Abstract
Osteoarthritis (OA) is a multidimensional health problem and a common chronic disease. It has a substantial impact on patient quality of life and is a common cause of pain and mobility issues in older adults. The functional limitations, lack of curative treatments, and cost to society all demonstrate the need for translational and clinical research. The use of OA models in mice is important for achieving a better understanding of the disease. Models with clinical relevance are needed to achieve 2 main goals: to assess the impact of the OA disease (pain and function) and to study the efficacy of potential treatments. However, few OA models include practical strategies for functional assessment of the mice. OA signs in mice incorporate complex interrelations between pain and dysfunction. The current review provides a comprehensive compilation of mouse models of OA and animal evaluations that include static and dynamic clinical assessment of the mice, merging evaluation of pain and function by using automatic and noninvasive techniques. These new techniques allow simultaneous recording of spontaneous activity from thousands of home cages and also monitor environment conditions. Technologies such as videography and computational approaches can also be used to improve pain assessment in rodents but these new tools must first be validated experimentally. An example of a new tool is the digital ventilated cage, which is an automated home-cage monitor that records spontaneous activity in the cages.
Abbreviations: CST, capacitance sensing technology; DVC, digital ventilated cage; MIA, mono-iodoacetate; OA, osteoarthritis; OARSI: osteoarthritis research society international; PWT, paw withdrawal time
Osteoarthritis (OA) is a multidimensional health problem and a common chronic disease.36 Functional limitations, the absence of curative treatments, and the considerable cost to society result in a substantial impact on quality of life.76 Historically, OA has been described as whole joint and whole peri-articular diseases and as a systemic comorbidity.9,111 OA consists of a disruption of articular joint cartilage homeostasis leading to a catabolic pathway characterized by chondrocyte degeneration and destruction of the extracellular matrix (ECM). Low-grade chronic systemic inflammation is also actively involved in the process.42,92 In clinical practice, mechanical pain, often accompanied by a functional decline, is the main reason for consultations. Recommendations to patients provide guidance for OA management.22, 33,49,86 Evidence-based consensus has led to a variety of pharmacologic and nonpharmacologic modalities that are intended to guide health care providers in managing symptomatic patients. Animal-based research is of tremendous importance for the study of early diagnosis and treatment, which are crucial to prevent the disease progression and provide better care to patients.
The purpose of animal-based OA research is 2-fold: to assess the impact of the OA disease (pain and function) and to study the efficacy of a potential treatment.18,67 OA model species include large animals such as the horse, goat, sheep, and dog, whose size and anatomy are expected to better reflect human joint conditions. However, small animals such as guinea pig, rabbit, mouse, and rat represent 77% of the species used.1,87 In recent years, mice have become the most commonly used model for studying OA. Mice have several advantageous characteristics: a short development and life span, easy and low-cost breeding and maintenance, easy handling, small joints that allow histologic analysis of the whole joint,32 and the availability of genetically modified lines.108 Standardized housing, genetically defined strains and SPF animals reduce the genetic and interindividual acquired variability. Mice are considered the best vertebrate model in terms of monitoring and controlling environmental conditions.7,14,15,87 Mouse skeletal maturation is reached at 10 wk, which theoretically constitutes the minimal age at which mice should be entered into an OA study.64,87,102 However, many studies violate this limit by testing mice at 8 wk of age.
Available models for OA include the following (Table 1): spontaneous naturally occurring OA (C57BL/6, BALB/c, STR/ort or genetically modified mice); chemically-induced (mainly mono-iodoacetate [MIA] injection); noninvasive (high fat diet or obesity-induced) consistent with the metabolic human OA;32,111 physical activity and exercise induced OA; noninvasive mechanical loading (repetitive mild loading and single-impact injury); and surgically induced (meniscectomy models or anterior cruciate ligament transection). The specific model used would be based on the goal of the study.7 For example, OA pathophysiology, OA progression, and OA therapies studies could use spontaneous, genetic, surgical, or noninvasive models. In addition, pain studies could use chemical models. Lastly, post-traumatic studies would use surgical or noninvasive models; the most frequently used method is currently destabilization of the medial meniscus,32 which involves transection of the medial meniscotibial ligament, thereby destabilizing the joint and causing instability-driven OA. An important caveat for mouse models is that the mouse and human knee differ in terms of joint size, joint biomechanics, and histologic characteristics (layers, cellularity),32,64 and joint differences could confound clinical translation.10
Table 1.
Mouse models of osteoarthritis.
| Models | Pros | Cons | |
|---|---|---|---|
| Spontaneous | Wild type mice7,9,59,67,68,70,72,74,80,85,87,115,118,119,120 | - Model of aging phenotype - The less invasive model - Physiological relevance: mimics human pathogenesis - No need for technical expertise - No need for specific equipment |
- Variability in incidence - Large number of animals at baseline - Long-term study: Time consuming (time of onset: 4 -15 mo) - Expensive (husbandry) |
| Genetically modified mice2,7,25,40,50,52,67,72,79,80, 89,120 | - High incidence - Earlier time of onset: 18 wk - No need for specific equipment - Combination with other models |
- Time consuming for the strain development - Expensive |
|
| Chemical- induced | Mono-iodoacetate injection7,11,46,47,60,66,90,91,101,128 | - Model of pain-like phenotype - To study mechanism of pain and antalgic drugs - Short-term study: Rapid progression (2-7 wk) - Reproducible - Low cost |
- Need for technical expertise - Need for specific equipment - Systemic injection is lethal - Destructive effect: does not allow to study the early phase of pathogenesis |
| Papain injection66,67,120 | - Short-term study: rapid progression - Low cost |
- Need for technical expertise - Need for specific equipment - Does not mimic natural pathogenesis |
|
| Collagenase injection7,65,67,98 | - Short-term study: rapid progression (3 wk) - Low cost |
- Need for technical expertise - Need for specific equipment - Does not mimic natural pathogenesis |
|
| Non-invasive | High-fat diet (Alimentary induced obesity model)5,8,43,45,57,96,124 | Model of metabolic phenotype No need for technical expertise No need for specific equipment Reproducible |
Long-term study: Time consuming (8 wk–9 mo delay) Expensive |
| Physical activity and exercise model45,73 | Model of post traumatic phenotype No need for technical expertise |
Long-term study: time consuming (18 mo delay) Expensive Disparity of results |
|
| Mechanical loading models Repetitive mild loading models Single-impact injury model7,16,23,24, 32,35,104,105,106 | Model of post traumatic phenotype Allow to study OA development Time of onset: 8-10 wk post injury Noninvasive |
Need for technical expertise Need for specific equipment Heterogeneity in protocol practices Repetitive anesthesia required or ethical issues |
|
| Surgical | Ovariectomy114 | Contested. | |
| Meniscectomy model7,32,63,67,87 | Model of post traumatic phenotype High incidence Short-term study: early time of onset (4 wk from surgery) To study therapies |
Need for technical expertise Need for specific equipment Surgical risks Rapid progression compared to human |
|
| Anterior cruciate ligament transection (ACLT)7,39,40,61,48,67,70,87,126 | Model of posttraumatic phenotype High incidence Short-term study: early time of onset (3-10 wk from surgery) Reproducible To study therapies |
Need for technical expertise Need for specific equipment Surgical risks Rapid progression compared to human |
|
| Destabilization of medial meniscus (DMM)7,32,39,40 | Model of post traumatic phenotype High incidence Short-term study: early time of onset (4 wk from surgery) To study therapies The most frequently used method |
Need for technical expertise Need for specific equipment Surgical risks Rapid progression compared to human |
Since all animal models have strengths and weaknesses, it is often best to plan using a number of models and techniques together to combine the results.
In humans, the lack of correlation between OA imaging assessment and clinical signs highlights the need to consider the functional data and the quality of life to personalize OA management. Clinical outcomes are needed to achieve 2 main goals: to assess the impact of the OA in terms of pain and function and to study the efficacy of treatments.65 Recent reviews offer few practical approaches to mouse functional assessment and novel approaches to OA models in mice.7,32,67,75,79,83,87, 100,120 This review will focus on static and dynamic clinical assessment of OA using automatic and noninvasive emerging techniques (Table 2).
Table 2.
Outcome options in mouse models of osteoarthritis
| Test name | Techniques | Kind of assessment | Output | Specific equipment required |
|---|---|---|---|---|
| Static measurement | ||||
| Von Frey filament testing | Calibrated nylon filaments of various thickness (and applied force) are pressed against the skin of the plantar surface of the paw in ascending order of force | Stimulus- evoked pain-like behavior Mechanical stimuli - Tactile allodynia The most commonly used test |
Latency to paw withdrawal and Force exerted are recorded |
Yes |
| Knee extension test | Apply a knee extension on both the intact and affected knee or Passive extension range of the operated knee joint under anesthesia |
Stimulus-evoked pain-like behavior | Number of vocalizations evoked in 5 extensions | None |
| Hotplate | Mouse placed on hotplate. A cutoff latency has been determined to avoid lesions | Stimulus-evoked pain-like behavior Heat stimuli- thermal sensitivity |
Latency of paw withdrawal | Yes |
| Righting ability | Mouse placed on its back | Neuromuscular screening | Latency to regain its footing | None |
| Cotton swab test | Bringing a cotton swab into contact with eyelashes, pinna, and whiskers | Stimulus-evoked pain-like behavior Neuromuscular screening |
Withdrawal or twitching response | None |
| Spontaneous activity | ||||
| Spontaneous cage activity | One by one the cages must be laid out in a specific platform | Spontaneous pain behavior Nonstimulus evoked pain Activity |
Vibrations evoked by animal movements | Yes |
| Open field analysis | Experiment is performed in a clear chamber and mice can freely explore | Spontaneous pain behavior Nonstimulus evoked pain Locomotor analysis |
Paw print assessment Distance traveled, average walking speed, rest time, rearing |
Yes |
| Gait analysis | Mouse is placed in a specific cage equipped with a fluorescent tube and a glass plate allowing an automated quantitative gait analysis | Nonstimulus evoked pain Gait analysis Indirect nociception |
Intensity of the paw contact area, velocity, stride frequency, length, symmetry, step width | Yes |
| Dynamic weight bearing system | Mouse placed is a specific cage. This method is a computerized capacitance meter (similar to gait analysis) | Nonstimulus evoked pain Weight-bearing deficits Indirect nociception |
Body weight redistribution to a portion of the paw surface | Yes |
| Voluntary wheel running | Mouse placed is a specific cage with free access to stainless steel activity wheels. The wheel is connected to a computer that automatically record data | Nonstimulus evoked pain Activity |
Distance traveled in the wheel | Yes |
| Burrowing analysis | Mouse placed is a specific cage equipped with steel tubes (32 cm in length and 10 cm in diameter) and quartz sand in Plexiglas cages (600 · 340x200 mm) | Nonstimulus evoked pain Activity |
Amount of sand burrowed | Yes |
| Digital video recordings | Mouse placed is a specific cage according to the tool | Nonstimulus evoked pain Or Evoked pain |
Scale of pain or specific outcome | Yes |
| Digital ventilated cage system | Nondisrupting capacitive-based technique: records spontaneous activity 24/7, during both light and dark phases directly from the home cage rack | Spontaneous pain behavior Nonstimulus evoked pain Activity-behavior |
Distance walked, average speed, occupation front, occupation rear, activation density. Animal locomotion index, animal tracking distance, animal tracking speed, animal running wheel distance and speed or rotation |
Yes |
| Challenged activity | ||||
| Rotarod test | Gradual and continued acceleration of a rotating rod onto which mice are placed | Motor coordination Indirect nociception |
Rotarod latency: riding time and speed with a maximum cut off. | Yes |
| Hind limb and fore grip strength | Mouse placed over a base plate in front of a connected grasping tool | Muscle strength of limbs | Peak force, time resistance | Yes |
| Wire hang analysis | Suspension of the mouse on the wire and start the time | Muscle strength of limbs: muscle function and coordination | Latency to fall gripping | None (self -constructed) |
Pain cannot be directly measured in rodents, so methods have been developed to quantify “pain-like” behaviors. The clinical assessment of mice should be tested both before and after the intervention (induced-OA ± administration of treatment) to take into account the habituation and establish a baseline to compare against.
Outcomes Measured to Assess Symptomatic OA
OA signs in mice comprise complex interactions between pain and dysfunction. Because pain cannot be directly measured in rodents, methods have been developed to quantify “pain-like” behaviors.30 Mice should be clinically assessed both before and after OA induction or administration of treatment both to account for habituation and to establish a baseline for subsequent comparisons.79 All outcomes are summarized in the Table 2. All static testing is based on pain assessment. Static measurements refer primarily to an assessment off evoked pain. Dynamic measurements require a monitoring of the mouse activity, which can be spontaneous or evoked.
Static measurements.
Static assessments of pain are one way to assess symptomatic OA. Rodents are particularly useful for statis measurements due to their small size and easy manipulation. However, in contrast to rats, mice are more active, which can make static assessments more difficult.83
One static test is the used of Von Frey filament to identify mechanical/tactile allodynia.17 Calibrated nylon filaments of various thickness (and applied force) are pressed against the skin of the plantar surface of the paw in ascending order of force.83,90 The stimulus should lead to a rapid withdrawal response. After a training and habituation period, latency to paw withdrawal and force exerted are recorded.100 The lowest amount of force inducing a response is the paw withdrawal threshold (PWT), expressed in grams.63 As in sham-operated mice, mice with a partial medial meniscectomy had a consistent decrease of the ipsilateral PWT at day 7 after surgery (from 0.56g to 0.45g).61 However, as compared with sham-operated mice, PWT remained low until day 56 in mice with meniscectomy, after which it fell from 0.45g to 0.24g. The effect of OA induction leads to an increase of allodynia with a clear decrease of withdrawal threshold.61 The Von Frey filament test sensitive with regard to analgesia testing in OA.123
The knee extension is another test used to assess pain in mice with OA.56,90,100,127 Various techniques can be considered. The knee can be extended on both the intact and the affected side and the number of vocalizations, indicating discomfort, evoked in 5 extensions can be counted.100 This is a relevant clinical measure because OA patients experience loss of knee range of motion that leads to discomfort.67,97 A surgically induced OA model created by resection of the medial collateral ligament and the medial meniscus in 8 wk old C57BL/6 male mice showed a significant impairment of knee extension starting from the third month after surgery; this difference from the control group was maintained until the sixth month.82 This study used passive extension range of the operated knee joint under anesthesia, measured the maximal and minimal angle of the mouse knee joint, and obtained results consistent with the Osteoarthritis Research Society International OARSI scoring, showing significant OA development in the operated mice.82 The reduction of extension angle leads to static limitations and also affects dynamic results.
The hotplate is a thermal sensitivity test.3,100 The latency of nociceptive reaction is measured by measuring PWT. After cruciate ligament transection, OA led to a longer PWT of mice on a hotplate, starting from 4 wk onward.109 Eight weeks after surgery, the response time in operated mice was approximately 7 s as compared with 4 s in the sham group.
Another global reflexive static test is neuromuscular screening using the cotton swab test. A cotton swab that is brought into contact with eyelashes, pinna, and whiskers should induce a withdrawal or twitching response.2 If not, reflexive behavior will be viewed as abnormal, indicating a behavioral characteristic of pain that can be associated with induced OA.
Righting ability can also be used as a general test The mouse is placed on its back and the time taken to regain upright posture is assessed and can be scored as normal, delayed, or abnormal.2
Dynamic measurements.
Spontaneous activity.
Biomechanical and functional assessments allow evaluation of the functional consequences of deficiencies or disabilities related to OA. Spontaneous cage activity can be measured automatically.32 This LABORAS (Laboratory Animal Behavior Observation Registration and Analysis System) records objective normal activity and OA-induced changes in locomotion (distance), climbing (hanging from a wire cage), feeding, grooming, rearing (standing on hind legs), head shakes, and nest-making as measures of behavior in mice.12,83,100 To use this system, cages must be arranged on a specific platform, which can be time-consuming, and the results can be skewed by handling the cages.121 As compared with human observation, software converts mouse movement into a data set that can be obtained and analyzed easily.122
The open field test consists of locomotor analysis with paw print assessment.27,109 The test is performed in an open space that mice can explore.109 Distance traveled, average walking speed, and rest time are related to horizontal locomotor activity.113 Some studies have assessed vertical locomotor activity by measuring rearing.113 Chronic pain leads to withdrawal and hypo-activity. Even if human observation introduces subjectivity, spontaneous pain behavior may be more clinically relevant than is evoked pain.83
The gait analysis or catwalk test is a method to indirectly assess pain. Automated quantitative gait analysis requires special equipment to measure the intensity of the paw contact area.32 OA causes gait changes and a smaller footprint area.109 Gait analysis includes velocity, stride frequency and length, symmetry, and step width.109 Parameters related to interlimb coordination can also be measured objectively. Pain can cause compensatory changes in joint load shifting, and the automated gait analysis system can assess body weight redistribution to a portion of the paw surface that is associated with pain.2,95,100 Computerized gait analysis provides nonbiased pain assessment. Gait analysis should include spatiotemporal, kinetic, and kinematic parameters.69
Voluntary wheel running has been used to assess pain in rodent. Data collection is completely automated, and the experimenter is not in the room during the assessment.26 However, multiple activity cages need individual randomization before the experiment.
Burrowing can also be evaluated. This has been done with the MIA model of OA to assess pain-related behavior and analgesic efficacy.13 Burrowing is an innate behavior and is reduced in rodents experiencing pain.13 Bilateral MIA injection in rats impairs burrowing behavior.13 The burrowing method has 2 phases: first, social facilitation during which rats are placed in pairs in a cage for 2 h on 2 consecutive days with a measurement of the amount of sand burrowed, and second placing a rat alone in the cage for 30 min per day and determining the average amount burrowed over 3 d to provide a baseline value for burrowing. Burrowing evaluation could also be useful in mice but the method must be optimized for mice before it can be used experimentally.121 Digital video recordings can also be used to assess facial grimace in rats and mice, which is not specific to OA but can be used to assess pain.71,88,116,117
Automated home-cage monitoring provides activity information directly from the home cage using electrical capacitance sensing technology (CST) of an electronic board placed under the home cage.55 The digital ventilated cage system (DVC) (Tecniplast S.p.A, Buguggiate (VA), Italy) records spontaneous activity using 12 electrodes spread as 3 × 4 grids in the cages. Electrodes continuously detect electrical capacitance every 250 ms during both light and dark phases directly from the home cage rack. This nondisruptive capacitive-based technique with several advantages including the reduction of animal handling and no need to set up an external data collection system.55,99 The system can collect data from thousands of home cages simultaneously; benefits are 2-fold (Figure 1). First, the DVC system can analyze mouse behavior global cage activity over time. As compared with conventional video metrics, individually housed mice CST metrics are highly correlated for distance walked, average speed, occupation front, occupation rear, and activation density. Currently validated DVC metrics are animal locomotion index, animal tracking distance, animal tracking speed, and running wheel distance and speed or rotation. Animal locomotion index is correlated with the activity pattern. The DVC system can also be used to monitor animal welfare.55 The system is can detect high activity levels that may signal aggression.38 Another use for the DVC system is to analyze the bedding status by monitoring increasing moisture due to urine and water bottle leakage. The range of capabilities of this technology can provide research, husbandry and welfare indicators. DVC systems will likely become an essential tool for many laboratories in the future.
Figure 1.
The digital ventilated cage system is an automated home-cage monitoring that continuously records spontaneous activity in the cages. The system can collect data from thousands of home cages simultaneously.
Challenged activity.
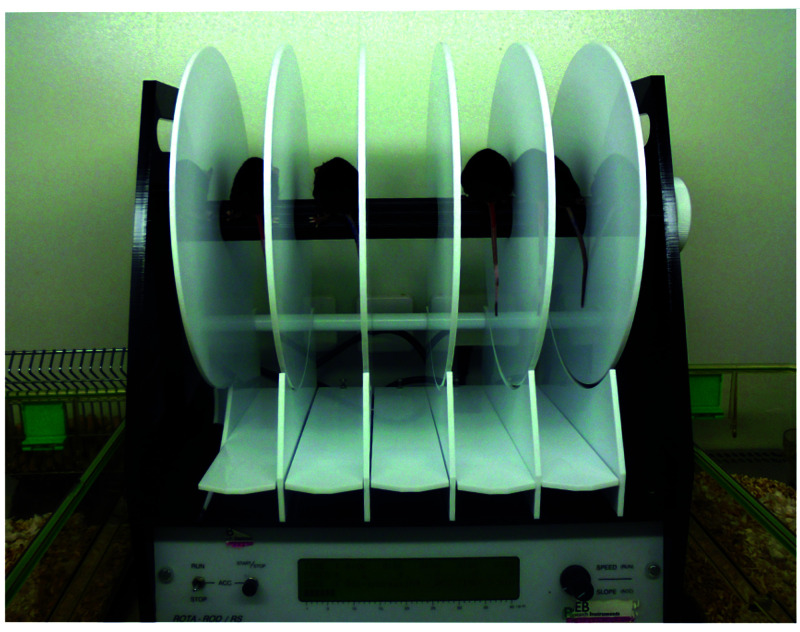
Motor dysfunction and indirect pain can be assessed using challenged exercises, although learning and motivation can mask true functional effects. The rotarod test is based on the gradual and continued acceleration of a rotating rod onto which mice are placed (Figure 2). Data collected are the riding times (the amount of time a mouse stays on the rod before falling off) and the speed (from 4 to 40 rpm) at which a mouse falls off.2,100,123 Mice are trained for 5 min at a constant speed of 4 rpm on the rotarod before the experimental trials begin. Each trial has a maximum time of 5 min. The trial consists of testing mice for a number of consecutive days (typically 3) with a minimum of 30 min intertrial rest.100,109 Rotarod creates a forced ambulation and involves motor coordination, balance, pain, sensorimotor skills, endurance, memory, and learning skills that could be limiting factors for the interpretation of the performance.10,100,109 OA decreases time on the rotarod.82,109 When cruciate ligament transection was performed on 8-wk-old male FVB/N mice and compared with the preoperative functional assessment, mice showed a postoperative motor dysfunction in rotarod analysis.109 Those functional changes were linked with histologic grading by OARSI; functional decline should occur concomitantly with the cartilage degeneration.109
Figure 2.
The rotarod test is based on a gradual and continued acceleration of a rotating rod onto which mice are placed. Data collected corresponds to the riding time (the amount of time each mouse stays on the rod before falling off or a passive rotation) and the speed (from 4 to 40 rpm).
Hind limb and fore grip strength are also challenge exercises that are commonly used in OA studies.2,29,79,93 Special equipment automatically measures the grip force of limbs of mice, including the peak force and time resistance. The mouse is placed over a base plate in front of a grasping tool (T-shaped or trapeze-shaped), and data are recorded. Three successive trials administered on the same day test the maximum force applied as the mouse is pulled away from the attachment of the grasping tool,2 allowing assessment of the effect of OA progression or pain medication on muscle strength. Wire hang analysis is another way to assess gripping ability, coordination and balance skills of mice. The measured parameter is the latency to fall.109
Discussion
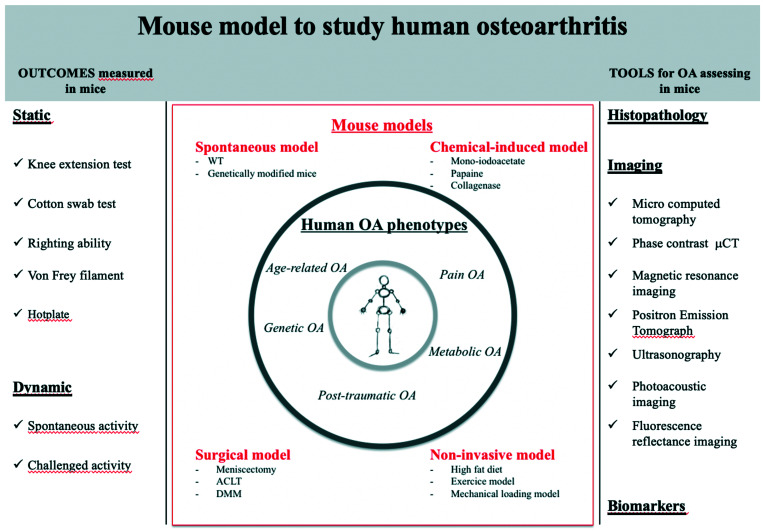
Additional OA research is necessary to meet the health challenges of patients and OA researchers will continue to need innovative models and technology in their studies. Figure 3 shows outcomes and tools available to study mouse model of OA. An animal model should be isomorphic, homologous, and predictive. In principle, the animal model should have the same signs of disease as humans; the pathophysiology and response to treatment should be comparable. At least 3 to 5 OA human phenotypes have been described: age-related OA, posttraumatic-OA, metabolic OA, genetic, and pain OA.9,107,111 However, interindividual heterogeneity is common among individuals with knee OA pain with regard to patient physical performance profiles.28 Patient profiles based on physical performance and movement-evoked pain were also significantly different in psychologic and somatosensory function.28 The identification of profiles supports the adjustment of the therapeutic plan to individual patients.
Figure 3.
This figure shows the most recent data on all aspects of OA mouse models to support choice of the best model in terms of objective, pathophysiological pathway, surrogate markers of progression, imaging techniques, and functional assessment.
OA has a complex physiopathology, and one single animal model will not mimic all of the components of the human disease.79,87,120 Some mouse models may replicate a phenotype without involving all the integrative pathways, and a perfect model does not exist. Therefore, the choice of rodent model must consider the objective of the study and the nature of the outcomes. Because all animal models have strengths and weaknesses, the use of several models and techniques considered together may provide the most useful results. For example, using a prey species as a model can be a weakness because prey animals such as rodents may not show obvious signs of pain4,79 to order avoid attracting predators. A link between this masking behavior and prey position in the food chain is difficult to confirm with robust data. In addition, the behavioral and functional tests used in OA studies may cause mice stress.6,14 Several assessments of pain and/or locomotion should be included in a study because mice may hide the signs of pain and distress. A summary of animal models used to study pain of OA with outcome measured longitudinally has been published to help promote use of the ARRIVE guidelines and achieve better cross-publication comparisons.10,79,112
The 3Rs principles of reduction, refinement, and replacement provide a framework for ethical decisions about using animals for scientific purposes.53 Refinement should be a constant concern of researchers from the point of experimental design (by improving animal welfare with appropriate housing and handling procedures, minimizing suffering through pain treatment, and terminating animals that reach humane endpoints)94 until the time of scientific publication (by reporting according to the ARRIVE guidelines).112 Consideration of analgesia and pain management is also important.15 Both pharmacologic and nonpharmacologic measures can be used to manage pain in mice with OA. Depending on the procedure, pain medication ranging from general anesthesia to analgesics can be administered before and after surgery in surgical models of OA. In addition, refined surgical procedures can be developed. For example, the destabilization of medial meniscus model is less invasive and considered more homologous to OA in human.39 Furthermore, assessment of modified surgical methods41, such as refining surgical small rodent models of OA for joint pathology and pain, indicates that pain behavior is not always present despite significant histopathologic changes during disease progression.40 This reflects the heterogeneity seen in human OA and therefore better mimics the human condition. Animals and human patients both experience pain as a symptom of OA.62 Two arguments are used by animal care and ethical committees to promote the use of analgesia. The first is that refining surgical models of osteoarthritis in rodents by using analgesics alters the pain phenotype but does not alter the joint.”41 Second, not using pain alleviating drugs in mice might alter the comparability to humans because the majority of human patients with OA take medicine for pain.43 We do not suggest that pain medication should be administrated in all OA studies, but consideration should be given to the issue and discussed both with ethical committees and within the OA scientific community.
Improved reporting of behavioral preclinical data will promote reproducibility.58 Identifying appropriate humane endpoints is essential to avoiding unnecessary suffering in research animals.14,94 Refinement could be achieved by using the earliest possible endpoints.31 The description of endpoints in the methods of publications can be improved by adhering to the ARRIVE guidelines112 and automated manuscript screening software can be used to detect inadequate reporting of methodology.125 In addition, including veterinarians in the research design is important.75
Refinement could also benefit from the use of new imaging technologies for OA assessment.84 Optimized for small animals, these imaging technologies include phase contrast μCT,37,51 photoacoustic imaging,19,81 and new fluorescent substrates.54,110 These imaging methods can be used to assess morphologic structural changes or molecular disease activity and to track the progression of damage in longitudinal studies.78 The ability to perform repeated observations on the same animal during disease progression can reduce both individual variation (due to using the same animal) and the number of animals needed (due to avoiding the need to terminate a statistically important number of animals at each interim time point). Finally, new technologies will allow nonlethal longitudinal monitoring of OA progression and support diagnosis, assessment of severity, and development of treatments, which are major concerns for the human disease.
Application of ethical principles also improves scientific knowledge and experimental outputs. Innovations, optimization of 3R rule, and scientific objectives are dynamically linked. The 3R’s have a positive impact on the reproducibility, reliability, and translatability of data from animal studies.77,103 Reporting of all the stimuli and conditions the animals experience is important because these features may interfere with disease development or intervention.64 Pain and pain medications may also affect some data outcomes. These features are challenges in research fields like OA.
In conclusion, new techniques such as the automated digital cage system will be useful to record spontaneous activity from thousands of home cages simultaneously and to monitor lab conditions. This system will become an essential research tool that provides scientific data and saves time. Environmental factors such as season, humidity, housing cage, time of day, sex, order of testing cage mates, mouse genotype, and experimenter identity can affect outcomes.20,21 New tools to improve pain assessment in rodents include videography and computational approaches but time will be required to assess these new tools.34
Acknowledgment
We thank Mrs and Mr Gittler for editorial assistance.
References
- 1.Ahern BJ, Parvizi J, Boston R, Schaer TP. 2009. Preclinical animal models in single site cartilage defect testing: a systematic review. Osteoarthritis Cartilage 17:705–713. 10.1016/j.joca.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Allen KD, Griffin TM, Rodriguiz RM, Wetsel WC, Kraus VB, Huebner JL, Boyd LM, Setton LA. 2009. Decreased physical function and increased pain sensitivity in mice deficient for type IX collagen. Arthritis Rheum 60:2684–2693. 10.1002/art.24783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ankier SI. 1974. New hot plate tests to quantify antinociceptive and narcotic antagonist activities. Eur J Pharmacol 27:1–4. 10.1016/0014-2999(74)90195-2. [DOI] [PubMed] [Google Scholar]
- 4.Arras M, Rettich A, Cinelli P, Kasermann HP, Burki K. 2007. Assessment of post-laparotomy pain in laboratory mice by telemetric recording of heart rate and heart rate variability. BMC Vet Res 3:16-26. 10.1186/1746-6148-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asou Y, Iwata M, Ochi H, Ailixiding M, Aibibula Z, Piao J, Jin G, Hara Y, Okawa A. 2016. Pleiotropic functions of high-fat diet in the etiology of osteoarthritis. PLoS One 11:e0162794. 10.1371/journal.pone.0162794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balcombe JP, Barnard ND, Sandusky C. 2004. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci 43:42–51. [PubMed] [Google Scholar]
- 7.Bapat S, Hubbard D, Munjal A, Hunter M, Fulzele S. 2018. Pros and cons of mouse models for studying osteoarthritis. Clin Transl Med 7:36. 10.1186/s40169-018-0215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berenbaum F, Griffin TM, Liu-Bryan R. 2016. Review: metabolic regulation of inflammation in osteoarthritis. Arthritis Rheumatol 69:9–21. 10.1002/art.39842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bijlsma JW, Berenbaum F, Lafeber FP. 2011. Osteoarthritis: an update with relevance for clinical practice. Lancet 377:2115–2126. 10.1016/S0140-6736(11)60243-2. [DOI] [PubMed] [Google Scholar]
- 10.Blaker CL, Clarke EC, Little CB. 2016. Using mouse models to investigate the pathophysiology, treatment, and prevention of post-traumatic osteoarthritis. J Orthop Res 35:424–439. 10.1002/jor.23343. [DOI] [PubMed] [Google Scholar]
- 11.Bove SE, Calcaterra SL, Brooker RM, Huber CM, Guzman RE, Juneau PL, Schrier DJ, Kilgore KS. 2003. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthritis Cartilage 11:821–830. 10.1016/S1063-4584(03)00163-8. [DOI] [PubMed] [Google Scholar]
- 12.Brent JM, Tian Z, Yao L, Huang J, Markova DZ, Shofer FS, Brice AK, Qin L, Scanzello CR, Vitale F, Chen Di, Zhang Yl. 2020. Functional deficits in mice expressing human interleukin 8. Comp Med 70:205–215. 10.30802/AALAS-CM-19-000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bryden LA, Nicholson JR, Doods H, Pekcec A. 2015. Deficits in spontaneous burrowing behavior in the rat bilateral monosodium iodoacetate model of osteoarthritis: an objective measure of pain-related behavior and analgesic efficacy. Osteoarthritis Cartilage 23:1605–1612. 10.1016/j.joca.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Burkholder T, Foltz C, Karlsson E, Linton CG, Smith JM. 2012. Health evaluation of experimental laboratory mice. Curr Protoc Mouse Biol 2:145–165. 10.1002/9780470942390.mo110217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carbone L. 2019. Ethical and IACUC considerations regarding analgesia and pain management in laboratory rodents. Comp Med 69:443–450. 10.30802/AALAS-CM-18-000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang JC, Christiansen BA, Murugesh DK, Sebastian A, Hum NR, Collette NM, Hatsell S, Economides AN, Blanchette CD, Loots GG. 2018. SOST/sclerostin improves posttraumatic osteoarthritis and inhibits MMP2/3 expression after injury. J Bone Miner Res 33:1105–1113. 10.1002/jbmr.3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. 1994. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63. 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 18.Chen D, Shen J, Zhao W, Wang T, Han L, Hamilton JL, Im H-J. 2017. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res 5:16044. 10.1038/boneres.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L, Ji Y, Hu X, Cui C, Liu H, Tang Y, Qi B, Niu Y, Xiang H, Yu A, Fan Q. 2018. Cationic poly-l-lysine-encapsulated melanin nanoparticles as efficient photoacoustic agents targeting to glycosaminoglycans for the early diagnosis of articular cartilage degeneration in osteoarthritis. Nanoscale 10:13471–13484. 10.1039/C8NR03791D. [DOI] [PubMed] [Google Scholar]
- 20.Chesler EJ, Wilson SG, Lariviere WR, Rodriguez-Zas SL, Mogil JS. 2002. Influences of laboratory environment on behavior. Nat Neurosci 5:1101–1102. 10.1038/nn1102-1101. [DOI] [PubMed] [Google Scholar]
- 21.Chesler EJ, Wilson SG, Lariviere WR, Rodriguez-Zas SL, Mogil JS. 2002. Identification and ranking of genetic and laboratory environment factors influencing a behavioral trait, thermal nociception, via computational analysis of a large data archive. Neurosci Biobehav Rev 26:907–923. 10.1016/S0149-7634(02)00103-3. [DOI] [PubMed] [Google Scholar]
- 22.Chevalier X, Henrotin Y, Osteoarthritis Committee of the French Society for Rheumatology . 2009. OARSI recommendations on knee and hip osteoarthritis: use with discernment. Joint Bone Spine 76:455–457. 10.1016/j.jbspin.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Christiansen BA, Anderson MJ, Lee CA, Williams JC, Yik JHN, Haudenschild DR. 2012. Musculoskeletal changes following non-invasive knee injury using a novel mouse model of post-traumatic osteoarthritis. Osteoarthritis Cartilage 20:773–782. 10.1016/j.joca.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Christiansen BA, Guilak F, Lockwood KA, Olson SA, Pitsillides AA, Sandell LJ, Silva MJ, van der Meulen MCH, Haudenschild DR. 2015. Non-invasive mouse models of post-traumatic osteoarthritis. Osteoarthritis Cartilage 23:1627–1638. 10.1016/j.joca.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clements KM, Price JS, Chambers MG, Visco DM, Poole AR, Mason RM. 2003. Gene deletion of either interleukin-1ß, interleukin-1ß -converting enzyme, inducible nitric oxide synthase, or stromelysin 1 accelerates the development of knee osteoarthritis in mice after surgical transection of the medial collateral ligament and partial medial meniscectomy. Arthritis Rheum 48:3452–3463. 10.1002/art.11355. [DOI] [PubMed] [Google Scholar]
- 26.Cobos EJ, Ghasemlou N, Araldi D, Segal D, Duong K, Woolf CJ. 2012. Inflammation-induced decrease in voluntary wheel running in mice: a non-reflexive test for evaluating inflammatory pain and analgesia. Pain 153:876–884. 10.1016/j.pain.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costello KE, Guilak F, Setton LA, Griffin TM. 2010. Locomotor activity and gait in aged mice deficient for type IX collagen. J Appl Physiol 109:211–218. 10.1152/japplphysiol.00056.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cruz-Almeida Y, Cardoso J, Riley JL, Goodin B, King CD, Petrov M, Bartley EJ, Sibille KT, Glover TL, Herbert MS, Bulls HW, Addison A, Staud R, Redden D, Bradley LA, Fillingim RB. 2017. Physical performance and movement-evoked pain profiles in community-dwelling individuals at risk for knee osteoarthritis. Exp Gerontol 98:186–191. 10.1016/j.exger.2017.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deacon RMJ. 2013. Measuring the strength of mice. J Vis Exp (76) e2610. 10.3791/2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deuis JR, Dvorakova LS, Vetter I. 2017. Methods used to evaluate pain behaviors in rodents. Front Mol Neurosci 10:284. 10.3389/fnmol.2017.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fairbanks CA, Goracke-Postle CJ. 2015. Neurobiological studies of chronic pain and analgesia: rationale and refinements. Eur J Pharmacol 759:169–181. 10.1016/j.ejphar.2015.03.049. [DOI] [PubMed] [Google Scholar]
- 32.Fang H, Beier F. 2014. Mouse models of osteoarthritis: modelling risk factors and assessing outcomes. Nat Rev Rheumatol 10:413–421. 10.1038/nrrheum.2014.46. [DOI] [PubMed] [Google Scholar]
- 33.Fernandes L, Hagen KB, Bijlsma JWJ, Andreassen O, Christensen P, Conaghan PG, Doherty M, Geenen R, Hammond A, Kjeken I, Lohmander LS, Lund H, Mallen CD, Nava T, Oliver S, Pavelka K, Pitsillidou I, da Silvva JA, de la Torre J, Zanoli G, Vliet Vlieland TPM. 2013. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis 72:1125–1135. 10.1136/annrheumdis-2012-202745. [DOI] [PubMed] [Google Scholar]
- 34.Fried NT, Chamessian A, Zylka MJ, Abdus-Saboor I. 2020. Improving pain assessment in mice and rats with advanced videography and computational approaches. Pain 161:1420–1424. 10.1097/j.pain.0000000000001843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furman BD, Strand J, Hembree WC, Ward BD, Guilak F, Olson SA. 2007. Joint degeneration following closed intraarticular fracture in the mouse knee: a model of posttraumatic arthritis. J Orthop Res 25:578–592. 10.1002/jor.20331. [DOI] [PubMed] [Google Scholar]
- 36.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. 2017. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016. Lancet 390:1211–1259. 10.1016/S0140-6736(17)32154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geith T, Brun E, Mittone A, Gasilov S, Weber L, Adam-Neumair S, Bravin A, Reiser M, Coan P, Horng A. 2018. Quantitative assessment of degenerative cartilage and subchondral bony lesions in a preserved cadaveric knee: propagation-based phase-contrast CT versus conventional MRI and CT. AJR Am J Roentgenol 210:1317–1322. 10.2214/AJR.17.18286. [DOI] [PubMed] [Google Scholar]
- 38.Giles JM, Whitaker JW, Moy SS, Fletcher CA. 2018. Effect of environmental enrichment on aggression in BALB/cJ and BALB/cByJ mice monitored by using an automated system. J Am Assoc Lab Anim Sci 57:236–243. 10.30802/AALAS-JAALAS-17-000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glasson SS, Blanchet TJ, Morris EA. 2007. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage 15:1061–1069. 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 40.Glasson SS. 2007. In vivo osteoarthritis target validation utilizing genetically modified mice. Curr Drug Targets 8:367–376. 10.2174/138945007779940061. [DOI] [PubMed] [Google Scholar]
- 41.Gowler PRW, Mapp PI, Burston JJ, Shahtaheri M, Walsh DA, Chapman V. 2020. Refining surgical models of osteoarthritis in mice and rats alters pain phenotype but not joint pathology. PLoS One 15:e0239663. 10.1371/journal.pone.0239663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greene MA, Loeser RF. 2015. Aging-related inflammation in osteoarthritis. Osteoarthritis Cartilage 23:1966–1971. 10.1016/j.joca.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gregori D, Giacovelli G, Minto C. 2018. Association of pharmacological treatments with long-term pain control in patients with knee osteoarthritis: a systematic review and meta-analysis. JAMA 320:2564–2579. 10.1001/jama.2018.19319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Griffin TM, Huebner JL, Kraus VB, Guilak F. 2009. Extreme obesity due to impaired leptin signaling in mice does not cause knee osteoarthritis. Arthritis Rheum 60:2935–2944. 10.1002/art.24854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Griffin TM, Huebner JL, Kraus VB, Yan Z, Guilak F. 2012. Induction of osteoarthritis and metabolic inflammation by a very high-fat diet in mice: effects of short-term exercise. Arthritis Rheum 64:443–453. 10.1002/art.33332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guingamp C, Gegout-Pottie P, Philippe L, Terlain B, Netter P, Gillet P. 1997. Mono-iodoacetate-induced experimental osteoarthritis: a dose-response study of loss of mobility, morphology, and biochemistry. Arthritis Rheum 40:1670–1679. 10.1002/art.1780400917. [DOI] [PubMed] [Google Scholar]
- 47.Harvey VL, Dickenson AH. 2009. Behavioural and electrophysiological characterisation of experimentally induced osteoarthritis and neuropathy in C57Bl/6 mice. Mol Pain 5:1744-8069-5-18. 10.1186/1744-8069-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayami T, Pickarski M, Zhuo Y, Wesolowski GA, Rodan GA, Duong LT. 2006. Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone 38:234–243. 10.1016/j.bone.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 49.Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, Towheed T, Welch V, Wells G, Tugwell P, American College of Rheumatology . 2012. Recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 64:465–474. 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 50.de Hooge ASK, van de Loo FAJ, Bennink MB, Arntz OJ, de Hooge P, van den Berg WB. 2005. Male IL-6 gene knock out mice developed more advanced osteoarthritis upon aging. Osteoarthritis Cartilage 13:66–73. 10.1016/j.joca.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 51.Horng A, Brun E, Mittone A, Gasilov S, Weber L, Geith T, Adam-Neumair S, Auweter SD, Bravin A, Reiser MF, Coan P. 2014. Cartilage and soft tissue imaging using X-rays: propagation-based phase-contrast computed tomography of the human knee in comparison with clinical imaging techniques and histology. Invest Radiol 49:627–634. 10.1097/RLI.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 52.Hu K, Xu L, Cao L, Flahiff CM, Brussiau J, Ho K, Setton LA, Youn I, Guilak F, Olsen BR, Li Y. 2006. Pathogenesis of osteoarthritis-like changes in the joints of mice deficient in type IX collagen. Arthritis Rheum 54:2891–2900. 10.1002/art.22040. [DOI] [PubMed] [Google Scholar]
- 53.Russell WMS, Burch RL. 1959. The principles of humane experimental technique. London (UK): Methuen & Co Ltd. [Google Scholar]
- 54.Hui Mingalone CK, Liu Z, Hollander JM, Garvey KD, Gibson AL, Banks RE, Zhang M, McAlindon TE, Nielsen HC, Georgakoudi I, Zeng L. 2018. Bioluminescence and second harmonic generation imaging reveal dynamic changes in the inflammatory and collagen landscape in early osteoarthritis. Lab Invest 98:656–669. 10.1038/s41374-018-0040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iannello F. 2019. Non-intrusive high throughput automated data collection from the home cage. Heliyon 5:e01454. 10.1016/j.heliyon.2019.e01454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Im H-J, Kim J-S, Li X, Kotwal N, Sumner DR, van Wijnen AJ, Davis FJ, Yan D, Levine B, Henry JL, Desevré J, Kroin JS. 2010. Alteration of sensory neurons and spinal response to an experimental osteoarthritis pain model. Arthritis Rheum 62:2995–3005. 10.1002/art.27608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iwata M, Ochi H, Hara Y, Tagawa M, Koga D, Okawa A, Asou Y. 2013. Initial responses of articular tissues in a murine high-fat diet-induced osteoarthritis model: pivotal role of the IPFP as a cytokine fountain. PLoS One 8:e60706. 10.1371/journal.pone.0060706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jacobs BY, Allen KD. 2019. Factors affecting the reliability of behavioral assessments for rodent osteoarthritis models. Lab Anim 54:317–329. 10.1177/0023677219867715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jaeger K, Selent C, Jaehme W, Mahr S, Goebel U, Ibrahim S, Vollmar B, Mueller-Hilke B. 2008. The genetics of osteoarthritis in STR/ort mice. Osteoarthritis Cartilage 16:607–614. 10.1016/j.joca.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 60.Kalbhen DA. 1987. Chemical model of osteoarthritis–a pharmacological evaluation. J Rheumatol 14:130–131. [PubMed] [Google Scholar]
- 61.Kamekura S, Hoshi K, Shimoaka T, Chung U, Chikuda H, Yamada T, Uchida M, Ogata N, Seichi A, Nakamura K, Kawaguchi H. 2005. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthritis Cartilage 13:632–641. 10.1016/j.joca.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 62.Klinck MP, Mogil JS, Moreau M, Lascelles BDX, Flecknell PA, Poitte T, Troncy E. 2017. Translational pain assessment: could natural animal models be the missing link? Pain 158:1633–1646. 10.1097/j.pain.0000000000000978. [DOI] [PubMed] [Google Scholar]
- 63.Knights CB, Gentry C, Bevan S. 2012. Partial medial meniscectomy produces osteoarthritis pain-related behaviour in female C57BL/6 mice. Pain 153:281–292. 10.1016/j.pain.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 64.van der Kraan PM. 2017. Factors that influence outcome in experimental osteoarthritis. Osteoarthritis Cartilage 25:369–375. 10.1016/j.joca.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 65.van der Kraan PM, Vitters EL, van Beuningen HM, van de Putte LB, van den Berg WB. 1990. Degenerative knee joint lesions in mice after a single intra-articular collagenase injection. A new model of osteoarthritis. J Exp Pathol (Oxford) 71:19–31. [PMC free article] [PubMed] [Google Scholar]
- 66.van der Kraan PM, Vitters EL, van de Putte LB, van den Berg WB. 1989. Development of osteoarthritic lesions in mice by “metabolic” and “mechanical” alterations in the knee joints. Am J Pathol 135:1001–1014. [PMC free article] [PubMed] [Google Scholar]
- 67.Kuyinu EL, Narayanan G, Nair LS, Laurencin CT. 2016. Animal models of osteoarthritis: classification, update, and measurement of outcomes. J Orthop Surg Res 11:19. 10.1186/s13018-016-0346-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kyostio-Moore S, Nambiar B, Hutto E, Ewing PJ, Piraino S, Berthelette P, Sookdeo C, Matthews G, Armentano D. 2011. STR/ort mice, a model for spontaneous osteoarthritis, exhibit elevated levels of both local and systemic inflammatory markers. Comp Med 61:346–355. [PMC free article] [PubMed] [Google Scholar]
- 69.Lakes EH, Allen KD. 2016. Gait analysis methods for rodent models of arthritic disorders: reviews and recommendations. Osteoarthritis Cartilage 24:1837–1849. https://doi.org/10.1016/ j.joca.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lampropoulou-Adamidou K, Lelovas P, Karadimas EV, Liakou C, Triantafillopoulos IK, Dontas I, Papaioannou NA. 2013. Useful animal models for the research of osteoarthritis. Eur J Orthop Surg Traumatol 24:263–271. 10.1007/s00590-013-1205-2. [DOI] [PubMed] [Google Scholar]
- 71.Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, Lacroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg AMJM, Ferrari MD, Craig KD, Mogil JS. 2010. Coding of facial expressions of pain in the laboratory mouse. Nat Methods 7:447–449. 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- 72.Lapveteläinen T, Hyttinen M, Lindblom J, Långsjö TK, Sironen R, Li SW, Arita M, Prockop DJ, Puustjärvi K, Helminen HJ. 2001. More knee joint osteoarthritis (OA) in mice after inactivation of one allele of type II procollagen gene but less OA after lifelong voluntary wheel running exercise. Osteoarthritis Cartilage 9:152–160. 10.1053/joca.2000.0370. [DOI] [PubMed] [Google Scholar]
- 73.Lapveteläinen T, Nevalainen T, Parkkinen JJ, Arokoski J, Kiraly K, Hyttinen M, Halonen P, Helminen HJ. 1995. Lifelong moderate running training increases the incidence and severity of osteoarthritis in the knee joint of C57BL mice. Anat Rec 242:159–165. 10.1002/ar.1092420204. [DOI] [PubMed] [Google Scholar]
- 74.Lapveteläinen T, Tiihonen A, Koskela P, Nevalainen T, Lindblom J, Király K, Halonen P, Helminen HJ. 1997. Training a large number of laboratory mice using running wheels and analyzing running behavior by use of a computer-assisted system. Lab Anim Sci 47:172–179. [PubMed] [Google Scholar]
- 75.Larson CM, Wilcox GL, Fairbanks CA. 2019. The study of pain in rats and mice. Comp Med 69:555–570. 10.30802/AALAS-CM-19-000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Le Pen C, Reygrobellet C, Gérentes I. 2005. Financial cost of osteoarthritis in France. The “COART” France study. Joint Bone Spine 72:567–570. 10.1016/j.jbspin.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 77.Lewis DI. 2019. Animal experimentation: implementation and application of the 3Rs. Emerg Top Life Sci 3:675–679. 10.1042/ETLS20190061. [DOI] [PubMed] [Google Scholar]
- 78.Lim NH, Wen C, Vincent TL. 2020. Molecular and structural imaging in surgically induced murine osteoarthritis. Osteoarthritis Cartilage 28:874–884. 10.1016/j.joca.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Little CB, Zaki S. 2012. What constitutes an “animal model of osteoarthritis”–the need for consensus? Osteoarthritis Cartilage 20:261–267. 10.1016/j.joca.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 80.Little CB, Hunter DJ. 2013. Post-traumatic osteoarthritis: from mouse models to clinical trials. Nat Rev Rheumatol 9:485–497. 10.1038/nrrheum.2013.72. [DOI] [PubMed] [Google Scholar]
- 81.Liu Z, Au M, Wang X, Chan P-MB, Lai P, Sun L, Zheng Y, Rong L, Wen C. 2019. Photoacoustic imaging of synovial tissue hypoxia in experimental post-traumatic osteoarthritis. Prog Biophys Mol Biol 148:12–20 10.1016/j.pbiomolbio.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 82.Makii Y, Asaka M, Setogawa S, Fujiki S, Hosaka Y, Yano F, Oka H, Tanaka S, Fukui N, Yanagihara D, Saito T. 2019. Alteration of gait parameters in a mouse model of surgically induced knee osteoarthritis. J Orthop Surg (Hong Kong) 26(2):1–7. 10.1177/2309499018768017. [DOI] [PubMed] [Google Scholar]
- 83.Malfait AM, Little CB, McDougall JJ. 2013. A commentary on modelling osteoarthritis pain in small animals. Osteoarthritis Cartilage 21:1316–1326. 10.1016/j.joca.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marenzana M, Vande Velde G. 2015. Refine, reduce, replace: imaging of fibrosis and arthritis in animal models. Best Pract Res Clin Rheumatol 29:715–740. 10.1016/j.berh.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 85.Mason RM, Chambers MG, Flannelly J, Gaffen JD, Dudhia J, Bayliss MT. 2001. The STR/ort mouse and its use as a model of osteoarthritis. Osteoarthritis Cartilage 9:85–91. 10.1053/joca.2000.0363. [DOI] [PubMed] [Google Scholar]
- 86.McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, Hawker GA, Henrotin Y, Hunter DJ, Kawaguchi H, Kwoh K, Lohmander S, Rannou F, Ross EM, Underwood M. 2014. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage 22:363–388. 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 87.McCoy AM. 2015. Animal models of osteoarthritis. Vet Pathol 52:803–818. 10.1177/0300985815588611. [DOI] [PubMed] [Google Scholar]
- 88.Miller AL, Leach MC. 2015. The mouse grimace scale: a clinically useful tool? PLoS One 10:e0136000. 10.1371/journal.pone.0136000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miller RE, Lu Y, Tortorella MD, Malfait A-M. 2013. Genetically engineered mouse models reveal the importance of proteases as osteoarthritis drug targets. Curr Rheumatol Rep 15:350. 10.1007/s11926-013-0350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miller RE, Malfait A-M. 2017. Osteoarthritis pain: what are we learning from animal models? Best Pract Res Clin Rheumatol 31:676–687. 10.1016/j.berh.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miyamoto S, Nakamura J, Ohtori S, Orita S, Omae T, Nakajima T, Suzuki T, Takahashi K. 2016. Intra-articular injection of mono-iodoacetate induces osteoarthritis of the hip in rats. BMC Musculoskelet Disord 17:132. 10.1186/s12891-016-0985-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mobasheri A, Matta C, Zákány R, Musumeci G. 2015. Chondrosenescence: definition, hallmarks and potential role in the pathogenesis of osteoarthritis. Maturitas 80:237–244. 10.1016/j.maturitas.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 93.Montilla-García Á, Tejada MÁ, Perazzoli G, Entrena JM, Portillo-Salido E, Fernández-Segura E, Cañizares FJ, Cobos EJ. 2017. Grip strength in mice with joint inflammation: A rheumatology function test sensitive to pain and analgesia. Neuropharmacology 125:231–242. 10.1016/j.neuropharm.2017.07.029. [DOI] [PubMed] [Google Scholar]
- 94.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. 2011. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington (DC): National Academies Press (US). [PubMed] [Google Scholar]
- 95.Neugebauer V, Han JS, Adwanikar H, Fu Y, Ji G. 2007. Techniques for assessing knee joint pain in arthritis. Mol Pain 3:8. 10.1186/1744-8069-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.O’Conor CJ, Griffin TM, Liedtke W, Guilak F. 2012. Increased susceptibility of Trpv4-deficient mice to obesity and obesity-induced osteoarthritis with very high-fat diet. Ann Rheum Dis 72:300–304. 10.1136/annrheumdis-2012-202272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ornetti P, Maillefert J-F, Laroche D, Morisset C, Dougados M, Gossec L. 2010. Gait analysis as a quantifiable outcome measure in hip or knee osteoarthritis: a systematic review. Joint Bone Spine 77:421–425. 10.1016/j.jbspin.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 98.Van Osch GJ, van der Kraan PM, Vitters EL, Blankevoort L, van den Berg WB. 1993. Induction of osteoarthritis by intra-articular injection of collagenase in mice. Strain and sex related differences. Osteoarthritis Cartilage 1:171–177. 10.1016/S1063-4584(05)80088-3. [DOI] [PubMed] [Google Scholar]
- 99.Pernold K, Iannello F, Low BE, Rigamonti M, Rosati G, Scavizzi F, Wang J, Raspa M, Wiles MV, Ulfhake B. 2019. Towards large scale automated cage monitoring - diurnal rhythm and impact of interventions on in-cage activity of C57BL/6J mice recorded 24/7 with a non-disrupting capacitive-based technique. PLoS One 14:e0211063. 10.1371/journal.pone.0211063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Piel MJ, Kroin JS, van Wijnen AJ, Kc R, Im H-J. 2014. Pain assessment in animal models of osteoarthritis. Gene 537:184–188. 10.1016/j.gene.2013.11.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pitcher T, Sousa-Valente J, Malcangio M. 2016. The monoiodoacetate model of osteoarthritis pain in the mouse. J Vis Exp (111). 10.3791/53746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Poole R, Blake S, Buschmann M, Goldring S, Laverty S, Lockwood S, Matyas J, McDougall J, Pritzker K, Rudolphi K, van den Berg W, Yaksh T. 2010. Recommendations for the use of preclinical models in the study and treatment of osteoarthritis. Osteoarthritis Cartilage 18 Suppl 3:S10–S16. 10.1016/j.joca.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 103.Poole T. 1997. Happy animals make good science. Lab Anim 31:116–124. 10.1258/002367797780600198. [DOI] [PubMed] [Google Scholar]
- 104.Poulet B. 2016. Non-invasive loading model of murine osteoarthritis. Curr Rheumatol Rep 18:40. 10.1007/s11926-016-0590-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Poulet B. 2017. Models to define the stages of articular cartilage degradation in osteoarthritis development. Int J Exp Pathol 98:120–126. 10.1111/iep.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Poulet B, Hamilton RW, Shefelbine S, Pitsillides AA. 2010. Characterizing a novel and adjustable noninvasive murine joint loading model. Arthritis Rheum 63:137–147. 10.1002/art.27765. [DOI] [PubMed] [Google Scholar]
- 107.Rahmati M, Nalesso G, Mobasheri A, Mozafari M. 2017. Aging and osteoarthritis: central role of the extracellular matrix. Ageing Res Rev 40:20–30. 10.1016/j.arr.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 108.Rosenthal N, Brown S. 2007. The mouse ascending: perspectives for human-disease models. Nat Cell Biol 9:993–999. 10.1038/ncb437. [DOI] [PubMed] [Google Scholar]
- 109.Ruan MZC, Patel RM, Dawson BC, Jiang M-M, Lee BHL. 2013. Pain, motor and gait assessment of murine osteoarthritis in a cruciate ligament transection model. Osteoarthritis Cartilage 21:1355–1364. 10.1016/j.joca.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Satkunananthan PB, Anderson MJ, De Jesus NM, Haudenschild DR, Ripplinger CM, Christiansen BA. 2014. In vivo fluorescence reflectance imaging of protease activity in a mouse model of post-traumatic osteoarthritis. Osteoarthritis Cartilage 22:1461–1469. 10.1016/j.joca.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sellam J, Berenbaum F. 2013. Is osteoarthritis a metabolic disease? Joint Bone Spine 80:568–573. 10.1016/j.jbspin.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 112.du Sert NP, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, Petersen OH, Rawle F, Reynolds P, Rooney K, Sena ES, Silberberg SH, Steckler T, Würbel H. 2020. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol 18:e3000410. 10.1371/journal.pbio.3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shetty YC, Patil AE, Jalgaonkar SV, Rege NN, Salgaonkar S, Teltumbde PA, Kshirsagar S, Koli PG, Brahma S. 2017. Intra-articular injections of ketamine and 25% dextrose improve clinical and pathological outcomes in the monosodium iodoacetate model of osteoarthritis. J Basic Clin Physiol Pharmacol 28:543–553. 10.1515/jbcpp-2016-0135. [DOI] [PubMed] [Google Scholar]
- 114.Sniekers YH, Weinans H, Bierma-Zeinstra SM, van Leeuwen JPTM, van Osch GJVM. 2008. Animal models for osteoarthritis: the effect of ovariectomy and estrogen treatment - a systematic approach. Osteoarthritis Cartilage 16:533–541. 10.1016/j.joca.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 115.Sokoloff L, Crittenden LB, Yamamoto RS, Jay GE. 1962. The genetics of degenerative joint disease in mice. Arthritis Rheum 5:531–546. 10.1002/art.1780050602. [DOI] [PubMed] [Google Scholar]
- 116.Sperry MM, Yu Y-H, Kartha S, Ghimire P, Welch RL, Winkelstein BA, Granquist EJ. 2020. Intra-articular etanercept attenuates pain and hypoxia from TMJ loading in the rat. J Orthop Res 38:1316–1326. 10.1002/jor.24581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sperry MM, Yu Y-H, Welch RL, Granquist EJ, Winkelstein BA. 2018. Grading facial expression is a sensitive means to detect grimace differences in orofacial pain in a rat model. Sci Rep 8:13894. 10.1038/s41598-018-32297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Staines KA, Poulet B, Wentworth DN, Pitsillides AA. 2017. The STR/ort mouse model of spontaneous osteoarthritis - an update. Osteoarthritis Cartilage 25:802–808. https://doi.org/10.1016/ j.joca.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stanescu R, Knyszynski A, Muriel MP, Stanescu V. 1993. Early lesions of the articular surface in a strain of mice with very high incidence of spontaneous osteoarthritic-like lesions. J Rheumatol 20:102–110. [PubMed] [Google Scholar]
- 120.Thysen S, Luyten FP, Lories RJU. 2015. Targets, models and challenges in osteoarthritis research. Dis Model Mech 8:17–30. 10.1242/dmm.016881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ueno H, Takahashi Y, Suemitsu S, Murakami S, Kitamura N, Wani K, Matsumoto Y, Okamoto M, Ishihara T. 2020. Effects of repetitive gentle handling of male C57BL/6NCrl mice on comparative behavioural test results. Sci Rep 10:3509. 10.1038/s41598-020-60530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Van de Weerd HA, Bulthuis RJA, Bergman AF, Schlingmann F, Tolboom J, Van Loo PLP, Remie R, Baumans V, Van Zutphen LFM. 2001. Validation of a new system for the automatic registration of behaviour in mice and rats. Behav Processes 53:11–20. 10.1016/S0376-6357(00)00135-2. [DOI] [PubMed] [Google Scholar]
- 123.Vonsy JL, Ghandehari J, Dickenson AH. 2012. Differential analgesic effects of morphine and gabapentin on behavioural measures of pain and disability in a model of osteoarthritis pain in rats. Eur J Pain 13:786–793. 10.1016/j.ejpain.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 124.Wei W, Bastiaansen-Jenniskens YM, Suijkerbuijk M, Kops N, Bos PK, Verhaar JAN, Zuurmond A-M, Dell’Accio F, van Osch GJVM. 2017. High fat diet accelerates cartilage repair in DBA/1 mice. J Orthop Res 35:1258–1264. 10.1002/jor.23280. [DOI] [PubMed] [Google Scholar]
- 125.Weissgerber T, Riedel N, Kilicoglu H, Labbé C, Eckmann P, ter Riet G, Byrne J, Cabanac G, Capes-Davis A, Favier B, Saladi S, Grabitz P, Bannach-Brown A, Schulz R, McCann S, Bernard R, Bandrowski A. 2021. Automated screening of COVID-19 preprints: can we help authors to improve transparency and reproducibility? Nat Med 27:6–7. 10.1038/s41591-020-01203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xie J, Zhang D, Lin Y, Yuan Q, Zhou X. 2018. Anterior cruciate ligament transection-induced cellular and extracellular events in menisci: implications for osteoarthritis. Am J Sports Med 46:1185–1198. 10.1177/0363546518756087. [DOI] [PubMed] [Google Scholar]
- 127.Yu YC, Koo ST, Kim CH, Lyu Y, Grady JJ, Chung JM. 2002. Two variables that can be used as pain indices in experimental animal models of arthritis. J Neurosci Methods 115:107–113. 10.1016/S0165-0270(02)00011-0. [DOI] [PubMed] [Google Scholar]
- 128.Zhang R-X, Ren K, Dubner R. 2013. Osteoarthritis pain mechanisms: basic studies in animal models. Osteoarthritis Cartilage 21:1308–1315. 10.1016/j.joca.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]