Abstract
Background
The use of central venous catheters is recognised as a risk factor for nosocomial infection. Prophylactic antibiotics may be effective in preventing catheter‐related blood stream infection in newborns but may also have the undesirable effect of promoting the emergence of resistant strains of micro‐organisms.
Objectives
To determine the effect of prophylactic antibiotics on mortality and morbidity in neonates with central venous catheters.
Search methods
Searches were done of the Cochrane Neonatal Review Group Specialised Register, MEDLINE from 1950 to April 2007, CINAHL from 1982 to April 2007, and the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 2 2007). Previous reviews (including cross references) were also searched.
Selection criteria
Randomised controlled trials or quasi‐randomised controlled trials of adequate quality in which either individual newborn infants or clusters of infants were randomised to receive prophylactic antibiotics (not including antifungals) versus placebo or no treatment. Infants must have had central venous catheters, been full term infants less than 28 days old or preterm infants up to 44 weeks (postmenstrual) corrected age.
Data collection and analysis
Criteria and methods used to assess the methodological quality of the trials: standard methods of the Cochrane Collaboration and its Neonatal Review Group were used. The review authors extracted data independently. Attempts were made to contact study investigators for additional information as required.
Main results
Three small studies have been included in this review. Prophylactic antibiotics in neonates with central venous catheters had no effect on overall mortality (typical RR 0.68, 95% confidence interval 0.31, 1.51). Prophylactic antibiotics in neonates with central venous catheters decreased the rate of proven bacterial sepsis (typical RR 0.38, 95% confidence interval 0.18, 0.82). Prophylactic antibiotics in neonates with central venous catheters decreased the rate of suspected or proven bacterial septicaemia (typical RR 0.40, 95% confidence interval 0.20, 0.78). No resistant organisms colonising infants were identified in any of the studies. No pooled data were available for other important outcome measures such as chronic lung disease or neurodevelopmental outcome.
Authors' conclusions
Prophylactic systemic antibiotics in neonates with a central venous catheter reduces the rate of proven or suspected septicaemia. However, this may not be clinically important in the face of no significant difference in overall mortality and the lack of data on long‐term neurodevelopmental outcome. Furthermore, there is a lack of data pertaining to the potentially significant disadvantages of this approach such as the selection of resistant organisms. The routine use of prophylactic antibiotics in infants with central venous catheters in neonatal units cannot currently be recommended.
Plain language summary
Prophylactic systemic antibiotics to reduce morbidity and mortality in neonates with central venous catheters
Central venous lines (long plastic tubes that have their tip ending in the big veins near the heart through which medicine and fluid can be given) are used in some newborn babies, particularly those that have been born too early or who are very sick. Babies with these lines are at risk of developing very serious blood infections, which may even cause death. Sometimes antibiotics are used to try to prevent these blood infections in babies with central venous lines. These preventive antibiotics may have unwanted side effects and could increase the likelihood of infections that are resistant to treatment. Therefore, it is possible that the risks of using antibiotics outweigh the potential benefits. Three small trials of 271 babies were included in this review. The results of these studies show that it is possible to reduce the chance of serious blood infection occurring, but that almost 10 babies need to be given preventive antibiotics to avoid one case of infection. There was no difference in the likelihood of death. There were not enough data on other important effects of the antibiotics or on the possible serious side effects. There was not much similarity between the studies included in this review. Therefore, there is currently not enough evidence to recommend routinely using antibiotics in babies with central venous lines.
Background
Central venous catheters are commonly used in the management of newborn infants who are preterm or have other potentially life‐threatening illness. The use of central venous catheters is recognised as a risk factor for nosocomial infection (Adams‐Chapman 2002; Apostolopoulou 2004; Chien 2002; Nagata 2002; Stoll 2002). Giving parenteral nutrition is an indication for the use of central venous catheters, and is also a risk factor for nosocomial infection in newborns (Adams‐Chapman 2002) and older patients (Hodge 2002). Nosocomial infection may be associated with significant morbidity and mortality (Nagata 2002; Stoll 2002). Nosocomial infection may lead to an increase in duration of respiratory illness, including chronic lung disease (Liljedahl 2004; Van Marter 2002), an increase in the need for respiratory support (Ogawa 1999; Stoll 2002), an increase in length of hospital stay (Isaacs 2003; Stoll 2002), and impaired neurodevelopmental outcome (Stoll 2004).
By virtue of their underlying illness, patients requiring central venous catheters may have impaired local and systemic defence mechanisms. Prematurity is recognised as a risk factor for late onset sepsis (Dear 1999), and it is often these infants in whom central venous catheters are used. Preterm neonates are at high risk of infection because of impaired immunity and central venous catheters may further increase this risk because they are foreign bodies. Central venous catheters are also commonly used in infants with major abdominal abnormalities or surgical conditions. Infants receiving total parenteral nutrition for surgical conditions have been shown to have an impaired immune response (Cruccetti 2003).
The Centers for Disease Control (CDC) provides recommendations for the prevention of catheter‐related infections when using central venous catheters. The CDC does not recommend the use of antimicrobial prophylaxis (O'Grady 2002). However, in some neonatal units it is standard practice to use antimicrobial prophylaxis in infants with central venous catheters in an attempt to reduce catheter colonisation and thereby reduce the risk of acquired infection. The choice of antibiotics depends on the most frequently encountered organisms, which may vary with local circumstances. Based on their own experience and retrospective analysis, Shaul et al (Shaul 1998) recommend antibiotic prophylaxis at the time of insertion of tunneled or implantable subcutaneous central venous catheters. In a randomised controlled trial, Henrickson et al (Henrickson 2000) demonstrated a reduction in infection following administration of antibiotic/heparin solutions to paediatric oncology patients with tunneled central venous catheters. They concluded that the prophylaxis was well tolerated and stated that it would not lead to an increase in emergence or spread of vancomycin‐resistant enterococcus since they did not find any in their study.
Prophylactic antibiotics may be effective in preventing catheter‐related blood stream infection in newborns, but may have the undesirable effect of promoting the emergence of resistant strains of micro‐organisms (Freij 1999) or of altering the pattern of isolates causing infection (Viudes 2002). A policy of prophylactic antibiotic use must take this possibility into account, and this possibility has been used as a basis for arguing against implementation of prophylactic antibiotics (Haas 2003; Isaacs 2000; Isaacs 2003; McGuire 2004). The emergence of resistant strains of organisms will develop over time and this resistance may vary depending upon the antibiotic.
Recent Cochrane systematic reviews on the use of prophylactic antibiotics for neonates with umbilical artery (Inglis 2004) and umbilical venous (Inglis 2005) catheters showed that there was no evidence from randomised trials to support or refute the use of prophylactic antibiotics when using such catheters in newborn infants. The following systematic review evaluates the use of prophylactic antibiotics for neonates with central venous catheters.
Objectives
To determine the effects of prophylactic antibiotics in neonates with central venous catheters on mortality and morbidity (especially septicaemia). We reviewed a policy of prophylactic antibiotics for the duration of catheterisation (or other fixed duration of antibiotic treatment) versus placebo or no treatment. Data permitting, subgroup analyses were done to determine whether results differ by: 1. term (> 37 weeks gestation) versus preterm (< 37 weeks gestation); 2. type of antibiotic (e.g., penicillins, macrolides, aminoglycosides, cephalosporins, or combinations); 3. type of catheter (e.g. peripherally inserted central catheter, surgically inserted or tunneled line).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials or some types of non‐randomised (i.e., quasi‐randomised) controlled trials of adequate quality in which either individual newborn infants or clusters of infants (such as separate neonatal units) were randomised to receive prophylactic antibiotics versus placebo or no treatment.
Types of participants
Neonates with central venous catheters: full term infants less than 28 days old; preterm infants up to 44 weeks postmenstrual age. Infants with umbilical venous catheters were not included (reviewed previously by Inglis 2005).
Types of interventions
Any systemic antibiotic (not including antifungals), or combination of antibiotics, versus placebo or no treatment.
Types of outcome measures
Primary:
Mortality (neonatal, at hospital discharge, or at one year)
Proven bacterial septicaemia (blood culture positive), suspected septicaemia or clinical septicaemia
Secondary:
Chronic lung disease (oxygen requirement at 36 weeks postmenstrual age)
Duration of ventilation (hours or days)
Duration of respiratory support (hours or days)
Duration of oxygen therapy (hours or days)
Duration of hospital stay (days)
Number of resistant organisms (i.e., species) causing infection, identified per time period per infant or per cluster unit
Number of resistant organisms (i.e., species) colonising infants in the study, identified per time period per infant
Number of resistant organisms (i.e., species) colonising all infants identified per time period per cluster unit
Neurodevelopmental outcome (cerebral palsy, sensorineural hearing loss, visual impairment and/or developmental delay ‐ at one year, 18 months, two years, or five years)
Search methods for identification of studies
See: Neonatal Group search strategy
The standard search strategy for the Cochrane Neonatal Review Group was used. Searches were done of the Cochrane Neonatal Review Group Specialised Register, MEDLINE from 1950 to April 2007, CINAHL from 1982 to April 2007, and the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 2 2007) using the following strategy:
MeSH search terms "Catheterization, Central Venous" OR the textwords ("central" AND ("cathet$" OR "cannul$")) OR "CVC" OR "CVL" OR "central vein catheter" OR "central venous catheter" OR "central vein line" OR "central venous line" OR "central line" OR "central catheter" OR "PICC" AND MeSH search term "Infant, newborn" OR the textwords "neonat$" OR "infant"' AND MeSH search term "Antibacterial Agents" OR the textword "antibiotic" AND MeSH search terms "Chemoprevention" OR "Antibiotic Prophylaxis" OR the textword "prophyl$".
Previous reviews (including cross references) were also searched. Searches were not restricted to publications in the English language or published data.
Data collection and analysis
Criteria and methods used to assess the methodological quality of the trials: standard methods of the Cochrane Collaboration and its Neonatal Review Group were used.
Two of the review authors worked independently to search for and assess trials for inclusion and methodological quality. Studies were assessed using the following key criteria: allocation concealment (blinding of randomisation), blinding of intervention, completeness of follow up and blinding of outcome measurement assigning a rating of 'Yes', 'No' or 'Can't tell' for each. The review authors extracted data independently. Differences were resolved by discussion. Attempts were made to contact study investigators (Cooke 1997; Garland 2005; Harms 1995; Spafford 1994; Baier 1998; and Kacica 1994) for additional information or data as required.
For pooled results: for continuous variables, weighted mean differences (WMD) and 95% confidence intervals were to be reported. For categorical outcomes, the relative risks (RR) and 95% confidence intervals are reported. For significant findings, the risk difference (RD) and number needed to treat (NNT) are reported.
Given enough included studies, each treatment effect was tested for heterogeneity to help determine suitability for pooling of results in a meta‐analysis. The fixed effects model was used for meta‐analysis. Where sufficient included studies were available, heterogeneity was assessed using the I squared test of heterogeneity, which is thought to be better at quantifying the heterogeneity than the chi‐squared test. Apart from the planned subgroup analyses detailed above, there were no other a priori specific potential causes of heterogeneity.
A sensitivity analysis was planned to see if results differed by quality of included studies (e.g., adequacy of randomisation, quasi‐randomised versus randomised) but was not performed due to insufficient data.
Results
Description of studies
Nine studies were identified. Four met eligibility criteria and three (Cooke 1997; Harms 1995; Spafford 1994) are included in this review. The fourth study (Graham 2003), has not been included due to a lack of data.
Cooke et al (Cooke 1997):
Population
All of the subjects in the study were very low birth weight infants receiving parenteral nutrition. The published paper included infants without central venous catheters. The first author provided data from the subgroup with central venous catheters (n = 72).
Interventions
The experimental group (n = 37) had low dose vancomycin (5 mg/kg) infused over one hour, twice a day. The control group (n = 35) received no treatment (i.e., no placebo was used).
Outcomes
Pre‐defined reported outcome measures were mortality, proven bacterial septicaemia, duration of ventilation, number of resistant organisms causing infection, and number of resistant organisms causing infection. Indications for a septic screen (consisting of FBC, CRP, aerobic and anaerobic blood cultures) included pyrexia, recurrent apnoea or bradycardia, lethargy, irritability, temperature instability, abdominal distension, and metabolic acidosis. In cases where coagulase negative staphylococcus (CONS) was grown in the blood culture, these results were regarded as true positives only if there was a concomitant rise in CRP (100% increase from baseline). Weekly surveillance swabs (throat, groin and rectum) were collected for bacterial culture.
Spafford et al (Spafford 1994): Attempts to contact the first author for additional information regarding our pre‐specified outcome measures were unsuccessful.
Population
All infants in the neonatal unit who had a peripherally inserted central venous catheter inserted (n = 70).
Interventions
The experimental group (n = 35) had vancomycin added to the parenteral nutrition solution at the concentration of 25 micrograms per mL. The control group (n = 35) had no vancomycin added to their parenteral nutrition.
Outcomes
Pre‐defined reported outcome measures were mortality, proven bacterial septicaemia, duration of hospital stay, number of resistant organisms causing infection, and brain stem auditory evoked responses (neurodevelopmental outcome). Infants were investigated for septicaemia if they had temperature instability, increased oxygen and ventilatory requirements, increased number and severity of apnoea or bradycardia, feeding intolerance, lethargy or blood pressure instability. Investigation of suspected sepsis included blood cultures obtained from a peripheral vein and drawn through the central venous catheter. On removal, catheters were sent for microbiologic evaluation (a drop of rinse solution onto blood agar or catheter tip rolled across surface of blood agar plate). Catheter related sepsis was defined as catheter specimen containing at least ten times the concentration of the same pathogen isolated from the peripheral sample.
Harms et al (Harms 1995): Attempts to contact the first author for additional information regarding our pre‐specified outcome measures were unsuccessful.
Population
All infants in the neonatal intensive care with central venous catheters (n = 148).
Interventions
The experimental group (n = 75) received amoxicillin (100 mg/kg/day) intravenously in three divided doses. The control group (n = 73) received no treatment (i.e., no placebo was used).
Outcomes
Pre‐defined reported outcome measures were mortality, proven bacterial septicaemia, suspected septicaemia, and number of resistant organisms causing infection. Twice weekly, a drop of fluid from the connecting hub was sent for bacteriologic examination. Leucocyte count, immature:total (I:T) neutrophil ratio, thrombocyte count and CRP were measured twice weekly and when clinically indicated. Catheter‐related septicaemia was defined as presence of clinical signs (apnoea, bradycardia, temperature instability, feeding problems, circulatory changes, lethargy), suspect laboratory findings (CRP > 0.6 mg/dL; I:T ratio > 0.16), and identical bacterial growth in peripheral vein blood culture and catheter tip. Suspected catheter‐related septicaemia was defined as presence of clinical signs and suspect laboratory findings but negative blood culture.
Four studies were excluded on the basis of wrong population for this review (Baier 1998; Kacica 1994; Moller 1993; Rackoff 1995) and one on the basis of wrong intervention for this review (Garland 2005) (see Table, Characteristics of Excluded Studies). Two of the studies (Baier 1998; Kacica 1994) may have included subpopulations relevant to this review, but attempts to contact the authors for relevant data were unsuccessful. See Table, Characteristics of excluded studies.
One study (Graham 2003) is awaiting further assessment. It was published in abstract form in the proceedings of the 2003 Pediatric Academic Societies meeting. A vancomycin flush technique, or placebo, was used in infants with birth weight less than 1500 g, greater than 48 hours of age, and with central venous access. The results provided in the abstract lack sufficient detail to include in this review at this time. Attempts to contact the authors for full details and data have been unsuccessful.
Risk of bias in included studies
All of the included studies randomised the individual patient. None were quasi‐randomised. Allocation concealment appeared adequate in two trials (Cooke 1997; Spafford 1994) but was unclear in the other. All had adequate follow‐up of at least one important outcome. In two studies (Cooke 1997; Harms 1995) the intervention and outcome assessments were not blind. In the other trial, those caring for the infants were unaware of study group assignment and outcome assessment was blind. The methodological quality of the studies by Cooke et al (Cooke 1997) and Harms et al (Harms 1995) was fair. The methodological quality of the study by Spafford et al (Spafford 1994) was good.
Effects of interventions
Three trials (Cooke 1997; Harms 1995; Spafford 1994), with a total of 271 infants met criteria for inclusion.
The results of individual studies are provided in the Table, Characteristics of included studies.
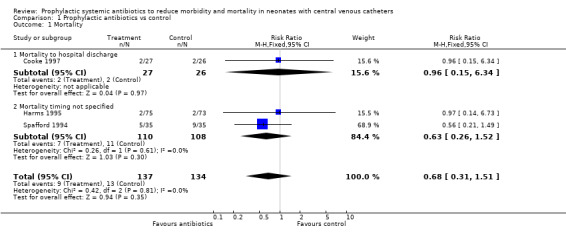
PROPHYLACTIC ANTIBIOTICS VERSUS CONTROL (Comparison 01): Primary outcomes: Mortality (Outcome 01.01): Mortality to hospital discharge (Outcome 01.01.01) Cooke et al (Cooke 1997) reported neonatal mortality and mortality to discharge but neither Harms et al (Harms 1995) nor Spafford et al (Spafford 1994) specified timing of death. Mortality timing not specified (Outcome 01.01.02) All three studies reported mortality, giving a total of nine deaths out of 137 treatment infants versus 13 out of 134 control infants.
Mortality at one year No study reported mortality at one year. No individual study found a significant difference in mortality rate. Meta‐analysis showed no significant difference in mortality (typical RR 0.68, 95% CI 0.31, 1.51).
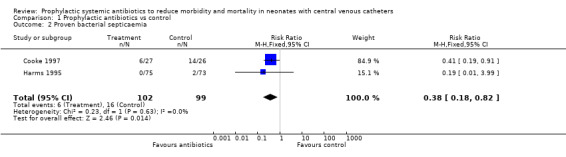
Septicaemia a. Proven bacterial septicaemia (Outcome 01.02) Two studies (Cooke 1997; Harms 1995) reported proven bacterial septicaemia rates, although their definitions differed. Cooke et al (Cooke 1997) reported septicaemia in 6/27 infants (22%) in the treatment group vs. 14/26 (54%) in the control group. They found no positive blood cultures that were deemed to be false. Harms et al (Harms 1995) found no cases of septicaemia in the treatment group vs. 2/73 infants (3%) in the control group. Numbers of positive blood cultures was not reported. Pooling the results of these studies showed 6/102 (6%) treatment infants and 16/99 (16%) control infants had proven bacterial septicaemia (typical RR 0.38, 95% CI 0.18, 0.82, typical RD ‐0.10, NNT 9.7). Spafford et al (Spafford 1994) reported rates of proven septicaemia due to Coagulase‐negative Staphylococci (CONS) only. The overall rates of septicaemia and positive blood cultures were not able to be determined from the published data.
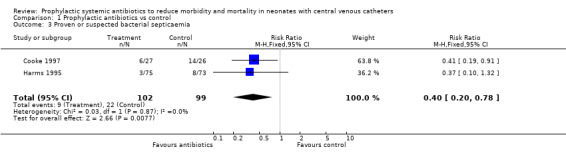
b. Proven or suspected bacterial septicaemia (Outcome 01.03) Nine infants (three in the treatment group and six in the control group) in the study by Harms et al (Harms 1995) had suspected or clinical septicaemia, not proven on blood culture. Therefore, proven or suspected bacterial septicaemia occurred in 9/102 treatment infants vs. 22/99 control infants (typical RR 0.40, 95% CI 0.20, 0.78, typical RD ‐0.13, NNT 7.5).
Secondary outcomes: Chronic lung disease No study reported this.
Duration of ventilation Cooke et al (Cooke 1997) found, in treatment and control groups respectively, a mean (SD) duration of ventilation of 14.1 (17.19) vs. 12.5 (17.55) days, and a median (range) duration of ventilation of 7 (0 ‐ 67) vs. 4.5 (0 ‐ 54) days. These data were not plotted because they were skewed due to a large number of the patients not being ventilated.
Duration of respiratory support No study reported this.
Duration of oxygen therapy No study reported this.
Duration of hospital stay Spafford et al (Spafford 1994) found no significant difference in duration of hospital stay (mean ± SE in treatment and control groups respectively 79 ± 12.3 days vs. 74 ± 8.8 days). These data were not plotted because they were skewed. No other study reported this outcome.
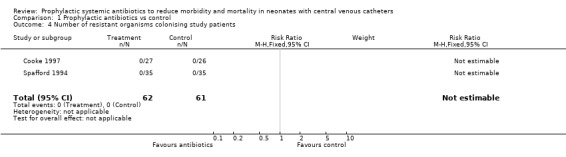
Number of resistant organisms causing infection, identified per time period per infant (Outcome 01.04) The two studies in which vancomycin was used (Cooke 1997; Spafford 1994) found no occurrences of infection due to vancomycin‐resistant organisms. Harms et al (Harms 1995) used amoxicillin. Of the two proven infections that occurred in that study, one was due to Staphylococcus aureus (sensitive to amoxicillin) and the other was due to Staphylococcus epidermidis (resistant to amoxicillin). It was expected, however, that CONS would be resistant to amoxicillin. Therefore, the results have not been pooled.
Number of resistant organisms causing infection, identified per time period per infant, identified per time period per infant Two studies (Cooke 1997; Spafford 1994) included routine surveillance of study infants for colonisation by resistant organisms. Neither study identified any resistant species during the study period. Number of resistant organisms (i.e., species) colonising all infants identified per time period per cluster unit (Outcome 01.11) No study included routine surveillance of all infants in the unit for colonisation by resistant organisms.
Neurodevelopmental outcome The only neurodevelopmental outcome reported was brain stem auditory evoked responses, in the study by Spafford et al (Spafford 1994). They reported that three infants, all from the control group, had significant hearing impairment. However, it was not possible to calculate the rate because three surviving infants in the study were not evaluated, and they do not state which group/s these infants were in. SUBGROUPS: TERM VS PRETERM: There was insufficient data to perform this subgroup analysis. All of the infants in the study by Cooke et al (Cooke 1997) were preterm. It was unclear from the reports of the other two studies whether they included some term infants.
TYPE OF ANTIBIOTIC: Glycopeptides vs penicillins In two trials (Cooke 1997; Spafford 1994) prophylactic vancomycin was given to a total of 62 infants. Harms et al (Harms 1995) gave prophylactic amoxicillin to 75 infants.
Mortality Of the 62 infants who received prophylactic vancomycin, seven died (11%). Two of the 75 infants given amoxicillin died (3%). Given the amount of clinical heterogeneity between the trials, no further analysis was done.
Proven bacterial septicaemia It was not possible to determine the overall rate of septicaemia in one of the studies (Spafford 1994) in which vancomycin was used. Excluding this study, there were 6/22 cases of proven infection in those receiving vancomycin and 0/75 cases in those receiving amoxicillin. Given the amount of clinical heterogeneity between the trials, no further analysis was done.
TYPE OF CATHETER All three included studies included only peripherally inserted central catheters.
Discussion
Three relatively small studies, with a great deal of clinical heterogeneity, were included in this review. Meta analysis demonstrated that routine use of prophylactic systemic antibiotics in neonates with a central venous catheter reduced the rate of proven septicaemia and the rate of proven or suspected septicaemia. However, no significant difference in mortality was demonstrated. Furthermore, there was a lack of data regarding the effect of systemic antibiotic prophylaxis on other important clinical outcomes such as chronic lung disease and neurodevelopmental outcome. There was also insufficient evidence available regarding significant adverse effects due to the use of prophylactic systemic antibiotics.
Of note is the relatively high background rate of septicaemia (16% in the control groups) in these studies. This was largely due to the extraordinarily high rate (54%) in the study by Cooke et al (Cooke 1997). The overall rate of neonatal septicaemia reported in the Australian and New Zealand Neonatal Network (ANZNN) for babies who met the admission criteria in 2004 was 10.3% (Abeywardana 2006). Cooke et al (Cooke 1997) used a low threshold for investigation for septicaemia (i.e., non‐specific clinical indications) and appeared to regard any organisms other than coagulase negative Staphylococcus (CONS) isolated in blood culture as true positives. Isolation of CONS was regarded as a true result only if accompanied by a two‐fold rise in CRP value. It would normally be expected that some instances of CONS‐positive blood cultures are false. However, this was not found, which casts some doubt on the validity of extrapolating the results to other units and clinical circumstances. Their rate of septicaemia may have been lower using more stringent criteria (although it was well below their expected rate of 70%, based on a prior study in the same unit) and, if so, may have increased the overall number needed to treat. Likewise, in units with much lower background rates of septicaemia, the number needed to treat to prevent an episode of septicaemia would potentially be much greater than that found in this review (NNT = 10).
Although results for certain outcomes were pooled, the degree of clinical heterogeneity between the included studies means that the findings should be interpreted with caution. While all three studies used low thresholds for investigation for septicaemia (a commonly used approach in neonatal units), their definitions of septicaemia varied greatly. One study (Cooke 1997) used CRP values, another (Harms 1995) used CRP values or immature:total neutrophil ratios and central line tip cultures, and the other (Spafford 1994) used cultures of blood drawn via central lines. Other sources of heterogeneity include inclusion and exclusion criteria (and therefore patient populations), intervention used, method of administration, and duration of intervention. Additionally, inclusion of the study awaiting further assessment (Graham 2003) could greatly alter the results of meta‐analysis and conclusions drawn.
In the study by Harms et al (Harms 1995), nine infants had their central venous line removed because of suspected septicaemia (i.e., they were clinically ill) but had negative blood cultures. Three had no clear source of infection following investigation, whereas the other six had a source diagnosed (pneumonia, necrotising enterocolitis or rotavirus infection). These six cases were included in assessing the outcome of proven or suspected bacterial septicaemia since they had already received clinically relevant management (i.e., line removal) for suspected bacterial septicaemia.
The emergence of resistant organisms following the liberal use of broad spectrum antibiotics is a major threat to infants in neonatal intensive care nurseries. None of the studies included in this review were able to adequately determine whether resistant organisms emerged as a result of the use of prophylactic antibiotics. Methods of surveillance varied between the studies and no study had routine unit‐wide surveillance. The studies may be of insufficient duration to detect the emergence of resistant organisms within the unit (Spafford 1994: 15 months; Harms 1995: 29 months; and Cooke 1997: 12 months). The only resistant organism causing infection reported by any of the three studies was a Staphylococcus epidermidis resistant to amoxicillin in the placebo arm of the study by Harms et al (Harms 1995). Resistance of this organism to amoxicillin is expected and does not indicate the emergence of antibiotic resistance in that unit.
Additional cost is required for antibiotic prophylaxis which included drug costs, administration costs and monitoring costs (e.g., antibiotic levels, surveillance swabs, audiology screening). The cost of treating infants with sepsis has also not been assessed in the included studies. The choice of prophylactic antibiotic depends on the most frequent organism/s causing catheter‐related blood stream infection. No single antibiotic will likely be effective in preventing all blood stream infections. More broad spectrum prophylaxis would require additional antibiotics. This may further complicate patient management, increase selection of resistant organisms, and add to costs.
Authors' conclusions
Implications for practice.
The results from three, small, heterogenous, randomised controlled trials (two of which were of poor quality) demonstrated that there is some evidence to suggest that prophylactic systemic antibiotics in neonates with central venous catheters can reduce the rates of proven septicaemia and proven or suspected septicaemia. However, this may not be important in the face of no significant difference in overall mortality and the lack of data on long‐term neurodevelopmental outcome. Furthermore, there is a lack of data pertaining to the significant disadvantages of such an approach such as the selection of resistant organisms. The routine use of prophylactic antibiotics in infants with central venous catheters cannot currently be recommended.
Implications for research.
There is a need for long‐term, good quality, double‐blind randomised controlled trials to fully assess the safety and efficacy of this practice. There is also a need to assess which method of administration of prophylactic antibiotics (e.g. flush versus addition to TPN), which antibiotic (e.g. amoxicillin versus vancomycin), which central venous catheters (e.g. peripherally inserted versus centrally inserted) and which infants (e.g. preterm vs. term) this practice would be best applied.
What's new
| Date | Event | Description |
|---|---|---|
| 13 April 2010 | Amended | Contact details updated. |
History
Protocol first published: Issue 4, 2006 Review first published: Issue 1, 2008
| Date | Event | Description |
|---|---|---|
| 11 September 2008 | Amended | Converted to new review format. |
| 14 September 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The review authors thank Dr Adrian Mattke for translating the paper by Moller et al (Moller 1993).
Data and analyses
Comparison 1. Prophylactic antibiotics vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 3 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.31, 1.51] |
| 1.1 Mortality to hospital discharge | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.15, 6.34] |
| 1.2 Mortality timing not specified | 2 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.26, 1.52] |
| 2 Proven bacterial septicaemia | 2 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.18, 0.82] |
| 3 Proven or suspected bacterial septicaemia | 2 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.20, 0.78] |
| 4 Number of resistant organisms colonising study patients | 2 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
Comparison 1 Prophylactic antibiotics vs control, Outcome 1 Mortality.
1.2. Analysis.
Comparison 1 Prophylactic antibiotics vs control, Outcome 2 Proven bacterial septicaemia.
1.3. Analysis.
Comparison 1 Prophylactic antibiotics vs control, Outcome 3 Proven or suspected bacterial septicaemia.
1.4. Analysis.
Comparison 1 Prophylactic antibiotics vs control, Outcome 4 Number of resistant organisms colonising study patients.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cooke 1997.
| Methods | Randomised controlled trial. Randomisation was by a "computer generated block design". Placebo was not used. Blinding of randomisation (allocation concealment): yes Blinding of intervention: no Completeness of follow up: yes Blinding of outcome measurement: no | |
| Participants | Single neonatal unit trial in Liverpool, United Kingdom from May 1993 to May 1994. VLBW infants receiving parenteral nutrition were considered eligible for inclusion. Exclusion criteria were postnatal age >= 2 weeks, serum creatinine >= 150 mmol/L and currently receiving antibiotic therapy. Most of the parenteral nutrition was given via silicon rubber intravenous long lines although in some patients peripheral intravenous cannulae were used. Therefore, the population of interest for this review is a subset of the total group studied. The first author of the study provided data for this subgroup of patients for our primary and secondary outcomes. The study included 72 infants (37 in the treatment group, 35 in the control group). The subgroup of infants with central venous catheters included 53 infants (27 in the treatment group, 26 in the control group). Outcomes for this subgroup are reported in this review. | |
| Interventions | Treatment Group; vancomycin 5 mg/kg infused over one hour, twice a day (n=37). Control Group; no treatment (n=35). Placebo was not used. | |
| Outcomes | Mortality ‐ early and late (before discharge) in each group. Proven bacterial septicaemia. Duration of ventilation. Number of resistant organisms causing infection. Number of resistant organisms colonising infants in study. | |
| Notes | Non‐published information provided by first author. All infants had daily FBC and CRP measurements. Weekly surveillance swabs (throat, groin and rectum) were collected for bacterial culture. Indications for a septic screen (FBC, CRP, and aerobic and anaerobic blood cultures) included pyrexia, recurrent apnoea or bradycardia, lethargy, irritability, temperature instability, abdominal distension, and metabolic acidosis. In cases where CONS was grown in blood culture it was regarded as a true positive only if there was a concomitant rise in CRP (100% increase from baseline).The intervention was stopped when parenteral nutrition was stopped or the infant reached 4 weeks of age. Peak and trough serum vancomycin levels were measured at the third dose and weekly thereafter. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Harms 1995.
| Methods | Randomised, controlled, sequential clinical trial, with evaluations every two months. Method of randomisation is unknown. Not blinded. Blinding of randomisation (allocation concealment): can't tell Blinding of intervention: no Completeness of follow up: yes Blinding of outcome measurement: no | |
| Participants | Single neonatal intensive care unit trial in Gottingen, Germany from August 1990 to November 1992. Infants having central venous catheters (all small‐diameter silicone) were considered eligible. Exclusion criteria are not stated. There were 148 infants (75 in the treatment group, 73 in the control group) recruited. Range of gestational age and birth weight is not stated. For each patient, only the initial catheter inserted was included in the data analysis. | |
| Interventions | Treatment Group; amoxicillin 100 mg/kg/day intravenously in three divided doses (n=75). Control Group; no treatment (n=73). Placebo was not used. | |
| Outcomes | Mortality ‐ timing of the death is not reported. Proven bacterial septicaemia. Suspected bacterial septicaemia or clinical septicaemia. | |
| Notes | We attempted, unsuccessfully, to contact the first author for further details and data. Catheters were used for administration of parenteral nutrition and all infants had heparin (1 unit/mL; minimum 50 units/day) added to the infusate. The catheters were removed when no longer required for parenteral nutrition administration, or when there were signs of serious infection, blockage or dislodgement. The catheter tip was sent for bacterial culture on removal. Twice weekly, a drop of fluid from the connecting hub was sent for bacteriologic examination. Leucocyte count, immature:total (I:T) neutrophil ratio, thrombocyte count and CRP were measured twice weekly and when clinically indicated. Catheter‐related septicaemia was defined as presence of clinical signs (apnoea, bradycardia, temperature instability, feeding problems, circulatory changes, lethargy), suspect laboratory findings (CRP >0.6 mg/dL; I:T ratio >0.16), and identical bacterial growth in peripheral vein blood culture and catheter tip. Suspected catheter‐related septicaemia was defined as presence of clinical signs and suspect laboratory findings but negative blood culture. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Spafford 1994.
| Methods | Double‐blind, randomised, controlled trial. A locally‐developed randomisation scheme was used. Sealed envelopes were used. Blinding of randomisation (allocation concealment): yes Blinding of intervention: yes Completeness of follow up: yes Blinding of outcome measurement: yes | |
| Participants | Single neonatal unit trial in Rochester, New York, United States from April 1991 to June 1992. All infants who had a central venous catheter inserted were considered eligible for inclusion. Catheters were inserted after a negative blood culture had been obtained and there was no evidence of acute infection. Exclusion criteria included renal dysfunction (not defined), and infants with Broviac, Hickman or umbilical venous catheters (i.e., all catheters in study subject were peripherally inserted central catheters). Group allocation was maintained for subsequent catheters. Catheters were used primarily for parenteral nutrition administration but some were also used for intravenous medication administration. Seventy infants (35 in each group) were included. No details about gestational age and birth weight are provided. | |
| Interventions | Treatment Group; parenteral nutrition solution containing 25 micrograms of vancomycin per mL (n=35). Control Group; parenteral nutrition with no vancomycin (n=35). | |
| Outcomes | Mortality ‐ timing of death not specified). Proven bacterial septicaemia ‐ CONS only. Duration of hospital stay. Number of resistant organisms causing infection. Number of resistant organisms colonising infants in study. Neurodevelopmental outcome ‐ brain stem auditory evoked potentials. | |
| Notes | We attempted, unsuccessfully, to contact the first author for further details and data. Blood urea nitrogen, creatinine and vancomycin levels were measured weekly and monitored by a physician not involved in patient care. Infants were investigated for sepsis if they had temperature instability, increased oxygen and ventilatory requirements, increased number or severity of apnoea or bradycardia, feeding intolerance, lethargy, or blood pressure instability. Investigation of suspected sepsis included blood cultures obtained from a peripheral vein and drawn through the central venous catheter. On removal, catheters were sent for microbiologic evaluation (a drop of rinse solution onto blood agar or catheter tip rolled across surface of blood agar plate). Catheter‐related sepsis was defined as catheter specimen containing at least 10 times the concentration of the same pathogen isolated from the peripheral sample. Brain stem auditory evoked potentials were obtained prior to discharge. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
CONS: coagulase‐negative Staphylococcus; CRP: C‐reactive protein; FBC: full blood count; SE: standard error; VLBW: very low birth weight
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baier 1998 | Wrong population. Study included infants without central venous catheters. Data specifically relating to the subgroup of infants with central venous catheters (12 of 38 subjects) were not available. We attempted, unsuccessfully, to contact trial investigator for these data. Randomised controlled trial studying the addition of vancomycin to parenteral nutrition (25 mcg/mL) versus no addition of vancomycin in 38 very low birth weight infants in the first 2 weeks of life. Infants were stratified by birth weight. Evaluation for sepsis was at the discretion of the treating physicians. Catheter tip was cultured following removal. Significant reductions in CONS bacteraemia, total bacteraemia, and length of hospital stay were reported. No vancomycin‐resistant CONS or enterococci were isolated during the study. |
| Garland 2005 | Wrong intervention. Studied the effect of using heparinised saline central venous catheter "lock" solution with or without the addition of vancomycin to the solution. The lock solution was withdrawn following a pre‐specified dwell time. Systemic antibiotic administration was not a part of the study design. Randomised controlled trial involving 85 infants with central venous catheters (42 treatment, 43 control). Serum vancomycin levels were monitored in the first 73 patients. Only 1 infant had vancomycin detected in serum. Catheters were not locked when infants were receiving systemic vancomycin therapy or continuous infusions of insulin or vasoactive drugs. Surveillance rectal and axillary swabs were obtained at study entry and at catheter removal. No vancomycin‐resistant organisms were identified. On clinical suspicion of sepsis blood cultures were collected from a peripheral vein and from the catheter; the catheter hub was also cultured. Catheters were removed at the discretion of the treating neonatologist. Bloodstream infections were classified as "definite CRBSI", "probable CRBSI", or "bloodstream infection without a source" according to degree of correlation between organisms identified in peripheral blood culture, catheter blood culture, and catheter hub or tip culture. There were 7 bloodstream infections in the treatment group vs 18 in the control group (RR 0.40; 95% CI: 0.19‐0.85; P = 0.01). This difference was mainly due to a reduction in the treatment group of definite CRBSI. |
| Kacica 1994 | Wrong population. Study appears to have included infants without central venous catheters. Inclusion criteria include "intravenous access needed for parenteral nutrition". Data specifically relating to the subgroup of infants with central venous catheters were not available. We attempted, unsuccessfully, to contact trial investigator for these data. Non‐blinded randomised, controlled trial comparing the effect of a continuous low dose vancomycin infusion (25 mcg/mL), added to the parenteral nutrition, versus no treatment in 141 VLBW infants (71 in treatment group, 70 in control). All were less than 2 weeks of age and not on antibiotic treatment at recruitment. Vancomycin was ceased when parenteral nutrition was no longer required or the infant reached 1 month of age. All infants had weekly serum creatinine level and urinalysis and those in the treatment group also had weekly full blood counts and monitoring of vancomycin levels. On suspicion of sepsis aerobic and anaerobic blood cultures were collected. Skin and rectal surveillance swabs were obtained at enrolment and at termination of the protocol. Infants in the treatment group had fewer evaluations for sepsis (44 vs 76; P < 0.05), fewer positive blood cultures (2 infants vs 26 infants; P < 0.001), shorter duration of catheterisation (21.4 +/‐ 14.3 days vs 27.8 +/‐ 21.6 days; P < 0.0001), and less time to full feeds (22.2 +/‐ 15.5 days vs 26.5 +/‐ 18.9 days; P < 0.01). No "shift to" vancomycin resistance was observed. |
| Moller 1993 | Wrong population. As the second phase of a three‐phase study, VLBW infants with any form of intravenous access were randomised to either prophylactic vancomycin (10 mg/kg/day in two doses) or no treatment. Published in German. The first phase involved the introduction of hygiene measures to the care of all VLBW infants. This had no significant impact on sepsis rates. Phase 2 involved the randomisation of all VLBW babies to treatment and control groups, as above. Nurses and "intensive care doctors" were blinded but the treating consultants were informed of group assignment. This showed a significant difference in rates of CONS sepsis (6/21 in control group vs 0/20 in treatment group; P = 0.012) but no significant difference in rates of Gram‐negative sepsis. Therefore, in phase 3 all VLBW infants were treated with vancomycin prophylaxis and those with pathogenic Gram‐negative organisms isolated from stool cultures were given a single dose of oral cefixime. |
| Rackoff 1995 | Wrong population. Randomised, controlled trial of heparin‐vancomycin vs heparin flush solutions in paediatric patients with central venous catheters. Patients had cancer or were receiving parenteral nutrition for bowel disorders. |
CONS: coagulase‐negative Staphylococcus; CRBSI: catheter‐related bloodstream infection; RR: relative risk; CI: confidence interval; VLBW: very low birth weight
Characteristics of studies awaiting assessment [ordered by study ID]
Graham 2003.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes |
Contributions of authors
GDI wrote the protocol MWD revised the protocol LAJ wrote the review GDI and MWD revised the review
Sources of support
Internal sources
Grantley Stable Neonatal Unit, Royal Brisbane and Women's Hospital, Brisbane, Australia.
Dept of Paediatrics and Child Health, University of Queensland, Brisbane, Australia.
Perinatal Research Centre, The University of Queensland, Royal Brisbane and Women's Hospital, Brisbane, Australia.
External sources
No sources of support supplied
Declarations of interest
None
Edited (no change to conclusions)
References
References to studies included in this review
Cooke 1997 {published and unpublished data}
- Cooke RWI, Nycyk A, Okuonghuae H, Shah V, Damjanovic V, Hart CA. Low‐dose vancomycin prophlyaxis reduces coagulase‐negative staphylococcal bacteraemia in very low birth weight infants. Journal of Hospital Infection 1997;37(4):297‐303. [DOI] [PubMed] [Google Scholar]
Harms 1995 {published data only}
- Harms K, Herting E, Kron M, Schiffmann H, Schulz‐Ehlbeck H. Randomized, controlled trial of amoxicillin prophylaxis for prevention of catheter‐related infections in newborn infants with central venous silicone elastomer catheters. The Journal of Pediatrics 1995;127(4):615‐9. [DOI] [PubMed] [Google Scholar]
Spafford 1994 {published data only}
- Spafford PS, Sinkin RA, Cox C, Reubens L, Powell KR. Prevention of central venous catheter‐realted coagulase‐negative staphylococcal sepsis in neonates. The Journal of Pediatrics 1994;125(2):259‐63. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Baier 1998 {published data only}
- Baier RJ, Bocchini JA Jr, Brown EG. Selective use of vancomycin to prevent coagulase‐negative staphylyococcal nasocomial bacteremia in high risk very low birth weight infants. The Pediatric Infectious Disease Journal 1998;17(3):179‐83. [DOI] [PubMed] [Google Scholar]
Garland 2005 {published data only}
- Garland JS, Alex CP, Henrickson KJ, McAuliffe TL, Maki DG. A vancomycin‐heparin lock solution for the prevention of nosocomial bloodstream infection in critically ill neonates with peripherally inserted central venous catheters: a prospective, randomised trial. Pediatrics 2005;116:e198‐205. [DOI] [PubMed] [Google Scholar]
Kacica 1994 {published data only}
- Kacica MA, Horgan MJ, Ochoa L, Sandleer R, Lepw ML, Venezia RA. Prevention of gram‐positive sepsis in neonates weighing less than 1500 grams. The Journal of Pediatrics 1994;125(2):253‐8. [DOI] [PubMed] [Google Scholar]
Moller 1993 {published data only}
- Moller JC, Rossa M, Nachtrodt G, Richter A, Tegtmeyer FK. Preventive antibiotic administration for prevention of nosocomial septicemia in very small premature infants (VLBW infants) [Praventive antibiotikagabe zur verhinderung nosokomialer septikamien bei sehr kleinen frugeborenen (VLBW infants)]. Klinische Padiatrie 1993;205:140‐4. [DOI] [PubMed] [Google Scholar]
Rackoff 1995 {published data only}
- Rackoff WR, Weiman M, Jakobowski D, Hirschl R, Stallings V, Bilodeau J, Danz P, et al. A randomised, controlled trial of the efficacy of a heparin and vancomycin solution in preventing central venous catheter infections in children. The Journal of Pediatrics 1995;127(1):147‐51. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Graham 2003 {published data only}
- Graham A, Finer NN. Vancomycin flush technique to prevent coagulase negative staphylococcus infection in VLBW infants: a prospective placebo‐controlled randomized trial. Pediatric Academic Societies Abstract Archive 2000 ‐ 2006. 2003:2120.
Additional references
Abeywardana 2006
- Abeywardana, Samanthi. Report of the Australian and New Zealand Neonatal Network 2004. Sydney: ANZNN, 2006:54. [Google Scholar]
Adams‐Chapman 2002
- Adams‐Chapman I, Stoll BJ. Prevention of nosocomial infections in the neonatal intensive care unit. Current Opinion in Pediatrics 2002;14:157‐64. [DOI] [PubMed] [Google Scholar]
Apostolopoulou 2004
- Apostolopoulou E, Lambridou M, Lambadaridis I. Nosocomial bloodstream infections in a neonatal intensive care unit. British Journal of Nursing 2004;13:806‐12. [DOI] [PubMed] [Google Scholar]
Chien 2002
- Chien L, Macnab Y, Aziz K, Andrews W, McMillan DD, Lee SK, Canadian Neonatal Network. Variations in central venous catheter‐related infection risks among Canadian neonatal intensive care units. Pediatric Infectious Disease Journal 2002;21:505‐11. [DOI] [PubMed] [Google Scholar]
Cruccetti 2003
- Cruccetti A, Pierro A, Uronen H, Klein N. Surgical infants on total parenteral nutrition have impaired cytokine responses to microbial challenge. Journal of Pediatric Surgery 2003;38:138‐42. [DOI] [PubMed] [Google Scholar]
Dear 1999
- Dear P. Infection in the newborn. In: Rennie JM, Roberton NRC editor(s). Textbook of Neonatology. 3rd Edition. Edinburgh: Churchill Livingstone, 1999:1109‐1202. [Google Scholar]
Freij 1999
- Freij BJ, McCracken Jr GH. Acute infections. In: Avery GB, Fletcher MA, MacDonald MG editor(s). Neonatology: pathophysiology and management of the newborn. 5th Edition. Philadelphia: Lippincott Williams & Wilkins, 1999:1189‐1230. [Google Scholar]
Haas 2003
- Haas NA, Haas SA. Central venous catheter techniques in infants and children. Current Opinion in Anaesthesiology 2003;16:291‐303. [DOI] [PubMed] [Google Scholar]
Henrickson 2000
- Henrickson KJ, Axtell RA, Hoover SM, Kuhn SM, Pritchett J, Kehl SC, et al. Prevention of central venous catheter‐related infections and thrombotic events in immunocompromised children by the use of vancomycin/ciprofloxacin/heparin flush solution: A randomized, multicenter double‐blind trial. Journal of Clinical Oncology 2000;18:1269‐78. [DOI] [PubMed] [Google Scholar]
Hodge 2002
- Hodge D, Puntis JW. Diagnosis, prevention, and management of catheter related bloodstream infection during long term parenteral nutrition. Archives of Disease in Childhood. Fetal and neonatal edition 2002;87:F21‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Inglis 2004
- Inglis GDT, Davies MW. Prophylactic antibiotics to reduce morbidity and mortality in neonates with umbilical artery catheters. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD004697.pub3] [DOI] [PubMed] [Google Scholar]
Inglis 2005
- Inglis GDT, Davies MW. Prophylactic antibiotics to reduce morbidity and mortality in neonates with umbilical venous catheters. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD005251.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Isaacs 2000
- Isaacs D. Rationing antibiotic use in neonatal units. Archives of Disease in Childhood. Fetal and neonatal edition 2000;82:F1‐F2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Isaacs 2003
- Isaacs D, Australasian Study Group for Neonatal Infections. A ten year, multicentre study of coagulase negative staphylococcal infections in Australasian neonatal units. Archives of Disease in Childhood. Fetal and neonatal edition 2003;88:F89‐F93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liljedahl 2004
- Liljedahl M, Bodin L, Schollin J. Coagulase‐negative staphylococcal sepsis as a predictor of bronchopulmonary dysplasia. Acta Pædiatrica 2004;93:211‐5. [DOI] [PubMed] [Google Scholar]
McGuire 2004
- McGuire W, Clerihew L, Fowlie PW. Infection in the preterm infant. BMJ 2004;329:1277‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nagata 2002
- Nagata E, Brito ASJ, Matsuo T. Nosocomial infections in a neonatal intensive care unit: incidence and risk factors. American Journal of Infection Control 2002;30:26‐31. [DOI] [PubMed] [Google Scholar]
O'Grady 2002
- O'Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, et al. Guidelines for the prevention of intravascular catheter‐related infections. The Hospital Infection Control Practices Advisory Committee, Centers for Disease Control and Prevention. Pediatrics 2002;110:e51. [DOI] [PubMed] [Google Scholar]
Ogawa 1999
- Ogawa Y, Shimizu H, Takasaki J, Nakamura T. Strategy for the prevention and treatment of chronic lung disease of the premature infant. Pediatric Pulmonology Supplement 1999;18:212‐5. [DOI] [PubMed] [Google Scholar]
Shaul 1998
- Shaul DB, Scheer B, Rokhsar S, Jones VA, Chan LS, Boody BA, et al. Risk factors for early infection of central venous catheters in pediatric patients. Journal of the American College of Surgeons 1998;186:654‐8. [DOI] [PubMed] [Google Scholar]
Stoll 2002
- Stoll BJ, Hansen N, Fanaroff AA, Wright, LL, Carlo WA, Ehrenkranz JA, et al. Late‐onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 2002;110:285‐91. [DOI] [PubMed] [Google Scholar]
Stoll 2004
- Stoll BJ, Hansen NI, Adams‐Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. Neurodevelopmental and growth impairment among extremely low‐birth‐weight infants with neonatal infection. JAMA 2004;292:2357‐65. [DOI] [PubMed] [Google Scholar]
Van Marter 2002
- Marter LJ, Dammann O, Allred EN, Leviton A, Pagano M, Moore M, et al. Chorioamnionitis, mechanical ventilation, and postnatal sepsis as modulators of chronic lung disease in preterm infants. Journal of Pediatrics 2002;140:171‐6. [DOI] [PubMed] [Google Scholar]
Viudes 2002
- Viudes A, Pemán J, Cantón E, Úbeda P, López‐Ribot JL, Gobernado M. Candidemia at a tertiary‐care hospital: epidemiology, treatment, clinical outcome and risk factors for death. European Journal of Clinical Microbiology and Infectious Diseases 2002;21:767‐74. [DOI] [PubMed] [Google Scholar]


