Abstract
Chronic visceral pain represents a major unmet clinical need with the severity of pain ranging from mild to so severe as to prevent individuals from participating in day-to-day activities and detrimentally affecting their quality of life. Although chronic visceral pain can be multifactorial with many different biological and psychological systems contributing to the onset and severity of symptoms, one of the major triggers for visceral pain is the exposure to emotional and physical stress. Chronic visceral pain that is worsened by stress is a hallmark feature of functional gastrointestinal disorders such as irritable bowel syndrome (IBS). Current pharmacological interventions for patients with chronic visceral pain generally lack efficacy and many are fraught with unwanted side effects. Cognitive behavioral therapy (CBT) has emerged as a psychotherapy that shows efficacy at ameliorating stress-induced chronic visceral pain; however, the molecular mechanisms underlying CBT remain incompletely understood. Preclinical studies in experimental models of stress-induced visceral pain employing environmental enrichment (EE) as an animal model surrogate for CBT are unraveling the mechanism by which environmental signals can lead to long-lasting changes in gene expression and behavior. Evidence suggests that EE signaling interacts with stress and nociceptive signaling. This review will (1) critically evaluate the behavioral and molecular changes that lead to chronic pain in IBS, (2) summarize the pharmacological and non-pharmacological approaches used to treat IBS patients, and (3) provide experimental evidence supporting the potential mechanisms by which CBT ameliorates stress-induced visceral pain.
Keywords: pain, environmental enrichment, behavioral therapy, visceral hypersensitivity, stress
Introduction
Irritable bowel syndrome (IBS) is a chronic gastrointestinal (GI) disorder that affects about 10–20% of the population of the USA (1). IBS is typically characterized by visceral hypersensitivity that presents as chronic abdominal pain accompanied by abnormal bowel habits such as diarrhea (IBS-D) or constipation (IBS-C) (1, 2), usually without any obvious histological damage. IBS is diagnosed by gastroenterologists using the ROME criteria (ROME IV) (3). The duration and severity of patient symptoms range from mild to severe enough to detrimentally affect the patients' quality of life (4, 5). The exact etiology of IBS is unclear and research has revealed that the cause of the disorder is multifactorial with many different biological and psychological systems contributing to the onset and severity of symptoms. The lack of understanding of the mechanisms behind IBS has caused a paucity in the development of effective pharmacological treatments of IBS. Stress is a significant risk factor for the emergence of chronic visceral pain in IBS and is often comorbid with other mood and anxiety disorders (6–9), suggesting that environmental factors and stimuli can influence visceral pain sensation in IBS.
Most pharmacological therapies to alleviate IBS symptoms show limited efficacy and can cause unwanted side effects (10, 11). Behavioral therapies have been employed to control the symptoms of mood and psychiatric disorders (12–14), and evidence suggests that behavioral therapies may have potential for the treatment of IBS. Cognitive behavioral therapy (CBT) is a form of psychosocial therapy used to change patterns of thought and emotions. CBT has been approved for a number of psychiatric disorders including anxiety, drug abuse, and depression (15–17). Clinical studies suggest that CBT may also be effective in ameliorating visceral pain disorders including IBS (18, 19). Unfortunately, CBT is not widely available for IBS patients due to a paucity of trained specialists, as well as the duration of treatment sessions. Moreover, the fact that the underlying mechanisms of CBT remain poorly understood continues to hinder the use of CBT to treat visceral pain. To unravel the underlying mechanisms of CBT, we and others have employed a rodent model of environmental enrichment (EE), the animal analog of CBT (20). In this review, we discuss the clinical presentation of chronic visceral pain, focusing on IBS. We will also explore the use of both pharmacological and non-pharmacological therapies in treating abdominal pain in IBS patients. We will summarize the most recent research findings on the neuronal and molecular changes that contribute to chronic visceral pain, and discuss the mechanisms underlying the efficacy of behavioral therapies including CBT in ameliorating chronic pain symptoms based on the latest data from experimental models.
Visceral Pain in IBS
Chronic visceral pain is defined as long-lasting, poorly localized pain emanating from the abdominal region (7, 12) and is a hallmark feature of IBS. Changes in the communication between the central nervous system and the enteric nervous system lead to visceral hypersensitivity and abdominal pain (12). Recent studies suggest that induction and maintenance of visceral hypersensitivity is a multifactorial process that may occur in both the peripheral and central nervous system. Peripherally, infectious agents or altered microbiota content can disrupt the normal functioning of the GI barrier and cause sensitization of nociceptive signals from the enteric nervous system to the brain (13, 14). Growing evidence suggests that psychological and psychosocial factors such as stress act as a key risk factor that triggers or exaggerates IBS symptoms, indicating a role of central regulation in GI function (21). Furthermore, early life stress, such as abuse, is associated with the development of IBS in adulthood (22, 23). Patients with IBS exhibit higher incidence of anxiety (15–45%) or depression (20–30%) (24). IBS patients also displayed significantly higher activity in brain regions involved in stress processing including the amygdala (25, 26). Pre-clinical studies have shown a critical role for corticotropin-releasing hormone (CRH) signaling in the induction of chronic visceral pain. CRH is a potent activator of the hypothalamic-pituitary-adrenal (HPA) axis which regulates the body's response to stress. Increased CRH expression activates the HPA axis leading to a release of cortisol. Under normal conditions, the released cortisol activates glucocorticoid receptors (GRs) in the hypothalamus to inhibit CRH production and reduce HPA activity. However, under chronic stress, persistent cortisol exposure causes alterations in GR signaling that inhibit this negative feedback loop leading to chronic activation of the HPA axis (27). In a female-specific rodent model, unpredictable early life stress uncoupled GR's control of CRH leading to increased CRH expression in the amygdala and increased visceral hypersensitivity (28). Adult stress and anxiety models of IBS use physical stimuli [restraint stress (29, 30), cold exposure (31), and forced swim test (32)] and psychological stimuli such as water avoidance stress (WAS) (27, 33) to induce visceral hypersensitivity. Chronic WAS specifically has been shown to decrease GR expression and increase CRH expression in the central nucleus of the amygdala (CeA) which induces visceral hypersensitivity that persists long after the stressor has been removed (20, 27, 34).
Experimental models of post-inflammatory IBS have revealed that previous inflammatory insult in the colon of rodents sensitizes afferent neurons to induce visceral hypersensitivity mediated by increased expression of nociceptive receptors including calcitonin gene-related peptide (CGRP) (35) and transient receptor potential cation channel subfamily V member 1 (TrpV1) (36) in the dorsal root ganglia cells (37, 38) leading to enhanced nociceptive transmission. Taken together, these studies demonstrate that changes in the processing of sensory signals in the central and peripheral nervous systems contribute to the pathophysiology of chronic visceral pain.
Pharmacological Therapies for IBS
IBS patients with mild symptoms are advised to make changes in their diet, lifestyle, and to take over-the-counter agents like laxatives and fiber supplements. The FDA has approved a number of pharmacological therapies for use in patients with moderate to severe IBS as shown in Table 1, highlighting its multifactorial etiology. Gastroprokinetics like linaclotide and lubiprostone which act by increasing fluid secretion in the small intestine have been approved for use in IBS-C patients. Linaclotide is a selective agonist of guanylate cyclase C (GC-C), which activates GC-C receptors on intestinal epithelial cells to increase secretion to the lumen (66, 67) Linaclotide is also involved in the modulation of afferent gut nerve activity to affect visceral nociception (66, 68, 69). Lubiprostone is a prostaglandin-derived bicyclic fatty acid, which eases constipation by increasing intraluminal chloride ion secretion, causing a passive influx of water and sodium to increase intestinal peristalsis and colonic laxation (40). Alosetron, cilansetron, and eluxadoline have been FDA-approved for use in IBS-D patients. Alosetron and cilansetron are 5-HT3 receptor antagonists that can aid in relaxing the colon and slowing lower GI motility while eluxadoline is a μ-opioid receptor agonist, which decreases GI transit by reducing muscle contractions and fluid secretion in the intestine. Both alosetron and eluxadoline have also been shown to relieve abdominal pain in IBS-D patients (45). Neuromodulators have also shown efficacy in controlling IBS symptoms. Low doses of tricyclic antidepressants such as amitriptyline, desipramine, and nortriptyline can be used to relieve abdominal pain in IBS patients (48–50, 52). Selective serotonin reuptake inhibitors like fluoxetine (52) and serotonin-noradrenergic reuptake inhibitors including duloxetine (53) and venlafaxine (54) are prescribed to IBS patients to attenuate abdominal pain. Delta ligand agents (selective voltage-gated calcium channel agonists) like gabapentin and pregabalin have also been used to treat visceral hypersensitivity and abdominal pain in IBS patients (51). Clinical trials also revealed that rifaximin (an antibiotic) can reduce abdominal pain sensation in adult IBS patients (55). Unfortunately, most of the therapies prescribed for IBS are intended primarily to control symptoms rather than treat the underlying causes. Pharmacological therapies for IBS also present with detrimental side effects. For example, linaclotide and lubiprostone can cause diarrhea in some patients (39, 67, 70), whereas eluxadoline has been reported to cause constipation, nausea, and abdominal pain, and has been associated with pancreatitis (45). The neuromodulators have neurological and mood-altering side effects which adversely affect the patients' quality of life (47, 50). These issues leave many patients with IBS unsatisfied with their current care.
Table 1.
A summary of the most common classes of pharmacological and non-pharmacological interventions used for controlling irritable bowel syndrome (IBS) symptoms, as well as their therapeutic effects.
| Classification | Intervention | Symptom management | |
|---|---|---|---|
| Pharmacological interventions | Gastroprokinetics | Linaclotide (39) Lubiprostone (40) |
Increases intestinal fluid secretion and transit in IBS-C (39, 41) Attenuates abdominal pain (40, 42) |
| 5-HT receptor antagonists | Alosetron (43) Cilansetron (44) |
Reduces intestinal fluid secretion and transit in IBS-D (43–46) Attenuates abdominal pain (44–46) |
|
| Opioid receptor agonists | Eluxadoline (45) | ||
| Tricyclic antidepressants (TCAs) | Amitriptyline (47) | Reduces diarrhea (47), Attenuates abdominal pain (47, 48) | |
| Desipramine (48, 49) | Reduces abdominal pain (48, 49) | ||
| Nortriptyline (49, 50) | Reduces abdominal pain (49, 50) | ||
| Delta ligand agents | GabapentinPregabalin (51) | Attenuates visceral hypersensitivity (50, 51) | |
| Selective serotonin reuptake inhibitors (SSRIs) | Fluoxetine | Attenuates abdominal pain Treats mood disorders (52) |
|
| Serotonin–noradrenaline reuptake inhibitor (SNRIs) | Duloxetine (53) Venlafaxine (54) |
Decreases abdominal pain (53, 54) | |
| Antibiotics | Rifaximin | Attenuates pain in IBS patients (55) | |
| Non-pharmacological interventions | Behavioral interventions | Cognitive behavioral therapy | Attenuates abdominal & Somatic pain Improved comorbid mood disorders (including anxiety, depression) (19, 56, 57) |
| Gut-directed hypnotherapy Meditation Mindfulness |
Attenuates abdominal pain Reduces colonic motility (58–60) |
||
| Alternative therapies | Acupuncture | Attenuates abdominal pain (61, 62), Improves GI motility (62–64) |
|
| Neurostimulation | Decreases abdominal pain in children with IBS (65) |
Emerging Non-Pharmacological Therapies for IBS
Behavioral therapies have historically been used to effectively control mood and anxiety disorders by countering the effects of stress. Because stress reactivity plays an important role in the pathophysiology of chronic visceral pain (7, 71), non-pharmacological interventions have been employed to control IBS symptoms including regular stress management, relaxation and mediation, mindfulness, gut-directed hypnotherapy, and CBT (72). Alternative therapies like acupuncture are also employed to reduce abdominal pain. Auricular neurostimulation therapy is a novel alternative therapy that has been shown to decrease abdominal pain in adolescents (65). CBT, gut-directed hypnotherapy, and acupuncture are the most researched techniques and the most well-known non-pharmacological techniques that have been employed for IBS.
Gut-Directed Hypnotherapy
Gut-directed hypnotherapy combines body relaxation and mental exercises to influence pain sensation in IBS patients. This requires the patient to be placed in a trance-like state of relaxation. In this state, the patient is given suggestions for how best to improve their IBS symptoms including relaxation and emotional control (73). Gut-directed hypnotherapy typically requires 12 daily sessions to achieve the maximum benefits from the treatment (58) including the ability to distinguish between abdominal sensations and thoughts of abdominal sensations (73). The original study conducted on 30 patients with severe IBS symptoms showed that gut-directed hypnotherapy over a 3-month period significantly improved IBS symptoms, with most patients reporting mild to no symptoms which were sustained long after completion of the treatment (74, 75). Subsequent studies have confirmed these findings (59, 76) and others have also showed that gut-directed hypnotherapy is capable of reversing extra-colonic symptoms of IBS including mood, work attitude, and psychic and physical well-being (77). A long-term study following 200 IBS patients who had been enrolled in a gut-directed hypnotherapy session revealed that most of these patients maintained the benefits of this therapy up to 5 years after their last session (78, 79). Pain relief is not only due to relaxation as studies have shown that specific hypnotic suggestions are usually required for pain relief (78) although that may not always be the case (80). Gut-directed hypnotherapy has been shown to alter emotions, cause reduced colonic motility (81), and normalize pain sensory thresholds in patients who were previously either hypersensitive or hyposensitive (60). Unfortunately, due to the inherent difficulties in creating an animal model for hypnotherapy, the molecular mechanisms underlying gut-directed hypnotherapy have yet to be delineated.
Acupuncture
Acupuncture involves the insertion of thin needles through skin at acupoints which communicate with specific visceral organs and have been shown to stimulate nerves, muscles, and connective tissue. Clinical trials on the efficacy of acupuncture in treating IBS symptoms have yielded mixed results (61, 63, 82, 83). Multiple clinical and preclinical trials suggest that acupuncture improves the quality of life and reduces pain perception in IBS patients (61, 84). However, other studies suggest that the benefits of acupuncture are due to a placebo response (63, 82). In animal studies, neurogenic inflammatory spots were found to correspond with acupoints in humans. The number and size of these spots were associated with the severity of visceral pain and evidence suggests that stimulating these neurogenic spots is sufficient to attenuate visceral hypersensitivity in rats (85).
Cognitive Behavioral Therapy
CBT has been shown to be an effective treatment option for IBS and is currently considered the gold standard for psychotherapy (86) with multiple studies showing CBT is capable of relieving IBS symptoms (87–89). CBT is a form of psychosocial intervention that uses behavioral and cognitive psychology to modify behavior, and alter dysfunctional thinking patterns and anxiety states to improve mental health (90). CBT was first pioneered by Beck et al. (91) and is based on the idea that maladaptive cognitive processes contribute to the potentiation of emotional and behavioral problems, and that changing these maladaptive thoughts and behaviors could lead to symptom relief. Different CBT protocols are mild to moderately effective in managing substance abuse such as cannabis and cocaine dependence (92). CBT-based coping skills were helpful in controlling nicotine addiction (93). CBT is also beneficial in controlling persistent psychotic symptoms of schizophrenia such as hallucinations and delusions (94), and there is evidence that CBT in addition to pharmacological approaches may be effective in patients with acute psychosis (95). In patients with depressive disorders, CBT is effective in reducing depressive and anxiety symptoms (96, 97). CBT in conjunction with medication was also more effective in treating chronic depression than CBT or medication alone (56). CBT is also used to treat various anxiety disorders and multiple studies have shown a medium to large effect size of CBT reducing symptoms of social anxiety (98, 99), panic (100), and post-traumatic stress disorders (101, 102). Meta-analysis of behavioral studies for generalized stress in patients found that CBT was one of the most effective therapeutic options in reducing stress (103, 104). CBT has been shown to decrease chronic pain and increase quality of life for patients suffering from fibromyalgia and musculoskeletal pain (57, 105, 106). CBT has also emerged as a prime candidate to attenuate IBS symptoms (89, 107).
Most forms of CBT treatment for IBS typically include a combination of education about the disorder, relaxation, and cognitive restructuring techniques used to address negative thinking patterns, symptom-related anxiety and hypervigilance, and stress reduction techniques based on the premise that if stress causes symptoms, then stress reduction may provide symptom relief (91). More recent iterations of CBT include practicing coping skills, problem-solving skills, de- catastrophizing skills, and exposure therapy (18).
Protocols involving exposure to an enriched environment (EE) have been developed as the rodent analog of CBT and are used to investigate the molecular effects of behavioral interventions in rodents. EE involves exposing animals to a novel environment with sufficient physical and social stimuli to promote cognitive and physiological well-being (108). EE typically uses some combination of physical (toys, platforms, and tunnels) and social cues as well as exercise motivators to simulate a stimulating novel environment (109, 110). EE has been studied extensively in the context of learning and memory where EE promotes hippocampal neuroplasticity and increases neuronal spine density to improve learning and memory (108, 111–113). Animal models of mood and depression disorders also suggest exposure to EE improves recovery from and decreases anxiety-like symptoms due to restraint and social defeat stress via hippocampal (114) and amygdalar (115) dependent mechanisms, respectively. Studies on neuropathic pain revealed that exposure to EE can reduce infant nerve injury-induced anxiety and depressive behavior in adolescent rodents (116). EE also reverses neuropathic pain in rodents (117) while either physical or social EE is sufficient to decrease the recovery time after induction of chronic inflammatory pain in rats (118). A recent study on felines showed that exposure to EE can decrease symptoms of idiopathic cystitis (119). Taken together, these studies demonstrated that EE was capable of reducing stress, anxiety, and visceral pain sensation highlighting a role of behavioral interventions as therapeutic candidates to attenuate stress-induced visceral pain. Our laboratory recently reported that exposing rats to EE 7 days before and 7 days during a WAS protocol persistently inhibited stress-induced visceral and somatic hypersensitivity long after cessation of EE (20). EE prevented the stress-induced decrease in GR expression in the CeA and inhibited CRH-induced potentiation of the stress response to block the development of visceral hypersensitivity (20).
Discussion
Clinical and preclinical studies clearly show that multiple factors are involved in the pathophysiology of visceral pain which might explain why finding the ideal therapy has been elusive. Current pharmacological treatments available for IBS focus on symptom relief rather than reversing the underlying pathology. Stress, anxiety, and inflammation cause changes in the expression of genes (GR, CRH, and TRPV1) in brain centers critical for stress reactivity and sensory neurotransmission (amygdala, dorsal horn). These alterations ultimately potentiate stress reactivity and sensitize nociceptive afferent fibers leading to visceral hypersensitivity in IBS. Behavioral therapies including hypnotherapy and CBT have already been used extensively as effective behavioral therapies for cognitive, mood, and neuropathic pain disorders and now clinical and pre-clinical trials are showing that behavioral therapies can also ameliorate chronic visceral pain (Table 1). Unfortunately, the lack of trained CBT specialists (120), difficulty of scheduling and maintaining weekly visits to the hospitals for patients (121), and a general misunderstanding of how behavioral therapies work have all contributed to the low adoption rate of CBT for IBS symptoms (122).
In recent years, interest in CBT-based treatment has increased leading to more clinical research and understanding of CBT techniques. Other variations of CBT have been developed to ease the burden of the patients having to constantly visit the clinic for CBT sessions. Studies have shown that patients can be trained to effectively practice CBT techniques remotely. Patients who attended an initial CBT session with a physician and then had subsequent follow-ups via the telephone reported improvements in their IBS symptoms similar to patients who visited a clinician for every session (123). CBT sessions delivered online were also capable of ameliorating IBS symptoms, although the treatment effects were less than with patients who at least one in-person session (124). Preclinical studies using EE have also revealed the molecular mechanisms underlying the effects of behavioral therapies, specifically how EE can reverse stress-induced alterations in the central and peripheral nervous systems to inhibit the development of chronic pain (Figure 1). Gut-directed hypnotherapy and CBT are behavioral therapies for IBS that show efficacy in reversing the underlying causes of the disorder including chronic stress reactivity and enhanced nociception. Other non-pharmacological techniques such as acupuncture have also shown some efficacy in controlling IBS symptoms although with mixed results.
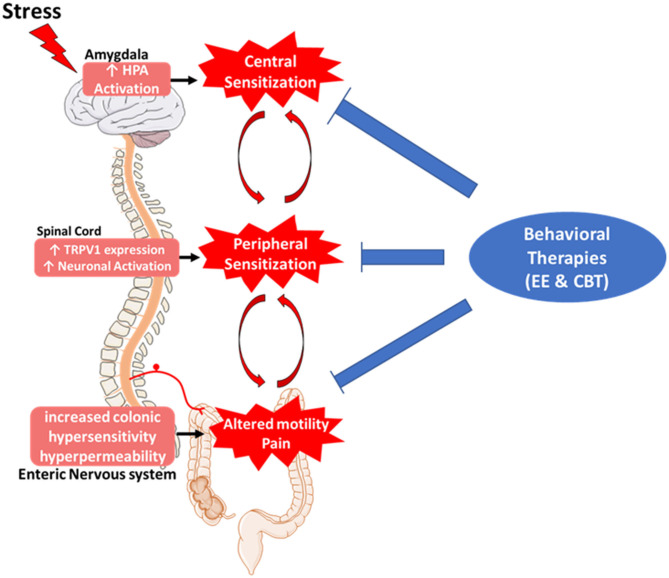
Figure 1.
Role of behavioral therapies in the pathophysiology of irritable bowel syndrome (IBS). IBS symptoms can be caused by bidirectional disruptions in the brain–gut axis. Chronic stress-induced hyperactivation of the hypothalamic-pituitary-adrenal axis in the brain leads to increased expression of pro-nociceptive genes and causes sensitization of nociceptive afferents in the spinal cord and enteric nervous systems leading to chronic visceral pain and altered motility. Pharmacological treatments are geared toward ameliorating visceral symptoms without treating the underlying neurological causes. Behavioral therapies show promise in inhibiting visceral pain as well as reversing enhanced stress reactivity and afferent sensitization in the central and peripheral nervous systems, respectively, leading to a more comprehensive and long-lasting relief of IBS symptoms.
One caveat when interpreting clinical data from behavioral studies is the risk of over-interpretation of placebo effects. Multiple studies have shown significant effects of psychological placebos in behavioral interventions for anxiety and depression (125, 126). It should be noted that the beneficial effects of the placebos were influenced mostly by patient expectations and desires and not the specific placebo (127). Research has also shown that appropriate “placebos” such as in-person meetings and encouragement can be used to improve the effectiveness of non-pharmacological therapies in treating IBS symptoms (126, 128).
In conclusion, non-pharmacological therapies have been shown to induce molecular and psychological changes that quantifiably reverse the pathophysiology of visceral pain in clinical and preclinical studies. Although more research is needed to unravel the mechanisms underlying CBT, experimental models employing EE are being used to determine the role of EE to influence gene expression (20). This review suggests that behavioral interventions could be a very effective therapy either on their own or in combination with other pharmaceutical options in treating IBS and should be offered more widely in IBS treatment programs.
Disclosure
Research in BG-V laboratory has grant funding from Ironwood Pharmaceuticals, Blue Therapeutics, and TEVA Pharmaceuticals.
Author Contributions
AO was the lead author in writing the review. TY used her expertise to write certain sections of the main text. BG-V mentored and supervised the team, providing critical input on the scope and topics included in the review. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. BG-V would like to acknowledge the long time and generous support from the Department of Veterans Affairs as the recipient of a Senior Research Career Scientist award (1IK6BX003610-01).
References
- 1.Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. (2012) 143:1179–87.e3. 10.1053/j.gastro.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. (2012) 10:712–21.e4. 10.1016/j.cgh.2012.02.029 [DOI] [PubMed] [Google Scholar]
- 3.Mujagic Z, Jonkers D, Hungin APS, de Wit NJ, Wensaas KA, Palka M, et al. Use of Rome criteria for the diagnosis of irritable bowel syndrome in primary care: a survey among European countries. Eur J Gastroenterol Hepatol. (2017) 29:651–6. 10.1097/MEG.0000000000000848 [DOI] [PubMed] [Google Scholar]
- 4.Inadomi JM, Fennerty MB, Bjorkman D. Systematic review: the economic impact of irritable bowel syndrome. Aliment Pharmacol Ther. (2003) 18:671–82. 10.1046/j.1365-2036.2003.t01-1-01736.x [DOI] [PubMed] [Google Scholar]
- 5.Cash B, Sullivan S, Barghout V. Total costs of IBS: employer and managed care perspective. Am J Manag Care. (2005) 11(1. Suppl):S7–16. [PubMed] [Google Scholar]
- 6.Hertig VL, Cain KC, Jarrett ME, Burr RLand Heitkemper MM. Daily stress and gastrointestinal symptoms in women with irritable bowel syndrome. Nurs Res. (2007) 56:399–406. 10.1097/01.NNR.0000299855.60053.88 [DOI] [PubMed] [Google Scholar]
- 7.Greenwood-Van Meerveld B, Johnson AC. Stress-induced chronic visceral pain of gastrointestinal origin. Front. Systems Neurosci. (2017) 11:86. 10.3389/fnsys.2017.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roohafza H, Bidaki EZ, Hasanzadeh-Keshteli A, Daghaghzade H, Afshar H, Adibi P. Anxiety, depression and distress among irritable bowel syndrome and their subtypes: an epidemiological population based study. Adv Biomed Res. (2016) 5:183. 10.4103/2277-9175.190938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maguen S, Madden E, Cohen B, Bertenthal D, Seal K. Association of mental health problems with gastrointestinal disorders in Iraq and Afghanistan veterans. Depress Anxiety. (2014) 31:160–5. 10.1002/da.22072 [DOI] [PubMed] [Google Scholar]
- 10.Peyton L, Greene J. Irritable bowel syndrome: current and emerging treatment options. P & T. (2014) 39:567–78. [PMC free article] [PubMed] [Google Scholar]
- 11.Mearin F, Malfertheiner P. Functional gastrointestinal disorders: complex treatments for complex pathophysiological mechanisms. Digestive Dise. (2017) 35(Suppl. 1):1–4. 10.1159/000485407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacy BE, Mearin F, Chang L, Chey WD, Lembo AJ, Simren M, et al. Bowel disorders. Gastroenterology. (2016) 150:1393–407.e5. 10.1053/j.gastro.2016.02.031 [DOI] [PubMed] [Google Scholar]
- 13.Distrutti E, Cipriani S, Mencarelli A, Renga B, Fiorucci S. Probiotics VSL#3 protect against development of visceral pain in murine model of irritable bowel syndrome. PLoS ONE. (2013) 8:e63893. 10.1371/journal.pone.0063893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barbara G, Cremon C, De Giorgio R, Dothel G, Zecchi L, Bellacosa L, et al. Mechanisms underlying visceral hypersensitivity in irritable bowel syndrome. Curr Gastroenterol Rep. (2011) 13:308–15. 10.1007/s11894-011-0195-7 [DOI] [PubMed] [Google Scholar]
- 15.Mueser KT, Rosenberg SD, Xie H, Jankowski MK, Bolton EE, Lu W, et al. A randomized controlled trial of cognitive-behavioral treatment for posttraumatic stress disorder in severe mental illness. J Consult Clin Psychol. (2008) 76:259–71. 10.1037/0022-006X.76.2.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiltsey Stirman S, Toder K, Crits-Cristoph P. New psychotherapies for mood and anxiety disorders. Can J Psychiatry. (2010) 55:193–201. 10.1177/070674371005500402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watkins LE, Sprang KR, Rothbaum BO. Treating PTSD: a review of evidence-based psychotherapy interventions. Front Behav Neurosci. (2018) 12:258. 10.3389/fnbeh.2018.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinsinger SW. Cognitive-behavioral therapy for patients with irritable bowel syndrome: current insights. Psychol Res Behav Manag. (2017) 10:231–7. 10.2147/PRBM.S120817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lackner JM, Jaccard J, Keefer L, Brenner DM, Firth RS, Gudleski GD, et al. Improvement in gastrointestinal symptoms after cognitive behavior therapy for refractory irritable bowel syndrome. Gastroenterology. (2018) 155:47–57. 10.1053/j.gastro.2018.03.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orock A, Louwies T, Yuan T, Greenwood-Van Meerveld B. Environmental enrichment prevents chronic stress-induced brain-gut axis dysfunction through a GR-mediated mechanism in the central nucleus of the amygdala. Neurogastroenterol Motil. (2020) 32:e13826. 10.1111/nmo.13826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drossman DA, Sandler RS, McKee DC, Lovitz AJ. Bowel patterns among subjects not seeking health care. Use of a questionnaire to identify a population with bowel dysfunction. Gastroenterology. (1982) 83:529–34. 10.1016/S0016-5085(82)80186-8 [DOI] [PubMed] [Google Scholar]
- 22.Chitkara DK, van Tilburg MA, Blois-Martin N, Whitehead WE. Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. Am J Gastroenterol. (2008) 103:765–74; quiz 775. 10.1111/j.1572-0241.2007.01722.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sansone RA, Sansone LA. Irritable bowel syndrome: relationships with abuse in childhood. Innov Clin Neurosci. (2015) 12:34–7. [PMC free article] [PubMed] [Google Scholar]
- 24.Lee C, Doo E, Choi JM, Jang SH, Ryu HS, Lee JY, et al. The increased level of depression and anxiety in irritable bowel syndrome patients compared with healthy controls: systematic review and meta-analysis. J Neurogastroenterol Motil. (2017) 23:349–62. 10.5056/jnm16220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D, Zhang X, Zhang X, Huang Z, Song Y. Magnetic resonance imaging analysis of brain function in patients with irritable bowel syndrome. BMC gastroenterol. (2017) 17:148. 10.1186/s12876-017-0673-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilder-Smith CH, Schindler D, Lovblad K, Redmond SM, Nirkko A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut. (2004) 53:1595–601. 10.1136/gut.2003.028514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myers B, Greenwood-Van Meerveld B. Differential involvement of amygdala corticosteroid receptors in visceral hyperalgesia following acute or repeated stress. Am J Physiol Gastrointest Liver Physiol. (2012) 302:G260–6. 10.1152/ajpgi.00353.2011 [DOI] [PubMed] [Google Scholar]
- 28.Prusator DK, Greenwood-Van >Meerveld B. Amygdala-mediated mechanisms regulate visceral hypersensitivity in adult females following early life stress: importance of the glucocorticoid receptor and corticotropin-releasing factor. Pain. (2017) 158:296–305. 10.1097/j.pain.0000000000000759 [DOI] [PubMed] [Google Scholar]
- 29.Yi L, Sun H, Ge C, Chen Y, Peng H, Jiang Y, et al. Role of insular cortex in visceral hypersensitivity model in rats subjected to chronic stress. Psychiatry Res. (2014) 220:1138–43. 10.1016/j.psychres.2014.09.019 [DOI] [PubMed] [Google Scholar]
- 30.Zhao J, Wang J, Dong L, Shi H, Wang Z, Ding H, et al. A protease inhibitor against acute stress-induced visceral hypersensitivity and paracellular permeability in rats. Eur J Pharmacol. (2011) 654:289–94. 10.1016/j.ejphar.2010.12.032 [DOI] [PubMed] [Google Scholar]
- 31.Yang CQ, Duan LP, Qiao PT, Zhao L, Guo LL. Increased VGLUT3 involved in visceral hyperalgesia in a rat model of irritable bowel syndrome. World J Gastroenterol. (2015) 21:2959–66. 10.3748/wjg.v21.i10.2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hubbard CS, Karpowicz JM, Furman AJ, da Silva JT, Seminowicz DA, Traub RJ. Estrogen-dependent visceral hypersensitivity following stress in rats: an fMRI study. Mol Pain. (2016) 12:1744806916654145. 10.1177/1744806916654145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bradesi S, Schwetz I, Ennes HS, Lamy CM, Ohning G, Fanselow M, et al. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. (2005) 289:G42–53. 10.1152/ajpgi.00500.2004 [DOI] [PubMed] [Google Scholar]
- 34.Makino S, Gold PW, Schulkin J. Corticosterone effects on corticotropin-releasing hormone mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Res. (1994) 640:105–12. 10.1016/0006-8993(94)91862-7 [DOI] [PubMed] [Google Scholar]
- 35.Plourde V, St-Pierre S, Quirion R. Calcitonin gene-related peptide in viscerosensitive response to colorectal distension in rats. Am J Physiol. (1997) 273(1 Pt 1):G191–6. 10.1152/ajpgi.1997.273.1.G191 [DOI] [PubMed] [Google Scholar]
- 36.Wiskur BJ, Tyler K, Campbell-Dittmeyer K, Chaplan SR, Wickenden AD, Greenwood-Van Meerveld B. A novel TRPV1 receptor antagonist JNJ-17203212 attenuates colonic hypersensitivity in rats. Methods Find Exp Clin Pharmacol. (2010) 32:557–64. 10.1358/mf.2010.32.8.1507853 [DOI] [PubMed] [Google Scholar]
- 37.Qian A, Song D, Li Y, Liu X, Tang D, Yao W, et al. Role of voltage gated Ca2+ channels in rat visceral hypersensitivity change induced by 2,4,6-trinitrobenzene sulfonic acid. Mol Pain. (2013) 9:15. 10.1186/1744-8069-9-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Q, Price DD, Caudle RM, Verne GN. Spinal NMDA NR1 subunit expression following transient TNBS colitis. Brain Res. (2009) 1279:109–20. 10.1016/j.brainres.2009.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chey WD, Lembo AJ, Lavins BJ, Shiff SJ, Kurtz CB, Currie MG, et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol. (2012) 107:1702–12. 10.1038/ajg.2012.254 [DOI] [PubMed] [Google Scholar]
- 40.Wilson N, Schey R. Lubiprostone in constipation: clinical evidence and place in therapy. Ther Adv Chronic Dis. (2015) 6:40–50. 10.1177/2040622314567678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eutamene H, Bradesi S, Larauche M, Theodorou V, Beaufrand C, Ohning G, et al. Guanylate cyclase C-mediated antinociceptive effects of linaclotide in rodent models of visceral pain. Neurogastroenterol Motil. (2010) 22:312–e84. 10.1111/j.1365-2982.2009.01385.x [DOI] [PubMed] [Google Scholar]
- 42.Mohammadi EN, Ligon CO, Silos-Santiago A, Ge P, Kurtz C, Higgins C, et al. Linaclotide attenuates visceral organ crosstalk: role of guanylate cyclase-C Activation in reversing bladder-colon cross-sensitization. J Pharmacol Expe Therapeutics. (2018) 366:274–81. 10.1124/jpet.118.248567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lacy BE, Nicandro JP, Chuang E, Earnest DL. Alosetron use in clinical practice: significant improvement in irritable bowel syndrome symptoms evaluated using the US Food and Drug Administration composite endpoint. Therapeutic Adv Gastroenterol. (2018) 11:1756284818771674. 10.1177/1756284818771674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chey WD, Cash BD. Cilansetron: a new serotonergic agent for the irritable bowel syndrome with diarrhoea. Expert Opin Investig Drugs. (2005) 14:185–93. 10.1517/13543784.14.2.185 [DOI] [PubMed] [Google Scholar]
- 45.Ozdener AE, Rivkin A. Eluxadoline in the treatment of diarrhea-predominant irritable bowel syndrome. Drug Des Devel Ther. (2017) 11:2827–40. 10.2147/DDDT.S127405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mayer EA, Bradesi S. Alosetron and irritable bowel syndrome. Expert Opin Pharmacother. (2003) 4:2089–98. 10.1517/14656566.4.11.2089 [DOI] [PubMed] [Google Scholar]
- 47.Drossman DA, Tack J, Ford AC, Szigethy E, Törnblom H, Van Oudenhove L. Neuromodulators for functional gastrointestinal disorders (disorders of gut-brain interaction): a rome foundation working team report. Gastroenterology. (2018) 154:1140–71.e1. 10.1053/j.gastro.2017.11.279 [DOI] [PubMed] [Google Scholar]
- 48.Morgan V, Pickens D, Gautam S, Kessler R, Mertz H. Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut. (2005) 54:601–7. 10.1136/gut.2004.047423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greenbaum DS, Mayle JE, Vanegeren LE, Jerome JA, Mayor JW, Greenbaum RB, et al. Effects of desipramine on irritable bowel syndrome compared with atropine and placebo. Dig Dis Sci. (1987) 32:257–66. 10.1007/BF01297051 [DOI] [PubMed] [Google Scholar]
- 50.Rahimi R, Nikfar S, Rezaie A, Abdollahi M. Efficacy of tricyclic antidepressants in irritable bowel syndrome: a meta-analysis. World J Gastroenterol. (2009) 15:1548–53. 10.3748/wjg.15.1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gale JD, Houghton LA. Alpha 2 Delta (α(2)δ) ligands, gabapentin and pregabalin: what is the evidence for potential use of these ligands in irritable bowel syndrome. Front Pharmacol. (2011) 2:28. 10.3389/fphar.2011.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Forootan H, Taheri A, Hooshangi H, Mohamamdi HR. Effects of fluoxetine, nortriptyline and amitriptyline in IBS patients. FEYZ. (2002) 6:49–55. [Google Scholar]
- 53.Brennan BP, Fogarty KV, Roberts JL, Reynolds KA, Pope HG, Jr, Hudson JI. Duloxetine in the treatment of irritable bowel syndrome: an open-label pilot study. Hum Psychopharmacol. (2009) 24:423–8. 10.1002/hup.1038 [DOI] [PubMed] [Google Scholar]
- 54.Chial HJ, Camilleri M, Ferber I, Delgado-Aros S, Burton D, McKinzie S, et al. Effects of venlafaxine, buspirone, and placebo on colonic sensorimotor functions in healthy humans. Clin Gastroenterol Hepatol. (2003) 1:211–8. 10.1053/jcgh.2003.50031 [DOI] [PubMed] [Google Scholar]
- 55.Saadi M, McCallum RW. Rifaximin in irritable bowel syndrome: rationale, evidence and clinical use. Ther Adv Chronic Dis. (2013) 4:71–5. 10.1177/2040622312472008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chan EKH. Efficacy of cognitive-behavioral, pharmacological, combined treatments of depression: a meta-analysis. ProQuest Information & Learning. (2006). p. 2218. [Google Scholar]
- 57.Castro MM, Daltro C, Kraychete DC, Lopes J. The cognitive behavioral therapy causes an improvement in quality of life in patients with chronic musculoskeletal pain. Arq Neuropsiquiatr. (2012) 70:864–8. 10.1590/S0004-282X2012001100008 [DOI] [PubMed] [Google Scholar]
- 58.Gonsalkorale WM. Gut-directed hypnotherapy: the Manchester approach for treatment of irritable bowel syndrome. Int J Clin. (2006) 54:27–50. 10.1080/00207140500323030 [DOI] [PubMed] [Google Scholar]
- 59.Lee HH, Choi YY, Choi MG. The efficacy of hypnotherapy in the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Neurogastroenterol Motil. (2014) 20:152–62. 10.5056/jnm.2014.20.2.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lea R, Houghton LA, Calvert EL, Larder S, Gonsalkorale WM, Whelan V, et al. Gut-focused hypnotherapy normalizes disordered rectal sensitivity in patients with irritable bowel syndrome. Aliment Pharmacol Ther. (2003) 17:635–42. 10.1046/j.1365-2036.2003.01486.x [DOI] [PubMed] [Google Scholar]
- 61.MacPherson H, Tilbrook H, Bland JM, Bloor K, Brabyn S, Cox H, et al. Acupuncture for irritable bowel syndrome: primary care based pragmatic randomised controlled trial. BMC Gastroenterol. (2012) 12:150. 10.1186/1471-230X-12-150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grundmann O, Yoon SL. Complementary and alternative medicines in irritable bowel syndrome: an integrative view. World J Gastroenterol. (2014) 20:346–62. 10.3748/wjg.v20.i2.346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schneider A, Enck P, Streitberger K, Weiland C, Bagheri S, Witte S, et al. Acupuncture treatment in irritable bowel syndrome. Gut. (2006) 55:649–54. 10.1136/gut.2005.074518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yin JJ, Chen DZ. Gastrointestinal motility disorders and acupuncture. Auton Neurosci. (2010) 157:31–7. 10.1016/j.autneu.2010.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krasaelap A, Sood MR, Li BUK, Unteutsch R, Yan K, Nugent M, et al. Efficacy of auricular neurostimulation in adolescents with irritable bowel syndrome in a randomized, double-blind trial. Clin Gastroenterol Hepatol. (2020) 18:1987–94.e2. 10.1016/j.cgh.2019.10.012 [DOI] [PubMed] [Google Scholar]
- 66.Busby RW, Bryant AP, Bartolini WP, Cordero EA, Hannig G, Kessler MM, et al. Linaclotide, through activation of guanylate cyclase C, acts locally in the gastrointestinal tract to elicit enhanced intestinal secretion and transit. Eur J Pharmacol. (2010) 649:328–35. 10.1016/j.ejphar.2010.09.019 [DOI] [PubMed] [Google Scholar]
- 67.Rey E, Mearin F, Alcedo J, Ciriza C, Delgado-Aros S, Freitas T, et al. Optimizing the use of linaclotide in patients with constipation-predominant irritable bowel syndrome: an expert consensus report. Adv Ther. (2017) 34:587–98. 10.1007/s12325-016-0473-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Potter LR Regulation and therapeutic targeting of peptide-activated receptor guanylyl cyclases . Pharmacol Ther. (2011) 130:71–82. 10.1016/j.pharmthera.2010.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Castro J, Harrington AM, Hughes PA, Martin CM, Ge P, Shea CM, et al. Linaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-C and extracellular cyclic guanosine 3',5'-monophosphate. Gastroenterology. (2013) 145:1334–46 e1–11. 10.1053/j.gastro.2013.08.017 [DOI] [PubMed] [Google Scholar]
- 70.Rao S, Lembo AJ, Shiff SJ, Lavins BJ, Currie MG, Jia XD, et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol. (2012) 107:1714–24. 10.1038/ajg.2012.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chaloner A, Greenwood-Van Meerveld B. Early life adversity as a risk factor for visceral pain in later life: importance of sex differences. Front Neurosci. (2013) 7:13. 10.3389/fnins.2013.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eriksson EM, Andrén KI, Kurlberg GK, Eriksson HT. Aspects of the non-pharmacological treatment of irritable bowel syndrome. World J Gastroenterol. (2015) 21:11439–49. 10.3748/wjg.v21.i40.11439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gaylord SA, Palsson OS, Garland EL, Faurot KR, Coble RS, Mann JD, et al. Mindfulness training reduces the severity of irritable bowel syndrome in women: results of a randomized controlled trial. Am J Gastroenterol. (2011) 106:1678–88. 10.1038/ajg.2011.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Whorwell PJ, Prior A, Faragher EB. Controlled trial of hypnotherapy in the treatment of severe refractory irritable-bowel syndrome. Lancet. (1984) 2:1232–4. 10.1016/S0140-6736(84)92793-4 [DOI] [PubMed] [Google Scholar]
- 75.Whorwell PJ, Prior A, Colgan SM. Hypnotherapy in severe irritable bowel syndrome: further experience. Gut. (1987) 28:423–5. 10.1136/gut.28.4.423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gonsalkorale WM, Houghton LA, Whorwell PJ. Hypnotherapy in irritable bowel syndrome: a large-scale audit of a clinical service with examination of factors influencing responsiveness. Am J Gastroenterol. (2002) 97:954–61. 10.1111/j.1572-0241.2002.05615.x [DOI] [PubMed] [Google Scholar]
- 77.Houghton LA, Heyman DJ, Whorwell PJ. Symptomatology, quality of life and economic features of irritable bowel syndrome–the effect of hypnotherapy. Aliment Pharmacol Ther. (1996) 10:91–5. 10.1111/j.1365-2036.1996.tb00181.x [DOI] [PubMed] [Google Scholar]
- 78.Gonsalkorale WM, Whorwell PJ. Hypnotherapy in the treatment of irritable bowel syndrome. Eur J Gastroenterol Hepatol. (2005) 17:15–20. 10.1097/00042737-200501000-00004 [DOI] [PubMed] [Google Scholar]
- 79.Gonsalkorale WM, Miller V, Afzal A, Whorwell PJ. Long term benefits of hypnotherapy for irritable bowel syndrome. Gut. (2003) 52:1623–9. 10.1136/gut.52.11.1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Palsson OS, Turner MJ, Johnson DA, Burnett CK, Whitehead WE. Hypnosis treatment for severe irritable bowel syndrome: investigation of mechanism and effects on symptoms. Dig Dis Sci. (2002) 47:2605–14. 10.1023/A:1020545017390 [DOI] [PubMed] [Google Scholar]
- 81.Whorwell PJ, Houghton LA, Taylor EE, Maxton DG. Physiological effects of emotion: assessment via hypnosis. Lancet. (1992) 340:69–72. 10.1016/0140-6736(92)90394-I [DOI] [PubMed] [Google Scholar]
- 82.Forbes A, Jackson S, Walter C, Quraishi S, Jacyna M, Pitcher M. Acupuncture for irritable bowel syndrome: a blinded placebo-controlled trial. World J Gastroenterol. (2005) 11:4040–4. 10.3748/wjg.v11.i26.4040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zheng Q, Wang J, Zheng H, Lu L, Zhou S, Hao X, et al. What types of patients with chronic diarrhea benefit more from acupuncture treatment? A secondary analysis of a randomized controlled trial. Eur J Integrative Med. (2020) 35:101098. 10.1016/j.eujim.2020.101098 [DOI] [Google Scholar]
- 84.Pei LX, Geng H, Chen H, Wu XL, Chen L, Zhou JL, et al. Acupuncture for irritable bowel syndrome: study protocol for a multicenter randomized controlled trial. Trials. (2018) 19:529. 10.1186/s13063-018-2922-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fan Y, Ryu Y, Zhao R, Bills KB, Steffensen SC, Yang CH, et al. Enhanced spinal neuronal responses as a mechanism for increased number and size of active acupoints in visceral hyperalgesia. Sci Rep. (2020) 10:10312. 10.1038/s41598-020-67242-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.David D, Cristea I, Hofmann SG. Why cognitive behavioral therapy is the current gold standard of psychotherapy. Front Psychiatry. (2018) 9:4. 10.3389/fpsyt.2018.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lackner JM, Jaccard J, Krasner SS, Katz LA, Gudleski GD, Blanchard EB. How does cognitive behavior therapy for irritable bowel syndrome work? A mediational analysis of a randomized clinical trial. Gastroenterology. (2007) 133:433–44. 10.1053/j.gastro.2007.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vollmer A, Blanchard EB. Controlled comparison of individual versus group cognitive therapy for irritable bowel syndrome. Behavior Therapy. (1998) 29:19–33. 10.1016/S0005-7894(98)80016-6 [DOI] [Google Scholar]
- 89.Lackner JM, Jaccard J, Krasner SS, Katz LA, Gudleski GD, Holroyd K. Self-administered cognitive behavior therapy for moderate to severe irritable bowel syndrome: clinical efficacy, tolerability, feasibility. Clin Gastroenterol Hepatol. (2008) 6:899–906. 10.1016/j.cgh.2008.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gaudiano BA. Cognitive-behavioural therapies: achievements and challenges. Evid Based Ment Health. (2008) 11:5–7. 10.1136/ebmh.11.1.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Beck AT. Cognitive therapy: nature and relation to behavior therapy. Behav Therapy. (1970) 1:184–200. 10.1016/S0005-7894(70)80030-2 [DOI] [Google Scholar]
- 92.Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. (2008) 165:179–87. 10.1176/appi.ajp.2007.06111851 [DOI] [PubMed] [Google Scholar]
- 93.Song F, Huttunen-Lenz M, Holland R. Effectiveness of complex psycho-educational interventions for smoking relapse prevention: an exploratory meta-analysis. J Public Health (Oxf). (2010) 32:350–9. 10.1093/pubmed/fdp109 [DOI] [PubMed] [Google Scholar]
- 94.Gould RA, Mueser KT, Bolton E, Mays V, Goff D. Cognitive therapy for psychosis in schizophrenia: an effect size analysis. Schizophr Res. (2001) 48:335–42. 10.1016/S0920-9964(00)00145-6 [DOI] [PubMed] [Google Scholar]
- 95.Zimmermann G, Favrod J, Trieu VH, Pomini V. The effect of cognitive behavioral treatment on the positive symptoms of schizophrenia spectrum disorders: a meta-analysis. Schizophr Res. (2005) 77:1–9. 10.1016/j.schres.2005.02.018 [DOI] [PubMed] [Google Scholar]
- 96.van Straten A, Geraedts AI. Verdonck-de Leeuw, Andersson G, Cuijpers P. Psychological treatment of depressive symptoms in patients with medical disorders: a meta-analysis. J Psychosom Res. (2010) 69:23–32. 10.1016/j.jpsychores.2010.01.019 [DOI] [PubMed] [Google Scholar]
- 97.Beltman MW, Voshaar RC, Speckens AE. Cognitive-behavioural therapy for depression in people with a somatic disease: meta-analysis of randomised controlled trials. Br J Psychiatry. (2010) 197:11–9. 10.1192/bjp.bp.109.064675 [DOI] [PubMed] [Google Scholar]
- 98.Fedoroff IC, Taylor S. Psychological and pharmacological treatments of social phobia: a meta-analysis. J Clin Psychopharmacol. (2001) 21:311–24. 10.1097/00004714-200106000-00011 [DOI] [PubMed] [Google Scholar]
- 99.Hofmann SG, Smits JA. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. (2008) 69:621–32. 10.4088/JCP.v69n0415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Furukawa TA, Watanabe N, Churchill R. Combined psychotherapy plus antidepressants for panic disorder with or without agoraphobia. Cochrane Database Syst Rev. (2007) 2007:Cd004364. 10.1002/14651858.CD004364.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bisson JI, Ehlers A, Matthews R, Pilling S, Richards D, Turner S. Psychological treatments for chronic post-traumatic stress disorder. Systematic review and meta-analysis. Br J Psychiatry. (2007) 190:97–104. 10.1192/bjp.bp.106.021402 [DOI] [PubMed] [Google Scholar]
- 102.Bisson J, Andrew M. Psychological treatment of post-traumatic stress disorder (PTSD). Cochrane Database Syst Rev. (2007) 2007:Cd003388. 10.1002/14651858.CD003388.pub3 [DOI] [PubMed] [Google Scholar]
- 103.Kim JH. [A meta-analysis of effects of job stress management interventions (SMIs)]. Taehan Kanho Hakhoe Chi. (2007) 37:529–39. 10.4040/jkan.2007.37.4.529 [DOI] [PubMed] [Google Scholar]
- 104.Richardson KM, Rothstein HR. Effects of occupational stress management intervention programs: a meta-analysis. J Occup Health Psychol. (2008) 13:69–93. 10.1037/1076-8998.13.1.69 [DOI] [PubMed] [Google Scholar]
- 105.Crofford LJ. Psychological aspects of chronic musculoskeletal pain. Best practice & research. Clin Rheumatol. (2015) 29:147–55. 10.1016/j.berh.2015.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bernardy K, Fuber N, Kollner V, Hauser W. Efficacy of cognitive-behavioral therapies in fibromyalgia syndrome - a systematic review and metaanalysis of randomized controlled trials. J Rheumatol. (2010) 37:1991–2005. 10.3899/jrheum.100104 [DOI] [PubMed] [Google Scholar]
- 107.Lackner JM, Mesmer C, Morley S, Dowzer C, Hamilton S. Psychological treatments for irritable bowel syndrome: a systematic review and meta-analysis. J Consult Clin Psychol. (2004) 72:1100–13. 10.1037/0022-006X.72.6.1100 [DOI] [PubMed] [Google Scholar]
- 108.Sampedro-Piquero P, Begega A. Environmental enrichment as a positive behavioral intervention across the lifespan. Current Neuropharmacol. (2017) 15:459–70. 10.2174/1570159X14666160325115909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Greenwood BN, Fleshner M. Exercise, learned helplessness, and the stress-resistant brain. Neuromolecular Med. (2008) 10:81–98. 10.1007/s12017-008-8029-y [DOI] [PubMed] [Google Scholar]
- 110.Slater AM, Cao L. A protocol for housing mice in an enriched environment. J Vis Exp. (2015) 2015:e52874. 10.3791/52874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Eckert MJ, Abraham WC. Effects of environmental enrichment exposure on synaptic transmission and plasticity in the hippocampus. Curr Top Behav Neurosci. (2013) 15:165–87. 10.1007/7854_2012_215 [DOI] [PubMed] [Google Scholar]
- 112.Clemenson GD, Henningfield CM, Stark CEL. Improving hippocampal memory through the experience of a rich minecraft environment. Front Behav Neurosci. (2019) 13:57. 10.3389/fnbeh.2019.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vazquez-Sanroman D, Sanchis-Segura C, Toledo R, Hernandez ME, Manzo J, Miquel M. The effects of enriched environment on BDNF expression in the mouse cerebellum depending on the length of exposure. Behav Brain Res. (2013) 243:118–28. 10.1016/j.bbr.2012.12.047 [DOI] [PubMed] [Google Scholar]
- 114.Schloesser RJ, Lehmann M, Martinowich K, Manji HK, Herkenham M. Environmental enrichment requires adult neurogenesis to facilitate the recovery from psychosocial stress. Mol Psychiatry. (2010) 15:1152–63. 10.1038/mp.2010.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ashokan A, Hegde A, Mitra R. Short-term environmental enrichment is sufficient to counter stress-induced anxiety and associated structural and molecular plasticity in basolateral amygdala. Psychoneuroendocrinology. (2016) 69:189–96. 10.1016/j.psyneuen.2016.04.009 [DOI] [PubMed] [Google Scholar]
- 116.Gong X, Chen Y, Chang J, Huang Y, Cai M, Zhang M. Environmental enrichment reduces adolescent anxiety- and depression-like behaviors of rats subjected to infant nerve injury. J Neuroinflammation. (2018) 15:262. 10.1186/s12974-018-1301-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vachon P, Millecamps M, Low L, Thompsosn SJ, Pailleux F, Beaudry F, et al. Alleviation of chronic neuropathic pain by environmental enrichment in mice well after the establishment of chronic pain. Behav Brain Funct. (2013) 9:22. 10.1186/1744-9081-9-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gabriel AF, Paoletti G, Della Seta D, Panelli R, Marcus MA, Farabollini F, et al. Enriched environment and the recovery from inflammatory pain: Social versus physical aspects and their interaction. Behav Brain Res. (2010) 208:90–5. 10.1016/j.bbr.2009.11.015 [DOI] [PubMed] [Google Scholar]
- 119.Buffington CA. Idiopathic cystitis in domestic cats–beyond the lower urinary tract. J Vet Intern Med. (2011) 25:784–96. 10.1111/j.1939-1676.2011.0732.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sinagra E, Romano C, Cottone M. Psychopharmacological treatment and psychological interventions in irritable bowel syndrome. Gastroenterol Res Pract. (2012) 2012:486067. 10.1155/2012/486067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tang QL, Lin GY, Zhang MQ. Cognitive-behavioral therapy for the management of irritable bowel syndrome. World J Gastroenterol. (2013) 19:8605–10. 10.3748/wjg.v19.i46.8605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Talley NJ, Owen BK, Boyce P, Paterson K. Psychological treatments for irritable bowel syndrome: a critique of controlled treatment trials. Am J Gastroenterol. (1996) 91:277–83. [PubMed] [Google Scholar]
- 123.Jarrett ME, Cain KC, Burr RL, Hertig VL, Rosen SN, Heitkemper MM. Comprehensive self-management for irritable bowel syndrome: randomized trial of in-person vs. combined in-person and telephone sessions. Am J Gastroenterol. (2009) 104:3004–14. 10.1038/ajg.2009.479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ljótsson B, Andersson G, Andersson E, Hedman E, Lindfors P, Andréewitch S, et al. Acceptability, effectiveness, and cost-effectiveness of internet-based exposure treatment for irritable bowel syndrome in a clinical sample: a randomized controlled trial. BMC Gastroenterol. (2011) 11:110. 10.1186/1471-230X-11-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Enck P, Zipfel S. Placebo Effects in Psychotherapy: A Framework. Frontiers in psychiatry, (2019) 10:456. 10.3389/fpsyt.2019.00456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jurinec N, Schienle A. Utilizing placebos to leverage effects of cognitive-behavioral therapy in patients with depression. J Affective Disorders. (2020) 277:779–84. 10.1016/j.jad.2020.08.087 [DOI] [PubMed] [Google Scholar]
- 127.Flik CE, Bakker L, Laan W, van Rood YRA J., Smout PM, de Wit NJ. Systematic review: the placebo effect of psychological interventions in the treatment of irritable bowel syndrome. World J Gastroenterol. (2017) 23:2223–33. 10.3748/wjg.v23.i12.2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhu Z, Zhang L, Jiang J, Li W, Cao X, Zhou Z, et al. Comparison of psychological placebo and waiting list control conditions in the assessment of cognitive behavioral therapy for the treatment of generalized anxiety disorder: a meta-analysis. Shanghai Arch Psychiatry. (2014) 26:319–31. 10.11919/j.issn.1002-0829.214173 [DOI] [PMC free article] [PubMed] [Google Scholar]