Abstract
A clinical isolate, Escherichia coli MG-1, isolated from a 4-month-old Vietnamese orphan child, produced a β-lactamase conferring resistance to extended-spectrum cephalosporins and aztreonam. In a disk diffusion test, a typical synergistic effect between ceftazidime or aztreonam and clavulanic acid was observed along with an unusual synergy between cefoxitin and cefuroxime. The gene for VEB-1 (Vietnamese extended-spectrum β-lactamase) was cloned and expressed in E. coli JM109. The recombinant plasmid pRLT1 produced a β-lactamase with a pI of 5.35 and conferred high-level resistance to extended-spectrum (or oxyimino) cephalosporins and to aztreonam. Vmax values for extended-spectrum cephalosporins were uncommonly high, while the affinity of the enzyme for ceftazidime and aztreonam was relatively low. blaVEB-1 showed significant homology at the DNA level with only blaPER-1 and blaPER-2. Analysis of the deduced protein sequence showed that VEB-1 is a class A penicillinase having very low levels of homology with any other known β-lactamases. The highest percentage of amino acid identity was 38% with PER-1 or PER-2, two uncommon class A extended-spectrum enzymes. Exploration of the genetic environment of blaVEB-1 revealed the presence of gene cassette features, i.e., (i) a 59-base element associated with blaVEB-1; (ii) a second 59-base element just upstream of blaVEB-1, likely belonging to the aacA1-orfG gene cassette; (iii) two core sites (GTTRRRY) on both sides of blaVEB-1; and (iv) a second antibiotic resistance gene 3′ of blaVEB-1, aadB. blaVEB-1 may therefore be the first class A extended-spectrum β-lactamase that is part of a gene cassette, which itself is likely to be located on a class 1 integron, as sulfamide resistance may indicate. Furthermore, blaVEB-1 is encoded on a large (>100-kb) transferable plasmid found in a Klebsiella pneumoniae MG-2 isolated at the same time from the same patient, indicating a horizontal gene transfer.
Although naturally susceptible to extended-spectrum cephalosporins, Escherichia coli strains may become resistant to these β-lactams by several mechanisms, mainly alterations in outer membrane proteins, overproduction of cephalosporinase (chromosomal or plasmid mediated), or production of an extended-spectrum β-lactamase (ESBL). ESBLs are enzymes capable of hydrolyzing oxyimino cephalosporins and monobactams.
Analysis of the known β-lactamase sequences has resulted in their being divided according to their amino acid sequences into four classes, designated A to D (1). Most of the ESBLs found so far in Enterobacteriaceae are Ambler class A β-lactamases. They are plasmid encoded, and the enzymes most commonly observed in E. coli are TEM derivatives and to a lesser extent SHV derivatives (28). The extension of their hydrolysis properties results from single amino acid changes within their catalytic sites. Epidemiological studies have revealed that these ESBLs are now disseminated worldwide (28). In addition to these ESBLs, non-SHV non-TEM derivative enzymes have been detected in E. coli: FEC-1 (27), CTX-M1 (MEN-1) (5, 6), CTX-M2 (6), PER-2 (7), and TOHO-1 (19). The structurally related CTX-M1, TOHO-1, and CTX-M2 have been isolated among rare enterobacterial isolates in Europe, while the spread of PER-2 among Enterobacteriaceae family members has been limited so far to South America (7). FEC-1, which was found in an E. coli strain in Japan, has so far only been biochemically characterized (27).
Along with a plasmid location, some β-lactamase genes may be encoded in gene cassettes that are present in the variable region of the integrons (15, 38, 45). The gene cassettes are discrete mobile units, and each comprises a gene, normally an antibiotic resistance gene and a recombination site that is recognized by an integrase (12, 38). The cassette-associated recombination sites known as 59-base elements (59-be) are located downstream of the genes and are of variable length (12, 46). The most highly conserved features of 59-be are a 7-bp core site with the consensus site GTTRRRY located at the right-hand end of the element (furthest from the 3′ end of the cassette-encoded gene) and an inverse core site with the consensus site RYYYAAC at the left-hand end (12, 46). The integrons most commonly isolated from antibiotic-resistant clinical isolates from members of the family Enterobacteriaceae and Pseudomonads belong to class 1. These class 1 integrons possess two conserved regions located on either side of the integrated gene cassettes. The 5′ conserved segment includes a gene, int1, encoding the integrase; attI, the cassette integration site; and the promoter Pant, which is responsible for expression of cassette genes. The 3′ conserved segment includes, along with two other open reading frames (ORFs), the sulfamide resistance determinant (sulI) (38). The existence of integrons and integron-associated genes explains how plasmids may accumulate a diversity of resistance genes. While some class D ESBLs are found on integrons (14, 37, 38), no class A ESBL has yet been found on the variable region of integrons (15).
The β-lactamase described in this report is a novel plasmid-encoded ESBL from an E. coli clinical isolate recovered from a 4-month-old Vietnamese child. E. coli MG-1 displayed resistance to both extended-spectrum cephalosporins and to aztreonam and showed typical synergy during a double disk assay when ceftazidime or aztreonam was placed next to a clavulanic acid disk on an agar plate. Its gene was cloned and sequenced, and the deduced protein sequence was compared with those of other class A β-lactamases. The enzymatic properties of the enzyme included high-level hydrolytic activity against extended-spectrum cephalosporins. We also characterized its plasmid determinant and its transferability to other Enterobacteriaceae spp. In addition, we provided evidence of gene transfer from E. coli to Klebsiella pneumoniae. Finally, its gene is located on a gene cassette which may itself be present on an integron.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this work are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or phenotype | Source or referencea |
|---|---|---|
| Strains | ||
| E. coli JM109 | endA1 hsdR17 gyrA96 Δ(lac proA) recA1 relA supE44 thi F′ (lacIqlacZΔM15 proAB+ traD36) | 52 |
| In vitro-obtained ciprofloxacin-resistant E. coli JM109 | Ciprofloxacin resistant | 37 |
| E. coli NCTC 50192 | 154-, 66-, 48-, and 7-kb reference plasmids | 37 |
| E. coli MG-1 | Extended spectrum cephalosporin resistant | This study |
| Plasmids | ||
| pBK-CMV phagemid | Neomycinr, kanamycinr | Stratagene (La Jolla, Calif.) |
| pBR322 | Recombinant plasmid containing the 560-bp SspI-PstI internal fragment of blaTEM-1 | 48 |
| pHUC37 | Recombinant plasmid containing the 435-bp PstI-NotI internal fragment of blaSHV-3 | 32 |
| pPZ1 | Recombinant plasmid containing the 1.1-kb SnaBI internal fragment of blaPER-1 | 34 |
| pPL1 | Recombinant plasmid containing the 450-bp SnaBI internal fragment of oxa18 | 37 |
| pRLT1 | Recombinant plasmid containing a 1.3-kb Sau3A fragment with blaVEB1 | This report |
| pNLT1 | Natural plasmid from E. coli MG-1 containing blaVEB1. Resistance phenotype: ESBL, Kan, Tm, AN, Net, Tet, Cm, Sul | |
| pNLT2 | Natural plasmid from E. coli MG-1 containing blaTEM-1. Resistance phenotype: TEM, Cm | |
| pNLT3 | Natural plasmid from K. pneumoniae MG-2 containing blaVEB1. Resistance phenotype: ESBL, Kan, Tm, AN, Net, Tet, Cm, Sul | |
| pRLT50 | Recombinant plasmid containing 1.4-kb genomic Sau3A fragment containing blaTEM-1 | This report |
Abbreviations: Kan, kanamycin; Tm, tobramycin; Net, netilmicin; AN, amikacin; Cm, chloramphenicol; Tet, tetracycline; Sul, sulfamide.
Antimicrobial agents and MIC determinations.
The antimicrobial agents used in this study were obtained in the form of standard laboratory powders and were used immediately after their solubilization. The agents and their sources were as follows: amoxicillin, clavulanic acid, and ticarcillin (Smith-Kline-French-Beecham, Nanterre, France); aztreonam and cefepime (Bristol-Myers Squibb, Paris La Défense, France); ceftazidime, cefuroxime, and cephaloridine (Glaxo, Paris, France); cefamandole, cefalotin, and moxalactam (Eli Lilly, Saint-Cloud, France); piperacillin and tazobactam (Lederle, Oullins, France); sulbactam and cefoperazone (Pfizer, Orsay, France); cefotaxime and cefpirome (Hoechst-Roussel, Paris, France); cefoxitin and imipenem (Merck Sharp and Dohme-Chibret, respectively, Paris, France); ceftriaxone (Hoffmann-La-Roche, Neuilly-sur-Seine, France); benzylpenicillin (Specia, Paris, France); and ciprofloxacin (Bayer, Paris, France). Antibiotic disks were used for routine antibiograms (Sanofi-Diagnostics Pasteur, Marnes-la-Coquette, France).
MICs were determined by an agar dilution technique on Mueller-Hinton agar (Diagnostics Pasteur) with an inoculum of 104 CFU per spot. All plates were incubated at 37°C for 18 h. MICs of β-lactams were determined alone or in combination with a fixed concentration of clavulanic acid (2 μg/ml).
Hybridization and PCR analyses.
Dot blots and Southern hybridizations were performed as described by the manufacturer by using the ECL nonradioactive kit (Amersham, Les Ulis, France). The probes (Table 1) consisted of the 1.1-kb SnaBI fragment from recombinant plasmid pPZ1 for blaPER-1, the 450-bp PstI-NotI fragment from recombinant plasmid pHUC37 for blaSHV-3, the 560-bp SspI-PstI fragment from plasmid pBR322 for blaTEM-1, or the 450-bp PstI-NotI fragment from recombinant plasmid pPL1 for oxa18. Standard PCR experiments were performed as described previously (40).
Plasmid content and mating-out assays.
Plasmid DNAs of E. coli MG-1 and K. pneumoniae MG-2 were prepared with the Qiagen plasmid DNA maxi kit (Qiagen, Courtaboeuf, France). Plasmid DNAs were analyzed by electrophoresis on a 0.8% agarose gel (Gibco-BRL-Life Technologies, Eragny, France) containing 0.15 μg of ethidium bromide (Pharmacia-Biotech, Orsay, France)/ml. Standard sizes of plasmid DNAs were extracted from E. coli NCTC 50192.
The extracted plasmid DNAs from either E. coli MG-1 or K. pneumoniae MG-2 were subjected to electroporation into E. coli JM109 according to the manufacturer’s instructions (Bio-Rad, Ivry-sur-Seine, France). Recombinant bacteria were plated onto Trypticase soy agar plates containing 100 μg of amoxicillin/ml. The plasmids were again extracted by using the Qiagen maxi columns kit (Qiagen), and the sizes were estimated by restriction endonuclease digestions (Pharmacia Biotech).
Direct transfer of resistance into ciprofloxacin-resistant E. coli JM109 obtained in vitro was attempted by liquid and solid mating-out assays at 30 and 37°C. Transconjugant selection was performed on Trypticase soy agar plates containing ciprofloxacin (3 μg/ml) and amoxicillin (100 μg/ml).
Cloning experiments and analysis of recombinant plasmids.
Genomic DNA of E. coli MG-1 was extracted as described previously (34). Fragments from Sau3AI partially digested genomic DNA were ligated into the BamHI site of pBK-CMV phagemid (Stratagene, La Jolla, Calif.) as previously described (34). The restriction enzymes as well as the ligase were from Pharmacia Biotech.
Recombinant plasmid DNA was prepared by using Qiagen columns (Qiagen), and plasmid maps were determined after double restriction analysis (40). Fragment sizes were estimated by comparison to the molecular weight standard 1-kb DNA ladder (Gibco-BRL-Life Technologies).
β-Lactamase preparation.
Cultures of E. coli expressing blaVEB-1 were grown overnight at 37°C in 100 ml of Trypticase soy broth with amoxicillin (100 μg/ml). Bacterial suspensions were disrupted by sonication (four times for 20 s at 20 KHz) and centrifuged (30 min, 20,000 × g, 4°C). The supernatant contained the crude enzyme extract. The enzyme was further purified by ion exchange chromatography using AGMP-1 resin (Bio-Rad) (34). The resin, in the form of ion chloride, was first treated with 0.1 M ammonia in water and then washed extensively with water. After absorption of the extracts, elution was performed with a 0.1 M NaCl solution. The active fractions were pooled, dialyzed extensively, and lyophilized. The relative molecular mass of partially purified β-lactamase obtained from E. coli JM109 harboring recombinant plasmid pRLT1 was estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis (24).
Isoelectric focusing.
Crude β-lactamase extracts were subjected to analytical isoelectric focusing on an ampholine polyacrylamide gel (pH 3.5 to 9.5) (Pharmacia Biotech) for 36 h at 10 W of constant power on a flatbed apparatus (FBE-3000; Pharmacia). The β-lactamase was visualized with an overlay of agar-iodine starch gel containing benzylpenicillin (0.01% [wt/vol]) in 0.1 M phosphate buffer (pH 7.0) (4). The pI of VEB-1 was determined by comparison to those of known β-lactamases.
Kinetic measurements.
Kinetic measurements were performed with a purified β-lactamase preparation extracted from E. coli harboring recombinant plasmid pRLT1. The kinetic constants of preparations were determined by the online computerized microacidimetric method at pH 7.0 and 37°C as previously described (23). As assessed by isoelectric focusing and sequencing, the enzyme preparation contained only a single β-lactamase activity. The Km was expressed in micromolar concentrations, and Vmax was expressed relative to that of benzylpenicillin (Vmax = 100). In the case of substrates with low or undetectable Vmax values, enzyme substrate affinity was measured as Ki (inhibition constant) rather than Km with cefotaxime as the substrate.
Inhibition of β-lactamase activity.
Various concentrations of clavulanic acid, sulbactam, tazobactam, imipenem, cefoxitin, and moxalactam were preincubated with the enzyme for 10 min at 37°C before testing the rate of cefotaxime hydrolysis and calculating the inhibition constant (Ki) (23).
DNA sequencing and protein analysis.
The 1.2-kb cloned DNA fragment from pRLT1 and the 1.4-kb cloned DNA fragment from pRLT50 were sequenced on both strands by using an Applied Biosystems sequencer (ABI 311). The nucleotide sequence and the deduced protein sequence were analyzed with software available over the Internet at the National Center of Biotechnology Information website (30a) and at Pedro’s BioMolecular Research Tools website (35a). Multiple sequence alignment of deduced peptide sequences was carried out over the Internet at the University of Cambridge website using the program ClustalW. The following 19 class A β-lactamases were compared to VEB-1: SHV-2 (18), TEM-3 (44), PSE-4 (8), SME-1 (30), NMC-A (29), KOXY (2), CTX-M-1 (6), TOHO-1 (19), CITDI (36), YENT (41), BLIP (31), CAKCC (25), ROB-1 (22), PC-1 (11), PER-1 (34), PER-2 (7), CFXA (35), CEPA (39), and CBLA (43). A dendrogram was derived from the multiple sequence alignment by a parsimony method using the phylogeny package PAUP (Phylogenetic Analysis Using Parsimony) version 3.0 (49).
Nucleotide sequence accession number.
The nucleotide sequence data reported in this paper will appear in the GenBank nucleotide database under the accession no. AF010416.
RESULTS
Origin of the E. coli MG-1 isolate.
E. coli MG-1 was isolated in 1996, at the Hôpital Antoine Béclère, Clamart (a suburb of Paris), France, from the pus of a 4-month-old Vietnamese boy hospitalized for severe respiratory problems. He was previously hospitalized in an intensive care unit in Vietnam. Antimicrobial regimens before admission were not documented, and the patient did not receive any antibiotic treatment prior to the isolation of the strain at the Hôpital Antoine Béclère. A routine antibiogram revealed high levels of resistance of E. coli MG-1 to amino, carboxy, and ureido-penicillins and to restricted and extended-spectrum cephalosporins (Table 2). E. coli MG-1 was also resistant to kanamycin, chloramphenicol, tetracycline, gentamicin, tobramycin, netilmicin, amikacin, trimethoprim, and trimethoprim-sulfamethoxazole. In the course of a systematic multiresistant-bacteria rectal screening, the same E. coli MG-1 was isolated along with a K. pneumoniae MG-2 strain presenting a similar resistance profile.
TABLE 2.
MICs of β-lactams for E. coli MG-1, E. coli JM109 harboring recombinant plasmids pRLT1 and pRLT50, and reference strain E. coli JM109
| Antibiotic(s) | MIC (μg/ml) for:
|
|||
|---|---|---|---|---|
| E. coli MG-1a | E. coli JM109 (pRLT1)b | E. coli JM109 (pRLT50)c | E. coli JM109 | |
| Amoxicillin | >512 | >512 | >512 | 2 |
| Amoxicillin-Clad | 128 | 8–16 | 256 | 1 |
| Ticarcillin | >512 | >512 | >512 | 2 |
| Ticarcillin-Cla | 256 | 4 | 256 | 2 |
| Piperacillin | 256 | 16 | 512 | 1 |
| Piperacillin-Cla | 4 | 2 | 64 | 1 |
| Cephalothin | 128 | 128 | 256 | 4 |
| Cephalothin-Cla | 4 | 4 | 4 | 4 |
| Cefamandole | 32 | 32 | 32–64 | 0.5 |
| Cefuroxime | 64 | 128 | 8 | <2 |
| Cefuroxime-Cla | 4 | 4 | 4 | <2 |
| Cefoxitin | 4 | 8 | 4 | 4 |
| Cefoxitin-Cla | 4 | 4 | 2 | 2 |
| Ceftazidime | 256 | 256 | 1 | 0.25 |
| Ceftazidime-Cla | 0.25 | 0.25 | 1 | 0.25 |
| Cefotaxime | 2 | 2 | 0.12 | 0.06 |
| Cefotaxime-Cla | 0.06 | 0.06 | 0.06 | 0.06 |
| Cefepime | 1 | 1 | 0.12 | 0.06 |
| Cefepime-Cla | 0.03 | 0.06 | 0.03 | 0.06 |
| Ceftriaxone | 2 | 4 | 0.12 | 0.06 |
| Moxalactam | 0.25 | 0.12 | 0.12 | 0.12 |
| Moxalactam-Cla | 0.25 | 0.12 | 0.12 | 0.25 |
| Aztreonam | 32 | 32 | 0.25 | 0.12 |
| Aztreonam-Cla | 0.12 | 0.12 | 0.25 | 0.06 |
| Imipenem | 0.25 | 0.25 | 0.12 | 0.12 |
E. coli MG-1 produces VEB-1 along with a TEM-1 penicillinase.
E. coli JM109 harboring recombinant plasmid pRLT1 produced the VEB-1 β-lactamase.
E. coli JM109 harboring recombinant plasmid pRLT50 produced the TEM-1 β-lactamase.
Cla, clavulanic acid at fixed concentration of 2 μg/ml.
Hybridizations and cloning of the ESBL gene.
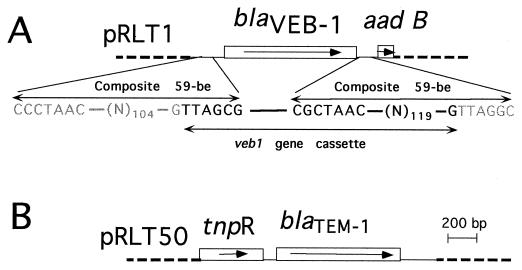
Preliminary hybridization experiments indicated that E. coli MG-1 harbored a TEM-derived resistance gene as indicated by a positive hybridization signal detected by a blaTEM- probe (data not shown). blaSHV-3, oxa18, and blaPER-1 probes failed to give positive hybridization signals. PCR amplification using TEM-1 intragenic primers and direct sequencing of the PCR product showed 100% identity with blaTEM-1. Since TEM-1 is not an ESBL, its presence might not explain the uncommon resistance phenotype. DNA from E. coli MG-1 was partially digested with restriction endonuclease Sau3AI and ligated to BamHI-digested plasmid pBK-CMV. The ligation product was transformed into E. coli JM109 by electroporation. Several recombinant colonies expressing one of the following two phenotypes were obtained: (i) a high level of resistance to amoxicillin, cephalothin, and ticarcillin, which was inhibited by clavulanic acid; or (ii) an extended-spectrum phenotype. The recombinant plasmids expressing each β-lactamase resistance phenotype were extracted and analyzed. The insert sizes ranged from 1.2 to 15 kb. Restriction maps were generated for both plasmids pRLT1 and pRLT50 containing, respectively, a 1.2-kb insert expressing the ESBL and a 1.3-kb insert expressing a penicillinase (pRLT50) (Fig. 1).
FIG. 1.
Schematic map of the recombinant plasmids pRLT1, encoding VEB-1, (A) and pRLT-50, encoding TEM-1 (B). The thin line represents the cloned inserts from E. coli MG-1 while the dotted lines indicate the vector pBK-CMV. The open boxes represent the genes, and the arrows indicate their translational orientation. The designations of the gene names are provided in the text. The core sites (GTTRRRY) along with the inverse core sites (RYYYAAC) are indicated along with the composite 59-BEs. The boundaries of veb1 gene cassette are indicated in bold, while the surrounding sequence is in gray.
Biochemical properties of VEB-1.
MICs of β-lactams for E. coli JM109 harboring recombinant plasmid pRLT50 showed mainly resistance to penicillins, while pRLT1 gave high MICs of aztreonam, ceftazidime, moxalactam, and cefuroxime. All β-lactam antibiotic MICs were lower in the presence of clavulanic acid (2 μg/ml).
Kinetic parameters of purified VEB-1 β-lactamase, obtained from an E. coli JM109 culture harboring recombinant plasmid pRLT1, showed strong hydrolytic activity against most antibiotics tested (Table 3). The activity against expanded-spectrum cephalosporins in general was very high except for ceftazidime and aztreonam (Table 3), while the hydrolytic activities against penicillins were much lower. The kinetic parameters of VEB-1 are characterized by low Km values for all the β-lactams tested (Table 3) except for ceftazidime and aztreonam. Steady-state inhibitory kinetic parameters (Ki) of VEB-1 β-lactamase with cefotaxime as substrate were as follows: cefoxitin, 15 nM; moxalactam, 18 nM; imipenem, 25 nM; clavulanic acid, 10 nM; sulbactam, 20 nM; tazobactam, 20 nM.
TABLE 3.
Steady-state kinetic parameters of VEB-1 β-lactamase
| Substrate | Vmaxrel | Km (μM) | Vmaxrel/Kma |
|---|---|---|---|
| Benzylpenicillin | 100 | 2.8 | 100 |
| Amoxicillin | 110 | 6.0 | 50 |
| Ticarcillin | 8 | 1.0 | 22 |
| Cephalothin | 700 | 6.0 | 325 |
| Cephaloridine | 2,300 | 12.0 | 533 |
| Cefamandole | 800 | 5.6 | 397 |
| Cefoperazone | 140 | 4.5 | 86 |
| Ceftriaxone | 2,900 | 22.0 | 366 |
| Cefotaxime | 4,300 | 38.0 | 314 |
| Cefuroxime | 2,000 | 24.0 | 230 |
| Ceftazidime | 8,000 | 460.0 | 47 |
| Aztreonam | 400 | 500.0 | 2 |
Values relative to that of benzylpenicillin, which was set at 100.
Analytical isoelectric focusing revealed that E. coli MG-1 had two β-lactamase activities with pIs of 5.4 and 5.35. E. coli JM109 harboring the recombinant plasmid pRLT1 had one β-lactamase activity with a pI of 5.35 (data not shown), while the recombinant plasmid pRLT50 had a β-lactamase activity with a pI of 5.4. The relative molecular mass of the cloned mature β-lactamase expressed from E. coli JM109 harboring pRLT1 was estimated by SDS-PAGE to be 30 kDa (data not shown).
Structural properties of blaVEB-1 and of its deduced protein sequence.
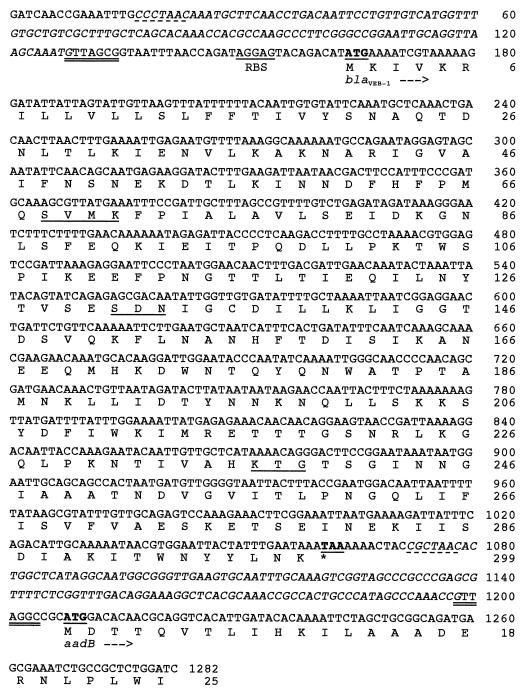
The cloned 1.3-kb genomic DNA of pRLT1 was sequenced on both strands. Analysis of coding regions revealed a sufficiently large ORF of 897 bp encoding a 299-amino-acid preprotein approximately 33 kDa in size. The DNA sequence of this gene, along with flanking sequences, is shown in Fig. 2. A BLAST search against the GenBank database using the DNA sequence of this gene revealed significant identity scores (54% over 260 bp) with blaPER-1 and blaPER-2 (7, 34). No other scores for known β-lactamase genes were found. The overall GC content of this gene, 45%, is typical of Enterobacteriaceae. The translation stop codon (TAA), found at positions 1071 to 1073, corresponded to the most common codon in E. coli and enterobacterial genes. No putative promoter sequence was detected by sequence analysis upstream of the β-lactamase gene.
FIG. 2.
Nucleotide sequence of the 1,282-bp fragment of pRLT1 containing the VEB-1 β-lactamase coding region. The deduced amino acid sequence is designated in single-letter code below the nucleotide sequence. Conserved motifs for class A enzymes are underlined. The start and stop codons of the various genes are shown in boldface and underlined. RBS, putative ribosomal binding site of blaVEB-1. The core sites (GTTRRRY) are double underlined, while the inverse core sites (RYYYAAC) are underscored with a dashed line. The composite 59-be’s are italicized.
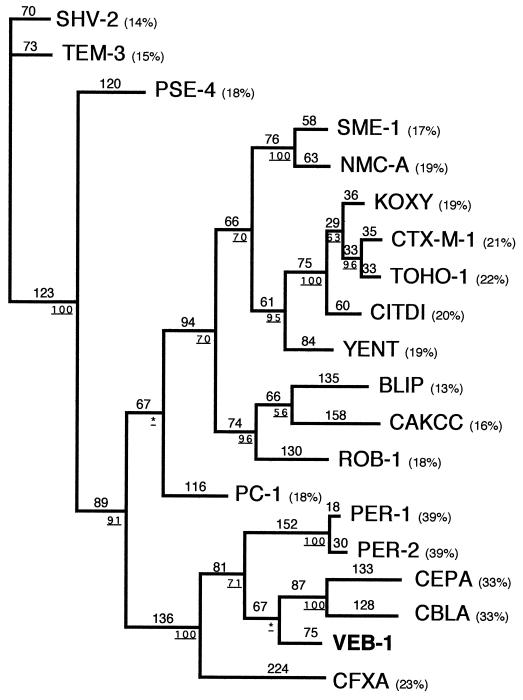
Within the deduced protein sequence, a serine-valine-methionine-lysine tetrad (S-X-X-K) was found at positions 70 to 73 (Fig. 2); it included the conserved serine and lysine amino acid residues characteristic of penicillin-binding proteins (20) or β-lactamases possessing a serine active site (21). Several other structural elements characteristic of class A β-lactamases were found, e.g., serine-aspartate-asparagine (S-D-N) at positions 130 to 132 and lysine-threonine-arginine (K-T-G) at positions 234 to 237 (Fig. 2). The deduced peptide sequence showed less than 20% amino acid identity with most known class A enzymes, with the highest percentage of identity being 38% with PER-1 and PER-2 (see Fig. 4), two ESBLs found primarily in P. aeruginosa (13, 34, 51) and in several Enterobacteriaceae, respectively (7). The enzyme is therefore a novel class A β-lactamase and was named VEB-1 (for Vietnamese extended-spectrum β-lactamase). A dendrogram analysis of VEB-1 with 17 class A β-lactamases showed that VEB-1 clearly clustered with PER-1, PER-2, CBLA, and CEPA, and to a lesser extent with CFXA.
FIG. 4.
Dendrogram obtained for 20 class A β-lactamases according to the parsimony method (49). Branch lengths are drawn to scale and are proportional to the number of amino acid changes. The percentages at branching points (underlined) refer to the number of times a particular node was found in 100 bootstrap replications (the stars indicate uncertainty of nodes with bootstrap values of less than 50%). The distance along the vertical axis has no significance. Abbreviations for β-lactamases are given in Materials and Method section. Percentages in parentheses are amino acid identities to VEB-1.
Analysis of the genetic environment of blaVEB-1 on pRLT1 revealed key signatures of gene cassettes. The presence of a core site, GTTAGCG, at positions 128 to 134 (Fig. 2) and the presence, 3′ of blaVEB-1, of an inverse core site, CGCTAAC, followed by the remainder of a 59-be strongly suggested that blaVEB-1 is encoded on a gene cassette and could thus be part of the variable region of an integron. The veb1 gene cassette is 1,059 bp long, and its 59-be is 133 bp long (Fig. 1 and 2). Exploration of the genetic environment of the veb1 gene cassette revealed the presence of a second antibiotic resistance gene, aadB, encoding an aminoglycoside adenyl transferase (17, 42), associated with a consensus 7-bp core site. Furthermore, the sequence upstream to the veb1 gene cassette is identical to part of a sequence from Tn2424 submitted to GenBank (AF047479) and not yet published. The alignment is to the end of a cassette (including the entire 59-be) containing two genes, aacA1 and orfG. Using primers specific to aacA1 and to blaVEB-1, we were able to amplify a 0.9-kb fragment from total DNA of E. coli MG-1 (data not shown), indicating that this aacA1-orfG cassette is indeed upstream of veb1 gene cassette.
The cloned 1.4-kb genomic DNA from pRLT50 was entirely sequenced on both strands. Coding region analysis revealed a sufficiently large ORF of 861 bp encoding a 286-amino-acid preprotein. A schematic representation of the ORFs and flanking sequences is shown in Fig. 2. A BLAST search against the GenBank database revealed 100% identity with a gene encoding the TEM-1 β-lactamase. The 1.4-kb insert had perfect homology with a plasmid, pJCD4, found in Neisseria gonorrhoeae (unpublished GenBank accession no. U20374) and with plasmid pCFF04 from K. pneumoniae (26). Indeed, the analysis of the sequence upstream of blaTEM-1 revealed the presence of tnpR, the resolvase gene of a Tn3 or Tn3-derived transposon (16). The sequence information did not allow us to discriminate between Tn3 and Tn3-derived transposons. One of these transposons, Tn1331, is present on plasmid pCFF04 and encodes oxa9 in addition to blaTEM-1 (50). However, this transposon was ruled out because the plasmid pNLT2 expresses only one β-lactamase, corresponding to TEM-1, as seen by isoelectric focusing (data not shown).
Plasmid analysis.
Plasmid DNA preparation from E. coli MG-1 revealed the presence of two distinct plasmids, pNLT1 and pNLT2. pNLT2 encoded blaTEM-1 and was not further characterized, whereas pNLT1 was >100 kb and coded for blaVEB-1, as shown by hybridization. Both plasmids were transferred by electroporation into E. coli JM109, resulting in the following phenotypes: ESBL, gentamicin, kanamycin, tobramycin, netilmicin, amikacin, chloramphenicol, tetracycline, and sulfamide resistance (for pNLT1) and penicillinase and chloramphenicol resistance (for pNLT2). These results indicated that both genes were plasmid borne and confirmed the hybridization results in the sense that the plasmid pNLT1 encoded blaVEB1. Furthermore, both plasmids were transferred by conjugation at a very high frequency (10−3 to 10−4) into an E. coli JM109 recipient strain (data not shown).
Rectal screening for multiresistant bacteria revealed the presence of the same E. coli MG-1 and a K. pneumoniae MG-2 presenting a similar resistance profile. PCR analysis using intragenic primers of blaVEB-1 indicated the presence of this gene in the K. pneumoniae strain. Furthermore, a single plasmid, pNLT3, similar in size to pNLT1 was present in the K. pneumoniae MG-2 strain. pNLT3, once conjugated into E. coli JM109 had the same associated markers and an identical restriction digestion pattern as pNLT1 (data not shown). These results indicate that the same plasmid is present in both enterobacterial strains.
DISCUSSION
This work was initiated with the observation that an E. coli clinical isolate showed an extended-spectrum resistance phenotype with a marked synergistic effect between clavulanic acid and ceftazidime. The enzyme responsible for the observed phenotype, VEB-1, has important functional similarities with ESBLs found in Enterobacteriaceae. The mature form of VEB-1 had a molecular mass of about 30 kDa as determined by SDS-PAGE and it belongs to Ambler class A β-lactamases (1) and to the Bush 2be class of enzymes (10).
Several interesting features emerged from the analysis of the sequence of blaVEB-1 and of the surrounding sequence. No obvious E. coli promoter sequence was identified 5′ to the coding sequence but instead, blaVEB1 displayed gene cassette features (15, 38). Based on sequence analysis and comparison to consensus sequences, several gene cassette signatures were identified. blaVEB1 is flanked at its 5′ end by the core site GTTAGCG (Fig. 1 and 2) and at its 3′ end by an imperfect inverted repeat of 127 bp called 59-be, possessing a perfect inverse core site immediately after the 3′ end of blaVEB1 CGCTAAC (38, 46), which corresponds to the start of the 59-be. The 59-be’s constitute a loosely related family of imperfect inverted repeats which differ from each other by their sequences and lengths (38). For the veb1 gene cassette, a longer than usual imperfect inverted repeat was found which has only 30% identity with the consensus 59-be sequence (46). Next to veb1, another gene cassette, aadB (17, 42), which confers resistance to gentamicin, kanamycin, and tobramycin, was found in same orientation as blaVEB1. This cassette starts with a perfect core site GTTAGGC and has 100% identity over the first 120 bp with other sequenced aadB gene cassettes. In fact, these cassettes are widespread and are mostly found on the variable region of integrons (38). Upstream of blaVEB-1, another 59-be belonging to the aacA1-orfG gene cassette was identified. The presence of gene cassette features, the lack of any obvious E. coli promoter in front of blaVEB1, the fact that blaVEB-1 is surrounded by two other gene cassettes, and the fact that pNLT1 confers resistance to sulfamides makes it likely that blaVEB1 is the first class A ESBL from E. coli located on the variable region of a class 1 integron. So far, only some extended-spectrum oxacillinase genes from Pseudomonas aeruginosa (14, 37) and one class B enzyme, IMP-1 (3), were found to be located on the integron. In order to have a conclusive answer, further analysis will be necessary to characterize the integrase and the nature of the integron.
In the E. coli MG-1 clinical strain, two natural plasmids were found. One of them, pNLT2, encoded the TEM-1 enzyme, which is part of a transposon, a derivative of Tn3 (26). This plasmid was not further analyzed. The second plasmid, pNLT1, encoded blaVEB-1. Both plasmids were transferable by conjugation into E. coli JM109. This is worrisome, since integrons and their associated gene cassettes have a tendency to spread rapidly, especially when they are located on conjugative plasmids. The finding of blaVEB-1 in a K. pneumoniae strain is a good illustration of the spread of this resistance gene to other bacteria.
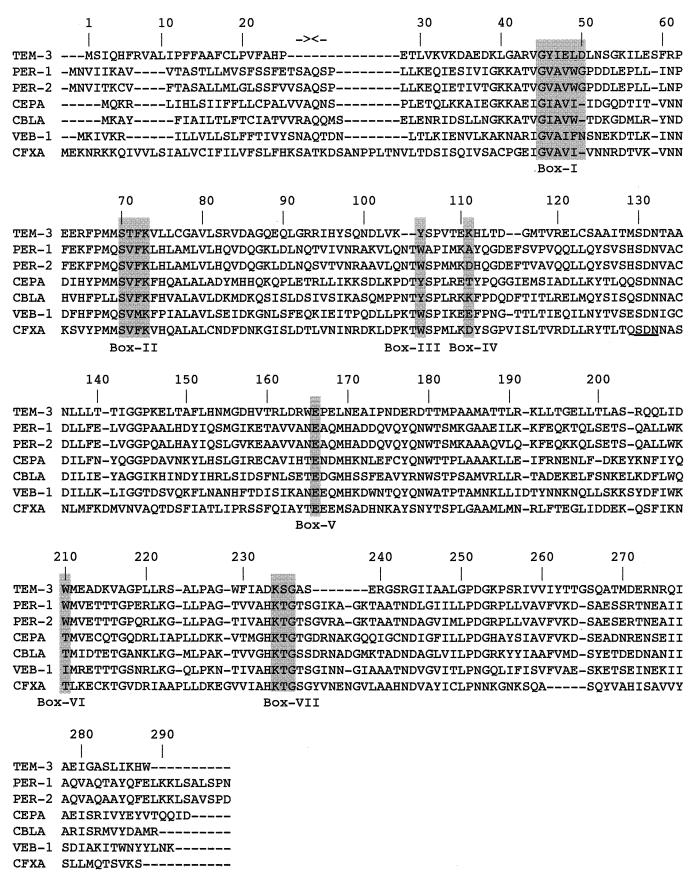
The protein alignment with 19 class A β-lactamases showed that VEB-1 shares the highest sequence identity (38%) with PER-1 and PER-2 (Fig. 3 and 4) from Salmonella typhimurium, Klebsiella sp., E. coli, and Proteus mirabilis strains from South America (7), and from S. typhimurium, Acinetobacter sp. (51), and P. aeruginosa, respectively (33). In addition, VEB-1 shares significant sequence identity with CBLA and CEPA, found in Bacteroides uniformis (43) and in Bacteroides fragilis, respectively (39). Interestingly, all these enzymes are ESBLs themselves and are plasmid mediated. VEB-1 is not a simple point mutant derivative from any known β-lactamase as are most of the described ESBLs from E. coli but rather belongs to a novel family or subgroup of class A ESBLs consisting of PER-1, PER-2, CEPA, and CBLA. The leader peptide cleavage site of PER-1 was found to be located between alanine and glutamine residues at ABL positions 22 and 23 (33). Interestingly, even though the leader peptides are very different, the two residues are conserved in the VEB-1 family members (Fig. 3), indicating that these amino acids may be important in the leader peptide cleavage site as well. As for PER-1, VEB-1 possesses highly conserved amino acid residues of the active-site serine enzymes that interact with β-lactam compounds (20, 21) (Fig. 3) and the SDN motif, which is known to be a structural block of the active site. It is interesting to note these homology regions are accountable for the observed homologies between VEB-1 and all the other class A enzymes.
FIG. 3.
Alignment of the amino acid sequence of VEB-1 with those of the closely related class A β-lactamases PER-1 (34), PER-2 (7), CBLA (43), CEPA (39), and CFXA (35) and to that of TEM-3. Dashes indicate gaps within the alignment. Standard numbering for class A enzymes according to Ambler (1) is indicated. The Roman numerals below the shaded boxes indicate conserved regions within the class A β-lactamases according to Joris et al. (21). The two facing arrows near the top of the figure indicate the cleavage site of the PER-1 leader peptide (34).
VEB-1 confers high-level resistance to amoxicillin, ticarcillin, piperacillin, cefotaxime, ceftazidime, and aztreonam which is reversed by clavulanic acid. Similar resistance profiles were observed with PER-1 (33, 34) and PER-2 (7). Detailed analysis of the VEB-1 amino acid sequence indicated important residues that may explain the observed ESBL phenotype. As observed for PER-1 (34), VEB-1 possesses only one cysteine, at position ABL 135. Therefore, these enzymes will not be able to form the disulfide bridge from ABL positions 77 to 123, as observed from biochemical and crystallographical observations in TEM (47), SHV, or CARB derivatives, nor the ABL positions 69 through 237 disulfide bridge in NMC-A (29) and SME-1 (30). The Ω loop, which extends from residues ABL 169 to 179, is a structural element encountered only in class A enzymes. This loop, even though present in VEB-1, is totally different from the one found in TEM-1. In this respect, VEB-1, along with PER-1, PER-2, CBLA, and CEPA, has a histidine residue at position ABL 170 instead of an asparagine. This asparagine, together with the glutamate ABL 166 and serine ABL 70, is involved in the positioning of the active-site water molecule. The particular phenotypic properties of VEB-1 and PER-1 may be connected to the presence of this histidine. Site-directed mutagenesis would be necessary to determine the precise function of this histidine. Furthermore, the KTG motif is known to be important in the activity of the enzyme (21). Threonine-serine residues found at positions ABL 237 and 238 are usually found in ESBLs (28) and thus are important in the extension of the substrate profile. However, a recent site-directed mutagenesis study (9) revealed that the S238G mutation has no effect on the activity of PER-1. The histidine at position ABL 233 is observed only in VEB-1 family members and CFXA (Fig. 3) (35). In all other class A enzymes, an aspartate residue is found at this position. In TEM-1, this aspartate 233 forms a salt bridge with arginine 222.
This work gives further insight on the complex genetic variety of β-lactamases and of their potential in spreading. The presence of the same enzyme in two different Enterobacteriaceae species from the same patient is a good illustration of how resistance genes can spread in natural conditions by using conjugative plasmids and integrons. Additionally, VEB-1 epidemiology studies among various gram-negative bacteria in Southeast Asian countries should be undertaken. The incidence and spread of other class A ESBLs in distant areas signal the ongoing evolution of novel enzymes beyond the TEM or SHV derivatives.
ACKNOWLEDGMENTS
L.P. and T.N. contributed equally to this work.
We thank P. Dubreuil for technical help.
This work was financed by grants from the Faculté de Médecine Paris-Sud, Université Paris XI (UPRES, JE, 2227), and the Institut Beecham, La Défense, France.
REFERENCES
- 1.Ambler R P. The structure of β-lactamases. Philos Trans R Soc Lond Ser B Biol Sci. 1980;289:321–331. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- 2.Arakawa Y, Ohta M, Kido N, Mori M, Ito H, Komatsu T, Fujii Y, Kato N. Chromosomal β-lactamase of Klebsiella oxytoca, a new class A enzyme that hydrolyzes broad spectrum β-lactam antibiotics. Antimicrob Agents Chemother. 1995;33:63–70. doi: 10.1128/aac.33.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arakawa Y, Murakami M, Suzuki K, Ito H, Wacharotayankun R, Ohsuka S, Kato N, Ohta M. A novel integron-like element carrying the metallo-β-lactamase gene blaIMP. Antimicrob Agents Chemother. 1995;39:1612–1615. doi: 10.1128/aac.39.7.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barthélémy M, Guionie M, Labia R. β-Lactamases: determination of their isoelectric points. Antimicrob Agents Chemother. 1978;13:695–698. doi: 10.1128/aac.13.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barthélémy M, Péduzzi J, Bernard H, Tancrède C, Labia R. Close amino acid sequence relationship between the new plasmid-mediated extended spectrum β-lactamase MEN-1 and chromosomally encoded enzymes of Klebsiella oxytoca. Biochim Biophys Acta. 1992;1122:15–22. doi: 10.1016/0167-4838(92)90121-s. [DOI] [PubMed] [Google Scholar]
- 6.Bauernfeind A, Stemplinger I, Jungwirth R, Ernst S, Casellas J M. Sequences of β-lactamase genes encoding CTX-M-1 (MEN-1) and CTX-M and relationship of their amino acid sequences with those of β-lactamases. Antimicrob Agents Chemother. 1996;40:509–513. doi: 10.1128/aac.40.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauernfeind A, Stemplinger I, Jungwirth R, Mangold P, Amann S, Akalin E, Ang O, Bal C, Casellas J M. Characterization of β-lactamase gene blaPER-2, which encodes an extended-spectrum class A β-lactamase. Antimicrob Agents Chemother. 1996;40:616–620. doi: 10.1128/aac.40.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boissinot M, Levesque R C. Nucleotide sequence of the PSE-4 carbenicillinase gene and correlations with the Staphylococcus aureus PC1 β-lactamase crystal structure. J Biol Chem. 1990;265:1225–1230. [PubMed] [Google Scholar]
- 9.Bouthors A-T, Dagoneau-Blanchard N, Naas T, Nordmann P, Jarlier V, Sougakoff W. Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 β-lactamase hydrolysing third-generation cephalosporins. Biochem J. 1998;330:1443–1449. doi: 10.1042/bj3301443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan P I. Nucleotide sequence of the Staphylococcus aureus PC1 β-lactamase gene. Nucleic Acids Res. 1986;14:5940. doi: 10.1093/nar/14.14.5940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collis C M, Hall R M. Site-specific deletion and rearrangement of integron insert genes catalyzed by the integron DNA integrase. J Bacteriol. 1992;174:1574–1585. doi: 10.1128/jb.174.5.1574-1585.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danel F, Hall L M C, Gur D, Akalin H E, Livermore D M. Transferable production of PER-1 β-lactamase in Pseudomonas aeruginosa. J Antimicrob Chemother. 1995;35:281–294. doi: 10.1093/jac/35.2.281. [DOI] [PubMed] [Google Scholar]
- 14.Hall L M C, Livermore D M, Gur D, Akova M, Akalin H E. OXA-11, an extended-spectrum variant of OXA-10 (PSE-2) β-lactamase from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1993;37:1637–1644. doi: 10.1128/aac.37.8.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall R M, Collis C M. Mobile gene cassettes and integrons: capture and spread of genes by site specific recombination. Mol Microbiol. 1995;15:593–600. doi: 10.1111/j.1365-2958.1995.tb02368.x. [DOI] [PubMed] [Google Scholar]
- 16.Heffron F, McCarthy B J, Ohtsubo H, Ohtsubo E. DNA sequence analysis of the transposon Tn3: three genes and three sites involved in the transposition of Tn3. Cell. 1979;18:1153–1163. doi: 10.1016/0092-8674(79)90228-9. [DOI] [PubMed] [Google Scholar]
- 17.Hollingshead S, Vapnek D. Nucleotide sequence analysis of a gene encoding a streptomycin/spectinomycin adenyltransferase. Plasmid. 1985;13:17–20. doi: 10.1016/0147-619x(85)90052-6. [DOI] [PubMed] [Google Scholar]
- 18.Huletsky A, Couture F, Levesque R C. Nucleotide sequence and phylogeny of SHV-2 β-lactamase. Antimicrob Agents Chemother. 1990;34:1725–1732. doi: 10.1128/aac.34.9.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishii Y, Ohno A, Taguchi H, Imajo S, Ishiguro M, Matsuzawa H. Cloning and sequence of the gene encoding a cefotaxime-hydrolyzing class A beta-lactamase isolated from E. coli. Antimicrob Agents Chemother. 1995;39:2269–2275. doi: 10.1128/aac.39.10.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joris B, Ghuysen J M, Dive G, Renard A, Dideberg O, Charlier P, Frère J M, Kelly J A, Boyington J C, Moews P C, Knox J R. The active-site-serine penicillin-recognizing enzymes as members of the Streptomyces R61 DD-peptidase family. Biochem J. 1988;250:313–324. doi: 10.1042/bj2500313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joris B, Ledent P, Dideberg O, Fonzé E, Lamotte-Brasseur J, Kelly J A, Ghuysen J M, Frère J M. Comparison of the sequences of class A β-lactamases and of the secondary structure elements of penicillin-recognizing proteins. Antimicrob Agents Chemother. 1991;35:2294–2301. doi: 10.1128/aac.35.11.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juteau J-M, Levesque R C. Sequence analysis and evolutionary perspectives of ROB-1 β-lactamases. Antimicrob Agents Chemother. 1990;34:1354–1359. doi: 10.1128/aac.34.7.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labia R, Guionie M, Barthélémy M, Philippon A. Properties of three carbenicillin hydrolysing β-lactamases (CARB) from Pseudomonas aeruginosa: identification of a new enzyme. J Antimicrob Chemother. 1981;7:49–56. doi: 10.1093/jac/7.1.49. [DOI] [PubMed] [Google Scholar]
- 24.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Lenzini M V, Ishihara H, Dusart J, Ogawara H, Joris B, Van Beemen J, Frère J M, Ghuysen J M. Nucleotide sequence of the gene encoding the active serine β-lactamase from Streptomyces cacaoi. FEMS Microbiol Lett. 1988;49:371–376. [Google Scholar]
- 26.Mabilat C, Lourencao-Vital J, Goussard S, Courvalin P. A new example of physical linkage between Tn1 and Tn21: the antibiotic multiple resistance region of plasmid pCFF04 encoding extended spectrum beta-lactamase TEM-3. Mol Gen Genet. 1992;235:113–121. doi: 10.1007/BF00286188. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto Y, Ikeda F, Kamimura T, Yokota Y, Mine Y. Novel plasmid-mediated β-lactamase from Escherichia coli that inactivates oxyimino-cephalosporins. Antimicrob Agents Chemother. 1989;32:1243–1246. doi: 10.1128/aac.32.8.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medeiros A A. Evolution and dissemination of β-lactamases accelerated by generations of β-lactam antibiotics. Clin Infect Dis. 1997;24(Suppl.):19–45. doi: 10.1093/clinids/24.supplement_1.s19. [DOI] [PubMed] [Google Scholar]
- 29.Naas T, Nordmann P. Analysis of a carbapenem-hydrolyzing class A β-lactamase from Enterobacter cloacae and its LysR type regulatory protein. Proc Natl Acad Sci USA. 1994;91:7693–7697. doi: 10.1073/pnas.91.16.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naas T, Vandel L, Sougakoff W, Livermore D M, Nordmann P. Cloning and sequence analysis of the gene for a carbapenem-hydrolysing class A β-lactamase, Sme-1, from Serratia marcescens S6. Antimicrob Agents Chemother. 1994;38:1262–1270. doi: 10.1128/aac.38.6.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30a.National Center of Biotechnology Information Website. 11 December 1998, revision date. [Online.] http://www.ncbi.nlm.nih.gov. [15 January 1999, last date accessed.]
- 31.Neugebauer K R, Sprengel R, Schaller H. Penicillinase from Bacillus licheniformis: nucleotide sequence of the gene and implications for the biosynthesis of a secretory protein in a gram-positive bacterium. Nucleic Acids Res. 1981;9:2577–2588. doi: 10.1093/nar/9.11.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicolas M H, Jarlier V, Honore N, Philippon A, Cole S T. Molecular characterization of the gene encoding SHV-3 β-lactamase responsible for transferable cefotaxime resistance in clinical isolates of Klebsiella pneumoniae. Antimicrob Agents Chemother. 1989;33:2096–2100. doi: 10.1128/aac.33.12.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nordmann P, Ronco E, Naas T, Duport C, Michel-Briand Y, Labia R. Characterization of a novel extended-spectrum β-lactamase from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1994;37:962–969. doi: 10.1128/aac.37.5.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nordmann P, Naas T. Sequence analysis of PER-1 extended-spectrum β-lactamase from Pseudomonas aeruginosa and comparison with class A β-lactamases. Antimicrob Agents Chemother. 1994;38:104–114. doi: 10.1128/aac.38.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parker A C, Smith C J. Genetic and biochemical analysis of a novel Ambler class A β-lactamase responsible for cefoxitin resistance in Bacteroides species. Antimicrob Agents Chemother. 1993;37:1028–1036. doi: 10.1128/aac.37.5.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35a.Pedrós BioMolecular Research Tools Website. 16 June 1995, revision date. [Online.] http://www.fmi.ch/biology/research_tools.html. [12 December 1998, last date accessed.]
- 36.Perilli M, Franceschini N, Segatore B, Amicosante G, Ovatore A, Duez C, Joris B, Frère J M. Cloning and nucleotide sequencing of the gene encoding the β-lactamase from Citrobacter diversus. FEMS Microbiol Lett. 1991;83:79–84. doi: 10.1016/0378-1097(91)90448-j. [DOI] [PubMed] [Google Scholar]
- 37.Philippon L N, Naas T, Bouthors A T, Barakett V, Nordmann P. OXA-18, a class D clavulanic-acid inhibited extended-spectrum β-lactamase from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1997;41:2188–2195. doi: 10.1128/aac.41.10.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Recchia G D, Hall R M. Gene cassettes: a new class of mobile elements. Microbiology. 1995;141:3015–3027. doi: 10.1099/13500872-141-12-3015. [DOI] [PubMed] [Google Scholar]
- 39.Rogers M B, Parker A C, Smith C J. Cloning and characterization of the endogenous cephalosporinase gene, cepA, from Bacteroides fragilis reveals a new subgroup of Ambler class A β-lactamases. Antimicrob Agents Chemother. 1993;37:2391–2400. doi: 10.1128/aac.37.11.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 41.Seoane A, Lobo J M G. Nucleotide sequence of new class A β-lactamase from the chromosome of Yersinia enterocolitica: implications for the evolution of class A β-lactamases. Mol Gen Genet. 1991;228:215–220. doi: 10.1007/BF00282468. [DOI] [PubMed] [Google Scholar]
- 42.Shaw K J, Rather P N, Hare R S, Miller G H. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol Rev. 1993;57:138–163. doi: 10.1128/mr.57.1.138-163.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith C J, Bennett T K, Parker A C. Molecular and genetic analysis of the Bacteroides uniformis cephalosporinase gene, cblA, encoding the species-specific β-lactamase. Antimicrob Agents Chemother. 1994;38:1711–1715. doi: 10.1128/aac.38.8.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sougakoff W, Goussard S, Courvalin P. The TEM-3 β-lactamase, which hydrolyses broad-spectrum cephalosporins, is derived from the TEM-2 penicillinase by two amino-acid substitutions. FEMS Microbiol Lett. 1988;56:343–348. [Google Scholar]
- 45.Stokes H W, Hall R M. A novel family of potentially mobile DNA elements encoding site-specific gene-integration functions: integrons. Mol Microbiol. 1989;3:1669–1683. doi: 10.1111/j.1365-2958.1989.tb00153.x. [DOI] [PubMed] [Google Scholar]
- 46.Stokes H W, O’Gorman D B, Recchia G D, Parsekhian M, Hall R M. Structure and function of 59-base element recombination sites associated with mobile gene cassettes. Mol Microbiol. 1997;3:1669–1683. doi: 10.1046/j.1365-2958.1997.6091980.x. [DOI] [PubMed] [Google Scholar]
- 47.Strynadka N C J, Adachi H, Jensen S E, Johns K, Sielecki A, Betzel C, Sutoh K, Amyes N G. Molecular structure of the acyl-enzyme intermediate in β-lactam hydrolysis at 1.7 angstroem resolution. Nature (London) 1992;359:700–705. doi: 10.1038/359700a0. [DOI] [PubMed] [Google Scholar]
- 48.Sutcliffe J G. Nucleotide sequence of the ampicillin resistance gene of Escherichia coli plasmid pBR322. Proc Natl Acad Sci USA. 1978;75:3737–3741. doi: 10.1073/pnas.75.8.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Swofford D L. PAUP (version 3.0): phylogenetic analysis using parsimony. Champaign, Ill: Illinois Natural History Survey; 1989. [Google Scholar]
- 50.Tolmasky M E, Crosa J H. Genetic organization of antibiotic resistance genes (aac(6′)-Ib, aadA, and oxa-9) in the multiresistance transposon Tn1331. Plasmid. 1993;29:31–40. doi: 10.1006/plas.1993.1004. [DOI] [PubMed] [Google Scholar]
- 51.Vahaboglu H, Ozturk R, Aygun G, Coskunkan F, Yaman A, Kaygusuz A, Leblebicioglu H, Balik I, Aydin K, Otkun M. Widespread detection of PER-1-type extended-spectrum β-lactamase among nosocomial Acinetobacter and P. aeruginosa isolates in Turkey: a nationwide multicenter study. Antimicrob Agents Chemother. 1997;41:2265–2269. doi: 10.1128/aac.41.10.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]