. Each outpatient visits and new hospital admission seeking healthcare in above departments will be screened for the eligibility of inclusion using the inclusion and exclusion criteria of the suspected case definition of pertussis by clinicians. Eligible ones will be ascertained and recorded as suspected case by study coordinator who assist with clinicians in SHs in enrolling cases using a standardised case reporting form (CRF) (online supplemental table 3). Among the suspected pertussis case recorded in SHs, convenient sampling method will be used to recruit cases for case–control study. We aim to enrol all hospital admissions and the first 1–3 outpatient visits each week in each hospital. After obtaining informed consent, study staff will conduct enrolment interviews, and collect nasopharyngeal (N/P) and blood specimens for each enrolled case.
Controls selection
At the middle of the study year when the sample size of cases reaches a half of the total (ie, n=500), a control is recruited in community of the study sites using approximate frequency matching, based on the following criteria, (1) similar proportion in sex strata; (2) similar proportion in age strata, ie, <1 year, 1–5 years, 6–19 years, 20–64 years and ≥65 years; (3) a control/case ratio of 2:1 and (4) no cough, running nose, shortness of breath, dyspnoea or other respiratory symptoms at enrolment nor have a record of healthcare for respiratory disease in previous 3 months before recruitment.
60-day follow-up of case
We will follow cases from the time of enrolment to a maximum time period of 60 days after enrolment. Follow-up will be conducted at second, fourth and eighth weeks after enrolment, with face-to-face interview if patient is currently hospitalised, or one telephone call each follow-up time if patient is discharged from hospital. At each follow-up visit/phone call, the study staff will ask about cough or other respiratory or systemic illness symptoms in the period since the last contact. If case is still symptomatic (ie, cough) during follow-up, they will be encouraged to visit their doctor who enrolled them in the SHs within 24 hours of contact. The doctor will check-up the patient’s health status and collect the swab and serum samples during the visit. If an enrolled patient does not want to visit the SHs, the study staff will arrange a household visit to collect the samples in the home.
Data collection from cases and controls
At enrolment, trained clinicians and the study coordinator will conduct face-to-face interview to collect sociodemographic, clinical and epidemiological data from cases and controls using a standardised CRF (online supplemental table 4). Demographic information includes household size (defined as a group of people who share a dinner table), average household income, rural or urban residence, age, alcohol consumption and smoking exposure and occupation, etc. A clinician will also examine all cases to document clinical signs and symptoms at enrolment, including cough characteristics (duration, paroxysms, post-tussive vomiting and exacerbation at night), body temperature, respiratory rate, heart rate, seizure, apnoea and other general respiratory symptoms, non-prescription antibiotic usage before visiting the doctor, blood test results and chest X-ray examinations. Vaccination history (ie, band, dosing, procedure and time of administration) of children aged ≤14 years is also collected by linkage of his/her individual records on immunisation in the national database (Childhood Immunisation Information Management System, CIIMS)35 or checking of vaccination certificate.
During follow-up visits, data on any current cough or respiratory symptoms, subjective severity of illness, illness duration, functional impairment, whether medical care was sought and outcomes since the last visits will be collected using CRFs (online supplemental table 5).
At the end of follow-up, medical charts of each hospitalised case will be reviewed by study staff to collect information on antibiotic treatment and outcomes during hospitalisation (ie, mechanical ventilation, ICU transfer and death) (online supplemental table 6).
The retrieval of electronic medical records and validation of the total number of suspected pertussis case
Since our case registry and enrolment is conducted in selective departments (ie, respiratory, paediatric, infectious disease and emergency departments) and on workdays in SHs, it is an incomplete record of the total number of suspected cases encountered in the whole hospital. It is essential to calibrate the registered number of suspected cases to equal the total. To do this, all hospital discharges or ambulatory visits coded for diagnosis under the International Classification of Diseases 10th Revision (ICD-10) codes A37, J00-J22, J40-J47, R05, R09.2, P22, P28.2, P28.3, P28.4 and P28.5 will be monitored on a daily basis as registry case, by hospital departments. At the end of the month, the complete EMRs records with the above diagnosis codes in the whole hospital will be abstracted from HIS of the SHs. This data will be used to calibrate the prospectively counting data of suspected case in the selective departments that conduct case enrolment to make a precise estimate of the total number of chronic/persistent cough illness outcomes in the studied population. Namely, through linking and comparing between the number of registry cases and the number of suspected pertussis case registered in the selected departments, we will calculate the Wcase. With this Wcase, we will narrow down the ICD-based EMRs records to the total number of suspected pertussis cases met our case definition in SHs (ie, the numerator of incidence).
Laboratory investigation
Specimen collection and transport
When patients meet our suspected pertussis case definition or are recruited controls, they, as well as symptomatic (cough) cases during follow-up contacts, will be sampled within 24 hours. Clinicians or nurses in SHs will be trained to collect nasopharyngeal swabs (N/P) and whole blood sample. Dacron or nylon swab will be used to collect N/P specimen to facilitate culture and PCR tests for B. pertussis.36 Collected swab specimens will be plated onto selective agar or placed in transport medium (Charcoal Agar, Thermo Fisher Scientific) immediately after sampling at the SHs. Whole blood without adding any anticoagulants (>4 mL for participants aged 5 years and older, and ≥2 mL for children aged<5 years) will be collected, and centrifuged to separate serum within 24 hours of collection. All collected swab and sera samples will be transported to the central laboratory of Chinese Centre for Disease Control and Prevention (China CDC), using a cold box to maintain a temperature of 4°C. During transportation, samples are packaged and transported in accordance with the provision of International Civil Aviation Organisation (ICAO) document Doc9284 and UN3373
Processing and storage of specimen
On arrival at the laboratory of China CDC, swab samples will be processed and prepared into three aliquots of swab supernatant, so will serum samples be. One of these aliquots will be analysed and the other two aliquots will be kept for future analyses. All aliquots will be stored at −70°C temperature until the time of analysis.
Laboratory testing
In the laboratory of China CDC, Charcoal Agars will be cultured to isolate B. pertussis using standard method recommended by China CDC37 and WHO.38 Swab supernatant will be analysed for B. pertussis, B. parapertussis, B. bronchiseptica and B. holmesii using PCR as recommended by US CDC.39 40 Sera samples that have a minimum volume of ≥1 mL will be tested for Anti-Ptx IgG titre using a commercially available diagnostic kit (Virion\Serion, Wurzburg, Germany) according to the manufacturer’s recommendations. To validate our laboratory methods and testing results, external quality assurance testing will be conducted to reach agreements with a reference laboratory on Bordetellae prior to study start. For serology testing, we use standard from the National Institute for Biological Standards and Control, London, UK (https://www.nibsc.org/products/brm_product_catalogue/detail_page.aspx?catid=18/146); and for PCR assays, the Wisconsin State Laboratory of Hygiene, Wisconsin, US (http://www.slh.wisc.edu/proficiency/training-and-competency/).
Suspected pertussis cases and controls that have B. pertussis Isolated, positive tests of swabs in any of samples collected during enrolment and follow-up, or for persons 3 years of age and over have a threefold or greater rise in anti-Ptx IgG antibody between sequential sera samples with at least one time point higher than 40 IU/mL of serum titre would be considered laboratory-confirmed pertussis.36 41
Data flow, management and analysis
The data collected in the study are centrally managed at China CDC, using an online data platform (http://eddc.chinacdc.cn/dap/). The completed CRFs will be entered into the information system by local study staff at the two sites and uploaded to data server through encrypted transmission via a Virtual Private Network set up by China CDC. The entered records are regularly checked for completeness, consistency and logical errors by data manager and the site’s coprinciple investigator who is responsible for authorisation, integrity, security and backup of database during data collection.
Statistical analysis
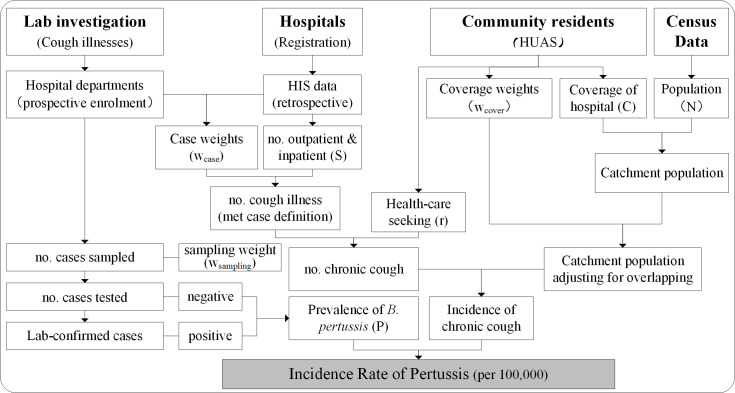
The collected data processing and key indicators based on which we calculate incidence are shown in figure 4. We will calculate the incidence of pertussis by age group and by settings with the following formula.
Figure 4.
Data flow chart and key indicators used to calculate incidence of pertussis. HUAS, Healthcare Utilisation and Attitudes Surveys.
Where Siinpatient and Sioutpatient indicate the registered number of inpatients and outpatient visits of cough illnesses at age group i, as obtained from HIS. is the weight used to adjust Siinpatient and Sioutpatient to meet our case definition in age group i. This weight is calculated from the results of the prospective case–control study as a ratio of suspected cases over registered cases of cough illnesses at the selective departments of SHs. Ni is the population size in age group i in census year 2020. is the weight used to adjust catchment population overlapping between participating hospitals from HUAS in age group i. It is calculated as the ratio of community residents who have the reported seeking medical care in the participating hospitals for the last episodes of their cough illness over the residents who have the willingness of healthcare-seeking in the participating hospitals, as obtained from the HUAS study. Ci is the proportion of population covered by participating hospitals in age group i, as measured in the HUAS study. It is calculated as the proportion of residents who report having the willingness of healthcare-seeking in the participating hospitals over the total no. of residents responded. is the proportion of community residents reporting seeking healthcare for their most recent episode of cough illnesses in age group i as measured in the HUAS study. AFi is the population AF of chronic/persistent cough due to B. pertussis infection in age group i, calculated based on case–control study using unconditional logistic regression model, as follows:
Pr(B. pertussis|Chronic cough) = is the prevalence of B. pertussis, calculated by dividing the number of laboratory-confirmed pertussis with the total number of chronic/persist cough tested. x1, x2, x3, …, xk are variables associated with the occurrence of chronic/persistent cough, including the presence of B. pertussis and other social and environmental factors significant at p<0.1 in univariate analysis.
The 95% CI of incidence is calculated with bootstrap method with 1000 replications. Besides incidence estimates, we will also explore factors associated with severe pertussis (defined as a composite outcome of death, sepsis, invasive ventilation and Intensive Care Unit transfer), by using multivariable logistic regression. Factors significantly associated with severe pertussis at p<0.1 in univariate analysis will be included in the model. The median age of children with pertussis will be calculated by type of vaccines, and factors predicting the age of pertussis breakthrough among children who had received DTP vaccination early in their life will be also studied by using Cox proportional hazards regression models. For sensitivity analysis, we will use a twofold or greater increase of anti-Ptx IgG antibody as the cut-off threshold for our serological assays and calculate incidence again.
Ethics and dissemination
This study is designed an observational study. The risk of harm is minimal and adverse medical events are not anticipated from the procedures involved in the study. The study protocol, CRF and consent form have been sent to and approved by China CDC’s Institutional Review Board (reference no. ICDC-202110).
The primary risk to participants is the loss of confidentiality. To help maintain confidentiality, all study investigators will sign a confidentiality agreement and receive appropriate ethics training. All interviews will be conducted at the study investigator’s office, and signed consent forms and completed survey forms will be locked in a secure file cabinet at the end of each day. A very limited number of trained study staff can have the key to the locked file cabinets. Participation in every aspect of the study will be voluntary, and for all new data collection, participants will be asked to provide written informed consent. Besides, collection of specimens may cause mild discomfort to the subject during the procedure, especially drawing blood from young children. To minimise invasive procedures during sample collection, swab and blood specimens will be collected by aseptic technique and we encourage the use of leftover sera during routine medical care at the time point of enrolment.
As a benefit of participating in the study, participants with pertussis will receive senior doctor consultation during treatment on how to limit transmissions among family members and coworkmates; patients enrolled in the study will have access to antibiotic susceptibility testing results should they have B. pertussis isolates acquired. This will give a guide on empirical antibiotic usages for physicians; moreover, the data generated in the study will be valuable to determine the burden of pertussis and explore risk factors for illness attributable to severe pertussis in children as well as adolescents/adults, which can be used by public health departments, healthcare providers and scientific group in China to inform policies making, implement disease control and prevention (ie, vaccination) and improve patient care, both at the sites level and national level. In general, the minimal risks associated with physical discomfort during blood and N/P sample collection are offset by the great benefit associated with the study’s ability to inform pertussis prevention and control strategies in China. On completion, results from this study will be disseminated via academic presentations and publication in peer-reviewed journals.
Discussion
PertussisChina is an innovative and a pilot of a laboratory-based and population-based active surveillance platform for vaccine-preventable bacterial diseases in China, which endeavours to establish a network of laboratories and hospitals using comparable and unified standards to provide up-to-date disease burden estimates and disease determinants for evaluating, prioritising and optimising the use of vaccines and for the development of new interventions against bacterial infections in the country. Pertussis is the first one of the several bacterial infections that we are planning to take this approach. In response to the changing epidemiology of pertussis in China,7 8 42 43 the 2019 summon of the National Immunization Advisory Committee submitted a motion to its members urging the modification of the current immunisation schedule of pertussis vaccine administered at 3, 4, 5 and 18–24 months44, to vaccinate children at 2, 4, 6 and 18–24 months instead and to add a fifth booster dose at 4–6 years of age; however, partly due to knowledge gaps existed in age-specific burden of pertussis, NIAC suspended its decision on this issue. To provide up-to-date evidence on disease burden of pertussis, this study will focus on age-specific incidence based on laboratory confirmation and will fill the data gaps on prospectively and actively collected incidence data and key information on illness severity and outcomes. We are expecting that data from this study can be served as background information augmenting NIDSS to inform NIAC’s recommendations on children vaccination and further quantify the benefit of adolescent/adult vaccination to protect infants from severe outcomes in future. There are several strengths of the study.
In this 1-year study, we will enrol suspected chronic/persistent cough patients (for infants aged less than 1 year, cough regardless of duration) from healthcare facilities in two sites of China, covering a censused population of 2.23 million. The catchment population utilising healthcare services at the SHs are well characterised and defined by HUAS, providing unbiased estimates of age-stratified total person-times observed in the cohort. The prevalence of cough in regarding of illness duration and proportion of people who do not seek healthcare are measured retrospectively by HUAS. Thus by comparing between data generated from HUAS in community and case registry in SHs, we will able to measure incidence by settings (ie, community, outpatient and inpatient), especially those in communities whose symptoms are mild or atypical after the waning of vaccine-induced immunity or those no healthcare are sought.2 Besides, all hospitalisations suspected of pertussis will be actively searched and prospectively enrolled in a timely manner in our SHs, serving as a complete and representative sample of pertussis occurred in the interested population that would have induced minimal selection bias. As for milder cases in ambulatory settings, sampling of patients with chronic/persistent cough in outpatient setting to conduct laboratory investigation is preferred. Misclassification of cases or recall bias will be minimised by the complex laboratory procedures (ie, culture, PCR and serology combined), unified data collection tools (ie, CRFs) and data collection process, that is, the 60-day of follow-up during which interesting events (eg, threefold titre raising) will be closely monitored by sequential sera samples. Using laboratory-confirmed pertussis as the outcome will allow us to specifically measure pertussis disease burden. To account for asymptomatic carriage of B. pertussis, we will recruit healthy control to investigate the proportion of population carrying B. pertussis in their upper respiratory tract and seropositivity, which could be useful for calculating population AF to adjust rate estimates. In addition, the prospective cohort will provide valuable follow-up data related to risk factors for severe illness (ie, adverse clinical outcomes of hospitalisation or death). Collection of the vaccination history (including band, dosing, procedure and time of administration) from study participants will help explore the breakthrough rates of B. pertussis infection among different type of vaccinees and investigate reasons of vaccination failure, by linkage of study subjects ≤14 years old with his/her individual records on immunisation in the national database. Finally, we will abstract EMR data from HIS, which serves as a complete and accurate record of cough illness outcomes occurred in SHs. The retrospectively collected EMR data will be validated by prospectively counting cases eligible for inclusion at selective departments of SHs on daily basis. Using data from the EMR will allow us to determine the size of outpatient and emergency department visits for cough illness in the studied population. For most of adults and fully immunised children and adolescents, their illness is generally mild and is most likely to be encountered at the ambulatory settings in which the diagnostic capacity is generally lacking.
Aside from acquiring incidence estimates, the prevalence and distribution of B. pertussis strains circulating in the population will be determined and characterised, which are reported to be evolving under the selection pressure from both vaccine and antibiotics in previous studies45 and are important data for the development of novel vaccine or new therapeutics in the country. For example, as a benefit of the study, we will create a representative national and well-characterised repository of strains and specimens that can be shared with other investigators for future research, the main antigenic and genotypic features of B. pertussis will be characterised by sequencing or other bio-molecular methods.
We realised that there are several limitations worthy of note in our study. First, we will not identify all pertussis that occur in our studied population since our case definition will not capture atypical and asymptomatic manifestations associated with B. pertussis infection. For example, previous studies showed that about 17.4% children46 and 20% adolescents/adults47 with B. pertussis infection had a cough duration less than 3 weeks, and other symptoms/signs used in the case definition, like spasmodic cough (63%), post-tussive vomiting (42%) and whoops (8%), were infrequently presented in adults,48 which will make incidence underestimated. It is argued that no symptom is sufficiently predictive for diagnosing pertussis49 and there was no case definition that has been proposed for purpose of studying disease burden of pertussis. After balancing at the sensitivity and specificity of case definition commonly recommended by WHO, the US and others50–52 and the available laboratory capacity and resources in the study, we finally adopted the current case definition that can be used to facilitate comparison of results between studies and countries. Second, our study period is a little short. Since pertussis has showed a cyclic pattern and peaked every 3–5 years,2,16 our study will not capture this feature. Moreover, our study is going to recruit cases in 2021–2022, right after COVID-19 pandemic. As the epidemiology of many respiratory infections has been reported changing as a result of widely implementation of non-pharmaceutical interventions (eg, wearing masks, social distancing and personal health protection)53 54 and the detained coverage of vaccines used in Expanded Programme on Immunisation during the pandemic.55 The impacts of COVID-19 outbreak on incidence estimates of pertussis are not foreseeable in the study. Future studies are upcoming depending on the results of this pilot. Finally, China is a big country with large variations in population density and across different climate, geographic and economic regions. Although we have paid careful attention to variables, like DTP3 vaccine coverage, childhood mortality and healthcare delivery pattern when selecting study sites, regions with the highest and lowest reported incidence of pertussis are generally not included. This may also influence the generalisability of the incidence estimates to extrapolate to other regions.
In summary, PertussisChina is an innovative study that uses unified protocol to generate up-to-date high-quality incidence data on pertussis. The study design can secure the precision of data collection and provide insights into the prospectively conducted studies that designed to augment passive surveillance in countries where resources is limited and data are currently lacking. When completed, the results coming out this study will provide valuable scientific data on the incidence, aetiology and risk factors for severe sequelae of pertussis to academic societies and the public health authorities, who is currently struggling and fighting against this burdensome disease worldwide.
Supplementary Material
Footnotes
JY, HH, YZ and YG contributed equally.
Contributors: ZS is the principal investigator on this study who conceived and critically revised the manuscript. JY, HH and YZ conceptualised and designed the study, wrote the first draft and contributed equally to this work. YG, JX, LX and YG designed the laboratory methods. XZ, QZ, YZ and XT wrote the statistical analysis plan. CC and ZC commented on and revised drafts of the manuscript. All authors contributed to reviewing, revising and approving the final manuscript.
Funding: This study received funding from the Beijing Municipal Natural Science Foundation of China (no. L192060), the National Natural Science Foundation of China (no. 81973106), the Ministry of Science and Technology of the People’s Republic of China (no. 2018Z×09737–007), the Chinese Preventive Medicine Association and the Sanofi Pasteur.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Consent obtained from parent(s)/guardian(s).
References
- 1.Pluta RM, Lynm C, Glass RM. Pertussis. JAMA 2010;304:922. 10.1001/jama.304.8.922 [DOI] [PubMed] [Google Scholar]
- 2.Kilgore PE, Salim AM, Zervos MJ, et al. Pertussis: microbiology, disease, treatment, and prevention. Clin Microbiol Rev 2016;29:449–86. 10.1128/CMR.00083-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.VanderEnde K, Gacic-Dobo M, Diallo MS, et al. Global Routine Vaccination Coverage - 2017. MMWR Morb Mortal Wkly Rep 2018;67:1261–4. 10.15585/mmwr.mm6745a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.PERISCOPE Consortium . Periscope: road towards effective control of pertussis. Lancet Infect Dis 2019;19:e179–86. 10.1016/S1473-3099(18)30646-7 [DOI] [PubMed] [Google Scholar]
- 5.Cherry JD. Epidemic pertussis in 2012--the resurgence of a vaccine-preventable disease. N Engl J Med 2012;367:785–7. 10.1056/NEJMp1209051 [DOI] [PubMed] [Google Scholar]
- 6.Choi YH, Campbell H, Amirthalingam G, et al. Investigating the pertussis resurgence in England and Wales, and options for future control. BMC Med 2016;14:121. 10.1186/s12916-016-0665-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Bambrick H, Mengersen K, et al. Resurgence of pertussis infections in Shandong, China: space-time cluster and trend analysis. Am J Trop Med Hyg 2019;100:1342–54. 10.4269/ajtmh.19-0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang H, Zhu T, Gao C, et al. Epidemiological features of pertussis resurgence based on community populations with high vaccination coverage in China. Epidemiol Infect 2015;143:1950–6. 10.1017/S095026881400260X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.GBD 2019 Diseases and Injuries Collaborators . Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet 2020;396:1204–22. 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeung KHT, Duclos P, Nelson EAS, et al. An update of the global burden of pertussis in children younger than 5 years: a modelling study. Lancet Infect Dis 2017;17:974–80. 10.1016/S1473-3099(17)30390-0 [DOI] [PubMed] [Google Scholar]
- 11.Li G, Zhang H, Zhou W, et al. Safety and immunogenicity of a diphtheria, tetanus, acellular pertussis and Haemophilus influenzae type B combination vaccine compared with separate administration of licensed equivalent vaccines in Chinese infants and toddlers for primary and booster immunization. Vaccine 2010;28:4215–23. 10.1016/j.vaccine.2010.03.061 [DOI] [PubMed] [Google Scholar]
- 12.Li RC, Li FX, Li YP, et al. Antibody persistence at 18-20 months of age and safety and immunogenicity of a booster dose of a combined DTaP-IPV//PRP∼T vaccine compared to separate vaccines (DTaP, PRP∼T and IPV) following primary vaccination of healthy infants in the people's Republic of China. Vaccine 2011;29:9337–44. 10.1016/j.vaccine.2011.09.131 [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Lei D, Zhang S. Acellular pertussis vaccines in China. Vaccine 2012;30:7174–8. 10.1016/j.vaccine.2012.10.009 [DOI] [PubMed] [Google Scholar]
- 14.Liang X. [Analysis of the situation of China’s immunization program]. The 2nd Annual Meeting of the Chinese Preventive Medicine Association & the 2nd Annual Meeting of the Global Chinese Public Health Association, Xianghe, Hebei, China, 2006. [Google Scholar]
- 15.World Health Organization . WHO-UNICEF estimates of DTP3 coverage (last update: 15-Jul-2018). who vaccine-preventable diseases: monitoring system 2018 global summary. World Health Organization, 2018. [Google Scholar]
- 16.Skoff TH, Hadler S, Hariri S. The epidemiology of nationally reported pertussis in the United States, 2000-2016. Clin Infect Dis 2019;68:1634–40. 10.1093/cid/ciy757 [DOI] [PubMed] [Google Scholar]
- 17.Carvalho CFA, Andrews N, Dabrera G, et al. National outbreak of pertussis in England, 2011-2012: a case-control study comparing 3-Component and 5-Component acellular vaccines with whole-cell pertussis vaccines. Clin Infect Dis 2020;70:200–7. 10.1093/cid/ciz199 [DOI] [PubMed] [Google Scholar]
- 18.Domenech de Cellès M, Magpantay FMG, King AA, et al. The impact of past vaccination coverage and immunity on pertussis resurgence. Sci Transl Med 2018;10. 10.1126/scitranslmed.aaj1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yih WK, Lett SM, des Vignes FN, et al. The increasing incidence of pertussis in Massachusetts adolescents and adults, 1989-1998. J Infect Dis 2000;182:1409–16. 10.1086/315863 [DOI] [PubMed] [Google Scholar]
- 20.Kmietowicz Z. Pertussis cases rise 10-fold among older children and adults in England and Wales. BMJ 2012;345:e5008. 10.1136/bmj.e5008 [DOI] [PubMed] [Google Scholar]
- 21.Guijun N, Yuan G, Dan W. [Epidemiology of pertussis in China, 2011-2017]. Chin J Vaccin Immun 2018;24. [Google Scholar]
- 22.Wang L, Wang Y, Jin S, et al. Emergence and control of infectious diseases in China. Lancet 2008;372:1598–605. 10.1016/S0140-6736(08)61365-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutter RW, Cochi SL. Pertussis hospitalizations and mortality in the United States, 1985-1988. evaluation of the completeness of national reporting. JAMA 1992;267:386–91. [PubMed] [Google Scholar]
- 24.Heininger U, Stehr K, Schmidt-Schläpfer G, et al. Bordetella pertussis infections and sudden unexpected deaths in children. Eur J Pediatr 1996;155:551–3. 10.1007/BF01957903 [DOI] [PubMed] [Google Scholar]
- 25.Ministry of Health . [National Guidelines and Recommandations on the Implementation of the Expanded Program on Immunization (EPI)] Beijing, 2007. Available: http://www.gov.cn/gzdt/2008-02/19/content_893572.htm
- 26.GBD 2015 LRI Collaborators . Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the global burden of disease study 2015. Lancet Infect Dis 2017;17:1133–61. 10.1016/S1473-3099(17)30396-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen R-chong, Lai K-fang, Liu C-li, et al. [An epidemiologic study of cough in young college students in Guangzhou]. Zhonghua Liu Xing Bing Xue Za Zhi 2006;27:123–6. [PubMed] [Google Scholar]
- 28.Wright SW, Edwards KM, Decker MD, et al. Pertussis infection in adults with persistent cough. JAMA 1995;273:1044–6. [PubMed] [Google Scholar]
- 29.Cornia PB, Hersh AL, Lipsky BA, et al. Does this coughing adolescent or adult patient have pertussis? JAMA 2010;304:890–6. 10.1001/jama.2010.1181 [DOI] [PubMed] [Google Scholar]
- 30.Nennig ME, Shinefield HR, Edwards KM, et al. Prevalence and incidence of adult pertussis in an urban population. JAMA 1996;275:1672–4. [PubMed] [Google Scholar]
- 31.Craig R, Kunkel E, Crowcroft NS, et al. Asymptomatic infection and transmission of pertussis in households: a systematic review. Clin Infect Dis 2020;70:152–61. 10.1093/cid/ciz531 [DOI] [PubMed] [Google Scholar]
- 32.Zhang Q, Li M, Wang L, et al. High-Resolution melting analysis for the detection of two erythromycin-resistant Bordetella pertussis strains carried by healthy schoolchildren in China. Clin Microbiol Infect 2013;19:E260–2. 10.1111/1469-0691.12161 [DOI] [PubMed] [Google Scholar]
- 33.Zhang Q, Yin Z, Li Y, et al. Prevalence of asymptomatic Bordetella pertussis and Bordetella parapertussis infections among school children in China as determined by pooled real-time PCR: a cross-sectional study. Scand J Infect Dis 2014;46:280–7. 10.3109/00365548.2013.878034 [DOI] [PubMed] [Google Scholar]
- 34.Linnemann CC, Bass JW, Smith MH. The carrier state in pertussis. Am J Epidemiol 1968;88:422–7. 10.1093/oxfordjournals.aje.a120903 [DOI] [PubMed] [Google Scholar]
- 35.L-s C, J-s Z, Cao L. Progress of Childhood Immunization Information Management System in China in 2011]. Chin J Vaccin Immun 2012;18:162–5. [Google Scholar]
- 36.van der Zee A, Schellekens JFP, Mooi FR. Laboratory diagnosis of pertussis. Clin Microbiol Rev 2015;28:1005–26. 10.1128/CMR.00031-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medical Administration Department of Ministry of Public Health . National guide to clinical laboratory procedures. fourth. Beijing: People’s Health Publishing House, 2015. [Google Scholar]
- 38.World Health Organization . Laboratory manual for the diagnosis of whooping cough caused by Bordetella pertussis-Bordetella parapertussis. World Health Organization, 2014. [Google Scholar]
- 39.Centers for Disease Control and Prevention . Best practices for health care professionals on the use of polymerase chain reaction (PCR) for diagnosing pertussis, 2011. [Google Scholar]
- 40.Tatti KM, Sparks KN, Boney KO, et al. Novel multitarget real-time PCR assay for rapid detection of Bordetella species in clinical specimens. J Clin Microbiol 2011;49:4059–66. 10.1128/JCM.00601-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riffelmann M, Thiel K, Schmetz J, et al. Performance of commercial enzyme-linked immunosorbent assays for detection of antibodies to Bordetella pertussis. J Clin Microbiol 2010;48:4459–63. 10.1128/JCM.01371-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu TC, Zhang J, Liu SQ, et al. Evaluation of immunisation strategies for pertussis vaccines in Jinan, China - an interrupted time-series study. Epidemiol Infect 2020;148:e26. 10.1017/S0950268820000102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Z, Liu S, Shu Y, et al. Severe Bordetella pertussis infection and vaccine issue in Chongqing, from 2012 to 2018. Int J Infect Dis 2019;84:102–8. 10.1016/j.ijid.2019.05.014 [DOI] [PubMed] [Google Scholar]
- 44.National Health Commission of the People’s Republic of China . Instructions and Vaccination Schedule for Vaccines Used in National Immunisation Program (2021 Edition): National Health Commission of the People’s Republic of China, 2021. Available: http://www.nhc.gov.cn/cms-search/xxgk/getManuscriptXxgk.htm?id=590a8c7915054aa682a8d2ae8199e222
- 45.Xu Z, Wang Z, Luan Y, et al. Genomic epidemiology of erythromycin-resistant Bordetella pertussis in China. Emerg Microbes Infect 2019;8:461–70. 10.1080/22221751.2019.1587315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heininger U, Klich K, Stehr K, et al. Clinical findings in Bordetella pertussis infections: results of a prospective multicenter surveillance study. Pediatrics 1997;100:E10. 10.1542/peds.100.6.e10 [DOI] [PubMed] [Google Scholar]
- 47.Hewlett EL, Edwards KM. Clinical practice. Pertussis--not just for kids. N Engl J Med 2005;352:1215–22. 10.1056/NEJMcp041025 [DOI] [PubMed] [Google Scholar]
- 48.Postels-Multani S, Schmitt HJ, Wirsing von König CH, et al. Symptoms and complications of pertussis in adults. Infection 1995;23:139–42. 10.1007/BF01793853 [DOI] [PubMed] [Google Scholar]
- 49.Cornia PB, Lipsky BA. Symptoms Associated With Pertussis Are Insufficient to Rule In or Rule Out the Diagnosis. Chest 2019;155:449–50. 10.1016/j.chest.2018.10.028 [DOI] [PubMed] [Google Scholar]
- 50.Moore A, Harnden A, Grant CC, et al. Clinically diagnosing Pertussis-associated cough in adults and children: chest guideline and expert panel report. Chest 2019;155:147–54. 10.1016/j.chest.2018.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prevention CfDCa . Pertussis (whooping cough) (Bordetella pertussis) 2020 case definition, 2020. Available: https://wwwn.cdc.gov/nndss/conditions/pertussis/case-definition/2020/ [Accessed 29 Apr 2021].
- 52.China MoHotPsRo . Diagnostic criteria for pertussis (2007 version). Beijing: People’s Medical Publishing House, 2007. [Google Scholar]
- 53.Cowling BJ, Ali ST, Ng TWY, et al. Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: an observational study. Lancet Public Health 2020;5:e279–88. 10.1016/S2468-2667(20)30090-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huh K, Jung J, Hong J, et al. Impact of Nonpharmaceutical interventions on the incidence of respiratory infections during the coronavirus disease 2019 (COVID-19) outbreak in Korea: a nationwide surveillance study. Clin Infect Dis 2021;72:e184–91. 10.1093/cid/ciaa1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu J, Yu W, Cao L, et al. Effectiveness of Catch-Up Vaccinations after COVID-19 Containment - China, 2020. China CDC Wkly 2020;2:968–74. 10.46234/ccdcw2020.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-053316supp001.pdf (246.9KB, pdf)