Significance
The CD4+ Treg response following acute Listeria infection is heterogeneous and deploys two distinct modes of suppression coinciding with initial pathogen exposure and resolution of infection. This bimodal suppression of CD8+ T cells during priming and contraction is mediated by separate Treg lineages. These findings make a significant contribution to our understanding of the functional plasticity inherent within Tregs, which allows these cells to serve as a sensitive and dynamic cellular rheostat for the immune system to prevent autoimmune pathology in the face of inflammation attendant to acute infection, enable expansion of the pathogen-specific response needed to control the infection, and reestablish immune homeostasis after the threat has been contained.
Keywords: FoxP3+ T regulatory cell, CD73, gap junction, cyclic AMP, Listeria monocytogenes
Abstract
CD4+ regulatory T cells (Tregs) must prevent immunopathology by cytotoxic CD8+ T lymphocytes (CTLs) responding to acute infection and restore immune homeostasis following pathogen clearance, yet little is known about the specific populations or mechanisms governing these discrete events. We found that acute Listeria monocytogenes (L. monocytogenes) infection produces a phenotypically and functionally complex Treg response comprising two separate suppressor cell subpopulations, with an early Treg peak occurring at 24 h postinfection and a later peak arising by day 7. The first wave of Tregs suppress primary CTL expansion via a contact-independent mechanism involving CD73-derived adenosine (Ado) production from extracellular adenosine monophosphate (5′-AMP), while the second originates from different precursors and acts throughout the contraction phase via contact-dependent gap junction transfer of 3′,5′-cyclic adenosine monophosphate (cAMP)—both potent inhibitors of T cell proliferation. We speculate that the early activation of CD73 on Tregs is enhanced in inflamed tissues due to high purine release from apoptotic cells, whereas late-phase gap junction–dependent Tregs rely more on cell number and less on tissue inflammation. This study importantly reveals that CTL priming and contraction phases are separately fine-tuned by developmentally distinct Treg lineages during an acute infection.
Tregs comprise a heterogeneous T cell population within the CD4+ compartment based on their organ/tissue niche occupancy, T cell receptor (TCR) repertoire, transcription factor profile, surface marker display, and mechanism of suppression (1–3). Natural CD4+ regulatory T cells (nTregs) develop in the thymus, coexpress the α-chain of the high-affinity IL-2 receptor (CD25) and the X-linked transcription factor forkhead box P3 (FoxP3), and are required for the maintenance of immune tolerance to self-antigens during homeostasis (4). Functional loss of Foxp3 has been shown to underlie a range of lymphoproliferative and autoimmune disorders in both mice and humans (5–8). FoxP3 expression can also be induced in conventional FoxP3−CD4+ T cells (Tconvs) under inflammatory or noninflammatory conditions, resulting in the generation of a second type of Treg called peripheral CD4+ regulatory T cells (pTregs) (9, 10).
In addition to a documented role in controlling self-reactivity, Tregs can also influence the magnitude and severity of acute and chronic infections through suppression of pathogen-specific Tconvs and CTLs (11, 12). Accordingly, clearance of microbial pathogens is promoted by depletion of nTreg and pTreg populations and/or inhibition of single suppressor pathways, albeit with an increased risk of autoimmune side effects (13). The specific effector mechanisms underlying Treg-mediated control in such cases appear to vary with different infections and distinct phases of the immune response. For example, IL-2 deprivation can alone inhibit CD4+ and CD8+ T cell responses during autoimmunity and Toxoplasma gondii, L. monocytogenes, and vaccinia virus infections (14, 15). During hepatitis C virus (HCV) infection, Treg suppression has been shown to be mainly dependent on TGF-β production and cell contact (16, 17). Other pathways are more controversial and context specific. IL-10 has been shown to contribute to Treg-mediated suppression of CD8+ T cells during L. monocytogenes infection of pregnant hosts (18). However, throughout acute lymphocytic choriomeningitis virus (LCMV Armstrong) infection, FoxP3+ Treg-derived IL-10 was shown conversely to “insulate” CD8+ T cells from proinflammatory signals in the primary response, indirectly promoting memory differentiation of these cells (19). There also appears to be both quantitative and temporal control of Tregs in acute L. monocytogenes infection, as the degree to which they inhibit protective CTLs depends on the initial infectious dose and associated early inflammatory cues (20), where the developing infection eventually overrides early baseline Treg function to allow for the rapid clonal expansion of CTLs 72 h after L. monocytogenes exposure (14, 21). There are currently significant gaps in our understanding of the quantitative and functional features of Treg development during priming/expansion versus contraction phases of an acute infection, although such information could meaningfully inform our understanding of immune protection versus pathology in this setting.
Prior studies suggest that the Treg response to acute infection may involve distinct, and perhaps contextual, phases of immunoregulatory function. We hypothesize that, in contrast to the stable population dynamics associated with chronic viral infection, the priming, expansion, and contraction of Tregs, Tconvs, and CTLs are coordinately regulated throughout the primary response to acute infection to prevent immune pathology and facilitate pathogen control. In this study, we have applied a variety of phenotypic, genetic, and functional tools to follow the dynamic evolution of the Treg response during acute L. monocytogenes infection and monitor its effect on pathogen-specific CTLs. Our results demonstrate that the CD8+ T cell response is regulated by developmentally distinct Treg populations that employ different suppressor mechanisms across different phases of the immune response against a single acute infection.
Results
Functional and Phenotypic Evolution of the Treg Response to Acute Listeria Infection.
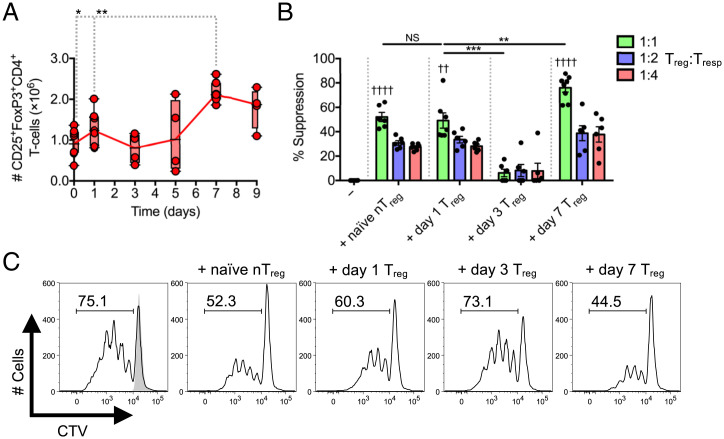
Intravenous (IV) infection of C57BL/6 hosts with L. monocytogenes ΔactA-Ova (an attenuated L. monocytogenes strain deleted for the actin assembly–inducing protein and expressing chicken ovalbumin) produces a distinct Treg response in terms of magnitude and kinetics, depending on the initial inoculum size (20). Acute infection with 1 × 106 colony-forming units (CFU) causes a rapid rise in the absolute number of Tregs measured at 24 h and the subsequent appearance of a second Treg peak at day 7 (Fig. 1A) (20). To evaluate the relative suppressive capacity of day 1 versus day 7 Tregs, we used a FoxP3-eGFP reporter system that allows for isolation of Tregs (22). CD4+FoxP3-eGFP+ Tregs were isolated at 1, 3, or 7 d following infection of Foxp3eGFP mice and tested for their ability to inhibit CD3/CD28-mediated proliferation of polyclonal CD45.1+CD8+ responder T cells (Tresps). The in vitro suppressive capacity of nTregs isolated from naïve Foxp3eGFP mice was comparable on a per-cell basis to Tregs isolated from mice 1 d postinfection (day 1 Treg) with ∼50% CD8+ Tresp suppression in 1:1 Treg:Tresp cocultures (Fig. 1 B and C). This cell-intrinsic suppressor activity was rapidly lost in Tregs isolated at day 3 postinfection compared to nTregs and day 1 Tregs paralleling the drop in total Treg absolute number (Fig. 1 A–C). Suppressor activity was again evident in Tregs isolated at day 7 postinfection (day 7 Treg), which displayed a significantly greater suppressive capacity per cell than that of Tregs isolated at earlier time points (days 0 to 1) (Fig. 1 B and C). These findings demonstrate that Treg activity varies over time during acute L. monocytogenes infection in that it is present at day 1 (priming phase) and at day 7 (contraction phase) but is notably absent during the period coincident with effector CD8+ T cell expansion (days 3 to 6).
Fig. 1.
Treg responses during Listeria infection display a biphasic kinetic pattern. C57BL/6 mice infected with L. monocytogenes ΔactA-Ova. (A) Number of CD25+FoxP3+CD4+ Tregs in spleens 0 to 9 d postinfection (n = 4 to 8 per group). (B and C) In vitro CD45.1+CD8+ Tresps proliferation/percent suppression at day 3 after coculture with CD45.2+CD4+FoxP3-eGFP+ Tregs isolated from naïve Foxp3eGFP mice and those that underwent 1, 3, or 7 d of infection. Indicated are titrated Treg:Tresp ratios after anti-CD3/CD28 stimulation (n = 4 per group). Reported in histograms are percentage and data from 1:1 Treg:Tresp cocultures. Mean ± SEM; (A and B) *P < 0.05, **P < 0.01, and ***P < 0.001 (Student’s t test); (B) ††P < 0.01 and ††††P < 0.0001 (one-way ANOVA relative to −Treg). CTV, CellTrace Violet; NS, not significant.
We next sought to investigate lineage relationships between the early (day 1) and later (day 7) Treg populations. We first performed differential kinetic labeling studies by intraperitoneally (IP) administering 5-ethynyl-2'-deoxyuridine (EdU) to Foxp3eGFP mice at the time of infection to allow tagging of day 1 Tregs followed by IP delivery of 5-bromo-2'-deoxyuridine (BrdU) at day 5 postinfection, just prior to the emergence of day 7 Tregs (SI Appendix, Fig. S1A). Examination of the CD4+FoxP3-eGFP− Tconv and CD4+FoxP3-eGFP+ Treg subsets showed that the majority of labeled populations were single positive for either EdU or BrdU, suggesting that proliferating CD4+ T cells present at either day 1 or day 7 largely arise through proliferation of different precursors (SI Appendix, Fig. S1 B and C). Minor populations (0.1 to 0.2%) of CD4+ T cells were consistently detected within Tconv (both EdUlo and EdUhi) and Treg (EdUhi) compartments that costained for BrdU (SI Appendix, Fig. S1 B and C), indicating that a small percentage of Tregs at day 7 could derive from day 1 Tregs. Despite appearing that day 1 Tregs decline in absolute number shortly after infection (Fig. 1A), it is also possible that early Tregs undergo extensive division, becoming entirely negative for EdU.
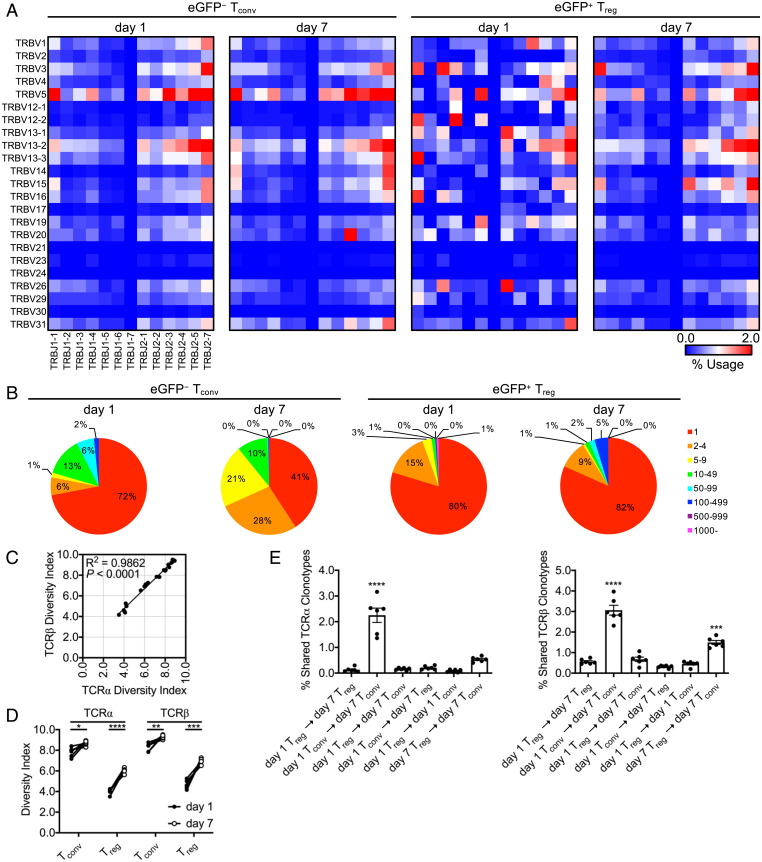
To further establish that day 1 and day 7 Tregs represent distinct populations, we compared the TCR repertoires of Tconv and Treg populations obtained via hemisplenectomy at these two time points within individual animals undergoing infection, thus allowing serial sampling of a given spleen. CD4+FoxP3-eGFP− Tconvs and CD4+FoxP3-eGFP+ Tregs were sorted at each time point, and TCR sequencing (TCR-Seq) was performed to determine the repertoire of V and J gene segments expressed by TCRα (Tra) and TCRβ (Trb) gene loci in each subset. In cells isolated at day 1 postinfection, interanimal average V and J gene segment family usage at the Tra (SI Appendix, Fig. S2) and Trb (Fig. 2A) loci were randomly distributed in both Tconvs and Tregs. By day 7, however, the usage of particular segment combinations increased. In day 7 Tconvs, we observed predominant usage of TRAV4D-4/TRAJ48, TRBV20/TRBJ2-3, and TRBV14/TRBJ2-7 that was not apparent in day 1 Tconvs and overall increased expression of combinations within TRBV5. Moreover, the pattern of gene segment family usage between Tconvs and Tregs, though distinct at day 1, more closely resembled each other by day 7 (Fig. 2A and SI Appendix, Fig. S2 and Table S1). As infection progressed, the number of clonal Trb CDR3 reads in both Tconv and Treg subsets increased in frequency from day 1 to day 7; however, Tconvs appeared to undergo more clonal expansion compared to Tregs. More repetitive CDR3 reads were apparent in Tregs at day 7, also suggesting that Tregs develop into larger, but fewer, clones compared to Tconvs (Fig. 2B). This was also consistent with increased overall TCR diversity in Tconvs that changed less over time versus observations in Tregs (Fig. 2 C and D). These results suggest that Tconv and Treg responses shift from a polyclonal to an oligoclonal repertoire at different rates and/or selectivities.
Fig. 2.
Splenic Tregs do not display clonal expansion during Listeria infection. Foxp3eGFP mice infected with L. monocytogenes ΔactA-Ova. Hemisplenectomy was performed at day 1 postinfection, and viable NK1.1−CD4+TCR-β+FoxP3-eGFP− (Tconv) and FoxP3-eGFP+ (Treg) cell populations were cell sorted from the dorsocranial lobe of the spleen. Corresponding cell populations were isolated at day 7 from the remaining ventral-caudal half of the spleen. Total RNA was isolated from all cells and subjected to TCR-Seq. (A) TCRβ Trb V (TRBV) and Trb J (TRBJ) gene segment family average usage among animals. (B) Representative number of Trb CDR3 clone reads. (C and D) Shannon–Weaver TCR diversity indices reported. (E) Intraanimal Venn analysis displaying shared Tra and Trb CDR3 clonotypes (n = 6 per group, 3 male and 3 female). Mean ± SEM. (C) R2/P (linear regression analysis); (D) *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 (Student’s t test); and (E) ***P < 0.001 and ****P < 0.0001 (one-way ANOVA).
Finally, we compared Tra and Trb CDR3 sequences shared between Tconvs and Tregs via intraanimal Venn analysis at day 1 and day 7. As expected, a strong clonal relationship existed between day 1 and day 7 Tconv subsets (as both shared TCRα and TCRβ clonotypes), supporting the notion that expansion of antigen-specific effector CD4+ T cells had occurred. Importantly, we did not observe any clonal similarity between day 1 and day 7 Treg populations, reinforcing our prior conclusion that these biphasic Treg populations are developmentally unrelated, yet retain the capacity to differentially suppress CTLs at distinct stages of the primary response to L. monocytogenes (Fig. 2E). Regardless of the origin of Tregs, our data clearly demonstrate that day 7 Tregs are not simply derived from increased expansion of the original Treg population present at day 1.
Early and Late Treg Development Is Linked to Specific Gene Expression Signatures.
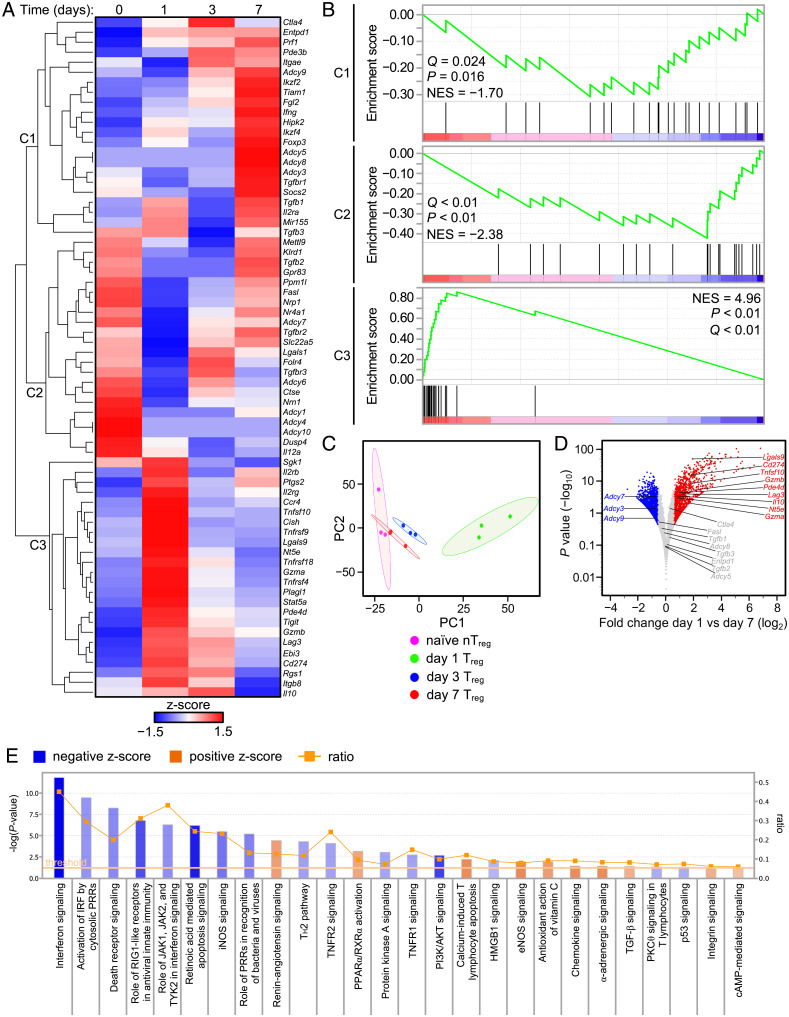
Having established that day 1 and day 7 Tregs were distinct in their ontogeny and TCR repertoire, we surveyed the suppressor mechanisms associated with these cells. To accomplish this, Foxp3eGFP mice were infected, and CD4+FoxP3-eGFP+ Tregs were isolated at days 0, 1, 3, and 7 for RNA sequencing (RNA-Seq). Unbiased hierarchical clustering of Treg-linked suppressor function genes identified highly dynamic and temporally regulated gene sets with the strongest expression of Treg-associated genes observed at days 0, 1, and 7. With the notable exception of Ctla4 (CTLA-4) and Il10 (IL-10), most Treg-associated genes were transcriptionally inactive at day 3, consistent with lack of functional suppression observed at this time point (Fig. 1 B and C and 3A). Gene set enrichment analysis (GSEA) of gene clusters 1 to 3 (C1-3) revealed that C2, highly expressed before infection, was largely down-regulated from days 1 to 3 postinfection only to be partially up-regulated as part of the subsequent day 7 signature. A similar pattern of gene up-regulation shared between C2 (day 0 nTreg) and C1 (day 7 Treg) suggested that late-arising day 7 Tregs reacquire some original “nTreg-like” transcripts but also induce transcription of new immunoregulatory genes at the contraction phase. In contrast, day 1 Tregs contained in the C3 gene cluster displayed a considerably enriched gene set (Fig. 3 A and B) that also segregated from all other time points using principal component analysis (PCA) (Fig. 3C).
Fig. 3.
Kinetic analysis of Treg RNA-Seq. Foxp3eGFP mice infected with L. monocytogenes ΔactA-Ova with total RNA isolated from NK1.1−CD4+TCR-β+FoxP3-eGFP+ Tregs at days 1, 3, and 7 postinfection or naïve animals. (A) RNA-Seq with hierarchical clustered, row-scaled log-transformed average reads per kilobase of transcript, per million mapped reads (RPKM) values. (B) GSEA against day 1 versus day 7 RNA-Seq comparisons performed with gene sets derived from clusters 1 to 3 (C1-3) in A. (C) PCA of Treg RNA-Seq. (D) Volcano plot of selected genes and log2 normalized expression with a Δ1.5 fold change cutoff against log10 normalized P values from day 1 versus day 7 comparisons. (E) IPA canonical pathways associated with day 1 versus day 7 comparisons (n = 3 per group). Reported are lymphocyte-related pathways and ratio of pathway coverage (P < 0.05 threshold for gene network overlap).
Major suppressor genes, including Ctla4, Tgfb1, Tgfb2, and Tgfb3 (TGF-β1/2/3), did not display a significant fold change in normalized expression between days 1 and 7. Day 1 Tregs had increased transcription of Lgals9 (Gal-9), Cd274 (PD-L1), Tnfsf10 (TRAIL), Gzmb (granzyme B), Lag3 (Lag-3), Il10, Nt5e (CD73), and Gzma (granzyme A), suggesting that these mechanisms may be relevant early after infection. In contrast, day 7 Tregs displayed increased expression of Adcy3, Adcy7, and Adcy9 (adenylyl cyclases 3/7/9) and a significant decrease in Pde4d (phosphodiesterase 4) (Fig. 3D). This in turn suggested that late rising Tregs may accumulate intracellular cAMP (23, 24).
Pathway analysis confirmed a significant role for cAMP in day 7 Treg function as several Adcy-linked pathways were up-regulated including peroxisome proliferator-activated receptor/retinoid X receptor (PPARα/RXRα) activation, eNOS signaling, α-adrenergic signaling, and cAMP-mediated signaling (Fig. 3E). In contrast, day 1 Tregs enriched for pathways related to innate immune signaling (interferon signaling and pattern recognition receptor [PRR] recognition of bacteria and viruses), apoptosis (death receptor signaling and retinoic acid–mediated apoptosis), abortive TCR signaling (protein kinase B [PI3K/AKT] and protein kinase A [PKA] signaling), and cell cycle inhibition (p53 signaling) consistent with its rapid induction but short lifespan (Fig. 3E). TGF-β1/2/3, despite not displaying a significant fold change in normalized expression between day 1 and day 7 Tregs, were not excluded in subsequent analyses because pathway analysis suggested TGF-β signaling and renin-angiotensin signaling (linked to TGF-β) were significantly up-regulated in day 7 Tregs (Fig. 3 D and E).
CD73 Expression Increases Early after Infection, while TGF-β Production and cAMP Accumulation Predominates during Contraction.
We next determined whether the dynamic gene expression patterns revealed by RNA-Seq correlated with protein expression and were functionally relevant. Treg CTLA-4 expression has been suggested to strip antigen presenting cells (APCs) of CD80/CD86; Lag-3 can bind major histocompatibility complex class II (MHC II) to modulate APC maturation and function; TRAIL, granzymes, and Gal-9 can induce apoptosis in Tresps; and IL-10 can exert antiinflammatory effects capable of negatively regulating T cell activation (1, 5, 25). Despite low levels of transcription at the Ctla4 locus, both day 1 and 7 Tregs displayed equal amounts of CTLA-4 protein. In contrast, we could not detect TRAIL, Gal-9, Lag-3, and granzyme B protein in Tregs directly ex vivo. Trace IL-10 production was detected after phorbol 12-myristate 13-acetate (PMA)/ionomycin restimulation of Tregs at various time points (SI Appendix, Fig. S3A). Antibody blockade of TRAIL, Gal-9, Lag-3, and IL-10R in Treg:Tresp cocultures did not reverse the suppression observed at either day 1 or day 7 (SI Appendix, Fig. S4A). Overall, these data indicate that most suppressor pathways differentially enriched in RNA-Seq and comparative GSEA related to direct Treg:Tresp interaction did not yield a functional response to a known suppressor mechanism but rather likely reflect Treg reactions to pro- and antiinflammatory cues at different time points.
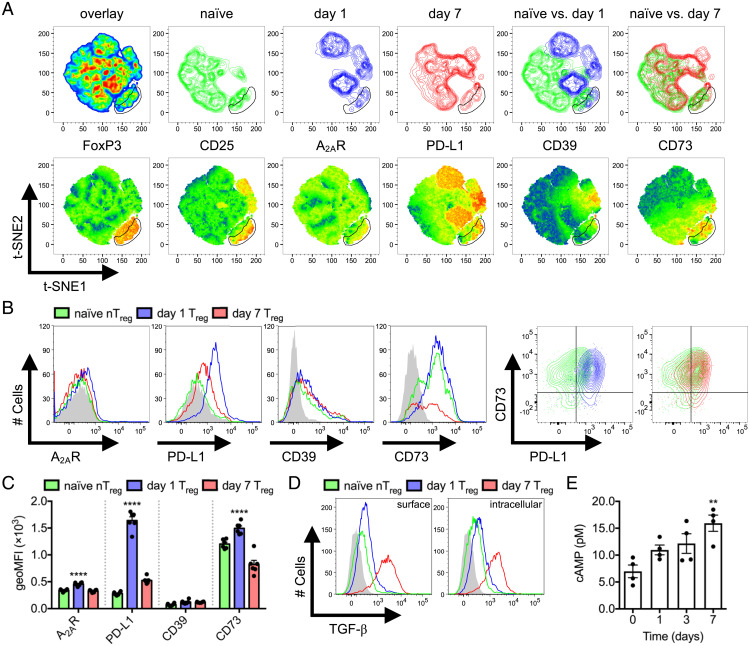
Tregs express high levels of surface CD39 and CD73 ectonucleotidases, which dephosphorylate adenosine diphosphate/triphosphate (5'-ADP/5'-ATP) and 5'-AMP, respectively. Extracellular Ado produced by Treg ectonucleotidase activity engages adenosine/A2A receptors (A2AR) on CD8+ T cells, whereas Treg intracellular cAMP can be directly transferred to the cytosol of CD8+ T cells via cell–cell contact. Both pathways separately lead to the inhibition of CTL proliferation, survival, and effector function due to the activation of PKA (23). Tregs can also use surface PD-L1 to suppress effector T cells by directly engaging the PD-1 inhibitory receptor (26). We evaluated protein expression of PD-L1, CD39, and CD73 as well as intracellular cAMP accumulation as genes associated with these suppressor pathways were differentially expressed in day 1 versus day 7 Tregs. The t-distributed stochastic neighbor embedding (t-SNE) clustering on total splenic CD4+ T cells isolated from naïve and day 1 versus day 7 infected mice was performed. Dimensional reduction using FoxP3, CD25, A2AR, PD-L1, CD39, and CD73 was sufficient to segregate the day 1 Treg clustering pattern from nTregs and day 7 Tregs within a CD25+FoxP3+CD4+ Treg gate on t-SNE plots (Fig. 4A). A2AR, CD73, and PD-L1 expression peaked in day 1 Tregs to varying degrees, consistent with RNA-Seq findings (Fig. 4 B and C). Although PD-L1 expression peaked at day 1, this occurred on both FoxP3− Tconvs and FoxP3+ Tregs (Fig. 4A), and importantly, antibody blockade studies did not reveal a contribution to in vitro immunosuppression (SI Appendix, Fig. S4A). In contrast, maximal CD73 expression was observed within FoxP3+ Treg populations. CD39 expression was apparent in only a small subset of FoxP3+ Tregs and activated CD25loPD-L1+ Tconvs (Fig. 4A), and its expression did not appear to vary throughout L. monocytogenes infection (Fig. 4 B and C). A separate t-SNE analysis of CD25+FoxP3+CD4+ Treg-gated populations also maintained that day 1 Tregs displayed a segregated clustering pattern based solely on A2AR, PD-L1, CD39, and CD73; however, CD39 was not coexpressed with CD73 (SI Appendix, Fig. S5).
Fig. 4.
Temporal variation of Treg effector mechanisms. Naïve compared to day 1 and 7 L. monocytogenes ΔactA-Ova–infected C57BL/6 mice. (A) t-SNE analysis of CD4+ T cells with FoxP3, CD25, A2AR, PD-L1, CD39, and CD73 dimensions. Density plots (Upper) and heatmap statistic displays of each input parameter (Lower) are displayed (n = 6 per group). (B and C) Expression of A2AR, PD-L1, CD39, and CD73 on Treg surface defined in A (n = 6 per group). (D) Expression of surface displayed and intracellular TGF-β by Tregs after PMA/ionomycin restimulation (n = 4 per group). (E) Intracellular FoxP3-eGFP+ Treg cAMP concentration after sorting from naïve Foxp3eGFP mice and those at days 1, 3, and 7 following L. monocytogenes ΔactA-Ova infection (n = 4 per group). Filled histograms represent fluorescence minus one (FMO). Mean ± SEM. (C and E) **P < 0.01 and ****P < 0.0001 (one-way ANOVA).
TGF-β contributes to Treg development and acts as a Treg-derived inhibitory cytokine (5). Although Tregs did not stain positive for surface TGF-β directly ex vivo (SI Appendix, Fig. S3A), day 7 Tregs significantly up-regulated both surface-displayed and intracellular TGF-β upon restimulation compared to nTreg, day 1 Treg (Fig. 4D), Tconv, and CTL subsets across all time points (SI Appendix, Fig. S3B). This is in direct contrast to the data generated by RNA-Seq where a transcriptional difference was not detected in the kinetics of TGF-β1/2/3 production across day 1 and day 7 Treg populations (Fig. 3D). RNA-Seq analysis also suggested that a dominant feature in the late rising day 7 Tregs included increased adenylyl cyclases 3/7/9 and reduced phosphodiesterase 4 expression (Fig. 3D), which we hypothesized would positively correlate with intracellular cAMP. Consistent with this, we observed a gradual increase in Treg cytosolic cAMP throughout the course of infection, with an increase in cAMP reaching significance by day 7 (Fig. 4E). Taken together, these data suggest that day 1 Tregs significantly up-regulate CD73 expression compared to nTregs, and day 7 Tregs preferentially increase TGF-β production and accumulate intracellular cAMP.
CD73 Enzymatic Conversion of 5′-AMP to Adenosine Is Dominant in the Early Phase.
CD73 expression on the cell surface does not strictly correlate with catabolic activity but is also synchronized with Treg TCR engagement (27). CD73 is a C-terminal glycosylphosphatidylinositol (GPI) anchored protein that exists in dimeric soluble and membrane-bound forms. When expressed on the cell surface, CD73 switches between open/closed conformations where the open form is receptive to catalysis and has at least two distinct states (ranging from 977 to 1,609 Å) when actively bound to Ado due to a fluid homodimerization interface. Further, 5′-ATP and 5′-ADP are known to be natural competitive inhibitors of CD73 (28, 29). This suggests that its activity is also influenced by the microenvironment and associated cell damage occurring early during infection.
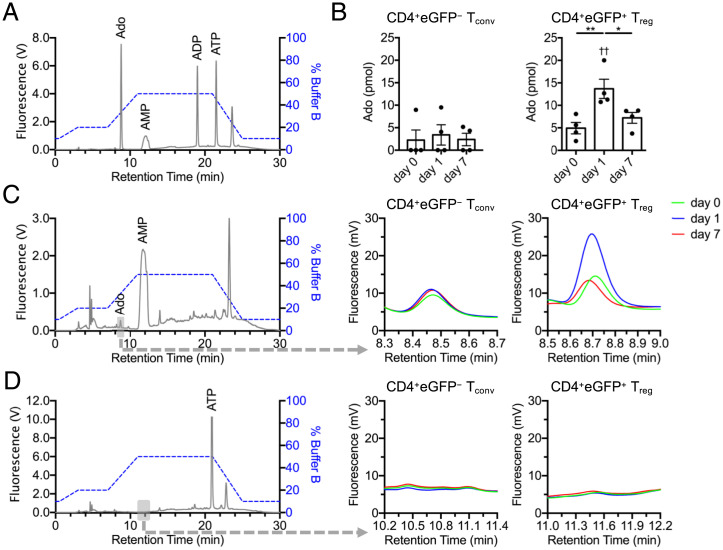
We therefore sought to determine whether there was a functional impact from the small increase in CD73 we observed in day 1 Tregs. Ado, 5′-AMP, 5′-ADP, and 5′-ATP can be converted into 1,N6-etheno-derivatized nucleotide analogs, which can be resolved by high-performance liquid chromatography (HPLC) and detected by fluorescence at 410 nm (Fig. 5A). To measure CD73 enzymatic activity, CD4+FoxP3-eGFP− Tconvs and CD4+FoxP3-eGFP+ Tregs were sorted from naïve and day 1 or day 7 infected Foxp3eGFP mice. Cells were seeded in transwells under serum-free conditions, and 10 μM 5′-AMP or 5′-ATP substrates were pulsed in the opposing chamber of each well (free of cells) to test for CD73-mediated 5′-AMP to Ado and CD39-mediated 5′-ATP to 5′-AMP hydrolysis, respectively. Chambers devoid of cells were mixed and aliquoted after 15 min of incubation, derivatized, and assessed via HPLC. Our analysis confirmed that, in association with a subtle increase in CD73 surface expression, day 1 Tregs had greater than twofold increased enzymatic activity in converting 5′-AMP to Ado compared to nTregs and day 7 Tregs. Tconvs did not display any CD73 activity (Fig. 5 B and C). We also could not detect any CD39 activity by this method in Tconv or Treg subsets (Fig. 5D). These findings support that day 1 Tregs are functionally hyperreactive to extracellular 5′-AMP during L. monocytogenes infection and can rapidly generate significant amounts of immunosuppressive Ado via CD73.
Fig. 5.
Tregs display increased CD73 enzymatic activity at day 1 postinfection. (A) A total of 10 pmol mixed 1,N6-etheno-derivatized nucleotide standards (Ado, 5′-AMP, 5′-ADP, and 5′-ATP) resolved by HPLC. (B–D) FoxP3-eGFP+ Tregs or FoxP3-eGFP− Tconvs sorted from naïve (day 0) and L. monocytogenes ΔactA-Ova–infected (days 1 and 7) Foxp3eGFP mice were placed in the upper chamber of a 96-well transwell plate. The bottom chamber was pulsed with either 10 μM 5′-AMP or 5′-ATP. (B and C) Concentration of Ado in the bottom chamber determined in cultures exposed to a 15-min 5′-AMP pulse for assessment of CD73 enzymatic activity, and (D) 5′-AMP similarly measured with a 15-min 5′-ATP pulse for measurement of CD39-mediated hydrolysis (n = 4 per group). Expanded retention times (gray box) for (C) Ado and (D) 5′-AMP are indicated. Mean ± SEM. (B) *P < 0.05 and **P < 0.01 (Student’s t test); ††P < 0.01 (one-way ANOVA).
cAMP and TGF-β Separately Regulate Priming and Contraction Phases of the CD8+ T Cell Response.
Given the possible relevance of differential TGF-β, CD73-released Ado, and cAMP production/accumulation, we investigated the contextual relevance of these suppressive mediators during bimodal Treg kinetics. We therefore evaluated the effect of blocking versus agonizing these pathways during in vitro CD3/CD28-based suppressor assays. TGF-β neutralization contributed equally to both day 1 and day 7 Tregs in vitro suppressor activity (SI Appendix, Fig. S4A), despite the fact that production and surface display of TGF-β was elevated by day 7 Tregs directly ex vivo compared to day 1 Tregs (Fig. 4D). We also noted that inclusion of anti–TGF-β in wells containing only CD8+ Tresps promoted additional proliferation over baseline responses to anti-CD3/CD28 agonism (SI Appendix, Fig. S4B), indicating that in vitro blockade of TGF-β may not accurately mirror Treg production and surface display in vivo due to autocrine production of TGF-β by CD8+ T cells after acute and chronic TCR ligation seen in this report (SI Appendix, Fig. S3B) and others (30, 31).
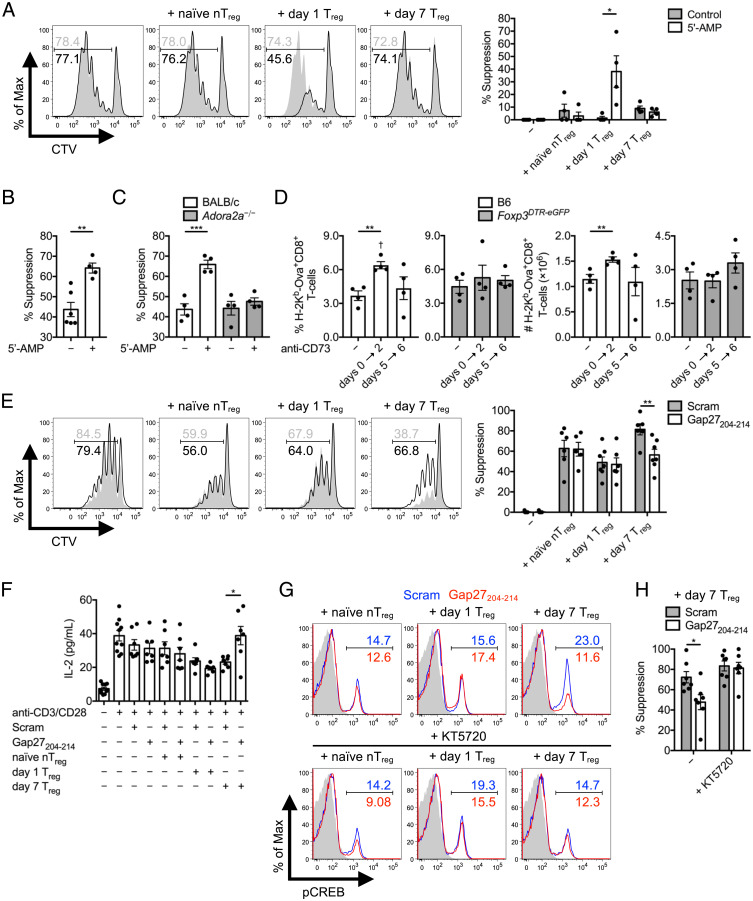
Ado binding to A2AR on CD8+ T cells activates adenylyl cyclases, which in turn induces the synthesis of intracellular cAMP—which is ultimately inhibitory to the activity of these cells (23). cAMP directly binds PKA in a 4:1 stoichiometry leading to separation of PKA catalytic and regulatory subunits, whereupon the catalytic subunits of PKA activate C-terminal Src kinase (CSK) via phosphorylation of Ser364. pCSK in turn phosphorylates lymphocyte-specific protein tyrosine kinase (Lck) at Tyr505 to directly inhibit TCR signaling in T cells (32). Indirect suppression of CD8+ Tresps by Treg CD73-derived Ado was therefore explored in this context. For this, CD4+FoxP3-eGFP+ Tregs sorted from naïve, day 1 infected, and day 7 infected Foxp3eGFP mice were placed in the upper chamber of transwell plates, above wells containing CD8+ Tresps. After CD3/CD28 stimulation of both subsets, CD8+ Tresp division was assessed. All Treg suppressor activity, including any mechanism related to TGF-β provision or surface display, was contact dependent at every time point in the absence of CD73 5′-AMP substrate. After addition of 5′-AMP, only day 1 Tregs displayed suppressor activity in the absence of cell contact (Fig. 6A). This phenotype was consistently apparent in day 1 Treg:Tresp cocultures with cell contact permitted (Fig. 6B). Importantly, Adora2a−/− CD8+ Tresp (lacking A2AR) division was not impeded after addition of 5′-AMP compared to isogenic BALB/c CD8+ Tresps when cocultured with day 1 Tregs (Fig. 6C). We also compared the timing of suppression in vitro to an in vivo CD73 antibody blockade approach. In vivo reduction in the magnitude of the day 7 peak Ova-specific CD8+ T cell response against L. monocytogenes ΔactA-Ova was apparent after early (days 0 to 2) but not late (days 5 to 6) blockade of CD73. The in vivo effect of anti-CD73–mediated inhibition of CTL responses was entirely dependent on the presence of Tregs, as depletion of Tregs by administration of diphtheria toxin (DT) to Foxp3DTR-eGFP mice negated this effect (Fig. 6D).
Fig. 6.
Day 1 versus day 7 bimodal Treg cAMP-mediated suppression. (A) CD45.2+CD4+FoxP3-eGFP+ Tregs sorted from naïve and day 1 or day 7 L. monocytogenes ΔactA-Ova–infected Foxp3eGFP mice were placed in the upper chamber of a 96-well transwell plate, with the bottom chamber containing naïve CTV-labeled CD45.1+CD8+ Tresps. At day 3 after anti-CD3/CD28 stimulation, CD45.1+CD8+ Tresp percent suppression in the presence or absence of 1 μM 5′-AMP was assessed (n = 4 per group). In vitro CD45.2+CD8+ Tresp proliferation of (B) C57BL/6 or (C) BALB/c compared to Adora2a−/− origin represented as percent suppression at day 3 after a cell-contact permissive, 1:1 coculture of naïve CTV-labeled CD45.2+CD8+ Tresps with CD45.1+CD4+FoxP3-eGFP+ Tregs isolated from day 1 infected CD45.1+ Foxp3eGFP mice in the presence or absence of 1 μM 5′-AMP (n = 4 per group). (D) The percent and absolute number of splenic Ova-specific CD8+ T cells at day 7 postinfection of C57BL/6 and Foxp3DTR-eGFP mice infected with L. monocytogenes ΔactA-Ova. DT injection dictated Treg depletion with control and early (days 0 to 2) versus late (days 5 to 6) CD73 blockade compared (n = 4 per group). (E) In vitro CD45.1+CD8+ Tresp percent suppression and (F) IL-2 detected in supernatant at day 3 after 1:1 coculture with CD45.2+CD4+FoxP3-eGFP+ Tregs isolated from naïve and day 1 or day 7 infected CD45.1+ Foxp3eGFP mice in the presence or absence of Gap27204–214 or scrambled control peptide (n = 6 to 10 per group). (G and H) Suppressor assay as in E and F with KT5720 selective inhibition of PKA with analysis of intracellular pCREB in CD45.1+CD8+ Tresps, and percent suppression exerted by day 7 Tregs (n = 7 per group). Numbers in histograms represent percentage. Mean ± SEM. (A–F and H) *P < 0.05, **P < 0.01, and ***P < 0.001 (Student’s t test); (D) †P < 0.05 (one-way ANOVA relative to negative control).
Cytoplasmic cAMP can be directly transferred from Tregs to Tresps via gap junction intercellular communication (GJIC) (33). Despite inhibiting TCR signaling in Tconvs and CTLs, cAMP does not inhibit TCR signals in Tregs, but rather enhances FoxP3 expression (34). As day 7 Tregs significantly accumulated intracellular cAMP compared to nTregs and day 1 Tregs (Fig. 4E), we determined whether this was critical for cell contact–dependent suppression. CD4+FoxP3-eGFP+ Tregs were sorted from naïve, day 1 infected, and day 7 infected Foxp3eGFP mice and placed in coculture with CD8+ Tresps in the presence of Gap27204–214 connexin mimetic peptide, known to interfere with gap junction formation and stability (33). As before, nTregs and day 1 Tregs displayed ∼50 to 60% suppression, and day 7 Tregs displayed ∼80% suppression in the presence of a sequence-scrambled control peptide. Blockade of GJIC with Gap27204–214 was sufficient to significantly reduce day 7 Treg suppression by ∼40 to 50%; however, GJIC blockade did not impact suppression mediated by nTregs or day 1 Tregs (Fig. 6E). GJIC blockade also led to an increased concentration of IL-2 in the supernatants of day 7 Treg:Tresp cocultures (Fig. 6F). Gap27204–214 inhibited phosphorylation of cAMP-responsive element binding protein (CREB) in Tresps, suggesting that cAMP was included among the contents transferred via GJIC (Fig. 6G, Upper). Lastly, addition of a PKA inhibitor (KT5720) completely accounted for GJIC-mediated pCREB and Gap27204–214 reversal of suppression in day 7 Treg:Tresp cocultures (Fig. 6 G and H). In vitro evidence therefore supported that day 1 Tregs provide abundant Ado in the surrounding extracellular microenvironment in regulating the priming of CTLs early after L. monocytogenes infection via a cell contact–independent mechanism. In contrast, day 7 Tregs are entirely dependent on cell contact–dependent suppression during the contraction phase of the CTL response and rely on cAMP GJIC transfer mechanisms (and likely elevated TGF-β production).
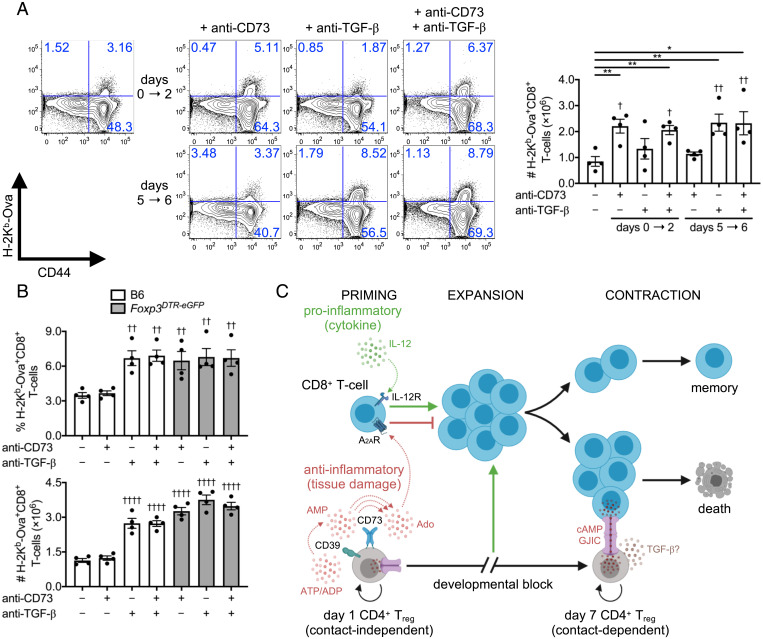
We questioned whether the timing of the distinct in vitro suppressor pathways translated to L. monocytogenes infection in vivo. Because GJIC cannot be blocked in vivo, we focused on evaluating the impact of single or dual TGF-β and CD73 antibody–mediated blockade administered early (days 0 to 2) or late (days 5 to 6) after infection. Splenic Ova-specific CD8+ T cell number was assessed by tetramer staining at day 7 postinfection. We found that early anti-CD73 boosted the number of Ova-specific CD8+ T cells. In contrast, anti–TGF-β led to increased antigen-specific CTL numbers at later time points. A combination of anti–TGF-β and anti-CD73 did not reveal any additive or synergistic effects (Fig. 7A). With DT-mediated depletion of Tregs in infected Foxp3DTR-eGFP mice, delayed TGF-β blockade alone or in combination with anti-CD73 did not impact Ova-specific CD8+ T cell frequency or absolute number (Fig. 7B). Therefore, the role of CD73 in Treg suppressor function was consistent with an early cell contact–independent mechanism on CD8+ T cells, whereas late inhibition of CTL responses by Tregs aligned with cell contact–dependent mechanisms in part involving TGF-β surface display and cAMP transfer. These data collectively support that distinct Treg populations and suppressor mechanisms arise to separately control priming and contraction phases of the CTL response during a single acute infection (Fig. 7C).
Fig. 7.
The CD73 pathway suppresses Ova-specific CD8+ T cell responses early after Listeria infection in vivo. (A) C57BL/6 mice infected with L. monocytogenes ΔactA-Ova. Absolute number of Ova-specific CD8+ T cells at 7 d postinfection in spleens with control and early (days 0 to 2) versus late (days 5 to 6) TGF-β and CD73 single and dual blockade compared (n = 4 per group). (B) Percent and absolute number of splenic Ova-specific CD8+ T cells at day 7 after L. monocytogenes ΔactA-Ova infection of C57BL/6 versus Foxp3DTR-eGFP mice. DT injection dictated specific Treg depletion with control and late (days 5 to 6) TGF-β and CD73 single and dual blockade compared (n = 4 per group). (C) Proposed model for biphasic Treg response to L. monocytogenes infection. Numbers in scatterplots represent percentage. Mean ± SEM. (A) *P < 0.05 and **P < 0.01 (Student’s t test); (A and B) †P < 0.05, ††P < 0.01, and ††††P < 0.0001 (one-way ANOVA relative to negative control).
Discussion
A wealth of studies has led to the view that Treg responses in infection, cancer, and autoimmunity are essentially monophasic (i.e., similar in terms of suppressor cells and molecular mechanisms). Here we demonstrate that Tregs display a sequential, biphasic expansion pattern in response to an infectious pathogen that is composed of developmentally distinct suppressor cell populations, which separately control the CD8+ T cell response during priming versus contraction stages. Following L. monocytogenes infection, day 1 Tregs arise in response to elevated microbial presence, activate a CD73-mediated suppressor mechanism, and rapidly produce Ado in order to fine-tune CD8+ T cell priming via cell contact–independent suppression. This first wave of regulation is transient, however, as the number of Tregs markedly decreases by day 3, as does their suppressive capacity per cell, commensurate with the primary expansion of pathogen-specific CD8+ T cells. When L. monocytogenes antigen is cleared and CD8+ T cell accumulation reaches its day 7 peak, a distinct population of Tregs appears that relies primarily on cell contact–dependent transfer of cAMP to initiate and/or in part support T cell contraction. These findings reveal an unexpected plasticity in the Treg response to acute infection, which employs distinct populations, expansion kinetics, and effector mechanisms early after pathogen sensing to avoid inflammation-induced immunopathology by CD8+ T cells versus late in the response to restore homeostasis. Importantly, our data show that early Treg-mediated suppression is short lived, presumably to avoid impeding the expansion of pathogen-specific CD8+ T cells that are needed for host defense once they have exerted an initial role in protecting the host from autoimmune- or inflammation-mediated pathology caused by systemic activation. Taken together, our findings reveal that, rather than being a unitary monophasic force opposing the induction of immunity, Treg-mediated regulation is more dynamic, varied, and coordinated than has been previously described.
Regarding the ontogeny of the early (day 1) versus late (day 7) Treg populations, we found no evidence of a developmental relationship using both lineage tracing (BrdU and EdU copulsing) and genetic (TCR-Seq) approaches, suggesting that these subpopulations arise from distinct progenitors. In previous work from our laboratory using the L. monocytogenes model, it was found that the day 1 Treg population is a mixture of activated nTregs and Tconvs that rapidly up-regulate FoxP3 and accumulate in the secondary lymphoid tissues of infected animals (20). The present work extends these findings through identification of the second day 7 Treg population that appears clonally distinct from day 1 Tregs and shows only ∼0.5 to 1.5% overlap in TCR clonotypes with Tconvs (Fig. 2E). We therefore hypothesize that the majority of day 7 Tregs are newly emergent nTregs that replace the original nTreg population present during homeostasis and early after infection (days 0 to 1). From a kinetic standpoint, it is also unlikely that day 1 Tregs are antigen-specific, whereas day 7 Tregs presumably contain a small fraction of clonotypes specific to L. monocytogenes.
The presence and suppressive capacity of day 1 Tregs are instead linked to the amount of inflammation, and not antigen, associated with increased initial L. monocytogenes exposure (20). More specifically, early neutrophil release of self-DNA as part of neutrophil extracellular trap (NET) generation has been suggested as a means by which tissue damage can be sensed in this model, enabling day 1 Tregs to fine-tune CTL responses to avoid immunopathology (20, 35, 36). We hypothesize that the generation of day 1 Tregs is a two-step process. In the first step, inflammatory cytokines and NET-derived self-DNA serve as initiating signals for Tconv to Treg conversion. In the second step, damage-associated adenine nucleotides are used as mediators of suppression. We have previously shown that, in the context of acute L. monocytogenes exposure, DNA released by neutrophils directly engages Toll-like receptor 9 (TLR9) on CD8α+ dendritic cells, which in turn is necessary and sufficient to support early day 1 Treg differentiation and accumulation (20). This occurs rapidly (in less than 6 h) and precedes the onset of tissue damage evidenced by later systemic rises in serum aspartate aminotransferase and alanine aminotransferase (AST/ALT) peaking at 2 d postinfection (20, 37). In this study, we can extend this mechanism by demonstrating that the rapidly generated Tregs utilize adenine nucleotides released from damaged tissues into the early phase of this immunoregulatory circuit. CD73 exerts early immunosuppression on CTL responses only in the presence of an intact Treg population. Day 1 Tregs express elevated levels of functional CD73 and suppress CD8+ Tresps in the absence of cell contact. We speculate that, as tissue damage leads to adenine nucleotide release, day 1 CD73+ Tregs respond by enzymatically increasing extracellular Ado concentration gradients. Once present in the extracellular milieu, Ado is poised to directly engage A2AR on CD8+ T cells in the local microenvironment to directly inhibit activation (23). Ado-mediated suppression early after infection may therefore endow day 1 Tregs with the ability to effectively monitor tissue damage and exert control on the amount of potentially autoreactive CD8+ T cells generated at priming.
Immunosuppression by Treg occurs through both cell contact–dependent and –independent mechanisms (1, 13, 38). Accordingly, we found both types operative within the regulatory circuit we describe. Whereas day 7 Tregs solely relied on cell contact–dependent mechanisms, including cAMP transfer via GJIC, day 1 Treg suppression was conversely mediated by a CD73 cell contact–independent mechanism. We propose that early cell contact–independent mechanisms are rapidly implemented to quickly counteract tissue damage when detected. These Tregs can be rapidly deployed to limit the activation of resident and/or nonresident memory T cells, known to be activated by bystander proinflammatory cytokines in the absence of antigen (39–41). We further speculate that cAMP transfer events act as part of a late phase restoration of homeostasis compared to the early burst of Treg Ado. Lastly, the kinetics of day 1 and day 7 Treg suppressor populations appear to be elegantly choreographed to accommodate the dynamics of the natural pathogen-specific CD8+ T cell response, with an early day 1 response that can protect the acutely infected host from immunopathology, a subsequent lull in Treg activity to permit the expansion of pathogen-specific CD8+ T cells, and a late day 7 phase of regulation to assist in the restoration of homeostasis (Fig. 7C). Of note, the in vitro suppression assay utilized herein only accounts for Treg:Tresp communication and does not account for the role of APCs, emphasizing the need for further research into the role of this latter population in light of its critical position in integrating innate signals during CD8+ T cell priming. Nevertheless, this report reveals insights into how Tregs function as a dynamic “rheostat” in adaptive immunity that is able to amplify versus inhibit CD8+ effector T cell responses at a time and in a manner that is most beneficial to the host.
In summary, this study shows that Treg immunosuppression of CD8+ T cells directed against an acute infection is dynamic and orchestrated with distinct phases that follow the rise and fall of the adaptive immune response. Importantly, these data define the discrete temporal and functional windows in which Tregs operate and give insights into the distinct effector mechanism(s) used in each. Rapid Treg plasticity is likely a common feature controlling cell-mediated immunity and is necessary to tightly resolve pathogenic threats while efficiently balancing self-reactivity. A closer examination and deeper analysis of this bimodal response may open up new research areas for understanding the complexities of Treg development, TCR specificity to self- versus foreign-antigen and overall relevance to immunosuppression, and the cues that dictate Treg function and presence. These findings may also aid in vaccination strategies to optimize the balance between CD8+ T cell priming and ultimate formation of memory.
Materials and Methods
Standard procedures for CD4+ and CD8+ T cell isolation and coculture, L. monocytogenes infection, hemisplenectomy, antibody/peptide/small molecule treatments, HPLC, enzyme-linked immunosorbent assay (ELISA), and fluorescence-activated cell sorting (FACS), as well as detailed descriptions of RNA-Seq/TCR-Seq experiments and data analysis are described in SI Appendix, SI Materials and Methods. Animals were used according to protocols approved by the La Jolla Institute for Immunology.
Supplementary Material
Acknowledgments
We thank Cheryl Kim and Denise Hinz at the La Jolla Institute for Immunology Imaging Facility for assisting with FACS sorting. SI Appendix, Fig. S1A and Fig. 7C were created with BioRender.com. NIH grant U01 DE028227 (to S.P.S.) supported this publication. NIH-funded equipment was supported by grants S10 RR027366 (BD FACSAria II cell sorters) and S10 OD016262 (Illumina HiSeq 2500 System).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2113329119/-/DCSupplemental.
Data Availability
RNA-Seq data have been deposited in NCBI GEO (GSE169741) (42). All other study data are included in the article and/or supporting information.
References
- 1.Sakaguchi S., Wing K., Onishi Y., Prieto-Martin P., Yamaguchi T., Regulatory T cells: How do they suppress immune responses? Int. Immunol. 21, 1105–1111 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S., Vignali D. A. A., Rudensky A. Y., Niec R. E., Waldmann H., The plasticity and stability of regulatory T cells. Nat. Rev. Immunol. 13, 461–467 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Wing J. B., Sakaguchi S., TCR diversity and Treg cells, sometimes more is more. Eur. J. Immunol. 41, 3097–3100 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Sakaguchi S., Sakaguchi N., Asano M., Itoh M., Toda M., Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155, 1151–1164 (1995). [PubMed] [Google Scholar]
- 5.Benoist C., Mathis D., Treg cells, life history, and diversity. Cold Spring Harb. Perspect. Biol. 4, a007021–a007021 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dons E. M., Raimondi G., Cooper D. K. C., Thomson A. W., Induced regulatory T cells: Mechanisms of conversion and suppressive potential. Hum. Immunol. 73, 328–334 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fontenot J. D., et al. , Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity 22, 329–341 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Ziegler S. F., FOXP3: Of mice and men. Annu. Rev. Immunol. 24, 209–226 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Kretschmer K., et al. , Inducing and expanding regulatory T cell populations by foreign antigen. Nat. Immunol. 6, 1219–1227 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Yadav M., Stephan S., Bluestone J. A., Peripherally induced tregs—Role in immune homeostasis and autoimmunity. Front. Immunol. 4, 232 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Josefowicz S. Z., Lu L.-F., Rudensky A. Y., Regulatory T cells: Mechanisms of differentiation and function. Annu. Rev. Immunol. 30, 531–564 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Togashi Y., Shitara K., Nishikawa H., Regulatory T cells in cancer immunosuppression—Implications for anticancer therapy. Nat. Rev. Clin. Oncol. 16, 356–371 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Belkaid Y., Tarbell K., Regulatory T cells in the control of host-microorganism interactions (*). Annu. Rev. Immunol. 27, 551–589 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Benson A., et al. , Microbial infection-induced expansion of effector T cells overcomes the suppressive effects of regulatory T cells via an IL-2 deprivation mechanism. J. Immunol. 188, 800–810 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chinen T., et al. , An essential role for the IL-2 receptor in Treg cell function. Nat. Immunol. 17, 1322–1333 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belkaid Y., Rouse B. T., Natural regulatory T cells in infectious disease. Nat. Immunol. 6, 353–360 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Cabrera R., et al. , An immunomodulatory role for CD4(+)CD25(+) regulatory T lymphocytes in hepatitis C virus infection. Hepatology 40, 1062–1071 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Rowe J. H., Ertelt J. M., Aguilera M. N., Farrar M. A., Way S. S., Foxp3(+) regulatory T cell expansion required for sustaining pregnancy compromises host defense against prenatal bacterial pathogens. Cell Host Microbe 10, 54–64 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laidlaw B. J., et al. , Production of IL-10 by CD4(+) regulatory T cells during the resolution of infection promotes the maturation of memory CD8(+) T cells. Nat. Immunol. 16, 871–879 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dolina J. S., et al. , TLR9 sensing of self-DNA controls cell-mediated immunity to Listeria infection via rapid conversion of conventional CD4+ T cells to Treg. Cell Rep. 31, 107249 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ertelt J. M., et al. , Foxp3+ regulatory T cells impede the priming of protective CD8+ T cells. J. Immunol. 187, 2569–2577 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J. M., Rasmussen J. P., Rudensky A. Y., Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 8, 191–197 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Klein M., Bopp T., Cyclic AMP represents a crucial component of Treg cell-mediated immune regulation. Front. Immunol. 7, 315 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peter D., Jin S. L. C., Conti M., Hatzelmann A., Zitt C., Differential expression and function of phosphodiesterase 4 (PDE4) subtypes in human primary CD4+ T cells: Predominant role of PDE4D. J. Immunol. 178, 4820–4831 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Safinia N., Scotta C., Vaikunthanathan T., Lechler R. I., Lombardi G., Regulatory T cells: Serious contenders in the promise for immunological tolerance in transplantation. Front. Immunol. 6, 438 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitazawa Y., et al. , Involvement of the programmed death-1/programmed death-1 ligand pathway in CD4+CD25+ regulatory T-cell activity to suppress alloimmune responses. Transplantation 83, 774–782 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Antonioli L., Pacher P., Vizi E. S., Haskó G., CD39 and CD73 in immunity and inflammation. Trends Mol. Med. 19, 355–367 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allard B., Longhi M. S., Robson S. C., Stagg J., The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets. Immunol. Rev. 276, 121–144 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knapp K., et al. , Crystal structure of the human ecto-5′-nucleotidase (CD73): Insights into the regulation of purinergic signaling. Structure 20, 2161–2173 (2012). [DOI] [PubMed] [Google Scholar]
- 30.Li M. O., Wan Y. Y., Sanjabi S., Robertson A.-K. L., Flavell R. A., Transforming growth factor-beta regulation of immune responses. Annu. Rev. Immunol. 24, 99–146 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Tinoco R., Alcalde V., Yang Y., Sauer K., Zuniga E. I., Cell-intrinsic transforming growth factor-beta signaling mediates virus-specific CD8+ T cell deletion and viral persistence in vivo. Immunity 31, 145–157 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wehbi V. L., Taskén K., Molecular mechanisms for cAMP-mediated immunoregulation in T cells—Role of anchored protein kinase A signaling units. Front. Immunol. 7, 222 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bopp T., et al. , Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J. Exp. Med. 204, 1303–1310 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wen A. Y., Sakamoto K. M., Miller L. S., The role of the transcription factor CREB in immune function. J. Immunol. 185, 6413–6419 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen S.-T., et al. , CLEC5A is a critical receptor in innate immunity against Listeria infection. Nat. Commun. 8, 299 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Witter A. R., Okunnu B. M., Berg R. E., The essential role of neutrophils during infection with the intracellular bacterial pathogen Listeria monocytogenes. J. Immunol. 197, 1557–1565 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miura T., et al. , Roles of endogenous cytokines in liver apoptosis of mice in lethal Listeria monocytogenes infection. FEMS Immunol. Med. Microbiol. 28, 335–341 (2000). [DOI] [PubMed] [Google Scholar]
- 38.Mills K. H. G., Regulatory T cells: Friend or foe in immunity to infection? Nat. Rev. Immunol. 4, 841–855 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Herndler-Brandstetter D., et al. , KLRG1+ effector CD8+ T cells lose KLRG1, differentiate into all memory T cell lineages, and convey enhanced protective immunity. Immunity 48, 716–729.e8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ingram J. T., Yi J. S., Zajac A. J., Exhausted CD8 T cells downregulate the IL-18 receptor and become unresponsive to inflammatory cytokines and bacterial co-infections. PLoS Pathog. 7, e1002273 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tough D. F., Sun S., Zhang X., Sprent J., Stimulation of memory T cells by cytokines. Vaccine 18, 1642–1648 (2000). [DOI] [PubMed] [Google Scholar]
- 42.J. S. Dolina et al., Systemic inflammation drives rapid conversion of conventional CD4+ T cells to Treg in vivo. NCBI Gene Expression Omnibus (GEO). https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE169741. Deposited 26 March 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-Seq data have been deposited in NCBI GEO (GSE169741) (42). All other study data are included in the article and/or supporting information.