Abstract
The origin and diversification of appendage types is a central question in vertebrate evolution. Understanding the genetic mechanisms that underlie fin and limb development can reveal relationships between different appendages. Here we demonstrate, using chemical genetics, a mutually agonistic interaction between Fgf and Shh genes in the developing dorsal fin of the channel catfish, Ictalurus punctatus. We also find that Fgf8 and Shh orthologs are expressed in the apical ectodermal ridge and zone of polarizing activity, respectively, in the median fins of representatives from other major vertebrate lineages. These findings demonstrate the importance of this feedback loop in median fins and offer developmental evidence for a median fin-first scenario for vertebrate paired appendage origins.
Keywords: evolutionary developmental biology, fibroblast growth factor signaling, Hedgehog signaling, unpaired fin, median fin
The fins of fishes are categorized as median (dorsal, caudal, and anal) or paired (pectoral and pelvic) (Fig. 1A), with paired fins appearing later in the fossil record and subsequently giving rise to the limbs of tetrapods (land vertebrates). How paired fins first arose is a long-standing, yet unresolved, question in vertebrate evolution (1, 2). The median fin hypothesis holds that the developmental genetic programs that pattern fins and limbs first evolved in midline appendages before being coopted to form the paired fins (3). In contrast, the gill arch hypothesis proposes that paired fins arose as modified gill arch outgrowths (4). These hypotheses predict different patterns of shared developmental programs among paired fins, limbs, median fins, and gill rays. One critical paired appendage growth mechanism, the fibroblast growth factor (Fgf)–Sonic hedgehog (Shh)-positive feedback loop, has been characterized in gill rays (5) but has not been reported in the median fins.
Fig. 1.
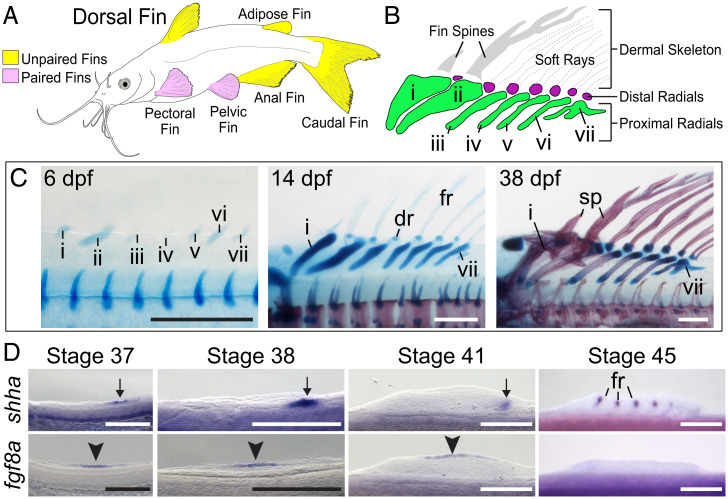
Development of the dorsal fin in the channel catfish. (A) Illustration of a channel catfish highlighting the paired (pink) and unpaired (yellow) fins. (B) Schematic of the channel catfish dorsal fin skeleton. Roman numerals indicate proximal radials. (C) Development of the channel catfish dorsal fin skeleton, showing cartilage in blue and bone in red at 6, 14, and 38 dpf; dr distal radial, fr fin ray, sp spine. (D) Expression of fgf8a in the AER (black arrowhead) and shha in the ZPA (black arrow) in the dorsal fin bud first appear at stage 37. Signal in the AER and ZPA is lost by stage 45, but shha is detected in the fin rays. Anterior to left, dorsal to top in all panels. Scale bars, 250 μm.
The Fgf–Shh feedback loop is a critical driver of appendage growth. In the developing appendage bud, Fgf ligands are expressed in the distal apical ectodermal ridge (AER), while Shh is expressed in the posterior mesenchyme in the zone of polarizing activity (ZPA) (6). Perturbation of either pathway leads to a reciprocal down-regulation of the other and diminished limb growth. The Fgf–Shh interaction is mediated by other genes and signaling pathways including Grem1 and Bmp4 (7). The importance of this mechanism is underscored by its ubiquitous presence in developing paired appendages, even in species that lack a morphological AER (8). Despite the importance of the Fgf–Shh feedback loop, it has yet to be assessed in the median fins. However, Shh is expressed in the posterior mesenchyme of median fins in skates (9), and similar regulatory elements drive ZPA Shh expression in paired and median fins (10). Additionally, Gli3–Shh interactions characteristic of limbs also function in paired and unpaired fins, suggesting multiple aspects of Shh regulation arose in unpaired fins prior to the origin of paired fins (11).
Results and Discussion
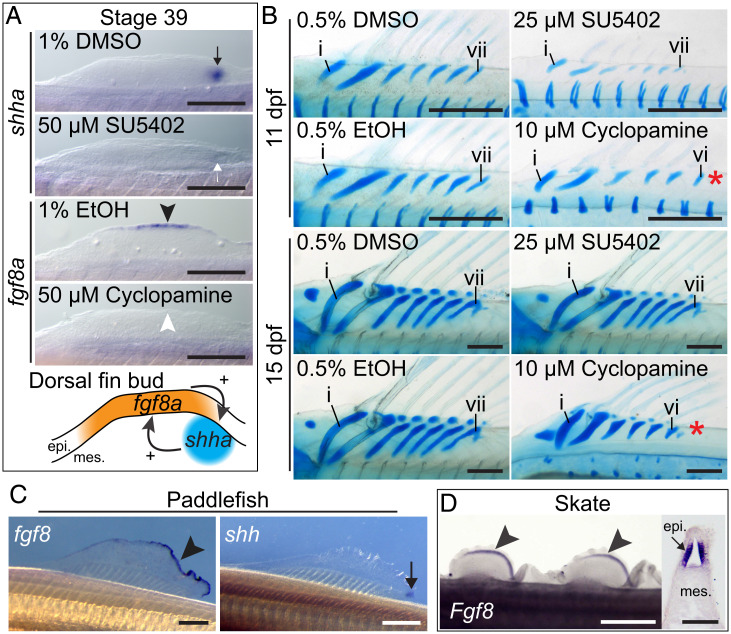
The dorsal fin of the channel catfish, Ictalurus punctatus, displays striking morphological anterior–posterior polarity due to the presence of anterior fin spines associated with enlarged proximal radials (Fig. 1 B and C). We find that fgf8a and shha are expressed in the dorsal fin in their respective AER and ZPA domains typical of paired appendages beginning at the first morphological indication of the fin bud at stage 37 (Fig. 1D). We treated larvae with 50 μM SU5402, an inhibitor of Fgf signaling, and assessed shha expression by in situ hybridization (Fig. 2A). We found ZPA shha expression was diminished in most treated embryos (6 of 10, 60%, absent in 3, reduced in 3) relative to controls (10 of 10, 100%). Reciprocally, when catfish were treated with 50 μM cyclopamine, a Hedgehog (Hh) signaling inhibitor, expression of fgf8a was diminished in the developing AER (7 of 9, 78%, absent in 5, reduced in 2) compared to controls (10 of 10, 100%). These experiments demonstrate that the mutually agonistic nature of Fgf8 and Shh signaling observed in paired appendages is also found in the median dorsal fin.
Fig. 2.
An Fgf–Shh positive feedback loop drives dorsal fin growth in channel catfish and may be ancestral for jawed vertebrates. (A) Perturbation of Fgf signaling by SU5402 reduced ZPA shha expression (white arrow) compared to controls (black arrow). Hh signaling inhibition by cyclopamine reduced AER fgf8a expression (white arrowhead) compared to controls (black arrowhead). Schematic illustrates mutually agonistic interactions between shha and fgf8a in the dorsal fin bud. (Scale bars, 250 μm.) (B) Inhibition of Fgf and Hh signaling impairs dorsal fin growth resulting in shorter proximal radials. Hh inhibition causes loss of endoskeletal elements along the anterior-posterior axis (red asterisk), resulting in six instead of seven proximal radials. (Scale bars, 250 μm.) (C) Expression of fgf8 in the AER (black arrowhead) and shh in the ZPA (black arrow) in the paddlefish dorsal fin at stage 45. (Scale bars, 500 μm.) (D) The dorsal fins of the skate express epithelial Fgf8 at stage 30 shown in whole mount (black arrowheads) and in section (black arrow). (White scale bar, 1 mm.; black scale bar, 50 μm.) epi., epithelium; mes., mesenchyme. Anterior to left, dorsal to top in all panels.
To determine the effect of Fgf–Hh feedback loop perturbation on dorsal fin morphology, we repeated the pharmacological experiments using lower drug dosages that permit survival to later stages. We found that treatment with 25 μM SU5402 caused reduction or absence of the dorsal fin endoskeleton (three of eight, 38%) compared to controls (five of five, 100%) at 11 d post fertilization (dpf) (Fig. 2B). However, SU5402-treated animals examined at 15 dpf displayed normal dorsal fin development (five of five, 100%) similar to controls (three of three, 100%), possibly due to catch up growth. Treatment with 10 μM cyclopamine resulted in reduced size of endoskeletal elements (seven of seven, 100%) relative to controls (five of five, 100%) at 11 dpf. Interestingly, cyclopamine-treated animals also exhibited a reduced number of proximal radials along the anterior–posterior axis, forming only six elements instead of seven (five of seven, 71%), while control animals develop the typical seven elements (five of five, 100%). This reduction is similar to that seen when Hh signaling is perturbed in the paired appendages (10). At 15 dpf, reductions in element length and number were still detected in cyclopamine-treated animals (four of four, 100%) but not in controls (three of three, 100%).
Next, we asked if Fgf8 and Shh orthologs are expressed in the developing median fins of species from other fish lineages. We examined the expression of these genes in a representative of the Chondrostei, a group comprising sturgeons and paddlefishes. In the American paddlefish, Polyodon spathula, we detected expression of fgf8 in the AER as well as shh in the ZPA in the dorsal fin at stage 45 (Fig. 2C). Among elasmobranchs, a group that includes sharks, rays, and skates, Shh is expressed in the ZPA in the developing dorsal fins of the little skate, Raja erinacea (9). We find that Fgf8 is also expressed in the AER of the dorsal fin of the little skate at stage 30 (Fig. 2D). These results suggest that the interaction of Fgf8 and Shh in median fins is phylogenetically wide-spread and likely represents the ancestral condition for jawed vertebrates.
Previous studies have searched for common patterning mechanisms between paired fins and more ancient structures, such as gill rays (5), the axial skeleton (12), and median fins (3). The presence of an Fgf–Shh positive feedback loop in unpaired fins removes an objection to the median fin hypothesis of paired appendage origins and suggests that this mechanism arose early in the prototypical vertebrate appendage, before the diversification of different fin types. Similar scenarios have been proposed for the origins of collinear Hox expression (3), Shh genomic regulation (10), and Gli3–Shh interactions (11) in vertebrate appendage patterning. Together these results indicate that a rather complete developmental program was already in place in early unpaired fins prior to the emergence and divergence of additional appendage types.
Materials and Methods
Catfish and paddlefish embryos were purchased from Osage Catfisheries. Skate embryos were purchased from the Marine Biological Laboratory. Catfish were treated with pharmacological inhibitors for 8 h beginning at stage 39. Experiments were assessed and approved by the University of Colorado at Boulder Institutional Animal Care and Use Committee. Details of animal care, in situ hybridization, staining, and pharmacological treatments are provided in SI Appendix.
Supplementary Material
Acknowledgments
The catfish illustration was based on a photograph kindly provided by Melissa McGaw. Kristen McDaniel and Rebecca Gonzalez helped process catfish eggs. Joseph M. Sanchez Jr. helped process catfish eggs and provided fish care. Alexander Cruz provided space and equipment for catfish husbandry. This work was supported by the NSF grants IBN-0446720, IOS-1121855, and IOS-1755305 (to D.W.S.); NSF grant IOS-1853949 (to M.C.D.); the Scientific Grant Agency of Slovak Republic (Vedecká Grantová Agentúra MŠVVaŠ SR a SAV [VEGA]) grant 1/0450/21 (to D.J.); and the Brinson Foundation (to N.H.S.).
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2120150119/-/DCSupplemental.
Data Availability
All study data are included in the main text and SI Appendix.
References
- 1.Coates M. I., The origin of vertebrate limbs. Dev. Suppl. 1994, 169–180 (1994). [PubMed] [Google Scholar]
- 2.Coates M. I., The evolution of paired fins. Theory Biosci. 122, 266–287 (2003). [Google Scholar]
- 3.Freitas R., Zhang G., Cohn M. J., Evidence that mechanisms of fin development evolved in the midline of early vertebrates. Nature 442, 1033–1037 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Gegenbaur C., Bell F. J., Elements of Comparative Anatomy (MacMillan and Co., London, 1878). [Google Scholar]
- 5.Gillis J. A., Dahn R. D., Shubin N. H., Shared developmental mechanisms pattern the vertebrate gill arch and paired fin skeletons. Proc. Natl. Acad. Sci. U.S.A. 106, 5720–5724 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niswander L., Jeffrey S., Martin G. R., Tickle C., A positive feedback loop coordinates growth and patterning in the vertebrate limb. Nature 371, 609–612 (1994). [DOI] [PubMed] [Google Scholar]
- 7.Zeller R., López-Ríos J., Zuniga A., Vertebrate limb bud development: Moving towards integrative analysis of organogenesis. Nat. Rev. Genet. 10, 845–858 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Doroba C. K., Sears K. E., The divergent development of the apical ectodermal ridge in the marsupial Monodelphis domestica. Anat. Rec. (Hoboken) 293, 1325–1332 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Dahn R. D., Davis M. C., Pappano W. N., Shubin N. H., Sonic hedgehog function in chondrichthyan fins and the evolution of appendage patterning. Nature 445, 311–314 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Letelier J., et al. , A conserved Shh cis-regulatory module highlights a common developmental origin of unpaired and paired fins. Nat. Genet. 50, 504–509 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Letelier J., et al. , The Shh/Gli3 gene regulatory network precedes the origin of paired fins and reveals the deep homology between distal fins and digits. Proc. Natl. Acad. Sci. U.S.A. 118, e2100575118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabin C., Laufer E., Hox genes and serial homology. Nature 361, 692–693 (1993). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the main text and SI Appendix.