Abstract
SARS-CoV-2 has exhibited varying pathogenesis in a variety of Mammalia family’s including Canidae, Mustelidae, Hominidae, Cervidae, Hyaenidae, and Felidae. Novel SARS-CoV-2 variants characterized by spike protein mutations have recently resulted in clinical and epidemiological concerns, as they potentially have increased infectious rates, increased transmission, or reduced neutralization by antibodies produced via vaccination. Many variants have been identified at this time, but the variant of continuing concern has been the Delta variant (B.1.617.2), due to its increased transmissibility and infectious rate. Felines vaccinated using an experimental SARS-CoV-2 spike protein-based veterinary vaccine mounted a robust immune response to the SARS-CoV-2 spike protein. Using a reporter virus particle system and feline serum, we have verified that vaccinated felines produce antibodies that neutralize the SARS-CoV-2 Wuhan strain and variant B.1.617.2 at comparable levels.
Keywords: SARS-CoV-2, Vaccine, Delta, Delta variant, Serum neutralization, Cat vaccine, Zoetis, Veterinary vaccine, Mink vaccine, Mink, Canine, Canine vaccine, B.1.617.2, Serum Neutralization
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the etiologic agent of coronavirus disease (COVID-19) and has been spreading globally, infecting both humans and diverse species of animals (Rabalski et al., 2021). One theory suggests the initial host of SARS-CoV-2 to be bats (Zhou et al., 2020); but cats, dogs, white-tailed deer, and mink have been proposed as other intermediate hosts or potential reservoirs (Mallapaty, 2021, Rabalski et al., 2021). Human to cat, and cat to cat transmission has been confirmed in a lab setting and in households where individuals have contracted COVID-19 (Jara et al., 2021). Since cats have been shown to infect other cats, there may be some risk of human transmission (McAloose et al., 2020). Human to dog transmission is well documented, but they often display mild symptoms, possibly accompanied with mild viral shedding (Hamer et al., 2020) when infected with the Wuhan strain. Animals belonging to the Mustelid family, specifically mink, have been shown to transmit SARS-CoV-2 to cats, dogs, and humans (Konishi, 2021, van Aart et al., 2021). All these species, and others, have shown typical symptomology and tested positive for SARS-CoV-2 RNA (Bonilla-Aldana et al., 2021). Infection with the SARS-CoV-2 variant viruses appear to induce typical symptomology which include neural and diabetic symptoms similar to what is observed in humans but increased severity (Ferasin et al., 2021, Yilmaz Celebi et al., 2022). The existence of reservoirs and cross-species transmission poses great epidemiological concern as this can result in higher rates of mutation leading to novel variants which could contribute to vaccine breakthrough infections.
Serum neutralization assays using a pseudovirus represent an in vitro standard for evaluating antibody protection in vaccinated individuals, as they measure a functional host immune response in a laboratory setting without the need for Biosafety level 3 facilities when using live wildtype viruses (Matusali et al., 2021, Nie et al., 2020). Recently, novel SARS-CoV-2 variants that can spread more quickly have emerged. There is evidence that some of these variants are less sensitive to antibody neutralization in Vitro, but it is not clear if it is significant enough to determine whether they can evade vaccine induced protection (Law et al., 2021). Most antibodies generated in response to infection from one variant are at least partially protective against other variants. Antibodies generated against B.1.1.7 (Alpha) show significant cross-neutralization of variant B.1.351 (Beta) and to a lesser extent, SARS-CoV-1 (Law et al., 2021). However, in vitro testing indicates that sera from vaccinated patients exhibit reduced neutralization activity against variants, particularly the variant B.1.351 originally from South Africa (Muik et al., 2021, Planas et al., 2021, Xie et al., 2021). Although the exact neutralizing antibody titers can vary depending on the pseudovirus infection, the fixed doses allow the direct comparison between viral variants and are predictors of protection. Neutralizing antibodies in convalescent patients correlate with their ability to bind the receptor binding domain (RBD) of the spike protein (Hall et al., 2021), although cellular immune responses are also likely to contribute to protection.
First detected in India, SARS-CoV-2 variant B.1.617.2 (Delta) has become the predominant circulating variant reported in over 100 countries drawing concern of breakthrough infections (Brown et al., 2021, Herlihy et al., 2021, Organization, 2021). Early studies have described longer persistence of genomic and subgenomic RNA in the upper respiratory tract, more severe gross lung lesions, and prolonged viral shedding when compared to other variants, which implies greater severity and transmissibility (Mohandas et al., 2021). Its increased transmissibility and severity of disease raises concerns about potential decreased vaccine efficacy in humans (Davis et al., 2021). Although it is not the only circulating variant, the ability for current vaccine protocols to prevent or mitigate infection with the Delta variant in humans or animals has become a focus of current research.
SARS-CoV-2 neutralizing antibodies have been shown to predict disease severity and survival (Garcia-Beltran et al., 2021) while displaying clear evidence of their protective capacity in preventing infection in animal models and humans (Addetia et al., 2020, Hassan et al., 2020, Yu et al., 2020). To date, there is limited data monitoring the level of neutralizing antibodies pre- and post-vaccination or how results compare with vaccine efficacy and protection (Kanji et al., 2021). This study tracks neutralizing antibodies in cats after first and second vaccinations with a recombinantly produced stabilized spike trimer based on the original Wuhan sequence, which has been formulated for use in animals. We report that neutralization titers against pseudovirus particles expressing the spike protein from either the original Wuhan virus or the Delta variant are very similar. These results suggest that vaccines based on the original spike sequence can induce a robust immune response that can neutralize the currently circulating Delta variant, if it becomes problematic in animals.
2. Materials and methods
2.1. Study design
Serum from seven SPF (Specific Pathogen Free) Domestic short hair cats approximately 19 months of age from Marshall Bioresources vaccinated with an adjuvanted recombinant stabilized spike trimer protein followed by a booster 21 days later and a single unvaccinated cat (0.9% Sodium Chloride) were evaluated. Serum was collected pre-inoculation, 20-, and 42-days post inoculation. Two viral spike proteins expressed in lentivirus particles were tested, the Wuhan-Hu-1 (GenBank QHD43416.1) and Indian variant B.1.617.2 (Delta). A pre-titered dose of pseudotyped reporter virus particles (RVPs) (Integral Molecular, Inc Cat#RVP-701 G/RVP-764 G) was incubated with serial dilutions (eight dilutions in a 2.5-fold step-wise method) of test serum samples in triplicate, in a final volume of 100 µL. The RVPs were then mixed with the serum in equal volume and incubated at 5% CO2, 37 °C for 1 h. Freshly typsinized clonal 293 T-hsACE2 cells (Integral Molecular, Inc Cat# C-HA102) expressing the human ACE2 receptor, were plated in a 96-well black/clear bottom cell culture plate (Costar Cat#3603) at a cell density of 2 × 105 cells/mL. The RVP/serum mix was then added to each well containing cells, gently mixed, and incubated in a humidified 5% CO2, 37 °C incubator for 72 h. In addition, each plate contained one set of control wells; cells (background), cells + virus (virus control), cells + virus + neutralizing serum (neutralizing control), and cells + virus + a non-neutralizing serum (non-neutralizing control). The GFP (Green fluorescent protein) signal was then read using a Cytation 5 cell imaging multi-mode reader (excitation 469 nm) and confirmed using fluorescence microscopy. Final TCID50 titers were determined using the Spearman-Karber method of calculation.
2.2. Statistical analysis
Values at the lower limit of quantification were replaced with ½ the lower limit of quantification for analysis. Logarithm2 transformed titers were analyzed with a general linear mixed model for repeated measures. The fixed effects in the model were variant, day of study and variant by day of study interaction. The random effects in the model were sample, sample by variant interaction and residual. Contrasts were used to compare variants at each day of study.
3. Results
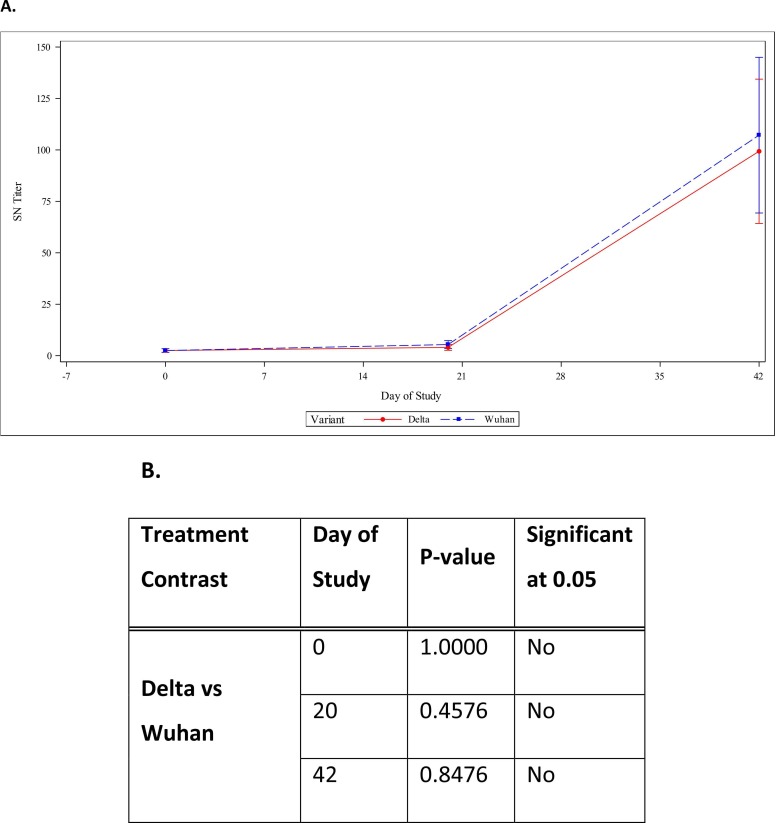
We tested SARS-CoV-2 GFP reporter virus particles that were created using a second-generation lentiviral system with clonal HEK293T-hsACE2 cells provided by Integral Molecular, with eight sera samples from adult specific pathogen free (SPF) domestic short hair cats. Prior to vaccination the neutralizing antibody titers for both the Wuhan strain and the Delta variant was < 5, which was our lowest serum dilution. At day 20, 5 of 7 animals had a neutralizing antibody titer > 5 against the Wuhan strain and 3 of 7 against the Delta variant (See Table 1). At 42 days post vaccination, 21 days post booster, all samples displayed a neutralization titer > 100 against both the Wuhan strain and the Delta variant. For all three days, the difference in titers between the Wuhan strain and the Delta variant was not considered statistically significant (See Fig. 1). The single unvaccinated negative control animal (ID M197855), which was a 0.9% sodium chloride placebo vaccination control had titers of < 5 for all time points for both RVPs.
Table 1.
SARS-CoV-2 pseudovirus based neutralization assay. β-serum neutralization titration of eight feline serum samples was used to determine the neutralization of two SARS-CoV-2 pseudoviruses pre and post vaccination.
| Viral Titer |
|||||||
|---|---|---|---|---|---|---|---|
|
Wuhan |
B.1.617.2 (Delta) |
||||||
| Day: |
0 |
20 |
42 |
0 |
20 |
42 |
|
| Animal ID: | Treatment: | ||||||
| M197766 | Vaccinated | < 5 | 6 | 309 | < 5 | < 5 | 124 |
| M197774 | Vaccinated | < 5 | 8 | 309 | < 5 | < 5 | 228 |
| M197898 | Vaccinated | < 5 | < 5 | 168 | < 5 | 8 | 309 |
| M198355 | Vaccinated | < 5 | 8 | 124 | < 5 | 8 | 124 |
| M198860 | Vaccinated | < 5 | 11 | 168 | < 5 | < 5 | 124 |
| M198886 | Vaccinated | < 5 | < 5 | 124 | < 5 | < 5 | 168 |
| M199212 | Vaccinated | < 5 | 11 | 168 | < 5 | 11 | 168 |
| M197855 | Unvaccinated | < 5 | < 5 | < 5 | < 5 | < 5 | < 5 |
Fig. 1.
Log base 2 statistical analysis of the Delta variant and Wuhan strain SARS-CoV-2 titrations A) Graphical data of day 0, day 20, and day 42 titers comparing the Delta variant and Wuhan strain of SARS-CoV-2. B) P-value observed differences in the two viral strains.
4. Discussion
Concerns have been raised that the current FDA (U.S. Food and Drug Administration) approved, or emergency use vaccines for humans may not be effective in protecting individuals from severe disease due to emerging SARS-CoV-2 variants (Davis et al., 2021). The variant of greatest concern currently is the B.1.617.2 (Delta) variant, which is reported to be both more transmissible and to cause more severe disease in humans (Mohandas et al., 2021). In this study, we immunized felines with an experimental SARS-CoV-2 spike protein-based vaccine (by Zoetis), demonstrated a robust immune response, and conducted a neutralizing serum comparison using RVPs for the original SARS-CoV-2 Wuhan strain and the B.1.617.2 (Delta) variant. There is a positive relationship between antibody titer and the number of vaccinations for both SARS-CoV-2 variants, with similar serum neutralizing titers at both 20 and 42 days with the Wuhan strain and Delta variant This data suggests a similar level of neutralizing antibodies were stimulated following the two dose vaccination series with the Zoetis SARS-CoV-2 vaccine in cats. These similar titers suggest that the vaccine should be as efficacious against the Delta variant as it is against the original Wuhan strain (Addetia et al., 2020, Hassan et al., 2020, Yu et al., 2020). Serious concerns have been raised about the Delta variant because it appears to be more infectious and result in severe disease, but this vaccine stimulates measurable cross-neutralization activity to the spike protein equivalent to what was seen with the original Wuhan strain of the virus. A limitation of this assay is the use of HEK293T cells that express human and not feline ACE2, but the two have previously been compared and are highly similar (Stout et al., 2020). Also, our internal controls have shown that both viral particles will infect with non-vaccinated cat serum and are inhibited in the presence of SARS-CoV-2 vaccinated cat serum.
There have been reports of non-terminal cases of canine infection (Shi et al., 2020), but the long-term effects of SARS-CoV-2 infection are currently unknown for canines. Mink, cats, and Felids are currently the only animals other than humans to routinely develop life threatening disease due to SARS-CoV-2 infection (Eckstrand et al., 2021, Kim et al., 2020, Shi et al., 2020). There have been multiple countries including Denmark, the Netherlands, and the USA that have reported clinical disease cases in farmed mink infected with SARS-CoV-2 (Boklund et al., 2021, Eckstrand et al., 2021). Some farms have resorted to culling animals because of the zoonotic potential that exists. The U.S. Department of Agriculture permitted a campaign that has provided mink farms access to this experimental vaccine to protect their farms from severe disease. Recently, there have been numerous felids that have died of COVID-19 in zoos in the United States and other countries (Giraldo-Ramirez et al., 2021). This study conducted by Zoetis suggest that these vaccinated animals should also be protected from serious disease caused by the Delta variant. Although this study did not address other variants, preliminary results from this study testing suggested that it may provide protection against multiple SARS-CoV-2 variants.
Compliance and ethical standards
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Conflict of interest
All authors are employed by Zoetis and the adjuvanted recombinant stabilized spike trimer protein vaccine is an Investigational Veterinary Product of the company with a business and/or financial interest.
References
- Addetia A., Crawford K.H.D., Dingens A., Zhu H., Roychoudhury P., Huang M.L., Greninger A.L. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. J. Clin. Microbiol. 2020;58(11) doi: 10.1128/JCM.02107-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boklund A., Hammer A.S., Quaade M.L., Rasmussen T.B., Lohse L., Strandbygaard B., Botner A. SARS-CoV-2 in Danish mink farms: course of the epidemic and a descriptive analysis of the outbreaks in 2020. Animals. 2021;11 doi: 10.3390/ani11010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla-Aldana D.K., Garcia-Barco A., Jimenez-Diaz S.D., Bonilla-Aldana J.L., Cardona-Trujillo M.C., Munoz-Lara F., Rodriguez-Morales A.J. SARS-CoV-2 natural infection in animals: a systematic review of studies and case reports and series. Vet. Q. 2021;41(1):250–267. doi: 10.1080/01652176.2021.1970280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C.M., Vostok J., Johnson H., Burns M., Gharpure R., Sami S., Laney A.S. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings - Barnstable County, Massachusetts, July 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70(31):1059–1062. doi: 10.15585/mmwr.mm7031e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C., Logan N., Tyson G., Orton R., Harvey W.T., Perkins J.S., investigators C.-D.V.C.S. Reduced neutralisation of the Delta (B.1.617.2) SARS-CoV-2 variant of concern following vaccination. PLoS Pathog. 2021;17(12) doi: 10.1371/journal.ppat.1010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstrand C.D., Baldwin T.J., Rood K.A., Clayton M.J., Lott J.K., Wolking R.M., Baszler T. An outbreak of SARS-CoV-2 with high mortality in mink (Neovison vison) on multiple Utah farms. PLoS Pathog. 2021;17(11) doi: 10.1371/journal.ppat.1009952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferasin L., Fritz M., Ferasin H., Becquart P., Corbet S., Ar Gouilh M., Leroy E.M. Infection with SARS-CoV-2 variant B.1.1.7 detected in a group of dogs and cats with suspected myocarditis. Vet. Rec. 2021;189(9) doi: 10.1002/vetr.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Beltran W.F., Lam E.C., Astudillo M.G., Yang D., Miller T.E., Feldman J., Balazs A.B. COVID-19-neutralizing antibodies predict disease severity and survival. Cell. 2021;184(2):476–488. doi: 10.1016/j.cell.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo-Ramirez S., Rendon-Marin S., Jaimes J.A., Martinez-Gutierrez M., Ruiz-Saenz J. SARS-CoV-2 clinical outcome in domestic and wild cats: a systematic review. Animals. 2021;11 doi: 10.3390/ani11072056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall V.J., Foulkes S., Charlett A., Atti A., Monk E.J.M., Simmons R., Group S.S. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN) Lancet. 2021;397(10283):1459–1469. doi: 10.1016/S0140-6736(21)00675-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer S.A., Pauvolid-Correa A., Zecca I.B., Davila E., Auckland L.D., Roundy C.M., Hamer G.L. Natural SARS-CoV-2 infections, including virus isolation, among serially tested cats and dogs in households with confirmed human COVID-19 cases in Texas, USA. bioRxiv. 2020 doi: 10.1101/2020.12.08.416339. [DOI] [Google Scholar]
- Hassan A.O., Case J.B., Winkler E.S., Thackray L.B., Kafai N.M., Bailey A.L., Diamond M.S. A SARS-CoV-2 infection model in mice demonstrates protection by neutralizing antibodies. Cell. 2020;182(3):744–753. doi: 10.1016/j.cell.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlihy R., Bamberg W., Burakoff A., Alden N., Severson R., Bush E., Stringer G. Rapid increase in circulation of the SARS-CoV-2 B.1.617.2 (Delta) variant - Mesa County, Colorado, April-June 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70(32):1084–1087. doi: 10.15585/mmwr.mm7032e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jara L.M., Ferradas C., Schiaffino F., Sanchez-Carrion C., Martinez-Vela A., Ulloa A., Zimic M. Evidence of neutralizing antibodies against SARS-CoV-2 in domestic cats living with owners with a history of COVID-19 in Lima - Peru. One Health. 2021;13 doi: 10.1016/j.onehlt.2021.100318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanji J.N., Bailey A., Fenton J., Ling S.H., Rivera R., Plitt S., Charlton C.L. Detection of SARS-CoV-2 antibodies formed in response to the BNT162b2 and mRNA-1237 mRNA vaccine by commercial antibody tests. Vaccine. 2021;39(39):5563–5570. doi: 10.1016/j.vaccine.2021.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.I., Kim S.G., Kim S.M., Kim E.H., Park S.J., Yu K.M., Choi Y.K. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe. 2020;27(5):704–709. doi: 10.1016/j.chom.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi T. SARS-CoV-2 mutations among minks show reduced lethality and infectivity to humans. PLoS One. 2021;16(5) doi: 10.1371/journal.pone.0247626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law J.L.M., Logan M., Joyce M.A., Landi A., Hockman D., Crawford K., Houghton M. SARS-COV-2 recombinant Receptor-Binding-Domain (RBD) induces neutralizing antibodies against variant strains of SARS-CoV-2 and SARS-CoV-1. Vaccine. 2021;39(40):5769–5779. doi: 10.1016/j.vaccine.2021.08.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallapaty S. The search for animals harbouring coronavirus - and why it matters. Nature. 2021;591(7848):26–28. doi: 10.1038/d41586-021-00531-z. [DOI] [PubMed] [Google Scholar]
- Matusali G., Colavita F., Lapa D., Meschi S., Bordi L., Piselli P., Inmi Covid-Laboratory T. SARS-CoV-2 serum neutralization assay: a traditional tool for a brand-new virus. Viruses. 2021;13(4) doi: 10.3390/v13040655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAloose D., Laverack M., Wang L., Killian M.L., Caserta L.C., Yuan F., Diel D.G. From people to panthera: natural SARS-CoV-2 infection in tigers and lions at the Bronx Zoo. mBio. 2020;11(5) doi: 10.1128/mBio.02220-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas S., Yadav P.D., Shete A., Nyayanit D., Sapkal G., Lole K., Gupta N. SARS-CoV-2 delta variant pathogenesis and host response in Syrian hamsters. Viruses. 2021;13(9) doi: 10.3390/v13091773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muik A., Wallisch A.K., Sanger B., Swanson K.A., Muhl J., Chen W., Sahin U. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine-elicited human sera. Science. 2021;371(6534):1152–1153. doi: 10.1126/science.abg6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie J., Li Q., Wu J., Zhao C., Hao H., Liu H., Wang Y. Quantification of SARS-CoV-2 neutralizing antibody by a pseudotyped virus-based assay. Nat. Protoc. 2020;15(11):3699–3715. doi: 10.1038/s41596-020-0394-5. [DOI] [PubMed] [Google Scholar]
- Organization, W.H., 2021. Weekly epidemiological update on COVID-19. Emergency Situational Updates. 49. 〈https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports〉.
- Planas D., Bruel T., Grzelak L., Guivel-Benhassine F., Staropoli I., Porrot F., Schwartz O. Sensitivity of infectious SARS-CoV-2 B.1.1.7 and B.1.351 variants to neutralizing antibodies. Nat. Med. 2021;27(5):917–924. doi: 10.1038/s41591-021-01318-5. [DOI] [PubMed] [Google Scholar]
- Rabalski L., Kosinski M., Smura T., Aaltonen K., Kant R., Sironen T., Grzybek M. Severe acute respiratory syndrome coronavirus 2 in farmed mink (Neovison vison), Poland. Emerg. Infect. Dis. 2021;27(9):2333–2339. doi: 10.3201/eid2709.210286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Wen Z., Zhong G., Yang H., Wang C., Huang B., Bu Z. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science. 2020;368(6494):1016–1020. doi: 10.1126/science.abb7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout A.E., Andre N.M., Jaimes J.A., Millet J.K., Whittaker G.R. Coronaviruses in cats and other companion animals: where does SARS-CoV-2/COVID-19 fit? Vet. Microbiol. 2020;247 doi: 10.1016/j.vetmic.2020.108777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Aart A.E., Velkers F.C., Fischer E.A.J., Broens E.M., Egberink H., Zhao S., Smit L.A.M. SARS-CoV-2 infection in cats and dogs in infected mink farms. Transbound. Emerg. Dis. 2021 doi: 10.1111/tbed.14173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., Liu Y., Liu J., Zhang X., Zou J., Fontes-Garfias C.R., Shi P.Y. Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine-elicited sera. Nat. Med. 2021;27(4):620–621. doi: 10.1038/s41591-021-01270-4. [DOI] [PubMed] [Google Scholar]
- Yilmaz Celebi M., Kiymet E., Boncuoglu E., Sahinkaya S., Cem E., Duzgol M., Devrim I. Evaluation of childhood hospitalization rates and degree of severity of SARS-CoV-2 variants, including B.1.1.7 (Alpha), B.1.315/P.1 (Beta/Gamma), and B.1.617.2 (Delta) J. Med. Virol. 2022 doi: 10.1002/jmv.27587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Tostanoski L.H., Peter L., Mercado N.B., McMahan K., Mahrokhian S.H., Barouch D.H. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020;369(6505):806–811. doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]