Background:
For women undergoing breast reconstruction after mastectomy, the comparative benefits and harms of implant-based reconstruction (IBR) and autologous reconstruction (AR) are not well known. We performed a systematic review with meta-analysis of IBR versus AR after mastectomy for breast cancer.
Methods:
We searched Medline, Embase, Cochrane CENTRAL, CINAHL, and ClinicalTrials.gov for studies from inception to March 23, 2021. We assessed the risk of bias of individual studies and strength of evidence (SoE) of our findings using standard methods.
Results:
We screened 15,936 citations and included 40 studies (two randomized controlled trials and 38 adjusted nonrandomized comparative studies). Compared with patients who undergo IBR, those who undergo AR experience clinically significant better sexual well-being [summary adjusted mean difference (adjMD) 5.8, 95% CI 3.4–8.2; three studies] and satisfaction with breasts (summary adjMD 8.1, 95% CI 6.1–10.1; three studies) (moderate SoE for both outcomes). AR was associated with a greater risk of venous thromboembolism (moderate SoE), but IBR was associated with a greater risk of reconstructive failure (moderate SoE) and seroma (low SoE) in long-term follow-up (1.5–4 years). Other outcomes were comparable between groups, or the evidence was insufficient to merit conclusions.
Conclusions:
Most evidence regarding IBR versus AR is of low or moderate SoE. AR is probably associated with better sexual well-being and satisfaction with breasts and lower risks of seroma and long-term reconstructive failure but a higher risk of thromboembolic events. New high-quality research is needed to address the important research gaps.
Takeaways
Question: What are the comparative benefits and surgical complications of implant-based reconstruction (IBR) versus autologous reconstruction (AR) after mastectomy?
Findings: In a large systematic review and meta-analysis, 40 studies met criteria. Patients who undergo AR experience better sexual well-being and satisfaction with breasts. AR is associated with a greater risk of venous thromboembolism, but IBR is associated with a greater risk of reconstructive failure and seroma in the long term (1.5–4 years). Other outcomes are comparable, or the evidence is insufficient to merit conclusions.
Meaning: Most evidence regarding IBR options is of low or moderate strength.
INTRODUCTION
Breast cancer is the most common new cancer diagnosis and the second most common cause of cancer death among women in the United States.1 Surgery is part of the standard treatment for most new breast cancer patients. Surgical options include mastectomy or breast conserving surgery (segmental mastectomy) followed by radiation. The percentage of women who elect to undergo breast reconstruction after mastectomy is increasing and, as of 2016, over 40% of women who underwent mastectomy had reconstruction, amounting to 137,808 women in 2020.1,2
Following mastectomy, breast reconstruction is considered to be associated with better quality of life than no reconstruction. It can be performed using autologous or implant-based (ie, alloplastic) techniques.3–5 In the United States, implant-based reconstruction (IBR) accounts for the vast majority (81%) of breast reconstruction procedures. These can be performed as a single-stage implant placement (13% of total reconstructions) or as a two-stage tissue expander placement followed by permanent implant exchange at a later date (68%). Autologous reconstruction (AR) is less common and represents approximately 19% of breast reconstruction procedures.6 AR has traditionally been associated with better patient satisfaction and quality of life outcomes but higher risks of both minor and major complications than IBR.7,8
Given the preference-sensitive nature of breast reconstruction, the evolving nature of the evidence, and the lack of an agreed-upon preferred surgical modality, we conducted a systematic review (SR) with meta-analysis to assess the benefits and surgical complications of IBR versus AR after mastectomy for breast cancer (or prophylaxis).
METHODS
This article is part of a larger SR, funded by the Agency for Healthcare Research and Quality (AHRQ) that addresses a range of questions related to breast reconstruction after mastectomy for breast cancer. The SR followed Evidence-based Practice Center (EPC) program methodology for reviews of comparative effectiveness research.9 The review protocol was registered in PROSPERO (registration number: CRD42020193183).
The full details of the SR methodology are provided in a companion article10 and in the full AHRQ report for the project.11 Briefly, based on discussions with panels of stakeholders and experts in the field, we prioritized specific benefits and surgical complications for the comparison between IBR and AR in women after mastectomy for treatment or prophylaxis against breast cancer. Examples of benefit outcomes included psychosocial well-being, sexual well-being, and satisfaction with breasts. Examples of surgical complications included necrosis, venous thromboembolism, seroma, and reconstructive failure.
In this article, we report the comparative studies [randomized controlled trials (RCTs) and nonrandomized comparative studies (NRCSs) with adequate statistical adjustment analyses] that addressed the comparison of IBR versus AR. The larger AHRQ SR also included single-group studies, from which we extracted data only on risks of surgical complications. The estimates from single-group studies are tabulated in the full AHRQ report of the SR11 and are not discussed further in this article.
We searched for studies in Medline (via PubMed), Embase, the Cochrane Central Register of Controlled Trials (CENTRAL), and CINAHL, and for unpublished studies in ClinicalTrials.gov, from database inception through March 23, 2021. We screened each identified record in duplicate using Abstrackr (http://abstrackr.cebm.brown.edu/). We extracted data from included studies into the Systematic Review Data Repository Plus (SRDR+) (http://srdrplus.ahrq.gov/). Data were extracted, and risk of bias was assessed by one researcher using standard tools. All extracted data were confirmed by a second, independent researcher. We assessed strength of evidence (SoE) using the AHRQ methodology.9 When feasible, for continuous outcomes, we made conclusions based on published estimates of minimal clinically important differences (MCIDs).
RESULTS
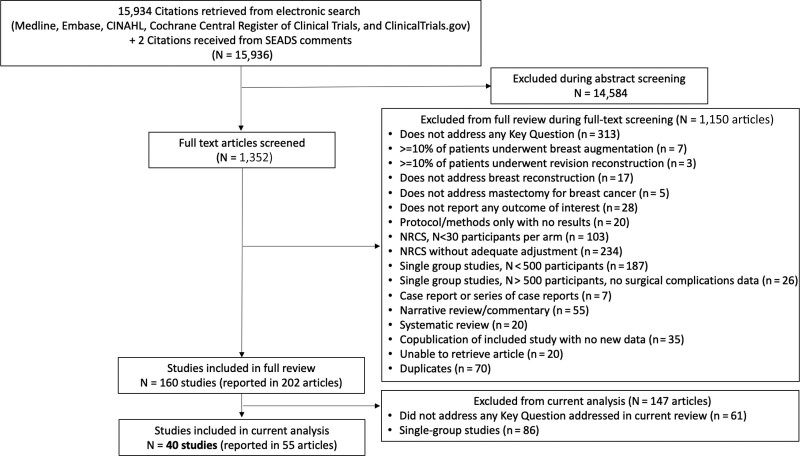
The search yielded 15,936 citations, of which 40 studies met eligibility criteria for this study (Fig. 1). The studies were published between 1989 and 2021, with 36 of 40 studies published in 2010 or more recently. They comprised two RCTs,12,13 and 38 adjusted NRCSs, reported in 53 articles.7,8,14–64 The two RCTs included a total of 223 patients in Sweden. One had a high risk of bias (due to incomplete outcome data and lack of blinding) and the other had a moderate risk (due to the lack of blinding). (See table 1, Supplemental Digital Content 1, which displays risk of bias assessment for RCTs. http://links.lww.com/PRSGO/B954.) The 38 NRCSs included a total of 121,302 patients. Among the 38 NRCSs, 10 NRCSs (26%) were prospective and 28 (74%) were retrospective. Twenty-five of the 38 NRCSs had a high risk of bias (mostly due to critical or serious risk of confounding and lack of blinding) and 13 had a moderate risk (mostly due to lack of blinding). (See table 2, Supplemental Digital Content 2, which displays risk of bias assessment for nonrandomized comparative studies (NRCSs), confounding, and selection bias. http://links.lww.com/PRSGO/B955 and See table 3, Supplemental Digital Content 3, which displays risk of bias assessment for NRCSs, assessment of remaining biases, quality, and overall risk of bias. http://links.lww.com/PRSGO/B956.)
Fig. 1.
PRISMA diagram depicting identification of studies in this SR.
Clinical Benefits
Physical Well-being
One RCT and five NRCSs reported data using seven different measurement instruments. (See table 4, Supplemental Digital Content 4, which displays continuous outcomes: physical well-being. http://links.lww.com/PRSGO/B957.)
Results were inconsistent across studies. Four studies used the BREAST-Q (0–100 scale; higher is better; MCID 3 points65). Tallroth et al. 202013 reported that patients randomized to AR had clinically significant better chest and upper body scores at 5.3 years of follow-up [mean difference (MD) 7.6, 95% confidence interval (CI) 0.30–14.9]. Among the NRCSs, one reported comparable scores at 2.2 years [adjusted mean difference (adjMD) −2.60, 95% CI −9.77 to 4.57]. However, the other two NRCSs reported that patients who underwent AR had clinically significant better physical well-being.
Psychosocial Well-being
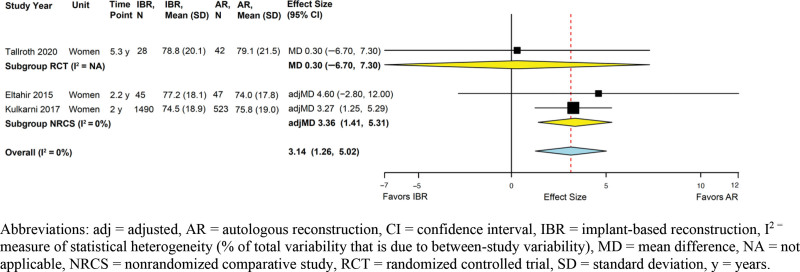
One RCT and four NRCSs reported data using six different measurement instruments. (See table 5, Supplemental Digital Content 5, which displays continuous outcomes: general quality of life, psychosocial well-being, sexual well-being, patient satisfaction with aesthetics, and patient satisfaction with outcome. http://links.lww.com/PRSGO/B958.) Results were generally comparable between IBR and AR groups. Across three studies (the RCT and two NRCSs with adjusted effect sizes) that used the BREAST-Q (MCID 4 points65), IBR and AR were associated with clinically comparable psychosocial well-being (summary adjMD 3.14, 95% CI 1.26–5.02; I2 = 0%) (Fig. 2).
Fig. 2.
Implant-based versus autologous breast reconstruction: meta-analysis of psychosocial well-being.
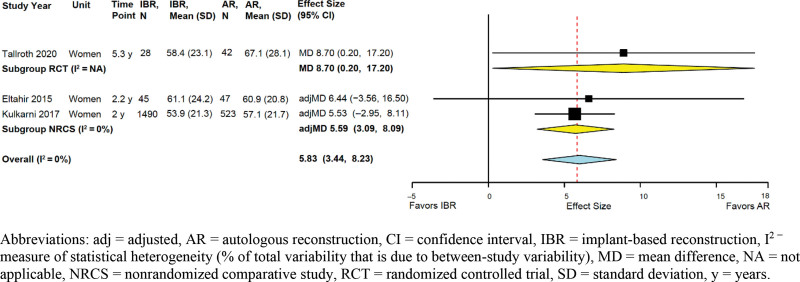
Sexual Well-being
One RCT and three NRCSs reported data using the BREAST-Q (MCID 5 points65) (see Table 5, supplemental Digital Content 5, http://links.lww.com/PRSGO/B958.). Across three studies (the RCT and two NRCSs with adjusted effect sizes), AR was associated with clinically significant better sexual well-being (summary adjMD 5.83, 95% CI 3.44–8.23; I2 = 0%) (Fig. 3).
Fig. 3.
Implant-based versus autologous breast reconstruction: meta-analysis of sexual well-being.
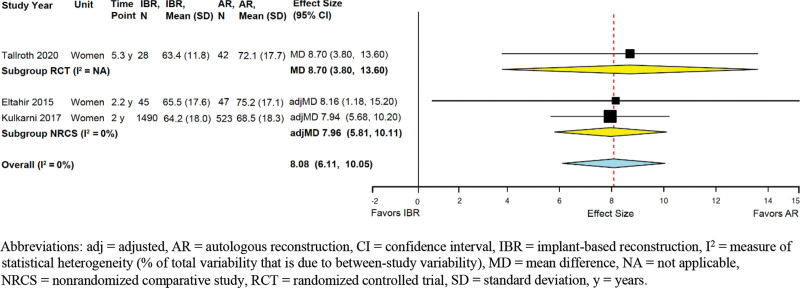
Satisfaction with Breasts
One RCT and six NRCSs reported data. The RCT and four NRCSs used the BREAST-Q (MCID 5 points65) (SDC 5, http://links.lww.com/PRSGO/B958). Across three studies (the RCT and two NRCSs with adjusted effect sizes), AR was associated with clinically greater satisfaction (summary adjMD 8.08, 95% CI 6.11–10.1; I2 = 0%) (Fig. 4). One NRCS also reported that at 2 years, the satisfaction advantage of AR over IBR existed also within the subgroups of women who underwent unilateral reconstruction (adjMD 9.85; P = 0.001) or bilateral reconstruction (adjMD 5.13; P = 0.001).
Fig. 4.
Implant-based versus autologous breast reconstruction: meta-analysis of satisfaction with breast aesthetics.
The other two NRCSs reported categorical data on satisfaction with breasts. One NRCS reported a comparable proportion of satisfied patients who underwent IBR or AR (adjusted odds ratio [adjOR] 0.85, 95% CI 0.36–1.63). However, the other NRCS reported that patients who underwent AR were more likely to be satisfied (adjOR 1.43, 95% CI 1.18–1.73).
Satisfaction with Surgical Outcome
One RCT and four NRCSs reported data. Results were inconsistent across studies (SDC 5, http://links.lww.com/PRSGO/B958). Three studies reported data using the BREAST-Q (MCID 5 points65), two studies reporting comparable satisfaction scores in the IBR and AR groups (the RCT: MD 2.9, 95% CI −3.1 to 8.9, and one NRCS: adjMD 4.9, 95% CI −3.1 to 12.9), and one NRCS reporting higher scores among patients with AR (P < 0.05; adjusted effect size not reported).
The other two NRCSs reported categorical data on satisfaction with the surgical outcome. One NRCS reported comparable proportions of satisfied patients who underwent IBR or AR (adjOR 0.69, 95% CI 0.45–1.67). However, the other NRCS reported that patients who underwent AR were more likely to be satisfied (adjOR 1.83, 95% 1.11–3.03).
General Quality of Life
Three NRCSs reported data using various instruments, but none reported adjusted effect sizes (SDC 5, http://links.lww.com/PRSGO/B958). For each instrument in each study, differences in general quality of life between IBR and AR were not statistically significant.
Mortality
One NRCS reported comparable risks of 9-year mortality in IBR and AR groups, both for overall mortality (adjOR 0.96, 95% CI 0.89–1.04) and breast cancer-specific mortality (adjOR 0.95, 95% CI 0.87–1.04). (See table 6, Supplemental Digital Content 6, which displays categorical outcomes: mortality, unplanned repeat hospitalizations, necrosis, wound dehiscence, delayed healing, seroma, and hematoma. http://links.lww.com/PRSGO/B959.)
Surgical Complications
Unplanned Repeat Hospitalizations
Three NRCSs reported data within 1 month (SDC 6, http://links.lww.com/PRSGO/B959). Two reported comparable risks of unplanned repeat hospitalizations, while the third reported comparable risks of unplanned emergency department visits overall (adjOR 1.11, 95% CI 0.91–1.25) as well as visits specifically for pain-related diagnoses (adjOR 1.11, 95% CI 0.83–1.67).
Unplanned Repeat Surgeries for Revision
Three NRCSs reported data, and results were inconsistent. (See table 7, Supplemental Digital Content 7, which displays categorical outcomes: unplanned repeat surgeries for revision, unplanned repeat surgeries for complications, pain, infections, and reconstructive failure. http://links.lww.com/PRSGO/B960.) Two reported that risks of unplanned repeat surgeries for revision were lower in the AR group than in the IBR group (one reported an adjOR of 0.72, 95% CI 0.50–1.06 at 4.9 years, and another reported that P = 0.003 at 1 year, adjusted effect size not reported), while the third reported the reverse at 2 years (adjORs ranging from 1.34 to 2.66 for various specific flaps in comparison with IBR).
Unplanned Repeat Surgeries for Complications
Three NRCSs reported data, and results were inconsistent (SDC 7, http://links.lww.com/PRSGO/B960). One NRCS reported that, compared with the AR group, risks were higher in the IBR direct-to-implant group (adjOR 2.03, 95% CI 1.03–3.98) and the IBR with tissue expanders group (adjOR 1.81, 95% CI 0.90–3.64) (time-points not reported). On the other hand, the other two NRCSs reported comparable risks (adjOR 1.08, 95% CI 0.88–1.32 at 1 month, and adjOR 0.63, 95% CI 0.29–1.37 at 4.9 years).
Necrosis
Four NRCSs reported data, and results were inconsistent (SDC 6, http://links.lww.com/PRSGO/B959). One reported that AR was associated with a lower risk (adjOR 0.31, 95% CI 0.11–0.86; time-point not reported), but another reported the reverse, albeit with an imprecise estimate at 4.3 years (adjOR 17.9, 95% CI 0.52–610.5). Two other NRCSs reported that risks were comparable (adjOR 0.66, 95% CI 0.38 to 1.16 at 1.9 years, and adjOR 0.83, 95% CI 0.19 to 3.50 at 10 years).
Infections
Six NRCSs reported data, and results were inconsistent (see Supplemental Digital Content 7, http://links.lww.com/PRSGO/B960). One reported that, at 6.3 years, AR was associated with higher risks of infection than single-staged IBR (adjOR 3.2, 95% CI 0.6–16.0) and two-staged IBR (adjOR 8.1, 95% CI 1.7–39.0). Another reported that, at 1 month, AR was associated with higher risks of infections overall (adjOR 1.40, 95% CI 1.01–1.96) and deep surgical site infections (adjOR 1.81, 95% CI 1.12–2.94) but not superficial surgical site infections (adjOR 1.20, 95% CI 0.81–1.76).
On the other hand, two other NRCSs reported that AR may be associated with lower risk of infection. One reported that patients in the deep inferior epigastric perforator flap group had a lower risk than the IBR group at 2 years (adjOR 0.44, 95% CI 0.2–0.78). Another reported a lower risk in the AR group (P < 0.001; adjusted effect size and time-point not reported).
Finally, two NRCSs reported imprecise estimates (adjOR 0.86, 95% CI 0.18–4.11 at 4.3 years, and adjOR 0.77, 95% CI 0.20–2.50 at 10 years).
Reconstructive Failure
Five NRCSs reported data (SDC 7, http://links.lww.com/PRSGO/B960). Two reported inconsistent data in the short term (1–1.3 months follow-up): NRCS reported that AR was associated with lower risk (adjOR 0.09, 95% CI 0.07–0.13), whereas another reported the reverse (adjOR 1.69, 95% CI 1.08–2.62).
However, three NRCSs reported that AR was associated with considerably lower risk in the long term (1.5–4 years follow-up). One NRCS reported a P value less than 0.001 (adjusted effect size not reported) at 1.5 years, and another NRCS reported an adjOR of 0.19 (95% CI 0.04–0.80) at 4 years. The third NRCS’s findings agreed with the other two, but data were reported separately for unilateral reconstructions (adjOR 0.12, 95% CI 0.04–0.36) and for bilateral reconstructions (adjOR 0.14, 95% CI 0.05–0.45).
Seroma
Two NRCSs reported data (SDC 6, http://links.lww.com/PRSGO/B959). Although they did not report adjusted effect sizes, both reported higher risks associated with IBR (P = 0.009 for seroma at 2.1 years and P < 0.001 for the composite outcome of seroma or hematoma at an unreported time-point).
Thromboembolic Events
Two RCTs and two NRCSs reported data (SDC 6, http://links.lww.com/PRSGO/B959). Neither RCT provided usable data because no thromboembolic events occurred. One NRCS reported comparable risks of deep vein thrombosis at 1 month (adjOR 0.99, 95% CI 0.41–2.41). This NRCS also reported that AR was associated with a statistically nonsignificant higher risk of pulmonary embolism at 1 month (adjOR 1.84, 95% CI 0.71–4.77). The other NRCS reported that AR was associated with higher risk of the composite outcome of deep vein thrombosis or pulmonary embolism at 3 months (adjOR 2.27, 95% CI 1.79 to 2.86).
DISCUSSION
Breast cancer reconstruction rates continue to increase every year in the United States, with more than 40% of women who undergo mastectomy opting for reconstruction.1 This is likely due to improved awareness of breast reconstruction techniques and their functional and psychological benefits.3 Given the preference-sensitive nature of breast reconstruction, the decision of a patient to pursue IBR versus AR is driven by a myriad of factors that include individual preference, resource availability, and suitability for surgery. Despite this, for many women, there is little consensus as to the optimal treatment choice, and long-term prospective data are largely lacking.
The goals of this SR were to thoroughly compare benefit outcomes and surgical complication profiles between IBR and AR. The evidence suggests that AR is probably associated with superior patient-reported benefit outcomes, specifically sexual well-being and satisfaction with breasts. Psychosocial well-being and physical well-being were either comparable or between-group differences were inconsistent across studies, respectively.
The meta-analysis findings of better sexual well-being and aesthetic satisfaction associated with AR are consistent with what has been considered conventional wisdom in this field.7,58 However, despite no demonstrable overall differences between IBR and AR in psychosocial and physical well-being, the subgroup of women who undergo AR with pedicled transverse rectus abdominis myocutaneous flaps may experience worse chest and upper body physical well-being than women who undergo IBR. These findings are consistent with multiple studies and highlight the potential morbidity associated with pedicled transverse rectus abdominis myocutaneous flaps, particularly when performed bilaterally.11
In addition to quality-of-life outcomes, this SR compared postoperative complication profiles between IBR and AR. Unfortunately, there was considerable inconsistency in reported complication outcomes. Repeat hospitalization for any reason after reconstruction served as a broad measure of complications within these studies. Our finding of no significant difference in unplanned repeat hospitalizations between AR and IBR is surprising given that the incidence of complications after breast reconstruction can be as high as 45%, and AR has been traditionally thought to lead to higher rates of complications in the first 30 days than IBR. Breast seroma risks may be higher with IBR than with AR, a finding consistent with the conventional wisdom that IBR, particularly with the use of acellular dermal matrices, poses a higher risk of seroma.66 However, there is insufficient evidence regarding most surgical complications.
One potentially concerning finding is the suggestion of higher risk of thromboembolic events with AR. AR, particularly when abdominally based, entails longer operative times, fascial plication, and abdominal flexing. Intuitively, these place a patient at a higher risk for deep vein thrombosis and pulmonary embolism. Efforts have been undertaken to educate surgeons and patients regarding best practices for prophylaxis against these complications. These efforts include perioperative anticoagulation guidelines and early mobilization.67
Finally, data for the comparative risks of reconstructive failure between AR and IBR were inconsistent at short-term follow-up of less than 6 weeks. However, at long-term follow-up beyond 1.5 years, IBR is probably associated with a greater risk of reconstructive failure than AR. These findings are consistent with other reports demonstrating increased rates of reoperation and reconstructive failure with IBR, particularly in the setting of postmastectomy radiation therapy and medical comorbidities.68
Given the relatively weak evidence addressing some outcomes for the choice between IBR and AR and the highly patient preference-sensitive nature of the decisions,69,70 we encourage clinicians to inform patients about the limitations of existing research. Among the limitations is that very little research has explicitly focused on patients whose mastectomy was performed for prophylactic (and not therapeutic) purposes. Therefore, the patient’s values and preferences, and the clinician’s expertise and experience are highly important.
The strengths of this SR pertain to the comprehensive methodology and in-depth stakeholder engagement of plastic surgeons, researchers, advocates, and patients. Importantly, this comparative effectiveness review provides a comprehensive focus on subjective patient-reported outcomes as well as complication profiles, which, taken together, provide critical evidence to support shared decision-making by patients and surgeons. Finally, in an effort to answer broad research questions, this SR covered a diverse range of reconstruction procedures and techniques. In doing so, the findings herein are broadly applicable to a range of techniques, implant types, and flap choices.
This SR’s limitations largely relate to the heterogeneity of the studies, generally low to moderate quality of evidence, and lack of consistent outcome reporting. Furthermore, we could not evaluate potential heterogeneity of treatment effects by timing of reconstruction, radiation therapy, and other factors because such analyses were not reported by the included studies. Additionally, we did not extract from studies information regarding surgeon experience. Differences in these factors both within and across studies could potentially influence results regarding satisfaction and complication profiles. Additionally, most studies were conducted in North America (United States and/or Canada; 55% of NRCSs) and, from limited reported data, women in the North American studies were mostly White. These factors may compromise the widespread applicability of our findings.
CONCLUSIONS
The evidence identified in this SR suggests that AR is probably associated with greater improvements in sexual well-being and satisfaction with aesthetic outcomes when compared with IBR. IBR may be associated with higher risk of seroma, whereas AR is probably associated with higher risks of thromboembolic events. Finally, AR is probably associated with more durable results; patients are probably less likely to experience reconstructive failure at long-term follow-up when compared with IBR. The results of this comprehensive review should provide valuable, long-term insights for both patients and surgeons. Patients who choose to undergo breast reconstruction after mastectomy should be informed about the benefits and potential harms of each procedure before making an informed decision.
ACKNOWLEDGMENTS
The authors of this article are responsible for its content. Statements in the report should not be construed as endorsement by AHRQ or U.S. Department of Health and Human Services. AHRQ retains a license to display, reproduce, and distribute the data and the report from which this article was derived under the terms of the agency’s contract with the author.
We would like to thank Binita Ashar, MD, Katelyn Donnelly, MPH, Phyllis Greenberger, MSW, Michele Manahan, MD, Priscilla McAuliffe, MD, PhD, Steven Nagel, MD, William Sikov, MD, Terence Myckatyn, MD, Anne Taylor, MD, Myelin Torres, MD, Edwin Wilkins, MD, and Sung Yoon, MD, who served as key informants and/or technical experts and helped us refine the research questions and develop the protocol. We are also grateful to the project’s Task Order Officer Jill Huppert, MD, MPH and the Acting EPC Program Director Christine Chang, MD, MPH at the Agency for Healthcare Research and Quality, Rockville, Maryland, and to the full systematic review report’s peer reviewers and Associate Editor Timothy Wilt, MD, MPH, at the Minnesota Evidence-based Practice Center, Minneapolis, Minnesota. We also thank Kristin Konnyu, MSc, PhD and Jonah Popp, PhD for their careful study screening and related contributions to the project.
Supplementary Material
Footnotes
Published online 11 March 2022.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. This project was funded under Contract No. HHSA 75Q80120D00001 from the Agency for Healthcare Research and Quality (AHRQ), U.S. Department of Health and Human Services.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Jonczyk MM, Jean J, Graham R, et al. Surgical trends in breast cancer: a rise in novel operative treatment options over a 12 year analysis. Breast Cancer Res Treat. 2019;173:267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Society of Plastic Surgeons. 2020 national plastic surgery statistics. Available at https://www.plasticsurgery.org/documents/News/Statistics/2020/plastic-surgery-statistics-full-report-2020.pdf. 2020.
- 3.Eltahir Y, Werners LLCH, Dreise MM, et al. Quality-of-life outcomes between mastectomy alone and breast reconstruction: comparison of patient-reported BREAST-Q and other health-related quality-of-life measures. Plast Reconstr Surg. 2013;132:201e–209e. [DOI] [PubMed] [Google Scholar]
- 4.Guyomard V, Leinster S, Wilkinson M. Systematic review of studies of patients’ satisfaction with breast reconstruction after mastectomy. Breast. 2007;16:547–567. [DOI] [PubMed] [Google Scholar]
- 5.Dean C, Chetty U, Forrest AP. Effects of immediate breast reconstruction on psychosocial morbidity after mastectomy. Lancet. 1983;1:459–462. [DOI] [PubMed] [Google Scholar]
- 6.American Society of Plastic Surgeons. 2018 plastic surgery statistics report. Available at https://www.plasticsurgery.org/documents/News/Statistics/2018/plastic-surgery-statistics-full-report-2018.pdf. 2018.
- 7.Pusic AL, Matros E, Fine N, et al. Patient-reported outcomes 1 year after immediate breast reconstruction: results of the Mastectomy Reconstruction Outcomes Consortium Study. J Clin Oncol. 2017;35:2499–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santosa KB, Qi J, Kim HM, et al. Long-term patient-reported outcomes in postmastectomy breast reconstruction. JAMA Surg. 2018;153:891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berkman ND, Lohr KN, Ansari M, et al. AHRQ methods for effective health care grading the strength of a body of evidence when assessing health care interventions for the Effective Health Care Program of the Agency for healthcare research and quality: an update. In: Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville, Md.: Agency for Healthcare Research and Quality (US); 2008. [PubMed] [Google Scholar]
- 10.Saldanha IJ, Broyles JM, Adam GP, et al. Implant-based breast reconstruction after mastectomy for breast cancer: a systematic review and meta-analysis. Plast Reconstr Surg Global Open. 2022;10:e4179 (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saldanha IJ, Cao W, Broyles JM, et al. Breast Reconstruction after Mastectomy: A Systematic Review and Meta-Analysis. Comparative Effectiveness Review No. 245. Rockville, Md.: Agency for Healthcare Research and Quality; 2021. Available at 10.23970/AHRQEPCCER245. Accessed July 16, 2021. [DOI] [PubMed] [Google Scholar]
- 12.Brorson F, Thorarinsson A, Kölby L, et al. Early complications in delayed breast reconstruction: a prospective, randomized study comparing different reconstructive methods in radiated and non-radiated patients. Eur J Surg Oncol. 2020;46:2208–2217. [DOI] [PubMed] [Google Scholar]
- 13.Tallroth L, Velander P, Klasson S. A short-term comparison of expander prosthesis and DIEP flap in breast reconstructions: a prospective randomized study. J Plast Reconstr Aesthet Surg. 2021;74:1193–1202. [DOI] [PubMed] [Google Scholar]
- 14.Abedi N, Ho AL, Knox A, et al. Predictors of mastectomy flap necrosis in patients undergoing immediate breast reconstruction: a review of 718 patients. Ann Plast Surg. 2016;76:629–634. [DOI] [PubMed] [Google Scholar]
- 15.Atisha D, Alderman AK, Lowery JC, et al. Prospective analysis of long-term psychosocial outcomes in breast reconstruction: two-year postoperative results from the Michigan Breast Reconstruction Outcomes Study. Ann Surg. 2008;247:1019–1028. [DOI] [PubMed] [Google Scholar]
- 16.Bennett KG, Qi J, Kim HM, et al. Comparison of 2-year complication rates among common techniques for postmastectomy breast reconstruction. JAMA Surg. 2018;153:901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brito IM, Fernandes A, Andresen C, et al. Patient satisfaction with breast reconstruction: how much do timing and surgical technique matter? Eur J Plast Surg. 2020;43:809–818.. [Google Scholar]
- 18.Chetta MD, Aliu O, Zhong L, et al. Reconstruction of the irradiated breast: a national claims-based assessment of postoperative morbidity. Plast Reconstr Surg. 2017;139:783–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen WA, Ballard TN, Hamill JB, et al. Understanding and optimizing the patient experience in breast reconstruction. Ann Plast Surg. 2016;77:237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dauplat J, Thivat E, Rouanet P, et al. ; STIC-RMI Working Group. Risk factors associated with complications after unilateral immediate breast reconstruction: a French prospective multicenter study. In Vivo. 2021;35:937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Araujo TB, Jue Xu M, Susarla SM, et al. Impact of prior unilateral chest wall radiotherapy on outcomes in bilateral breast reconstruction. Plast Reconstr Surg. 2016;138:575e–580e. [DOI] [PubMed] [Google Scholar]
- 22.Eltahir Y, Werners LLCH, Dreise MM, et al. Which breast is the best? Successful autologous or alloplastic breast reconstruction: patient-reported quality-of-life outcomes. Plast Reconstr Surg. 2015;135:43–50. [DOI] [PubMed] [Google Scholar]
- 23.Fischer JP, Fox JP, Nelson JA, et al. A longitudinal assessment of outcomes and healthcare resource utilization after immediate breast reconstruction-comparing implant- and autologous-based breast reconstruction. Ann Surg. 2015;262:692–699. [DOI] [PubMed] [Google Scholar]
- 24.Fischer JP, Nelson JA, Cleveland E, et al. Breast reconstruction modality outcome study: a comparison of expander/implants and free flaps in select patients. Plast Reconstr Surg. 2013;131:928–934. [DOI] [PubMed] [Google Scholar]
- 25.Fischer JP, Wes AM, Nelson JA, et al. Propensity-matched, longitudinal outcomes analysis of complications and cost: comparing abdominal free flaps and implant-based breast reconstruction. J Am Coll Surg. 2014;219:303–312. [DOI] [PubMed] [Google Scholar]
- 26.Garvey PB, Villa MT, Rozanski AT, et al. The advantages of free abdominal-based flaps over implants for breast reconstruction in obese patients. Plast Reconstr Surg. 2012;130:991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ha JH, Hong KY, Lee HB, et al. Oncologic outcomes after immediate breast reconstruction following mastectomy: comparison of implant and flap using propensity score matching. BMC Cancer. 2020;20:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hangge PT, Jogerst K, Mohsen A, et al. Making an informed choice: which breast reconstruction type has the lowest complication rate? Am J Surg. 2019;218:1040–1045. [DOI] [PubMed] [Google Scholar]
- 29.Jiang YZ, Liu YR, Yu KD, et al. Immediate postmastectomy breast reconstruction showed limited advantage in patient survival after stratifying by family income. PLoS One. 2013;8:e82807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kouwenberg CAE, de Ligt KM, Kranenburg LW, et al. Long-term health-related quality of life after four common surgical treatment options for breast cancer and the effect of complications: a retrospective patient-reported survey among 1871 patients. Plast Reconstr Surg. 2020;146:1–13. [DOI] [PubMed] [Google Scholar]
- 31.Kouwenberg CAE, Kranenburg LW, Visser MS, et al. The validity of the EQ-5D-5L in measuring quality of life benefits of breast reconstruction. J Plast Reconstr Aesthet Surg. 2019;72:52–61. [DOI] [PubMed] [Google Scholar]
- 32.Kulkarni AR, Pusic AL, Hamill JB, et al. Factors associated with acute postoperative pain following breast reconstruction. JPRAS Open. 2017;11:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laporta R, Sorotos M, Longo B, et al. Breast reconstruction in elderly patients: risk factors, clinical outcomes, and aesthetic results. J Reconstr Microsurg. 2017;33:257–267. [DOI] [PubMed] [Google Scholar]
- 34.Lee KT, Bang SI, Pyon JK, et al. Method of breast reconstruction and the development of lymphoedema. Br J Surg. 2017;104:230–237. [DOI] [PubMed] [Google Scholar]
- 35.Lee KT, Pyon JK, Bang SI, et al. Does the reconstruction method influence development of mastectomy flap complications in nipple-sparing mastectomy? J Plast Reconstr Aesthet Surg. 2013;66:1543–1550. [DOI] [PubMed] [Google Scholar]
- 36.Lei C, Xu L, Xu F, et al. Patient satisfaction in one-stage immediate breast reconstruction after mastectomy: a multi-center comparative patient evaluation of prosthesis, LDMF, and TRAM techniques. Medicine (Baltimore). 2020;99:e19991. [DOI] [PubMed] [Google Scholar]
- 37.Liu C, Momeni A, Zhuang Y, et al. Outcome analysis of expander/implant versus microsurgical abdominal flap breast reconstruction: a critical study of 254 cases. Ann Surg Oncol. 2014;21:2074–2082. [DOI] [PubMed] [Google Scholar]
- 38.Mak JC, Kwong A. Complications in post-mastectomy immediate breast reconstruction: a ten-year analysis of outcomes. Clin Breast Cancer. 2020;20:402–407. [DOI] [PubMed] [Google Scholar]
- 39.McCarthy CM, Mehrara BJ, Long T, et al. Chest and upper body morbidity following immediate postmastectomy breast reconstruction. Ann Surg Oncol. 2014;21:107–112. [DOI] [PubMed] [Google Scholar]
- 40.Merchant SJ, Goldstein L, Kruper LL. Patterns and trends in immediate postmastectomy reconstruction in California: complications and unscheduled readmissions. Plast Reconstr Surg. 2015;136:10e–19e. [DOI] [PubMed] [Google Scholar]
- 41.Mioton LM, Smetona JT, Hanwright PJ, et al. Comparing thirty-day outcomes in prosthetic and autologous breast reconstruction: a multivariate analysis of 13,082 patients? J Plast Reconstr Aesthet Surg. 2013;66:917–925. [DOI] [PubMed] [Google Scholar]
- 42.Mlodinow AS, Ver Halen JP, Lim S, et al. Predictors of readmission after breast reconstruction: a multi-institutional analysis of 5012 patients. Ann Plast Surg. 2013;71:335–341. [DOI] [PubMed] [Google Scholar]
- 43.Momeni A, Fox JP. Venous thromboembolism after surgical treatment of breast cancer. Ann Plast Surg. 2018;80:188–192. [DOI] [PubMed] [Google Scholar]
- 44.Naoum GE, Oladeru OT, Niemierko A, et al. Optimal breast reconstruction type for patients treated with neoadjuvant chemotherapy, mastectomy followed by radiation therapy. Breast Cancer Res Treat. 2020;183:127–136. [DOI] [PubMed] [Google Scholar]
- 45.Naoum GE, Salama L, Niemierko A, et al. Single stage direct-to-implant breast reconstruction has lower complication rates than tissue expander and implant and comparable rates to autologous reconstruction in patients receiving postmastectomy radiation. Int J Radiat Oncol Biol Phys. 2020;106:514–524. [DOI] [PubMed] [Google Scholar]
- 46.Nasser JS, Huetteman HE, Chung TT, et al. Unplanned emergency department visits within 30 days of mastectomy and breast reconstruction. Plast Reconstr Surg. 2018;142:1411–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nelson JA, Voineskos SH, Qi J, et al. Elective revisions after breast reconstruction: results from the mastectomy reconstruction outcomes consortium. Plast Reconstr Surg. 2019;144:1280–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palve JS, Luukkaala TH, Kääriäinen MT. Predictive risk factors of complications in different breast reconstruction methods. Breast Cancer Res Treat. 2020;182:345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qin Q, Tan Q, Lian B, et al. Postoperative outcomes of breast reconstruction after mastectomy: a retrospective study. Medicine (Baltimore). 2018;97:e9766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roth RS, Lowery JC, Davis J, et al. Persistent pain following postmastectomy breast reconstruction: long-term effects of type and timing of surgery. Ann Plast Surg. 2007;58:371–376. [DOI] [PubMed] [Google Scholar]
- 51.Roth RS, Qi J, Hamill JB, et al. Is chronic postsurgical pain surgery-induced? A study of persistent postoperative pain following breast reconstruction. Breast. 2018;37:119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shiraishi M, Sowa Y, Fujikawa K, et al. Factors associated with chronic pain following breast reconstruction in Japanese women. J Plast Surg Hand Surg. 2020;54:317–322. [DOI] [PubMed] [Google Scholar]
- 53.Simon P, Barrou J, Cohen M, et al. Types of mastectomies and immediate reconstructions for ipsilateral breast local recurrences. Front Oncol. 2020;10:567298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taylor EM, Wilkins EG, Pusic AL, et al. Impact of unilateral versus bilateral breast reconstruction on procedure choices and outcomes. Plast Reconstr Surg. 2019;143:1159e–1168e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tong WMY, Baumann DP, Villa MT, et al. Obese women experience fewer complications after oncoplastic breast repair following partial mastectomy than after immediate total breast reconstruction. Plast Reconstr Surg. 2016;137:777–791. [DOI] [PubMed] [Google Scholar]
- 56.Weichman KE, Clavin NW, Miller HC, et al. Does the use of biopatch devices at drain sites reduce perioperative infectious complications in patients undergoing immediate tissue expander breast reconstruction? Plast Reconstr Surg. 2015;135:9e–17e.. [DOI] [PubMed] [Google Scholar]
- 57.Weichman KE, Hamill JB, Kim HM, et al. Understanding the recovery phase of breast reconstructions: patient-reported outcomes correlated to the type and timing of reconstruction. J Plast Reconstr Aesthet Surg. 2015;68:1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilkins EG, Hamill JB, Kim HM, et al. Complications in postmastectomy breast reconstruction: one-year outcomes of the Mastectomy Reconstruction Outcomes Consortium (MROC) Study. Ann Surg. 2018;267:164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woo KJ, Lee KT, Mun GH, et al. Effect of breast reconstruction modality on the development of postmastectomy shoulder morbidity. J Plast Reconstr Aesthet Surg. 2018;71:1761–1767. [DOI] [PubMed] [Google Scholar]
- 60.Wu ZY, Han HH, Kim HJ, et al. A propensity score-matched comparison of recurrence outcomes after immediate implant vs autologous flap reconstruction in patients receiving neoadjuvant chemotherapy for breast cancer. Breast Cancer Res Treat. 2021;187:417–425.. [DOI] [PubMed] [Google Scholar]
- 61.Xu F, Sun H, Zhang C, et al. Comparison of surgical complication between immediate implant and autologous breast reconstruction after mastectomy: a multicenter study of 426 cases. J Surg Oncol. 2018;118:953–958. [DOI] [PubMed] [Google Scholar]
- 62.Yueh JH, Slavin SA, Adesiyun T, et al. Patient satisfaction in postmastectomy breast reconstruction: a comparative evaluation of DIEP, TRAM, latissimus flap, and implant techniques. Plast Reconstr Surg. 2010;125:1585–1595. [DOI] [PubMed] [Google Scholar]
- 63.Yueh JH, Slavin SA, Bar-Meir ED, et al. Impact of regional referral centers for microsurgical breast reconstruction: the New England perforator flap program experience. J Am Coll Surg. 2009;208:246–254. [DOI] [PubMed] [Google Scholar]
- 64.Zhang L, Jin K, Wang X, et al. The impact of radiotherapy on reoperation rates in patients undergoing mastectomy and breast reconstruction. Ann Surg Oncol. 2019;26:961–968. [DOI] [PubMed] [Google Scholar]
- 65.Voineskos SH, Klassen AF, Cano SJ, et al. Giving meaning to differences in BREAST-Q scores: minimal important difference for breast reconstruction patients. Plast Reconstr Surg. 2020;145:11e–20e. [DOI] [PubMed] [Google Scholar]
- 66.Jordan SW, Khavanin N, Kim JYS. Seroma in prosthetic breast reconstruction. Plast Reconstr Surg. 2016;137:1104–1116. [DOI] [PubMed] [Google Scholar]
- 67.Astanehe A, Temple-Oberle C, Nielsen M, et al. An enhanced recovery after surgery pathway for microvascular breast reconstruction is safe and effective. Plast Reconstr Surg Glob Open. 2018;6:e1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Manyam BV, Shah C, Woody NM, et al. Long-term complications and reconstruction failures in previously radiated breast cancer patients receiving salvage mastectomy with autologous reconstruction or tissue expander/implant-based reconstruction. Breast J. 2019;25:1071–1078. [DOI] [PubMed] [Google Scholar]
- 69.Keirns CC, Goold SD. Patient-centered care and preference-sensitive decision making. JAMA. 2009;302:1805–1806. [DOI] [PubMed] [Google Scholar]
- 70.Lee CN, Ubel PA, Deal AM, et al. How informed is the decision about breast reconstruction after mastectomy?: A prospective, cross-sectional study. Ann Surg. 2016;264:1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]