Abstract
Objective:
To determine the physiologic changes of salivary flow rate, pH, and buffer capacity and the levels of Streptococcus mutans and Lactobacillus spp in patients undergoing fixed orthodontic treatment.
Materials and Methods:
The study included 23 patients scheduled for fixed orthodontic therapy. All subjects received equal braces, bands, and brackets, bonded with the same material. Stimulated saliva samples were taken before placement of the appliance, and at weeks 6, 12, and 18 during the therapy. Salivary flow rate and salivary pH were measured, and the salivary buffer capacity was determined. Saliva samples were cultivated on selective microbial agar for microorganism detection.
Results:
A significant (P < .05) increase in stimulated salivary flow rate and salivary pH was found. The salivary levels of S mutans and Lactobacillus spp also inscreased significantly (P < .05), and the major peak was at week 12 of fixed orthodontic therapy.
Conclusion:
The 6th to 12th week of orthodontic therapy is the period of the most intensive intraoral growth of S mutans and Lactobacillus spp and a time of very intensive salivary functions and physiologic response.
Keywords: Streptococcus mutans, Lactobacillus spp, Saliva physiology
INTRODUCTION
Wearing orthodontic appliances has been known to induce intraoral changes, such as increased plaque accumulation and elevated bacterial colonization along with potential enamel demineralization and a harmful effect on periodontal tissues.1 Intraoral environmental change, such as the placement of a fixed orthodontic appliance, leads to an increase in the volume and number of bacteria within dental plaque2 and may shift a healthy bacterial community to one that is able to cause disease.3 However, several studies of interactions among orthodontic material, microorganisms, and saliva have not detected associations between orthodontic appliances and clinical and microbial outcomes.4–7
People experience changes in salivary function over time, and these changes have a long-term clinical significance.8 Although numerous studies of salivary function have used different methods to measure salivary function and corresponding microbial interactions, a relationship between salivary functions and outcomes related to bacterial counts in saliva or plaque or caries has been shown but not explained.9–11 This may be attributable to a lack of data on a person's salivary function in healthy state, so that it is difficult to determine how the salivary function adjusts to new intraoral circumstances, such as placement of fixed orthodontic appliances.
Although some studies have detected associations between fixed orthodontic appliances, microbial outcomes, and measures of salivary function, the results are not consistent. Chang et al.12 found an increase in stimulated salivary flow rate, pH, buffer capacity, and levels of Streptococcus mutans and Lactobacillus spp after 3 months of active treatment. The findings of Kanaya et al.13 were similar, except that salivary pH decreased in their study.13 Other authors have detected the oral carriage of coliform species,14 and periodontal pathogens15 among patients with fixed orthodontic appliances, but did not record changes in salivary functions. Li et al.16 found that during the first month of fixed orthodontic treatment, the whole saliva flow rate and concentrations of some saliva ions increased significantly, but were at normal levels after 3 months. Sanpei et al.17 found changes in salivary Lactobacillus spp levels but no changes in salivary flow rates, buffer capacity, and S mutans levels during and after active orthodontic treatment.
The aim of this study was to determine the physiologic changes of salivary flow rate, pH, and buffer capacity in relation to salivary levels of S mutans and Lactobacillus spp in healthy patients before and during therapy with fixed orthodontic appliances.
SUBJECTS AND METHODS
Subjects
Subjects for this study were patients scheduled for fixed orthodontic therapy in the Department of Orthodontics, Dental clinic of Clinical Hospital Zagreb, Croatia. All experimental procedures were conducted in accordance with the Declaration of Helsinki's recommendations guiding physicians in biomedical research involving human subjects. The study was approved by the Ethical Committee of the Dental School, University of Zagreb, Croatia. All participants and their parents or guardians received written information about the aims and design of the study and signed a written informed consent form.
The criteria for inclusion were as follows: permanent dentition period, moderate crowding, age 12 to 17 years, good general health, general dentistry completed, and consent to participate. Criteria for exclusion were diagnosed diabetes mellitus, autoimmune connective tissue diseases, epilepsy, any syndrome, systemic or prolonged prescribed medication, antibiotic therapy, any failure in brackets during 18 week period. A total of 23 subjects met the criteria for inclusion. A power analysis conducted during the planning of the study showed that a sample size of 20 would provide sufficient statistical power to discriminate changes in the categorical variables under study. The orthodontic process in all subjects was started with 0.012-in NiTi, followed with 0.016-in NiTi after 6 weeks and 0.016-in × 0.022-in NiTi for the next 6 weeks. Subjects were required to establish good oral hygiene status. Subjects were counseled regarding oral hygiene maintenance and tooth brushing before treatment and on each visit. All subjects used fluoride-containing toothpaste. None used supplementary fluoride during study. None of them received any periodontal procedure before or during active orthodontic treatment.
Materials, Equipment, and Supplies
All subjects received equal braces, four bands and 20 brackets from the same manufacturer (Forestadent, Pforzheim, Germany), bonded with the same adhesive material (Transbond XT Light, 3M Unitek, Monrovia, CA) without fluoride. A halogen light source was used for polymerization (applied for 20 seconds per bracket). Wire ligatures were used. For salivary analysis we used a handheld digital pH meter (Piccolo plus ATC pH meter, Hannah Instruments, Woonsocket, RI); a Cultura incubator (Ivoclar Vivadent, Schaan, Lichenstein Liechtenstein); and a CRT bacteria test kit, including mitis-salivarius with bacitracin agar, Rogosa agar, NaHCO3 tablets, pipettes, and vials (Ivoclar Vivadent, Schaan Liechtenstein).
Methods
Salivary samples were collected 2 to 4 weeks before fixed appliance treatment a baseline assessment. Salivary samples were then taken at week 6, 12, and 18 weeks after placement of fixed orthodontic appliances. The orthodontic process at each stage was consistent for all subjects, so the stages among the samples were almost the same at every investigation. The time of day for sample collection was consistent for each subject during the study.8,12 Subjects were instructed not to eat or to drink for at least one hour before sample collection and to brush their teeth once in the morning on the day of salivary collection.8,12 Subjects sat in a dental chair, slightly bent forward, and chewed bilaterally on a standardized piece of paraffin wax. The stimulated saliva was collected by having the subject spit for 10 minutes into a sterile plastic graduated cup with 1-mL gradation marks. Collected salivary volume was measured in milliliters, and salivary flow rate was calculated based on a collection time of 10 minutes (milliliters per minute).12,17 The first salivary pH was measured using a handheld digital pH meter with incorporated automatic temperature compensation.12 Two milliliters of collected saliva sample was treated with 0.1 mL of 0.1M acetic acid, and the pH was measured again.12 The downward pH change was calculated by subtracting the treated salivary pH from the first salivary pH measured,12 and salivary buffer capacity was calculated according to buffer capacity defined as Cbuffer = −Δn(acid) /(V1 • ΔpH), where −Δn(acid) is the amount of acid added, V1 is the volume of saliva, and ΔpH is the pH change induced by adding acid.
For determining S mutans salivary levels, mitis-salivarius with bacitracin agar12,13,17 (CRT bacteria selective agar, Ivoclar Vivadent) was used according to the manufacturer's instructions: 1 mL of saliva, immediately after collection, was inoculated on agar, closed in the test vial containing NaHCO3 tablet, and placed in an incubator at 37°C (99°F) for 48 hours. The number of S mutans per milliliter of saliva was estimated by comparing the colony density on the growth substrate with the evaluation chart provided by the manufacturer (counts categorized as follows: 0, no colonies detected; 1, <105 colony-forming units [CFU]/mL; 2, ≥105 CFU/mL). The same procedure was used to determine Lactobacillus spp salivary levels, but using selective Rogosa agar12,17 from the same manufacturer.
Statistical Analysis
Descriptive statistics and the χ2 test were used to determine age and gender differences, respectively. The Friedman analysis of variance (ANOVA) test procedure was used to test the overall level of significance for the data. The Bonferroni test, following a significant Friedman ANOVA result, was used to identify at which measurement times the differences in scores were observed. The nonparametric Wilcoxon matched-pairs test, following a significant Friedman ANOVA result, was used to determine the significance of changes at individual time points for salivary flow rate, pH, buffer capacity, and number of S mutans and Lactobacillus spp colonies in saliva before and after insertion of orthodontic appliances and during the 18 weeks of the study. The Spearman rank-order correlation test was used to find possible associations between dependent and independent variables. A value of P < .05 was considered statistically significant. For statistical analysis software Statistica (Statistica 7.1, StatSoft, Inc Tulsa, OK) was used.
RESULTS
A total of 23 subjects met the criteria for inclusion, 9 males (39.1%) and 14 females (60.8%); mean age was 14.04 ± 1.52 (range = 12–17 years). Data for salivary flow rate, pH, buffer capacity, levels of S mutans and Lactobacillus spp obtained before placement of fixed orthodontic appliances and at 6, 12, and 18 weeks after the placement were tested using the χ2 test and the Spearman rank-order correlation test regarding gender and age subgroups of subjects, respectively. There were no significant differences regarding gender and age.
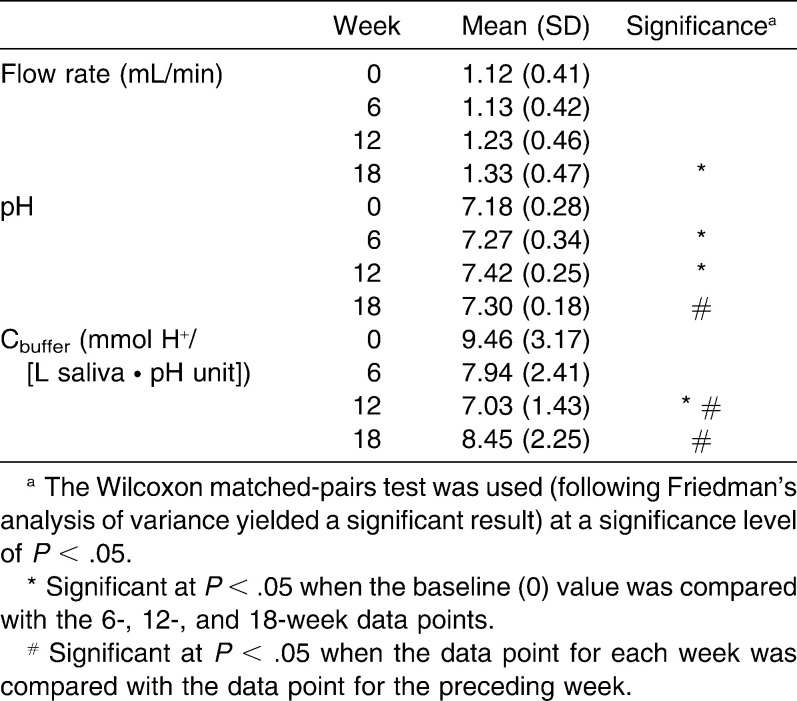
Summary data for salivary flow rate, pH, and buffer capacity are presented in Table 1. The overall level of significance for the data presented was previously analyzed with a Friedman procedure (N = 23; degrees of freedom [df] = 3; χ2flow rate = 10.33; χ2pH = 8.61; χ2buffer capacity = 16.11; P < .05) and yielded a significant result. Salivary flow rate significantly increased after 18 weeks of orthodontic therapy with fixed appliances (P < .05). Salivary pH significantly increased after first 6 weeks, continued to increase during the next 6 weeks (P < .05), and between week 12 and week 18 decreased toward baseline. A significant decrease in salivary buffer capacity at week 12 of orthodontic therapy with fixed appliances preceded an increase of salivary flow at week 18. Salivary buffer capacity increased significantly at week 18 of orthodontic therapy compared with week 12. Higher salivary flow rate before placement of appliances was significantly related to higher salivary flow rate at weeks 6, 12, and 18 after the placement of appliances (rweek6 = .838; rweek12 = .727; rweek18 = .721; P < .05). Higher salivary flow rate before treatment was significantly related to higher buffer capacity at week 12 after the placement of appliances (r = .426; P < .05).
Table 1.
Summary Data for Salivary Flow Rate, pH, and Buffer Capacity of Saliva Before (week 0, baseline) and 6, 12, and 18 Weeks after Insertion of Orthodontic Appliances (n = 23)
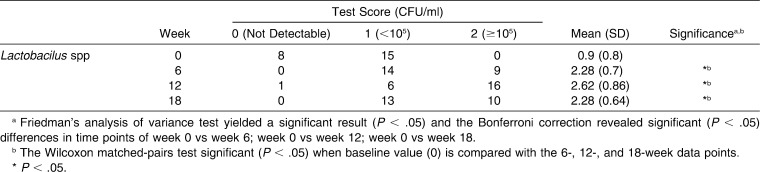
Salivary S mutans and Lactobacillus spp counts before and during 18 weeks of orthodontic therapy are presented in Tables 2 and 3. The overall level of significance for salivary S mutans and salivary Lactobacillus spp data was analyzed using a Friedman procedure (N = 23; df = 3; χ2S mutans = 20.48; χ2Lactobacillus spp = 33.93; P < .05) and yielded a significant result. Salivary S mutans increased in 21 of 23 subjects and showed no change in 2 subjects.
Table 2.
Summary Data for Salivary Streptococcus mutans in Milliliters of Saliva Before (week 0, baseline) and 6, 12, and 18 Weeks after Insertion of Orthodontic Appliances (n = 23)
Table 3.
Summary Data for Salivary Lactobacilus spp in Milliliters of Saliva Before (week 0, baseline) and 6, 12, and 18 Weeks after Insertion of Orthodontic Appliances (n = 23)
A Wilcoxon test for salivary S mutans in milliliters of saliva before and after the placement of orthodontic appliances, over 18 weeks, revealed a significant increase after first 6 weeks of appliance placement; the highest mean values were at week 12 compared to baseline. Salivary Lactobacillus spp increased in 22 of 23 subjects and showed no change in one subject. A Wilcoxon test for salivary Lactobacillus spp in milliliters of saliva before and after insertion of orthodontic appliances, over 18 weeks, revealed a significant increase after the first 6 weeks of appliance placement; the highest mean values were at week 12 compared to baseline.
DISCUSSION
Published studies on the association between orthodontic treatment and changes in oral microflora and salivary functions are neither numerous nor unambiguous.12,13,17,18 Because saliva provides a general protective effect, clinically significant changes in salivary functions may be considered an etiologic factor that contributes to the development or prevention of dental caries. Our study presented new data on the duration of salivary microbial changes elicited by the placement of fixed orthodontic appliances. In our study, as in some other investigations,12,13 a significant increase in cariogenic microorganisms S mutans and Lactobacillus spp in saliva was found after commencing fixed orthodontic therapy. In this study, the first significant increase of S mutans and Lactobacillus spp in saliva, as a result of an unfavorable effect of fixed appliances on oral hygiene, was detected as early as 6 weeks after the intraoral placement of fixed orthodontic appliances, and the highest levels were at the 12th week of therapy. Other authors have reported a significant salivary microbial increase only at week 12, but they only followed their patients for 12 weeks of therapy.12,13
A new and valuable result offered in our study is the number of colony-forming units of S mutans and Lactobacillus spp in saliva of orthodontic patients after 18 weeks of orthodontic therapy: it was still higher than the baseline amount but lower compared to week 12. All patients in our study received oral hygiene instructions and maintained their oral hygiene using fluoride toothpaste, as they had used before orthodontic therapy. None of the patients received supplementary fluoride, professional cleaning, or other antimicrobial preventive measures, so the decrease of the salivary levels of S mutans and Lactobacillus spp after 12th week of orthodontic therapy is caused by intraoral characteristics of patients. Changes of salivary functions shown in our study as a physiologic response to disturbed intraoral homeostasis had a successful antimicrobial effect. The clinical significance of the decrease of the salivary levels of S mutans and Lactobacillus spp after the 12th week of orthodontic therapy is yet to be determined, but we suggest, based on our results, that the success of antimicrobial preventive measures for orthodontic patients may be improved by proper timing, and it should take place between weeks 6 and 12 of orthodontic therapy, which is then the number of S mutans and Lactobacillus spp increase in saliva.
The peak microbial changes at week 12 were followed by salivary changes in our study. Baseline values for salivary flow rate and salivary pH in this study are in accordance with salivary values for healthy caries-free children in the study of O'Sullivan and Curzon.19 Increasing salivary flow rate and pH in this study appeared at week 12 or later, highlighting that the period of fixed orthodontic therapy is important in possible caries-onset complications or in successful host caries defense. Previous studies showed that the application of orthodontic appliances, dentures, and maxillary occlusal splints increased the salivary flow rate.12,20,21 Also, the salivary flow rate increased significantly with the weight and dimension of the stimulus,22 which may explain why some authors who applied fewer braces on subjects could not detect salivary flow changes.17 Our study presented an interesting correlation in salivary flow rate during fixed orthodontic therapy and before orthodontic treatment: although all of our subjects received equal appliances, for an equal time, causing equal stimulation on mechanoreceptors, some of them developed a higher increase in salivary flow rate than others. Our findings are in accordance with those of Ulukapi et al.,18 which showed that fixed orthodontic appliances are not the sole factor for a patient's caries risk during orthodontic treatment.18 There are some new salivary characteristics to be found and explained.
Increased salivary flow is followed by increased buffer capacity,12,23 although some authors have reported a decrease in buffer capacity.24 Recent work of Bardow et al.25 showed stimulated whole-saliva buffer capacity measured without loss of CO2. In our study, although a different method was used, data for stimulated whole-saliva buffering capacity before orthodontic therapy are similar, with some slightly higher; this is likely due to the age difference of the subjects. In addition, in this study baseline data for stimulated whole-saliva buffer capacity is in accordance with the findings of Siudikiene et al.,26 who found high buffer capacity in healthy children aged 10–15 years. In our study, an increase in salivary buffer capacity following increased salivary flow was detected when values were compared to week 12, not to baseline. Our findings are in agreement with the studies of Ulukapi et al.,18 who found a significant increase in salivary flow rate and no significant change in buffer capacity, which suggests that salivary flow rate is more sensitive to the placement of orthodontic appliances than buffer capacity.17
Moritsuka et al.27 showed that higher salivary flow rate correlates with higher buffering capacity and that the quantitative saliva assessment is useful as a screening method to identify patients with a low buffering capacity. In our study, we found no correlation of salivary flow rate and buffering capacity at baseline. Also in our study, higher salivary flow rate before placement of appliances was significantly related to the higher buffer capacity at week 12 after the placement of appliances; this is in agreement with the conclusion of Moritsuka et al.27
CONCLUSION
The 6th to 12th week of orthodontic therapy is a period of the most intensive intraoral growth of S mutans and Lactobacillus spp and a time of very intensive salivary functions physiologic response.
Acknowledgments
This work was supported by the Croatian Ministry of Science, Education and Sport (grant 065-0650444-0436).
REFERENCES
- 1.Boersma J. G, Van der Veen M. H, Lagerweij M. D, Bokhout B, Prahl-Andersen B. Caries prevalence measured with QLF after treatment with fixed orthodontic appliances: influencing factors. Caries Res. 2005;39:41–47. doi: 10.1159/000081655. [DOI] [PubMed] [Google Scholar]
- 2.Rosenbloom R. G, Tinanoff N. Salivary Streptococcus mutans levels in patients before, during, and after orthodontic treatment. Am J Orthod Dentofacial Orthop. 1991;100:35–37. doi: 10.1016/0889-5406(91)70046-Y. [DOI] [PubMed] [Google Scholar]
- 3.Marsh P. D. Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res. 1994;8:263–271. doi: 10.1177/08959374940080022001. [DOI] [PubMed] [Google Scholar]
- 4.Forsberg C. M, Brattström V, Maimberg E, Nord C. E. Ligature wires and elastomeric rings: two methods of ligation, and their association with microbial colonization of Streptococcus mutans and lactobacilli. Eur J Orthod. 1991;13:416–420. doi: 10.1093/ejo/13.5.416. [DOI] [PubMed] [Google Scholar]
- 5.Fournier A, Payant L, Bouclin R. Adherence of Streptococcus mutans to orthodontic brackets. Am J Orthod Dentofacial Orthop. 1998;114:414–417. doi: 10.1016/s0889-5406(98)70186-6. [DOI] [PubMed] [Google Scholar]
- 6.Ahn S. J, Kho H. S, Lee S. W, Nahm D. S. Roles of salivary proteins on the adherence of oral streptococci to various orthodontic brackets. J Dent Res. 2002;81:411–415. doi: 10.1177/154405910208100611. [DOI] [PubMed] [Google Scholar]
- 7.Badawi H, Evansa R. D, Wilson M, Ready D, Noara J. H, Pratten J. The effect of orthodontic bonding materials on dental plaque accumulation and composition in vitro. Biomaterials. 2003;24:3345–3350. doi: 10.1016/s0142-9612(03)00166-2. [DOI] [PubMed] [Google Scholar]
- 8.Rudney J. D, Pan Y, Chen R. Streptococcal diversity in oral biofilms with respect to salivary function. Arch Oral Biol. 2003;48:475–493. doi: 10.1016/s0003-9969(03)00043-8. [DOI] [PubMed] [Google Scholar]
- 9.Carlen A, Olsson J, Borjesson A. C. Saliva-mediated binding in vitro and prevalence in vivo of Streptococcus mutans. Arch Oral Biol. 1996;41:35–39. doi: 10.1016/0003-9969(95)00099-2. [DOI] [PubMed] [Google Scholar]
- 10.Carlen A, Olsson J, Ramberg P. Saliva mediated adherence, aggregation and prevalence in dental plaque of Streptococcus mutans, Streptococcus sanguis and Actinomyces spp., in young and elderly humans. Arch Oral Biol. 1996;41:1133–1140. doi: 10.1016/s0003-9969(96)00094-5. [DOI] [PubMed] [Google Scholar]
- 11.Carlen A, Bratt P, Stenudd C, Olsson J, Stromberg N. Agglutinin and acidic proline-rich protein receptor patterns may modulate bacterial adherence and colonization on tooth surfaces. J Dent Res. 1998;77:81–90. doi: 10.1177/00220345980770011301. [DOI] [PubMed] [Google Scholar]
- 12.Chang H. S, Walsh L. J, Freer T. J. The effect of orthodontic treatment on salivary flow, pH, buffer capacity, and levels of mutans streptococci and lactobacilli. Aust Orthod J. 1999;15:229–234. [PubMed] [Google Scholar]
- 13.Kanaya T, Kaneko N, Amaike C, Fukushima M, Morita S, Miyazaki H, Saito I. The effect of orthodontic appliances on levels of Streptococcus mutans, Streptococcus sobrinus and microbial flora in saliva. Int Congr Ser. 2005;1284:189–190. [Google Scholar]
- 14.Hägg U, Kaveewatcharanont P, Samaranayake Y. H, Samaranayake L. P. The effect of fixed orthodontic appliances on the oral carriage of Candida species and Enterobacteriaceae. Eur J Orthod. 2004;26:623–629. doi: 10.1093/ejo/26.6.623. [DOI] [PubMed] [Google Scholar]
- 15.Sallum E. J, Nouer D. F, Klein M. I, Goncalves R. B, Machion L, Sallum A. W, Sallum E. A. Clinical and microbiologic changes after removal of orthodontic appliances. Am J Orthod Dentofacial Orthop. 2004;126:363–366. doi: 10.1016/j.ajodo.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Hu B, Liu Y, Ding G, Zhang C, Wang S. The effects of fixed orthodontic appliances on saliva flow rate and saliva electrolyte concentrations. J Oral Rehabil. 2009;36:781–785. doi: 10.1111/j.1365-2842.2009.01993.x. [DOI] [PubMed] [Google Scholar]
- 17.Sanpei S, Endo T, Shimooka S. Caries risk factors in children under treatment with sectional brackets. Angle Orthod. 2010;80:509–514. doi: 10.2319/072909-431.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ulukapi H, Koray F, Efes B. Monitoring the caries risk of orthodontic patients. Quintessence Int. 1997;28:27–29. [PubMed] [Google Scholar]
- 19.O'Sullivan E. A, Curzon M. E. J. Salivary factors affecting dental erosion in children. Caries Res. 2000;34:82–87. doi: 10.1159/000016574. [DOI] [PubMed] [Google Scholar]
- 20.Yurdukoru B, Terzioglu H, Yilmaz T. Assessment of whole saliva flow rate in denture wearing patients. J Oral Rehabil. 2001;28:109–112. doi: 10.1046/j.1365-2842.2001.00624.x. [DOI] [PubMed] [Google Scholar]
- 21.Miyawaki S, Katayama A, Tanimoto Y, Araki Y, Fujii A, Yashiro K, Takano-Yamamoto T. Salivary flow rates during relaxing, clenching, and chewing-like movement with maxillary occlusal splints. Am J Orthod Dentofacial Orthop. 2004;126:367–370. doi: 10.1016/j.ajodo.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 22.Brand H. S, Ligtenberg A. J. M, Bots C. P, Nieuw Amerongen A. V. Secretion rate and buffer capacity of whole saliva depend on the weight of the mechanical stimulus. Int J Dent Hygiene. 2004;2:137–138. doi: 10.1111/j.1601-5037.2004.00081.x. [DOI] [PubMed] [Google Scholar]
- 23.Heintze U, Birkhed D, Björn H. Secretion rate and buffer effects of resting and stimulated whole saliva as a function of age and sex. Swed Dent J. 1983;7:227–238. [PubMed] [Google Scholar]
- 24.Dawes C. Rhythms in salivary flow rate and composition. Int J Chronobiol. 1974;2:253–279. [PubMed] [Google Scholar]
- 25.Bardow A, Moe D, Nyvad B, Nauntofte B. The buffer capacity and buffer systems of human whole saliva measured without loss of CO2. Arch Oral Biol. 2000;45:1–12. doi: 10.1016/s0003-9969(99)00119-3. [DOI] [PubMed] [Google Scholar]
- 26.Siudikiene J, Machiulskiene V, Nyvad B, Tenovuo J, Nedzelskiene I. Dental caries increments and related factors in children with type 1 diabetes mellitus. Caries Res. 2008;42:354–362. doi: 10.1159/000151582. [DOI] [PubMed] [Google Scholar]
- 27.Moritsuka M, Kitasako Y, Burrow M. F, Ikeda M, Tagami J, Nomura S. Quantitative assessment for stimulated saliva flow rate and buffering capacity in relation to different ages. J Dent. 2006;34:716–720. doi: 10.1016/j.jdent.2006.01.004. [DOI] [PubMed] [Google Scholar]