Abstract
The topoisomerase IV subunit A gene, parC homolog, has been cloned and sequenced from Pseudomonas aeruginosa PAO1, with cDNA encoding the N-terminal region of Escherichia coli parC used as a probe. The homolog and its upstream gene were presumed to be parC and parE through sequence homology with the parC and parE genes of other organisms. The deduced amino acid sequence of ParC and ParE showed 33 and 32% identity with that of the P. aeruginosa DNA gyrase subunits, GyrA and GyrB, respectively, and 69 and 75% identity with that of E. coli ParC and ParE, respectively. The putative ParC and ParE proteins were overexpressed and separately purified by use of a fusion system with a maltose-binding protein, and their enzymatic properties were examined. The reconstituted enzyme had ATP-dependent decatenation activity, which is the main catalytic activity of bacterial topoisomerase IV, and relaxing activities but had no supercoiling activity. So, the cloned genes were identified as P. aeruginosa topoisomerase IV genes. The inhibitory effects of quinolones on the activities of topoisomerase IV and DNA gyrase were compared. The 50% inhibitory concentrations of quinolones for the decatenation activity of topoisomerase IV were from five to eight times higher than those for the supercoiling activities of P. aeruginosa DNA gyrase. These results confirmed that topoisomerase IV is less sensitive to fluoroquinolones than is DNA gyrase and may be a secondary target of new quinolones in wild-type P. aeruginosa.
Bacterial DNA topoisomerases are enzymes responsible for controlling the topological states of DNA in DNA replication and transcription (23). They act upon DNA to alter the level of supercoiling, as well as to catenate and decatenate chromosomes (7, 28). Four DNA topoisomerases have been isolated from Escherichia coli: topoisomerase I (44), DNA gyrase (11), topoisomerase III (6), and topoisomerase IV (18). DNA gyrase and topoisomerase IV are classified as type II topoisomerases based on similarities in amino acid sequences and enzymatic mechanisms. The mechanism of these enzymes involves DNA cleavage and DNA strand passage through the break, followed by rejoining of the cleaved DNA (36). DNA gyrase is unique among known DNA topoisomerases because of its ability to introduce negative supercoils into DNA molecules (11). DNA gyrase, a heterotetramer, is composed of two subunits, GyrA and GyrB, which are encoded by the gyrA and gyrB genes, respectively (1, 41, 45). GyrA is responsible for the DNA strand binding, cleavage, and rejoining, and GyrB is responsible for ATPase activity. The N-terminal region of GyrA is where the covalent attachment of a tyrosine residue to the 5′ end of cleaved DNA is formed (14).
Topoisomerase IV, the other type II DNA topoisomerase, is encoded by the parC and parE genes in E. coli (18). Topoisomerase IV was reported previously to relax superhelical DNA and to decatenate kinetoplast DNA (19, 34, 35). Unlike gyrase, it shows no supercoiling activity. In vitro studies using purified ParC and ParE proteins showed that the decatenation activity of topoisomerase IV was five times more effective than its relaxing activity (15).
The ParC and ParE proteins are homologous to GyrA (36%) and GyrB (40%), respectively, in E. coli, and the amino acids around the DNA-binding site (tyrosine at position 122 in GyrA) are particularly well conserved (18, 19, 34). In spite of the high sequence homology between the respective DNA gyrase and topoisomerase IV subunit genes, they cannot complement each other (19, 34). The genes encoding homologs of E. coli DNA gyrase and topoisomerase IV subunits have since been identified in many phylogenetic branches of bacteria (16).
Type II topoisomerases have become critical targets of drugs for the treatment of various diseases. Bacterial type II topoisomerases have proven to be important targets for two classes of antimicrobial agents; the A subunit is considered to be a target of quinolones, whereas the B subunits are considered to be that of coumarins (26). Quinolone antibacterial agents have been used in therapy for various bacterial infections (8, 26). In vitro and in vivo studies showed that the activity of DNA gyrase and topoisomerase IV is inhibited by quinolones (8). DNA gyrase is a primary target of quinolones in the gram-negative species, such as E. coli, Neisseria gonorrhoeae, and Haemophilus influenzae (3, 12, 13, 21). For the E. coli enzymes, the inhibition of the decatenating activity of topoisomerase IV requires a 15- to 50-times-higher concentration of quinolones than does the inhibition of the supercoiling activity of DNA gyrase (15). In contrast, the topoisomerase activity of topoisomerase IV is more sensitive than that of DNA gyrase to some quinolones such as levofloxacin and ciprofloxacin in Staphylococcus aureus (43).
Pseudomonas aeruginosa is an opportunistic human pathogen and is intrinsically resistant to a wide variety of antibiotics, because of the low outer membrane permeability and drug efflux systems (32). Especially in patients with cystic fibrosis, emergence of antibiotic-resistant P. aeruginosa strains is observed (29). The major mechanisms of bacterial resistance to quinolones are the modifications of the target sites of DNA gyrase and topoisomerase IV. Alterations in DNA gyrase or topoisomerase IV caused by mutations in the so-called quinolone resistance-determining region (QRDR) (47) of gyrA or parC appear to provide the resistance in many species of bacteria (8). The gyrA gene of P. aeruginosa was identified by Kureishi et al. (22), and many mutations of the QRDR of gyrA have been found in the quinolone-resistant P. aeruginosa (5, 22, 46). Although many studies have focused on DNA gyrase (17, 20, 25, 48, 49), the studies on topoisomerase IV are less advanced for quinolone-resistant P. aeruginosa. Recently, Nakano et al. (31) determined QRDR sequences of the gyrA and parC genes of 22 clinical isolates of P. aeruginosa and reported that the accumulation of alterations in GyrA and the simultaneous presence of alterations in ParC may be associated with the development of higher-level fluoroquinolone resistance. However, it remains to be determined whether topoisomerase activity of DNA gyrase is more sensitive to inhibition by quinolones than that of topoisomerase IV in P. aeruginosa.
Insofar as P. aeruginosa is an important bacterium for ecology and infectious disease, we attempted to clarify the role of topoisomerase IV in the mechanism of action of quinolone on P. aeruginosa. We report here the sequence of topoisomerase IV parC and parE genes of P. aeruginosa PAO1. We focused on and compared the inhibitory activities of quinolones against gyrase and topoisomerase IV purified by the same method from P. aeruginosa PAO1.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture media.
P. aeruginosa PAO1 was used to construct genomic libraries and as a reference strain having wild-type DNA gyrase and topoisomerase IV. E. coli MC1061 and plasmids pUC19 and pUC118 were used to construct libraries and to subclone DNA inserts. Plasmid pCRII (Invitrogen, San Diego, Calif.) was employed to clone PCR products in E. coli JM109. Supercoiled pBR322 plasmid DNA (Boehringer Mannheim GmbH, Mannheim, Germany) and kinetoplast DNA (Nippongene, Toyama, Japan) were used for enzyme assays. Bacteria were grown routinely in Luria-Bertani broth or on Luria-Bertani agar plates (27). SOC medium (Gibco BRL, Grand Island, N.Y.) was used for transformation. The antibiotic used for plasmid selection in E. coli was ampicillin (50 μg/ml). All other chemicals were purchased from Sigma-Aldrich Japan (Tokyo, Japan). Plasmid preparation, agarose gel electrophoresis, DNA ligation, transformation, and other cloning procedures were done by standard methods (37).
Southern blot analysis.
Chromosomal DNA or cloned DNA fragments were digested with restriction enzymes, separated by 0.8% agarose gel electrophoresis, and blotted onto Hybond-N+ membranes (Amersham Pharmacia Biotech) according to standard procedures (37). Filters were hybridized to 32P-radiolabeled DNA probes obtained by random priming with the Quick Prime kit (Amersham Pharmacia Biotech) with [α-32P]dCTP. After hybridization, filters were washed twice in 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.5% sodium dodecyl sulfate (SDS) for 2 h at 65°C.
PCR amplification and DNA sequence determination and analysis.
The oligonucleotide primers used for PCR amplification were synthesized in our laboratories and are listed in Table 1. The amplification procedure comprised denaturation at 92°C for 2 min; this was followed by 35 cycles including denaturation for 1 min at 92°C, annealing for 1 min at 55°C, and polymerization for 1 to 5 min at 68 or 72°C. The reactions were performed in a final volume of 50 μl with 2.5 U of LA Taq DNA polymerase (Takara, Kyoto, Japan). DNA fragments were subcloned into plasmid pUC19 and sequenced by the dideoxy chain termination method (38) with a T7 sequencing kit (Amersham Pharmacia Biotech) according to the manufacturer’s instructions and by a Pharmacia automatic sequencer. DNA and protein sequences were analyzed by use of the GENETYX program (Software Development Co., Ltd., Tokyo, Japan).
TABLE 1.
Nucleotide sequences of primers used in PCR
| Primer | Nucleotide sequence | Position | Comment |
|---|---|---|---|
| Pr-PAPARE01 | 5′-ATGATCGCCTGGAATCCTTTCCCG-3′ | parE 1310–1285 | Amplification of a 1.5-kb fragment containing the 5′ region of parE |
| Pr-PAPARC05 | 5′-TCATCACCCCGCGCGCCGACCTGCAG-3′ | parC 683–708 | |
| Pr-PAPARC06 | 5′-TCTCCGTGAGGGATCCATGAGCGAAT-3′ | parC −16–10 | Amplification of a 2.5-kb fragment containing parC |
| Pr-PAPARC07 | 5′-ACCAGCGCCAAAGCTTCAGAGGCAGA-3′ | parC 2450–2425 | |
| Pr-PAPARE03 | 5′-TTGACTGTCTAGAGACCCCATGGCTA-3′ | parE −19–7 | Amplification of a 1.9-kb fragment containing parE |
| Pr-PAPARE04 | 5′-GAACAAGCTTAGGATGGCCAGCAGGC-3′ | parE 1922–1897 | |
| Pr-PAGYRA31 | 5′-GAAAAAGGATCTAGACTTCTC-3′ | gyrA −21–1 | Amplification of a 2.8-kb fragment containing gyrA |
| Pr-PAGYRA32 | 5′-CCGAAGCTTACTCTTCGTT-3′ | gyrA 2778–2759 | |
| Pr-PAGYB103 | 5′-ACGACCATCGGGAATTCAGCATGAGCGAGA-3′ | gyrB −20–5 | Amplification of a 2.2-kb fragment containing gyrB |
| Pr-PAGYB104 | 5′-CCGTATCCAAGCTTCCTGGCGCAA-3′ | gyrB 2502–2478 |
Construction of fusion plasmids.
Four sets of 26-mer oligonucleotide primers were designed to allow amplification of parC, parE, gyrA, and gyrB genes (Table 1). These genes were digested by BamHI-HindIII (parC), XbaI-HindIII (parE and gyrA), or EcoRI-HindIII (gyrB); ligated with the pMAL-c2 plasmid (New England Biolabs, Beverly, Mass.), yielding plasmids pMPPC203 (parC), pMPPE72 (parE), pMPGA417 (gyrA), and pMPGB512 (gyrB), respectively; and used to produce the fusion protein.
Purification of the ParC, ParE, GyrA, and GyrB proteins.
Proteins encoded by the parC, parE, gyrA, and gyrB genes were purified by a protein fusion and purification system for maltose-binding protein (MBP) fusion proteins (New England Biolabs). Purification of the fusion proteins was carried out according to the manufacturer’s protocol.
Inhibitory activities of quinolones against topoisomerase IV and DNA gyrase.
Supercoiled pBR322 plasmid DNA was purchased from Boehringer Mannheim GmbH and was relaxed by topoisomerase I (Fermentas Ltd., Vilnius, Lithuania) before testing for the supercoiling activity of DNA gyrase. Inhibitory activities of quinolones against type II topoisomerases were assayed electrophoretically as described previously (2).
Determination of MICs.
P. aeruginosa PAO1 was cultivated overnight at 37°C in Mueller-Hinton broth, and the MICs of quinolones were determined by the standard agar dilution method with Mueller-Hinton agar (Difco). The inoculum size was approximately 104 CFU/spot. The MIC was defined as the lowest drug concentration that prevented visible bacterial growth of the inoculum after incubation for 18 h at 37°C.
Nucleotide sequence accession numbers.
The nucleotide sequence data of parC and parE will appear in the DDBJ, EMBL, and GenBank nucleotide sequence databases with the following respective accession numbers: AB003428 and AB003429.
RESULTS
Cloning of the parC and parE homologs from P. aeruginosa PAO1.
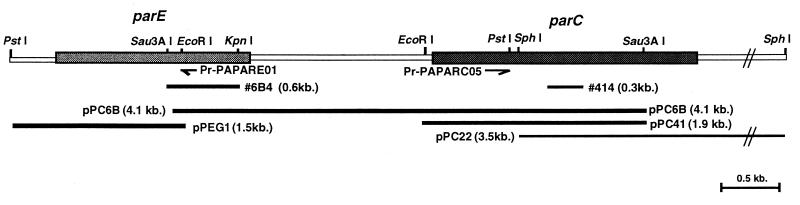
The genomic DNA from P. aeruginosa PAO1 was partially digested with Sau3AI. The digested DNA was size fractionated (2.0 to 6.5 kb) and ligated into pUC19, which was digested with BamHI. Transformants derived from E. coli MC1061 transformed with the resultant plasmids were screened with the probe of 0.6-kb E. coli KL-16 parC (N-terminal region) (positions 1 to 599 in parC). Plasmids were isolated from colonies that showed a hybridization signal, and plasmid pPC6B (4.1-kb insert in pUC19) and plasmid pPC41 (1.9-kb insert in pUC19) were isolated (Fig. 1). DNA sequence analysis indicated that plasmid pPC6B contained parts of the parC N-terminal and parE C-terminal regions and that plasmid pPC41 contained part of the parC N-terminal region (Fig. 1).
FIG. 1.
Restriction map of the parC and parE genes in P. aeruginosa PAO1 and alignment of plasmid clones. The parC and parE genes are indicated by shaded regions. Plasmid pPC6B (3.8-kb insert in pUC19) and plasmid pPC41 (2.0-kb insert in pUC19) were obtained by colony hybridization with the 0.6-kb probe encoding the N-terminus of E. coli parC. Plasmid pPC22, containing a 3.5-kb SphI insert, was isolated from a size-selected library with a 0.3-kb fragment (no. 414) as probe. Plasmid pPEG1 was obtained by PCR of a 4.7-kb PstI fragment.
To obtain full-length parC and parE genes, we performed Southern blot analysis. SphI digestion of genomic DNA from P. aeruginosa PAO1 produced a single band of 3.5 kb that hybridized with part of the P. aeruginosa parC N-terminal (no. 414; positions 1105 to 1315 in parC; 0.3 kb) probe. DNA fragments after SphI digestion were ligated into pUC19 digested with SphI, and the resulting plasmids were transformed into E. coli MC1061. After screening with probe 414, plasmid pPC22 (3.5-kb insert in pUC19) was isolated from a colony that showed a hybridization signal. The subsequent sequence analysis revealed that plasmid pPC22 contained part of the parC C-terminal region (Fig. 1).
P. aeruginosa PAO1 genomic DNA after PstI digestion contained a single band of 4.7 kb that hybridized with probe 6B4 (positions 1168 to 1794 of parE). The genomic DNA digested with PstI was size fractionated and circularized by self-ligation with T4 DNA ligase. Self-ligated circular DNA was used as a PCR template to obtain a fragment corresponding to part of the parE N-terminal region. PCR was done with forward primer Pr-PAPARC05 and reverse primer Pr-PAPARE01 (Table 1). The PCR product was ligated into pCRII, and plasmid pPEG1 was isolated (Fig. 1).
Nucleotide sequence of the P. aeruginosa parC and parE homologs.
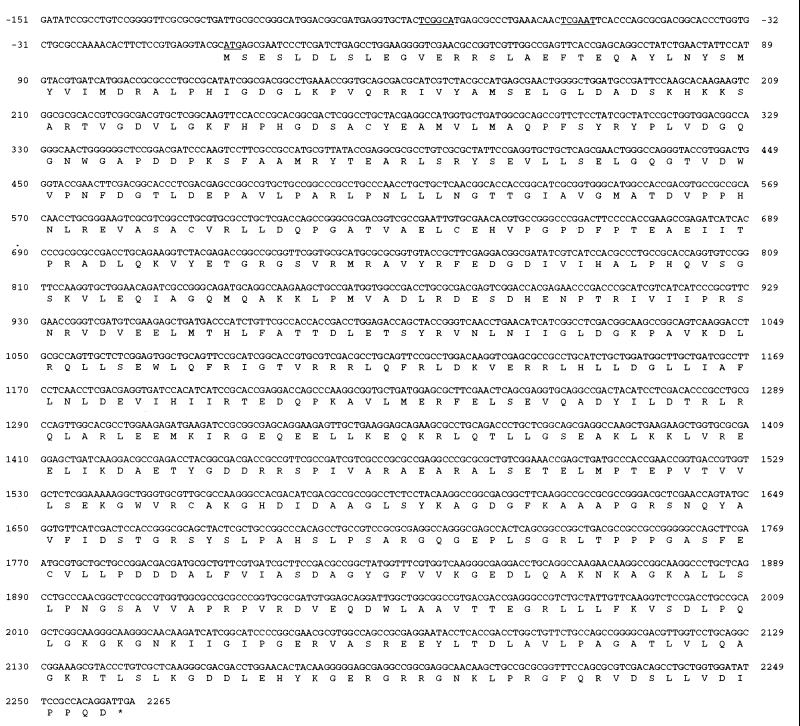
The DNA fragments shown in Fig. 1 were subcloned for sequence analysis. The subcloned plasmids were sequenced by the dideoxy chain termination method with either vector-specific primers or primers chosen from the internal sequence. An open reading frame (ORF1) of 2,265 nucleotides coded for a polypeptide of 754 amino acids (Fig. 2) with a calculated molecular mass of 83.3 kDa. Putative −10 (TCGAAT) and −35 (TCGGCA) regions and ribosome binding signals were found upstream of the initiation ATG codon. The deduced protein had a general amino acid identity of 33% with P. aeruginosa GyrA (22) and exhibited homology with known topoisomerase IV subunit A proteins of E. coli (18, 34), Salmonella typhimurium (24), H. influenzae (10), S. aureus (i.e., GrlA) (9), Streptococcus pneumoniae (33), Bacillus subtilis (i.e., GrlA) (accession no. Z73234), and N. gonorrhoeae (3) at 69, 68, 64, 31, 33, 31, and 44%, respectively (Table 2). These results suggested that ORF1 might be identified as parC. P. aeruginosa ParC-like protein was compared with ParC of E. coli and GrlA of S. aureus. A region with high homology was found in the N-terminal DNA breakage-reunion region of P. aeruginosa ParC-like protein and its counterparts. The catalytic tyrosine residue present in the active site of the type II topoisomerases was identified putatively as Tyr-127 in P. aeruginosa ParC by alignment of a conserved AAMRYTE sequence with catalytic Tyr-120 of E. coli ParC. Serine (equivalent to Ser-80 in E. coli ParC and S. aureus GrlA) in the QRDR sequence was at amino acid position 87 (reported as Ser-80 by Nakano et al. [31]). From the results, it was concluded that ORF1 might be the parC gene of P. aeruginosa PAO1.
FIG. 2.
Nucleotide sequence and deduced amino acid sequence of the P. aeruginosa parC region. A methionine initiation codon and putative −10 and −35 regions are shown by underlining. An asterisk indicates the translation stop codon.
TABLE 2.
Comparison of protein homology among P. aeruginosa topoisomerase IV and its counterparts
| Bacterium | % Homology of P. aeruginosa
|
|
|---|---|---|
| Subunit A (ParC) | Subunit B (ParE) | |
| E. coli | 69 | 75 |
| S. typhimurium | 68 | 73 |
| H. influenzae | 64 | 70 |
| N. gonorrhoeae | 44 | NDa |
| S. aureus | 31 | 33 |
| S. pneumoniae | 33 | 36 |
| B. subtilis | 31 | 37 |
| P. aeruginosa (DNA gyrase) | 33 | 32 |
ND, not determined.
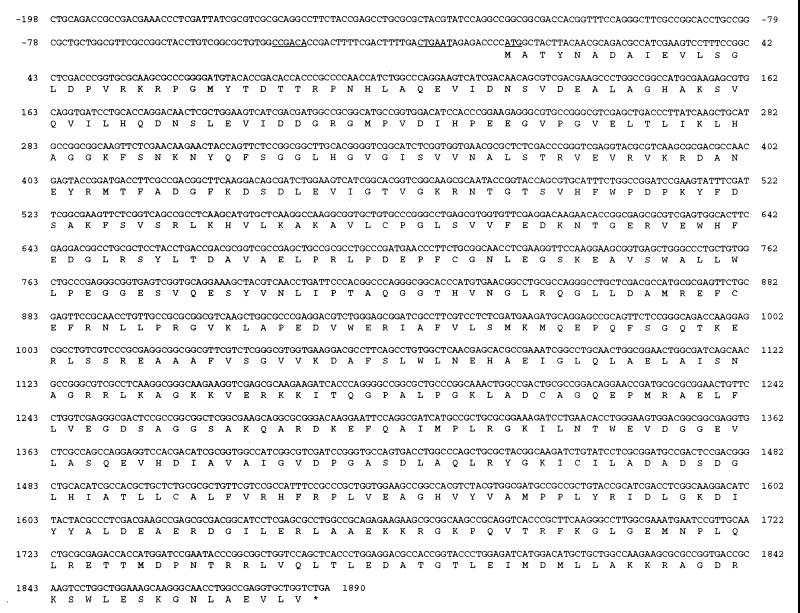
Analysis of the sequenced regions upstream of the putative parC gene revealed a region homologous with the parE sequence of E. coli: an open reading frame (ORF2) of 1,890 nucleotides coding for a polypeptide of 629 amino acids with a predicted molecular mass of 69.2 kDa. Putative −10 (CTGAAT) and −35 (CCGACA) promoter regions were found upstream of the initiation ATG codon (Fig. 3). The deduced amino acid sequence exhibited 75% identity with the ParE subunit of E. coli (18, 34). Comparison of ORF2 with the GyrB subunits of P. aeruginosa (accession no. AB00581) and E. coli (1, 45) revealed 32% identity with each of them. On this basis, the 629-residue P. aeruginosa protein was identified putatively as ParE. The P. aeruginosa ParE homolog is identical to known ParE proteins of S. typhimurium (40), H. influenzae (10), S. aureus (i.e., GrlB) (9), S. pneumoniae (33), and B. subtilis (i.e., GrlB) (accession no. Z73234) at 70, 64, 33, 37, and 36%, respectively (Table 2). Compared with its counterparts, P. aeruginosa ParE showed highly conserved EGDSA and N-terminal sequences, including the G-loop ATP-binding moiety.
FIG. 3.
Nucleotide sequence and deduced amino acid sequence of the P. aeruginosa parE region. The P. aeruginosa parE nucleotide sequence is shown along with the predicted amino acid sequence. Symbols are the same as those defined for Fig. 2.
Purification of P. aeruginosa topoisomerase IV subunits.
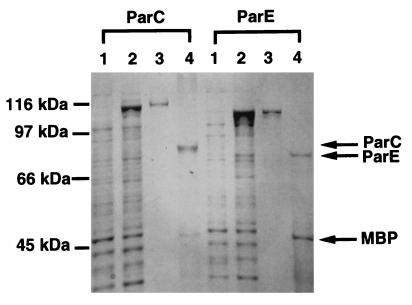
The putative ParC and ParE proteins were overexpressed and were purified separately with a fusion system with MBP and then analyzed by SDS-polyacrylamide gel electrophoresis (Fig. 4). The putative parC and parE genes from P. aeruginosa PAO1 were cloned into pMAL-c2, a tac promoter-based expression vector, yielding plasmids pMPPC203 and pMPE72, respectively. E. coli MC1061 harboring pMPPC203 and pMPPE72 overproduced MBP-ParC and MBP-ParE proteins, respectively, after induction with isopropyl-β-d-thiogalactopyranoside (IPTG) (Fig. 4, lanes 2). MBP-ParC and MBP-ParE were purified by affinity chromatography (Fig. 4, lanes 3), and the ParC and ParE proteins were then obtained by digestion with protease factor Xa (Fig. 4, lanes 4). The molecular size of the purified proteins was in agreement with the molecular weight calculated from the deduced protein sequences of ParC and ParE.
FIG. 4.
SDS-polyacrylamide gel electrophoresis analysis of ParC and ParE. Proteins at various purification steps were electrophoresed in an SDS–12.5% polyacrylamide gel and stained with Coomassie brilliant blue. Lanes 1, soluble extracts from uninduced cells; lanes 2, soluble extracts from IPTG-induced cells, lanes 3, affinity-purified MBP-ParC or MBP-ParE protein; lanes 4, factor Xa digest of MBP-ParC or MBP-ParE.
Enzymatic activities of P. aeruginosa topoisomerase IV.
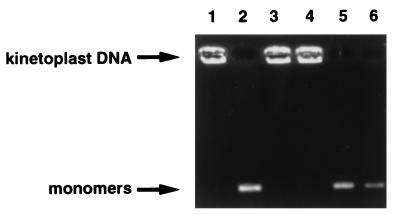
Putative ParC and ParE were examined for decatenating activity (Fig. 5). Enzymatic activity was dependent on ATP, Mg2+ (data not shown), and the presence of both subunits (Fig. 5, lane 2), because the omission of ATP (Fig. 5, lane 4) or either single subunit (Fig. 5, lanes 1 and 3) did not lead to DNA decatenation activity. In addition, relaxing activity of superhelical DNA was detected with the combination of ParC and ParE (results not shown). No supercoiling activity was detected with ATP when relaxed DNA was incubated with ParC, ParE, or both (data not shown), indicating that the combination of ParC and ParE catalyzes reactions similarly to E. coli and other topoisomerase IV proteins (19, 34).
FIG. 5.
Topoisomerase IV activities of ParC and ParE proteins. Lane 1, P. aeruginosa ParC (1 U); lane 2, P. aeruginosa ParC (1 U) and P. aeruginosa ParE (1 U); lane 3, P. aeruginosa ParE (1 U); lane 4, P. aeruginosa ParC (1 U) and P. aeruginosa ParE (1 U) but with ATP omitted; lane 5, P. aeruginosa ParC (1 U) and E. coli ParE (1 U); lane 6, E. coli ParC (1 U) and P. aeruginosa ParE (1 U).
When P. aeruginosa ParC or ParE was combined with E. coli ParE or ParC, respectively, the decatenating activity was detected (Fig. 5, lanes 5 and 6). These results indicate that a complementation occurs between topoisomerase IV subunits of P. aeruginosa and E. coli in vitro.
Comparison of inhibitory activities of quinolones against DNA gyrase and topoisomerase IV.
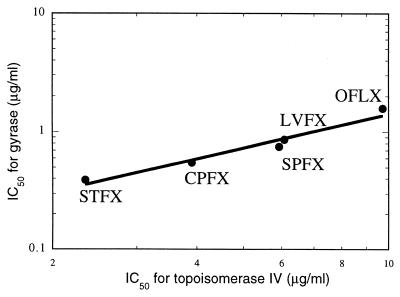
The inhibitory effects of quinolones on the topoisomerase activities of topoisomerase IV and DNA gyrase were determined by quantitative electrophoresis with kinetoplast DNA and relaxed DNA as a substrate. The 50% inhibitory concentrations (IC50s) of quinolones were calculated from the quantification of bands corresponding to fully decatenated substrate or supercoiled DNA. MICs and the IC50s of quinolones on the topoisomerase activities are shown in Table 3. The IC50s against the decatenation of topoisomerase IV were higher than those against the supercoiling activity of DNA gyrase. Among the quinolones tested, sitafloxacin showed the highest level of inhibitory activity against DNA gyrase and topoisomerase IV. There was a high correlation between the inhibitory effects of the quinolones on bacterial growth and the inhibitory activity against the topoisomerase activity of DNA gyrase (correlation coefficient, 0.942) and of topoisomerase IV (correlation coefficient, 0.972). The correlation between the IC50s of quinolones against DNA gyrase and topoisomerase IV is presented in Fig. 6. The inhibitory activities of quinolones against type II topoisomerases were correlated well, with the correlation coefficient being 0.977.
TABLE 3.
Inhibitory activities of quinolones against topoisomerase IV and DNA gyrase
| Drug | MIC (μg/ml)a | IC50 (μg/ml)
|
|
|---|---|---|---|
| DNA gyrase, supercoiling | Topoisomerase IV, decatenation | ||
| Sitafloxacin | 0.10 | 0.42 | 2.34 |
| Levofloxacin | 0.39 | 0.88 | 6.08 |
| Ofloxacin | 0.78 | 1.47 | 9.72 |
| Ciprofloxacin | 0.10 | 0.55 | 3.90 |
| Sparfloxacin | 0.39 | 0.75 | 5.93 |
Tested against P. aeruginosa PAO1.
FIG. 6.
Correlation between inhibition of topoisomerase IV and that of DNA gyrase. Abbreviations: STFX, sitafloxacin; CPFX, ciprofloxacin; SPFX, sparfloxacin; LVFX, levofloxacin; OFLX, ofloxacin.
DISCUSSION
A P. aeruginosa parC homolog was cloned and sequenced from P. aeruginosa PAO1, by use of cDNA encoding the N-terminal region of E. coli parC as a probe. The homolog and its upstream gene were identified putatively as parC and parE, respectively, through sequence homology with other parC and parE genes. The parC homolog of 2,265 bp coded for a protein of 754 amino acids. The deduced amino acid sequence of the ParC homolog showed 69% identity with that of E. coli ParC and 33% identity with that of P. aeruginosa GyrA. The parE homolog of 1,890 bp encoded 629 amino acids, and this gene product was 75% identical to E. coli ParE and 32% identical to P. aeruginosa GyrB.
The putative ParC and ParE proteins were overexpressed and separately purified with a fusion system involving an MBP, and their enzymatic properties were examined. The combined putative ParC and ParE proteins catalyzed decatenation and relaxing reactions but had no supercoiling reaction. Not only from the sequence homologies but also from the characteristics of the enzyme, isolated P. aeruginosa genes were confirmed as parC and parE genes of P. aeruginosa topoisomerase IV.
When P. aeruginosa ParC or ParE was combined with E. coli ParE or ParC, respectively, decatenation activity was detected in vitro. This result is not surprising given the high degree of homology between the P. aeruginosa and E. coli ParC proteins and the fact that P. aeruginosa GyrA protein can functionally complement the E. coli GyrA protein in vivo (22). However, when S. aureus GrlA or GrlB was combined with E. coli ParE or ParC, respectively, no decatenation activity was detected (4), and the temperature-sensitive phenotype of S. typhimurium parC and parE mutants was complemented by the S. aureus grlA and grlB genes only when the two genes were coexpressed (9).
The decatenation activity of P. aeruginosa topoisomerase IV was inhibited by quinolones. There was a high correlation between the inhibitory activity against the topoisomerase activity of DNA gyrase and that of topoisomerase IV.
Sitafloxacin (DU-6859a), a newly developed quinolone antibacterial agent, showed more potent activity against a wide spectrum of bacteria (30, 39, 42) than did levofloxacin and ciprofloxacin. Our previous study (20) showed the inhibition by sitafloxacin of purified DNA gyrases from only clinical isolates of P. aeruginosa. In this study, sitafloxacin had a lower MIC against P. aeruginosa PAO1 than most of the other quinolones and the lowest IC50s for DNA gyrase and topoisomerase IV of P. aeruginosa among the quinolones tested, and a good correlation existed between the inhibitory effects on bacterial growth (MICs) and those on the type II topoisomerases of P. aeruginosa PAO1. From these results, sitafloxacin appears to have higher activity against P. aeruginosa than do other available quinolones, probably because of its higher inhibitory effects against type II topoisomerases.
In this study, we purified topoisomerase IV and DNA gyrase of P. aeruginosa PAO1 in the same manner and compared the inhibitory activities of quinolones against the purified enzymes. The supercoiling activity of DNA gyrase was more sensitive to quinolones than was the decatenation activity of topoisomerase IV. Our results, obtained by enzymatic methods, support the view that DNA gyrase is the primary target of new quinolones and have shown that topoisomerase IV may act as a secondary target in the quinolone-susceptible P. aeruginosa strain.
ACKNOWLEDGMENTS
We thank Yuki Nagano for preparation of the primers. We are thankful to Kenji Hayata for sharing with us the sequence of the gyrB gene of P. aeruginosa.
REFERENCES
- 1.Adachi T, Mizuuchi M, Robinson E, Appella E, O’Dea M, Gellert M, Mizuuchi K. DNA sequence of the E. coli gyrB gene: application of a new sequencing strategy. Nucleic Acids Res. 1987;15:771–783. doi: 10.1093/nar/15.2.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akasaka T, Kurosaka S, Uchida Y, Tanaka M, Sato K, Hayakawa I. Antibacterial activities and inhibitory effects of sitafloxacin (DU-6859a) and its optical isomers against type II topoisomerases. Antimicrob Agents Chemother. 1998;42:1284–1287. doi: 10.1128/aac.42.5.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belland R J, Morrison S G, Ison C, Huang W M. Neisseria gonorrhoeae acquires mutations in analogous regions of gyrA and parC in fluoroquinolone-resistant isolates. Mol Microbiol. 1994;14:371–380. doi: 10.1111/j.1365-2958.1994.tb01297.x. [DOI] [PubMed] [Google Scholar]
- 4.Blanche F, Cameron B, Bernard F X, Maton L, Manse B, Ferrero L, Ratet N, Lecoq C, Goniot A, Bisch D, Crouzet J. Differential behaviors of Staphylococcus aureus and Escherichia coli type II DNA topoisomerases. Antimicrob Agents Chemother. 1996;40:2714–2720. doi: 10.1128/aac.40.12.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cambau E, Perani E, Dib C, Petinon C, Trias J, Jarlier V. Role of mutations in DNA gyrase genes in ciprofloxacin resistance of Pseudomonas aeruginosa susceptible or resistant to imipenem. Antimicrob Agents Chemother. 1995;39:2248–2252. doi: 10.1128/aac.39.10.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dean F, Krasnow M A, Otter R, Matzuk M M, Spengler S J, Cozzarelli N R. Escherichia coli type-1 topoisomerases: identification, mechanism, and role in recombination. Cold Spring Harbor Symp Quant Biol. 1983;2:769–777. doi: 10.1101/sqb.1983.047.01.088. [DOI] [PubMed] [Google Scholar]
- 7.Drlica K. Control of bacterial DNA supercoiling. Mol Microbiol. 1992;6:425–433. doi: 10.1111/j.1365-2958.1992.tb01486.x. [DOI] [PubMed] [Google Scholar]
- 8.Drlica K, Zhao X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol Mol Biol Rev. 1997;61:377–392. doi: 10.1128/mmbr.61.3.377-392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrero L, Cameron B, Manse B, Lagneaux D, Crouzet J, Famechon A, Blanche F. Cloning and primary structure of Staphylococcus aureus DNA topoisomerase IV: a primary target of fluoroquinolones. Mol Microbiol. 1994;13:641–653. doi: 10.1111/j.1365-2958.1994.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 10.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J F, Dougherty B A, Merrick J M, et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 11.Gellert M, Mizuuchi K, O’Dea M H, Nash H A. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci USA. 1976;73:3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Georgiou M, Munoz R, Roman F, Canton R, Gomez-Lus R, Campos J, De La Campa A G. Ciprofloxacin-resistant Haemophilus influenzae strains possess mutations in analogous positions of GyrA and ParC. Antimicrob Agents Chemother. 1996;40:1741–1744. doi: 10.1128/aac.40.7.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heisig P. Genetic evidence for a role of parC mutations in development of high-level fluoroquinolone resistance in Escherichia coli. Antimicrob Agents Chemother. 1996;40:879–885. doi: 10.1128/aac.40.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horowitz D, Wang J. Mapping the active site tyrosine of Escherichia coli DNA gyrase. J Biol Chem. 1987;262:5339–5348. [PubMed] [Google Scholar]
- 15.Hoshino K, Kitamura A, Morrissey I, Sato K, Kato J, Ikeda H. Comparison of inhibition of Escherichia coli topoisomerase IV by quinolones with DNA gyrase inhibition. Antimicrob Agents Chemother. 1994;38:2623–2627. doi: 10.1128/aac.38.11.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang W M. Bacterial diversity based on type II DNA topoisomerase genes. Annu Rev Genet. 1996;30:79–107. doi: 10.1146/annurev.genet.30.1.79. [DOI] [PubMed] [Google Scholar]
- 17.Inoue Y, Sato K, Fujii T, Hirai K, Inoue M, Iyobe S, Mitsuhashi S. Some properties of subunits of DNA gyrase from Pseudomonas aeruginosa PAO1 and its nalidixic acid-resistant mutant. J Bacteriol. 1987;169:2322–2325. doi: 10.1128/jb.169.5.2322-2325.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato J, Nishimura Y, Imamura R, Niki H, Hiraga S, Suzuki H. New topoisomerase essential for chromosome segregation in E. coli. Cell. 1990;63:393–404. doi: 10.1016/0092-8674(90)90172-b. [DOI] [PubMed] [Google Scholar]
- 19.Kato J, Suzuki H, Ikeda H. Purification and characterization of DNA topoisomerase IV in Escherichia coli. J Biol Chem. 1992;267:25676–25684. [PubMed] [Google Scholar]
- 20.Kitamura A, Hoshino K, Kimura Y, Hayakawa I, Sato K. Contribution of the C-8 substituent of DU-6859a, a new potent fluoroquinolone, to its activity against DNA gyrase mutants of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:1467–1471. doi: 10.1128/aac.39.7.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumagai Y, Kato J, Hoshino K, Akasaka T, Sato K, Ikeda H. Quinolone-resistant mutants of Escherichia coli DNA topoisomerase IV parC gene. Antimicrob Agents Chemother. 1996;40:710–714. doi: 10.1128/aac.40.3.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kureishi A, Diver J M, Beckthold B, Schollaardt T, Bryan L E. Cloning and nucleotide sequence of Pseudomonas aeruginosa DNA gyrase gyrA gene from strain PAO1 and quinolone-resistant clinical isolates. Antimicrob Agents Chemother. 1994;38:1944–1952. doi: 10.1128/aac.38.9.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luttinger A. The twisted ’life’ of DNA in the cell: bacterial topoisomerases. Mol Microbiol. 1995;15:601–606. doi: 10.1111/j.1365-2958.1995.tb02369.x. [DOI] [PubMed] [Google Scholar]
- 24.Luttinger A, Springer A, Schmid M. A cluster of genes that affects nucleoid segregation in Salmonella typhimurium. New Biol. 1991;3:687–697. [PubMed] [Google Scholar]
- 25.Masecar B L, Celesk R A, Robillard N J. Analysis of acquired ciprofloxacin resistance in a clinical strain of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990;34:281–286. doi: 10.1128/aac.34.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maxwell A. DNA gyrase as a drug target. Trends Microbiol. 1997;5:102–109. doi: 10.1016/S0966-842X(96)10085-8. [DOI] [PubMed] [Google Scholar]
- 27.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 28.Miller W G, Simons R W. Chromosomal supercoiling in Escherichia coli. Mol Microbiol. 1993;10:675–684. doi: 10.1111/j.1365-2958.1993.tb00939.x. [DOI] [PubMed] [Google Scholar]
- 29.Mouton J W, den Hollander J G, Horrevorts A M. Emergence of antibiotic resistance amongst Pseudomonas aeruginosa isolates from patients with cystic fibrosis. J Antimicrob Chemother. 1993;31:919–926. doi: 10.1093/jac/31.6.919. [DOI] [PubMed] [Google Scholar]
- 30.Nakane T, Iyobe S, Sato K, Mitsuhashi S. In vitro antibacterial activity of DU-6859a, a new fluoroquinolone. Antimicrob Agents Chemother. 1995;39:2822–2826. doi: 10.1128/aac.39.12.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakano M, Deguchi T, Kawamura T, Yasuda M, Kimura M, Okano Y, Kawada Y. Mutations in the gyrA and parC genes in fluoroquinolone-resistant clinical isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1997;41:2289–2291. doi: 10.1128/aac.41.10.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nikaido H. Multidrug efflux pumps of gram-negative bacteria. J Bacteriol. 1996;178:5853–5859. doi: 10.1128/jb.178.20.5853-5859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan X S, Fisher L M. Cloning and characterization of the parC and parE genes of Streptococcus pneumoniae encoding DNA topoisomerase IV: role in fluoroquinolone resistance. J Bacteriol. 1996;178:4060–4069. doi: 10.1128/jb.178.14.4060-4069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng H, Marians K J. Escherichia coli topoisomerase IV: purification, characterization, subunit structure, and subunit interactions. J Biol Chem. 1993;268:24481–24490. [PubMed] [Google Scholar]
- 35.Peng H, Marians K J. Decatenation activity of topoisomerase IV during oriC and pBR322 DNA replication in vitro. Proc Natl Acad Sci USA. 1993;90:8571–8575. doi: 10.1073/pnas.90.18.8571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roca J. The mechanisms of DNA topoisomerases. Trends Biochem Sci. 1995;20:156–160. doi: 10.1016/s0968-0004(00)88993-8. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 38.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato K, Hoshino K, Tanaka M, Hayakawa I, Osada Y. Antimicrobial activity of DU-6859, a new potent fluoroquinolone, against clinical isolates. Antimicrob Agents Chemother. 1992;36:1491–1498. doi: 10.1128/aac.36.7.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Springer A L, Schmid M B. Molecular characterization of the Salmonella typhimurium parE gene. Nucleic Acids Res. 1993;21:1805–1809. doi: 10.1093/nar/21.8.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swanberg S, Wang J. Cloning and sequencing of the Escherichia coli gyrA gene coding for the A subunit of DNA gyrase. J Mol Biol. 1987;197:729–736. doi: 10.1016/0022-2836(87)90479-7. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka M, Hoshino K, Hohmura M, Ishida H, Kitamura A, Sato K, Hayakawa I, Nishino T. Effect of growth conditions on antimicrobial activity of DU-6859a and its bactericidal activity determined by the killing curve method. J Antimicrob Chemother. 1996;37:1091–1102. doi: 10.1093/jac/37.6.1091. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka M, Onodera Y, Uchida Y, Sato K, Hayakawa I. Inhibitory activities of quinolones against DNA gyrase and topoisomerase IV purified from Staphylococcus aureus. Antimicrob Agents Chemother. 1997;41:2362–2366. doi: 10.1128/aac.41.11.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J C. Interaction between DNA and an Escherichia coli protein omega. J Mol Biol. 1971;55:523–533. doi: 10.1016/0022-2836(71)90334-2. [DOI] [PubMed] [Google Scholar]
- 45.Yamagishi J, Yoshida H, Yamayoshi M, Nakamura S. Nalidixic acid-resistant mutations of the gyrB gene of Escherichia coli. Mol Gen Genet. 1986;204:367–373. doi: 10.1007/BF00331012. [DOI] [PubMed] [Google Scholar]
- 46.Yonezawa M, Takahata M, Matsubara N, Watanabe Y, Narita H. DNA gyrase gyrA mutations in quinolone-resistant clinical isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:1970–1972. doi: 10.1128/aac.39.9.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshida H, Bogaki M, Nakamura M, Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrA gene of Escherichia coli. Antimicrob Agents Chemother. 1990;34:1271–1272. doi: 10.1128/aac.34.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshida H, Nakamura M, Bogaki M, Nakamura S. Proportion of DNA gyrase mutants among quinolone-resistant strains of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990;34:1273–1275. doi: 10.1128/aac.34.6.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshida T, Muratani T, Iyobe S, Mitsuhashi S. Mechanisms of high-level resistance to quinolones in urinary tract isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1994;38:1466–1469. doi: 10.1128/aac.38.7.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]