Abstract
Approximately two-third of the compounds in the pharmaceutical industry were developed through combinatorial chemistry and high throughput screening of particulate solids. Poor solubility and bioavailability of these pharmaceuticals are challenging attributes confronted by a formulator during product development. Hence, substantial efforts have been directed into the research on particle generation techniques. Although the conventional methods, such as crushing or milling and crystallization or precipitation, are still used; supercritical fluid technology introduced in the mid-1980s presents a new method of particle generation. Supercritical fluid processes not only produce micro- and nanoparticles with a narrow size distribution, they are also employed for the microencapsulation, cocrystallization, and surface coating with polymer. Recognized as a green technology, it has emerged as successful variants chiefly as Rapid Expansion of supercritical solutions (RESS), Supercritical anti-solvent (SAS) and Particles from Gas Saturated Solution (PGSS) depending upon type of solvent, solute, antisolvent and nebulization techniques. Being economical and eco-friendly, supercritical fluid technolgy has garnered considerable interest both in academia and industry for modification of physicochemical properties such as particle size, shape, density and ultimately solubility. The current manuscript is a comprehensive update on different supercritical fluid processes used for particle generation with the purpose of solubility enhancement of drugs and hence bioavailability.
Keywords: Supercritical fluid techniques, Micronization, nanonization, solubility, bioavailability, composite particles
Introduction
In the last decade, supercritical fluid (SCF) processes have been extensively utilized for pursuing chemical reactions, extraction, crystallization, precipitation, purification, and development of micro- and nanoparticles. Considerable research efforts are being made to modify solubility and improvement of bioavailability of poorly soluble drugs through SCF. As a well known fact the bioavailability of drugs depends on the absorption through gastrointestinal tract that is in turn governed by their solubility and dissolution. Thus the particle size is of utmost importance. Conventional methods such as crushing, milling, crystallization and precipitation are commonly utilized for particle development in the pharmaceutical industry. Each of these methods has their own set of limitations. SCF technology presents an innovative approach for particle formation that evades most of the downsides related to the traditional methods [1]. Hence, the technology has firmly positioned itself in the pharmaceutical arena. SCF finds a vital application in the development of dry powder inhalers (mean particle size of 2 to 5 μm) that accurately deliver precise dose to the lungs. Furthermore, SCF technology can be explored for the development of sustained and controlled release systems via microencapsulation [2, 3], coating and formation of composite particles [4]. This technology is eco-friendly, green process that generates less waste during operation and produces fine product at minimum cost.
The accuracy presented by SCF processes permits micronization of drug particles, often to submicron stage. The SCF processes can generate nanosuspensions of particles to the tune of 5-2,000 nm [5]. The technology acts as a re‐precipitation aid for rapid and uniform nucleation of the solute in all its variants of fine particle formation. The performance efficiency of this technology is based on proper solvent selection and by adjusting critical parameters (temperature and pressure) during operations (Fig. 1).
Figure 1.
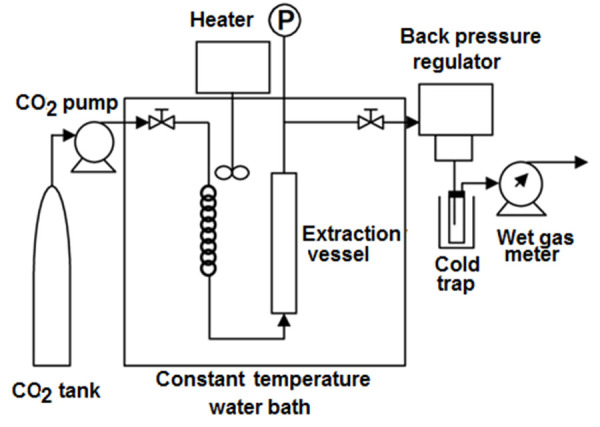
Scheme of the SCF technology process
As documented in the literature, Rapid Expansion of Supercritical Solutions (RESS), Supercritical Anti Solvent (SAS) and Particles from Gas Saturated Solutions (PGSS) are the frequently employed methods not only for the fabrication of monodisperse fine powders, but also to control crystal polymorphism. It is also established that RESS can be used for CO2 soluble molecules, while SAS can process non-soluble molecules. However, the selection is not so simple and a good knowledge of operating conditions and phase equilibrium thermodynamics is required.
SCF methods not only produce micro- and nanoparticles of uniform size distribution, but are extensively applied for microencapsulation and polymeric surface coating on drug crystals, cocrystallization with excipients and development of soluble complexes with cyclodextrin. Nijlen et al. [6] demonstrated significant reduction in particle size of artemisinin and improved dissolution rate when processed with SCF technology. Table 1 lists various SCF techniques that can be used for solubility enhancement based on particle generation.
Table 1. Protagonists of supercritical fluid technology.
| Processing Component | Process/ Acronym |
|---|---|
| Solvent | Rapid expansion of supercritical solution (RESS) |
| Rapid expansion of supercritical solution into a liquid solvent (RESOLV) | |
| Rapid expansion of supercritical solution into an aqueous solution (RESS-AS) | |
| Rapid expansion of supercritical solution with a non-solvent (RESS-N) | |
| Anti-solvent | Gas anti-solvent (GAS) |
| Supercritical anti-solvent (SAS) | |
| Aerosol Solvent Extraction System (ASES) | |
| Particles by Compressed Anti-solvent (PCA) | |
| Solution Enhanced Dispersion by Supercritical Fluids (SEDS) | |
| Solute | Particles from Gas-Saturated Solutions (PGSS) |
| Depressurization of an Expanded Liquid Organic Solution (DELOS) |
SCF processes find vital applications in almost all drug delivery routes, such as oral, intravenous, ophthalmic, pulmonary, transdermal, and implants. Revercheon et al. [7] emphasized the construction of various nanostructures i.e. nanofibers, nanotubes, nanowires, nanoparticles and other nano-constructions using supercritical fluid-based techniques. Byrappa et al. [8] explored the adaptive properties of SCF for the synthesis of advanced nanomaterials including carbon nanotubes, fullerenes, magnetic particles, quantum dots, phosphors, nanocomposites (peptide/hydroxyapatite), and gold nanoshells for drug delivery and other biomedical applications such as imaging, sensing, and cancer theranostics. The review elaborates the applications of Rapid Expansion of Supercritical Solutions (RESS), Supercritical Anti Solvent (SAS) and Particles from Gas Saturated Solutions (PGSS) for the generation of micro- and nano sized drug particles and composite particles. The write up also entails other SCF technologies that have been used for solubility enhancement of poorly water soluble drugs.
RESS for particle generation
RESS is composed of two steps, first to dissolve the solid compound in a supercritical fluid, and the second step results in the formation of particles by the virtue of supersaturation. The supercritical fluid (CO2) is allowed to pump at required pressure and temperature to the solid substance contained in the extraction chamber. The supercritical fluid expands adiabatically in the vessel, triggering a downfall of temperature and pressure, leading to the formation of fine particles [9, 10]. Hasty expansion of the supercritical solution causes reduction in the density and hence particle precipitation with minimum residual solvent occurs (Fig. 2).
Figure 2.
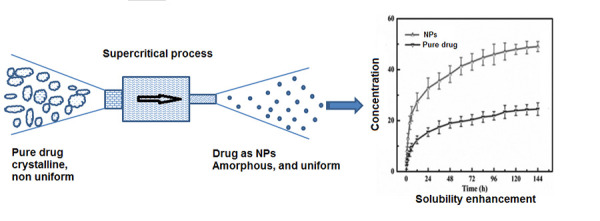
RESS process depicted diagrammatically for solubility enhancement of a hypothetical drug
Charoenchaitrakool et al. [11] aimed at micronization of racemic ibuprofen and examined the dissolution rate of the micronized product in a buffered solution. The solubility data at various temperatures was modeled using Peng-Robinson equations of state with Vander Waals mixing rules. The solubility of (S)-ibuprofen exhibited solubility in CO2 similar to (R)-form. The aggregated particles were easily dispersed by ultrasonication in water. The degree of crystallinity was slightly decreased and the intrinsic dissolution rate was higher than the original form. Likewise the amorphous nanoparticles of cefuroxime axetil were produced directly by RESS technology, without any additive. The nanoparticles obtained were between 158 and 513 nm. More than 90% of the nanoparticles dissolved in 3 min and complete dissolution occurred within 20 min, while the commercial drug achieved only about 50% dissolution in 60 min [12].
Bioavailability of the pharmaceutical substances is very important for their activity. In case of necessity, bioavailability can be improved by reducing the particle size of the drugs. Hezave and Esmaeilzadeh [13] aimed to manufacture fine particles of diclofenac and optimize the RESS conditions for generating uniform particles. Micronization resulted in the average particle size between 10.92 and 1.33 μm. The morphology of the processed particles changed to quasi-spherical while the virgin particles of diclofenac were irregular. Similarly, the reduction in particle size of digitoxin was achieved by Atila et al., [14] and response surface method was used to optimize the process parameters. The particle size of digitoxin was decreased from 0.2-8 μm to 68-458 nm by RESS technique and 97% of the particles were below 200 nm depending on the different experimental conditions.
The solubility of the drug substance in supercritical CO2 has a major effect on the average diameter of the particles prepared by RESS process. This was proved by Kim et al. when they used RESS for the preparation of ultra-fine drug particles using supercritical CO2, with no organic solvent. Three drug substances (lidocaine, griseofulvin, benzoic acid) with different solubility in supercritical CO2 were used, and orifice disks and capillary tubes were adapted as an expansion device. The solubilities of drug substances in supercritical CO2 and the effects of various operating parameters on the characteristics of the particles prepared by RESS process were experimentally investigated. The solubility of the drug substance in supercritical CO2 had a major effect on the average diameter of the particle prepared by RESS process, and the particle diameter decreased with the solubility for all the drugs and operating conditions [15]. In another report, the particle size of raloxifene was reduced from 45 μm to 19 nm by RESS process, the smallest of which was 18.93 nm. In addition, dissolution rate study indicated that a 7-fold increase in dissolution rate could be obtained by particle size reduction of raloxifene using RESS. Response Surface Methodology was used for the optimization of the results and it was demonstrated that the smallest particle size could be achieved at a temperature of 50 °C, pressure of 17.7 MPa and a spray distance of 10 cm [16].
In addition to particle size reduction, RESS can also be used to achieve microencapsulation and surface coating of an active substance particle with a polymer or co-crystallization with excipients or host molecules like cyclodextrins. Kim et al. [17] used RESS process to produce polymeric microparticles loaded with naproxen for drug delivery applications. Poly (L‐lactic acid) (L‐PLA), naproxen, and a mixture of naproxen and L‐PLA were dissolved in supercritical CO2 and precipitated by the RESS process. Composite particles appeared as a naproxen core encapsulated in a polymer coating. Mishima et al. [18] reported a new method — Rapid Expansion from Supercritical Solution with a non-solvent (RESS-N) for forming polymer microparticles containing proteins such as lysozyme and lipase. A suspension of protein in CO2 was made that contained a cosolvent and a dissolved polymer amongst poly (ethylene glycols), poly (methyl methacrylate), poly (L-lactic acid), poly (DL-lactide-co-glycolide) and PEG–poly(propylene glycol) (PPG)–PEG triblock copolymer. The solubilities of these polymers in CO2 increased significantly with low-molecular-weight alcohols as cosolvents. The wide applications of RESS namely micronization and nanonization of APIs and generation of composite particles is further summarized in Table 2.
Table 2. A cross section of micronization and nanonization of some drugs using RESS technology.
| API | Objective | Research highlight | Ref |
|---|---|---|---|
| Coumarin | Coumarin nanoparticles were prepared and the effect of temperature, pressure, spray distance and nozzle diameter on particle size and solubility was assessed. | A considerable decline in particle size was observed from 40.35 μm to 21.37 nm thereby affecting solubility. Quadrupolar cubic plus association theory and perturbed-chain polar statistical associating fluid theory were applied to interpret the solubility data. | [19] |
| Ibuprofen, aspirin and griseofulvin | RESS was used to reduce particle size. The solubility study of poorly soluble drugs was performed employing five cubic equation of state (EoS) with two mixing rules. | Based on the calculated solubilities, two of the most accurate equations of state are PR-vdW and PR-KM with less absolute percent deviation than the other EoS for all systems | [20] |
| Ipriflavone | The solubility of ipriflavone was enhanced through the RESS process using supercritical CO2. | Results outlined improved solubility of ipriflavone in supercritical CO2. Additionally, the particle size was reduced to 4.4 μm from the original 30.9 μm utilizing RESS process. | [21] |
| Letrozole | RESS with solid cosolvent (RESS-SC) was employed to precipitate nanoparticles of letrozole | Obtained findings suggested enhanced solubility of letrozole (7.1 times) in the ternary phase with solid co-solvent application in RESS process. The average particle size was reduced 30 nm to 19 nm. | [22] |
| Aprepitant | Effect of parameters i.e. pressure, temperature, spraying distance and nozzle diameter was studied on the nanoparticles morphology. | Significant reduction in the particle size (micrometer to nanometer) was observed for the nanoparticles developed through RESS-SC method. The dissolution rate of aprepitant was increased by 8.2 times, suggesting improved solubility of the drug. | [23] |
| TBTPP (5, 10, 15,20-tetrakis (3, 5-bis- tri fluoro methyl phenyl porphyrin | RESS process was investigated applying numerical modeling for particle formation of TBTPP. | Peng-Robinson EoS with Kwak-Mansoori mixing rules were applied after the optimization of pressure and temperature. The improved solubility was measured by numerical modeling and the results were compared with experimental data. | [24] |
| Progesterone | Fine progesterone particles were produced and the solubility was analyzed by varying temperature and pressure and compared with a well known model. | The solubility studies were correlated with empirical density-based models and the Peng-Robinson equation of state model. It was found to be improved range of 5.3 × 10− 5–8.9 × 10− 4, with submicron size. | [25] |
| Paracetamol | A novel fluidized-bed coating process using RESS was described for the coating of fine particles. | Microspheroidal catalyst particles (average particle size 56 μm) were used as the core particles. Supercritical CO2 solution of paraffin was expanded through the nozzle into the bed that was fluidized by air. The coating mass and coating rates were measured by a sampling method. A stable coating of fine particles was achieved without the formation of agglomerates at room temperature | [26] |
| Ibuprofen and nicotinamide | RESS was used as a means of simultaneous micronization and cocrystallization of drugs with poor aqueous solubility. | 1:1 cocrystals of ibuprofen and nicotinamide with high purity were produced. The specific surface area of RESS cocrystals was increased by almost tenfold in comparison to cocrystals produced by slow solvent evaporation and the mean dissolution time of ibuprofen from RESS cocrystals was decreased. For drugs with dissolution- limited bioavailability, RESS cocrystallization may be a superior approach in comparison to established cocrystallization techniques. | [27] |
SAS process for particle generation
The SAS process is a highly useful for the micronization and nanonization of synthetic drugs and natural compounds. This method refers to the precipitation of compounds in a provided supercritical fluid. The selected supercritical fluid should be essentially miscible with solvent whereas the solute should be insoluble in supercritical anti-solvents. To process SAS, selection of solvent depends upon two types of requisites, first is good miscibility with CO2 i.e. ethanol, acetone, toluene; and second is the solubility of solute to be precipitated. Indeed, the solvent must usually belong to class 3 (non-toxic) of the pharmaceutical guidelines. In any case, the amount of residual solvent in the crystallized powder must not exceed 5000 ppm.
Many pharmaceuticals have been processed using SAS and derived processes (SEDS, PCA, ASES). A very broad range of molecules can be used namely antibiotics, proteins, biopolymers, paracetamol, salbutamol, naproxen, ascorbic acid etc [28]. Kordikowski et al. [29] worked with sulfathiazole, a compound that exhibits five different polymorphs. Using a semi-continuous SAS process with methanol and CO2, the researchers were able to produce pure polymorph by controlling the flow rate of methanol and the temperature. Three pure polymorphs I, III and IV could be obtained by choosing the right temperature while the flow rate, and the ratio of methanol: CO2 had less influence on the polymorphs. The method is widely studied today because of its potential industrial applications.
A semi-continuous SAS precipitation has been used to produce rifampicin micro- and nanoparticles with controlled particle size and particle size distribution; using different liquid solvents. The best micronization results were obtained using dimethyl sulfoxide at 400 °C. The nanoparticles with mean diameter ranging from 0.4 to 1 μm were obtained at a pressure of ≥ 120 bars, while microparticles with mean diameter ranging from 2.5 to 5 μm were obtained at pressures between 90 and 110 bars. The morphology of rifampicin precipitates was different too. Nanoparticles connected in small aggregates were obtained at pressures higher than 120 bars, whereas, spherical single microparticles were obtained at lower operating pressures [30].
In a research work, a swirl mixer was employed to produce the micronized curcumin with polyvinyl pyrrolidone (PVP) by the SAS process to improve the bioavailability of curcumin. The effects of operating parameters such as curcumin: PVP ratio, feed concentration, temperature, pressure, and CO2 flow rate were investigated. The result showed that the optimal condition for the production of curcumin-PVP particles were at curcumin:PVP ratio of 1:30, feed concentration of 5 mg/mL, temperature of 40 °C, pressure of 15 MPa, and CO2 flow rate of 15 mL/min. Curcumin-PVP particles (< 150 nm) were completely soluble in aqueous solution to form a clear yellow solution unlike poorly soluble raw curcumin. The solubility of curcumin-PVP particles was 2.34 μg/mL whereas that of raw curcumin was 0.006 μg/mL after 12 h. The reason attributable was that the water-soluble polymer (PVP) can modify the surface properties of the particle and thus enhance the solubility of curcumin in aqueous solution [31].
SAS process was used for telmisartan (BCS class II drug) in a variety of ways including micronization, amorphization and solid dispersion. Solid dispersions were prepared using HPMC and PVP at 1:0.5, 1:1, and 1:2 weight ratios of drug to polymer, and pure telmisartan was also treated using the SAS process. After the SAS process, all samples were converted to the amorphous form and were hundreds nm in size. Solubility and dissolution rate were increased compared to the raw material. Though the drug’s solubility increased with increase in the amount of polymer used; the dissolution rate decreased with increasing polymer concentration. Processed pure telmisartan showed higher drug release than its original form, even though it had lower solubility compared to other solid dispersions. The authors concluded that that after controlling the formulation of solid dispersion, the SAS process could be a promising approach for improving the solubility and dissolution rate of telmisartan [32].
The SAS process was used to modify the solid state characteristics of fluconazole by preparing its polymorphs by varying the temperature, pressure and solvents. Fluconazole anhydrate form I was obtained at low temperature (40 °C) and anhydrate form II was obtained at high temperature (80 °C). Not much difference was found in solubility of the polymorphs [33]. The same research group improved dissolution rate of poorly water-soluble drug, cilostazol, using SAS process. In particular, the mean particle size and distribution were markedly influenced by drug solution concentration during SAS process. Moreover, the drug did not change its crystal form and the operating parameters probably controlled the 'crystal texture'. The micronized particles exhibited a 6.5 fold increase in dissolution rate compared to the unprocessed cilostazol [34]. Likewise, amorphous SAS treated nanoparticles of atorvastatin calcium showed increased bioavailability of atorvastatin owing to their nano-dimensional size that offered high solubility and increased dissolution rate. The oral absorption of amorphous atorvastatin calcium nanoparticles in rats was obviously higher when compared with the crystalline atorvastatin calcium after a single dose of 25 mg/kg. The AUC0-12h of the amorphous atorvastatin calcium nanoparticles (179 nm) was 2.1 times that of unprocessed drug. Thus, the SAS process for generation of amorphous atorvastatin calcium nanoparticles is a promising method for enhancing their bioavailability [35].
The SAS process can also be utilized to establish co-precipitation of two different compounds and to form beta cyclodextrin complexes of poorly soluble drugs. The inclusion complex of apigenin-hydroxypropyl -beta-cyclodextrin was prepared through a SAS process and its bioavailability was evaluated. The inclusion complex exhibited improved wettability and the dissolution of apigenin inclusion complex was significantly enhanced. The inclusion complex demonstrated an enhanced oral bioavailability of approximately 6 fold when compared to pure apigenin, in rats [36]. Table 3 summarizes few additional applications of SAS process in improving solubility, dissolution or drug release of certain poorly soluble pharmaceuticals.
Table 3. A compilation of reports on solubility enhancement of poorly soluble drugs affected by SAS technology.
| API | Objective | Outcome | Ref |
|---|---|---|---|
| Tolfenamic acid | SAS parameters were evaluated for solid state property modification and improvement of dissolution profile of tolfenemic acid. | SAS technology was efficient in modifying the solid-state. It produced microparticles with improved dissolution behavior. | [37] |
| N-acetyl-cysteine | The study aimed to micronize N-acetylcysteine by the anti-solvent SEDS technique. | Micronized N-acetylcysteine presented prominent biological activity (100 times) depicted by lower minimum inhibitory concentration compared to non-micronized N-acetylcysteine | [38] |
| Curcumin | Curcumin based dye extract was developed employing SAS. Eudragit® L100, Pluronic® 127 and tween 20 were added to improve the aqueous solubility and stability. | Formulation of a soluble curcumin was carried out for food application. Highest aqueous stability and solubility was observed at pH 4. The mean diameter and zeta potential of the amorphous curcumin particles was 5667.4 nm and 11.21 mV respectively. | [39] |
| Warfarin | To determine solubility of warfarin in supercritical CO2 using SAS | Regular crystals of warfarin with a mean particle size of 6.6 μm were produced | [40] |
| Irbesartan | To improve the dissolution of irbesartan through solid dispersions using SAS concept. | The crystalline state of the drug was transformed into the amorphous state. The dissolution was enhanced after formation of irbesartan solid dispersions | [41] |
| Azithromycin | Solid dispersions of azithromycin were developed utilizing variable amounts of PEG 6000, sorbitol, SLS and Poloxamer 188, | The amorphous solid dispersions of azithromycin demonstrated enhanced solubility with PEG 6000 and SLS. | [42] |
The SAS technology offers reasonable morphology, high drug loading and improved bioavailability but occasionally leads to particle aggregation that can be resolved by use of ultrasonic processor [43]. Among the various SAS based micronization techniques reported, the SEDS (solution enhanced dispersion by supercritical fluids) is an efficient process for the development of uniform sized nanoparticles [44]. York and Hanna developed modified SAS or SEDS process in 1996. This revised technique curtails the drawbacks and limitations of SAS and produces completely dried, uniform, narrow sized particles with low residual organic solvent [45]. In this modified SAS system, initially the drug and excipients are allowed to dissolve in an appropriate organic solvent followed by rigorous agitation. Thereafter, the blended components come in contact with the supercritical fluid. Especially designed coaxial nozzle in SEDS process efficiently sprays the components at high pressure that results in micronized uniform particles (Fig. 3).
Figure 3.
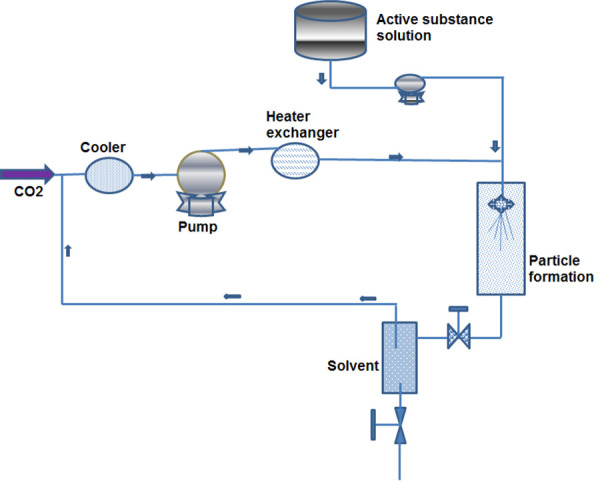
Operational design of SEDS for the formation of micronized particles
SEDS has been employed to enhance dissolution of baicalein via micronization of baicalein. SEDS reduced the particle size from 25.335 μm to 0.614 μm and the particle morphology was transformed to flakes in comparison to original rod shaped crystals. The in vitro release studies demonstrated 50% of the drug release in approximately 20 min, and over 80% of the baicalein was released in 60 min. However, the original powder exhibited a much slower dissolution rate, and less than 25% of the baicalein was released in 60 min. The faster dissolution rate of baicalein microparticles was attributed to the reduced particle size of the baicalein and the extremely large specific surface area [46]. Likewise, the micronization of resveratrol via SEDS enhanced its solubility by approximately 2.8 times and the dissolution rate by 1.8 fold. The antioxidant efficacy of the resultant product was also enhanced significantly [47]. SEDS has been investigated to enhance solubility of bixin [48] and the dissolution rate of aescin by 5.5. fold [49].
The process can also be utilized for development of solid dispersion of poorly water soluble drugs. Water-soluble PVP and astaxanthin nanocoprecipitates were successfully prepared by SEDS precipitation. It was found that the operating pressure, temperature, PVP:astaxanthin ratio, and Z-isomer content of astaxanthin affected the particle size and the astaxanthin content in the coprecipitates. The researchers selectively used Z-isomers of astaxanthin as it has higher bioavailability and antioxidant capacity, than the E-isomer. The authors predicted that use of PVP-Z-isomers of astaxanthin coprecipitates would improve its functionality [50].
Lee et al. [51] investigated the application of SEDS to improve aqueous solubility of and rographolide through particle engineering. The sticky crude Andrographis paniculata extract was precipitated into powder from CO2-acetone system and CO2-acetone:ethanol (1:1 v/v) system. The modification of aqueous solubility of andrographolide was then attempted by manipulating its precipitation process. A. paniculata powder precipitated from CO2-acetone system at 150 bar and 40 °C consisted of large, irregularly shaped, less crystalline particles with the highest andrographolide aqueous solubility (two fold increment compared to crude extract). Complete dissolution of andrographolide from A. paniculata powder precipitated from CO2-acetone system was achieved within 90 min. Based on the higher aqueous solubility and dissolution of andrographolide, and different morphology observed from the less crystalline A. paniculata powder precipitated from CO2-acetone system, it was concluded that fewer impurities could have co-precipitated with andrographolide. Conclusively, the SEDS process offers many advantages over conventional SAS technique such as requirement of less solvent, applicability for thermosensitive materials and less concentration of residual solvent in the product.
PGSS for particle generation
PGSS is a favorable technique for polymeric encapsulation of drugs, proteins and peptides as fine particles without employing organic solvents. In this process, carriers such as polymers are melted with the dissolved or suspended active pharmaceutical ingredient (API) contained within them (Fig. 4). The resulting product is then equilibrated with supercritical-CO2 and expanded through a nozzle in an expansion chamber in order to form fine and porous composite particles [52]. Several carriers and APIs have been micronized using a PGSS process [53, 54]. For example, ibuprofen has been successfully micronized with different carrier materials such as polyethylene glycol (PEG) 6000 [55], poloxamers, Gelucire1 and glyceryl monostearate [56]. PEG 4000 has also been used as a carrier for the micronization of poorly water-soluble drugs [57].
Figure 4.
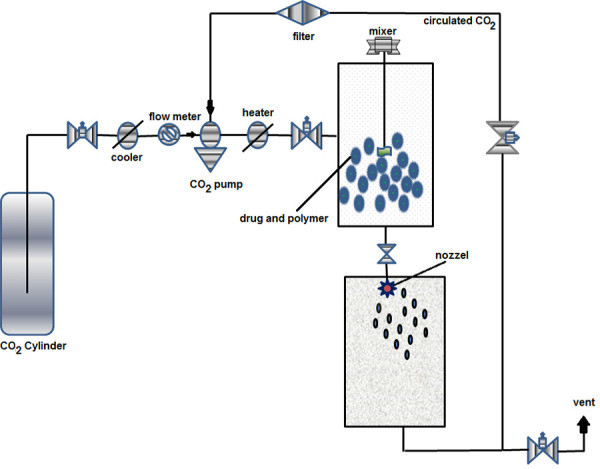
Schematic illustration of working of PGSS (Particles from Gas Saturated Solution)
Another particle generation process close to PGSS has been described by Sievers et al. [58] that consists of production of a dense fine droplet aerosol plume followed by a drying step. The aim of this process was to obtain fine particles usable in a dry powder inhaler form. This patented process has been used with lactose, for developing dry powder inhaler of anti asthamatic drugs: albuterol sulfate and cromolyn sodium. The product comprised of fine spherical particles in the range of 0.1– 3 μm making them suitable for inhalation.
In the experiments carried out in a pre-expansion pressure range from 100-200 bar, the particle size of nifedipine was reduced from 50 to 15 μm. At higher pressures smaller particles were formed. With the particle size reduction the dissolution rate increased to some extent, but the anticipated effective surface area was probably reduced by the drug's hydrophobicity and agglomeration of the particles during micronization [59]. Pestieau et al. [60] optimized PGSS process for a fenofibrate lipid-based solid dispersion formulation. The researchers aimed to develop a formulation containing fenofibrate and Gelucire1 50/13 (Gattefossé, France) in order to improve the oral bioavailability of the drug. The PGSS process was optimized according to the in vitro drug dissolution profile obtained using a biphasic dissolution test. Based on the fact that the propensity of drug precipitation during in vitro testing of lipid-based formulations can serve as a potential indicator for the in vivo performance of a drug, the authors predicted less time to reach Cmax and its sustainment for longer period for PGSS derived solid dispersion than conventional solid dispersions. The authors also deduced that these formulations may avoid the precipitation of this poorly water- soluble drug in vivo. Furthermore, an increase in apparent solubility induced by the formulation used can lead to an enhancement of a drug's permeability through biological membranes. Thus, improvement of the oral bioavailability of fenofibrate should be more pronounced with the PGSS formulation as a result of supersaturation being maintained for longer period.
Fenofibrate solid dispersions were also investigated by Krananja et al. [61]. The authors used PGSS process for the carrier materials: Brij 5100 and polyethylene glycol PEG 4000, for the incorporation of the insoluble drugs nimodipine, fenofibrate, and o-vanillin with the purpose of improving their bioavailability and dissolution rate. The authors however, reported primarily the influence of processing parameters of PGSS on process yield, particle size distribution, loading efficiency and dissolution rates. The general conclusion was that with increasing pre-expansion pressure, the mean particle size of nimodipine-Brij S100, vanillin-Brij S100, and vanillin-PEG 4000 solid dispersion(s) decreased. In the case of a mixture of fenofibrate-Brij S100, the anticipated effective surface area was slightly reduced with pressure as a result of agglomeration. The loading efficiency of drugs in carriers was high and the particles obtained were irregular in shape. The authors deduced that a combination of factors, including particle size reduction and interaction between drug(s) and hydrophilic carrier(s), contributed to enhancing the dissolution rates of precipitated solid particles. On average, a 3.5-fold greater amount of nimodipine was dissolved in 1 h from solid dispersions, compared to unprocessed nimodipine. Dissolution profiles were compared with a different f1 factor and a similarity factor f2. It was confirmed that the dissolution character of processed o-vanillin and fenofibrate by PGSS was different from that of unprocessed o-vanillin and fenofibrate.
The PGSS process is not limited to production of solid dispersions; the technique also finds applications in production of a variety of composite particles namely solid lipid particles, microparticles and microcapsules that have potential to modulate drug release and thereby bioavailability. Table 5 presents a cross section of such research reports.
Table 5. A cross-section of research reports on micronized particles using discrete SCF technology.
| SCF Process | API | Purpose | Highlight | Ref |
|---|---|---|---|---|
| DELOS | ibuprofen and naproxen | Micronization and determination of solubility | Ibuprofen showed same solubility profile, both in CO2-expanded ethanol and CO2–expanded acetone mixtures; whereas the naproxen solubility was greatly dependent on the nature of the solvent i.e. high in CO2–expanded ethanol. | [88] |
| RESOLV | Gambogic acid | Nanoparticles of gambogic acid were prepared to improve solubility. | Results outlined successfully preparation of nanosuspension of gambogic acid. Extended solubility data was correlated with density-based models that suggested enhanced bioavailability and antineoplastic efficacy of nanosized gambogic acid. | [89] |
| DELOS | Phytosterol | Nanonization and decrease crystallinity of phytosterol to modify solubility | Phytosterol nanoparticles were formulated through fast cooling, The crystallinity of the impregnated phytosterols was found to decrease in comparison to the pure phytosterol that modified water solubility. | [90] |
| RESOLV | Poly (l-lactide) (PLLA) nanoparticles loaded with retinyl palmitate | Nanoparticles of PLLA retinyl palmitate were developed with Pluronic F127, F68 and sodium dodecyl sulfate | Spherical PLLA- retinyl palmitate nanoparticles were prepared that possessed mean size of 40–110 nm with improved solubility and great retinyl palmitate loading. | [91] |
| RESOLV | Fenofibrate | Precipitation and stabilization as ultrafine particles of fenofibrate | The mean particle size was approximately 3 μm, which suggested improved solubility. The particles were found to be stable for 24 h. | [92] |
| GAS | Resveratrol [REMOVED HYPERLINK FIELD] and isoniazid, nicotinamide | Co-crystals of resveratrol were prepared with isoniazid and nicotinamide using CO2 antisolvent | The developed co-crystals exhibited enhanced bioavailability when compared to original resveratrol. | [93] |
| GAS | 5-fluorouracil | Halloysite clay nanocarrier was developed to obtain high drug loading of 5-fluorouracil | Prepared nanocarrier loaded with 5-fluorouracil released significantly high drug release at pH 7.4 owing to improve solubility. | [94] |
| SSI | Quercetin | Quercetin was impregnated on Silica to enhance solubility | Several parameters i.e. temperature, time, pressure, and different cosolvents in the supercritical impregnation process were reported influential for quercetin solubility. | [95] |
| SSI | Promogran | Promogran was embedded on a spilanthol-enriched extract to modify solubility | Jambu extract that is completely soluble in fluid phase, was used to demonstrate enhanced solubility of promogran. A 4 h processing period was used for complete dissolution of the extract in SCF. | [96] |
| ASES | Irbesartan | Development of Irbesartan micro-particles and its composite micro-particles | Results highlighted modified solubility (7.5 times) and dissolution rate of Irbesatan microparticles compared to pure drug. | [97] |
| ASES | β-sitosterol | Preparation of submicroparticles of β-sitosterol | Powdered submicroparticles of β-sitosterol exhibited improved solubility. | [98] |
| ASES | Itraconazole | Preparation of solid-inclusion complexes of itraconazole with HP-β-CD | ASES-processed ITR-HP-β-CD inclusion complex solid powder showed 90% drug dissolution within 10 minutes. | [99] |
PGSS process is simpler in operation than other techniques as the therapeutic substance need not be necessarily solubilized in the supercritical fluid (CO2). It requires low solvent gas supplies and pressure for operative purposes as compared to other processes. PGSS process can be employed to develop inorganic powders into multifunctional pharmaceutical compounds. However, precautions need to be taken while processing thermolabile substances.
Other supercritical fluid techniques for enhancement of solubility
Rapid expansion of a supercritical solution into a liquid solvent (RESOLV)
When the traditional RESS is modified by expanding the supercritical solution into a liquid solvent instead of ambient air it is termed as rapid expansion of a supercritical solution into a liquid solvent (RESOLV). This technique can be used for the production of nanoscale particles (less than 50 nm in average diameter) from a CO2 soluble polymer [69]. The development of versatile methods for the preparation of homogeneously distributed nanoscale drug particles and their stable aqueous suspension is still a major challenge, despite the extensive effort based on traditional techniques.
RESOLV was employed for the production of drug nanoparticles of two model drugs: ibuprofen and naproxen, which are somewhat soluble in supercritical CO2 and practically insoluble in water. The RESOLV process yielded aqueous suspensions of homogeneously distributed ibuprofen (average size of 40 nm in diameter and a size distribution standard deviation of 8.5 nm) and naproxen nanoparticles of average size of 64 nm in diameter and a size distribution standard deviation of 10 nm. The nanoparticles agglomerated to form larger aggregates on a longer time scale. The agglomeration can be minimized by the presence of a stabilization agent, e. g. poly (N-vinyl-2-pyrrolidone) in the aqueous suspension. The technique may serve as a “clean” way for nanosizing the drug particles and the preparation of stable suspensions thereof for formulation and other delivery related requirements, specifically addressing the bioavailability issues [70].
Depressurization of an Expanded Liquid Organic Solution (DELOS)
Polymorphism is very common in pharmaceutical drug substances since their solubility and bioavailability are determined by the crystalline structure adopted by the solid drugs [71]. In addition, the drug substance will in most cases be handled as a solid in some stages of the manufacturing process, and its handling and stability properties may depend on the solid phase. Consequently, the control of the production of a given solid polymorph is of the utmost importance in such commercial applications and industries. In a study by Sala et al. [72] it was observed that the precipitation of pure monoclinic E form of stearic acid was favored by DELOS process, a kinetically controlled crystallization in which high supersaturation levels are rapidly achieved. Remarkably, the DELOS process for the first time provided a pure polymorphic monoclinic E form, without the presence of traces from other polymorphs that always are present when using other kinetically driven methods, such as the conventional fast cooling. On the contrary, the C polymorph was preferentially obtained by the thermodynamically controlled GAS technique in which the increase of the solution supersaturation is slow or low supersaturation levels are attained.
DELOS can be used as a route to obtain nutraceutical products that might show enhanced functional properties. Phytosterols are absorbed to a much smaller extent in the body compared to cholesterol and they interfere with the intestinal absorption of cholesterol. DELOS methodology has the potential to process phytosterols into micrometer or submicrometer particles, which is not possible with conventional technologies. Moreno-Calvo et al. [73] processed β-sitosterol through DELOS thereby reducing the crystals from 188 μm to <6.5 μm, with a narrow size distribution at all processing conditions employed. The new phase showed higher chemical purity and higher crystallinity than the native mixture. The authors recommend further studies to confirm the expected enhancement of absorption and bioavailability of β-sitosterol.
Aerosol Solvent Extraction System (ASES)
ASES methodology has been used extensively for processing pharmaceuticals and biopolymers and is capable of producing micrometer sized or nanosized particles with low levels of residual solvent [74]. Furthermore, in the ASES process the particle size and morphology can be easily modulated by the optimization of the processing parameters. The therapeutic applications of silybin, an antihepatotoxic polyphenolic substance, are strongly limited by its poor solubility and low bioavailability. The issues can be addressed by designing nanodrug via ASES. In the process, water soluble and biocompatible PVP was used to improve the dissolution rate and bioavailability of silybin. The size of the silybin PVP nanodrug was to the tune of 100 to 300 nm. Compared with raw silybin, the nanodrug had low crystallinity and hence showed solubility enhancement by more than 8-fold and hence in dissolution too [74].
Copper-indomethacin is a non-steroidal anti-inflammatory drug currently available for veterinary use. Its application is limited to oral formulations because of its poor solubility in biocompatible solvents. Meure et al. [75] prepared microspheres of PVP and copper-indomethacin that ranged in size from 50 nm to 4 μm. A coprecipitate containing 10%wt copper-indomethacin and 90%wt PVP was found to be at least 93 times more soluble in ethanol than factory-grade copper-indomethacin. The significance of these results is that the coprecipitate of copper-indomethacin may be used for parenteral applications [75].
Rao et al. [76] suggested enhancement of the dissolution rate, apparent solubility and oral bioavailability of tadalafil by nanosized amorphous particles prepared by using antisolvent precipitation. Optimization of processing parameters yielded amorphous tadalafil solid dispersion of approximately 5-10 μm. The solid dispersion obtained using the optimized process conditions exhibited 8.5 times faster dissolution rates in the first minute of dissolution, 22 times greater apparent solubility at 10 min and a 3.67-fold increase in oral bioavailability than the unprocessed tadalafil.
Supercritical solvent impregnation (SSI)
Among the different approaches that employ supercritical fluids for pharmaceutical purposes, polymer impregnation techniques have been recently used in literature to achieve the impregnation of many kinds of polymers namely, poly(lactic-co-glycolic acid), ethyl/methyl cellulose, poly(dimethylsiloxane), poly(methyl methacrylate, etc. with a drug [77]. In particular, reports where PVP was impregnated with some crystalline drugs (i.e. carbamazepine, ibuprofen, ketoprofen) can be found [78-80]. In these processes, the crystalline drug is dissolved by supercritical CO2 and thus conveyed through the swollen polymeric matrix until the partition equilibrium takes place between the phases. The encapsulated particles display extensive solubility, better diffusion and substantial dissolution profile owing to the supercritical fluid that plays a role of cosolvent.
In a study by Banchero et al. [81] successful impregnation was reported for all the PVP K15-piroxicam systems at 300 bar and 100 °C. Good results in terms of acceleration in the drug release were obtained with the PVP K15-piroxicam system. The best result was obtained for the impregnated sample containing a piroxicam amount equal to 11.3%, which released 94.7% of the drug after 10 min, with respect to 7.8% released by the corresponding physical mixture after the same period of time.
Gas Antisolvent process (GAS)
In the GAS processes, a solute is dissolved in solvent and loaded into a crystallizer. The solution is then expanded by injecting carbon dioxide into the crystallizer. A sharp reduction of solute solubility in liquid phase is observed and subsequently particle precipitation occurs. This technique is used for drugs with low solubility in the supercritical fluid. The mean particle size and particle size distribution are controlled by GAS variables. Various drugs have been micronized by GAS process namely, microparticle production of carbamazepine [82], controlled crystallization of β-carotene [83], caffeine [84], phenanthrene [85] and paracetamol [86]. Control of GAS processing variables resulted in a decrease in ampicillin particle size from 359 to 260 nm. The mean particle size was 425 nm for the lowest pressure (9 MPa). When the pressure was increased, a smaller mean particle size (220 nm) was obtained. The smaller mean particle size was observed at the lower temperature and low solute concentration [87].
All the detailed SCF techniques are virtuous alternatives for micronization and nanonization of drugs that require particle engineering for modification and improvement of solubility. SCF processes are frequently utilized to formulate readily solubilized drug carrier systems i.e. microparticles, nanoparticles, inclusion complexes, solid dispersions, macromolecular powders and microporous foams. Some of these have been elaborated in Table 4.
Table 4. A cross section of composite particles of drugs prepared using PGSS process.
| API and excipients | Strategy | Result highlight (s) | Ref |
|---|---|---|---|
| Curcumin, tristearin, soyphosphatidyl-holine, DMSO | Curcumin embedded solid lipid particles were developed via PGSS with less quantity of organic solvent. | The use of helium in the process of PGSS, for designing of lipid mixture exhibited improvement in the biopharmaceutical properties and therapeutic value of curcumin. | [62] |
| S-(+)-ibuprofen, Poloxamers, Gelucire, Glyceryl monostearate(GMS) | PGSS was employed for the enhancement of solubility of (+)-ibuprofen using hydrophilic or hydrophobic carrier. | Spherical and porous particles (50-200 μm) with 90% encapsulation efficiency were produced. The solubility of ibuprofen was significantly enhanced with poloxamer in the gastro-intestinal fluids; gelucire and GMS did not enhance the solubility of ibuprofen. | [63] |
| Ketoprofen, Glyceryl monooleate (GMO), Gelucire 43/01™, Geleol™ and Gelucire 50/13™ |
For production of structured lipid carriers a liquid glycerolipid (GMO), was incorporated into three solid glycerolipids with hydrophilic-lipophilic balance ranging from 1 to 13 and compared with solid lipid particles. | Irregular porous microparticles with a wide particle size distribution were obtained. The drug loading capacity of the structured lipid carriers increased as the GMO content in the particles increased, achieving a maximum encapsulation efficiency of 97% for the 3:1 mass ratio. The structured lipid carriers presented an immediate release of ketoprofen from its matrix with higher permeation through a mucous-membrane model, while solid lipid particles presented controlled release of the drug with less permeation capacity. | [64] |
| Quercetin, soybean, lecithin, and pluronic L64 | To modify bioavailability of quercetin through microencapsulation | More homogenous lyophilized less crystalline encapsulated quercetin particles were reported with enhanced bioavailability | [65] |
| β-carotene, poly-(ε-caprolactone) | β- carotene was encapsulated in poly- caprolactones, and precipitated out using PGSS process. | Small, regular, uniform, microencapsulated β-carotene particles in the size range of 100 –600 μm were obtained, that demonstrated enhanced solubility. | [66] |
| 1,3-diphenyl-2-propenone (chalcone) | Microparticles of chalcone alone and with lipid carriers were developed via PGSS and the solubility was analyzed. | The lipid carriers influenced the solubility of trans-chalcone in simulated gastric and intestinal fluids, without addition of enzymes. | [67] |
| Omega-3 PUFA-rich salmon oil and astaxanthin | Microparticles of omega-3 PUFA-rich salmon oil in PEG-6000 were developed through PGSS concept. | Developed microparticles showed significant thermogravimetric stability up to 350 °C. Moreover, in vitro release of oil in fluids stimulating gastric conditions was faster than in distilled water. | [68] |
Conclusions
Supercritical fluid techniques for micronization and solubility enhancement have been progressively applied in pharmaceutical industry. The characteristic advantages of SCF technology include non-toxicity, eco-friendly and flexibility that make it suitable for green chemistry. SCF processes are proven promising strategies to develop and design drug delivery system of those drugs whose solubility and bioavailability is significantly low. Moreover, SCF technologies are also utilized for formulating drug carrier owing to unique solvent features that can be readily modified by altering operating temperature and pressure. Several issues still remain i.e. the influence of operating parameters on the characteristics of the particle produced (size, morphology, polymorphism), the comprehension of the fluid dynamics, the nucleation phenomenon, the crystal growth under provided conditions etc. Whatsoever, the technology has arrived and is promising green option for pharmaceutical development.
Footnotes
Conflict of interest: The author declares no conflict of interest.
References
- [1].Baldelli A, Boraey MA, Nobes DS, Vehring R. Analysis of the particle formation process of structured microparticles. Mol Pharm. 12 (2015) 2562–73. [DOI] [PubMed] [Google Scholar]
- [2].Tomasko DL, Li H, Liu D, Han X, Wingert MJ, Lee LJ, et al. A Review of CO2 applications in the processing of polymers. Ind Eng Chem Res. 42 (2003) 6431–56. [Google Scholar]
- [3].Mishima K. Biodegradable particle formation for drug and gene delivery using supercritical fluid and dense gas. Adv Drug Deliv Rev. 60 (2008) 411–32. [DOI] [PubMed] [Google Scholar]
- [4].Tien Y.C., Su C.S., Lien L.H., Chen YP.. Recrystallization of erlotinib hydrochloride and fulvestrant using supercritical antisolvent process. The Journal of Supercritical Fluids b (2010) 292-299 [Google Scholar]
- [5].Yasuji T, Takeuchi H, Kawashima Y. Particle design of poorly water-soluble drug substances using supercritical fluid technologies. Adv Drug Deliv Rev. 60(3) (2008) 388–98. [DOI] [PubMed] [Google Scholar]
- [6].Van Nijlen TV, Brennan K, Mooter GV, Blaton N, Kinget R, Augustijns P. Improvement of the dissolution rate of artemisinin by means of supercritical fluid technology and solid dispersions. Int J Pharm. 254 (2003) 173–81. [DOI] [PubMed] [Google Scholar]
- [7].Reverchon E, Adami R. Nanomaterials and supercritical fluids. J Supercrit Fluids. 37 (2006) 1–22. [Google Scholar]
- [8].Byrappa K, Ohara S, Adschiri T. Nanoparticles synthesis using supercritical fluid technology-towards biomedical applications. Adv Drug Deliv Rev. 60 (2008) 299–327. [DOI] [PubMed] [Google Scholar]
- [9].Sauceau M, Fages J, Letourneau JJ, Richon D. A novel apparatus for accurate measurements of solid solubilities in supercritical phases. Ind Eng Chem Res. 39 (2000) 4609–14. [Google Scholar]
- [10].Sauceau M, Letourneau JJ, Richon D, Fages J. Solid compound solubilities in SC–CO2 and cosolvent: development of enhanced density-based models. Fluid Phase Equilib. 208 (2003) 99–113. [Google Scholar]
- [11].Charoenchaitrakool M, Dehghani F, Foster NR, Chan HK. Micronization by rapid expansion of supercritical solutions to enhance the dissolution rates of poorly water soluble pharmaceuticals. Ind Eng Chem Res. 39 (2000) 4794–802. [Google Scholar]
- [12].Varshosaz J, Hassanzadeh F, Mahmoudzadeh M, Sadeghi A. Preparation of cefuroxime axetil nanoparticles by rapid expansion of supercritical fluid technology. Powder Technol. 189 (2009) 97–102. [Google Scholar]
- [13].Hezave AZ, Esmaeilzadeh F. The effects of RESS parameters on the diclofenac particle size. Adv Powder Technol. 22 (2011) 587–95. [Google Scholar]
- [14].Atila C, Yildiz N, Caliml A. Particle size design of digitoxin in supercritical fluids. J Supercrit Fluids. 51 (2010) 404–11. [Google Scholar]
- [15].Kim J-T, Kim H-L, Ju C-S. Micronization and characterization of drug substances by RESS with supercritical CO2. Korean J Chem Eng. 27 (2010) 1139–44. [Google Scholar]
- [16].Keshavarz A, Karimi-Sabet J, Fattahi A, Golzary AA, Rafiee-Tehrani M, Dorkoosh FA. Preparation and characterization of raloxifene nanoparticles using rapid expansion of supercritical solution (RESS). J Supercrit Fluids. 63 (2012) 169–79. [Google Scholar]
- [17].Kim JH, Paxton TE, Tomasko DL. Microencapsulation of naproxen using rapid expansion of supercritical solutions. Biotechnol Prog. 12 (1996) 650–61. [Google Scholar]
- [18].Mishima K, Matsuyama K, Tanabe D, Yamauchi S, Young TJ, Johnston KP. Microencapsulation of proteins by rapid expansion of supercritical solution with a non-solvent. AIChE J. 46 (2000) 857–65. [Google Scholar]
- [19].Sodeifian G. [REMOVED HYPERLINK FIELD] N.S. Ardestani, S.A. Sajaadian, H.S. Panah, Experimental measurements and thermodynamic modeling of Coumarin-7 solid solubility in supercritical carbon dioxide: Production of nanoparticles via RESS method. Fluid Phase Equilib. 483 (2019) 122–43. [Google Scholar]
- [20].Bagheri H, Mansoori GA, Hashemipour H. A novel approach to predict drugs solubility in supercritical solvents for RESS process using various cubic EoS-mixing rule. J Mol Liq. 261 (2018) 174–88. [Google Scholar]
- [21].Wang B., Shaansu C.. Solid solubility measurement of ipriflavone in supercritical carbon dioxide and microparticle production through the rapid expansion of supercritical solutions process. Journal of CO2 Utilization 37 (2020) 285-294. [Google Scholar]
- [22].Sodeifian G, Sajadian SA. Solubility measurement and preparation of nanoparticles of an anticancer drug (Letrozole) using rapid expansion of supercritical solutions with solid cosolvent (RESS-SC). J Supercrit Fluids. 133 (2018) 239–52. [Google Scholar]
- [23].Seyed GS, Daneshyan ASS. Preparation of aprepitant nanoparticles (efficient drug for coping with the effects of cancer treatment) by rapid expansion of supercritical solution with solid cosolvent (RESS-SC). J Supercrit Fluids. 140 (2018) 72–84. [Google Scholar]
- [24].Bagheri H, Mipour HH, Mirzaie M. Investigation on hydrodynamic and formation of nano particle by RESS process: the numerical study. J Mol Liq. 281 (2019) 490–505. [Google Scholar]
- [25].Huang Z, Guo Y-H, Miao H, Teng L-J. Solubility of progesterone in supercritical carbon dioxide and its micronization through RESS. Powder Technol. 258 (2014) 66–77. [Google Scholar]
- [26].Tsutsumi A, Nakamoto S, Mineo T, Yoshida KA. A novel fluidized-bed coating of fine particles by rapid expansion of supercritical fluid solution. Powder Technol. 85 (1995) 275–8. [Google Scholar]
- [27].Müllers KC, Paisana M, Wahl MA. Simultaneous Formation and Micronization of Pharmaceutical Cocrystals by Rapid Expansion of Supercritical Solutions (RESS). Pharm Res. 32 (2015) 702–13. [DOI] [PubMed] [Google Scholar]
- [28].Jung J, Perrut M. Particle design using supercritical fluids: literature and patent survey. J Supercrit Fluids. 20 (2001) 179–219. [Google Scholar]
- [29].Kordikowski A, Shekunov B, York P. Crystallisation of sulfathiazole polymorphs using CO2. International Society for the Advancement of Supercritical Fluids. 1 (2000) 117–22. [Google Scholar]
- [30].Reverchon E, Marco I, Porta G. Rifampicin microparticles production by supercritical antisolvent precipitation. Int J Pharm. 243 (2002) 83–91. [DOI] [PubMed] [Google Scholar]
- [31].Chhouk K, Kanda WH, Kawasaki S-I, Goto M. Micronization of curcumin with biodegradable polymer by supercritical anti-solvent using micro swirl mixer. Front Chem Sci Eng. 12 (2018) 184–93. [Google Scholar]
- [32].Park J, Cho W, Cha KH, Ahn J, Han K, Hwang SJ. Solubilization of the poorly water soluble drug, telmisartan, using supercritical anti-solvent (SAS) process. Int J Pharm. 441 (2013) 50–5. [DOI] [PubMed] [Google Scholar]
- [33].Park HJ, Kim MS, Lee S, Kim JS, Woo JS, Park JS, et al. Recrystallization of fluconazole using the supercritical antisolvent (SAS) process. Int J Pharm. 328 (2007) 152–60. [DOI] [PubMed] [Google Scholar]
- [34].Kim MS, Lee S, Park JS, Woo JS, Hwang SJ. Micronization of cilostazol using supercritical antisolvent (SAS) process: effect of process parameters. Powder Technol. 177 (2007) 64–70. [Google Scholar]
- [35].Kim MS, Jin SJ, Kim JS, Park HJ, Song HS, Neubert RH, et al. Preparation, characterization and in vivo evaluation of amorphous atorvastatin calcium nanoparticles using supercritical antisolvent (SAS) process. Eur J Pharm Biopharm. 69 (2008) 454–65. [DOI] [PubMed] [Google Scholar]
- [36].Huang Y, Zu Y, Zhao X, Wu M, Feng Z, Deng Y, et al. Preparation of inclusion complex of apigenin-hydroxypropyl-beta-cyclodextrin by using supercritical antisolvent process for dissolution and bioavailability enhancement. Int J Pharm. 511 (2016) 921–30. [DOI] [PubMed] [Google Scholar]
- [37].Chen HH, Su CS, Liu JJ, Sheu MT. Solid-state property modification and dissolution rate enhancement of tolfenamic acid by supercritical antisolvent process. J Supercrit Fluids. 101 (2015) 17–23. [Google Scholar]
- [38].Aguiar GPS, Marcon M, Mocelin R, Herrmann AP, Chaves LMPC, Piato Al, et al. Micronization of N-acetylcysteine by supercritical fluid: Evaluation of in vitro and in vivo biological activity. J Supercrit Fluids. 130 (2017) 282–91. [Google Scholar]
- [39].Arango-Ruiz Á, Martin A, Cosero MJ, Jimenez C, Londono J. Encapsulation of curcumin using supercritical antisolvent (SAS) technology to improve its stability and solubility in water. Food Chem. 258 (2018) 156–63. [DOI] [PubMed] [Google Scholar]
- [40].Ciou J-M, Wang B-C. C-S, Su, J-J. Liu, M-T. Sheu. Measurement of solid solubility of warfarin in supercritical carbon dioxide and recrystallization study using supercritical antisolvent process. Adv Powder Technol. 29 (2018) 479–87. [Google Scholar]
- [41].Adeli E. The use of supercritical anti-solvent (SAS) technique for preparation of Irbesartan-Pluronic® F-127 nanoparticles to improve the drug dissolution. Powder Technol. 298 (2016) 65–72. [Google Scholar]
- [42].Adeli E. A comparative evaluation between utilizing SAS supercritical fluid technique and solvent evaporation method in preparation of azithromycin solid dispersions for dissolution rate enhancement. J Supercrit Fluids. 87 (2014) 9–21. [Google Scholar]
- [43].Campardelli R, Adami R, Porta GD, Reverchon E. Nanoparticle precipitation by supercritical assisted injection in a liquid antisolvent. Chem Eng J. 192 (2012) 246–51. [Google Scholar]
- [44].Li S, Zhao Y. Preparation of zein nanoparticles by using solution-enhanced dispersion with supercritical CO2 and elucidation with computational fluid dynamics. Int J Nanomedicine. 12 (2017) 3485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kankala RK, Chen B-Q, Liu CG, Tang H-X, Wang S-B, Chen A-Z. Solution-enhanced dispersion by supercritical fluids: an ecofriendly nanonization approach for processing biomaterials and pharmaceutical compounds. Int J Nanomedicine. 13 (2018) 4227–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yan T, Zhixiang YC, Huang WD, Miao H, Zhang Y. Preparation and characterization of baicalein powder micronized by the SEDS process. J Supercrit Fluids. 104 (2015) 177–89. [Google Scholar]
- [47].Aguiar GPS, Arcari BD, Chaves MPC, Magro CD, Boschetto DL, Piato AL, et al. Micronization of trans-resveratrol by supercritical fluid: Dissolution, solubility and in vitro antioxidant activity. Ind Crops Prod. 112 (2018) 1–5. [Google Scholar]
- [48].Suo QL, He WZ, Huang YC, Huang C, Pingli C, Hong HL, et al. Micronization of the natural pigment-bixin by the SEDS process through prefilming atomization. Powder Technol. 154 (2005) 110–5. [Google Scholar]
- [49].Wang J, Zhang K, Zhou D, Ge F, Zhao Y. Aescin nanoparticles prepared using SEDS: Composition stability and dissolution enhancement. J Supercrit Fluids. 130 (2017) 267–72. [Google Scholar]
- [50].Kaga K, Honda M, Adachi T, Honjo M, Goto M. [REMOVED HYPERLINK FIELD][REMOVED HYPERLINK FIELD] Nanoparticle formation of PVP-astaxanthin inclusion complex by solution-enhanced dispersion by supercritical fluids (SEDS): Effect of PVP and astaxanthin Z-isomer content. J Supercrit Fluids. 136 (2018) 44–51. [Google Scholar]
- [51].Lee SY, Abdullah LC, Rahman RA, Abas F, Tan WK. [REMOVED HYPERLINK FIELD] G.H. Chong. Solution enhanced dispersion by supercritical fluids (SEDS): An approach in particle engineering to modify aqueous solubility of andrographolide from Andrographis paniculata. Chem Eng Res Des. 138 (2018) 176–89. [Google Scholar]
- [52].Hakuta Y, Hayashi H, Arai K. Fine particle formation using supercritical fluids. Curr Opin Solid State Mater Sci. 7 (2003) 341–51. [Google Scholar]
- [53].Jung J, Perrut M. Particle design using supercritical fluids: literature and patent survey. J Supercrit Fluids. 20 (2001) 179–219. [Google Scholar]
- [54].Knez Z, Škerget M, Knez HM, Cucek D. Particle formation using sub- and supercritical fluids. In: A. Fan V. (Ed.), Supercritical Fluid Technology for Energy and Environmental Applications. Elsevier, Boston, 2014, pp. 31–67 [Google Scholar]
- [55].Chen W, Hu X, Hong Y, Su Y, Wang H, Li J. Ibuprofen nanoparticles prepared by a PGSSTM-based method. Powder Technol. 245 (2013) 241–50. [Google Scholar]
- [56].Fraile M, Martín Y, Deodato D, Rodriguez-Rojo S, Nogueira ID, Simplício AL, et al. Production of new hybrid systems for drug delivery by PGSS (Particles from Gas Saturated Solutions) process. J Supercrit Fluids. 81 (2013) 226–35. [Google Scholar]
- [57].Weidner E, Steiner R, Knez Z. Powder generation from polyethylene glycols with compressible fluids, In: Von Rohr P.R., Trepp C., (Eds.) Process Technology Proceedings 12, High Pressure Chemical Engineering. Netherlands, Elsevier, 1996, pp. 223-228. [Google Scholar]
- [58].Sievers RE, Milewski PD, Sellers SP, Miules BA, Korte BJ, Kusek KD, et al. Supercritical and near-critical carbon dioxide assisted low-temperature bubble drying. Ind Eng Chem Res. 39 (2000) 4831–6. [Google Scholar]
- [59].Božić PS, Srčič S, Knez Z, Kerc J. Improvement of nifedipine dissolution characteristics using supercritical CO2. Int J Pharm. 148 (1997) 123–30. [Google Scholar]
- [60].Pestieau A, Krier F, Lebrun P, Brouwers A, Streel B, Evrard B. Optimization of a PGSS process for a fenofibrate lipid-based solid dispersion formulation. Int J Pharm. 485 (2015) 295–305. [DOI] [PubMed] [Google Scholar]
- [61].Krananja G, Knez Z, Kotnik P, Ljubec B, Knez M. Formulation of nimodipine, fenofibrate, and o-vanillin with Brij S100 and PEG 4000 using the PGSS™ process. J Supercrit Fluids. 135 (2018) 245–53. [Google Scholar]
- [62].Pedro AS, Villa SD, Calicet P, Melo SABV, Albuquerque EC, Bertucco A, et al. Curcumin-loaded solid lipid particles by PGSS technology. J Supercrit Fluids. 107 (2016) 534–41. [Google Scholar]
- [63].Fraile M, Martin A, Deodato D, Rodriguez-Rojo S, Nogueira ID, Simplicio AL, et al. Production of new hybrid systems for drug delivery by PGSS. J Supercrit Fluids. 81 (2013) 226–35. [Google Scholar]
- [64].Gonçalves VSS, Matias AA, Rodríguez-Rojo S, Nogueira ID, Duarte CMM. Supercritical fluid precipitation of ketoprofen in novel structured lipid carriers for enhanced mucosal delivery-a comparison with solid lipid particles. Int J Pharm. 495 (2015) 302–11. [DOI] [PubMed] [Google Scholar]
- [65].Levai G, Martin A, Moro A, Matias AA, Goncalves VSS, Bronze MR. Production of encapsulated quercetin particles using supercritical fluid technologies. Powder Technol. 317 (2017) 142–53. [Google Scholar]
- [66].Paz E, Martin A, Duarte MM, Cocero MJ. Formulation of β-carotene with poly-(ε-caprolactones) PGSS process. Powder Technol. 217 (2012) 77–83. [Google Scholar]
- [67].Sousa ARS, Silva R, Tay FH, Simplicio AL, Kazarian SG, Duarte CMM. Solubility enhancement of trans-chalcone using lipid carriers and supercritical CO2 processing. J Supercrit Fluids. 48 (2009) 120–5. [Google Scholar]
- [68].Haq M, Chun BS. Microencapsulation of omega-3 polyunsaturated fatty acids and astaxanthin-rich salmon oil using particles from gas saturated solutions (PGSS) process. Lebensm Wiss Technol. 92 (2018) 523–30. [Google Scholar]
- [69].Meziani MJ, Pathak P, Hurezeanu R, Thies MC, Enick RM, Sun YP. Supercritical fluid processing technique for nanoscale polymer particles. Angew Chem Int Ed Engl. 43 (2004) 704–7. [DOI] [PubMed] [Google Scholar]
- [70].Pathak P, Meziani MJ, Desai T, Sun YP. Nanosizing Drug Particles in Supercritical Fluid Processing. J Am Chem Soc. 2004;•••:10842–3. [DOI] [PubMed] [Google Scholar]
- [71].Price CP, Grzesiak AL, Matzger AJ. Crystalline polymorph selection and discovery with polymer heteronuclei. J Am Chem Soc. 127 (2005) 5512–7. [DOI] [PubMed] [Google Scholar]
- [72].Sala S, Elizondo E, Moreno E, Calvet T, Cuevas-Diarte MA, Ventosa N, et al. Kinetically driven crystallization of a pure polymorphic phase of stearic acid from CO2-expanded solutions. Cryst Growth Des. 10 (2010) 1226–32. [Google Scholar]
- [73].Moreno-Calvo E, Temelli F, Cordoba A, Masciocchi N, Veciana J, Ventosa N. A new microcrystalline phytosterol polymorph generated using CO2-expanded solvents. Cryst Growth Des. 14 (2014) 58–68. [Google Scholar]
- [74].Teng W, Wang J, Foster NR, Wen N, Zhang J. Preparation of silybin-poly (vinyl pyrrolidone) nanodrugs by using the aerosol solvent extraction system for improving drug solubility. Ind Eng Chem Res. 53 (2014) 10519–24. [Google Scholar]
- [75].Meure LA, Warwik B, Dehgnani F, Regtop HL, Foster NR. Increasing copper indomethacin solubility by coprecipitation with poly (vinylpyrrolidone) using the aerosol solvent extraction system. Ind Eng Chem Res. 43 (2004) 1103–12. [Google Scholar]
- [76].Rao Q, Qiu Z, Zhenyu T, Zhang J, Luo D, Pan P, et al. Enhancement of the apparent solubility and bioavailability of tadalafil nanoparticles via antisolvent precipitation. Eur J Pharm Sci. 128 (2019) 222–31. [DOI] [PubMed] [Google Scholar]
- [77].Guney O, Akgerman A. Synthesis of controlled-release products in supercritical medium. AIChE J. 48 (2002) 856–66. [Google Scholar]
- [78].Sethia S, Squillante E. Solid dispersion of carbamazepine in PVP K30 by conventional solvent evaporation and supercritical methods. Int J Pharm. 272 (2004) 1–10. [DOI] [PubMed] [Google Scholar]
- [79].Kazarian SG, Martirosyan GG. Spectroscopy of polymer drug formulations processed with supercritical fluids: in situ ATR-IR and Raman study of impregnation of ibuprofen into PVP. Int J Pharm. 232 (2002) 81–90. [DOI] [PubMed] [Google Scholar]
- [80].Manna L, Banchero M, Sola D, Ferri A, Ronchetti S, Sicardi S. Impregnation of PVP microparticles with ketoprofen in the presence of supercritical CO2. J Supercrit Fluids. 42 (2007) 378–84. [Google Scholar]
- [81].Banchero M, Manna L, Ronchetti S, Campanelli P, Ferri A. Supercritical solvent impregnation of piroxicam on PVP at various polymer molecular weights. J Supercrit Fluids. 49 (2009) 271–8. [Google Scholar]
- [82].Moneghini M, Kikic I, Voinovich D, Perissutti B, Filipovic-Grcic J. Processing of carbamazepine-PEG 4000 solid dispersions with supercritical carbon dioxide: preparation, characterization and in vitro dissolution. Int J Pharm. 222 (2001) 129–38. [DOI] [PubMed] [Google Scholar]
- [83].Cocero MJ, Ferrero S. Crystallization of β-carotene by a GAS process in batch effect of operating conditions. J Supercrit Fluids. 22 (2002) 237–45. [Google Scholar]
- [84].Park S, Yeo S. Recrystallization of caffeine using gas antisolvent process. J Supercrit Fluids. 47 (2008) 85–92. [Google Scholar]
- [85].Bakhbakhi Y, Rohani S, Charpentier PA. Micronization of phenanthrene using the gas antisolvent process. 1. Experimental study and Use of FTIR. Ind Eng Chem Res. 44 (2005) 7337–44. [Google Scholar]
- [86].Fusaro F, Mazzotti M, Muhrer G. Gas antisolvent recrystallization of paracetamol from acetone using compressed carbon dioxide as antisolvent. Cryst Growth Des. 4 (2004) 881–9. [Google Scholar]
- [87].Esfandiari N, Ghoreishi SM. Ampicillin nanoparticles production via supercritical CO2 gas antisolvent process. AAPS PharmSciTech. 16 (2015) 1263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Munto M, Ventosa N, Sala S, Veciana J. Solubility behaviors of ibuprofen and naproxen drugs in liquid “CO2–organic solvent” mixtures. J Supercrit Fluids. 47 (2008) 147–53. [Google Scholar]
- [89].Xiang ST, Kankala RK, Wang SB, Chen AZ. Solubility measurement and RESOLV-assisted nanonization of gambogic acid in supercritical carbon dioxide for cancer therapy. J Supercrit Fluids. 150 (2019) 147–55. [Google Scholar]
- [90].Ubeyitogullari A, Ciftci ON. Generating phytosterol nanoparticles in nanoporous bioaerogels via supercritical carbon dioxide impregnation: Effect of impregnation conditions. J Food Eng. 207 (2017) 99–107. [Google Scholar]
- [91].Sane A, Limtrakul J. Formation of retinyl palmitate-loaded poly (l-lactide) nanoparticles using rapid expansion of supercritical solutions into liquid solvents (RESOLV). J Supercrit Fluids. 51 (2009) 230–7. [Google Scholar]
- [92].Dalvi SV, Azad MA, Dave R. Precipitation and stabilization of ultrafine particles of fenofibrate in aqueous suspensions by RESOLV. Powder Technol. 236 (2013) 75–84. [Google Scholar]
- [93].Pessoa AS, Pablo G, Aguiar S, Vladimir J, Adailton O, Ortoluzzi JB, et al. Precipitation of resveratrol-isoniazid and resveratrol-nicotinamide cocrystals by gas antisolvents. J Supercrit Fluids. 145 (2019) 93–102. [Google Scholar]
- [94].Harikrishnan S, Sedev R, Beh C, Priest C. Loading of 5-fluorouracil onto Halloysite nanotubes for targeted drug delivery using a subcritical gas antisolvent process (GAS). J Supercrit Fluids. 159 (2020) 104756. https://doi.org/10.1016/j.supflu.2020.104756. 10.1016/j.supflu.2020.104756 [DOI] [Google Scholar]
- [95].Casas IG, Crampon C, Montes A, Pereyra C, Martinez EJ. Supercritical CO2 impregnation of silica microparticles with quercetin. J Supercrit Fluids. 143 (2019) 157–61. [Google Scholar]
- [96].Silva CV, Pereira VJ, Costa GMN, Albuquerque ECMC, Melo SABV, Sousa HC, et al. Supercritical solvent impregnation/deposition of spilanthol-enriched extracts into a commercial collagen/cellulose-based wound dressing. J Supercrit Fluids. 133 (2018) 503–11. [Google Scholar]
- [97].Yan TY, Zhang Y, Ji M, Wang Z, Yan T. Preparation of irbesartan composite microparticles by supercritical aerosol solvent extraction system for dissolution enhancement. J Supercrit Fluids. 153 (2019) 104594. https://doi.org/10.1016/j.supflu.2019.104594. 10.1016/j.supflu.2019.104594 [DOI] [Google Scholar]
- [98].Yu W, Xia F, Jin H, Lin C, Zhao Y, Jiang S, et al. Production of submicroparticles of β-sitosterol using an aerosol solvent extraction system. Chin J Chem Eng. 16 (2008) 956–60. [Google Scholar]
- [99].Lee SY, Jung I, Kim JK, Lim GB, Ryu JH. Preparation of itraconazole/HP-β-CD inclusion complexes using supercritical aerosol solvent extraction system and their dissolution characteristics. J Supercrit Fluids. 44 (2008) 400–8. [Google Scholar]


