Abstract
Fulminant myocarditis (FM) is an uncommon syndrome characterized by sudden and severe hemodynamic compromise secondary to acute myocardial inflammation, often presenting as profound cardiogenic shock, life-threatening ventricular arrhythmias and/or electrical storm. FM may be refractory to conventional therapies and require mechanical circulatory support (MCS). The immune system has been recognized as playing a pivotal role in the pathophysiology of myocarditis, leading to an increased focus on immunosuppressive treatment strategies. Recent data have highlighted not only the fact that FM has significantly worse outcomes than non-FM, but that prognosis and management strategies of FM are heavily dependent on histological subtype, placing greater emphasis on the role of endomyocardial biopsy in diagnosis. The impact of subtype on severity and prognosis will likewise influence how aggressively the myocarditis is managed, including whether MCS is warranted. Many patients with refractory cardiogenic shock secondary to FM end up requiring MCS, with venoarterial extracorporeal membrane oxygenation demonstrating favorable survival rates, particularly when initiated prior to the development of multiorgan failure. Among the challenges facing the field are the need to more precisely identify immunopathophysiological pathways in order to develop targeted therapies, and the need to better optimize the timing and management of MCS to minimize complications and maximize outcomes.
Fulminant myocarditis (FM) is the most severe manifestation of acute myocarditis (AM), an acute inflammatory myocardial disease most often triggered by viral infections or autoimmune disorders.[1,2] It represents an uncommon syndrome characterized by various clinical manifestations such as severe acute heart failure (HF), cardiogenic shock (CS), ventricular arrhythmias, or sudden death. This narrative review provides a summary of the definition, physiopathology, etiologies, and diagnosis of FM, as well as the rationale and evidence supporting the use of temporary mechanical circulatory support (MCS) and specific immunosuppressive therapies. We also discuss future key research questions and challenges for this complex disease.
DEFINITION
AM is an inflammatory disease of the heart of recent onset (e.g., < one month), generally presenting in young, healthy individuals, with a male prevalence, with a wide spectrum of clinical severity: from asymptomatic or minor symptoms to overt CS or sudden death. [3] FM refers to the latter, with acute HF, CS and/or severe arrhythmic disturbances. The original definition for FM included only lymphocytic myocarditis (LM) and the diagnosis of FM was made almost exclusively on autopsies.[2,4] Recently, Ginsberg, et al.[5] proposed a new, more practical, clinical definition of FM to align with clinical practice, focusing on acute symptom onset (< two weeks) with severe hemodynamic impairment requiring either inotropes or temporary MCS. Currently, the most accepted definition of FM requires acute illness (< one month from symptom onset), hemodynamic compromise due to CS or electrical storm, and need for hemodynamic support (inotropes or temporary MCS) in the absence of an ischemic cause or other pre-existing cardiomyopathies.[6]
EPIDEMIOLOGY
The incidence of AM and FM is difficult to quantify given the wide variation of clinical presentations and challenges in establishing the diagnosis. Currently, approximately 33% of the patients initially labeled as myocardial infarction with non-obstructed coronary arteries are later diagnosed as AM. Based on the Global Burden disease registry, referring to 2019, in the age between 20 years and 44 years, when myocarditis can commonly occur, the rate of myocarditis is 6.1 per 100,000 in men and 4.4 per 100,000 in women.[7] Regarding FM, data are scarcer, even if it has been estimated that the prevalence among patients admitted with AM is between 5% and 10%.[3,8] In one of the largest series of FM requiring MCS across 13 centers over six years, only 57 patients were identified.[9] Similarly, in an international, multicenter study spanning almost 19 years, there were only 220 histologically-confirmed cases of AM, 165 cases of which were classified as FM.[10]
PATHOPHYSIOLOGY
Although not completely elucidated, the pathophysiology of AM has traditionally been divided into three phases:
Viral Phase. Historically, it has been believed that AM is initiated by either a reactivation of a dormant virus (i.e., parvovirus B19) or an introduction of a new cardiotropic virus (i.e., coxsackievirus) in the host myocardium (virus-induced myocarditis). This phase is usually short (e.g., few days) and is frequently missed from a clinical perspective.[11,12] Data from endomyocardial biopsy (EMB) from patients with AM and FM show a high prevalence of parvovirus B19,[1,13] although its pathogenic role is still discussed. Nowadays, it is believed that common respiratory viruses (influenza viruses, rhinoviruses and coronaviruses) can trigger AM through an immune-mediated LM.[1,14]
Immune Activation. Although viral replication within the myocardium can be injurious in itself, most of the tissue damage results from an unregulated host immune response in an attempt to eliminate the infected cells.[15] This phase is characterized by the activation of virus-specific T lymphocytes.[12] T cells clonally expand to neutralize the original antigen, which could be a myocardial protein instead, leading to greater myocardial destruction by molecular mimicry.[16] Interleukin-6 is thought to play a key role in this inflammatory cascade, with subsequent activation of B lymphocytes and production of antibodies.[17] This phase usually lasts for days or weeks,[12] even if in most cases resolve spontaneously. An alternative explanation is that in virus-triggered AM, there is a molecular mimicry between viral and cardiac antigens, that might result in autoreactive T cell infiltration in the myocardium in predisposed individuals, without the need for viral infiltration of the heart.[6,18]
Myopathy Phase. In most cases of AM, the immune response is properly modulated and adaptative, with the eventual elimination of viral replication, when present, allowing the myocardium to heal without sequelae. However, in some cases, the infection is not well-controlled and the inflammation persists, leading to myocardial necrosis, ventricular remodeling, and dilated cardiomyopathy.[15] The persistent release of cytokines activates metalloproteinases, which cleave and digest the extracellular framework of the myocardium.[19] Eventually, myocardial fibrosis may develop, sharing common molecular pathways with other cardiomyopathies.[20]
ETIOLOGY: SPECIFIC ETIOLOGICAL SUBTYPES
AM can broadly be characterized as an inflammatory myocarditis, the etiologies of which are presented in Table 1, along with their respective clinical presentations, treatments, and outcomes.
Table 1. Main histological subtypes of fulminant myocarditis.
| Subtype of fulminant myocarditis | Pathology | Incidence | Etiology | Clinical presentation/Diagnosis | Treatment |
| Lymphocytic myocarditis | Small mononuclear cells (CD3+ T lymphocytes) | The most frequent
histological subtype |
Viruses
Drugs/toxins Autoimmune |
Wide range of presentations
The most frequent subtype in asymptomatic patients |
Frequently self-limited
In fulminant myocarditis, mechanical circulatory support as supportive care No clear evidence of immunosuppressants |
| Eosinophilic myocarditis | Eosinophilic infiltrate | Rare: unknown | Hypersensitivity (antibiotics, clozapine, carbamazepine)
Churg-Strauss Hypereosinophilic syndrome Parasitic infections (Toxocara canis) |
From asymptomatic to fulminant myocarditis or Loeffler cardiomyopathy
Fever, skin rash if hypersensitivity Peripheral eosinophilia not always present Frequent intraventricular thrombosis |
Identifying cause
High-dosing corticosteroids +/− cyclophosphamide, albendazole, imatinib, azathioprine or methotrexate Mechanical circulatory support (mainly in hypersensitivity and idiopathic forms) |
| Giant cell myocarditis | Large multinuclear cells in the absence of well-formed granuloma
Degranulated eosinophils |
Uncommon | Unknown
Autoimmune disorders frequently associated (25%) |
Young, healthy adults
Severe heart failure, refractory cardiogenic shock Frequent arrhythmic disturbances (atrioventricular block, ventricular tachycardia, ventricular fibrillation) |
Aggressive, combined, immunosuppressants treatment: cardiogenic shock + cyclosporine-based treatment
Rabbit anti-thymocyte immunoglobulin Muromunab, azathioprine: second-line treatment Frequent need of mechanical circulatory support |
| Immune checkpoints inhibitors-checkpoint myocarditis | Similar to a high-grade cardiac rejection: T cell mediated injury | Less than 1% of treated patients | Immune checkpoints inhibitors (nivolumab, pembrolizumab) | Life-threatening arrhythmic disturbances (atrioventricular block, refractory ventricular tachycardia), multiorgan failure
Early presentation after initiation of immune checkpoints inhibitors (< 6 weeks) Possible other organ involvement (liver, lung, etc) |
Withdrawal of the drug
High dosing of corticosteroids Mechanical circulatory support |
Lymphocytic Myocarditis
LM is the most frequent form of AM, characterized by myocardial infiltration of mononuclear cells and left ventricular dysfunction. In a biopsy-proven cohort, LM represented 71% and 72% of the FM and non-FM groups, respectively.[10] It is important to distinguish LM from other inflammatory myocarditis, as its outcome tends to be better than that of giant cell myocarditis or other non-specific subtypes of AM.
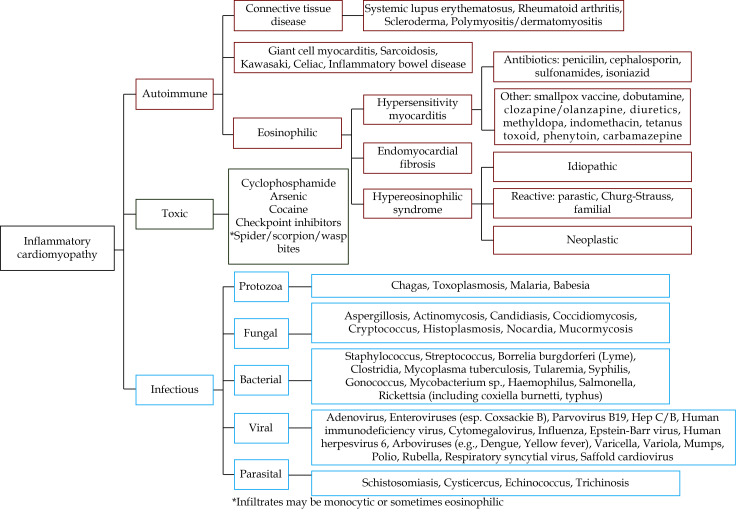
There are three broad etiological categories typically responsible for LM (Figure 1): pathogens (mainly viruses, either by direct or indirect immune-mediated myocardial injury), drugs or toxins, and autoimmune disorders.[2] Viral infections are the most frequent cause of LM, accounting for approximately 30%−40%.[11,21] Other causative pathogens, such as bacteria (e.g., chlamydia, rickettsia), protozoa, and fungi have also been described.[12]
Figure 1.
Primary causes and associated subcategories of acute myocarditis.
Reprinted from Trachtenberg, et al.[113] Copyright© 2017, the American Heart Association, Inc.
The pathophysiological mechanisms of myocardial injury include either the direct viral tropism (virus-mediated myocarditis) or molecular mimicry due to cross-reactivity with host myocardial proteins (virus-triggered myocarditis). Typically, enteroviruses (e.g., coxsackievirus) depend on viral-mediated myocardial injury, whereas respiratory viral infections (e.g., influenza and severe acute respiratory syndrome coronavirus 2) usually trigger immune-mediated AM, with the viruses themselves typically undetectable in the myocardium.[22,23] Parvovirus B19 and human herpesvirus 6 represent viral pathogens that can cause myocardial injury by both mechanisms.[6]
LM is the subtype most likely to present with self-limited disease without the need for specific treatment.[24] Even fulminant LM has been reported to recover spontaneously despite an initial need for temporary MCS. Medical treatment in this AM subtype could be directed toward the virus itself or the subsequent inflammatory cascade. Antiviral approaches with interferon have infrequently been attempted in humans, albeit with promising results,[25] although most of these cases involved chronic or subacute disease and excluded FM. The role of corticosteroids in fulminant LM remains unclear and warrants further investigations,[26] even if cases series have demonstrated interesting results.[27,28]
Influenza Virus
Influenza virus may induce myocarditis, reportedly in the form of LM. With influenza viral antigens or genetic material rarely detected in the myocardium itself, the likely mechanism of injury is indirect damage (i.e., cytokine storm, endothelial cell dysfunction, and inflammation-mediated injury) rather than direct cardiac tropism.[29,30] Acute and fulminant forms have been reported as a result of either influenza A or B, with several case reports and series published after the pandemic influenza A (H1N1) in 2009.[31,32] Notably, influenza B-related FM was associated with favorable outcomes despite severe hemodynamic impairment, early onset of life-threatening arrhythmias, and need for venoarterial extracorporeal membrane oxygenation (VA-ECMO).[31] In one international cohort of influenza A-associated AM, 36 of 58 patients had a fulminant presentation, with 17 patients requiring temporary MCS.[33]
Severe Acute Respiratory Syndrome Coronavirus 2
The pathophysiology behind coronavirus disease 2019 (COVID-19)-related myocardial injury and subsequent CS, has not yet been fully elucidated.[34] Both direct tropism and indirect injury due to cytokine storm have been proposed, with the latter seeming to predominate.[35–37] The presence of cardiac injury has been independently associated with a 4-fold increased risk of mortality in patients infected with COVID-19.[38] Given the complex pathophysiology, risk of complications, and uncertain outcomes, the role of VA-ECMO has not been firmly established and should be individualized on a case-by-case basis by an interdisciplinary team. Similar to the multisystem inflammatory syndrome described in children,[39] corticosteroids and intravenous immunoglobulins have been proposed in adults.[40] Lastly, recent case series reported myocarditis-like illness 2–4 days after mRNA (Moderna or Pfizer-BioNTech) or adenovirus (Janssen) COVID-19 vaccination.[41,42] Although the link between COVID-19 vaccination and myocarditis remains circumstantial, and a mechanism has not been established, the clinical course of vaccine-associated myocarditis-like illness appears favorable, with resolution of symptoms in all patients.[41,42]
Giant Cell Myocarditis (GCM)
GCM is an uncommon but highly lethal form of FM in young healthy adults,[11] with approximately 20% of patients having pre-existing autoimmune disorders.[43] Patients typically present with severe acute HF, often leading to refractory CS. Arrhythmic disturbances, mostly ventricular tachycardia or complete atrio-ventricular block, are common.[44] Arrhythmias at initial presentation have been associated with an increased long-term risk of life-threatening arrhythmias (50% at five years).[45] As delays in both diagnosis and targeted treatment can lead to dismal outcomes, patients with typical features (e.g., rapidly evolving HF, arrhythmias, and lack of response to medical treatment) should promptly undergo EMB.[6,46,47] In the largest cohort of biopsy-confirmed FM, Ammirati, et al.[10] demonstrated a strong correlation between giant cell histology and short and long-term prognosis, compared to LM and EMB. Notably, sensitivity of EMB may be less than 60% when performed early in the disease course[48] and may warrant repeating if initial EMB is negative but suspicion of GCM remains high.[45] Histopathologically, GCM is a diffuse or multifocal lymphocytic infiltrate with multinucleated giant cells associated with myocardial damage. Degranulated eosinophils are also frequently observed.[2] Acute HF can develop rapidly in GCM, thus necessitating close monitoring of hemodynamics and early consideration of MCS.[48] Given the very low likelihood of spontaneous myocardial recovery and high risk of death or heart transplant within the first year of diagnosis,[48–54] prompt and aggressive treatment with immunosuppression is an essential component of management. Cooper, et al.[50] showed that combined immunosuppressants significantly prolonged survival or time to transplant from 3.0 months to 12.3 months. The combination of steroids and rabbit anti-thymocyte globulin has been recently examined, showing promising results on left ventricular recovery.[54] Similarly, cyclosporine-based immunosuppressive therapy has a pivotal role in the treatment of GCM,[50,52] with muromunab or azathioprine considered second-line treatments.[55]
Eosinophilic Myocarditis (EM)
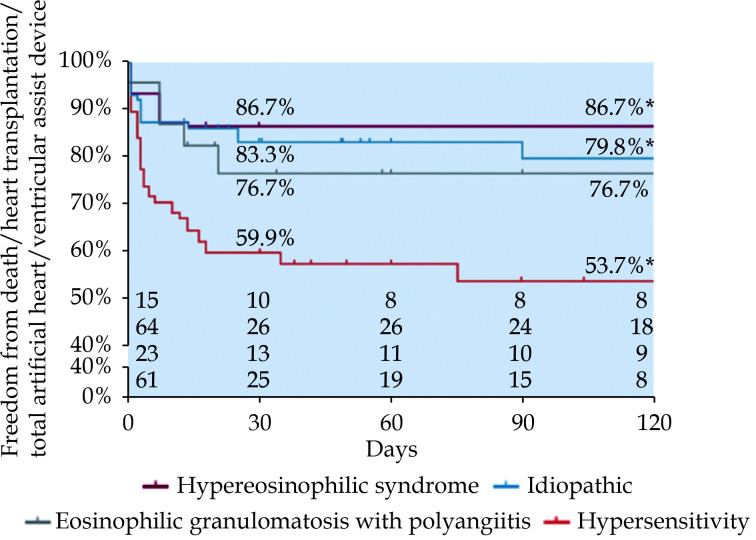
EM represents a rare form of myocarditis characterized by myocardial eosinophilic infiltration, often with accompanying peripheral eosinophilia. In a recent review of 179 patients with EM, an associated systemic disorder was found in 64% of cases;[56] drug-hypersensitivity was most common (34%), followed by Churg-Strauss syndrome (13%) and hypereosinophilic syndrome (HES) (8%).[2,56] Hypersensitivity-related EM was associated with the highest mortality rate (36%) (Figure 2), in part explained by the high prevalence of cardiac arrest during the acute phase.[56]
Figure 2.
Outcomes of different etiologies leading to eosinophilic myocarditis.
Kaplan-Meier survival free from death, heart transplantation, total artificial heart, and long-term ventricular assist device. *Presented as unadjusted significant differences between the groups (P < 0.05), after Bonferroni’s test only idiopathic or undefined versus hypersensitivity eosinophilic myocarditis remains significant. Reprinted from Brambatti, et al.[56]
The clinical presentation varies from paucisymptomatic to acute FM (also called acute necrotizing EM) to chronic restrictive cardiomyopathy (classically called Loeffler cardiomyopathy).[6] Usually, EM presents in middle-aged patients, often presenting with dyspnea or chest pain. ST-segment deviations, increased troponins, and peripheral eosinophilia are common findings, although the absence of eosinophilia does not rule out the diagnosis of EM.[56–58] In fact, the more fulminant form of EM, with higher associated mortality, lacks peripheral eosinophilia 35%−40% of the time.[56] Notably, the late gadolinium enhancement (LGE) pattern on cardiac magnetic resonance imaging (MRI) is more often subendocardial, unlike most myocarditis which typically show epicardial LGE.[56,59] Cardiac MRI may also help identify intraventricular thrombi, which have frequently been associated with HES-related EM.
Given the need for targeted therapies in EM, EMB plays an important role in diagnosis and management.[6] However, due to relatively high rates of intracardiac thrombosis, echocardiography or cardiac MRI if feasible should rule out ventricular thrombi before performing EMB. It is also relevant to identify the underlying trigger of EM to help guide management. Despite a lack of clinical trials studying the effect of steroids in EM, high-dose steroids should be given to those with hypersensitivity-related EM, in addition to withdrawal of the offending agent.[56,60] The combination of steroids with additional treatments may be warranted for particular etiologies, such as cyclophosphamide in Churg-Strauss-related EM, imatinib in the myeloproliferative variant of platelet-derived growth factor receptor A-associated HES, or albendazole in EM associated with Toxocara canis infection. In some forms of HES, therapeutic anticoagulation may be considered due to the increased risk of intraventricular thrombi.[61]
The need for MCS in fulminant forms of EM ranges between 2% and 20% of patients with wide variation across EM subtypes. The hypersensitivity and idiopathic forms seem to be associated with higher rates of temporary MCS (19.7% and 15.6%, respectively).[56] Interestingly, myocardial recovery can be quite high in temporary MCS-supported patients treated with concomitant corticosteroids, with survival approaching 90%.[62,63] However, the overall rate of death or heart transplant in the fulminant forms is reported to be up to 26% in recent series.[10]
AM Associated with Immune Checkpoints Inhibitors (ICI) or Other Novel Cancer Therapies
Chemotherapy-associated cardiac toxicity may manifest as myocarditis. ICI-induced myocarditis is a newly recognized, potentially fatal form of AM.[64] Despite being reported in less than 1% of treated patients,[65,66] the wider use of novel cancer therapies and recent characterization of this entity has led to increasing awareness and diagnosis. ICI are blocking antibodies targeting immune checkpoints (i.e., cytotoxic T-lymphocyte antigen 4, programmed death receptor 1, and its ligand) that in turn activate T cells responsible for myocardial injury. The pattern of T cell-mediated injury has an appearance that mimics high-grade cardiac transplant rejection.
The fulminant form of ICI-associated AM typically presents with life-threatening arrhythmic disturbances (e.g., complete atrio-ventricular block and refractory ventricular tachycardia), leading to multiorgan failure and death.[64] Most patients present early after treatment initiation, generally within the first six weeks.[66] The underlying immune activation may lead also to other organ injury, such as hepatitis, pneumonitis, and, commonly, myositis. The latter may precede cardiac involvement and should prompt a workup for skeletal muscle involvement, including biopsy, as indicated.[67]
Recommended treatment includes withdrawal of the offending agent, high-dose steroids, and hemodynamic support, with temporary MCS if necessary.[2] Second-line treatment options include alemtuzumab, anti-thymocyte globulin, and abatacept. Outcomes are variable, but mortality may be as high as 50%.[67]
DIAGNOSIS
Clinical Evaluation
A thorough history and physical exam is important to help characterize both the etiology and severity. Patients with AM usually report chest pain (which may also represent concomitant pericarditis), dyspnea, or palpitations. Syncope occurs in 6% of cases, and sudden death may be the initial manifestation.[68] A history of exposure to drugs or infections should be considered, as should the presence of autoimmune or systemic inflammatory disorders, given their presence in 7.2% of patients with AM, including 15.4% of severe cases.[3] In patients with acute HF symptoms or CS, it is important to identify signs of tissular hypoperfusion that may impact clinical management.[2,69]
Electrocardiogram (ECG)
ECG is neither specific nor sensitive, with 85% of cases having an abnormal finding. The most common abnormality is ST-segment elevation which could mimic an acute coronary syndrome.[70] Other ECG abnormalities may include widening of the QRS interval, atrio-ventricular block, bradyarrhythmia, or tachyarrhythmia, including ventricular tachycardia or ventricular fibrillation, with malignant arrhythmias conferring the highest risk. PR-segment depression may be seen when there is concomitant pericarditis. AM is typically not associated with the presence of Q waves or reciprocal changes.
Laboratory Tests
Typically, patients with AM present with high levels of cardiac troponins. There is a mild correlation between the extent of myocardial damage and the magnitude of troponin levels.[71] Importantly, cardiac troponin does not help differentiate AM from myocardial ischemia, and low troponin levels do not rule out myocarditis,[68] even if higher levels of creatine kinase-myocardial band isoenzyme has been associated with poor prognosis, specifically lower likelihood of weaning form temporary MCS in patients with FM for peak values of creatine kinase-myocardial band isoenzyme above 185 U/I.[72] Natriuretic peptides are often elevated and are related to outcomes.[2,73] C-reactive protein is non-specific but elevated in most cases.[3] Eosinophilia, when present, may suggest EM. The presence of autoantibodies may be indicative of autoimmune-mediated AM. Recently, it has been identified a novel and specific microRNA for the diagnosis of AM, able to distinguish AM from acute myocardial infarction, and with appealing applications in the near future.[74] In fulminant presentations, extra-cardiac organ dysfunction may manifest as elevations in lactate (which should always be assessed early), hepatic enzymes, bilirubin, creatinine or coagulation parameters (e.g., prolonged prothrombin time or partial thromboplastin time).
Echocardiography
Echocardiography is one of the cornerstones of AM evaluation. Uncomplicated AM is defined by left ventricular ejection fraction (LVEF) > 50%, which is the finding in nearly three-quarters of cases. [6] LVEF on admission is closely associated with outcomes.[3] Segmental wall motion abnormalities can be seen, mostly in the inferior and lateral walls, without a typical coronary distribution.[73] Other findings include pericardial effusion, right ventricular dysfunction, or wall thickening and mottling.[75] It is worth highlighting that LVEF can rapidly change in AM, requiring close and prompt monitoring, thus even patients with initial LVEF > 50% can have an unfavorable evolution, especially in the first 24−48 h since admission. The importance of echocardiography becomes even more important in the presence of hemodynamic compromise.
Magnetic Resonance Imaging
Cardiac MRI has emerged as a useful diagnostic tool for AM, particularly in uncomplicated patients, with the ability to quantify alterations in myocardial signal, providing data about left ventricular function, edema, and LGE.[59,76] Cardiac MRI is currently indicated in patients presenting with chest pain, abnormal levels of cardiac troponin, and non-obstructed coronary arteries, referred to as myocardial infarction with non-obstructed coronary arteries, in order to distinguish between ischemic and non-ischemic myocardial injury.[77] The expanding use of cardiac MRI has contributed to the detection of a higher incidence of uncomplicated AM. However, cardiac MRI may not be feasible in patients with FM, owing to clinical instability and the incompatibility between MRI and MCS. MRI may be more relevant after medical stabilization, with the goal of characterizing the presence, extent, and location of inflammation and fibrosis.
In general, MRI cannot determine the histological subtype of AM, although the location of inflammation, such as basal septum in cardiac sarcoidosis, may be suggestive.[78] Typically, the distribution of LGE in AM is epicardial, as opposed to an endocardial-to-epicardial distribution seen in chronic ischemic disease.[59] MRI can be useful in the longitudinal assessment of AM, with the persistence of LGE associated with worse outcomes, including recurrence of myocarditis.[79]
Endomyocardial Biopsy
The role of EMB in AM remains controversial.[2] The American Heart Association/American College of Cardiology/European Society of Cardiology consensus statement from 2007 considers EMB a Class I indication for: (1) unexplained new-onset HF of less than two weeks duration associated with hemodynamic compromise; or (2) unexplained new-onset HF of two weeks to three months duration associated with a dilated left ventricular and new bradyarrhythmia (Mobitz type II or complete heart block), new ventricular arrhythmias, or a failure to respond to standard care within one to two weeks of diagnosis.[47] In 2016, the American Heart Association released a revised statement recommending EMB for patients with rapidly progressive HF in whom there is a high suspicion of an etiology that can be only confirmed by myocardial histology.[80] In 2020, an expert consensus document recommended EMB for patients with AM and CS, acute HF, ventricular arrhythmias, or advanced atrioventricular block, especially in case of mildly or non-dilated left ventricular and recent onset of symptoms.[6] Importantly, the authors of that consensus document suggest that EMB is no longer useful in most patients with LVEF > 40%, especially if in the absence of symptoms or acute HF, but still relevant for patients with severe left ventricular systolic dysfunction or FM. Based on the existing guidelines and consensus statements, we suggest that EMB be considered when a specific diagnosis is needed to guide or tailor therapy, including cases of CS with normal coronary arteries with clinical suspicion of AM and most cases of FM. [2]
Despite the existing guidance, the rates of EMB are perhaps understandably lower than recommended,[9,48] likely owing, at least in part, to hemodynamic instability and a higher risk of complications in these patients. EMB-related complication rates correlate with center experience and volume, with rates as low as 1%−2% in experienced centers, versus nearly 9% in low-volume centers.[81,82] Risk may be compounded by the need for multiple biopsy specimens in order to increase yield when myocardial involvement is patchy. Sensitivity of EMB may be increased by directing the procedure with cardiac MRI, when feasible.[2]
MANAGEMENT
Temporary MCS
FM often consists of acute HF progressing to CS, typically requiring inotropic agents to achieve hemodynamic stabilization. However, inotropes may induce or aggravate tachyarrhythmias, which may, in turn, necessitate early implementation of temporary MCS, including extracorporeal life support.
Temporary MCS as a bridge-to-recovery is a reasonable consideration for FM; patients tend to be young with few preexisting comorbidities, FM is usually self-limited with a high rate of recovery and infrequent need for transplantation, and duration of temporary MCS is typically < 7−10 days. The use of temporary MCS for FM markedly increased since its first use in 1990, [83,84] but the type of temporary MCS and best timing of implantation are still a matter of debate. With extra-cardiac end-organ failure independently associated with higher mortality,[85,86] the objective of temporary MCS is to intervene timely and prior to the development of multiorgan failure, while allowing time for cardiac recovery. Identifying the proper candidacy for and timing of temporary MCS may be facilitated through the involvement of multidisciplinary shock teams, where available. Because most fulminant LM recovers spontaneously, the duration of temporary MCS support is usually short. However, in the absence of myocardial recovery after 10−15 days, patients should be considered for bridging to long-term mechanical support or heart transplant, as appropriate.
Extracorporeal life support, typically VA-ECMO, is the quickest and most straightforward way to provide full hemodynamic support, and the results from several case series have suggested that early implementation of ECMO may be associated with better outcomes.[85,87,88] However, any potential survival benefit has to be weighed against the risk of complications associated with extracorporeal support.[89] In addition, the increase in afterload from VA-ECMO may require additional device support (e.g., intra-aortic balloon pump, Impella® systems) for left ventricular unloading.[90–93] Microaxial percutaneous ventricular assist devices (i.e., Impella® systems) are increasingly being used in this setting to unload the left ventricle, reduce myocardial oxygen consumption, and mitigate injury to the inflamed myocardium, with preliminary data from small case series suggesting favorable outcomes.[94] Of note, in FM patients with ventricular wall edema and thickening with a resultant small left ventricular cavity, caution should be taken in using devices that create negative drainage pressure, as suction phenomena may occur.
Immunosuppressive Therapy
In many cases of FM, immunosuppressive therapy is an essential component of management, particularly for EM, GCM, cardiac sarcoidosis, and autoimmune-associated FM. There has been a recent trend towards the use of combination therapy, with corticosteroids as the mainstay.[50] In the acute phase of FM, other agents such as intravenous immunoglobulins, cyclophosphamide, and rituximab, are used. Plasmapheresis is sometimes used, especially in cases of antiphospholipid syndrome.[95–97] In the absence of an identified systemic inflammatory disease state warranting specific therapy, there are no data to guide treatments for LM.[2] The Myocarditis Treatment Trial,[98] aiming to assess the efficacy of immunosuppressants in AM, reported no clinical benefit from immunosuppression in LM, although the rarity of the disease makes it difficult to conduct large randomized controlled trials aimed at answering this question.
It remains controversial whether steroids should be given in LM with negative viral polymerase chain reaction (PCR) studies. The European Society of Cardiology Working Group on Myocardial and Pericardial Diseases recommends that immunosuppressants be started only after ruling out active viral infection on EMB by PCR,[68] although there is no consensus among experts whether it is necessary to perform viral studies on all EMB specimens.[6] Although the presence of parvovirus B19, the virus most frequently identified in the myocardium, does not appear to impact prognosis, the presence of other viruses (e.g., cytomegalovirus, adenovirus, enterovirus) may have prognostic implications, and their presence should preclude the use of immunosuppressants. In general, immunosuppressants should be considered in LM with negative viral PCR on EMB, or those with parvovirus B19 or human herpesvirus 6, with ongoing usage dependent on the initial response and presence or trajectory of a viral load, if present.[6] Furthermore, it is still debated if viral search in the myocardium can guide the choice to administer immunosuppression therapy in patients with FM.[99] In fact, in the setting of lymphocytic FM only parvovirus B19 has been found in a large recent registry. At present, the role of a routine viral genome search on EMB in guiding immunosuppression in patients with FM remains largely to be proven, also considering that PCR technique is time consuming with an average time to response varying from 36 h to 72 h.[99] Of note, when there is a high suspicion for GCM, corticosteroids should be administered promptly, as their usage will not obscure EMB results.[2]
OUTCOMES OF FM AND TEMPORARY MCS
Historically, there had been a perception that FM had better outcomes than non-FM.[24] More recently, a cohort of AM out of Italy demonstrated that FM was associated with worse outcomes, with lower LVEF at follow-up, and increased long-term mortality and need for heart transplant.[27] These findings were confirmed in an international registry of 220 cases of biopsy-proven myocarditis,[10] with higher rates of cardiac death and heart transplant at 60 days in FM compared to non-FM (28.0% vs. 1.8%, P < 0.01), a trend that persisted at seven-year follow-up (47.7% vs. 10.4%, P < 0.01). These findings were consistent in the subgroup of patients with LM.
VA-ECMO is considered first-line therapy in FM-related CS refractory to inotropes, with overall survival ranging from 47% to 83% despite the high severity of illness in this patient population.[9,83,100–102] Table 2 summarizes the existing literature on cohorts or case series (> 10 patients) of FM managed with temporary MCS (predominantly VA-ECMO). Compared to other etiologies of CS, VA-ECMO for FM has been associated with lower mortality.[85,103]
Table 2. Studies including more than ten adult patients with fulminant myocarditis treated with VA-ECMO and/or Impella®.
| Authors | Time | Patients, n | Age, yrs | Type of MCS | Biopsy performed | Time of support, day | Etiology | Complications | Outcomes |
| Data are presented as means ± SD. *Presented as median (interquartile range). **Presented as multicenter studies. ***Presented as regarding the complete fulminant myocarditis population. BiV: biventricular; eCPR: extracorporeal-cardiopulmonary resuscitation; EM: eosinophilic myocarditis; EMB: endomyocardial biopsy; GCM: giant cell myocarditis; HTx: heart transplant; IABP: intraao
rtic balloon pump; LM: lymphocytic myocarditis; LVAD: left ventricular assist device; MCS: mechanical circulatory support; NA: non-applicable; RRT: renal replacement therapy; VA-ECMO: venoarterial extracorporeal membrane oxygenation; VAP: ventilator-associated pneumonia. | |||||||||
| Aoyama N, et al.[114] | 1989–2000 | 52 | 48 ± 16 | Percutaneous cardiopulmonary support | NA (43 patients diagnosed on EMB) | NA | Idiopathic: 34
Viral: 14 Eosinophilic: 2 GCM: 2 |
NA | Survival and return to normal life: 57.7 % |
| Mirabel M, et al.[115] | 2002–2009 | 35 | NA | VA-ECMO | 61% (25 patients) | NA | LM: 20
GCM: 2 EM: 2 |
NA | Survival to discharge: 68.6% |
| Ishida K, et al.[116] | 1995–2010 | 20 | 45 ± 19 | VA-ECM | 60% | NA | NA | NA | Survival to discharge: 60% |
| Asaumi Y, et al.[117] | 1993–2001 | 14 | 38 ± 15 | VA-ECMO (43% IABP) | 64% | 5.4 (1.7–7.1)* | NA | NA | ECMO weaning and acute survival: 71% |
| Lorusso R, et al.[9] | 2008–2013 | 57 | 38 ± 12 | VA-ECMO (65% IABP)
eCPR: 21% |
26.3% | 9.9 ± 19 | Idiopathic: 80.6%
Viral: 17.5% Autoimmune: 1.7% |
Major complications in 70% | ECMO weaning: 75%
Survival to hospital discharge: 72% |
| Montero S, et al.[48] | 2002–2016 | 13 | 44 (21–76)* | VA-ECMO: 85%
LVAD: 8% BiV MEDOS: 8% eCPR: 15% |
38% | 10 (1–13)* | GCM: 100% | RRT: 61%
Hemorrhage: 61% VAP: 46% Bacteremia: 46% |
69% survival at 90-days & one year post-symptom onset
0% survival free from HTx at one year after symptom onset |
| Beurtheret S, et al.[118] | 2005–2009 | 14 | NA | VA-ECMO | NA | NA | NA | NA | Survival to discharge: 65% |
| Wu MY, et al.[103] | 2003–2010 | 16 | NA | VA-ECMO
eCPR: 31% |
NA | NA | Viral: 62%
Unknown: 32% Postpartum: 6% |
NA | Survival to discharge: 87.5% |
| Diddle JW, et al.[86]** | 1995–2014 | 147 | 31 (21–47)* | VA-ECMO: 91%
eCPR: 21% |
NA | 5.7 (2.9–8.6)* | Viral: 7%
Other: 93% |
NA | ECMO weaning: 69%
Survival to hospital discharge: 61% |
| Ammirati E, et al.[10] | 2001–2018 | 73
2 9 |
NA | VA-ECMO
Impella® Other |
100% | 8.5 (5–15)* | LM: 72.7%
GCM: 14.5% EM: 11.5% Sarcoidosis: 1.2%*** |
NA | Death on MCS: 13.9%
Recovery with MCS: 24.8% |
| Annamalai SK, et al.[119] | 2009–2016 | 34 | 42 ± 17 | Impella® (concomitant VA-ECMO in 6%) | 32% | 3.8 ± 3.1 | NA | Hemolysis: 12%
Anemia requiring transfusion: 18% RRT: 32% Limb ischemia: 9% Stroke: 6% Vascular complications: 12% |
Survival to hospital discharge: 62% |
Particular consideration should be given to GCM, in light of its high rates of mortality and need for heart transplantation. A French cohort of fulminant GCM supported with temporary MCS showed 100% of mortality or heart transplant at one year from symptom onset. In such cases, prompt initiation of VA-ECMO is often needed, in combination with aggressive immunosuppression. In the absence of rapid clinical improvement, candidacy for heart transplant should be considered early,[48] despite relapse having been described.[104,105]
KEY RESEARCH QUESTIONS AND CHALLENGES
Improvements in FM management and outcomes are likely to come from two directions: optimization of immune modulation and advancements in mechanical support.[99] There is growing interest in modulating the immune response or promoting regulatory elements of the immune system. Anakinra (an IL-1beta receptor antagonist) has been linked to reductions in inflammation and fibrosis in preclinical studies,[106] and a randomized controlled trial is ongoing ( https://www.clinicaltrials.gov, Unique Identifier: NCT03018834). Additionally, a clinical trial of secukinumab, an anti-IL-17 monoclonal antibody, has been proposed.[107] Other treatments that offer promise include cell-based therapies, as mesenchymal stromal cells have been shown to have immunomodulatory and cardioprotective effects in mouse models of myocarditis.[108] Lastly, aldosterone antagonists, cannabidiol, antagomirs, and modulators of gut microbiota are alternative ways of immunomodulation that have become recent focuses of interest.[107] There remains a need for randomized clinical trials to assess the utility and efficacy of these therapies, particularly focusing on AM or FM rather than subacute cardiomyopathies.[2]
The second potential area for improvement in FM-related CS is in the management and optimization of MCS strategies. The need for, timing, and optimal mode of left ventricular unloading, including whether the combination of VA-ECMO and Impella® is better than ECMO alone, remain to be determined.[109] Additional improvements in outcomes may come from the prevention of MCS-related complications. Percutaneous cannulation[110] and awake VA-ECMO[111,112] may be among the strategies that could help avoid some of the complications observed in this patient population, thereby improving outcomes of mechanically supported patients with FM.
CONCLUSIONS
FM represents a growing and challenging field with complex interrelations between etiology, pathophysiology, management, and treatment. Due to the complexity, heterogeneity, and rarity of FM, there remain challenges in the establishment of protocols for diagnosis (especially the role of EMB) and treatment, including how best to tailor immunosuppressive regimens and optimize of timing and management of MCS. Future design and performance of randomized clinical trials may benefit from collaboration with international networks that specialize in extracorporeal support, such as the Extracorporeal Life Support Organization (www.elso.org) and the International ECMO Network (www.internationalecmonetwork.org).
ACKNOWLEDGMENTS
All authors had no conflicts of interest to disclose.
References
- 1.Ammirati E, Varrenti M, Veronese G, et al Prevalence and outcome of patients with acute myocarditis and positive viral search on nasopharyngeal swab. Eur J Heart Fail. 2021;23:1242–1245. doi: 10.1002/ejhf.2247. [DOI] [PubMed] [Google Scholar]
- 2.Kociol RD, Cooper LT, Fang JC, et al Recognition and initial management of fulminant myocarditis: a scientific statement from the American Heart Association. Circulation. 2020;141:e69–e92. doi: 10.1161/CIR.0000000000000745. [DOI] [PubMed] [Google Scholar]
- 3.Ammirati E, Cipriani M, Moro C, et al Clinical presentation and outcome in a contemporary cohort of patients with acute myocarditis: multicenter Lombardy registry. Circulation. 2018;138:1088–1099. doi: 10.1161/CIRCULATIONAHA.118.035319. [DOI] [PubMed] [Google Scholar]
- 4.Ammirati E, Cipriani M, Camici PG New concepts in fulminant myocarditis and risk of cardiac mortality. Oncotarget. 2017;8:84624–84625. doi: 10.18632/oncotarget.21393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ginsberg F, Parrillo JE Fulminant myocarditis. Crit Care Clin. 2013;29:465–483. doi: 10.1016/j.ccc.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Ammirati E, Frigerio M, Adler ED, et al Management of acute myocarditis and chronic inflammatory cardiomyopathy: an expert consensus document. Circ Heart Fail. 2020;13:e007405. doi: 10.1161/CIRCHEARTFAILURE.120.007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roth GA, Mensah GA, Johnson CO, et al Global burden of cardiovascular diseases and risk factors, 1990−2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ammirati E, Veronese G, Bottiroli M, et al Update on acute myocarditis. Trends Cardiovasc Med. 2021;31:370–379. doi: 10.1016/j.tcm.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lorusso R, Centofanti P, Gelsomino S, et al Venoarterial extracorporeal membrane oxygenation for acute fulminant myocarditis in adult patients: a 5-year multi-institutional experience. Ann Thorac Surg. 2016;101:919–926. doi: 10.1016/j.athoracsur.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Ammirati E, Veronese G, Brambatti M, et al Fulminant versus acute nonfulminant myocarditis in patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2019;74:299–311. doi: 10.1016/j.jacc.2019.04.063. [DOI] [PubMed] [Google Scholar]
- 11.Cooper LT Jr Myocarditis. N Engl J Med. 2009;360:1526–1538. doi: 10.1056/NEJMra0800028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kindermann I, Barth C, Mahfoud F, et al Update on myocarditis. J Am Coll Cardiol. 2012;59:779–792. doi: 10.1016/j.jacc.2011.09.074. [DOI] [PubMed] [Google Scholar]
- 13.Veronese G, Ammirati E, Brambatti M, et al Viral genome search in myocardium of patients with fulminant myocarditis. Eur J Heart Fail. 2020;22:1277–1280. doi: 10.1002/ejhf.1738. [DOI] [PubMed] [Google Scholar]
- 14.Belkaya S, Kontorovich AR, Byun M, et al Autosomal recessive cardiomyopathy presenting as acute myocarditis. J Am Coll Cardiol. 2017;69:1653–1665. doi: 10.1016/j.jacc.2017.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dennert R, Crijns HJ, Heymans S Acute viral myocarditis. Eur Heart J. 2008;29:2073–2082. doi: 10.1093/eurheartj/ehn296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Heuser JS, Cunningham LC, et al Mimicry and antibody-mediated cell signaling in autoimmune myocarditis. J Immunol. 2006;177:8234–8240. doi: 10.4049/jimmunol.177.11.8234. [DOI] [PubMed] [Google Scholar]
- 17.Abe S, Okura Y, Hoyano M, et al Plasma concentrations of cytokines and neurohumoral factors in a case of fulminant myocarditis successfully treated with intravenous immunoglobulin and percutaneous cardiopulmonary support. Circ J. 2004;68:1223–1226. doi: 10.1253/circj.68.1223. [DOI] [PubMed] [Google Scholar]
- 18.Rose NR Learning from myocarditis: mimicry, chaos and black holes. F1000Prime Rep. 2014;6:25. doi: 10.12703/P6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JK, Zaidi SH, Liu P, et al A serine elastase inhibitor reduces inflammation and fibrosis and preserves cardiac function after experimentally-induced murine myocarditis. Nat Med. 1998;4:1383–1391. doi: 10.1038/3973. [DOI] [PubMed] [Google Scholar]
- 20.Jana S, Zhang H, Lopaschuk GD, et al Disparate remodeling of the extracellular matrix and proteoglycans in failing pediatric versus adult hearts. J Am Heart Assoc. 2018;7:e010427. doi: 10.1161/JAHA.118.010427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fung G, Luo H, Qiu Y, et al Myocarditis. Circ Res. 2016;118:496–514. doi: 10.1161/CIRCRESAHA.115.306573. [DOI] [PubMed] [Google Scholar]
- 22.Bratincsák A, El-Said HG, Bradley JS, et al Fulminant myocarditis associated with pandemic H1N1 influenza A virus in children. J Am Coll Cardiol. 2010;55:928–929. doi: 10.1016/j.jacc.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Veronese G, Cipriani M, Bottiroli M, et al Fulminant myocarditis triggered by OC43 subtype coronavirus: a disease deserving evidence-based care bundles. J Cardiovasc Med (Hagerstown) 2020;21:529–531. doi: 10.2459/JCM.0000000000000989. [DOI] [PubMed] [Google Scholar]
- 24.McCarthy RE 3rd, Boehmer JP, Hruban RH, et al Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N Engl J Med. 2000;342:690–695. doi: 10.1056/NEJM200003093421003. [DOI] [PubMed] [Google Scholar]
- 25.Mirić M, Vasiljević J, Bojić M, et al Long-term follow up of patients with dilated heart muscle disease treated with human leucocytic interferon alpha or thymic hormones initial results. Heart. 1996;75:596–601. doi: 10.1136/hrt.75.6.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winter MP, Sulzgruber P, Koller L, et al Immunomodulatory treatment for lymphocytic myocarditis: a systematic review and meta-analysis. Heart Fail Rev. 2018;23:573–581. doi: 10.1007/s10741-018-9709-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ammirati E, Cipriani M, Lilliu M, et al Survival and left ventricular function changes in fulminant versus nonfulminant acute myocarditis. Circulation. 2017;136:529–545. doi: 10.1161/CIRCULATIONAHA.117.026386. [DOI] [PubMed] [Google Scholar]
- 28.Turgeon PY, Massot M, Beaupré F, et al Effect of acute immunosuppression on left ventricular recovery and mortality in fulminant viral myocarditis: a case series and review of literature. CJC Open. 2020;3:292–302. doi: 10.1016/j.cjco.2020.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan HY, Yamada H, Chida J, et al Up-regulation of ectopic trypsins in the myocardium by influenza A virus infection triggers acute myocarditis. Cardiovasc Res. 2011;89:595–603. doi: 10.1093/cvr/cvq358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teijaro JR, Walsh KB, Cahalan S, et al Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011;146:980–991. doi: 10.1016/j.cell.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hékimian G, Jovanovic T, Bréchot N, et al When the heart gets the flu: fulminant influenza B myocarditis: a case-series report and review of the literature. J Crit Care. 2018;47:61–64. doi: 10.1016/j.jcrc.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Ukimura A, Ooi Y, Kanzaki Y, et al A national survey on myocarditis associated with influenza H1N1pdm 2009 in the pandemic and postpandemic season in Japan. J Infect Chemother. 2013;19:426–431. doi: 10.1007/s10156-012-0499-z. [DOI] [PubMed] [Google Scholar]
- 33.Ukimura A, Satomi H, Ooi Y, et al Myocarditis associated with influenza a H1N1pdm 2009. Influenza Res Treat. 2012;2012:351979. doi: 10.1155/2012/351979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Babapoor-Farrokhran S, Gill D, Walker J, et al Myocardial injury and COVID-19: possible mechanisms. Life Sci. 2020;253:117723. doi: 10.1016/j.lfs.2020.117723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akhmerov A, Marbán E COVID-19 and the heart. Circ Res. 2020;126:1443–1455. doi: 10.1161/CIRCRESAHA.120.317055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knowlton KU Pathogenesis of SARS-CoV-2 induced cardiac injury from the perspective of the virus. J Mol Cell Cardiol. 2020;147:12–17. doi: 10.1016/j.yjmcc.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindner D, Fitzek A, Bräuninger H, et al Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5:1281–1285. doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi S, Qin M, Shen B, et al Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dufort EM, Koumans EH, Chow EJ, et al Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Angus DC, Derde L, Al-Beidh F, et al Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020;324:1317–1329. doi: 10.1001/jama.2020.17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muthukumar A, Narasimhan M, Li QZ, et al In-depth evaluation of a case of presumed myocarditis after the second dose of COVID-19 mRNA vaccine. Circulation. 2021;144:487–498. doi: 10.1161/CIRCULATIONAHA.121.056038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosner CM, Genovese L, Tehrani BN, et al Myocarditis temporally associated with COVID-19 vaccination. Circulation. 2021;144:502–505. doi: 10.1161/CIRCULATIONAHA.121.055891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blauwet LA, Cooper LT Idiopathic giant cell myocarditis and cardiac sarcoidosis. Heart Fail Rev. 2013;18:733–746. doi: 10.1007/s10741-012-9358-3. [DOI] [PubMed] [Google Scholar]
- 44.Kandolin R, Lehtonen J, Kupari M Cardiac sarcoidosis and giant cell myocarditis as causes of atrioventricular block in young and middle-aged adults. Circ Arrhythm Electrophysiol. 2011;4:303–309. doi: 10.1161/CIRCEP.110.959254. [DOI] [PubMed] [Google Scholar]
- 45.Kandolin R, Lehtonen J, Salmenkivi K, et al Diagnosis, treatment, and outcome of giant-cell myocarditis in the era of combined immunosuppression. Circ Heart Fail. 2013;6:15–22. doi: 10.1161/CIRCHEARTFAILURE.112.969261. [DOI] [PubMed] [Google Scholar]
- 46.Caforio AL, Marcolongo R, Jahns R, et al Immune-mediated and autoimmune myocarditis: clinical presentation, diagnosis and management. Heart Fail Rev. 2013;18:715–732. doi: 10.1007/s10741-012-9364-5. [DOI] [PubMed] [Google Scholar]
- 47.Cooper LT, Baughman KL, Feldman AM, et al The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation. 2007;116:2216–2233. doi: 10.1161/CIRCULATIONAHA.107.186093. [DOI] [PubMed] [Google Scholar]
- 48.Montero S, Aissaoui N, Tadié JM, et al Fulminant giant-cell myocarditis on mechanical circulatory support: management and outcomes of a French multicentre cohort. Int J Cardiol. 2018;253:105–112. doi: 10.1016/j.ijcard.2017.10.053. [DOI] [PubMed] [Google Scholar]
- 49.Cooper LT Jr, Berry GJ, Shabetai R Idiopathic giant-cell myocarditis: natural history and treatment. Multicenter Giant Cell Myocarditis Study Group Investigators. N Engl J Med. 1997;336:1860–1866. doi: 10.1056/NEJM199706263362603. [DOI] [PubMed] [Google Scholar]
- 50.Cooper LT Jr, Hare JM, Tazelaar HD, et al Usefulness of immunosuppression for giant cell myocarditis. Am J Cardiol. 2008;102:1535–1539. doi: 10.1016/j.amjcard.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ekström K, Lehtonen J, Kandolin R, et al Long-term outcome and its predictors in giant cell myocarditis. Eur J Heart Fail. 2016;18:1452–1458. doi: 10.1002/ejhf.606. [DOI] [PubMed] [Google Scholar]
- 52.Maleszewski JJ, Orellana VM, Hodge DO, et al Long-term risk of recurrence, morbidity and mortality in giant cell myocarditis. Am J Cardiol. 2015;115:1733–1738. doi: 10.1016/j.amjcard.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 53.Senderek T, Malecka B, Ząbek A, et al Fulminant heart failure due to giant cell myocarditis affecting the left ventricle. Postepy Kardiol Interwencyjnej. 2015;11:351–353. doi: 10.5114/pwki.2015.55613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suarez-Barrientos A, Wong J, Bell A, et al Usefulness of rabbit anti-thymocyte globulin in patients with giant cell myocarditis. Am J Cardiol. 2015;116:447–451. doi: 10.1016/j.amjcard.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 55.Veronese G, Ammirati E, Cipriani M, et al Fulminant myocarditis: characteristics, treatment, and outcomes. Anatol J Cardiol. 2018;19:279–286. doi: 10.14744/AnatolJCardiol.2017.8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brambatti M, Matassini MV, Adler ED, et al Eosinophilic myocarditis: characteristics, treatment, and outcomes. J Am Coll Cardiol. 2017;70:2363–2375. doi: 10.1016/j.jacc.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 57.Morimoto S, Kubo N, Hiramitsu S, et al Changes in the peripheral eosinophil count in patients with acute eosinophilic myocarditis. Heart Vessels. 2003;18:193–196. doi: 10.1007/s00380-003-0721-0. [DOI] [PubMed] [Google Scholar]
- 58.Sohn IS, Park JC, Chung JH, et al A case of acute eosinophilic myopericarditis presenting with cardiogenic shock and normal peripheral eosinophil count. Korean J Intern Med. 2006;21:136–140. doi: 10.3904/kjim.2006.21.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferreira VM, Schulz-Menger J, Holmvang G, et al Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 60.Ammirati E, Stucchi M, Brambatti M, et al Eosinophilic myocarditis: a paraneoplastic event. Lancet. 2015;385:2546. doi: 10.1016/S0140-6736(15)60903-5. [DOI] [PubMed] [Google Scholar]
- 61.Kontani M, Takashima S, Okura K, et al Survival after acute necrotizing eosinophilic myocarditis complicating a massive left ventricular mural thrombus: a case report. J Cardiol. 2007;50:127–133. [PubMed] [Google Scholar]
- 62.Cooper LT, Zehr KJ Biventricular assist device placement and immunosuppression as therapy for necrotizing eosinophilic myocarditis. Nat Clin Pract Cardiovasc Med. 2005;2:544–548. doi: 10.1038/ncpcardio0322. [DOI] [PubMed] [Google Scholar]
- 63.Howell E, Paivanas N, Stern J, et al Treatment of acute necrotizing eosinophilic myocarditis with immunosuppression and mechanical circulatory support. Circ Heart Fail. 2016;9:e003665. doi: 10.1161/CIRCHEARTFAILURE.116.0036. [DOI] [PubMed] [Google Scholar]
- 64.Johnson DB, Balko JM, Compton ML, et al Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375:1749–1755. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mahmood SS, Fradley MG, Cohen JV, et al Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71:1755–1764. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moslehi JJ, Salem JE, Sosman JA, et al Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391:933. doi: 10.1016/S0140-6736(18)30533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salem JE, Manouchehri A, Moey M, et al Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–1589. doi: 10.1016/S1470-2045(18)30608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Caforio AL, Pankuweit S, Arbustini E, et al Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648, 2648a–2648d. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 69.Shauer A, Gotsman I, Keren A, et al Acute viral myocarditis: current concepts in diagnosis and treatment. Isr Med Assoc J. 2013;15:180–185. doi: 10.5152/balkanmedj.2012.115. [DOI] [PubMed] [Google Scholar]
- 70.Younis A, Matetzky S, Mulla W, et al Epidemiology characteristics and outcome of patients with clinically diagnosed acute myocarditis. Am J Med. 2020;133:492–499. doi: 10.1016/j.amjmed.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 71.Gilotra NA, Minkove N, Bennett MK, et al Lack of relationship between serum cardiac troponin I level and giant cell myocarditis diagnosis and outcomes. J Card Fail. 2016;22:583–585. doi: 10.1016/j.cardfail.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 72.Matsumoto M, Asaumi Y, Nakamura Y, et al Clinical determinants of successful weaning from extracorporeal membrane oxygenation in patients with fulminant myocarditis. ESC Heart Fail. 2018;5:675–684. doi: 10.1002/ehf2.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ammirati E, Veronese G, Cipriani M, et al Acute and fulminant myocarditis: a pragmatic clinical approach to diagnosis and treatment. Curr Cardiol Rep. 2018;20:114. doi: 10.1007/s11886-018-1054-z. [DOI] [PubMed] [Google Scholar]
- 74.Blanco-Domínguez R, Sánchez-Díaz R, de la Fuente H, et al A novel circulating microRNA for the detection of acute myocarditis. N Engl J Med. 2021;384:2014–2027. doi: 10.1056/NEJMoa2003608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Felker GM, Boehmer JP, Hruban RH, et al Echocardiographic findings in fulminant and acute myocarditis. J Am Coll Cardiol. 2000;36:227–232. doi: 10.1016/S0735-1097(00)00690-2. [DOI] [PubMed] [Google Scholar]
- 76.Gräni C, Eichhorn C, Bière L, et al Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J Am Coll Cardiol. 2017;70:1964–1976. doi: 10.1016/j.jacc.2017.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pasupathy S, Beltrame JF Refining the role of CMR imaging in MINOCA. JACC Cardiovasc Imaging. 2021;14:1784–1786. doi: 10.1016/j.jcmg.2021.03.024. [DOI] [PubMed] [Google Scholar]
- 78.Birnie DH, Nery PB, Ha AC, et al Cardiac sarcoidosis. J Am Coll Cardiol. 2016;68:411–421. doi: 10.1016/j.jacc.2016.03.605. [DOI] [PubMed] [Google Scholar]
- 79.Aquaro GD, Ghebru Habtemicael Y, Camastra G, et al Prognostic value of repeating cardiac magnetic resonance in patients with acute myocarditis. J Am Coll Cardiol. 2019;74:2439–2448. doi: 10.1016/j.jacc.2019.08.1061. [DOI] [PubMed] [Google Scholar]
- 80.Bozkurt B, Colvin M, Cook J, et al Current diagnostic and treatment strategies for specific dilated cardiomyopathies: a scientific statement from the American Heart Association. Circulation. 2016;134:e579–e646. doi: 10.1161/CIR.0000000000000455. [DOI] [PubMed] [Google Scholar]
- 81.Singh V, Mendirichaga R, Savani GT, et al Comparison of utilization trends, indications, and complications of endomyocardial biopsy in native versus donor hearts (from the Nationwide Inpatient Sample 2002 to 2014) Am J Cardiol. 2018;121:356–363. doi: 10.1016/j.amjcard.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 82.Bennett MK, Gilotra NA, Harrington C, et al Evaluation of the role of endomyocardial biopsy in 851 patients with unexplained heart failure from 2000–2009. Circ Heart Fail. 2013;6:676–684. doi: 10.1161/CIRCHEARTFAILURE.112.000087. [DOI] [PubMed] [Google Scholar]
- 83.Thiagarajan RR, Barbaro RP, Rycus PT, et al Extracorporeal Life Support Organization registry international report 2016. ASAIO J. 2017;63:60–67. doi: 10.1097/MAT.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 84.Shah M, Patnaik S, Patel B, et al Trends in mechanical circulatory support use and hospital mortality among patients with acute myocardial infarction and non-infarction related cardiogenic shock in the United States. Clin Res Cardiol. 2018;107:287–303. doi: 10.1007/s00392-017-1182-2. [DOI] [PubMed] [Google Scholar]
- 85.Combes A, Leprince P, Luyt CE, et al Outcomes and long-term quality-of-life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit Care Med. 2008;36:1404–1411. doi: 10.1097/CCM.0b013e31816f7cf7. [DOI] [PubMed] [Google Scholar]
- 86.Diddle JW, Almodovar MC, Rajagopal SK, et al Extracorporeal membrane oxygenation for the support of adults with acute myocarditis. Crit Care Med. 2015;43:1016–1025. doi: 10.1097/CCM.0000000000000920. [DOI] [PubMed] [Google Scholar]
- 87.Choi KH, Yang JH, Hong D, et al Optimal timing of venoarterial-extracorporeal membrane oxygenation in acute myocardial infarction patients suffering from refractory cardiogenic shock. Circ J. 2020;84:1502–1510. doi: 10.1253/circj.CJ-20-0259. [DOI] [PubMed] [Google Scholar]
- 88.Lee HH, Kim HC, Ahn CM, et al Association between timing of extracorporeal membrane oxygenation and clinical outcomes in refractory cardiogenic shock. JACC Cardiovasc Interv. 2021;14:1109–1119. doi: 10.1016/j.jcin.2021.03.048. [DOI] [PubMed] [Google Scholar]
- 89.Cheng R, Hachamovitch R, Kittleson M, et al Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1, 866 adult patients. Ann Thorac Surg. 2014;97:610–616. doi: 10.1016/j.athoracsur.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 90.Donker DW, Brodie D, Henriques JPS, et al Left ventricular unloading during veno-arterial ECMO: a simulation study. ASAIO J. 2019;65:11–20. doi: 10.1097/MAT.0000000000000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Meani P, Gelsomino S, Natour E, et al Modalities and effects of left ventricle unloading on extracorporeal life support: a review of the current literature. Eur J Heart Fail. 2017;19:84–91. doi: 10.1002/ejhf.850. [DOI] [PubMed] [Google Scholar]
- 92.Russo JJ, Aleksova N, Pitcher I, et al Left ventricular unloading during extracorporeal membrane oxygenation in patients with cardiogenic shock. J Am Coll Cardiol. 2019;73:654–662. doi: 10.1016/j.jacc.2018.10.085. [DOI] [PubMed] [Google Scholar]
- 93.Schrage B, Becher PM, Bernhardt A, et al Left ventricular unloading is associated with lower mortality in patients with cardiogenic shock treated with venoarterial extracorporeal membrane oxygenation: results from an international, multicenter cohort study. Circulation. 2020;142:2095–2106. doi: 10.1161/CIRCULATIONAHA.120.048792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tschöpe C, Van Linthout S, Klein O, et al Mechanical unloading by fulminant myocarditis: LV-IMPELLA, ECMELLA, BI-PELLA, and PROPELLA concepts. J Cardiovasc Transl Res. 2019;12:116–123. doi: 10.1007/s12265-018-9820-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Griveas I, Sourgounis A, Visvardis G, et al Immunoadsorption in lupus myocarditis. Ther Apher Dial. 2004;8:281–285. doi: 10.1111/j.1526-0968.2004.00165.x. [DOI] [PubMed] [Google Scholar]
- 96.Pagnoux C Plasma exchange for systemic lupus erythematosus. Transfus Apher Sci. 2007;36:187–193. doi: 10.1016/j.transci.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 97.Xing ZX, Yu K, Yang H, et al Successful use of plasma exchange in fulminant lupus myocarditis coexisting with pneumonia: a case report. World J Clin Cases. 2020;8:2056–2065. doi: 10.12998/wjcc.v8.i10.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mason JW, O’Connell JB, Herskowitz A, et al A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators. N Engl J Med. 1995;333:269–275. doi: 10.1056/NEJM199508033330501. [DOI] [PubMed] [Google Scholar]
- 99.Veronese G, Ammirati E, Chen C, et al Management perspectives from the 2019 Wuhan international workshop on fulminant myocarditis. Int J Cardiol. 2021;324:131–138. doi: 10.1016/j.ijcard.2020.10.063. [DOI] [PubMed] [Google Scholar]
- 100.Carroll BJ, Shah RV, Murthy V, et al Clinical features and outcomes in adults with cardiogenic shock supported by extracorporeal membrane oxygenation. Am J Cardiol. 2015;116:1624–1630. doi: 10.1016/j.amjcard.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 101.Kawahito K, Murata S, Yasu T, et al Usefulness of extracorporeal membrane oxygenation for treatment of fulminant myocarditis and circulatory collapse. Am J Cardiol. 1998;82:910–911. doi: 10.1016/S0002-9149(98)00503-7. [DOI] [PubMed] [Google Scholar]
- 102.Pages ON, Aubert S, Combes A, et al Paracorporeal pulsatile biventricular assist device versus extracorporal membrane oxygenation-extracorporal life support in adult fulminant myocarditis. J Thorac Cardiovasc Surg. 2009;137:194–197. doi: 10.1016/j.jtcvs.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 103.Wu MY, Lee MY, Lin CC, et al Resuscitation of non-postcardiotomy cardiogenic shock or cardiac arrest with extracorporeal life support: the role of bridging to intervention. Resuscitation. 2012;83:976–981. doi: 10.1016/j.resuscitation.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 104.Patel PM, Saxena A, Wood CT, et al Outcomes of mechanical circulatory support for giant cell myocarditis: a systematic review. J Clin Med. 2020;9:3905. doi: 10.3390/jcm9123905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vaidya GN, Czer LSC, Luthringer D, et al Heart transplantation for giant cell myocarditis: a case series. Transplant Proc. 2021;53:348–352. doi: 10.1016/j.transproceed.2020.10.047. [DOI] [PubMed] [Google Scholar]
- 106.Kraft L, Erdenesukh T, Sauter M, et al Blocking the IL-1β signalling pathway prevents chronic viral myocarditis and cardiac remodeling. Basic Res Cardiol. 2019;114:11. doi: 10.1007/s00395-019-0719-0. [DOI] [PubMed] [Google Scholar]
- 107.Tschöpe C, Ammirati E, Bozkurt B, et al Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. 2021;18:169–193. doi: 10.1038/s41569-020-00435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Van Linthout S, Savvatis K, Miteva K, et al Mesenchymal stem cells improve murine acute coxsackievirus B3-induced myocarditis. Eur Heart J. 2011;32:2168–2178. doi: 10.1093/eurheartj/ehq467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Combes A, Price S, Slutsky AS, et al Temporary circulatory support for cardiogenic shock. Lancet. 2020;396:199–212. doi: 10.1016/S0140-6736(20)31047-3. [DOI] [PubMed] [Google Scholar]
- 110.Danial P, Hajage D, Nguyen LS, et al Percutaneous versus surgical femoro-femoral veno-arterial ECMO: a propensity score matched study. Intensive Care Med. 2018;44:2153–2161. doi: 10.1007/s00134-018-5442-z. [DOI] [PubMed] [Google Scholar]
- 111.Martín Badía I, Pagliarani Gil P, Pérez Vela JL, et al Awake VA-ECMO in cardiogenic shock: an experience with future potential. Rev Esp Cardiol (Engl Ed) 2020;73:851–853. doi: 10.1016/j.recesp.2020.02.012. [DOI] [PubMed] [Google Scholar]
- 112.Montero S, Huang F, Rivas-Lasarte M, et al Awake venoarterial extracorporeal membrane oxygenation for refractory cardiogenic shock. Eur Heart J Acute Cardiovasc Care. 2021;10:585–594. doi: 10.1093/ehjacc/zuab018. [DOI] [PubMed] [Google Scholar]
- 113.Trachtenberg BH, Hare JM Inflammatory cardiomyopathic syndromes. Circ Res. 2017;121:803–818. doi: 10.1161/CIRCRESAHA.117.310221. [DOI] [PubMed] [Google Scholar]
- 114.Aoyama N, Izumi T, Hiramori K, et al National survey of fulminant myocarditis in Japan: therapeutic guidelines and long-term prognosis of using percutaneous cardiopulmonary support for fulminant myocarditis (special report from a scientific committee) Circ J. 2002;66:133–144. doi: 10.1253/circj.66.133. [DOI] [PubMed] [Google Scholar]
- 115.Mirabel M, Luyt CE, Leprince P, et al Outcomes, long-term quality of life, and psychologic assessment of fulminant myocarditis patients rescued by mechanical circulatory support. Crit Care Med. 2011;39:1029–1035. doi: 10.1097/CCM.0b013e31820ead45. [DOI] [PubMed] [Google Scholar]
- 116.Ishida K, Wada H, Sakakura K, et al Long-term follow-up on cardiac function following fulminant myocarditis requiring percutaneous extracorporeal cardiopulmonary support. Heart Vessels. 2013;28:86–90. doi: 10.1007/s00380-011-0211-8. [DOI] [PubMed] [Google Scholar]
- 117.Asaumi Y, Yasuda S, Morii I, et al Favourable clinical outcome in patients with cardiogenic shock due to fulminant myocarditis supported by percutaneous extracorporeal membrane oxygenation. Eur Heart J. 2005;26:2185–2192. doi: 10.1093/eurheartj/ehi411. [DOI] [PubMed] [Google Scholar]
- 118.Beurtheret S, Mordant P, Paoletti X, et al Emergency circulatory support in refractory cardiogenic shock patients in remote institutions: a pilot study (the cardiac-RESCUE program) Eur Heart J. 2013;34:112–120. doi: 10.1093/eurheartj/ehs081. [DOI] [PubMed] [Google Scholar]
- 119.Annamalai SK, Esposito ML, Jorde L, et al The Impella microaxial flow catheter is safe and effective for treatment of myocarditis complicated by cardiogenic shock: an analysis from the Global cVAD registry. J Card Fail. 2018;24:706–710. doi: 10.1016/j.cardfail.2018.09.007. [DOI] [PubMed] [Google Scholar]