Abstract
Cidofovir is the first nucleoside monophosphate analogue currently being used for the treatment of human cytomegalovirus (HCMV) retinitis in individuals with AIDS. Unfortunately, the period of therapy with the use of this compound may be limited due to the possible emergence of serious irreversible nephrotoxic effects. New drugs with improved toxicity profiles are needed. The goal of this study was to investigate the anticytomegaloviral properties and drug-induced toxicity of a novel phosphonate analogue, namely, (−)-2-(R)-dihydroxyphosphinoyl-5-(S)-(guanin-9′-yl-methyl) tetrahydrofuran (compound 1), in comparison with those of cidofovir. The inhibitory activities of both compounds on HCMV propagation in vitro were similar against the AD 169 and Towne strains, with 50% inhibitory concentrations ranging from 0.02 to 0.17 μg/ml for cidofovir and <0.05 to 0.09 μg/ml for compound 1. A clinical HCMV isolate that was resistant to ganciclovir and that had a known mutation within the UL54 DNA polymerase gene and a cidofovir-resistant laboratory strain derived from strain AD 169 remained sensitive to compound 1, whereas their susceptibilities to ganciclovir and cidofovir were reduced by 33- and 10-fold, respectively. Both compound 1 and cidofovir exhibited equal potencies in an experimentally induced murine cytomegalovirus (MCMV) infection in mice, with a prevention or prolongation of mean day to death at dosages of 1.0, 3.2, and 10.0 mg/kg of body weight/day. In cytotoxicity experiments, compound 1 was found to be generally more toxic than cidofovir in cell lines Hs68, HFF, and 3T3-L1 (which are permissive for HCMV or MCMV replication) but less toxic than cidofovir in MRC-5 cells (which are permissive for HCMV replication). Drug-induced toxic side effects were noticed for both compounds in rats and guinea pigs in a 5-day repeated-dose study. In guinea pigs, a greater weight loss was noticed with cidofovir than with compound 1 at dosages of 3.0 and 10.0 mg/kg/day. An opposite effect was detected in rats, which were treated with the compounds at relatively high dosages (up to 100 mg/kg/day). Compound 1 and cidofovir were nephrotoxic in both rats and guinea pigs, with the epithelium lining the proximal convoluted tubules in the renal cortex being the primary target site. The incidence and the severity of the lesions were found to be dose dependent. The lesions observed were characterized by cytoplasm degeneration and nuclear modifications such as karyomegaly, the presence of pseudoinclusions, apoptosis, and degenerative changes. In the guinea pig model, a greater incidence and severity of lesions were observed for cidofovir than for compound 1 (P < 0.001) with a drug regimen of 10 mg/kg/day.
Human cytomegalovirus (HCMV) is one of the most common opportunistic infections in immunocompromised individuals such as AIDS patients (22, 29, 34) and organ transplant recipients (31). Despite a reduction of the incidence of AIDS-related opportunistic infections in patients under highly active antiretroviral treatment (23, 47), HCMV retinitis development or progression remains an important risk factor, and attention should be paid to this risk factor in these individuals (24). The treatment of HCMV infections is difficult because few therapeutic options are available. At present ganciclovir [GCV; 9-(2′-hydroxy-1(hydroxymethyl) ethoxymethyl) guanine], foscarnet (PFA), and cidofovir [(S)-1-(3-hydroxy-2-phosphonylmethoxypropyl) cytosine) have been approved for the treatment of HCMV infections (12, 22, 35). Although the treatment of HCMV infections with these drugs has produced clinical improvement in a large proportion of patients, the drugs suffer from poor oral bioavailability, low potency (12, 22, 35), the development of drug resistance in the clinic (3, 11, 46), and dose-limiting toxicities (22, 33, 37); and for treatments with some of these drugs, the patients need to be confined in a hospital.
Of the three approved drugs, cidofovir is the one that has been the most recently advanced for the treatment of HCMV. Cidofovir is a member of a new class of antiviral agents (phosphonylmethyl ether acyclic nucleotide analogues) first described by De Clercq et al. (16). Cidofovir, like other members of this class of compounds, was shown to possess potent activity against a wide spectrum of viruses, especially against the human herpesviruses (16, 49), adenoviruses (2, 17), vaccinia virus (15), hepatitis B virus (18, 49), and papillomavirus (16, 25, 43). The anti-HCMV mechanism of action of cidofovir is via a diphosphate metabolite which selectively inhibits the HCMV DNA polymerase either by competitive inhibition or via a reduction in the viral DNA synthesis efficiency after incorporation of the drug metabolites into elongating DNA chains (48). Unfortunately, due to issues of drug-induced toxicity and the development of virus resistant to cidofovir, continued discovery and development of novel anti-HCMV agents are warranted.
Cidofovir and other phosphonate analogues are not subjected to phosphorylation by virally encoded enzymes, whereas GCV and acyclovir are modified as precursors of the active form of the drug by the HCMV UL97 gene product (phosphotransferase) and by the thymidine kinase in herpes simplex virus type 1 (HSV-1) (25). Therefore, this class of molecules can be used to treat patients infected with HCMV isolates that have developed resistance to GCV through a UL97 mutation(s), which seems to be the most frequent genotype observed in clinical HCMV isolates selected after a prolonged use of GCV (11, 44). However, cross-resistance between GCV and cidofovir could become a concern since the existence of HCMV strains resistant to GCV through UL54 mutations obtained in vitro or in HCMV strains from patients with AIDS is well documented (9, 41, 45). Moreover, double resistance to GCV and PFA in clinical HCMV strains isolated from patients with AIDS has been reported (3, 38), and in another case report, high-level GCV-resistant strains with modifications in both the UL97 and the UL54 genes were found to be cross-resistant to cidofovir (41). At this time, however, cidofovir has been found to be effective against the majority of GCV-resistant clinical isolates studied (44) as well as PFA-resistant viruses generated in vitro (42).
One of the advantages of cidofovir over GCV and PFA in the treatment of HCMV retinitis in AIDS patients is its long intracellular half-life (1, 20), which translates into intermittent intravenous administration; and to date, resistance to cidofovir in the clinic as a result of treatment has not been described (10). However, while efficacy and drug resistance evaluations have shown some of the advantages of the use of phosphonate nucleoside analogues either instead of other anti-HCMV agents or in combination with other anti-HCMV agents (32), toxicity studies have raised some serious questions around the particular use of cidofovir. Toxicology evaluations with various animal species have demonstrated that nephrotoxicity is the major side effect associated with the use of cidofovir (19), with the proximal tubular epithelial cell being the primary target site. The rate of drug uptake is believed to be responsible for the accumulation of cidofovir at toxic levels in the renal tubular cells, and this causes substantial necrosis. Attempts have been made to circumvent the risk of renal injury. For example, the coadministration of a high dose of probenecid and saline hydration as well as a less frequent cidofovir dosing regimen have been shown to reduce the nephrotoxic effect of cidofovir in vivo (14, 35). In addition, a cyclic ester prodrug form of cidofovir has been demonstrated to have a lower nephrotoxicity than cidofovir in animal models (6, 13) and to have anti-HCMV activity in vitro and anti-HSV-2 activity in vivo similar to those of cidofovir (6). It is clear from these studies that potent, less toxic HCMV inhibitors which are preferably active against virus resistant to current chemotherapy are needed.
In the present study our efforts centered on identifying potent phosphonate nucleoside analogues that are active against HCMV, that are as potent or more potent than cidofovir, and that have more favorable toxicity profiles than existing anti-HCMV agents. The most promising compound evaluated in these efforts is a guanine phosphonate analogue, (−)-2-(R)-dihydroxyphosphinoyl-5-(S)-(guanin-9′-yl-methyl) tetrahydrofuran (compound 1), which was tested and whose activity was compared with that of cidofovir against both laboratory and drug-resistant strains of HCMV in vitro and in vivo. In addition, we have analyzed the effects of these two phosphonate nucleoside analogues on the proximal tubular epithelial cells of rats and guinea pigs. The results of the current studies demonstrate that compound 1 has an efficacy profile equal to that of cidofovir and, most importantly, that it has decreased nephrotoxicity compared to that of cidofovir in guinea pigs, suggesting that less toxic derivatives of phosphonate nucleoside analogs are achievable.
MATERIALS AND METHODS
Compounds.
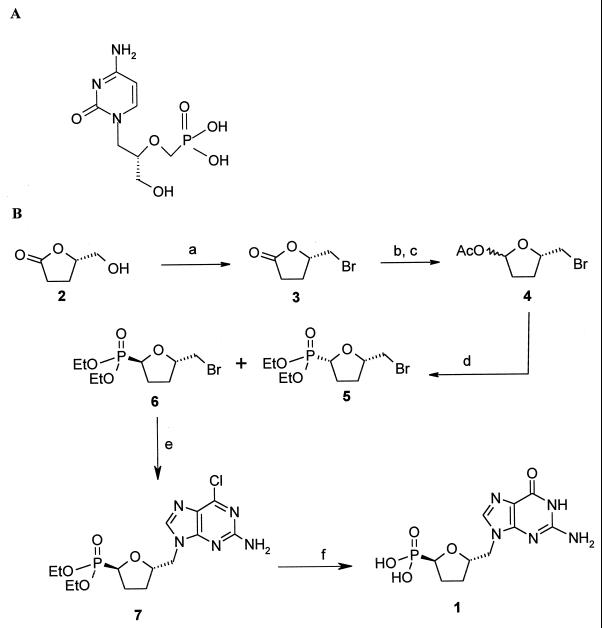
Cidofovir (Fig. 1A) was synthesized by the procedure of Bronson et al. (7), while compound 1 was prepared as described by Chan et al. (8). Briefly, our approach for the preparation of compound 1 is depicted in Fig. 1B. The commercially available (Aldrich) compound (−)-(5S)-5-(hydroxymethyl)-tetrahydrofuran-2-one (compound 2) was converted to the corresponding bromide (compound 3) as reported previously (30). Reduction of the lactone followed by acetylation under standard conditions gave compound 4 in good yields as a 1:1 mixture of isomers. The phosphonate group was then introduced by a Lewis acid-catalyzed Arbuzov reaction; thus, treatment of compound 4 with TiCl4 in methylene chloride at −30°C followed by treatment with triethylphosphite gave phosphonates (compound 5) in an 80% yield as a 1:1 mixture of cis and trans isomers. The mixture of bromophosphonates (compounds 5 and 6) was readily separable by flash chromatography (10 to 20% acetone in hexanes), and the relative stereochemistry was assigned by a Nuclear Overhauser Effect experiment with the corresponding bromophosphonic acids. Condensation of the bromide (compound 6) with 2-amino-6-chloropurine in the presence of Cs2CO3 (50) in dimethylformamide at 95°C gave 6-chloropurine (compound 7) in a 42% yield. The phosphonate ester was deprotected by treatment with excess bromotrimethylsilane, followed by concomitant hydrolysis of the resulting trimethylsilyl ester and the 6-chloropurine to the guanine compound (compound 1) by refluxing in water; the phosphonic acid was sufficiently acidic to smoothly effect the last transformation.
FIG. 1.
Chemical structure of cidofovir (A) and chemical synthesis of (−)-2-(R)-dihydroxyphosphinoyl-5-(S)-(guanin-9′-yl-methyl) tetrahydrofuran (compound 1) (B). a, CBr4, PPh3, MeCN, 84%; b, diisobutylaluminum hydride, toluene, −78°C, 77%; c, Ac2O, pyr, 4-(dimethylamino)pyridine, CH2Cl2, 86%; d, TiCl4, P(OEt)3, CH2Cl2, 86%; e, Cs2CO3, 2-amino-6-chloropurine, dimethylformamide 95°C, 42%; f, bromotrimethylsilane, CH2Cl2 and then water, 100°C, 60%.
Compound 1 and cidofovir were stored at −20°C in a sterile saline solution at concentrations of 2 and 1 mg/ml, respectively.
Cells and viruses.
Primary newborn human fibroblasts (Hs68 cells; ATCC CRL 1635), mouse embryo 3T3-L1 cells (ATCC CL 173), and HCMV AD 169 (ATCC VR 538) were obtained from the American Type Culture Collection (ATCC; Rockville, Md.). Murine cytomegalovirus (MCMV) Smith was kindly provided by L. C. Loh, University of Saskatchewan, Saskatoon, Saskatchewan, Canada. Cells were passaged in Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies Inc., Gaithersburg, Md.) supplemented with 10% fetal bovine serum (FBS; Hyclone Laboratories Inc., Logan, Utah) and 2 mM glutamine (Life Technologies Inc.). Penicillin and streptomycin (Life Technologies Inc.) were added at final concentrations of 500 U/ml and 50 μg/ml, respectively. Hs68 cells were used at between passages 13 and 24 for the plaque reduction assay.
Plaque reduction assays. (i) HCMV.
The efficacies of the compounds against various HCMV isolates were evaluated with Hs68, MRC-5, and HFF cells as described previously (27). Human fibroblast Hs68 cells were plated at a density of 1.5 × 105 cells/well in 2 ml of DMEM–10% FBS and were incubated overnight in 5% CO2–air at 37°C in 12-well tissue culture dishes (no. 25815; Corning Costar Corp., Orneonta, N.Y.). The medium was then removed and the cells were washed and then infected with 0.5 ml of DMEM–2% FBS containing approximately 125 PFU of HCMV AD 169 per ml. After adsorption at 37°C for 2 h, the inoculum was removed and the monolayer was overlaid with 1 ml of DMEM–2% FBS containing the test compounds at concentrations ranging from 0.001 to 1.0 μg/ml for cidofovir and 0.001 to 2.0 μg/ml for compound 1. After 7 days of incubation, the cells were fixed with 1 volume of 8% formaldehyde in water for 30 min, at which time the solution was removed and the cell monolayers were stained with crystal violet (2%)–ethanol (20%) for a few seconds. The cells were rinsed with tap water and dried, and the monolayers were examined for the presence of plaques under an inverted microscope with ×40 magnification. The MRC-5 cell line was used to test the efficacies of the antiviral agents against AD 169, P8, C8704, C8805-37, and D16 (4, 39), while the HFF cell line was used to test the efficacies of the antiviral agents against isolates resistant to AD 169, Towne, PFA, cidofovir, and 2-bromo-5,6-dichloro-1-β-D-ribofuranosyl benzimidazole (BDCRB), as described previously (36, 51).
(ii) MCMV.
The methodology used for the MCMV plaque reduction assay is basically the same as that described above for the HCMV plaque reduction assay with the Hs68 cell line, except that the mouse embryonic fibroblast cells 3T3-L1 were seeded at 1.8 × 105 cells/well and 125 PFU/well of MCMV was used for the infection, followed by a period of incubation of 3 days at 37°C.
HCMV yield reduction assay.
The compounds were tested for their effects on HCMV replication by a yield reduction assay as described by Zou et al. (51). Briefly, HFF cells were seeded in a 96-well cluster dishes and were infected on the following day with HCMV Towne at a multiplicity of infection (MOI) of 0.5 PFU/cell, followed, after viral adsorption, by the addition of compounds at final concentrations ranging from 0.01 to 10 μg/ml. Infection was allowed to proceed for 7 days at 37°C, at which time the plates were subjected to one cycle of freezing and thawing. Aliquots of the cell lysates were then used to reinfect, by serial dilution, a 96-well monolayer culture of HFF cells. After a 7-day period of incubation at 37°C, the plaques were enumerated and the virus titer was determined.
Cytotoxicity determination.
The in vitro toxicity profiling of the test compounds in Hs68 and 3T3-L1 cells was performed by measuring the levels of [3H]thymidine incorporation into exponentially growing cells. A total of 1,000 cells/well were seeded in a 96-well cluster plate in a volume of 0.150 ml of culture medium. After incubation for 18 h at 37°C (5% CO2), the supernatant was removed and was replaced with compounds diluted in DMEM–10% FBS. Six concentrations of drug (3.2 to 100 μg/ml) were tested in triplicate. After a 72-h period of incubation, a volume of 0.050 ml of a 10-μCi/ml solution of [methyl-3H]thymidine (2 Ci/mmol) in culture medium was added and the cells were incubated for an additional 18 h at 37°C. The cells were then washed with phosphate-buffered saline and trypsinized for 2 min, collected on a fiberglass filter with a Tomtec cell harvester (Tomtec, Orange, Conn.), dried at 37°C for 1 h, and then placed into a bag with 4.5 ml of liquid scintillation cocktail (Wallac Oy, Turku, Finland). The radioactivity was then measured with a liquid scintillation counter (1450-Microbeta; Wallac Oy). Cytotoxicity was determined with stationary HFF and MRC-5 cells as described previously (21, 27, 51).
In vivo evaluation of drug efficacy.
Female BALB/c mice (Simonsen Laboratories, Gilroy, Calif.) weighing 8 to 10 g were infected intraperitoneally (i.p.) with a 106 50% cell culture infectious dose as described previously (40). This virus concentration was equivalent to a dose that was lethal for 90% of the animals used in the study. The compounds were administered to the infected mice i.p. once a day at doses of 0.1, 0.32, 1.0, 3.2, and 10 mg/kg of body weight for 6 days starting at 8 h postinfection. The evaluation of efficacy was based on the prevention of MCMV-induced death and prolongation of the mean day to death.
Toxicology studies.
In vivo toxicity studies were performed with rats and guinea pigs. The animals were obtained from Charles River Laboratories, St-Constant, Quebec, Canada. The test compounds were administered i.p. once daily for 5 consecutive days at 25, 50, 75, and 100 mg/kg of body weight to four groups of 200- to 210-g CD male rates (four rats per group, one group per dosage) and by subcutaneous injection at 0.3, 1.0, 3.0, and 10 mg/kg of body weight to four groups of 250- to 270-g Hartley male guinea pigs (four animals per group, one group per dosage). The average daily body weight of the animals was recorded during the 5 days of drug dosing. The animals were killed and necropsied at day 6 after drug treatment. The kidneys were removed and submitted for gross and histopathological examinations. Longitudinal and transverse kidney sections were processed and stained with either hematoxylin and eosin or periodic acid-Schiff. Quantitative measurements of the incidence of nuclear degeneration, pseudoinclusions, apoptosis, and karyomegaly in the outer cortex region were made. To better evaluate the severity of the lesions induced by the compounds, scores of their incidence in each of six high-magnification (×40) fields were recorded manually with a Clay Adams counter, and these scores were used to calculate the mean incidence and standard deviation per animal and treatment group. Statistical analysis was performed with GraphPad Prism software, version 2.0 (GraphPad Software Inc., San Diego, Calif.) from the mean ± standard deviation of the mean. Differences were considered significant when P was <0.05. Toxicity data were compared by one-way analysis of variance, followed by the Bonferroni test for multiple comparisons between the two compounds and among doses.
RESULTS
A series of tetrahydrofuran phosphonate analogues was prepared and investigated for their potential activities against HCMV. Only the guanine derivatives showed promising antiviral activity (8), with the most active being a trans isomer analogue, compound 1 (Fig. 1). Compound 1 was synthesized as described in Materials and Methods by the procedure of Chan et al. (8).
Cytotoxicity studies.
Drug-induced cytotoxic effects were investigated in several cell lines permissive for HCMV and MCMV replication by various assays including a [3H]thymidine uptake assay with exponentially growing cells and by visual examination of stationary cells. Compound 1 was found to be generally more toxic than cidofovir in Hs68 and 3T3-L1 cells ([3H]thymidine uptake assay) and in HFF cells (visual examination) but less toxic in MRC-5 cells (visual examination) (Table 1).
TABLE 1.
In vitro cytotoxicity profile of cidofovir and compound 1
| Cell line | CC50 (μg/ml)a
|
|
|---|---|---|
| Cidofovir | Compound 1 | |
| Hs68b | 50 ± 9 | <10 |
| MRC-5c | 19 | 28 |
| HFFd | >10 | 10 |
| 3T3-L1b | 12.2 ± 0.9 | 5.4 ± 0.9 |
CC50, 50% cytotoxic concentration.
Thymidine uptake assay with exponentially growing cells. The data are presented as the average of three or more experiments with the standard deviation.
Visual examination of stationary cells. MRC-5 cells were observed microscopically for morphological changes due to drug-induced cytotoxicity by using seven concentrations of test compound in duplicate. No variation around the reported values was noticed.
Visual examination of stationary HFF cells was performed in six separate experiments. On a one to four-plus basis of scoring the cytopathology, no variation around the reported values was observed.
In vitro efficacy studies against herpesviruses.
Compound 1 and cidofovir were found to be generally equipotent against laboratory-derived HCMV AD 169 and Towne as well as against MCMV Smith (Table 2). They were both also more active than GCV when they were tested by the plaque reduction assay. The low-passage isolate P8 and GCV-resistant clinical isolates C8704 and C8805-37 were all susceptible to both compound 1 and cidofovir (Table 2). Interestingly, a cidofovir-resistant laboratory strain (1117r3-1-2) and a GCV-resistant clinical isolate (D16) remained sensitive to compound 1, whereas their susceptibilities to cidofovir were reduced (Table 2). A 10- and a 33-fold increase in the 50% inhibitory concentration (IC50) was observed with cidofovir against HCMV 111r3-1-2 and D16, respectively. HCMV strains with a relatively high level of resistance to PFA and BDCRB remained susceptible to cidofovir and compound 1 (Table 2).
TABLE 2.
In vitro activities of GCV, PFA, BDCRB, cidofovir, and compound 1 against various cytomegalovirus isolates by the plaque reduction assay
| Isolate | Cell line | IC50 (μg/ml)a
|
||||
|---|---|---|---|---|---|---|
| GCV | PFA | BDCRB | Cidofovir | Compound 1 | ||
| AD 169 | Hs68 | 0.3 ± 0.05 | NDb | ND | 0.02 ± 0.003 | 0.09 ± 0.002 |
| AD 169 | MRC-5 | 2.2 ± 0.2 | ND | ND | 0.04 ± 0.006 | 0.11 ± 0.04 |
| P8c | MRC-5 | 2.5 ± 0.2 | ND | ND | 0.4 ± 0.04 | 0.2 ± 0.08 |
| C8704c | MRC-5 | 7.6 ± 0.6 | ND | ND | 0.14 ± 0.04 | 0.3 ± 0.07 |
| C8805-37c | MRC-5 | 12.0 ± 0.14 | ND | ND | 0.33 ± 0.07 | 0.32 ± 0.09 |
| D16c | MRC-5 | 28.0 ± 5.0 | ND | ND | 13.0 ± 0.0 | 0.4 ± 0.04 |
| AD 169 | HFF | ND | 29 | ND | 0.2 | 0.08 |
| Towne | HFF | 0.75 ± 0.25 | ND | 0.37 ± 0.14 | 0.16 ± 0.02 | 0.08 ± 0.0 |
| 4760recPolA1-1-1d | HFF | ND | 75 | ND | 0.2 | 0.2 |
| 1117r3-1-2d | HFF | ND | 35 | ND | 2.0 | 0.06 |
| D-10 C4d | HFF | ND | ND | >10 | 0.11 | 0.07 |
| MCMVe | 3T3-L1 | 1.0 ± 0.08 | ND | ND | 0.03 ± 0.004 | 0.05 ± 0.004 |
The IC50 was defined as the concentration of compound that resulted in a 50% reduction in plaque number compared to the number observed in control samples without drug. IC50s were calculated from dose-response curves obtained after linearly regressing the percent reduction of plaques against drug concentrations. For the experiments performed with the Hs68 fibroblasts, the data are presented as the averages of three or more experiments with the standard deviation. For the experiments performed with the MRC-5 and HFF cells, all dose-response curves for all HCMV strains were constructed by using seven and eight drug concentrations, respectively, in duplicate. For MCMV Smith, all dose-response curves were constructed by using six drug concentrations, in duplicate.
ND, not determined.
The following low-passage HCMV strains were used to infect MRC-5 cells: P8, which is GCV sensitive; C8704 and C8805-37, which are GCV resistant due to a UL97 phosphotransferase gene mutation (5, 44); and D16, which is GCV resistant through a mutation within the UL54 DNA polymerase gene (46).
HCMV 4760recPolA1-1-1 (genotype unknown) is PFA resistant and is derived from a clinical isolate (3), 1117r73-1-2 (genotype unknown) is cidofovir resistant and is derived from strain AD 169, and D-10 C4 (D344E and Q204R mutations within the UL89 and UL56 genes, respectively) is 2,5,6-trichloro-1-(β-d-ribofuranosyl)benzimidazole and BDCRB resistant and is derived from strain Towne (26).
Smith strain.
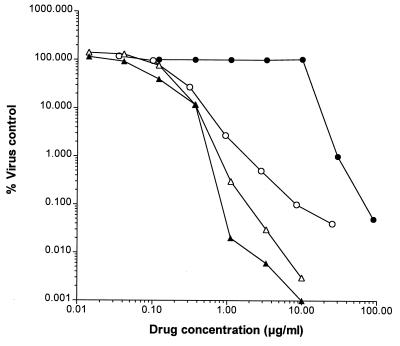
We next compared the efficacies of GCV, cidofovir, and compound 1 in a virus yield assay with a high MOI (0.5) of HCMV Towne. In this assay the antiviral activities of the three compounds were comparable, with IC90s of 0.39 μg/ml observed for compound 1 and cidofovir and an IC90 of 0.50 μg/ml observed for GCV (Fig. 2).
FIG. 2.
Anti-HCMV activities of compound 1, cidofovir, GCV, and PFA in the virus yield reduction assay. HFF cells were infected at an MOI of 0.5 and were then treated with compound 1 (▴), cidofovir (▵), GCV (○), and PFA (●) at concentrations ranging from 0.01 to 100 μg/ml for a period of 7 days at 37°C. Thereafter, infected-treated cells were subjected to one freeze-thaw cycle and then the virus titer in the cell lysates was determined as described in Materials and Methods.
In vivo efficacy studies against MCMV.
Mice lethally infected with MCMV were treated i.p. with dosages ranging from 0.1 to 10 mg/kg/day. A study of the maximum tolerated dose was initially performed to ensure the validity of the results. These studies determined that the drug regimens used were not lethally toxic to the uninfected mice; a weight gain in the animals used as toxicity controls was observed with compound 1 at 10 mg/kg/day (data not shown). When administered at dosages of 1, 3.2, and 10 mg/kg/day, both compound 1 and cidofovir had approximately equal anti-MCMV activities in the mouse model used in this study (Table 3). Animal death was significantly prevented or delayed in animals treated with these drug concentrations compared to the times to death for the control infected animals which did not receive treatment.
TABLE 3.
Effects of cidofovir and compound 1 on an experimentally induced cytomegalovirus infection in mice
| Compound | Dosage (mg/kg/day) | No. of survivors/total no. | Mean day to deatha |
|---|---|---|---|
| Saline | 1/20 | 3.9 | |
| Compound 1 | 0.1 | 1/10 | 4.7 ± 1.0b |
| 0.32 | 0/10 | 4.3 ± 0.3 | |
| 1.0 | 6/10 | 5.3 ± 0.5b | |
| 3.2 | 10/10 | >21 ± 0.0c | |
| 10 | 10/10 | >21 ± 0.0c | |
| Cidofovir | 0.1 | 0/10 | 4.1 ± 0.3 |
| 0.32 | 0/10 | 4.1 ± 0.3 | |
| 1.0 | 7/10 | 9.0 ± 2.9b | |
| 3.2 | 10/10 | >21 ± 0.0c | |
| 10 | 10/10 | >21 ± 0.0c |
A total of 10 infected mice were used treated with each dosage of drug, and 20 mice were used as placebo (saline)-treated controls. The data are presented as mean day to death with the standard deviation.
P < 0.05.
P < 0.01.
Toxicology studies with rats and guinea pigs.
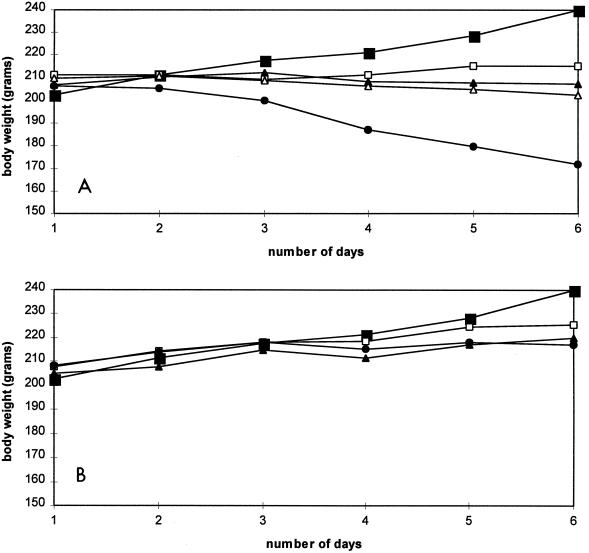
Two studies were undertaken to compare the in vivo toxicities of compound 1 and cidofovir. In one study, five groups of rats were treated once daily for 5 consecutive days by i.p. injection of saline or various doses of compounds ranging from 25 to 100 mg/kg. The animals were killed at day 6 after drug treatment. No animal deaths were recorded. In groups receiving compound 1, a dose-dependent reduction in body weight gain was noticed in the animals treated with the concentrations tested, with a 30% weight loss at day 6 for rats treated with compound 1 at 100 mg/kg (Fig. 3A). With the same drug concentration, only a 10% loss in body weight was observed for animals treated with cidofovir (Fig. 3B).
FIG. 3.
(A) Compound 1-related weight changes in rats. Rats were injected i.p. once a day for 5 days with a dose of 0 (■), 25 (□), 50 (▴), 75 (▵), or 100 (●) mg of compound 1 per kg. The averages of quadriplicates are presented. The standard deviation was less than 10% for each point. (B) Cidofovir-related weight changes in rats. Rats were injected i.p. once a day for 5 days with a dose of 0 (■), 25 (□), 50 (▴), or 100 (●) mg of cidofovir per kg. The averages of quadriplicates are presented. The standard deviation was less than 10% for each point.
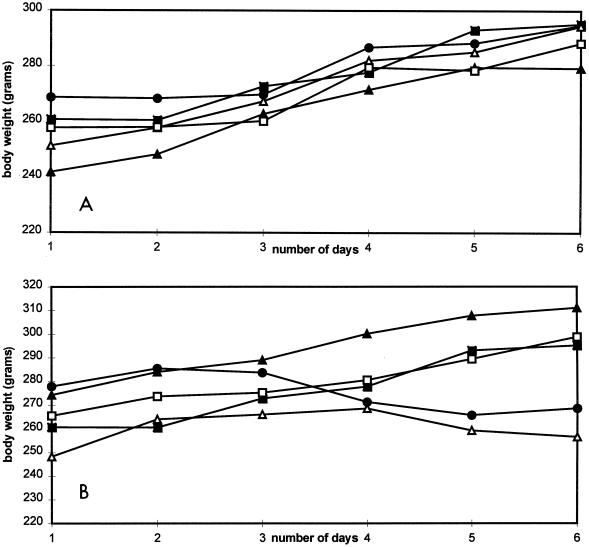
Guinea pigs are known to be sensitive to cidofovir-induced toxic side effects (28). Therefore, in the second study five groups of guinea pigs were also treated once daily for 5 consecutive days subcutaneously with saline or various doses of compounds ranging from 0.3 to 10 mg/kg. The animals were killed at day 6 after drug treatment. No animals died during the course of the experiment. In groups receiving compound 1 (Fig. 4A), no significant reduction in body weight gain at any dose used up to 6 days posttreatment was observed, whereas in groups receiving cidofovir at 10 mg/kg, an approximate 10% loss was observed (Fig. 4B).
FIG. 4.
(A) Compound 1-related weight changes in guinea pigs. Guinea pigs were injected subcutaneously once a day for 5 days with a dose of 0 (■), 0.3 (□), 1.0 (▴), 3.0 (▵), or 10 (●) mg of compound 1 per kg. The averages of quadriplicates are presented. The standard deviation was less than 10% for each point. (B) Cidofovir-related weight changes in guinea pigs. Guinea pigs were injected subcutaneously once a day for 5 days with a dose of 0 (■), 0.3 (□), 1.0 (▴), 3.0 (▵), or 10 (●) mg of cidofovir per kg. The averages of quadriplicates are presented. The standard deviation was less than 10% for each point.
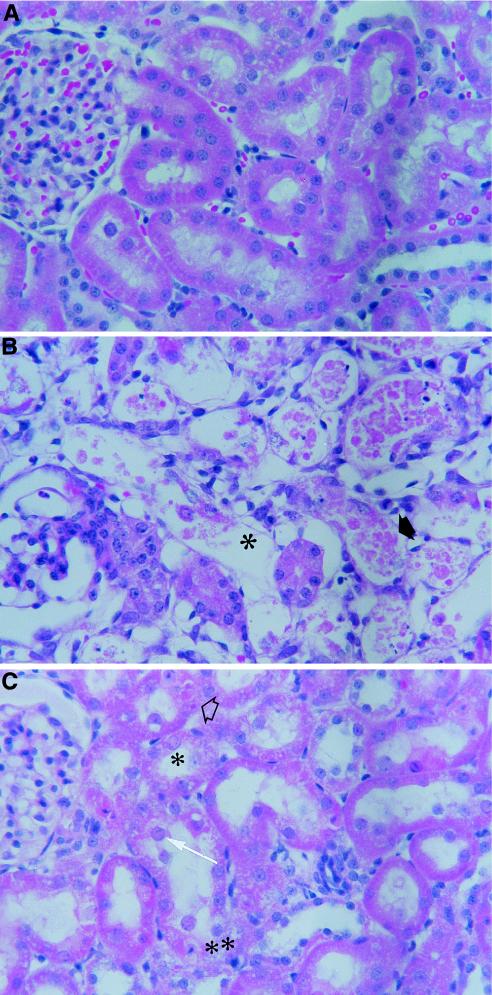
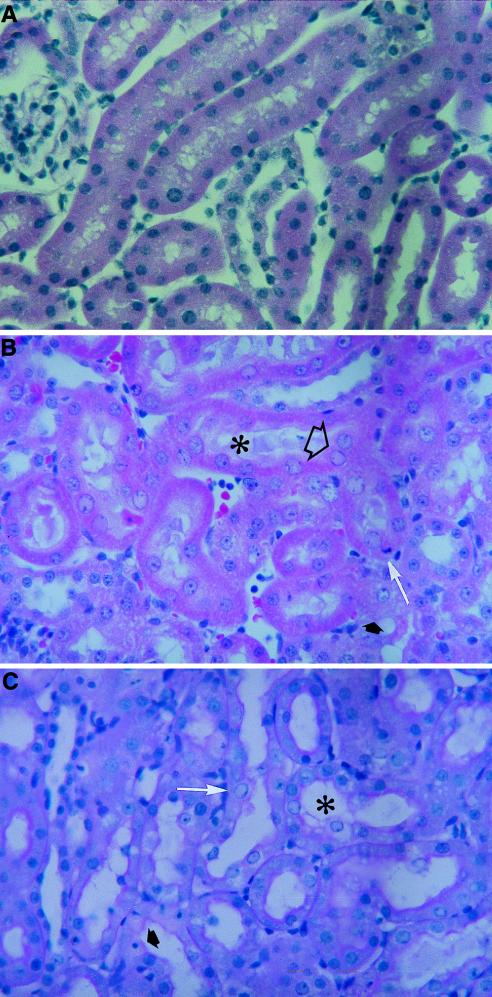
Histopathological examination of the rat and guinea pig kidneys revealed that for both compounds the drug-induced lesions occurred in a dose-dependent manner and were characterized by similar qualitative cytoplasmic and/or nuclear changes in the proximal tubules (Fig. 5 and 6). Cytoplasmic degeneration was characterized by reduced staining intensity, loss of the usual cytoplasmic granularity, microvacuolation, swelling with protrusion into the lumen, sloughing of the cytoplasm or cells into the lumen, or combinations thereof. Nuclear changes included nuclear enlargement (karyomegaly) and apoptosis. Karyomegaly was associated in some cases with eosinophilic pseudoinclusions in the nucleoplasm and in other cases with nuclear degenerative changes consisting of hydropic degeneration of the nucleus, condensation and margination of the chromatin, loss of the nucleoplasm, and dissolution of the nucleus. Apoptosis was characterized either by a markedly condensed nucleus and strongly eosinophilic cytoplasm or by multiple, small, dark nuclear fragments surrounded by a small amount of strongly eosinophilic cytoplasm. In guinea pigs and rats given cidofovir at 10 mg/kg/day (Fig. 5) and 100 mg/kg/day (Fig. 6), respectively, the proximal tubules were severely affected and had considerable epithelial cell sloughing and denudation of the basal lamina. Qualitatively, a less severe effect was noticed with compound 1.
FIG. 5.
Histopathology analysis of kidneys from guinea pigs treated with cidofovir and compound 1. The animals were administered a solution of saline (A), a dose of 10 mg of cidofovir per kg (B), or a dose of 10 mg of compound 1 per kg (C) i.p. once daily for 5 consecutive days. (B) The severe tubular nephropathy was characterized by widespread necrosis and sloughing of the lining epithelium (➧) and denudation of the basal lamina (✻). (C) The tubular nephropathy was characterized by cytoplasmic eosinophilic droplets (➱) and nuclear changes consisting of karyomegaly (➞), eosinophilic inclusions (✻), and apoptosis (✻✻).
FIG. 6.
Histopathology analysis of kidneys from rats treated with cidofovir and compound 1. The animals were administered a solution of saline (A), a dose of 100 mg of cidofovir per kg (B), or a dose of 100 mg of compound 1 per kg (C) subcutaneously once daily for 5 consecutive days. (B) Cytoplasmic changes included effacing of the fine structural details (➧) and shedding into the lumen (✻). Nuclear changes consisted of karyomegaly and eosinophilic pseudoinclusions (➱) and apoptosis (➞). (C) Cytoplasmic changes included mild vacuolation and perinuclear hydropic degeneration (✻). Nuclear changes consisted of karyomegaly (➞) and apoptosis (➧).
The quantitative measurements of the key lesions in drug-treated guinea pigs demonstrated that the incidences and severities of the lesions were statistically greater for cidofovir, especially when the drugs were given at a concentration of 10 mg/kg/day (P < 0.001), than those of the lesions caused by compound 1 (Table 4), supporting the view that the former compound is more nephrotoxic than the latter one in this animal species. Statistically significant (P < 0.05) dose-dependent cell injury was also observed for both compounds. Similar degrees of renal toxicity were also observed in rats (Table 4).
TABLE 4.
Histopathological evaluations of rat and guinea pig kidneys
| Lesion or histo-pathology | Histopathology score for the following guinea pig treatment groupsa:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.0 mg/kg/day
|
0.3 mg/kg/day
|
1.0 mg/kg/day
|
3.0 mg/kg/day
|
10.0 mg/kg/day
|
||||||
| Cidofovir | Compound 1 | Cidofovir | Compound 1 | Cidofovir | Compound 1 | Cidofovir | Compound 1 | Cidofovir | Compound 1 | |
| Apoptosis | 0.0 ± 0.0 | 0.0 ± 0.0 | 2.2 ± 1.7 | 1.7 ± 1.4 | 3.6 ± 2.6 | 2.1 ± 1.4 | 8.3 ± 2.2 | 0.8 ± 0.9 | 68.4 ± 9.8 | 0.3 ± 0.4 |
| Total karyomegaly | 1.5 ± 0.8 | 1.5 ± 0.8 | 17.3 ± 3.3 | 11.3 ± 3.0 | 15.3 ± 3.5 | 7.5 ± 3.1 | 24.2 ± 6.1 | 3.9 ± 2.3 | 27.0 ± 6.7 | 2.2 ± 1.4 |
| Pseudoinclusions | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.2 | 0.9 ± 0.9 | 1.0 ± 1.0 | 1.1 ± 0.9 | 0.4 ± 0.5 | 0.5 ± 0.1 | 0.0 ± 0.0 | 0.1 ± 0.1 |
| Nuclear degeneration | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.7 ± 0.9 | 0.7 ± 0.9 | 1.0 ± 0.9 | 1.1 ± 1.0 | 7.6 ± 4.1 | 0.1 ± 0.2 | 21.1 ± 4.6 | 0.0 ± 0.0 |
| Histopathology score for the following rat treatment groupsa:
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.0 mg/kg/day
|
25.0 mg/kg/day
|
50.0 mg/kg/day
|
75.0 mg/kg/day
|
100.0 mg/kg/day
|
|||||
| Cidofovir | Compound 1 | Cidofovir | Compound 1 | Cidofovir | Compound 1 | Cidofovir | Compound 1 | Cidofovir | Compound 1 |
| 0.0 ± 0.0 | 0.0 ± 0.0 | 5.1 ± 2.7 | 1.6 ± 1.3 | 3.6 ± 4.7 | 2.1 ± 1.6 | NDb | 3.3 ± 1.5 | 2.5 ± 1.7 | 4.8 ± 2.7 |
| 2.4 ± 0.9 | 2.4 ± 0.9 | 31.5 ± 6.4 | 17.9 ± 6.3 | 29.1 ± 9.4 | 17.2 ± 5.3 | ND | 21.0 ± 4.3 | 21.2 ± 7.9 | 14.7 ± 4.7 |
| 0.0 ± 0.0 | 0.0 ± 0.0 | 4.7 ± 2.6 | 1.2 ± 1.4 | 2.9 ± 1.9 | 2.0 ± 1.2 | ND | 2.3 ± 1.3 | 3.2 ± 2.1 | 1.1 ± 0.9 |
| 0.0 ± 0.0 | 0.0 ± 0.0 | 23.8 ± 5.3 | 10.2 ± 4.6 | 24.7 ± 8.4 | 10.7 ± 3.4 | ND | 14.7 ± 3.7 | 16.8 ± 6.6 | 12.5 ± 4.3 |
The values are the means and standard deviations of the scores of the incidence of the observed lesions in each of six high-magnification fields. Counts were obtained from the proximal convoluted tubules at the outer cortex region of the kidneys as described in the Materials and Methods.
ND, not determined.
DISCUSSION
A series of cyclic phosphonate nucleoside analogues was previously synthesized and evaluated for anti-HCMV activity (8). In this report, we have described a comparative analysis of the anticytomegaloviral activity and toxicity of one representative of this class of compound, compound 1, with those of cidofovir.
The results obtained from these studies determined that compound 1 has potency against HCMV in vitro and MCMV in vivo equal to that of cidofovir. Since compound 1, like cidofovir, is a nucleotide phosphonate analogue, its mechanism of action should be similar to that of cidofovir. In order to address the mechanism of action issue (i.e., inhibition of viral DNA polymerase) and to determine if there are advantages to using compound 1 over other known anti-HCMV nucleosides, various viral strains known to contain specific mutations within the UL54 and UL97 genes were evaluated for their susceptibilities to compound 1. From these studies, no cross-resistance to existing compounds was seen. Clinical HCMV isolates characterized by mutations within the UL97 phosphotransferase gene, which is known to impair the first step of phosphorylation of GCV into an active metabolite (44), were found to remain sensitive to compound 1, like the low-passage clinical isolate P8, indicating that this phosphorylation pathway is not a prerequisite or could be by-passed as for cidofovir (Table 2). Another HCMV strain used in these studies, D16, has been reported to exhibit a GCV, (S)-9-(3-hydroxy-2-phosphonylmethoxypropyl) adenine, and cidofovir resistance phenotype (25). In addition, both viral isolate D16 and HCMV 1117r3-1-2, for which cidofovir IC50s are 33- and 10-fold higher than those for wild-type isolates P8 and AD 169, respectively (Table 2), were found to remain sensitive to compound 1. There was also no significant change in strain D-10 C4’s susceptibility to compound 1. These data taken together suggest that while compound 1 is a nucleoside analogue, its activity against HCMV in culture is not affected by HCMV mutations derived from other known anti-HCMV agents affecting the UL54, UL56, UL89, and UL97 gene products. These data suggest that compound 1 has a mechanism of action different from that of cidofovir, even though both anti-HCMV agents may share a common molecular target. In terms of anti-HCMV activity against the laboratory-derived Towne strain, cidofovir and compound 1 had no significant loss of potency in the virus yield assay compared to that in the plaque reduction assay, suggesting that these compounds have MOI-independent anticytomegaloviral activities. There is an indication that the antiviral activity of compound 1 is not restricted only to HCMV. Testing of compound 1 against HSV-1 revealed that compound 1 has an IC50 comparable to that of acyclovir (data not shown).
Our results from histopathology studies with rat and guinea pig kidneys demonstrated that one of the primary target sites for both compound 1 and cidofovir is the epithelial cell of the proximal convoluted tubules. In the case of cidofovir, this result is consistent with published data on the nephrotoxicity of cidofovir (28). It is generally believed that the mechanism of cidofovir-induced toxicity in the renal proximal convoluted tubule cells of rats, guinea pigs, cynomolgus monkeys, and humans is related to the accumulation of relatively high concentrations of drug or a drug-related choline metabolite inside the cells. This would apparently be the result of a faster drug uptake at the basolateral membrane site compared to the rate of drug efflux at the luminal side of the cells. The fact that compound 1 has a lower nephrotoxic side effect than cidofovir could be due to a lower level of uptake or an increased level of efflux, or both, at the proximal convoluted tubules, resulting in a reduced amount of drug inside the cells. The presence of different compound 1 metabolites that have lower levels of toxicity could be an additional explanation.
In conclusion, we have reported here on a novel guanine phosphonate analogue with a potency comparable to that of cidofovir against HCMV and MCMV and with a potentially greater safety index in vivo. Additional investigations need to be performed in order to evaluate the potential inhibitory effect of compound 1 on cellular DNA polymerases as well as to characterize the intracellular metabolism pathways of this nucleoside analogue.
ACKNOWLEDGMENTS
The assistance of Nathalie Turcotte and Paul Nguyen-Ba in carrying out the synthesis and in providing the reference material, respectively, is gratefully acknowledged. We also thank Marie-Josee Gilbert, Christine Pelletier, Chantal Boudreau, Dominique Barbeau, and Martine Hamel for technical support.
REFERENCES
- 1.Aduma P, Connelly M C, Srinivas R V, Fridland A. Metabolic diversity and antiviral activities of acyclic nucleoside phosphonates. Mol Pharmacol. 1995;47:816–822. [PubMed] [Google Scholar]
- 2.Baba M, Mori S, Shigeta S, DeClercq E. Selective inhibitory effect of (S)-9-(3-hydroxy-2-phosphonylmethoxy-propyl)adenine and 2′-nor-cyclic GMP on adenovirus replication in vitro. Antimicrob Agents Chemother. 1987;31:331–339. doi: 10.1128/aac.31.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldanti F, Underwood M R, Stanat S C, Biron K K, Chou S, Sarasini A, Silini E, Gerna G. Single amino acid changes in the DNA polymerase confer foscarnet resistance and slow-growth phenotype, while mutations in the UL97-encoded phosphotransferase confer ganciclovir resistance in three double-resistant human cytomegalovirus strains recovered from patients with AIDS. J Virol. 1996;70:1390–1395. doi: 10.1128/jvi.70.3.1390-1395.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnard J A, Huffman J H, Sidwell R W, Reist E J. Selective inhibition of cytomegalovirus by 9-(3′-ethylphosphono-1′-propyloxy-methyl)guanine. Antivir Res. 1993;22:77–89. doi: 10.1016/0166-3542(93)90086-x. [DOI] [PubMed] [Google Scholar]
- 5.Biron K K. Ganciclovir-resistant human cytomegalovirus clinical isolates: resistance mechanisms and in vitro susceptibility to antiviral agents. Transplant Proc. 1991;23:162–167. [PubMed] [Google Scholar]
- 6.Bischofberger N, Hitchcock M J, Chen M S, Barkhimer D B, Cundy K C, Kent K M, Lacy S A, Lee W A, Li Z H, Mendel D B, Smee D F, Smith J L. 1-[(S)-2-Hydroxy-2-oxo-1,4,2-dioxaphosphorinan-5-yl)methyl]cytosine, an intracellular prodrug for (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine with improved therapeutic index in vivo. Antimicrob Agents Chemother. 1994;38:2387–2391. doi: 10.1128/aac.38.10.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bronson J J, Ghazzouli I, Hitchcock M J M, Webb R R, Kern E R, Martin J C. Synthesis and antiviral activity of nucleoside analogues bearing the (S)-(3-hydroxy-2-phosphonylmethoxy)propyl moiety attached to adenine, guanine, and cytosine. In: Martin J C, editor. Nucleotide analogues as antiviral agents. Washington, D.C: American Chemical Society; 1989. pp. 88–102. [Google Scholar]
- 8.Chan, L., N. Turcotte, P. Nguyen-Ba, L. Yuen, D. Barbeau, J. Bedard, and M. Hamel. Identification of novel nucleotide phosphonate analogues with potent anti-HCMV activity. Submitted for publication. [DOI] [PubMed]
- 9.Cherrington J M, Allen S J W, Mulato A S, Miner R, Drew W L, Chen M S. Sensitivities of human cytomegalovirus (HCMV) clinical isolates to cidofovir. Antivir Res. 1998;26:A319. [Google Scholar]
- 10.Cherrington J M, Miner R, Hitchcock M J, Lalezari J P, Drew W L. Susceptibility of human cytomegalovirus to cidofovir is unchanged after limited in vivo exposure to various regiments of drug. J Infect Dis. 1996;173:987–992. doi: 10.1093/infdis/173.4.987. [DOI] [PubMed] [Google Scholar]
- 11.Chou S, Erice A, Jordan M C, Vercellotti G M, Michels K R, Talarico C L, Stanat S C, Biron K K. Analysis of the UL97 phosphotransferase coding sequence in clinical cytomegalovirus isolates and identification of mutations conferring ganciclovir resistance. J Infect Dis. 1995;171:576–583. doi: 10.1093/infdis/171.3.576. [DOI] [PubMed] [Google Scholar]
- 12.Chrisp P, Clissold S P. Foscarnet: a review of its antiviral activity, pharmacokinetic properties and therapeutic use in immunocompromised patients with cytomegalovirus retinitis. Drugs. 1991;41:104–129. doi: 10.2165/00003495-199141010-00009. [DOI] [PubMed] [Google Scholar]
- 13.Cundy K C, Bidgood A M, Lynch G, Shaw J P, Griffin L, Lee W A. Pharmacokinetics, bioavailability, metabolism and tissue distribution of cidofovir (HPMPC) and cyclic HPMPC in rats. Drug Metab Dispos. 1996;24:745–752. [PubMed] [Google Scholar]
- 14.Cundy K C, Li Z H, Lee A. Effect of concomitant probenecid on the tissue distribution and urinary excretion of HPMPC in preclinical models. Pharm Res. 1994;11:S449. [Google Scholar]
- 15.De Clercq E. Trends in the development of new antiviral agents for the chemotherapy of infections caused by herpesviruses and retroviruses. Rev Med Virol. 1995;5:149–164. [Google Scholar]
- 16.De Clercq E, Holy A, Rosenberg I, Sakuma T, Balzarini J, Maudgal P C. A novel selective broad-spectrum anti-DNA virus agent. Nature. 1986;323:464–467. doi: 10.1038/323464a0. [DOI] [PubMed] [Google Scholar]
- 17.Gordon Y J, Romanowski E, Araullo-Cruz T. Topical HPMPC inhibits adenovirus type 5 in the New Zealand rabbit ocular replication model. Invest Ophthal Vis Sci. 1994;35:4135–4143. [PubMed] [Google Scholar]
- 18.Heijtink R A, Kruining J, De Wilde A G, Bazarini J, De Clercq E, Schalm S W. Inhibitory effects of acyclic nucleoside phosphonates on human hepatitis B virus and duck hepatitis virus infections in tissue culture. Antimicrob Agents Chemother. 1994;38:2180–2182. doi: 10.1128/aac.38.9.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hitchcock M J M, Lacy S A, Lindsey J R, Kern E R. The cyclic congener of cidofovir has reduced nephrotoxicity in three species. Antivir Res. 1995;26:A358. [Google Scholar]
- 20.Ho H-T, Woods K L, Bronson J J, De Boeck H, Martin J C, Hitchcock M J. Intracellular metabolism of the antiherpes agent (S)-1-(3-hydroxy-2-(phosphonylmethoxy)propyl)-cytosine. Mol Pharmacol. 1992;41:197–202. [PubMed] [Google Scholar]
- 21.Huffman J H, Sidwell R W, Barnard D L, Morrison A, Otto M J, Hill C L, Schinazi R F. Influenza virus-inhibitory effects of a series of germanium and silicon centred polyoxometalates. Antivir Chem Chemother. 1997;8:75–83. [Google Scholar]
- 22.Jacobson M A. Treatment of cytomegalovirus retinitis in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1997;337:105–114. doi: 10.1056/NEJM199707103370207. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson M A, French M. Altered natural history of AIDS-related opportunistic infections in the era of potent combination antiretroviral therapy. AIDS. 1998;12:S157–S163. [PubMed] [Google Scholar]
- 24.Jacobson M A, Zegans M, Pavan P R, O’Donnell J J, Sattler F, Rao N, Owens S, Pollard R. Cytomegalovirus retinitis after initiation of highly active antiretroviral therapy. Lancet. 1997;349:1443–1445. doi: 10.1016/S0140-6736(96)11431-8. [DOI] [PubMed] [Google Scholar]
- 25.Kimberlin D W, Coen D M, Biron K K, Cohen J I, Lamb R A, McKinlay M, Emini E A, Whitley R J. Molecular mechanisms of antiviral resistance. Antivir Res. 1995;26:369–401. doi: 10.1016/0166-3542(95)00027-j. [DOI] [PubMed] [Google Scholar]
- 26.Krosky P M, Underwood M N, Turk S R, Feng K W H, Jain R K, Ptark R G, Westerman A C, Biron K K, Townsend L B, Drach J C. Resistance of human cytomegalovirus to benzimidazole ribonucleotides maps to two open reading frames: UL89 and UL56. J Virol. 1998;92:4721–4728. doi: 10.1128/jvi.72.6.4721-4728.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis A F, Drach J C, Fennewald S M, Huffman J H, Ptak R G, Sommadossi J P, Revankar G R, Rando R F. Inhibition of human cytomegalovirus in culture by alkenyl guanine analogs of the thiazolo[4,5-d]pyrimidine ring system. Antimicrob Agents Chemother. 1994;38:2889–2895. doi: 10.1128/aac.38.12.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li S B, Yang Z H, Feng J S, Fong C K Y, Lucia H L, Hsiung G D. Activity of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)-cytosine (HPMPC) against guinea pig cytomegalovirus infection in cultured cells and in guinea pigs. Antivir Res. 1990;13:237–252. doi: 10.1016/0166-3542(90)90069-j. [DOI] [PubMed] [Google Scholar]
- 29.Macher A M, Reichert C M, Straus S E, Longo D L, Parrillo J, Lane H C, Fauci A S, Rook A H, Manischewitz J F, Quinnan G V J. Death in the AIDS patient: role of cytomegalovirus. N Engl J Med. 1983;309:1454. doi: 10.1056/NEJM198312083092312. [DOI] [PubMed] [Google Scholar]
- 30.Mattes H, Benezra C. Synthesis of a model hapten with cyclohexanediol and alpha-methylene-gamma-butyrolactone groups, a synthetic analogue of poison ivy and tulipalin allergens connected with a carbon chain. J Org Chem. 1988;53:2732–2737. [Google Scholar]
- 31.Meyers J D. Infection in bone marrow transplant recipients. Am J Med. 1986;81:27–38. doi: 10.1016/0002-9343(86)90511-5. [DOI] [PubMed] [Google Scholar]
- 32.Mulato A S, Cherrington J M, Chen M S. Anti-HCMV activity of cidofovir in combination with antiviral compounds and immunosuppressive agents: in-vitro analyses. Antivir Chem Chemother. 1996;7:203–208. [Google Scholar]
- 33.Palestine A G, Polis M A, DeSmet M D, Baird B F, Falloon J, Kovacs J A, Davey R T, Zurlo J J, Zunich K M, Davis M. A randomized, controlled trial of foscarnet in the treatment of cytomegalovirus retinitis in patients with AIDS. Appl Microbiol. 1991;22:797–801. doi: 10.7326/0003-4819-115-9-665. [DOI] [PubMed] [Google Scholar]
- 34.Pertel P, Hirschtick R, Phair J, Poggensee L, Murphy R. Risk of developing cytomegalovirus retinitis in persons infected with the human immunodeficiency virus. J Acquired Immune Defic Syndr. 1992;5:1069–1074. [PubMed] [Google Scholar]
- 35.Polis M A, Spooner K M, Baird B F, Manischewitz J F, Jaffe H S, Fisher P E, Falloon J, Davey R T J, Kovacs J A, Walker R E, Whitcup S M, Nussenblatt R B, Lane H C, Masur H. Anticytomegaloviral activity and safety of cidofovir in patients with human immunodeficiency virus infection and cytomegalovirus viruria. Antimicrob Agents Chemother. 1995;39:882–886. doi: 10.1128/aac.39.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prichard M N, Shipman C., Jr A three-dimensional model to analyze drug-drug interactions. Antivir Res. 1990;14:181–206. doi: 10.1016/0166-3542(90)90001-n. [DOI] [PubMed] [Google Scholar]
- 37.Ringden O, Lonnqvist B, Paulin T, Ahlmen J, Klintmalm G, Wahren B, Lernestedt J O. Pharmacokinetics, safety and preliminary clinical experiences using foscarnet in the treatment of cytomegalovirus infections in bone marrow and renal transplant recipients. J Antimicrob Chemother. 1986;17:373–387. doi: 10.1093/jac/17.3.373. [DOI] [PubMed] [Google Scholar]
- 38.Sarasini A, Baldanti F, Furione M, Percevalle E, Brerra R, Barbi M, Gerna G. Double resistance to ganciclovir and foscarnet of four human cytomegalovirus strains recovered from AIDS patients. J Med Virol. 1995;47:237–244. doi: 10.1002/jmv.1890470309. [DOI] [PubMed] [Google Scholar]
- 39.Sidwell R W, Huffman J H. Use of disposable micro tissue culture plates for antiviral and interferon induction studies. Appl Microbiol. 1971;22:797–801. doi: 10.1128/am.22.5.797-801.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sidwell R W, Smee D F, Warren R P, Huffman J H, Gilbert B J, Burger R A, Pearson F C. Murine cytomegalovirus-inhibitory effects of ImuVert. Antivir Res. 1993;20:279–292. doi: 10.1016/0166-3542(93)90072-q. [DOI] [PubMed] [Google Scholar]
- 41.Smith I L, Cherrington J M, Jiles R E, Fuller M D, Freeman W R, Spector S A. High-level resistance of cytomegalovirus to ganciclovir is associated with alterations in both the UL97 and DNA polymerase genes. J Infect Dis. 1997;176:69–77. doi: 10.1086/514041. [DOI] [PubMed] [Google Scholar]
- 42.Snoeck R, Andrei G, De Clercq E. Human cytomegalovirus (HCMV) strains selected under selective pressure of phosphonoformate (PFA) are resistant for both PFA and phosphonylmethoxyethyl derivatives in vitro. Antivir Res. 1995;26:A320. [Google Scholar]
- 43.Snoeck R, Van Ranst M, Andrei G, De Clercq E, De Wit S, Poncin M, Clumeck N. Treatment of anogenital papillomavirus infections with an acyclic nucleoside phosphonate analogue. N Engl J Med. 1995;333:943–944. doi: 10.1056/NEJM199510053331418. [DOI] [PubMed] [Google Scholar]
- 44.Stanat S C, Reardon J E, Erice A, Jordan M C, Drew W L, Biron K K. Ganciclovir-resistant cytomegalovirus clinical isolates: mode of resistance to ganciclovir. Antimicrob Agents Chemother. 1991;35:2191–2197. doi: 10.1128/aac.35.11.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sullivan V, Biron K K, Talarico C, Stanat S C, Davis M, Pozzi L M, Coen D M. A point mutation in the human cytomegalovirus DNA polymerase gene confers resistance to ganciclovir and phosphonylmethoxyalkyl derivatives. Antimicrob Agents Chemother. 1993;37:19–25. doi: 10.1128/aac.37.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tatarowicz W A, Lurain N S, Thompson K D. A ganciclovir-resistant clinical isolate of human cytomegalovirus exhibiting cross-resistance to other DNA polymerase inhibitors. J Infect Dis. 1992;166:904–907. doi: 10.1093/infdis/166.4.904. [DOI] [PubMed] [Google Scholar]
- 47.Whitley R J, Jacobson M A, Friedberg D N, Holland G N, Jabs D A, Dieterich D T, Hardy W D, Polis M A, Deutsch T A, Feinberg J, Spector S A, Walmsley S, Drew W L, Powderly W G, Griffiths P D, Benson C A, Kessler H A. Guidelines for the treatment of cytomegalovirus diseases in patients with AIDS in the era of potent antiretroviral therapy: recommendations of an international panel. International AIDS Society-USA. Arch Intern Med. 1998;158:957–969. doi: 10.1001/archinte.158.9.957. [DOI] [PubMed] [Google Scholar]
- 48.Xiong X, Smith J L, Kim C, Huang E, Chen M S. Kinetic analysis of the interaction of cidofovir diphosphate with human cytomegalovirus DNA polymerase. Biochem Pharmacol. 1996;51:1563–1567. doi: 10.1016/0006-2952(96)00100-1. [DOI] [PubMed] [Google Scholar]
- 49.Yokota T, Mochizuki S, Konno K, Mori S, Shigeta S, De Clercq E. Inhibitory effects of selected antiviral compounds on human hepatitis B virus DNA synthesis. Antimicrob Agents Chemother. 1991;35:394–397. doi: 10.1128/aac.35.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu K L, Bronson J J, Yang H, Patick A, Alam M, Brankovan V, Datema R, Hitchcock M J M, Martin J C. Synthesis and antiviral activity of methyl derivatives of 9-[2-(phosphonomethyl)ethyl]guanine. J Med Chem. 1988;35:2958–2969. doi: 10.1021/jm00094a005. [DOI] [PubMed] [Google Scholar]
- 51.Zou R, Drach J C, Townsend L B. Design, synthesis, and antiviral evaluation of 2-substituted 4,5-dichloro- and 4,6-dichloro-1-β-d-ribofuranosylbenzimidazoles as potential agents for human cytomegalovirus infections. J Med Chem. 1997;40:802–810. doi: 10.1021/jm960533b. [DOI] [PubMed] [Google Scholar]