Abstract
Objectives:
People living with HIV (PLWH) experience an increased burden of coronary artery disease (CAD) believed to be related, in part, to an interplay of chronically increased inflammation and traditional risk factors. Recent trials suggest cardiovascular benefits of the anti-inflammatory, colchicine, in HIV-seronegative CAD patients. However, the impact of colchicine on impaired vascular health, as measured by coronary endothelial function (CEF), an independent contributor to CAD, has not been studied in PLWH. We tested the hypothesis that colchicine improves vascular health in PLWH.
Design:
This was a randomized, placebo-controlled, double-blinded trial in 81 PLWH to test whether low-dose colchicine (0.6mg daily) improves CEF over eight- to twenty four-weeks.
Methods:
Coronary and systemic endothelial function and serum inflammatory markers were measured at baseline, and at 8 and 24 weeks. The primary endpoint was CEF, measured as the change in coronary blood flow from rest to that during isometric handgrip exercise, an endothelial-dependent stressor, measured with noninvasive MRI at 8 weeks.
Results:
Colchicine was well tolerated and not associated with increased adverse events. However, there were no significant improvements in coronary or systemic endothelial function or reductions in serum inflammatory markers at 8 or 24 weeks with colchicine as compared to placebo.
Conclusions:
In PLWH with no history of CAD, low-dose colchicine was well tolerated but did not improve impaired coronary endothelial function, a predictor of cardiovascular events. These findings suggest that an anti-inflammatory approach using colchicine in PLWH does not improve vascular health, the central, early driver of coronary atherosclerosis.
Keywords: coronary endothelial function, inflammation, HIV, coronary artery disease, clinical trial
Introduction:
Although survival in people living with HIV (PLWH) has significantly improved with the use of antiretroviral therapy (ART), they now experience an increasing burden of chronic diseases, including coronary artery disease (CAD) [1]. The increased CAD risk in PLWH may be due to the impact of chronic inflammation, a critical driver of atherosclerosis which is increased in PLWH [2]. There is recent interest in evaluating anti-inflammatory strategies to limit the adverse pathophysiologic consequences of inflammation which promote coronary atherosclerosis and its complications in HIV seronegative populations [3]. Coronary endothelial function (CEF) is impaired early in the atherosclerotic process, and this drives CAD, is an independent predictor of future cardiovascular events, and improves with protective interventions [4, 5]. However, randomized placebo-controlled trials examining the role of inflammation in the pathogenesis of atherosclerosis in PLWH on contemporary ART are scarce [6] but have direct clinical implications for the prevention and management of cardiovascular disease in PLWH.
Several factors limited the evaluation of the importance of systemic and local inflammation in the pathogenesis of CAD in PLWH. First, some anti-inflammatory strategies suppress immune function and are a potential safety concern for PLWH. Second, although current imaging methods document the presence of anatomic CAD that developed over years, a noninvasive methodology to identify and quantify coronary endothelial dysfunction, a central, early mechanism contributing to the pathogenesis of CAD, was not available. It is now possible to overcome those hurdles and test the impact of an anti-inflammatory intervention in HIV-associated CAD.
Colchicine is an anti-inflammatory agent approved by the FDA more than 50 years ago and is now used to treat gout and recurrent pericarditis. Colchicine reduces inflammatory biomarkers in HIV-seronegative individuals [7] and low dose colchicine (LDC) was recently reported to reduce cardiovascular events by more than 65% (p<0.001) in stable CAD patients [8]. Thus, LDC offers an untested but clinically available, relatively inexpensive, anti-inflammatory medication to probe the impact of inflammation on CAD pathogenesis in PLWH.
Inflammation enhances the development and progression of coronary atherosclerosis via several mechanisms, but endothelial dysfunction is believed to be one common result of these mechanisms. Abnormal coronary endothelial function (CEF) plays a pivotal role in the development, progression, and clinical manifestations of CAD and endothelial dysfunction is an independent predictor of cardiovascular events and improves with successful medical interventions [4, 9]. Coronary endothelial-dependent function was previously assessed only invasively in the catheterization laboratory by changes in coronary arterial diameter and flow in response to endothelial-dependent vasomotor interventions. We developed and validated the first noninvasive MRI method to study CEF [10] and this approach allows safe, reproducible CEF studies in low risk, stable populations and in the same individuals over time. In addition, the vasoreactive responses measured by MRI-CEF are primarily nitric oxide-mediated [11].
To test the hypothesis that an anti-inflammatory strategy improves vascular health in PLWH, we conducted a randomized, placebo-controlled, double-blind, NIH-sponsored trial exploiting non-invasive CEF measures to determine whether colchicine improves impaired CEF over 8 and 24 weeks in PLWH with no clinical CAD.
Methods:
This randomized, placebo-controlled, double-blinded clinical trial was approved by the Johns Hopkins Medicine Institutional Review Board and complies with the Declaration of Helsinki. All study participants provided written informed consent. HIV-seropositive people on stable ART with no clinical CAD were recruited from the outpatient clinics at Johns Hopkins Medicine and at University of Maryland (Table 1). Potential participants underwent screening MRI to measure CEF and those with abnormal CEF (defined as a change in coronary blood flow (CBF) during isometric handgrip exercise (IHE) of ≤7ml/min from the resting value in at least one coronary segment) [10] underwent additional screening measures described in detail in Supplement. After completing all screening procedures, qualifying subjects were randomly assigned 1:1 by the Johns Hopkins Investigational Pharmacy to either LDC: colchicine (0.6mg daily) or placebo orally once daily. The investigators and study participants were blinded to the study drug assignment. Enrollment began January 11th, 2016 and the trial ended May 1st, 2019. Additional details appear in the Supplement and the trial was registered at www.clinicaltrials.gov (NCT02624180).
Table 1: Demographics and clinical characteristics of study participants.
There were no significant differences in the clinical and demographic variables between groups.
| Baseline Characteristics of the Trial Participants | ||
|---|---|---|
| Characteristic | Colchicine (n=43) | Placebo (n=38) |
| Median age (IQR)-yr | 54.66 (45.0–60.7) | 52.0 (45.2–57.4) |
| Female sex-no. (%) | 9 (20.9) | 8 (21.1) |
| White, non-Hispanic-no. (%) | 8 (18.6) | 11 (29.0) |
| Black-no. (%) | 33 (76.7) | 26 (68.4) |
| Hispanic or other nonwhite race-no. (%) | 2 (4.7) | 1 (2.6) |
| Current smoker-no. (%) | 14 (33.3) | 13 (34.2) |
| Alcohol use-no. (%) | 26 (61.9) | 25 (67.6) |
| Median body-mass index (IQR) | 28.2 (24.9–31.7) | 27.3 (24.8–29.4) |
| Hypertension-no. (%) | 20 (47.6) | 15 (39.5) |
| Diabetes-no. (%) | 4 (9.5) | 1 (2.6) |
| Median LDL Cholesterol (IQR)-mg/dL | 98.5 (81.8–120.5) | 105 (81.5–127.0) |
| Median Triglycerides (IQR)-mg/dL | 100 (76.5–148.5) | 95 (83.8–134.8) |
| Median C-reactive protein level (IQR)- mg/liter | 1.7 (0.8–4.7) | 1.3 (0.9–2.7) |
| GFR ≥60-no. (%) | 42 (97.7) | 35 (92.1) |
| HIV Viral load log <1.30. (%) | 28 (66.7) | 21 (56.8) |
| HIV quant log value ≤ 20 copies/ml. (%) | 28 (66.7) | 22 (59.5) |
| Use of ACE inhibitor or ARB-no. (%) | 4 (9.3%) | 2 (5.3%) |
| Use of statin-no. (%) | 19 (44.2%) | 12 (31.6%) |
| Use of beta-blocker-no. (%) | 13 (30.2%) | 7 (18.4%) |
| Use of antiplatelet or antithrombotic agent-no. (%) | 0 (0%) | 0 (0%) |
Study Population:
Participants were clinically stable on ART and were receiving guideline–recommended medical therapy. The inclusion criteria were: a.) patients of either gender who were ≥21 years of age (no upper age limit), b.) HIV-seropositive and taking stable ART (no change in ART regimen in the prior 3 months), c.) undetectable HIV viral load (plasma HIV RNA concentration <100 copies/mL), d.) abnormal CEF at baseline (≤7ml/min change in CBF from rest to that during IHE). Exclusion criteria included history of clinical CAD, acute coronary syndrome, myocardial infarction or revascularization, estimated glomerular filtration rate <45ml/min, moderate-severe hepatic disease, CD4 cell count<200/mm3, women of childbearing potential, and taking protease inhibitors, cobicistat or CYP3A4 inhibitors. Other exclusion criteria are detailed in the online Supplement.
Study procedures:
Initial baseline evaluation consisted of history, physical exam, and blood draw. Patients underwent MRI for CEF measures and brachial ultrasound for flow mediated dilatation (FMD) at baseline, prior to study drug administration, and again after 8 and 24 weeks of study-drug administration. Study drug compliance was assessed by questionnaire and pill count at the 8, 16 and 24-week follow-up visits.
MRI methods for coronary endothelial function (CEF):
Patients underwent MRI studies of CEF in the fasting state at baseline, and at 8- and 24-weeks using MRI methodology at rest and during continuous IHE as previously described [10]. In particular, coronary MRI was repeated at 8 and 24 weeks with a protocol identical to that at baseline with special attention taken to evaluate the same coronary segments in follow-up visits as those studied at baseline, using anatomic coronary landmarks as in prior studies [12]. Detailed MRI parameters were previously published [10], and further details are available in the Supplement. Images were analyzed blinded to study-drug assignment and clinical information. CEF was measured by change in cross-sectional area (CSA), coronary flow velocity (CFV), and coronary blood flow (CBF), as previously described [10]. Our prior studies using this methodology demonstrated low intra- and inter-observer variability with good reproducibility over eight weeks [11].
Systemic Endothelial Function and Inflammatory Biomarkers:
Brachial flow mediated dilatation (FMD) and velocity were measured in the fasting state using standard techniques and analyzed in blinded fashion. Inflammatory biomarkers were measured at the University of Vermont (Supplement Table 1).
Demographic and baseline clinical characteristics were summarized using descriptive statistics for all participants. The primary analysis used an intent-to-treat approach. The primary efficacy endpoint was the % change in CBF from rest to IHE at the end of 8 weeks of the colchicine or placebo administration periods. The secondary efficacy endpoints included the IHE stress-induced change in CSA after 8 weeks of treatment, and change in CSA and CBF with IHE after 24 weeks of treatment. Further statistical details and methods are in the Supplement.
Sample size calculation:
Details of the sample size calculations appear in the online Supplement. Briefly, the principal outcome was the change in coronary blood flow (CBF) from rest to that during IHE at 8 weeks in the LDC and placebo groups. We chose CBF because it a.) reflects both macrovascular and microvascular changes related to the endothelial-dependent IHE stressor, b.) offers a large dynamic range in responses between healthy participants and PLWH, c.) is reproducible over 8-weeks [11], and d.) because endothelial function is an independent predictor of atherosclerotic progression and clinical events [4]. The 8 week time was chosen to minimize confounding events occurring over longer times and because improvements in endothelial function have been observed in PLWH in as little as 4 weeks following the initiation of ART [13] and as little as 8 weeks of treatment with statins [14]. We powered the sample size calculations on the hypothesis that an increase in the IHE-CBF response in PLWH to 50% of that of healthy subjects would occur with LDC, because such a change would be both biologically significant and consistent with the changes in CEF in CAD patients with statins [15]. We assumed that there would be no increase in the 8-week CBF response to IHE in the placebo-administered PLWH and that there would be a +20% increase in the LDC-administered PLWH. With a sample size of 35 in each group, the power would be 92% (alpha=0.05, two-sided test) to detect such a difference in CBF-IHE response between the placebo and LDC groups [16]. However, if as many as 20% dropped out, or declined to return for MRI, there would still be approximately 28 in each group, resulting in 85% power.
Statistical Analysis:
The statistical analysis was performed according to the intent-to-treat principle. To examine whether CEF in the LDC and placebo groups differed, the group results were compared using a two-sample t-test if the data were normally distributed or appropriate non-parametric testing if data were non-normally distributed. The primary outcome variable was change in CBF from rest to IHE stress at 8 weeks. Linear regression was used to account for residual confounding and changes that occurred during the interim. Data are expressed as mean ± standard error unless otherwise specified, and further statistical details are presented in the Supplement. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Role of the funding source:
The study sponsors played no role in the study design, data collection, analysis or writing of the report.
Results:
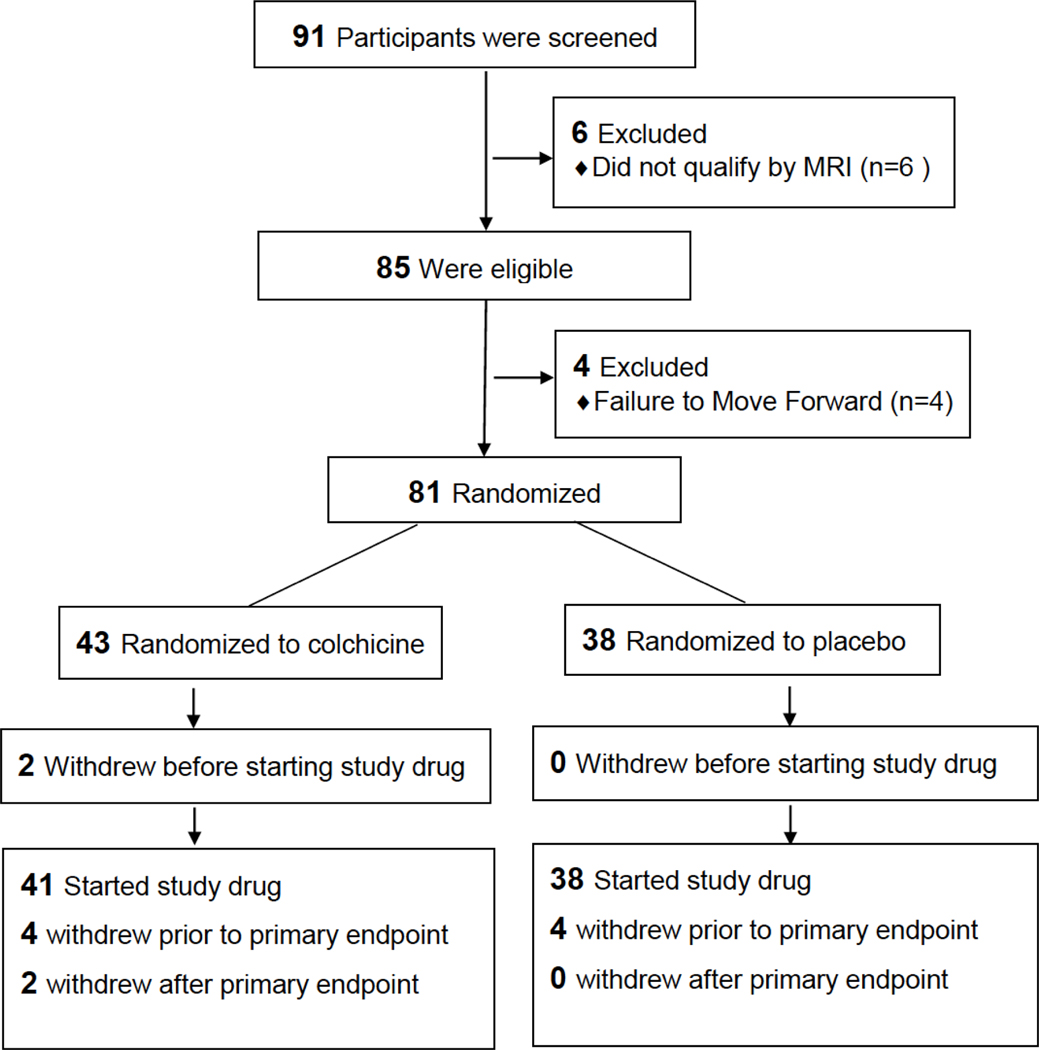
The mean age of the study population at the time of randomization was 51.6 ± 1.2 years and 21% were women, with a mean BMI of 27.7 ± 0.5 kg/m2. The mean baseline hsCRP level for the entire cohort was 3.6 ± 0.9 mg/L and the mean low-density lipoprotein cholesterol level (LDL) was 104 ± 4.4 mg/dL. Ninety-one participants underwent screening MRI, 85 were eligible by MRI evidence of abnormal CEF, and 81 were enrolled. There were no significant differences in baseline clinical and demographic characteristics between subjects randomized to the two study groups (Table 1). Two participants randomized to colchicine withdrew before receiving study drug. Only one person was removed from the study for non-compliance. Serum colchicine levels were measured at 8 weeks in all subjects randomized to colchicine and detected in all subjects except for one. The disposition of subjects during the trial is shown in Figure 1. A total of 37 participants for colchicine and 34 participants for placebo were included in the final analysis of the primary endpoint by intention to treat analysis.
Figure 1:
Trial Flow Chart
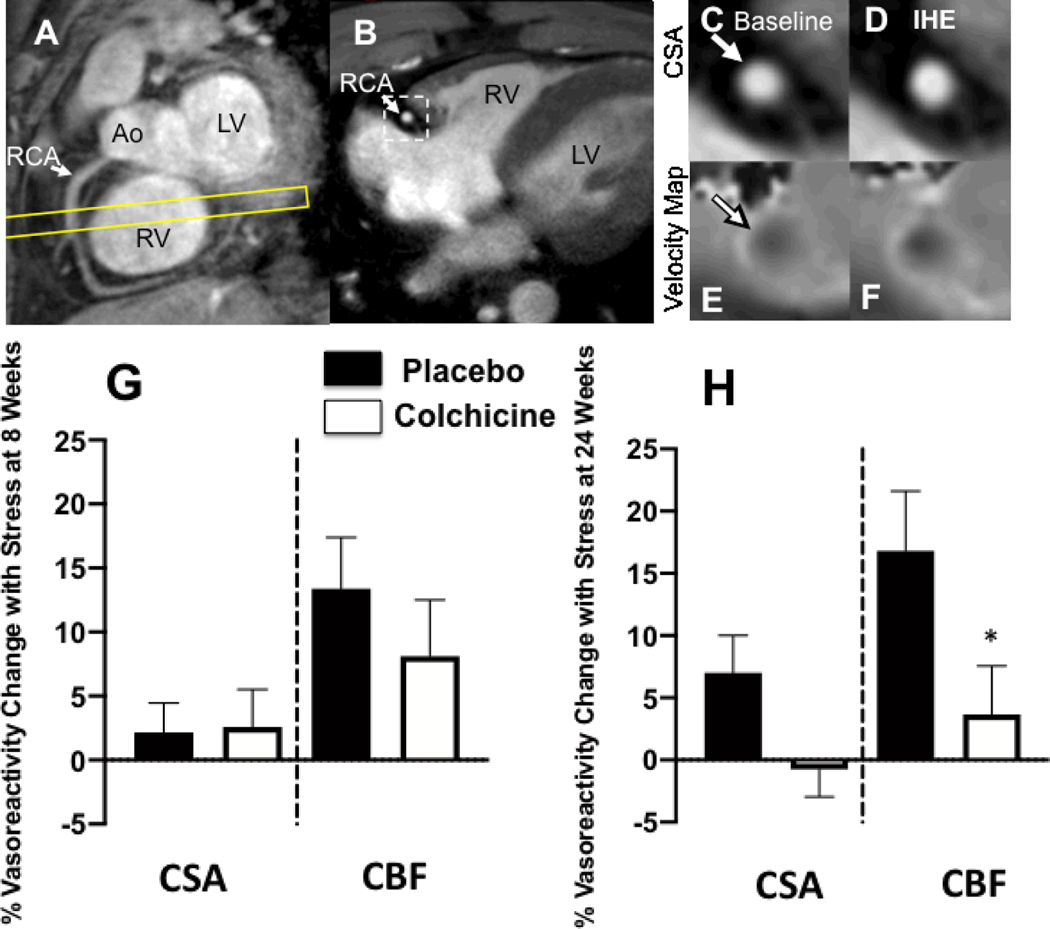
Representative cardiac MR images are shown in Figure 2. At baseline for the entire randomized cohort, the mean percent change in CBF with IHE for qualifying coronary segments and for all coronary segments were −3.5%±1.8% and +9.3%±2.4%, respectively. The mean percent change in CSA with IHE for qualifying coronary segments was −2.4%±1.4% and for all coronary segments was +2.0%±1.3%. The CBF and CSA findings in this cohort are consistent with coronary endothelial dysfunction measures previously reported in PLWH [17, 18].
Figure. 2:
Representative coronary artery MRI images for CEF. (A) A scout MRI obtained parallel to the right coronary artery (RCA) is shown with the location for subsequent cross-sectional imaging (yellow outline). (B) Image acquired along the yellow-outlined region in (A) with RCA in cross-section (white arrow). The dotted rectangle in B is magnified in subsequent panels and shows the region analyzed for cross-sectional area at rest (C) and during isometric handgrip exercise (IHE), D). Flow velocity images of the same segment at rest (E) and during IHE (F) using a phase contrast technique wherein signal darkness increases only slightly during IHE, indicating an impaired response. G and H: Relative changes (%) in coronary artery cross sectional area (CSA), and coronary blood-flow (CBF) using MRI during isometric handgrip exercise at 8 weeks (G) and 24 weeks (H). Percent change in coronary vasoreactive parameters with IHE are shown for those on colchicine (red) and placebo (blue). Error bars indicate standard error of the mean. There were no significant differences in coronary endothelial function parameters between the placebo and anti-inflammatory treatment at the 8 week (primary) en point. % CBF change was lower in the colchicine than placebo group (*p=0.05) at 24 weeks. Ao=aorta; LV=left ventricle, RV=right ventricle.
At eight weeks, the primary endpoint for the study, the change in CBF with IHE, and the secondary endpoint, the change in CSA with IHE, for all coronary segments did not differ between the LDC and placebo groups (Fig 2G). Specifically, the mean IHE-induced percentage change in CBF for all segments following 8 weeks of LDC was +8.1%±4.4% and following eight weeks of placebo was +13.4%±4.0% (p=NS, Fig 2G). Similarly, there was no significant difference in mean % CSA change with IHE at 8 weeks between groups (Fig 2G). In addition, there was no CEF difference between the groups at 8 weeks if only qualifying coronary segments were included in the analysis. At 24 weeks, there was still no benefit of colchicine relative to placebo on CEF, although the CBF change was lower in the colchicine group compared to placebo (Fig 2H). Other CEF endpoints are presented in Supplement Table 3.
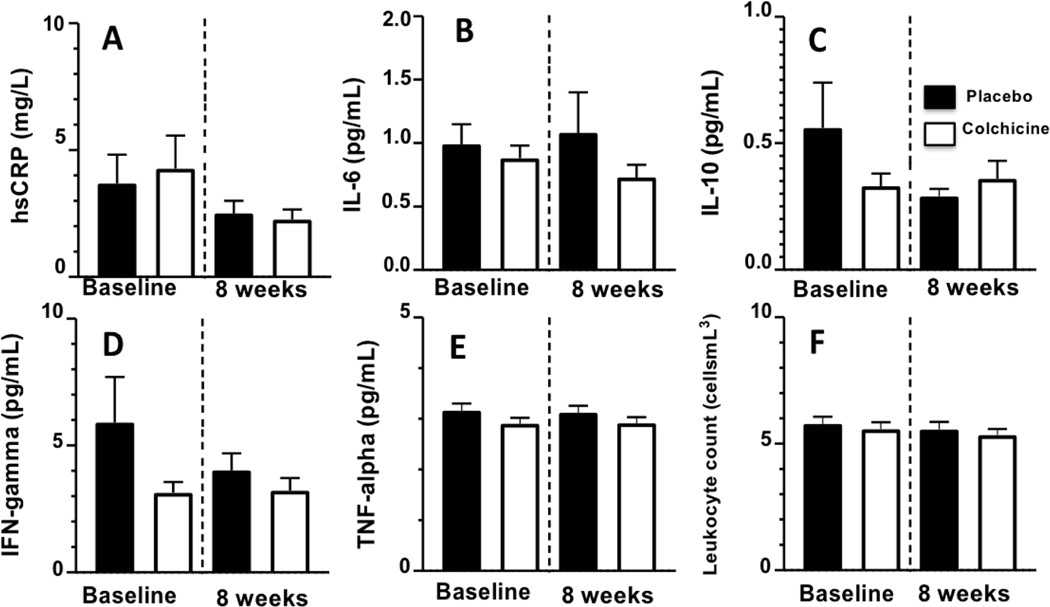
At baseline, brachial FMD was 4.5%±0.4% for all study participants and did not differ between groups (Supplement Table 3). There were also no differences in brachial FMD between groups after either 8 weeks or 24 weeks of study drug administration. There were also no significant group differences between the change from baseline to eight weeks in hsCRP, interleukin-6 (IL-6), and other inflammatory biomarkers (see Figure 3 and Supplement Table 3).
Figure 3:
Bar graphs showing serum biomarkers (mean values) at baseline and at 8 weeks after randomization to colchicine (red bars) or placebo (blue bars) in people living with HIV. Error bars indicate standard error of the mean. There were no significant differences in level of inflammatory biomarkers (A-F) between groups.
Overall, LDC was relatively well tolerated (Table 2). The most common adverse events in the colchicine group were gastrointestinal disorders (colchicine: N=12, placebo: N=9), minor infections, and joint and muscle aches, though none were significantly different between the LDC and placebo groups except for infections, which were higher in placebo. There were no serious AEs during the course of the study in the colchicine group and two in the placebo group (Table 2). The reasons for premature withdrawal from the study due to an AE are presented in Supplement Table 2. There were no significant changes in white blood cell count, creatinine, LDL cholesterol or CD4 cell count compared to baseline at 8 or 24 weeks.
Table 2:
Adverse events in colchicine and placebo groups. A laboratory value qualified for an adverse event whenever the value dropped below the Johns Hopkins Hospital reference range if the baseline value was within the reference range. GFR=glomerular filtration rate
| Adverse Event or Abnormal Laboratory Value | Placebo (n=38) | Colchicine (n=41) | p-value |
|---|---|---|---|
| Adverse Events | 80 | 75 | |
| Infection (respiratory or other) | 19 | 9 | 0.009 |
| Gastrointestinal disorder | 9 | 12 | 0.57 |
| Joint/Muscle Soreness/Stiffness | 9 | 8 | 0.65 |
| Fatigue | 1 | 1 | 1 |
| Extremity Swelling | 1 | 0 | 0.48 |
| Dental Pain/Infection | 3 | 0 | 0.11 |
| Skin irritation | 5 | 2 | 0.25 |
| Vision | 0 | 1 | 1 |
| Physical Injury | 3 | 1 | 0.35 |
| Increased Aspartate Amino Trans > 37U/L | 6 | 7 | 0.88 |
| Increased Alanine Amino Trans > 40U/L | 5 | 7 | 0.63 |
| Decreased White Blood Cell below 4.50K/cu mm | 4 | 7 | 0.52 |
| Decreased Hematocrit below 41.0% | 8 | 10 | 0.72 |
| Decreased GFR below 60 mL/min/1.73qm | 7 | 10 | 0.52 |
| Serious Adverse Event | 0 | 2 | 0.23 |
Discussion:
In summary, we performed a randomized, double-blinded, placebo-controlled clinical trial, and found that low dose colchicine (0.6mg daily) did not improve coronary endothelial dysfunction in PLWH on stable ART as compared to placebo. In addition, we observed that LDC did not reduce serum markers of inflammation or improve peripheral endothelial function as measured by brachial FMD in the short term (8 weeks) or longer term (24 weeks) in PLWH. In this cohort, LDC administration was relatively well tolerated and was not associated with significantly more adverse events or serious adverse events compared to placebo.
Coronary endothelial function is an important index of vascular health and a predictor of future cardiovascular events [4]. Moreover, prior invasive studies of CEF showed that medications that reduce cardiovascular outcomes, such as statins, rapidly improve CEF [19] [20]. We used previously developed and validated non-invasive MRI methods to evaluate CEF,[21] which is abnormal during the early development of atherosclerosis [10]. Studies have shown that MRI-IHE measures of CEF reflect nitric oxide-mediated coronary endothelial vasoreactivity with good short- and intermediate-term reproducibility [10, 11]. Recently, we reported that the PCSK9 inhibitor, evolocumab, improves CEF measured with these techniques in as little as one to six weeks, including in PLWH, showing that the MRI-IHE approach can detect rapid CEF improvements in response to treatment in PLWH [12].
There is growing recognition of the role of inflammation in CAD [22], and as a result renewed interest in anti-inflammatory strategies to combat atherosclerosis in disease states with heightened inflammation such as HIV in part because patients with increased inflammation are at elevated risk for cardiovascular events [23]. Although statins have anti-inflammatory properties and reduce cardiovascular mortality [24], statins alone do not fully suppress inflammation in many patients [25]. To answer the question of whether an anti-inflammatory medication improves coronary endothelial dysfunction in PLWH, we chose to study colchicine, an agent with anti-inflammatory properties that does not significantly affect lipids. LDC is an appealing choice to suppress inflammation in PLWH because it has been used for decades to treat inflammatory diseases and, in some populations, reduces inflammatory biomarkers (when given twice daily), improves systemic endothelial function, and is associated with fewer cardiac events [26].
Several recent trials evaluated the ability of LDC to reduce cardiovascular events in HIV-seronegative people with CAD. In the LoDoCo trial (Low Dose Colchicine), treatment with LDC (0.5 mg daily) in patients without HIV and with stable ischemic heart disease significantly reduced cardiac events as compared to those who did not receive colchicine at 3 years follow-up [27]. Another trial, LoDoCo-MI evaluated the effect of colchicine vs. placebo in acute MI patients with elevated CRP (defined as >2 mg/L), and observed that LDC did not reduce CRP levels significantly one month post MI [28]. The recent COLCOT trial randomized acute MI patients to LDC vs. placebo and reported a reduction in a composite endpoint of CV events driven by reduced incidence of stroke and hospitalization for angina in the LDC group [29]. However, the inflammatory states in the setting of acute MI as studied in COLCOT compared to the stable PLWH participants in the present study are not equivalent; the median hsCRP in COLCOT was over 4 mg per liter, substantially higher than that in our study participants (median hsCRP=1.5 mg/L) [29]. The finding that LDC did not significantly reduce serum inflammatory markers such as CRP or IL-6 in this study in PLWH with no history of heart disease is consistent with the findings in other randomized clinical trials (i.e. LODOCO-MI, COLCOT) that reported a neutral effect of colchicine 0.5mg daily on inflammatory biomarkers. More recently the LoDoCo2 trial in 5522 HIV-seronegative patients with chronic CAD showed that colchicine reduced a composite primary end-point of cardiovascular death, spontaneous myocardial infarction, ischemic stroke, or ischemia-driven coronary revascularization events (6.8% vs 9.6%, P<0.001) but increased the risk of death from noncardiovascular causes [30]. Biomarkers of inflammation were not reported. In the current study, we used a dose of LDC similar to that used in other trials in HIV-seronegative individuals (0.6mg daily), but show for the first time that LDC does not improve coronary endothelial dysfunction, peripheral endothelial function or reduce circulating inflammatory biomarkers in PLWH after 8 to 24 weeks.
There were no significant differences in serious adverse events experienced during treatment with colchicine compared to placebo. All reported AEs were only mild-moderate in severity, and these findings confirm prior studies of the safety and tolerability of LDC and provide important safety data for LDC in PLWH. Only two people withdrew from the study after starting study drug due to an AE (one in the placebo group and one in the colchicine group), and none withdrew due to laboratory abnormalities.
The present study was not powered for clinical outcomes but instead evaluated imaging approaches to non-invasively evaluate the CEF response to LDC over 24 weeks. The cohort size was justified with sample size estimates (see Supplement) based on prior published studies of MRI measures of CEF [11, 31], and prior studies showed that the MRI-IHE approach is capable of detecting even small changes in CEF and is reproducible over time [11]. Moreover, we very recently observed significant improvements in MRI-detected CEF within six-weeks of PCSK9 inhibition in only 19 PLWH [12] but here we observed no change in MRI-detected CEF in the 37 subjects randomized to colchicine who completed 8 weeks of therapy. Other trials have also used surrogate endpoints to test for cardiovascular benefit of interventions in PLWH. Such trials include, among others, the impact of statins on coronary plaque morphology[32], the effect of aspirin on immune activation[33] as well as trials of Factor Xa inhibitors[34] and of pro-biotics [35]. We believe that our randomized trial is the first in PLWH to use the endpoint of coronary endothelial function, an established predictor of future cardiovascular events and barometer of vascular health.
It is possible that one reason for the disparate results we report and that of a prior study demonstrating reduced cardiovascular events in HIV uninfected individuals with colchicine is that the individuals in the prior study were followed for a median of three years[27]. Moreover, we observed no trend between the two study groups for a colchicine benefit, suggesting that a larger sample size was unlikely to have resulted in significant differences with LDC as well. Because levels of inflammation in PLWH on stable ART are higher than those of people without HIV, it is also possible that the dose of colchicine was not sufficient to suppress inflammation adequately. The absence of changes in inflammatory biomarkers in PLWH with this dose of colchicine is consistent with this possibility. When this cohort was stratified post-hoc as those with high versus those with low inflammation, there was perhaps a trend for better CEF after 8 weeks of colchicine compared to placebo in those with CRP level>2 mg/L at baseline; however, this difference was not statistically significant. Another limitation was the inability of LDC to reduce inflammation as measured by serum inflammatory markers; however, these findings are consistent with prior studies in stable CAD patients [27].
In summary, this study is the first to evaluate the effects of an anti-inflammatory approach using colchicine on coronary artery vascular health in PLWH on stable ART. Colchicine was generally well tolerated; however, this treatment approach did not improve coronary endothelial dysfunction, a well-established index of coronary vascular health. Thus although colchicine is inexpensive and commonly available with a reasonable safety profile and prior large clinical trials to date suggest the benefits for cardiovascular disease in HIV-seronegative populations with established coronary disease, we do not find evidence that colchicine (0.6mg daily) improves coronary artery or systemic vascular health in PLWH. Thus, it is not clear that the cardiovascular benefits of colchicine in HIV-seronegative individuals extend to PLWH. Until large, long-term randomized trials of colchicine or other anti-inflammatory medications in PLWH become available, clinical practice for attenuating increased cardiovascular risk in PLWH should continue to focus on conventional risk factors.
Supplementary Material
Acknowledgements:
Funding: National Institutes of Health (HL125059, HL147660), the American Heart Association (AHA 17GRNT33670943) and the Clarence Doodeman Endowment in Cardiology at Johns Hopkins University School of Medicine. Colchicine levels were determined by the Analytical Pharmacology Core of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins [NIH grants P30CA006973 and UL1TR003098, and the Shared Instrument Grant S10OD020091]. Dr. Hays is supported by the Magic that Matters Fund of Johns Hopkins Medicine, NIH/NHLBI 1R01HL147660, and the Johns Hopkins Center for AIDS Research (P30AI094189).
Footnotes
Declaration of interests: There are no conflicts of interests from any of the authors.
References
- 1.Boccara F, Lang S, Meuleman C, Ederhy S, Mary-Krause M, Costagliola D, et al. HIV and Coronary Heart Disease: Time for a Better Understanding. Journal of the American College of Cardiology 2013; 61(5):511–523. [DOI] [PubMed] [Google Scholar]
- 2.Nou E, Lo J, Grinspoon SK. Inflammation, immune activation, and cardiovascular disease in HIV. AIDS 2016; 30(10):1495–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 2017; 377(12):1119–1131. [DOI] [PubMed] [Google Scholar]
- 4.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation 2000; 101(16):1899–1906. [DOI] [PubMed] [Google Scholar]
- 5.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation 2007; 115(10):1285. [DOI] [PubMed] [Google Scholar]
- 6.Hsue PY, Ribaudo HJ, Deeks SG, Bell T, Ridker PM, Fichtenbaum C, et al. Safety and Impact of Low-dose Methotrexate on Endothelial Function and Inflammation in Individuals With Treated Human Immunodeficiency Virus: AIDS Clinical Trials Group Study A5314. Clin Infect Dis 2019; 68(11):1877–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nidorf M, Thompson PL. Effect of colchicine (0.5 mg twice daily) on high-sensitivity C-reactive protein independent of aspirin and atorvastatin in patients with stable coronary artery disease. Am J Cardiol 2007; 99(6):805–807. [DOI] [PubMed] [Google Scholar]
- 8.Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-Dose Colchicine for Secondary Prevention of Cardiovascular Disease. Journal of the American College of Cardiology 2013; 61(4):404–410. [DOI] [PubMed] [Google Scholar]
- 9.Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, et al. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med 1986; 315(17):1046–1051. [DOI] [PubMed] [Google Scholar]
- 10.Hays AG, Hirsch GA, Kelle S, Gerstenblith G, Weiss RG, Stuber M. Noninvasive visualization of coronary artery endothelial function in healthy subjects and in patients with coronary artery disease. J Am Coll Cardiol 2010; 56(20):1657–1665. [DOI] [PubMed] [Google Scholar]
- 11.Hays AG, Iantorno M, Soleimanifard S, Steinberg A, Schar M, Gerstenblith G, et al. Coronary vasomotor responses to isometric handgrip exercise are primarily mediated by nitric oxide: a noninvasive MRI test of coronary endothelial function. American journal of physiology Heart and circulatory physiology 2015; 308(11):H1343–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leucker TM, Gerstenblith G, Schar M, Brown TT, Jones SR, Afework Y, et al. Evolocumab, a PCSK9-Monoclonal Antibody, Rapidly Reverses Coronary Artery Endothelial Dysfunction in People Living With HIV and People With Dyslipidemia. J Am Heart Assoc 2020:e016263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torriani FJ, Komarow L, Parker RA, Cotter BR, Currier JS, Dubé MP, et al. Endothelial Function in Human Immunodeficiency Virus-Infected Antiretroviral-Naive Subjects Before and After Starting Potent Antiretroviral TherapyThe ACTG (AIDS Clinical Trials Group) Study 5152s. Journal of the American College of Cardiology 2008; 52(7):569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hürlimann D, Chenevard R, Ruschitzka F, Flepp M, Enseleit F, Béchir M, et al. Effects of statins on endothelial function and lipid profile in HIV infected persons receiving protease inhibitor-containing anti-retroviral combination therapy: a randomised double blind crossover trial. Heart 2006; 92(1):110–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Treasure CB, Klein JL, Weintraub WS, Talley JD, Stillabower ME, Kosinski AS, et al. Beneficial effects of cholesterol-lowering therapy on the coronary endothelium in patients with coronary artery disease. New England Journal of Medicine 1995; 332(8):481–487. [DOI] [PubMed] [Google Scholar]
- 16.Mukerjee R, Wu CFJ. A modern theory of factorial design. Springer; 2006. [Google Scholar]
- 17.Iantorno M, Soleimanifard S, Schar M, Brown TT, Bonanno G, Barditch-Crovo P, et al. Regional coronary endothelial dysfunction is related to the degree of local epicardial fat in people with HIV. Atherosclerosis 2018; 278:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iantorno M, Hays AG, Schar M, Krishnaswamy R, Soleimanifard S, Steinberg A, et al. Simultaneous Noninvasive Assessment of Systemic and Coronary Endothelial Function. Circ Cardiovasc Imaging 2016; 9(3):e003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Treasure CB, Klein JL, Weintraub WS, Talley JD, Stillabower ME, Kosinski AS, et al. Beneficial effects of cholesterol-lowering therapy on the coronary endothelium in patients with coronary artery disease. N Engl J Med 1995; 332(8):481–487. [DOI] [PubMed] [Google Scholar]
- 20.Wassmann S, Faul A, Hennen B, Scheller B, Bohm M, Nickenig G. Rapid effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition on coronary endothelial function. Circ Res 2003; 93(9):e98–103. [DOI] [PubMed] [Google Scholar]
- 21.Schar M, Soleimanifard S, Bonanno G, Yerly J, Hays AG, Weiss RG. Precision and accuracy of cross-sectional area measurements used to measure coronary endothelial function with spiral MRI. Magn Reson Med 2019; 81(1):291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, et al. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N Engl J Med 2019; 380(8):752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med 2002; 347(20):1557–1565. [DOI] [PubMed] [Google Scholar]
- 24.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350(15):1495–1504. [DOI] [PubMed] [Google Scholar]
- 25.Ridker PM, Morrow DA, Rose LM, Rifai N, Cannon CP, Braunwald E. Relative efficacy of atorvastatin 80 mg and pravastatin 40 mg in achieving the dual goals of low-density lipoprotein cholesterol <70 mg/dl and C-reactive protein <2 mg/l: an analysis of the PROVE-IT TIMI-22 trial. J Am Coll Cardiol 2005; 45(10):1644–1648. [DOI] [PubMed] [Google Scholar]
- 26.Crittenden DB, Lehmann RA, Schneck L, Keenan RT, Shah B, Greenberg JD, et al. Colchicine use is associated with decreased prevalence of myocardial infarction in patients with gout. J Rheumatol 2012; 39(7):1458–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol 2013; 61(4):404–410. [DOI] [PubMed] [Google Scholar]
- 28.Hennessy T, Soh L, Bowman M, Kurup R, Schultz C, Patel S, et al. The Low Dose Colchicine after Myocardial Infarction (LoDoCo-MI) study: A pilot randomized placebo controlled trial of colchicine following acute myocardial infarction. Am Heart J 2019; 215:62–69. [DOI] [PubMed] [Google Scholar]
- 29.Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N Engl J Med 2019; 381(26):2497–2505. [DOI] [PubMed] [Google Scholar]
- 30.Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, et al. Colchicine in Patients with Chronic Coronary Disease. N Engl J Med 2020. [DOI] [PubMed] [Google Scholar]
- 31.Hays AG, Iantorno M, Schar M, Mukherjee M, Stuber M, Gerstenblith G, et al. Local coronary wall eccentricity and endothelial function are closely related in patients with atherosclerotic coronary artery disease. J Cardiovasc Magn Reson 2017; 19(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lo J, Lu MT, Ihenachor EJ, Wei J, Looby SE, Fitch KV, et al. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Lancet HIV 2015; 2(2):e52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Brien M, Montenont E, Hu L, Nardi MA, Valdes V, Merolla M, et al. Aspirin attenuates platelet activation and immune activation in HIV-1-infected subjects on antiretroviral therapy: a pilot study. J Acquir Immune Defic Syndr 2013; 63(3):280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baker JV, Wolfson J, Peterson T, Mooberry M, Gissel M, Mystakelis H, et al. Factor Xa Inhibition Reduces Coagulation Activity but Not Inflammation Among People With HIV: A Randomized Clinical Trial. Open Forum Infect Dis 2020; 7(2):ofaa026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reikvam DH, Meyer-Myklestad MH, Troseid M, Stiksrud B. Probiotics to manage inflammation in HIV infection. Curr Opin Infect Dis 2020; 33(1):34–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.