Abstract
Cardiogenic shock (CS) following acute myocardial infarction (AMI) is a major challenge in cardiovascular care. Mortality remains high with 40%−50% after thirty days. Randomised controlled trials (RCTs) play a key role to generate evidence on optimal care in this field. However, the number of completed or ongoing RCTs is still relatively low compared to the gaps in evidence. Challenges in the conduct of these trials are in particular the selection of patients and ethical issues in the informed consent process. When determining eligibility criteria, special attention should be paid to the severity of CS, to the inclusion of patients with cardiac arrest and to potential age limits. Median age of AMI-CS patients is increasing. Age limits are therefore controversial as it is important to include elderly patients in RCTs in order to make the results generalisable and to address the special needs of this group. As patients with AMI-CS are in most cases unable to provide informed consent themselves, a step-wise approach with acute consent by a legal representative or independent physicians and later informed consent by the patient if possible might be established depending on regularities of the respective ethical review board and country legislation. Multicenter studies should be sought to generate adequate power.
Cardiogenic shock (CS) following acute myocardial infarction (AMI) remains a major challenge in cardiovascular care. Between 5% and 13% of patients with AMI develop CS, resulting in 60,000 to 70,000 patients being affected each year in Europe.[1–4] Despite major advances in interventional and intensive care treatment, mortality of AMI-CS remains high, reaching 40%−50% at thirty days after hospital admission.[5,6] Short-term mortality of elderly patients with AMI-CS is particularly high with up to 79% in patients aged ≥ 75 years.[7] Clinical trials, especially in randomised designs, play a key role to a better understanding and treatment guidance in AMI-CS.
RANDOMISED CONTROLLED TRIALS IN AMI-CS
Although there are numerous randomised controlled trials (RCTs) in the field of acute cardiovascular care and especially AMI, patients with CS were generally excluded from the vast majorities of these studies. The landmark SHOCK (Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock) trial generated the first large randomised data in the field of AMI-CS.[8] Including a total of 302 patients, the study showed a significant medium- and long-term survival benefit of early revascularization in patients with ST-segment elevation myocardial infarction (STEMI) and CS. Many subanalyses were derived from the dataset.
Several RCTs followed, rarely exceeding a study population of 30−40 patients. Among the larger trials were the TRIUMPH (Tilarginine Acetate Injection in a Randomized International Study in Unstable Myocardial Infarction Patients With Cardiogenic Shock) trial (n = 398), showing no benefit of treatment with the nitric oxide synthase inhibitor tilarginine,[9] the IABP-SHOCK II (Intraaortic Balloon Pump in Cardiogenic Shock II) trial (n = 600), demonstrating no clinically beneficial effect of routine intraaortic balloon pumping use,[5] and the CULPRIT-SHOCK (Culprit Lesion Only PCI Versus Multivessel PCI in Cardiogenic Shock) trial (n = 706), showing superiority of culprit lesion only percutaneous coronary intervention in multivessel disease compared to an immediate multivessel percutaneous coronary intervention approach.[6] These studies resulted in substantial changes in American and European guidelines over time.[10–12]
However, the amount of evidence derived from RCTs in AMI-CS during the past decades cannot be considered satisfactory and is even more insufficient in elderly patients as those were often excluded. For this reason, the American Heart Association refrained from issuing guidelines on the treatment of CS, but decided to publish a scientific statement including a chapter highlighting the need for research.[13] According to this, current main research questions for RCTs in AMI-CS are listed in Table 1.
Table 1. Research needs in acute myocardial infarction complicated by cardiogenic shock which should be addressed by randomised controlled trials*.
| Therapeutic target parameters (e.g., blood pressure, cardiac index, PaO2, PaCO2, body temperature) |
| *Adopted from van Diepen, et al.[13] |
| Optimal fluid management |
| Optimal vasopressor and inotropic regimens |
| Antiplatelet and anticoagulant therapy |
| Access site for invasive angiography and percutaneous coronary intervention |
| Complication prevention in mechanical circulatory support |
| Utility and timing of percutaneous or durable mechanical support devices |
| Optimal mechanical ventilation modes and targets |
| Early versus late discussion of palliative care |
CHALLENGES WHEN CONDUCTING RCTS IN AMI-CS
In 2015, van Diepen, et al.[14] analyzed the proportion of RCTs in cardiovascular intensive care to RCTs in intensive care overall: only about 5% of the registered studies covered cardiovascular care. Further the study showed exemplary for Canada, that only 2% of intensive care studies funded by the Canadian Institutes for Health Research were cardiac.
What Are The Challenges of RCTs in AMI-CS?
First, these come with challenges of RCTs in general, which include funding, design and securing of sufficient recruitment rates. Specifically in AMI-CS, challenges involve: (1) the complexity and heterogeneity of patients together with an often necessary multicentre approach to generate adequate power; and (2) the frequent incapability of patients to provide informed consent.
Challenges in design
Patient selection.
When designing RCTs in AMI-CS, there is tension between the applicability to a large proportion of real-world patients in order to fill gaps in evidence-based care and the heterogeneity of these patients.
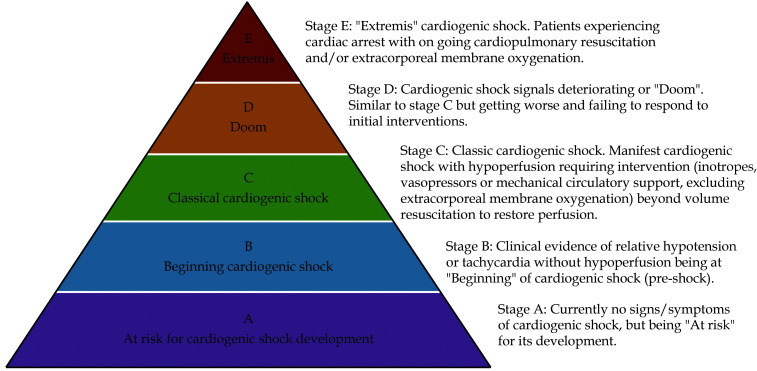
A major source of heterogeneity comes from the severity of CS. The classification by the Society for Cardiovascular Angiography and Interventions may help to illustrate the different stages of CS (Figure 1). Individual systemic response, extent of intensive care treatment and outcome reasonably differ between the stages.[15,16]
Figure 1.
Cardiogenic shock pyramid according to the classification proposed by the Society for Cardiovascular Angiography and Interventions.
By permission of Oxford University Press on behalf of the European Society of Cardiology.
Patient selection is therefore a key design issue. With regard to trials with highly invasive study interventions such as the use of mechanical circulatory support systems, the inclusion of patients in lower shock stages might not be reasonable as potential benefits outweigh the risks of the procedure. Conversely, the inclusion of patients in very severe shock stages with the need for resuscitation in trials on physiologic target parameters (e.g., blood pressure) may not be reasonable.
A further challenge in patient selection is age restriction. The median age of AMI-CS patients is about 69 years,[6,15] with a likely increase within the upcoming years as a result of demographic changes. Patients aged ≥ 75 years are significantly more likely to be affected by AMI than younger patients (15%−25% of STEMI admissions), even though this age group represents less than 10% of the total population.[7] Nevertheless, old or very old patients were often excluded from RCTs, either by eligibility criteria or in practice by the individual investigators. This was partly justified by the difficulty to objectify a possible multimorbidity, as well as the (presumed) will of the patient and the individual prognosis. However, there is an ongoing need to include these patients into RCTs. If necessary, dedicated studies should be designed to address the needs for this subset of patients. An exception, when age restriction is reasonable, can be highly invasive study interventions, such as the use of extracorporeal life support. In this setting, the extent of age limits is being extensively discussed, since retrospective studies have shown that mortality in case of extracorporeal life support therapy in old patients approaches 95%.[17]
Another special group of interest when designing RCTs are patients with cardiac arrest (CA). Up to 50% of AMI-CS patients experience CA.[5,6] At the same time, two thirds of CA patients experience CS with the need for vasopressor therapy.[18] Outcome of AMI-CS with CA is significantly worse compared to those without CA.[19] The prognosis of patients with CA is often determined by the extent of anoxic brain injury. This aspect should be considered in the early phase of trial planning as the outcome of CA patients with relevant anoxic brain injury will likely oftentimes not be dependent on the study intervention. This might partly explain neutral trial results.[20] During analyses the dichotomization into the presence or absence of CA might also be not sufficient, as patients with CA differ significantly, especially when accounting for the duration of no-flow/low-flow time and the extent of post-CA syndrome including multi-organ failure.
Primary endpoint definition.
The choice of the primary endpoint is crucial, as the results should lead to recommendations in clinical practice or for future trials. However, the target sample size is inversely proportional to the expected difference in the primary endpoint. Primary endpoints such as mortality are desirable, but may not be useful if the recruitment rate in the planned time window is not realistic according to the case number calculation. Combined or surrogate endpoints might be considered in this case. In combined endpoints, it is desirable that the primary outcome is not strongly driven by the ‘weakest’ endpoint component.[21] As an alternative, pilot phases of trials can be implemented. Sample size calculation also depends on the choice for a superiority or non-inferiority design.[22]
Multicenter design.
For hard clinical endpoints, a multicenter design is almost always necessary in AMI-CS to ensure sufficient recruitment. However, the organizational and financial effort should not be underestimated. Further, the recruitment effort is then distributed among many investigators and needs often continuous encouragement. This can be promoted by a pragmatic design where possible and a manageable scope of documentation. It is important that the potential study centres have sufficient experience in the treatment of CS patients, as this is known to be a significant predictor of outcome.[23,24]
Internal and external validity.
In all RCTs, internal validity should be maximized by keeping biases to a minimum. However, particularly in AMI-CS RCTs, blinding of investigators is often not possible due to the nature of the respective study intervention. Attrition bias should be diminished by ensuring seamless follow-up of the individual patient during the whole study period. The choice to perform the analysis between groups as intention-to-treat or per-protocol has impact on internal validity, but also on applicability of the trial results in clinical routine.
The lack of generalisability (also external validity) is often a major point of criticism in RCTs.[21,25] Next to the choice of statistical analyses, eligibility criteria has significant impact on generalisability, as mentioned above. Analyses with retrospective or prospective application of eligibility criteria on registry all-comers data can give impressions on the proportion of AMI-CS patients covered before planning RCTs. Multicentre design, including different health-care systems might enhance generalisability. Surrogate outcomes should be used with caution, as the correlation with clinical outcomes might be overestimated and external validity can be diminished.[21]
Challenges in informed consent
Approximately 80%–90% of AMI-CS patients present comatose/sedated or with impaired mental status, impeding the capability to provide informed consent.[6,26] Studies showed that even in fully oriented patients on intensive care units only 30% were able to recall their given consent and the purpose of the trial 10−12 days after study inclusion.[27] Providing consent is therefore a complex ethical issue in this field, enhanced by the acuity of potential study interventions.[28]
Ethical regularities vary significantly between countries and institutional review boards. Some countries do not allow the inclusion of non-consenting patients at all. This has to be kept in mind in the setting of international multicenter RCTs. In other countries, each study centre or region has its own ethical review board, which is entrusted with the individual review of the studies. This might result into a large number of differing consent forms within one trial. When the inclusion of non-conscious patients is possible, a step-wise consent model is often used. This includes an initial consent by either a legal representative or independent physicians and a subsequent informed consent given by the patient as soon as he gains the capability to do so.
CONCLUSIONS
RCTs in AMI-CS are feasible and the number of adequately powered trials is increasing. However, the need for trials currently exceeds the planned and ongoing RCTs. The challenges of conducting these RCTs are manifold. In particular, patient selection and ethical issues pose special challenges.
ACKNOWLEDGMENTS
All authors had no conflicts of interest to disclose.
References
- 1.Aissaoui N, Puymirat E, Tabone X, et al Improved outcome of cardiogenic shock at the acute stage of myocardial infarction: a report from the USIK 1995, USIC 2000, and FAST-MI French nationwide registries. Eur Heart J. 2012;33:2535–2543. doi: 10.1093/eurheartj/ehs264. [DOI] [PubMed] [Google Scholar]
- 2.Jeger RV, Radovanovic D, Hunziker PR, et al Ten-year trends in the incidence and treatment of cardiogenic shock. Ann Intern Med. 2008;149:618–626. doi: 10.7326/0003-4819-149-9-200811040-00005. [DOI] [PubMed] [Google Scholar]
- 3.Rathod KS, Koganti S, Iqbal MB, et al Contemporary trends in cardiogenic shock: incidence, intra-aortic balloon pump utilisation and outcomes from the London Heart Attack Group. Eur Heart J Acute Cardiovasc Care. 2018;7:16–27. doi: 10.1177/2048872617741735. [DOI] [PubMed] [Google Scholar]
- 4.Thiele H, Allam B, Chatellier G, et al Shock in acute myocardial infarction: the Cape Horn for trials? Eur Heart J. 2010;31:1828–1835. doi: 10.1093/eurheartj/ehq220. [DOI] [PubMed] [Google Scholar]
- 5.Thiele H, Zeymer U, Neumann FJ, et al Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287–1296. doi: 10.1056/NEJMoa1208410. [DOI] [PubMed] [Google Scholar]
- 6.Thiele H, Akin I, Sandri M, et al PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017;377:2419–2432. doi: 10.1056/NEJMoa1710261. [DOI] [PubMed] [Google Scholar]
- 7.Alexander KP, Newby LK, Armstrong PW, et al Acute coronary care in the elderly, part II: ST-segment elevation myocardial infarction: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115:2570–2589. doi: 10.1161/CIRCULATIONAHA.107.182616. [DOI] [PubMed] [Google Scholar]
- 8.Hochman JS, Sleeper LA, Webb JG, et al Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med. 1999;341:625–634. doi: 10.1056/NEJM199908263410901. [DOI] [PubMed] [Google Scholar]
- 9.Alexander JH, Reynolds HR, Stebbins AL, et al Effect of tilarginine acetate in patients with acute myocardial infarction and cardiogenic shock: the TRIUMPH randomized controlled trial. JAMA. 2007;297:1657–1666. doi: 10.1001/jama.297.15.joc70035. [DOI] [PubMed] [Google Scholar]
- 10.O’Gara PT, Kushner FG, Ascheim DD, et al 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–e140. doi: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 11.Ibanez B, James S, Agewall S, et al 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 12.Collet JP, Thiele H, Barbato E, et al 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 13.van Diepen S, Katz JN, Albert NM, et al Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136:e232–e268. doi: 10.1161/CIRCULATIONAHA.117.029532. [DOI] [PubMed] [Google Scholar]
- 14.van Diepen S, Granger CB, Jacka M, et al The unmet need for addressing cardiac issues in intensive care research. Crit Care Med. 2015;43:128–134. doi: 10.1097/CCM.0000000000000609. [DOI] [PubMed] [Google Scholar]
- 15.Jentzer JC, van Diepen S, Barsness GW, et al Cardiogenic shock classification to predict mortality in the cardiac intensive care unit. J Am Coll Cardiol. 2019;74:2117–2128. doi: 10.1016/j.jacc.2019.07.077. [DOI] [PubMed] [Google Scholar]
- 16.Jentzer JC Understanding cardiogenic shock severity and mortality risk assessment. Circ Heart Fail. 2020;13:e007568. doi: 10.1161/CIRCHEARTFAILURE.120.007568. [DOI] [PubMed] [Google Scholar]
- 17.de Waha S, Graf T, Desch S, et al Outcome of elderly undergoing extracorporeal life support in refractory cardiogenic shock. Clin Res Cardiol. 2017;106:379–385. doi: 10.1007/s00392-016-1068-8. [DOI] [PubMed] [Google Scholar]
- 18.Callaway CW, Donnino MW, Fink EL, et al Part 8: Post-cardiac arrest care: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132:S465–S482. doi: 10.1161/CIR.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Omer MA, Tyler JM, Henry TD, et al Clinical characteristics and outcomes of STEMI patients with cardiogenic shock and cardiac arrest. JACC Cardiovasc Interv. 2020;13:1211–1219. doi: 10.1016/j.jcin.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Jentzer JC, van Diepen S, Henry TD Understanding how cardiac arrest complicates the analysis of clinical trials of cardiogenic shock. Circ Cardiovasc Qual Outcomes. 2020;13:e006692. doi: 10.1161/CIRCOUTCOMES.120.006692. [DOI] [PubMed] [Google Scholar]
- 21.Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?” Lancet 2005; 365: 82–93.
- 22.Zhong B How to calculate sample size in randomized controlled trial? J Thorac Dis. 2009;1:51–54. doi: 10.3978/j.issn.2072-1439.2009.12.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaefi S, O’Gara B, Kociol RD, et al Effect of cardiogenic shock hospital volume on mortality in patients with cardiogenic shock. J Am Heart Assoc. 2015;4:e001462. doi: 10.1161/jaha.114.001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vallabhajosyula S, Dunlay SM, Barsness GW, et al Hospital-level disparities in the outcomes of acute myocardial infarction with cardiogenic shock. Am J Cardiol. 2019;124:491–498. doi: 10.1016/j.amjcard.2019.05.038. [DOI] [PubMed] [Google Scholar]
- 25.Feinstein AR, Horwitz RI Problems in the “evidence” of “evidence-based medicine”. Am J Med. 1997;103:529–535. doi: 10.1016/S0002-9343(97)00244-1. [DOI] [PubMed] [Google Scholar]
- 26.Burns KE, Zubrinich C, Tan W, et al Research recruitment practices and critically ill patients. A multicenter, cross-sectional study (the Consent Study) Am J Respir Crit Care Med. 2013;187:1212–1218. doi: 10.1164/rccm.201208-1537OC. [DOI] [PubMed] [Google Scholar]
- 27.Chenaud C, Merlani P, Ricou B Informed consent for research in ICU obtained before ICU admission. Intensive Care Med. 2006;32:439–444. doi: 10.1007/s00134-005-0059-4. [DOI] [PubMed] [Google Scholar]
- 28.Ecarnot F, Quenot JP, Besch G, et al Ethical challenges involved in obtaining consent for research from patients hospitalized in the intensive care unit. Ann Transl Med. 2017;5:S41. doi: 10.21037/atm.2017.04.42. [DOI] [PMC free article] [PubMed] [Google Scholar]