Abstract
Background
The risk of SARS-CoV-2 infection and severity with disease modifying therapies (DMTs) in multiple sclerosis (MS) remains unclear, with some studies demonstrating increased risks of infection with B-cell-depleting (anti-CD20) therapies and severity, while others fail to observe an association. Most existing studies are limited by a reliance on ‘numerator’ data (i.e., COVID-19 cases) only.
Objective
To assess the risks of COVID-19 by DMT, this study aimed to assess both ‘numerator’ (patients with SARS-CoV-2 infection) and ‘denominator’ data (all patients treated with DMTs of interest) to determine if any DMTs impart an increased risk of SARS-CoV-2 infection or disease severity.
Methods
We systematically reviewed charts and queried patients during clinic encounters in the NYU MS Comprehensive Care Center (MSCCC) for evidence of COVID-19 in all patients who were on the most commonly used DMTs in our clinic (sphingosine-1-phosphate receptor (S1P) modulators (fingolimod/siponimod), rituximab, ocrelizumab, fumarates (dimethyl fumarate/diroximel fumarate), and natalizumab). COVID-19 status was determined by clinical symptoms (CDC case definition) and laboratory testing where available (SARS-CoV-2 PCR, SARS-CoV-2 IgG). Multivariable analyses were conducted to determine predictors of infection and severe disease (hospitalization or death) using SARS-CoV-2 infected individuals per DMT group and all individuals on a given DMT as denominator.
Results
We identified 1,439 MS patients on DMTs of interest, of which 230 had lab-confirmed (n = 173; 75.2%) or suspected (n = 57; 24.8%) COVID-19. Infection was most frequent in those on rituximab (35/138; 25.4%), followed by fumarates (39/217; 18.0%), S1P modulators (43/250; 17.2%), natalizumab (36/245; 14.7%), and ocrelizumab (77/589; 13.1%). There were 14 hospitalizations and 2 deaths. No DMT was found to be significantly associated with increased risk of SARS-CoV-2 infection. Rituximab was a predictor of severe SARS-CoV-2 infection among patients with SARS-CoV-2 infection (OR 6.7; 95% CI 1.1-41.7) but did not reach statistical significance when the entire patient population on DMT was used (OR 2.8; 95% CI 0.6-12.2). No other DMT was associated with an increased risk of severe COVID-19.
Conclusions
Analysis of COVID-19 risk among all patients on the commonly used DMTs did not demonstrate increased risk of infection with any DMT. Rituximab was associated with increased risk for severe disease.
Keywords: Multiple Sclerosis, COVID-19, disease severity, disease-modifying therapy
Abbreviations: MS, Multiple Sclerosis; DMT, Disease modifying therapy; MSCCC, Multiple Sclerosis comprehensive care center; OR, Odds ratio; CI, Confidence Interval; FUM, Fumarates; S1P, Sphingosine-1-phosphate receptor; NAT, Natalizumab; OCR, Ocrelizumab; RTX, Rituximab
1. Introduction
Whether any disease modifying therapy (DMT) for multiple sclerosis (MS) increases the risk for SARS-CoV-2 infection or severe disease (hospitalization/death) has not been resolved to date (Chaudhry, Bulka et al., 2020; Hughes, Whitley et al., 2020; Loonstra, Hoitsma et al., 2020; Louapre, Collongues et al., 2020; Möhn, Konen et al., 2020; Parrotta, Kister et al., 2020; Safavi, Nourbakhsh et al., 2020; Sahraian, Azimi et al., 2020; Alonso, Silva et al., 2021; Arrambide, Llaneza-González et al., 2021; Bsteh, Assar et al., 2021; Esmaeili, Abbasi et al., 2021; Fragoso, Schiavetti et al., 2021; Langer-Gould, Smith et al., 2021; Mantero, Abate et al., 2021; Naghavi, Kavosh et al., 2021; Pérez, Zhang et al., 2021; Reder, Centonze et al., 2021; Rm, Em et al., 2021; Salter, Fox et al., 2021; Sen, Karabudak et al., 2021; Sepúlveda, Llufriu et al., 2021; Simpson-Yap, De Brouwer et al., 2021; Sormani, De Rossi et al., 2021; Spelman, Forsberg et al., 2021; Sormani, Schiavetti et al., 2022). Some studies show increased risk of infection or severe COVID-19 with anti-CD20 therapies (Möhn, Konen et al., 2020; Safavi, Nourbakhsh et al., 2020; Sahraian, Azimi et al., 2020; Esmaeili, Abbasi et al., 2021; Fragoso, Schiavetti et al., 2021; Langer-Gould, Smith et al., 2021; Naghavi, Kavosh et al., 2021; Pérez, Zhang et al., 2021; Reder, Centonze et al., 2021; Salter, Fox et al., 2021; Simpson-Yap, De Brouwer et al., 2021; Sormani, De Rossi et al., 2021; Spelman, Forsberg et al., 2021; Sormani, Schiavetti et al., 2022) while others fail to detect an increased risk (Chaudhry, Bulka et al., 2020; Hughes, Whitley et al., 2020; Loonstra, Hoitsma et al., 2020; Louapre, Collongues et al., 2020; Parrotta, Kister et al., 2020; Alonso, Silva et al., 2021; Arrambide, Llaneza-González et al., 2021; Bsteh, Assar et al., 2021; Mantero, Abate et al., 2021; Rm, Em et al., 2021; Sen, Karabudak et al., 2021; Sepúlveda, Llufriu et al., 2021). The discrepant conclusions between these studies may be due in part to methodological limitations. Studies that examined COVID-19 risk across DMTs frequently relied on numerator data only, i.e., did not account for the frequency with which each DMT is used in the respective MS population. To properly assess the risks per DMT, one needs both numerator data (the number of COVID-19 cases occurring on a given DMT) as well the denominator data (total number of patients on that DMT).
The few studies that used both numerator and denominator data to estimate relative risk of SARS-CoV-2 infection by DMT are limited by incomplete data capture. For example, the analysis of the IBM Explorys database (>72 million patients in the US) identified an increased risk of infection with anti-CD20 therapies, but could not yield “strong conclusions” about the effect of DMT on hospitalization and deaths due to the very low incidence of severe disease, as well as cases in general (likely an undercount of mildly symptomatic, asymptomatic, or unconfirmed SARS-CoV-2infections) (Reder, Centonze et al., 2021). Moreover, only 20% of MS patients in this dataset had an open DMT prescription, which is a surprisingly low percentage for MS patients and underscores gaps in the data (Reder, Centonze et al., 2021). An Iranian study from an early phase of the pandemic that utilized numerator and denominator data had a small total number of COVID cases (n=68), likely due to the requirement of positive SARS CoV-2 PCR or lung CT scan supporting the diagnosis of COVID-19, and showed an increased rate of SARS-CoV-2infection in patients on rituximab (Sahraian, Azimi et al., 2020).
To overcome some of these methodologic limitations, we set out to systematically collect both numerator (number of COVID-19 cases on DMTs of interest including both clinician-diagnosed and lab-confirmed cases) and denominator (number of patients on DMTs of interest) data for the most common DMTs prescribed in the NYU Multiple Sclerosis Comprehensive Care Center (NYU MSCCC). We present the adjusted risks of SARS-CoV-2 infection and of severe disease per DMT among our MS patients receiving fumarates, sphingosine-1-phosphate receptor (S1P) modulators, natalizumab, and two anti-CD20 therapies (rituximab and ocrelizumab) during the first 15 months of the COVID-19 pandemic (February 2020 to May 2021).
2. Methods
Inclusion criteria for the denominators were: clinician-diagnosed MS; actively followed at the NYU MSCCC (at least 2 clinic visits in the past 2 years); and on treatment with a commonly used DMT during the period between February 2020 and May 2021. The commonly used DMTs in our practice were: 1. fumarates (FUM: dimethyl fumarate and diroximel fumarate), 2. Sphingosine-1-phosphate receptor modulators (S1P modulators: fingolimod and siponimod), 3. natalizumab (NAT), 4. ocrelizumab (OCR), and 5. rituximab (RTX). Fumarates and S1P modulators were each analyzed as a medication class, because the number of patients on siponimod and diroximel fumarate were too small for separate analysis. Patients on rituximab and ocrelizumab were analyzed separately since adequate number of patients were available. Patients on other DMTs (e.g., platform injectables, teriflunomide, alemtuzumab, cladribine) were not included in this study as the number of patients on these therapies in our practice was too small to enable meaningful statistical comparisons.
Patients who contacted the center or were seen during routine clinic visits between February 2020 and May 2021 were queried regarding SARS-CoV-2 infection using a standardized instrument that included questions regarding common COVID-19 symptoms (per CDC clinical case definition criteria), such as fever, cough, chills, loss of taste and smell, and dyspnea, and if they had been formally diagnosed with COVID-19 either clinically by a medical professional and/or underwent laboratory testing for SARS CoV-2 (a copy of the lab report was required for the case to be defined as ‘laboratory-confirmed’). Additionally, we undertook a systematic chart review of all actively followed MS patients receiving DMTs of interest to identify patients with symptoms of COVID-19 and laboratory findings indicative of current or prior SARS-CoV-2 infection. All patients diagnosed with COVID-19 by a healthcare provider based on clinical criteria (Centers for Disease Control and Prevention) or had positive SARS-CoV-2 PCR/antibodies were included in the numerator data (COVID-19 cases). Patients with COVID-19 clinical diagnosis, but lacking laboratory confirmation were defined as ‘suspected,’ while patients with positive SARS-CoV-2 PCR or SARS-CoV-2-specific antibody were defined as ‘laboratory-confirmed.’ ‘Severe COVID-19’ was defined as hospitalization and/or death. Comorbidities relevant to COVID-19 severity were collected for COVID-19 cases (numerators) only: cancer; cardiovascular disease; cerebrovascular disease; chronic kidney disease; chronic liver disease; chronic lung disease; diabetes; hypertension, and obesity (Centers for Disease Control and Prevention 2021). For hospitalized patients, inpatient records were reviewed when available. Vaccination status was not collected, as no vaccines were available at the start of the study period.
Descriptive statistics (frequency distribution for categorical variables and mean, SD, median, interquartile range, minimum, and maximum for continuous variables) were calculated for both numerator and denominator data. Descriptive statistics and Chi-Square/Fisher Exact tests were used for exploratory analyses. Univariate logistic regression was used to screen variables with a p-value criterion of p < 0.05 for entry into the model selection procedure. Multivariable logistic regression model was performed with the following variables as predictors: age, race/ethnicity, and DMT to calculate risk of infection. The risk of severe COVID-19 was calculated for the entire sample (severe COVID-19 on a given DMT per all individuals on given DMT) as well as for those with any SARS-CoV-2 infection (severe COVID-19 on a given DMT per all COVID-19 cases on a given DMT). Age, race/ethnicity, DMT, and BMI where available (BMI was only collected for COVID-19 cases), were used as predictors to assess risk of severe disease. Analysis of maximum likelihood estimates was also done. A sensitivity analysis was conducted for “laboratory-confirmed COVID-19” cases only. Fumarates were used as a reference group, similar to prior studies, because fumarates have not been identified to impart an increased risk of infection and are frequently used in clinical practice (Mehta, Miller et al., 2019; Naghavi, Kavosh et al., 2021; Reder, Centonze et al., 2021; Simpson-Yap, De Brouwer et al., 2021).
NYU Grossman School of Medicine Institutional Review Board approved the study. Informed consent was not required for this retrospective study.
3. Data Availability
Anonymized data can be made available upon request for research purposes by submitting a request to the corresponding author.
4. Results
We identified 1,439 MS patients on DMTs of interest (mean age: 41.2 ± 12.1 years; 71% female, 54% non-White). Demographics and disease characteristics of patients on each of the five DMT groups are shown in Table 1 .
Table 1.
Demographics for patients per DMT group.
| FUM | S1P | NTZ | OCR | RTX | ||
|---|---|---|---|---|---|---|
| Total patients per DMT | 217 | 250 | 245 | 589 | 138 | |
| Age (yrs, mean ± SD) | 46.3 ± 11.4 | 40.6 ± 11.0 | 40.0 ± 12.8 | 40.0 ± 11.6 | 41.1 ± 13.0 | |
| Race/Ethnicity | White | 55% | 50% | 44% | 40% | 42% |
| Black | 18% | 19% | 28% | 26% | 30% | |
| Other | 19% | 20% | 22% | 24% | 19% | |
| Hispanic | 8% | 11% | 7% | 10% | 10% | |
| Public Insurance | 28% | 22% | 34% | 41% | 49% |
Legend:
FUM: dimethyl fumarate, diroximel fumarate; S1P: fingolimod, siponimod; NTZ: natalizumab; OCR: ocrelizumab; RTX: rituximab.
We identified 230 patients in our sample with SARS-CoV-2 infection – 173 (75%) were laboratory-confirmed and 57 (25%) clinically-suspected. The average age of patients with SARS-CoV-2 infection was 39.9 ± 12.3 years, 75% were female, and 57% were non-White. Demographics and disease characteristics of COVID-19 cases by DMT group are shown on Table 2 . The proportion of infected patients was highest for rituximab (25%), followed by fumarates (18%), S1P modulators (17%), natalizumab (15%), and ocrelizumab (13%). The proportion of lab-confirmed cases was lower in patients on anti-CD20 (69% for ocrelizumab and 60% for rituximab) than in patients on other therapies (87% fumarates, 84% S1P modulators, and 81% natalizumab). The proportion of patients with at least one COVID-19 relevant comorbidity was lowest in the rituximab group (20%) and highest in the fumarate group (38%). Mean duration on the current therapy was approximately 4.5 years for all DMT except for ocrelizumab, for which the mean duration on current therapy was 1.8 years.
Table 2.
Demographics and MS Characteristics of COVID-19 Cases by Disease Modifying Therapy.
| FUM1 | S1P | NTZ | OCR | RTX | ||
|---|---|---|---|---|---|---|
| COVID-19 Cases/Total patients per DMT | 39/217 (18%) | 43/250 (17%) |
36/245 (15%) |
77/589 (13%) |
35/138 (25%) |
|
| Lab confirmed2 | 87% | 84% | 81% | 69% | 60% | |
| Age (mean ± SD) | 44.6 ± 12.2 | 40.2 ± 11.7 | 37.3 ± 13.9 | 40.5 ± 11.4 | 35.4 ± 11.9 | |
| Age Groups | 0-29 yrs | 13% | 26% | 28% | 19% | 34% |
| 30-49 yrs | 46% | 49% | 56% | 58% | 51% | |
| 50-64 yrs | 33% | 26% | 17% | 21% | 11% | |
| ≥65 yrs | 6% | 0 | 0 | 1% | 3% | |
| Race/Ethnicity | White | 54% | 35% | 44% | 42% | 46% |
| Black | 26% | 30% | 25% | 19% | 23% | |
| Other | 13% | 21% | 17% | 18% | 11% | |
| Hispanic | 8% | 14% | 14% | 21% | 20% | |
| Current Tx Duration (yrs, mean ± SD)3 | 3.9 ± 2.6 | 4.4 ± 2.6 | 5.1 ± 4.0 | 1.8 ± 1.3 | 4.6 ± 3.4 | |
| Co-morbidity4 | 38% | 21% | 19% | 31% | 20% | |
| BMI ≥30 (%) | 39% | 26% | 31% | 39% | 31% | |
| Ambulation status | Fully Ambulatory | 72% | 67% | 75% | 61% | 74% |
| Impaired | 13% | 19% | 11% | 17% | 6% | |
| Cane | 5% | 5% | 8% | 10% | 9% | |
| Walker | 5% | 7% | 3% | 9% | 6% | |
| Non-ambulatory5 | 5% | 2% | 3% | 3% | 6 % | |
| Public Insurance (%) | 33% | 28% | 33% | 44% | 54% |
Legend:
Reference group.
PCR or serum antibody/total cases reviewed.
Data regarding current treatment duration only available for those with SARS-CoV-2 infection.
Percentage of patients with any COVID-19-relevant co-morbidity: Cancer, Cardiovascular disease; Cerebrovascular disease; Chronic kidney disease; Chronic liver disease; Chronic lung disease; Diabetes; Hypertension.
Non-ambulatory specifies patients who are in wheelchairs or bedbound.
FUM: dimethyl fumarate, diroximel fumarate; S1P: fingolimod, siponimod; NTZ: natalizumab; OCR: ocrelizumab; RTX: rituximab.
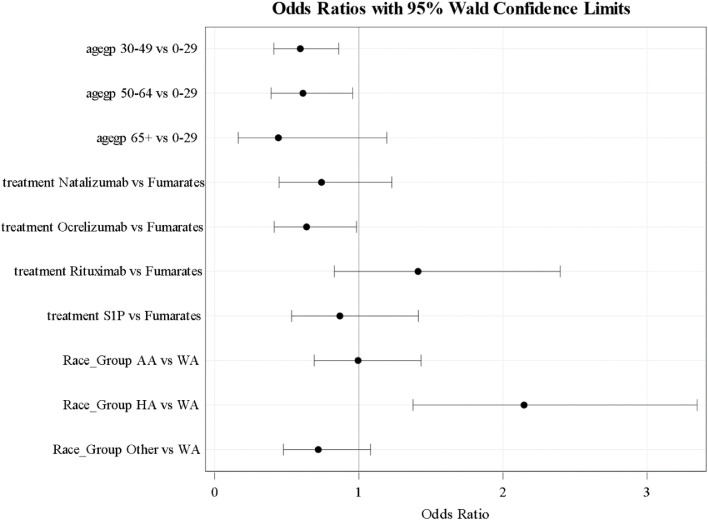
Multivariable logistic analysis revealed a higher risk of infection was associated with Hispanic ethnicity (OR 2.72; 95% CI 1.38-3.35) as compared to Whites; and decreased risk in age groups 30-49 (OR 0.59; 95% CI 0.41-0.86) and 50-64 (OR 0.61; 95% CI 0.39-0.96) relative to the 0-29 age group as shown in Fig. 1 . Sensitivity analysis for predictors of SARS-CoV-2infection using only laboratory-confirmed COVID-19 cases yielded similar results (data not shown).
Fig. 1.
Multivariate analysis of predictors of SARS-CoV-2 infection.
Fifteen COVID-19 cases had severe disease: 14 were hospitalized (6.1% of COVID-19 cases), and 2 died (0.8% of all COVID-19 cases; 1 death each occurred at home and hospital). Characteristics of severe COVID-19 cases by DMT group are shown in Table 3 . The percentage of severe COVID-19 relative to all COVID-19 cases on a given DMT was highest for rituximab group (14%), followed by fumarates (8%), S1P modulators (5%), ocrelizumab (5%) and natalizumab (3%). A multivariable model that included those with SARS-CoV-2 infection (severe COVID-19 on a given DMT per all COVID-19 cases on a given DMT) identified African-American and Hispanic race/ethnicity relative to Whites (OR 4.4; 95% CI 1.0-18.5; OR 5.4; 95% CI 1.1-28.5, respectively); older age (OR 1.1; 95% CI 1.0-1.1), and rituximab (OR 6.7; 95% CI 1.1-41.7), but not ocrelizumab (OR 0.9; 95% CI 0.2-4.9) relative to fumarates as variables associated with higher risk of severe disease. We analyzed whether comorbid conditions were associated with severe COVID-19 in univariate analyses, but because these analyses did not demonstrate significance, this variable was not included in the final multivariate model.
Table 3.
Hospitalization and/or death due to COVID-19 Cases Demographics by Disease Modifying Therapy.
| FUM1 | S1P | NTZ | OCR | RTX | ||
|---|---|---|---|---|---|---|
| Total2 | 3/39 | 2/43 | 1/36 | 4/77 | 5/35 | |
|
Age (yrs, mean ± SD) (range) |
46.7 ± 12.7 (32-54) |
61.5 ± 0.7 (61, 62) | 60.0 | 43.5 ± 12.6 (31-60) | 40.0 ± 12.4 (23-55) | |
| Race/Ethnicity (n) | White | 1 | 0 | 0 | 0 | 3 |
| Black | 2 | 1 | 1 | 1 | 1 | |
| Other | 0 | 0 | 0 | 0 | 1 | |
| Hispanic | 0 | 1 | 0 | 3 | 0 | |
|
Tx Duration (yrs, mean ± SD) |
3.7 ± 3.2 | 4.0 ± 2.8 | 12.0 | 3.0 ± 2.7 | 5.2 ± 2.3 | |
| Co-morbidity (n)3 | 1 | 2 | 1 | 2 | 1 | |
| BMI ≥30 (n) | 2 | 2 | 1 | 2 | 2 | |
| Assistive Device (n) | Fully Ambulatory | 2 | 0 | 1 | 2 | 2 |
| Impaired | 0 | 0 | 0 | 0 | 0 | |
| Cane | 0 | 0 | 0 | 0 | 1 | |
| Walker | 1 | 1 | 0 | 2 | 0 | |
| Non-ambulatory4 | 0 | 1 | 0 | 0 | 2 | |
| Public Insurance (n) | 1 | 2 | 0 | 0 | 4 |
Legend:
Reference group.
Total hospitalizations and deaths/total COVID-19 cases.
Percentage of patients with any COVID-19-relevant co-morbidity: Cancer, Cardiovascular disease; Cerebrovascular disease; Chronic kidney disease; Chronic liver disease; Chronic lung disease; Diabetes; Hypertension.
Non-ambulatory specifies patients who are in wheelchairs or bedbound.
FUM: dimethyl fumarate, diroximel fumarate; S1P: fingolimod, siponimod; NTZ: natalizumab; OCR: ocrelizumab; RTX: rituximab.
In the univariate analysis of severe COVID-19 relative to the total number of patients treated with the given DMT, we again identified Hispanic ethnicity as a predictor of severe COVID-19 (3.1% of severe COVID-19 for Hispanics vs 0.3% for White; p = 0.004). Rituximab was not a predictor of severe disease (3.6% severe disease for RTX v. 1.4% for fumarates; p = 0.27), nor were any other DMTs predictive of severe disease in this univariate analysis (percentage of severe disease in S1P modulators was 0.80%, natalizumab 0.41%, and ocrelizumab 0.68%). In a multivariable analysis adjusted for age in which the total population was used (severe COVID-19 on a given DMT per all individuals on given DMT), only Hispanic ethnicity was associated with higher odds of severe disease (OR 6.2; 95% CI 1.5-26.2). In this analysis, rituximab had higher odds for severe disease -2.8, but this was not was not statistically significant (95% CI 0.6-12.2).
5. Discussion
Lack of consistency in identifying DMT associated with worse COVID-19 prognosis is likely due to several factors; among them is the choice of reference drug. When computing odds ratios, so called Wald statistics are commonly used which take the maximum likelihood estimate of the slope (beta coefficient) divided by its standard error. The estimate of the beta coefficient is the added risk of infection relative to one of the other DMTs or other categories included in that specific grouping of covariates. For example, ocrelizumab was the only DMT that was significantly likely to be associated with decreased infection when compared fumarates (OR 0.64; 95% CI 0.41-0.98), but not when compared with S1P (OR 1.63; 95% CI 0.97-2.72). If ocrelizumab was chosen as reference, the odds ratio for rituximab (OR 2.3; CI 1.32-4.40) and to natalizumab (OR 1.9; 95% CI 1.12-3.23) were statistically significant, while comparison with S1P modulators (OR 1.63; 95% CI 0.97-2.72) was not significant. As these comparisons demonstrate, the choice of reference group and statistical analyses may lead to different conclusions regarding the effect of DMT on SARS-CoV-2 infection and severity that have been published in the literature to date.
While rituximab was associated with severe disease in some analyses, similar findings were not replicated with ocrelizumab in several studies (Sahraian, Azimi et al., 2020; Salter, Fox et al., 2021; Simpson-Yap, De Brouwer et al., 2021) despite a similar mechanism of action. Neither age nor presence of comorbidities could explain the difference between the two anti-CD20 agents in our study. In fact, both age (RTX mean age 35.7 ± 11.9 years v. OCR 40.5 ± 11.4 years; p = 0.04, 95% CI 0.13-9.46) and frequency of comorbid conditions (RTX 20% v. OCR 31.2%; p = 0.319) were lower for RTX than OCR. It is possible that the discrepancy in COVID-19 outcomes is due to the longer median treatment duration of RTX (4.6 ± 3.4 v. OCR 1.8 ± 1.3 years; p = 0.18; 95% CI -5.78-1.07), which increases the risk of secondary hypogammaglobulinemia (Tallantyre, Whittam et al., 2018; Vollmer, Vollmer et al., 2020), infection (Perriguey, Maarouf et al., 2022), and hospitalization from infections (Spelman, Forsberg et al., 2021). Alternatively, rituximab may cause a more pronounced immune defect than ocrelizumab per dose, in which case we would expect difference in risk of COVID-19 severity between RTX and OCR to persist independent of treatment duration. It is also possible that differences between RTX and OCR are unrelated to treatment and were unaccounted for in the multivariable analysis (i.e., unknown confounders).
There are several other potential explanations for why the effect of anti-CD20 therapies are more evident in some studies than others. Ascertainment bias is an important potential source of influence in both the present and prior similar studies. Patients on DMTs that are regarded to be more immunosuppressive and therefore considered ‘high risk’ for COVID-19 complications (e.g., anti-CD20 therapies) may be more prone to get testing for their symptoms and to report COVID-19 diagnosis to their provider than patients who are on presumably ‘lower risk’ DMT (e.g., fumarates). There may be some evidence for ascertainment bias in our data as there was a higher proportion of suspected (but not lab-confirmed) infections with the two anti-CD20 therapies compared to other DMTs (33.9% for anti-CD20 versus 15.3% for other DMTs; p = 0.001). However, it is also possible that anti-CD20-treated patients, which are much less likely to generate SARS CoV-2 specific antibodies following infection (Ali, Dwyer et al., 2021; Bigaut, Kremer et al., 2021; Bsteh, Dürauer et al., 2021; Disanto, Sacco et al., 2021; Louapre, Ibrahim et al., 2021; Sabatino, Mittl et al., 2021; Sharifian-Dorche, Sahraian et al., 2021; Sormani et al., 2021; van Kempen, Strijbis et al., 2021; Wallach and Picone, 2021), are therefore more likely to remain ‘suspected but not confirmed given the lack of detectable antibody results with commercially available tests following infection. Furthermore, patients on infusion therapies tend to have more frequent contact with the physicians and therefore more opportunities to report an infection. In our study, we sought to mitigate against this bias by systematically reviewing all active patients on all DMTs independent of DMTs’ presumed risk for COVID-19 complications.
A limitation of our study is that we did not systematically assess the incidence of SARS-CoV-2 infection in our patient population. As this was not our clinical practice, we are not able to report on the seroprevalence of asymptomatic infections in our patient populations. However, we have encountered asymptomatic patients who had positive SARS-CoV-2 antibody tests, either because they requested this test, were tested by outside providers, or were tested as part of unrelated research studies. These incidentally discovered asymptomatic cases were included in the analyses. However, it was not the aim of our study to comprehensively assess rate of asymptomatic infection, and our numbers almost certainly reflect an undercount of asymptomatic cases.
The crude death rate in our study, 0.87%, is among the lowest reported for MS cohorts (Chaudhry, Bulka et al., 2020; Hughes, Whitley et al., 2020; Loonstra, Hoitsma et al., 2020; Louapre, Collongues et al., 2020; Möhn, Konen et al., 2020; Parrotta, Kister et al., 2020; Sahraian, Azimi et al., 2020; Alonso, Silva et al., 2021; Arrambide, Llaneza-González et al., 2021; Bsteh, Assar et al., 2021; Esmaeili, Abbasi et al., 2021; Moghadasi, Mirmosayyeb et al., 2021; Pérez, Zhang et al., 2021; Prosperini, Tortorella et al., 2021; Reder, Centonze et al., 2021; Salter, Fox et al., 2021; Sepúlveda, Llufriu et al., 2021; Sharifian-Dorche, Sahraian et al., 2021; Sormani, De Rossi et al., 2021; Spelman, Forsberg et al., 2021). This may be due to the fact that we excluded untreated patients, who tend to be older, more disabled, and to have a higher number of comorbidities and more frequently developed severe disease (Möhn, Konen et al., 2020; Naghavi, Kavosh et al., 2021; Simpson-Yap, De Brouwer et al., 2021). Another possible reason may be related to anti-CD20 dosing adjustments implemented in our clinic at the onset of the pandemic. Due to concern for increased severity of COVID-19 with anti-CD20 therapies at the start of the pandemic, we delayed infusions in stable patients, sometimes extending the interval between doses by up to a year or, on occasion, even longer. As time from infusion may be an independent predictor of COVID-19 severity (Langer-Gould, Smith et al., 2021), it is possible that the practice of delaying infusions may have led to better outcomes in patients on anti-CD20 therapies in our clinic than would be expected with the standard 6-month dosing intervals.
Improvement in COVID-19 management over the course of this study such as introduction of monoclonal antibodies to reduce hospitalization and use of dexamethasone in hospitalized patients, may also account for lower severity and fatality rates (Pérez, Zhang et al., 2021; Rm, Em et al., 2021). In addition, increased accessibility to testing led to improved detection of mild cases, which was not possible during the early phase of pandemic. An estimated 78% of cases in the United States were undocumented in 2020, so non-hospitalized cases were mostly undetected (Sen, Yamana et al., 2021). Finally, three vaccinations for COVID-19 received emergency use authorization (EUA) during the latter half of this study period (US Food & Drug Administration (FDA); US Food & Drug Administration (FDA); US Food & Drug Administration (FDA)), and patients on anti-CD20 therapies in particular were encouraged to vaccinate early. Of the 39 SARS-CoV-2 infections occurring between January 2021 (when SARS-CoV-2 vaccines started to become available to select patients) to May 2021 (end of our study period), a total of only 10 individuals reached fully-vaccinated status (defined as 2 weeks after completion of primary vaccine series), and only 3 of these individuals had breakthrough infections after becoming fully vaccinated. Given these small numbers, it is difficult to draw conclusions as to whether vaccination may have preferentially protected patients on some DMTs more than others. It is unlikely that vaccination had impacted our analyses, as patients who were infected with SARS CoV-2 were overwhelmingly infected during pre-vaccination period or did not reach fully-vaccinated status during this early phase of vaccination period.
Among limitations of our study is a relatively small sample size, which may have precluded detection of statistically significant differences for severe outcomes on anti-CD20 agents and other DMTs (type 2 errors). We would need a sample size of 1,141 per each DMT group to detect an increase in likelihood of death from 2% to 4%, with an α of 0.05, a β of 0.2 and a power of 0.8 (Korsukewitz, Reddel et al., 2020). Ultimately, relatively low fatality and hospitalization rates across all DMTs in our study is reassuring. Another limitation was the lack of access to gold standard PCR SARS-CoV-2 testing in the early phases of the pandemic, which we sought to mitigate by assessing SARS CoV-2 antibody titers in patients with a history of possible infection. Another limitation is possibility of missing an infection if it occurred after last clinic visit or contact with the NYU MSCCC; we sought to decrease this possibility by encouraging all patients to report infections to us as soon as possible.
We also examined other patient characteristics as risk factors for SARS-CoV-2 infection or severe disease. Hispanic ethnicity and younger age were predictors of infection in our study, which may be a reflection of the difficulty with which younger and Hispanic patients, many of whom may be ‘essential workers,’ live in more crowded conditions, and were unable to socially isolate to limit their exposure to the SARS-CoV-2 virus compared to older and more resource-rich patients. Hispanic ethnicity was also a risk factor for SARS-CoV-2 infection and complications in other studies from our area (Thompson, Baumgartner et al., 2020; Jin, Nesbitt et al., 2021) and in the United States (Magesh, John et al., 2021). Older age, obesity, and worse ambulation status are known risk factor for severe disease, and these findings were generally reflected in our sample as well (Chaudhry, Bulka et al., 2020; Louapre, Collongues et al., 2020; Etemadifar, Nouri et al., 2021; Pérez, Zhang et al., 2021; Salter, Fox et al., 2021). Some risk factors for COVID-19 severity such as presence of certain comorbidities and insurance status (as a proxy for socio-economic status) (Guan, Liang et al., 2020; Oh, Choi et al., 2021) were not observed in our study, likely due to a limited sample size and a relatively young population. Due to lack of resources, we limited in-depth chart review for relevant comorbidities to the 230 ‘numerator cases’ and did not collect these data on all 1,439 ‘denominator’ patients in our practice.
In conclusion, our single-center analysis of SARS-CoV-2 infection and severe disease in MS patients, does not suggest that any DMT increases the risk of infection. We did observe an association between severe infection and rituximab in some of our analyses. We did not observe increased risk of COVID-19 complications with ocrelizumab, which may be due to differences in the extent of B-cell depletion under different therapies, or due to duration of therapy, or other factors. Given the fact that anti-CD20 therapies have been identified as a risk factor for worse outcomes in other studies (Möhn, Konen et al., 2020; Safavi, Nourbakhsh et al., 2020; Sahraian, Azimi et al., 2020; Esmaeili, Abbasi et al., 2021; Fragoso, Schiavetti et al., 2021; Langer-Gould, Smith et al., 2021; Naghavi, Kavosh et al., 2021; Pérez, Zhang et al., 2021; Reder, Centonze et al., 2021; Salter, Fox et al., 2021; Simpson-Yap, De Brouwer et al., 2021; Sormani, De Rossi et al., 2021; Spelman, Forsberg et al., 2021; Sormani, Schiavetti et al., 2022), it is prudent to recommend extra precautions in B-cell depleted individuals, including booster vaccinations as recommended by federal health authorities, maintaining work-from-home conditions when feasible during times of local surges in COVID-19 cases, as well as extending dosing intervals between infusions or switch to non-anti-CD20 DMTs.
Disclosures: The institution of TES received support for a clinical fellowship from the National Multiple Sclerosis Society and Biogen. CS has received personal compensation as a consultant and/or speaker Bureau for Biogen, Genzyme, & BMS (Celgene). LC has received personal compensation for serving as a Consultant for Johnson & Johnson. The institution of LC has received research support from Biogen Idec. GC has received personal compensation for serving as a Consultant for Biodelivery Sciences International, Biogen, Click Therapeutics, Genzyme, Genentech, GW Pharmaceuticals, Immunic, Klein-Buendel Incorporated, Medimmune/Viela Bio, Medday, Merck/Serono, Neurogenesis LTD, Novartis, Osmotica Pharmaceuticals, Perception Neurosciences, Recursion/Cerexis Pharmaceuticals, Regeneron, Reckover Pharmaceuticals, Roche, TG Therapeutics and for serving as an Editor, Associate Editor, or Editorial Advisory Board Member for JASN. LK has received personal compensation for serving as a Consultant for Biogen, Janssen, Gerson Lerhman and served on a Scientific Advisory or Data Safety Monitoring board for Sanofi and Biogen, Board of Directors for Cleveland Clinic. The institution of LK has received research support from Biogen and NMSS. IK has received personal for serving as a Consultant for Genentech-Roche. The institution of IK has received research support from Genentech. The institution of IK has received research support from Biogen and CMSC, NMSS. LZR has received personal compensation for serving on a Scientific Advisory or Data Safety Monitoring board for Biogen, Genentech, Novartis. The institution of LZR has received research support from Biogen, Genentech and CMSC. The following report no disclosures: M. M., D. G., A. P., V. S., Z. R., G. Z., D. G.
Funding
This work was supported by an investigator-initiated grant from The Consortium of Multiple Sclerosis Centers.
Acknowledgements
None
Footnotes
The corresponding author takes full responsibility for the data, the analyses and interpretation, and the conduct of the research; the corresponding author has full access to all of the data; and corresponding author has the right to publish any and all data.
References
- Ali A., Dwyer D., Wu Q., Wang Q., Dowling C.A., Fox D.A., Khanna D., Poland G.A., Mao-Draayer Y. Characterization of humoral response to COVID mRNA vaccines in multiple sclerosis patients on disease modifying therapies. Vaccine. 2021;39(41):6111–6116. doi: 10.1016/j.vaccine.2021.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso R., Silva B., Garcea O., Diaz P.E.C., dos Passos G.R., Navarro D.A.R., Valle L.A.G., Salinas L.C.R., Negrotto L., Luetic G., Tkachuk V.A., Míguez J., de Bedoya F.H.D., Goiry L.G., Sánchez N.E.R., Burgos M., Steinberg J., Balbuena M.E., Alvarez P.M., López P.A., Ysrraelit M.C., León R.A., Cohen A.B., Gracia F., Molina O., Casas M., Deri N.H., Pappolla A., Patrucco L., Cristiano E., Tavolini D., Nadur D., Granda A.M.T., Weiser R., Cassará F.P., Sinay V., Rodríguez C.C., Lazaro L.G., Menichini M.L., Piedrabuena R., Escobar G.O., Carrá A., Chertcoff A., Pujols B.S., Vrech C., Tarulla A., Carvajal R., Mainella C., Becker J., Peeters L.M., Walton C., Serena M.A., Nuñez S., Rojas J.I. COVID-19 in multiple sclerosis and neuromyelitis optica spectrum disorder patients in Latin America: COVID-19 in MS and NMOSD patients in LATAM. Multiple Sclerosis and Related Disorders. 2021 doi: 10.1016/j.msard.2021.102886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrambide G., Llaneza-González M.Á., Costa-Frossard França L., Meca-Lallana V., Díaz E.F.-., Moreno-Torres I., García-Domínguez J.M., Ortega-Suero G., Ayuso-Peralta L., Gómez-Moreno M., Sotoca-Fernández J.J., Caminero-Rodríguez A.B., Rodríguez de Antonio L.A., Corujo-Suárez M., Otano-Martínez M.A., Pérez-Miralles F.C., Reyes-Garrido V., Ayuso-Blanco T., Balseiro-Gómez J.J., Muñoz-Pasadas M., Pérez-Molina I., Arnal-García C., Domingo-Santos Á., Guijarro-Castro C., Íñiguez-Martínez C., Téllez Lara N., Castellanos-Pinedo F., Castillo-Triviño T., Cerdán-Santacruz D.M., Pérez-Sempere Á., Torres B.S., Álvarez de Arcaya A., Costa-Arpín E., Durán-Ferreras E., Fragoso-Martínez M., González-Platas M., Landete Pascual L., Millán-Pascual J., Oreja-Guevara C., Meca-Lallana J.E. SARS-CoV-2 Infection in Multiple Sclerosis. Results of the Spanish Neurology Society Registry. 2021;8(5):e1024. doi: 10.1212/NXI.0000000000001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigaut K., Kremer L., Fabacher T., Lanotte L., Fleury M.-C., Collongues N., de Seze J. Impact of Disease-Modifying Treatments of Multiple Sclerosis on Anti–SARS-CoV-2 Antibodies. An Observational Study. 2021;8(5):e1055. doi: 10.1212/NXI.0000000000001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bsteh G., Assar H., Hegen H., Heschl B., Leutmezer F., Di Pauli F., Gradl C., Traxler G., Zulehner G., Rommer P., Wipfler P., Guger M., Enzinger C., Berger T. COVID-19 severity and mortality in multiple sclerosis are not associated with immunotherapy: Insights from a nation-wide Austrian registry. PLoS One. 2021;16(7) doi: 10.1371/journal.pone.0255316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bsteh G., Dürauer S., Assar H., Hegen H., Heschl B., Leutmezer F., Pauli F.D., Gradl C., Traxler G., Zulehner G., Rommer P., Wipfler P., Guger M., Höftberger R., Enzinger C., Berger T. "Humoral immune response after COVID-19 in multiple sclerosis: A nation-wide Austrian study. Mult Scler: 2021 doi: 10.1177/13524585211049391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention National Notifiable Diseases Surveillance System (NNDSS) Coronavirus Disease 2019 (COVID-19) Coronavirus Disease. 2019 https://ndc.services.cdc.gov/case-definitions/coronavirus-disease-2019-2021/ (COVID-19) 2021 Case Definition " Retrieved November 23 2021, from. [Google Scholar]
- Centers for Disease Control and Prevention (2021). “Science Brief: Evidence used to update the list of underlying medical conditions that increase a person's risk of severe illness from COVID-19”. [PubMed]
- Chaudhry F., Bulka H., Rathnam A.S., Said O.M., Lin J., Lorigan H., Bernitsas E., Rube J., Korzeniewski S.J., Memon A.B., Levy P.D., Schultz L., Javed A., Lisak R., Cerghet M. COVID-19 in multiple sclerosis patients and risk factors for severe infection. J Neurol Sci. 2020;418 doi: 10.1016/j.jns.2020.117147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disanto G., Sacco R., Bernasconi E., Martinetti G., Keller F., Gobbi C., Zecca C. Association of Disease-Modifying Treatment and Anti-CD20 Infusion Timing With Humoral Response to 2 SARS-CoV-2 Vaccines in Patients With Multiple Sclerosis. JAMA Neurology. 2021 doi: 10.1001/jamaneurol.2021.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaeili S., Abbasi M.H., Abolmaali M., Mojtahed M., Alavi S.N.R., Soleimani S., Mokhtari M., Hatam J., Khotbehsara S.T., Motamed M.R., Joghataei M.T., Mirzaasgari Z., Moghaddasi M. Rituximab and risk of COVID-19 infection and its severity in patients with MS and NMOSD. BMC Neurol. 2021;21(1):183. doi: 10.1186/s12883-021-02218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etemadifar M., Nouri H., Maracy M.R., Akhavan Sigari A., Salari M., Blanco Y., Sepúlveda M., Zabalza A., Mahdavi S., Baratian M., Sedaghat N. Risk factors of severe COVID-19 in people with multiple sclerosis : A systematic review and meta-analysis. Rev Neurol (Paris) 2021 doi: 10.1016/j.neurol.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragoso Y.D., Schiavetti I., Carmisciano L., Ponzano M., Steinberg J., Treviño-Frenk I., Ciampi E., Vecino M.C.A., Correa E.P., Carcamo C., Gomes S., Pimentel M.L.V., Santos G.A.C., Vrech C., Winckler T.C.A., Sormani M.P. Coronavirus disease 2019 in Latin American patients with multiple sclerosis. Mult Scler Relat Disord. 2021;55 doi: 10.1016/j.msard.2021.103173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W-j, Liang W-h, Zhao Y, Liang H-r, Chen Z-s, Li Y-m et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. European Respiratory Journal. 2020;55(5):2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed]
- Hughes R., Whitley L., Fitovski K., Schneble H.M., Muros E., Sauter A., Craveiro L., Dillon P., Bonati U., Jessop N., Pedotti R., Koendgen H. COVID-19 in ocrelizumab-treated people with multiple sclerosis. Mult Scler Relat Disord. 2020;49 doi: 10.1016/j.msard.2020.102725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D.K., Nesbitt D.J., Yang J., Chen H., Horowitz J., Jones M., Vandergaast R., Carey T., Reiter S., Russell S.J., Kyratsous C., Hooper A., Hamilton J., Ferreira M., Deng S., Straus D., Baras A., Hillyer C.D., Luchsinger L.L. Seroprevalence of anti-SARS-CoV-2 antibodies in a cohort of New York City metro blood donors using multiple SARS-CoV-2 serological assays: Implications for controlling the epidemic and “Reopening. PLOS ONE. 2021;16(4) doi: 10.1371/journal.pone.0250319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsukewitz C., Reddel S.W., Bar-Or A., Wiendl H. Neurological immunotherapy in the era of COVID-19 — looking for consensus in the literature. Nature Reviews Neurology. 2020;16(9):493–505. doi: 10.1038/s41582-020-0385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer-Gould A., Smith J.B., Li B.H. Multiple sclerosis, rituximab, and COVID-19. Ann Clin Transl Neurol. 2021;8(4):938–943. doi: 10.1002/acn3.51342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loonstra F.C., Hoitsma E., van Kempen Z.L., Killestein J., Mostert J.P. COVID-19 in multiple sclerosis: The Dutch experience. Multiple Sclerosis Journal. 2020;26(10):1256–1260. doi: 10.1177/1352458520942198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louapre C., Collongues N., Stankoff B., Giannesini C., Papeix C., Bensa C., Deschamps R., Créange A., Wahab A., Pelletier J., Heinzlef O., Labauge P., Guilloton L., Ahle G., Goudot M., Bigaut K., Laplaud D.-A., Vukusic S., Lubetzki C., De Sèze J., investigators f.t.C. Clinical Characteristics and Outcomes in Patients With Coronavirus Disease 2019 and Multiple Sclerosis. JAMA Neurology. 2020;77(9):1079–1088. doi: 10.1001/jamaneurol.2020.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louapre C., Ibrahim M., Maillart E., Abdi B., Papeix C., Stankoff B., Dubessy A.-L., Bensa-Koscher C., Créange A., Chamekh Z., Lubetzki C., Marcelin A.-G., Corvol J.-C., Pourcher V. Anti-CD20 therapies decrease humoral immune response to SARS-CoV-2 in patients with multiple sclerosis or neuromyelitis optica spectrum disorders. Journal of Neurology, Neurosurgery & Psychiatry: jnnp-2021-326904. 2021 doi: 10.1136/jnnp-2021-326904. [DOI] [PubMed] [Google Scholar]
- Magesh S., John D., Li W.T., Li Y., Mattingly-App A., Jain S., Chang E.Y., Ongkeko W.M. Disparities in COVID-19 Outcomes by Race, Ethnicity, and Socioeconomic Status: A Systematic-Review and Meta-analysis. JAMA Netw Open. 2021;4(11) doi: 10.1001/jamanetworkopen.2021.34147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantero V., Abate L., Basilico P., Balgera R., Salmaggi A., Nourbakhsh B., Cordano C. COVID-19 in dimethyl fumarate-treated patients with multiple sclerosis. J Neurol. 2021;268(6):2023–2025. doi: 10.1007/s00415-020-10015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D., Miller C., Arnold D.L., Bame E., Bar-Or A., Gold R., Hanna J., Kappos L., Liu S., Matta A., Phillips J.T., Robertson D., von Hehn C.A., Campbell J., Spach K., Yang L., Fox R.J. Effect of dimethyl fumarate on lymphocytes in RRMS: Implications for clinical practice. Neurology. 2019;92(15):e1724–e1738. doi: 10.1212/WNL.0000000000007262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghadasi A.N., Mirmosayyeb O., Barzegar M., Sahraian M.A., Ghajarzadeh M. The prevalence of COVID-19 infection in patients with multiple sclerosis (MS): a systematic review and meta-analysis. Neurol Sci. 2021;42(8):3093–3099. doi: 10.1007/s10072-021-05373-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möhn N., Konen F.F., Pul R., Kleinschnitz C., Prüss H., Witte T., Stangel M., Skripuletz T. Experience in Multiple Sclerosis Patients with COVID-19 and Disease-Modifying Therapies: A Review of 873 Published Cases. J Clin Med. 2020;9(12) doi: 10.3390/jcm9124067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghavi S., Kavosh A., Adib I., Shaygannejad V., Arabi S., Rahimi M., Mazaheri S., Ashtari F. COVID-19 infection and hospitalization rate in Iranian multiple sclerosis patients: what we know by May 2021. Multiple Sclerosis and Related Disorders. 2021 doi: 10.1016/j.msard.2021.103335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh T.K., Choi J.-W., Song I.-A. Socioeconomic disparity and the risk of contracting COVID-19 in South Korea: an NHIS-COVID-19 database cohort study. BMC Public Health. 2021;21(1):144. doi: 10.1186/s12889-021-10207-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrotta E., Kister I., Charvet L., Sammarco C., Saha V., Charlson R.E., Howard J., Gutman J.M., Gottesman M., Abou-Fayssal N., Wolintz R., Keilson M., Fernandez-Carbonell C., Krupp L.B., Zhovtis Ryerson L. COVID-19 outcomes in MS: Observational study of early experience from NYU Multiple Sclerosis Comprehensive Care Center. Neurol Neuroimmunol Neuroinflamm. 2020;7(5) doi: 10.1212/NXI.0000000000000835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez C.A., Zhang G.Q., Li X., Huang Y., Lincoln J.A., Samudralwar R.D., Gupta R.K., Lindsey J.W. COVID-19 severity and outcome in multiple sclerosis: Results of a national, registry-based, matched cohort study. Mult Scler Relat Disord. 2021;55 doi: 10.1016/j.msard.2021.103217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perriguey M., Maarouf A., Stellmann J.-P., Rico A., Boutiere C., Demortiere S., Durozard P., Pelletier J., Audoin B. Hypogammaglobulinemia and Infections in Patients With Multiple Sclerosis Treated With Rituximab. Neurology - Neuroimmunology Neuroinflammation. 2022;9(1):e1115. doi: 10.1212/NXI.0000000000001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosperini L., Tortorella C., Haggiag S., Ruggieri S., Galgani S., Gasperini C. Increased risk of death from COVID-19 in multiple sclerosis: a pooled analysis of observational studies. Journal of Neurology. 2021 doi: 10.1007/s00415-021-10803-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reder A.T., Centonze D., Naylor M.L., Nagpal A., Rajbhandari R., Altincatal A., Kim M., Berdofe A., Radhakrishnan M., Jung E., Sandrock A.W., Smirnakis K., Popescu C., de Moor C. COVID-19 in Patients with Multiple Sclerosis: Associations with Disease-Modifying Therapies. CNS Drugs. 2021;35(3):317–330. doi: 10.1007/s40263-021-00804-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rm M., Em C., Wj R. COVID-19 in Multiple Sclerosis: Clinically reported outcomes from the UK Multiple Sclerosis Register. Multiple Sclerosis and Related Disorders. 2021 doi: 10.1016/j.msard.2021.103317. [DOI] [PubMed] [Google Scholar]
- Sabatino, J. J., K. Mittl, W. Rowles, K. McPolin, J. V. Rajan, C. R. Zamecnik, R. Dandekar, B. D. Alvarenga, R. P. Loudermilk, C. Gerungan, C. M. Spencer, S. A. Sagan, D. G. Augusto, J. Alexander, J. A. Hollenbach, M. R. Wilson, S. S. Zamvil and R. Bove (2021). “Impact of multiple sclerosis disease-modifying therapies on SARS-CoV-2 vaccine-induced antibody and T cell immunity.” medRxiv.
- Safavi F., Nourbakhsh B., Azimi A.R. B-cell depleting therapies may affect susceptibility to acute respiratory illness among patients with multiple sclerosis during the early COVID-19 epidemic in Iran. Mult Scler Relat Disord. 2020;43 doi: 10.1016/j.msard.2020.102195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahraian M.A., Azimi A., Navardi S., Ala S., Naser Moghadasi A. Evaluation of the rate of COVID-19 infection, hospitalization and death among Iranian patients with multiple sclerosis. Mult Scler Relat Disord. 2020;46 doi: 10.1016/j.msard.2020.102472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter A., Fox R.J., Newsome S.D., Halper J., Li D.K.B., Kanellis P., Costello K., Bebo B., Rammohan K., Cutter G.R., Cross A.H. Outcomes and Risk Factors Associated With SARS-CoV-2 Infection in a North American Registry of Patients With Multiple Sclerosis. JAMA Neurology. 2021;78(6):699–708. doi: 10.1001/jamaneurol.2021.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen P., Yamana T.K., Kandula S., Galanti M., Shaman J. Burden and characteristics of COVID-19 in the United States during 2020. Nature. 2021;598(7880):338–341. doi: 10.1038/s41586-021-03914-4. [DOI] [PubMed] [Google Scholar]
- Sen S., Karabudak R., Schiavetti I., Demir S., Ozakbas S., Tutuncu M., Petek Balci B., Turan O.F., Uzunkopru C., Koseoglu M., Yetkin M.F., Gunduz T., Gumus H., Kale Icen N., Carmisciano L., Terzi M., Acar P., Gungor Dogan I., Baba C., Tuncer A., Uygunoglu U., Sormani M.P., Efendi H., Siva A. The outcome of a national MS-Covid-19 study: What the Turkish MS cohort reveals? Multiple Sclerosis and Related Disorders. 2021;52 doi: 10.1016/j.msard.2021.102968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepúlveda M., Llufriu S., Martínez-Hernández E., Català M., Artola M., Hernando A., Montejo C., Pulido-Valdeolivas I., Martínez-Heras E., Guasp M., Solana E., Llansó L., Escudero D., Aldea M., Prats C., Graus F., Blanco Y., Saiz A. Incidence and Impact of COVID-19 in MS. A Survey From a Barcelona MS Unit. 2021;8(2):e954. doi: 10.1212/NXI.0000000000000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifian-Dorche M., Sahraian M.A., Fadda G., Osherov M., Sharifian-Dorche A., Karaminia M., Saveriano A.W., Piana R.La, Antel J.P., Giacomini P.S. COVID-19 and disease-modifying therapies in patients with demyelinating diseases of the central nervous system: A systematic review. Mult Scler Relat Disord. 2021;50 doi: 10.1016/j.msard.2021.102800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson-Yap S., De Brouwer E., Kalincik T., Rijke N., Hillert J.A., Walton C., Edan G., Moreau Y., Spelman T., Geys L., Parciak T., Gautrais C., Lazovski N., Pirmani A., Ardeshirdavanai A., Forsberg L., Glaser A., McBurney R., Schmidt H., Bergmann A.B., Braune S., Stahmann A., Middleton R., Salter A., Fox R.J., van der Walt A., Butzkueven H., Alroughani R., Ozakbas S., Rojas J.I., van der Mei I., Nag N., Ivanov R., Sciascia do Olival G., Dias A.E., Magyari M., Brum D., Mendes M.F., Alonso R.N., Nicholas R.S., Bauer J., Chertcoff A.S., Zabalza A., Arrambide G., Fidao A., Comi G., Peeters L. Associations of Disease-Modifying Therapies With COVID-19 Severity in Multiple Sclerosis. Neurology. 2021;97(19):e1870–e1885. doi: 10.1212/WNL.0000000000012753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., et al. SARS-CoV-2 serology after COVID-19 in multiple sclerosis: An international cohort study. Multiple Sclerosis Journal. 2021;0(0) doi: 10.1177/13524585211035318. [DOI] [PubMed] [Google Scholar]
- Sormani M.P., De Rossi N., Schiavetti I., Carmisciano L., Cordioli C., Moiola L., Radaelli M., Immovilli P., Capobianco M., Trojano M., Zaratin P., Tedeschi G., Comi G., Battaglia M.A., Patti F., Salvetti M. Disease-Modifying Therapies and Coronavirus Disease 2019 Severity in Multiple Sclerosis. Ann Neurol. 2021;89(4):780–789. doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., Schiavetti I., Carmisciano L., Cordioli C., Filippi M., Radaelli M., Immovilli P., Capobianco M., De Rossi N., Brichetto G., Cocco E., Scandellari C., Cavalla P., Pesci I., Zito A., Confalonieri P., Marfia G.A., Perini P., Inglese M., Trojano M., Brescia Morra V., Tedeschi G., Comi G., Battaglia M.A., Patti F., Salvetti M. COVID-19 Severity in Multiple Sclerosis. Putting Data Into Context. 2022;9(1):e1105. doi: 10.1212/NXI.0000000000001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spelman T., Forsberg L., McKay K., Glaser A., Hillert J. Increased rate of hospitalisation for COVID-19 among rituximab-treated multiple sclerosis patients: A study of the Swedish multiple sclerosis registry. Multiple Sclerosis Journal. 2021;0(0) doi: 10.1177/13524585211026272. [DOI] [PubMed] [Google Scholar]
- Tallantyre E.C., Whittam D.H., Jolles S., Paling D., Constantinesecu C., Robertson N.P., Jacob A. Secondary antibody deficiency: a complication of anti-CD20 therapy for neuroinflammation. Journal of Neurology. 2018;265(5):1115–1122. doi: 10.1007/s00415-018-8812-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C.N., Baumgartner J., Pichardo C., Toro B., Li L., Arciuolo R., Chan P.Y., Chen J., Culp G., Davidson A., Devinney K., Dorsinville A., Eddy M., English M., Fireteanu A.M., Graf L., Geevarughese A., Greene S.K., Guerra K., Huynh M., Hwang C., Iqbal M., Jessup J., Knorr J., Lall R., Latash J., Lee E., Lee K., Li W., Mathes R., McGibbon E., McIntosh N., Montesano M., Moore M.S., Murray K., Ngai S., Paladini M., Paneth-Pollak R., Parton H., Peterson E., Pouchet R., Ramachandran J., Reilly K., Sanderson Slutsker J., Van Wye G., Wahnich A., Winters A., Layton M., Jones L., Reddy V., Fine A. COVID-19 Outbreak - New York City, February 29-June 1, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(46):1725–1729. doi: 10.15585/mmwr.mm6946a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Food & Drug Administration (FDA). (November 23, 2021). "Comirnaty and Pfizer-BioNTech COVID-19 Vaccine." Retrieved November 23, 2021, from https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/comirnaty-and-pfizer-biontech-covid-19-vaccine.
- US Food & Drug Administration (FDA). (November 23, 2021). "Janssen COVID-19 Vaccine." Retrieved November 23, 2021, from https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/janssen-covid-19-vaccine.
- US Food & Drug Administration (FDA). (November 19, 2021). "Moderna COVID-19 Vaccine." November 23, 2021, from https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine.
- van Kempen Z.L.E., Strijbis E.M.M., Al M.M.C.T., Steenhuis M., Uitdehaag B.M.J., Rispens T., Killestein J. SARS-CoV-2 Antibodies in Adult Patients With Multiple Sclerosis in the Amsterdam MS Cohort. JAMA Neurology. 2021;78(7):880–882. doi: 10.1001/jamaneurol.2021.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer B., Vollmer T., Corboy J., Alvarez E. Evaluation of Risk Factors in Developing Lymphopenia and Hypogammaglobulinemia in Anti-CD20 Treated Multiple Sclerosis Patients (5218) Neurology. 2020;94(15 Supplement):5218. [Google Scholar]
- Wallach A.I., Picone M.A. The presence of SARS-CoV2 antibodies in MS patients. Mult Scler Relat Disord. 2021;50 doi: 10.1016/j.msard.2021.102793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data can be made available upon request for research purposes by submitting a request to the corresponding author.