Abstract
Ophthalmic study of collagen CVII hypomorphic mice is uniquely challenging due to the strain’s published survival rate to weaning of 24%. Because chronic ocular fibrosis requires time to develop, optimizing the survival rate is of critical importance. In this study, standard husbandry practices were enhanced by the addition of sterilized diet and drug delivery gels, acidified water, irradiated food pellets, cellulose fiber bedding, minimal handling, removal of siblings within 2-3 wk from birth, and a preferred housing location. Survival rates per breeding cycle, sex, weight, and cause of early euthanasia were recorded and analyzed over 43 mo. Overall, 49% of mice survived to weaning and 76% of weaned mice survived to 20 wk of age. Corneal opacities were seen in 65% of mice by 20 wk, but only 10% of eyes showed the sustained opacification that was indicative of fibrosis. Corneal opacities occurred at the same rate as in humans with epidermolysis bullosa. 66% of the mice showed weight loss at 11 wk. Males required early euthanasia 4 times more often than did females. Euthanasia was required for urinary obstruction due to penile prolapse in 88% of males. With our enhanced care protocol, hypomorphic mice in our colony survived at twice the published rate. With this revised husbandry standard, experiments planned with termination endpoints of 14 wk for males and 17 wk for females are more likely to reach completion.
Abbreviations: DEB, dystrophic epidermolysis bullosa; EB, epidermolysis bullosa; CVII, collagen VII
Introduction
Epidermolysis bullosa (DEB) is a rare and disfiguring disease known as “the worst disease you’ve never heard of.”7 This devastating condition causes blistering in multiple organ systems including the skin, eyes, larynx, gastrointestinal, and genitourinary systems. Patients with EB suffer from constant pain, disfigurement, vision loss, apnea, esophageal obstructions, poor weight gain, and urinary obstruction.15,16,27 Limb deformities, loss of fingers and toes, and impaired mobility may also occur in childhood due to excessive subepithelial fibrosis.4,14 A disease specific therapy is not available for DEB; all current treatments are supportive.11
The subtype of EB that we study is known as dystrophic EB (DEB) and is caused by genetic mutation of the Col7a1 gene, which encodes type VII collagen (CVII). CVII forms anchoring fibrils that secure stratified squamous epithelium to the underlying basement membrane.19 As of 2016, epidemiologic data found the incidence of DEB was 3.26 per million live births in the United States.12 The more severe type of disease, known as recessive DEB (RDEB), causes painful corneal disease in 41% of patients and vision loss in 25%.29 At present, no disease-specific therapies are available. The standard of ophthalmic care is simply ocular lubrication, which is insufficient to alleviate suffering and vision loss.11
Efforts are currently underway to replete CVII, including phase II-III trials of recombinant human CVII protein and gene therapies. Our goal is to develop a topical ophthalmic therapy to reduce painful ocular blistering and vision loss from corneal fibrosis. The foremost challenge in this endeavor is survival of the animal model. Knock-out mice with complete CVII deficiency have been created, but these strains express severe disease and die within the first 7 d of life. The only model that survives up to 150 d without treatment is the hypomorphic collagen VII-deficient mouse (also known as knock-out, KO mice, or Col7a1flNeo/flNeo), but survival rates for this strain are low.2 While CVII deficiency occurs naturally in sheep, dogs, cats, and rats, no known colonies of large animals with CVII deficiency are available to date.2,10
The hypomorphic mouse (Col7a1tm1Lbt; MGI ID: 3796248)25 was created in 2008. In this mouse, the Col71a gene was disrupted by using a cassette insertion technique that generates a frame-shift mutation.18 The induced mutation causes the mouse to produce only 10% of normal collagen VII levels in the skin and 35% to 58% of normal CVII levels in the cornea.3 Heterozygous breeders for these mice are phenotypically normal, with no external abnormalities to the eye, no problems with skin, fur, or limbs, and no blisters anywhere on the body. Previous studies report high attrition rates, with 67% death before 28 d, and 8.5% after 28 d, leaving only 24.5% of offspring weaned and available for experiments.18 Given the high loss rate before 28 d, the number of mice that must be generated, even for small experiments, is quite high. For example, an experiment using 10 mice would require 40 mutants (hypomorphs) and would extraneously produce 120 heterozygous and wildtype mice. Not only are large colonies expensive to maintain, but the low production rate of this strain is a rate-limiting factor in discovery and progress in EB research. Therefore, any strategies that improve survival rates are of tremendous benefit to the field and are consistent with “refinement of techniques” according to the 3Rs.35
We report here our experience after 43 mo of breeding and caring for these mice. Special husbandry practices were introduced for this colony at its initiation to optimize longevity of this delicate mouse strain. The enhanced care protocol was based on our prior experience with high attrition strains, like the RR strain, in which fewer than 70% of pups survive until weaning age and 50% of dams lose pups, and the CFTR S489× strain, which has a 90% mortality rate of untreated mice by 40 d.9,37 In addition, we document the natural course of ocular disease in these mice and examine factors that influence early death.
To date, no publications have reported the age at which corneal scarring develops in these mice. For humans with DEB, the cumulative risk of scarring is 30% by age 9, and 50% by age 12.13 According to calculations that estimate age equivalence of mice and human, a 42-d-old mouse is comparable to an 11.5-y-old child, and 9 mouse days are equivalent to 1 human year.8 By this equation, a 6.25-wk-old mouse is equivalent to a 12-y-old child. If 50% of children have scarring by age 12 y, we expected that 50% of hypomorphic mice would show scarring by 6 wk. However, our pilot study showed that no hypomorphic mice (n = 10) had corneal opacities on photographs before 10 wk of age.
Materials and Methods
All animal work was performed at Tufts University, an AAALAC-accredited institution, and was reviewed and approved by the Tufts University Institutional Animal Care and Use Committee.
Twelve heterozygous collagen VII deficient mice (Col7a1flNeo/WT, MGI:Col7a1tm1Lbt) on a mixed C57BL/6 and 129/Sv background were the gift of Dr. Alexander Nystrom (University of Freiburg, Germany).25 Details of the insertional mutation used to create this strain are published elsewhere.18
Enhanced care protocol.
In anticipation of a high attrition rate, we altered our standard care protocol (Table 1) for these mice. The mice were fed pelleted rodent diet (Envigo, Teklad 2918, Indianapolis, IN) and a nutritionally fortified dietary supplement with animal source protein (DietGel 76A, Clear H2O, Westbrook, ME). Pellets were γ-irradiated using a cobalt-60 source at a minimum dose of 20 kGy to 50 kGy and the dietary supplement was sterile to avoid pathogen exposure. Water was acidified with hydrochloric acid to a pH between 2.8 - 3.2 to limit growth of Pseudomonas spp. and was provided ad libitum. A water gel (HydroGel, Clear H2O) and food pellets were placed on the cage floor to allow easier access. Bedding was exclusively cellulose fiber (Carefresh) and was changed once per week for CVII knockouts. Food was replenished twice weekly. Breeder cages were changed once every 2 wk and replenished with food twice a week if litters were present. Minimal cage and mouse manipulations were accomplished by restricting access to pre-weanling animals and breeders to 2 animal care staff with additional training in changing cages and providing additional diet. No investigative staff were permitted access to animals prior to weaning. The colony was placed on a wall-mounted rack, away from doors and foot traffic within the facility. Mice were housed in microisolation caging on ventilated racks with 70 air changes per hour, 0.3 supply and 0.25 exhaust (Thoren, Hazleton, PA), in small mouse cages model #9 (Thoren [cage size 7.70 × 12.17 × 5.875 inches]) on a 14-h light/10-h dark cycle, 68 to 76 °F room temperature range, and 30% to 70% humidity.
Table 1.
Standard verse enhanced care protocol.
| Standard Protocol | Enhanced Care Protocol | Difference | Potential Benefit |
|---|---|---|---|
| Soft breakfast cereal | DietGel 76A | Nutritionally balanced, irradiated | Improved nutrition, lowers risk of infection |
| Mouse Chow | Irradiated Chow | Irradiated | Reduces spread of pathogens |
| Corncob bedding | Cellulose (Carefresh) bedding | Soft on paws and eyes | Decreases injury to limbs and eyes |
| Unacified water | Acidified water | Limits Pseudomonas | Lowers risk of infection |
| Water by bottle | Hydrogel | Accessible water (on floor of cage) | Provides access if limited mobility |
| Cull siblings, wean late (5-6 wk) | Increased attention and time with mother | Enhanced wellbeing, increased access to milk | |
| Lacrilube ointment for penile prolapse | Lowers risk of early euthanasia | ||
| Preferred location in animal housing | Away from doors and activity benches | Decreases stress on mothers and newborns |
Genotyping is typically performed between 5-12 d. All heterozygous and wild type mice are culled when results are received, and homozygous hypomorphs are kept with mother until 5-6 wk or large enough to wean (average 12.5 grams). Food, water, and bedding are changed weekly. Each cage contains 2-4 mice. DietGel is exchanged twice weekly. The animal room is Hepa-filtered. Access to hypomorph cages are restricted to 2 staff members with special care training. The colony is kept in a quiet corner away from doors and activity benches. Survival rates declined when the colony was housed next to an access door but improved when moved to a quieter area.
We conducted health surveillance for mouse pathogens quarterly by serology and/or PCR using dirty bedding sentinels and semiannually via PCR by testing exhaust air dust for pinworms and fur mites. The following agents are tested for and excluded in this facility: mouse parvovirus, minute virus of mice, mouse hepatitis virus, murine rotavirus, mouse theilovirus, mouse adenovirus, reovirus, pneumonia virus of mice, Sendai virus, ectromelia, lymphocytic choriomeningitis virus, K virus, polyoma virus Citrobacter rodentium, Mycoplasma pulmonis, Clostridium piliforme, Salmonella spp., Corynebacterium kutscheri, Streptobacillus moniliformis, Streptococcus pneumoniae, fur mites (Myobia musculi, Myocoptes musculinus, and Radfordia affinis), pinworms (Aspicularis tetraptera and Syphacia obvelata), Giardia spp., and Cryptosporidium spp. The facility does not exclude mouse norovirus; Beta hemolytic Streptococcus spp. groups A, B, C, and G; Helicobacter spp.; Rodentibacter heylii; Rodentibacter pneumonotropicus; Spironucleus muris; or Entamoeba spp.; these agents may be present in the colonies.
Breeding was performed in using pairs of heterozygous males and females. Males were never removed from breeding cages. Genetic status of offspring was determined by PCR genotyping of distal tail tissue of pups.22 Breeding production was carefully monitored and recorded, with new breeders replacing one or both parents as needed. Reproductive performance was evaluated for the colony by “breeding rounds.” Each round corresponds to the litter number produced by all breeders. For example, breeding round 3 corresponds to all breeders’ third litter. If KO mice offspring were too small to wean while the second litter arrived, both litters would be nursed together until they were large enough to wean (at least 4 g).
After approximately every 3 to 4 litters, a decrease in breeding production was noted. At these times, the breeding line was “refreshed” by mating the Col7a1 heterozygous males with either a female C57BL/6J (B6) or 129S1/SvImJ (129) mouse. The strain of the female mouse (heterozygous KO or either B6 or 129) alternated between each “refresh.” This mating scheme was consistent with the practices of the donating lab.18
Extended weaning time.
Hypomorphs (Col7a1flNeo/flNeo) were identified by PCR for the presence of the Col7a1 gene from tissue obtained from the distal tail obtained between 5 to 12 d of life. After genotype confirmation (approximately 10 to 17 d of life), littermates were removed to allow the mother to focus on the hypomorphs. Hypomorph pups were socially housed and separated by sex after removal from mother. Pups remained with their mothers for 35 d, whereas typical weaning in our facility occurs at 21 to 28 d.
Data collection and analysis.
Survival data per breeding cycle was recorded from August 2016 to February 2020. Weights of Col7a1flNeo/flNeo KO mice were recorded weekly from May 2019 to May 2020. Most mice were used for experiments and euthanized at 2 to 4 wk after experimental treatment (at 10 to 12 wk old). Twenty-one mice were observed without experimentation from ages 8 to 20 wk. For these mice, slit-lamp evaluation and ophthalmic photography were performed at 8, 12, 16, 18, and 20 wk. No statistical analysis or statistical comparisons were made due to the lack of literature available for Col7a1 mice survival.
Analgesia and humane endpoints.
Mice that appeared to have ocular pain (inability to open eyes, swelling/redness of the eyes) received analgesia (topical meloxicam 5 mg/kg eyedrops) as needed and were monitored daily. Humane endpoints for eye pain were an inability to open eyes spontaneously for more than 72 h after surgery (during this study some mice were used in independent experiments that required central corneal de-epithelialization and/or sub-conjunctival injections). Clinical signs of painful eye disease requiring euthanasia are very dry eyes, dullness of the globes, ulcerations, and perforation. Our protocol requires euthanasia of mice that cannot engage in normal activities or show ocular perforation, greater than 15% weight loss from established adult body weight, body condition score (BCS) less than 2 out of 5, lethargy, or penile prolapse leading to urinary obstruction.38 Due to the occurrence of penile prolapse in males, mice with mild to moderate symptoms need extra observation. Daily monitoring for each mouse was required for urine expression and lubricant application.
Results
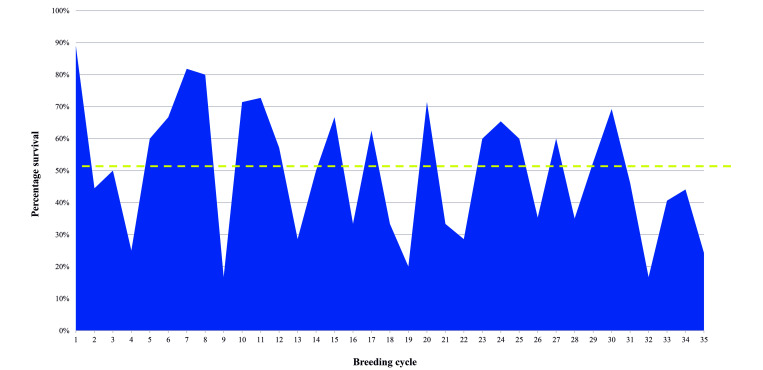
Over 43 mo, 480 hypomorphic mice were genotyped and 226/480 (48%) survived to the extended weaning age of 5 wk. Of these, 118 were female and 103 were male (5 had no sex recorded). The female to male ratio was 1.14:1. The annual survival rates for the weaning of hypomorphic mice ranged from 44% to 51%, compared to the original 20% survival rate. The average survival per litter was 50%. The average number of hypomorphs weaned per breeding cage was 0.9 ± 0.5, after removal from mother. On average, each experiment used 6 mice, requiring 8 breeding cages per experiment (Table 2). The colony was “refreshed” with either B6 or 129 females after breeding cycles 9 and 22, which doubled the survival of the next breeding cycle (Figure 1). The period between refreshing or breeding of Col7a1 heterozygous females with B6 or 129 females was 14 mo for the first year, 16 mo for the second year, and 13 mo for the third year.
Table 2.
Number of hypomorphic mice produced and weaned.
| Hypomorphic Mice Data | N | Survival Rate % |
|---|---|---|
| Produced | 480 | — |
| Weaned | 226 | 47.1% |
| Females | 118 | 52.2% |
| Males | 103 | 45.6% |
| Sex not recorded | 5 | 2.2% |
| Weaned/produced | N/n | |
| Year 1 (14 mo) | 67 of132 | 50.8% |
| Year 2 (16 mo) | 47 of 93 | 50.5% |
| Year 3 (13 mo) | 112 of 255 | 43.9% |
| Mean survival rate per year | 48.4% | |
| Mean (SD) | Range | |
| Breeding cages per cycle | 8.0 ± 4.4 | 3-17 |
| Hypomorphs weaned per cycle | 6.5 ± 4.5 | 1-17 |
| Average hypomorphs per breeder | 0.9 ± 0.5 |
Time period 8/2016-2/2020. Duration 42.6 mo (3.6 y). Survival rate is calculated as total number of animals weaned over total animals produced (47.1%) and the mean survival rate per year (48.4%) are comparable to the average survival rate per breeding round (50%- see Figure 1). Cycle refers to breeding round.
Figure 1.
Percentage of hypomorphs that survived past 5 wk (weaning age). A total of 35 breeding rounds occurred over 43 mo. The average survival rate per breeding cycle was 50% (dash line) which is double the previously reported rate of 24.5%. Colony was refreshed when survival declined—specifically after cycles 9, 23, and 32. The average time between refreshing lines was 13.2 mo.
Survival to 20 wk without intervention.
Of the mice that underwent observation with weekly eye examinations, 17 of 21 (76%) survived to 20 wk, which was the predetermined study endpoint. Nine of 21 (43%) were male, and 12 of 21 (57%) were female. Humane endpoints necessitated the euthanasia of 4 of 9 males (44%) and 1 of 12 females (8%) prior to study endpoints, at a mean age of 14 ± 5 wk for males and 16 wk for the 1 female.
Weight loss occurs by 11 wk in most mice.
The weights of 97 mice were recorded weekly for up to 18 wk. Mean weight increased with age until 8 wk; thereafter, weight gain slowed. The average weight was 16.0 grams (g) by 8 wk of age, and 17.2 g by 18 wk. A decline from peak adult weight occurred at weeks 6 to 8 for 13 of 50 (26%) and at weeks 9 to 11 for 20 of 50 (40%). Of the remaining mice, 11 of 47 (23%) lost weight between 12 to 15 wk. After 15 wk, only 4 mice remained, and they showed no weight loss.
Corneal opacities increase with age.
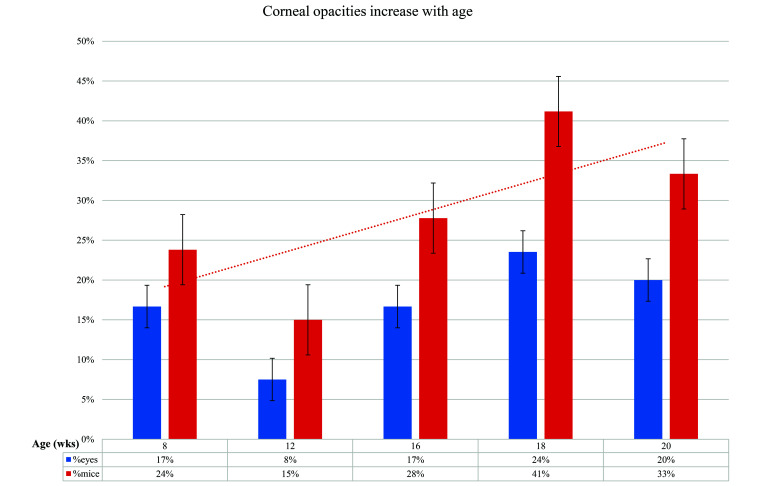
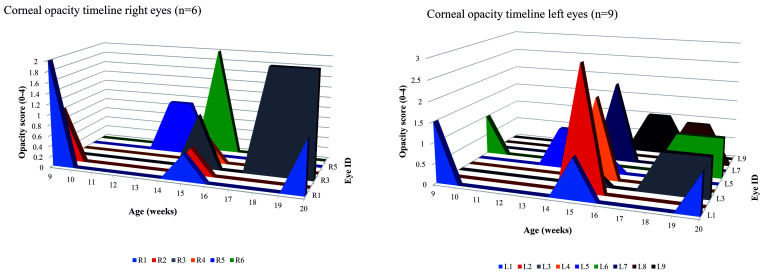
A cohort of 21 mice were examined weekly from 8 to 20 wk; at baseline, 5 of 21 (24%) of mice showed corneal opacification in one or both eyes. Of the 17 mice that survived to 20 wk, 11 of 17 (64%) showed corneal opacification in one or both eyes. Figure 2 shows the trend of more frequent corneal opacity with age, peaking at 18 wk. Opacities were seen in 15 of 22 (68%) eyes, but the opacification was intermittent in most eyes (12 of 15, 67%). Only 3 of 15 (33%) of eyes showed sustained opacification that started at 18 wk and was still present or worse by 20 wk. Individual eye results are shown in Figure 3.
Figure 2.
Corneal opacities increase with age. Peak opacity prevalence occurred at 18 wk. The 33% to 41% incidence of opacity in young adulthood reflects the human prevalence of corneal scarring in RDEB which averages 37%.
Figure 3.
Opacity timelines for right and left eyes (n = 15). Eyes with opacities at any time point are shown (eyes that never opacified are not shown). Intermittent opacity, indicating edema, was seen in 67% (12/15). Sustained opacity from 18 wk to 20 wk was seen in 33% (3/15) indicating scarring.
Impact of sex on death rate and penile prolapse rates.
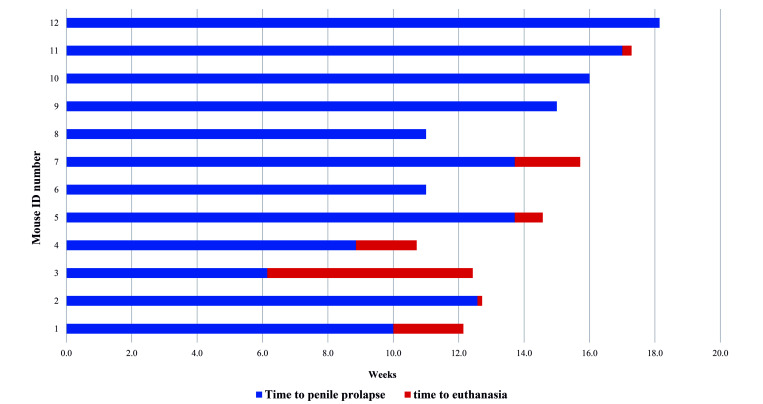
The rate of premature death, defined as required euthanasia or spontaneous death prior to completion of the designated experiment, was recorded for 50 mice. Of these, 10 of 50 (20%) were female, and 40 of 50 (80%) were male. The most common humane endpoint requiring euthanasia for males was penile prolapse causing urinary obstruction, which occurred in 35 of 40 of males (88%). No males were found dead, and the remaining 5 of 40 (12%) males were euthanized due to poor body condition or a body condition score of less than 2 out of 5.38 The mean age of penile prolapse was 12.2 ± 3.3 wk, while the average age for euthanasia due to penile prolapse was 17.6 ± 6.2 wk. Figure 4 shows the progression of penile prolapse in 12 mice from onset of prolapse to the time of early euthanasia (resulting in failure to complete the experiment). The time between diagnosis and euthanasia was 1.2 ± 1.7 wk with treatment. Treatment included daily manual urine expression and application of a lubricant to the penis; 7 of the 12 (58%) required euthanasia within 2 d of diagnosis due to the severity of prolapse at presentation. The overall rate of penile prolapse in males was 35 of 103 (34%). Euthanasia in females was primarily due to poor body score.
Figure 4.
Time to euthanasia following diagnosis of penile prolapse. Male animals were monitored weekly for penile prolapse. This figure shows only animals that required early euthanasia for penile prolapse. The mean time between diagnosis and euthanasia was 1.2 ± 1.7 wk.
Discussion
Major findings.
The survival rate to weaning age in our breeding facility was 48.4% over 43 mo, which is nearly double the reported rate of 24.5%.18 The enhanced care protocol devised by our animal care team likely improved the survival rate of hypomorphic mice. At times, we required up to 24 breeding cages to produce 6 to 8 mice for an experiment. Refreshing the breeders with B6 or 129 mice when survival dipped below 30% doubled the survival rate for the next breeding cycle. This intervention was necessary every 13 to 16 mo.
Females survive 4 times longer than males, because males develop penile prolapse leading to urinary obstruction. While most males require euthanasia within 2 d of penile prolapse diagnosis, some males survived for 6 wk. On average, their weights fell after 8 wk of age.
Corneal opacities were seen in 24% on slit-lamp exam at 8 wk and incidence of opacities increased with age. By 18 wk, corneal opacities (suggestive of scarring) can occur at an incidence similar to humans with DEB.
Standard compared with enhanced care.
The standard of care for hypomorphic mice previously had included a soft cereal diet made daily, clean bottled water ad libitum, prolonged housing with siblings, and corncob bedding. For this study, we made the following changes in addition to the standard of care to lengthen survival: provided sterilized rodent chow along with a diet supplement and water gel, acidified water, and cellulose bedding, culled siblings before weaning to decrease competition for maternal care, extended time of weaning to 5 wk, places colony in a quiet location, and treated prolapsed penises topically with an artificial tears eye ointment (Refresh, Irvine, CA; Puralube, Dechra, Overland Park, KS). Together, these changes reduce potential pathogen exposure, stress, and sibling competition to offer hypomorphic mice a greater chance of survival past weaning age.
The addition of supplementary nutritional gel improves survival in some strains of mice. For example, the commonly used C57BL/6 strain is known for high loss of first litters.40 Male C57Bl/6J pups that received supplements in forms of dough (Transgenic Dough Diet, Bio-Serv, Frenchtown, NJ), gel (DietGel Boost, ClearH20, Portland, ME) and chow (irradiated extruded diet, Teklad Global 2912, Harlan, Madison, WI) showed a cumulative average daily weight gains of 0.64 ± 0.01 g/d in the dough group, whereas the control group had 0.58 ± 0.01 g/d during the development phase.6 No statistically significant changes or clinical benefit was found in using gel as compared with the control diet because.6 No morbidity or mortality was observed in the study, although the reported mortality through commercial breeders for these mice is 13%.6 However, the effects of supplements differ based on strain; in 3 strains of mice that were studied after radiation (C57BL/6, NRG and CD45.1), supplements reduced mortality rate and adverse clinical signs of radiation in C57BL/6, but not in NRG or CD45.1 strains.23 We anticipated that hypomorphic mice would benefit from fortified supplements because the diet is easier to ingest than pellets and less likely to lead to gastrointestinal obstruction or injury. In patients with DEB, the entire gastrointestinal tract can be affected by blistering, from the oral mucosa to the anus. The teeth are also affected; therefore, problems with deglutition are common. Gastroesophageal reflux and esophageal strictures occur in 75% and 66% (respectively) of patients and contribute to poor weight gain.17 These problems ultimately lead to malnutrition and wasting, which are seen by 10 y of age in 50% of children with generalized recessive DEB.5,31 Fortified supplements may improve survival by providing an easy to ingest, hygienic, and nutritionally complete formulation.
Exposure to pathogens was further reduced by irradiation of food pellets. This method has been shown to effectively limit viral infection from murine norovirus and parvovirus in rodent facilities.1 Irradiation likely limits other pathogens as well. Unsterilized feed was cited as the likely cause of a widespread outbreak of murine parvovirus in Johns Hopkins University School of Medicine, Baltimore, MD in 2009.39 After irradiation of feed was implemented, subsequent studies showed a reduction in murine parvovirus levels among endemically infected colonies.32 Autoclaving is another method of sterilization but is not ideal because the process can lead to elevated acrylamide levels in rodent feed and genotoxicity compared with irradiated pellets.24
Acidifying water reduces Pseudomonas aeruginosa and coliform bacteria in mice.26 It is also suspected to improve intestinal barrier function and protect mice against gut-derived sepsis from Pseudomonas.41 In certain mouse strains (for example, CLN3(−/−) mice, which develop Batten disease), acidified water not only decreased infection but also changed the composition of gut microbiota and improved motor function.21 By reducing the potential for infection or illness, using acidified water can improve mouse survival.
Housing and littermate changes.
The colony was housed in a location with relatively low foot traffic and noise. We observed an immediate decrease in survival from 46% to 17% during a 2-mo period after the colony was moved to a high-traffic area near a procedural room. When moved back to the quieter location, survival increased to 41% and 44% for the next 2 breeding cycles respectively. The adverse impact of auditory stress on litter size noise was noted in a study that compared auditory stress to physical restraint and placebo.20 The study reported that dams who were exposed to a loud 3000 Hz tone exhibited more signs of stress than did those who experienced physical restraint. Examination of uteri revealed that loud noises caused higher rates of embryo resorption.20 A similar effect was reported in a study that analyzed the effects of construction noise on reproduction after implantation of 15 embryos into female Swiss Webster mice.30 After just 1 h per day of noise exposure during the first week after parturition, the number of stillborn pups increased by 4%.30 Additional investigations studied the effects of vacuum cleaner noise on behavior and fecal corticosterone metabolites in 10 female C57BL/6Cr mice.21 One 1 h of vacuuming did not produce an acute stress response in stress-sensitive behavioral tests but did cause a diurnal variation of corticosterone levels in synchrony with exposure to noise.21 Similar findings with spectral analysis showed that vacuum noise is multitonal, and audiograms of young C57BL/6 and newly-weaned CD1 mice show recognition of low frequency noise from vacuuming.28 Although noise can be stressful for mice, this is not the case for all sounds; some common sounds may not be audible to mice.33 For example, construction noise from a jackhammer and shot blaster did not increase sound pressure levels inside cages,33 suggesting that sounds that are audible and loud to humans may not be perceived similarly by mice.
We reduced litter sizes after genotyping because smaller litters have been shown to promote growth in rabbits and rats.33 Preweaning growth rates of 91 litters equaling 281 rabbit kits (average litter size: 4.1 ± 1.3) and 636 rat pups from 65 litters (average litter size: 9.8 ± 3.6) have shown that litter size correlated negatively with growth rate, with a growth difference of 43.2 g between 2 and 7 individuals.34 The number of siblings was the most important factor in growth rate of pups.34 These authors concluded that fewer siblings allow the dam to concentrate on the remaining pups, thereby improving the survival rate until weaning.
Penile prolapse limits long-term experimentation.
When planning studies, scientists should consider the allocation of these mice according to sex. In humans, no known sex difference affects survival or disease severity, but in hypomorphic mice, penile prolapse and urinary obstruction can lead to early euthanasia and thereby disrupt experimental designs. Knowledge of the early euthanasia rate for males and the age of onset for penile prolapse allows appropriate allocation of mice with regard to sex. For example, males can be used for short-term studies requiring survival up to 14 wk, and females can be used for longer-term experiments up to 20 wk. These sex differences are particularly important in ocular research, because corneal fibrosis by slit lamp assessment peaks at 18 wk.
Corneal opacities.
Corneal opacities in this strain can represent numerous histologic abnormalities including ulceration, epithelial hyperplasia, stromal fibrosis, edema, inflammation, dysplasia, neovascularization, and blistering. In prior studies, histologic changes were demonstrated in 89% of eyes, yet only 2 of 9 (22%) mice showed grossly visible corneal opacities on nonslit lamp photographs.3 In the current study, we performed slit-lamp examination, which gave far greater detail than could be seen on nonslit lamp photographs. This likely provided greater sensitivity in identifying external changes. In the prior study, no mice under 10 wk of age showed opacities, whereas our current study showed opacities in 24% of mice at 9 wk.3 While published ophthalmic data on these mice are available, human studies have demonstrated an increased risk of corneal scarring with increasing age in DEB.13
The permanence of a corneal opacity suggests its etiology. In our mice, intermittent opacification was seen frequently during the 12-wk observation period, but often resolved within 1 to 2 wk, suggesting that most opacities are due to a transient edema, inflammation, ulceration, or blistering. Only one-third of eyes that were opacified at 18 wk remained opacified at 20 wk. The stable appearance of the cornea over 3 wk suggests that more permanent changes occurred, likely stromal fibrosis, epithelial hyperplasia, or dysplasia.
Limitations.
Because this was a retrospective observational study, the impact of prospectively applying the enhanced care protocol is unknown. Due to the high value of this delicate mouse strain, justification of use standard care for the sole purpose of examining attrition rate would be difficult. That said, investigating the relative contribution of each facet of the enhanced care protocol could provide valuable information for management of high-attrition strains.
Analysis of weight was the most reliable up to 11 wk of age. After 15 wk, only 4 of 97 (4%) mice remained (others had been used for experiments). Therefore, we cannot make meaningful conclusions regarding decline in weight beyond 15 wk.
Regarding data on penile prolapse, only 17 of 40 (40%) of males with penile prolapse had the date of prolapse onset recorded; therefore, the age of onset is not generalizable. Likewise, the date of euthanasia due to penile prolapse was only available for 11 of 40 (30%) of males. As a result, the age of euthanasia with respect to the onset of prolapse is not clear. The mean age of prolapse onset (17.6 ± 6.2 wk) was calculated from limited data and had a large standard deviation. These data should be viewed with caution. Future studies will include closer monitoring of this important outcome.
Conclusions.
Survival to older ages is critical for the study of ocular disease because corneal scarring, the critical component of this model for the study of DEB, increases with age. With our enhanced care protocol, hypomorphic mice in our colony survived at double the published rate. Critical findings on survival rate per breeding cage, timing of female refreshment, anticipated age of declining weight and health, and knowing the causes of early death will help DEB researchers plan long-term studies with greater confidence. Corneal changes consistent with edema and blistering were common and transient in most mice. Fibrosis occurs naturally in this mouse strain after 18 wk of age and was observed throughout the study in our mice; therefore, studies of antifibrotic interventions may require long-term observation. Knowledge of the sex-dependent and long-term survival rates provides guidance for future study design. We currently terminate studies of chronic fibrosis at 14 wk for males and 17 to 18 wk for females. This strategy allows us to retain nearly all mice until the planned end of the experiment.
Acknowledgments
The authors gratefully acknowledge Alexander Nyström, who generously gifted breeders for this study.
References
- 1.Adams SC, Myles MH, Tracey LN, Livingston RS, Schultz CL, Reuter JD, Leblanc M. 2019. Effects of pelleting, irradiation, and autoclaving of rodent feed on MPV and MNV infectivity. J Am Assoc Lab Anim Sci 58:542–550. 10.30802/AALAS-JAALAS-18-000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruckner-Tuderman L, McGrath JA, Robinson EC, Uitto J. 2010. Animal models of epidermolysis bullosa: update 2010. J Invest Dermatol 130:1485–1488. 10.1038/jid.2010.75. [DOI] [PubMed] [Google Scholar]
- 3.Chen VM, Shelke R, Nystrom A, Laver N, Sampson JF, Zhiyi C, Bhat N, Panjwani N. 2018. Collagen VII deficient mice show morphologic and histologic corneal changes that phenotypically mimic human dystrophic epidermolysis bullosa of the eye. Exp Eye Res 175:133–141. 10.1016/j.exer.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Cianfarani F, De Domenico E, Nystrom A, Mastroeni S, Abeni D, Baldini E, Ulisse S, Uva P, Bruckner-Tuderman L, Zambruno G, Castiglia D, Odorisio T. 2019. Decorin counteracts disease progression in mice with recessive dystrophic epidermolysis bullosa. Matrix Biol 81:3–16. 10.1016/j.matbio.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Colomb V, Bourdon-Lannoy E, Lambe C, Sauvat F, Hadj Rabia S, Teillac D, De Prost Y, Bodemer C. 2012. Nutritional outcome in children with severe generalized recessive dystrophic epidermolysis bullosa: a short- and long-term evaluation of gastrostomy and enteral feeding. Br J Dermatol 166:354–361. 10.1111/j.1365-2133.2011.10592.x. [DOI] [PubMed] [Google Scholar]
- 6.Craig AM, Graham ML. 2020. Characterization of different commercial dietary supplements in the peri-weaning period on consumption and growth performance in C57Bl/6J mice. Animals 10:1284. 10.3390/ani10081284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DEBRA. Accessed online 2020. About EB. https://www.debra.org.
- 8.Dutta S, Sengupta P. 2016. Men and mice: relating their ages. Life Sci 152:244–248. 10.1016/j.lfs.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 9.Eckman EA, Cotton CU, Kube DM, Davis PB. 1995. Dietary changes improve survival of CFTR S489X homozygous mutant mouse. Am J Physiol 269:L625–L630. 10.1152/ajplung.1995.269.5.L625. [DOI] [PubMed] [Google Scholar]
- 10.Eden KB, Peterson A, Payne HR, Corapi WV, Mansell J, Hoffman AR. 2016. Congenital dystrophic epidermolysis bullosa (DEB) in Sprague Dawley rats: a case series. Vet Dermatol 27:122–e34. 10.1111/vde.12293. [DOI] [PubMed] [Google Scholar]
- 11.El Hachem M, Zambruno G, Bourdon-Lanoy E, Ciasulli A, Buisson C, Hadj-Rabia S, Diociaiuti A, Gouveia CF, Hernandez-Martin A, de Lucas Laguna R, Dolenc-Voljc M, Tadini G, Salvatori G, De Ranieri C, Leclerc-Mercier S, Bodemer C. 2014. Multicentre consensus recommendations for skin care in inherited epidermolysis bullosa. Orphanet J Rare Dis 9:76. 10.1186/1750-1172-9-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fine JD. 2016. Epidemiology of inherited epidermolysis bullosa based on incidence and prevalence estimates from the National Epidermolysis Bullosa Registry. JAMA Dermatol 152:1231–1238. 10.1001/jamadermatol.2016.2473. [DOI] [PubMed] [Google Scholar]
- 13.Fine JD, Johnson LB, Weiner M, Stein A, Cash S, Deleoz J, Devries DT, Suchindran C. 2004. Eye involvement in inherited epidermolysis bullosa: experience of the National Epidermolysis Bullosa Registry. Am J Ophthalmol 138:254–262. 10.1016/j.ajo.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 14.Fine JD, Johnson LB, Weiner M, Suchindran C. 2004. Assessment of mobility, activities and pain in different subtypes of epidermolysis bullosa. Clin Exp Dermatol 29:122–127. 10.1111/j.1365-2230.2004.01428.x. [DOI] [PubMed] [Google Scholar]
- 15.Fine JD, Mellerio JE. 2009. Extracutaneous manifestations and complications of inherited epidermolysis bullosa. J Am Acad Dermatol 61:367–384. 10.1016/j.jaad.2009.03.052. [DOI] [PubMed] [Google Scholar]
- 16.Fine JD, Mellerio JE. 2009. Extracutaneous manifestations and complications of inherited epidermolysis bullosa: part II. other organs. J Am Acad Dermatol 61:387–402. 10.1016/j.jaad.2009.03.053. [DOI] [PubMed] [Google Scholar]
- 17.Freeman EB, Koglmeier J, Martinez AE, Mellerio JE, Haynes L, Sebire NJ, Lindley KJ, Shah N. 2008. Gastrointestinal complications of epidermolysis bullosa in children. Br J Dermatol 158:1308–1314. 10.1111/j.1365-2133.2008.08507.x. [DOI] [PubMed] [Google Scholar]
- 18.Fritsch A, Loeckermann S, Kern JS, Braun A, Bosl MR, Bley TA, Schumann H, von Elverfeldt D, Paul D, Erlacher M, Berens von Rautenfeld D, Hausser I, Fassler R, Bruckner-Tuderman L. 2008. A hypomorphic mouse model of dystrophic epidermolysis bullosa reveals mechanisms of disease and response to fibroblast therapy. J Clin Invest 118:1669–1679. 10.1172/JCI34292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gipson IK, Spurr-Michaud SJ, Tisdale AS. 1987. Anchoring fibrils form a complex network in human and rabbit cornea. Invest Ophthalmol Vis Sci 28:212–220. [PubMed] [Google Scholar]
- 20.Jafari Z, Faraji J, Mirza Agha B, Metz GAS, Kolb BE, Mohajerani MH. 2017. The adverse effects of auditory stress on mouse uterus receptivity and behaviour. Sci Rep 7:4720. 10.1038/s41598-017-04943-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen K, Hahn NE, Palme R, Saxton K, Francis DD. 2010. Vacuum-cleaner noise and acute stress responses in female C57BL/6 mice (Mus musculus). J Am Assoc Lab Anim Sci 49:300–306. [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson TB, Langin LM, Zhao J, Weimer JM, Pearce DA, Kovacs AD. 2019. Changes in motor behavior, neuropathology, and gut microbiota of a Batten disease mouse model following administration of acidified drinking water. Sci Rep 9:14962. 10.1038/s41598-019-51488-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jumanca OI, Palmer J. 2015. Critical care of sub-lethal irradiated transgenic mice using a complete soft food formula-DietGel76A. J Pharmacol Toxicol Methods 71:46–53. 10.1016/j.vascn.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Kurtz DM, Glascoe R, Caviness G, Locklear J, Whiteside T, Ward T, Adsit F, Lih F, Deterding LJ, Churchwell MI, Doerge DR, Kissling GE. 2018. Acrylamide production in autoclaved rodent feed. J Am Assoc Lab Anim Sci. 10.30802/AALAS-JAALAS-18-000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laboratory J. [Internet]. 2021. Col7a1. Mouse genome informatics. Available at: http://www.informatics.jax.org/allele/MGI:3796248#.
- 26.McPherson CW. 1963. Reduction of pseudomonas aeruginosa and coliform bacteria in mouse drinking water following treatment with hydrochloric acid or chlorine. Lab Anim Care 13:737–744. [PubMed] [Google Scholar]
- 27.Mellado F, Fuentes I, Palisson F, Vergara JI, Kantor CA. 2018. Ophthalmologic approach in epidermolysis bullosa: a cross-sectional study with phenotype-genotype correlations. Cornea 37:442–447. 10.1097/ICO.0000000000001525. [DOI] [PubMed] [Google Scholar]
- 28.Naff KA, Riva CM, Craig SL, Gray KN. 2007. Noise produced by vacuuming exceeds the hearing thresholds of C57Bl/6 and CD1 mice. J Am Assoc Lab Anim Sci 46:52–57. [PubMed] [Google Scholar]
- 29.Rashad R, Weed MC, Quinn N, Chen VM. 2020. Extended wear bandage contact lenses decrease pain and preserve vision in patients with epidermolysis bullosa: case series and review of literature. Ocul Immunol Inflamm 28:379–383. 10.1080/09273948.2019.1587472. [DOI] [PubMed] [Google Scholar]
- 30.Rasmussen S, Glickman G, Norinsky R, Quimby FW, Tolwani RJ. 2009. Construction noise decreases reproductive efficiency in mice. J Am Assoc Lab Anim Sci 48:363–370. [PMC free article] [PubMed] [Google Scholar]
- 31.Reimer A, Hess M, Schwieger-Briel A, Kiritsi D, Schauer F, Schumann H, Bruckner-Tuderman L, Has C. 2019. Natural history of growth and anaemia in children with epidermolysis bullosa: a retrospective cohort study. Br J Dermatol 182:1437–1448. 10.1111/bjd.18475. [DOI] [PubMed] [Google Scholar]
- 32.Reuter JD, Livingston R, Leblanc M. 2011. Management strategies for controlling endemic and seasonal mouse parvovirus infection in a barrier facility. Lab Anim (NY) 40:145–152. 10.1038/laban0511-145. [DOI] [PubMed] [Google Scholar]
- 33.Reynolds RP, Kinard WL, Degraff JJ, Leverage N, Norton JN. 2010. Noise in a laboratory animal facility from the human and mouse perspectives. J Am Assoc Lab Anim Sci 49:592–597. [PMC free article] [PubMed] [Google Scholar]
- 34.Rodel HG, Prager G, Stefanski V, von Holst D, Hudson R. 2008. Separating maternal and litter-size effects on early postnatal growth in two species of altricial small mammals. Physiol Behav 93:826–834. 10.1016/j.physbeh.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 35.Russell WMSB. R.L. [Internet]. 1959. The principles of humane experimental technique. Available at: https://caat.jhsph.edu/principles/the-principles-of-humane-experimental-technique.
- 36.Shimizu Y, Ishida T, Hosomichi J, Kaneko S, Hatano K, Ono T. 2013. Soft diet causes greater alveolar osteopenia in the mandible than in the maxilla. Arch Oral Biol 58:907–911. 10.1016/j.archoralbio.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Suto J, Yamanaka H, Sekikawa K. 2002. Genetic analysis of inferior nurturing ability in RR mice. Reproduction 123:52–58. 10.1530/rep.0.1230052. [DOI] [PubMed] [Google Scholar]
- 38.Ullman-Cullere MH, Foltz CJ. 1999. Body condition scoring: a rapid and accurate method for assessing health status in mice. Lab Anim Sci 49:319–323. [PubMed] [Google Scholar]
- 39.Watson J. 2013. Unsterilized feed as the apparent cause of a mouse parvovirus outbreak. J Am Assoc Lab Anim Sci 52:83–88. [PMC free article] [PubMed] [Google Scholar]
- 40.Weber EM, Algers B, Würbel H, Hultgren J, Olsson IA. 2012. Influence of strain and parity on the risk of litter loss in laboratory mice. Reprod Domest Anim 48:292–296. 10.1111/j.1439-0531.2012.02147.x. [DOI] [PubMed] [Google Scholar]
- 41.Wu L, Kohler JE, Zaborina O, Akash G, Musch MW, Chang EB, Alverdy JC. 2006. Chronic acid water feeding protects mice against lethal gut-derived sepsis due to Pseudomonas aeruginosa. Curr Issues Intest Microbiol 7:19–28. [PubMed] [Google Scholar]