Abstract
Purpose of review
The intestine is the most immunologically complex solid organ allograft with the greatest risk of both rejection and graft-versus-host disease (GVHD). High levels of immunosuppression are required, further increasing morbidity. Due to low volume of transplants and few centers with experience, there is paucity of evidence-based, standardized, and effective therapeutic regimens. We herein review the most recent data about immunosuppression, focusing on novel and emerging therapies.
Recent findings
Recent data are moving the field towards increasing use of basilixumab and consideration of alemtuzumab for induction and inclusion of mTOR inhibitors and antimetabolites for maintenance. For rejection, we highlight novel roles for TNFα inhibition, α4β7 integrin inhibition, microbiome modulation, desensitization protocols, and tolerance induction strategies. We also highlight emerging novel therapies for GVHD, especially the promising role of Janus kinase inhibition.
Summary
New insights into immune pathways associated with rejection and GVHD in intestinal allografts have led to an evolution of therapies from broad-based immunosuppression to more targeted strategies that hold promise for reducing morbidity from infection, rejection, and GVHD. These should be the focus of further study to facilitate their widespread use.
Keywords: Intestinal transplantation, rejection, graft-versus-host disease, immunosuppression, immunomodulation
INTRODUCTION
The intestine is the most immunologically complex solid organ allograft. It has the highest incidence of acute cellular rejection (ACR), exceeding 60% in some groups, requiring high levels of immunosuppression, which predisposes recipients to elevated rates of infections, graft-versus-host disease (GVHD), and post-transplant lymphoproliferative disorder (PTLD)[1, 2]. These challenges are compounded by the paucity of data regarding optimal regimens for induction and maintenance immunosuppression as well as for treating rejection and GVHD. Current regimens are largely determined by the experience and independent evaluation of protocols within high volume centers and vary widely between centers. Consequently, outcomes after intestinal transplantation (ITx) have not markedly improved over the past decade[1]. However, recent insights into specific immunologic pathways are guiding development of novel and targeted immunomodulatory strategies, providing more effective treatment and mitigating the undesirable consequences of over-immunosuppression. We herein review the most recent evidence supporting the use of both classical and novel agents for induction and maintenance immunosuppression as well as treatment of rejection and GVHD after ITx, focusing especially on emerging therapeutics and novel immunomodulatory strategies (see Figure 1 for summary).
Figure 1:
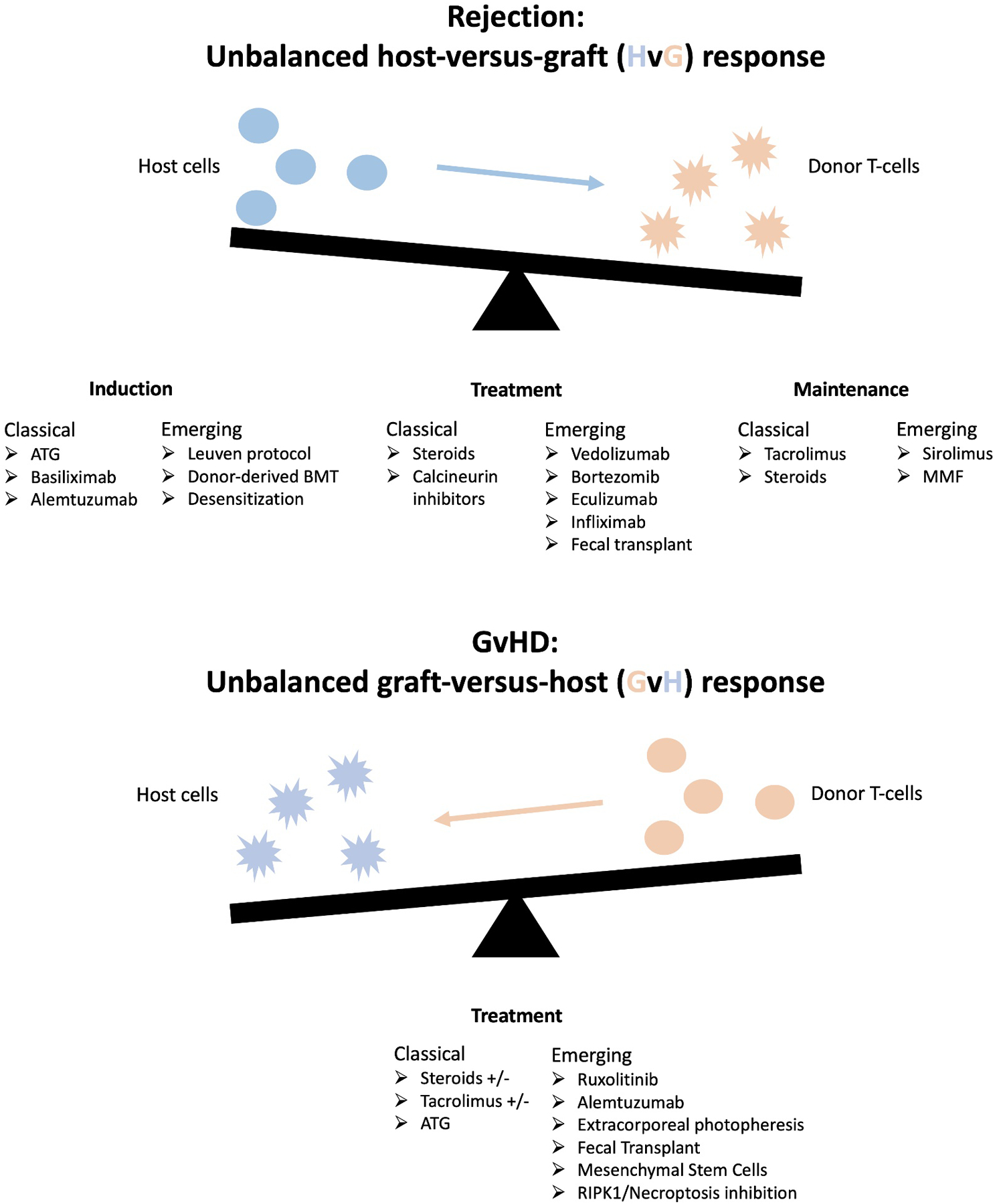
Summary of therapeutics discussed in this review and their location on the axis of classical and novel induction, maintenance, and treatment options after intestinal transplantation.
INDUCTION IMMUNOSUPPRESION
Classical therapies
Anti-thymocyte globulin (ATG)
T cell depletion with ATG, either equine-derived ATGAM (Pfizer) or rabbit-derived Thymoglobulin (Genzyme), is a mainstay of induction immunosuppression for ITx and is used as a primary induction agent in many high-volume centers, especially because the intestine contains more lymphocytes than any other solid organ [3]. In addition to markedly reducing rates of ACR, the use of ATG in combination with steroids and rituximab has been shown to reduce rates of chronic rejection and PTLD[4]. A comparative study of induction agents favored such a regimen for providing the most favorable balance in mitigating rates of infection and ACR[5].
Basiliximab
Basiliximab, a monoclonal antibody targeting the interleukin-2 receptor, is an induction agent of choice in many centers for unsensitized ITx recipients[6]. Inhibition of interleukin-2 promotes the engraftment of regulatory T cells and enables minimization of steroids and global T cell depleting agents[7]. Basiliximab induction therapy has been shown to reduce the incidence of acute rejection after ITx without increasing the incidence of infectious complications[8].
Alemtuzumab
Alemtuzumab is a monoclonal antibody targeting CD52, a glycoprotein found on mature T cells and monocyte-derived dendritic cells. Larger studies have demonstrated that the use of tacrolimus in combination with alemtuzumab led to excellent 1-year survival with low rejection rates[9]. However, alemtuzumab has been associated with higher rates of PTLD[10] and infection[5, 11] in some series.
Comparing classical therapies
In the absence of trials directly comparing the major classical induction therapies, the current consensus is based on comparison of large single-center series. Our interpretation of the data is that thymoglobulin is the safest and most established induction agent for most patients, despite the risk of infectious complications. For unsensitized patients, basilixumab is a good option for preventing rejection while avoiding side-effects associated with more aggressive depletion. Alemtuzumab may effectively attenuate both rejection and GVHD, but data remain too sparse to assess whether this outweighs increased infectious and malignant risks.
Emerging Strategies/Agents
Leuven Protocol
The Leuven immunomodulatory protocol was designed to minimize rejection and requirement for broad-based immunosuppression by promoting a regulatory environment utilizing donor-specific blood transfusion, avoiding high-dose steroids and calcineurin inhibitors, and minimizing ischemia-reperfusion injury and endotoxin translocation. The initial report of clinical implementation in 13 patients resulted in 11 patients surviving off parenteral nutrition with a median follow-up of 3.5 years with 92% 5-year graft survival and elevated levels of circulating regulatory T cells[7].
Donor-Derived Bone Marrow Infusions
Combined bone marrow/intestine transplantation as a strategy of tolerance induction is an extension of successful trials with other solid organs[12]. A recent report of spontaneous operational tolerance in an intestinal graft recipient indicates that tolerance is more than a theoretical possibility, even with the highly immunogenic intestine[13]. We are not aware of trials utilizing non-myeloablative conditioning, however we are conducting a novel clinical trial of intestine transplant with delayed donor bone marrow infusion without conditioning (Columbia University/Ossium Health). The scientific rationale is that donor CD34+ progenitor cells originating from the graft emigrate to recipient bone marrow, where they take up long-term residence and provide a stable source of donor-derived T cells. Moreover, engraftment is facilitated by cytotoxic donor-derived T cells with graft-versus-host reactivity that emigrate from the graft into recipient bone marrow, where they “make space” for engraftment by selectively attacking recipient hematopoietic stem cells without systemic GVHD (so-called “Lymphohematopoietic Graft-Versus-Host Response” or LGVHR)[14]. The point of maximal LGVHR is approximately 11 days after transplantation. Donor bone marrow infusion is timed to the point of maximal LGVHR to facilitate permanent long-term multilineage macrochimerism and, ideally, tolerance.
Desensitization
It has historically been prohibitively difficult to find cross-match-negative donors for some recipients with high levels of donor-specific antibody (DSA). Recently, individual centers have trialed desensitization protocols. Illinois and New York groups have recently published promising case reports using a combination of medical desensitization (plasmapheresis, IVIG, rituximab, bortezomib) [15, 16] and immunoadsorption through temporary reperfusion of the donor spleen[17]. Further work is required to evaluate and optimize such protocols for broader use.
MAINTENANCE IMMUNOSUPPRESION
Maintenance immunosuppression is the most standardized aspect of the intestinal transplant regimen. The pillars are tacrolimus, particularly high-dose (>15 ng/ml in the first 3–6 months[18]), and steroids. Recently, the mTOR inhibitor sirolimus and the antimetabolite mycophenolate mofetil (MMF) have been added to some protocols. Sirolimus is increasingly used at later post-transplant timepoints, especially because of concern for early wound complications, and has recently shown advantages for controlling rejection with lower risk of renal injury, hemolytic anemia, neuroroxicity, thrombotic microangiopathy, and potentially GVHD[19]. MMF has also been employed to reduce nephrotoxicity associated with long-term use of calcineurin inhibitors. When used in combination with tacrolimus and low-dose steroids, improved graft survival and lower rates of allorejection have been observed[20]. However, GI side effects and cytopenias associated with its use have limited widespread adoption.
TREATMENT OF ALLOREJECTION
Classical therapies
First line treatment of intestinal ACR is high-dose steroid pulse, often with increased calcineurin or mTOR inhibitor levels. ATG is reserved for steroid-refractory or severe rejection but increases the risk of opportunistic infection and PTLD[21].
Antibody mediated rejection (AMR), characterized by the presence of DSA, occasional C4d deposition in mucosa, and serosal vasculopathy, can occur early or late after intestinal transplantation and lead to graft dysfunction and loss. While it is occasionally possible to treat successfully with strategies targeting antibody (IVIG, plasmapheresis) or B cells (rituximab), better strategies are necessary due to frequently refractory disease[22].
Emerging Strategies/Agents
Vedolizumab
Vedolizumab is an antibody targeting the T lymphocyte gut-homing α4β7 integrin and is used for treatment of inflammatory bowel disease (IBD). With effectiveness in this population, off-label use of vedolizumab for successful treatment of ACR after ITx was recently reported by Trentadue et al.[23]. However, the effectiveness of this agent has not been systematically evaluated, and further studies are required to assess its utility prior to widespread adoption.
Infliximab
While vedolizumab prevents the trafficking of activated recipient T cells to the donor intestine, a novel role has recently been described for the use of infliximab for the suppression of inflammation caused by the T cells already residing in the gut. In a potentially practice-changing study, Kroemer et al. demonstrated that the response of intestinal rejection to treatment is more dependent on the ability to deplete T cells in the graft rather than the blood. Terminally differentiated effector memory T cells, similar to the CCR6+/CD45RO+/CD62L+ FACS phenotype and Th17 cytokine profile (TNFα/IL-17 double-positive) seen in active IBD, persisted in the grafts of patients with thymoglobulin-resistant rejection, even after being depleted in the blood. Just as these cells respond to TNFα-blockade by infliximab in severe refractory IBD, 14 of 14 ITx patients with thymoglobulin-refractory rejection showed improved clinical and histological score after treatment with infliximab[24]. Besides making the case for clinical trials incorporating infliximab into the treatment algorithm for refractory intestinal rejection, this study also raises interesting questions about which aspects of the Th17 pathway are inflammatory rather than protective, since blocking IL-17 entirely causes increased inflammation in some cases, likely because IL-17 has functions that are essential for protection from intestinal pathogens and for maintaining homeostasis between the gut mucosa and commensals through various pathways involving IL-22, IL-10, serum amyloid A, STAT3, and IL-10[25]. Thus, another important recent concept about novel immunomodulatory treatments after intestinal transplantation is that, unlike TNFα-blockade, which preferentially targets pathological Th17 responses, IL-17 blockade is not a promising direction and, more broadly, that it is important to consider the range of cytokine functions when blocking entire pathways.
Bortezomib
As a proteasome inhibitor selectively targeting plasma cells, bortezomib has been used successfully for AMR in kidney transplant recipients. Its use in intestinal transplantation is case-reportable, with effectiveness against AMR balanced by increased viral infection risk[26, 27]. Bortezomib is therefore promising but requires further evaluation.
Eculizumab
There is a single report of successful treatment of AMR with DSA using eculizumab, a monoclonal antibody against complement C5[28]. Further investigation is warranted.
Probiotics/Fecal Microbiota Transplant (FMT)
Disruptions in the gut microbiome have been associated with intestinal inflammatory states, including IBD[29], graft rejection[30], and GVHD[31]. It remains unknown if the etiology is differential abundance of certain beneficial or pathological microbes or changes in overall diversity or whether dysbiosis is the cause or consequence of these disease states. However, recent work demonstrates that restoring homeostasis between luminal microbes and mucosal defenses, using probiotics[32] or FMT[33], reduces intestinal inflammation, and one animal study linked restoration of normal flora with resolution of intestinal rejection[34]. Whether these interventions have similar benefit in human intestinal rejection is a subject ripe for investigation.
TREATMENT OF GRAFT-VERSUS-HOST DISEASE
The incidence of GVHD after intestinal transplant ranges from approximately 5–15%, far exceeding that of other solid organ transplants[2, 35, 36]. Increased graft volume (especially liver-containing or colon-including grafts) is the most definitive risk factor, with other risk factors, such as recipient age and certain induction regimens, varying between centers[35, 36, 37]. GVHD is a major contributor to morbidity and mortality that is frequently refractory to modern medical management, with greater than 50% mortality in some series[35, 37].
Certain induction and maintenance immunosuppressive regimens may affect the subsequent incidence of GVHD. In one series, early use of sirolimus was protective against subsequent GVHD[35]. In a large case series from the University of Miami, the use of alemtuzumab was shown to significantly decrease the later incidence of GVHD when used as part of induction therapy for ITx[36]. Its role in the treatment of acute or chronic GVHD following ITx remains to be investigated.
Steroids
In the absence of clinical trials or multicenter studies, there is no current standard of care treatment regimen for GVHD, even within individual intestinal transplant programs[36]. Corticosteroids remain the cornerstone of both acute and chronic GVHD treatment after all forms of transplantation, including intestinal[35], but outcomes are variable[38, 39].
There is also variability in the management of maintenance immunosuppression, with some programs showing good responses to GVHD in patients in whom tacrolimus levels were increased, with the goal of suppressing alloreactive donor cells[40]. Conversely, reduction in immunosuppression levels is another strategy, particularly in patients at low risk of rejection[38], with the goal of allowing the host immune system to mitigate the activity of donor-derived lymphocytes[35, 36]. No consensus strategy exists.
ATG
T cell depletion with ATG is used as either first or second-line therapy for GVHD depending on the severity of presentation and the center-specific practices. Thymoglobulin rather than ATGAM is by far the more commonly utilized option for both induction and GVHD treatment in ITx recipients, perhaps because it has been preferentially adopted for use in solid organ transplantation due to superior T cell depletion and decreased adverse events when used both for induction therapy[41] and treatment of rejection[42]. However, some programs that utilize rabbit ATG for induction use horse ATG for subsequent GVHD since it has been shown that anti-idiotype antibodies may develop and decrease the effectiveness of T cell depletion[43].
We also note that some centers infuse ATG into organ donors prior to procurement based on animal model data showing decreased rates of GVHD[44]. We are not aware of any clinical data showing benefits or detriments specifically attributed to this practice, and a recent large case series demonstrated no effect[35].
Emerging therapies for refractory GVHD
Ruxolitinib
The FDA recently approved the Janus kinase (JAK) inhibitor ruxolitinib for the treatment of steroid-refractory acute GVHD by blocking the JAK-STAT-mediated inflammatory cytokine pathway. Its use following ITx is thus far limited to two case reports of GVHD refractory to steroids and T cell depletion. Both patients ultimately succumbed to their disease, but one showed good response to therapy until treatment was discontinued due to bone marrow suppression and polymicrobial infections, after which disease recurred[35, 45]. The authors concluded that ruxolitinib might better improve outcomes if used before the onset of advanced disease and are currently trialing its use as a second-line therapy after steroids but before T cell depletion[35].
Extracorporeal photopheresis
Extracorporeal photopheresis (ECP) inactivates peripheral blood mononuclear cells collected via leukapheresis by exposure to the photosensitization agent 8-methoxypsoralen and subsequent UVA irradiation and reinfusion. Although successful ECP treatment of otherwise-refractory GVHD after HSCT is well-described, only two reports document the potential benefit of ECP for treatment of GVHD after ITx. Peripheral blood chimerism decreased markedly in both patients, and one patient, whose disease was less advanced, recovered[46, 47]. These two cases indicate that ECP could be an adjunctive therapy in cases of GVHD with high donor chimerism after ITx, but further study is required.
Fecal Microbiota Transplantation
The relationship of dysbiosis and intestinal inflammation was discussed above. Under similar reasoning, FMT has been proposed as a treatment for intestinal GVHD after HSCT and has recently demonstrated efficacy in pilot studies[31, 48]. There are no published cases of FMT for the treatment of GVHD following ITx in humans. We believe that investigation is warranted.
Hypothetical therapies
Both mesenchymal stem cells (MSCs) and receptor interacting protein kinase 1 (RIPK1) inhibition hold hypothetical benefit for ITx recipients. MSCs have immunoregulatory properties and have been used to achieve complete remission of steroid-resistant acute GVHD following HSCT[49]. Variants of the autophagy mediator ATG16L1 have been linked to intestinal GVHD after HSCT[50] and IBD[51]. Blocking the necroptosis factor RIPK1 in these cases has prevented intestinal inflammation in experimental models and may be effective in humans. MSC infusion has been trialed as part of induction therapy in a small cohort of ITx recipients without showing any benefit for either GVHD or rejection[52], while RIPK1 inhibition has not yet been attempted.
CONCLUSION
Rejection and GVHD are both high risk and often difficult to treat after ITx. This is compounded by the lack of established therapeutic protocols and the paucity of adequately powered studies due to the low-volume and geographically scattered nature of its practice. This review briefly summarizes established therapies and highlights emerging immunotherapies that may hold future promise. We advocate that these be the focus of future study.
KEY POINTS.
Basilixumab and alemtuzumab should be considered for induction therapy in addition to thymoglobulin, and sirolimus and mycophenalate mofetil should be considered for maintenance therapy in addition to steroids and tacrolimus.
Desensitization and tolerance induction protocols may hold promise for selected patients.
Recent evidence indicates that infliximab and vedolizumab are compelling novel therapies for refractory rejection and Janus kinase inhibition for GVHD after intestinal transplantation.
Recent work indicates a role for manipulation of the microbiome as a focus of novel therapies for both rejection and GVHD after intestinal transplantation.
ACKNOWLEDGEMENTS
The authors thank Dr. Mercedes Martinez for her expert review.
FINANCIAL SUPPORT AND SPONSORSHIP
This work was supported by NIH grant K23 AI 156026 (J.W.), the Nelson Family Transplant Innovation Award (J.W.), the Columbia University Department of Surgery (J.W.), and a Summer Research Fellowship (A.S.) from the NIH Grant T35 DK 093430.
Footnotes
CONFLICTS OF INTEREST
None
REFERENCES
- 1.Horslen SP, Smith JM, Ahn Y, et al. OPTN/SRTR 2019 Annual Data Report: Intestine. Am J Transplant. 2021 Feb;21 Suppl 2:316–55. [DOI] [PubMed] [Google Scholar]
- 2.Ganoza A, Mazariegos GV, Khanna A. Current status of graft-versus-host disease after intestinal transplantation. Curr Opin Organ Transplant. 2019. Apr;24(2):199–206. [DOI] [PubMed] [Google Scholar]
- *3.Gondolesi GE. Induction in Intestinal Transplantation. Transplantation. 2020. Oct;104(10):1999–2000. [DOI] [PubMed] [Google Scholar]; This is a brief, high-yield expert commentary on the multiple benefits of thymoglobulin induction therapy for ITx.
- 4.Vianna RM, Mangus RS, Fridell JA, et al. Induction immunosuppression with thymoglobulin and rituximab in intestinal and multivisceral transplantation. Transplantation. 2008. May 15;85(9):1290–3. [DOI] [PubMed] [Google Scholar]
- 5.Trevizol AP, David AI, Dias ER, et al. Intestinal and multivisceral transplantation immunosuppression protocols--literature review. Transplant Proc. 2012. Oct;44(8):2445–8. [DOI] [PubMed] [Google Scholar]
- 6.Elsabbagh AM, Hawksworth J, Khan KM, et al. Long-term survival in visceral transplant recipients in the new era: A single-center experience. Am J Transplant. 2019. Jul;19(7):2077–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ceulemans LJ, Braza F, Monbaliu D, et al. The Leuven Immunomodulatory Protocol Promotes T-Regulatory Cells and Substantially Prolongs Survival After First Intestinal Transplantation. Am J Transplant. 2016. Oct;16(10):2973–85. [DOI] [PubMed] [Google Scholar]
- 8.Sudan DL, Chinnakotla S, Horslen S, et al. Basiliximab decreases the incidence of acute rejection after intestinal transplantation. Transplant Proc. 2002. May;34(3):940–1. [DOI] [PubMed] [Google Scholar]
- 9.Nishida S, Levi DM, Moon JI, et al. Intestinal transplantation with alemtuzumab (Campath-1H) induction for adult patients. Transplant Proc. 2006. Jul-Aug;38(6):1747–9. [DOI] [PubMed] [Google Scholar]
- 10.Devine K, Ranganathan S, Mazariegos G, et al. Induction regimens and post-transplantation lymphoproliferative disorder after pediatric intestinal transplantation: Single-center experience. Pediatr Transplant. 2020. Aug;24(5):e13723. [DOI] [PubMed] [Google Scholar]
- 11.Zanfi C, Lauro A, Cescon M, et al. Daclizumab and alemtuzumab as induction agents in adult intestinal and multivisceral transplantation: rejection and infection rates in 40 recipients during the early postoperative period. Transplant Proc. 2010. Jan-Feb;42(1):35–8. [DOI] [PubMed] [Google Scholar]
- *12.Issa F, Strober S, Leventhal JR, et al. The Fourth International Workshop on Clinical Transplant Tolerance. Am J Transplant. 2021. Jan;21(1):21–31. [DOI] [PubMed] [Google Scholar]; This is the most up-to-date summary of current tolerance induction protocols for solid organ transplantation.
- 13.Kroemer A, Khan K, Kaufman SS, et al. Operational tolerance in intestinal transplantation. Am J Transplant. 2021. Feb;21(2):876–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **14.Fu J, Zuber J, Shonts B, et al. Lymphohematopoietic graft-versus-host responses promote mixed chimerism in patients receiving intestinal transplantation. J Clin Invest. 2021. Apr 15;131(8). [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the most sophisticated mechaistic analysis to date of the immunobiology by which donor cells from the intestinal graft migrate to and engraft in recipient mone-marrow, contributing to prolonged mixed-chierism and decreased ITx rejection. This analysis could guide future thinking about novel immunomodulatory therapies for ITx.
- 15.Santeusanio AD, Moon J, Nair V, et al. Is There a Role for Desensitization in Intestinal Transplantation? Prog Transplant. 2019. Sep;29(3):275–8. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Roca R, Tzvetanov IG, Jeon H, et al. Successful living donor intestinal transplantation in cross-match positive recipients: Initial experience. World J Gastrointest Surg. 2016. Jan 27;8(1):101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *17.Spaggiari M, Lichvar A, Tzvetanov I, et al. Temporary Deceased Donor Splenic Transplant Prior to Intestinal Transplantation: A New Strategy for Desensitization? Transplant Proc. 2021. Oct;53(8):2602–8. [DOI] [PubMed] [Google Scholar]; Highlights recent advances in ability to overcome the barrier of high levels of donor-specific antibody, providing new possibilities for highly-sensitized recipients.
- 18.Santeusanio AD, Gu A, Weinberg AD, et al. Tacrolimus time-in-therapeutic range is associated with freedom from acute rejection and graft failure following intestinal transplantation. Clin Transplant. 2021. Jun;35(6):e14291. [DOI] [PubMed] [Google Scholar]
- *19.Andres AM, Talayero P, Alcolea Sanchez A, et al. Delayed introduction of sirolimus in paediatric intestinal transplant recipients: indications and long-term benefits. Transpl Int. 2021. Oct;34(10):1895–907. [DOI] [PubMed] [Google Scholar]; This refernce makes astrong case for the increasing utility of sirolimus for ITx, which may change the practice patterns for centers that have not historically used this terapy routinely.
- 20.Courbage S, Canioni D, Talbotec C, et al. Beyond 10 years, with or without an intestinal graft: Present and future? Am J Transplant. 2020. Oct;20(10):2802–12. [DOI] [PubMed] [Google Scholar]
- 21.Huard G, Schiano TD, Moon J, et al. Severe acute cellular rejection after intestinal transplantation is associated with poor patient and graft survival. Clin Transplant. 2017. May;31(5). [DOI] [PubMed] [Google Scholar]
- 22.Wu GS, Zhao QC, Li ZS, et al. Successful Rescue of Late-onset Antibody-mediated Rejection 12 Years After Living-donor Intestinal Transplantation: A Case Report. Transplant Proc. 2017. Jan - Feb;49(1):232–6. [DOI] [PubMed] [Google Scholar]
- **23.Trentadue G, Kats-Ugurlu G, Blokzijl T, et al. Safe and Successful Treatment of Acute Cellular Rejection of an Intestine and Abdominal Wall Transplant With Vedolizumab. Transplant Direct. 2020. Feb;6(2):e527. [DOI] [PMC free article] [PubMed] [Google Scholar]; Introduces the use of vedolizumab as a novel therapy for rejection after ITx. One of the first truly-novel therapies to be put into practical use in several years and likely will change standard-of-care practices.
- **24.Kroemer A, Belyayev L, Khan K, et al. Rejection of intestinal allotransplants is driven by memory T helper type 17 immunity and responds to infliximab. Am J Transplant. 2021. Mar;21(3):1238–54. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is both a clinical study demonstrating the efficacy of infliximab for refractory acute cellular rejection after ITx and a novel immunological analysis of the mechanisms by which it is effective. Has the potential to change standard-of-care as well as advance understanding of the rejection and inflammatory patheays unique to the intestine.
- 25.Sano T, Kageyama T, Fang V, et al. Redundant cytokine requirement for intestinal microbiota-induced Th17 cell differentiation in draining lymph nodes. Cell Rep. 2021. Aug 24;36(8):109608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujiwara S, Wada M, Kudo H, et al. Effectiveness of Bortezomib in a Patient With Acute Rejection Associated With an Elevation of Donor-Specific HLA Antibodies After Small-Bowel Transplantation: Case Report. Transplant Proc. 2016. Mar;48(2):525–7. [DOI] [PubMed] [Google Scholar]
- 27.Island ER, Gonzalez-Pinto IM, Tsai HL, et al. Successful treatment with bortezomib of a refractory humoral rejection of the intestine after multivisceral transplantation. Clin Transpl. 2009:465–9. [PubMed] [Google Scholar]
- 28.Fan J, Tryphonopoulos P, Tekin A, et al. Eculizumab Salvage Therapy for Antibody-Mediated Rejection in a Desensitization-Resistant Intestinal Re-Transplant Patient. Am J Transplant. 2015. Jul;15(7):1995–2000. [DOI] [PubMed] [Google Scholar]
- 29.Gevers D, Kugathasan S, Denson LA, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014. Mar 12;15(3):382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh PL, Martinez I, Sun Y, et al. Characterization of the ileal microbiota in rejecting and nonrejecting recipients of small bowel transplants. Am J Transplant. 2012. Mar;12(3):753–62. [DOI] [PubMed] [Google Scholar]
- 31.Kakihana K, Fujioka Y, Suda W, et al. Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood. 2016. Oct 20;128(16):2083–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *32.Cristofori F, Dargenio VN, Dargenio C, et al. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front Immunol. 2021;12:578386. [DOI] [PMC free article] [PubMed] [Google Scholar]; Excellent summary of the pathophysiology of inflammation associated with loss of homeostasis between intestinal microbes and mucosal defenses, an emerging concept and the potential source of novel therapies.
- 33.Burrello C, Garavaglia F, Cribiu FM, et al. Therapeutic faecal microbiota transplantation controls intestinal inflammation through IL10 secretion by immune cells. Nat Commun. 2018. Dec 5;9(1):5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Q, Zhang Q, Wang C, et al. Fish oil enhances recovery of intestinal microbiota and epithelial integrity in chronic rejection of intestinal transplant. PLoS One. 2011;6(6):e20460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *35.Kaufman SS, Hussan E, Kroemer A, et al. Graft Versus Host Disease After Intestinal Transplantation: A Single-center Experience. Transplant Direct. 2021. Aug;7(8):e731. [DOI] [PMC free article] [PubMed] [Google Scholar]; The most complete case-series to date of GVHD after ITx, including new insights into risk factors and efficacy of various therapeutic regimens. Also includes largest series of ITx patient treated with ruxolitinib for GVHD.
- *36.Vianna R, Farag A, Gaynor JJ, et al. Association of Alemtuzumab Induction With a Significantly Lower Incidence of GVHD Following Intestinal Transplantation: Results of 445 Consecutive Cases From a Single Center. Transplantation. 2020. Oct;104(10):2179–88. [DOI] [PubMed] [Google Scholar]; Provides new insights into potentially protective effects of alemtuzumab when used as induction therapy that warrant further examination.
- 37.Clouse JW, Kubal CA, Fridell JA, et al. Post-intestine transplant graft-vs-host disease associated with inclusion of a liver graft and with a high mortality risk. Clin Transplant. 2019. Jan;33(1):e13409. [DOI] [PubMed] [Google Scholar]
- 38.Mazariegos GV, Abu-Elmagd K, Jaffe R, et al. Graft versus host disease in intestinal transplantation. Am J Transplant. 2004. Sep;4(9):1459–65. [DOI] [PubMed] [Google Scholar]
- 39.Wu G, Selvaggi G, Nishida S, et al. Graft-versus-host disease after intestinal and multivisceral transplantation. Transplantation. 2011. Jan 27;91(2):219–24. [DOI] [PubMed] [Google Scholar]
- 40.Shin CR, Nathan J, Alonso M, et al. Incidence of acute and chronic graft-versus-host disease and donor T-cell chimerism after small bowel or combined organ transplantation. J Pediatr Surg. 2011. Sep;46(9):1732–8. [DOI] [PubMed] [Google Scholar]
- 41.Brennan DC, Flavin K, Lowell JA, et al. A randomized, double-blinded comparison of Thymoglobulin versus Atgam for induction immunosuppressive therapy in adult renal transplant recipients. Transplantation. 1999. Apr 15;67(7):1011–8. [DOI] [PubMed] [Google Scholar]
- 42.Gaber AO, First MR, Tesi RJ, et al. Results of the double-blind, randomized, multicenter, phase III clinical trial of Thymoglobulin versus Atgam in the treatment of acute graft rejection episodes after renal transplantation. Transplantation. 1998. Jul 15;66(1):29–37. [DOI] [PubMed] [Google Scholar]
- 43.Jol-van der Zijde CM, Bredius RG, Jansen-Hoogendijk AM, et al. IgG antibodies to ATG early after pediatric hematopoietic SCT increase the risk of acute GVHD. Bone Marrow Transplant. 2012. Mar;47(3):360–8. [DOI] [PubMed] [Google Scholar]
- 44.Bakonyi A, Berho M, Ruiz P, et al. Donor and recipient pretransplant conditioning with nonlethal radiation and antilymphocyte serum improves the graft survival in a rat small bowel transplant model. Transplantation. 2001. Sep 27;72(6):983–8. [DOI] [PubMed] [Google Scholar]
- **45.Ghobrial S, Gonzalez C, Yazigi N, et al. Efficacy and feasibility of ruxolitinib in chronic steroid-refractory GVHD in a pediatric intestine transplant. Pediatr Transplant. 2021. May;25(3):e13836. [DOI] [PubMed] [Google Scholar]; First report of efficacy of ruxolitinib for GVHD after ITx in a way that may change practices and that should generate further trials.
- 46.Houston BL, Yan M, Tinckam K, et al. Extracorporeal photopheresis in solid organ transplant-associated acute graft-versus-host disease. Transfusion. 2016. Apr;56(4):962–9. [DOI] [PubMed] [Google Scholar]
- 47.Lauro A, Arpinati M, Zanfi C, et al. Extracorporeal photopheresis for chronic GVHD: case report after adult bowel-abdominal wall transplantation. Transplantation. 2013. Jul 27;96(2):e9–e10. [DOI] [PubMed] [Google Scholar]
- *48.van Lier YF, Davids M, Haverkate NJE, et al. Donor fecal microbiota transplantation ameliorates intestinal graft-versus-host disease in allogeneic hematopoietic cell transplant recipients. Sci Transl Med. 2020. Aug 12;12(556). [DOI] [PubMed] [Google Scholar]; Excellent recent mechanistic analysis of the attenuation of intestinal GVHD by FMT after HSCT that provides rationale for trials of FMT for GVHD agter ITx. Also an excellent illustration of the way in which changes in homeostasis between intestinal microbes and mucosal defenses can precipitate inflammation in the gut and even systemically.
- 49.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008. May 10;371(9624):1579–86. [DOI] [PubMed] [Google Scholar]
- 50.Matsuzawa-Ishimoto Y, Hine A, Shono Y, et al. An intestinal organoid-based platform that recreates susceptibility to T-cell-mediated tissue injury. Blood. 2020. Jun 25;135(26):2388–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011. Jun 15;474(7351):307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dogan SM, Kilinc S, Kebapci E, et al. Mesenchymal stem cell therapy in patients with small bowel transplantation: single center experience. World J Gastroenterol. 2014. Jul 7;20(25):8215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]


