Abstract
Background
Azapirones are a group of drugs that work at the 5‐HT1A receptor and are used to treat patients suffering from generalized anxiety disorder (GAD). However, several studies have shown conflicting results. Whether azapirones are useful as first line treatment in general anxiety disorders still needs to be answered.
Objectives
To assess the efficacy and the acceptability of azapirones for the treatment of GAD.
Search methods
Initiallyt the Cochrane Collaboration Depression, Anxiety and Neurosis Controlled Trials Register (CCDANCTR) and The Cochrane Central Register of Controlled Trials (CENTRAL) were searched, incorporating results of group searches of MEDLINE (1966 to June 2005), EMBASE (1980 to June 2005), CINAHL (1982 to June 2005), PsycLIT (1974 to June 2005), PSYNDEX (1977 to June 2005), and LILACS (1982 to June 2005). Subsequently the revised Cochrane Collaboration Depression, Anxiety and Neurosis Controlled Trials Registers (CCDANCTR‐Studies and CCDANCTR‐References) were searched on 21‐10‐2005. Reference lists of relevant papers and major text books of anxiety disorder were examined. Authors, other experts in the field and pharmaceutical companies were contacted for knowledge of suitable trials, published or unpublished. Specialist journals concerning azapirones were handsearched.
Selection criteria
Randomized controlled trials of azapirones, including buspirone versus placebo and/or other medication and/or psychological treatment, were included. Participants were males and females of all ages with a diagnosis of generalized anxiety disorder.
Data collection and analysis
Data were extracted from the original reports independently by CC, MA and MT. The main outcomes studied were related to the objectives stated above. Data were analysed for generalized anxiety disorder versus placebo, versus other medication and versus psychological treatment separately. Data were analysed using Review Manager Version 4.2.7.
Main results
Thirty six trials were included in the review, reporting on 5908 participants randomly allocated to azapirones and/or placebo, benzodiazepines, antidepressants, psychotherapy or kava kava. Azapirones, including buspirone, were superior to placebo in treating GAD. The calculated number needed to treat for azapirones using the Clinical Global Impression scale was 4.4 (95% confidence interval (CI) 2.16 to 15.4). Azapirones may be less effective than benzodiazepines and we were unable to conclude if azapirones were superior to antidepressants, kava kava or psychotherapy. Azapirones appeared to be well tolerated. Fewer participants stopped taking benzodiazepines compared to azapirones. The length of studies ranged from four to nine weeks, with one study lasting 14 weeks.
Authors' conclusions
Azapirones appeared to be useful in the treatment of GAD, particularly for those participants who had not been on a benzodiazepine. Azapirones may not be superior to benzodiazepines and do not appear as acceptable as benzodiazepines. Side effects appeared mild and non serious in the azapirone treated group. Longer term studies are needed to show that azapirones are effective in treating GAD, which is a chronic long‐term illness.
Keywords: Humans, Anti‐Anxiety Agents, Anti‐Anxiety Agents/adverse effects, Anti‐Anxiety Agents/therapeutic use, Antidepressive Agents, Antidepressive Agents/therapeutic use, Anxiety Disorders, Anxiety Disorders/drug therapy, Anxiety Disorders/therapy, Benzodiazepines, Benzodiazepines/therapeutic use, Buspirone, Buspirone/therapeutic use, Psychotherapy, Pyrimidines, Pyrimidines/therapeutic use, Randomized Controlled Trials as Topic
Plain language summary
Azapirones for generalized anxiety disorder (GAD)
Generalized anxiety disorder is one of the most common anxiety disorders and can be costly if unrecognized or left untreated. Azapirones are a group of drugs that work at the 5‐HT1A receptor and are used to treat patients suffering from GAD. This systematic review evaluates the effectiveness of azapirones compared to other treatments. From the results of 36 randomized controlled trials, azapirones appear to be superior to placebo in short‐term studies (four to nine weeks) but may not be superior to benzodiazepines. We were unable to conclude if azapirones were superior to antidepressants, psychotherapy or kava kava. As GAD is generally chronic in nature, conclusions about azapirones' long‐term efficacy are not able to be made and longer term trials are needed.
Background
Generalized anxiety disorder (GAD) is characterized by excessive, pervasive and uncontrollable worry. Associated symptoms include irritability, restlessness and concentration problems. The overall lifetime prevalence of GAD in community samples range from 5 to 6 % and in similar populations the one year prevalence rates range from 2.2 to 4.9 % (Kessler 1994). When left undiagnosed and untreated, GAD can result in high social costs (Bond 2002).
Patients diagnosed as having GAD may be treated with some form of psychotherapy, associated or not with the use of medication (Feighner 1989; Borkovec 1993). Benzodiazepines are the drugs most commonly prescribed and their efficacy and relative safety are well established (Shader 1993), although antidepressants are also being used more commonly (Kapczinski 2005). In particular, data from a pooled analysis of five studies have shown venlafaxine XR to be an effective treatment for GAD (Meoni 2001).
The long term use of benzodiazepines is associated with physical dependency, withdrawal symptoms and important side effects such as ataxia, sedation and memory deficits (Lydiard 1997). The side effects of benzodiazepines have prompted the search for new treatments for GAD. It has been reported that the anxiolytic properties of azapirones are similar to those of benzodiazepines (Ballenger 2001). Azapirones are a group of drugs, for example buspirone, gepirone and ipsapirone, that work at the 5‐HT1A receptor; they do not present abuse potential or withdrawal symptoms (Cohn 1986).
Initial findings in patients with GAD showed that azapirones had an efficacy similar to benzodiazepines (Strand 1990). Subsequent studies using DSM‐III criteria showed that buspirone was not superior to placebo in GAD patients (Cohn 1986; Olajide 1987). It was also pointed out by some authors that patients who were treated with benzodiazepines were not satisfied when switching to buspirone (Goa 1986). Indeed, recent findings suggest that GAD patients previously treated with benzodiazepines are more likely to benefit from treatment with antidepressants than with buspirone (Rickels 2000). In a meta‐analysis of buspirone (Gammans 1992), results showed that patients with GAD, plus coexisting depressive symptoms of at least moderate intensity, exhibited significantly greater improvement with buspirone compared to placebo treatment. Long‐term studies are lacking.
However, several studies have shown conflicting results and whether azapirones are useful as first line treatment in general anxiety disorders still needs to be answered.
OUTLINE OF THIS SERIES OF REVIEWS Several treatments have been proposed for GAD. In this series of systematic reviews, they have been grouped as follows:
(1) Psychotherapy; (2) Antidepressants; (3) Azapirones (including buspirone); (4) Benzodiazepines; (5) Other treatments
In this review, we propose to assess the role of azapirones in the treatment of GAD.
Objectives
To investigate the efficacy and acceptability of azapirones in the treatment of generalized anxiety disorder.
Methods
Criteria for considering studies for this review
Types of studies
Prospective randomized controlled trials.
Types of participants
Males and females diagnosed with generalized anxiety disorder.
Types of interventions
(1) Azapirone alone (2) Azapirone combined with other drugs and/or psychological treatment
Control intervention: (1) Placebo (2) Benzodiazepine or antidepressant (3) Different azapirones (4) Psychological treatment (5) Kava kava
Types of outcome measures
(1) Hamilton Anxiety Scale (HAM‐A) (Hamilton 1959) end point means and standard deviations (SD) (i) Clinical Global Impression (CGI) Scale end point scores of one or two (much or very much improved)
(2) Acceptability of the treatment as measured by: (i) Number of people dropping out during the trial (ii) Specific side effects
Search methods for identification of studies
See: Collaborative Review Group search strategy
(1) Electronic databases Initially the Cochrane Collaboration Depression, Anxiety and Neurosis Controlled Trials Register (CCDANCTR) and The Cochrane Controlled Clinical Trials Register (CCTR), incorporating results of group searches of MEDLINE (1966‐June 2005), EMBASE (1980‐June 2005), CINAHL (1982‐June 2005), PsycLIT (1974‐June 2005), PSYNDEX (1977‐June 2005), and LILACS (1982‐June 2005) were searched using the following terms: Azapirones or Buspirone or Ipsapirone or Gepirone or Lesopitron or Tandospirone and Generalized Anxiety Disorder and Psychological treatment or Psychological placebo.
(2) Reference checking The reference lists of all identified randomized controlled trials, other relevant papers and major textbooks on anxiety disorder were checked.
(3) Handsearching The journal Depression and Anxiety was handsearched.
(4) Personal communication The authors of randomized controlled trials included in the review and other recognized experts in the field were contacted and asked if they had knowledge of any other studies, published or unpublished, relevant to the review. Pharmaceutical companies marketing azapirone products were requested to provide relevant published and unpublished data (see CCDAN group policy).
(5) To supplement and confirm these results we also searched the revised CCDAN registers on 21‐10‐2005
CCDANCTR‐Studies was searched using the terms: Diagnosis = "Generalized Anxiety" and Intervention = Buspirone or Ipsapirone or Gipirone or Lesopitron or Tandospirone
CCDANCTR‐References was searched using the terms Free‐Text = "Generalized Anxiety" and Free‐Text = Azaprione or buspirone or ipsapirone or Gepirone or Lesopitron or Tandospirone.
Data collection and analysis
Selection of trials and data extraction Studies generated by the search strategies were checked to ensure that they met the previously defined inclusion criteria. Three reviewers (CC, MA, MT) independently extracted data concerning participant characteristics, intervention details and outcome measures from the included studies. Any disagreements were resolved by consensus.
Assessment of study quality The methodological quality of the included studies was assessed according to the Cochrane criteria for quality assessment (Higgins 2005). It is based on the evidence of a strong relationship between the potential for bias in the results and the allocation concealment and is defined as below:
A. Low risk of bias (adequate allocation concealment) B. Moderate risk of bias (unclear method of allocation concealment) C. High risk of bias (inadequate allocation concealment)
For the purpose of the analysis in this review, trials were included if they met the criteria A or B as described in the Cochrane Handbook.
Analysis In the statistical analysis, the relative risk (RR) and 95% confidence interval (CI) for dichotomous and continuous variables were calculated using the random‐effects model, as it takes into account any study differences (even if there is no statistically significant heterogeneity) and gives the same result as the fixed‐effects model, when there is no between‐study variance. Results were analyzed using the Review Manager Software 4.2.4. In the efficacy analysis, the number needed to treat (NNT) was also calculated using 95% CI and studies ranging from 4 to 9 weeks of follow‐up were entered to provide a similar length of treatment. The NNT expresses the number of patients that must be treated in order to achieve one response, when compared to the control group.
Data on continuous outcomes are frequently skewed, where the mean is not the centre of the distribution. The statistics for meta‐analysis are thought to be able to cope with some skew, but were formulated for parametric data. To avoid this potential risk, means and standard deviations were reported and changed data were not included, which can be more problematic (Altman 1996).
Heterogeneity in the results of the trials was assessed both by inspection of graphical presentations and by calculating a test for heterogeneity. Possible reasons for heterogeneity were not predetermined but once it was determined that heterogeneity was present, different studies were examined for both methodological differences but also for different medication effects. This was assessed by looking at separate subgroups of trials.
Results
Description of studies
Reasons for excluding studies (1) Missing data (Bourin 1995; Bouvard 1998; Fontaine 1987; Petracca 1990; Rickels 1988; Rolland 2000; Rouillon 1995; Schweizer 1990; Sramek 1996; Tyrer 1984; Yevich 1990) (2) Non‐standardized criteria (Goldberg 1982; Rickels 1982; Strand 1990; Wheatley 1982) (3) Substance comorbidities (Tollefson 1991) (4) Non‐randomized criteria (Lucki 1987; Olajide 1987; Rickels 2000; Shah 1991; Sontheimer 2001; van Laar 1992) (5) Could not locate (Caetano 1990; Costa e Silva 1987; Shen 1999; van Amerongen 1991)
Search We retrieved 62 clinical trials (non‐duplicate) in which azapirones were used to treat GAD.
Thirty six trials assessing azapirones in adult GAD patients used diagnostic criteria for GAD and had data which could be included in this review (5908 patients in total). The 36 trials included:
Four trials comparing azapirones versus placebo reported HAM‐A means and SDs at the end of the trial (Chiaie 1995; Borison 1990; Ross 1987; Majercsik 2003).
Three trials comparing azapirones versus benzodiazepines reported HAM‐A means and SDs at the end of the trial (Borison 1990; Cohn 1986; Ross 1987).
Two trials comparing azapirones versus placebo (Harto 1988; Sramek 1996a) including one utilizing buspirone (Sramek 1996a), reported CGI rates of one or two at the end of the trial which were included in the final analysis.
Eight trials reporting dropout rates, including six which were used analyzing buspirone versus placebo (Cutler 1994; Enckelmann 1991; Laakmann 1998; Lader 1998; Pecknold 1989; Sramek 1996a) and two additional studies analyzing azapirones (Borison 1990; Harto 1988), were entered into the final analysis.
Fourteen trials reporting dropout rates, including 12 which were used for analyzing buspirone versus benzodiazepines (Ansseau 1990; Cohn 1986a; Enckelmann 1991; Fabre 1987; Feighner 1982; Harto 1988; Laakmann 1998; Lemone 1996; Pecknold 1989; Ramchandran 1990; Boral 1989; Sacchetti 1994) and two additional studies (Borison 1990; Rickels 1997) analyzing azapirones versus benzodiazepines, were entered into the final analysis.
One trial (Davidson 1999) comparing buspirone versus venlafaxine XR was handled separately.
One trial (Bond 2002) comparing buspirone versus psychotherapy was handled separately.
One trial (Chiaie 1995) in which participants were initially stabilized on a benzodiazepine and then were randomized to either buspirone or placebo was handled separately.
One trial (Murphy 1989) examining early and late withdrawal of buspirone and diazepam over a 14‐week period will be commented on separately.
One trial (Boerner 2003) comparing buspirone versus kava kava was handled separately.
The number of studies identified using different search strategies: Electronic databases, 37 studies identified; reference checking, 0 identified; handsearching, 0 identified; personal communication, 6 additional studies identified; librarian searched the CCDANCTR‐Studies, 22 identified.
Design All the studies were described as randomized and used a parallel group design. The duration of the active treatment versus placebo phases of each trial ranged from four to eight weeks for most with the exception of one trial (Boerner 2003) lasting nine weeks and one trial (Murphy 1989) lasting 14 weeks. All trials had a washout period before the active treatment phases.
Setting All trials studied outpatients from psychiatric clinics or from the community.
Participants All trials included for the main comparisons used either ICD‐10, DSM‐III, DSM‐III‐R or DSM‐IV criteria for the diagnosis of GAD. The study populations were reasonably comparable. The number of participants randomized ranged from 18 to 735.
Outcomes All trials used symptom scales in assessing treatment effects. The HAM‐A scale (Hamilton 1959) was used if end means and SDs were reported. Three dichotomous outcomes were used in this review including: a CGI scoring of one or two, specific side effects (whenever a side effect appeared in at least 10% of patients compared to any comparison group), and drop out rates.
Risk of bias in included studies
All RCTs were classified as 'B', not giving information on allocation concealment. We are still awaiting further details from most of the authors. All trials reported the randomization procedure without any information on allocation concealment.
Effects of interventions
Efficacy analysis
(1) Azapirones versus placebo
(a) HAM‐A The efficacy analysis intimally included four trials comparing azapirones versus placebo where HAM‐A means and SDs could be extracted at the end of the trial (Chiaie 1995; Borison 1990; Majercsik 2003; Ross 1987). When all four studies were entered into the meta‐analysis, the test for heterogeneity was positive with a P value of < 0.00001. Once the studies with a different design (Chiaie 1995; Majercsik 2003) were taken out and the studies using ipsapirone (Borison 1990) and buspirone (Ross 1987) were separately evaluated, the heterogeneity was non‐significant.
(i) Ipsapirone The only study available (N = 18), showed superiority compared to placebo by HAM‐A: the calculated weighted mean difference (WMD)was ‐4.3 (95% CI ‐7.33 to ‐1.27) (P = 0.005).
Buspirone The one study (N = 21) entered into the analysis, showed unclear results as to whether buspirone was better or not than placebo using reported HAM‐A (Cohn 1986): WMD (fixed) 0.4 (95% CI ‐5.62 to 6.42) (P = 0.9).
(ii) Buspirone The study by Chiaie (N = 38), was superior to placebo with a reported WMD (fixed) of ‐7.52 (95% CI ‐9.89 to ‐5.15) (P < 0.00001).
Buspirone The study by Majercsik (N = 52) was superior to placebo with a reported WMD (fixed) of ‐3.73 (95% CI ‐4.01 to ‐3.45) (P < 0.0001).
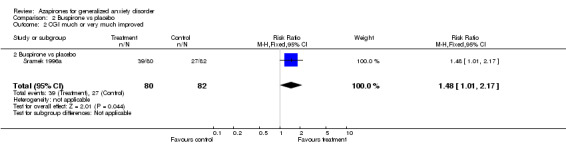
(b) CGI The efficacy analysis included two trials (N = 187) comparing azapirones (Harto 1988; Sramek 1996a) and one with buspirone versus placebo (Sramek 1996a).They reported CGI rates of one or two at the end of the trial. In general, short‐term treatment response was more likely in patients receiving azapirones versus placebo: Relative risk (RR) 1.74 (95% CI 1.21 to 2.5) (P = 0.003) and the calculated NNT was 4.4 (95% CI 2.16 to 15.4) utilizing CGI results (the two studies were six weeks in duration).
(i) Buspirone's one study (N = 162) reported favourably utilizing CGI, 1.48 RR (95% CI 1.01 to 2.17) (P = 0.04).
(2) Azapirones versus benzodiazepines
(a) HAM‐A The efficacy analysis initially included three trials comparing azapirones versus benzodiazepines where HAM‐A means and SDs could be extracted at the end of the trial (Borison 1990; Cohn 1986; Ross 1987). When all three studies were entered into the meta‐analysis, the test for heterogeneity was positive with a P value of 0.001. Once the studies were separated, analysed by azapirone (ipsapirone and buspirone) as well as by the benzodiazepine used (diazepam and lorazepam), the heterogeneity was non‐significant. In general, it is unclear if azapirones were better than benzodiazepines and in some cases benzodiazepines were better.
(i) Diazepam (Borison 1990) (N = 17) WMD 3.8 (95% CI 1.46 to 6.14) (P = 0.001) was superior to ipsapirone utilizing HAM‐A.
(ii) Lorazepam (Cohn 1986) (N = 40) WMD 1.1 (95% CI 0.29 to 1.91) (P = 0.008) and alprazolam (Cohn 1986) (N = 39) WMD 1.1 (95% CI 0.28 to 1.92) (P = 0.009) were superior to buspirone but buspirone compared to diazepam showed inconclusive results (Ross 1987) (N = 19) WMD ‐0.20 (95% CI ‐7.45 to 7.05) (P = 0.96).
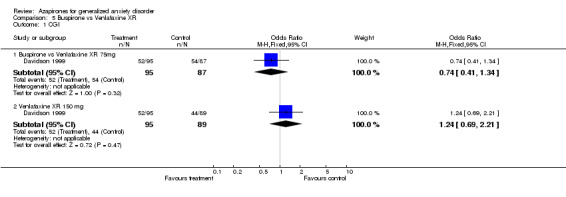
(3) Buspirone versus venlafaxine XR One study (Davidson 1999) showed non‐conclusive results utilizing CGI: Venlafaxine XR, 75 mg, N = 182; RR 0.74 (95% CI 0.41 to 1.34) and venlafaxine XR, 150 mg, N = 184, RR 1.24 (95% CI 0.69 to 2.21) (P = 0.47) but does appear from visualizing the graph that 150 mg may be a superior compared to 75 mg.
(4) Azapirones versus psychotherapy One study (Bond 2002) showed that all four treatment groups (N = 60) (two by two treatment groups: buspirone versus placebo and psychotherapy versus non‐directive treatment) were significantly improved from baseline but no difference between groups was noted.
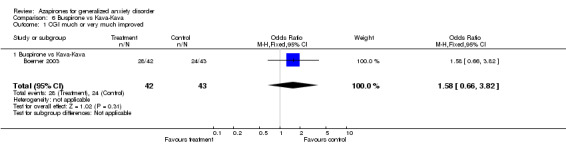
(5) Buspirone versus kava kava One study (Boerner 2003) showed non‐conclusive results utilizing CGI: RR 1.58 (95% CI 0.66 to 3.82). Side effect profiles were not statistically significant between the two drugs.
Acceptability
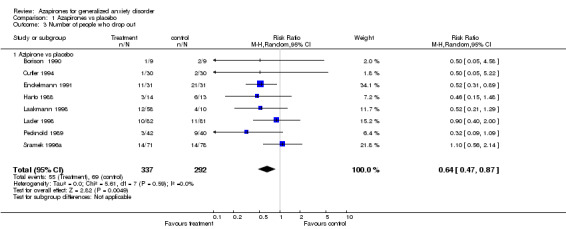
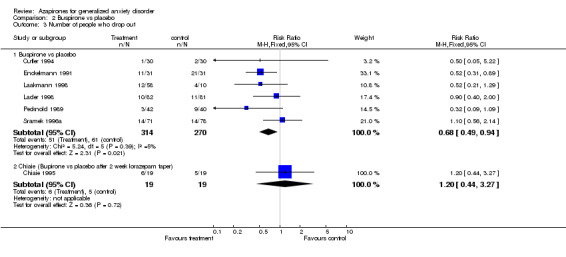
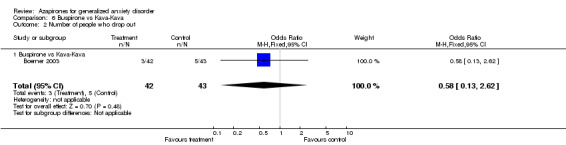
(a) Drop Out rates Significantly fewer participants dropped out who were on azapirones compared to placebo. The RR for any azapirone was 0.64 (95% CI 0.47 to 0.87) (P = 0.005). Similarly, when buspirone was considered alone, fewer participants on buspirone dropped out compared to placebo. The RR for buspirone was 0.68 (95% CI 0.49 to 0.94) (P = 0.02). In the study by Chiaie, no differences were noted in dropout rates with the RR (fixed) being 1.2 (95% CI 0.44 to 3.27) (P value of 0.72). Significantly fewer participants dropped out on benzodiazepines compared to azapirones. The RR favouring benzodiazepines over azapirones was 1.30 (95% CI 1.01 to 1.79) (P = 0.04). Similarly, when buspirone was considered alone, results were found to show a significant difference favouring benzodiazepines over buspirone. The RR favouring benzodiazepines over buspirone was 1.24 (95% CI 1.01 to 1.52) (P = 0.04). Buspirone compared to venlafaxine XR showed no significant differences between those who dropped out on buspirone versus venlafaxine XR 75 mg or 150 mg. The RR for buspirone versus venlafaxine XR 75mg was 0.98 (95% CI 0.6 to 1.60) (P = 0.92) and versus venlafaxine XR 150 mg was 0.7 (95% CI 0.45 to 1.09) (P = 0.12). Also, buspirone compared to kava kava showed no significant difference between those who dropped out. The RR for buspirone versus kava kava was 0.58 (95% CI 0.13 to 2.62) (P = 0.48).
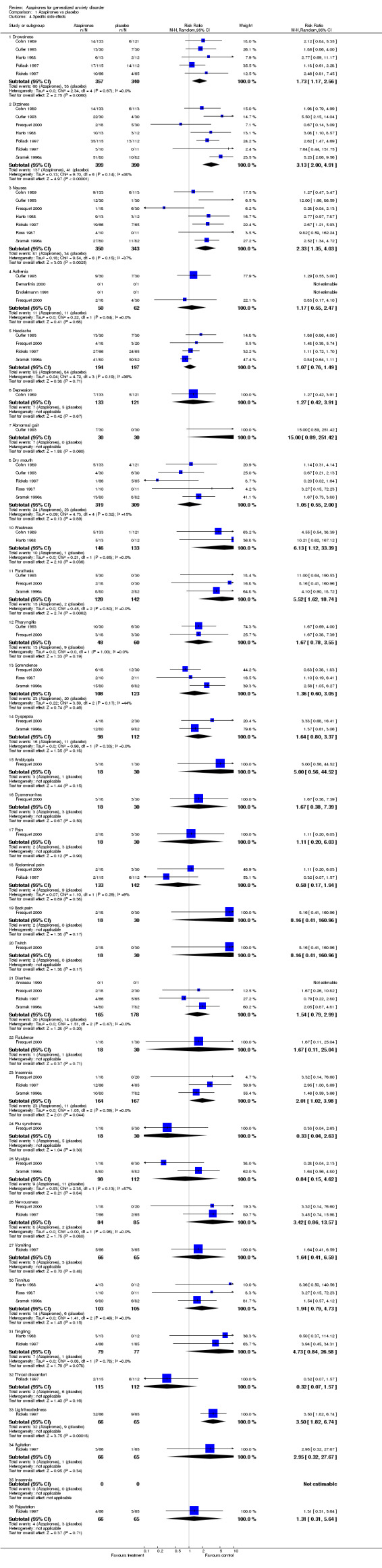
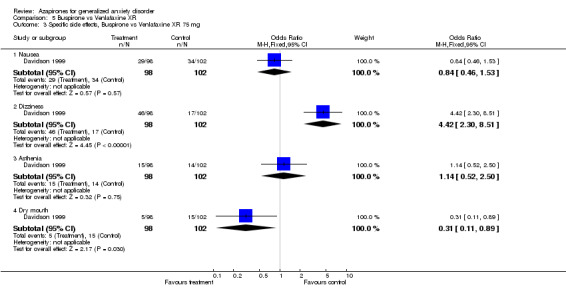
(b) Side effects Overall, no study reported significant side effects as a problem. Overall, side effects were more common in the drug treated groups than in the placebo treated groups. Those on azapirones reported more drowsiness, dizziness, nausea, weakness, parathesis, insomnia, and lightheadedness than those on placebo. Those on buspirone reported more dizziness and nausea compared to those on placebo. In the one study (Chiaie 1995) that utilized stabilizing patients on a benzodiazepine and then randomizing to buspirone versus placebo with a two week taper during randomizations, no significant withdrawal symptoms were noted. Withdrawal symptoms were more pronounced in the placebo versus buspirone at week one into the randomized phase but were not significant by the end of the randomized phase. Those on azapirones reported less fatigue, depression, sleepiness, sleep problems and dry mouth compared to benzodiazepines, while those on benzodiazepines reported less nausea and dizziness compared to those on azapirones. Those on buspirone reported less drowsiness, fatigue, nervousness, depression, insomnia and sleep problems compared to benzodiazepines, while those on benzodiazepines reported less nausea and dizziness compared to buspirone. In the one extension trial with a taper off (Cutler 1993; Mandos 1995), 25% of those on ipsapirone showed rebound symptoms compared to 40 % of those on lorazepam (P < 0.001). In the trial that discontinued either diazepam or buspirone at either six or twelve weeks (Murphy 1989), neither group had worsening symtoms of anxiety but those on diazepam did show withdrawal symptoms at six weeks compared to those on buspirone (P < 0.001). Those on buspirone reported less dry mouth compared to venlafaxine while those on venlafaxine reported less dizziness compared to venlafaxine. No differences in side effects were reported between buspirone and kava kava.
Discussion
Efficacy This review showed that azapirones, as indicated by the majority of the data, were effective in the treatment of patients with GAD compared to placebo. The calculated NNT for azapirones, as a group using CGI, was 4.4 (95% CI 2.16 to 15.4) in the trials lasting six weeks. This means that about five patients would have to be treated to cause one additional clinical improvement. A limitation was the small number of studies that we were able to include in the analysis.
Patients taking azapirones did not do as well as those on benzodiazepines, as reported by about 60 % of the studies available for analysis. However, a limitation was the small number of studies that were able to be included partially secondary to the issue of heterogeneity. This was due to a lack of reported measure results and heterogeneity in the different studies.
One study assessed the use of buspirone versus placebo in those who had been taking benzodiazepines (Chiaie 1995). Participants were able to tolerate being switched to either buspirone or placebo with less withdrawal side effects at week one if on buspirone. The results showed that buspirone was superior to placebo. From the analysis mentioned earlier in this section, it would be useful to know how patients did on buspirone compared to another benzodiazepine in this situation as opposed to placebo.
One study showed that those who were tapered off ipsapirone did better as far as rebound anxiety symptoms compared to lorazepam (Cutler 1993).
One study assessed withdrawal symptoms and rebound anxiety between diazepam and buspirone, both at six weeks and twelve weeks (Murphy 1989). Those on buspirone had no withdrawal symtoms at six weeks compared to diazepam. Also, of interest, is that diazepam and buspirone were both effective in treating GAD, but buspirone did not show equal efficacy until six weeks demonstrating a more rapid improvement on diazepam.
Only one study assessed the use of antidepressants versus azapirones (Davidson 1999). The results were inconclusive.
Only one study assessed the use of psychotherapy versus azapirones (Bond 2002). The results showed that all treatments were effective and that psychotherapy may be similarly effective as buspirone. Based on the small numbers, superiority of a specific treatment was not able to be demonstrated. Psychotherapy, usually incorporating some form of cognitive behavioral therapy, has been commonly used for the treatment of GAD (Borkovec 1993).
One study assessed the use of kava kava versus buspirone (Boerner 2003). Results were inconclusive as to whether buspirone was superior or not to kava kava. A recent Cochrane Review (Pittler 2001) did show kava kava to be useful for the treatment of anxiety. One limitation to this study was that the dosing of buspirone was generally higher than that used in the study and makes interpretation of results somewhat difficult. If a more usual dosing regiment had been used, it is possible buspirone may have been superior to kava kava.
Acceptability Drop out rates
This review showed that azapirones, including buspirone, had less drop outs compared to placebo, as indicated by the majority of the data. This suggests that azapirones, although benzodiazepines may fair better, have a good acceptability among GAD patients. Some forms of treatment, particularly when drugs are used which induce strong activating effects in the beginning of the treatment, are likely to induce high dropout rates (Wingerson 1992). The compliance to the azapirones treatment did not seem to be affected by side effects, which were more frequent in the treated group.
One study comparing buspirone versus placebo in those who had been on benzodiazepines (Chiaie 1995) showed non‐conclusive results as far as drop out rates.
On the other hand, the majority of the data supported less dropouts on benzodiazepines including buspirone compared to azapirones.
One study assessing the use of antidepressants compared to venlafaxine (Davidson 1999) showed non‐conclusive results as far as drop out rates.
One study assessing buspirone compared kava kava showed non‐conclusive results (Boerner 2003).
Side effects The side effects reported did not appear to be serious and it is noteworthy that all of the reported common side effects in those on azapirones versus benzodiazepines were also bodily symptoms i.e. nausea, dizziness and lightheadedness, frequently described by patients suffering from GAD. Apart from the more conservative hypothesis, where side effects are considered 'de novo' symptoms, one has to bear in mind that azapirones may exacerbate some of the bodily symptoms which GAD patients present at baseline. Alternatively, azapirones may induce non specific changes in the way these patients appraise their own bodily sensations, which are interpreted according to the cognitive bias inherent to GAD. In addition, those on azapirones compared to benzodiazepines reported less depression as a side effect. This may be important in treating those with anxious and depressive symptoms which are common in this population (Sramek 1996a). For those coming off treatments, those on azapirones may have less rebound compared to benzodiazepines and may be an easier medication selection in certain populations where non‐compliance is an issue.
Generalization of the results Data presented in this review included GAD patients with possible concomitant depressive symptoms but without comorbid substance misuse as well as the possible recent use of benzodiazepines. One should be able to apply this to the general GAD population, who has concomitant depressive symptoms but without a lot of other comorbidity. One should be cautious in applying this review to those with GAD who have already been on a benzodiazepine as this review shows that benzodiazepines may be superior and it is unclear to predict how this group would do. In addition, caution needs to be taken as all the studies are short term and long term conclusions are not able to be made.
Authors' conclusions
Implications for practice.
The available evidence suggests that azapirones may be useful in treating those with GAD particularly in those who have not been on benzodiazepines. Azapirones compared to benzodiazepines, however, may not be as well tolerated. It is unclear if azapirones are as effective as venlafaxine XR 150 mg, anxiety management (psychotherapy), or kava kava as we were unable to draw definitive conclusions based on current data. Again, caution must be used as all the studies are short term in nature and GAD is a chronic illness (Bruce 2005). Also the dosing of buspirone in the kava kava study may overestimate kava kava's effect.
Implications for research.
As GAD is a chronic illness, longer term studies are needed to address whether azapirones continue to successfully treat GAD.
The onset of GAD commonly precedes the onset of major depressive disorder. Some authors argue that major depression may develop as a complication of GAD, probably as a result of the burn out induced by chronic anxiety. Increased risk for secondary depression is likely to continue for many years after the onset of primary GAD and continues only when GAD is active as opposed to remitted (Wittchen 1994). It remains unclear whether the treatment of GAD with agents such as azapirones could prevent outcomes such as major depression, dysthymia, panic disorder and other associated comorbidities. If this hypothesis holds true, one could start to think about what is now thought in terms of comorbidities as degrees of severity of expression of a common biological diathesis for anxiety and mood disorders.
What's new
| Date | Event | Description |
|---|---|---|
| 1 November 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 4, 1999 Review first published: Issue 3, 2006
| Date | Event | Description |
|---|---|---|
| 23 May 2006 | New citation required and conclusions have changed | Substantive amendment |
Notes
"This review was passed on to the current reviewers following publication of the original protocol. The protocol for the current review was amended and the review subsequently completed. The protocol for this review is not, therefore, identical to the initially published protocol (5HT‐1 agonists for generalized anxiety) authored by Flavio Kapczinski. Review published issue 3, 2006"
Please note that the current version of this review contravenes Cochrane's commercial sponsorship policy (revised 2014). The review is being updated by a team who meet the requirements of the policy; the updated version is scheduled for publication in 2016.
Data and analyses
Comparison 1. Azapirones vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HAM‐A score | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Azapirones vs Placebo | 4 | 135 | Mean Difference (IV, Random, 95% CI) | ‐4.48 [‐6.86, ‐2.10] |
| 1.2 Ipsapirone vs placebo | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐4.30 [‐7.33, ‐1.27] |
| 2 CGI much or very much improved | 2 | 187 | Risk Ratio (M‐H, Random, 95% CI) | 2.35 [0.72, 7.72] |
| 2.1 Azipirone vs placebo | 2 | 187 | Risk Ratio (M‐H, Random, 95% CI) | 2.35 [0.72, 7.72] |
| 3 Number of people who drop out | 8 | 629 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.47, 0.87] |
| 3.1 Azipirone vs placebo | 8 | 629 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.47, 0.87] |
| 4 Specific side effects | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Drowsiness | 5 | 697 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [1.17, 2.56] |
| 4.2 Dizziness | 7 | 789 | Risk Ratio (M‐H, Random, 95% CI) | 3.13 [2.00, 4.91] |
| 4.3 Nausea | 7 | 693 | Risk Ratio (M‐H, Random, 95% CI) | 2.33 [1.35, 4.03] |
| 4.4 Asthenia | 4 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.55, 2.47] |
| 4.5 Headache | 4 | 391 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.76, 1.49] |
| 4.6 Depression | 1 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.42, 3.91] |
| 4.7 Abnormal gait | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 15.0 [0.89, 251.42] |
| 4.8 Dry mouth | 5 | 628 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.55, 2.00] |
| 4.10 Weakness | 2 | 279 | Risk Ratio (M‐H, Random, 95% CI) | 6.13 [1.12, 33.39] |
| 4.11 Parathesia | 3 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 5.52 [1.62, 18.74] |
| 4.12 Pharyngitis | 2 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.78, 3.55] |
| 4.13 Somnolence | 3 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.60, 3.05] |
| 4.14 Dyspepsia | 2 | 210 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.80, 3.37] |
| 4.15 Amblyopia | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 5.00 [0.56, 44.52] |
| 4.16 Dysmenorrhea | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.38, 7.39] |
| 4.17 Pain | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.20, 6.03] |
| 4.18 Abdominal pain | 2 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.17, 1.94] |
| 4.19 Back pain | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 8.16 [0.41, 160.96] |
| 4.20 Twitch | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 8.16 [0.41, 160.96] |
| 4.21 Diarrhea | 4 | 343 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.79, 2.99] |
| 4.22 Flatulence | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.11, 25.04] |
| 4.23 Insomnia | 3 | 331 | Risk Ratio (M‐H, Random, 95% CI) | 2.01 [1.02, 3.98] |
| 4.24 Flu syndrome | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 2.63] |
| 4.25 Myalgia | 2 | 210 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.15, 4.62] |
| 4.26 Nervousness | 2 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 3.42 [0.86, 13.57] |
| 4.27 Vomiting | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.41, 6.59] |
| 4.30 Tinnitus | 3 | 208 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [0.79, 4.73] |
| 4.31 Tingling | 2 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 4.73 [0.84, 26.58] |
| 4.32 Throat discomfort | 1 | 227 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.07, 1.57] |
| 4.33 Lightheadedness | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 3.50 [1.82, 6.74] |
| 4.34 Agitation | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 2.95 [0.32, 27.67] |
| 4.35 Insomnia | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.36 Palpatation | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.31, 5.64] |
1.1. Analysis.

Comparison 1 Azapirones vs placebo, Outcome 1 HAM‐A score.
1.2. Analysis.

Comparison 1 Azapirones vs placebo, Outcome 2 CGI much or very much improved.
1.3. Analysis.
Comparison 1 Azapirones vs placebo, Outcome 3 Number of people who drop out.
1.4. Analysis.
Comparison 1 Azapirones vs placebo, Outcome 4 Specific side effects.
Comparison 2. Buspirone vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HAM‐A score | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Buspirone vs placebo | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐5.62, 6.42] |
| 1.2 Chiaie (Bupirone vs placebo after 2 week lorazepam taper) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐7.52 [‐9.89, ‐5.15] |
| 1.3 Majercsik | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐3.73 [‐4.01, ‐3.45] |
| 2 CGI much or very much improved | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.01, 2.17] |
| 2.2 Buspirone vs placebo | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.01, 2.17] |
| 3 Number of people who drop out | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Buspirone vs placebo | 6 | 584 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.49, 0.94] |
| 3.2 Chiaie (Bupirone vs placebo after 2 week lorazepam taper) | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.44, 3.27] |
| 4 Specific side effects | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Drowsiness | 2 | 481 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.84, 2.49] |
| 4.2 Depression | 1 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.42, 3.91] |
| 4.3 Dizziness | 3 | 635 | Risk Ratio (M‐H, Random, 95% CI) | 3.18 [1.82, 5.56] |
| 4.4 Throat discomfort | 1 | 227 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.07, 1.57] |
| 4.5 Dry mouth | 3 | 437 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [0.79, 3.05] |
| 4.6 Nausea | 3 | 429 | Risk Ratio (M‐H, Random, 95% CI) | 2.16 [1.14, 4.10] |
| 4.7 Fatigue | 1 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.23, 1.45] |
| 4.8 Headache | 2 | 416 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.36, 6.02] |
| 4.9 Weakness | 1 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 4.55 [0.54, 38.39] |
| 4.10 Abdominal pain | 1 | 227 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.07, 1.57] |
| 4.11 Somnolence | 2 | 183 | Risk Ratio (M‐H, Random, 95% CI) | 2.15 [0.97, 4.79] |
| 4.12 Tinnitus | 2 | 183 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.64, 4.22] |
| 4.13 Insomnia | 1 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.59, 3.66] |
| 4.14 Myalgia | 1 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.56, 4.80] |
| 4.15 Diarrhea | 1 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 2.05 [0.87, 4.81] |
| 4.16 Dyspepsia | 1 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.61, 3.06] |
| 4.17 Paresthesia | 1 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 4.1 [0.90, 18.72] |
2.1. Analysis.

Comparison 2 Buspirone vs placebo, Outcome 1 HAM‐A score.
2.2. Analysis.
Comparison 2 Buspirone vs placebo, Outcome 2 CGI much or very much improved.
2.3. Analysis.
Comparison 2 Buspirone vs placebo, Outcome 3 Number of people who drop out.
2.4. Analysis.
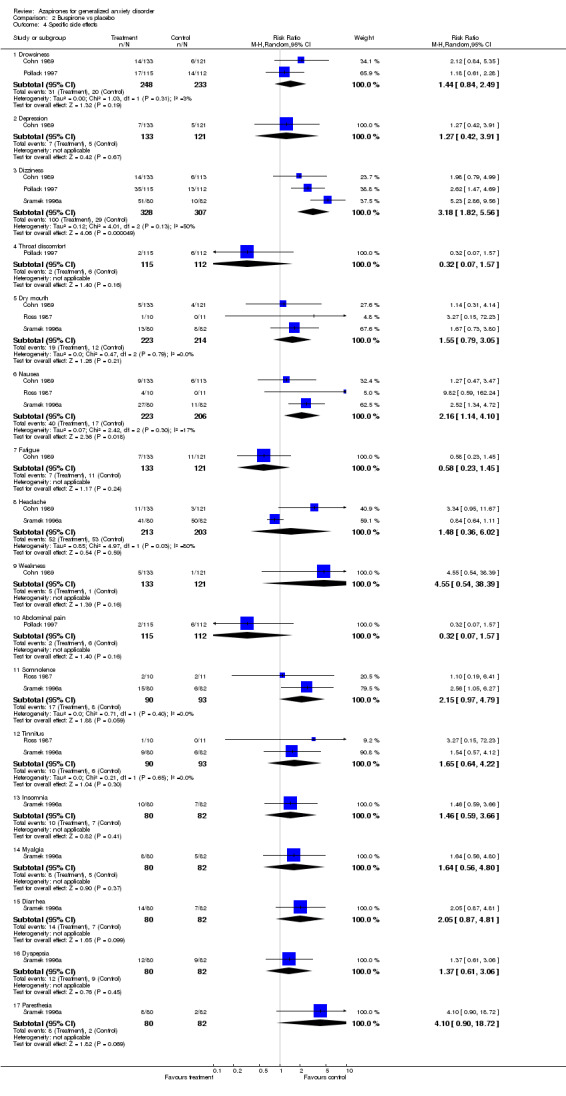
Comparison 2 Buspirone vs placebo, Outcome 4 Specific side effects.
Comparison 3. Azapirone vs benzodiazepine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HAM‐A score | 3 | 75 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐1.08, 0.76] |
| 2 HAM‐A score, ipsapirone vs diazepam | 1 | 17 | Mean Difference (IV, Random, 95% CI) | 3.80 [1.46, 6.14] |
| 2.2 Ipsapirone vs diazepam | 1 | 17 | Mean Difference (IV, Random, 95% CI) | 3.80 [1.46, 6.14] |
| 4 Number of people who drop out | 14 | 1594 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.05, 1.61] |
| 4.1 Azipirone vs benzodiazepine | 14 | 1594 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.05, 1.61] |
| 5 Specific side effects | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Confusion | 1 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.12, 3.92] |
| 5.2 Lethargy | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.08 [0.00, 1.34] |
| 5.3 Nausea | 5 | 497 | Risk Ratio (M‐H, Random, 95% CI) | 2.95 [1.73, 5.04] |
| 5.4 Decreased cognition | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.01, 4.11] |
| 5.5 Dizziness | 4 | 355 | Risk Ratio (M‐H, Random, 95% CI) | 1.90 [1.15, 3.15] |
| 5.6 Headaches | 5 | 443 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.75, 2.51] |
| 5.7 Restlessness | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.8 Lightheadedness | 3 | 245 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.29, 8.08] |
| 5.9 Fatigue | 5 | 609 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.21, 0.54] |
| 5.10 Infection | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [0.21, 21.36] |
| 5.11 Constipation | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.07, 15.66] |
| 5.12 Coryza | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 3.15 [0.14, 72.88] |
| 5.13 Faintness | 1 | 2 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.14 Gastrointestinal distress | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.02, 8.10] |
| 5.15 Nervousness | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.48, 4.39] |
| 5.16 Tremor | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.01, 4.11] |
| 5.17 Weakness | 2 | 285 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.15, 1.12] |
| 5.18 Depression | 4 | 909 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.08, 0.47] |
| 5.19 Asthenia | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.8 [0.68, 4.74] |
| 5.20 Dry mouth | 5 | 531 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.22, 0.84] |
| 5.21 Anorexia | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.02, 1.09] |
| 5.22 Abnormal gait | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.57, 5.36] |
| 5.23 Paresthesia | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 11.00 [0.64, 190.53] |
| 5.24 Phayngitis | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 2.5 [0.88, 7.10] |
| 5.25 Rhinitis | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.18, 3.07] |
| 5.26 Amblyopia | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.6 [0.16, 2.29] |
| 5.27 Insomnia | 1 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [0.91, 6.53] |
| 5.28 Sleepiness | 3 | 428 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.07, 0.55] |
| 5.29 Agitation | 1 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [0.33, 28.54] |
| 5.30 Diarrhea | 1 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.16, 1.60] |
| 5.31 Vomiting | 1 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 2.54 [0.51, 12.62] |
| 5.32 Palpatation | 1 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.02, 1.37] |
| 5.33 Tingling | 1 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.26, 3.89] |
| 5.34 Tinnitus | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | 2.73 [0.12, 59.57] |
| 5.35 Unsteadiness | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.01, 6.62] |
| 5.36 Sleep problems | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.08, 0.81] |
3.1. Analysis.

Comparison 3 Azapirone vs benzodiazepine, Outcome 1 HAM‐A score.
3.2. Analysis.
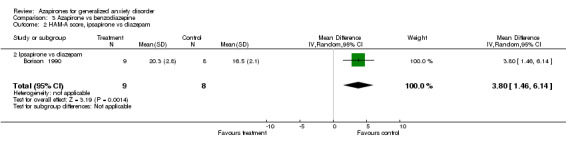
Comparison 3 Azapirone vs benzodiazepine, Outcome 2 HAM‐A score, ipsapirone vs diazepam.
3.4. Analysis.

Comparison 3 Azapirone vs benzodiazepine, Outcome 4 Number of people who drop out.
3.5. Analysis.

Comparison 3 Azapirone vs benzodiazepine, Outcome 5 Specific side effects.
Comparison 4. Buspirone vs benzodiazepine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HAM‐A score, buspirone vs diazepam | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐7.45, 7.05] |
| 1.2 Buspirone vs diazepam | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐7.45, 7.05] |
| 2 HAM‐A score, buspirone vs lorazepam | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [0.29, 1.91] |
| 2.1 Buspirone vs lorazepam | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [0.29, 1.91] |
| 3 HAM‐A score, buspirone vs alprazolam | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [0.28, 1.92] |
| 4 Number of people who drop out | 12 | 1444 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.01, 1.52] |
| 4.2 Buspirone vs benzodiazepine | 12 | 1444 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.01, 1.52] |
| 5 Specific side effects, buspirone | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Nausea | 3 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [1.14, 7.09] |
| 5.2 Decreased cognition | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.11] |
| 5.3 Dizziness | 3 | 295 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.28 [1.15, 4.54] |
| 5.4 Headaches | 3 | 573 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.76, 2.63] |
| 5.5 Restlessness | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.6 Drowsiness | 3 | 621 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.21, 0.41] |
| 5.7 Lethargy | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.34] |
| 5.8 Lighheadedness | 2 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.27, 2.52] |
| 5.9 Fatigue | 4 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.13, 0.45] |
| 5.10 Infection | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [0.21, 21.36] |
| 5.11 Constipation | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.07, 15.66] |
| 5.12 Coryza | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.15 [0.14, 72.88] |
| 5.13 Faintness | 1 | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.14 Gastrintestinal distress | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.02, 8.10] |
| 5.15 Nervousness | 2 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.06, 0.47] |
| 5.16 Tremor | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.11] |
| 5.17 Weakness | 2 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.15, 1.12] |
| 5.18 Depression | 4 | 909 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.12, 0.39] |
| 5.19 Insomnia | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.03, 0.63] |
| 5.20 Dry mouth | 3 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.22, 1.26] |
| 5.21 Sleepiness | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.10, 1.17] |
| 5.22 Tinnitus | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [0.12, 59.57] |
| 5.23 Unsteadiness | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.01, 6.62] |
| 5.24 Sleep Problems | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.08, 0.81] |
4.1. Analysis.
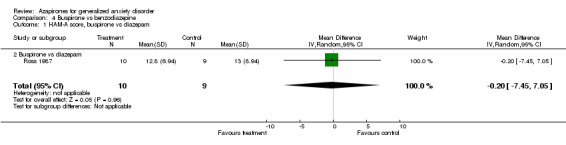
Comparison 4 Buspirone vs benzodiazepine, Outcome 1 HAM‐A score, buspirone vs diazepam.
4.2. Analysis.

Comparison 4 Buspirone vs benzodiazepine, Outcome 2 HAM‐A score, buspirone vs lorazepam.
4.3. Analysis.
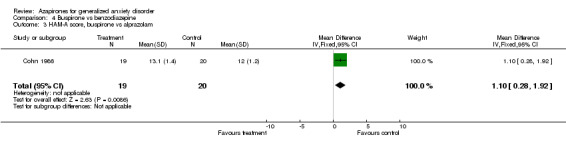
Comparison 4 Buspirone vs benzodiazepine, Outcome 3 HAM‐A score, buspirone vs alprazolam.
4.4. Analysis.

Comparison 4 Buspirone vs benzodiazepine, Outcome 4 Number of people who drop out.
4.5. Analysis.

Comparison 4 Buspirone vs benzodiazepine, Outcome 5 Specific side effects, buspirone.
Comparison 5. Buspirone vs Venlafaxine XR.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CGI | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Buspirone vs Venlafaxine XR 75mg | 1 | 182 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.41, 1.34] |
| 1.2 Venlafaxine XR 150 mg | 1 | 184 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.69, 2.21] |
| 2 Number of people who drop out | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Buspirone vs Venlafaxine XR 75 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.50, 1.88] |
| 2.2 Buspirone vs Venlafaxine XR 150 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.32, 1.13] |
| 3 Specific side effects, Buspirone vs Venlafaxine XR 75 mg | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Nausea | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.46, 1.53] |
| 3.2 Dizziness | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.42 [2.30, 8.51] |
| 3.3 Asthenia | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.52, 2.50] |
| 3.4 Dry mouth | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.11, 0.89] |
5.1. Analysis.
Comparison 5 Buspirone vs Venlafaxine XR, Outcome 1 CGI.
5.2. Analysis.

Comparison 5 Buspirone vs Venlafaxine XR, Outcome 2 Number of people who drop out.
5.3. Analysis.
Comparison 5 Buspirone vs Venlafaxine XR, Outcome 3 Specific side effects, Buspirone vs Venlafaxine XR 75 mg.
Comparison 6. Buspirone vs Kava‐Kava.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CGI much or very much improved | 1 | 85 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.66, 3.82] |
| 1.1 Buspirone vs Kava‐Kava | 1 | 85 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.66, 3.82] |
| 2 Number of people who drop out | 1 | 85 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.13, 2.62] |
| 2.1 Buspirone vs Kava‐Kava | 1 | 85 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.13, 2.62] |
| 3 Sepcific side effects, Buspirone vs Kava‐Kava | 1 | 85 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.13, 2.62] |
| 3.1 common cold, pharyngtis, bronchitis | 1 | 85 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.13, 2.62] |
6.1. Analysis.
Comparison 6 Buspirone vs Kava‐Kava, Outcome 1 CGI much or very much improved.
6.2. Analysis.
Comparison 6 Buspirone vs Kava‐Kava, Outcome 2 Number of people who drop out.
6.3. Analysis.

Comparison 6 Buspirone vs Kava‐Kava, Outcome 3 Sepcific side effects, Buspirone vs Kava‐Kava.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ansseau 1990.
| Methods | 1. Randomized 2. Double blind 3. 2 parallel groups: ‐Buspirone 15‐60mg/d ‐Oxazepam 45‐180mg/d 4. Duration: 6 weeks with 1 week washout period on placebo 5. Analysis: end‐point data using ANOVA |
|
| Participants | 1. Diagnosis: GAD (DSM‐III) 2. N = 26 3. Age (mean, SD): Buspirone 38.9 (9.3) Oxazepam 41.7 (12.3) Sex: 46.2% females Setting: outpatients History: Evidence of contraindication for a benzodiazepine anxiolytic, serious or ncontrolled medical illness, benzodiazepine withdrawal symptoms of any degree, or major depressive symptomatology |
|
| Interventions | 1. Buspirone (N = 14) 2. Oxazepam (N = 12) |
|
| Outcomes | 1. dropout rates 2. HAM‐A 3. Anxiety subscale of the Association for Metodology and Documentation in Psychatry 4. CGI |
|
| Notes | (a) Means and SD not available on HAM‐A (b) CGI ratings not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Boerner 2003.
| Methods | 1. Randomized 2. Double blind 3. Parallel groups ‐Kava LI 150 400mg ‐Buspirone 10mg ‐Opipramol 100mg 4. Duration: 8 weeks ‐ acute treatment 1 week ‐ follow‐up withdrawal symptoms and early relapse with a 1‐7 day open run‐in phase; psychotropic medications withdrawn two weeks prior to screening and psychotherapeutic intervention stopped four weeks prior to screening 5. Analysis: LOCF |
|
| Participants | 1. Diagnosis: GAD (ICD‐10) 2. N = 127 3. Age (differences of means and p value) Kava‐Kava = ‐4.45 Buspirone = 5.05 (p=0.26) Sex: 84.3% females Setting: outpaients History: excluded any concurrent ICD‐10 diagnoses of depressive episode, panic disorder, phobias, obsessive‐compulsive disorder, hypochondria, substance‐related disorders, mental disorders of organic origin, psychotic mental disorders; history of seizure disorder or use of anticonvulsants; serious unstable acute or chronic medical illness; seriously impaired hepatic function or renal failure; blood cound deviations >10% outside limits; pregnancy, breast‐feeding, or use of inadequate methods for birth control in fertile women; concurrent initiaion of hormonal contraception or other treatments with sex hormones; allergy or hypersensitivity to study medications; positive urine drug screen, participation in another clinical trial during the preceeding six weeks |
|
| Interventions | 1. Buspirone 10mg/d (N = 42) 2. Kava‐kava (N = 43) |
|
| Outcomes | 1. dropout rates 2. Ham‐A 3. CGI‐I 4. Boerner Anxiety Scale 5. Self‐Rating Anxiety Scale 6. Self‐Rating scale for well‐being 7. Sleep questionnaire 8.Quality‐of‐life questionnaire |
|
| Notes | (a) Supported by a grant from Lichtwer Pharma AG (b) HAM‐A not included in final analysis as end point means and SD not included |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Bond 2002.
| Methods | 1. Randomized 2. Both therapies done by same therapist; rater was blind 3. Four treatment groups in a 2 X 2 design: ‐Buspirone 15‐30 mg/d and anxiety management training (AMT) ‐Buspirone 15‐30 mg/d and non‐directive therapy (NDT) ‐Placebo and anxiety management training ‐placebo and non‐directive therapy 4. Duration: 8 weeks with no psychotropic drugs within the past 6 weeks or benzodiazepines within the past six months prior 5. Analysis: LOCF; ANOVA and MANOVA |
|
| Participants | 1. Diagnosis: GAD (DSM‐III‐R) 2. N = 60 3. Age (mean and SD) Buspirone and AMT = 33.4 (14.5) Buspirone and NDT = 29.4 (5.8) Placebo and AMT = 36.2 (10.3) Placebo and NDT = 34.6 (10.4) Sex: 45% females Setting: outpatients History: excluded any significant psychiatric disorder other than GAD |
|
| Interventions | 1. Buspirone and AMT (N=11) 2. Buspirone and NDT (N=7) 3. Placebo and AMT (N=12) 4. Placebo and NDT (N=14) |
|
| Outcomes | 1. dropout rates 2. HAM‐A 3. Hospital Anxiety and Depression Scale 4. Zung anxiety scale 5. General health questionnaire 6. Cognitive check list 7. Mood rating scale |
|
| Notes | Suppored by Bristol‐Meyers Squibb | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Boral 1989.
| Methods | 1. Randomized 2. Double blind 3. Two parallel group: ‐Buspirone 15‐30mg/d ‐Diazepam 15‐30mg/d 3. Duration: 4 weeks with one week washout prior 4. Analysis: LOCF |
|
| Participants | 1. Diagnosis: GAD (DSM‐III) 2. N = 80 3. Age (mean and SEM): Buspirone: 37.2 (2.11) Diazepam: 37.7 (1.94) Sex: 40% females Setting: outpatients History: excluded any significant psychiatric disorder other than GAD |
|
| Interventions | 1. Buspirone (N = 40) 2. Diazepam (N = 40) |
|
| Outcomes | 1. dropout rates 2. HAM‐A 3. HAM‐D 4. Raskin Scale for Depression 5. Covi Scale for Anxiety 6. Lipman‐Rickels Symtom Checklist (SCL‐56) 7. Profile of Mood States (POMS) |
|
| Notes | HAM‐A not entered into final analysis as SDs not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Borison 1990.
| Methods | 1. Randomized 2. Double blind 3. Four parallel groups: ‐ placebo, ‐ Ipsapirone 15 mg/d ‐ Ipsapirone 30 mg/d ‐ diazepam: 15 mg/d 4. Duration: 4 weeks with a single‐blind placebo washout for 1 week prior 5. Analysis: LOCF; ANCOVA |
|
| Participants | 1. Diagnosis: GAD (DSM‐III) 2. N = 34 3. Age (mean and SD): 43.2 (6.8) Sex: 44.1% females Setting: outpatients History: excluded any significant psychiatric disorder other than GAD |
|
| Interventions | 1. Placebo (N = 9) 2. Diazepam (N = 8) 3. Ipsapirone 15 mg/d (N = 9) 4. Ipsapirone 30 mg/d (N = 8) |
|
| Outcomes | 1. dropout rates 2. HAM ‐A |
|
| Notes | Data on treatment group Ipsapirone 30mg was disregarded by the trial´s authors because there were too many dropouts | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Boyer 1993.
| Methods | 1. Randomized 2. Double blind 3. Four paralell groups: ‐ placebo ‐ Ipsapirone 15 mg/d ‐ Ipsapirone 30 mg/d ‐ diazepam: 15 mg/d 4. Duration: 4 weeks with one week run in period with placebo prior 5. Analysis: LOCF (excluded if double‐blind treatment period less than 5 days) |
|
| Participants | 1. Diagnosis: GAD (DSM‐III) 2. N = 249 No significative differences between groups in terms of sex and age. Setting: outpatients History: excluded any significant psychiatric disorder other than GAD |
|
| Interventions | 1. Placebo (N = 60) 2. Ipsapirone 15 mg/d (N = 63) 3. Ipsapirone 30 mg/d (N =65) 4. Diazepam (N = 61) |
|
| Outcomes | 1. dropout rates 2. HAM ‐A 3. CGI scores 4. Zung Self‐Rating Scale for Anxiety 5 HAM‐D |
|
| Notes | (a) Data on side effects were present only for the treatment group Ipsapirone 30
mg/d (b) Data on CGI and HAM‐A not entered secondary to lack of reported data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Chiaie 1995.
| Methods | 1. Randomized 2. Double blind 3. Two Parallel groups: ‐placebo ‐Buspirone 15mg/d 4. Duration: 0‐5 weeks‐ Lorazepam 3‐5 mg/d 6 weeks randomized with 1st 2 weeks randomization Lorazepam was tapered off weeks 12 and 13 single blind placebo 5. Analysis: two‐way ANOVA; LOCF |
|
| Participants | 1. Diagnosis: GAD (DSM‐III‐R) 2. N=44 3. Age (mean and SD): 42.5 (11.5) Sex: 61.4% females Seting: outpatients History: excluded any significan psychiatric disorder other than GAD |
|
| Interventions | 1. Placebo (N= 22) 2. Buspirone (N= 22) |
|
| Outcomes | 1. dropout rates 2. HAM‐A 3. HAM‐D 4. CGI scores 5. State‐Trait Anxiety Inventory (STAI‐X1) 6. Zung and Eddy Self‐Rating Scale of Anxiety Symptoms (SASA) 7. Trait X‐2 form of the STAI 8. Rome Depression Inventory |
|
| Notes | (a) CGI not entered into final analysis secondary to lack of data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
Cohn 1986.
| Methods | 1. Randomized 2. Double blind 3. Three parallel groups: ‐Buspirone 15‐50mg/d ‐Alprazolam 1.5‐5mg/d ‐Lorazepam 1‐10mg/d 4. Duration: 4 weeks with 4‐7 placebo washout period beforehand 5. Analysis: Cochran‐Mantel‐Haenszel test used endpoint analysis of categoric responses |
|
| Participants | 1. Diagnosis: GAD (DSM‐III) 2. N=60 3. Age (mean): Buspirone = 40.5 Alprazolam = 35.9 Lorazepam = 38.8 Sex: 55% females Setting: outpatients |
|
| Interventions | 1. Buspirone (N = 20) 2. Alprazolam (N = 20) 3. Lorazepam (N = 20) |
|
| Outcomes | 1. HAM‐A 2. Raskin Depression Scale 3. Covi Anxiety Scale 4. physician's questionnaire measuring global psychopathology |
|
| Notes | (a)Statistical analysis support from Mead Johnson Pharmaceutical Group (b) Alprazolam used in the analysis using HAM‐A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Cohn 1986a.
| Methods | 1. Randomized 2. Double blind 3. 2 paralle groups: ‐Buspirone 10‐60mg/d ‐Cloraepate 15‐90mg/d 4. Duration: 4 weeks with 4‐7 single‐blind washout 5. Analysis: end point data using ANOVA; subgroup of axous patients accompanied by depression with HAM‐D of 18 or more also analyzed |
|
| Participants | 1. Diagnosis: GAD (DSM‐III) 2. N=293 3. Age (mean for all patients): 38.3 Sex: 83% females Setting: outpatients History: exluded women who were pregnant or not using reliable means of contraception, any significant renal, liver, or cardiovascular disease, significant findings on physical examination, electrocardiography, or clinical laboratory evaluation or manifestations of psychosis, borderline states, severe behavioral disorder, organic mental disorders, or serious psychosomatic disorders, patients who had received any central nervous system‐active medication within seven days or any neuroleptic agent, antidepressant, or investigational drug witin four weeks of the study |
|
| Interventions | 1. Buspirone (N = 218) 2. Clorazepate (N = 75) |
|
| Outcomes | 1. dropout rates 2. HAM‐A 3. HAM‐D 4. Physician's Questionnaire 5. Lipman‐Rickels Symptom Checklist (SCL‐56) 6. Profile of Mood States (POMS) 7. Sleep Evaluation Questionnaire |
|
| Notes | (a) Means and SD not avaliable on HAM‐A | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Cohn 1989.
| Methods | 1. Randomized 2. Double blind 3. Three parallel groups ‐ placebo ‐ Buspirone 5‐60 mg/d ‐ Diazepam 5‐60 mg/d 4. Duration: 4 weeks with a 4‐7 day wash‐out period prior 5. Analysis: ANOVA, Logistic regression used to analyze the phsician's and patients's final assessments |
|
| Participants | 1. Diagnosis: GAD (DSM‐III) 2. N = 367 3. Age (mean): placebo = 39 (12.8) Diazepam = 38 (13.1) Buspirone = 39 (11.2) Sex: 100% females Setting: outpatients History: excluded any significant psychiatric disorder other than GAD |
|
| Interventions | 1. Placebo (N = 121) 2. Diazepam (N = 113) 3. Buspirone (N = 133) |
|
| Outcomes | 1. dropout rates 2. HAM ‐A 3. 5‐point final assessment scale (patient´s and physicians´s) |
|
| Notes | HAM‐A not entered into final analysis as SD not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Cutler 1993.
| Methods | 1. Randomized 2. Double blind 3. Three parallel groups ‐ placebo ‐ Ipsapirone 10‐30 mg/d ‐ Lorazepam 2‐6 mg/d 4. Duration: 4 weeks ‐ acute treatment 4 weeks ‐ extended treatment 2 weeks ‐ withdrawal period with a 1‐week placebo lead‐in prior 5. Analysis: LOCF |
|
| Participants | 1. Diagnosis: GAD (DSM‐III) 2. N = 90 3. Age (mean): placebo = 32.9 Lorazepam = 29.1 Ipsapirone = 31.2 Sex: 62.2% females Setting: outpatients History: excluded any significant psychiatric disorder other than GAD |
|
| Interventions | 1. Placebo (N = 30) 2. Lorazepam (N = 30) 3. Ipsapirone (N = 30) |
|
| Outcomes | 1. dropout rates 2. CGI scores 3. HAM ‐A 4. Physisicans Withdrawal Checklist 5. Zung Anxiety Scale 6. HAM‐D |
|
| Notes | (a) CGI and HAM‐A not entered into final analysis as means and SDs not reported; (b) only HAM‐A for Ipsapirone vs benzodiazepine entered secondary to means and SDs reported‐week 8 used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Cutler 1994.
| Methods | 1. Randomized 2. Double blind 3. Four parallel groups: ‐placebo ‐Ipsapirone 2.5 mg/d ‐Ipsapirone 5.0 mg/d ‐Ipsapirone 7.5 mg/d 4. Duration: 4 weeks with a 1‐week placebo run‐in 5. Analysis: LOCF |
|
| Participants | 1. Diagnosis: GAD (DSM‐III) 2. N=267 3. Age (mean and range) 39.6 (20‐61) Sex: 27% females Setting: outpatients History: excluded any significant psychiatric disorder other than GAD |
|
| Interventions | 1. Ipsapirone 2.5 mg (N = 67) 2. Ipsapirone 5.0 mg (N = 64) 3. Ipsapirone 7.5 mg (N = 67), 4. Placebo (N = 69) |
|
| Outcomes | 1. dropout rates 2. HAM‐A 3. Zung Anxiety Rating Scale 4. CGI scores |
|
| Notes | (a) CGI not entered into final analysis as data not reported 2. HAM‐A not entered into final analysis as SDs not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Davidson 1999.
| Methods | 1. Randomized 2. Double blind 3. Four parallel groups ‐ placebo, ‐ Venlafaxine XR 75 mg/d ‐ Venlafaxine XR 150 mg/d ‐ Buspirone 30 mg/d 4. Duration: 8 weeks without any medications 14‐30 days prior depending on medication 5. Analysis: LOCF |
|
| Participants | 1. Diagnosis: GAD (DSM‐IV) 2. N = 365 efficacy analysis; N=405 safety analysis 3. Age (mean and SD): placebo = 39 (11) Venlafaxine XR 75 mg/d = 38(10) Venlafaxine XR 150 mg/d = 37 (11) Buspirone 30 mg/d = 37(10) Sex: 61.4% females Setting: outpatients History: excluded any significant psychiatric disorder other than GAD |
|
| Interventions | 1. Placebo (N = 98) 2. Venlafaxine XR 75 mg/d (N = 87) 3. Venlafaxine XR 150 mg/d (N = 87) 4. Buspirone mg/d N= 93 |
|
| Outcomes | 1. dropout rates 2. CGI scores 3. HAM ‐A endpoint scores 4. Patient‐rated hospital anxiety and depression scale 5. Covi Anxiety Scale 6. Raskin Depression Scale | |
| Notes | Supported by Wyeth‐Ayerst Research | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Demartinis 2000.
| Methods | 1. Randomized 2. Double blind 3. Three parallel groups ‐ placebo ‐ Buspirone 15‐30 mg/d (mean 20 mg/d) ‐benzodiazepines (mean 20 mg/d) 4. Duration: 4 weeks with a 1‐week, single‐blind placebo washout period prior 5. Analysis: LOCF |
|
| Participants | 1. Diagnosis: GAD (DSM‐IV) 2. N = 735 3. Age (range): placebo = 38 (18‐66) benzodiazepines = 37 (18‐67) buspirone = 39 (19‐64) Sex: 62.5% females Setting: outpatients |
|
| Interventions | 1. Placebo (N = 235) 2. Benzodiazepines (N = 248) 3. Buspirone (N = 252) |
|
| Outcomes | 1. dropout rates 2. CGI scores 3. HAM ‐A |
|
| Notes | (a) Included subgroups regarding prior benzodiazepine use: (1) no prior use, (2)
remote use (> 1 month benzodiazepine‐free) and (3) recent use (benzodiazepine use in
the past month). Recent use used to report side effects. (b) adverse effevts with Buspirone as a function of prior Benzodiazeines treatment group are presented (c) Supported in part by Brystol Meyers Squibb (d) HAM‐A not entered into analysis as SDs not available (e) CGI scores not entered into analysis as data not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Enckelmann 1991.
| Methods | 1. Randomized 2. Double blind 3. Three parallel groups ‐ placebo ‐ Alprazolam 1.5‐4 mg/d ‐ Buspirone 15‐40 mg/d 4. Duration: 6 weeks with a 3‐7 day washout period with placebo prior 5. Analysis: data from patients still in the study at the time point (week 2, 4, or 6) included in the analysis |
|
| Participants | 1. Diagnosis: GAD (DSM‐III) 2. N = 94 Age (mean and range): 35 (19‐65) Sex: 49% females Setting: outpatients History: excluded any significant psychiatric disorder other than GAD |
|
| Interventions | 1. Placebo (N = 31) 2. Alprazolam (N = 32) 3. Buspirone (N = 31 |
|
| Outcomes | 1. dropout rates 2. HAM ‐A 3. HAM‐D 4. Raskin Depression Scale 5. Covi‐Anxiety Scale 6. SCL 90 7. Physician Global Improvement Scale |
|
| Notes | (a) CGI scores not entered into final analysis as data not available (b) HAM‐A scores not included into final analyses as means and SDs not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Fabre 1987.
| Methods | 1. Randomized 2. Double blind 3. 2 parallel groups: ‐Buspirone 10‐50mg/d ‐Diazepam 10‐50mg/d 4. Duration: 4 weeks with a single‐blind lacebo for four to seven days prior 5. Analysis: Analysis of variance technique; all patients considered evaluable for safety and those who completed at least seven days were considered evaluable for efficacy |
|
| Participants | 1. Diagnosis: GAD (DSM‐III) 2. N = 156 3. Age (% 18‐40, >40) ‐Buspirone (89,11) ‐Diazepam (85, 15) Sex: 47% females Setting: outpatients History: escluded any clinically significant reexisting kidney, liver, or cardiovascular disease, abnormal physical exam, laboratory studies or electrocardiograms, psychosis, borderline states, severe behvior disorders, substance abuse, or serious psychosomatic disorders |
|
| Interventions | 1. Buspirone (N = 88) 2. Diazepam (N = 33) |
|
| Outcomes | 1. dropout rates 2. HAM‐A 3. HAM‐D 4. CGI 5. Lipman‐Rickels Symptoms Checklist 6. Sleep Evaluation Questionnaire 7. Patient's Global Impressions 8. Profile of Mood States |
|
| Notes | (a) HAM‐A and CGI not entered into final analysis as data not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Feighner 1982.
| Methods | 1. Randomized 2. Double blind 3. Two parallel groups: ‐Buspirone (10‐50mg/d) ‐Diazepam 10‐50mg/d 4. Duration: 4 weeks preceded by a 4‐7 day placebo washout 5. Analysis: Major parametric analyses included split‐plot analysis of covariance on the individual factor scores and the total scores and one‐way ANOVA |
|
| Participants | 1. Diagnosis: GAD (DSM‐III) 2. N = 100 (efficacy analysis); 118 (safety analysis) 3. Age (mean): ‐Buspirone = 38.5 ‐Diazepam = 38.8 Sex: 77% females Setting: outpatients History: excluded any significant psychiatric disorder other thatn GAD |
|
| Interventions | 1. Buspirone (N = 89) 2. Diazepam (N = 29) |
|
| Outcomes | 1. dropout rates 2. HAM‐A 3. HAM‐D 4. Lipman‐Rickels Symptom Checklist (SCL‐56) 5. Profile of Mood States 6. Patients' and physicians' global impressions 7. Patients' ratings of sleep 8. Covi Anxiety Scale 9. Raskin Depression Scale |
|
| Notes | (a) Supported by Mead Johnson Pharmaceutical Division (b) HAM‐A not entered into final analysis secondary to means and SDs not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Feighner 1989.
| Methods | 1. Randomized 2. Double blind 3. Two parallel groups: ‐placebo ‐Buspirone 10mg‐60mg/d Six double‐blind studies were pooled from the FDA‐approved New Drug Application database for buspirone 4. Duration: 4 weeks with 4‐7 day waskout period 5. Analysis: ANOVA |
|
| Participants | 1. Diagnosis: GAD (DSM‐III) 2. N = 459 3. Age (mean): placebo = 38.4 Buspirone = 39.3 Sex: 64% females Setting: outpatients History: excluded any significant psychiatric disorder other than GAD |
|
| Interventions | 1. Placebo (N = 225) 2. Buspirone (N = 234) |
|
| Outcomes | 1. HAM‐A 2. Covi Anxiety Scale 3. Raskin Depression Scale |
|
| Notes | HAM‐A not entered into final analysis as means and SDs not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Fontaine 1993.
| Methods | 1. Randomized 2. Double blind 3. Two parallel groups: ‐Buspirone 15mg/d ‐Buspirone 30mg/d 4. 6 weeks with 2‐week placebo washout period prior 5. Analysis: one‐way ANOVA |
|
| Participants | 1. Diagnosis: GAD (DSM‐III) 2. N = 38 3. Age (mean and SD) ‐Buspirone 15mg = 39 (9.3) ‐Buspirorone 30mg = 42.6 (11.9) Sex: 42.1% females Setting: outpatients Hstory: excluded any significant psychiatric disorder other than GAD |
|
| Interventions | 1. Buspirone 15mg (N = 19) 2. Buspirone 30 mg (N = 19) |
|
| Outcomes | 1. dropout rates 2. HAM‐A 3. CGI‐S 4. Physician's Questionnaire, and adverse effect scale 5. Self‐Rating Symptom Scale (SCL‐56) 6. State‐Trait Anxiety Inventory |
|
| Notes | Supported by Bristol‐Meyers Squibb | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Fresquet 2000.
| Methods | 1. Randomized 2. Double blind 3. Three parallel groups ‐ placebo ‐ Lesopitron 40‐80 mg/d ‐ Lorazepam 2‐4 mg/d 4. Duration: 6 weeks with one week placebo lead‐in 5. Analysis: LOCF |
|
| Participants | 1. Diagnosis: GAD (DSM‐IV) 2. N = 68 Age (mean and range): placebo: 34.3 (22‐52) Lesopitron: 39.6 (21‐58) Lorazepam: 36.4 (20‐54) Sex: 51,5% females Setting: outpatients History: excluded any significant psychiatric disorder other than GAD |
|
| Interventions | 1. Placebo (N = 20) 2. Lorazepam (N = 30) 3. Lesopitron (N = 18) |
|
| Outcomes | 1. dropout rates 2. HAM‐A 3. CGI Scores |
|
| Notes | (a) This trial was supported by Laboratorios Dr. Esteve S. A., Barcelona, Spain (b) Duration: presented data on a subset of patients with prior documented episodes of GAD or anxiety disorder NOS (c) HAM‐A was entered into analysis secondary to SDs not being reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Harto 1988.
| Methods | 1. Randomized 2. Double blind 3. Two parallel groups ‐ placebo gepirone 30‐60 mg/d 4. Duration: 6 weeks; no psychotropic drugs (except chloral hydrate) within 14 days of randomizatin and investigational drugs and benzodiazepines were prohibited within 28 days prior 5. Analysis: ANOVA |
|
| Participants | 1. Diagnosis: GAD (DSM‐III) 2. N = 30 3. Age (mean and SD): placebo = 39.9 (3.8) gepirone: 44.7 (3.3) Sex: 33.1 % females Setting: outpatients History: excluded any significant psychiatric disorder other than GAD |
|
| Interventions | 1. Placebo (N = 12 2. Gepirone (N = 13) |
|
| Outcomes | 1. dropout rates 2. HAM‐A 3. CGI Scores |
|
| Notes | HAM‐A was not included in the final analysis as means and SD not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Jacobson 1985.
| Methods | 1. Randomized 2. Double blind 3. Two parallel groups: ‐Buspirone 10‐40 mg/d ‐Diazepam 10‐40 mg/d 4. Duration: 4 weeks with a 7‐day placebo washout period prior 5. Analysis: end point using ANOVA and ochran‐Mantel‐Haenszel approach |
|
| Participants | 1. Diagnosis: GAD (DSM‐III) 2. N = 66 3. Age (mean and range): 40.8 (23‐64) Sex: 23% females Setting: outpatients History: excluded any significant psychiatric disorder other than GAD |
|
| Interventions | 1. Buspirone (N = 51) 2. Diazepam (N = 15) |
|
| Outcomes | 1. dropout rates 2. HAM‐A 3. HAM‐D 4. Raskin/Covi scale 5. physician's global ratings 6. doctor's disposition form 7. Lipan‐Reckels symptom checklist‐56 8. Proofile of mood states 9. Sleep evuation form and the sociodemographic inventory |
|
| Notes | Study supported from Bristol‐Meyers Company | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Laakmann 1998.
| Methods | 1. Randomized 2. Double blind 3. Three parallel groups ‐ placebo ‐ Lorazepam 3 mg/d ‐ Buspirone 15 mg/d 4. Duration: 4 weeks ‐ acute treatment 2 weeks ‐ tapering 4 weeks ‐ withdrawal period with a 3‐7 day wash out period prior 5. Analysis: LOCF |
|
| Participants | 1. Diagnosis: GAD (DSM‐III) 2. N = 125 3. Age (mean and SD): placebo = 42.4 (11.5) Lorazepam = 51 (9.9) Buspirone = 49 (10.3) Sex: 64% females Setting: outpatients History: excluded any significant psychiatric disorder other than GAD |
|
| Interventions | 1. Placebo (N = 10) 2. Lorazepam (N = 57) 3. Buspirone (N = 58) |
|
| Outcomes | 1. dropout rates 2. CGI scores 3. HAM ‐A 4. Covi Anxiety Scale 5. Raskin Anxiety Scale 6. STAI X2 ‐ State Trait Anxiety Inventory |
|
| Notes | (a) HAM‐A data not used in analysis secondary to lack of reported numbers (b) CGI not entered into analysis as data not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Lader 1998.
| Methods | 1. Randomized 2. Double blind 3. Three parallel groups ‐ placebo ‐ hydroxyzine 50 mg/d ‐ Buspirone 20 mg/d) 4. Duration: 4 weeks with 7 day run‐in period with single‐blind placebo prior 5. Analysis: LOCF |
|
| Participants | 1. Diagnosis: GAD (DSM‐IV) 2. N = 244 3. Age (mean): placebo = 40 (11) buspirone: 41 (12) hydroxyzine: 42 (11) Sex: 69.3% females Setting: outpatients History: excluded any significant psychiatric disorder other than GAD |
|
| Interventions | 1. Placebo (N = 81) 2. Hydroxyzine (N = 81) 3. Buspirone (N = 82) |
|
| Outcomes | 1. dropout rates 2. HAM ‐A 3. CGI scores 4. Montgomery‐Asberg Depression rating Scale 5. Ferreri Anxiety Rating Diagram 6. Echelle Dyscontrole Comportemental (EDC) 7. Hospital Anxiety and Depression Scale 8. Tyrer Withdrawal Symptom Scale |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Lemone 1996.
| Methods | 1. Randomized 2. Double Blind 3. 4 Parallel groups: ‐Buspirone 30mg/d steady dose after first week maintained for 2 weeks and withdrawn abruptly and replaced with placebo for 4 weeks ‐Clobazam 30mg with abrupt withdrawal followed by placebo for 4 weeks ‐Clobazam 30mg/d maintained for 2 weeks and withdrawn over 3 week period replaced with placebo for 1 week ‐Lorazepam 3mg after first week, maintained for 2 weeks and withdrawn over 3 week period and replaced with placebo for 1 week 4. Duration: 7 weeks active and withdrawal and one week observation for full study period of 8 weeks 5. Analysis: LOCF |
|
| Participants | 1. Diagnosis: GAD (DSM‐III) 2. N = 128 3. Age (mean, SD): 39.9 (11.1) Sex: not stated Setting: outpatients History: excluded comorbid psychiatric or medical disorders |
|
| Interventions | 1. Buspirone (N = 33) 2. Lorazepam (N = 33) 3. Clobazam (abrupt withdrawal) (N = 30) 4. Clobazam (slow withdrawal) (N = 27) |
|
| Outcomes | 1. dropout rates 2. HAM‐A 3. Tyrer Scale |
|
| Notes | (a) HAM‐A data not included as end point means and SDs not included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Majercsik 2003.
| Methods | 1. Randomized 2. Double Blind 3. Parallel groups: ‐Placebo ‐Buspirone 30mg/d 4. Duration: six weeks 5. Analysis: LOCF ANOVA |
|
| Participants | 1. Diagnosis: GAD (DSM‐IV) 2. N = 52 3. Age (mean, SD): 81.05 (1.01) Sex: 100% male Setting: not states History: excluded those who had received any anxiolytic medication in the 6‐month period leading up to the study and was not sufficiently debilitated to warrant exclusion from the study |
|
| Interventions | 1. Placebo (N = 19 patients) 2. Buspirone (N = 33) |
|
| Outcomes | 1. HAM‐A | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Murphy 1989.
| Methods | 1. Randomized 2. Double blind 3. 2 parallel groups: ‐Buspirone (5‐20mg/d) ‐Diazepam (5‐20mg/d) 4. Duration: one‐half took active drug for six weeks and then switched to placebo while the other half took active medication for 12 weeks and placebo for two weeks 5. Analysis: ANOVA using 44 of the 51 patients (five excluded who were lost after the initial assessment and two (allocated to buspirone) after the second visit |
|
| Participants | 1. Diagnosis: GAD (DSM‐III) 2. N = 51 3. Age (mean, range): Diazepam (late withdrawal) 37.5 (17‐61) Diazepam, early withdrawal 37.6 (21‐55) Buspirone, late withdrawal 34.7 (25‐54) Buspirone, early withdrawal 33.3 (20‐51) Drop‐outs 35.6 (19‐60) Sex: 67% females Setting: outpatients History: No psychotropic 3 weeks prior |
|
| Interventions | 1. Diazepam, late withdrawal (N = 10) 2. Diazepam, early withdrawal (N = 10) 3. Busprione, late withdrawal (N = 10) 4. Buspirone, early withdrawal (N = 10) 5. Drop‐outs (N = 11) (3 allocated to diazepam and 4 to buspirone); four remaining unreported allocation |
|
| Outcomes | 1. dropout rates 2. Comprehensive Psychopathological Rating Scale |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Pecknold 1985.
| Methods | 1. Randomized 2. Double blind 3. Three parallel groups ‐ placebo ‐ Diazepam 5‐40 mg/d ‐ Buspirone 5‐40 mg/d) 4. Duration: 4 weeks with seven day washout period with placebo prior 5. Analysis: Two‐Way ANOVA and Cochran‐Mantel‐Haenszel test |
|
| Participants | 1. Diagnosis: GAD (DSM‐III) 2. N = 60 3. Age: Buspirone = 34.71 Diazepam = 35.81 Placebo = 35.33 Sex: 65% females Setting: outpatients History: excluded any significant psychiatric disorder other than GAD |
|
| Interventions | 1. Placebo (N = 18) 2. Diazepam (N = 21) 3. Buspirone (N = 21) |
|
| Outcomes | 1. dropout rates 2. SCL‐56 3. HAM ‐A 5. CGI |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Pecknold 1989.
| Methods | 1. Randomized 2. Double blind 3. Three parallel groups ‐ placebo ‐ Diazepam 10‐40 mg/d ‐ Buspirone 10‐40 mg/d) 4. Duration: 4 weeks with seven day washout period prior 5. Analysis: ANOVA and Cochran‐Mantel‐Haenszel test |
|
| Participants | 1. Diagnosis: GAD (DSM‐III) 2. N = 125 3. Age: mean and range: Diazepam = 35 Buspirone 35 placebo: 34.1 4.Sex: 64% females 5. Setting: outpatients 6. History: excluded any significant psychiatric disorder other than GAD |
|
| Interventions | 1. Placebo (N = 40) 2. Diazepam (N = 43) 3. Buspirone (N = 42) |
|
| Outcomes | 1. dropout rates 2. SCL‐56 3. HAM ‐A 4. Global Psychopathology Rating |
|
| Notes | (a) Supported by Brystol Meyers Squibb (b) HAM‐A not included in analysis secondary to SDs not being reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Pollack 1997.
| Methods | 1. Randomized 2. Double blind 3. Four parallel groups ‐ placebo ‐ Abecarnil high dosage 7.5‐22.5 mg/d ‐ Abecarnil low dosage 3.0‐9.0 mg/d ‐ Buspirone 15 ‐ 45 mg/d 4. Duration: acute treatment: 6 weeks optional maintainance treatment: 18 weeks withdrawal period: 3 weeks placebo run‐in for week prior to randomization 5. Analysis: ANOVA and Cochran‐Manel‐Haenszel |
|
| Participants | 1. Diagnosis: GAD (DSM‐III‐R) 2. N = 464 3. Age (mean) Abecarnil low dosage = 38 Abecarnil high dosage: 41.3 Buspirone: 37 placebo: 39.2 Sex: 53% females Setting: outpatients History: excluded any significant psychiatric disorder other than GAD |
|
| Interventions | 1. Placebo (N = 112) 2. Abecarnil high (N = 115) 3. Abecarnil low (N = 116) 4. Buspirone (N = 115) |
|
| Outcomes | 1. dropout rates 2. HAM ‐A 3. CGI Scores 4. Physician´s Withdrawal Checklist 5. Covi anxiety scale 6. HAM‐D 7. Raskin depression scale |
|
| Notes | (a) Supported by Sandoz and Schering Berlin (b) HAM‐A not used in final analysis‐means and SDs not reported (c) CGI not used in final anaylsis‐end scores not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Ramchandran 1990.
| Methods | 1. Randomized 2. Double blind 3. Two parallel groups: ‐Buspirone 15mg/d ‐Diazepam 15mg/d 4. Duration: 4 weeks with one week washout period prior 5. Analysis: Data on continuous variables were subjected to Student's t test and the categorical data was analyzed statistically using either the chi‐square test or Fisher's exact probabilty test |
|
| Participants | 1. Diagnosis: GAD (DSM‐III) 2. N = 80 3. Age (mean and SE): Buspirone 25.8 (1.53) Diazepam 28.0 (1.31) Sex: 25.5% females Setting: outpatients History: excluded any significant psychiatric disorder other than GAD |
|
| Interventions | 1. Buspirone (N = 40) 2. Diazepam (N = 40) |
|
| Outcomes | 1. dropout rates 2. HAM‐A 3. HAM‐D 4. Lipman‐Rickets symptom check list 5. Profile of mood states 6. Raskin scale for depression 7. Covi scale for anxiety |
|
| Notes | (a) HAM‐A not entered into final analysis as means and SDs not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Rickels 1997.
| Methods | 1. Randomized 2. Double blind 3. Three parallel groups ‐ placebo ‐ Diazepam 10‐45 mg/d (mean = 19.5 mg/d) ‐ Gepirone 10‐45 mg/d (mean = 19.4 mg/d) 4. Duration: 8 weeks with one week washout period and 2 week post‐8 week abrupt shift under single‐blind conditions for 2 weeks on placebo 5. Analysis: LOCF |
|
| Participants | 1. Diagnosis: GAD (DSM‐III) 2. N = 198 3. Age (mean and SD): 41 (12) Sex: 61% females Setting: outpatients History: excluded any significant psychiatric disorder other than GAD |
|
| Interventions | 1. Placebo (N = 65) 2. Diazepam (N = 67) 3. Gepirone (N = 66) |
|
| Outcomes | 1. dropout rates 2. HAM ‐A 3. CGI Scores 4. Physicians Withdrawal Checklist |
|
| Notes | (a) Supported by Brystol Meyers Squibb (b) HAM‐A not enterered into final analysis secondary to means and SDs not being reported (c) CGI not entered into final analysis‐data not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Ross 1987.
| Methods | 1. Randomized 2. Double blind 3. Three parallel groups ‐ placebo ‐ Diazepam (5‐40 mg/d) ‐ Buspirone (5‐40 mg/d) 4. Duration: 4 weeks with a single‐blind placebo washout one week prior 5. Analysis: ANOVA |
|
| Participants | 1. Diagnosis: GAD (DSM‐III) 2. N = 30 3. Age (mean and range): placebo = 36.2 (24‐52) Buspirone = 38 (20‐61) Diazepam = 37.2 (26‐ 46) Sex: no significant differences Setting: outpatients History: excluded any significant psychiatric disorder other than GAD |
|
| Interventions | 1. Placebo (N = 11) 2. Buspirone (N = 10) 3. Diazepam (N = 9) |
|
| Outcomes | 1. dropout rates 2. HAM‐A 3. CGI Scores 4. SCL‐56 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Sacchetti 1994.
| Methods | 1. Randomized 2. Double blind 3. Four parallel groups: ‐Buspirone 15‐30mg/d ‐Lorazepam 3‐6mg/d ‐Diazepam 15‐30mg/d Bromazepam 9‐18mg/d 4. Duration: four weeks with no continuous anxiolytic medications for the two weeks preceding the screening visit 5. Analysis: ANOVA ‐ |
|
| Participants | 1. Diagnosis: GAD (DSMIII) 2. N = 335 3. Age (mean and SD) Buspirone (N = 58) 43 (13) Buspirone (N = 57) 38 (14) Buspirone (N = 56) 44 (13) Lorazepam 45 (11) Diazepam 39 (12) Bromazepam 47 (10) Sex: no significant differences Setting: outpatient History: excluded any significant psychiatric disorder other than GAD |
|
| Interventions | 1. Buspirone (N = 171) 2. Lorazepam (N = 55) 3. Diazepam (N = 56) 4. Bromazepam (N = 53) |
|
| Outcomes | 1. dropout rates 2. HAM‐A 3. CGI 4. Global Self Rating Scale |
|
| Notes | (a) HAM‐A and CGI were not entered into final analysis as means and SDs not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Sramek 1996a.
| Methods | 1. Randomized 2. Double blind 3. Two parallel groups: ‐placebo ‐Buspirone 15‐45mg/d 4. Duration: 6 weeks with 7 day placebo lead‐in time 5. Analysis: LOCF |
|
| Participants | 1. Diagnosis: GAD (DSM III) with co‐exisitng mild depressive symptoms HAM‐A score 2 or
3 on the "depressed mood" item 2. N = 162 3. Age (mean and SE) placebo = 37.7 (1.3) Buspirone = 38.1 (1.3) Sex: 55.6% females Setting: outpatients History: excluded any significant psychiatric disorder other than GAD and mild depression |
|
| Interventions | 1. Placebo (N = 82) 2. Buspirone (N = 80) |
|
| Outcomes | 1. dropout rates 2. HAM‐A 3. CGI physician and patient‐rated 4. HAM‐D |
|
| Notes | (a) Supported by Bristol‐Myers Squibb Pharmaceutical Research Institute (b) HAM‐A not entered into final analysis as data reported as reduction in score and end scores not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Sramek 1997.
| Methods | 1. Randomized 2. Double blind 3. Two parrallel groups: ‐Buspirone 60 mg/d ‐Buspirone 90 mg/d 4. Duration: 8 weekds with 7‐day placebo lead‐in phase 5. Analysis: patient data included if the patient completed at least 3 weeks of the double‐masked treatment phase (120/137) |
|
| Participants | 1. Diagnosis: GAD (DSM‐III‐R) 2. N = 137 3. Age (mean and SD) Buspirone 60 mg/d = 33.6 (10.4) Buspirone 90 mg/d = 31.3 (8.5) Sex: 46.7% females Setting: outpatients History excluded any significant psychiatric disorder other than GAD |
|
| Interventions | 1. Buspirone 60mg/d (N = 68) 2. Buspirone 90 mg/d (N = 69) |
|
| Outcomes | 1. dropout rates 2. HAM‐A 3. CGI 4. Zung Self‐Rating Anxiety Scale 5. HAM‐D |
|
| Notes | Study supported by Bristol‐Myers Squibb | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bourin 1995 | In abstract and Patients section, describe 43 patients with demographics and then in study procedure, talk about 60 patients without demographic data; confusing to interpret |
| Bouvard 1998 | Missing data on number in each arm with minimal demographic data available |
| Caetano 1990 | Could not locate this article |
| Costa e Silva 1987 | Could not locate this article |
| Fontaine 1987 | This trial do not present the number of patients within each group for all parameteres studied. |
| Goldberg 1982 | Diagnostic criteria used: DSM II |
| Lucki 1987 | Non‐randomized trial |
| Olajide 1987 | Non‐ramdomized trial; cross‐over allocation |
| Petracca 1990 | Dosing of medications beginning point recorded but only flexible dosing after that and not a final range |
| Rickels 1982 | A formal diagnosis of Generalized Anxiety Disorder was not made |
| Rickels 1988 | Generalized Anxiety Disorder and Panic Disorder; results not reported by disorder |
| Rickels 2000 | Non‐randomized trial |
| Rolland 2000 | Minimal demographic data reported and results not reported; dropouts not reported by treatment and results of measured not reported by HAM‐A scores or CGI improved or much improved |
| Rouillon 1995 | Missing reported data as far as drop outs, side effects, and end point HAM‐A scores and SD that can be used in the analysis |
| Schweizer 1990 | Numbers in treatment groups not included |
| Shah 1991 | Unclear randomization |
| Shen 1999 | Not able to locate this article |
| Sontheimer 2001 | No randomization |
| Sramek 1996 | Phase I trial with unclear statistical analysis procedures |
| Strand 1990 | Standardized criteria for GAD not used |
| Tollefson 1991 | This trial includes alcohol abuse/dependency as comorbidies |
| Tyrer 1984 | Data not separated out by diagnoses of GAD |
| van Amerongen 1991 | Not able to locate this article |
| van Laar 1992 | |
| Wheatley 1982 | Criteria used are not standardized acccording DSM / ICD diagnostic criteria. |
| Yevich 1990 | No demographic data and results only repoted by bar graph, dropouts and side effect data not reported |
Contributions of authors
Cheryl Chessick: Conceived the review, designed the review, coordinated the review and data collection, analyzed and interpreted the data and wrote the review. Michael Allen and Michael Thase: helped with reviewing the data, analyzing the data and interpretation of the data and helped with the writing of the review. FK , AC , MSL, JS: Worked on performing previous work that was the foundation of the current study
Sources of support
Internal sources
CNPq, Brazil.
University of Colorado Health Sciences Center, USA.
External sources
No sources of support supplied
Declarations of interest
None
Edited (no change to conclusions)
References
References to studies included in this review
Ansseau 1990 {published data only}
- Ansseau, M, Papart, P, Gérard, MA, Von Frenckell, R, Franck, G. Controlled comparison of Buspirone and Oxazepam in Generalized Anxiety. Neuropsychobiology 1990‐91;24:74‐78. [DOI] [PubMed] [Google Scholar]
Boerner 2003 {published data only}
- *Boerner R, Sommer H, Berger W, Kuhn U, Schmidt U, Mannel M. Kava‐Kava extract LI 150 is as effective as Opipramol and Buspirone in Generalised Anxiety Disorder‐An 8‐week randomized, double‐blind multi‐cenre clinical trial in 129 out‐patients. Phytomedicine 2003;10(Supplement IV):38‐49. [DOI] [PubMed] [Google Scholar]
Bond 2002 {published data only}
- Bond AJ, Wingrove J, Curran HV, Lader MH. Treatment of generalised anxiety disorder with a short course of psychological therapy, combined with buspirone or placebo. Journal of Affective Disorders 2002;72:267‐271. [DOI] [PubMed] [Google Scholar]
Boral 1989 {published data only}
- Boral GC, Bandopadhaya G, Ike VG, Jha RJ, Navani SR, Bowalekar SK, Raghu CN. Double‐Blind, Randomized Clinical Evaluation of Buspirone and Diazepam in Generalized Anxiety Disorders. Advances In Therapy 1989;6(3):112‐124. [Google Scholar]
Borison 1990 {published data only}
- *Borison, RL, Albrecht, JW, Diamond, BI. Efficacy and safety of a putative anxyiolitic agent: Ipsapirone. Psychopharmacology Bulletin 1990;6(26):207‐209. [PubMed] [Google Scholar]
Boyer 1993 {published data only}
- *Boyer, WF, Feighner, JP. A lacebo‐controlled double‐blind multicenter trial of two doses of ipsapirone versus diazepam in generalized anxiety disorder. International Clinical Psychopharmacology 1993;8:173‐176. [DOI] [PubMed] [Google Scholar]
Chiaie 1995 {published data only}
- Chiaie, RD, Pancheri, P, Casacchia, M, Stratta, P, Kotzalidis, GD, Zibellini, M. Assessment of the efficacy of Buspirone in patients affected by generalized anxiety disorder, shifting to Buspirone from prior treatment with Lorazepam: a placebo‐controlled, double blind study. Journal of Clinical Psychopharmacology 1995;15(1):12‐19. [DOI] [PubMed] [Google Scholar]
Cohn 1986 {published data only}
- *Cohn, JB, Wilcox, CS. Low‐sedation potential of Buspirone compared with Alprazolam and Lorazepam in the treatment of anxious patients: A double‐blind study. The Journal of Clinical Psychiatry 1986;47(8):409‐412. [PubMed] [Google Scholar]
Cohn 1986a {published data only}
- Cohn J, Bowden C, Fisher J, Rodos J. Double‐blind comparison of buspirone and clorazepate in anxious outpatients. American Journal of Medicine 1986;80(3B):10‐16. [DOI] [PubMed] [Google Scholar]
Cohn 1989 {published data only}
- Cohn, J, Rickels, K. A pooled, double‐blind comparison of the effects of buspirone, diazepam and placebo in women with chronic anxiety. Current Medical Research and Opinion 1989;11(5):304‐320. [DOI] [PubMed] [Google Scholar]
Cutler 1993 {published data only}
- *Cutler, NR, Sramek, JJ, Wardle, TS, Hesselink, JMK, Roeschen, JK. The Safety and Efficacy of Ipsapirone vs. Lorazepam in outpatients with Gneralized Anxiety Disorder (GAD): Single Site Findings from a Multicenter Trial. Psychopharmacology Bulletin 1993;29(2):303‐308. [PubMed] [Google Scholar]
Cutler 1994 {published data only}
- *Cutler, NR, Hesselinnk, JM, Sramek, JJ. A phase II multicenter dose‐finding, efficacy and safety trial of Ipsapirone in outpatients with generalized anxiety disorder. Progress in Neuro‐psychopharmacology & Biological Psychiatry 1994;18:447‐463. [DOI] [PubMed] [Google Scholar]
Davidson 1999 {published data only}
- *Davidson, JRT, DuPont, RL, Hedges, D, Haskins, JT. Efficacy, safety and tolerability of Venlafaxine Extended Release and Buspirone in outpatients with Generalized Anxiety Disorder. The Journal of Clinical Psychiatry 1999;60(8):528‐535. [DOI] [PubMed] [Google Scholar]
Demartinis 2000 {published data only}
- DeMartinis, N, Runn, M, Rickels, K, mandos, L. Prior Benzodiazepine Use and Buspirone Response in the Treatment of Generalized Anxiety Disorder. The Journal of Clinical Psychiatry 2000;61(2):91‐94. [DOI] [PubMed] [Google Scholar]
Enckelmann 1991 {published data only}
- Enkelmann, R. Alprazolam versus Buspirone in the treatment of outpatients with Genralized Anxiety Disorder. Psychopharmacology 1991;105:428‐432. [DOI] [PubMed] [Google Scholar]
Fabre 1987 {published data only}
- Fabre L. Double‐Blind Comparison of Buspirone with Diazepam in Anxious Patients. Current Therapeutic Research, Clinical & Experimental 1987;41(5):751‐758. [Google Scholar]
Feighner 1982 {published data only}
- Feighner, JP, Merideth, CH, Gordon AH. A Double‐Blind Comparison of Buspirone and Diazepam in Outpatients with Generalized Anxiety Disorder. The Journal of Clinical Psychiatry 1982;43(12 [sec 2]):103‐107. [PubMed] [Google Scholar]
Feighner 1989 {published data only}
- Feighner, JP, Cohn, JB. Analysis off Individual Symptoms in Generalized Anxiety ‐ a Pooled, Multistudy, Double‐Blind Evaluation of Buspirone. Neuropsychobiology 1989;21:124‐130. [DOI] [PubMed] [Google Scholar]
Fontaine 1993 {published data only}
- Fontaine R, Napoliellow MJ. Double‐Blind Comparison of Buspirone 10 mg BID versus Buspirone 5mg TID in Generalized Anxiety Disorder. Current Therapeutic Research 1993;54(2):254‐261. [Google Scholar]
Fresquet 2000 {published data only}
- Fresquet, A, Sust, M, Lloret, A, Murphy, MF, Carter, FJ, Campbell, GM, Marion‐Landais, G. Efficacy and safety of Lesopitron in Outpatients with Generalized Anxiety Disorder. The Annals of Pharmacotherapy 2000;34:147‐153. [DOI] [PubMed] [Google Scholar]
Harto 1988 {published data only}
- Harto, NE, Roland, J, Branconnier, MA, Spera, KF, Dessain, EC. Clinical Profile of Gepirone, a Nonbenzodiazepine Anxiolytic. Psychopharmacology Bulletin 1988;24(1):154‐160. [PubMed] [Google Scholar]
Jacobson 1985 {published data only}
- Jacobson, AF, Dominguez, RZ, Goldstein, BJ, Steinbook, RM. Comparison of Buspirone and Diazepam in Generalized Anxiety Disorder. Pharmacotherapy 1985;5(5):290‐296. [DOI] [PubMed] [Google Scholar]
Laakmann 1998 {published data only}
- *Laakmann, G, Schule, C, Lorkowski, G, Baghai, T. Buspirone and Lorazepam in the treatment of generalized anxiety disorder in outpatients. Psychopharmacology 1998;136:357‐366. [DOI] [PubMed] [Google Scholar]
Lader 1998 {published data only}
- *Lader, M, Scotto, JC. A multicentre double‐blind comparison of hydroxizine, buspirone and placebo in patients with GAD. Psychopharmacology 1998;139:402‐406. [DOI] [PubMed] [Google Scholar]
Lemone 1996 {published data only}
- Lemoine P, Rouillon F, Pouget D. Efficacy and withdrawal of clobazam, lorazepam and buspirone in the treatment of anxiety disorders [Efficacite et sevrage du clobazam comparativement au lorazepam et a la buspirone dans le traitement du trouble anxiete generalisee]. Encephale 1996;22(6):461‐7. [PubMed] [Google Scholar]
Majercsik 2003 {published data only}
- Majercsik E, Haller J, Leveleki C, Baranyi J, Halasz J, Rodgers R. The effect of social factors on the axiolytic efficacy of buspirone in male rats, male mice, and men. Prgress in Neuro‐Psychopharmacology & Biological Psychiatry 2003;27:1187‐1199. [DOI] [PubMed] [Google Scholar]
Murphy 1989 {published data only}
- Murphy S, Owen R, Tyrer P. Comparative Assessment of Efficacy and Withdrawal symptoms After 6 ad 12 Weeks' Treatment with Diazepam or Buspirone. British Journal of Psychiatry 1989;154:529‐534. [DOI] [PubMed] [Google Scholar]
Pecknold 1985 {published data only}
- Pecknold, JC, Familamiri, P, Chang, H, Wilson, R, Alarcia, J, McClure, J. Buspirone: Anxiolytic?. Progress in Neuro‐psychopharmacology & Biological Psychiatry 1985;9:638‐642. [DOI] [PubMed] [Google Scholar]
Pecknold 1989 {published data only}
- Pecknold, JC, Matas, M, Howarth, BG, Ross, C, Swinson, R, Vezeau, C, Ungar, W. Evaluation of Buspirone as an antianxiety agent: Buspirona and Diazepam versus Placebo. Canadian Journal of Psychiatry 1989;34:766‐771. [DOI] [PubMed] [Google Scholar]
Pollack 1997 {published data only}
- Pollack, MH, Worthington, JJ, Manfro, GG, Otto, MW, Zucker, BG. Abecarnil for the Treatment of Generalized Anxiety Disorder: A Placebo‐Controlled Comparison of Two Dosage Ranges of Abecarnil and Buspirone. The Journal of Clinical Psychiatry 1997;58(suppl 11):19‐23. [PubMed] [Google Scholar]
Ramchandran 1990 {published data only}
- Ramchandran, V, Thirunavukarasu, M, Oke, VG, Jha, J, Navani, SR, Bowalekar, SK, Raghu, SN. Comparative Clinical Evaluation of Buspirone and Diazepam in Generalized Anxiety Disorder. Current Therapeutic Research 1990;47(3):502‐510. [Google Scholar]
Rickels 1997 {published data only}
- *Rickels, K, Schweizer, E, DeMartinis, N, Mandos, L, Mercer, C. [Gepirone and Diazepam in Generalized Anxiety Disorder: A Placebo‐Controlled Trial]. Journal of Clinical Psychopharmacology 1997;17(4):272‐277. [DOI] [PubMed] [Google Scholar]
Ross 1987 {published data only}
- Ross, CA, Matas, M. A Clinical Trial of Buspirone and Diazepam in the Treatment of Generalized Anxiety Disorder. Canandian Journal of Psychiatry 1987;32:351‐355. [DOI] [PubMed] [Google Scholar]
Sacchetti 1994 {published data only}
- Sacchetti E, Zerbini O, Banfi F. Tansella M. Overlap of Buspirone with Lorazepam, Diazepam and Bromazepam in Patients with Generalized Anxiety Disorder: Findings from a Controlled, Multicentre, Double‐Blind Study. Human Psychopharmacology 1994;9:409‐422. [Google Scholar]
Sramek 1996a {published data only}
- Sramek JJ, Tansma M, Suri A, Hornig‐Rohan M, Amsterdam JD, Stahl SM, Weisler RH, Cutler NR. Efficacy of Buspirone in Generalized Anxiety Disorder with Coexisting Mild Depressive Symptoms. The Journal of Clinical Psychiatry 1996;57:287‐291. [PubMed] [Google Scholar]
Sramek 1997 {published data only}
- Sramek JJ, Frackiewicz EJ, Cutler NR. Efficacy and Safety of Two Dosing Regimens of Buspirone in the Treatment of Outpatients with Persistent Anxiety. Clinical Therapeutics 1997;19(3):498‐506. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bourin 1995 {published data only}
- Bourin M, Malinge M. Controlled comparison of the effects and abrupt discontinuation of buspirone and lorazepam. Progress in Neuro‐Psychopharmacology and Biological Psychiatry 1995;19:567‐575. [DOI] [PubMed] [Google Scholar]
Bouvard 1998 {unpublished data only}
- Bouvard M. Buspirone in Adolescents with Anxiety Disorders. American Psychiatric Association, Annual Meeting. 1998.
Caetano 1990 {published data only}
- Caetano D, Oliveira J. Double blind comparative study between bsupirone and lorazepam in the treatment of generalized anxiety. Jornal Brasileiro de Psiquiatria 1990;39(1):43‐46. [Google Scholar]
Costa e Silva 1987 {published data only}
- Costa e Silva J, Ruschel S. A double‐blind comparison between buspirone vs bromazepm in outpatients with symptoms of anxiety. Jornal Brasileiro de Psiquiatria 1987;36(4):253‐8. [Google Scholar]
Fontaine 1987 {published data only}
- Fontaine, R, Reaudry, P, Beauclair, L, Chouinard, G. Comparison of withdrawal of Buspirone and Diazepam: A placebo‐controlled study. Progress in Neuro‐Psychopharmacol & Biological Psychiatry 1987;11:189‐197. [DOI] [PubMed] [Google Scholar]
Goldberg 1982 {published data only}
- Goldberg, HL, Finnerty, R. Comparison of Buspirone in Two Separate Studies. The Journal of Clinical Psychaitry 1982;43(12[sec 2]):87‐92. [PubMed] [Google Scholar]
Lucki 1987 {published data only}
- Lucki I, Rickels K, Giesecke M, Geller A. Differential effects of the anxiolytic drugs, diazepam and buspirone, on memory function. British Journal of Clinical Pharmacology 1987;23(2):207‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Olajide 1987 {published data only}
- Olajide, D, Lader, M. A Comparison of Buspirone, Diazepam and Placebo in Patients with Chronic Anxiety States. Journal of Clinical Psychopharmacology 1987;7(3):148‐152. [PubMed] [Google Scholar]
Petracca 1990 {published data only}
- Petracca, A, Nisita, C, McNair, D, Melis, GB, Guerani, G, Cassano, GB. Treatment of Generalized Anxiety Disorder: Preliminary Clinical Experience With Buspirone. The Journal of Clinical Psychiatry 1990;51(9 [suppl]):31‐39. [PubMed] [Google Scholar]
Rickels 1982 {published data only}
- Rickels K, Weisman K, Norstad N, Singer M, Stoltz D, Brown A, Danton J. Buspirone and diazepam in anxiety: a controlled study. Journal of Clinical Psychiatry 1982;43(12 Pt 2):81‐6. [PubMed] [Google Scholar]
Rickels 1988 {published data only}
- Rickels K, Schweizer E, Csanalosi I, Case W, Chung H. Long‐term Treatment of Anxiety and Risk of Withdrawal. Archives of General Psychiatry 1988;45(5):444‐450. [DOI] [PubMed] [Google Scholar]
Rickels 2000 {published data only}
- Rickels, K, DeMartinis, N, Garcia‐España, F, Greeblatt, DJ, Mandos, LA, Rynn, M. Imipramine and Buspirone in Treatment of Patients With Generalized Anxiety Disorder Who are Discontinuing Long‐Term Benzodiazepine Therapy. The American Journal of Psychiatry 2000;157(12):1973‐1979. [DOI] [PubMed] [Google Scholar]
Rolland 2000 {published data only}
- Rolland P, Kablinger A, Brannon G, Freeman A. Treatment of Generalised Anxiety Disorder with Venlafaxine XR. Clinical Drug Investigation 2000;19(2):163‐165. [Google Scholar]
Rouillon 1995 {published data only}
- Rouillon F. A double‐blind controlled study of PCR 7060 vs buspirone in the treatment of generalised anxiety disorder. European Neuropsychopharmacology 1995;5(3):367. [Google Scholar]
Schweizer 1990 {published data only}
- Schweizer E, Rickels K, Csanalosi I, London J, Turner D. A Placebo‐Conrolled Study of Enciprazine in the Treatment of Generalized Anxiety Disorder: A Preliminary Report. Psychopharmacology Bulletin 1990;26(2):215‐217. [PubMed] [Google Scholar]
Shah 1991 {published data only}
- Shah, AV, Parulkar, GB, Mattoo, V, Bowalekar, SK, Raghu, CN. Clinical evaluation of Buspirone and Diazepam in Generalized Anxiety Disorders. Current Therapeutic Research 1991;50(6):827‐834. [Google Scholar]
Shen 1999 {published data only}
- Shen J, Zhong X. Clinical study on buspirone hydrochloride treated for generalized anxiety disorder. Journal of Chinese Civil Administration Medicine 1999;11(2):70‐2. [Google Scholar]
Sontheimer 2001 {published data only}
- Sontheimer DL, Ables AZ. Is Imipramine or Buspirone Treatment Effective in Patients Wishing to Discontinue Long‐Term Benzodiazepine Use?. Family Practice 2001;50(3):203. [PubMed] [Google Scholar]
Sramek 1996 {published data only}
- *Sramek, JJ, Fresquet A, Gaston M‐L, Hourani J, Jhee SS, Martinez L, Jensen C, Bolles K, Carrington A, Cutler N. Establishing the Maximum Tolerated Dose of Lesopitron in Patients With Generalized Anxiety Disorder: A Bridging Study. Journal of Clinical Psychopharmacology 1996;16(6):454‐458. [DOI] [PubMed] [Google Scholar]
Strand 1990 {published data only}
- Strand, M, Hetta, J, Rosen, A, Sorensen, S, Malmstrom, R, Fabian, C, Marits, K, Vetterskog, K, Liljestrand, AG, Hegen, C. A Double‐Blind Controlled Trial in Primary Care Patients With Generalized Anxiety: A Comparison Between Buspirone and Oxazepam. The Journal of Clinical Psychiatry 1990;51(9 [suppl]):40‐45. [PubMed] [Google Scholar]
Tollefson 1991 {published data only}
- *Tollefson, GD, Lancaster, SP, Montague‐Clouse, J. The Association of Buspirone ands Its Metabolite 1‐pyrimidinylpiperazine in the Remission of Comorbid Anxiety with Depressive Features and Alcohol Dependency. Psychopharmacology Bulletin 1991;27(2):163‐170. [PubMed] [Google Scholar]
Tyrer 1984 {published data only}
- Tyrer P, Owen R. Anxiety in primary care: is short‐term drug treatment appropriate?. Journal of Psychiatric Research 1984;18(1):73‐8. [DOI] [PubMed] [Google Scholar]
van Amerongen 1991 {published data only}
- Amerongen A, Viel J, Konopka C, Berdeaux G. French multicenter dose‐ranging trial of ipsapirone in GAD patients: a randomized, double‐blind parallel group placebo‐controlled trial. 5th World Congress of Biological Psychiatry. Florence, 9‐14 June, 1991.
van Laar 1992 {published data only}
- Laar M, Volkerts E, Willigenburg A. Therapeutic efects and effects on actual driving performance of chronically administered buspirone and diazepam in anxious outpatients. Journal of Clinical Psychopharmacology 1992;12(2):86‐95. [PubMed] [Google Scholar]
Wheatley 1982 {published data only}
- Wheatley, D. Buspirone: Multicenter Efficacy Study. The Journal of Clinical Psychiatry 1982;43(12[sec 2]):92‐94. [PubMed] [Google Scholar]
Yevich 1990 {published data only}
- Yevich JP, Eison MD, Eison AS, Taylor DP, Yocca FD, VanderMaelen CP, Riblet LA, Robinson DS, Roberts Dl, Temple DL. Gepirone hydrochloride: preclinical pharmacology and recent clinical findings. Current and Future Trends in Anticonvulsant, Anxiety, and Stroke Therapy 1990;361:443‐51. [PubMed] [Google Scholar]
Additional references
Altman 1996
- Altman DG, Bland JM. Detecting skeweness fromsummary information. British Medical Journal 1996;313(7066):1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ballenger 2001
- Ballenger, JC, Davidson, JRT, Lecrubier, Y, Nutt, DJ, Borkovec, T, Rickels K, Stein, D, Wittchen, H. Consensus statement on Generalized Anxiety Disorder from the Tenth Internation Consensus Group on Depression and Anxiety. The Journal of Clinical Psychiatry 2001;62(suppl 11):53‐58. [PubMed] [Google Scholar]
Borkovec 1993
- Borkovec TD, Costello E. Efficacy of applied relaxation and cognitive behavioral therapy in the treatment of generalized anxiety disorder. Journal Consulting and Clinical psychology 1993;61:611‐19. [DOI] [PubMed] [Google Scholar]
Brawman‐Mintzer 2001
- Brawman‐Mintzer O. Pharmacological treatment of Generalized Anxiety Disorder. The Psychiatric Clinics of North America 2001;24(1):119‐137. [DOI] [PubMed] [Google Scholar]
Bruce 2005
- Bruce SE, Yonker KA, Otto MW, Eisen JL, Weisberg RB, Pagano M, Shea MT, Keler MB. Influence of Psychiatric Comorbidity on Recovery and Recurrence in Generalized Anxiety Disorder, Social Phobia, and Panic Disorder: A 12‐Year Prospective Study. American Journal of Psychiatry 2005;162:1179‐1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gammans 1992
- Gammans RE, Stringfellow JC, Hvizdos AJ, Seidehamel RJ, Cohn JB, Wilcox CS, Fabre LF, Pecknold Jc, Smith Wt, Rickels K. Use of buspirone in patients with generalized anxiety disorde and coexising depressive symtoms. A meta‐analysis of eight randomized, controlled studies. Neuropsychobiology 1992;24(4):193‐201. [DOI] [PubMed] [Google Scholar]
Goa 1986
- Goa KL, Ward A 32. Buspirone. A preliminary review of its pharmacological properties and therapeutic efficacy as an anxiolytic.. Drugs (New Zealand) 1986;2:114‐129. [DOI] [PubMed] [Google Scholar]
Hamilton 1959
- Hamilton, M. The assessment of anxiety states by rating. British Journal of Medical Psychology 1959;32:50‐55. [DOI] [PubMed] [Google Scholar]
Higgins 2005
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Intrventions 4.2.4 [updated March 2005]. In: The Cochrane Library, Issue 2, 2005. Chichester, UK: John Wiley & Sons, Ltd.. [Google Scholar]
Kapczinski 2005
- Kapczinski F, Lima MS, Souza S, Cunha A, Schmitt R. Antidepressants for generalized anxiety disorder (Review). The Cochrane Collaboration 2005. [DOI] [PubMed] [Google Scholar]
Kessler 1994
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen H‐U, Kendler KS. Lifetime and 12‐month prevalence of DSM‐III‐R psychiatric disorers in the United States: results from the National Comorbidity Survey. Archives of General Psychiatry 1994;51:8‐19. [DOI] [PubMed] [Google Scholar]
Lydiard 1997
- Lydiard, RB, Ballenger, JC, Rickels, K. A Double‐Blind Evaluation of the Safety and Efficacy of Abecarnil, Alprazolam and Placebo in Outopatients with Generalized Anxiety Disorder. The Journal of Clinical Psychiatry 1997;58(suppl 11):11‐18. [PubMed] [Google Scholar]
Mandos 1995
- Mandos LA, Rickels K, Cutler N, Roeschen J, Keppel Hesselink JM, Schweizer E. Placebo‐controlled comparison of the clinical effects of rapid discontinuation of ipsapirone and lorazepam after 8 weeks of treatment for generalized anxiety disorder. International Clinical Psychopharmacology 1995;10:251‐256. [DOI] [PubMed] [Google Scholar]
Meoni 2001
- Meoni P: Salina E, Yves B, Hackett D. Pattern of Symptom Improvement Following Treatment with Venlafaxine XR in Patients with Generalized Anxiety Disorder. The Journal of Clinical Psychiatry 2001;62(11):888‐893. [DOI] [PubMed] [Google Scholar]
Pittler 2001
- Pittler MH, Ernst E. Kava extract for treating anxiety (Cochrane Review). Cochrane Database Systematic Review 2001, Issue 4. [CD003383] [DOI] [PubMed] [Google Scholar]
Shader 1993
- Shader, RI, Greenblatt, DJ. Use of benzodiazepines in anxiety disorders. New England Journal of Medicine 1993;328:1398‐1405. [DOI] [PubMed] [Google Scholar]
Wingerson 1992
- Wingerson D, Nguyen C, Roy‐Byrne PP. Chlomipramine treatment for Generalized Anxiety Disorder [Journal of Clinical Psychopharmacology]. 1992; Vol. 12, issue 3:214‐215. [PubMed]
Wittchen 1994
- Wittchen, HU, Zhao, S, Kessler, RC, Eaton, WW. DSM III‐R generalized anxiety disorder in the national comorbidity survey. Archives of General Psychiatry 1994;51:355‐364. [DOI] [PubMed] [Google Scholar]


