Abstract
Background: Little is known regarding the clinical impact of treatment and treatment duration of probiotic VSL#3 on gut and microbiome function in irritable bowel syndrome (IBS). As part of a safety trial, we assessed the effect of VSL#3 treatment duration on abdominal pain, stooling, gut permeability, microbiome composition and function.
Methods: Adults with IBS were randomized into an open label trial to receive the probiotic VSL#3 for 4 or 8 weeks. Adverse events, abdominal pain, and stooling patterns were recorded daily. Gut permeability, fecal bile acid levels, and microbiome composition were profiled at baseline and after treatment.
Results: Fifteen subjects completed the trial (4-week: n = 8; 8-week: n = 7). Number of pain episodes decreased in both groups (P = 0.049 and P = 0.034; 4- vs. 8-week, respectively). Probiotic organisms contained in VSL#3 were detected in feces by whole shotgun metagenomic sequencing analysis and relative abundances of Streptococcus thermophilus, Bifidobacterium animalis, Lactobacillus plantarum, and Lactobacillus casei subsp. paraccasei correlated significantly with improved abdominal pain symptoms and colonic permeability at study completion. Although abdominal pain correlated significantly with the detection of probiotic species at study completion, a composite view of gut microbiome structure showed no changes in community diversity or composition after VSL#3 treatment.
Conclusions: Probiotic organisms identified in stool correlated significantly with improvement in colonic permeability and clinical symptoms, prompting future studies to investigate the mechanistic role of VSL#3 and colonic permeability in IBS pathophysiology in a larger randomized controlled trial.
Clinical Trial Registration: www.clinicaltrials.gov, Identifier: NCT00971711.
Keywords: irritable bowel syndrome, VSL#3, probiotic, permeability, microbiome, bile acids
Introduction
Irritable bowel syndrome (IBS) is common at all ages, from young children to adults, and the impact on quality of life can be significant (1–4). Understanding of the pathogenesis of IBS has evolved greatly over the past several decades. Once considered a disorder caused by abnormalities of gut smooth muscle, visceral hypersensitivity, and/or central nervous system hypervigilance, the past decade has seen a change in focus to include both central and peripheral mechanisms in the pathophysiology of IBS. Such peripheral mechanisms include abnormal colonic transit and rectal evacuation, intestinal intraluminal irritants, alterations in the gut microbiome, enteroendocrine cell dysfunction, low grade mucosal inflammation, and/or bile acid malabsorption (5, 6).
Alterations in gut microbiome community composition, as well as metabolites generated as a function of microbial metabolism (e.g., bile acids) have become ripe areas of study given the findings of alterations in some patients with IBS (7–10). These observations appear to have clinical relevance based on reports that probiotics can be efficacious as treatment for patients with IBS in both children and adults (7, 8, 11). Of these, there is evidence of efficacy based on randomized trials of VSL#3 in the treatment of IBS in children and adults (12–15).
The exact mechanism(s) whereby probiotics produce benefit in patients with IBS is still in question. Certain combination probiotics have been noted to alter gut microbial composition in humans, supporting the hypothesis that probiotics work by replacing or altering component bacteria of the gut (16, 17). In contrast, previous studies in humans using VSL#3 have shown disparate results with regard to its effects on gut microbiota composition, possibly related to differences in the duration of treatment or other factors (18–20). Similarly, some adults and children with IBS may have increased gut permeability which appears in some cases to relate to abdominal pain symptoms (21–23). To our knowledge, whether VSL#3 alters gut permeability in humans has not been studied.
In general, probiotics are thought to be safe except in the case of patients who are critically ill and/or immunocompromised (24). However, a recent review questions the presumed favorable risk/benefit ratio of treating IBS with probiotics (25). To our knowledge, no study of VSL#3 in adult patients with IBS has focused on potential side effects. In previous clinical trials of VSL#3 in IBS, it is not clear how adverse events were captured or reported (12–15, 19), although we previously reported no significant adverse events when the closely related probiotic VisBiome was administered to autistic children with functional gastrointestinal disorders (26).
We were requested by the United States Food and Drug Administration (FDA) to carry out a thorough safety study of VSL#3 in adults with IBS prior to its use in a randomized study in children with IBS. We made use of this opportunity to investigate in adult IBS patients with matched fecal omics data the effects of two dosing durations of VSL#3 on gut permeability, recovery of probiotic organisms and changes in gut microbiome composition determined by shotgun metagenomic sequencing, and metabolome (bile acid) profile alterations.
Methods
Study Design
The study was an investigator-initiated, phase I, open-label trial mandated by the FDA to assess the safety of VSL#3 in adults with IBS. Informed consent was obtained from the participants and the study was approved by the Baylor College of Medicine Institutional Review Board. The study was registered as clinicaltrials.gov identifier NCT00971711.
Patients were seen by a gastroenterologist (BA) who obtained a medical history and performed a physical exam to confirm the IBS diagnosis using Rome III criteria. Subjects who met entry criteria were randomized (1:1) to receive VSL#3 for either 4 or 8 weeks using a random number generator (www.randomization.com). Assignments were kept in sealed envelopes and were opened and recorded by the research pharmacy when dispensing the probiotic (Table 1).
Table 1.
Schedule of study events.
| Action/event (days related to study drug) | Prior to | During/after |
|---|---|---|
| study drug | study drug | |
| Gastroenterologist visit (history and physical exam) | −7 | |
| Rome III questionnaire | −7 | 56 or 84 |
| Informed consent | −7 | |
| Medical exam | −14 or −7 | |
| Pregnancy test | −2 to 0 | |
| Stool microbiome/bile acid analysis | −7 | 28 or 56 |
| GI permeability test | −7 | 28 or 56 |
| VSL#3 administration | 0-28 or 0-56 | |
| Pain/stooling diary | −7 to −1 | 0-42 or 0-70 |
| AE and SAE diary | −7 to −1 | 0-42 or 0-70 |
| Follow up call for symptoms and AE | 56 or 84 |
A stool was collected for microbiome and bile acid analysis, and a GI permeability test was carried out prior to and after 4 or 8 weeks of probiotic administration. Weekly telephone calls were made to participants by the study staff to ensure compliance and assess any potential adverse events. While receiving the probiotic, participants kept a pain/stooling diary (Supplementary Table 1), recorded any concomitant medication use, and kept an adverse event (AE) and serious adverse event (SAE) diary (Supplementary Table 2).
A follow-up telephone call was made 1 month after completion of the probiotic treatment to inquire about any adverse events. To aid in assessment of compliance, participants were asked to return empty packets to study staff.
Subjects
For recruitment, advertisements were placed in local papers and radio stations, and in gastroenterologists' offices. The consent form, along with the Rome III IBS module and the Functional Bowel Disorder Severity Index (27, 28), were mailed out to the participants to complete at home (Supplementary Table 1). Upon receipt of the consent form and the questionnaires by the investigators, the participants were screened further by telephone to assess eligibility. Inclusion/exclusion criteria listed in Supplementary Table 3 were applied. Subjects who passed screening were seen by an adult gastroenterologist (BA) as described above.
Probiotic
The VSL#3 (Danisco®, Madison, WI) clinical trial product was obtained in December, 2011 and is made up of four strains of Lactobacillus (L. casei subsp. paracasei, L. plantarum, L. acidophilus, and L. delbrueckii subsp. bulgaricus), three strains of Bifidobacterium (B. longum, B. infantis, and B. breve), one strain of Streptococcus thermophilus, and starch. Although previously described as separate species, B. infantis and B. longum are now considered to be biotypes or subspecies of the same organism (B. longum). Lactobacillus bulgaricus in VSL#3 has been re-classified as L. helveticus (29, 30). Subjects took two packets daily (900 billion CFU) for either 4 or 8 weeks. The VSL#3 was mixed in a cold food or non-carbonated beverage.
Stool Collection
A stool sample was collected prior to administration of the probiotic and on the final study day. Stools were collected in a self-sealing container placed over the toilet. Specimens then were placed in a freezer and transported by courier to the Texas Children's Microbiome Center and stored at −80°C until processed as previously described (31).
Microbiome Analysis
Bacterial DNA was extracted using the PowerSoil DNA Isolation kit (MO BIO Laboratories, Carlsbad, CA, USA), with the Human Microbiome Project modifications to the manufacturer's protocol (32). Shotgun metagenomic sequence libraries (WGS) were generated and sequenced by the Human Genome Sequencing Center at Baylor College of Medicine (Houston, TX, USA). Each DNA sample was processed following a 101-bp, paired-end sequence library construction protocol with an average insert length of 200-bp, and was sequenced on the HiSeq2000 platform (Illumina Inc., San Diego, CA, USA). Raw sequence reads were quality filtered by VSEARCH (version 2.9) (33) using the options of—fastq_truncee of 1 and—fastq_minlen of 90. Taxonomic characterization of filtered reads was performed using the MetaPhlan2 package (34). Species profiles were used for alpha-diversity analysis using Shannon index, and was also used for beta-diversity analysis using principal coordinate analysis for abundance-weighted Jaccard distance profiling. ANOSIM test with 999 permutations was used to test the significance of sample groups, i.e., baseline vs. treated samples. Mann-Whitney tests with false discovery rate (Benjamini-Hochberg correction) were used to compare differences in the relative abundance of bacterial taxa including individual VSL#3 component species or the VSL#3 mixture as a whole between baseline and treatment groups. Spearman correlation analysis with Benjamini-Hochberg correction was performed when analyzing clinical variables with the relative abundance of individual VSL#3 component species.
Permeability Test
Prior to starting the probiotic and at the end of the study treatment period, participants underwent a gastrointestinal permeability test (35). In brief, participants drank 100 mL of a solution containing sucrose (10 g), lactulose (5 g), mannitol (1 g), and swallowed a pill containing sucralose (1 g) followed by 240 ml of water after at least a 4-h fast after dinner. Subjects voided before drinking the solution and were told to avoid alcohol and non-steroidal antiinflammatory drugs 24 h prior to and during the permeability test. Each time the subject subsequently voided, urine was placed in separate containers. Thymol (33 μl of a 10% solution) was added as a preservative to each container provided to the participants. After the first morning void, participants were again allowed to eat and drink (with the exception of foods containing sucralose). Urine was collected for a total of 24 h starting from the time of ingestion of the sugars. The urine was placed in the freezer and transported by courier to Texas Children's Hospital after all urine had been collected. High-performance liquid chromatography was carried out to quantify the individual sugars as we and others have described (36). Percent sucrose recovery overnight was used to define gastric permeability and the lactulose/mannitol ratio and percent sucralose recovery over 24 h were used to define small intestinal and colonic permeability, respectively (37).
Bile Acid Analysis
Stools from each treatment group were collected for bile acid analysis. Hot methanol bile acid extracts were analyzed by the Baylor College of Medicine Miraca Genetics Laboratory which provided quantitative measures of primary, secondary and conjugated bile acids [7α-hydroxy-4-cholestan-3-one—C4, HCO, cholic acid (CA), chenodeoxycholic acid (CDCA), glycocholic acid (GCA), taurocholic acid (TCA), glycochenodeoxycholic acid (GCDCA), taurochenodeoxycholic acid (TCDCA), deoxycholic acid (DCA), lithocholic acid (LCA), glycodeoxycholic acid (GDCA), taurodeoxycholic acid (TDCA). Additional bile acids measured included: ursodeoxycholic acid (UDCA, ursodiol) and tauroursodeoxycholic acid (TUDCA)]. Bile acid concentrations (ng/ml) were correlated with clinical symptoms, intestinal permeability and microbiome features as described in the statistics section.
Pain and Stool Diaries
Subjects kept diaries of pain episodes, stools, and adverse events during a 1-week baseline period, during VSL#3 administration, and for 2 weeks after completion of probiotic administration. The data was called to a 24-h phone line as previously described (38). Subjects recorded all pain episodes they experienced in a day, documenting time, duration, and body location. Subjects also reported all stools in a day, noting whether or not they had a stool, if there was pain associated with stooling, and the type of stool as described by the Bristol Stool Scale (39). Thus, participants in the 4-week group recorded up to 49 days of pain episodes and stools, and those in the 8-week group up to 77 days.
Adverse Events
Adverse events were recorded daily using Supplementary Table 2. Subjects were called by study coordinators weekly for 4 or 8 weeks to assess any adverse events which may have occurred during the prior week, while taking, and for 2 weeks after completion of VSL#3. Follow-up at 1 month after study completion occurred via telephone call from the study coordinator. As per the FDA, adverse events were defined as “any untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product and which does not necessarily have to have a causal relationship with this treatment.”
General Statistics
Statistics were analyzed using SPSS® 23 (IBM Corporation, Armonk, NY). Total number of pain episodes over the study periods, mean pain rating over the study periods, and mean of the maximum ratings of pain per day over the study periods were compared using Mann-Whitney U-tests. Assessment of pain data in the 4- and 8-week groups combined was carried out using Wilcoxon Signed Ranks tests. Number of bowel movements, number of days with no bowel movement, and median stool type were compared using Mann-Whitney U-tests. Data are shown as mean ± SD. Pearson and Spearman correlations between probiotic and microbial species, bile acids concentrations, abdominal pain frequency and intestinal permeability in each treatment group were conducted. Benjamini-Hochberg false discovery rate correction was applied to compare microbiome correlations and group differences.
Results
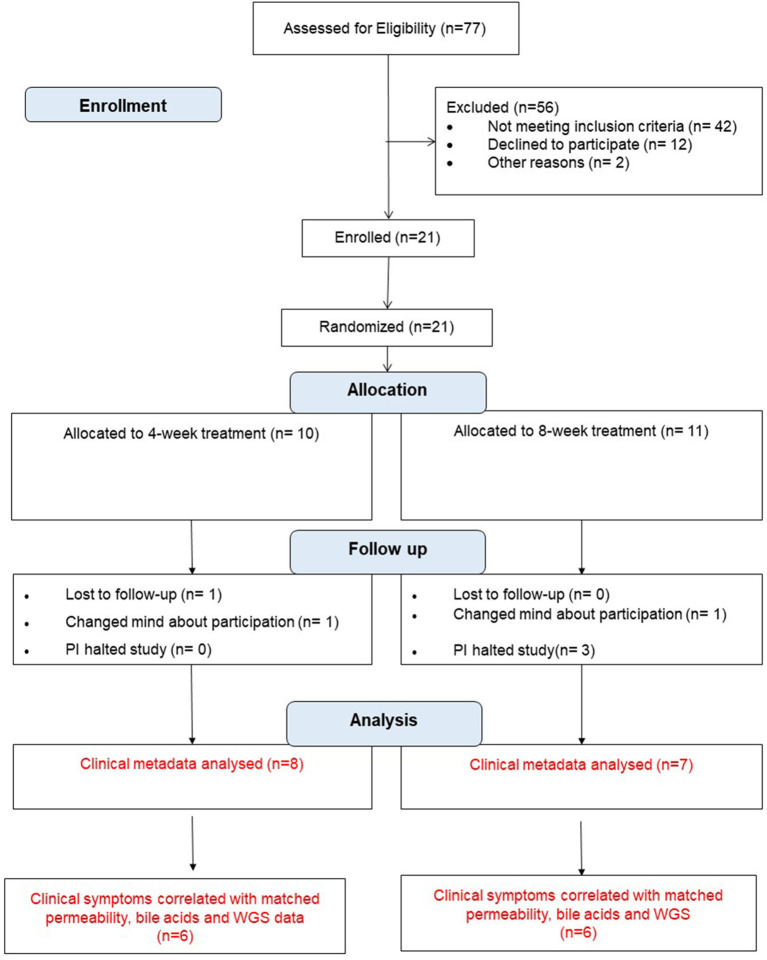
Of the 77 individuals screened, 21 were enrolled and provided product safety data (Figure 1). Demographic and clinical characteristics of the participants are presented in Table 2. The first two participants did not provide urine or stool samples as these collections were added to the study design after the trial began. Three participants dropped out of the study. Three participants were halted on the study when it was discovered that the research pharmacy had dispensed expired VSL#3 to them. Parenthetically, samples of the expired product were sent back to the manufacturer (Danisco) for analysis which revealed that the product still contained the appropriate colony forming units (CFU) of bacteria (i.e., matched the Certificate of Analysis). Therefore, permission was obtained from the FDA to continue the study. Recruitment was carried out between January 2011 and January 2014.
Figure 1.
Consort diagram of probiotic VSL#3 treatment in patients with irritable bowel syndrome.
Table 2.
Demographic and clinical characteristics of 4- and 8-weeks treatment group.
| 4-week treatment group | 8-week treatment group | |
|---|---|---|
| (n = 8) | (n = 7) | |
| Demographic and clinical characteristics | ||
| Age (years) | 36 ± 10 | 36 ± 9 |
| Gender | ||
| Male | 2 | 2 |
| Female | 6 | 5 |
| Ethnicity | ||
| Non-hispanic | 7 | 6 |
| Hispanic | 1 | 1 |
| Race | ||
| Caucasian | 3 | 3 |
| African American | 3 | 3 |
| Asian | 1 | 0 |
| Hispanic | 1 | 1 |
| BMI | 25 ± 5.3 | 25 ± 5.4 |
| IBS severity score | 249.8 ± 85.5 | 242.6 ± 66.9 |
| Starting pain severity | 37.4 ± 24.1 | 42.4 ± 20.3 |
| IBS subtype | ||
| Constipation | 2 | 2 |
| Diarrhea | 0 | 1 |
| Mixed | 6 | 4 |
*Mean ± SD.
Pain and Stool Diaries
Total number of pain episodes, mean pain rating over the study periods, and mean of the maximum ratings of pain per day over the study periods pre- and post-VSL#3 administration were not different between the 4- and 8-week groups (Table 3). However, assessing 4- and 8-week groups together, there were significant decreases in the number of pain episodes and mean of the maximum pain rating after VSL#3 administration as compared to before probiotic (P = 0.007 and P = 0.004, respectively). Assessed individually, the number of pain episodes was significantly decreased after VSL#3 administration in both 4- and 8-week groups (P = 0.049 and P = 0.034, respectively). Additionally, when assessed individually, the 8-week group had a decrease in the mean of the maximum pain rating after VSL#3 administration (P = 0.018). The number of bowel movements per day, number of days without a bowel movement, and mean stool type did not differ between groups (Table 3).
Table 3.
Pain and stooling results of 4- and 8-weeks group.
| Parameter | 4-week group | 8-week group | ||
|---|---|---|---|---|
| Baseline | Post-treatment | Baseline | Post-treatment | |
| Pain and stooling results | ||||
| Pain | ||||
| Number of pain episodes | 6.8 ± 5.0‡ | 2.0 ± 2.4# | 6.4 ± 4.2 | 2.0 ± 2.7# |
| Mean pain rating | ||||
| Mean of maximum pain ratings | 3.8 ± 1.7 | 2.1 ± 2.4* | 3.9 ± 2.2 | 1.5 ± 1.8* |
| Stooling | ||||
| Number of bowel movements per day | 1.5 ± 0.6 | 1.4 ± 0.9 | 1.1 ± 0.8 | 1.3 ± 0.9 |
| Number of days with no bowel movement | 1.3 ± 1.3 | 1.1 ± 1.4 | 1.9 ± 2.4 | 1.8 ± 2.8 |
| Median bristol stool scale type | 3.9 ± 1.6 | 4.3 ± 1.3 | 3.2 ± 1.9 | 3.4 ± 1.4 |
‡Mean ± SD.
*P = 0.049 for post-treatment change within each group.
#P = 0.034 for post-treatment change within each group.
Microbiome Analysis of the Pure VSL#3 Product
Of the 15 study participants who completed the probiotic trial with clinical metadata, only 12 subjects provided sufficient fecal material to facilitate matched deep shotgun metagenomic sequencing (WGS) and bile acid profiling. WGS analysis was performed using MetaPhlan2 to positively identify the presence of VSL#3 containing L. casei subsp. paracasei (38.93%), L. helveticus (2.45%), L. plantarum (1.20%), L. acidophilus (1.36%), S. thermophilus (32.89%), B. animalis (11.14%), and B. breve (11.33%). The abundance of B. longum listed as a probiotic species in VSL#3 was 0.00148% indicating that this organism is barely detected.
Microbiome Analysis–Whole Genome Shotgun Sequencing
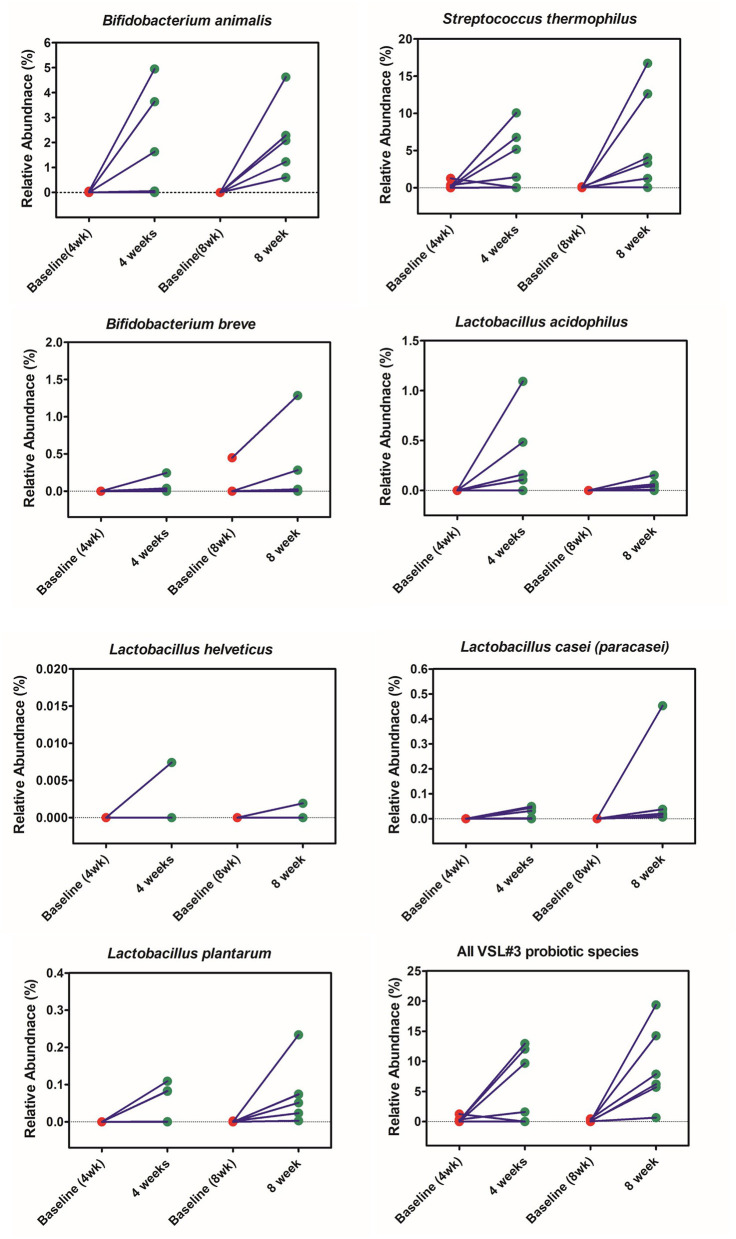
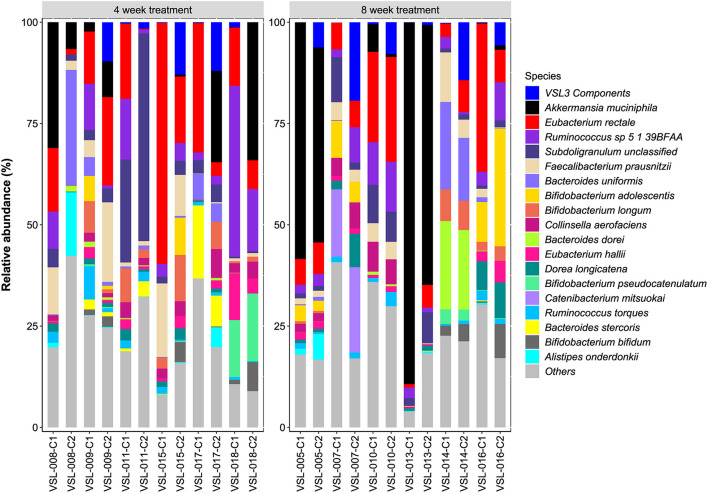
The probiotic species found in VSL#3 (guided by our WGS analysis of the ingested pure product) increased in relative abundance in stool after both 4- and 8-weeks of treatment (Figure 2). The relative abundance of individual VSL#3 species was not affected by treatment duration but when the two treatment groups were considered together to facilitate statistical power, significant enrichment of B. breve, B. animalis, L. plantarum, L. casei, L. acidophilus, and S. thermophilus was observed (P < 0.05 for each; Mann-Whitney Rank test with FDR correction). Of note, B. animalis and S. thermophilus were highly enriched after probiotic administration, whereas Lactobacillus species were detected at lower relative abundance after either 4 or 8 weeks. The relative abundance of several microbiome community members were altered following VSL#3 consumption, although these shifts were not consistent enough across subjects to be statistically significant (Figure 3). As recently reported (40), unique host and microbiota-dependent factors determine empiric probiotic colonization in humans and analysis of individual subjects in this study supported this corresponding microbiota heterogeneity.
Figure 2.
Relative abundance of organisms found in VSL#3 in stool after both 4- and 8-weeks of treatment.
Figure 3.
Species-level relative abundances of community members after both 4- and 8-weeks of treatment.
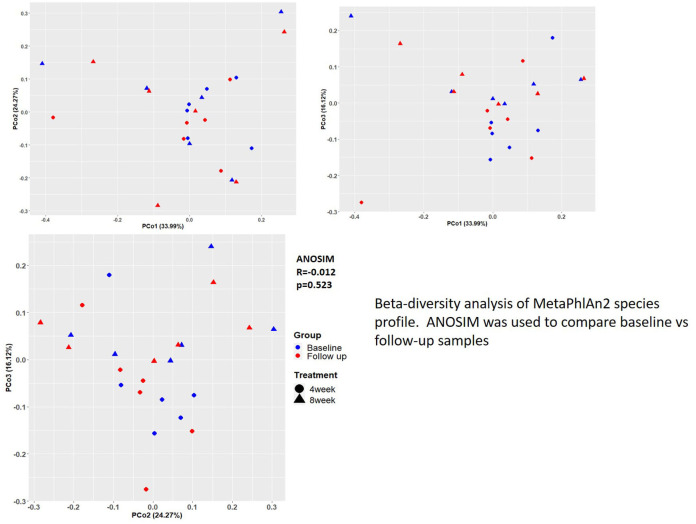
Although significant changes were found for several VSL#3-related species, beta-diversity analysis of MetaPhlAn2 species profile supported the notion that probiotic administration did not significantly alter fecal microbiota community diversity (Figure 4). Spearman correlations measured for identified VSL#3 species abundances of L. plantarum, L. casei subsp. paraccasei, B. animalis, and S. thermophilus with the number of pain episodes were rho = −0.59 (p = 0.003, BH corrected p = 0.030), rho = −0.67 (p = 0.0004, BH corrected p = 0.010), rho = −0.64 (p = 0.001, BH corrected p = 0.018) and rho = −0.34 (p = 0.118, BH corrected p = 0.42), respectively, demonstrating significant inverse correlations between probiotic species abundance in feces with reported pain frequency.
Figure 4.
Beta-diversity analysis of MetaPhlAn2 species profile. ANOSIM was used to compare baseline vs. follow-up samples.
Gastrointestinal Permeability
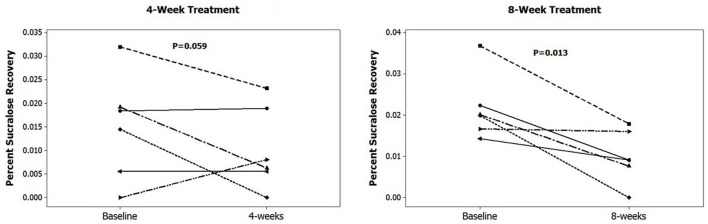
There was a trend for colonic permeability (percent sucralose recovery) to correlate positively with number of pain episodes but statistical significance was not achieved (rho = 0.36; raw p = 0.09). Similarly, colonic permeability decreased with VSL#3 probiotic treatment duration and reached statistical significance after 8-weeks of probiotic treatment (Figure 5). These trends need to be validated in larger randomized trials in order to explore possible disease mechanism.
Figure 5.
Colonic permeability (percent sucralose recovery) of probiotic VSL#3 treatment in 4- and 8-weeks group.
Bile Acids
Bile acid profiles did not change significantly between baseline and after probiotic treatment when applying multiple testing corrections (data not shown), and require a sufficiently powered trial to explore possible trends that we identified. In particular, unconjugated secondary bile acids associated negatively with colonic permeability (rho = −0.39; raw p = 0.064, BH corrected p = 0.491), whereas, primary bile acids associated positively with stool type (rho = 0.48; raw p = 0.020, BH corrected p = 0.550).
Adverse Events
Safety data included all 21 subjects. No deaths, infections with component bacteria, unscheduled IBS-related health care visits, change in frequency or severity of events, or reported causality occurred in subjects while taking VSL#3.
There were 88 AEs reported (Supplementary Table 4); all were rated as not serious according to the FDA rating scale. The AEs were deemed not related to VSL#3 as the symptoms reported as AEs are common symptoms in IBS. Bloating was the most common AE reported followed by having ≥ 4 stools in a 24-h period.
Serious Adverse Events
Two SAEs were reported during the study in the same subject who was admitted to the hospital for vaginal bleeding due to uterine fibroids and required administration of blood products. These SAEs, the first of which occurred while the subject was on VSL#3, were deemed to be unrelated to the probiotic.
Discussion
Many gaps still remain in our understanding of how probiotics impact the gut microbiome in patients with IBS. Our study aimed to comprehensively assess the pre- and post-VSL#3 gut response at the level of the microbiome using deep resolution WGS, metabolome (bile acids), and the gut barrier integrity (permeability).
VSL#3 has been shown previously to be effective in improving symptoms in adults and children with IBS in double blind, randomized trials (12–14). However, an understanding of the mechanism(s) responsible remain elusive. The response of the microbiome to probiotics seems to hold promise in helping to understand the mechanisms by which gut microbiota contribute to symptoms in IBS. Only one previous study, to our knowledge, examined the gut microbiome response to VSL#3 administration. Michail et al. did not find the gut microbiome to be altered after 8 weeks of treatment (19). However, their study employed microarray technology which potentially could limit their ability to detect changes (19). In our own study of Visbiome—a closely related probiotic mixture to VSL#3—we found significant improvement in the quality of life score (PedQL) in autistic children that correlated with detectable Lactobacillus spp abundance in fecal specimens (26). However, this 16S rDNA amplicon-based study lacked the species resolution provided by the WGS sequencing employed in the current study to identify specific probiotic strains associated with these health benefits. Although our WGS analysis did not identify robust VSL#3-induced compositional changes in the gut microbiome of IBS patients, probiotic species L. plantarum, L. casei subsp. paraccasei, B. animalis, and S. thermophilus were found to correlate significantly with number of pain episodes. These probiotic species also were associated with trends in reduced colonic permeability, although a larger randomized trial of IBS patients validating probiotic correlations with colonic permeability and abdominal pain symptoms is required. It is not clear how permeability is linked mechanistically with abdominal pain (21), but this could relate to direct microbial-regulation of the epithelial barrier, allowing access to submucosal immune cells which in turn can activate sensory nerves directly (41, 42). Probiotic and microbiome species also have the potential to directly relay nociceptive signals to sensory intrinsic afferent neurons in the intestine.
To our knowledge, gut permeability has not been measured following VSL#3 administration to adults with IBS. Animal models of gut inflammation have suggested that administration of VSL#3 is associated with decreased gut permeability, perhaps acting through increases in the expression of phosphorylated p38 and ERK and/or preventing apoptosis and maintaining tight junction protein expression (43, 44). A number of studies have shown that increased gut permeability can be related to abdominal pain symptoms in IBS (45). Whether the improvement in IBS symptoms reported with VSL#3 and other probiotics relates directly to normalization of permeability awaits further studies.
Several of the VSL#3 species identified in the fecal specimens have the potential to transform bile acids. It is possible that altered metabolic activity of the microbiota leads to an imbalance in fecal bile acid profiles in IBS cases, as we and others recently have reported (46). Although our study was underpowered to make statistical claims, we found trends that merit further investigation in larger trials indicating that taurochenodeoxycholic acid is associated with increased abdominal pain and colonic permeability, which could reflect a direct bile acid interaction on epithelial tight junction activity. In a prior omics study of IBS children we observed a similar abdominal pain association with taurochenodeoxycholic acid (47), and deconjugation by VSL#3 could constitute a protective mechanism as this is a bile acid species that is also known to disrupt epithelial barrier integrity.
From the abundance profile of individual patients at species level, we found that the sum of the relative abundances of probiotic species increased after 4 or 8 weeks of VSL#3 administration. Specifically, B. animalis and S. thermophilus were more abundant than the other VSL#3-related species and both demonstrated negative correlations with pain frequency, although the latter organism did not meet statistical rigor after multiple-testing correction. Although, S. thermophilus has not been associated previously with pain resolution in clinical trials, one previous clinical trial demonstrated B. animalis DN-173 to be associated with improved health-related quality of life and abdominal pain in adults IBS patients (46). Several other clinical studies also indicate that B. longum, which is not detected in VSL#3 product used in this study, is also a potential species for pain management in IBS patients (48).
The primary bile acids (CA and CDCA) are synthesized from cholesterol in the liver and are then released and transported to the small intestine, where a minority amount enters the large intestine for conversion to secondary bile acids by the gut microbiota. Bile acids play a role in IBS pathophysiology and in some IBS patients abdominal pain is linked with bile acid malabsorption (44, 45, 49). Lactobacilli and Bifidobacteria subspecies are able to de-conjugate and absorb bile acids, as well as perform bile acid biotransformation which may detoxify species that are linked with increased intestinal permeability and pain (50). Dior et al., demonstrated bile acid deconjugation activity was decreased in IBS (51). Although we observed trends in bile acid profiles that support a potential pathogenic role in modulating abdominal pain and colonic permeability, these results did not meet the statistical rigor to establish precedent for bile-acid mediated protection by VSL#3.
In summary, our results show that 4-week treatment with VSL#3 is as effective as 8-weeks in populating the gut with probiotic organisms. Amelioration of abdominal pain correlated with abundance of probiotic organisms detected in study participants, indicating that future studies should focus on identifying probiotic signals to the host. We have previously reported in a constipated animal model that Lactobacillus probiotic retention is critical in communicating effectively with the host, in this case via modulation of visceral opioid receptors (52). Our study had some limitations, given the relatively small sample size and the open trial design. Strengths of our study included the deep-resolution metagenomics used for probiotic and structural microbiome characterization, with simultaneous physiological measurement of gut permeability and bile acid profiles. The permeability results would not have been affected by the open label nature of our study, and sets the stage for a future double blind randomized controlled trial to control for significant placebo effects observed in IBS patients.
Data Availability Statement
The datasets presented in this study can be found at https://www.ncbi.nlm.nih.gov/bioproject/PRJNA725223.
Ethics Statement
The studies involving human participants were reviewed and approved by Baylor College of Medicine Institutional Review Board. The study was registered as clinicaltrials.gov identifier NCT00971711. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This study was supported in part by R01 NR05337 and R34 AT006986, U01-AI24290, P01-AI152999 R01-AI10091401, UH2DK093990 and UH3DK083990 from the National Institutes of Health, the Daffy's Foundation, the USDA/ARS under Cooperative Agreement No. 58-3092-0-001, and P30 DK56338 which funds the Texas Medical Center Digestive Disease Center.
Author Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work is a publication of the USDA/ARS Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine and Texas Children's Hospital. The contents do not necessarily reflect the views or policies of the USDA, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We gratefully acknowledge Danisco®, Madison, WI for provision of the VSL#3.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpain.2021.691689/full#supplementary-material
References
- 1.Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. (2015) 313:949-58. 10.1001/jama.2015.0954 [DOI] [PubMed] [Google Scholar]
- 2.van Tilburg MA, Hyman PE, Walker L, Rouster A, Palsson OS, Kim SM, et al. Prevalence of functional gastrointestinal disorders in infants and toddlers. J Pediatr. (2015) 166:684-9. 10.1016/j.jpeds.2014.11.039 [DOI] [PubMed] [Google Scholar]
- 3.Lewis ML, Palsson OS, Whitehead WE, van Tilburg MAL. Prevalence of functional gastrointestinal disorders in children and adolescents. J Pediatr. (2016) 177:39-43.e3. 10.1016/j.jpeds.2016.04.008 [DOI] [PubMed] [Google Scholar]
- 4.Sperber AD, Dumitrascu D, Fukudo S, Gerson C, Ghoshal UC, Gwee KA, et al. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: a Rome Foundation working team literature review. Gut. (2017) 66:1075-82. 10.1136/gutjnl-2015-311240 [DOI] [PubMed] [Google Scholar]
- 5.Camilleri M. Peripheral mechanisms in irritable bowel syndrome. N Engl J Med. (2013) 368:578-9. 10.1056/NEJMc1214185 [DOI] [PubMed] [Google Scholar]
- 6.Camilleri M. Advances in understanding of bile acid diarrhea. Exp Rev Gastroenterol Hepatol. (2014) 8:49-61. 10.1586/17474124.2014.851599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simren M, Barbara G, Flint HJ, Spiegel BM, Spiller RC, Vanner S, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. (2013) 62:159-76. 10.1136/gutjnl-2012-302167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayer EA, Savidge T, Shulman RJ. Brain-gut microbiome interactions and functional bowel disorders. Gastroenterology. (2014) 146:1500-12. 10.1053/j.gastro.2014.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bajor A, Tornblom H, Rudling M, Ung KA, Simren M. Increased colonic bile acid exposure: a relevant factor for symptoms and treatment in IBS. Gut. (2015) 64:84-92. 10.1136/gutjnl-2013-305965 [DOI] [PubMed] [Google Scholar]
- 10.Slattery SA, Niaz O, Aziz Q, Ford AC, Farmer AD. Systematic review with meta-analysis: the prevalence of bile acid malabsorption in the irritable bowel syndrome with diarrhoea. Aliment Pharmacol Ther. (2015) 42:3-11. 10.1111/apt.13227 [DOI] [PubMed] [Google Scholar]
- 11.Vanuytsel T, Tack JF, Boeckxstaens GE. Treatment of abdominal pain in irritable bowel syndrome. J Gastroenterol. (2014) 49:1193-205. 10.1007/s00535-014-0966-7 [DOI] [PubMed] [Google Scholar]
- 12.Guandalini S, Magazzu G, Chiaro A, La Balestra V, Di Nardo G, Gopalan S, et al. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr. (2010) 51:24-30. 10.1097/MPG.0b013e3181ca4d95 [DOI] [PubMed] [Google Scholar]
- 13.Kim HJ, Vazquez Roque MI, Camilleri M, Stephens D, Burton DD, Baxter K, et al. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil. (2005) 17:687-96. 10.1111/j.1365-2982.2005.00695.x [DOI] [PubMed] [Google Scholar]
- 14.Kim HJ, Camilleri M, McKinzie S, Lempke MB, Burton DD, Thomforde GM, et al. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. (2003) 17:895-904. 10.1046/j.1365-2036.2003.01543.x [DOI] [PubMed] [Google Scholar]
- 15.Wong RK, Yang C, Song GH, Wong J, Ho KY. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: a randomized double-blinded placebo study. Dig Dis Sci. (2015) 60:186-94. 10.1007/s10620-014-3299-8 [DOI] [PubMed] [Google Scholar]
- 16.Kajander K, Myllyluoma E, Rajilic-Stojanovic M, Kyronpalo S, Rasmussen M, Jarvenpaa S, et al. Clinical trial: multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment Pharmacol Ther. (2008) 27:48-57. 10.1111/j.1365-2036.2007.03542.x [DOI] [PubMed] [Google Scholar]
- 17.Yoon JS, Sohn W, Lee OY, Lee SP, Lee KN, Jun DW, et al. Effect of multispecies probiotics on irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Gastroenterol Hepatol. (2014) 29:52-9. 10.1111/jgh.12322 [DOI] [PubMed] [Google Scholar]
- 18.Brigidi P, Vitali B, Swennen E, Bazzocchi G, Matteuzzi D. Effects of probiotic administration upon the composition and enzymatic activity of human fecal microbiota in patients with irritable bowel syndrome or functional diarrhea. ResMicrobiol. (2001) 152:735-41. 10.1016/S0923-2508(01)01254-2 [DOI] [PubMed] [Google Scholar]
- 19.Michail S, Abernathy FW, Harshavadran K, Stolfi A. VSL#3 use in a double-blinded, placebo-controlled pilot trial in diarrhea-predominant irritable bowel syndrome. Gastroenterology. (2010) 138:S230. 10.1016/S0016-5085(10)61045-1 [DOI] [Google Scholar]
- 20.Kim SE, Choi SC, Park KS, Park MI, Shin JE, Lee TH, et al. Change of fecal flora and effectiveness of the short-term VSL#3 probiotic treatment in patients with functional constipation. J Neurogastroenterol Motil. (2015) 21:111-20. 10.5056/jnm14048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. (2009) 146:41-6. 10.1016/j.pain.2009.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shulman RJ, Jarrett ME, Cain KC, Broussard EK, Heitkemper MM. Associations among gut permeability, inflammatory markers, and symptoms in patients with irritable bowel syndrome. J Gastroenterol. (2014) 49:1467-76. 10.1007/s00535-013-0919-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shulman RJ, Eakin MN, Czyzewski DI, Jarrett M, Ou CN. Increased gastrointestinal permeability and gut inflammation in children with functional abdominal pain and irritable bowel syndrome. J Pediatr. (2008) 153:646-50. 10.1016/j.jpeds.2008.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Didari T, Solki S, Mozaffari S, Nikfar S, Abdollahi M. A systematic review of the safety of probiotics. Expert Opin Drug Saf. (2014) 13:227-39. 10.1517/14740338.2014.872627 [DOI] [PubMed] [Google Scholar]
- 25.Bennett WE, Jr. Quantitative risk-benefit analysis of probiotic use for irritable bowel syndrome and inflammatory bowel disease. Drug Saf. (2016) 39:295-305. 10.1007/s40264-015-0349-x [DOI] [PubMed] [Google Scholar]
- 26.Arnold LE, Luna RA, Williams K, Chan J, Parker RA, Wu Q, et al. Probiotics for gastrointestinal symptoms and quality of life in autism: a placebo-controlled pilot trial. J Child Adolesc Psychopharmacol. (2019) 29:659-69. 10.1089/cap.2018.0156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drossman DA, Li Z, Toner BB, Diamant NE, Creed FH, Thompson D, et al. Functional bowel disorders. A multicenter comparison of health status and development of illness severity index. Dig Dis Sci. (1995) 40:986-95. 10.1007/BF02064187 [DOI] [PubMed] [Google Scholar]
- 28.Sperber AD, Carmel S, Atzmon Y, Weisberg I, Shalit Y, Neumann L, et al. Use of the functional bowel disorder severity index (FBDSI) in a study of patients with the irritable bowel syndrome and fibromyalgia. Am J Gastroenterol. (2000) 95:995-8. 10.1111/j.1572-0241.2000.01977.x [DOI] [PubMed] [Google Scholar]
- 29.Sakata S, Kitahara M, Sakamoto M, Hayashi H, Fukuyama M, Benno Y. Unification of Bifidobacterium infantis and Bifidobacterium suis as Bifidobacterium longum. Int J Sys Evolution Microbiol. (2002) 52:1945-51. 10.1099/00207713-52-6-1945 [DOI] [PubMed] [Google Scholar]
- 30.Mattarelli P, Bonaparte C, Pot B, Biavati B. Proposal to reclassify the three biotypes of Bifidobacterium longum as three subspecies: Bifidobacterium longum subsp. longum subsp. nov., Bifidobacterium longum subsp. infantis comb. nov. and Bifidobacterium longum subsp. suis comb. nov. Int J Sys Evolution Microbiol. (2008) 58:767-72. 10.1099/ijs.0.65319-0 [DOI] [PubMed] [Google Scholar]
- 31.Saulnier DM, Riehle K, Mistretta TA, Diaz MA, Mandal D, Raza S, et al. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology. (2011) 141:1782-91. 10.1053/j.gastro.2011.06.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aagaard K, Petrosino J, Keitel W, Watson M, Katancik J, Garcia N, et al. The human microbiome project strategy for comprehensive sampling of the human microbiome and why it matters. FASEB J. (2013) 27:1012-22. 10.1096/fj.12-220806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rognes T, Flouri T, Nichols B, Quince C, Mahé F. VSEARCH: a versatile open source tool for metagenomics. PeerJ. (2016) 4:e2584. 10.7717/peerj.2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Truong DT, Franzosa EA, Tickle TL, Scholz M, Weingart G, Pasolli E, et al. MetaPhlAn2 for enhanced metagenomic taxonomic profiling. Nat Methods. (2015) 12:902-3. 10.1038/nmeth.3589 [DOI] [PubMed] [Google Scholar]
- 35.McOmber ME, Ou CN, Shulman RJ. Effects of timing, sex, and age on site-specific gastrointestinal permeability testing in children and adults. J Pediatr Gastroenterol Nutr. (2010) 50:269-75. 10.1097/MPG.0b013e3181aa3aa9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shulman RJ, Schanler RJ, Lau C, Heitkemper M, Ou CN, Smith EO. Early feeding, antenatal glucocorticoids, and human milk decrease intestinal permeability in preterm infants. Pediatr Res. (1998) 44:519-23. 10.1203/00006450-199810000-00009 [DOI] [PubMed] [Google Scholar]
- 37.Meddings JB, Gibbons l. Descrimination of site-specific alterations in gastrointestinal permeability in the rat. Gastroenterology. (1998) 114:83-92. 10.1016/S0016-5085(98)70636-5 [DOI] [PubMed] [Google Scholar]
- 38.Shulman RJ, Eakin MN, Jarrett M, Czyzewski DI, Zeltzer LK. Characteristics of pain and stooling in children with recurrent abdominal pain. J Pediatr Gastroenterol Nutr. (2007) 44:203-8. 10.1097/01.mpg.0000243437.39710.c0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. ScandJ Gastroenterol. (1997) 32:920-4. 10.3109/00365529709011203 [DOI] [PubMed] [Google Scholar]
- 40.Zmora N, Zilberman-Schapira G, Suez J, Mor U, Dori-Bachash M, Bashiardes S, et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell. (2018) 174:1388-405.e21. 10.1016/j.cell.2018.08.041 [DOI] [PubMed] [Google Scholar]
- 41.Aguilera-Lizarraga J, Florens MV, Viola MF, Jain P, Decraecker L, Appeltans I, et al. Local immune response to food antigens drives meal-induced abdominal pain. Nature. (2021) 590:151-6. 10.1038/s41586-020-03118-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McVey Neufeld KA, Mao YK, Bienenstock J, Foster JA, Kunze WA. The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse. Neurogastroenterol Motil. (2013) 25:183-e88. 10.1111/nmo.12049 [DOI] [PubMed] [Google Scholar]
- 43.Dai C, Zhao DH, Jiang M. VSL#3 probiotics regulate the intestinal epithelial barrier in vivo and in vitro via the p38 and ERK signaling pathways. Int J Mol Med. (2012) 29:202-8. 10.3892/ijmm.2011.839 [DOI] [PubMed] [Google Scholar]
- 44.Mennigen R, Nolte K, Rijcken E, Utech M, Loeffler B, Senninger N, et al. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol. (2009) 296:G1140-9. 10.1152/ajpgi.90534.2008 [DOI] [PubMed] [Google Scholar]
- 45.Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. (2012) 303:G775-85. 10.1152/ajpgi.00155.2012 [DOI] [PubMed] [Google Scholar]
- 46.Guyonnet D, Chassany O, Ducrotte P, Picard C, Mouret M, Mercier CH, et al. Effect of a fermented milk containing Bifidobacterium animalis DN-173 010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults in primary care: a multicentre, randomized, double-blind, controlled trial. Aliment Pharmacol Ther. (2007) 26:475-86. 10.1111/j.1365-2036.2007.03362.x [DOI] [PubMed] [Google Scholar]
- 47.Hollister EB, Oezguen N, Chumpitazi BP, Luna RN, Weidler EM, Rubio-Gonzales M, et al. Leveraging human microbiome features to diagnose and stratify children with irritable bowel syndrome. J Mol Diagn. (2019) 21:449–61. 10.1016/j.jmoldx.2019.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pratt C, Campbell MD. The effect of Bifidobacterium on reducing symptomatic abdominal pain in patients with irritable bowel syndrome: a systematic review. Probiot Antimicrob Proteins. (2020) 12:834-9. 10.1007/s12602-019-09609-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol. (2014) 30:332-8. 10.1097/MOG.0000000000000057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruiz L, Margolles A, Sánchez B. Bile resistance mechanisms in Lactobacillus and Bifidobacterium. Front Microbiol. (2013) 4:396. 10.3389/fmicb.2013.00396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dior M, Delagreverie H, Duboc H, Jouet P, Coffin B, Brot L, et al. Interplay between bile acid metabolism and microbiota in irritable bowel syndrome. Neurogastroenterol Motil. (2016) 28:1330-40. 10.1111/nmo.12829 [DOI] [PubMed] [Google Scholar]
- 52.Hegde S, Lin YM, Fu Y, Savidge T, Shi XZ. Precision Lactobacillus reuteri therapy attenuates luminal distension-associated visceral hypersensitivity by inducing peripheral opioid receptors in the colon. Pain. (2020) 161:2737-49. 10.1097/j.pain.0000000000001967 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found at https://www.ncbi.nlm.nih.gov/bioproject/PRJNA725223.