Abstract
Background
People with pes cavus frequently suffer foot pain, which can lead to significant disability. Despite anecdotal reports, rigorous scientific investigation of this condition and how best to manage it is lacking.
Objectives
To assess the effects of interventions for the prevention and treatment of pes cavus.
Search methods
We searched the Cochrane Neuromuscular Disease Group Specialized Register (17 August 2010), MEDLINE (January 1966 to August 2010), EMBASE (January 1980 to August 2010), CINAHL (January 1982 to August 2010), AMED (January 1985 to August 2010) and reference lists of articles. We contacted experts in the field to identify additional published or unpublished data.
Selection criteria
We included all randomized and quasi‐randomised controlled trials of interventions for the treatment of pes cavus. We included trials aimed at preventing or correcting the pes cavus deformity.
Data collection and analysis
Two authors independently selected papers, assessed trial quality and extracted data.
Main results
Four trials were included in the review. One new trial of botulinum toxin was identified in the updated search. Only one trial of custom‐made foot orthoses fully met the inclusion criteria. Three additional studies (botulinum toxin, footwear and off‐the‐shelf foot orthoses), all assessing secondary outcomes were included. We could not pool data used in the four studies due to heterogeneity of diagnostic groups and outcome measures. The one trial that fully met the inclusion criteria investigated the treatment of pes cavus pain in 154 adults over three months. The trial showed a significant reduction in the level of foot pain with custom‐made foot orthoses versus sham orthoses (WMD 10.90; 95% CI 3.21 to 18.59). Furthermore, a significant improvement in self‐reported foot function and physical functioning was reported with custom‐made foot orthoses. There was no difference in reported adverse events following the allocation of custom‐made or sham orthoses. Secondary biomechanical outcomes improved with the use of custom‐made foot orthoses and footwear (pedobarography), but not with intramuscular injections of botulinum toxin (radiographic) or off‐the‐shelf foot orthoses (electromyography).
Authors' conclusions
This updated review shows that custom‐made foot orthoses are significantly more beneficial than sham orthoses for treating foot pain associated with pes cavus in a variety of clinical populations. We also show that some secondary biomechanical outcomes improve with custom‐made foot orthoses and footwear, but not with botulinum toxin or off‐the‐shelf foot orthoses. There is an absence of evidence for any other type of intervention for the treatment or prevention of pes cavus.
Plain language summary
Interventions for the prevention and treatment of pes cavus (high‐arched foot deformity)
Pes cavus is characterised by an excessively high medial longitudinal arch (the arch on the inside of the foot) and is typically defined as a high‐arched or supinated foot type. Population based studies suggest the prevalence of pes cavus is approximately 10%, and its cause is primarily neuromuscular (for example Charcot‐Marie‐Tooth disease) or idiopathic (unknown) in nature. It has been estimated that 60% of people with cavus feet will experience chronic foot pain at some time in their life, most commonly beneath the forefoot (for example metatarsalgia, sesamoiditis) or heel (for example plantar fasciitis). Conditions such as these are thought to be the result of abnormal pressure distribution across the sole of the foot during walking. Many conservative therapies and surgical procedures have been recommended for cavus‐related foot pain. In particular, foot orthoses (aids applied and worn on the outside of the body to support the bony structures) customised to an individual's foot shape are increasingly prescribed by podiatrists, physiotherapists, orthopaedic surgeons and rehabilitation specialists for people with pes cavus pain. This updated review analysed four relevant trials, but only one fully met the inclusion criteria. This trial with 154 adults showed that custom‐made foot orthoses can reduce and redistribute plantar foot pressure and subsequently decrease foot pain by approximately 75%. Some biomechanical outcomes, such as pressure distribution, improve with custom‐made foot orthoses and footwear, but many other biomechanical outcomes, such as foot alignment or muscle activity, do not improve with botulinum toxin or off‐the‐shelf foot orthoses, respectively. More research is needed to determine the effectiveness of other interventions for people with painful high‐arched feet.
Background
Pes cavus is a multiplanar foot deformity characterised by an abnormally high medial longitudinal arch. It also commonly features a varus (inverted) hindfoot, a plantarflexed (downward) position of the first metatarsal, an adducted (internally directed) forefoot and claw toes. The resultant deformity is usually bilateral and rigid leading to difficulty with fitting footwear and decreased walking tolerance.
The term pes cavus encompasses a broad spectrum of foot deformities. Three main types of pes cavus are regularly described in the literature: pes cavovarus, pes calcaneovarus and ‘pure’ pes cavus. Cavovarus, the most common type of pes cavus, is seen primarily in neuromuscular disorders such as Charcot‐Marie‐Tooth disease, and in cases of unknown aetiology, conventionally termed as ‘idiopathic’. Cavovarus presents with the calcaneus in varus, the first metatarsal plantarflexed and a claw‐toe deformity. In the calcaneocavus foot, which is seen primarily following poliomyelitis, the calcaneus is dorsiflexed and the forefoot is plantarflexed. In ‘pure’ pes cavus the calcaneus is neither dorsiflexed or in varus, and is highly‐arched due to a plantarflexed position of the forefoot on the rearfoot. A combination of any or all of these elements can also be seen in a ‘combined’ type of pes cavus that may be further categorised as flexible or rigid. Despite various presentations and descriptions of pes cavus, all are characterised by an abnormally high medial longitudinal arch, gait disturbances and resultant foot pathology.
While much attention has been given to the plight of the pathological pes planus, or 'flat foot', little is known about the other extreme in foot type, the high‐arched foot, or pes cavus. People with pes cavus frequently suffer foot pain, which can lead to significant disability. Despite anecdotal reports, rigorous scientific investigation of this condition and how best to manage it is lacking. This review examines the effectiveness of established and experimental interventions for the prevention and treatment of pes cavus.
Epidemiology of pes cavus
There are few good estimates of the prevalence of pes cavus. There has been only one community‐based study of its prevalence in the general population. An Indian study of 1,846 healthy people aged 16 to 65 years found that 10% had high‐arched feet, as assessed on static footprint (Sachithanandam 1995).
Aetiology of pes cavus
The aetiology and mechanisms underlying pes cavus are complex and not well understood. Factors considered influential in the development of pes cavus include muscle weakness and imbalance in neuromuscular disease (Burns 2005b), residua of congenital clubfoot (Pandey 2002), post‐traumatic bone malformations (Horne 1984), peroneus longus tendon laceration (Carroll 1999), contracture of the plantar fascia and shortening of the Achilles tendon (Dickson 1939).
Foot and ankle pain in pes cavus
Individuals with pes cavus frequently report foot pain, which can lead to significant disability (Badlissi 2005). The range of complaints reported in the literature include forefoot metatarsal pain and plantar heel pain (Burns 2005a), pain under the sesamoid region (Helliwell 1995), painful callosities (Aktas 2000), ankle arthritis (Fortin 2002) and Achilles tendonitis (Giannini 2002). Other symptoms believed to be related to pes cavus include shoe‐fitting problems (Aktas 2000), lateral ankle instability (Barrie 2001), lower limb stress fractures (Korpelainen 2001), knee pain (Dahle 1991), iliotibial band friction syndrome (Williams 2001) and osteoarthritis of the hip (Reilly 2006).
Interventions for the prevention and treatment of pes cavus
Successful prevention and treatment of pes cavus foot pain and associated disability is clinically challenging. While conservative and surgical approaches have been described in the literature, no firm evidence exists for any approach. Conservative management of people with pes cavus typically involves strategies to reduce and redistribute plantar pressure loading through the use of foot orthoses and specialised cushioned footwear (Statler 2005). Other non‐surgical rehabilitation approaches include stretching and strengthening of tight and weak muscles, debridement of plantar callosities and strategies to improve balance (Manoli 2005).
There are also numerous surgical approaches described in the literature aimed at correcting and preventing the progression of the deformity by re‐balancing and reconstructing the foot. Surgical procedures fall into three main groups: (1) soft‐tissue procedures (for example plantar fascia release, Achilles tendon lengthening, tendon transfer); (2) osteotomies or removing a wedge‐shaped portion of the bone (for example metatarsal, midfoot or calcaneal); and (3) bone‐stabilising procedures (for example triple arthrodesis, which consists of fusion of the three joints in the foot and ankle: the talocalcaneal, talonavicular and calcaneocuboid) (Wines 2005). There are few reports of long‐term follow‐up of patients undergoing surgery for cavus foot deformities.
The evidence base of interventions for the prevention and treatment of pes cavus is currently weak. This review was undertaken because of the complex nature of pes cavus, the apparently high incidence of pain and chronic morbidity in people with this condition, and the limited scientific data on all aspects of its prevention and treatment.
Objectives
To assess the effects of interventions for the prevention and treatment of pes cavus.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised and quasi‐randomised controlled trials of interventions for the treatment of pes cavus. We also included trials aimed at preventing or correcting the cavus foot deformity.
Types of participants
Due to the variety of assessment methods, we included studies on participants of all ages who were described as having pes cavus deformities of any aetiology and type.
Types of interventions
Any intervention aimed at preventing or treating pes cavus. These might have included but were not limited to the following:
Appliances: for example foot orthoses, footwear.
Physical therapies: for example joint range of motion exercises, muscle stretching and strengthening, electrotherapeutic modalities, debridement of plantar callosities.
Medications: for example non‐steroidal, anti‐inflammatory drugs (NSAIDs), corticosteroid injections.
Surgery: for example soft‐tissue and bony procedures.
Types of outcome measures
The primary outcome was the level of a quantifiable measure of foot pain, or the change in the level of pain three months after intervention. We also considered any similarly defined outcome measure used to evaluate foot pain. Studies with different follow‐up periods were combined with appropriate adjustments if the assumption of steady rates of change could be justified.
We also assessed the data three months after the intervention for the following:
Functional improvement: for example activities of daily living, disability measures.
Biomechanical improvement: for example kinetic, kinematic, electromyographic, radiographic.
Health‐related quality of life.
Patient satisfaction.
Adverse events of any treatment regimen.
Again studies with different follow‐up periods were combined with appropriate adjustments if the assumption of steady rates of change could be justified.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Neuromuscular Disease Group Specialized Register (17 August 2010) for randomised trials using the following search terms: foot deformities, cavus, cavovarus, supinated, high arch, foot malformation. We adapted this strategy to search the MEDLINE (January 1966 to August 2010), EMBASE (January 1980 to August 2010), CINAHL (January 1982 to August 2010), AMED (January 1985 to August 2010) and reference lists of articles. Non‐English reports were included and we contacted the authors and known experts in the field to identify additional published or unpublished data.
For MEDLINE, EMBASE, AMED, CINAHL search strategies please see Appendix 1, Appendix 2, Appendix 3, Appendix 4 respectively.
Data collection and analysis
Selection of studies
Two review authors (JB and KL) independently checked the titles and abstracts of the articles identified by the search. Two review authors (JB and KL) assessed the risk of bias in the selected articles using a standardised grading system, and independently decided upon inclusion. Disagreements about whether a study should be included were resolved by discussion until consensus was reached.
Data extraction and management
Two review authors (JB and KL) extracted data from the trials and wrote to trial authors for further information as necessary. One review author (JB) entered data into Review Manager and a second review author (KL) checked the data entry .
Assessment of risk of bias in included studies
We used a standardised grading system to assess risk of bias in the trials. This took into account: (1) sequence generation; (2) concealment of allocation; (3) blinding; (4) incomplete data; (5) selective outcome reporting; (6) other issues. A risk of bias table was created to present a description of what was reported within the published report for each criterion and to assign a judgment relating to the risk of bias for that entry. 'Yes' indicated a low risk of bias, 'No' indicated a high risk of bias and 'Unclear' indicated an unclear or unknown risk of bias; or if an entry is not relevant to the study at hand. A 'Risk of bias summary' Figure was generated using Review Manager 5 to present all of the judgements in a cross‐tabulation of study by entry.
Data synthesis
If there was more than one trial with a specific treatment or prevention approach we calculated a pooled estimate of the treatment effect across the trials using the Cochrane statistical package Review Manager. Where possible, we calculated weighted mean differences and 95% confidence intervals (CIs) for all continuous outcomes, and relative risks and 95% CIs for dichotomous outcomes, using a fixed‐effect model. We tested for heterogeneity across trials, and if found, performed a sensitivity analysis by repeating the calculation after omitting trials with low scores on individual quality items and repeated the analysis, using a random‐effects model if the heterogeneity remained.
Subgroup analysis and investigation of heterogeneity
If the data were available we compared the effect of interventions for the following subgroups:
Age: ≤16 years, >16 years.
Pes cavus aetiology: neuromuscular disease (Charcot‐Marie‐Tooth disease, myopathies such as Duchenne muscular dystrophy, neurologic conditions affecting the spinal cord such as congenitally tethered cord, conditions affecting the brain such as cerebral palsy), residua of congenital clubfoot, post‐traumatic, idiopathic.
Studies with different follow‐up periods were presented separately to assess the effect of follow‐up period and also combined with appropriate adjustments if the assumption of steady rates of change could be justified.
Results
Description of studies
The original search of possibly relevant references on each database were as follows: Cochrane Neuromuscular Disease Group Register: 7; MEDLINE: 389; EMBASE: 914; CINAHL: 102; AMED: 81, EBMReviews: 73; SPORTdiscuss: 18. Handsearches and contact with known experts in the field identified one unpublished study. Review authors JB and KL selected a total of eight full‐length possibly relevant studies and one unpublished study for evaluation. Three studies, published in detail in peer‐reviewed journals, were excluded because they were non‐randomised (Bellomo 1982; Bus 2004; Olmsted 2004) and three studies were excluded because they did not include participants with pes cavus (Bus 2004; Butler 2006; Simkin 1989) (see Characteristics of excluded studies).
When we repeated the searches in August 2010, one new reference was obtained from the Cochrane Neuromuscular Disease Group Register, 228 from MEDLINE, 58 from EMBASE, 140 from CINAHL and 24 from AMED. EBMReviews and SPORTdiscuss were not updated because they did not identify any studies in addition to those found in the original search. Hand searches and contact with known experts in the field identified one unpublished study. Review authors JB and KL selected a total of five full‐length possibly relevant studies and one unpublished study for evaluation. One published study was excluded because it was non‐randomised (Mubarak 2009) and four studies were excluded because they did not include participants with pes cavus (Butler 2005; Butler 2007; Molloy 2009; Knapik 2010) (see Characteristics of excluded studies).
Overall, five trials were identified by the authors as randomised controlled trials of interventions for the prevention or treatment of pes cavus (Burns 2006; Hertel 2005; Kavros 2005; Wegener 2008; Burns 2010) (see Characteristics of included studies). However, in one trial evaluating a pneumatic compression device (Aircast Airheel, Aircast, Inc., Summit New Jersey, USA) versus an off‐the shelf orthotic device (1st Step, Wrymark, Inc., St. Louis, USA) for the treatment of heel pain in participants with a range of foot types (normal, pes cavus and pes planus), data were not provided in the published report for the subgroup of seven participants with a cavus foot type (Kavros 2005). We contacted the author for additional data but he was unwilling to provide the information.
Only one trial fulfilled all selection criteria (Burns 2006). The trial evaluated the effect of custom‐made foot orthoses against sham orthoses on the level of chronic musculoskeletal foot pain three months from baseline in a community sample of 154 adults with pes cavus of mixed aetiology: 21 neuromuscular (16 Charcot‐Marie‐Tooth disease, four poliomyelitis, one polyneuropathy) and 133 idiopathic. This trial also evaluated efficacy in terms of functional change, biomechanical improvement (plantar pressure), health‐related quality of life and adverse events. The custom‐made foot orthoses were moulded from neutral‐suspension plaster casts of the feet, and the orthoses were fabricated from 3‐mm polypropylene using a computer aided design–computer‐aided manufacturing milling machine to a standardised prescription that had been previously developed and pilot tested (Burns 2006).
Whilst a further three trials were included (Hertel 2005; Wegener 2008; Burns 2010), they did not fulfil all selection criteria. The three trials assessed only secondary biomechanical outcomes. Two were performed with a cross‐over design with no follow‐up period (Hertel 2005; Wegener 2008), and one with a between‐legs parallel design with a 24‐month follow‐up period (Burns 2010). Hertel et al. investigated the effect of three off‐the‐shelf orthotic devices (medial posted, neutral and laterally posted; Superfeet footbed orthotics, Superfeet Worldwide LLC, Ferndale, Washington, USA) against no orthotic device on surface electromyography of the quadriceps (vastus medialis and lateralis) and gluteus medius during selected exercises (squat, step down, vertical jump) in 10 healthy young adults with pes cavus (Hertel 2005). Wegener et al. evaluated the effect of two commercially available running footwear models commonly recommended for the athlete with pes cavus: Asics Nimbus VI (Asics Oceania Pty Ltd, Sydney, Australia) and Brooks Glycerin 3 (Texas Peak Pty Ltd, Melbourne, Australia) against a control shoe (Dunlop Volley, Pacific Dunlop Ltd, Melbourne, Australia) on in‐shoe plantar pressure whilst running in 22 healthy adults (Wegener 2008). Burns et al. tested the safety and effectiveness of 6‐monthly intramuscular injections of botulinum toxin type‐A (Dysport, Ipsen Pty Ltd, Victoria, Australia) in the posterior tibialis and peroneus longus muscles on preventing progression of pes cavus in 10 children (20 legs ‐ see analysis 2.1‐2.8) aged 3 to 14 years with Charcot‐Marie‐Tooth disease type 1A (CMT1A) (Burns 2010). Each child had a randomly allocated treated and control leg and the trial was single blind. Primary outcome was radiographic alignment at 24‐months. Secondary outcomes were objective clinical measures of foot structure, ankle flexibility and strength.
Risk of bias in included studies
Details of the scoring for overall study quality can be found in the 'Risk of bias' tables. The review authors' judgments about each risk of bias item for included studies are shown in Figure 1.
1.
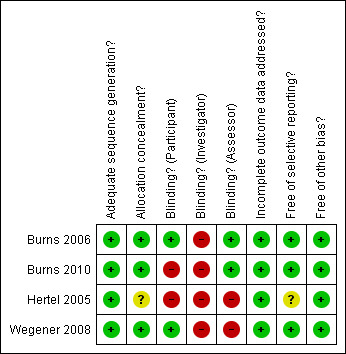
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
Due to the limited number of trials and the differences in the type of studies reported, data were not pooled.
Primary outcome measure
Change in the level of foot pain three months after intervention
Only one of the randomised trials (Burns 2006) investigated the treatment of pes cavus foot pain. This study of 154 participants over a follow‐up period of three months showed a significant reduction in the level of foot pain, measured using the validated 100‐point Foot Health Status Questionnaire (Bennett 1998), with custom‐made foot orthoses versus sham orthoses (WMD 10.90; 95% CI 3.21 to 18.59), (seeTable 1 and Analysis 1.1). No other randomised trials have investigated the treatment or prevention of foot pain in people with pes cavus.
1. Custom foot orthoses on pain, function, quality of life and plantar pressure.
| Outcome measures | No. of participants | Sham orthoses | Custom orthoses | Statistical method | Effect size |
| Change in foot pain at three months | 154 | 20.30 (22.70) | 31.20 (25.80) | WMD (fixed), 95% CI | 10.90 (3.21to 18.59) |
| Change in foot function at three months | 154 | 14.60 (20.60) | 25.60 (27.20) | WMD (fixed), 95% CI | 11.0 (3.35 to 18.65) |
| Change in physical function at three months | 154 | 2.60 (14.60) | 12.10 (19.30) | WMD (fixed), 95% CI | 9.50 (4.07 to 14.93) |
| Change in general health at three months | 154 | 3.00 (20.80) | 3.50 (18.40) | WMD (fixed), 95% CI | 0.50 (‐5.70 to 6.70) |
| Change in vitality at three months | 154 | 3.00 (15.20) | 8.50 (17.80) | WMD (fixed), 95% CI | 5.50 (0.26 to 10.74) |
| Change in social function at three months | 154 | 6.20 (16.20) | 8.70 (20.10) | WMD (fixed), 95% CI | 2.50 (‐3.28 to 8.28) |
| Change in pressure‐time integral (N.s/cm2, whole foot) at baseline | 154 | ‐1.60 (1.70) | ‐4.50 (2.70) | WMD (fixed), 95% CI | ‐2.90 (‐3.62 to ‐2.18) |
| Change in pressure‐time integral (N.s/cm2, rearfoot) at baseline | 154 | ‐0.70 (0.80) | ‐1.90 (1.40) | WMD (fixed), 95% CI | ‐1.20 (‐1.56 to ‐0.84) |
| Change in pressure‐time integral (N.s/cm2, midfoot) at baseline | 154 | ‐0.20 (0.60) | 0.30 (2.20) | WMD (fixed), 95% CI | 0.50 (‐0.02 to 1.02) |
| Change in pressure‐time integral (N.s/cm2, forefoot) at baseline | 154 | ‐1.40 (2.00) | ‐3.20 (2.90) | WMD (fixed), 95% CI | ‐1.80 (‐2.59to ‐1.01) |
| Adverse events at three months | 154 | 12/79 | 7/75 | RR (fixed), 95% CI | 0.61 (0.26 to 1.48) |
1.1. Analysis.
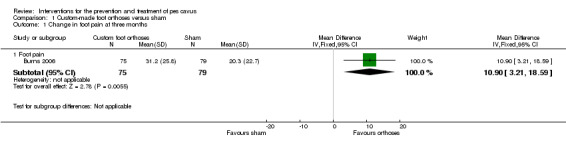
Comparison 1 Custom‐made foot orthoses versus sham, Outcome 1 Change in foot pain at three months.
Secondary outcomes measures
Functional improvement
Only one study (Burns 2006) investigated the effect of an intervention on functional improvement. In the trial of 154 participants evaluated over a follow‐up period of three months, a significant improvement in foot function was reported on the Foot Health Status Questionnaire with custom‐made foot orthoses compared to sham orthoses (WMD 11.00; 95% CI 3.35 to 18.65), (see Table 1). The foot function domain of the Foot Health Status Questionnaire asks questions about how much a patient’s feet: cause difficulties with work or activities; limit the kind of work; limit walking; limit climbing stairs, on a series of 5‐point scales. In the same trial physical functioning, measured with the 100‐point Medical Outcomes Short Form‐36 (SF‐36), was also shown to improve more with custom‐made foot orthoses compared to the sham orthoses (WMD 9.50; 95% CI 4.07 to 14.93), (see Table 1). The physical functioning domain of the SF‐36 asks questions about how much a patient’s health limits: vigorous activities, such as running; moderate activities, such as cleaning the house; lifting or carrying bags of shopping; climbing a steep hill; climbing one flight of stairs; getting up from a sitting position; walking more than a kilometre; walking one hundred meters; showering or dressing. No other trial measured functional improvement (for example activities of daily living, disability measures).
Biomechanical improvement
All four included trials reported biomechanical outcomes (Burns 2006; Hertel 2005; Wegener 2008; Burns 2010). One trial (Burns 2006) evaluating the pressure reducing/redistributing qualities of custom‐made foot orthoses versus sham reported a reduction of in‐shoe plantar pressure (pressure‐time integral, N.s/cm2) for the whole foot (WMD ‐2.90; 95% CI ‐3.62 to ‐2.18), rearfoot (WMD ‐1.20; 95% CI ‐1.56 to ‐0.84) and forefoot (WMD ‐1.80; 95% CI ‐2.59 to ‐1.01) (see Table 1). While the custom‐made foot orthoses increased midfoot plantar pressure compared to the sham, the change was not significant (WMD 0.50; 95% CI ‐0.02 to 1.02) (see Table 1).
In another trial (Wegener 2008) evaluating in‐shoe plantar pressure (peak pressure (kPa), pressure‐time integral (kPa.s) and force (% Body Weight), two commercial types of running footwear routinely recommended for athletes with pes cavus [Asics Nimbus VI (Asics Oceania Pty Ltd, Sydney, Australia) and Brooks Glycerin 3 (Texas Peak Pty Ltd, Melbourne, Australia)] were compared to a control footwear condition (Dunlop Volley, Pacific Dunlop Ltd, Melbourne, Australia) in 22 athletes running at least 20 kilometres per week. They showed that both types of running footwear significantly reduced peak plantar pressure (kPa) compared to a control shoe for all regions of the foot (whole foot, rearfoot, midfoot and forefoot) (see Table 2). Furthermore, the pressure‐time integral was reduced by both types of running footwear compared to the control for the whole and forefoot (see Table 2). However, force did not differ significantly between footwear conditions for any region of the foot (see Table 2).
2. Running footwear on plantar pressure.
| Outcome measure | No. of participants | 1. Control | 2. Asics Nimbus | 3. Brooks Glycerin | Statistical method | Effect size (1 versus 2) | Effect size (1 versus 3) | Effect size (2 versus 3) |
| Peak pressure (kPa, whole foot) | 22 | 513.4 (78.9) | 399.4 (88.6) | 361.2 (82.2) | WMD (Fixed), 95% CI | ‐114.00 (‐163.58 to ‐64.42) | ‐152.20 (‐199.81 to ‐104.59) | ‐38.20 (‐88.70 to 12.30) |
| Peak pressure (kPa, rearfoot) | 22 | 358.1 (173.8) | 240.9 (91.9) | 264.4 (90.5) | WMD (Fixed), 95% CI | ‐117.20 (‐199.35 to ‐35.05) | ‐93.70 (‐175.58 to ‐11.82) | 23.50 (‐30.40 to 77.40) |
| Peak pressure (kPa, midfoot) | 22 | 168.6 (68.1) | 126.3 (31.0) | 131.4 (34.4) | WMD (Fixed), 95% CI | ‐42.30 (‐73.57to ‐11.03) | ‐37.20 (‐69.08 to ‐5.32) | 5.10 (‐14.25to 24.45) |
| Peak pressure (kPa, forefoot) | 22 | 464.2 (106.4) | 386.1 (100.0) | 340.8 (89.4) | WMD (Fixed), 95% CI | ‐78.10 (‐139.12to ‐17.08) | ‐123.40 (‐181.47to ‐65.33) | ‐45.30 (‐101.35to 10.75) |
| Pressure time integral (kPa.s, whole foot) | 22 | 69.9 (12.4) | 55.6 (12.2) | 51.7 (9.7) | WMD (Fixed), 95% CI | ‐14.30 (‐21.57to ‐7.03) | ‐18.20 (‐24.78to ‐11.62) | ‐3.90 (‐10.41to 2.61) |
| Pressure time integral (kPa.s, rearfoot) | 22 | 19.8 (10.9) | 17.2 (6.9) | 18.8 (7.6) | WMD (Fixed), 95% CI | ‐2.60 (‐7.99 to 2.79) | ‐1.00 (‐6.55to 4.55) | 1.60 (‐2.69 to 5.89) |
| Pressure time integral (kPa.s, midfoot) | 22 | 15.3 (7.7) | 14.4 (3.9) | 14.8 (4.4) | WMD (Fixed), 95% CI | ‐0.90 (‐4.51to 2.71) | ‐0.50 (‐4.21to 3.21) | 0.40 (‐2.06 to 2.86) |
| Pressure time integral (kPa.s, forefoot) | 22 | 63.9 (13.2) | 50.3 (12.3) | 46.0 (9.6) | WMD (Fixed), 95% CI | ‐13.60 (‐21.14 to ‐6.06) | ‐17.90 (‐24.72 to ‐11.08) | ‐4.30 (‐10.82 to 2.22) |
| Force (%Body Weight, whole foot) | 22 | 226.2 (23.1) | 217.1 (20.4) | 219.4 (17.2) | WMD (Fixed), 95% CI | ‐9.10 (‐21.98 to 3.78) | ‐6.80 (‐18.83 to 5.23) | 2.30 (‐8.85to 13.45) |
| Force (%Body Weight, rearfoot) | 22 | 97.4 (43.3) | 90.3 (34.9) | 95.9 (30.3) | WMD (Fixed), 95% CI | ‐7.10 (‐30.34 to 16.14) | ‐1.50 (‐23.58 to 20.58) | 5.60 (‐13.71 to 24.91) |
| Force (%Body Weight, midfoot) | 22 | 25.6 (12.3) | 30.0 (7.0) | 28.6 (8.3) | WMD (Fixed), 95% CI | 4.40 (‐1.51 to 10.31) | 3.0 (‐3.20 to 9.20) | ‐1.40 (‐5.94 to 3.14) |
| Force (%Body Weight, forefoot) | 22 | 188.0 (21.5) | 176.4 (24.3) | 175.9 (20.6) | WMD (Fixed), 95% CI | ‐11.60 (‐25.16 to 1.96) | ‐12.10 (‐24.54 to 0.34) | ‐0.50 (‐13.81 to 12.81) |
In the third trial (Hertel 2005), the effect of three types of off‐the‐shelf foot orthoses (medial posted, neutral and laterally posted (Superfeet footbed orthotics, Superfeet Worldwide LLC, Ferndale, Washington, USA) compared to no orthotic condition on electromyography (percentage of maximum electromyographic activity (% Normalized iEMG)) of the vastus medialis, vastus lateralis and gluteus medialis was investigated in 10 healthy adults with pes cavus. Compared to the no orthotic condition, no significant changes in any of the electromyographic outcomes of muscle activity were found (seeTable 3).
3. Off‐the‐shelf foot orthoses on upper leg EMG during selected exercises.
| Outcome measure | No. of participants | 1. No orthoses | 2. Medial orthoses | 3. Neutral orthoses | 4. Lateral orthoses | Statistical method | Effect size (1 versus 2) | Effect size (1 versus 3) | Effect size (1 versus 4) |
| Vastus Medialis EMG during squat | 10 | 1.14 (0.98) | 1.19 (0.94) | 1.24 (1.15) | 1.22 (1.00) | WMD (fixed), 95% CI | 0.05 ‐0.79 to 0.89) | 0.10 (‐0.84 to 1.04) | 0.08 (‐0.79 to 0.95 |
| Vastus Medialis EMG during stepdown | 10 | 0.99 (0.67) | 1.33 (1.36) | 1.27 (1.23) | 1.42 (1.49) | WMD (fixed), 95% CI | 0.34 (‐0.60 to 1.28) | 0.28 (‐0.59 to 1.15) | 0.43 (‐0.58to 1.44) |
| Vastus Medialis EMG during vertical jump | 10 | 1.15 (0.54) | 1.32 (0.88) | 1.26 (0.70) | 1.28 (0.73) | WMD (fixed), 95% CI | 0.17 (‐0.47to 0.81) | 0.11 (‐0.44to 0.66) | 0.13 (‐0.43to 0.69) |
| Vastus Lateralis EMG during squat | 10 | 1.07 (0.63) | 0.95 (0.42) | 0.99 (0.47) | 0.97 (0.47) | WMD (fixed), 95% CI | ‐0.12 (‐0.59to 0.35) | ‐0.08 (‐0.57to 0.41) | ‐0.10 (‐0.59to 0.39) |
| Vastus Lateralis EMG during stepdown | 10 | 0.98 (0.56) | 1.08 (0.60) | 1.09 (0.65) | 1.13 (0.70) | WMD (fixed), 95% CI | 0.10 (‐0.41to 0.61) | 0.11 (‐0.42to 0.64) | 0.15 (‐0.41to 0.71) |
| Vastus Lateralis EMG during vertical jump | 10 | 1.31 (1.31) | 1.25 (0.62) | 1.27 (0.62) | 1.28 (0.54) | WMD (fixed), 95% CI | ‐0.06 (‐0.96to 0.84) | ‐0.04 (‐0.94 to 0.86) | ‐0.03 (‐0.91to 0.85) |
| Gluteus Medius EMG during squat | 10 | 0.66 (0.26) | 0.67 (0.24) | 0.69 (0.26) | 0.70 (0.30) | WMD (fixed), 95% CI | 0.01 (‐0.21to 0.23) | 0.03 (‐0.20 to 0.26) | 0.04 (‐0.21to 0.29) |
| Gluteus Medius EMG during stepdown | 10 | 0.62 (0.23) | 0.74 (0.39) | 0.72 (0.33) | 0.74 (0.44) | WMD (fixed), 95% CI | 0.12 (‐0.16 to 0.40) | 0.10 (‐0.15 to 0.35) | 0.12 (‐0.19to 0.43) |
| Gluteus Medius EMG during vertical jump | 10 | 0.90 (0.34) | 1.02 (0.41) | 0.96 (0.35) | 1.05 (0.45) | WMD (fixed), 95% CI | 0.12 (‐0.21to 0.45) | 0.06 (‐0.24to 0.36) | 0.15 (‐0.20to 0.50) |
In the fourth trial (Burns 2010) evaluating 24‐months of intramuscular injections of botulinum toxin for pes cavus, there was no effect on radiographic alignment, Foot Posture Index, ankle dorsiflexion range of motion or foot strength (seeTable 4).
4. Botulinum toxin type‐A on radiographic alignment, Foot Posture Index, ankle dorsiflexion range of motion, foot strength.
| Outcome Measure | No. of participants | Control Leg | BoNT‐A Leg | Statistical Method | Effect Size |
| Change of calcaneal‐first metatarsal angle | 10 | ‐3.2 (4.2) | ‐2.2 (3.3) | WMD (fixed), 95% CI | 1.00 (‐2.31 to 4.31) |
| Change of tibia‐calcaneal angle | 10 | ‐2.1 (3.7) | ‐1.0 (2.9) | WMD (fixed), 95% CI | 1.10 (‐1.81 to 4.01) |
| Change of Foot Posture Index | 10 | 1.3 (2.5) | ‐0.2 (2.4) | WMD (fixed), 95% CI | ‐1.50 ‐3.65 to 0.65) |
| Change of ankle dorsiflexion range of motion | 10 | 5.3 (7.4) | 3.1 (6.3) | WMD (fixed), 95% CI | ‐2.20 ‐8.22 to 3.82) |
| Change of dorsiflexion foot strength | 10 | 19.5 (22.7) | 19.9 (24.8) | WMD (fixed), 95% CI | 0.40 (‐20.44 to 21.24) |
| Change of plantarflexion foot strength | 10 | 50.6 (58.1) | 61.5 (59.9) | WMD (fixed), 95% CI | 10.90 (‐40.82 to 62.62) |
| Change of inversion foot strength | 10 | 28.8 (40.4) | 35.8 (35.2) | WMD (fixed), 95% CI | 7.00 (‐26.21 to 40.21) |
| Change of eversion foot strength | 10 | 31.0 (34.5) | 24.8 (26.0) | WMD (fixed), 95% CI | ‐6.20 (‐32.98 to 20.58) |
Health‐related quality of life
Only one trial (Burns 2006) investigated the effect of an intervention on the measurement of health‐related quality of life. In the prospective trial of 154 participants with foot pain followed over a period of three months, a significant improvement in vitality was reported on the Medical Outcomes Short Form‐36 (SF‐36) with custom‐made foot orthoses compared to the sham orthoses (WMD 5.50; 95% CI 0.26 to 10.74), (see Table 1). In the same trial there were no significant changes in general health (WMD 0.50; 95% CI ‐5.70 to 6.70) or social functioning (WMD 2.50; 95% CI ‐3.28 to 8.28) (see Table 1) between custom‐made foot orthoses and the sham orthoses. No other trials investigated health‐related quality of life in people with pes cavus.
Patient satisfaction
This was not measured in any of the trials included.
Adverse events of any treatment regimen
Minor adverse events such as additional foot pain, ankle instability and skin irritation were reported by one trial (Burns 2006). There was no difference in reported adverse events following the allocation of custom‐made foot orthoses (9%) or the sham orthoses (15 %) (RR 0.61; 95% CI 0.26 to 1.48), (see Table 1). Another trial (Burns 2010) reported more frequent trips and falls in two children during the 24‐month trial of intramuscular injections of botulinum toxin, consistent with the natural history of CMT1A. There were no serious adverse events (Burns 2010). No other studies reported adverse events.
Subgroup analysis
No trials reported data for participants under and over the age of 16 years. Data were available from the authors of one trial (Burns 2006) to perform a subgroup analysis of pes cavus aetiology for the primary outcome measure. In a subgroup of 16 participants with pes cavus as a result of Charcot‐Marie‐Tooth disease, custom‐made foot orthoses improved mean (SD) foot pain by 26.9 (35.9) points (using the Foot Health Status Questionnaire, 0 to 100 scale) compared to 14.0 (32.4) points with the sham orthoses. In 133 participants with idiopathic pes cavus, custom‐made foot orthoses improved mean foot pain by 32.0 (24.9) points versus 22.0 (21.1) points with the sham orthoses. In both subgroups, custom‐made foot orthoses were more effective than the sham intervention at improving chronic musculoskeletal foot pain. Insufficient data were available for the other aetiological subgroups (four poliomyelitis, one polyneuropathy).
Discussion
This systematic review was conducted and subsequently updated in order to evaluate the effectiveness of interventions for the treatment and prevention of pes cavus. Only one trial investigated the primary outcome and four trials investigated secondary biomechanical outcomes.
Primary outcome measure
Change in the level of foot pain three months after intervention
The one trial evaluating this outcome (Burns 2006), showed that custom‐made foot orthoses were more effective than sham orthoses at reducing foot pain in a community sample of 154 adults with pes cavus attributed to a variety of aetiologies (21 neuromuscular (16 Charcot‐Marie‐Tooth disease, 4 poliomyelitis, 1 polyneuropathy) and 133 idiopathic). Foot pain scores improved by 74% with custom‐made foot orthoses compared to 43% with the sham orthoses. From the authors' experience this difference was clinically worthwhile although it does not reflect how many participants completely recovered to 'normal' levels of foot pain. Resolution of foot pain to normal levels, i.e. a score of > 85 Foot Health Status Questionnaire points, was reported by 20 (27%) participants fitted with custom‐made foot orthoses and 12 (15%) with sham orthoses (RR 1.76; 95% CI 0.92 to 3.34).
The trial was adequately powered and methodological quality was high. There was high compliance in both groups (approximately 80%) and only one participant dropped out during the three months follow‐up (99% completion). However, the investigator was not blinded, which may have resulted in a risk of performance and detection (assessor) bias. In some trials, especially orthotic and footwear trials, double blinding is difficult or impossible. In this study the authors commented that blinding of the investigator was not appropriate because of the potential need for ongoing contact with the participants concerning adverse events. To reduce potential bias, many of the outcome measures were self‐reported (foot pain, functional improvement and health‐related quality of life) and the investigator was blinded to all data entry and processing by a research assistant not involved in participant contact. Nevertheless, these results are consistent with evidence from observational studies and expert opinion that custom‐made foot orthoses are an effective treatment for cavus‐related foot pain (Manoli 2005; Statler 2005).
Subgroup analysis by aetiology demonstrated a similar effect size for custom‐made foot orthoses for participants with pes cavus due to Charcot‐Marie‐Tooth disease and pes cavus aetiology regarded as idiopathic. Given that the cost was US$ 87 for the custom‐made foot orthoses and US$ 2 for the sham orthoses, for people presenting to the clinician with painful cavus feet, custom‐made foot orthoses may be a cost‐effective treatment option for chronic musculoskeletal foot pain. However, a cost‐benefit analysis would be required to confirm this assertion.
Secondary outcomes measures
Functional improvement
Only one trial (Burns 2006) investigated functional improvement and showed that custom‐made foot orthoses were more effective than sham orthoses at improving foot function and physical functioning in 154 adults with pes cavus attributed to a variety of aetiologies. Function scores improved by 45% with custom‐made foot orthoses compared to 24% with the control. As mentioned above, the trial was adequately powered and methodological quality was high, and although the chief investigator was not blinded to treatment allocation, functional improvement was self‐assessed. In trials in which key outcomes are self‐assessed e.g. Foot Health Status Questionnaire or Medical Outcomes Short Form‐36 (SF‐36), the assessor was considered to be blind if the participant was blind.
Biomechanical improvement
Painful conditions of the cavus foot and ankle are thought to be associated with abnormal pressure distribution on the plantar surface of the foot (Burns 2005a). Insights into the mechanism of custom‐made orthotic therapy were provided by the plantar pressure data in one trial (Burns 2006). Overall, the custom‐made foot orthoses reduced plantar pressure by 26% compared to a 9% reduction with the sham orthoses. Specifically, the custom‐made orthotic device was shown to increase pressure at the midfoot and decrease pressure at the rearfoot and forefoot, supporting preliminary hypotheses that the mechanism of pain relief is by reduction and redistribution of plantar pressure (Redmond 2000). A small reduction in pressure may also have explained the improvement of foot pain in the control group receiving sham orthoses.
Further insight into pressure reducing interventions was examined in another trial (Wegener 2008). The cross‐over trial investigated the effect of two popular commercially available running shoe models (Asics Nimbus VI and Brooks Glycerin 3), designed and recommended for athletes with cavus feet, on in‐shoe plantar pressure distribution. In 22 athletes with a cavus foot type, peak pressures were significantly different between shoe conditions for all foot regions (whole foot, rearfoot, midfoot, forefoot). Peak pressures were reduced in both the Asics Nimbus and Brooks Glycerin compared to the control shoe (Dunlop Volley). Similar patterns were observed for pressure‐time integrals, although force did not differ significant between footwear conditions. While the sample size was small, the trial was adequately powered and methodological quality was generally high. However, the investigator and the outcomes assessor were not blinded, which may have resulted in a risk of performance and detection bias respectively. Furthermore, an important methodological limitation was the cross‐over design with no follow‐up period. Nevertheless, these results are consistent with evidence from observational studies and expert opinion in other clinical populations, such as diabetes mellitus, that athletic footwear can modify and improve plantar pressure distribution (Hennig 1995; Perry 1995).
An evaluation of the effects of different types of off‐the‐shelf foot orthoses (medial posted, neutral and laterally posted) on electromyography was reported in one trial (Hertel 2005). No significant differences were reported for any of the electromyographic outcomes (vastus medialis, vastus lateralis, gluteus medius). This trial was small (N = 10), and methodological quality was low, with no blinding or concealed allocation. These results provide limited insight into the effect of off‐the‐shelf foot orthoses for pes cavus. Further research is needed to determine the effect of any intervention on muscular activity in people with a cavus foot deformity.
The trial evaluating a potential preventative intervention for pes cavus in children with CMT1A (Burns 2010) found no measurable effect of 24‐months of intramuscular injections of botulinum toxin in the tibialis posterior and peroneus longus muscles on radiographic alignment, or clinical measures of foot structure, ankle flexibility or muscle strength using highly reliable and validated age‐appropriate instruments. The leg injected with botulinum toxin for 24‐months had a less cavoid radiographic alignment by, on average approximately one degree, compared to the control leg. Such a small change has questionable clinical significance. This trial was small (N = 10 participants ‐ 20 legs. See Analysis 2.1 to Analysis 2.8), but methodological quality was high, with outcome assessor blinding and concealed allocation. This experimental trial provides some evidence that 24‐months of botulinum toxin injections in the posterior tibialis and peroneus longus is safe and well‐tolerated in children with CMT1A, but does not have any significant effect on the progression of the disease or foot deformity.
2.1. Analysis.
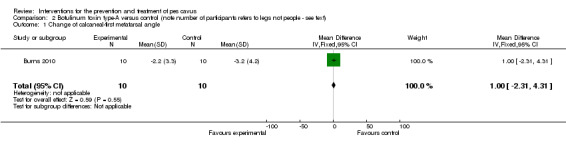
Comparison 2 Botulinum toxin type‐A versus control (note number of participants refers to legs not people ‐ see text), Outcome 1 Change of calcaneal‐first metatarsal angle.
2.8. Analysis.
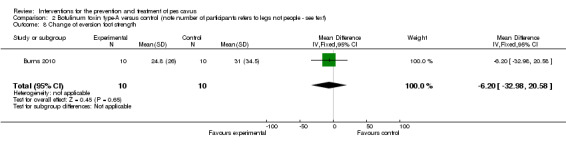
Comparison 2 Botulinum toxin type‐A versus control (note number of participants refers to legs not people ‐ see text), Outcome 8 Change of eversion foot strength.
Health‐related quality of life
Health‐related quality of life scores are becoming an important primary outcome measure for clinical trials of people with foot pain and disability. The only trial (Burns 2006) investigating health‐related quality of life measures showed that custom‐made foot orthoses were more effective than sham orthoses at improving vitality in 154 adults with pes cavus attributed to a variety of aetiologies. While health‐related quality of life data generally favoured the group receiving custom‐made foot orthoses, there was no evidence that general health or social functioning was improved by custom‐made foot orthoses in people with pes cavus.
Patient satisfaction
This was not measured in any of the trials included and should be considered in future trials as an important outcome measure to assist cost‐benefit analyses.
Adverse effects of any treatment regimen
One trial (Burns 2006) reported minor adverse events (additional foot pain, ankle instability and skin irritation) for both intervention groups: custom‐made foot orthoses (9%) and sham orthoses (15%). However, there was no significant difference in reported adverse events following either custom‐made foot orthoses or the sham intervention during the three‐month follow‐up. Another trial (Burns 2010) reported more frequent trips and falls in two children during the 24‐month trial of intramuscular injections of botulinum toxin, consistent with the natural history of CMT1A. No other trial reported any adverse effects.
Authors' conclusions
Implications for practice.
In this updated review we show that custom‐made foot orthoses are significantly more beneficial than sham orthoses for treating chronic musculoskeletal foot pain associated with pes cavus in a variety of clinical populations. We also show that some secondary biomechanical outcomes, such as plantar pressure, improve with custom‐made foot orthoses and footwear, but that foot alignment, flexibility and strength do not improve with botulinum toxin, and that upper leg electromyography does not improve with off‐the‐shelf foot orthoses. There is an absence of evidence for any other type of intervention for the treatment or prevention of foot pain in people with pes cavus.
Implications for research.
Given the high prevalence of cavus‐related foot pain and disability, more well‐designed trials are needed to determine the efficacy of other therapies for patients with painful pes cavus. Common treatments of foot pain and disability requiring scientific evaluation are footwear; joint range of motion exercises; muscle stretching and strengthening; electrotherapeutic modalities; debridement of plantar callosities, medications and soft‐tissue/bony surgical procedures.
What's new
| Date | Event | Description |
|---|---|---|
| 19 October 2010 | New search has been performed | Searches updated to August 2010. One new study included. Minor change to conclusions. |
History
Protocol first published: Issue 3, 2006 Review first published: Issue 4, 2007
| Date | Event | Description |
|---|---|---|
| 20 May 2008 | Amended | Converted to new review format. |
| 21 August 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors are grateful for the assistance of Professor Richard Hughes, Kate Jewitt, Janice Fernandes and Tony Swan of the Cochrane Neuromuscular Disease Group. Editorial support from the Cochrane Neuromuscular Disease Group was funded by the TREAT NMD European Union Grant 036825.
Appendices
Appendix 1. MEDLINE (OvidSP) search strategy
1 randomized controlled trial.pt. 2 controlled clinical trial.pt. 3 randomized.ab. 4 placebo.ab. 5 drug therapy.fs. 6 randomly.ab. 7 trial.ab. 8 groups.ab. 9 or/1‐8 10 (animals not (animals and humans)).sh. 11 9 not 10 12 Foot Deformities/ 13 cavus.mp. 14 cavovarus.mp. 15 supinated.mp. 16 high arch$.mp. 17 foot deformit$.tw. 18 foot malformation$.tw. 19 or/12‐18 (2996) 20 11 and 19 (263)
Appendix 2. EMBASE (OvidSP) search strategy
1 crossover‐procedure/ 2 double‐blind procedure/ 3 randomized controlled trial/ 4 single‐blind procedure/ 5 (random$ or factorial$ or crossover$ or cross over$ or cross‐over$ or placebo$ or (doubl$ adj blind$) or (singl$ adj blind$) or assign$ or allocat$ or volunteer$).tw. 6 or/1‐5 7 human/ 8 6 and 7 9 nonhuman/ or human/ 10 6 not 9 11 8 or 10 12 Foot Deformities/ 13 cavus.mp. 14 cavovarus.mp. 15 supinated.mp. 16 high arch$.mp. 17 foot deformit$.mp. 18 foot malformation$.mp. 19 or/12‐18 20 11 and 19
Appendix 3. AMED (OvedSP) search strategy
1 Randomized controlled trials/ 2 Random allocation/ 3 Double blind method/ 4 Single‐Blind Method/ 5 exp Clinical Trials/ 6 (clin$ adj25 trial$).tw. 7 ((singl$ or doubl$ or treb$ or trip$) adj25 (blind$ or mask$ or dummy)).tw. 8 placebos/ 9 placebo$.tw. 10 random$.tw. 11 research design/ 12 Prospective Studies/ 13 meta analysis/ 14 (meta?analys$ or systematic review$).tw. 15 control$.tw. 16 (multicenter or multicentre).tw. 17 ((study or studies or design$) adj25 (factorial or prospective or intervention or crossover or cross‐over or quasi‐experiment$)).tw. 18 or/1‐17 19 Foot Deformities/ 20 cavus.mp. 21 cavovarus.mp. 22 supinated.mp. 23 high arch$.mp. 24 foot deformit$.mp. 25 foot malformation$.mp. 26 or/19‐25 27 18 and 26
Appendix 4. CINAHL (EBSCOhost) search strategy
S26 S18 and S25 S25 S19 or S20 or S21 or S22 or S23 or S24 S24 high arch* S23 supinated S22 cavovarus S21 cavus S20 foot deformit* or foor malformation* S19 (MH "Foot Deformities") S18 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 S17 ABAB design* S16 TI random* or AB random* S15 ( TI (cross?over or placebo* or control* or factorial or sham? or dummy) ) or ( AB (cross?over or placebo* or control* or factorial or sham? or dummy) ) S14 ( TI (clin* or intervention* or compar* or experiment* or preventive or therapeutic) or AB (clin* or intervention* or compar* or experiment* or preventive or therapeutic) ) and ( TI (trial*) or AB (trial*) ) S13 ( TI (meta?analys* or systematic review*) ) or ( AB (meta?analys* or systematic review*) ) S12 ( TI (single* or doubl* or tripl* or trebl*) or AB (single* or doubl* or tripl* or trebl*) ) and ( TI (blind* or mask*) or AB (blind* or mask*) ) S11 PT ("clinical trial" or "systematic review") S10 (MH "Factorial Design") S9 (MH "Concurrent Prospective Studies") or (MH "Prospective Studies") S8 (MH "Meta Analysis") S7 (MH "Solomon Four‐Group Design") or (MH "Static Group Comparison") S6 (MH "Quasi‐Experimental Studies") S5 (MH "Placebos") S4 (MH "Double‐Blind Studies") or (MH "Triple‐Blind Studies") S3 (MH "Clinical Trials+") S2 (MH "Crossover Design") S1 (MH "Random Assignment") or (MH "Random Sample") or (MH "Simple Random Sample") or (MH "Stratified Random Sample") or (MH "Systematic Random Sample")
Data and analyses
Comparison 1. Custom‐made foot orthoses versus sham.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in foot pain at three months | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Foot pain | 1 | 154 | Mean Difference (IV, Fixed, 95% CI) | 10.90 [3.21, 18.59] |
Comparison 2. Botulinum toxin type‐A versus control (note number of participants refers to legs not people ‐ see text).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change of calcaneal‐first metatarsal angle | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐2.31, 4.31] |
| 2 Change of tibia‐calcaneal angle | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 1.1 [‐1.81, 4.01] |
| 3 Change of Foot Posture Index | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐3.65, 0.65] |
| 4 Change of ankle dorsiflexion range of motion | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐8.22, 3.82] |
| 5 Change of dorsiflexion foot strength | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐20.44, 21.24] |
| 6 Change of plantarflexion foot strength | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 10.90 [‐40.82, 62.62] |
| 7 Change of inversion foot strength | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 7.00 [‐26.21, 40.21] |
| 8 Change of eversion foot strength | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐6.20 [‐32.98, 20.58] |
2.2. Analysis.
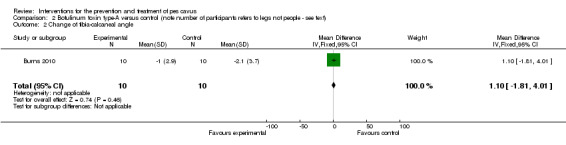
Comparison 2 Botulinum toxin type‐A versus control (note number of participants refers to legs not people ‐ see text), Outcome 2 Change of tibia‐calcaneal angle.
2.3. Analysis.
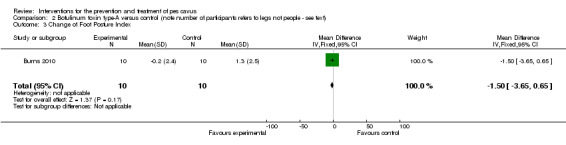
Comparison 2 Botulinum toxin type‐A versus control (note number of participants refers to legs not people ‐ see text), Outcome 3 Change of Foot Posture Index.
2.4. Analysis.
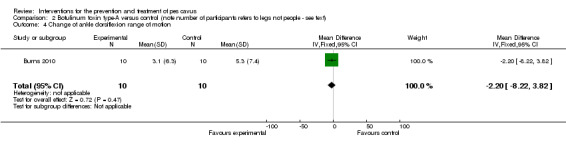
Comparison 2 Botulinum toxin type‐A versus control (note number of participants refers to legs not people ‐ see text), Outcome 4 Change of ankle dorsiflexion range of motion.
2.5. Analysis.
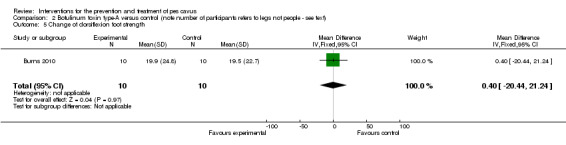
Comparison 2 Botulinum toxin type‐A versus control (note number of participants refers to legs not people ‐ see text), Outcome 5 Change of dorsiflexion foot strength.
2.6. Analysis.
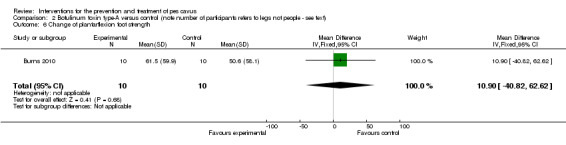
Comparison 2 Botulinum toxin type‐A versus control (note number of participants refers to legs not people ‐ see text), Outcome 6 Change of plantarflexion foot strength.
2.7. Analysis.
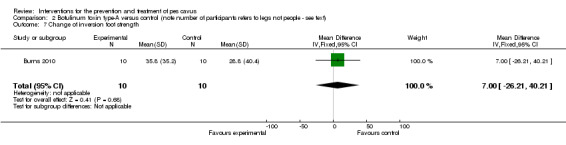
Comparison 2 Botulinum toxin type‐A versus control (note number of participants refers to legs not people ‐ see text), Outcome 7 Change of inversion foot strength.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Burns 2006.
| Methods | Prospective randomized, single‐blind, sham controlled trial. | |
| Participants | 154 adults with chronic foot pain. Pes cavus was defined by a Foot Posture Index score of –2 or less, which is 2 standard deviations below the reported normal mean of +5 (Redmond 2006). | |
| Interventions | Custom‐made foot orthoses versus sham orthoses. | |
| Outcomes | Foot pain, functional limitation, health‐related quality of life, in‐shoe plantar pressure at 3 months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Using a computer random number generator |
| Allocation concealment? | Low risk | Central allocation by telephone by a third party not involved in the study |
| Blinding? Participant | Low risk | Participants were blinded to treatment allocation for the duration of the study |
| Blinding? Investigator | High risk | Blinding of the investigator was not appropriate because of the potential need for ongoing contact with the participants concerning adverse effects |
| Blinding? Assessor | Low risk | No blinding, but primary outcome measure was self‐reported and not likely to be influenced by lack of blinding |
| Incomplete outcome data addressed? All outcomes | Low risk | Only one missing outcome data |
| Free of selective reporting? | Low risk | Protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
| Free of other bias? | Low risk | The study appears to be free of other sources of bias. Explicit inclusion criteria, no baseline differences |
Burns 2010.
| Methods | Prospective randomized, single‐blind, experimental trial. | |
| Participants | 10 children aged 3 to 14 years with Charcot‐Marie‐Tooth disease type 1A. Pes cavus was defined weight bearing by a Foot Posture Index score between –12 and –1 (Redmond 2006) | |
| Interventions | One leg received intramuscular injections at six‐monthly intervals of botulinum toxin type‐A in the posterior tibialis and peroneus longus muscles, and one leg received no intervention. | |
| Outcomes | Primary outcome was radiographic alignment at 24‐months. Secondary outcomes were objective clinical measures of foot structure, ankle flexibility and strength. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | A simple randomization sequence was determined by coin‐toss |
| Allocation concealment? | Low risk | Central allocation by Paediatric Neurologist not involved in recruitment |
| Blinding? Participant | High risk | Each child/parent was aware of the leg selection for treatment |
| Blinding? Investigator | High risk | The injecting physician was aware of the leg selection for treatment |
| Blinding? Assessor | Low risk | The principal investigator conducting all outcome measures was blinded to the injected leg |
| Incomplete outcome data addressed? All outcomes | Low risk | No missing data |
| Free of selective reporting? | Low risk | Protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
| Free of other bias? | Low risk | The study appears to be free of other sources of bias. Explicit inclusion criteria, no baseline differences |
Hertel 2005.
| Methods | Experimental, randomized cross‐over trial. | |
| Participants | 10 healthy young adults. Cavus feet were subjectively categorised as those having a high medial longitudinal arch accompanied by either excessive rearfoot varus and/or excessive forefoot valgus. | |
| Interventions | Three off‐the‐shelf orthotic combinations (neutral post, medial post, lateral post) versus no orthotic device. | |
| Outcomes | Electromyography of 3 muscles (vastus medialis, vastus lateralis and gluteus medius) during 3 activities (squat, step down and vertical jump). | |
| Notes | No follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Latin square design |
| Allocation concealment? | Unclear risk | Insufficient information to permit judgement |
| Blinding? Participant | High risk | No blinding |
| Blinding? Investigator | High risk | No blinding |
| Blinding? Assessor | High risk | No blinding |
| Incomplete outcome data addressed? All outcomes | Low risk | No missing data |
| Free of selective reporting? | Unclear risk | Insufficient information to permit judgement |
| Free of other bias? | Low risk | The study appears to be free of other sources of bias. Explicit inclusion criteria, no baseline differences |
Wegener 2008.
| Methods | Experimental, randomized, single‐blind, cross‐over trial. | |
| Participants | 22 healthy athletic adults. Pes cavus was defined weight bearing by a Foot Posture Index score between –12 and –1 (Redmond 2006) | |
| Interventions | Two common off‐the‐shelf running footwear models versus a control footwear. | |
| Outcomes | In‐shoe plantar pressure during running. | |
| Notes | No follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Using a computer random number generator |
| Allocation concealment? | Low risk | Allocation by opaque envelopes |
| Blinding? Participant | Low risk | Participants were blinded to the manufacturer and model of each shoe condition to reduce preference bias |
| Blinding? Investigator | High risk | The chief investigator who recruited and assessed the participants was not blinded, to enable footwear fitting and data collection |
| Blinding? Assessor | High risk | The chief investigator who recruited and assessed the participants was not blinded, to enable footwear fitting and data collection |
| Incomplete outcome data addressed? All outcomes | Low risk | No missing data |
| Free of selective reporting? | Low risk | Study protocol is not available but it is clear that the published report (and thesis) include all expected outcomes |
| Free of other bias? | Low risk | The study appears to be free of other sources of bias. Explicit inclusion criteria, no baseline differences |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bellomo 1982 | Not a randomized controlled trial. |
| Bus 2004 | Not a randomized controlled trial and does not include pes cavus. |
| Butler 2005 | Does not include participants with pes cavus |
| Butler 2006 | Does not include participants with pes cavus |
| Butler 2007 | Does not include participants with pes cavus |
| Kavros 2005 | Pes cavus subgroup data (N = 7) not published. Author unwilling to provide additional data. |
| Knapik 2010 | Does not include participants with pes cavus |
| Molloy 2009 | Does not include participants with pes cavus |
| Mubarak 2009 | Not a randomized controlled trial. |
| Olmsted 2004 | Not a randomized controlled trial. |
| Simkin 1989 | Excludes participants with pes cavus. |
Differences between protocol and review
One new trial of botulinum toxin was discovered in the new search in August 2010.
Contributions of authors
J. Burns wrote the first draft. All authors agreed on the final text.
Sources of support
Internal sources
Faculty of Health Sciences, The University of Sydney, Australia.
Department of Podiatry, La Trobe University, Melbourne, Australia.
Institute for Neuroscience & Muscle Research, The Children's Hospital at Westmead, Sydney, Australia.
External sources
No sources of support supplied
Declarations of interest
J. Burns, J. Crosbie and R. Ouvrier have been involved in studies that may be eligible for consideration in this review.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Burns 2006 {published data only}
- Burns J, Crosbie J, Ouvrier R, Hunt A. Effective orthotic therapy for the painful cavus foot: a randomized controlled trial. Journal of the American Podiatric Medical Association 2006;96(3):205‐11. [DOI] [PubMed] [Google Scholar]
Burns 2010 {published and unpublished data}
- Burns J, Scheinberg A, Ryan MM, Rose KJ, Ouvrier RA. Randomized trial of botulinum toxin to prevent pes cavus progression in pediatric CMT1A. Muscle & Nerve 2010;42(2):262‐7. [DOI] [PubMed] [Google Scholar]
Hertel 2005 {published and unpublished data}
- Hertel J, Sloss BR, Earl JE. Effect of foot orthotics on quadriceps and gluteus medius electromyographic activity during selected exercises. Archives of Physical Medicine and Rehabilitation 2005;86(1):26‐30. [DOI] [PubMed] [Google Scholar]
Wegener 2008 {published and unpublished data}
- Wegener C, Burns J, Penkala S. Effect of neutral‐cushioned running shoes on plantar pressure loading and comfort in athletes with cavus feet: a crossover randomized controlled trial. American Journal of Sports Medicine 2008;36(11):2139‐46. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bellomo 1982 {published data only}
- Bellomo F, Carannante G, Negretto R. Treatment of external pes cavus with long‐acting muscular stimulants. Minerva Ortopedica 1982;33(11):1107‐9. [Google Scholar]
Bus 2004 {published data only}
- Bus SA, Ulbrecht JS, Cavanagh PR. Pressure relief and load redistribution by custom‐made insoles in diabetic patients with neuropathy and foot deformity. Clinical Biomechanics 2004;19(6):629‐38. [DOI] [PubMed] [Google Scholar]
Butler 2005 {unpublished data only}
- Butler RJ. Interaction of arch type and footwear on running mechanics. Interaction of arch type and footwear on running mechanics PhD Thesis. Delaware: University of Delaware, 2005. [Google Scholar]
Butler 2006 {published data only}
- Butler RJ, Davis IS, Hamill J. Interaction of arch type and footwear on running mechanics. American Journal of Sports Medicine 2006;Dec; 34(12):1998‐2005. [DOI] [PubMed] [Google Scholar]
Butler 2007 {published data only}
- Butler RJ, Hamill J, Davis I. Effect of footwear on high and low arched runners' mechanics during a prolonged run. Gait & Posture 2007;26(2):219‐25. [DOI] [PubMed] [Google Scholar]
Kavros 2005 {published data only}
- Kavros SJ. The efficacy of a pneumatic compression device in the treatment of plantar fasciitis. Journal of Applied Biomechanics 2005;21(4):404‐13. [DOI] [PubMed] [Google Scholar]
Knapik 2010 {published data only}
- Knapik JJ, Brosch LC, Venuto M, Swedler DI, Bullock SH, Gaines LS, et al. Effect on injuries of assigning shoes based on foot shape in air force basic training. American Journal of Preventive Medicine 2010;38(1 Suppl):S197‐211. [DOI] [PubMed] [Google Scholar]
Molloy 2009 {published data only}
- Molloy JM, Christie DS, Teyhen DS, Yeykal NS, Tragord BS, Neal MS, et al. Effect of running shoe type on the distribution and magnitude of plantar pressures in individuals with low‐ or high‐arched feet. Journal of the American Podiatric Medical Association 2009;99(4):330‐8. [DOI] [PubMed] [Google Scholar]
Mubarak 2009 {published data only}
- Mubarak SJ, Valin SE. Osteotomies of the foot for cavus deformities in children. Journal of PediatricOrthopaedics 2009;29(3):294‐9. [DOI] [PubMed] [Google Scholar]
Olmsted 2004 {published data only}
- Olmsted LC, Hertel J. Influence of foot type and orthotics on static and dynamic control. Journal of Sports Rehabilitation 2004;13:54‐6. [Google Scholar]
Simkin 1989 {published data only}
- Simkin A, Leichter I, Giladi M, Stein M, Milgrom C. Combined effect of foot arch structure and an orthotic device on stress fractures. Foot and Ankle International 1989;10(1):25‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Aktas 2000
- Aktas S, Sussman MD. The radiological analysis of pes cavus deformity in Charcot Marie Tooth disease. Journal of Pediatric Orthopedics 2000;9(2):137‐40. [DOI] [PubMed] [Google Scholar]
Badlissi 2005
- Badlissi F, Dunn JE, Link CL, Keysor JJ, McKinlay JB, Felson DT. Foot musculoskeletal disorders, pain, and foot‐related functional limitation in older persons. Journal of American Geriatric Society 2005;53(6):1029‐33. [DOI] [PubMed] [Google Scholar]
Barrie 2001
- Barrie JL, McLoughlin C, Robem N, Nuttal R, Lishman J, Wardle F, et al. Ankle instability in pes cavus. Journal of Bone and Joint Surgery (British Volume) 2001;83‐B(Suppl 3):339. [Google Scholar]
Bennett 1998
- Bennett PJ, Patterson C, Wearing S, Baglioni T. Development and validation of a questionnaire designed to measure foot‐health status. Journal of the American Podiatric Association 1998;88(9):419‐28. [DOI] [PubMed] [Google Scholar]
Burns 2005a
- Burns J, Crosbie J, Hunt A, Ouvrier R. The effect of pes cavus on foot pain and plantar pressure. Clinical Biomechanics 2005;20(9):877‐82. [DOI] [PubMed] [Google Scholar]
Burns 2005b
- Burns J, Redmond A, Crosbie J, Ouvrier R. Quantification of muscle strength and imbalance in neurogenic pes cavus, compared to health controls, using hand‐held dynamometry. Foot and Ankle International 2005;26(7):540‐4. [DOI] [PubMed] [Google Scholar]
Carroll 1999
- Carroll KL, Shea KG, Stevens PM. Posttraumatic cavovarus deformity of the foot. Journal of Pediatric Orthopedics 1999;19(1):39‐41. [PubMed] [Google Scholar]
Dahle 1991
- Dahle LK, Mueller M, Delitto A, Diamond JE. Visual assessment of foot type and relationship of foot type to lower extremity injury. Journal of Orthopaedic and Sports Physical Therapy 1991;14(2):70‐4. [DOI] [PubMed] [Google Scholar]
Dickson 1939
- Dickson FD, Diveley RL. Functional disorders of the foot. Second Edition. Philadelphia: J.B. Lippincott Company, 1939. [Google Scholar]
Fortin 2002
- Fortin PT, Guettler J, Manoli A. Idiopathic cavovarus and lateral ankle instability: recognition and treatment implications relating to ankle arthritis. Foot and Ankle International 2002;23(11):1031‐7. [DOI] [PubMed] [Google Scholar]
Giannini 2002
- Giannini S, Ceccarelli F, Benedetti MG, Faldini C, Grandi G. Surgical treatment of adult idiopathic cavus foot with plantar fasciotomy, naviculocuneiform arthrodesis, and cuboid osteotomy. A review of thirty‐nine cases. Journal of Bone and Joint Surgery (American Volume) 2002;84‐A(Suppl 2):62‐9. [DOI] [PubMed] [Google Scholar]
Helliwell 1995
- Helliwell TR, Tynan M, Hayward M, Klenerman L, Whitehouse G, Edwards RH. The pathology of the lower leg muscles in pure forefoot pes cavus. Acta Neuropathologica 1995;89(6):552‐9. [DOI] [PubMed] [Google Scholar]
Hennig 1995
- Hennig EM, Milani TL. In‐shoe pressure distribution for running in various types of footwear. Journal of Applied Biomechanics 1995;11(3):299‐310. [Google Scholar]
Horne 1984
- Horne G. Pes cavovarus following ankle fracture. A case report. Clinical Orthopaedics and Related Research 1984;184:249‐50. [PubMed] [Google Scholar]
Korpelainen 2001
- Korpelainen R, Orava S, Karpakka J, Siira P, Hulkko A. Risk factors for recurrent stress fractures in athletes. American Journal of Sports Medicine 2001;29(3):304‐10. [DOI] [PubMed] [Google Scholar]
Manoli 2005
- Manoli A 2nd, Graham B. The subtle cavus foot, "the underpronator," a review. Foot and Ankle International 2005;26(3):256‐63. [DOI] [PubMed] [Google Scholar]
Pandey 2002
- Pandey S. Neglected clubfoot. The Foot 2002;12(3):123‐41. [Google Scholar]
Perry 1995
- Perry JE, Ulbrecht JS, Derr JA, Cavanagh PR. The use of running shoes to reduce plantar pressures in patients who have diabetes. The Journal of Bone and Joint Surgery 1995;77(12):1819‐28. [DOI] [PubMed] [Google Scholar]
Redmond 2000
- Redmond A, Lumb PS, Landorf K. Effect of cast and noncast foot orthoses on plantar pressure and force during normal gait. Journal of the American Podiatric Medical Association 2000;90(9):441‐9. [DOI] [PubMed] [Google Scholar]
Redmond 2006
- Redmond AC, Crosbie J, Ouvrier RA. Development and validation of a novel rating system for scoring standing foot posture: the FootPosture Index. Clinical Biomechechanics 2006;21:89‐98. [DOI] [PubMed] [Google Scholar]
Reilly 2006
- Reilly KA, Barker KL, Shamley D, Sandall S. Influence of foot characteristics on the site of lower limb osteoarthritis. Foot and Ankle International 2006;27(3):206‐11. [DOI] [PubMed] [Google Scholar]
Sachithanandam 1995
- Sachithanandam V, Joseph B. The influence of footwear on the prevalence of flat foot. A survey of 1846 skeletally mature persons. Journal of Bone and Joint Surgery (British Volume) 1995;77(2):254‐7. [PubMed] [Google Scholar]
Statler 2005
- Statler TK, Tullis BL. Pes cavus. Journal of the American Podiatric Medical Association 2005;95(1):42‐52. [DOI] [PubMed] [Google Scholar]
Williams 2001
- Williams DS, 3rd, McClay IS, Hamill J. Arch structure and injury patterns in runners. Clinical Biomechanics 2001;16(4):341‐7. [DOI] [PubMed] [Google Scholar]
Wines 2005
- Wines AP, Chen D, Lynch B, Stephens MM. Foot deformities in children with hereditary motor and sensory neuropathy. Journal of Pediatric Orthopedics 2005;25(2):241‐4. [DOI] [PubMed] [Google Scholar]


