Purpose of review
To critically appraise new insights into HDL structure and function in type 1 diabetes (T1DM) and type 2 diabetes (T2DM).
Recent findings
In young T1DM patients with early renal impairment and a high inflammatory score, both HDL antioxidative activity and endothelial vasodilatory function were impaired, revealing a critical link between HDL dysfunction, subclinical vascular damage, systemic inflammation and end organ damage. HDL may inhibit development of T2DM by attenuating endoplasmic reticulum (ER) stress and apoptotic loss of pancreatic β-cells, an effect due in part to ABC transporter-mediated efflux of specific oxysterols with downstream activation of the hedghehog signalling receptor, Smoothened. The apoM-sphingosine-1-phosphate complex is critical to HDL antidiabetic activity, encompassing protection against insulin resistance, promotion of insulin secretion, enhanced β-cell survival and inhibition of hepatic glucose production. Structure-function studies of HDL in hyperglycemic, dyslipidemic T2DM patients revealed both gain and loss of lipidomic and proteomic components. Such changes attenuated both the optimal protective effects of HDL on mitochondrial function and its capacity to inhibit endothelial cell apoptosis. Distinct structural components associated with individual HDL functions.
Summary
Extensive evidence indicates that both the proteome and lipidome of HDL are altered in T1DM and T2DM, with impairment of multiple functions.
Keywords: dyslipidemia, glycaemic burden, HDL subspecies, lipidome, pancreatic β cells, proteome
INTRODUCTION
The potential causal relationships between HDL and the pathogenesis of vascular inflammation and premature atherosclerosis on the one hand, and between HDL and glucose homeostasis on the other, remain the subject of debate; these questions are of pertinence to both type 1 diabetes (T1DM) and type 2 diabetes (T2DM) given the high cardiovascular risk with which they are associated [1–3,4▪,5–7,8▪,9▪]. Considering the paucity of evidence for causal links between HDL functionality and the development of T1DM or T2DM, the objectives of this review are to summarise progress in our understanding of the impact of perturbed glucose homeostasis and dyslipidaemia in T1DM and T2DM on the structure and functionality of HDL particles.
Box 1.
no caption available
FUNCTIONALITY OF HDL PARTICLES: THE PROTEOME AND LIPIDOME
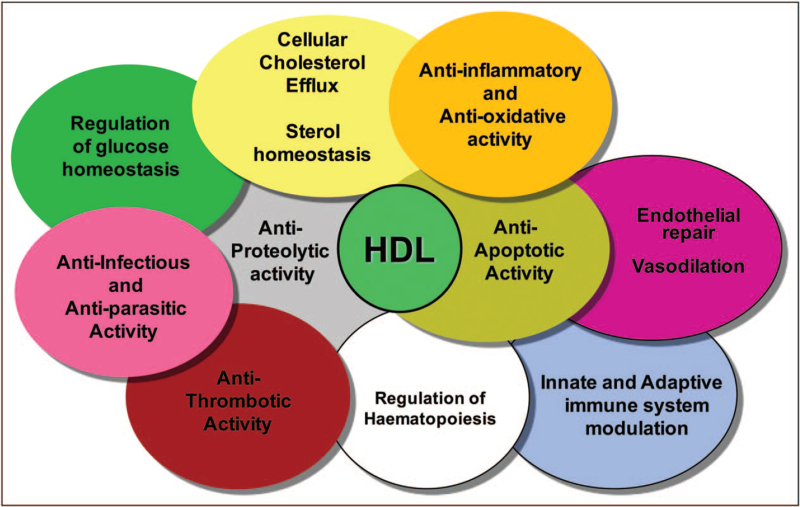
A wide spectrum of biological activities – which have been equated with diverse functions in vivo – and which may all be of potential relevance to hyperglycaemia, inflammation and vascular dysfunction in diabetes depending on the metabolic and pathophysiological contexts – are exerted by HDL particles. Such activities include cellular efflux of cholesterol (CEC) in the context of reverse cholesterol transport, a spectrum of anti-inflammatory activities, antiprotease activity, antioxidative activity, antiapoptotic activity, antithrombotic and antiplatelet activity, endothelial protection and repair including endothelial cell progenitor homeostasis, proangiogenic activity, activity integral to innate and adaptive immunity, antiinfectious and antiparasitic activities as exemplified by implication in reverse lipopolysaccharide transport and by antitrypanosomic activity, antidiabetic activity and regulation of glucose homeostasis, and transport and tissue delivery of small noncoding RNAs, respectively (Fig. 1) [9▪,10,11,12▪,13,14,15▪▪,16▪,17▪▪,18▪–20▪,21]. These multifarious activities frequently involve cellular signal transduction, and are relevant to multiple cell types, including endothelial cells, immunoinflammatory cells such as monocytes and monocyte-derived macrophages, pancreatic β cells, hepatocytes, adipocytes and myocytes and smooth muscle cells [9▪,10,11,12▪,13,14,15▪▪,16▪,17▪▪,18▪–20▪,21].
FIGURE 1.
Schematic summary of the wide spectrum of biological activities displayed by human HDL particles. This schematic does not imply that all such activities are transported by one and the same HDL particle; indeed, HDL particles are highly heterogeneous in terms of their structure, metabolism and functionality.
HDL particles equally function as a transport platform for key biologically active molecules, and notably sphingosine-1-phosphate (S1P), hormones such as oestradiol and thyroxine, carotenoid precursors of vitamin A, vitamin E, potent antioxidative lipids including plasmalogens and lycopene, and miRNAs, among which miRNA-223 may act as a coordinator of cholesterol homeostasis [12▪,22,23,24▪▪].
Consideration of the nature of the starting biological material and the methodologies employed for isolation of total HDL, or HDL subfractions and /or their subspecies, is critical to evaluation of their function [25–27]. HDLs are frequently isolated as lipoproteins in the density interval 1.063–1.21 g/ml by flotational ultracentrifugation; as in some precipitation techniques, this multistep procedure exposes HDL to high ionic strength [26]. Partial loss of small apolipoproteins and oxidative modification of lipid and protein components may occur; rigorous quality control and characterisation of the isolated HDL fraction(s) is a prerequisite [26]. Moreover, HDL subfractions prepared by differing methods may not contain the same populations of particle subspecies [25,26].
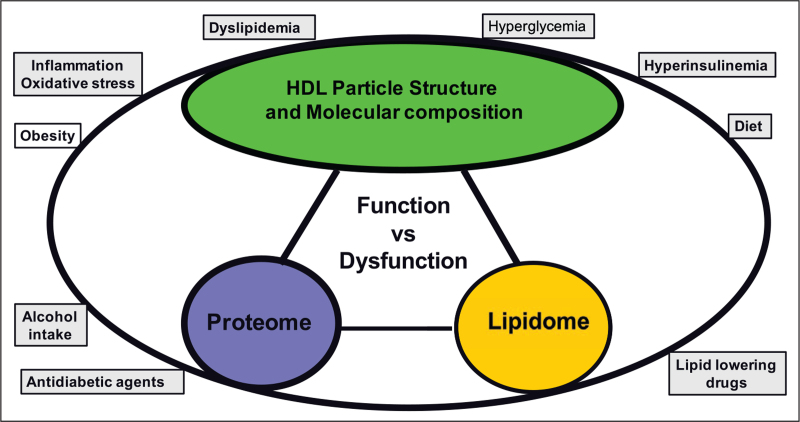
In the context of the marked heterogeneity of HDL particles in diabetes, substantial evidence supports the contention not only that both the proteome and lipidome are integral to the overall biological activity expressed by a given HDL particle, but also that the plasma pool of HDL consists of a highly diverse spectrum of individual particle species, each defined by distinct lipid and protein components (Fig. 2) [25–27,28▪▪,29–32,33▪▪,34]. Some 95 proteins (and potentially up to 200) constitute the HDL proteome; the plasma abundance of many of these proteins is insufficient to permit one copy per HDL particle, thereby suggesting that specific proteins may be bound to distinct particle species, which may then exert specific functions [31,32,33▪▪]. Thus, the capacity of an HDL particle to integrate several proteins may be limited by size and surface constraints, as in the case of small dense HDL3 [25,26,30–32,33▪▪]. The HDL lipidome is equally highly complex [34], state-of-the-art mass spectrometric analyses revealing that more than 400 molecular lipid species are detectable (Meikle PJ and Chapman MJ, unpublished data). The working hypothesis that a specific biological function of HDL might be mediated by a distinct particle subspecies defined by a specific cluster(s) of bound proteins and lipids is now plausible; the possibility that a single particle species might exert more than one function cannot be excluded [18▪,30–32,33▪▪]. In a similar manner to the proteome, the lipid composition of HDL particles differs between subfractions and between subspecies and is an intimate feature of their functionality [28▪▪,29,34].
FIGURE 2.
The lipidome and proteome of HDL in normolipidemic, normoglycemic healthy subjects are integral to expression of the optimal biological activities of HDL particles which are equated with its functionality. The lipidome and proteome are however markedly altered in both type 1 diabetes and type 2 diabetes, leading to functional impairment, that is, dysfunctionality. The lipidome is composed of more than 400 molecular lipid species (PJ Meikle and MJ Chapman, unpublished observations) while the proteome is composed of at least 95 distinct protein species. Multiple distinct particle subfractions are now recognised with the circulating HDL pool, which now extends to distinct particle subspecies with defined protein and lipid components. Lipid–lipid, lipid–protein and protein–protein interactions ensure the integrity and stability of HDL particles; such interactions may confer conformational changes in major apolipoproteins such as apo-AI and apo-AII, which may in turn impact functionality. The lipidome, proteome and functionality of HDL are impacted by their metabolic environment, major factors including dyslipidaemia and dysglycaemia.
The specific function(s) with which an HDL particle is endowed may well relate to processes involved in its formation. Whether particles constituting the HDL spectrum in human plasma result primarily by maturation through progressive lipidation of a preβ, lipid-poor particle as classically proposed, or whether individual HDL particle size species principally originate in the liver, are metabolically stable and undergo only minor remodelling to larger or smaller particles as recently reported, remains indeterminate; moreover, these processes may be altered in both T1DM and T2DM [1,11,35]. Thus, the enlarged hepatic pool of triglyceride in T2DM may result in direct production of HDL with elevated triglyceride content. Particle size stability in plasma does not however preclude dynamic exchange and transfer of both apolipoproteins and lipids; indeed, transfer of free cholesterol-rich and phospholipid-rich surface remnants from triglyceride-rich lipoproteins (TGRL) and remnants contribute significantly to the HDL lipid pool under normal conditions, although such transfer is diminished (−25%) in T2DM [35,36▪].
DYNAMIC ASPECTS OF HDL METABOLISM, STRUCTURE AND FUNCTIONALITY IN DIABETES
The compositional and functional features of HDL particles in a single plasma sample are a ‘photo-shot’ in time of the summation of integrated processes of particle production, intravascular metabolism and remodelling by lipases and lipid transfer proteins, and cellular interactions. Such features are directly impacted by the metabolic environment. Indeed, major factors which may significantly modify HDL structure and function in the context of diabetes include hyperglycaemia, insulin resistance, hyperinsulinemia, insulin therapy, dyslipidaemia, inflammation and oxidative stress, obesity, dietary components and alcohol intake; lipid-lowering pharmacotherapy – and potentially antidiabetic agents – may equally impact HDL metabolism, composition and function (Fig. 2) [7,17▪▪,19▪,22,26,36▪].
HDL FUNCTIONALITY IN TYPE 1 DIABETES
T1DM, involving autoimmune-mediated destruction of pancreatic β cells, uniquely features insulin therapy from its inception, with the goal of controlling glucose homeostasis. Indeed, an excessive glycaemic burden is a major driver of systemic and tissular oxidative stress, endothelial dysfunction and vascular inflammation, end organ damage such as nephropathy and retinopathy, and ASCVD; its effects are exacerbated by synergism with concomitant risk factors [5]. Nonenzymatic glycation of plasma proteins by advanced glycosylation end-products in poorly controlled T1DM thus underlies covalent modification of major HDL apolipoproteins such as apo-AI, with subsequent altered metabolism and attenuation of function [8▪,9▪,10,26]. Equally, elevated oxidative stress results in glycoxidation of HDL proteins and peroxidation of HDL lipids. Quantitatively, lipoprotein profiles in T1DM show variability as a function of the degree of glycaemic control and additional factors but may be quasi-normal when glycaemic status is well controlled [8▪,9▪,10]. Insulin therapy upregulates expression of lipoprotein lipase however, leading to efficient lipolysis of TGRL and supranormal levels of HDL-C.
Early findings in recent-onset T1DM indicated that HDL display both anti-inflammatory and immunoregulatory activities [10]. By contrast, HDL cholesterol efflux capacity (CEC) is significantly decreased early in the disease course independently of glucose control [9▪]. Recently a shift to a larger mean HDL particle size (∼10%) accompanied by lower HDL particle numbers (∼30%) was reported in T1DM patients relative to matched controls; this latter parameter was independently associated with significant enhancement in total HDL-mediated CEC in recent-onset T1DM, but not with ABCA1-independent CEC [37]. Discrepancies are frequent in reported findings for CEC in T1DM and may in part be explained by differences between the averaged activity of all particles in a total HDL fraction as compared with the activity in distinct subfractions or subspecies and preferably expressed on a per particle basis (see below), by differences in the cell systems used for in-vitro experimentation and in the biological and therapeutic status of the patients such as glycaemic burden. Study of HDL function in T1DM therefore requires strict definition of the temporal/longitudinal stage of the condition, of comorbidities and of biological, clinical and therapeutic status, conditions which have rarely been satisfied in published investigations.
The capacity of HDL to remove and detoxify lipid hydroperoxides associated with either modified LDL or cell membranes, a metric of antioxidative activity, is consistently impaired in T1DM patients, and is observed independently of the degree of glycaemic control [9▪]. This effect appears to be linked to reduction in HDL-associated paraoxonase-1 (PON1) activity, although the endogenous substrate of this enzyme is indeterminate.
In a novel study of HDL function in T1DM in subsets of individuals in two small cohorts with long-term T1DM with or without microvascular and macrovascular complications, individuals without vascular complications exhibited higher levels of medium-sized HDL particles (M-HDL); such protected individuals equally displayed higher levels of HDL-associated PON1 mass and activity, which were in part transported by M-HDL [38▪]. No additional functional distinctions were however observed in the long-term protected T1DM individuals. Furthermore, evidence for alterations in the HDL proteome in young T1DM subjects versus matched controls in relation to the degree of glycaemic control has provided evidence to support the contention that the HDL proteome is altered [39]. A small number of specific HDL proteins were altered in T1DM independently of glycaemic control, whereas others were partially or totally corrected with optimal glycaemic control. While the relationship of these changes, principally involving enrichment in protease inhibitors, to HDL functionality remains speculative, nonetheless they highlight the impact of glycaemic burden on the HDL proteome in T1DM. Such proteomic alterations are accompanied by changes in the HDL lipidome, which frequently feature both triglyceride enrichment and elevation in ratios of free cholesterol/phosphatidylcholine and sphingomyelins/phosphatidylcholine, the latter (sphingomyelins/phosphatidylcholine) indicative of enhanced HDL surface rigidity; the former (free cholesterol/phosphatidylcholine) may result from enhanced transfer of cholesterol-rich TGRL surface fragments during lipolysis, or attenuated cholesteryl ester transfer protein (CETP)-mediated lipid transfer activity, or diminished cholesterol esterification by lecithin:cholesterol acyltransferase (LCAT), or a combination of these [9▪,10,36▪]. In addition, significant depletion (up to 15%) in S1P cargo was detected in both HDL2 and HDL3 subfractions of long-term T1DM subjects; given the critical role of S1P in endothelial cell signalling and in stimulation of nitric oxide synthesis, such a reduction may contribute to impaired endothelial vasorelaxation (see discussion below) [9▪].
Young T1DM subjects with renal impairment represent a very high-risk group for future cardiovascular and renal events, and are characterised by dyslipidaemia, systemic inflammation perturbed vascular homeostasis which typically progresses to endothelial dysfunction and ultimately to premature ASCVD. Earlier studies established that HDL in T1DM exhibited an impaired anti-inflammatory activity [10]. In an innovative study, Chiesa et al.[40] evaluated the interplay between the antioxidative activity of HDL and its capacity to protect nitric oxide bioavailability with inflammatory status and in-vivo endothelial function (as flow-mediated dilation of the brachial artery) in a cohort of adolescents with T1DM (n = 70) and early renal dysfunction and compared them with matched control subjects. Using the albumin/creatinine ratio as a measure of renal function, an increased inflammatory risk score and HDL dysfunction were characteristic of patients with early renal impairment. HDL dysfunction was detected as a diminished capacity to inhibit in-vitro superoxide anion production and as reduction in PON-1 activity; in addition, reduction in HDL-mediated nitric oxide availability was observed. Importantly, endothelial dysfunction was limited to patients with renal impairment accompanied by both a high inflammatory risk score and elevated HDL-C level. Surprisingly, glycaemic burden was not a factor in contributing to the dysfunctional HDL phenotype. Despite limited insight into causality, this study nonetheless highlights the clinically significant link between HDL dysfunctionality, subclinical vascular damage, systemic inflammation and early renal impairment in T1DM [40].
HDL FUNCTIONALITY IN TYPE 2 DIABETES
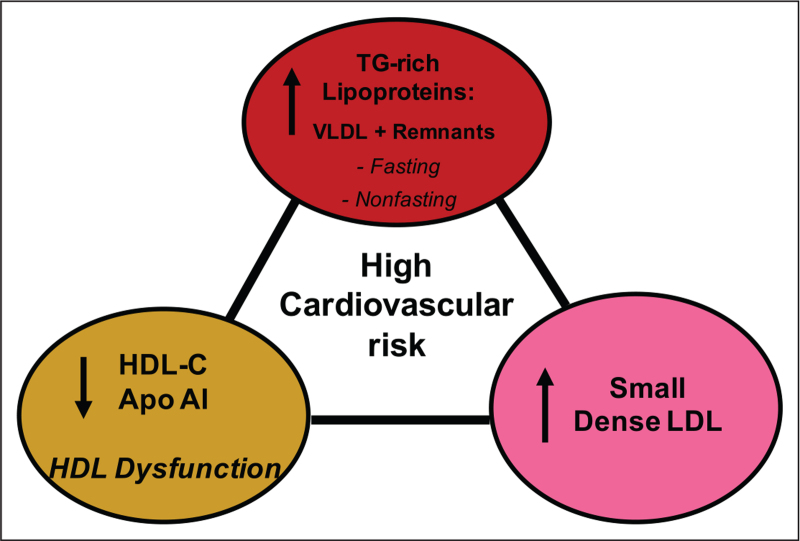
A marked dyslipidaemic phenotype is typical of T2DM in both the fasting and postprandial states, featuring hypertriglyceridemia and subnormal levels of triglyceride-rich HDL (as HDL-C) with defective function (Fig. 3); the metabolism of the circulating TGRL, LDL and HDL pools is intimately linked via the action of lipases [lipoprotein lipase (LPL), hepatic lipase (HL) and endothelial lipase (EL)], lipid transfer proteins [CETP and phospholipid transfer protein (PLTP)], LCAT and apolipoprotein exchange and transfer. The recent finding that free cholesterol transfer from TGRL during lipolysis is attenuated may further enhance the elevated ratio of triglyceride to free cholesterol in T2DM HDL; the latter represents a biomarker of HDL dysfunction [1,7,26,36▪,41–44]. Despite the classical paradigm that insulin resistance causes dyslipidaemia however, it remains conjectural as to whether components of the atherogenic dyslipidaemia themselves favour development of insulin resistance, as glucose intolerance, hyperinsulinemia and beta cell dysfunction evolve concomitantly with perturbation of lipid metabolism [41].
FIGURE 3.
Schematic representation of the major components of the atherogenic dyslipidemia typical of patients displaying type 2 diabetes with poor glycemic control. This dyslipidemia features hypertriglyceridemia involving elevated levels of VLDLs and remnants, a predominance of small, cholesterol-poor dense LDL in the LDL particle profile and subnormal levels of HDL and apoAI which exhibit impaired functionality. Elevated flux of free fatty acids from adipose tissue to the liver as a result of peripheral insulin resistance in type 2 diabetes are a major metabolic driver of hepatic VLDL production and thus of this dyslipidemic phenotype.
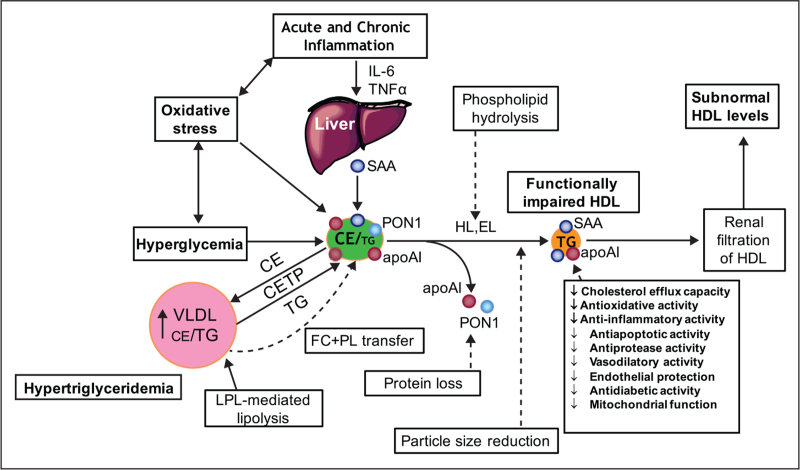
Profound HDL dysfunction is typical of T2DM patients, and is exacerbated primarily by the degree of hypertriglyceridemia, hyperglycaemic burden, and chronic systemic and tissular inflammation with concomitant elevation in oxidative stress (Fig. 4) [17▪▪,26,42–44,45▪▪]. Nonenzymatic glycation of HDL proteins resulting from poorly controlled glycaemia, together with oxidative modification of both proteins and lipids, are prominent as potentially causal factors in inducing HDL dysfunction; such dysfunction is extensively documented, and involves attenuated CEC and impaired antioxidative, anti-inflammatory, antiapoptotic, vasoprotective and vasodilator activities [17▪▪,19▪,42–44,45▪▪]. Again, activities monitored in the total HDL fraction may differ significantly from those in HDL subfractions. Alterations equally occur in small noncoding RNA transport by HDL in T2DM, which results from altered export from pancreatic beta cells [24▪▪]. Indeed, the export process for miR-375-3p to HDL is inversely regulated by glucose-stimulated insulin secretion and appears to be independent of cholesterol homeostasis [24▪▪,46▪]. Given the potential action of HDL-associated miRNAs in modulating gene expression in target cells, with for example impact on vascular function, it is of relevance that diabetic nephropathy specifically attenuated HDL-mediated delivery of proangiogenic miR-132-3p to endothelial cells [24▪▪,47].
FIGURE 4.
Altered metabolism of HDL in type 2 diabetes with hyperglycaemia and insulin resistance, hypertriglyceridemia and acute and/or chronic systemic inflammation associated with elevated oxidative stress. These factors act in a synergistic manner to modify the lipidome and proteome of HDL particles, resulting in impaired functionality. Chronic inflammation characteristic of type 2 diabetes is associated with elevated plasma levels of IL-6. As a result, the liver and other tissues may produce serum amyloid A, which binds to HDL, displaces apoA-I, paraoxonase-1 and other protein components. Oxidative stress may lead to oxidation of both lipids and proteins. Hyperglycaemia, through the formation of advanced glycosylation end products, results in glycation of HDL proteins, including apoAI, with impaired biological activity. Elevated lipid transfer activity of CETP in hypertriglyceridemia enhances the triglyceride content of HDL with depletion of cholesteryl esters. Attenuated transfer of surface lipolytic fragments of VLDL to the HDL pool occurs in T2DM; these fragments consist mainly of free cholesterol and phospholipids. Lipolysis of HDL lipids by hepatic and endothelial lipases produces small, dense HDL with impaired functionality. Small HDLs are filtered by the kidney with subsequent fall in circulating HDL levels.
Current investigations of the impact of T2DM on HDL functionality are focussed on alterations in the lipidomic and proteomic components of distinct HDL subfractions or subspecies and their impact on function. With respect to CEC, isolation of large, medium and small HDL particles from T2DM patients and matched controls revealed that, on a per particle basis, ABCA1-mediated efflux to small HDL was selectively attenuated (−23%) [48▪]. This finding likely was explained by reduced content of SERPINA1 (serpin family member 1, equally termed α1-antitrypsin), an antiprotease and phospholipid-binding protein, in small HDL in T2DM, as in-vitro SERPINA1 enrichment of small HDL partially compensated for its deficient efflux activity [48▪]. As covalent modification of apo-AI or apo-AII or other HDL apolipoproteins by glycation, carbamylation, nitration or oxidation leads to marked attenuation of biological activity, the potential contribution of posttranslational apolipoprotein modification to these experimental findings is indeterminate; a similar qualification frequently applies to studies of HDL functionality in diabetes [17▪▪,46▪,48▪,49].
In an integrated approach, Cardner et al.[50▪▪] defined the relationship between the structure (lipidomic and proteomic profiles) of ultracentrifugally isolated HDL from obese adult T2DM patients without coronary heart disease (n = 46) and five distinct functions: CEC, inhibition of endothelial cell apoptosis, inhibition of beta cell apoptosis, rescue of mitochondrial membrane potential and mitochondrial respiration, with either reference plasma (for CEC) or reconstituted HDL as calibrators; data were compared with those in a group of healthy control subjects. Importantly, this cohort was hyperglycaemic (HbA1c, 54 ± 11 nmol/mol), mildly hypertriglyceridemia (triglycerides, 2 ± 1.5 mmol) and exhibited subnormal HDL-C levels (1.2 ± 0.3 mmol). A high prevalence of both lipid-lowering (61% statin use) and antidiabetic medications was evident; the effects of such polymedication on HDL structure and function in this T2DM cohort are therefore indeterminate. The HDL particle profile showed a loss of large particles and preferential increase in small, triglyceride-rich HDL. These findings are not inconsistent with those of Mora et al.[51] who, in a prospective study in a cohort of 1687 women with 13-year follow-up involving nuclear magnetic spectroscopic analysis, observed that small HDL particles were correlated positively with future risk of T2DM, and that large HDL were inversely associated after adjustment for established risk factors. By contrast, only a minor fall occurred in total HDL particle numbers (<5%; P < 0.001).
Overall, HDL in the T2DM cohort of Cardner et al.[50▪▪] showed loss of specific lipids and proteins with gain of others; a net loss of proteins was observed. Thus, proteome depletion in apo-AIV, PON1, PON3, apo-D, apo-E, apo-F, apo-J and apo-M was associated with increase in contents of serum amyloid SAA1 (serum amyloid A) and SAA2, apo-CII, apo-CIII and fibrinogen. In this context, it is relevant that loss of some beneficial functional proteins may be magnified by gain of others with deleterious action; for example, apo-AIV enhances insulin secretion, whereas apo-CIII adversely induces pancreatic islet inflammation and promotes β-cell death [17▪▪]. In the lipidome, enrichment in phosphatidylethanolamine species occurred at the expense of depletion in ether-phosphatidylcholines, lysophosphatidylcholines, phosphatidylinositols, sphingomyelins and the 18 : 2 species of cholesteryl ester. T2DM HDL was dysfunctional in displaying decreased capacity to lower the mitochondrial potential of myotubes (MMM), an inability to promote maximal respiration of brown adipocytes (MRBA) and an attenuated ability to inhibit starvation-induced apoptosis of human aortic endothelial cells (SIAP). No change was seen in CEC in T2DM HDL as compared with control subjects, apo-AI concentration representing the principal determinant. Furthermore, CEC clustered poorly with other HDL functions and did not constitute a surrogate for them. Moreover, individual functions of HDL were poorly correlated with each other, suggesting that they may be determined by distinct structural components. Significantly, non-CEC functions were typically associated with minor HDL proteins, and notably apo-CIII and S100A9 for MMM, apo-F and cathepsin D for MRBA, apoL1 for endoplasmic reticulum-stress-induced apoptosis, and glycosylphosphatidyl-inositol specific phospholipase D1 (GLPD1) with SIAP. Three novel determinants of HDL function were identified, the sphingadienine-based sphingomyelin sphingomyelins 42.3 and GLPD1 for inhibition of SIAP, and apo-F, a lipid transfer inhibitor, for MRBA. Nonetheless, their precise functional roles remain speculative. Overall, these studies are seminal in demonstrating that (i) several biological activities of HDL are independent of each other, and are notably independent of CEC, (ii) lipidomic and proteomic components of HDL are modified in T2DM and underlie impaired functionality and (iii) HDL dysfunctionality in T2DM involves diverse biological activities [50▪▪].
The gain of SAA by HDL in T2DM is noteworthy; the origin of this acute phase protein lies in both hepatic and nonhepatic tissues [50▪▪,52▪▪]. As HDL-bound SAA appears to be biologically inert, HDL may serve as a transport vehicle to sequester SAA secreted from diverse tissues and thus protect the host from uncontrolled inflammation and tissue damage. Indeed, free SAA may stimulate multiple inflammatory processes during tissue injury or acute infection [52▪▪]. Binding of SAA to HDL displaces apo-AI and other apolipoproteins; nonetheless, the potential for SAA to modify CEC and the antioxidative and anti-inflammatory actions of HDL is subject to debate [52▪▪]. Definition of mechanisms regulating the dissociation of SAA from HDL in chronic diseases such as T2DM then becomes paramount to understanding of its pathological effects.
In-vivo turnover studies of HDL proteins may provide insight into mechanisms underlying alterations in the proteome of HDL in T2DM as observed above [53]. Thus, in a recent in-vivo kinetic investigation using 2H2O enrichment of body water coupled with mass spectrometry in new-onset, insulin-naïve, diet-controlled T2DM subjects (n = 9) with mild hyperglycaemia, the fractional catabolic rates of apo-AII, apo-J, apo-AIV, complement C3, transthyretin and vitamin D-binding protein were significantly increased; by contrast the half-life of PON1 was prolonged, but associated with reduced activity and mass and suggestive of a lower production rate [53]. Significantly, the proinflammatory index of HDL was a strong determinant of apo-AII flux. This proof-of-concept study clearly indicates that the in-vivo dynamics of HDL proteins are perturbed at a very early stage of T2DM, with potential consequences for their steady state concentrations.
ANTIDIABETIC FUNCTIONS OF HDL: EFFECTS OF TYPE 2 DIABETES
Is HDL function relevant to glycaemic control and to lipotoxicity in pancreatic β cells in T2DM?
The antidiabetic properties of HDL include inhibition of pancreatic β-cell apoptosis induced by endoplasmic reticulum stress or by inhibition of native-induced or oxidised LDL-induced activation of the c-Jun N-terminal kinase pathway, improvement in glycaemic control due to enhanced glucose-stimulated insulin synthesis and secretion from β cells, improved insulin sensitivity, improved glucose uptake into heart and skeletal muscle and enhanced proliferation of β cells [11,15▪▪,17▪▪,26,45▪▪,46▪,54▪]. Apo-AI is intimately involved in these activities, which are independent of the ATP-binding cassette transporters ABCA1 and ABCG1 [54▪,55▪▪,56]. Whether these antidiabetic actions of HDL may afford β-cell protection at early stages of T1DM or T2DM remains conjectural.
A key mechanistic question concerns the potential relevance of HDL-mediated CEC via ABC transporters to these findings, or whether effects of HDL on intracellular cholesterol metabolism or receptor-mediated cell signalling are implicated, or both [15▪▪,46▪,54▪,55▪▪,56]. First, improvement in beta cell function by apo-AI occurred by a cholesterol-independent mechanism and was equally independent of ABCA1 and ABCG1 transporters in a genetically modified mouse model [56]. Second, the innovative study of Yalcinkaya et al.[15▪▪] in the immortalised rat β-cell line INS-1e revealed that both native human plasma HDL and reconstituted HDL prevented endoplasmic reticulum stress-dependent apoptosis induced by thapsigargin (TPSG), an inhibitor of the sarco/endoplasmic reticulum. This effect involved cellular efflux of oxysterols, notably 24-hydroxycholesterol (24-OHC), to HDL; moreover, supplementation of HDL with 24-OHC increased its cytoprotective action. Indeed, sterol efflux was essential, as silencing of ABC transporters abrogated the capacity of HDL to prevent TPSG-induced cell death. Employing siRNA or pharmacologically mediated inhibition, the role of the hedgehog signalling receptor, smoothened (SMO), was equally evaluated. SMO inhibition attenuated the antiapoptotic effects of HDL. Finally, nuclear translocation of the SMO-activated transcription factor GL-1 was inhibited by endoplasmic reticulum stress but restored by both 24-OHC and HDL. Together, these insightful findings indicate that the cytoprotective effect of HDL on model β cells involves the mobilisation of oxysterols for direct activation of the hedgehog signalling receptor SMO. Furthermore, the capacity of HDL to ensure pancreatic β-cell homeostasis of both cholesterol and oxysterols is integral to HDL-mediated protection of β-cell function.
Do HDL subfractions differ in their capacity to positively impact insulin metabolism in pancreatic β cells? In the insulinoma MIN-6 cell line, hydrated density-defined human HDL subfractions HDL2a-3c were similar in their capacity to induce insulin secretion or to maintain insulin content under experimental conditions involving low, normal or high glucose levels [57]. Importantly therefore, all HDL subfractions may act as both insulin secretagogues (at low glucose) and insulin secretion enhancers (at high glucose) in MIN-6 cells.
Might defined lipid/protein complexes within HDL particles be involved in its antidiabetic action?
S1P, a sphingolipid mediator, regulates diverse functions in a wide range of cell types via the high-affinity G protein-coupled receptor system, involving receptors S1P1–5, and is anchored to HDL particles by its chaperone, apolipoprotein M [58]. A substantial body of evidence supports a direct role for the S1P/apo-M complex in exerting antidiabetic actions; such actions may extend to its role in regulating diverse inflammatory processes [16▪,26,58,59▪▪,60]. The actions of the S1P/apo-M complex in HDL encompass protection against insulin resistance in the liver, adipose tissue and skeletal muscle, promotion of insulin secretion, enhanced pancreatic β-cell survival and inhibition of hepatic glucose production [16▪,26,58,59▪▪]. Protective action against development of insulin resistance appears to involve activation of insulin signalling pathways, including the AKT and AMPK pathways, and equally upregulation of SIRT1 protein levels to improve mitochondrial function [16▪,58,59▪▪,60]. To what degree the ‘antidiabetic tandem’ of apo-M and S1P can exert protection against development of insulin resistance in man remains subject to debate [16▪].
Plasma apo-M levels are moderately reduced in T2DM (<10%; P < 0.001) [61▪]. Most importantly however, apo-M activity is altered at high glucose levels due to glycation and polymerisation in vitro, reducing its capacity to bind S1P [61▪]. Such an effect can be anticipated to attenuate the pleiotropic activities of the apo-M/S1P complex, including antiapoptotic, cell proliferative and vasorelaxant actions as well as maintenance of vascular permeability. Apo-M polymers were not however detected in diabetic subjects [61▪].
Finally, established evidence clearly indicates impairment of several additional HDL functions in T2DM of relevance to β-cell survival and insulin homeostasis, including CEC, antioxidative and anti-inflammatory actions (Fig. 4) [44,45▪▪,46▪,47,48▪,49,50▪▪,52▪▪,54▪,61▪,62,63].
CONCLUSION
Despite the contrasting quantitative changes in HDL (as HDL-C) in T1DM as compared with T2DM, current data attest to the impaired functionality of HDL particles in both these chronic cardiometabolic disorders. Glycaemic burden is a principal factor in such dysfunction in both T1DM and T2DM; this effect is amplified in T2DM – and to a lesser degree in T1DM – by atherogenic dyslipidaemia (Fig. 3) [63]. HDL dysfunctionality is therefore gaining traction as an innovative therapeutic target for prevention of the premature, accelerated vascular disease typical of both T1DM and T2DM, and thus for attenuation or even correction of the complex pathophysiological processes which underlie microangiopathy and macroangiopathy in these chronic conditions.
The current critical review has highlighted new insights into the relationships between HDL structural components and defined functions. Indeed, the preparative fractionation of HDL subspecies, the characterisation of their proteome and lipidome, and the definition of their functionality is becoming a reality. Furthermore, we have initial insights into alterations in the proteolipidome which are associated with impairment in HDL functionality in both T1DM and T2DM. Accumulating evidence in these disorders highlights the functional impairment of small dense HDL. It is therefore of significance that apoAI-rich, small dense HDL (HDL3c) were identified earlier as particles with potent capacity for ABCA1 transporter-mediated cellular cholesterol efflux and antioxidative, anti-inflammatory, antiapoptotic and antithrombotic activities in healthy normolipidaemic, nondiabetic, nonobese subjects [13,21,29,33▪▪,58,60,64,65]. The phosphosphingolipidome of these particles is distinct, featuring phosphatidylserine and S1P enrichment for example [29,64].
These insights prompt novel therapeutic approaches to future targeting of HDL dysfunctionality. To initiate such a strategy, further intense research is urgently needed to define both the individual structure of subspecies within the HDL3c subfraction and their specific functions. The in-vivo characteristics of each of these subspecies, such as plasma residence time, would provide insight into their intravascular metabolism and stability in a suitable animal model, such as the rabbit with enhanced hepatic lipase activity [66]. On the basis of such knowledge, reconstitution of proteo-lipidomically and functionally defined HDL particles becomes feasible. ApoAI-containing HDL particles transporting the apoM-S1P complex could be prioritised, particularly given that their functions are multiple, extending to antidiabetic and anti-inflammatory actions and endothelial maintenance and vasodilation.
The infusion of such reconstituted HDL featuring recombinant proteins and a defined lipidome portends the normalisation of specific aspects of HDL dysfunctionality in diabetes, potentially with beneficial clinical sequelae such as stabilisation or even regression of vascular disease or pancreatic β-cell protection, or both. Effective background treatment of dyslipidaemic patients with diabetes at high cardiovascular risk with antidiabetic and lipid-lowering agents will be essential in order to optimise the clinical benefit of such innovative therapy.
Acknowledgements
None.
Financial support and sponsorship
The cited studies of the Author were supported by a research grant from Pfizer (Grant WS285961-6027/ID #53234871) and by continuous research funding from the National Institute of Health and Medical Research (INSERM), Sorbonne/Pierre and Marie Curie University, Fondation de la Recherche Medicale, the City of Paris, ARLA (Association for Research on Lipoproteins and Atherogenesis), and NSFA (Nouvelle Societé Francaise de l’Athérosclerose).
Conflicts of interest
There are no conflicts of interest for the preparation of this review.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Chapman MJ, Ginsberg HN, Amarenco P, et al. European Atherosclerosis Society Consensus Panel. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J 2011; 32:1345–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asztalos BF, Schaefer EJ. HDL in atherosclerosis: actor or bystander? Atheroscler Suppl 2003; 4:21–29. [DOI] [PubMed] [Google Scholar]
- 3.Madsen CM, Nordestgaard BG. Is it time for new thinking about high-density lipoprotein? Arterioscler Thromb Vasc Biol 2018; 38:484–486. [DOI] [PubMed] [Google Scholar]
- 4▪.Madsen CM, Varbo A, Nordestgaard BG. Novel insights from human studies on the role of high-density lipoprotein in mortality and noncardiovascular disease. Arterioscler Thromb Vasc Biol 2021; 41:128–140. [DOI] [PubMed] [Google Scholar]; An insightful review of observational data supporting an association between extreme high HDL-C and elevated mortality in major noncardiovascular disorders.
- 5.Schofield J, Ho J, Soran H. Cardiovascular risk in type 1 diabetes mellitus. Diabetes Ther 2019; 10:773–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation 2019; 140:e596–e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barter PJ. The causes and consequences of low levels of high-density lipoproteins in patients with diabetes. Diabetes Metab J 2011; 35:101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8▪.Vergès B. Cardiovascular disease in type 1 diabetes: a review of epidemiological data and underlying mechanisms. Diabetes Metab 2020; 46:442–449. [DOI] [PubMed] [Google Scholar]; An informative review of factors promoting ASCVD in type 1 diabetes (T1DM), with identification of underlying pathophysiological mechanisms.
- 9▪.Vergès B. Dyslipidemia in type 1 diabetes: a masked danger. Trends Endocrinol Metab 2020; 31:422–434. [DOI] [PubMed] [Google Scholar]; Quantitative, qualitative and functional abnormalities of HDL in T1DM in relation to the degree of glycemic control.
- 10.Ganjali S, Dallinga-Thie GM, Simental-Mendía LE, et al. HDL functionality in type 1 diabetes. Atherosclerosis 2017; 267:99–109. [DOI] [PubMed] [Google Scholar]
- 11.Rye KA, Barter PJ. Cardioprotective functions of HDLs. J Lipid Res 2014; 55:168–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12▪.Ben-Aicha S, Badimon L, Vilahur G. Advances in HDL: much more than lipid transporters. Int J Mol Sci 2020; 21:732. [DOI] [PMC free article] [PubMed] [Google Scholar]; Focus on the functions of HDL particles in relation to their role as transporters of diverse biologically active molecules.
- 13.Keul P, Polzin A, Kaiser K, et al. Potent anti-inflammatory properties of HDL in vascular smooth muscle cells mediated by HDL-S1P and their impairment in coronary artery disease due to lower HDL-S1P: a new aspect of HDL dysfunction and its therapy. FASEB J 2019; 33:1482–1495. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka S, Couret D, Tran-Dinh A, et al. High- density lipoproteins during sepsis: from bench to bedside. Crit Care 2020; 24:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15▪▪.Yalcinkaya M, Kerksiek A, Gebert K, et al. HDL inhibits endoplasmic reticulum stress-induced apoptosis of pancreatic beta-cells in vitro by activation of Smoothened. J Lipid Res 2020; 61:492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cellular mechanisms implicated in the protection of pancreatic β cells against endoplasmic reticulum stress and apoptotic β-cell death are identified and highlight the role of HDL-mediated homeostasis of oxysterols in activating the hedgehog signalling receptor, smoothened.
- 16▪.Yalcinkaya M, von Eckardstein A. Apolipoprotein M and sphingosine-1-phosphate: a potentially antidiabetic tandem carried by HDL. Diabetes 2020; 69:859–861. [DOI] [PMC free article] [PubMed] [Google Scholar]; Insights into the potential protective roles of apoM and sphingosine-1-phosphate (S1P) in diabetes, obesity and insulin resistance.
- 17▪▪.Cochran BJ, Ong KL, Manandhar B, Rye KA. High density lipoproteins and diabetes. Cells 2021; 10:850. [DOI] [PMC free article] [PubMed] [Google Scholar]; A comprehensive and insightful review of the lipid and protein components underlying the antidiabetic function of HDL, and of the impact of T1DM and type 2 diabetes (T2DM) upon them.
- 18▪.Bonacina F, Pirillo A, Catapano AL, Norata GD. HDL in immune-inflammatory responses: implications beyond cardiovascular diseases. Cells 2021; 10:1061. [DOI] [PMC free article] [PubMed] [Google Scholar]; The immunomodulatory function of HDL is considered in the context of immune-inflammatory disorders.
- 19▪.Rohatgi A, Westerterp M, von Eckardstein A, et al. HDL in the 21st century: a multifunctional roadmap for future HDL research. Circulation 2021; 143:2293–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]; Insights into unanswered questions in HDL biology.
- 20▪.Verdi J, Zipkin R, Hillman E, et al. Inducible germline IgMs bridge trypanosome lytic factor assembly and parasite recognition. Cell Host Microbe 2020; 28:79–88.e4. [DOI] [PubMed] [Google Scholar]; HDL complexes termed trypanosome lytic factors (TLF) 1 and 2 respectively contain apoL1 with a role in lysis, and haptoglobin-related protein (HpR) which functions as a ligand for a parasite receptor. TLF2 contains bound immunoglobulin M antibodies which interact with HpR and alternate surface trypanosome proteins to facilitate parasite entry.
- 21.Du XM, Kim MJ, Hou L, et al. HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export. Circ Res 2015; 116:1133–1142. [DOI] [PubMed] [Google Scholar]
- 22.Orsoni A, Thérond P, Tan R, et al. Statin action enriches HDL3 in polyunsaturated phospholipids and plasmalogens and reduces LDL-derived phospholipid hydroperoxides in atherogenic mixed dyslipidemia. J Lipid Res 2016; 57:2073–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goulinet S, Chapman MJ. Plasma LDL and HDL subspecies are heterogenous in particle content of tocopherols and oxygenated and hydrocarbon carotenoids. Relevance to oxidative resistance and atherogenesis. Arterioscler Thromb Vasc Biol 1997; 17:786–796. [DOI] [PubMed] [Google Scholar]
- 24▪▪.Vickers KC, Michell DL. HDL-small RNA export, transport, and functional delivery in atherosclerosis. Curr Atheroscler Rep 2021; 23:38. [DOI] [PMC free article] [PubMed] [Google Scholar]; A seminal review of the export, transport and delivery of the full small RNA signature of circulating HDL, their cellular targets and downstream actions.
- 25.Shah AS, Tan L, Long JL, Davidson WS. Proteomic diversity of high-density lipoproteins: our emerging understanding of its importance in lipid transport and beyond. J Lipid Res 2013; 54:2575–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kontush A, Chapman MJ. High-density lipoproteins: structure, metabolism, function and therapeutics. Hoboken, NJ: John Wiley & Sons; 2012. [Google Scholar]
- 27.Sacks FM, Jensen MK. From high-density lipoprotein cholesterol to measurements of function: prospects for the development of tests for high-density lipoprotein functionality in cardiovascular disease. Arterioscler Thromb Vasc Biol 2018; 38:487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28▪▪.Niisuke K, Kuklenyik Z, Horvath KV, et al. Composition-function analysis of HDL subpopulations: influence of lipid composition on particle functionality. J Lipid Res 2020; 61:306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of the earliest studies demonstrating the implication of the lipidome in functionality (as CEC and antioxidative activity) in five HDL subpopulations isolated from subjects stratified based on the level of functionality in preβ-1 particles.
- 29.Camont L, Lhomme M, Rached F, et al. Small, dense high-density lipoprotein-3 particles are enriched in negatively charged phospholipids: relevance to cellular cholesterol efflux, antioxidative, antithrombotic, anti-inflammatory, and antiapoptotic functionalities. Arterioscler Thromb Vasc Biol 2013; 33:2715–2723. [DOI] [PubMed] [Google Scholar]
- 30.Furtado JD, Yamamoto R, Melchior JT, et al. Distinct proteomic signatures in 16 HDL (high-density lipoprotein) subspecies. Arterioscler Thromb Vasc Biol 2018; 38:2827–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davidson WS, Silva RA, Chantepie S, et al. Proteomic analysis of defined HDL subpopulations reveals particle-specific protein clusters: relevance to antioxidative function. Arterioscler Thromb Vasc Biol 2009; 29:870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davidson WS, Shah AS. High-density lipoprotein subspecies in health and human disease: focus on type 2 diabetes. Methodist Debakey Cardiovasc J 2019; 15:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33▪▪.Davidson WS, Cooke AL, Swertfeger DK, Shah AS. The difference between high density lipoprotein subfractions and subspecies: an evolving model in cardiovascular disease and diabetes. Curr Atheroscler Rep 2021; 23:23. [DOI] [PMC free article] [PubMed] [Google Scholar]; An innovative summary of progress in our understanding of the relationships of component-specific HDL particle subspecies to their functionality, with focus on apoC3 and the apoM-S1P complex in T2DM.
- 34.Kontush A, Lhomme M, Chapman MJ. Unraveling the complexities of the HDL lipidome. J Lipid Res 2013; 54:2950–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mendivil CO, Furtado J, Morton AM, et al. Novel pathways of apolipoprotein A-I metabolism in high-density lipoprotein of different sizes in humans. Arterioscler Thromb Vasc Biol 2016; 36:156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36▪.Feng M, Darabi M, Tubeuf E, et al. Free cholesterol transfer to high-density lipoprotein (HDL) upon triglyceride lipolysis underlies the U-shape relationship between HDL-cholesterol and cardiovascular disease. Eur J Prev Cardiol 2020; 27:1606–1616. [DOI] [PubMed] [Google Scholar]; Using a novel assay to quantitate transfer of free cholesterol from triglyceride-rich lipoproteins to HDL during lipoprotein lipase-mediated lipolysis in vitro, such transfer was shown to be attenuated in T2DM, potentially contributing to the subnormal HDL levels typical of this disorder.
- 37.Ahmed MO, Byrne RE, Pazderska A, et al. HDL particle size is increased and HDL-cholesterol efflux is enhanced in type 1 diabetes: a cross-sectional study. Diabetologia 2021; 64:656–667. [DOI] [PubMed] [Google Scholar]
- 38▪.Vaisar T, Kanter JE, Wimberger J, et al. High concentration of medium-sized HDL particles and enrichment in HDL paraoxonase 1 associate with protection from vascular complications in people with long-standing type 1 diabetes. Diabetes Care 2020; 43:178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]; Elevated levels of medium-sized HDL particles and of HDL-associated paraoxonase-1 (PON1) likely contribute to protection from the microvascular and macrovascular complications in T1DM.
- 39.Gourgari E, Ma J, Playford MP, et al. Proteomic alterations of HDL in youth with type 1 diabetes and their associations with glycemic control: a case–control study. Cardiovasc Diabetol 2019; 18:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiesa ST, Charakida M, McLoughlin E, et al. Elevated high-density lipoprotein in adolescents with type 1 diabetes is associated with endothelial dysfunction in the presence of systemic inflammation. Eur Heart J 2019; 40:3559–3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li N, Fu J, Koonen DP, et al. Are hypertriglyceridemia and low HDL causal factors in the development of insulin resistance? Atherosclerosis 2014; 233:130–138. [DOI] [PubMed] [Google Scholar]
- 42.Chapman MJ, Le Goff W, Guerin M, Kontush A. Cholesteryl ester transfer protein: at the heart of the action of lipid-modulating therapy with statins, fibrates, niacin, and cholesteryl ester transfer protein inhibitors. Eur Heart J 2010; 31:149–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kontush A, Chapman MJ. Why is HDL functionally deficient in type 2 diabetes? Curr Diab Rep 2008; 8:51–59. [DOI] [PubMed] [Google Scholar]
- 44.Hui N, Barter PJ, Ong KL, Rye KA. Altered HDL metabolism in metabolic disorders: insights into the therapeutic potential of HDL. Clin Sci (Lond) 2019; 133:2221–2235. [DOI] [PubMed] [Google Scholar]
- 45▪▪.Robert J, Osto E, von Eckardstein A. The endothelium is both a target and a barrier of HDL's protective functions. Cells 2021; 10:1041. [DOI] [PMC free article] [PubMed] [Google Scholar]; A comprehensive treatise on the HDL-mediated protection of multiple endothelial functions implicating diverse molecular modes of action; such actions are perturbed in both T1DM and T2DM.
- 46▪.Bonilha I, Zimetti F, Zanotti I, et al. Dysfunctional high-density lipoproteins in type 2 diabetes mellitus: molecular mechanisms and therapeutic implications. J Clin Med 2021; 10:2233. [DOI] [PMC free article] [PubMed] [Google Scholar]; Structural and compositional changes in the HDL lipidome and proteome, together with physicochemical changes in particle profile, are intimately associated with the transformation of HDL into dysfunctional particles in T2DM.
- 47.Florijn BW, Duijs JMGJ, Levels JH, et al. Diabetic nephropathy alters the distribution of circulating angiogenic microRNAs among extracellular vesicles, HDL, and Ago-2. Diabetes 2019; 68:2287–2300. [DOI] [PubMed] [Google Scholar]
- 48▪.He Y, Ronsein GE, Tang C, et al. Diabetes impairs cellular cholesterol efflux from ABCA1 to small HDL particles. Circ Res 2020; 127:1198–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]; Comparison of the capacity of large, medium and small HDL particles to mediate CEC via the ABC transporters revealed that small HDL accounted for most of CEC activity in total HDL on a per particle basis; such activity was attenuated in T2DM, and could likely be explained by loss of SERPINA1, a phospholipid binding protein endowed with antiprotease and anti-inflammatory activity.
- 49.Lui DTW, Cheung CL, Lee ACH, et al. Carbamylated HDL and mortality outcomes in type 2 diabetes. Diabetes Care 2021; 44:804–809. [DOI] [PubMed] [Google Scholar]
- 50▪▪.Cardner M, Yalcinkaya M, Goetze S, et al. Structure-function relationships of HDL in diabetes and coronary heart disease. JCI Insight 2020; 5:e131491. [DOI] [PMC free article] [PubMed] [Google Scholar]; In an innovative study, lipidomic and proteomic data from total plasma HDL fraction of control and T2DM subjects were interfaced with five in-vitro functions (CEC, inhibition of endothelial cell apoptosis, inhibition of beta cell apoptosis, rescue of mitochondrial membrane potential and mitochondrial respiration). Proteome depletion in apoAIV, PON1, PON3, apoD, apoE, apoF, apoJ and apoM was associated with increase in contents of serum amyloid SAA1 (serum amyloid A) and SAA2, apoCII, apoCIII and fibrinogen; overall, a net protein loss occurred. Except for CEC, all HDL functions were impaired. Individual functions were poorly correlated with each other, suggesting that they may be determined by distinct structural components.
- 51.Mora S, Otvos JD, Rosenson RS, et al. Lipoprotein particle size and concentration by nuclear magnetic resonance and incident type 2 diabetes in women. Diabetes 2010; 59:1153–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52▪▪.Webb NR. High-density lipoproteins and serum amyloid A (SAA). Curr Atheroscler Rep 2021; 23:7. [DOI] [PMC free article] [PubMed] [Google Scholar]; The gain of the acute phase protein, SAA, by HDL in T2DM is noteworthy. As HDL-bound SAA appears to be biologically inert, HDL may serve as a transport vehicle to sequester SAA secreted from diverse tissues and thus protect the host from uncontrolled inflammation and tissue damage.
- 53.Kheniser KG, Osme A, Kim C, et al. Temporal dynamics of high-density lipoprotein proteome in diet-controlled subjects with type 2 diabetes. Biomolecules 2020; 10:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54▪.Manandhar B, Cochran BJ, Rye KA. Role of high-density lipoproteins in cholesterol homeostasis and glycemic control. J Am Heart Assoc 2020; 9:e013531. [DOI] [PMC free article] [PubMed] [Google Scholar]; A valuable treatise on the mechanisms underlying the relationship of HDL-mediated cholesterol homeostasis to insulin sensitivity and β-cell function in both T1DM and T2DM.
- 55▪▪.Nilsson O, Del Giudice R, Nagao M, et al. Apolipoprotein A-I primes beta cells to increase glucose stimulated insulin secretion. Biochim Biophys Acta Mol Basis Dis 2020; 1866:165613. [DOI] [PubMed] [Google Scholar]; Can apoAI prime β cells to enhance insulin secretion? ApoAI increased glucose-stimulated insulin secretion in INS-1E rat clonal beta cells and isolated murine islets via induction of an increased reservoir of insulin granules at the cell membrane. Several mechanisms contributed to this effect, including ApoA-I internalization into β cells, PDX1 nuclear translocation and elevated levels of proinsulin processing enzymes.
- 56.Hou L, Tang S, Wu BJ, et al. Apolipoprotein A-I improves pancreatic β-cell function independent of the ATP-binding cassette transporters ABCA1 and ABCG1. FASEB J 2019; 33:8479–8489. [DOI] [PubMed] [Google Scholar]
- 57.Ochoa-Guzmán A, Guillén-Quintero D, Muñoz-Hernández L, et al. The influence of high-density lipoprotein (HDL) and HDL subfractions on insulin secretion and cholesterol efflux in pancreatic derived β-cells. J Endocrinol Invest 2021; 44:1897–1904. [DOI] [PubMed] [Google Scholar]
- 58.Obinata H, Hla T. Sphingosine 1-phosphate and inflammation. Int Immunol 2019; 31:617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59▪▪.Kurano M, Tsukamoto K, Shimizu T, et al. Protection against insulin resistance by apolipoprotein M/sphingosine-1-phosphate. Diabetes 2020; 69:867–881. [DOI] [PubMed] [Google Scholar]; Are circulating levels of HDL-associated apoM and S1P associated with glucose intolerance and insulin resistance? Using genetically modified murine models and overexpression or knockdown of apoM in in-vitro cell systems, apoM-S1P was shown to exert protection against development of insulin resistance by activation of insulin signalling and by enhancement of mitochondrial function.
- 60.Ruiz M, Frej C, Holmér A, et al. High-density lipoprotein-associated apolipoprotein M limits endothelial inflammation by delivering sphingosine-1-phosphate to the sphingosine-1-phosphate receptor 1. Arterioscler Thromb Vasc Biol 2017; 37:118–129. [DOI] [PubMed] [Google Scholar]
- 61▪.Kobayashi T, Kurano M, Nanya M, et al. Glycation of HDL polymerizes apolipoprotein M and attenuates its capacity to bind to sphingosine 1-phosphate. J Atheroscler Thromb 2021; 28:730–741. [DOI] [PMC free article] [PubMed] [Google Scholar]; Treatment of HDL with either high glucose levels or methylglyoxal in vitro led to formation of homo polymers and hetero polymers of apoM in HDL, with decreased binding of S1P. Such polymers were not however detected in diabetic subjects. Further research on the biological activity of apoM is warranted as a function of glycemic burden.
- 62.Sedgeman LR, Beysen C, Ramirez Solano MA, et al. Beta cell secretion of miR-375 to HDL is inversely associated with insulin secretion. Sci Rep 2019; 9:3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gomez Rosso L, Lhomme M, Meroño T, et al. Poor glycemic control in type 2 diabetes enhances functional and compositional alterations of small, dense HDL3c. Biochim Biophys Acta Mol Cell Biol Lipids 2017; 1862:188–195. [DOI] [PubMed] [Google Scholar]
- 64.Kontush A, Therond P, Zerrad A, et al. Preferential sphingosine-1-phosphate enrichment and sphingomyelin depletion are key features of small dense HDL3 particles: relevance to antiapoptotic and antioxidative activities. Arterioscler Thromb Vasc Biol 2007; 27:1843–1849. [DOI] [PubMed] [Google Scholar]
- 65.Kontush A, Chantepie S, Chapman MJ. Small, dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Arterioscler Thromb Vasc Biol 2003; 23:1881–1888. [DOI] [PubMed] [Google Scholar]
- 66.Rashid S, Trinh DK, Uffelman KD, et al. Expression of human hepatic lipase in the rabbit model preferentially enhances the clearance of triglyceride-enriched versus native high-density lipoprotein apolipoprotein A-I. Circulation 2003; 107:3066–3072. [DOI] [PubMed] [Google Scholar]