Purpose of review
Improving care of individuals with familial hypercholesteremia (FH) is reliant on the synthesis of evidence-based guidelines and their subsequent implementation into clinical care. This review describes implementation strategies, defined as methods to improve translation of evidence into FH care, that have been mapped to strategies from the Expert Recommendations for Implementing Change (ERIC) compilation.
Recent findings
A search using the term ‘familial hypercholesterolemia’ returned 1350 articles from November 2018 to July 2021. Among these, there were 153 articles related to improving FH care; 1156 were excluded and the remaining 37 were mapped to the ERIC compilation of strategies: assess for readiness and identify barriers and facilitators [9], develop and organize quality monitoring systems [14], create new clinical teams [2], facilitate relay of clinical data to providers [4], and involve patients and family members [8]. There were only 8 of 37 studies that utilized an implementation science theory, model, or framework and two that explicitly addressed health disparities or equity.
Summary
The mapping of the studies to implementation strategies from the ERIC compilation provides a framework for organizing current strategies to improve FH care. This study identifies potential areas for the development of implementation strategies to target unaddressed aspects of FH care.
Keywords: cascade screening, familial hypercholesterolemia, identification, implementation science
INTRODUCTION
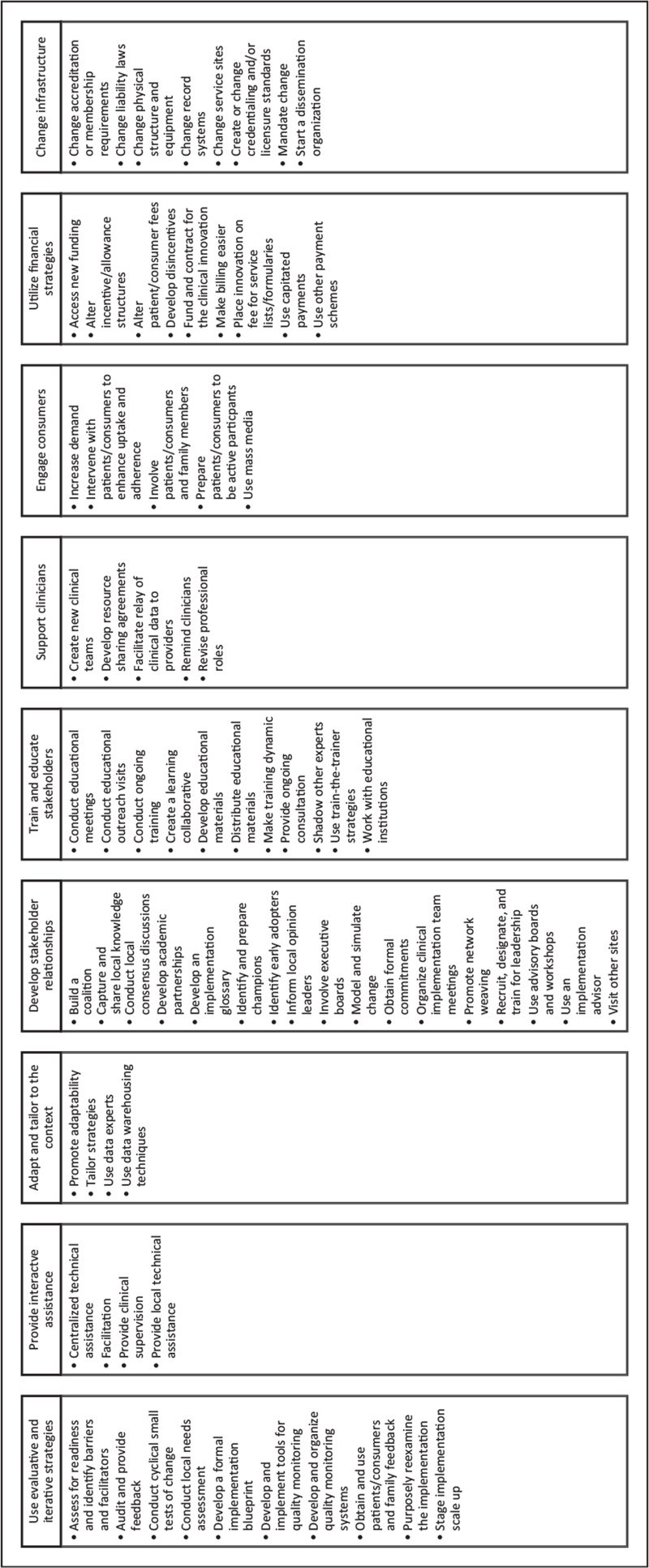
Improving care of individuals with familial hypercholesteremia (FH) is reliant on the synthesis of evidence-based guidelines and their subsequent implementation into clinical care. Recent Cholesterol Guidelines provide evidence-based clinical guidance for caring for individuals with FH [1,2]. However, not all these recommendations have been implemented into clinical care (e.g., systematic identification of individuals with FH [3]). The field of implementation science supplies theories, models, and frameworks for the development and implementation of strategies to reduce the time from discovery to translation into clinical practice [4,5]. Compilations of implementation strategies, defined as ‘methods or techniques used to enhance the adoption, implementation, and sustainability of a clinical program or practice’ [6], have been developed, such as the Expert Recommendation for Implementation Change (ERIC) [7] and Effective Practice and Organization of Care (EPOC) [8]. The purpose of ERIC and EPOC was to develop a list of commonly used implementation strategies and then to create a standard naming schema for those strategies accompanied by standardized definitions that could be modified for specific studies. Figure 1 provides a list of 73 ERIC strategies categorized into nine overarching themes. This review describes implementation strategies, defined as methods to improve translation of evidence into FH care, that have been mapped to standardized compilation of strategies.
FIGURE 1.
List of the 73 Expert Recommendations for Implementing Change (ERIC) implementation strategies categorized by nine overarching themes.
Box 1.
no caption available
METHODS
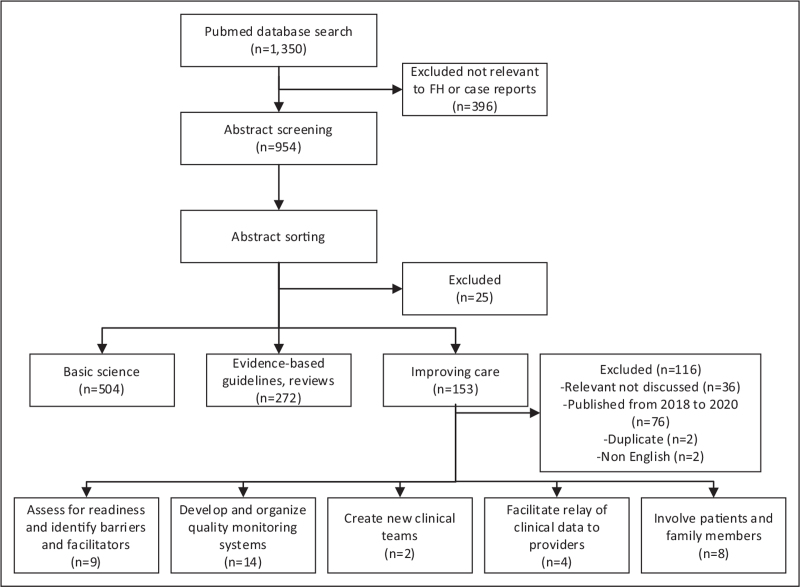
We conducted a scoping review of the literature focused on studies to improve care for individuals with FH [9]. We searched PubMed from November 1, 2018 to July 31, 2021 to identify all relevant articles that were published after the release of the 2018 AHA/ACC Multi-Society Cholesterol Guidelines [1]. This search returned 1350 articles when using key words associated with ‘familial hypercholesterolemia’ (PubMed search strategy Table 1). During phase 1 of abstract screening, studies were excluded that were case reports, or articles not relevant to FH. During phase 2 of abstract screening, studies were sorted into three categories: findings in basic science (i.e., discovery), evidence-based guidelines/reviews, and suggestions for improving care for individuals with FH. All abstract screening was completed by a single reviewer. The focus of this review was only articles in the latter category which included any studies that explored aspects related to implementation of an evidence-based intervention for adults. Included full text articles were categorized into one of the 73 implementation strategies from the ERIC compilation. The ERIC compilation was selected as the standardized list of implementation strategies because the identified strategies in the articles reviewed better aligned with this compilation. Figure 2 depicts the article review process and categorization. Each article was coded if they utilized an implementation science theory, model, or framework or focused on health disparities or equity by identifying barriers to care or strategies to reduce care variation in certain populations.
Table 1.
PubMed search strategy
| PubMed search strategy |
| “Hyperlipoproteinemia Type II’[Mesh] OR “familial hypercholesterolaemia’[All Fields] OR “hyperlipoproteinemia type ii’[Mesh] OR (“hyperlipoproteinemia’[All Fields] AND “type’[All Fields] AND “ii’[All Fields]) OR “hyperlipoproteinemia type ii’[All Fields] OR (“familial’[All Fields] AND “hypercholesterolemia’[All Fields]) OR “familial hypercholesterolemia’[All Fields]. |
FIGURE 2.
Flow diagram of articles included in the review.
RESULTS
Of the 1350 articles found, 954 abstracts were sorted into three categories: basic science (n = 504), evidence-based guidelines/reviews (n = 272) and improving care (n = 153). Of the 153 articles in the improving care category, 116 were excluded, as they were relevant but either did not map to implementation strategies, focused on pediatric care, published between 2018 and 2020, duplicates, or not available in English. The remaining 37 were categorized into the following implementation strategies: ‘assess for readiness and identify barriers and facilitators’ [9], ‘develop and organize quality monitoring systems’ [14], ‘create new clinical teams’ [2], ‘facilitate relay of clinical data to providers’ [4], and ‘involve patients and family members’ [8]. Table 2 lists and defines the mapped ERIC implementation strategies. There were only 8 of 37 studies that utilized an implementation science theory, model, or framework (three of the eight were published by the first author of this manuscript) and two that explicitly addressed health disparities or equity. Table 3 details the studies included in the review categorized by the ERIC compilation of strategies and coded for including an implementation science theory, model, or framework and mention of health disparities or equity.
Table 2.
Categorization and definitions of implementation strategies to the Expert Recommendations for Implementing Change (ERIC) compilation
| Implementation strategy | Number of studies | Definition |
| Develop and organize quality monitoring systems | 14 | Develop and improve diagnostic performance of tools to identify individuals with FH |
| Assess for readiness and identify barriers and facilitators | 9 | Assess healthcare organizations and providers to determine their degree of readiness to implement and barriers and enablers to FH care |
| Involved patients/consumers and family members | 8 | Engage or include patients and families to improve FH care |
| Create new clinical teams | 2 | Change who serves on the clinical team, adding different disciplines, and different skills to the FH care team |
| Facilitate relay of clinical data to providers | 4 | Provide data using integrated modes of communication to improve FH care |
FH, familial hypercholesteremia.
Table 3.
Description of studies included in the review
| Study | Year | Design | Country | Implementation strategy | Implementation science theory, model, framework | Health disparities or equity focusa |
| Assess for readiness and identify barriers and facilitators (n = 9) | ||||||
| Jones et al. | 2021 | Qualitative analysis | United States | Focus groups with individuals with FH and providers on the acceptability, appropriateness, and feasibility of identification and cascade screening methods for FH | Conceptual Model of Implementation Research | |
| Jones et al. | 2020 | Qualitative analysis | United States | Interviews and focus groups with individuals with FH and providers to discuss barriers and facilitators and develop potential solutions to improve treatment approaches | Practical, Robust Implementation and Sustainability Model | |
| Kawasaki et al. | 2021 | Prepost | Japan | Genetic literacy education program for providers | ||
| Miller et al. | 2021 | Qualitative analysis | United States | Interviews with key informants regarding barriers and recommendations to improve FH screening | Reach, Effectiveness, Adoption, Implementation, and Maintenance | |
| Mszar et al. | 2021 | Cross sectional | United States | Survey based on the health belief model to understand self-efficacy, perceived barriers to care and health-promoting behaviors across cardiovascular risk factors | Health Belief Model | Yes |
| Schwiter et al. | 2020 | Cross sectional | United States, International | Survey of perspectives regarding direct contact as an approach for cascade screening of relatives | ||
| Wand et al. | 2020 | Cross sectional | United States | Survey of clinically diagnosed FH patients regarding intention to obtain genetic testing | ||
| Wong et al. | 2021 | Cross sectional | United States | Survey of primary care physicians and cardiologists regarding perceptions and barriers to use of PCSK9 inhibitors in FH | ||
| Unim et al. | 2020 | Cross sectional | Canada | Survey of healthcare workers on barriers to genetic testing | ||
| Develop and organize quality monitoring systems (n = 14) | ||||||
| Abul-Husn et al. | 2021 | Cross sectional | United States | Population genetic screening | Yes | |
| Akyea et al. | 2020 | Cross sectional | United Kingdom | EHR data screening tool (FAMCAT) | ||
| Akyea et al. | 2020 | Diagnostic accuracy | United Kingdom | Machine learning algorithm | ||
| Birnbaum et al. | 2021 | Prospective cohort | United States | EHR data screening tool (MEDPED primary) | ||
| Buchanan et al. | 2020 | Cross sectional | United States | Population genetic screening | ||
| David et al. | 2021 | Cross sectional | United States | Population genetic screening | ||
| Ingoe et al. | 2021 | Cross sectional | United Kingdom | EHR data screening tool (Simon Broome primary) | ||
| Grzymski et al. | 2020 | Cross sectional | United States | Population genetic screening | ||
| Kawame et al. | 2021 | Noncontrolled | Japan | Population genetic screening | ||
| Pepplinkhuizen et al. | 2020 | Cross sectional | Netherlands | EHR data screening tool (DLCN primary) | ||
| Pina et al. | 2020 | Diagnostic accuracy | Sweden and Italy | Machine learning algorithm (compared to DLCN) | ||
| Sabatel-Perez et al. | 2021 | Cross sectional | Spain | EHR data screening tool (DLCN primary) | ||
| Sheth et al. | 2021 | Cross sectional | United States | Machine learning algorithm | ||
| Zamora et al. | 2021 | Cross sectional | Spain | EHR data screening tool (7 different phenotype algorithms were tested) | ||
| Create new clinical teams (n = 2) | ||||||
| Jones et al. | 2021 | Cross sectional | United States | Implementation and evaluation of a multidisciplinary lipid clinic | Reach, effectiveness, adoption, implementation, and maintenance | |
| Wilkinson et al. | 2020 | Cross sectional | United Kingdom | Implementation and evaluation of a nurse-led lipid clinic | ||
| Facilitate relay of clinical data to providers (n = 4) | ||||||
| Bangash et al. | 2020 | Qualitative analysis | United States | Interview and survey with providers for development and implementation of a CDS tool | Conceptual Framework of Implementation Research | |
| Ellis et al. | 2020 | Cross sectional | Australia | Impact of genetic risk scores | ||
| Gallo et al. | 2021 | Cross sectional | France | Contribution of coronary calcium scores to SAFEHEART-RE | ||
| Ramos et al. | 2020 | Cross sectional | Spain | Performance of the SIDIAP-FHP score compared to SAFEHEART-RE | ||
| Involved patients and family members (n = 8) | ||||||
| Baldry et al. | 2021 | Prepost | United States | Motivational interviewing and extended parallel process model | ||
| Benatar et al. | 2020 | Qualitative | New Zealand | Family visit with healthcare professionals and initiation of a family Facebook® page to discuss family implications of an FH result | ||
| Descamps et al. | 2020 | Cross sectional | Belgium | Probands were screened by specialist and met DLCN score ≥6 and then relatives were visited for screening | ||
| Gidding et al. | 2020 | Cross sectional | United States | Individuals were recruited from the FH CASACDE® Registry to undergo genetic testing and their first-degree relatives could also receive testing | ||
| Kinnear et al. | 2020 | Qualitative analysis | United Kingdom | Theory informed behavior change intervention to improve adherence to dietary and physical activity guidelines for individuals with FH | Behavior change wheel and Theoretical domains framework | |
| Kinnear et al. | 2020 | Cross sectional | United Kingdom | Results of feasibility trial of the intervention to improve adherence to dietary and physical activity guidelines | Behavior change wheel and Theoretical domains framework | |
| McGowan et al. | 2021 | Prepost | United States | FH Foundation directly engaged with FH probands and relatives | ||
| Neuner et al. | 2020 | Cross sectional | United States | Probands were identified via web-based risk assessment service (MeTree) linked to EHR information or EHR query alone, if positive, relatives were invited to receive genetic testing | ||
CDS, clinical decision support; DLCN, Dutch Lipid Clinic Network criteria; EHR, electronic health records; FH, familial hypercholesteremia; MEDPED, Make Early Diagnosis to Prevent Early Deaths; SAFEHEART-RE, Spanish FH Cohort Study risk equation.
Focus on health disparities or equity by identifying barriers to care or strategies to reduce care variation in certain populations.
Assess for readiness and identify barriers and facilitators
Lack of a systematic and sustainable approach to identifying individuals with FH leads to delays in care [10]. A survey of providers found significant barriers to providers offering genetic testing to their patients and barriers that providers perceived patients having to the acceptability of genetic testing including limited coverage by insurance companies, availability of personnel to explain and order testing, and lack of access to genetic counseling professionals [11]. However, when individuals with a clinical diagnosis of FH were surveyed three factors were associated with their willingness to undergo genetic testing. These factors included aversion to FH genetic information, curiosity regarding medical and family history, psychological reassurance of genetic testing intent [12].
These barriers identified by providers and patients have led researchers to develop educational strategies to improve uptake of genetic testing. The implications of a genetic literacy program to address these barriers found that providers improved their understanding about genetics and ability to provide accurate knowledge and advice while promoting genetic literacy to patients [13]. Similarly, for cascade screening of relatives, an international survey explored perspectives of patients on indirect and direct contact approaches for cascade screening and found that a majority of individuals supported direct outreach by their provider to their relatives to share their FH result [14]; however, this approach is seldom used.
Barriers and facilitators to improving access to care for FH [15▪▪] and treatment approaches for FH exist [16▪▪]. Articles included share similar findings: awareness of FH is poor, guidelines are complex and changing, and a focused supportive effort is needed to improve FH management [15▪▪,16▪▪]. A recent study found 30% of young patients with FH had poor adherence to lipid-lowering therapies, the main reason being lack of motivation. A survey of primary care physicians and cardiologists found several factors influencing prescribing of PCSK9 inhibitors: clinical type (cardiologist more likely to order) and practice setting and location (urban and academic centers more likely to order) [17].
Assessment of stakeholder readiness to implement is important for successful uptake of an evidence-based intervention [18▪▪,19▪▪]. Focus groups with stakeholders that addressed willingness to use novel identification processes including automated approaches (i.e., machine learning) and cascade screening methods for FH, including chatbots and direct contact. They found these methods were acceptable, appropriate, and feasible if they fit into the clinician workflow [19▪▪].
Develop and organize quality monitoring systems
Four studies implemented the existing clinical diagnostic criteria into their healthcare system electronic health records (EHRs) as a screening tool to identify previously unrecognized individuals with FH. Similar rates of individuals requiring additional diagnostic screening for FH were found: 1 in 245 (7468/1 831 658) met the Make Early Diagnoses Prevent Early Deaths (MEDPED) criteria [20], 1 in 150 (303/45 123) met the Simon Broome (SB) Criteria [21], and 1 in 183 (269/49 321) [21] and 1 in 119 (351/41 937) [22] met the Dutch Lipid Clinic Network Criteria (DLCN). The screening positive rate for FH was higher, 1 in 5 (84/469), when the DLCN criteria were applied to EHRs of those with known severe hypercholesterolemia [23]. Diagnostic evaluation for FH in individuals identified by these EHR screening initiatives found 18–36% met clinical criteria [21–23]. However, the percentage of these individuals with a genomic risk variant for FH ranged from 25 to 68% depending on the study [20,21,23] meaning that using genetics as the sole indicator for a diagnosis of FH would miss many individuals who met clinical diagnostic criteria.
Instead of utilizing the traditional clinical diagnostic criteria, some have implemented specific algorithms that use clinical data available in the EHR [24,25]. The most efficient of the seven algorithms tested that could be translated into clinical practice identified 840 patients with FH [24]. Another study found the FH case ascertainment identification tool (FAMCAT) algorithm to have a high level of discrimination (area under the curve [AUC] = 0.844, 95% confidence interval [CI] = 0.834–0.854) and performed better when compared to the manual scoring of the SB criteria (AUC = 0.730, 95% CI = 0.719 to 0.741) and DLCN Score (AUC = 0.766, 95% CI = 0.755 to 0.778) [25].
The use of machine learning approaches to identify individuals with FH is novel and positive results from these studies provide insight into their capabilities to help close the FH identification gap [26,27]. A machine learning algorithm that utilized five different approaches (logistic regression, random forest, gradient boosting machines, neural networks, and ensemble learning) had high predictive accuracy (AUC > 0.89) [26]. Three machine learning algorithm approaches (classification tree, gradient boosting machine, and neural network) were found to perform better than applying the DLCN criteria alone [27]. There is still more to learn on how to successfully move from identification approaches to implementation into clinical care. A study utilizing the FH Foundation's FIND FH machine learning algorithm (random forest) identified 5006 screened positive patients but only 153 were seen for clinical confirmation [28]. Implementation at the healthcare system level will be required to fully realize the potential of information-technology based tools.
Five healthcare systems have implemented population genetic screening approaches to identify unselected individuals with risk for genetic disease including Tier 1 genetic conditions (designated by the Centers for Disease Control and Prevention's Office of Public Health Genomics [29]) including FH [30▪,31–34]. Each of these population screening approaches performs exome sequencing, links exome data to EHR systems, returns actionable results, and allows for recontact for future studies. To date, these programs have identified participants with variants in three genes associated with FH (LDLR, APOB, PCSK9). Rates of identification: Mt. Sinai 8 in 692, Geisinger 93 in 64 392, Healthy Nevada 102 in 26 906, NorthShore 29 in 9797, Japan 23 of 215 participants. Very few individuals knew about their genetic risk prior to return from one of these programs: Mt. Sinai 1 in 8, Geisinger 0 in 93, Healthy Nevada 3 in 102, and NorthShore and Japan not reported.
Create new clinical teams
Articles reporting creation of a multidisciplinary lipid clinic composed of different specialists to improve care of individuals with FH showed this approach to be effective. One clinic found high levels of uptake in genetic counseling and subsequent testing for FH (25% with a genetic risk result for FH (6/24)), and intensification of lipid-lowering therapy that resulted in a 79 mg/dl reduction in average LDL-C (n = 12, P < 0.001) and 75% (9/12) achieving LDL-C target goals [35▪▪]. Another lipid clinic study utilized the SB Criteria to identify individuals with definite and possible FH and found that 100% of patients with definite FH and 25% (34/134) of those with possible FH had a genetic risk variant [36].
Facilitate relay of clinical data to providers
Clinical data that is imperative to the care of individuals with FH should be communicated quickly and in a way that is usable by providers. Clinical decision support tools can be used to prompt providers to identify and treat individuals; however, information on the format, placement, content, timing and frequency, and level of alert urgency/prioritization is key to their uptake [37▪▪]. Once prompted, clinicians should be familiar with the different risk scores used to predict cardiovascular disease including a genetic risk score [38] and risk models [39,40]. A genetic risk score was found to be associated with increased odds of cardiovascular disease (variant positive odd ratio [OR] = 3.3; 95% CI 1.3–8.2 and variant negative OR = 1.8; 95% CI 1.0–3.3) [38]. A clinical risk model was found to have fair fit in primary (C-statistic: 0.71; 95% CI: 0.68–0.75) and secondary prevention (0.65; 95% CI 0.60–0.70) patients [39]. When including coronary artery calcium scoring to a traditional risk model there was significantly improved prediction of cardiovascular disease (AUC 0.884, 95% CI 0.871−0.894 compared to 0.793, 95% CI 0.779–0.818) [40].
Involve patients and family members
Strategies to involve patients and family members in the care process are important. The Netherlands implemented a large cascade screening program for family members of individuals who presented to lipid clinics throughout the country. Several publications highlight the success of this government-sponsored program in identifying family members with FH [41]. Norway has implemented the second most successful cascade screening program [42].
Belgium initiated a national pilot project for cascade screening by recruiting probands with DLCN scores ≥6 from specialty care and then visiting their relatives to collect relevant clinical data and obtain a sample for genetic testing [43]. In this study, the FH diagnosis was made either via DLCN or MEDPED and they found 127 probands with FH and subsequently screened 156 relatives [43]. New Zealand implemented a direct contact approach by hosting a hui, a social gathering, that was organized to inform extended family members about the proband's genetic risk variant that included doctors and nurses from a local health practice, extended family members, and elders to discuss how to best manage and access testing and treatment [44]. A closed Facebook group was initiated that housed the family tree (of consented individuals) and offered information to relatives including a family letter for relatives to show their healthcare providers and information about testing and treatment [44]. This approach reached 17 family members from one family [44]. In the United Kingdom, one study tested a 1-h family-based appointment followed up with telephone calls [45▪▪]. This intervention found minimal impact on physical activity but improvements in cardiovascular disease risk factors including reduction in LDL-C [46].
The United States has initiated a few pilot cascade screening programs. The FH Foundation recruited CASCADE FH registry participants who did not previously have genetic testing via the patient portal to obtain free genetic testing [47]. Of the 435 eligible, 110 underwent genetic testing, the majority were female, White, with a median age of 52 years [47]. Sixty-four had a positive genetic test for the familial variant and only three relatives consented to undergoing genetic testing [47]. Another study consented individuals to receive genetic testing for FH by evaluating cholesterol results from a web-based risk assessment service (MeTree [48]) linked to EHR information or EHR query alone to identify probands and then confirmed personal or family history of early coronary artery disease without previous genetic testing [49]. Of the 106 probands that met criteria, 53 underwent genetic testing and two had positive results [49]. The two positive probands gave 4 relatives information and subsequently underwent genetic testing with two having positive results [49]. Motivational interviewing and the extended parallel process model with probands has been tested as an intervention to improve cascade screening and found on average 2.23 new relatives were contacted and 2.46 were screened [50]. A feasibility study based on core principles from the Dutch model, found that when the FH Foundation served as the agency to directly engage with 11 FH probands, they were able to engage nine relatives [51].
CONCLUSION
The categorization of the studies in this review of implementation strategies from the ERIC compilation provided a framework for organizing current strategies to improve FH care. Strategies described in this review have been shown to improve identification and adherence to guideline recommendations for individuals with FH. Included studies were only mapped to 5 of the 73 implementation strategies from ERIC compilation. This identifies potential areas for research and development of implementation strategies to target unaddressed aspects to improve FH care. In addition, only 8 of the 37 studies included utilized an implementation science theory, model, or framework and only two addressed health disparities and equity in FH care. Application of implementation science and categorization of strategies are important to understanding their benefit and tailoring future strategies to improve care for any cardiovascular condition.
Acknowledgements
Katrina Romagnoli, MLIS, PhD, medical librarian, for assisting with the literature search.
Financial support and sponsorship
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute (number K12HL137942, the National Cancer Institute (number P50CA244431), the National Institute of Diabetes and Digestive and Kidney Diseases (numbers P30DK092950 and R25DK123008), the Centers for Disease Control and Prevention (number U48DP006395). The findings and conclusions in this paper are those of the authors and do not necessarily represent the official positions of the National Institutes of Health or the Centers for Disease Control and Prevention.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019; 139:e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis 2019; 290:140–205. [DOI] [PubMed] [Google Scholar]
- 3.Uchmanowicz I, Hoes A, Perk J, et al. Optimising implementation of European guidelines on cardiovascular disease prevention in clinical practice: what is needed? Eur J Prev Cardiol 2021; 28:426–431. [DOI] [PubMed] [Google Scholar]
- 4.Bauer MS, Kirchner J. Implementation science: what is it and why should I care? Psychiatry Res 2020; 283:112376. [DOI] [PubMed] [Google Scholar]
- 5.Oxford University Press, Brownson RC. Dissemination and implementation research in health: translating science to practice. 2017. [Google Scholar]
- 6.Proctor EK, Powell BJ, McMillen JC. Implementation strategies: recommendations for specifying and reporting. Implement Sci 2013; 8:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci 2015; 10:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mowatt G, Grimshaw JM, Davis DA, Mazmanian PE. Getting evidence into practice: the work of the Cochrane Effective Practice and Organization of Care Group (EPOC). J Contin Educ Health Prof 2001; 21:55–60. [DOI] [PubMed] [Google Scholar]
- 9.Peters MDJ, Godfrey CM, Khalil H, et al. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc 2015; 13:141–146. [DOI] [PubMed] [Google Scholar]
- 10.Gidding SS, Champagne MA, de Ferranti SD, et al. The agenda for familial hypercholesterolemia: a scientific statement from the American Heart Association. Circulation 2015; 132:2167–2192. [DOI] [PubMed] [Google Scholar]
- 11.Unim B, De Vito C, Hagan J, et al. The provision of genetic testing and related services in Quebec, Canada. Front Genet 2020; 11:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wand H, Sturm AC, Erby L, et al. Genetic testing preferences and intentions in patients with clinically diagnosed familial hypercholesterolemia. J Genet Counsel 2020; 29:919–927. [DOI] [PubMed] [Google Scholar]
- 13.Kawasaki H, Kawasaki M, Iki T, Matsuyama R. Genetics education program to help public health nurses improve their knowledge and enhance communities’ genetic literacy: a pilot study. BMC Nurs 2021; 20:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwiter R, Brown E, Murray B, et al. Perspectives from individuals with familial hypercholesterolemia on direct contact in cascade screening. J Genet Counsel 2020; 29:1142–1150. [DOI] [PubMed] [Google Scholar]
- 15▪▪.Mszar R, Buscher S, McCann D, et al. Perceived barriers to care, and health-promoting behaviors among Franco-Americans across cardiovascular risk factors: a cross-sectional study. Am J Health Promot 2021; 35:703–707. [DOI] [PubMed] [Google Scholar]; A cross-sectional survey to assess prevalence of perceived barrieres to accessing healthcare services, self-efficacy, and health-promoting behaviors based on the Health Belief Model of Franco-Americans and French Canadians. Franco-Americans reported significant barriers to accessing healthcare services. This article highlights the need to address health diaparities and equity in FH care.
- 16▪▪.Jones LK, Sturm AC, Seaton TL, et al. Barriers, facilitators, and solutions to familial hypercholesterolemia treatment. PLoS One 2020; 15:e0244193. [DOI] [PMC free article] [PubMed] [Google Scholar]; This was a multisite qualitative study with 33 participants to understand barriers, facilitators and solutions to improve FH treatment approaches. This study utilized the Practical, Robust Implementation and Substantiability Model (PRISM) in the development and analyze of the focus groups and interviews.
- 17.Wong ND, Bang M, Block RC, et al. Perceptions and barriers on the use of proprotein subtilisin/kexin type 9 inhibitors in heterozygous familial hypercholesterolemia (from a survey of primary care physicians and cardiologists). Am J Cardiol 2021; 152:57–62. [DOI] [PubMed] [Google Scholar]
- 18▪▪.Miller DM, Gaviglio A, Zierhut HA. Development of an implementation framework for overcoming underdiagnoses of familial hypercholesterolemia in the USA. Public Health Genomics 2021; 24:110–122. [DOI] [PubMed] [Google Scholar]; This qualitative study conducted interviews to investigate the implementation of FH screening program guided by the Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) framework.
- 19▪▪.Jones LK, Walters N, Brangan A, et al. Acceptability, appropriateness, and feasibility of automated screening approaches and family communication methods for identification of familial hypercholesterolemia: stakeholder engagement results from the IMPACT-FH study. J Pers Med 2021; 11: [DOI] [PMC free article] [PubMed] [Google Scholar]; This qualitative study utilized the conceptual model of implementation research to develop and analyze focus groups conducted with patients and providers on the acceptability, appropriateness, and feasibility of identification and cascade screening methods for individuals with FH.
- 20.Birnbaum RA, Horton BH, Gidding SS, et al. Closing the gap: identification and management of familial hypercholesterolemia in an integrated healthcare delivery system. J Clin Lipid 2021; 15:347–357. [DOI] [PubMed] [Google Scholar]
- 21.Ingoe L, Potter A, Musson S, et al. Improving the identification of patients with a genetic diagnosis of familial hypercholesterolaemia in primary care: a strategy to achieve the NHS long term plan. Atherosclerosis 2021; 325:38–45. [DOI] [PubMed] [Google Scholar]
- 22.Pepplinkhuizen S, Ibrahim S, Vink R, et al. Electronic health records to facilitate continuous detection of familial hypercholesterolemia. Atherosclerosis 2020; 310:83–87. [DOI] [PubMed] [Google Scholar]
- 23.Sabatel-Pérez F, Sánchez-Prieto J, Becerra-Muñoz VM, et al. Improving familial hypercholesterolemia index case detection: sequential active screening from centralized analytical data. J Clin Med 2021; 10:749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zamora A, Paluzie G, García-Vilches J, et al. Massive data screening is a second opportunity to improve the management of patients with familial hypercholesterolemia phenotype. Clin Investig Arterioscler 2021; 33:138–147. [DOI] [PubMed] [Google Scholar]
- 25.Akyea RK, Qureshi N, Kai J, et al. Evaluating a clinical tool (FAMCAT) for identifying familial hypercholesterolaemia in primary care: a retrospective cohort study. BJGP Open 2020; 4: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akyea RK, Qureshi N, Kai J, Weng SF. Performance and clinical utility of supervised machine-learning approaches in detecting familial hypercholesterolaemia in primary care. NPJ Digit Med 2020; 3:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pina A, Helgadottir S, Mancina RM, et al. Virtual genetic diagnosis for familial hypercholesterolemia powered by machine learning. Eur J Prevent Cardiol 2020; 27:1639–1646. [DOI] [PubMed] [Google Scholar]
- 28.Sheth S, Lee P, Bajaj A, et al. Implementation of a machine-learning algorithm in the electronic health record for targeted screening for familial hypercholesterolemia: a quality improvement study. Circ Cardiovasc Qual Outcomes 2021; 14:e007641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Centers for Disease Control and Prevention. Tier 1 genomics applications and their importance to public health. 2014. Available at: https://www.cdc.gov/genomics/implementation/toolkit/tier1.htm. [Accessed 1 September 2021] [Google Scholar]
- 30▪.Abul-Husn NS, Soper ER, Braganza GT, et al. Implementing genomic screening in diverse populations. Genome Med 2021; 13:17. [DOI] [PMC free article] [PubMed] [Google Scholar]; Population-based genomic screening program that returns clinically relevant results to participating individuals in a culturally diverse population. This study found that younger participants, women, and Hispanic/Latinx were more likely to opt out of receiving genomic results.
- 31.Buchanan AH, Lester Kirchner H, Schwartz MLB, et al. Clinical outcomes of a genomic screening program for actionable genetic conditions. Genet Med 2020; 22:1874–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grzymski JJ, Elhanan G, Morales Rosado JA, et al. Population genetic screening efficiently identifies carriers of autosomal dominant diseases. Nat Med 2020; 26:1235–1239. [DOI] [PubMed] [Google Scholar]
- 33.David SP, Dunnenberger HM, Ali R, et al. Implementing primary care mediated population genetic screening within an integrated health system. J Am Board Fam Med 2021; 34:861–865. [DOI] [PubMed] [Google Scholar]
- 34.Kawame H, Fukushima A, Fuse N, et al. The return of individual genomic results to research participants: design and pilot study of Tohoku Medical Megabank Project. J Hum Genet 2021; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 35▪▪.Jones LK, McMinn M, Kann D, et al. Evaluation of a multidisciplinary lipid clinic to improve the care of individuals with severe lipid conditions: a RE-AIM framework analysis. Implement Sci Commun 2021; 2:32. [DOI] [PMC free article] [PubMed] [Google Scholar]; This cross-sectional study of a newly implemented multidisciplinary lipid clinic (MDLC) used RE-AIM to evaluate the implementation. Despite limited reach and adoption of the MDLC, was found a large intervention effect that included improved diagnosis, increased prescribing of guideline-recommended treatments, and clinically significant reduction of lipid levels.
- 36.Wilkinson B, George E, Horton S, et al. A service evaluation: impact of nurse-led regional familial hypercholesterolaemia service on a hospital adult lipid clinic. Br J Nurs 2020; 29:1206–1208. [DOI] [PubMed] [Google Scholar]
- 37▪▪.Bangash H, Pencille L, Gundelach JH, et al. An implementation science framework to develop a clinical decision support tool for familial hypercholesterolemia. J Pers Med 2020; 10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]; This qualitative study utilized the conceptual framework of implementation research to ellicit provider perspectives into the development and implementation of a clinical decision support tool to aid in the identification and management of individuals with FH.
- 38.Ellis KL, Hooper AJ, Pang J, et al. A genetic risk score predicts coronary artery disease in familial hypercholesterolaemia: enhancing the precision of risk assessment. Clin Genet 2020; 97:257–263. [DOI] [PubMed] [Google Scholar]
- 39.Ramos R, Masana L, Comas-Cufí M, et al. Derivation and validation of SIDIAP-FHP score: a new risk model predicting cardiovascular disease in familial hypercholesterolemia phenotype. Atherosclerosis 2020; 292:42–51. [DOI] [PubMed] [Google Scholar]
- 40.Gallo A, Pérez de Isla L, Charrière S, et al. The added value of coronary calcium score in predicting cardiovascular events in familial hypercholesterolemia. JACC Cardiovasc Imaging 2021; S1936-878X(21)00501-5. [DOI] [PubMed] [Google Scholar]
- 41.Umans-Eckenhausen MA, Defesche JC, Sijbrands EJ, et al. Review of first 5 years of screening for familial hypercholesterolaemia in the Netherlands. Lancet 2001; 357:165–168. [DOI] [PubMed] [Google Scholar]
- 42.Leren TP, Manshaus T, Skovholt U, et al. Application of molecular genetics for diagnosing familial hypercholesterolemia in Norway: results from a family-based screening program. Semin Vasc Med 2004; 4:75–85. [DOI] [PubMed] [Google Scholar]
- 43.Descamps OS, Rietzschel E, Laporte A, et al. Feasibility and cost of FH cascade screening in Belgium (BEL-CASCADE) including a novel rapid rule-out strategy. Acta Cardiol 2021; 76:227–235. [DOI] [PubMed] [Google Scholar]
- 44.Benatar J, Evile T, Wihongi H. Hui: a partnership in practice in familial hypercholesterolemia. N Z Med J 2020; 133:63–70. [PubMed] [Google Scholar]
- 45▪▪.Kinnear FJ, Wainwright E, Bourne JE, et al. The development of a theory informed behaviour change intervention to improve adherence to dietary and physical activity treatment guidelines in individuals with familial hypercholesterolaemia (FH). BMC Health Serv Res 2020; 20:27. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study used the behavior change wheel and the theoretical domains framework to develop a theory informed intervention to improve adherence to dietary and physcial activity for individuals with FH.
- 46.Kinnear FJ, Lithander FE, Searle A, et al. Reducing cardiovascular disease risk among families with familial hypercholesterolaemia by improving diet and physical activity: a randomised controlled feasibility trial. BMJ Open 2020; 10:e044200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gidding SS, Sheldon A, Neben CL, et al. Patient acceptance of genetic testing for familial hypercholesterolemia in the CASCADE FH Registry. J Clin Lipidol 2020; 14: 218-23.e2. [DOI] [PubMed] [Google Scholar]
- 48. MeTree software. Available at: https://precisionmedicine.duke.edu/researchers/precision-medicine-programs/risk-assessment/family-history/metree-software. [Accessed 1 September 2021] [Google Scholar]
- 49.Neuner J, Dimmock D, Kirschner ALP, et al. Results and lessons of a pilot study of cascade screening for familial hypercholesterolemia in US primary care practices. J Gen Intern Med 2020; 35:351–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baldry E, Redlinger-Grosse K, MacFarlane I, et al. Outcomes from a pilot genetic counseling intervention using motivational interviewing and the extended parallel process model to increase cascade cholesterol screening. J Genet Couns 2021; doi: 10.1002/jgc4.1466. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 51.McGowan MP, Cuchel M, Ahmed CD, et al. A proof-of-concept study of cascade screening for Familial Hypercholesterolemia in the US, adapted from the Dutch model. Am J Prev Cardiol 2021; 6:100170. [DOI] [PMC free article] [PubMed] [Google Scholar]