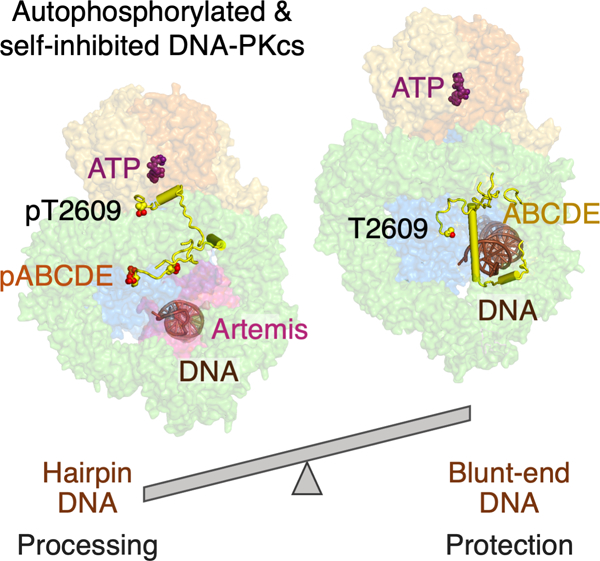
Summary
The DNA-dependent protein kinase (DNA-PK) initially protects broken DNA ends, but then promotes their processing during non-homologous end-joining (NHEJ). Before ligation by NHEJ, DNA hairpin ends generated during V(D)J recombination must be opened by the Artemis nuclease together with autophosphorylated DNA-PK. Structures of DNA-PK bound to DNA before and after phosphorylation, and in complex with Artemis and a DNA hairpin, reveal an essential functional switch. When bound to open DNA ends in its protection mode, DNA-PK is inhibited for cis-autophosphorylation of the so-called ABCDE cluster but activated for phosphorylation of other targets. In contrast, DNA hairpin ends promote cis-autophosphorylation. Phosphorylation of four Thr residues in ABCDE leads to gross structural rearrangement of DNA-PK, widening the DNA-binding groove for Artemis recruitment and hairpin cleavage. Meanwhile, Artemis locks DNA-PK into the kinase-inactive state. Kinase activity and autophosphorylation of DNA-PK are regulated by different DNA ends, feeding forward to coordinate NHEJ events.
Keywords: Artemis, Ku70, Ku80, DNA-PKcs, hairpin, NHEJ
Graphical Abstract
eTOC blurb
Liu et al. find that DNA-PK bound to a blunt-end DNA can autophosphorylate in cis (~33%) or phosphorylate other targets in trans (67%) while protecting the DNA end. A DNA hairpin shifts the equilibrium toward autophosphorylation, leading to dramatic conformational changes of DNA-PK, exposing DNA-end, Artemis recruitment, and kinase self-inhibition.
Introduction
Non-homologous end joining (NHEJ) is the major pathway for repair of DNA double-strand breaks induced by endogenous processes as well as exogenous DNA damage. DNA-dependent protein kinase (DNA-PK), composed of the 469 kD catalytic unit (DNA-PKcs) and the Ku70/80 heterodimer, is the platform and controller of DNA-end sensing, protection, processing, pairing and ligation in NHEJ (Jette and Lees-Miller, 2015; Meek et al., 2004). DNA-PKcs is a member of the PIKK (Phosphatidylinositol 3-kinase related kinase) family, which includes mTOR, ATR, ATM and SMG1 (Baretic and Williams, 2014). These protein kinases are key signaling molecules of cellular fitness and critical regulators of metabolism and biogenesis. With a simpler target than others, the main phosphorylation substrate of DNA-PKcs is itself. Mice can survive without DNA-PKcs, but Ala mutation of the main phosphorylation sites or elimination of the kinase activity of DNA-PKcs causes embryonic lethality and extreme radiosensitivity in cell lines (Jiang et al., 2015; Zhang et al., 2011). These studies reveal that DNA-PK protects DNA ends from degradation and loss of genetic information, but failure of DNA-PK autophosphorylation prevents NHEJ factors from accessing and repairing these ends. Despite extensive studies, how phosphorylation by PIKKs is regulated and how autophosphorylation of DNA-PKcs alters its macromolecular interactions have remained opaque.
NHEJ not only ligates broken DNA ends, but also opens DNA hairpin ends, which are generated by the RAG1/2 recombinase as obligate intermediates in the first step of V(D)J recombination (Roth, 2014), and completes end joining in antigen receptor gene rearrangement (Ma et al., 2002; Zhao et al., 2020). Artemis, a member of the β-CASP family with the metallo-beta-lactamase fold (Callebaut et al., 2002), is the only known nuclease that can open DNA hairpins in vertebrates (Ma et al., 2002). Defects in Artemis activity result in immunodeficiency (Moshous et al., 2001; Rooney et al., 2002). In the presence of DNA-PKcs, Artemis also can efficiently remove 5´ overhangs (Ma et al., 2002). The structure of human Artemis with a disordered DNA has been reported (Karim et al., 2020). The N-terminal 361 of the total 692 residues are folded into the nuclease domain, but the C-terminal regulatory region (Niewolik et al., 2006) is unstructured. In the presence of a DNA hairpin end, DNA-PK phosphorylates itself as well as the C-terminal portion of Artemis (Ma et al., 2002). But phosphorylation of Artemis is not required for the endonuclease activity of Artemis; instead, the hairpin-opening activity requires Artemis and autophosphorylated DNA-PK (Goodarzi et al., 2006; Ma et al., 2002).
Structures of DNA complexed with unphosphorylated DNA-PK holo-enzyme, either in the inactive or activated state, reveal that the DNA end and terminal 15 bp are bound and protected by DNA-PKcs, while the adjacent 16 bp internal DNA flank is stabilized by Ku70/80 (Chaplin et al., 2021; Chen et al., 2021b; Yin et al., 2017). DNA-PKcs is composed of two large roughly concentric HEAT repeat rings, N-HEAT and M-HEAT, and the FATKIN head (including the kinase domain and FAT regulatory region), which is conserved in all PIKKs (Chaplin et al., 2021; Chen et al., 2021b; Sibanda et al., 2017). A DNA-binding groove formed between the two HEAT rings at the bottom of DNA-PKcs continues the binding tunnel of Ku70/80 to accommodate over 30 bp of DNA (Chen et al., 2021b).
The principal autophosphorylation sites in DNA-PKcs, T2609, T2638, and T2647, are collectively known as the ABCDE cluster, which forms an extension of the clasp that closes the M-HEAT ring at the neck under the FATKIN head. Autophosphorylation of ABCDE loosens the DNA-PK interaction with DNA and allows NHEJ factors to process and ligate DNA ends (Block et al., 2004; Crowe et al., 2020; Ding et al., 2003; Goodarzi et al., 2006; Meek, 2020; Reddy et al., 2004). In the available DNA-PK structures, which are all unphosphorylated and devoid of ATP, ABCDE is largely disordered and situated either outside the HEAT rings near the kinase domain (Chen et al., 2021b) or spread inside the HEAT rings (Chen et al., 2021a; Sibanda et al., 2017; Yin et al., 2017). Autophosphorylation of a second patch, PQR, restrains Artemis nuclease activity from excessive DNA-end processing (Cui et al., 2005). As both monomeric and dimeric DNA-PK complexes, as well as DNA-PK dimerized via the NHEJ factors (Ligase IV, XRCC4 and XLF), have been observed (Chaplin et al., 2021; Chen et al., 2021a; Chen et al., 2021b; Graham et al., 2018; Hepburn et al., 2021), whether autophosphorylation occurs in cis within a DNA-PK monomer (Lu et al., 2008) or in trans in a dimer of DNA-PK (Chen et al., 2021a; Meek et al., 2007) is still under debate.
We previously reported structures of inactive and active forms of DNA-PK, with the substrate peptide-binding groove either blocked by the PIKK-regulatory domain (PRD) (complexes I-V) or accessible, with a raised FATKIN head (complex VI) (Chen et al., 2021b, video S4), all without ATP. To understand how autophosphorylation of DNA-PKcs is regulated and how DNA-PK both activates and restrains Artemis, we have determined cryoEM structures, at resolutions up to 2.7 Å, of (1) DNA-PK protecting a DNA blunt end before autophosphorylation, (2) conformational changes of DNA-PK upon cis-autophosphorylation of the ABCDE cluster, (3) recruitment of Artemis for DNA hairpin opening, and (4) self-inhibition of the ABCDE-phosphorylated DNA-PK. These structures together with biochemical and cellular data reveal how DNA-PK responds differently to different DNA ends, the duality of DNA-PKcs autophosphorylation, and its consequences in NHEJ. What we find here probably implies a general mechanism for PIKK function and regulation.
Results
Structures of phosphorylated DNA-PK with and without Artemis and DNA hairpin
To capture phosphorylated DNA-PK in complex with Artemis and a DNA end, we monitored in parallel the time course of DNA-PK autophosphorylation and DNA cleavage by Artemis of either a hairpin end or an open end with a 5´ overhang (Fig. S1). In both cases, autophosphorylation of DNA-PKcs occurred first, reaching a plateau by 10 min at 37°C, but DNA cleavage by Artemis only started after 5 min and did not plateau until beyond 60 min (Fig. 1A). The separate phases of these two enzymatic events provided us a window to structurally capture autophosphorylated DNA-PK complexed with Artemis before the DNA hairpin was cleaved.
Figure 1. Autophosphorylation of DNA-PK activates Artemis.
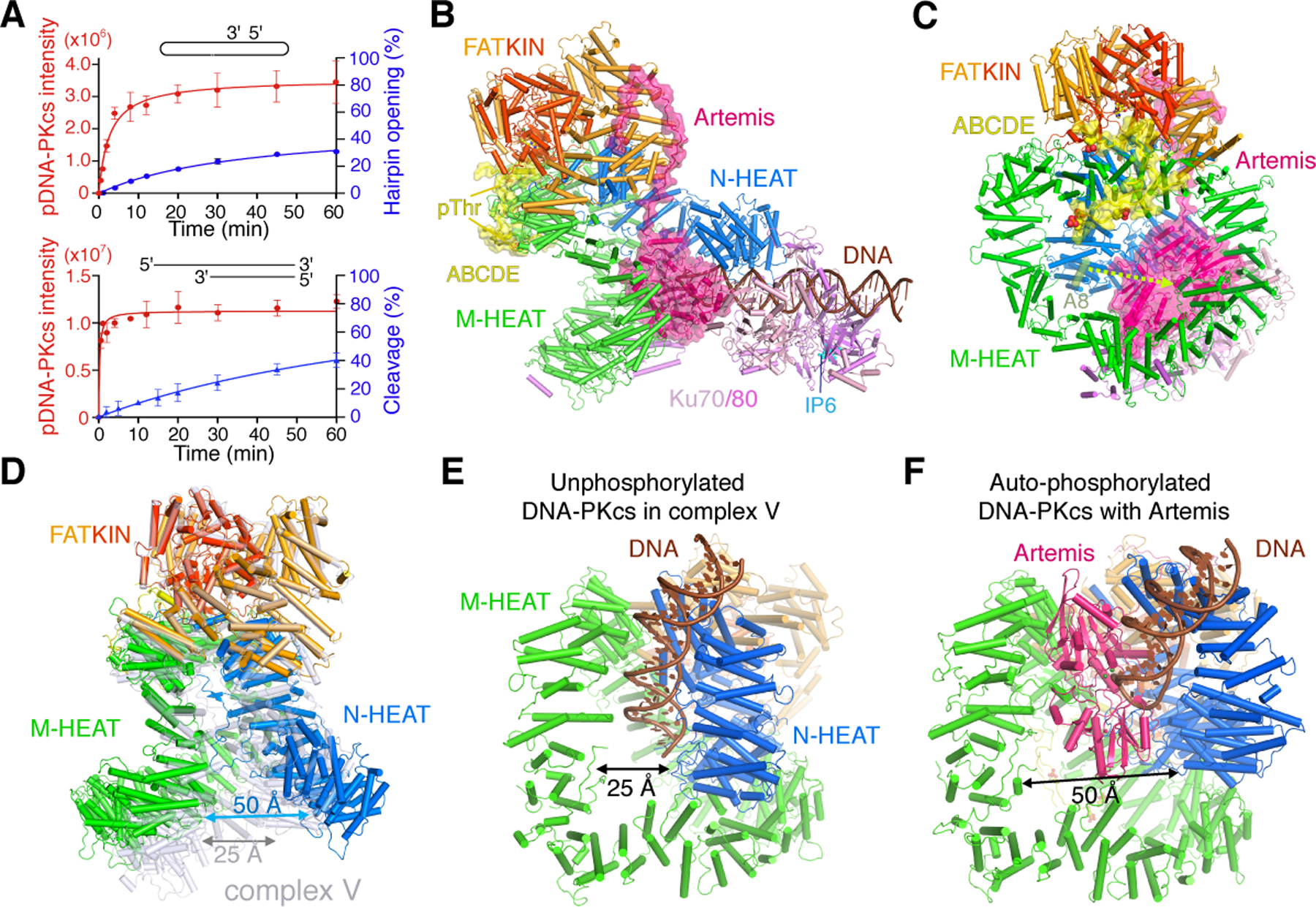
A. The time course of phosphorylation of DNA-PK and DNA cleavage by Artemis. With hairpin end or 5´-overhang DNA, phosphorylation clearly occurs before DNA cleavage. B. Overall structure of DNA-PK, Artemis and DNA complex (side view). Artemis and the ABCDE patch are shown with pink and yellow semi-transparent molecular surface, respectively. The IP6 bound to Ku70/80 (as cyan balls) is indicated. C. The front view of DNA-PK complexed with Artemis and hairpin DNA. Phosphorylated T2609, T2638, T2645 and T2647 are shown as yellow (C), orange (P) and red (O) spheres. The shift of helix A8 from complex V (semitransparent green) to contacting Artemis is indicated by a dashed lemon arrowhead. D. Structural changes of phosphorylated DNA-PK. DNA-PKcs in the Artemis complex (multicolor) is superimposed on the unphosphorylated form of complex V (PDB: 7K1Y, grey). E. The DNA binding groove is closed in complex V before autophosphorylation. F. The two HEAT rings are opened wide after ABCDE autophosphorylation to expose the DNA end and accommodate Artemis.
For structural analysis of DNA hairpin opening, we froze a reaction mixture of DNA-PK, Artemis, and DNA with hairpins on both ends, after 12 min of incubation at 37°C with 0.3 mM ATP and 5 mM Mg2+ (see Methods). CryoEM analysis revealed that the complex of DNA-PK, Artemis and hairpin DNA was the dominant species (Fig. S2). The structure of the complete complex was determined at 3.0 Å, with Artemis and FATKIN domain locally refined to 2.7 Å (Table S1, Fig. 1B-C). The unprecedented high resolution also reveals an inositol hexakisphosphate (IP6) co-purified with Ku70/80 from human cells (Fig. 1B, S2M). The IP6 is bound opposite to the DNA-binding channel, as previously identified by biochemical and mutational analyses (Cheung et al., 2008; Ma and Lieber, 2002). In this structure, the Thr residues in ABCDE are phosphorylated and well ordered. Different from all known DNA-PK structures, the two HEAT rings, N (N-terminal) and M (middle), which form the DNA-binding groove, have opened wide by pivoting at the neck that links them to the FATKIN head (Fig. 1D-F) (Movie S1). Artemis is inserted between M-HEAT and the DNA substrate, while the DNA remains bound to N-HEAT and Ku.
In parallel, we briefly incubated DNA-PK bound to a blunt DNA end with ATP and Mg2+, and obtained the structure of autophosphorylated DNA-PK without Artemis at 3.6 Å (Fig. S3, Methods, Table S1). In this structure (termed complex VIII) DNA-PKcs is very similar to that in the Artemis-DNA-PK complex (Fig. S4A), but the DNA and associated Ku and the very N terminus of DNA-PKcs are mobile and difficult to trace, which agrees with observations that autophosphorylated DNA-PKcs releases bound DNA and Ku (Jette and Lees-Miller, 2015). The opening of N-HEAT and reduced DNA binding by phosphorylated DNA-PKcs in complex VIII mirror the weak initial DNA binding by unphosphorylated DNA-PKcs in complex I (Chen et al., 2021b). N-HEAT and the top of M-HEAT outside of ABCDE patch in complex I and VIII are similar (Fig. S4B-C), while autophosphorylation most significantly changes the lower two thirds of M-HEAT, where DNA binds.
Autophosphorylation occurs in cis and self-inhibits DNA-PKcs kinase
In both structures after ATP incubation, the ABCDE patch of DNA-PKcs (aa 2580–2780) is phosphorylated at T2609, T2638, T2645 and T2647 (Fig. S2K). T2645 is a minor phosphorylation site but found in human tissues (www.phosphosite.org/siteAction.action?id=31693). Phosphorylation of ABCDE transforms it from being disordered (Chen et al., 2021a; Chen et al., 2021b; Neal et al., 2014; Sibanda et al., 2017; Yin et al., 2017) to a square pair of jaws. With its phosphates serving as teeth, ABCDE bites the top rim of the M-HEAT ring and lifts M-HEAT toward the FATKIN head (Fig. 1C, 2A). The upper jaw contains pT2609; the lower jaw has the other three phosphorylation sites. Each phosphate interacts with positively charged side chains (Fig. 2B-D). The phosphorylated pT2638 surrounded by R2773, R2776 and K1042 fastens the ABCDE extension to the M-HEAT ring (Fig. 2A). The lift of M-HEAT by ABCDE opens the two HEAT rings (Fig. 1D-F, S4D), which undergo Slinky-like as well as rigid-body movement (Movie S1). The groove for DNA binding, which is ~25 Å wide before autophosphorylation, expands to over 50 Å in width with or without Artemis (Fig. 1D-F) and exposes the DNA end after autophosphorylation.
Figure 2. Re-modeling of DNA-PKcs by ABCDE autophosphorylation.
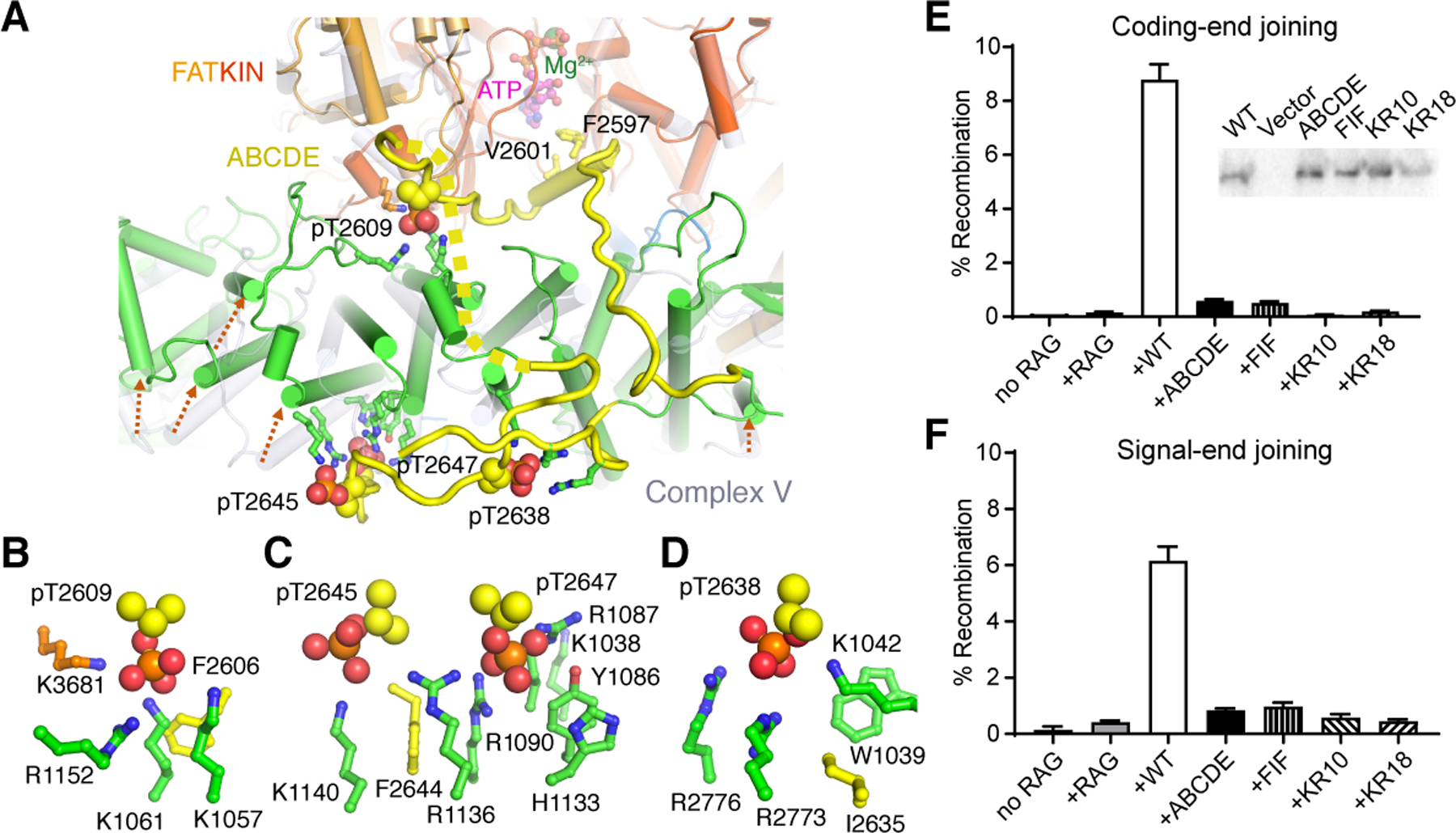
A. The phosphorylated ABCDE binds the top rim of M-HEAT (aa 1090 – 1160). ABCDE is colored yellow, M-HEAT green, and FAT orange. The phosphates are shown as orange (P) and red (O) spheres. B-D. Zoom-in view of each phosphorylated site in the ABCDE patch. pT2745 (B), pT2645 and pT2647 (C), and pT2638 (D) are surrounded by positively charged sidechains. The hydrophobic residue preceding each phosphorylation site is also shown and labeled. Residues that bind pThr are mutated in two clusters, KR10 (R1031 to K1074) and KR18 (KR10 plus Y1086 to R1152). Twelve of the 18 residues mutated are shown as green and blue sticks, and the remaining six are on the periphery of pThr binding and solvent exposed. E-F. Episomal assays of WT DNA-PKcs (WT), Ala mutations of ABCDE phosphorylation sites (ABCDE), the adjacent hydrophobic residues (FIF), and binding sites of phosphorylated Thr (KR10 and KR18) in joining coding (E) and signal ends (F) generated by RAG1/2 (RAG). Western blot of WT and mutant DNA-PKcs expression is shown as an insert in panel E.
Based on recent phylogenetic studies, phosphate-binding K1042, K1061, H1133, R1136 and W1039 are highly conserved (Lees-Miller et al., 2021). We also noted that the third residue preceding each phosphorylation site, which is always hydrophobic and bulky (Lees-Miller et al., 2021) (www.biorxiv.org/content/10.1101/2021.04.26.441429v1), interacts with the aliphatic arms of Lys and Arg that coordinate the phosphate (Fig. 2B-D). Ala substitution of F2606, I2635, and F2644 preceding T2609, T2638, and T2647 (mutant termed FIF) or Ala replacement of 10 or 18 Lys and Arg residues, which coordinate pT2609, pT2638, pT2645 and pT2647 (mutants termed KR10 of R1031 to K1074, and KR18 of R1031 to R1152) (Fig. 2B-D), results in severe deficits in both V(D)J coding-end (hairpin) and signal-end (blunt) joining (Fig. 2E-F). The fact that joining of both coding (hairpin) and signal ends (blunt) by the FIF, KR10 or KR18 mutant DNA-PKcs are as defective as with Ala mutation of the ABCDE phosphorylation sites (Fig. 2E-F) indicates that blocking the dramatic conformational changes that results from phospho-ABCDE binding to M-HEAT is functionally equivalent to blocking phosphorylation itself.
Numerous previous studies have concluded that ABCDE phosphorylation is required to allow access to DNA ends for both end processing factors and the ligase complex, and that ABCDE autophosphorylation is requisite for efficient NHEJ (Crowe et al., 2020; Ding et al., 2003; Jiang et al., 2015; Neal et al., 2014; Zhang et al., 2011). We show here that ABCDE phosphorylation leads to the N- and M-heat ring opening, which is mediated by association of the phosphorylated sites and preceding hydrophobic residues with the positively charged pockets lined by Lys and Arg residues (Fig. 2), thus making the DNA end accessible to repair factors. Our structure and mutational analysis provide the molecular mechanism for previous cellular observations.
Although an ATP molecule occupies the kinase active site in both phosphorylated structures, the substrate peptide-binding groove is blocked by the closed PRD on one end and by residues 2594–2603 of ABCDE on the other end (Fig. 2A, S2L). Therefore, after ABCDE is phosphorylated, DNA-PKcs is self-inhibited and cannot phosphorylate other targets. The very close approach (10 Å) of ABCDE to ATP in the kinase active site in both structures suggests that autophosphorylation occurs within a single DNA-PK complex in cis, as biochemical analysis indicated (Lu et al., 2008).
The FATKIN domain in autophosphorylated DNA-PKcs is indistinguishable from that in the inactive DNA-PK complex V (PDB:7K1N) (Chen et al., 2021b); the two are superimposable with a RMSD of 1.5 Å over 1205 pairs of Cα atoms (Fig. S4D). Transition from unphosphorylated Complex V to VIII (or to Artemis-DNA-PK) involves a 40 Å translation and 30° rotation of the DNA along with Ku70/80 and the first half of N-HEAT (aa 1–350) as a block (Fig. S4E). In the presence of Artemis the DNA is shifted along its helical axis by 4 bp outward, away from the center of the double HEAT rings, and the hairpin end is relinquished to Artemis (Fig. 1).
Activation of Artemis
The diamond-shaped Artemis is wedged crosswise between M-HEAT and DNA with the DNA hairpin end captured in the nuclease active site, and the scissile phosphate coordinating two Mg2+ ions necessary for the DNA cleavage (Fig. 3A-B). N-HEAT of DNA-PKcs fortifies the DNA hairpin binding by Artemis. Lengthwise, Artemis is bound by the well-folded C-terminal region (CTR) of Ku80 and the αβ domain of Ku70, which contacts M-HEAT and DNA in the unphosphorylated DNA-PK complexes (Chen et al., 2021b). Although the DNA hairpin end contains a fully complementary sequence, two nucleotides on each side of the hairpin turn are unpaired and the 3rd base pair from the hairpin is distorted (Fig. 3B-C). The scissile phosphate is between the 1st and 2nd nucleotide on the 5´ side of the duplex, so cleavage by Artemis would lead to a 3´ overhang of two nucleotides, consistent with in vivo observations (Lafaille et al., 1989).
Figure 3. Activation of Artemis by autophosphorylated DNA-PK on a hairpin-end DNA.
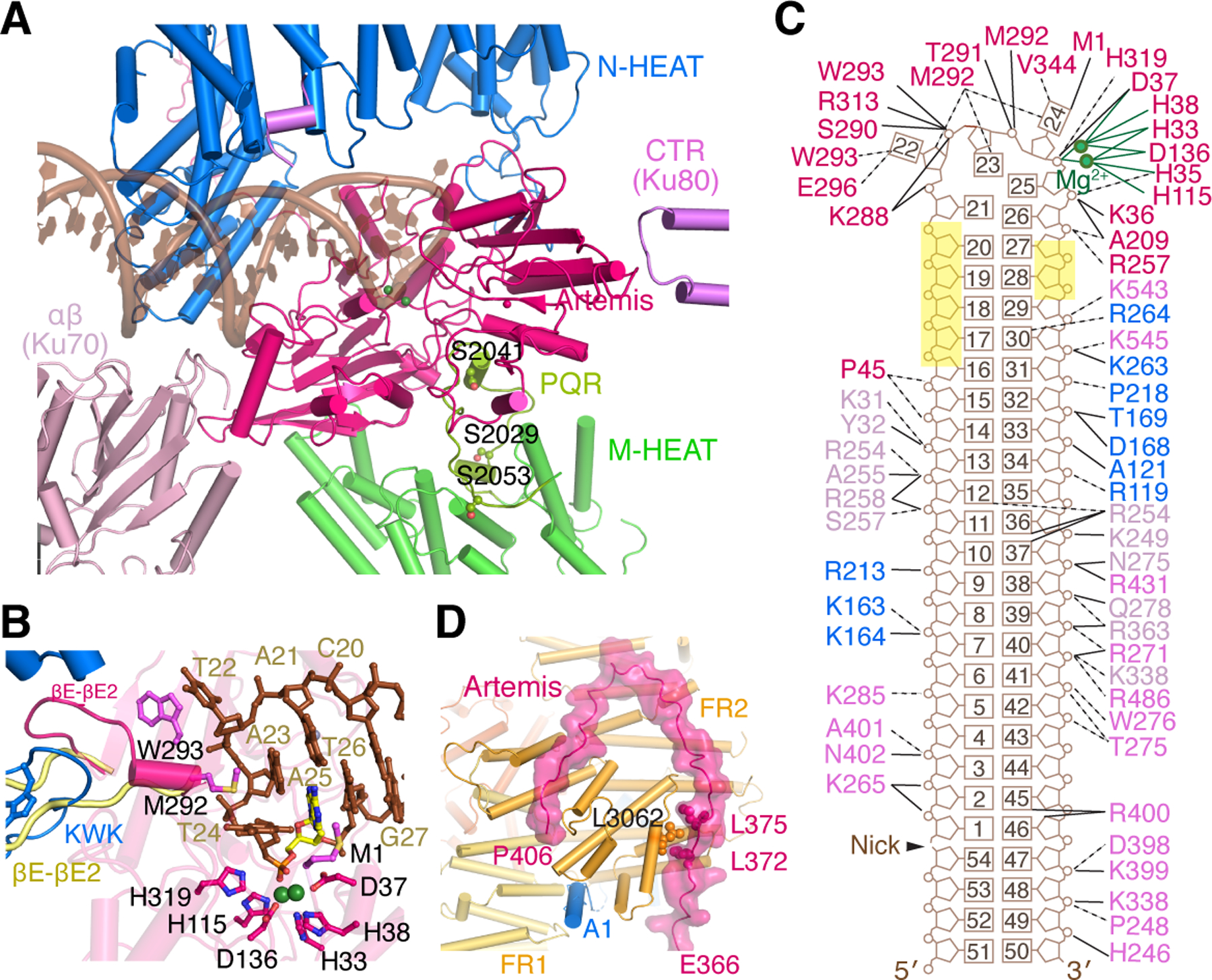
Zoom-in view of interactions between Artemis and DNA-PK with hairpin DNA. The PQR patch (aa 2000–2060), which is unphosphorylated and colored yellow-green, interacts with Artemis on the backside from DNA binding. B. DNA hairpin end in the Artemis active site. The catalytic residues are shown as magenta sticks. Mg2+ ions are shown as green spheres. The nucleotide containing the scissile phosphate is highlighted in yellow. The βE-βE2 loop of apo-Artemis (aa 293–303, shown in pale yellow after structural superposition) is remodeled by the KWK (aa 503–521) and adjacent loop (aa 447–452) of DNA-PKcs. The Artemis βE-βE2 loop forms a helix and contacts the unpaired DNA hairpin end with M292 and W293, which are shown as magenta sticks. C. Diagram of DNA interactions made by Artemis, DNA-PKcs, and Ku. Labels are colored in the same scheme as in panel A. The nucleotides with no protein contact and exposed to solvent are highlighted in yellow. D. Zoom-in view of the extended peptide of Artemis (aa 361–406) reaching to FATKIN of DNA-PKcs, stabilizing the inactive state. Helix A1 of DNA-PKcs (blue) is wedged between FR1 and FR2, further stabilizing the inactive state. L3062 of DNA-PKcs, which is sandwiched by L372 and L375 of Artemis, must be critical for the protein complex formation, as the L3062R mutation leads to SCID.
The nuclease core of Artemis (aa 3–361) is superimposable with the crystal structures of Artemis (PDB: 6WO0, 6WNL, 6TT5 and 7ABS) with an RMSD of 0.8 Å over 333 pairs of Cα atoms (Karim et al., 2020; Yosaatmadja et al., 2021) (Fig. S5). Residues beyond the nuclease domain, aa 398 to 403, have been identified to be critical for DNA-PKcs binding (Niewolik et al., 2006), but they are absent from the crystal structures. In the complex with DNA-PK, although the C-terminal one-third of Artemis remains disordered, Artemis reveals an extended structure of residues 362–406, stretching 40 Å from the DNA binding groove to reach the FATKIN head and contacting its FR1, FR2 and FR3 subdomains (Fig. 3D). Juxtaposition of the disordered C-terminal regulatory region of Artemis with FATKIN of DNA-PKcs in the single complex renders phosphorylation of Artemis in cis probable. Interestingly, L3062 of DNA-PKcs is sandwiched between L372 and L375 of Artemis. This hydrophobic cluster must be important for Artemis recruitment, as the L3062R mutant DNA-PKcs has been implicated in SCID (severe combined immunodeficiency) in human patients, and is defective in Artemis processing of V(D)J coding ends (van der Burg et al., 2009).
The detailed structure reveals that Artemis does not bind the duplex portion of the DNA, and has direct contacts with only 6 nucleotides, 4 in the unpaired loop of the hairpin end (T22, A23, T24 and A25) and 2 additional (T26 and A27) on the cleavage side (Fig. 3C). Except for two hydrophobic interactions with DNA bases, the interactions of Artemis with DNA are polar, involving the phospho-sugar backbone, which supports the non-sequence-specific nature of DNA cleavage. The DNA duplex, over 22 bp, is bound by DNA-PK, which explains why Artemis alone is devoid of endonuclease activity. Five consecutive nucleotides (position 17–21) 5´ to the hairpin loop make no protein contact (Fig. 3C), and this “opening” may accommodate a DNA hairpin of varied loop size or an extended 5´-overhang for Artemis to cleave (Fig. S1).
Around the catalytic center of Artemis, the most significant structural change occurs in the βE-βE2 loop (aa 290–303) in the β-CASP domain (Karim et al., 2020), which is re-arranged from being partially exposed in the absence of DNA to becoming a short α-helix and clamping onto the DNA hairpin (Fig. 3B, S5). In the crystal structure of Artemis without DNA, this loop is rooted in the hydrophobic core by M292, W293 and F294. The N-HEAT repeat 11 of DNA-PKcs (aa 509–520), which is named KWK for K518, W519 and K520, flips it open and exposes M292 and W293 for interactions with two unpaired bases on the 3´ side of the scissile phosphate (Fig. 3B). The flipped βE-βE2 loop is further stabilized by the N-HEAT repeats 9 (aa 398–406) and 10 (aa 447–451) of DNA-PKcs. In the unphosphorylated Complex V, these three HEAT repeats (9–11) bind the blunt DNA end (Chen et al., 2021b). Autophosphorylation of DNA-PK frees the DNA end from these HEAT repeats, which then bind and reshape Artemis with both hydrophobic and hydrophilic interactions (Fig. 3A-B). The hairpin end-binding by the remodeled βE-βE2 loop may explain why Artemis exhibits 5´ exonuclease activity on single-stranded DNA in the absence of DNA-PKcs (Ma et al., 2002). Next to loop βE-βE2, loops βA-αB (aa 206–211) and βD-αF (aa 256–263) of Artemis also undergo structural changes and bind the minor groove at the DNA-hairpin end (Fig. S5). The first two residues, M1 and S2 at the N terminus, which are absent in the crystal structures of Artemis (PDB: 6WNL, 6WO0 and 7AF1), are very close to the catalytic center and interact with the unpaired base associated with the scissile phosphate (Fig. 3B-C). These conformational changes, particularly in loop βE-βE2, suggest how the hairpin endonuclease is activated by DNA-PK.
Artemis sequesters the autophosphorylated and self-inhibited DNA-PK
When the HEAT rings open upon ABCDE phosphorylation, two DNA-PKcs α-helices A1 and A2 (aa 814–837) of N-HEAT are displaced from DNA binding (Chen et al., 2021b) and relocated 40 Å away to the FATKIN head, with helix A1 wedged between FR1 and FR2 subdomains (Fig. 3D). This A1-binding site exists only in the inactive conformation of DNA-PKcs and is eliminated in the activated state when the FATKIN head is raised (Fig. S4F). The particular conformation of FR1 and FR2, with helix A1 bound between them, is transmitted to FR3 and FR4, keeping the PRD loop closed and preventing substrate peptides binding to the kinase active site. Displacement of helix A1 from DNA and opening of the HEAT rings allow Artemis to bind a hairpin end. In return, Artemis stabilizes the DNA in the open DNA-PK structure and also the inactive conformation of DNA-PKcs.
Opening of the two HEAT rings from the neck (12 o’clock position) down to the DNA binding groove (6 o’clock) also releases Helix A8 (aa 2034–2048), which contains two PQR phosphorylation sites and is wedged between the two HEAT rings at the 9 o’clock position before ABCDE phosphorylation (Chen et al., 2021b) (Fig. 1D). In the complex with Artemis and DNA hairpin, the previously partially disordered PQR patch becomes traceable in its entirety from E1995 to Q2057. Helix A8 binds the back side of Artemis (opposite the DNA-binding surface) by hydrophobic interactions (L2036, M2040, F2043, F2045 and V2049). The phosphorylation sites S2029, S2041 and S2053 of PQR are clearly unmodified, and are sequestered by interactions with Artemis, which is consistent with the observation that hairpin DNA ends do not promote PQR phosphorylation (Meek, 2020) (Fig. 3A). After the DNA hairpin end is opened, Artemis association with the cleaved DNA and DNA-PK is probably reduced, so PQR phosphorylation may take place more readily. Phosphorylated PQR, particularly at S2029 and S2041 (Fig. 3A), potentially disrupts Artemis-DNA-PKcs interactions and dissociates the two. This probably explains how phospho-mimetic mutations of PQR limit excessive DNA-end processing (Cui et al., 2005). In contrast, Ala mutations of the phosphorylation sites in PQR may prolong the Artemis association with DNA-PK even after the hairpin end is cleaved, and lead to extended trimming of DNA ends (Cui et al., 2005). The prolonged association of Artemis with the PQR-unphosphorylated form of DNA-PKcs may partially overcome the requirement of ABCDE phosphorylation (Neal et al., 2014) for accessibility of a hairpin end, because loose DNA binding by N-HEAT in the unphosphorylated complex I (Chen et al., 2021b) is similar to that in the ABCDE-phosphorylated complexes (Fig. S4B-C).
Dual function of DNA-PK in protecting or making DNA ends accessible
Analysis by cryoEM of DNA-PK bound to blunt end DNA in the presence of ATP reveals two major structural species (Fig. S3). The first is as described above (complex VIII) with ABCDE phosphorylated, and DNA and Ku70/80 partially released from DNA-PKcs. In the second, the DNA-bound DNA-PK is in the activated state but unphosphorylated, similar to our previously reported Complex VI (Chen et al., 2021b) (Fig. 4A-B, S6). But the ABCDE patch, instead of being disordered and situated near the kinase domain as in Complex VI, has moved inside the HEAT rings and can be traced and modeled, except for residues 2611–2652. The DNA end is covered, as found in the long-range (LR) complex with LigIV-XRCC4 and XLF at 4.6 Å resolution (Chen et al., 2021a). We determined this holo-enzyme structure at 3.3 Å resolution (Fig. S3) and termed it Complex VII.
Figure 4. Structure of Complex VII.
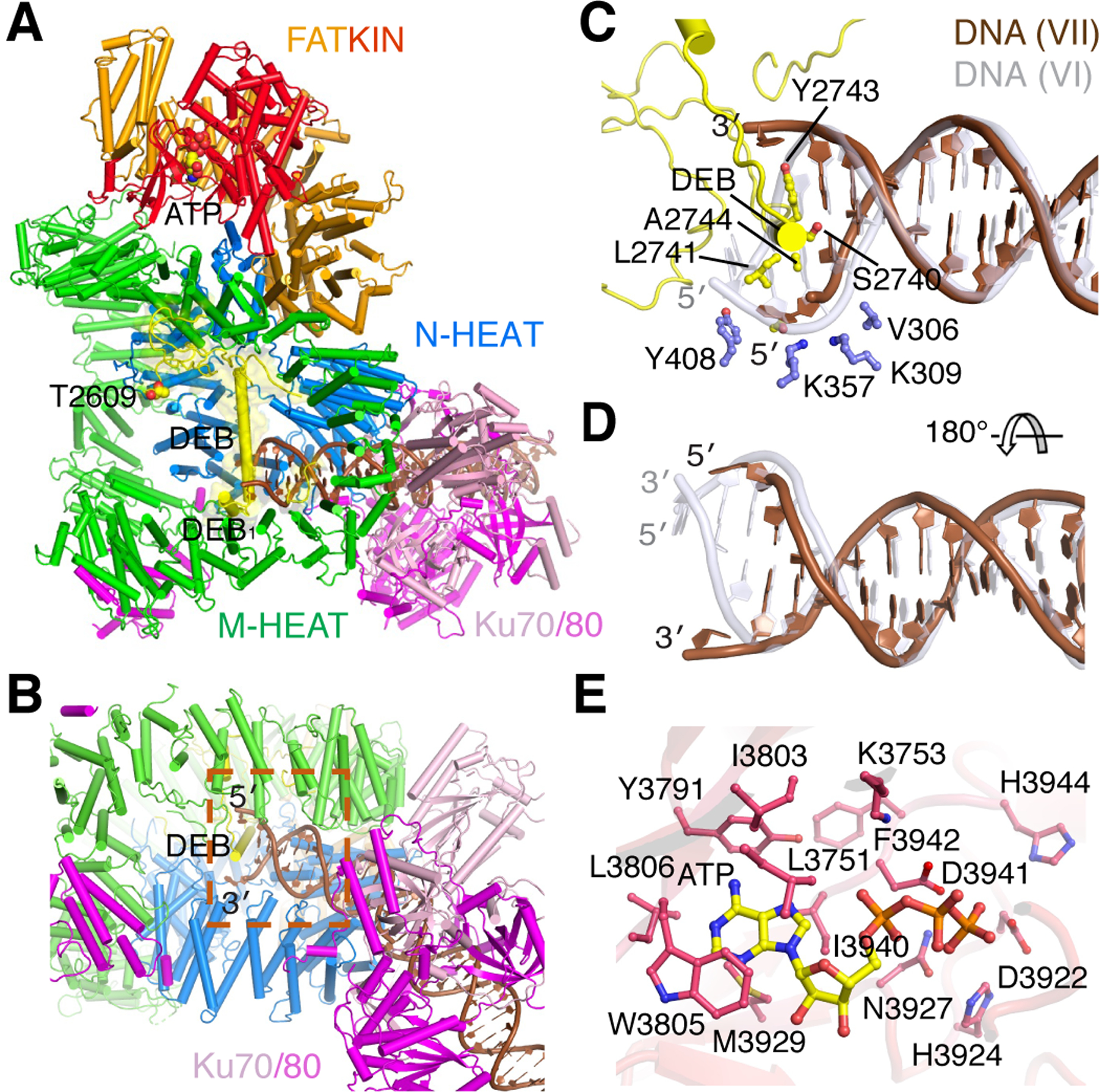
A. The overall structure with the unphosphorylated ABCDE patch (yellow) covering the DNA blunt end. B. The local structure of the DNA end sandwiched between two HEAT rings and protected by the long helix DEB of ABCDE. C. Zoom-in view of protein-DNA interactions at the blunt end. The first nucleotide of each strand is unpaired. DNA in Complex VI is shown as semitransparent grey after superposition of DNA-PK. D. The DNA in complex VII is shifted outward by 2 bp along the helical contour. E. The active site of DNA-PKcs is occupied by ATP. The adenine base is cocooned by hydrophobic residues. The catalytic residues are in proximity for phosphorylation of a substrate peptide.
In Complex VII, the DNA duplex shifts outward by 2 bp compared to Complex VI (Fig. 4C-D) (Movie S2), and an extension of the ABCDE patch replaces N-HEAT in binding the DNA end. The long α-helix (aa 2737–2764, called DEB for DNA-End Blocking (Chen et al., 2021a), separates the first base pair of the blunt DNA end and stacks with the second base pair via Y2743 and A2744. The nucleotide at the very end of each DNA strand is flipped out (Fig. 4C). By comparison, in the LR complex the 5´ end is recessed, so only the duplex with a 3´ overhang was modeled (Chen et al., 2021a). Here we observe that the first nucleotide on the 5´ end is encased between N-HEAT and the long α-helix DEB (Fig. 4C). On the 3´ end, a gap between ABCDE and M-HEAT can easily accommodate an overhang of unrestricted length (Fig. 4B-C). DNA-PK thus protects DNA ends, either blunt or with a 3´ overhang in the form of complex VII.
Previously DNA-PK complexed with the blunt-end DNA in the absence of ATP was prepared at 4°C, and only complex VI was observed, while complex VII was absent. Thermal energy perhaps helps to melt the terminal DNA base pair and promote complex VII formation at 37°C. To confirm the existence of unphosphorylated complex VII, structures of DNA-PK bound to the blunt-end DNA and briefly incubated with the non-hydrolyzable ATP analog, AMPPNP, and Mg2+ at 37°C were determined (Fig. S7, Methods). In contrast to the incubation with ATP, where complexes VII (unphosphorylated) and VIII (phosphorylated) were observed in 4:3 molar ratio (Fig. S3), incubation with AMPPNP resulted in complex VI with AMPPNP and VII-like structures at a ratio of 1:2 (Fig. S7, Table S1). AMPPNP doesn’t change the structure of complex VI, in which ABCDE is near the kinase domain for cis autophosphorylation and the DNA end is partially protected by N-HEAT (Chen et al., 2021b). Complex VI is most probably the precursor of autophosphorylated complex VIII (Fig. 5A). In the presence of ATP, complex VI was not detected because of its conversion to complex VIII by autophosphorylation (Fig. 5B). The autophosphorylation reaction perturbed the equilibrium between complex VI and VII and led to lower percentage of VII (57%) with ATP than with AMPPNP (67%). The distribution of these blunt-end DNA complexes indicates that end protection by ABCDE (complex VII) is preferred over the ready-to-be or already autophophorylated forms (VI and VIII), which lead to exposure and processing of the DNA end.
Figure 5. Dual function of DNA-PK.
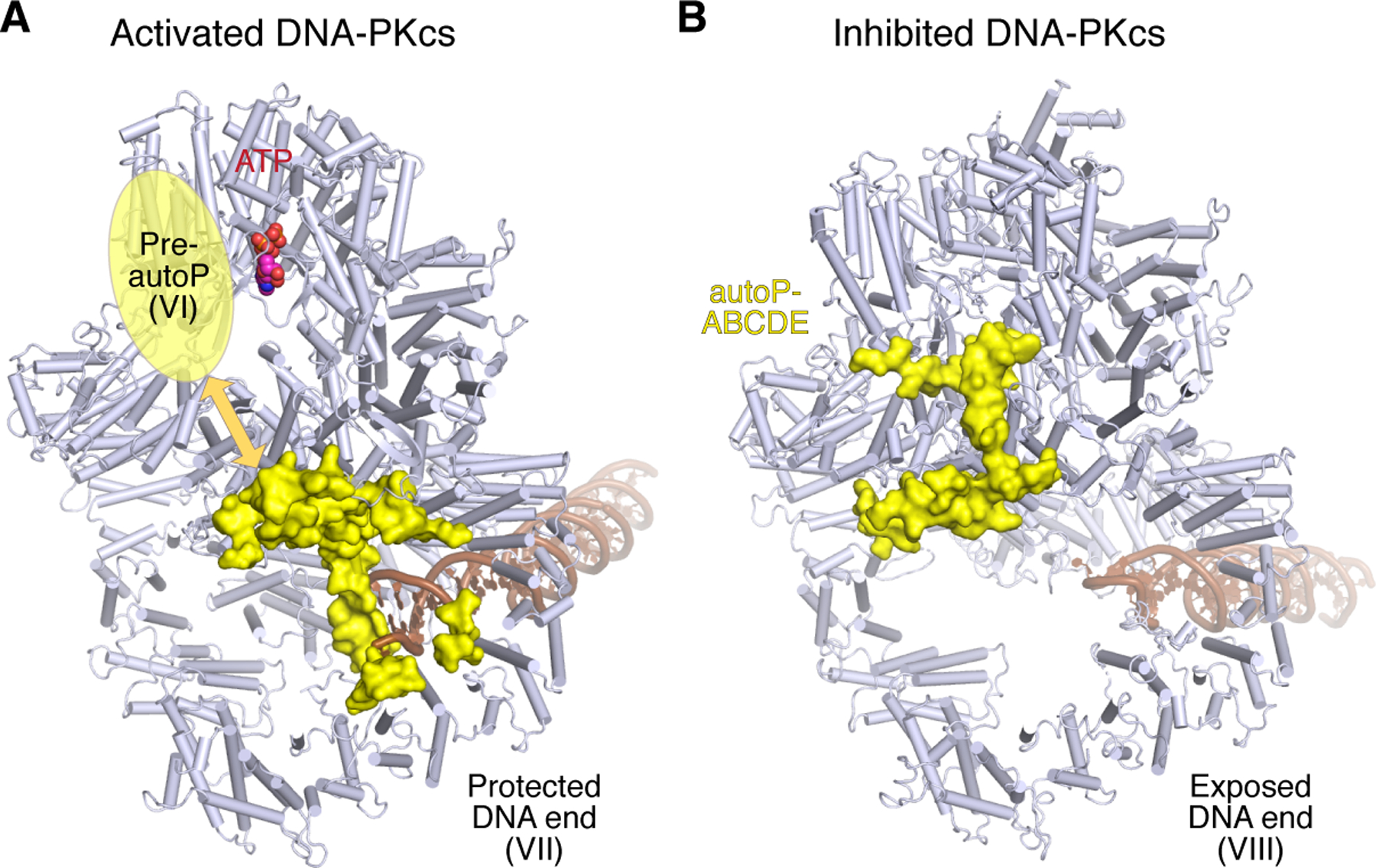
A. When bound to DNA blunt end, DNA-PKcs is in equilibrium between DNA-end blocking (complex VII) and ABCDE autophosphorylation-ready (complex VI) form. In complex VII, the kinase is activated, but ABCDE and surrounding region (yellow) is sequestered by the DNA end. In the presence of a hairpin end, DNA-PK can form only complex VI. B. Autophosphorylation of ABCDE leads to gross structural changes of DNA-PKcs (complex VIII) with the phosphorylated ABCDE (yellow) biting the top rim of M-HEAT and exposing the DNA end to Artemis and other NHEJ repair factors. Except for ABCDE, DNA-PKcs is represented by light blue cartoon diagram. DNA is shown as brown tube-and-ladder.
In Complex VII, the ABCDE phosphorylation cluster is anchored inside the HEAT rings by T2609, Q2610 and R2653, which are traceable but over 60 Å away from the kinase active site (Fig. 4A). A similarly long distance (>60 Å), which cannot be covered by seven (T2647 to R2653) or sixteen residues (T2638 to R2653), is also observed in the dimerized DNA-PK (Chen et al., 2021a). Although in Complex VII the kinase is activated as in complex VI, with an ATP (or AMPPNP) occupying the active site and the substrate peptide-binding site accessible (Fig. 4E, S3G), autophosphorylation of ABCDE is unlikely to occur either in cis or in trans because of the >60Å distance separating the substrate and the catalytic site.
The activated kinase in complex VII, however, could phosphorylate other targets in trans, such as Ku70/80 and PQR of DNA-PKcs. When DEB blocks the blunt DNA end in complex VII, a short helix before DEB, DEB1 (aa 2723–2730), replaces the helix A7 of PQR observed in Complex VI to interact with M-HEAT (Fig. 4A). The PQR patch along with its surrounding residues (aa 2002–2081) is disordered in Complex VII. So PQR phosphorylation is possible, and likely to occur in trans because PQR is over 90 Å away from the kinase domain of the same polypeptide chain. Phosphorylated PQR is expected to have little influence on the stability of Complex VII.
DNA ends determine the phosphorylation state of DNA-PK
We envision that Complex VII, in which ABCDE (DEB) is engaged in protecting an open DNA end and cannot be autophosphorylated, and complex VI, which is ready for autophosphorylation, are in equilibrium when DNA-PK is bound to blunt end DNA (Fig. 5). A specific type of DNA end may favor one complex over the other. Unlike blunt-end DNA, a hairpin end cannot stack with DEB and a 5´-overhang is not easily accommodated in complex VII. Therefore these DNA ends are more likely to form complex VI leading to rapid autophosphorylation and activation of Artemis. In contrast, a blunt end, or DNA with a 3´-overhang, would favor complex VII formation and DNA end protection. Indeed, in the presence of Artemis and a DNA hairpin or 5´-overhang end, DNA-PKcs was autophosphorylated efficiently (Fig. 1A). Similar results were also obtained when we assayed the kinase activity of DNA-PK in the absence of Artemis (Fig. 6A-B). DNA-PKcs autophosphorylation was fastest in the presence of hairpin DNA ends (complex VI only) and slowest with blunt ends and 3´-overhangs (preferably forming complex VII). Moreover, formation of complex VII, which slows down autophosphorylation of DNA-PKcs, promotes phosphorylation of other targets. For example, Ku70/80 of other DNA-PK complexes is more heavily phosphorylated in trans with open DNA ends than hairpin DNA (Fig. 6, S1). Because phosphorylated ABCDE blocks substrate binding to DNA-PKcs, formation exclusively of complex VI with hairpin DNA, and the resulting rapid autophosphorylation of ABCDE, explains the loss of other phosphorylation in the presence of hairpin DNA (Meek, 2020; Smider et al., 1998).
Figure 6. DNA end-dependent DNA-PK kinase and Artemis nuclease activity.
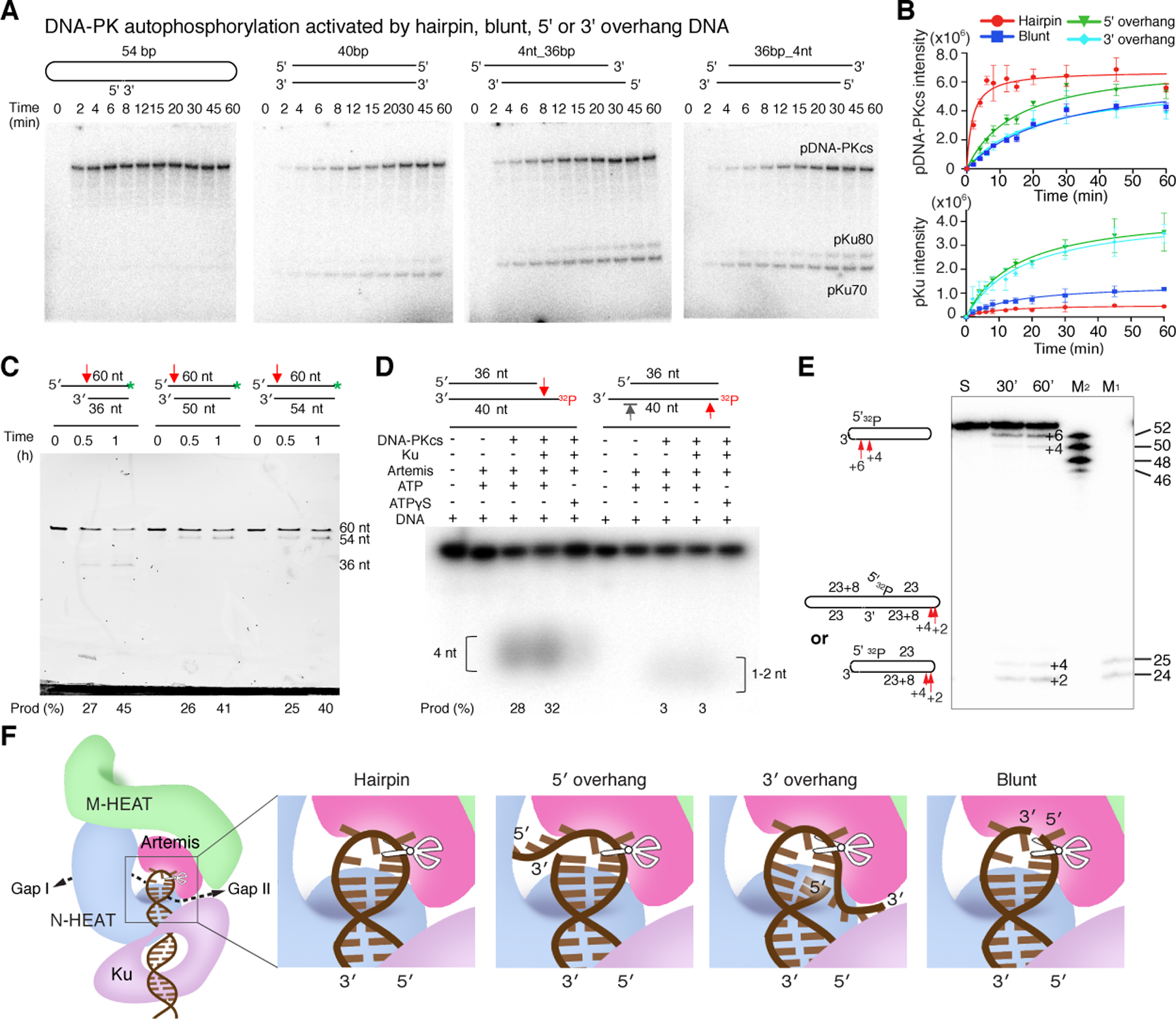
A. Different DNA-PK kinase activity on various DNA substrates. Hairpin, blunt, 5´ and 3´ overhang DNA used in the assay are diagrammed. B. Plot of triplicate measurement of DNA end-dependent autophosphorylation of DNA-PKcs and trans-phosphorylation of Ku70/80. Hairpin DNA end activates cis autophosphorylation of DNA-PKcs more than any other type of DNA end. After autophosphorylation of the ABCDE patch, DNA-PKcs is self-inhibited and exhibits very low kinase activity towards Ku70/80. C. Cleavage of 36–54 bp DNA duplexes with 24-nt 5´-overhang, both 5´- and 3´-overhangs (6 and 4 nt, respectively), and 6-nt 5´-overhang. The endonuclease activity in removing the 5´-overhang is similar in all three. D. Cleavage activity of Artemis-DNA-PK on 36-bp duplex with a 4-nt 5´- or 3´-overhang, or blunt-end DNA. The bottom strand is 5´−32P labeled. The 5´-overhang endonuclease activity is comparable to the longer overhangs shown in panel A. No endonuclease activity is detectable on the 3´-overhang (marked by grey arrowhead), while weak 5´ end trimming is observed on the blunt end (right half). E. Cleavage of the dumbbell-shaped hairpin-end DNA by Artemis and DNA-PK at 37°C. Both hairpin opening and cleavage of the half DNA substrate (an 8-nt 3´-overhang) occurred. According to oligonucleotide markers (M1 and M2), hairpin opening results in 2- and 4-nt 3´ overhang; the 8- nt 3´ overhang is trimmed to 4- or 6-nt. F. Structure-based diagram of how Artemis cleaves different DNA substrates.
Coupled with the efficient ABCDE autophosphorylation and activation of Artemis by DNA-PK, we observed efficient cleavage of hairpin DNA and 5´ overhangs of 4, 6 or 24 nt (Fig. 6C-D). With poorer autophosphorylation, a blunt end DNA was cleaved only moderately, and a 4-nt 3´ overhang not at all by Artemis (Fig. 6C-D). With our particular DNA sequence, Artemis opens the hairpin to a 2-nt 3´ overhang more frequently than a 4-nt overhang (Fig. 6E).
Discussion
The nuclease activity of Artemis and DNA-PK
Artemis has been characterized as having not only hairpin-opening endonuclease activity, but also an endonuclease capable of processing 5´ or 3´ overhangs, or branched or Y shaped DNA (Chang and Lieber, 2016). Artemis even has weak 5´ nuclease activity on blunt-end DNA (Fig. 6D) (Yannone et al., 2008). The detailed structure of Artemis-DNA interactions, and the alteration of hairpin loop-binding by phosphorylated DNA-PK, confirm that many of these activities are variations of the endonuclease activity intrinsic to Artemis, achieved by molding different substrates into the DNA-binding surface of Artemis and DNA-PK (Fig. 6F). The Artemis-DNA interface observed here is related yet different from that in the absence of DNA-PK (Fig. S5B) (Yosaatmadja et al., 2021). Aided by DNA-PK, a 5´-overhang DNA can be accommodated by Artemis and DNA-PK similarly to a hairpin end (as observed here), and a 5´-overhang DNA longer than 4 nt can protrude out through the gap without protein contact (Fig. 3C, 6F). This binding mode of 5´ overhangs is supported by the finding of blunt-end cleavage products when the overhang length varies from 4 to 24 nt (Fig. 6C). The 5´ end trimming of blunt end DNA by Artemis may be a result of 1 or 2 bp unpairing that forms a discontinuous hairpin-like loop (Fig. 6F). To cleave a 3´ overhang, the single-stranded DNA needs to be at least 6 nt in length to reach the Artemis active site (Fig. 6F). Indeed, the 8-nt 3´ overhang is trimmed to 4 or 6 nt, while the 4-nt 3´ overhang is untouched by Artemis (Fig. 6D-E).
DNA end determines the autophosphorylation and kinase activity of DNA-PK
In this study we find that DNA-PKcs can differentiate different types of DNA ends: hairpin, blunt, or with overhangs. DNA-PK forms either a “protective” (VII) or “processing” (VI) complex, to promote trans phosphorylation of other targets (VII) or cis autophosphorylation of ABCDE (VI) (Fig. 7). With a hairpin end, DNA-PK seems to form exclusively the processing complex, in which DNA-PK autophosphorylates itself rapidly, and also phosphorylates Artemis, probably in cis (in the same complex) (Fig. S1), but does not allow cis phosphorylation of Ku70/80, which is too far away from the kinase active site (over 100 Å) and hindered by the bound DNA. Upon autophosphorylation of ABCDE, DNA-PK assists Artemis to open the DNA hairpin end. Interestingly, in this state the DNA-PKcs kinase is self-inhibited against further phosphorylation, which may underlie the absence of Ku70/80 phosphorylation in trans (Fig. S1, 6). With open DNA ends, the two forms (complex VI and VII) of DNA-PK can co-exist at equilibrium (Fig. 7). In the protection mode, autophosphorylation of ABCDE is inhibited because the phosphorylation sites are trapped inside the HEAT rings by the DNA end (Fig. 6A-B). But the activated kinase can phosphorylate Ku70/80, presumably in trans (Fig. 6A-B). Phosphorylation of other targets in trans by DNA-PK enhances NHEJ, negatively regulates ATM, and coordinates the cellular response to DNA damage (Gustafsson et al., 2014; Zhou et al., 2017; Zhou and Paull, 2013).
Figure 7. DNA-PK serves as the NHEJ scaffold and moderator.
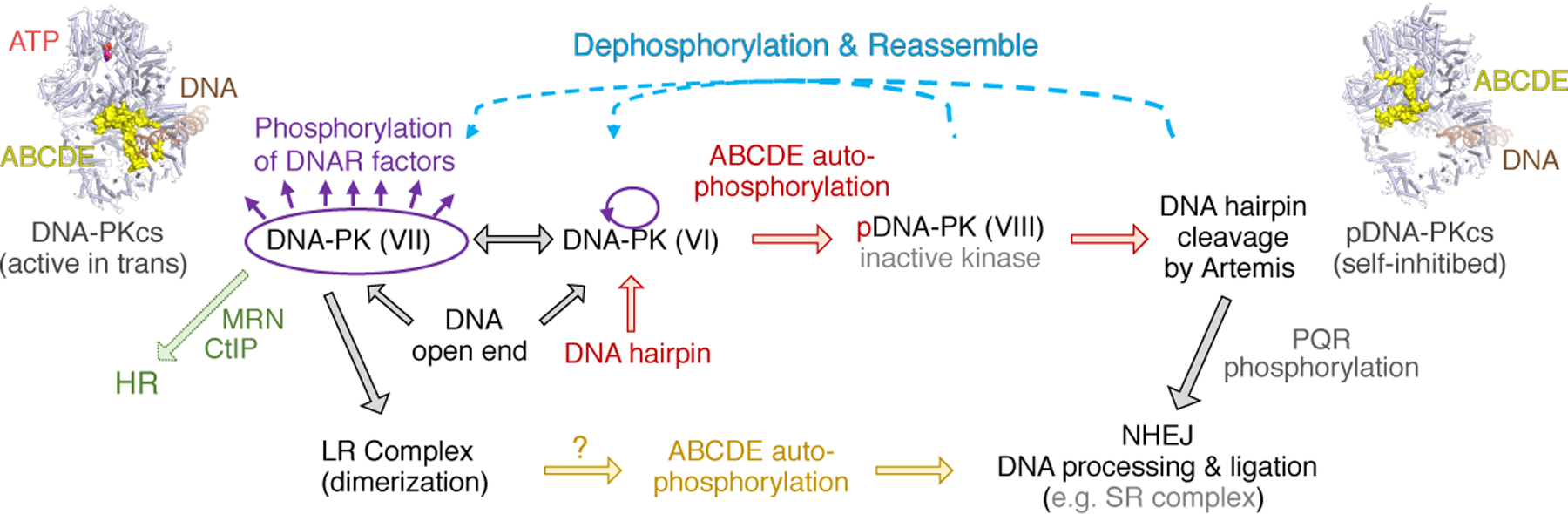
Structures of DNA-PKcs with bound DNA before and after autophosphorylation of ABCDE are shown in the upper left and right corner, respectively. ABCDE phosphorylation (transforming from complex VI to VIII) is essential for exposing the bound DNA hairpin end for Artemis to cleave. Afterwards, DNA ends may be processed and joined directly by the NHEJ pathway. PQR phosphorylation prevents excessive DNA cutting by Artemis. DNA-PK bound to DNA open ends, e.g. resulting from ionizing radiation, may form complex VI or VII. In complex VII, a blunt or 3´-overhang end is covered by ABCDE, and the activated DNA-PK can phosphorylate other proteins. Complex VII may transit to complex VI and undergo autophosphorylation and directly enter the NHEJ process, or form the LR complex as an intermediate to NHEJ. If ABCDE autophosphorylation fails, DNA ends would be stuck to DNA-PK and need alternative pathways, e.g. homologous recombination (HR), to repair them.
Regulatory mechanism of PIKK kinases
In addition to the cis vs. trans phosphorylation activity of DNA-PKcs induced by different DNA ends (Fig. 7), we also discern a mechanism of self-inhibition of DNA-PKcs kinase resulting from autophosphorylation of ABCDE. As the ATP binding site is always open (Langer et al., 2020; Williams et al., 2020; Yang et al., 2017), and ATP is bound to activated as well as inactive DNA-PKcs (Fig. 1, 4), the kinase activity appears to be regulated by the accessibility of the substrate binding site. The FATKIN domain, which is conserved among PIKKs and plays a determining role in the kinase state, is subject to structural modulations by the DNA-binding A1 helix and the helix before the ABCDE phosphorylation sites within DNA-PKcs. FATKIN can also be influenced by partner proteins via protein-protein interactions, for example, the extended C-terminal peptide of Artemis (Fig. 4D) or XRCC4 (Chen et al., 2021a). We suppose that the mechanism for activating and inhibiting DNA-PKcs kinase activity by alteration of the FATKIN structure is general among PIKKs. Activation of ATM and ATR has been suggested to involve dissociation of dimeric FATKIN heads (Baretic and Williams, 2014; Williams et al., 2020). On the other hand, IP6 has been reported to bind several PIKKs (Gat et al., 2019) and stimulates NHEJ by binding to Ku70/80 (Cheung et al., 2008; Hanakahi et al., 2000; Ma and Lieber, 2002). In our hands, IP6 has no discernible effect on the kinase or nuclease activity in agreement with the observation that IP6 is already bound to the Ku70/80 in all of our DNA-PK structures (Fig. S2M) (Chen et al., 2021b). However, IP6 is absent in the Ku70/80 crystal structures and some DNA-PK structures (Chen et al., 2021a; Nemoz et al., 2018; Walker et al., 2001), which may result from different expression and purification conditions. Given the natural high concentrations of IP6 in mammalian cells (10–100 µM) (Szwergold et al., 1987), IP6 may be coopted into non-globular proteins for structural stability.
The extensive structural changes of the two HEAT rings upon DNA-PKcs autophosphorylation highlight the intrinsic plasticity of such HEAT repeats, which are main structural components of PIKKs. Both Slinky-like and rigid-body movements enable the over 40 Å translocation and 30° rotation of the protein and DNA block in the DNA-PK complex (Fig. 1, S4). This gross rearrangement demonstrates the capacity of HEAT repeats to adapt to new environment (autophosphorylation) and serve diverse functional roles, for example to enclose and activate Artemis nuclease.
Involvement of phosphatase and dephosphorylation of DNA-PKcs in NHEJ
The natural sequence of DNA end-joining in V(D)J recombination is that hairpin opening occurs first (Fig. 7), followed by DNA-end trimming, synthesis, and ligation (Helmink et al., 2011). Our structural analysis confirms that hairpin opening by Artemis depends on ABCDE phosphorylation (Meek, 2020) and occurs without the formation of either a long-range (LR) or short-range (SR) complex with LigIV, XRCC4 and XLF, as they have been defined by single-molecule analyses (Graham et al., 2018; Stinson et al., 2020) and confirmed by cryoEM structures (Chaplin et al., 2021; Chen et al., 2021a). After hairpin opening, an LR complex may form next, in which ABCDE is unphosphorylated (Reddy et al., 2004) (Fig. 4–5), but then autophosphorylation of ABCDE is necessary for DNA-PKcs to yield the DNA ends and for the SR complex to form and ligation to take place. In addition to the possibility that fresh unphosphorylated DNA-PKcs could replace phosphorylated DNA-PKcs to form the LR complex that pairs DNA ends, ABCDE may be dephosphorylated between hairpin DNA end opening and LR complex formation, or possibly the ligation complex may form without the LR complex as an intermediate (Fig. 7). In support of a de-phosphorylation step, protein phosphatase PP6, which targets DNA-PKcs, is known to enhance DNA repair and resistance to ionizing radiation (Dziegielewski et al., 2020; Hosing et al., 2012), and in vivo ABCDE phosphorylation is short-lived and difficult to detect (Douglas et al., 2002). However, the second route is also possible, because Artemis physically interacts with LigIV (De Ioannes et al., 2012), and thus could recruit LigIV/XRCC4 to DNA-PK for DNA-end ligation. Regardless of how these later steps are organized, our results show clearly that Artemis can function outside, and presumably before, the formation of either the long range or short range NHEJ complexes.
Repair pathway choices of double-strand DNA breaks
In addition to NHEJ, homologous recombination (HR) and alternative end joining mediated by DNA pol θ are well established pathways for repair of DNA double-strand breaks. Outside of G1 phase and V(D)J recombination, DNA-PK can protect open DNA ends temporarily, and upon autophosphorylation release the DNA end for processing and repair by HR. Blocking ABCDE phosphorylation by Ala mutation of phosphorylation sites, or inactivation of the kinase, leads to DNA-PK trapped on broken DNA ends and failure of NHEJ. DNA-PK stuck on DNA ends may be released by the endonuclease activity of MRN and CtIP, leading to repair by homologous recombination or alternative end-joining pathways (Fig. 7) (Crowe et al., 2020; Deshpande et al., 2020). In summary, our results here show how the abundant DNA-PK regulates itself by means of its structural plasticity and autophosphorylation, and coordinates NHEJ and other pathways to repair broken DNA ends.
Limitations of the study
Auto-phosphorylation of DNA-PKcs is significantly enhanced by Artemis in the presence of a long 5´-overhang DNA (Fig. 1A and 6). How Artemis stimulates the kinase activity and how Artemis and DNA-PK cooperate to cleave 5´ overhangs await future studies. Furthermore, it is not yet clear how DNA-PKcs auto-phosphorylation coordinates DNA end ligation during NHEJ.
STAR METHODS
RESOURCE AVAILABILITY
Lead Contact
Email contact for further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Wei Yang (weiy@niddk.nih.gov).
Material Availability
All reagents generated in this study are available upon request from the Lead Contact without restriction.
Data and Code Availability
There is no new sequence data associated with this work. Original gels of kinase activity and DNA cleavage have been deposited at Mendeley and are publicly available as of the date of publication. The DOI is listed in the key resource table.
This paper does not report any original code. The structures and cryoEM maps have been deposited with PDB and EMDB with accession codes of the Artemis complex 7SGL (PDB) and 25113 (EMDB), and locally refined maps EMD-25114 (FATKIN) and EMD-25115 (DNA and Artemis-Ku-N-HEAT); accession codes of complexes VII and VIII 7SU3 and 7SUD (PDB), 25439 and 25440 (EMDB); accession codes of complexes VIIa, VIIb and VIb 25112 (EMDB), 25111 (EMDB) and 25110 (EMDB). The DOI is listed in the key resource table.
Any additional information required to reanalyze the data reported in this paper is available from the Lead Contact upon request.
KEY RESOURCE TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, Peptides, and Recombinant Proteins | ||
| Human DNA-PKcs (full-length) | This paper | Residues 1–4128 |
| Human Ku70/80 | This paper | Residues 1–609 and 1–732 |
| Human Artemis | This paper | Residues 1–692 |
| Deposited Data | ||
| DNA-PK-Artemis complex, cryoEM (3.0 Å) | This paper | PDB: 7SGL EMDB: EMD-25113 |
| FATKIN domain of DNA-PK-Artemis (2.7 Å) | This paper | EMDB: EMD-25114 |
| DNA-Artemis-Ku-N-heat of DNA-PK-Artemis (2.9 Å) | This paper | EMDB: EMD-25115 |
| DNA-PK-DNA complex VII, cryoEM (3.3 Å) | This paper | PDB: 7SU3 EMDB: EMD-25439 |
| DNA-PK-DNA complex VIII, cryoEM (3.6 Å) | This paper | PDB: 7SUD EMDB: EMD-25440 |
| DNA-PK-DNA complex VIIa, cryoEM (4.2 Å) | This paper | EMDB: EMD-25112 |
| DNA-PK-DNA complex VIIb, cryoEM (4.2 Å) | This paper | EMDB: EMD-25111 |
| DNA-PK-DNA complex VIb, cryoEM (4.4 Å) | This paper | EMDB: EMD-25110 |
| Original gel scans | Mendeley Data | http://dx.doi.org/10.17632/t3525wy6gk.1 |
| Experimental Models: Cell Lines | ||
| HeLa-S3 cells | National Cell Culture Center | https://cellculturecompany.com/national-cell-culture-center/ |
| HEK293T cells | ATCC | CRL-3216 |
| DNA-PKcs deficient V3 cells | Katheryn Meek’s lab | N/A |
| Oligonucleotides | ||
| See DNA preparation in Table S2 | This paper | N/A |
| Recombinant DNA | ||
| Ku70 in pLEXm plasmid | This paper | pWY2799 |
| Ku80 in pLEXm plasmid | This paper | pWY2802 |
| Artemis in pLEXm plasmid | This paper | pWY2332 |
| Software and Algorithms | ||
| PyMol | Schrodinger | https://pymol.org/2/ |
| Prism-8 | GraphPad Software | https://www.graphpad.com/ |
| CCP4 | CCP4 | http://www.ccp4.ac.uk |
| PHENIX | (Adams et al., 2010) | https://www.phenix-online.org |
| COOT | (Emsley, 2010) | https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/ |
| UCSF Chimera | (Pettersen et al., 2004) | https://www.cgl.ucsf.edu/chimera/ |
| cryoSPARC | (Punjani et al., 2017) | https://cryosparc.com/ |
| RELION | (Scheres, 2012); (Fernandez-Leiro and Scheres, 2017) | https://www2.mrc-lmb.cam.ac.uk/relion |
| SerialEM | (Mastronarde, 2005) | https://bio3d.colorado.edu/SerialEM/ |
EXPERIMENTAL MODEL AND SUBJECT DETAILS
DNA-PKcs was purified from HeLa-S3 epithelial suspension adapted cells that were purchased from National Cell Culture Center, Minneapolis, MN. Expression of Ku and Artemis were performed in HEK293T cells that were cultured in Freestyle 293 expression medium (GIBCO) at 37°C and 5% CO2. DNA-PKcs deficient V3 cells used in episomal end-joining assay were cultured in alpha minimum essential medium (α-MEM) with 10% fetal calf serum, penicillin-streptomycin, and ciprofloxacin (complete medium) and maintained at 37°C with 5% CO2 (Neal et al., 2011).
METHOD DETAILS
Protein and DNA preparation
DNA-PKcs was purified from HeLa cells (purchased from National Cell Culture Center, Minneapolis, MN) as previously described (Chen et al., 2021b). Briefly, the nuclear extracts were prepared according to a standard protocol (Abmayr et al., 2006) and then fractionated with ammonium sulfate and purified by chromatography over DEAE Sepharose FF column (GE Healthcare), HiTrap Heparin HP column (GE Healthcare), Mono Q 10/100 GL anion exchange column (GE Healthcare) and Superose 6 10/300 GL size exclusion column (GE Healthcare). Purified DNA-PKcs was stored in 50 mM HEPES pH 7.9, 300 mM KCl, 1 mM DTT, 1mM EDTA and 50% glycerol at –80℃. All protein purification steps (including Ku and Artemis purification described below) were carried out at 4℃, and protease inhibitors (1 mM PMSF, 1 μM pepstatin, 10 μg/ml aprotinin, 5 μg/ml leupeptin) were added to all buffers.
Ku70/80 protein was over-expressed and purified from HEK293T cells as previously described (Chen et al., 2021b). The cell lysate was first purified over an amylose affinity column. After removal of the N-terminal His8-MBP tag on Ku70 by PreScission Protease (recognizing LEVLFQ/GP, “/” indicating the protease cleavage site), Ku70/80 was further purified over a Mono Q 10/100 GL anion exchange column (GE Healthcare) and stored in 20 mM HEPES pH 7.9, 150 mM KCl, 1 mM EDTA, 1 mM DTT, and 50% glycerol at −80℃.
The protocol for expression and purification of Artemis is similar to that of Ku70/80 except for an additional gel-filtration purification step. In brief, the full-length Artemis gene was cloned into a modified pLEXm vector with a C-terminal PreScission cleavage site followed by an MBP tag, and expressed in HEK293T cells. MBP fused Artemis was first purified by amylose affinity column (NEB), and the eluate was digested with PreScission Protease. Artemis was further purified by a Mono Q 10/100 GL column (GE Healthcare) in buffer containing 25 mM HEPES pH 7.9, 100 to 1000 mM KCl, 1 mM DTT and 5% glycerol. Fractions containing Artemis were pooled and purified over a Superdex 200 Increase 10/300 GL column (GE Healthcare) in buffer containing 25 mM HEPES pH 7.9, 100 mM KCl, 1 mM DTT and 5% glycerol. The peak fractions were concentrated and stored in 50% glycerol at −80℃.
All DNA oligonucleotides (Table S2) were purchased from IDT (Integrated DNA Technologies, Coralville, IA) and purified using 8–15% TBE-urea PAGE gels in small gel cassettes (Life Technologies). The purified oligonucleotides were then desalted using Glen Gel-Pak columns (Glen Research) and stored in sterile ultra-pure H2O at −20℃. To make DNA duplexes, oligos (Table S2) were annealed in 20 mM Tris-HCl (pH 8.0), 50 mM NaCl and 0.5 mM EDTA buffer in a Thermocycler by heating to 95°C for 5 min and cooling to 4°C over 1 hour.
Sample preparation and cryo-EM data collection
To assemble the DNA-PK-Artemis complex, DNA-PKcs, Ku70/80, Artemis and the symmetric hairpin DNA were mixed in a molar ratio of 1:2:2:2, dialyzed into buffer containing 25 mM HEPES pH 7.5, 100 mM KCl and 1mM DTT and concentrated to 0.3 mg/ml of total protein. Cryo-EM samples were prepared using C-Flat R1.2/1.3 holey carbon grids (EMS) with a 3-nm layer of home-made carbon film. Before depositing the sample onto a cryoEM grid, 1/10 volume of 10× concentrated ATP/Mg2+ solution (3 mM ATP, 50 mM MgCl2 and 0.5% β-OG in 25 mM HEPES pH 7.5, 100 mM KCl, 1mM DTT) was added to the protein mixture and incubated at 37℃ for 12 min. 3 μl of protein mixture was applied to a grid suspended in a FEI Vitrobot operated at 4℃ with 100% humidity. After 10 s, the grid was blotted with force 1 for 2 s and plunged into liquid ethane cooled by liquid nitrogen. To assemble the DNA-PK complex with ATP or AMPPNP, purified DNA-PKcs, Ku70/80 and 40 bp symmetrized blunt end DNA were mixed at the molar ratio of 1:1:1 in 25 mM HEPES pH 7.9, 100 mM KCl, 1mM DTT, 10 mM MgCl2 and 200 µM ATP or AMPPNP. The protein concentration of the mixture was adjusted to 0.4 mg/ml, incubated at 37℃ for 1 min and then immediately loaded on QUANTIFOIL R 1.2/1.3 (Gold, 300 mesh) grids with a continuous layer of 2 nm carbon film, 3 µl sample per grid at 100% humidity and 37°C in a FEI Vitrobot, blotted for 4 s, and flash-frozen in liquid ethane. Prolonged incubation with ATP without Artemis would result in complete dissociation of DNA and mixed phosphorylated population and conformational species.
For the DNA-PK-Artemis and DNA-PK-ATP samples, 7993 and 6089 micrographs were collected, respectively, on a Titan Krios electron microscope with a Gatan K3 Summit direct electron detector operated at 300 kV in the super-resolution mode of 105K nominal magnification (calibrated pixel size of 0.4165 Å, corresponding to 0.833 Å at the sample level), at the Multi-Institute Cryo-EM Facility (MICEF) of NIH. For the DNA-PK-AMPPNP sample, 5105 micrographs were collected on the Glacios electron microscope with a Gatan K3 Summit direct electron detector operated at 200 kV in the super-resolution mode of 36K nominal magnification (calibrated pixel size of 0.58 Å, corresponding to 1.16 Å at the sample level), at NIDDK, NIH.
Structure determination and model refinement
MotionCor2 (Zheng, 2017) was used for drift correction, with dose-weighting applied, and the pixel size was binned to 0.833 Å/pixel (Titan Krios datasets) or 1.16 Å/pixel (Glacios dataset). CTF (contrast transfer function) was estimated with the dose-unweighted micrographs using Gctf (Zhang, 2016). Particle picking was performed using Gautomatch (developed by Kai Zhang; https://www.mrc-lmb.cam.ac.uk/kzhang/Gautomatch). cryoSPARC V3.2 (Punjani, 2017) was used to generate the initial maps, and the two-dimensional (2D) projections were used for template-based particle picking. 2D classifications were performed with RELION 3.1 to remove obviously bad particles, and both RELION 3.1 and cryoSPARC V3.2 were further used for 3D classifications to sort out different conformations. The selected particles from 3D classification belonging to each conformation were subjected to auto-refinement, CTF refinement and Bayesian polishing were done in RELION 3.1. All reported resolutions were determined based on the “gold standard” of the 0.143 Fourier shell correlation (FSC) criterion (Swint-Kruse, 2005). Local resolution was estimated using ResMap (Kucukelbir et al., 2014). For model building, the 3.7 Å DNA-PK complex VI (PDB: 7K0Y) and Artemis nuclease domain (PDB: 6WO0) were used as initial models. We first fit the coordinates into the cryo-EM maps using Chimera (Pettersen et al., 2004), and then manually adjusted and rebuilt the models according to the cryo-EM density in COOT (Emsley, 2010). Real-space refinement in Phenix (Adams et al., 2010) was used to refine the models, and MolProbity (Chen et al., 2010) was used to validate the final models. The refinement statistics are summarized in Table S1. The detailed classifications and map qualities of the structures reported in this manuscript are shown in supplemental figures (Fig. S2 and S3).
Kinase and nuclease activity assays
The DNAs used in the kinase assay were prepared according to the section on “DNA substrate preparation” (Table S2). The kinase reaction system contained 30 nM DNA-PKcs, 30 nM Ku70/80, 30 nM DNA with or without 30 nM Artemis, in a reaction buffer containing 25 mM HEPES pH 7.5, 100 mM KCl, 1mM DTT, 0.1 mM EDTA, 50 ng/μl BSA, 0.3 mM ATP (containing 32P-γ-ATP), 5 mM MgCl2. The reaction mixture was incubated at 37℃. Equal volumes of reaction product were collected at each time point and mixed with 2 × SDS-PAGE loading buffer (100 mM Tris-Cl pH 6.8, 4% (w/v) SDS, 0.2% (w/v) bromophenol blue, 20% (v/v) glycerol, 200 mM DTT) to stop the reaction. Radioactively phosphorylated proteins were separated on 3%–8% SDS-PAGE and visualized on a Typhoon PhosphorImager. The phosphorylated DNA-PKcs and Ku70/80 bands were quantified using ImageQuant NL (GE Healthcare). All assays were performed in triplicate.
The DNA substrates used in nuclease assays are listed in Table S2. 40nt-5overhang-1, 40nt-3overhang-1, and 54nt-hairpin oligos were 5´ labeled with 32P-γ-ATP using T4 kinase, and then annealed with the complementary oligos. 60nt-FAM was annealed with 36nt-blunt-2, 50nt-5overhang-2 and 54nt-5overhang-2 to form three kinds of 5´ overhang dsDNA. The nuclease reaction system contained 30 nM DNA-PKcs, 30 nM Ku70/80, 30 nM Artemis and 30 nM DNA in a reaction buffer containing 25 mM HEPES pH 7.5, 100 mM KCl, 1mM DTT, 0.1 mM EDTA, 50 ng/μl BSA, 0.3 mM ATP and 5 mM MgCl2. Reactions were carried out at 37℃ and stopped with 2× loading dye (98% formamide, 0.05% bromophenol blue, 20 mM EDTA). The samples were heated at 95℃ for 5 min and resolved on 16% mini TBE-Urea gels (8 × 10 cm), except for Figure 6E. For Figure 6E, samples were resolved on a 16% sequencing gel (30 × 40 cm) and two markers (M1 and M2) were used to accurately indicate the length of cleavage products. The 32P or FAM labeled DNA bands were detected on a Typhoon PhosphorImager and quantified using ImageQuant NL (GE Healthcare).
Episomal end-joining assays
The FIF (to Ala) mutant DNA-PKcs expression construct was generated by inserting a synthetic gene block encoding F2606A, I2635A, and F2644A substitutions between PshA1 and FseI restriction sites. The KR10 DNA-PKcs expression construct was generated by inserting a synthetic gene block encoding R1031A, R1034A, K1038A, W1039A, K1042A, K1051A, K1057, K1061A, H1069A, and K1074A substitutions between SpeI and BstAPI. The KR18 DNA-PKcs expression construct was generated by inserting a synthetic gene block encoding the KR10 substitutions as well as Y1086A, R1087A, R1090A, H1133A, R1136A, K1140A, K1150A and R1152A between SpeI and SalI. Episomal end-joining assays were performed as described previously (Meek, 2020). Briefly, to assess V(D)J coding and signal joints, DNA-PKcs deficient V3 cells were co-transfected with either the 290-RFP/CFP coding joint or 289-RFP/CFP signal joint substrates, with either no RAGs, RAGs only, or RAGs plus wild type or mutant DNA-PKcs constructs. WT and mutant DNA-PKcs expressions were confirmed by Western blot. Results were compiled from at least four experiments; two tailed unpaired T test comparing FIF, ABCDE, KR10, KR18 and wild type DNA-PKcs: p<.0001 for both coding and signal joint assays.
QUANTIFICATION AND STATISTICAL ANALYSIS
Kinase, nuclease and cell-based assays
Means and standard deviation (S.D.) of triplicates are shown.
ADDITIONAL RESOURCES
Titan Krios electron microscopes
The microscopes belong to Multi-Institute Cryo-EM Facility (MICEF) of NIH (Bethesda, MD).
Supplementary Material
Movie S2. DNA moves outward along its helical axis by 2 bp from complex VI to complex VII, Related to Figure 4
DNA-PKcs is depicted in green cartoon, Ku70/80 in light and dark pink, and the blunt end DNA in brown. Two different views are shown. At the end of each viewing angle, DNA-PKcs in complex VII is colored in rainbow fashion from the blue N terminus to red C terminus. The DEB helix that splits the first base pair and covers the DNA end is colored yellow. DNA-PKcs is more compact in complex VII than in complex VI and appears to “shrink” when transforming from complex VI to VII.
Movie S1. Structural changes of DNA-PKcs before (PDB: 7K1N) and after autophosphorylation of ABCDE (PDB: 7SGL), Related to Figure 1
The DNA-PKcs protein is colored from blue N terminus to red C terminus in a rainbow gradient. The four phosphorylated Thr residues (2609, 2638, 2645 and 2647) are shown as orange and red spheres. Helices A1 and A8, which undergo large motions, are labeled in the first frame. Two orthogonal views are shown side-by-side. This animation is based on superposition of the FATKIN domain.
Highlights.
A DNA hairpin activates DNA-PKcs to autophosphorylate its ABCDE sites in cis.
ABCDE-phosphorylated DNA-PKcs opens up dramatically and exposes DNA end.
Artemis is sandwiched by phosphorylated DNA-PKcs to cleave DNA hairpin
DNA-PK protects blunt DNA ends and phosphorylates other targets in trans.
ACKNOWLEDGEMENTS
This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (M.G., DK036167; W.Y., DK036147 and DK036144), the USDA National Institute of Food and Agriculture (K.M., 1019208) and Public Health Service (K.M., AI048758 and AI147634).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
The authors declare no competing interests.
References
- Abmayr SM, Yao T, Parmely T, and Workman JL (2006). Preparation of nuclear and cytoplasmic extracts from mammalian cells. Curr Protoc Mol Biol Chapter 12, Unit 12 11. 10.1002/0471142727.mb1201s75. [DOI] [PubMed] [Google Scholar]
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. (2010). PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66, 213–221. S0907444909052925 [pii] 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baretic D, and Williams RL (2014). PIKKs--the solenoid nest where partners and kinases meet. Curr Opin Struct Biol 29, 134–142. 10.1016/j.sbi.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Block WD, Yu Y, Merkle D, Gifford JL, Ding Q, Meek K, and Lees-Miller SP (2004). Autophosphorylation-dependent remodeling of the DNA-dependent protein kinase catalytic subunit regulates ligation of DNA ends. Nucleic Acids Res 32, 4351–4357. 10.1093/nar/gkh761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callebaut I, Moshous D, Mornon JP, and de Villartay JP (2002). Metallo-beta-lactamase fold within nucleic acids processing enzymes: the beta-CASP family. Nucleic Acids Res 30, 3592–3601. 10.1093/nar/gkf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HH, and Lieber MR (2016). Structure-Specific nuclease activities of Artemis and the Artemis: DNA-PKcs complex. Nucleic Acids Res 44, 4991–4997. 10.1093/nar/gkw456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin AK, Hardwick SW, Liang S, Kefala Stavridi A, Hnizda A, Cooper LR, De Oliveira TM, Chirgadze DY, and Blundell TL (2021). Dimers of DNA-PK create a stage for DNA double-strand break repair. Nat Struct Mol Biol 28, 13–19. 10.1038/s41594-020-00517-x. [DOI] [PubMed] [Google Scholar]
- Chen S, Lee L, Naila T, Fishbain S, Wang A, Tomkinson AE, Lees-Miller SP, and He Y (2021a). Structural basis of long-range to short-range synaptic transition in NHEJ. Nature 593, 294–298. 10.1038/s41586-021-03458-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen VB, Arendall WB 3rd, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, and Richardson DC (2010). MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr 66, 12–21. 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Xu X, Chen Y, Cheung JC, Wang H, Jiang J, de Val N, Fox T, Gellert M, and Yang W (2021b). Structure of an activated DNA-PK and its implications for NHEJ. Mol Cell 81, 801–810 e803. 10.1016/j.molcel.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung JC, Salerno B, and Hanakahi LA (2008). Evidence for an inositol hexakisphosphate-dependent role for Ku in mammalian nonhomologous end joining that is independent of its role in the DNA-dependent protein kinase. Nucleic Acids Res 36, 5713–5726. 10.1093/nar/gkn572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe JL, Wang XS, Shao Z, Lee BJ, Estes VM, and Zha S (2020). DNA-PKcs phosphorylation at the T2609 cluster alters the repair pathway choice during immunoglobulin class switch recombination. Proc Natl Acad Sci U S A 10.1073/pnas.2007455117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Yu Y, Gupta S, Cho YM, Lees-Miller SP, and Meek K (2005). Autophosphorylation of DNA-dependent protein kinase regulates DNA end processing and may also alter double-strand break repair pathway choice. Mol Cell Biol 25, 10842–10852. 10.1128/MCB.25.24.10842-10852.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ioannes P, Malu S, Cortes P, and Aggarwal AK (2012). Structural basis of DNA ligase IV-Artemis interaction in nonhomologous end-joining. Cell Rep 2, 1505–1512. 10.1016/j.celrep.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande RA, Myler LR, Soniat MM, Makharashvili N, Lee L, Lees-Miller SP, Finkelstein IJ, and Paull TT (2020). DNA-dependent protein kinase promotes DNA end processing by MRN and CtIP. Sci Adv 6, eaay0922. 10.1126/sciadv.aay0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Reddy YV, Wang W, Woods T, Douglas P, Ramsden DA, Lees-Miller SP, and Meek K (2003). Autophosphorylation of the catalytic subunit of the DNA-dependent protein kinase is required for efficient end processing during DNA double-strand break repair. Mol Cell Biol 23, 5836–5848. 10.1128/mcb.23.16.5836-5848.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas P, Sapkota GP, Morrice N, Yu Y, Goodarzi AA, Merkle D, Meek K, Alessi DR, and Lees-Miller SP (2002). Identification of in vitro and in vivo phosphorylation sites in the catalytic subunit of the DNA-dependent protein kinase. Biochem J 368, 243–251. 10.1042/BJ20020973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziegielewski J, Bonkowska MA, Poniecka EA, Heo J, Du K, Crittenden RB, Bender TP, Brautigan DL, and Larner JM (2020). Deletion of the SAPS1 subunit of protein phosphatase 6 in mice increases radiosensitivity and impairs the cellular DNA damage response. DNA Repair (Amst) 85, 102737. 10.1016/j.dnarep.2019.102737. [DOI] [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, and Cowtan K (2010). Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66, 486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Leiro R, and Scheres SHW (2017). A pipeline approach to single-particle processing in RELION. Acta Crystallogr D Struct Biol 73, 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gat Y, Schuller JM, Lingaraju M, Weyher E, Bonneau F, Strauss M, Murray PJ, and Conti E (2019). InsP6 binding to PIKK kinases revealed by the cryo-EM structure of an SMG1-SMG8-SMG9 complex. Nat Struct Mol Biol 26, 1089–1093. 10.1038/s41594-019-0342-7. [DOI] [PubMed] [Google Scholar]
- Goodarzi AA, Yu Y, Riballo E, Douglas P, Walker SA, Ye R, Harer C, Marchetti C, Morrice N, Jeggo PA, and Lees-Miller SP (2006). DNA-PK autophosphorylation facilitates Artemis endonuclease activity. EMBO J 25, 3880–3889. 10.1038/sj.emboj.7601255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham TGW, Carney SM, Walter JC, and Loparo JJ (2018). A single XLF dimer bridges DNA ends during nonhomologous end joining. Nat Struct Mol Biol 25, 877–884. 10.1038/s41594-018-0120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson AS, Abramenkovs A, and Stenerlow B (2014). Suppression of DNA-dependent protein kinase sensitize cells to radiation without affecting DSB repair. Mutat Res 769, 1–10. 10.1016/j.mrfmmm.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Hanakahi LA, Bartlet-Jones M, Chappell C, Pappin D, and West SC (2000). Binding of inositol phosphate to DNA-PK and stimulation of double-strand break repair. Cell 102, 721–729. 10.1016/s0092-8674(00)00061-1. [DOI] [PubMed] [Google Scholar]
- Helmink BA, Tubbs AT, Dorsett Y, Bednarski JJ, Walker LM, Feng Z, Sharma GG, McKinnon PJ, Zhang J, Bassing CH, and Sleckman BP (2011). H2AX prevents CtIP-mediated DNA end resection and aberrant repair in G1-phase lymphocytes. Nature 469, 245–249. 10.1038/nature09585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepburn M, Saltzberg DJ, Lee L, Fang S, Atkinson C, Strynadka NCJ, Sali A, Lees-Miller SP, and Schriemer DC (2021). The active DNA-PK holoenzyme occupies a tensed state in a staggered synaptic complex. Structure 29, 467–478 e466. 10.1016/j.str.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosing AS, Valerie NC, Dziegielewski J, Brautigan DL, and Larner JM (2012). PP6 regulatory subunit R1 is bidentate anchor for targeting protein phosphatase-6 to DNA-dependent protein kinase. J Biol Chem 287, 9230–9239. 10.1074/jbc.M111.333708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jette N, and Lees-Miller SP (2015). The DNA-dependent protein kinase: A multifunctional protein kinase with roles in DNA double strand break repair and mitosis. Prog Biophys Mol Biol 117, 194–205. 10.1016/j.pbiomolbio.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Crowe JL, Liu X, Nakajima S, Wang Y, Li C, Lee BJ, Dubois RL, Liu C, Yu X, et al. (2015). Differential phosphorylation of DNA-PKcs regulates the interplay between end-processing and end-ligation during nonhomologous end-joining. Mol Cell 58, 172–185. 10.1016/j.molcel.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim MF, Liu S, Laciak AR, Volk L, Koszelak-Rosenblum M, Lieber MR, Wu M, Curtis R, Huang NN, Carr G, and Zhu G (2020). Structural analysis of the catalytic domain of Artemis endonuclease/SNM1C reveals distinct structural features. J Biol Chem 295, 12368–12377. 10.1074/jbc.RA120.014136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucukelbir A, Sigworth FJ, and Tagare HD (2014). Quantifying the local resolution of cryo-EM density maps. Nat Methods 11, 63–65. 10.1038/nmeth.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafaille JJ, DeCloux A, Bonneville M, Takagaki Y, and Tonegawa S (1989). Junctional sequences of T cell receptor gamma delta genes: implications for gamma delta T cell lineages and for a novel intermediate of V-(D)-J joining. Cell 59, 859–870. 10.1016/0092-8674(89)90609-0. [DOI] [PubMed] [Google Scholar]
- Langer LM, Gat Y, Bonneau F, and Conti E (2020). Structure of substrate-bound SMG1–8-9 kinase complex reveals molecular basis for phosphorylation specificity. Elife 9. 10.7554/eLife.57127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees-Miller JP, Cobban A, Katsonis P, Bacolla A, Tsutakawa SE, Hammel M, Meek K, Anderson DW, Lichtarge O, Tainer JA, and Lees-Miller SP (2021). Uncovering DNA-PKcs ancient phylogeny, unique sequence motifs and insights for human disease. Prog Biophys Mol Biol 163, 87–108. 10.1016/j.pbiomolbio.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Shimazaki N, Raval P, Gu J, Watanabe G, Schwarz K, Swanson PC, and Lieber MR (2008). A biochemically defined system for coding joint formation in V(D)J recombination. Mol Cell 31, 485–497. 10.1016/j.molcel.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, and Lieber MR (2002). Binding of inositol hexakisphosphate (IP6) to Ku but not to DNA-PKcs. J Biol Chem 277, 10756–10759. 10.1074/jbc.C200030200. [DOI] [PubMed] [Google Scholar]
- Ma Y, Pannicke U, Schwarz K, and Lieber MR (2002). Hairpin opening and overhang processing by an Artemis/DNA-dependent protein kinase complex in nonhomologous end joining and V(D)J recombination. Cell 108, 781–794. 10.1016/s0092-8674(02)00671-2. [DOI] [PubMed] [Google Scholar]
- Mastronarde DN (2005). Automated electron microscope tomography using robust prediction of specimen movements. J Struct Biol 152, 36–51. [DOI] [PubMed] [Google Scholar]
- Meek K (2020). Activation of DNA-PK by hairpinned DNA ends reveals a stepwise mechanism of kinase activation. Nucleic Acids Res 48, 9098–9108. 10.1093/nar/gkaa614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek K, Douglas P, Cui X, Ding Q, and Lees-Miller SP (2007). trans Autophosphorylation at DNA-dependent protein kinase’s two major autophosphorylation site clusters facilitates end processing but not end joining. Mol Cell Biol 27, 3881–3890. 10.1128/MCB.02366-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek K, Gupta S, Ramsden DA, and Lees-Miller SP (2004). The DNA-dependent protein kinase: the director at the end. Immunol Rev 200, 132–141. 10.1111/j.0105-2896.2004.00162.x. [DOI] [PubMed] [Google Scholar]
- Moshous D, Callebaut I, de Chasseval R, Corneo B, Cavazzana-Calvo M, Le Deist F, Tezcan I, Sanal O, Bertrand Y, Philippe N, et al. (2001). Artemis, a novel DNA double-strand break repair/V(D)J recombination protein, is mutated in human severe combined immune deficiency. Cell 105, 177–186. 10.1016/s0092-8674(01)00309-9. [DOI] [PubMed] [Google Scholar]
- Neal JA, Dang V, Douglas P, Wold MS, Lees-Miller SP, and Meek K (2011). Inhibition of homologous recombination by DNA-dependent protein kinase requires kinase activity, is titratable, and is modulated by autophosphorylation. Mol Cell Biol 31, 1719–1733. 10.1128/MCB.01298-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal JA, Sugiman-Marangos S, VanderVere-Carozza P, Wagner M, Turchi J, Lees-Miller SP, Junop MS, and Meek K (2014). Unraveling the complexities of DNA-dependent protein kinase autophosphorylation. Mol Cell Biol 34, 2162–2175. 10.1128/MCB.01554-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoz C, Ropars V, Frit P, Gontier A, Drevet P, Yu J, Guerois R, Pitois A, Comte A, Delteil C, et al. (2018). XLF and APLF bind Ku80 at two remote sites to ensure DNA repair by non-homologous end joining. Nat Struct Mol Biol 25, 971–980. 10.1038/s41594-018-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewolik D, Pannicke U, Lu H, Ma Y, Wang LC, Kulesza P, Zandi E, Lieber MR, and Schwarz K (2006). DNA-PKcs dependence of Artemis endonucleolytic activity, differences between hairpins and 5’ or 3’ overhangs. J Biol Chem 281, 33900–33909. 10.1074/jbc.M606023200. [DOI] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, and Ferrin TE (2004). UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem 25, 1605–1612. 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Punjani A, Rubinstein JL, Fleet DJ, and Brubaker MA (2017). cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296. [DOI] [PubMed] [Google Scholar]
- Reddy YV, Ding Q, Lees-Miller SP, Meek K, and Ramsden DA (2004). Non-homologous end joining requires that the DNA-PK complex undergo an autophosphorylation-dependent rearrangement at DNA ends. J Biol Chem 279, 39408–39413. 10.1074/jbc.M406432200. [DOI] [PubMed] [Google Scholar]
- Rooney S, Sekiguchi J, Zhu C, Cheng HL, Manis J, Whitlow S, DeVido J, Foy D, Chaudhuri J, Lombard D, and Alt FW (2002). Leaky Scid phenotype associated with defective V(D)J coding end processing in Artemis-deficient mice. Mol Cell 10, 1379–1390. 10.1016/s1097-2765(02)00755-4. [DOI] [PubMed] [Google Scholar]
- Roth DB (2014). V(D)J Recombination: Mechanism, Errors, and Fidelity. Microbiol Spectr 2. 10.1128/microbiolspec.MDNA3-0041-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres SH (2012). RELION: implementation of a Bayesian approach to cryo-EM structure determination. J Struct Biol 180, 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibanda BL, Chirgadze DY, Ascher DB, and Blundell TL (2017). DNA-PKcs structure suggests an allosteric mechanism modulating DNA double-strand break repair. Science 355, 520–524. 10.1126/science.aak9654. [DOI] [PubMed] [Google Scholar]
- Smider V, Rathmell WK, Brown G, Lewis S, and Chu G (1998). Failure of hairpin-ended and nicked DNA To activate DNA-dependent protein kinase: implications for V(D)J recombination. Mol Cell Biol 18, 6853–6858. 10.1128/MCB.18.11.6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson BM, Moreno AT, Walter JC, and Loparo JJ (2020). A Mechanism to Minimize Errors during Non-homologous End Joining. Mol Cell 77, 1080–1091 e1088. 10.1016/j.molcel.2019.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swint-Kruse L, and Brown CS (2005). Resmap: automated representation of macromolecular interfaces as two-dimensional networks. Bioinformatics 21, 3327–3328. [DOI] [PubMed] [Google Scholar]
- Szwergold BS, Graham RA, and Brown TR (1987). Observation of inositol pentakis- and hexakis-phosphates in mammalian tissues by 31P NMR. Biochem Biophys Res Commun 149, 874–881. 10.1016/0006-291x(87)90489-x. [DOI] [PubMed] [Google Scholar]
- van der Burg M, Ijspeert H, Verkaik NS, Turul T, Wiegant WW, Morotomi-Yano K, Mari PO, Tezcan I, Chen DJ, Zdzienicka MZ, et al. (2009). A DNA-PKcs mutation in a radiosensitive T-B-SCID patient inhibits Artemis activation and nonhomologous end-joining. J Clin Invest 119, 91–98. 10.1172/JCI37141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JR, Corpina RA, and Goldberg J (2001). Structure of the Ku heterodimer bound to DNA and its implications for double-strand break repair. Nature 412, 607–614. 10.1038/35088000. [DOI] [PubMed] [Google Scholar]
- Williams RM, Yates LA, and Zhang X (2020). Structures and regulations of ATM and ATR, master kinases in genome integrity. Curr Opin Struct Biol 61, 98–105. 10.1016/j.sbi.2019.12.010. [DOI] [PubMed] [Google Scholar]
- Yang H, Jiang X, Li B, Yang HJ, Miller M, Yang A, Dhar A, and Pavletich NP (2017). Mechanisms of mTORC1 activation by RHEB and inhibition by PRAS40. Nature 552, 368–373. 10.1038/nature25023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannone SM, Khan IS, Zhou RZ, Zhou T, Valerie K, and Povirk LF (2008). Coordinate 5’ and 3’ endonucleolytic trimming of terminally blocked blunt DNA double-strand break ends by Artemis nuclease and DNA-dependent protein kinase. Nucleic Acids Res 36, 3354–3365. 10.1093/nar/gkn205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Liu M, Tian Y, Wang J, and Xu Y (2017). Cryo-EM structure of human DNA-PK holoenzyme. Cell Res 27, 1341–1350. 10.1038/cr.2017.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosaatmadja Y, Baddock HT, Newman JA, Bielinski M, Gavard AE, Mukhopadhyay SMM, Dannerfjord AA, Schofield CJ, McHugh PJ, and Gileadi O (2021). Structural and mechanistic insights into the Artemis endonuclease and strategies for its inhibition. Nucleic Acids Res 49, 9310–9326. 10.1093/nar/gkab693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K (2016). Gctf: Real-time CTF determination and correction. J Struct Biol 193, 1–12. 10.1016/j.jsb.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Yajima H, Huynh H, Zheng J, Callen E, Chen HT, Wong N, Bunting S, Lin YF, Li M, et al. (2011). Congenital bone marrow failure in DNA-PKcs mutant mice associated with deficiencies in DNA repair. J Cell Biol 193, 295–305. 10.1083/jcb.201009074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Rothenberg E, Ramsden DA, and Lieber MR (2020). The molecular basis and disease relevance of non-homologous DNA end joining. Nat Rev Mol Cell Biol 10.1038/s41580-020-00297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SQ, Palovcak E, Armache JP, Verba KA, Cheng Y, and Agard DA (2017). MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat Methods 14, 331–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Lee JH, Jiang W, Crowe JL, Zha S, and Paull TT (2017). Regulation of the DNA Damage Response by DNA-PKcs Inhibitory Phosphorylation of ATM. Mol Cell 65, 91–104. 10.1016/j.molcel.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, and Paull TT (2013). DNA-dependent protein kinase regulates DNA end resection in concert with Mre11-Rad50-Nbs1 (MRN) and ataxia telangiectasia-mutated (ATM). J Biol Chem 288, 37112–37125. 10.1074/jbc.M113.514398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie S2. DNA moves outward along its helical axis by 2 bp from complex VI to complex VII, Related to Figure 4
DNA-PKcs is depicted in green cartoon, Ku70/80 in light and dark pink, and the blunt end DNA in brown. Two different views are shown. At the end of each viewing angle, DNA-PKcs in complex VII is colored in rainbow fashion from the blue N terminus to red C terminus. The DEB helix that splits the first base pair and covers the DNA end is colored yellow. DNA-PKcs is more compact in complex VII than in complex VI and appears to “shrink” when transforming from complex VI to VII.
Movie S1. Structural changes of DNA-PKcs before (PDB: 7K1N) and after autophosphorylation of ABCDE (PDB: 7SGL), Related to Figure 1
The DNA-PKcs protein is colored from blue N terminus to red C terminus in a rainbow gradient. The four phosphorylated Thr residues (2609, 2638, 2645 and 2647) are shown as orange and red spheres. Helices A1 and A8, which undergo large motions, are labeled in the first frame. Two orthogonal views are shown side-by-side. This animation is based on superposition of the FATKIN domain.
Data Availability Statement
There is no new sequence data associated with this work. Original gels of kinase activity and DNA cleavage have been deposited at Mendeley and are publicly available as of the date of publication. The DOI is listed in the key resource table.
This paper does not report any original code. The structures and cryoEM maps have been deposited with PDB and EMDB with accession codes of the Artemis complex 7SGL (PDB) and 25113 (EMDB), and locally refined maps EMD-25114 (FATKIN) and EMD-25115 (DNA and Artemis-Ku-N-HEAT); accession codes of complexes VII and VIII 7SU3 and 7SUD (PDB), 25439 and 25440 (EMDB); accession codes of complexes VIIa, VIIb and VIb 25112 (EMDB), 25111 (EMDB) and 25110 (EMDB). The DOI is listed in the key resource table.
Any additional information required to reanalyze the data reported in this paper is available from the Lead Contact upon request.
KEY RESOURCE TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, Peptides, and Recombinant Proteins | ||
| Human DNA-PKcs (full-length) | This paper | Residues 1–4128 |
| Human Ku70/80 | This paper | Residues 1–609 and 1–732 |
| Human Artemis | This paper | Residues 1–692 |
| Deposited Data | ||
| DNA-PK-Artemis complex, cryoEM (3.0 Å) | This paper | PDB: 7SGL EMDB: EMD-25113 |
| FATKIN domain of DNA-PK-Artemis (2.7 Å) | This paper | EMDB: EMD-25114 |
| DNA-Artemis-Ku-N-heat of DNA-PK-Artemis (2.9 Å) | This paper | EMDB: EMD-25115 |
| DNA-PK-DNA complex VII, cryoEM (3.3 Å) | This paper | PDB: 7SU3 EMDB: EMD-25439 |
| DNA-PK-DNA complex VIII, cryoEM (3.6 Å) | This paper | PDB: 7SUD EMDB: EMD-25440 |
| DNA-PK-DNA complex VIIa, cryoEM (4.2 Å) | This paper | EMDB: EMD-25112 |
| DNA-PK-DNA complex VIIb, cryoEM (4.2 Å) | This paper | EMDB: EMD-25111 |
| DNA-PK-DNA complex VIb, cryoEM (4.4 Å) | This paper | EMDB: EMD-25110 |
| Original gel scans | Mendeley Data | http://dx.doi.org/10.17632/t3525wy6gk.1 |
| Experimental Models: Cell Lines | ||
| HeLa-S3 cells | National Cell Culture Center | https://cellculturecompany.com/national-cell-culture-center/ |
| HEK293T cells | ATCC | CRL-3216 |
| DNA-PKcs deficient V3 cells | Katheryn Meek’s lab | N/A |
| Oligonucleotides | ||
| See DNA preparation in Table S2 | This paper | N/A |
| Recombinant DNA | ||
| Ku70 in pLEXm plasmid | This paper | pWY2799 |
| Ku80 in pLEXm plasmid | This paper | pWY2802 |
| Artemis in pLEXm plasmid | This paper | pWY2332 |
| Software and Algorithms | ||
| PyMol | Schrodinger | https://pymol.org/2/ |
| Prism-8 | GraphPad Software | https://www.graphpad.com/ |
| CCP4 | CCP4 | http://www.ccp4.ac.uk |
| PHENIX | (Adams et al., 2010) | https://www.phenix-online.org |
| COOT | (Emsley, 2010) | https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/ |
| UCSF Chimera | (Pettersen et al., 2004) | https://www.cgl.ucsf.edu/chimera/ |
| cryoSPARC | (Punjani et al., 2017) | https://cryosparc.com/ |
| RELION | (Scheres, 2012); (Fernandez-Leiro and Scheres, 2017) | https://www2.mrc-lmb.cam.ac.uk/relion |
| SerialEM | (Mastronarde, 2005) | https://bio3d.colorado.edu/SerialEM/ |


