Abstract
Background
In the past, progesterone has been advocated for prevention of pre‐eclampsia. Although progestogens were never widely used for this purpose in clinical practice, new hypothesis have emerged which suggest progesterone may have a role in promoting immunological tolerance between the fetus and mother, and may reduce risk of pre‐eclampsia.
Objectives
To assess the effects of progesterone during pregnancy on risk of pre‐eclampsia and its complications.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 January 2011) and the metaRegister of Controlled Trials (2 June 2010).
Selection criteria
Randomised trials evaluating progesterone or other progestogen during pregnancy for prevention of pre‐eclampsia and its complications.
Data collection and analysis
Two review authors independently assessed studies for inclusion and extracted data.
Main results
We included four trials of variable quality (1445 women). Three trials compared progesterone injections, and one compared progestogen vaginal gel, with no progesterone. There was insufficient evidence to demonstrate any clear differences between the two groups on risk of pre‐eclampsia (three trials, 1277 women; risk ratio (RR) 1.25, 95% confidence interval (CI) 0.95 to 1.63), death of the baby (four trials, 2594 babies; RR 1.34, 95% CI 0.78 to 2.31), preterm birth (three trials, 1313 women; RR 1.01, 95% CI 0.93 to 1.10), small‐for‐gestational‐age babies (one trial, 168 women; RR 0.82, 95% CI 0.19 to 3.57), major congenital defects (three trials, 2436 babies; one trial, no events, two trials RR 1.19 , 95% CI 0.31 to 4.52), or any other outcome reported. There were no reported cases of masculinisation of female babies (one trial, 128 women).
Long‐term follow‐up for the children has been reported in one trial, but we have excluded these data from the review as 54% were lost to follow‐up at one year and 80% at 16 years.
In one trial comparing progesterone injections with placebo, over 60% of women in both the progesterone and placebo groups had side effects which were generally mild and most often limited to the injection site.
Authors' conclusions
There is insufficient evidence for reliable conclusions about the effects of progesterone for preventing pre‐eclampsia and its complications. Therefore, progesterone should not be used for this purpose in clinical practice at present.
Plain language summary
Progesterone for preventing pre‐eclampsia and its complications
No good evidence that giving the hormone progesterone to pregnant women will help women and babies avoid the problems of pre‐eclampsia.
Pre‐eclampsia is a serious complication of pregnancy occurring in about 2% to 8% of women. It is identified by increased blood pressure and protein in the urine, but women often suffer no symptoms initially. It can, through constriction of the blood vessels in the placenta, interfere with food and oxygen passing to the baby, thus inhibiting the baby's growth and causing the baby to be born too soon. Women can be affected through problems in their kidneys, liver, brain, and clotting system. One theory is that is that pre‐eclampsia might be associated with a shortage of progesterone, and so it has been suggested that giving women progesterone during pregnancy might help them to avoid pre‐eclampsia. The review found four trials involving 1445 women. There were insufficient data be to be able to say if progesterone helped, and there was very little information on potential adverse outcomes. So progesterone should not be used in pregnancy for the purpose of trying to reduce the incidence of pre‐eclampsia, and further testing is needed.
Background
Description of the condition
Hypertension (high blood pressure) is common during pregnancy. Around 10% of women will have raised blood pressure at some point before delivery. The hypertensive disorders of pregnancy comprise a spectrum of conditions that is usually classified into four categories: (i) gestational hypertension, a rise in blood pressure during the second half of pregnancy; (ii) pre‐eclampsia, usually hypertension with proteinuria (protein in urine) during the second half of pregnancy; (iii) chronic hypertension, a rise in blood pressure prior to pregnancy or before 20 weeks' gestation, and (iv) pre‐eclampsia superimposed on chronic hypertension (NHBPEP 2000). For women with uncomplicated mild to moderate hypertension, pregnancy outcome is similar to that for women with normal blood pressure. Outcome deteriorates if blood pressure is very high or if pre‐eclampsia develops. Pre‐eclampsia is a multisystem disorder, involving the liver, kidneys, brain, and placenta. It affects 2% to 8% of pregnancies (WHO 1988), and is associated with a substantive increase in morbidity and mortality for both the woman and her baby (DH 2002). Complications for the mother may include eclampsia (seizures), stroke, liver or kidney failure, and abnormal blood clotting, and problems for the baby include poor growth and preterm birth.
The cause of pre‐eclampsia is uncertain, but current belief is that reduced blood supply to the placenta leads to abnormal function of endothelial cells, possibly as a result of oxidative stress. Endothelial dysfunction results in generalised vasoconstriction, platelet activation and thrombosis, and decreased plasma volume, with subsequently reduced blood supply to multiple organs. Pre‐eclampsia is discussed in more detail in the generic protocol for this review (Generic Protocol 05).
Description of the intervention
Progesterone is a hormone which plays an essential role in reproduction, both in the regulation of the menstrual cycle, and in the maintenance of pregnancy. It is used for a range of gynaecological problems such as heavy uterine bleeding, fertility control, and postmenopausal hormone replacement. In addition, it has been suggested that progesterone may have a role in the treatment of premenstrual syndrome (Ford 2009), threatened miscarriage (Haas 2008; Wahabi 2007), and preterm birth (Dodd 2006).
During pregnancy, progesterone stimulates the growth and differentiation of endometrium to allow implantation, induces immunological tolerance to the fetus, and inhibits uterine contractions (Szekeres‐Bartho 1992). Progesterone appears, at least in part, to influence the vascular adaptations of normal pregnancy by decreasing responsiveness of blood vessels to vasoconstrictors and inducing vasodilatation (Buhimschi 1995; Ito 1992; Liao 1996; Radwanska 1993).
A number of progesterone derivatives are now commercially available. These include dydrogesterone, hydroxyprogesterone, medroxyprogesterone, norethisterone, norgestrel, desogestrel, norgestimate, gestodene, and levonorgestrel. Progestogens may be administered orally, as suppositories or pessaries, by local patches, or as injections. However, progesterone itself is given as injections, vaginal pessaries or rectal suppositories, as absorption from the oral route is unreliable.
Whilst progesterone is not known to be harmful during pregnancy, there is little information about its long‐term safety for the fetus or mother. Progesterone does not cause masculinisation of the female fetus, although this is a concern with some of the other synthetic progestogens. Progestogens with more androgenic activity, which may cause masculinisation, should not be used during pregnancy. Side effects of progestogens include symptoms such as bloating, fluid retention, weight gain, breast tenderness, headache, nausea, acne, hirsutism, skin reactions, and depression.
How the intervention might work
In the past, administration of progesterone has been suggested for prevention and treatment of pre‐eclampsia. In the 1930s, Robson and Paterson observed that a toxic state, convulsions, and death could be produced experimentally in pregnant rabbits if progesterone secretion was reduced in the second half of pregnancy, by removal of the embryo. It was suggested that the 'toxaemia' of pregnancy (pre‐eclampsia) might also be associated with progesterone insufficiency (Robson 1937). Results from several case series were not particularly encouraging (Bennett 1939; Marsden 1937; Robson 1937; Young 1937), and the hypothesis seemed to have been discounted. Two decades later, Katharina Dalton reported the observation that women who developed pre‐eclampsia had a high incidence of premenstrual syndrome when they were not pregnant. She also observed that women who experienced what she referred to as 'toxaemic symptoms' such as tiredness, depression, nausea, irritability and headache in early pregnancy were more likely to go on to develop pre‐eclampsia (Dalton 1960). These symptoms she considered to be similar to those of premenstrual syndrome. She therefore suggested that premenstrual syndrome was akin to pre‐eclampsia, and that both were related to progesterone deficiency. The hypothesis that progesterone reduced the risk of pre‐eclampsia was tested by Dalton (Dalton 1954; Dalton 1957; Dalton 1962), but the use of progesterone for prevention of pre‐eclampsia has never become widespread in clinical practice.
More recently, a new hypothesis has emerged about the potential role of progesterone in the development of pre‐eclampsia. Special placental cells called cytotrophoblasts invade into maternal blood vessels during early placental development. These cells express a protein called HLA‐G which is thought to play a key role in enhancing immunological tolerance between the "foreign" fetal tissue and maternal cells (Kovats 1990). Studies indicate that HLA‐G is reduced in the placenta and serum of women with pre‐eclampsia (Yie 2004), and therefore may play a role in impaired placentation leading to the development of pre‐eclampsia. Progesterone has been shown to increase the expression of HLA‐G protein in placental cytotrophoblast cells (Yie 2006), which supports the hypothesis that progesterone may reduce the risk of pre‐eclampsia.
Maternal serum progesterone levels increase in normal pregnancy, but a deficiency of progesterone has not been found in association with pre‐eclampsia (Parker 1976; Tamimi 2003; Zeisler 2000).
Why it is important to do this review
Progesterone has been proposed for prevention of pre‐eclampsia in the past, and again, more recently. The evidence to support this hypothesis has been weak, but new data on the role of progesterone in promoting immunological tolerance appear plausible. Progesterone has never been widely used in clinical practice for this purpose; nevertheless, it remains relevant to review the evidence about the effects of progesterone, and other progestogens, when used for prevention of pre‐eclampsia.
Other Cochrane reviews have assessed the role of progesterone for prevention of preterm birth (Dodd 2006) and of miscarriage (Haas 2008). Other interventions for prevention of pre‐eclampsia are listed in the generic protocol for this review (Generic Protocol 05).
Objectives
To assess the effects of progesterone, or any other progestogen, for prevention of pre‐eclampsia and its complications.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised trials evaluating progesterone or other progestogens for prevention of pre‐eclampsia. We excluded trials with quasi‐random design and crossover design.
Types of participants
We included pregnant women with normal blood pressure or high blood pressure without proteinuria, regardless of gestation at trial entry. If possible, we grouped women on the basis of their risk of developing pre‐eclampsia at trial entry as follows.
(1) Women with normal blood pressure
(a) High risk: defined as having one or more of the following: diabetes, renal disease, thrombophilia, autoimmune disease, previous severe or early onset pre‐eclampsia, or multiple pregnancy. (b) Moderate risk: defined as none of the above, but having either previous pre‐eclampsia that was not severe or early onset (or severity unspecified), or a first pregnancy and at least one of the following: teenager or over 35 years age, family history of pre‐eclampsia, obesity (body mass index greater than 30), increased sensitivity to Angiotensin II, positive roll‐over test, abnormal uterine artery doppler scan. (c) Low risk: defined as pregnancy that does not qualify as either high or moderate risk. (d) Undefined risk: when the risk is unclear or not specified.
(2) Women with high blood pressure, without proteinuria
These women are all at high risk of developing pre‐eclampsia. They fall into two groups. (a) Gestational hypertension: hypertension detected for the first time after 20 weeks' gestation, in the absence of proteinuria. (b) Chronic hypertension: essential or secondary hypertension detected prior to pregnancy or before 20 weeks' gestation. Some women with chronic hypertension may have longstanding proteinuria due to their underlying disease. We included these women, as their proteinuria was not due to pre‐eclampsia.
We excluded women if they had established pre‐eclampsia.
If a trial included women with pre‐eclampsia as well as those with non‐proteinuric hypertension (gestational or chronic), where possible, we planned to include only the women with non‐proteinuric hypertension in the review. For trials that did not report results separately for the two groups, we planned to include the data but present it as a separate subgroup. However, we did not find any such trials.
Types of interventions
We included the following comparisons:
any progestogen versus placebo or no intervention;
any progestogen versus any other intervention for preventing pre‐eclampsia; and
one type of progestogen versus another progestogen, during pregnancy, if appropriate.
We included all types of progestogen preparations, as well as different dosage regimens and routes of administration.
We excluded trials if the intended duration of therapy at trial entry was less than seven days. This was done mainly to exclude studies evaluating short‐term physiological effects of progesterone, and also because it is unlikely that such short therapy could influence pregnancy outcomes, based on what is known of the pathophysiology of pre‐eclampsia.
Types of outcome measures
We included the following outcomes. The definitions used for each outcome are summarised below. We have described trials that used acceptable variations of these definitions, or that did not define their outcomes were also included, and definitions, where available, in the table Characteristics of included studies. If an important outcome was not reported, we attempted to contact the authors.
For the woman
Primary outcome
(1) Pre‐eclampsia: hypertension (blood pressure at least 140 mmHg systolic or 90 mmHg diastolic) with proteinuria (at least 300 mg protein in a 24‐hour urine collection or 1+ on dipstick). For women with chronic hypertension and proteinuria at trial entry, pre‐eclampsia was defined as sudden worsening of proteinuria and/or hypertension, or other signs and symptoms of pre‐eclampsia after 20 weeks' gestation.
Secondary outcomes
(2) Death: during pregnancy or up to 42 days after the birth. (3) Severe morbidity related to pre‐eclampsia including: eclampsia, liver or renal failure, haemolysis elevated liver enzymes and low platelets (HELLP) syndrome, disseminated intravascular coagulation, stroke, and pulmonary oedema. We have reported these outcomes individually, and as a composite measure where the information is available. (4) Severe pre‐eclampsia: pre‐eclampsia with two or more signs or symptoms of severe disease, such as severe hypertension, severe proteinuria (usually 3 g/24 h, or 3+ on dipstick), visual disturbances, exaggerated tendon reflexes, upper abdominal pain, impaired liver function tests, high serum creatinine, low platelets, fetal growth restriction, or reduced liquor volume. (5) Early onset of pre‐eclampsia: pre‐eclampsia before 34+0 weeks. (6) Severe hypertension: blood pressure at least 160 mmHg systolic or 110 mmHg diastolic. (7) Gestational hypertension: new onset of hypertension after 20 weeks' gestation. (8) Use of antihypertensive drugs or need for additional antihypertensive drugs. (9) Abruption of the placenta or antepartum haemorrhage. (10) Elective delivery: induction of labour or elective caesarean section. (11) Caesarean section: emergency plus elective. (12) Postpartum haemorrhage: blood loss of 500 ml or more. (13) Side effects related to progesterone such as skin reactions, depression, etc, progesterone stopped due to side effects. (14) Use of health service resources: antenatal clinic visits, visit to day care unit, antenatal hospital admission, intensive care. (15) Women's experiences and views of progesterone.
For the child
Primary outcomes
(1) Death: including all deaths before birth and up to discharge from hospital. (2) Preterm birth: birth before 37+0 weeks' gestation. (3) Small‐for‐gestational age: growth below the third centile, or lowest centile reported.
Secondary outcomes
(4) Apgar score at five minutes: low (less than seven) and very low (less than four) or lowest reported. (5) Endotracheal intubation or use of mechanical ventilation. (6) Neonatal morbidity: respiratory distress syndrome, chronic lung disease, sepsis, retinopathy of prematurity, and intraventricular haemorrhage. (7) Long‐term growth and development: blindness, deafness, seizures, poor growth, neurodevelopmental delay, and cerebral palsy. (8) Side effects associated with progesterone. (9) Use of hospital resources: admission to neonatal intensive care unit, duration of hospital stay after delivery.
Economic outcome
(1) Costs to health service resources: short term and long term for both mother and baby.
(2) Costs to the woman, her family, and society associated with exercise.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (31 January 2011).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly search of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
In addition, we searched the metaRegister of Controlled Trials for planned and ongoing trials (2 June 2010) (see:Appendix 1 for search terms used).
In the previous version of the review, we searched CENTRAL (The Cochrane Library 2006, Issue 2), and EMBASE (1974 to August 2005) using the search strategies listed in Appendix 2.
Searching other resources
We searched the reference lists of relevant reports.
We did not apply any language restrictions.
Data collection and analysis
For the methods used when assessing the trials identified in the previous versions of this review, seeAppendix 3.
For this update, we used the following methods when assessing the trials identified by the updated search (Nassar 2008; Rouse 2007; STOPPIT 2009; Uckele 2010).
Selection of studies
Two review authors independently assessed all the potentially eligible studies for inclusion. We resolved any differences in opinion by discussion.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors independently extracted data, using the form. We resolved any discrepancies through discussion. When information was unclear or not available, we contacted the authors of the original reports to provide further details.
We entered data into the Review Manager software (RevMan 2008), and checked for accuracy.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009). We resolved any disagreement by discussion.
(1) Random sequence generation (checking for possible selection bias)
We describe for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator),
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number) or,
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We describe for each included study the method used to conceal allocation to interventions prior to assignment and assess whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3) Blinding of participants and personnel (checking for possible performance bias)
We describe for each included study the methods used, if any, to blind study participants, personnel and outcome assessors from knowledge of which intervention a participant received. We consider studies to be at low risk of bias if they were blinded, or if we judge that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel;
low, high or unclear risk of bias for outcome assessors
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We state whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported, or was supplied by the trial authors, we re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (where less than 5% of participants were excluded from the analyses);
high risk of bias (5% to 20% of participants were excluded from the analyses);
unclear risk of bias.
We excluded studies if more than 20% of participants were excluded from the analysis.
We have analysed data based on the group to which the participants were randomised, regardless of whether they received the allocated intervention or not.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by 1 to 5 above)
We described for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Handbook (Higgins 2009). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. We planned to explore overall risk of bias by conducting a sensitivity analyses ‐ see 'Sensitivity analysis'.
Measures of treatment effect
Dichotomous data
For dichotomous data, we have presented results as summary risk ratios (RR) with 95% confidence intervals (CI).
Continuous data
For continuous data, we have presented results as the mean difference if outcomes are measured in the same way between trials. The standardised mean difference is used to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
If cluster‐randomised trials are included in the analyses along with individually randomised trials in future updates, we will adjust their sample sizes using the methods described in the Handbook (Higgins 2009) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If ICCs from other sources are used, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC if appropriate. If both cluster‐randomised trials and individually‐randomised trials are identified, we will synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and if we consider the interaction between the effect of intervention and the choice of randomisation unit to be unlikely.
This review includes some studies which recruited only women with multiple pregnancy (Rouse 2007; STOPPIT 2009). For these studies, and outcomes which used the number of babies as the denominator, we adjusted the sample size by using the cluster trial methods specified above, where each woman was regarded as a randomised cluster. We obtained an ICC from the STOPPIT 2009 trial for analyses. An ICC was not available from Rouse 2007 so we used the ICC from STOPPIT 2009 to analyse the study data from Rouse 2007, as we considered the trials sufficiently similar. we meta‐analysed effect estimates and their standard errors using the generic inverse‐variance method in RevMan RevMan 2008. Data for the outcomes from these trials are available separately in additional tables in the review (Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7).
1. Data table: fetal or neonatal death.
| Study | Progesterone | Control | Comments | ||
| Events | Total | Events | Total | ||
| STOPPIT 2009 | 14 | 494 | 10 | 494 | ICC = 0.87 |
| Rouse 2007 | 34 | 650 | 22 | 660 | ICC = 0.87 |
| Hauth 1983 | 3 | 80 | 3 | 88 | |
| Dalton 1962 | 1 | 62 | 3 | 66 | |
| Total | 52 | 1286 | 38 | 1308 | |
ICC: intracluster correlation co‐efficient
2. Data table: serious neonatal morbidity.
| Outcome | Study | Progesterone | Control | Comments | ||
| Events | Total | Events | Total | |||
| Retinopathy of prematurity | Rouse 2007 | 0 | 632 | 0 | 648 | No events so RR not estimable ‐ study not part of RevMan Analysis. |
| Respiratory distress syndrome | Rouse 2007 | 96 | 632 | 87 | 648 | ICC = 0.87 |
| Sepsis | Rouse 2007 | 24 | 632 | 26 | 648 | ICC = 0.87 |
| Necrotising enterocolitis | Rouse 2007 | 3 | 632 | 4 | 648 | ICC = 0.87 |
| Bronchopulmonary dysplasia | Rouse 2007 | 19 | 632 | 17 | 648 | ICC = 0.87 |
| Intraventricular haemorrhage | Rouse 2007 | 7 | 632 | 6 | 648 | ICC = 0.87 |
| Periventricular leukomalacia | Rouse 2007 | 5 | 632 | 6 | 648 | ICC = 0.87 |
| Seizures | Rouse 2007 | 5 | 632 | 5 | 648 | ICC = 0.87 |
| Mechanical ventilation | Rouse 2007 | 70 | 632 | 77 | 648 | ICC = 0.87 |
ICC: intracluster correlation co‐efficient
3. Data table: admission to neonatal intensive care unit.
| Study | Progesterone | Control | Comments | ||
| Events | Total | Events | Total | ||
| STOPPIT 2009 | 167 | 494 | 158 | 494 | ICC = 0.87 |
| Total | 167 | 494 | 158 | 494 | |
ICC: intracluster correlation co‐efficient
4. Data table: Apgar score at five minutes less than seven.
| Study | Progesterone | Control | Comments | ||
| Events | Total | Events | Total | ||
| Rouse 2007 | 27 | 632 | 33 | 648 | ICC = 0.87 |
| Total | 27 | 632 | 33 | 648 | |
ICC: intracluster correlation co‐efficient
5. Data table: any congenital malformations.
| Study | Progesterone | Control | Comments | ||
| Events | Total | Events | Total | ||
| Hauth 1983 | 3 | 80 | 2 | 88 | |
| Rouse 2007 | 3 | 632 | 4 | 648 | ICC = 0.87 |
| STOPPIT 2009 | 0 | 494 | 0 | 494 | No events so RR not estimable ‐ study not part of RevMan Analysis. |
| Total | 6 | 1206 | 6 | 1230 | |
ICC: intracluster correlation co‐efficient
6. Data table: patent ductus arteriosus.
| Study | Progesterone | Control | Comments | ||
| Events | Total | Events | Total | ||
| Rouse 2007 | 18 | 632 | 31 | 648 | ICC = 0.87 |
| Total | 18 | 632 | 31 | 648 | |
ICC: intracluster correlation co‐efficient
7. Data table: masculinisation of the female fetus.
| Study | Progesterone | Control | Comments | ||
| Events | Total | Events | Total | ||
| Dalton 1962 | 0 | 62 | 0 | 66 | No events so RR not estimable ‐ study not part of RevMan Analysis. |
| Total | 0 | 62 | 0 | 66 | |
In the future, if it is not possible to obtain enough information to make any adjustment for the effects of multiple pregnancies, we will analyse the data as if babies from multiple pregnancies were independent, using the number of babies as the denominator. This will give an unbiased result but the width of the confidence intervals will be underestimated.
We will also acknowledge any heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit if appropriate.
Dealing with missing data
For included studies, we noted levels of attrition. We will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis, when appropriate, once sufficient data become available.
For all outcomes, we have carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempt to include all participants randomised to each group in the analyses, and analysed all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if I² was greater than 30% and either T² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
Once there are 10 or more studies in the meta‐analysis, we would explore reporting biases (such as publication bias) using funnel plots. We would assess funnel plot asymmetry visually, and use formal tests for funnel plot asymmetry. For continuous outcomes we would use the test proposed by Egger 1997, and for dichotomous outcomes we would use the test proposed by Harbord 2006. If we detected asymmetry in any of these tests or by a visual assessment, we would perform an exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2008). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects would differ between trials, or if substantial statistical heterogeneity was detected, we would use random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. We would treat the random‐effects summary as the average range of possible treatment effects and discuss the clinical implications of treatment effects differing between trials. If the average treatment effect would not be clinically meaningful then we would not combine trials.
If we used random‐effects analyses, we would present the results as the average treatment effect with its 95% confidence interval, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
We based pre‐specified subgroup analyses on the:
risk of women at trial entry: high, moderate, low, or undefined;
gestation at trial entry: at and before 20 weeks' gestation, or after 20 weeks' gestation.
We will include only the primary outcomes listed above in the subgroup analyses. We will conduct these subgroup analyses once sufficient data become available.
For fixed‐effect inverse variance meta‐analyses, we would assess differences between subgroups by interaction tests. For random‐effects and fixed‐effect meta‐analyses using methods other than inverse variance, we would assess differences between subgroups by inspection of the subgroups’ confidence intervals; non‐overlapping confidence intervals would indicate a statistically significant difference in treatment effect between the subgroups.
Where appropriate, we would also use subgroup analyses to investigate any substantial heterogeneity, if we detected this using the methods specified above.
Sensitivity analysis
We planned sensitivity analyses:
to investigate heterogeneity, by excluding studies at high risk of bias, for example those with inadequate sequence generation or concealment of allocation, or high levels of missing data;
for studies with cluster‐randomisation, to assess the impact of alternative values for the ICC.
We will undertake these analyses if appropriate, once sufficient data become available.
Results
Description of studies
We included four studies with 1445 women in this review. Details of the studies are available in the table of Characteristics of included studies.
Two studies were conducted in the United Kingdom (Dalton 1962; STOPPIT 2009) and two in the USA (Hauth 1983; Rouse 2007). Two were multicentre trials (Rouse 2007; STOPPIT 2009).
Participants were recruited between 16 and 28 weeks' gestation. In one study, women had normal blood pressure (Dalton 1962), in another (STOPPIT 2009), less than 1% had high blood pressure, and in the other two, blood pressure at trial entry was not reported. Two studies recruited twin pregnancies only (Rouse 2007; STOPPIT 2009).The risk of developing pre‐eclampsia for women at trial entry is unclear in two trials, and was high risk in the other two studies.
Progesterone was given by intramuscular injection in three studies; in one (Dalton 1962) dosage was from 300 mg daily to 50 mg on alternate days, depending on changes in intensity of 'toxaemic' symptoms, and in the other two studies, 17 alpha‐hydroxyprogesterone was given 1000 mg (Hauth 1983), or 250 mg weekly (Rouse 2007). In the fourth study (STOPPIT 2009), progesterone was given as a vaginal gel of 90 mg daily. Three were placebo controlled trials, but for the fourth study (Dalton 1962), women in the control group were offered simple treatments such as alkalis, analgesics, sedatives, and antihistamines for the relief of their symptoms. It was not reported how many women received these alternative interventions. Outcomes reported included development of pre‐eclampsia, which in Dalton 1962 was defined as blood pressure above 140/90 mmHg with either oedema or albuminuria after 28 weeks. As the definition of pre‐eclampsia no longer includes oedema, the number of women reported to have developed pre‐eclampsia is likely to be an overestimate. In the review, we therefore used the number of women with proteinuria as a better estimate of the number with pre‐eclampsia. In Rouse 2007, data were reported as 'hypertensive disorder' and included both gestational hypertension and pre‐eclampsia, but data were not available separately for the two outcomes so it has been reported under pre‐eclampsia in this review, but this may be an overestimation. Side effects were reported in three studies, but data have been excluded from two studies because of attrition above 23% in one (STOPPIT 2009) and because side effects have been reported for the progesterone group only in the other (Dalton 1962). For the children, a range of outcomes have been reported. Development at age one year, and intelligence and psychosexual personality at 16 years of age have been reported, but the data are excluded from this review due to large losses to follow‐up (Dalton 1962).
We excluded five studies from the review: three were not randomised trials (Dalton 1976; Sammour 1975; Sammour 1982), and two of these recruited women with established pre‐eclampsia. The fourth excluded study was a quasi‐randomised study (El‐Zibdeh 2005), and in the fifth, we were unable to use data as presented (Hartikainen 1980). Other trials evaluating progesterone during pregnancy were not considered to be potentially eligible for this review, as they focused on preterm birth or miscarriage and did not consider pre‐eclampsia or gestational hypertension as outcomes.
Risk of bias in included studies
Two included studies are of uncertain quality because of inadequate reporting of methods used for randomisation and allocation concealment. Two studies were of good quality. The outcome assessment was blinded for all studies, and the participants were also blinded in all except Dalton 1962. The follow‐up was complete for Hauth 1983, and attrition was around 1% and balanced in both groups for Rouse 2007 and STOPPIT 2009; but in Dalton 1962, 22 participants (15%) were excluded from analysis for pregnancy outcome for various reasons. Although long‐term follow‐up of the children was attempted, there is considerable potential for bias due to large losses to follow‐up (54% at one year and 80% at 16 years of age).
Heterogeneity was not statistically significant for any of the outcomes reported.
Effects of interventions
Four trials with 1445 women compared progesterone with no progesterone.
Outcomes for women
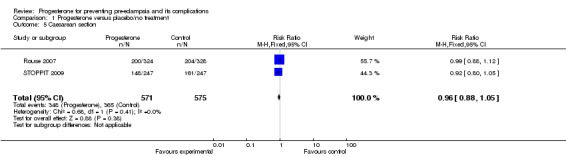
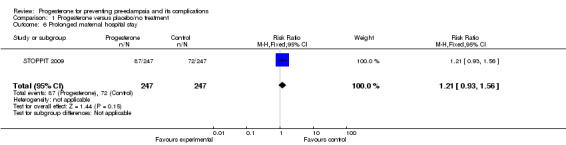
There were insufficient data to demonstrate any clear differences between the two groups on the risk of pre‐eclampsia (three trials; 1277 women; RR 1.25, 95% CI 0.95 to 1.63) or pregnancy‐induced hypertension (one trial, 168 women; RR 0.92, 95% CI 0.42 to 2.01). The effect of progesterone was also unclear on the risk of caesarean section (two trials, 1146 women; RR 0.96, 95% CI 0.88 to 1.05), and prolonged hospital stay (one trial, 494 women; RR 1.21, 95% CI 0.93 to 1.56). There were no maternal deaths in the one trial that reported this outcome (STOPPIT 2009). No other maternal outcomes were reported.
Outcomes for baby
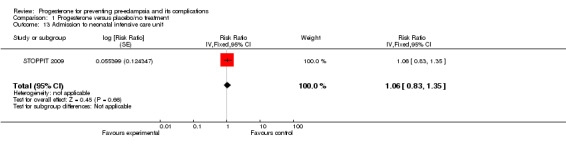
Similarly, there were insufficient data for any reliable conclusions about the potential effects of progesterone on the RR of stillbirth or neonatal death (four trials, 2594 babies; RR 1.34, 95% CI 0.78 to 2.31), and small‐for‐gestational‐age babies (one trial, 168 babies; RR 0.82, 95% CI 0.19 to 3.57). There was no clear difference in the risk of serious neonatal morbidity in the one trial that reported this outcome (Rouse 2007), or in the risk of preterm birth (three trials, 1313 women; RR 1.01, 95% CI 0.93 to 1.10), admission to the neonatal intensive care unit (one trial, 988 babies; RR 1.06, 95% CI 0.83 to 1.35), or Apgar scores (one trial, 1280 babies; RR 0.84, 95% CI 0.43 to 1.65).
Adverse effects
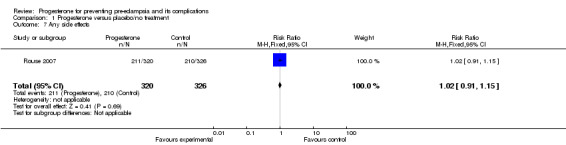
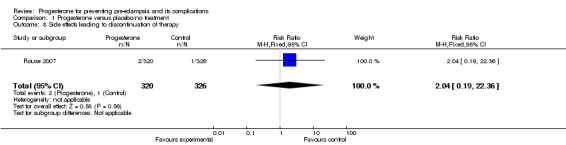
There were no reported cases of masculinisation of the female fetus (one trial, 128 women), and the effects of progesterone on risk of major congenital malformations (not defined) is unclear (three trials, 2436 babies: one trial, no events, two trials RR 1.19, 95% CI 0.31 to 4.52). Progesterone injections were associated with urticarial skin reactions in 18% of women in Dalton 1962, but in this study the comparison group did not receive an injection. In Rouse 2007, more than 60% of women in both the progesterone and placebo groups had side effects that were generally mild and most often limited to the injection site, but led to the discontinuation of injections in three women (two in the progesterone group and one in the placebo group) who had more intense reactions.
Discussion
The aim of this review was to assess the effects of progesterone on prevention of pre‐eclampsia and its complications. Of the four included studies, one (Dalton 1962) aimed to test the hypothesis that women at risk of pre‐eclampsia developed 'toxaemic symptoms' associated with progesterone deficiency, and so administration of progesterone would reduce these symptoms and prevent the onset of pre‐eclampsia. The other three primarily assessed the effects of progesterone on prevention of preterm birth, but also reported data for pre‐eclampsia as a secondary outcome (albeit that these data were not in a format that enabled them to be incorporated into the review for all studies (Hauth 1983)). Two studies were of uncertain methodological quality, and two were of good quality. Compliance with medication was assessed in two studies. Women took around 95% of their medication in one study (Rouse 2007), but in another only 58% of women reported taking at least 80% or the treatment (STOPPIT 2009). There is insufficient evidence for any reliable conclusions about the benefits or risks associated with progesterone therapy for prevention of pre‐eclampsia or its consequences.
In one study (Dalton 1962), about one‐fifth of women receiving progesterone injections had an urticarial skin reaction, all of which responded to antihistamines, but there was no control group in that study. In Rouse 2007, over 60% of women in both the progesterone and placebo groups had side effects but there was no significant difference between the two groups in risk of side effects, and these appeared to be related to administration of injections. Although data on side effects haven't been included from STOPPIT 2009 due to attrition, even when progesterone or placebo was given vaginally, over 45% of women in both groups reported a wide range of side effects ranging from bloating and nausea to increased vaginal discharge. As many of the side effects associated with progesterone use are similar to those of pregnancy (bloating, fluid retention, breast tenderness, weight gain, nausea, headache dizziness, drowsiness, depression and skin reactions), to elucidate any differences reliably would require well‐controlled and blinded comparisons.
There was no clear difference in the risk of congenital malformations between the two groups. Although data on long‐term follow‐up of some children born to women recruited (Dalton 1962) have been reported, there is considerable potential for bias due to large losses to follow‐up. Less than half the children were available for assessment at one year, and only 20% were available at 16 years of age. Data at one year were collected by a questionnaire administered either by a 'clinic doctor' or by a health visitor. At 16 years, children were assessed by an interviewer in their own homes. At age one year, it was reported that 92% of children in the progesterone group were able to stand alone compared to 68% of controls, and 62% were walking compared to 35% of controls. There were no apparent differences between the two groups in talking or teeth eruption. One boy in the progesterone group had small testes, whilst in the control group two had undescended testes and three had breast engorgement. Assessment at 16 years of age did not appear to show any significant differences between the two groups with regards to intelligence, perception, personality or psychosexual identity, although these data must be interpreted with caution due to the large losses to follow‐up.
An additional limitation is that progesterone administration requires frequent intramuscular injections. Its use in clinical practice could only be justified if supported by clear evidence of benefit, and adequate reassurance about safety, in both the short and the long term, for both the child and the woman. Currently such evidence is not available, and so progesterone should not be used for prevention of pre‐eclampsia and its complications outside of clinical trials.
Authors' conclusions
Implications for practice.
There is insufficient evidence to recommend progesterone for prevention of pre‐eclampsia and its complications. Progesterone should therefore not be used for this purpose in clinical practice at present.
Implications for research.
As new and plausible hypotheses have emerged, further trials of progesterone specifically to prevent pre‐eclampsia may be justified. As there is a resurgence of interest in progesterone for prevention of preterm birth, those planning trials to evaluate progesterone for women at risk of preterm birth should ensure that pre‐eclampsia is one of the outcomes reported.
What's new
| Date | Event | Description |
|---|---|---|
| 31 January 2011 | New search has been performed | Search updated and four new studies found (11 citations). Two studies added to included studies (Rouse 2007; STOPPIT 2009), and two studies added to Ongoing studies (Nassar 2008; Uckele 2010). Conclusions not changed. The methods of the review were also updated in line with the updated methods for the Pregnancy and Childbirh Group. |
History
Protocol first published: Issue 2, 2005 Review first published: Issue 4, 2006
| Date | Event | Description |
|---|---|---|
| 20 September 2008 | Amended | Converted to new review format. |
Acknowledgements
We would like to thank Graeme Maclennan, Jane Norman, Elizabeth Thom, and Dweight Rouse for providing unpublished data for two trials (Rouse 2007; STOPPIT 2009).
Appendices
Appendix 1. Search terms used in the metaRegister
The following terms were searched for separately and each set of search results was screened for relevant reports:
pre‐eclampsia, pih, HELLP, eclampsia, toxemia, toxaemia
Appendix 2. Additional search strategies
Authors searched CENTRAL (The Cochrane Library 2006, Issue 2), and EMBASE (1974 to August 2005) by combining the terms progestins, progestogens, progestogen, progesterone, norethisterone, norethindrone, dydrogesterone, medroxyprogesterone, and levonorgestrel with the CENTRAL and EMBASE search strategies listed in the generic protocol (Generic Protocol 05).
Appendix 3. Methods used to assess trials included in previous versions of this review
The following methods were used to assess Dalton 1962; Hauth 1983.
Selection of studies
Two review authors independently assessed potentially eligible studies for inclusion. Any differences in opinion were resolved by discussion.
Assessment of study quality
Two review authors independently assessed the quality of each trial using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2005). Methods used for generation of the randomisation sequence are described for each trial, where possible. Each study was assessed for quality of concealment of allocation, completeness of follow up, and blinding. If data were missing, we attempted to contact trialists to provide further details.
(1) Allocation concealment
A quality score for concealment of allocation was assigned to each trial, using the following criteria: (A) adequate concealment of allocation, such as telephone randomisation, consecutively numbered, sealed opaque envelopes; (B) unclear whether concealment of allocation was adequate; (C) inadequate concealment of allocation such as open random‐number tables, sealed envelopes that were not numbered and opaque.
(2) Completeness of follow up Completeness of follow up was assessed using the following criteria: (A) less than 5% of participants excluded from analysis; (B) 5% to 10% of participants excluded from analysis; (C) more than 10% and up to and including 20% of participants excluded from analysis.
Studies were excluded if: (1) more than 20% of participants were excluded from the analysis; (2) more than 10% of participants were not analysed in their randomised groups and it was not possible to restore participants to the correct group; (3) more than 10% difference in loss of participants between groups.
Data were analysed based on the group to which the participants were randomised, regardless of whether they received the allocated intervention or not. Where data were missing, clarification was sought from the authors.
(3) Blinding
Blinding was assessed using the following criteria:
blinding of participants (yes/no/unclear or unspecified);
blinding of caregiver (yes/no/unclear or unspecified);
blinding of outcome assessment (yes/no/unclear or unspecified).
Data extraction and data entry
Two review authors independently extracted data. We entered data onto the Review Manager software (RevMan 2003), and these were double checked for accuracy.
Statistical analyses
We carried out statistical analyses using Review Manager (RevMan 2003). Results are presented as summary relative risk with 95% confidence intervals. At present, only one trial is included, but when sufficient data become available, we will assess heterogeneity between trials using the I² statistic. In the absence of significant heterogeneity, we will pool results using a fixed‐effect model. If substantial heterogeneity is detected (I² more than 50%), we will explore possible causes and perform subgroup analyses for the main outcomes. Heterogeneity that is not explained by subgroup analyses may be modelled using a random‐effects analysis, if appropriate.
Sensitivity analyses
We planned to do a sensitivity analysis to explore the effects of trial quality based on concealment of allocation, by excluding studies with clearly inadequate allocation concealment (rated C). This analysis will be undertaken once sufficient data are available.
Subgroup analyses
Prespecified subgroup analyses for the main outcomes were based on the:
risk of women at trial entry: high, moderate, low, or undefined;
gestation at trial entry: at and before 20 weeks' gestation, or after 20 weeks' gestation.
These subgroup analyses will be conducted once sufficient data become available.
Data and analyses
Comparison 1. Progesterone versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pre‐eclampsia | 3 | 1277 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.95, 1.63] |
| 2 Pregnancy‐induced hypertension | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.42, 2.01] |
| 3 Eclampsia | 1 | 494 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Maternal death | 1 | 494 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Caesarean section | 2 | 1146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.88, 1.05] |
| 6 Prolonged maternal hospital stay | 1 | 494 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.93, 1.56] |
| 7 Any side effects | 1 | 646 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.91, 1.15] |
| 8 Side effects leading to discontinuation of therapy | 1 | 646 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.19, 22.36] |
| 9 Fetal or neonatal death | 4 | Risk Ratio (Fixed, 95% CI) | 1.34 [0.78, 2.31] | |
| 10 Preterm birth | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Preterm birth < 37 weeks | 3 | 1313 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.93, 1.10] |
| 10.2 Preterm birth < 34 weeks | 1 | 490 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.91, 1.79] |
| 10.3 Preterm birth < 32 weeks | 1 | 655 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.82, 1.66] |
| 10.4 Preterm birth < 28 weeks | 1 | 655 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.75, 2.32] |
| 11 Small‐for‐gestational‐age baby | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.19, 3.57] |
| 12 Serious neonatal morbidity | 1 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| 12.1 Respiratory distress syndrome | 1 | Risk Ratio (Fixed, 95% CI) | 1.13 [0.78, 1.63] | |
| 12.2 Sepsis | 1 | Risk Ratio (Fixed, 95% CI) | 0.95 [0.45, 1.99] | |
| 12.3 Necrotising enterocolitis | 1 | Risk Ratio (Fixed, 95% CI) | 0.77 [0.10, 5.92] | |
| 12.4 Bronchopulmonary dysplasia | 1 | Risk Ratio (Fixed, 95% CI) | 1.15 [0.47, 2.77] | |
| 12.5 Intraventricular haemorrhage | 1 | Risk Ratio (Fixed, 95% CI) | 1.20 [0.27, 5.27] | |
| 12.6 Periventricular leukomalacia | 1 | Risk Ratio (Fixed, 95% CI) | 0.85 [0.17, 4.30] | |
| 12.7 Seizures | 1 | Risk Ratio (Fixed, 95% CI) | 1.03 [0.19, 5.55] | |
| 12.8 Mechanical ventilation | 1 | Risk Ratio (Fixed, 95% CI) | 0.93 [0.61, 1.41] | |
| 13 Admission to neonatal intensive care unit | 1 | Risk Ratio (Fixed, 95% CI) | 1.06 [0.83, 1.35] | |
| 14 Apgar score at 5 minutes < 7 | 1 | Risk Ratio (Fixed, 95% CI) | 0.84 [0.43, 1.65] | |
| 15 Congenital malformations | 2 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| 15.1 Patent ductus arteriosus | 1 | Risk Ratio (Fixed, 95% CI) | 0.60 [0.27, 1.30] | |
| 15.2 Any major congenital malformations | 2 | Risk Ratio (Fixed, 95% CI) | 1.19 [0.31, 4.52] |
1.1. Analysis.
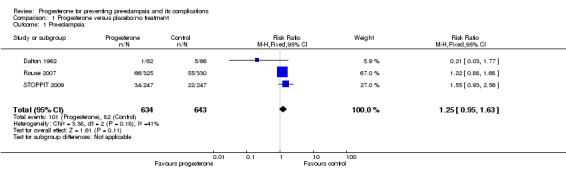
Comparison 1 Progesterone versus placebo/no treatment, Outcome 1 Pre‐eclampsia.
1.2. Analysis.
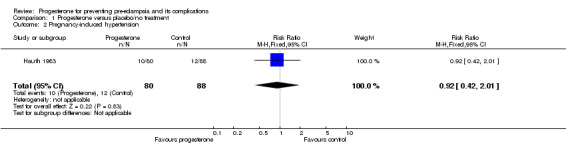
Comparison 1 Progesterone versus placebo/no treatment, Outcome 2 Pregnancy‐induced hypertension.
1.3. Analysis.
Comparison 1 Progesterone versus placebo/no treatment, Outcome 3 Eclampsia.
1.4. Analysis.
Comparison 1 Progesterone versus placebo/no treatment, Outcome 4 Maternal death.
1.5. Analysis.
Comparison 1 Progesterone versus placebo/no treatment, Outcome 5 Caesarean section.
1.6. Analysis.
Comparison 1 Progesterone versus placebo/no treatment, Outcome 6 Prolonged maternal hospital stay.
1.7. Analysis.
Comparison 1 Progesterone versus placebo/no treatment, Outcome 7 Any side effects.
1.8. Analysis.
Comparison 1 Progesterone versus placebo/no treatment, Outcome 8 Side effects leading to discontinuation of therapy.
1.9. Analysis.
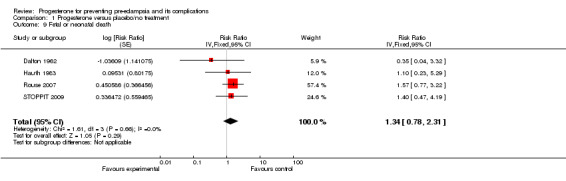
Comparison 1 Progesterone versus placebo/no treatment, Outcome 9 Fetal or neonatal death.
1.10. Analysis.
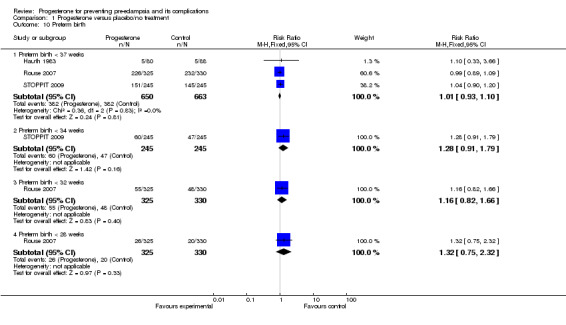
Comparison 1 Progesterone versus placebo/no treatment, Outcome 10 Preterm birth.
1.11. Analysis.
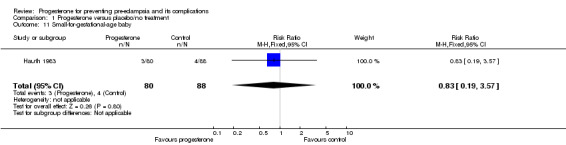
Comparison 1 Progesterone versus placebo/no treatment, Outcome 11 Small‐for‐gestational‐age baby.
1.12. Analysis.
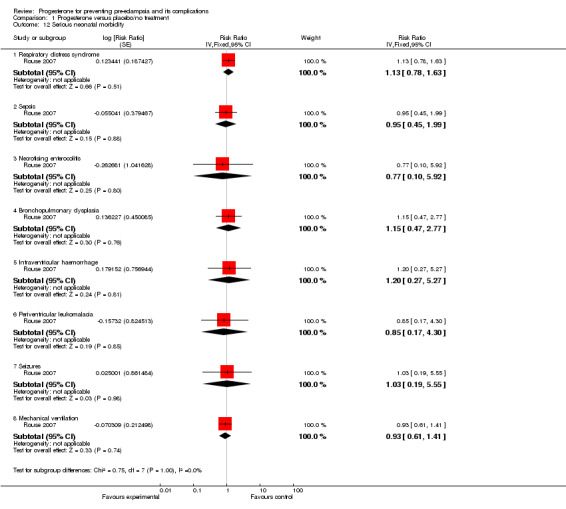
Comparison 1 Progesterone versus placebo/no treatment, Outcome 12 Serious neonatal morbidity.
1.13. Analysis.
Comparison 1 Progesterone versus placebo/no treatment, Outcome 13 Admission to neonatal intensive care unit.
1.14. Analysis.
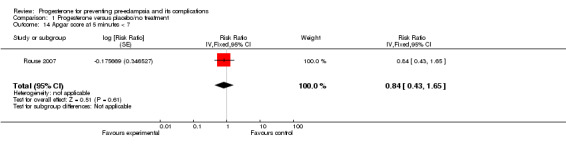
Comparison 1 Progesterone versus placebo/no treatment, Outcome 14 Apgar score at 5 minutes < 7.
1.15. Analysis.
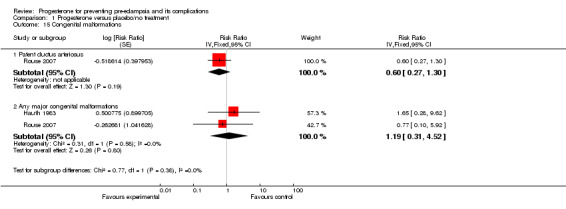
Comparison 1 Progesterone versus placebo/no treatment, Outcome 15 Congenital malformations.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dalton 1962.
| Methods | Randomisation: women allocated 'at random' to 2 groups. No further information. Allocation concealment: 'numbered envelope system'. No further information. Follow up: 22/150 (15%) excluded from analysis: 14 from progesterone arm (3 raised BP at booking, 2 withdrew booking, 1 abortion, 8 opted out), and 8 from control arm (3 raised BP at booking, 3 withdrew booking, 1 abortion, 1 opted out). Blinding: participant and caregiver not blinded, and blinding of outcome assessment not reported. | |
| Participants | 128 women between 16‐28 weeks' gestation with 2 or more 'toxaemic symptoms' (nausea, vomiting, lethargy, backache, headache, vertigo, fainting, cramp, or paraesthesia), blood pressure < 140/90 mmHg and no albuminuria. | |
| Interventions | Progesterone: 100 mg IM daily or on alternate days for 1 week. On subsequent visits, dose and frequency of injection adjusted depending on symptoms (range 300 mg daily to 50 mg on alternate days). Progesterone stopped if symptoms disappeared (in one‐third of the participants, before 34 weeks) or when labour started. Control: simple medication to relieve symptoms based on individual needs: such as alkalis, analgesics, sedatives, antihistamines. Administered as mixtures, tablets, capsules, or powders. | |
| Outcomes | Woman: PE (BP > 140/90 mmHg + either oedema or albuminuria after 28 weeks); side effects in progesterone group only. Baby: stillbirth or neonatal death. | |
| Notes | 385 women interviewed, 150 eligible and agreed to recruitment. Definition of PE included oedema, so these data likely to be overestimated (2 in progesterone arm vs 7 in control arm). Numbers with proteinuria (1 vs 5) likely to be better estimate of PE, and used for review. Child development at age 1 year; intelligence and personality at 16 years of age reported. Data excluded due to large losses to follow up (54% at 1 year and 80% at 16 years). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Inadequate information for assessment. |
| Allocation concealment (selection bias) | Unclear risk | Inadequate information for assessment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants and personnel not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition 15% but appears to be balanced across groups. |
| Selective reporting (reporting bias) | High risk | No data on serious maternal morbidity. Side effects reported for progesterone group only. |
| Other bias | High risk | Although long‐term follow up attempted, large losses to follow‐up. |
Hauth 1983.
| Methods | Randomisation: women 'randomised' to either of 2 treatments. Allocation concealment: no information. Follow up: complete (A). Blinding: double blind. | |
| Participants | 168 women between 16‐20 weeks' gestation on active duty in the USA Air Force. | |
| Interventions | Progesterone: 17 alpha‐hydroxyprogesterone caproate 1000 mg IM weekly up to 36 completed weeks. Control: placebo with castor oil, benzyl benzoate, and benzyl alcohol IM weekly. | |
| Outcomes | Woman: PIH (not defined); post‐term pregnancy. Baby: perinatal death (if BW >/= 500 g, stillbirth or neonatal death in first 28 days); preterm birth (not defined); SGA (not defined); BW < 2500 g; major congenital defects (not defined). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Inadequate information for assessment. |
| Allocation concealment (selection bias) | Unclear risk | No information available. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Use of placebo, 'double blind'. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition. |
| Selective reporting (reporting bias) | Unclear risk | No information on serious maternal morbidity. |
| Other bias | High risk | Outcome PIH not defined. |
Rouse 2007.
| Methods | Randomisation: urn method, centrally stratified by clinical centre. Allocation concealment: boxes, no other information. Follow up: 6/661 (0.9%) lost to follow‐up (2 in progesterone group, 4 in placebo group). Blinding: placebo controlled, blinding of participants, caregivers, and research personnel. | |
| Participants | 655 women between 16‐20+3 weeks' gestation with twin pregnancy. Excluded: serious fetal anomaly, fetal death before 12 weeks, monoamniotic placenta, suspected twin‐twin transfusion syndrome, planned progesterone treatment after 16 weeks or cervical cerclage, major uterine anomaly, chronic medical disease |
|
| Interventions | Progesterone: 17 alpha‐hydroxyprogesterone caproate 250 mg IM injections weekly.
Control: identical placebo (castor oil) IM injections weekly. Duration of treatment in both groups ‐ weekly up to 34+6 weeks, or until delivery (whichever first). |
|
| Outcomes | Woman: tocolytic therapy; cerclage placement; hypertensive disorder (gestational hypertension or pre‐eclampsia); chorioamnionitis; caesarean delivery; side‐effects in progesterone group only. Baby: composite delivery or fetal death before 35 weeks' gestation (primary outcome); time to fetal death or delivery; composite of serious adverse fetal or neonatal outcomes (retinopathy of prematurity, RDS, sepsis, stage 2/3 NEC, bronchopulmonary dysplasia, grade 3/4 IVH, periventricular leukomalacia); birthweight; major malformation; Apgar score; patent ductus arteriosus; mechanical ventilation; pneumonia; seizures. | |
| Notes | Compliance 94.5% in progesterone group and 95% in placebo group. Women recruited from 14 sites in the USA, between April 2004 and February 2006. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate. Urn randomisation, stratified by clinical centre. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. Treatment and placebo packaged according to the randomisation sequence, but no further information. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Adequate. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few losses to follow‐up (6/661), balanced in both groups. |
| Selective reporting (reporting bias) | Low risk | Yes. |
| Other bias | Low risk | No ICC available from published data. |
STOPPIT 2009.
| Methods | Randomisation: permuted blocks of mixed sizes with minimisation. Allocation concealment: telephone randomisation, packs in sealed opaque coverings in pharmacy. Follow up: 6/500 (1.2%) lost to follow‐up (3 in progesterone group, 3 in placebo group). Blinding: placebo controlled, blinding of participant and personnel. | |
| Participants | 494 women at 24 weeks' gestation with twin pregnancy; gestation and chorionicity confirmed by scan before 20 weeks. Excluded: fetal abnormality, contra‐indication to progesterone, planned cervical suture, planned delivery before 34 weeks, twin‐twin transfusion syndrome. |
|
| Interventions | Progesterone: progesterone gel inserted vaginally 90 mg daily from 24 weeks for 10 weeks. Control: placebo gel vaginally daily from 24 weeks for 10 weeks. | |
| Outcomes | Woman: gestation at delivery, method of delivery; duration of stages of labour; duration of hospital stay; persistent maternal disability; life threatening complication; maternal mortality; hypertensive disorder (gestational hypertension and pre‐eclampsia); chorioamnionitis; side effects; maternal satisfaction. Baby: composite delivery or fetal death before 34 weeks' gestation (primary outcome); admission to NICU; duration of stay in NICU; intrauterine death; neonatal death; congenital anomaly. | |
| Notes | Recruitment in 9 UK hospitals. Compliance: 287/494 (58%) women returned diaries indicating they had taken 80% or more of their medication. Remainder either did not return diaries or stopped early because they had a preterm birth, because they were told to stop, or because they did not comply with treatment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate. Permuted blocks of randomly mixed sizes, with minimisation based on chorionicity and hospital. |
| Allocation concealment (selection bias) | Low risk | Adequate. Telephone randomisation, packs in sealed opaque coverings and kept in pharmacy. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Adequate. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few losses to follow‐up (6/500), and balanced in both groups. |
| Selective reporting (reporting bias) | Low risk | Yes. |
| Other bias | Low risk | Yes. |
BP: blood pressure BW: birthweight ICC: intracluster correlation co‐efficient IM: intramuscular IVH: intraventricular haemorrhage NEC: necrotising enterocolitis NICU: neonatal intensive care unit PE: pre‐eclampsia PIH: pregnancy‐induced hypertension SGA: small‐for‐gestational age VS: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Dalton 1976 | Not a randomised trial. Methods: cohort study. Participants: 65 children of women who had received progesterone during pregnancy, 71 children of women with normal pregnancies (next born in birth register), and 25 children of women with pre‐eclampsia (next born in birth register). Intervention: progesterone injections at least 50 mg per week IM in mothers for relief of symptoms. Outcomes: educational attainments of children at 17‐20 years of age. |
| El‐Zibdeh 2005 | Quasi‐randomised trial. Methods: allocation to treatment group according to the day of week. Participants: 180 women < 35 years of age with at least 3 consecutive miscarriages of unknown aetiology. Interventions: 3‐arm trial: dydrogesterone 10 mg bd orally versus hCG 5000 IU IM every 4 days versus no intervention. Outcomes: miscarriage, perinatal death, APH, preterm labour, IUGR, pre‐eclampsia, congenital malformations, fetal distress, caesarean section, PPH. |
| Hartikainen 1980 | Data on PIH not reported in a format that can be used in the review. Methods: 2 groups, double blind, placebo controlled study. Participants: 77 women with twin pregnancy between 28‐33 weeks' gestation. Interventions: 17 alpha‐hydroxyprogesterone caproate 250 mg weekly IM from 28‐37 weeks versus placebo. Outcomes: duration of pregnancy, preterm birth, IOL for PIH or cholestasis, perinatal mortality, birthweight, neonatal complications. |
| Sammour 1975 | Not a randomised trial. Women with established pre‐eclampsia. Methods: observational study. Participants: 40 women with pre‐eclampsia (cases) and 10 normal pregnant women (controls). Intervention: progesterone injections, 200 mg daily for up to 6 weeks. Outcomes: changes in blood pressure, proteinuria, urinary output, weight, oedema, and biochemical outcomes. |
| Sammour 1982 | Not a randomised trial. Women with established pre‐eclampsia. Methods: observational study. Participants: women with severe pre‐eclampsia (cases) and normal pregnant women (controls). Intervention: progesterone injections 600 mg daily for 1‐6 weeks. Outcomes: changes in blood pressure, proteinuria, urinary output, weight, oedema, and biochemical outcomes. |
APH: antepartum haemorrhage bd: twice a day IM: intramuscular IOL: induction of labour IU: international units IUGR: intrauterine growth restriction PIH: pregnancy‐induced hypertension PPH: postpartum haemorrhage
Characteristics of ongoing studies [ordered by study ID]
Nassar 2008.
| Trial name or title | Prevention of preterm delivery in twin pregnancies by 17 alpha‐hydroxyprogesterone caproate. |
| Methods | Randomised, double blind trial. |
| Participants | Viable twin pregnancy, current pregnancy between 16 weeks and 20 weeks of gestation. |
| Interventions | 17‐alpha Hydroxyprogesterone Caproate 250 mg IM weekly or castor oil weekly IM injections. |
| Outcomes | Primary
Frequency of delivery prior to completed 37 weeks of gestation (259 days). Secondary Delivery before 35 weeks of gestation, delivery before 32 weeks of gestation, admission during current pregnancy for preterm labour. Need for tocolytic therapy in current pregnancy, need for corticosteroids to enhance fetal lung maturity, route of delivery. Obstetrical complications (antepartum and intrapartum) of pregnancy, indicated preterm deliveries, neonatal outcome variables (birthweight < 2500 grams. Birthweight < 1500 grams, fetal death, antepartum or intrapartum, neonatal intensive care unit admissions, respiratory distress syndrome. |
| Starting date | October 2006. |
| Contact information | Anwar H Nassar, MD, +961‐1‐340460 ext 5607, an21@aub.edu.lb |
| Notes |
NCT00141908 Study is close to completing recruitment ‐ personal communication with trialists, September 2010. |
Uckele 2010.
| Trial name or title | Oral progesterone and low‐dose aspirin in the prevention of pre‐eclampsia. |
| Methods | Randomised double blind trial. |
| Participants | Pregnant women between 18 and 45 years with a previous history of pre‐eclampsia in the preceding pregnancy. |
| Interventions | Aspirin and progesterone versus aspirin and placebo. |
| Outcomes | Pre‐eclampsia. |
| Starting date | July 2008. |
| Contact information | John.Uckele@beaumont.edu, Evie.Russell@beaumont.edu (Michigan, USA) |
| Notes |
IM: intramuscular
Contributions of authors
The protocol for this review was based on the Generic Protocol of interventions for preventing pre‐eclampsia and its complications, which was drafted by Shireen Meher and Lelia Duley, with contributions from the Prevention of Pre‐eclampsia Review Authors. Shireen Meher and Lelia Duley independently assessed trials for inclusion. Shireen Meher extracted and entered data into the Review Manager software and Lelia Duley double checked them for accuracy.
Shireen Meher and Lelia Duley drafted the review.
Sources of support
Internal sources
The University of Liverpool, UK.
University of Oxford, UK.
External sources
Health Technology Assessment, UK.
Medical Research Council, UK.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Dalton 1962 {published data only}
- Dalton K. Ante‐natal progesterone and intelligence. British Journal of Psychiatry 1968;114:1377‐82. [DOI] [PubMed] [Google Scholar]
- Dalton K. Controlled trials in the prophylactic value of progesterone in the treatment of pre‐eclamptic toxaemia. Journal of Obstetrics and Gynaecology of the British Commonwealth 1969;3:463‐8. [DOI] [PubMed] [Google Scholar]
- Lynch A, Mychalkiw W. Prenatal progesterone II. Its role in the treatment of pre‐eclamptic toxaemia and its effect on the offspring's intelligence: a reappraisal. Early Human Development 1978;2(4):323‐39. [DOI] [PubMed] [Google Scholar]
- Lynch A, Mychalkiw W, Hutt SJ. Prenatal progesterone I. Its effect on development and on intellectual and academic achievement. Early Human Development 1978;2(4):305‐22. [DOI] [PubMed] [Google Scholar]
Hauth 1983 {published data only}
- Hauth JC, Gilstrap LC, Brekken AL, Hauth JM. The effect of 17‐alpha‐hydroxyprogesterone caproate on pregnancy outcome in an active‐duty military population. American Journal of Obstetrics and Gynecology 1983;146:187‐90. [DOI] [PubMed] [Google Scholar]
Rouse 2007 {published and unpublished data}
- Caritis S, Rouse D. A randomized controlled trial of 17‐hydroxyprogesterone caproate (17‐OHPC) for the prevention of preterm birth in twins. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S2. [Google Scholar]
- Caritis SN, Simhan H. Relationship of 17‐alpha hydroxyprogesterone caproate (17‐OHPC) concentrations and gestational age at delivery in twins. 55th Annual Meeting of the Society of Gynecologic Investigation; 2008 March 26‐29; San Diego, USA. 2008:Abstract no: 139.
- Durnwald C. The impact of cervical length on risk of preterm birth in twin gestations. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S10. [Google Scholar]
- Horton A, Gyamfi C. 17‐alpha hydroxyprogesterone caproate does not increase the risk of gestational diabetes in singleton and twin pregnancies. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse DJ, Caritis SN, Peaceman AM, Sciscione A, Thom EA, Spong CY, et al. A trial of 17 alpha‐hydroxyprogesterone caproate to prevent prematurity in twins. New England Journal of Medicine 2007;357(5):454‐61. [DOI] [PubMed] [Google Scholar]
- Simhan HN, Caritis SN. The effect of 17‐alpha hydroxyprogesterone caproate (17‐OHPC) on maternal plasma CRP levels in twin pregnancies. 55th Annual Meeting of the Society of Gynecologic Investigation; 2008 March 26‐29; San Diego, USA. 2008:Abstract no: 140.
- US National Institutes of Health. Trial of progesterone in twins and triplets to prevent preterm birth (STTARS). http://www.clinicaltrials.gov/ct/gui/show/NCT00099164 (accessed 25 August 2005).
STOPPIT 2009 {published and unpublished data}
- Norman JE. Double blind randomised placebo controlled study of progesterone for the prevention of preterm birth in twins (STOPPIT) (ongoing trial). National Research Register (www.nrr.nhs.uk) (accessed 6 July 2006).
- Norman JE, Mackenzie F, Owen P, Mactier H, Hanretty K, Cooper S, et al. Progesterone for the prevention of preterm birth in twin pregnancy (STOPPIT): a randomised, double‐blind, placebo‐controlled study and meta‐analysis. Lancet 2009;373(9680):2034‐40. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Dalton 1976 {published data only}
- Dalton K. Prenatal progesterone and educational attainments. British Journal of Psychiatry 1976;129:438‐42. [DOI] [PubMed] [Google Scholar]
El‐Zibdeh 2005 {published data only}
- El‐Zibdeh MY. Dydrogesterone in the reduction of recurrent spontaneous abortion. Journal of Steroid Biochemistry & Molecular Biology 2005;97(5):431‐4. [DOI] [PubMed] [Google Scholar]
Hartikainen 1980 {published data only}
- Hartikainen‐Sorri A, Kauppila A, Tuimala R. Inefficacy of 17 alpha‐hydroxyprogesterone caproate in prevention of prematurity in twin pregnancy. Obstetrics & Gynecology 1980;56:692‐5. [PubMed] [Google Scholar]
Sammour 1975 {published data only}
- Sammour MB, El‐Kabarity H, Khalifa AS. Progesterone therapy in pre‐eclamptic toxaemia. Acta Obstetricia et Gynecologica Scandinavica 1975;54(3):195‐202. [DOI] [PubMed] [Google Scholar]
Sammour 1982 {published data only}
- Sammour MB, El‐Makhzangy MN, Fawzy MM, Schindler A. Progesterone therapy in pregnancy induced hypertension therapeutic value and hormonal profile. Clinical and Experimental Hypertension. Part B, Hypertension in Pregnancy 1982;1(4):455‐78. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Nassar 2008 {published data only}
- Nassar AH. Prevention of preterm delivery in twin pregnancies by 17 alpha hydroxyprogesterone caproate. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 10 February 2009). [NCT00141908]
Uckele 2010 {published data only}
- Uckele JE. Oral progesterone and low dose aspirin in the prevention of preeclampsia. metaRegister of Controlled Trials (http://www.controlled‐trials.com/mrct/trial/454661/preeclampsia) (accessed 2 June 2010).
Additional references
Bennett 1939
- Bennett FO. Progesterone in pre‐eclamptic toxaemia. New Zealand Medical Journal 1939;38:11. [Google Scholar]
Buhimschi 1995
- Buhimschi I, Yallampalli C, Chwalisz K, Garfield RE. Pre‐eclampsia‐like conditions produced by nitric oxide inhibition: effects of L‐arginine, D‐arginine and steroid hormones. Human Reproduction 1995;10:2723‐30. [DOI] [PubMed] [Google Scholar]
Dalton 1954
- Dalton K. Similarity of symptomatology of premenstrual syndrome and toxaemia of pregnancy and their response to progesterone. British Medical Journal 1954;2:1071‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dalton 1957
- Dalton K. Toxaemia of pregnancy treated with progesterone during the symptomatic stage. British Medical Journal 1957;2:378‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dalton 1960
- Dalton K. Early symptoms of pre‐eclamptic toxaemia. Lancet 1960;1:198‐9. [DOI] [PubMed] [Google Scholar]
DH 2002
- Department of Health, Scottish Executive Health Department and Department of Health, Social Services, Public Safety. Northern Ireland. Why mothers die. The sixth report on confidential enquiries into maternal deaths in the United Kingdom 2000‐2002. London: RCOG Press, 2002. [Google Scholar]
Dodd 2006
- Dodd JM, Flenady V, Cincotta R, Crowther CA. Prenatal administration of progesterone for preventing preterm birth in women considered to be at risk of preterm birth. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD004947.pub2] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ford 2009
- Ford O, Lethaby A, Roberts H, Mol BWJ. Progesterone for premenstrual syndrome. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD003415.pub3] [DOI] [PubMed] [Google Scholar]
Generic Protocol 05
- Meher S, Duley L, on behalf of the Prevention of Pre‐eclampsia Cochrane Review authors. Interventions for preventing pre‐eclampsia and its consequences: generic protocol. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD005301] [DOI] [Google Scholar]
Haas 2008
- Haas DM, Ramsey PS. Progestogen for preventing miscarriage. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD003511.pub2] [DOI] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [DOI] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
Ito 1992
- Ito M, Nakamura T, Yoshimura T, Koyama H, Okamura H. The blood pressure response to infusions of angiotensin II during normal pregnancy: relation to plasma angiotensin II concentration, serum progesterone level, and mean platelet volume. American Journal of Obstetrics and Gynecology 1992;166(4):1249‐53. [DOI] [PubMed] [Google Scholar]
Kovats 1990
- Kovats S, Main EK, Librach C, Stubblebine M, Fisher SJ, DeMars R. A class I antigen HLA‐G, expressed in human trophoblasts. Science 1990;248:220‐3. [DOI] [PubMed] [Google Scholar]
Liao 1996
- Liao QP, Buhimschi IA, Saade G, Chwalisz K, Garfield RE. Regulation of vascular adaptation during pregnancy and post‐partum: effects of nitric oxide inhibition and steroid hormones. Human Reproduction 1996;11:2777‐84. [DOI] [PubMed] [Google Scholar]
Marsden 1937
- Marsden GB. Treatment with progesterone of eight cases of pre‐eclampsia. British Medical Journal 1937;2:1221. [Google Scholar]
NHBPEP 2000
- Gifford RW Jr, August PA, Cunningham G, Green LA, Lindhemier MD, McNellis D, et al. Report of the national high blood pressure education program working group on high blood pressure in pregnancy. American Journal of Obstetrics and Gynecology 2000;183 Suppl:1‐22. [PubMed] [Google Scholar]
Parker 1976
- Parker CR Jr, Everett RB, Quirk JG Jr, Walley PJ, Gant NF. Hormone production during pregnancy in the primigravid patient. I. Plasma levels of progesterone and 5‐alpha‐pregnane‐3,20‐dione throughout pregnancy of normal women and women who developed pregnancy‐induced hypertension. American Journal of Obstetrics and Gynecology 1979;135(6):778‐82. [PubMed] [Google Scholar]
Radwanska 1993
- Radwanska E. The role of reproductive hormones in vascular disease and hypertension. Steroids 1993;58:605‐10. [DOI] [PubMed] [Google Scholar]
RevMan 2003 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 4.2 for Windows. Oxford, England: The Cochrane Collaboration, 2003.
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Robson 1937
- Robson JM, Paterson SJ. Progesterone in pre‐eclamptic toxaemia. British Medical Journal 1937;1:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Szekeres‐Bartho 1992
- Szekeres‐Bartho J, Szekeres J. Immunosuppression by progesterone in pregnancy. Boca Raton, FL, USA: CRC Press, 1992. [Google Scholar]
Tamimi 2003
- Tamimi R, Lagiou P, Vatten LJ, Mucci L, Trichopoulos D, Hellerstein S, et al. Pregnancy hormones, pre‐eclampsia, and implications for breast cancer risk in the offspring. Cancer Epidemiology, Biomarkers and Prevention 2003;12(7):647‐50. [PubMed] [Google Scholar]
Wahabi 2007
- Wahabi HA, Abed NF, Elawad M. Progestogen for treating threatened miscarriage. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD005943.pub2] [DOI] [PubMed] [Google Scholar]
WHO 1988
- World Health Organization International Collaborative Study of Hypertensive Disorders in Pregnancy. Geographic variation in the incidence of hypertension in pregnancy. American Journal of Obstetrics and Gynecology 1988;158:80‐3. [PubMed] [Google Scholar]
Yie 2004
- Yie S, Li L, Li Y, Xiao R, Librach L. HLA‐G protein concentrations in maternal serum and placental tissue are decreased in preeclampsia. American Journal of Obstetrics and Gynecology 2004;191(2):525‐9. [DOI] [PubMed] [Google Scholar]
Yie 2006
- Yie S, Li L, Li G, Xiao R, Librach L. Progesterone enhances HLA‐G gene expression in JEG‐3 choriocarcinoma cells and human cytotrophoblasts in vitro. Human Reproduction 2006;21(1):46‐51. [DOI] [PubMed] [Google Scholar]
Young 1937
- Young J. The habitual abortion and stillbirth syndrome and late pregnancy toxaemia: vitamin E and the prolan‐progesterone mechanism. British Medical Journal 1937;1:953. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zeisler 2000
- Zeisler H, Sator MO, Joura EA. Serum levels of progesterone in patients with preeclampsia. Wiener Klinische Wochenschrift 2000;112(8):362‐4. [PubMed] [Google Scholar]


