Abstract
Preclinical studies suggest palbociclib may enhance radiation effects, with limited data on myelosuppression severity. We present real-world experience characterizing 247 patients with advanced breast cancer on palbociclib comparing radiation-exposed (n = 47) to unexposed (n = 200). We observed significantly lower absolute lymphocyte counts with radiation exposure. Hematological toxicities did not result in additional treatment interruption or dose reductions in exposed vs. unexposed patients.
Background:
Palbociclib is a cyclin-dependent kinase (CDK) 4/6 inhibitor with a primary toxicity of myelosuppression, especially neutropenia, due to cytostatic CDK6 inhibition on bone marrow. Preclinical studies suggest palbociclib may enhance radiation toxicity, but this was only evaluated in limited case series of palliative radiotherapy and not specific to radiation targeting bony metastases.
Patients and Methods:
This was a single institution retrospective cohort study. We included female patients who initiated palbociclib for advanced breast cancer between 2015 and 2019. The primary exposure was receipt of palliative radiation to bony metastases within 1 year prior to starting palbociclib. The primary outcome was the incidence and severity of myelosuppression during cycle one. Secondary outcomes include treatment interruptions and cycle 2 dose reductions, with subgroup analysis of radiation timing, type, dose, and location.
Results:
Of the 247 patients, 47 received radiation to bone metastases. Only absolute lymphocyte count (ALC) after cycle one of palbociclib was significantly lower in the group receiving radiation (median ALC 0.84 vs. 1.10 K/mm3, P < .001), with similar rates of neutropenia, anemia, and thrombocytopenia. Patients who received ≥ 10 fractions radiation were more likely to have cycle one interrupted than those receiving shorter radiation courses (42.9% vs. 11.1%, P = .03). No radiation characteristics were associated with other hematologic toxicities or dose reduction.
Conclusion:
Palliative bone radiation within 1 year prior to palbociclib initiation was associated with greater lymphopenia during the first cycle than patients unexposed to radiation, but not neutropenia, anemia, or thrombocytopenia that would modify treatment.
Keywords: CDK inhibitors, Hormone receptor-positive, Human epidermal growth factor receptor 2-negative, Metastatic breast cancer, Endocrine therapy
Introduction
Cyclin-dependent kinase (CDK) 4/6 inhibitors are highly selective and reversible inhibitors that act to block retinoblastoma phosphorylation, a cell cycle regulator that prevents premature division by binding to E2F transcription factors responsible for gene expression during S-phase.1 The targeted therapy class includes palbociclib (approved 2015), ribociclib (approved 2017), and abemaciclib (approved 2017).2–4 These agents are given concurrently with endocrine therapy in hormone receptor positive (HR+), human epidermal growth factor receptor 2-negative (Her2-) advanced breast cancer and have demonstrated significant improvements in progression-free survival and overall survival in first and second-line settings.5–16
Myelosuppression, especially neutropenia, is the most frequent class toxicity of CDK4/6 inhibitors. The underlying mechanism is attributed to a cytostatic effect in the bone marrow, as cell cycle arrest without apoptosis has been demonstrated in vitro.17 This is supported by low rates of febrile neutropenia ≤ 2.5% across all CDK4/6 inhibitor trials, as well as the median duration of grade 3 or 4 neutropenia being 7 days and resolving rapidly with drug hold.7–14 Toxicity is also dose-proportionate, with dose reduction effectively reducing future neutropenia and indicating an absence of cumulative toxicity.
The incidence of hematologic adverse events varies by degree of CDK6 targeting due to higher CDK6 expression in bone marrow and its key role regulating transcription underlying hematopoietic and leukemic stem cell activation.18 Thus, CDK4/6 inhibitors palbociclib and ribociclib have a higher risk of hematologic toxicity than abemaciclib which is 14-fold more selective for CDK4.19 Across the PALOMA-1, PALOMA-2, and PALOMA-3 clinical trials, all grade neutropenia occurred with palbociclib in 80% to 83% of patients; 55% to 56% of patients experienced grade 3 neutropenia and 10% to 11% developed grade 4 neutropenia.10,11,14 Because of these adverse effects, palbociclib is typically part of a 28-day treatment cycle being given daily for 21 days followed by a 7 day rest period.2 Dose reduction is recommended for any occurrence of grade 4 neutropenia (Absolute Neutrophil Count [ANC] < 500/mm3) and is recommended for grade 3 neutropenia (ANC 500–1000/mm3) only if occurring with concurrent febrile events or infection.2
Another potential hematologic factor for patients on CDK4/6 inhibitors is the use of radiotherapy for palliation of bone metastases. Hematopoietic stem cells in the bone marrow are among the most radiosensitive cells in the body and historic data suggests that radiation doses as low as 2 to 4 gray (Gy) delivered within 1 to 3 days can reduce bone marrow cellularity and proliferation.20,21 By arresting the cell cycle in G1 phase CDK4/6 inhibitors may sensitize cells to radiotherapy, as cells are more radiosensitive in G phase.22 PALOMA-2 and PALOMA-3 allowed palliative radiotherapy earlier than two weeks before randomization and during the trial with palbociclib interruption one day before and resumption one week after radiation.3,4 In a hematological safety analysis of PALOMA-2, 53% of patients experienced grade 3/4 neutropenia without a difference for prior radiation exposure (odds ratio 0.984, P = 0.936); however timing of radiation and sites of radiation were not evaluated.23
Given that palliative radiotherapy to bone is a common treatment used for symptom control in advanced breast cancer, the aim of this study was to evaluate the impact of prior palliative bone radiation exposure on the hematological effects of palbociclib. Further, this research evaluates the impact of radiation timing, anatomical site, and dose on myelosuppression, treatment interruptions, and dose reductions.
Patients and Methods
Study Design
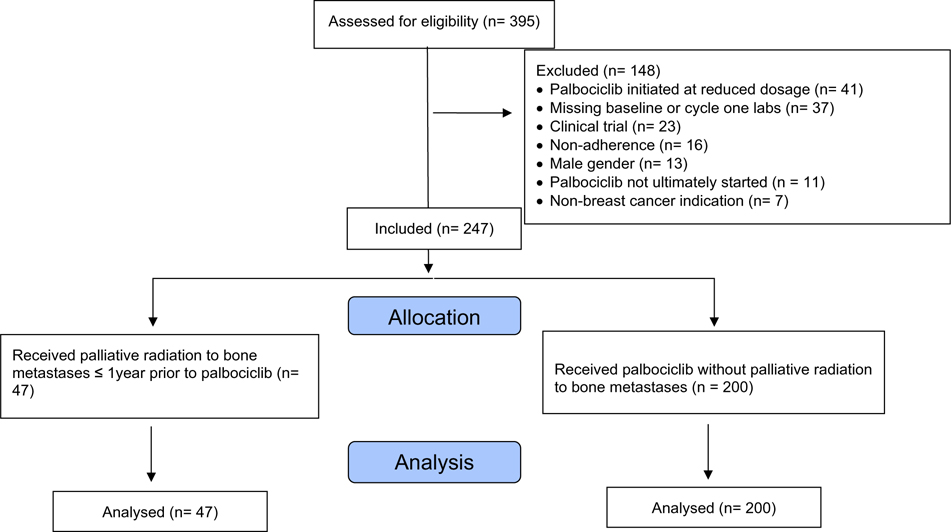
A retrospective cohort study was conducted at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, with patients identified via shared electronic health record Epic (Epic Systems Corporation; Verona, WI; Hyperspace version August 2019). Patients were included if they were female, followed by a Johns Hopkins Medicine provider as their longitudinal oncologist, and initiated palbociclib 125 mg for HR+, Her2- advanced breast cancer between February 2015 and November 2019. Exclusion criteria included prior CDK4/6 inhibitor exposure, bone marrow involvement, concurrent cytotoxic therapy, history of hematologic malignancy or autoimmune cytopenias, male gender, lack of baseline or cycle one lab results, dosing, or treatment interruption for reasons other than hematologic toxicity such as gastrointestinal toxicity, fatigue, or non-adherence (Fig. 1). The study was approved by the Johns Hopkins Medicine Institutional Review Board.
Figure 1.
Inclusion/exclusion diagram.
Data Collection
Demographics collected included age at palbociclib initiation, race, gender, menopausal status, Eastern Cooperative Oncology Group (ECOG) performance status, tumor histology, areas of metastasis, palbociclib line of therapy, prior chemotherapy, targeted therapy, or immunotherapy history, concurrent endocrine therapy, and concurrent systemic corticosteroids. Radiation therapy history was collected if radiation to bone metastases occurred within 1 year prior to palbociclib initiation or concurrently within cycle one1 of palbociclib. Days between radiation and palbociclib initiation (considered zero if concurrent), radiation modality, anatomical location, and dose in gray (Gy) per fractions were also evaluated. Absolute neutrophil count (ANC), absolute lymphocyte count (ALC), hemoglobin, and platelet count were collected at baseline prior to palbociclib initiation and throughout cycle one, graded according to the Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 by the lowest value.24 Treatment interruptions or cycle two dose reductions due to hematologic toxicity were also assessed.
Statistical Analysis
Descriptive statistics were used to characterize patient demographics and treatment history. All data was non-parametric. Chi-square or Fisher’s exact test were utilized to compare between categorical groups; Wilcoxon rank-sum test was performed for continuous data with two groups, and Kruskal-Wallis was used for continuous variables with more than two groups. Univariate logistic regression was used to determine predictors of hematologic toxicity, with any variables identified as statistically significant included in multiple logistic regression to control for multiple factors. An alpha level of < 0.05 was considered statistically significant for all tests, and statistical analysis was performed with Stata (StataCorp LLC; College Station, TX; version 15.1).
Results
Of the 247 patients who met criteria for inclusion, 47 had received radiation to bone metastases within a year prior to palbociclib initiation or concurrently (Table 1). Four patients received concurrent radiation therapy, which completed within the first half of their initial palbociclib cycle. Patients who had radiation were most commonly treated within 3 months of initiating palbociclib, received conventional radiation, had radiation of spinal or pelvic metastases, and received either doses of 20 Gy over 5 fractions or 30 Gy over 10 fractions (Table 2), with 22 (47%) having all of these characteristics. Patients who received intensity-modulated radiation therapy or stereotactic body radiation therapy were more likely to have had prior chemotherapy for metastatic disease than those who received conventional radiotherapy (55.6% vs. 15.8%, P = 0.02), but were less likely to have had 10 or more fractions in their radiation course (25.0% vs. 68.5%, P = 0.04). Patients who received radiotherapy were significantly less likely to have had prior chemotherapy delivered as neoadjuvant/adjuvant treatment (P = 0.044), but there was no significant difference in the line of metastatic therapy for which palbociclib was used.
Table 1.
Population Characteristics
| Without Bone Radiation (n = 200) | Bone Radiation (n = 47) | |
|---|---|---|
| Median age at palbociclib start, years (range) | 60 (27–90) | 56 (34–84) |
| Menopausal status, n (%) | ||
| Premenopausal | 41 (21) | 11 (23) |
| Postmenopausal | 159 (80) | 36 (77) |
| Race, n (%) | ||
| White | 130 (65) | 30 (64) |
| Black | 44 (22) | 12 (26) |
| Asian | 12 (6) | 3 (6) |
| Other | 14 (7) | 2 (4) |
| ECOG performance status, n (%) | ||
| 0 | 118 (59) | 19 (40) |
| 1 | 63 (32) | 21 (45) |
| ≥ 2 | 19 (10) | 7 (15) |
| Tumor histology, n (%) | ||
| Ductal carcinoma | 109 (55) | 34 (72) |
| Lobular carcinoma | 41 (21) | 6 (13) |
| Mixed ductal/lobular | 14 (7) | 4 (9) |
| Other/unknown | 36 (18) | 3 (6) |
| Metastatic disease sites, n (%) | ||
| Bone only | 90 (45) | 35 (74) |
| Visceral ± bone | 110 (55) | 12 (26) |
| Endocrine therapy ± ovarian suppression, n (%) | ||
| Aromatase inhibitor | 140 (70) | 27 (57) |
| Fulvestrant | 56 (28) | 20 (43) |
| Tamoxifen | 4 (2) | 0 |
| Metastatic therapy line, n (%) | ||
| 1 | 117 (59) | 20 (43) |
| 2 | 61 (31) | 19 (40) |
| ≥ 3 | 22 (11) | 8 (17) |
| Prior chemotherapy, n (%) | ||
| None | 95 (48) | 30 (64) |
| Neoadjuvant or adjuvant treatment only | 73 (37) | 6 (13) |
| Metastatic ± prior early stage treatment | 32 (16) | 11 (23) |
Abbreviations: ECOG = Eastern Cooperative Oncology Group.
Table 2.
Palliative Bone Radiation Characteristics
| Radiation Characteristics | Bone Radiation (n = 47) |
|---|---|
| Months between radiation completion and palbociclib initiation, n (%) | |
| < 1 | 16 (34) |
| 1 to < 3 | 17 (36) |
| 3 to < 6 | 5 (11) |
| 6 to < 12 | 9 (19) |
| Location of radiation, n (%) | |
| Spine alone | 21 (45) |
| Pelvis alone | 12 (26) |
| Spine and pelvis | 4 (9) |
| Femur | 4 (9) |
| Humerus | 3 (6) |
| Other axial bones | 3 (6) |
| Radiation type, n (%) | |
| Conventional radiation therapy | 38 (81) |
| Stereotactic body radiation therapy | 5 (11) |
| Intensity-modulated radiation therapy | 3 (6) |
| Unknown | 1 (2) |
| Total dose in gray per fractions, n (%) | |
| 20 Gy per 5 fractions | 11 (23) |
| ≤ 30 Gy per < 10 fractions | 7 (15) |
| 30 Gy per 10 fractions | 23 (49) |
| ≥ 30 Gy per > 10 fractions | 5 (11) |
| Unknown | 1 (2) |
Abbreviations: Gy = Gray.
Baseline ANC, ALC, and platelet count were significantly lower in those who received bone metastasis-directed radiation compared to patients without such radiation (Table 3). Following the first cycle of palbociclib, ANC, ALC, platelet count, and hemoglobin were all significantly lowered compared to baseline. However, ALC was the only parameter with a significant difference between the groups with and without radiation for both any-grade and severe toxicity. Univariable logistic regression found decreasing days between radiation and palbociclib initiation was associated with lymphopenia, as was having total radiation Gy ≥ 30 with fractions ≥ 10. However, when controlling for baseline ALC with multivariable logistic regression, only time between radiation and initiation was significant for development of lymphopenia (P = 0.04) and not radiation dose, type, or location. No differences between groups or association with radiation characteristics were identified with other hematologic toxicities.
Table 3.
Myelosuppression Adverse Effects
| Without Bone Radiation (n = 200) | Bone Radiation (n = 47) | P value | |
|---|---|---|---|
| Absolute neutrophil count, median (IQR) K/mm3 | |||
| Baseline prior to palbociclib | 3.61 (2.62–4.90) | 3.23 (2.59–3.74) | 0.049 |
| Lowest value during cycle 1 | 1.05 (0.78–1.44) | 0.98 (0.70–1.20) | 0.22 |
| Neutropenia grade, n (%) | |||
| None | 47 (24) | 7 (15) | 0.60 |
| 1 or 2 | 64 (32) | 16 (34) | |
| 3 | 77 (39) | 24 (43) | |
| 4 | 12 (6) | 4 (9) | |
| Absolute lymphocyte count, median (IQR) K/mm3 | |||
| Baseline prior to palbociclib | 1.56 (1.17–2.06) | 1.16 (0.80–1.61) | 0.001 |
| Lowest value during cycle 1 | 1.10 (0.81–1.49) | 0.84 (0.44–1.14) | < 0.001 |
| Lymphopenia grade, n (%) | |||
| None | 103 (52) | 13 (28) | < 0.001 |
| 1 or 2 | 85 (42) | 22 (47) | |
| 3 | 14 (7) | 9 (19) | |
| 4 | 0 (0) | 3 (6) | |
| Hemoglobin, median (IQR) K/mm3 | |||
| Baseline prior to palbociclib | 12.6 (11.5–13.2) | 12.1 (11.3–12.8) | 0.08 |
| Lowest value during cycle 1 | 11.5 (10.6–12.3) | 11.2 (10.4–12.1) | 0.16 |
| Anemia grade, n (%) | |||
| None | 75 (38) | 13 (28) | 0.55 |
| 1 or 2 | 118 (59) | 33 (70) | |
| 3 | 7 (4) | 1 (2) | |
| 4 | 0 (0) | 0 (0) | |
| Platelets, median (IQR) K/mm3 | |||
| Baseline prior to palbociclib | 256 (216–312) | 237 (168–272) | 0.02 |
| Lowest value during cycle 1 | 173 (133–221) | 169 (138–216) | 0.76 |
| Thrombocytopenia grade, n (%) | |||
| None | 124 (62) | 30 (64) | 0.43 |
| 1 or 2 | 75 (38) | 16 (34) | |
| 3 | 1 (1) | 1 (2) | |
| 4 | 0 (0) | 0 (0) | |
| Cycle 1 treatment interruption, n (%) | 68 (34) | 14 (30) | 0.58 |
| Cycle 2 dose reduction, n (%) | 64 (32) | 13 (28) | 0.56 |
Abbreviations: IQR = interquartile range.
Palbociclib treatment interruption was similar between the groups with and without bone radiation. Radiation patients who received at least 10 fractions were more likely to have cycle one interrupted than those with shorter radiation courses (42.9% vs. 11.1%, P = 0.03). However, dose reduction due to hematologic toxicity was similar across all groups and radiation characteristic subgroups. No patients with concurrent radiation and palbociclib had radiotherapy interrupted or suspended.
Discussion
To our knowledge, these data represent the largest cohort study of hematologic interactions between prior palliative radiation and palbociclib and includes comparison to a cohort without radiation treatment. In contrast to prior reports (Table 4), our study is specific to radiation to bone metastases, which may have been particularly impactful on hematological toxicity given the cytostatic mechanism of CDK4/6 inhibitors on bone marrow proliferation.
Table 4.
Review of Studies
| Study | Patients, n | CDK 4/6 Inhibitor(s) | Radiation | Radiation Sites | Radiation Technique | Toxicity |
|---|---|---|---|---|---|---|
| Kawamoto et al | 1 | Palbociclib | Concurrent | Bone (1) | Conformal | • Colitis: Grade 3 (1) • Onset: 3 days after completion of conformal RT to left iliac bone and sacrum (30 Gy in 10 fractions) • Descending colon wall thickening on CT and erosion and angiectasis on colonoscopy • Time to Resolution: 3 weeks |
| Dasgupta et al | 1 | Palbociclib | Concurrent | Bone (1) | Conventional | • Colitis: Grade 3 (1) • Onset: 5 days after completion of conventional RT to left pelvis and proximal femur (30 Gy in 10 fractions) • Generalized thickening of colon predominantly near rectum, sigmoid and descending colon on CT and fragile mucosa on colonoscopy with inflammatory changes on biopsy • Given mesalamine 10 mg daily for 1 week, 2.5 mg daily for 1 week • Time to Resolution: 3 weeks |
| Messer et al | 1 | Ribociclib | Concurrent | Locoregional (1) | Conformal | • Esophagitis: Grade 3 (1) • Dermatitis: Grade 3(1) • Onset: During RT with conformal RT to left neck (60 Gy in 20 fractions) • Given viscous lidocaine and pain medications for esophagitis. Given silver sulfadiazine and petrolatum ointment for dermatitis. • Time to Resolution: 1 month |
| Kalash et al | 3 | Palbociclib | Concurrent | Lung (2) Locoregional (1) |
NA | • Pulmonary Fibrosis (3) • Steroid-refractory, atypical radiation pneumonitis (2) • Symptoms improved with palbociclib discontinuation |
| Hans et al | 5 | Palbociclib | Concurrent | Bone (4) Visceral (1) |
EBRT (4) Liver radiosurgery (1) |
• Neutropenia: Grade 3 (2) • Anemia: Grade 3 (1) • Thrombocytopenia: Grade 3 (2) • Mucositis: Grade 2 (1), Grade 1 (1) |
| Meattini et al | 5 | Ribociclib | Concurrent | Bone (5) Visceral (3) |
VMAT (1) 3DCRT (4) |
• Neutropenia: Grade 3–4 (1) • Diarrhea: Grade 3–4 (1) • Vomiting: Grade 3–4 (1) |
| Figura et al | 15 | Palbociclib (10) Abemaciclib (5) |
Concurrent (14) Sequential (28) |
Brain (42) | SRS (26) FSRT (16) |
• Radiation necrosis (2) • Attributed to re-irradiation of lesions • Managed with steroids and bevacizumab |
| Ippolito et al | 16 | Palbociclib (13) Ribociclib (3) |
Concurrent | Bone (22) Locoregional (2) |
3DCRT (19) VMAT (3) IMRT (2) |
• Neutropenia: Grade 4 (1), Grade 3 (4), Grade 2 (2) • Thrombocytopenia: Grade 1 (2) • Anemia: Grade 1 (3) • Dermatitis: ≥ Grade 2 (1), Grade 1 (1) • Fatigue: Grade 1 (1) • Radiation interruption (2) • Palbociclib interruption (2) • Palbociclib dose reduction (1) |
| Chowdhary et al | 16 | Palbociclib | Concurrent (5) Sequential (11) |
Bone (11) Brain (4) Visceral (1) |
3DCRT (15) SBRT (2) IMRT (2) WBRT (3) fSRS (1) |
• Leukopenia: Grade 2 (1), Grade 1 (3) • Neutropenia: Grade 2 (1) • Thrombocytopenia: Grade 1 (1) • Fatigue: Grade 1 (5) • Dermatitis: Grade 1 (3) • Nausea: Grade 1 (1) |
| Stauder et al | 18 | Palbociclib | Concurrent (15) Sequential (3) |
Bone (10) Brain (6) Soft Tissue (2) |
NA | • Increased mucosal toxicity • No ≥ Grade 2 AE attributable to RT |
| Beddok et al | 30 | Palbociclib | Concurrent | Locoregional (9) Bone (24) Brain (2) Choroid (1) |
IMRT (10) 3DCRT (24) SBRT (1) |
• Dermatitis: Grade 3 (1), Grade 2 (1), Grade 1 (10) • Neutropenia: Grade 3 (2), Grade 2 (7), Grade 1 (3) • Dysphagia: Grade 2 (1), Grade 1 (2) • Pain: Grade 3 (1), Grade 1 (7) • Colitis: Grade 1 (1) • Radiation interruption (2) • Palbociclib interruption (3) |
| Ratosa et al | 46 | Palbociclib (30) Ribociclib (15) Abemaciclib (1) |
Concurrent (16) Sequential (30) |
Locoregional (2) Bone (5) Visceral (7) Brain (3) |
2DRT (11) 3DCRT (41) IMRT/VMAT (1) Helical (2) SBRT/CK (7) |
• ≥ Grade 3 AEs were observed in 6.5%, 15.2%, and 23.9% before RT, 2 weeks after RT, and 6 weeks after radiation with increases noted in neutropenia and leukopenia |
| Meattini et al | 85 | Palbociclib (63) Ribociclib (22) |
Concurrent (14) Sequential (11) vs. No-RT (60) |
NA | NA | RT vs No-RT (P value) • CDK4/6i Dose reduction (1.0) • CDK4/6i Discontinuation (1.0) • ≥ Grade 2 Neutropenia (0.057) • Any Grade AE (1.0) • ≥ Grade 2 AE (0.096) |
| Our study | 247 | Palbociclib (247) | Sequential (44) Concurrent (3) vs. No-RT (200) |
Bone (47) | Conventional (38) SBRT (5) IMRT (3) Unknown (1) |
RT vs. No-RT (P value) • Grade 3 Neutropenia (0.60) • Thrombocytopenia (0.43) • Palbociclib interruption (0.58) • Palbociclib dose reduction (0.56) |
Abbreviations: 3DCRT = 3-dimensional conformational radiation therapy; AE = adverse effects; CDK4/6i = cyclin-dependent kinase 4/6 inhibitor; IMRT = intensity modulated radiation therapy; NA = not available; No-RT = no radiation; RT = radiation; SRS = stereotactic radiosurgery; VMAT = volumetric modulated arc therapy; WBRT= whole brain radiation.
Study design of initiation after radiation was decided for practicality of data collection and interpretation of consistent palbociclib exposure. As palbociclib arrests the cell cycle in G1 phase and sensitizes cells to radiotherapy, initiation timing prior to or concurrently with radiation may have greater toxicity than palbociclib following radiation as studied. The subgroup receiving radiation concurrently was too small to address whether specific timing within the palbociclib administration cycle may have a differential effect. Meattini et al examined 85 patients on CDK4/6 inhibitors with 14 given concurrently with radiation and another 11 given sequentially following radiation compared to 60 without prior radiation.25 They reported that no difference was seen in palbociclib dose reduction, palbociclib discontinuation, and any grade adverse effect including neutropenia in the radiation exposed vs. unexposed cohorts. Our findings are similar except for observing a significant decrease in ALC post-radiation prior to palbociclib initiation that persisted in the radiation exposed cohort compared to the unexposed. This is attributed to the lymphocyte lineage generally having a longer nadir period following radiation before recovery compared to cells with faster turnover such as granulocytes and platelets.26,27 The majority of patients receiving radiation were within 3 months of palbociclib initiation and may reflect a transient overlap in post-radiation nadir and palbociclib initiation that is not due to sensitization. However, radiation-induced lymphopenia can persist for years in some cases and is suspected to involve loss of compensatory proliferation, with potential for hematologic interactions with palbociclib outside the most frequent 1- to 2-month timeframe of lymphocyte recovery.28 These results only reflect palbociclib initiation and cannot address whether the ALC difference remains significant in future cycles. Radiation-induced lymphopenia has also been implicated as a poor prognostic factor for disease free survival, but the impact of lymphopenia from CDK4/6 inhibitors is currently unknown. There is no recommended monitoring or management for lymphopenia with palbociclib, and our results do not suggest a need to delay palbociclib initiation following palliative bone radiation.
Ratosa et al reported grade ≥ 3 adverse effects with CDK4/6 inhibitors were observed in 6.5% of patients two weeks before radiotherapy, 15.2% of patients two weeks after radiotherapy, and 23.9% of patients six weeks after radiation, with increases noted in neutropenia and leukopenia.29 However, the authors attributed the increase in toxicity to CDK4/6 inhibitors instead of radiation as patients were early in therapy with a median 3 cycles at the start of radiation. The findings of our study support that the neutrophil decrease observed after initiation is characteristic of the CDK4/6 inhibitors and not radiation, as a significant decrease in ANC and platelets post-radiation compared to patients who did not receive radiation was not persistent after palbociclib initiation. Chowdhary et al reported no toxicity difference between palbociclib timing in relation to pre-, post-, or concurrent radiation.30 A comparable series of 16 patients with palliative radiotherapy and concurrent palbociclib by Ippolito et al found prior severe neutropenia was not associated with worse neutropenia during radiation.22 Together, this also supports that delaying palbociclib initiation following palliative radiotherapy to bone metastases is not necessary even with prior hematologic toxicity.
The distribution of bone marrow by anatomic bony site was characterized by Hayman and colleagues, with highest areas including the thoracic spine (19.9%), lumbar spine (16.6%), sacrum (9.2%), and pelvis (25.3%).31 Despite high rates of radiation to these areas, we did not find differences based on anatomical site where radiation was administered. There was also no difference observed among those with multiple sites of radiotherapy to bone, though assessment was limited by low patient volume. Similarly, a case series of 16 patients by Chowdhary et al examining initiation of palliative radiation in close proximity to palbociclib reported no difference in toxicity based on axial, pelvic, or other irradiation site.30 However, it did not include patients with multiple radiotherapy sites or describe radiation field size. Our study did not assess soft tissue radiation exposure, but lymphocytes are also sensitive to radiation in both the blood and lymphatic tissue.26 Radiotherapy targeting soft tissue or even bone with larger field sizes could damage circulating lymphocytes or contribute to other toxicities, and cannot be assumed to have similar tolerability when close to palbociclib initiation.
The retrospective nature of the study is a limitation, as it is possible there were unmeasured differences between patients who received palliative bone radiation and those without prior radiotherapy such as relatively more advanced disease. While known bone marrow involvement was an exclusion criterion, patients receiving palliative radiation may have had greater disease burden in the bones and bone marrow either unknown or undocumented. Pretreatment is another factor which was unbalanced between the groups, as the proportion of patients with only one line of metastatic therapy was less in the radiotherapy group than those without radiation. However, this was not a statistically significant difference. We did not review history for benign hematologic conditions that could affect blood counts, and details of radiation field size were not available to better quantify the amount of irradiated marrow. Moreover, treatment decisions may have reflected physician preference and have been applied inconsistently. This could have contributed to the greater frequency of cycle one interruption for patients receiving ≥ 10 fractions of radiotherapy, despite no association with greater toxicity. It may also explain the higher rates of cycle two dose reduction for hematologic toxicity observed in our cohort compared to the historical rates in the pooled PALOMA analysis, despite having similar rates of cycle one treatment interruption due to adverse effects.23
An additional limitation is that the study did not extend beyond cycle two, as cumulative or latent toxicity was not commonly observed in the PALOMA trials and would be complicated by cycle two dose reduction.14 While limiting review to the first cycle may be too narrow to detect differences in additive radiation and palbociclib effect, the high rates of overall hematologic toxicity do not suggest a lack of response to palbociclib. The ongoing phase 2 ASPIRE trial (NCT03691493) of palbociclib plus radiation for bone metastases and phase 2 CLEAR trial (NCT03750396) of endocrine therapy plus local therapy for oligometastatic breast cancer will provide prospective data for safety and efficacy of CDK4/6 inhibitors.32,33
Conclusion
Prior palliative radiation to bony metastases within 1 year prior to palbociclib initiation was not associated with increased rates of neutropenia, anemia, or thrombocytopenia during the first cycle compared to patients without radiation. While any-grade and severe lymphopenia were significantly more likely in patients who received radiation, it did not require a modified treatment approach and both cycle one treatment interruption and dose reduction for cycle two were similar between those with and without palliative radiation. These findings are consistent with prior studies that have demonstrated tolerability without additive toxicity, and do not suggest the need for a delay between delivery of palliative bone radiotherapy and initiation of palbociclib.
Clinical Practice Points.
Case series suggest concurrent CDK4/6 inhibitor and radiotherapy are generally well tolerated without an increase in hematologic toxicity.
Our study of sequential palbociclib following radiotherapy similarly found no increase in rates of neutropenia, anemia, and thrombocytopenia in the radiation cohort but did observe increased rates of lymphopenia. While the impact of lymphopenia is an ongoing subject of research and concern, particularly regarding viral infection risk and disease prognosis, it has no current role in toxicity monitoring for palbociclib or other CDK 4/6 inhibitors.
Radiation patients with ≥ 10 fractions were more likely to have cycle one interrupted than with shorter radiation courses. Radiation characteristics such as time between completion of radiation and initiation of palbociclib, radiation dose, or location of radiation were not associated with other hematologic toxicities or cycle two dose reduction.
These results support the safety of initiating palbociclib in short interval after delivery of bone radiotherapy.
Acknowledgments
We thank Stefanie Houseknecht, PharmD, BCOP, and Michael Goldenhorn, PharmD, MBA for their technical assistance. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declare the following financial interests/personal relationships: KTL: Received research grants to institution from Pfizer Inc. and has collaborated with Eli Lilly and Company in the role of an unpaid consultant in the past 12 months. VS: Received research grants to institution from Abbvie, Biocept, Pfizer Inc., Novartis, and Puma Biotechnology. Member, Data Safety Monitoring Board, Immunomedics, Inc. SRA: Received research grant from Radiation Oncology Institute.
References
- 1.Spring LM, Zangardi ML, Moy B, Bardia A. Clinical management of potential toxicities and drug interactions related to cyclin-dependent kinase 4/6 inhibitors in breast cancer: practical considerations and recommendations. Oncologist. 2017;22:1039–1048. doi: 10.1634/theoncologist.2017-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ibrance (palbociclib) Tablets [Prescribing Information]. New York, NY: Pfizer Inc; 2019. [Google Scholar]
- 3.Kisqali (ribociclib) Tablets [Prescribing Information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2018. [Google Scholar]
- 4.Verzenio (abemaciclib) Tablets [Prescribing Information]. Indianapolis, IN: Eli Lilly and Company; 2019. [Google Scholar]
- 5.Sledge GW Jr, Toi M, Neven P, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol. 2019;6:116–124. doi: 10.1001/jamaoncol.2019.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeMichele A, Cristofanilli M, Brufsky A, et al. Abstract P1–19-02: Overall survival for first-line palbociclib plus letrozole vs letrozole alone for HR+/HER2- metastatic breast cancer patients in US real-world clinical practice. Cancer Res. 2020;80(suppl 4) P1-19-02 LP-P1-19-02. doi: 10.1158/1538-7445.SABCS19-P1-19-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36:2465–2472. doi: 10.1200/JCO. [DOI] [PubMed] [Google Scholar]
- 8.Tripathy D, Im SA, Colleoni M, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018;19:904–915. doi: 10.1016/S1470-2045(18)30292-4. [DOI] [PubMed] [Google Scholar]
- 9.Sledge GW, Toi M, Neven P, et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35:2875–2884. doi: 10.1200/JCO. [DOI] [PubMed] [Google Scholar]
- 10.Verma S, Bartlett CH, Schnell P, et al. Palbociclib in combination with fulvestrant in women with hormone receptor-positive/HER2-negative advanced metastatic breast cancer: detailed safety analysis from a multicenter, randomized, placebo-controlled, phase III study (PALOMA-3). Oncologist. 2016;21:1165–1175. doi: 10.1634/theoncologist.2016-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 12.Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, n patients with refractory HR+/HER2- metastatic breast cancer. Clin Cancer Res. 2017;23:5218–5224. doi: 10.1158/1078-0432.CCR-17-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hortobagyi GN, Stemmer SM, Burris HA, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018;29:1541–1547. doi: 10.1093/annonc/mdy155. [DOI] [PubMed] [Google Scholar]
- 14.Finn RS, Crown JP, Ettl J, et al. Efficacy and safety of palbociclib in combination with letrozole as first-line treatment of ER-positive, HER2-negative, advanced breast cancer: Expanded analyses of subgroups from the randomized pivotal trial PALOMA-1/TRIO-18. Breast Cancer Res. 2016;18. doi: 10.1186/s13058-016-0721-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slamon DJ, Neven P, Chia S, et al. Overall survival with ribociclib plus fulvestrant in advanced breast Cncer. N Engl J Med. 2019;382:514–524. doi: 10.1056/NEJMoa1911149. [DOI] [PubMed] [Google Scholar]
- 16.Hurvitz SA, Im S-A, Lu Y-S, et al. Phase III MONALEESA-7 trial of premenopausal patients with HR+/HER2- advanced breast cancer (ABC) treated with endocrine therapy ± ribociclib: Overall survival (OS) results. J Clin Oncol. 2019;37(suppl 18) LBA1008–LBA1008. [Google Scholar]
- 17.Malumbres M, Sotillo R, Santamaría D, et al. Mammalian cells cycle without the D-type cyclin-dependent kinases CDK4 and CDK6. Cell. 2004;118:493–504. doi: 10.1016/j.cell.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Scheicher R, Hoelbl-Kovacic A, Bellutti F, et al. CDK6 as a key regulator of hematopoietic and leukemic stem cell activation. Blood. 2015;125:90–101. doi: 10.1182/blood-2014-06-584417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torres-Guzmán R, Calsina B, Hermoso A, et al. Preclinical characterization of abemaciclib in hormone receptor positive breast cancer. Oncotarget. 2017;8:69493–69507. doi: 10.18632/oncotarget.17778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sykes MP, Chu FC, Savel H, Bonadonna G, Mathis H. The effects of varying dosages of irradiation upon sternal-marrow regeneration. Radiology. 1964;83:1084–1088. doi: 10.1148/83.6.1084. [DOI] [PubMed] [Google Scholar]
- 21.Sykes MP, Savel H, Chu FCH, Bonadonna G, Farrow J, Mathis H. Long-term effects of therapeutic irradiation upon bone marrow. Cancer. 1964;17:1144–1148. doi:. [DOI] [PubMed] [Google Scholar]
- 22.Ippolito E, Greco C, Silipigni S, et al. Concurrent radiotherapy with palbociclib or ribociclib for metastatic breast cancer patients: preliminary assessment of toxicity. Breast. 2019;46:70–74.doi: 10.1016/j.breast.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Diéras V, Rugo HS, Schnell P, et al. Long-term pooled safety analysis of palbociclib in combination with endocrine therapy for HR1/HER2- advanced breast cancer. J Natl Cancer Inst. 2019;111. doi: 10.1093/jnci/djy109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Common Terminology Criteria for Adverse Events (CTCAE) v5.0; 2017. 1–147. Available at: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. Accessed: August 22, 2019. Published November 27, 2017.
- 25.Meattini I, Scoccimarro E, Saieva C, et al. Impact of metastases directed radiation therapy on CDK4/6 inhibitors dose reduction and treatment discontinuation for metastatic HR+/HER2- breast cancer (MBC). J Clin Oncol. 2020;38:562. doi: 10.1200/JCO.2020.38.15_suppl.562. [DOI] [Google Scholar]
- 26.Fliedner TM, Friesecke I, Beyrer K. Medical Management of Radiation Accidents: Manual on the Acute Radiation Syndrome. Plymouth, UK: Latimer Trend & Company Ltd; 2001. [Google Scholar]
- 27.Sini C, Fiorino C, Perna L, et al. Dose-volume effects for pelvic bone marrow in predicting hematological toxicity in prostate cancer radiotherapy with pelvic node irradiation. Radiother Oncol. 2016;118:79–84. doi: 10.1016/j.radonc.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 28.Sun GY, Wang SL, Song YW, et al. Radiation-induced lymphopenia predicts poorer prognosis in patients with breast cancer: a post hoc analysis of a randomized controlled trial of postmastectomy hypofractionated radiation therapy. Int J Radiat Oncol Biol Phys. 2020;108:277–285. doi: 10.1016/j.ijrobp.2020.02.633. [DOI] [PubMed] [Google Scholar]
- 29.Ratosa I, Orazem M, Scoccimarro E, et al. Cyclin-dependent kinase 4/6 inhibitors combined with radiotherapy for patients with metastatic breast cancer. Clin Breast Cancer. 2020;20:495–502. doi: 10.1016/j.clbc.2020.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Chowdhary M, Sen N, Chowdhary A, et al. Safety and efficacy of palbociclib and radiation therapy in patients with metastatic breast cancer: initial results of a novel combination. Adv Radiat Oncol. 2019;4:453–457. doi: 10.1016/j.adro.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayman JA, Callahan JW, Herschtal A, et al. Distribution of proliferating bone marrow in adult cancer patients determined using FLT-PET imaging. Int J Radiat Oncol Biol Phys. 2011;79:847–852. doi: 10.1016/j.ijrobp.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 32.Torres M. Radiation therapy, palbociclib, and hormone therapy in treating breast cancer patients with bone metastasis (ASPIRE). Available at: https://clinicaltrials.gov/ct2/show/NCT03691493?term=nct03691493&draw=1&rank=1. Accessed: March 8, 2021.
- 33.Jeong J. Local treatment in ER-positive/HER2-negative oligo-metastatic breast cancer (CLEAR). Available at: https://clinicaltrials.gov/ct2/show/NCT03750396?cond=nct03750396&draw=2&rank=1. Accessed: December 8, 2021.