Abstract
Ethambutol (EMB) is the most frequent “fourth drug” used for the empiric treatment of Mycobacterium tuberculosis and a frequently used drug for infections caused by Mycobacterium avium complex. The pharmacokinetics of EMB in serum were studied with 14 healthy males and females in a randomized, four-period crossover study. Subjects ingested single doses of EMB of 25 mg/kg of body weight under fasting conditions twice, with a high-fat meal, and with aluminum-magnesium antacid. Serum was collected for 48 h and assayed by gas chromatography-mass spectrometry. Data were analyzed by noncompartmental methods and by a two-compartment pharmacokinetic model with zero-order absorption and first-order elimination. Both fasting conditions produced similar results: a mean (± standard deviation) EMB maximum concentration of drug in serum (Cmax) of 4.5 ± 1.0 μg/ml, time to maximum concentration of drug in serum (Tmax) of 2.5 ± 0.9 h, and area under the concentration-time curve from 0 h to infinity (AUC0–∞) of 28.9 ± 4.7 μg · h/ml. In the presence of antacids, subjects had a mean Cmax of 3.3 ± 0.5 μg/ml, Tmax of 2.9 ± 1.2 h, and AUC0–∞ of 27.5 ± 5.9 μg · h/ml. In the presence of the Food and Drug Administration high-fat meal, subjects had a mean Cmax of 3.8 ± 0.8 μg/ml, Tmax of 3.2 ± 1.3 h, and AUC0–∞ of 29.6 ± 4.7 μg · h/ml. These reductions in Cmax, delays in Tmax, and modest reductions in AUC0–∞ can be avoided by giving EMB on an empty stomach whenever possible.
Ethambutol (EMB) is the most frequently used “fourth drug” for the empiric treatment of tuberculosis (3). The standard short-course treatment of tuberculosis consists of isoniazid (INH), rifampin (RIF), and pyrazinamide (PZA), plus either EMB or streptomycin until susceptibility data are available (3). Also, EMB is frequently used for the treatment of infections caused by Mycobacterium avium complex (2). Limited information exists regarding the pharmacokinetics of EMB in healthy or infected individuals or regarding the effect of food or antacids on the gastrointestinal absorption of the drug (1, 12–15, 17–19, 21, 22). We examined the pharmacokinetics of EMB in healthy volunteers under fasting conditions (two replicates), with food, and with an aluminum-magnesium hydroxide antacid. This study describes the concentrations in serum and the pharmacokinetic behavior under optimal conditions, and the results can be used as benchmarks for comparison with those for samples obtained in other clinical settings.
(Part of this study was presented at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Ontario, Canada, 28 September to 1 October 1997 [20].)
MATERIALS AND METHODS
We conducted a four-period, randomized crossover study of EMB. The study protocol followed the guidelines of the Helsinki Declaration of 1975 and its amendments and was approved by the institutional review board at Millard Fillmore Hospital, Buffalo, N.Y. Written informed consent was obtained from each subject before the study. Sixteen healthy female and male volunteers were scheduled to participate. Subjects were eligible to participate if they were >18 years of age and were determined to be in good health as assessed by history, physical examination, and laboratory studies including serum chemistries, complete blood count with differential, and 12-lead electrocardiogram. Individuals were excluded if they had histories of a major disease of the kidneys (estimated creatinine clearance [CLCR], ≤50 ml/min), the liver (transaminases, alkaline phosphatase, or bilirubin ≥2 times normal), or the cardiovascular system (New York class I to IV heart failure) or a hematocrit ≤36% at screening. They were also excluded if they had known gastrointestinal diseases that might affect the absorption of the drugs; known positive human immunodeficiency virus serology; AIDS; or histories of adverse reactions to INH, RIF, PZA, EMB, or related drugs. They were also excluded if they weighed >130% of ideal body weight, were pregnant or nursing, or donated blood within 30 days prior to the study (4). The subjects agreed to refrain from the use of prescription or nonprescription drugs (including vitamins) and alcohol during the entire study period. Women who were taking oral contraceptives at the start of the study were allowed to continue these during the study. They were required to agree to use additional contraceptive methods during the study period and for a week after the last dose of RIF. At the conclusion of the study, each subject underwent a brief physical examination and had blood drawn for serum chemistry and hematology, and female subjects had a repeat pregnancy test.
Experimental design.
Sixteen subjects were randomized in four blocks of four subjects. The four treatments were fasting conditions (twice, to determine intrasubject variability), a high-fat meal, and aluminum-magnesium antacid. The subjects were housed at the study center from 10 h before to 24 h after dosing and returned for the 36- and 48-h collections. After eating a light snack prior to 2300, they fasted overnight. For three of the treatments, they continued to fast for 4 h after the dose. On one of these three fasting occasions, they also took 30 ml of aluminum-magnesium hydroxide (Mylanta) 9 h before dosing, at the time of the dose, after meals, and at bedtime postdose. For the fourth treatment, they consumed the standard Food and Drug Administration high-fat breakfast beginning 0.25 h before dosing. This meal consisted of 8 oz of whole milk, two scrambled eggs, two strips of bacon, two slices of toast with two butter pads, and one hash brown potato patty. The meal provided an estimated 53 g of carbohydrate, 33 g of protein, and 51 g of fat, for 792 kcal, 57% as fat. For all four treatments, subjects received single oral doses of 25 mg of EMB (median, 1,950 mg; dosed to the nearest 100 mg, with scored 500-mg tablets [Wyeth-Lederle, Philadelphia, Pa.) per kg of body weight with 240 ml of tap water. They also received 300 mg of INH, 600 mg of RIF, and 30 mg of PZA (median, 2,386 mg) per kg. Doses for all treatment periods were based on the subjects’ prestudy weights. The subjects were allowed to ingest water ad libitum after the doses were given, and identical, nutritionally balanced meals were provided to all subjects during the remainder of the study period. There was a 14-day washout between each study period.
Sample collection.
A 20-gauge angiocatheter was inserted into a forearm vein for the collection of blood samples and was maintained patent with a dilute heparin solution (10 to 15 U/ml). Two milliliters of blood was withdrawn and discarded prior to collection of each blood sample (12 ml) into plain red-top vacuum tubes. Serial blood samples for serum drug concentration analyses were collected at 0, 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, 12, 16, 24, 36, and 48 h after the doses. Samples were allowed to clot for 30 min and then centrifuged at 2,500 to 3,000 × g for 10 min. Serum samples were then harvested and frozen at ≤−70°C until assay.
Urine samples were collected within 30 min of dosing (baseline). Subsequently, all urine was collected from 0 to 12 h and from 12 to 24 h. Samples were kept refrigerated during the period of collection. The total volume was measured at the end of the collection period, and 10-ml aliquots from each collection were frozen at ≤−70°C until assay.
Sample analysis.
All assays were performed with a validated assay on a Hewlett-Packard (Wilmington, Del.) model 5890 Series II gas chromatograph with a Hewlett-Packard model 5971A mass selective detector. The serum standard curves for EMB ranged from 0.05 to 10 μg/ml. The absolute recovery of EMB from serum was 95.8%. The within-day precision (percent coefficient of variation [% CV]) of validation quality control (QC) samples was 2.2 to 4.1%, and the overall validation precision was 2.8 to 3.3%. The urine standard curves for EMB ranged from 0.05 to 50 μg/ml, with similar reproducibility. EMB urine samples were diluted 1:10 prior to extraction. The assay error pattern was determined from QC samples assayed over the course of the study. A line was fitted to the plot of the QC standard deviations (y) versus their means (x) at the three QC ranges (low, medium, and high). The assay error pattern used for the subsequent nonlinear regression was variance (standard deviation squared [SD2]) = (0.024 + 0.024x)2 (11).
Pharmacokinetic analysis.
Concentrations in serum below the quantification lower limit were treated as zeros in averaging the concentrations at a given collection time. The observed maximal serum concentration (Cmax) and the time at which it occurred (Tmax) were determined for each subject by inspection of the serum concentration-versus-time graphs. The area under the serum concentration-versus-time curve (AUC) from time zero to the time of the last quantifiable concentration (AUC0–t*) was determined by the linear trapezoidal rule. The last quantifiable concentration was designated C*. The AUC from time zero to infinity (AUC0–∞) was determined as AUC0–t* + C*/β, with β determined by ADAPT II (see below). The potential for accumulation of these drugs with multiple doses was evaluated by the principle of superposition (8). The accumulation of EMB with eight daily doses was simulated with the median serum concentration data from 0 to 24 h (first fasting treatment) and extrapolated from 24 h to day 8 with the median β.
Compartmental analysis.
ADAPT II software was used to construct candidate pharmacokinetic models, with nonlinear least-squares regression, weighted by the inverse of the assay variance, as described above (6). The Akaike information criteria were used to discriminate among candidate models, and a two-compartment model with apparent zero-order absorption was selected. The model included the zero-order absorption time (Tabs [milligrams per hour]), the absorption lag time (Tlag [hours]), the volume in the central compartment (V1 [liters per kilogram]), the intercompartmental transfer rate constant (K21 [1/h]), and the α and β elimination rate constants (1/h). The rate constant K10 was calculated as [(α × β)/K21], K12 was calculated as [(α + β) − K21 − K10], and total body clearance (CL [liters per hour]) was calculated as (V1 × K10). The steady-state volume of distribution (VSS [liters per kilogram]) was calculated as [V1 × (1 + K21/K12)]. The terminal elimination half-life (t1/2) was calculated as ln(2)/β.
D-optimal sampling time analysis was performed by using ADAPT II software and the compartmental parameter estimates. The linear assay error pattern described above was used. Sampling times were analyzed with the parameters Tabs, VSS, and β over the period 0.5 to 24.0 h, with various initial sampling times and sampling time constraints. A two-sample strategy (achieved by fixing Tabs and fitting only VSS and β) and a three-sample strategy (achieved by fitting all three parameters) were tested. In addition, an analysis of Cmax was performed over the period 0.5 to 4.0 h, calculating the maximum, median, and minimum percentages for the measured concentration divided by Cmax. CLCR was calculated by the method of Cockroft and Gault (5).
The amount of EMB recovered in the urine was calculated as the measured volume of urine multiplied by the corresponding EMB concentration. Total recovery (milligrams) was calculated as the sum of the recoveries from the collection periods 0 to 12 h and 12 to 24 h, and the percent dose recovered was calculated as total recovery divided by dose multiplied by 100%. Renal clearance (CLR) was calculated as total recovery divided by AUC0–24.
Statistical analysis.
Data analysis was performed with JMP version 3.1.6 (SAS Institute, Cary, N.C.), with supplemental analyses done with Excel version 4.0 (Microsoft, Seattle, Wash.). Frequency distributions (JMP) included plots of the data, distribution curves to test for normality, parametric and nonparametric measures of central tendency and dispersion, and the Shapiro-Wilk W test for normality. Means are reported ± the SD. The percent CV was calculated as SD/mean multiplied by 100%. Differences among the treatment groups were determined with an analysis of variance model that tested differences based on period, treatment, sequence, and subject (sequence). Pairwise differences across the four treatments were evaluated with individual linear contrasts. Bioequivalence criteria were tested according to the 1992 Food and Drug Administration guidelines (7). Cmax and AUC0–∞ were log transformed and were analyzed with the analysis of variance model described above. Mean estimates and standard errors were obtained from the linear contrasts, and these were used to calculate the geometric means and the lower and upper 90% confidence limits. Comparison treatments were considered bioequivalent to the reference treatment (fasting treatment 2) if the comparison parameter 90% lower limit was ≥80% and the upper limit was ≤125%.
Correlation analysis (JMP) was performed across the subject and outcome variables by nonparametric techniques (Spearman rho). The dependence of outcome variables (the pharmacokinetic parameters) upon subject characteristics (demographic data such as age, weight, CLCR, etc.) was determined with y by x analyses, one parameter at a time (JMP). Subsequently, models with multiple x variables were constructed by forward addition and backward deletion. Differences between groups (JMP) were determined by the analysis of log likelihood with the Pearson chi-square statistic (contingency tables). Student’s t test or analysis of variance (two or more than two groups, respectively) of normally distributed data (one-way layouts and linear regression), the Wilcoxon or the Kruskal-Wallis tests (rank sums) for nonnormally distributed data (one-way layouts), and the whole-model test table with chi-square statistic (logistic regression). Differences between groups or correlations between parameters and covariates were considered statistically significant at P ≤0.05.
RESULTS
Fourteen subjects completed all four treatments: six white females, three black males, and five white males. The remaining subjects dropped out for personal reasons. The mean age was 39.1 ± 7.4 years, and the mean weight was 79.3 ± 13.2 kg. The subjects received a mean EMB dose of 1,936 ± 343 mg (24.9 ± 0.4 mg/kg). All subjects denied the use of any nonprotocol medications during the study period. CLCR estimates were a mean of 103 ± 25 ml/min.
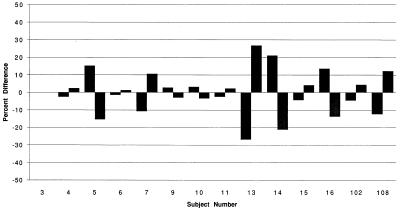
The absorption characteristics for EMB with the four treatments are described in Table 1, and the corresponding mean EMB serum concentration-versus-time profiles across the 14 subjects are shown in Fig. 1. Under fasting conditions, variability in absorption of EMB was small (Table 1) and the individual results were quite reproducible (Fig. 2). The mean EMB Cmax was significantly reduced by antacids (−29%) and, to a lesser extent, by food (−17%) (P = 0.0003). The mean EMB Tmax was increased by antacids (+17%) and, to a greater extent, by food (+29%) (P = 0.0787). The mean EMB AUC0–∞ was modestly decreased by antacids (−10%) and minimally by food (−4%) (P = 0.1625). With the bioequivalence criteria, food did not significantly affect the Cmax (90% confidence interval [CI], 88.3 to 100.0%) or the AUC0–∞ (90% CI, 96.4 to 103.6%). Antacids did not significantly affect the Cmax (90% CI, 83.6 to 94.6) or AUC0–∞ (90% CI, 93.2 to 100.2).
TABLE 1.
The absorption characteristics for EMB for 14 subjects across the four treatments
| Group |
Cmax (μg/ml)
|
Tmax (h)
|
AUC0–48 (μg · h/ml)
|
AUC0–∞ (μg · h/ml)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | % CV | Range | Mean | % CV | Range | Mean | % CV | Range | Mean | % CV | Range | |
| Fasting 1 | 4.52 | 22.8 | 1.96–5.97 | 2.46 | 35.0 | 1.5–4.0 | 27.2 | 15.5 | 17.1–33.4 | 28.9 | 16.4 | 8.35–35.8 |
| Fasting 2 | 4.58 | 32.1 | 1.79–6.85 | 2.50 | 26.0 | 1.5–4.0 | 29.0 | 19.2 | 20.1–38.5 | 30.7 | 19.1 | 21.7–41.1 |
| Antacid | 3.27 | 15.3 | 2.37–3.87 | 2.93 | 42.3 | 1.5–6.0 | 24.9 | 18.6 | 9.63–34.4 | 27.5 | 21.5 | 20.6–39.7 |
| Fed | 3.83 | 22.0 | 2.66–5.04 | 3.21 | 41.7 | 2.0–6.0 | 27.5 | 16.2 | 21.5–35.3 | 29.6 | 15.7 | 22.2–39.0 |
FIG. 1.
Mean EMB serum concentrations for 14 subjects across the four treatments.
FIG. 2.
Pairings of individual 2.5-h serum concentrations for 14 subjects between the two fasting treatments.
Simulated multiple daily doses of EMB with the median β value showed that, on day 4, after 6 to 7 EMB half-lives, the 2.5-h EMB concentration (Cmax) was 7.7% higher than that on day 1 but only 1.8% higher than that on day 2 and 0.4% higher than that on day 3. Subsequent simulated Cmax values remained constant. Given the day 1 Cmax range of 2.0 to 6.0 μg/ml (first fasting treatment [Table 1]), the calculated steady-state Cmax range for EMB (∼25-mg/kg dose) was 2.1 to 6.4 μg/ml.
Table 2 shows the parameter estimates for EMB following the 25-mg/kg dose as calculated with ADAPT II (first fasting treatment). The various parameter estimates were not significantly different across the four treatments. Tabs was somewhat longer in the feeding treatment, but this did not reach statistical significance (P = 0.0856).
TABLE 2.
Parameter values obtained with ADAPT II for 14 subjects for the first fasting treatment
| Parameter | Mean | % CV | Range |
|---|---|---|---|
| Tabs (mg/h) | 3.03 | 40.3 | 1.35–5.34 |
| Tlag (h) | 0.26 | 41.3 | 0.07–0.45 |
| V1 (liters/kg) | 3.87 | 50.8 | 0.34–7.16 |
| VSS (liters/kg) | 6.10 | 59.8 | 0.37–14.3 |
| CL (liters/h) | 90.0 | 33.7 | 55.1–163 |
| CL (liters/h/kg) | 1.14 | 20.2 | 0.85–1.72 |
| K12 (1/h) | 0.54 | 155 | 0.10–3.35 |
| K21 (1/h) | 0.16 | 64.6 | 0.05–0.42 |
| K10 (1/h) | 0.55 | 146 | 0.16–3.25 |
| α (1/h) | 1.18 | 140 | 0.30–6.72 |
| t1/2α (h) | 1.18 | 61.1 | 0.10–2.31 |
| β (1/h) | 0.07 | 44.5 | 0.03–0.12 |
| t1/2β (h) | 12.1 | 42.1 | 5.56–22.02 |
The D-optimal sampling times for all subjects over the period 0.5 to 24.0 h were 0.6 and 11 h for the two-sample strategy and 0.5, 6.2, and 6.2 h (duplicate) for the three-sample strategy if the entire interval was available for sampling. D-optimal sampling times were not affected by changes in the initial sampling times chosen but did change based on the constraints placed on the sampling times. Restriction of the D-optimal sampling period from Tmax to 24 h resulted in the selection of Tmax as the first sampling time. Table 3 shows that the 2.5-h sample came closest to Cmax for the greatest number of the 14 subjects, followed by the 2- and 3-h samples.
TABLE 3.
Concentrations collected from 0.25 to 4.0 h expressed as percentages of Cmax
| Time postdose (h) | %
|
||
|---|---|---|---|
| Highest | Median | Lowest | |
| 0.25 | 15 | 2 | 1 |
| 0.50 | 48 | 16 | 8 |
| 0.75 | 55 | 31 | 15 |
| 1.00 | 90 | 39 | 17 |
| 1.50 | 100 | 65 | 19 |
| 2.00 | 100 | 80 | 20 |
| 2.50 | 100 | 80 | 50 |
| 3.00 | 100 | 80 | 34 |
| 4.00 | 100 | 64 | 20 |
The recovery of EMB in the urine is shown in Table 4. Most of the urinary excretion of EMB occurred during the first 12 h postdose, with about 30% of the dose recovered unchanged in the urine over 24 h. Intersubject variability was small. The total recovery of EMB in the urine, the percentage of the dose recovered in the urine, and the CLR were not different across the four treatments. Subjects receiving antacid treatment had the lowest recovery in the period 0 to 12 h (415 mg versus first fasting result of 498 mg) but the highest recovery in the period 12 to 24 h (123 mg versus first fasting result of 102 mg, P < 0.03 for each comparison), resulting in a total recovery comparable to those of the other treatments.
TABLE 4.
Recovery of EMB in urine over 24 h (first fasting treatment)
| Parametera | Mean | % CV | Range |
|---|---|---|---|
| Xu 0–12 h (mg) | 498 | 29 | 276–785 |
| Xu 12–24 h (mg) | 102 | 35 | 36–147 |
| Xu total (mg) | 599 | 27 | 316–900 |
| % Dose recovered | 31 | 16 | 24–38 |
| CLR (liters/h) | 25 | 23 | 18–37 |
Xu, drug concentration in urine.
The EMB results were analyzed with JMP, and the nonparametric measures of association are reported (Spearman rho). Cmax and Tmax showed a modest negative correlation (r = −0.5192, P = 0.0571), with early absorbers showing the higher Cmax values. AUC0–∞ was somewhat higher in those with higher serum creatinine levels (r = 0.7603, P = 0.0016), but it did not correlate with CLCR (r = −0.1736, P = 0.5528). The EMB CL correlated with CLCR (r = 0.5341, P = 0.0492) and with EMB CLR (r = 0.5385, P = 0.0470). EMB pharmacokinetic parameters were not dependent upon age, gender, or race in this group of 14 subjects.
DISCUSSION
Determinations of the absolute bioavailability of EMB from the tablets versus an intravenous dosage form were not performed. All parameters were estimated assuming F = 1.
EMB was not rapidly absorbed, with most Tmax values near 2.5 h. Similar results were described by Lee et al., who studied six healthy volunteers (two females and four males) and determined Tmax values of 2.8 ± 0.7 h (12). They also determined Cmax values of 4.0 ± 0.8 μg/ml following smaller doses of 15 mg/kg, given as tablets. Under fasting conditions, variability across our 14 subjects was small, especially for the Cmax and AUC0–∞ values, and was highly reproducible between the two fasting treatments. Concentrations in serum increased by 7.7% over 4 days of simulated dosing. However, sampling as early as the second day of treatment will reflect steady-state serum concentrations. EMB taken with food or antacids was bioequivalent to EMB taken in the fasting state.
Antacids reduced the EMB Cmax by 29% and reduced the EMB AUC0–∞ by 10%. Therefore, antacids should be avoided near the time of EMB dosing. These findings are generally consistent with the work of Mattila et al., who measured EMB serum concentrations 2, 4, and 10 h postdose (15). In our study, food reduced the EMB Cmax by 17% but reduced the EMB AUC0–∞ by only 4%. Ameer et al. previously showed a similar lack of effect by food on the EMB AUC, although the standardized meal given to their subjects was not described (1). Their paper does not describe the effect on Cmax or Tmax. Place and Thomas found slightly higher serum concentrations in the nonfasted state, again, without a detailed description of the study conditions (21). Therefore, it may be preferable to give EMB on an empty stomach whenever possible. However, when this is not possible, the absorption of EMB should still be adequate. This may facilitate the dosing of EMB with other drugs such as nelfinavir, ritonavir, saquinavir, or rifapentine that are preferentially given with food, assuming that there are no direct interactions with these drugs (16, 23).
While a two-compartment model with first-order absorption produced good results, the two-compartment model with apparent zero-order absorption was superior based on the Akaike information criteria. The small Tlag corresponds to the first sampling time. This is consistent with the assay’s low limit of quantification, which produced measurable concentrations for nearly all subjects and treatments from 0.25 to 48 h. EMB displayed a large V1 and VSS, which in part reflects its binding to erythrocytes and its uptake by macrophages (12, 14, 17). Both Peets and Lee found erythrocyte/plasma concentration ratios of 1.1 to 1.8 in vitro, with higher ratios described by Peets for human subjects (12, 17). Entry into macrophages is particularly useful, since a portion of the total body burden of Mycobacterium tuberculosis and M. avium complex is found within macrophages. Previous estimates of the EMB V1 and VSS have been smaller (12, 13). Our CL estimates were quite similar across the 14 subjects, whether or not normalized to body weight. These also were larger than previously described (12, 13).
The EMB serum concentrations clearly displayed a biexponential decline, with a median t1/2α of about 1.3 h and a t1/2β of about 12.4 h. Visual inspection of the serum concentration-versus-time data shows an apparent decrease in the slope occurring about 12 h postdose. Similar findings were described previously by Lee et al. (12, 13). Following oral doses, they described a decline of concentrations in serum over the first 12 h postdose, with a least-squares regression t1/2 of 4.0 ± 0.5 h. They showed a second phase from 12 to 24 h with a least-squares regression t1/2 of 8.8 ± 2.2 h. Differences between our curve fitting techniques and theirs account for the discrepancies in the apparent t1/2s. Reanalysis of our data by their techniques produced a t1/2 over the first 12 h of 3.3 ± 0.8 h and a secondary t1/2 over 12 to 48 h of 13.9 ± 2.1 h. EMB’s long terminal t1/2 renders it suitable for once-daily dosing, particularly since it is used against the slow-growing M. tuberculosis and M. avium, which have doubling times of ≥24 h.
The clearance of EMB has been described as occurring predominantly through renal mechanisms. We recovered only 30% of the single oral doses unchanged in the urine over 24 h. The serum AUC0–24 represented a median 83% of the AUC0–∞, so longer collection periods might have resulted in roughly 36% recovery, assuming that CLR is constant over the range of concentrations in serum. The completeness of oral absorption, and other sources of elimination, including metabolites, were not determined in this study. In two studies, Lee et al. documented 24-h urinary recoveries of 53% after oral EMB doses and 73% after intravenous EMB doses (12). The reasons for lower recovery in our study are not apparent but may include incomplete absorption from the oral doses here versus the intravenous doses used by Lee. Also, there may be some differences in specificity between the two methods of detection used. Renal dysfunction has been shown previously to have a significant impact on the CL of EMB, and frequency of dosing should be reduced in patients with renal dysfunction (18, 24).
If the sampling times were not restricted to begin at Tmax, the EMB D-optimal sampling times included a point during the absorptive phase and one point in the elimination phase, but not the true Cmax. The three-point strategy produced identical sampling times for the second and third parameters. Samples collected at 2.5 h postdose captured most of the Cmax values and would be preferred for that parameter.
EMB has modest activity against both M. tuberculosis and M. avium (9, 10). Using radiometric techniques, Heifets determined the MIC of EMB to be 1 to 4 μg/ml against M. tuberculosis and to be 4 to 8 μg/ml against M. avium (9). Against an isolate of M. tuberculosis having a MIC of 1 μg/ml, the EMB Cmax/MIC ratio may range from 2:1 to 6:1, with a time above MIC from 5 to 12 h. However, EMB barely achieves inhibitory concentrations in serum against M. avium, even with good absorption. Since the absorption of EMB has been shown to be poor in patients with AIDS, EMB doses higher than 25 mg/kg may be required for some of these patients in order to inhibit the pathogens (19).
To conclude, the concentrations in serum found in this study were consistent with those previously described. In contrast, our V1, VSS, and CL estimates were larger than previously described. The kinetic behavior of EMB was consistent between the two fasting treatments. Food had a minimal effect on the absorption of EMB, while antacids should be avoided near the time of EMB dosing. Samples drawn between 2 and 3 h postdose approach Cmax for most subjects, and samples drawn as early as day 2 of daily EMB therapy will produce concentrations in serum that approach steady-state values.
ACKNOWLEDGMENT
This study was supported, in part, by NIH grant 1 RO1 AI37845.
REFERENCES
- 1.Ameer B, Polk R E, Kline B J, Grisafe J P. Effect of food on ethambutol absorption. Clin Pharm. 1982;1:156–158. [PubMed] [Google Scholar]
- 2.American Thoracic Society. Diagnosis and treatment of disease caused by nontuberculosis mycobacteria. Am J Respir Crit Care Med. 1997;156:S1–S25. doi: 10.1164/ajrccm.156.2.atsstatement. [DOI] [PubMed] [Google Scholar]
- 3.American Thoracic Society/Centers for Disease Control and Prevention. Treatment of tuberculosis and tuberculosis infection in adults and children. Am J Respir Crit Care Med. 1994;149:1359–1374. doi: 10.1164/ajrccm.149.5.8173779. [DOI] [PubMed] [Google Scholar]
- 4.Anonymous. 1983 Metropolitan height and weight tables. Stat Bull Metrop Life Found. 1983;64:3–9. [PubMed] [Google Scholar]
- 5.Cockroft D W, Gault M H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;10:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 6.D’Argenio D, Schumitzky A. ADAPT II user’s guide. Biomedical Simulations Resource. Los Angeles, Calif: University of Southern California; 1992. [Google Scholar]
- 7.Food and Drug Administration. Bioavailability and bioequivalence requirements. Fed Regist. 1992;57:17997–18001. [Google Scholar]
- 8.Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. New York, N.Y: Marcel Dekker, Inc.; 1982. [Google Scholar]
- 9.Heifets L B. Antituberculosis drugs: anti-microbial activity in vitro. In: Heifets L B, editor. Drug susceptibility in the chemotherapy of mycobacterial infections. Boca Raton, Fla: CRC Press, Inc.; 1991. pp. 13–58. [Google Scholar]
- 10.Iwainsky H. Mode of action, biotransformation and pharmacokinetics of antituberculosis drugs in animals and man. In: Bartmann K, editor. Antituberculosis drugs. Berlin, Germany: Springer-Verlag; 1988. pp. 399–553. [Google Scholar]
- 11.Jelliffe R W. ASCP clinical pharmacology check sample 10, no. DM 89-4 (DM 56). Chicago, Ill: American Society of Clinical Pathologists; 1989. Explicit determination of laboratory assay error patterns: a useful aid in therapeutic drug monitoring; pp. 1–6. [Google Scholar]
- 12.Lee C S, Gambertoglio J G, Brater D C, Benet L Z. Kinetics of oral ethambutol in the normal subject. Clin Pharmacol Ther. 1977;22:615–621. doi: 10.1002/cpt1977225part1615. [DOI] [PubMed] [Google Scholar]
- 13.Lee C S, Brater D C, Gambertoglio J G, Benet L Z. Disposition kinetics of ethambutol in man. J Pharmacokinet Biopharm. 1980;8:335–346. doi: 10.1007/BF01059382. [DOI] [PubMed] [Google Scholar]
- 14.Liss R H, Letouneau R J, Schepis J P. Disposition of ethambutol in primate tissues and cells. Am Rev Respir Dis. 1981;123:529–532. doi: 10.1164/arrd.1981.123.5.529. [DOI] [PubMed] [Google Scholar]
- 15.Mattila M J, Linnoila M, Seppälä T, Koskinen R. Effect of aluminum hydroxide and glycopyrronium on the absorption of ethambutol and alcohol in man. Br J Clin Pharm. 1978;5:161–166. doi: 10.1111/j.1365-2125.1978.tb01618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owens R C, Keung A C F, Gardner S, Eller M G, Eller S J, Weir S J, Nicolau D P. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Pharmacokinetic and food effect evaluation of rifapentine in subjects seropositive for the human immunodeficiency virus, abstr. A2; p. 1. [Google Scholar]
- 17.Peets E A, Sweeney W M, Place V A, Buyske D A. The absorption, excretion, and metabolic fate of ethambutol in man. Am Rev Respir Dis. 1965;91:51–58. doi: 10.1164/arrd.1965.91.1.51. [DOI] [PubMed] [Google Scholar]
- 18.Peloquin C A. Antituberculosis drugs: pharmacokinetics. In: Heifets L B, editor. Drug susceptibility in the chemotherapy of mycobacterial infections. Boca Raton, Fla: CRC Press, Inc.; 1991. pp. 59–88. [Google Scholar]
- 19.Peloquin C A. Using therapeutic drug monitoring to dose the antimycobacterial drugs. Clin Chest Med. 1997;18:79–87. doi: 10.1016/s0272-5231(05)70357-9. [DOI] [PubMed] [Google Scholar]
- 20.Peloquin C A, Bulpitt A E, Jaresko G S, Jelliffe R W, Nix D E. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Effect of food and antacids on the pharmacokinetics (PK) of ethambutol (EMB) and pyrazinamide (PZA), abstr. A3; p. 1. [Google Scholar]
- 21.Place V A, Thomas J P. Clinical pharmacology of ethambutol. Am Rev Respir Dis. 1963;87:901–904. doi: 10.1164/arrd.1963.87.6.901. [DOI] [PubMed] [Google Scholar]
- 22.Place V A, Peets E A, Buyske D A, Little R R. Metabolic and special studies of ethambutol in normal volunteers and tuberculosis patients. Ann N Y Acad Sci. 1966;135:775–795. doi: 10.1111/j.1749-6632.1966.tb45522.x. [DOI] [PubMed] [Google Scholar]
- 23.U.S. Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. Fed Regist. 1997;62:118. [Google Scholar]
- 24.Varughese A, Brater D C, Benet L Z, Lee C S. Ethambutol kinetics in patients with impaired renal function. Am Rev Respir Dis. 1986;134:34–38. doi: 10.1164/arrd.1986.134.1.34. [DOI] [PubMed] [Google Scholar]