Abstract
Background
Patent ductus arteriosus (PDA) is associated with increased mortality and morbidity in preterm infants. Prophylactic indomethacin results in the reduction in significant PDA, need for surgical ligation, severe intraventricular hemorrhage and serious pulmonary hemorrhage without modifying long‐term neurosensory outcomes. Little is known about the effectiveness and safety of prophylactic surgical closure of the PDA in extremely low birth weight (ELBW) infants.
Objectives
To determine the effect of prophylactic surgical ligation of the PDA on mortality and morbidities of preterm infants less than 1000 g at birth as compared to no prophylaxis or prophylaxis with cyclooxygenase inhibitors.
Search methods
We searched MEDLINE (1966 to December 2006), EMBASE (1980 to December 2006), the Cochrane Central Register of Controlled Trials (The Cochrane Library, Issue 4, 2006), and abstracts of annual meetings of the Society for Pediatric Research (1995 to 2006). This search was updated in March 2010.
Selection criteria
Randomized or quazi‐randomised controlled trials that enrolled infants ≤ 28 weeks gestation or ≤ 1000 g at birth who were on assisted ventilation and/or supplemental oxygen without clinical signs of hemodynamic significant PDA were considered. Trials addressing prophylactic surgical ligation of the PDA (i.e. procedure done during the first 72 hours of life) versus no intervention or cyclooxygenase inhibitor prophylaxis were included.
Data collection and analysis
We used the standard methods of the Cochrane Neonatal Review Group. For dichotomous outcomes, relative risk (RR) and its associated confidence interval were calculated. For continuous outcomes, treatment effect was expressed as mean difference and its calculated standard deviation.
Main results
Only one eligible study that enrolled 84 ELBW infants was identified. Prophylactic surgical ligation of the PDA resulted in a statistically significant reduction of severe stage II or III necrotizing enterocolitis (NEC) [RR 0.25, 95% CI (0.08,0.83), p value 0.02, NNT 5]. The study found no statistically significant difference in mortality, severe grade III and IV intraventricular hemorrhage (IVH), BPD, and retinopathy of prematurity (ROP).
Authors' conclusions
Prophylactic surgical ligation of the PDA did not decrease mortality or BPD in ELBW infants. A significant reduction of stage II or III NEC was noted. Based on the current evidence, the high rate of spontaneous closure, availability of effective safe medical therapies, and the potential short and long‐term complications of surgical ligation, the use such prophylactic surgical therapy is not indicated in the management of the preterm infants.
Plain language summary
Prophylactic surgical ligation of patent ductus arteriosus for prevention of mortality and morbidity in extremely low birth weight infants
There is no evidence to support the use of prophylactic surgical ligation of the patent ductus arteriosus (PDA) in the management of the preterm infants. The ductus arteriosus is a blood vessel that is open during fetal life and connects blood flow from the vessel that supplies blood to the lungs to the major vessel that supplies blood to the body (the aorta). This blood vessel can stay open after birth leading to a condition called patent ductus arteriosus (PDA). PDA is one of the most common cardiac conditions in babies who are born very early (premature) and can lead to significant complications and death. Prophylactic (very early) closure of the ductus arteriosus (within 72 hours after birth) can be achieved medically or surgically. Llittle is known about the effectiveness and safety of very early surgical closure (ligation). The review found that surgical ligation in preterm infants reduced the risk of severe necrotizing enterocolitis (NEC), a gastrointestinal disease that mostly affects premature infants involving infection and inflammation of the bowel (intestine); however, early surgical ligation did not decrease the risk of death, chronic lung disease and other major complications of preterm infants. In view of the lack of significant benefit and growing data suggesting the potential harm of such treatment modality, current evidence does not support the use of early surgical ligation of PDA in the management of preterm infants.
Background
Description of the condition
Patent ductus arteriosus (PDA) is the most common cardiac condition among preterm infants. It affects approximately 40 to 55% of preterm infants born at less than 29 weeks gestation and/or weighing under 1500 g at birth (Reller 1993; Trus 1993; Mouzinho 1991). In spite of significant advances in neonatal care over the last two decades, the approach to PDA management remains one of the most controversial topics in neonatal medicine.
PDA remains hemodynamically significant in preterm infants for a variety of reasons including decreased ductal sensitivity to the partial pressure of oxygen, increased circulating prostaglandin E2, and increased ductal tissue sensitivity to prostaglandin E2 and nitric oxide (Clyman 2000; Clyman 1998; Clyman 1990; Brook 1995). A hemodynamically significant PDA is associated with left to right shunting of systemic blood with subsequent pulmonary overcirculation and a diastolic steal that results in hypoperfusion of vital organs. In very low birth weight (VLBW) infants, symptomatic PDA increases the risk of prolonged ventilation and oxygen requirements, pulmonary hemorrhage (Kluckow 2000), and bronchopulmonary dysplasia (BPD) (Rojas 1995; Brown 1979; Bancalari 2003). The diastolic steal is associated with renal hypoperfusion (Hammerman 1995), intestinal ischemia, necrotizing enterocolitis (NEC), reduced middle cerebral artery blood flow velocity (Martin 1982; Nestrud 1980; Shimada 1994; Weir 1999), and decreased superior vena cava (SVC) flow with concurrent increased risk of intraventricular hemorrhage (IVH) (Duddell 1984; Osborn 2003 ). If not managed appropriately, complications of symptomatic PDA may lead to death (Cotton 1979).
Description of the intervention
The increased number of surviving preterm infants has led to a higher number of infants requiring medical or surgical intervention for the PDA . Standard therapies include fluid restriction and use of cyclooxygenase inhibitors such as indomethacin or ibuprofen. Surgical ligation is used when medical treatment fails or is contraindicated (Clyman 1996; Kitterman 1972; Wagner 1984). There is no consensus as to what is the best time of treatment and the optimal management strategy among this high‐risk vulnerable population.
Prophylactic indomethacin has been shown to have favorable intermediate outcomes, such as reductions in the risk of significant PDA, the need for surgical ligation, severe intraventricular hemorrhage (Schmidt 2001; Fowlie 2002), and pulmonary hemorrhage (AlFaleh 2004). Prophylactic indomethacin has not been shown to alter long‐term outcomes including the rate of survival without neurosensory impairment at 18 months of corrected age (Schmidt 2001). Given the limitation of medical treatment, prophylactic surgical closure of the PDA may be a reasonable alternative in a high‐risk population, since it results in ultimate ductal closure and, therefore, might prevent associated neonatal morbidities.
Routine surgical ligation of a PDA refractory to medical treatment is widely practiced (Palder 1987; Pokharel 1998; Satur 1991; Chen 1999). However, there is no clear evidence that surgical ligation of a hemodynamically significant PDA is associated with improved long‐term outcomes in preterm infants. Furthermore, surgery may potentially result in significant morbidities such as pneumothorax, hypothermia, intra‐operative bleeding, phrenic nerve palsy, wound infection, vocal cord palsy and thoracic scoliosis. On the other hand, there are reports suggesting that surgical ligation may be the preferred first line of treatment when compared to indomethacin in preterm infants less than 800 g (Palder 1987; Trus 1993).
Why it is important to do this review
Even though there may be a general consensus with respect to the use of indomethacin as the initial therapy for a symptomatic PDA while reserving surgical ligation for indomethacin failures, this therapeutic approach may not represent the optimal management of PDA in extremely low birth weight infants (ELBW), the population at greatest risk for PDA (Malviya 2003).
Objectives
The primary objective of this systematic review was to identify and summarize evidence from randomised controlled trials investigating the effectiveness and safety of prophylactic surgical ligation of the PDA on mortality and morbidities in preterm infants less than 1000 g at birth as compared to no prophylaxis or to the use of prophylactic cyclooxygenase inhibitors.
The secondary objective was to investigate the role of prophylactic ligation in a subgroup of high‐risk infants < 750 g at birth.
Methods
Criteria for considering studies for this review
Types of studies
Only randomised and quasi‐randomized controlled trials were included.
Types of participants
Infants < 28 weeks gestation or less than 1000 g at birth who were on assisted ventilation and/or supplemental oxygen without clinical signs of a hemodynamically significant PDA.
Types of interventions
Prophylactic surgical ligation of the patent ductus arteriosus (i.e. procedure done during the first 72 hours of life in asymptomatic ELBW infants) versus no prophylactic intervention or medical prophylaxis (cyclooxygenase inhibitors) without dose specification.
Types of outcome measures
Primary outcomes
All cause neonatal mortality at 36 weeks postmenstrual age.
Bronchopulmonary dysplasia (BPD) defined as oxygen requirement at 36 weeks postmenstrual age.
Secondary outcomes
a) Any intraventricular hemorrhage (IVH); b) severe grade III, IV IVH as per Papile criteria (Papile 1978).
Severe NEC (stage II or more) as per Bell's criteria (Bell 1978).
Periventricular leukomalacia (PVL) defined as cystic changes in the periventricular area.
a) Retinopathy of prematurity (ROP) (defined by ICORP classification: any ROP and severe ROP stage 3 or worse); b) any ROP.
Nosocomial sepsis, defined as positive bacterial blood or cerebrospinal fluid cultures taken beyond five days of age.
Time to establish full enteral feeds (days).
Duration of ventilation and supplemental oxygen (days).
Duration of hospital stay (days).
Neurodevelopmental impairment i.e. rates of cerebral palsy, cognitive delay defined as a Mental Development Index score of less than 70 (2 SD below the mean of 100) on the Bayley Scales of Infant Development II (Bayley 1993), deafness, blindness or their composite reported at 18 months corrected age or later.
Pneumothora.
Vocal cord palsy.
Search methods for identification of studies
Electronic searches
The standard search strategy for the Cochrane Neonatal Review Group was used. Randomized and quasi‐randomized controlled trials that evaluated prophylactic surgical ductal closure in the management of premature infants were identified from OVID MEDLINE‐National Library of Medicine (1966 to December 2006) using the following subject headings (MeSH) and text word terms: "neonate(s), newborn(s), infant(s), patent ductus arteriosus or PDA, ligation, indomethacin, ibuprofen, cyclooxygenase inhibitors and publication type 'controlled trial'. No language restrictions were applied.
Other databases were searched including: EMBASE (1980 to December 2006), the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 4, 2006). Two review authors performed the electronic database search independently. A manual search of the abstract books published from the Society of Pediatric Research (SPR) and the European Society of Pediatric Research (ESPR) for the period of 1985 to 2006 was performed.
In March, 2010, we updated the search as follows: MEDLINE, CINAHL, EMBASE and CENTRAL (The Cochrane Library) were searched from 2006 to 2010. Search terms: neonate(s), newborn(s), infant(s), patent ductus arteriosus or PDA, ligation, indomethacin, ibuprofen, cyclooxygenase inhibitors and publication type 'controlled trial'. No language restrictions were applied.
Searching other resources
Additional citations were sought using references in articles retrieved from searches. Subject experts were contacted to identify the unpublished and ongoing studies. Authors of the published trials were contacted to clarify or provide additional information. Two review authors independently screened candidate articles to check the eligibility for inclusion in the review.
Clinical trials registries were also searched for ongoing or recently completed trials (clinicaltrials.gov; controlled‐trials.com; and who.int/ictrp).
Data collection and analysis
Selection of studies
Retrieved articles were independently assessed for eligibility by two review authors. Discrepancies were resolved by discussion and consensus.
Data extraction and management
Data abstracted independently by two review authors. Discrepancies were resolved by discussion and consensus. Where data were incomplete, the primary investigator was contacted for further information and clarifications.
Assessment of risk of bias in included studies
Standard methods of the Cochrane Collaboration and its Neonatal Group were used to assess the methodological quality (validity criteria) of the trials. For each trial, information was sought regarding the method of randomization, blinding and reporting of all outcomes of all the infants enrolled in the trial. Each criteria was assessed as yes, no, can't tell. This information was entered in the table "Characteristics of Included Studies".
For the updated review in 2010, the Risk of Bias table was completed. The two review authors independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions. Any disagreement was resolved by discussion.
The Risk of Bias table addressed the following questions:
1. Sequence generation: Was the allocation sequence adequately generated?
For each included study, we described the method used to generate the allocation sequence as: adequate (any truly random process e.g. random number table; computer random number generator); inadequate (any nonrandom process e.g. odd or even date of birth; hospital or clinic record number); or unclear.
2. Allocation concealment: Was allocation adequately concealed?
For each included study, we described the method used to conceal the allocation sequence as: adequate (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes); inadequate (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or unclear.
3. Blinding of participants, personnel and outcome assessors: Was knowledge of the allocated intervention adequately prevented during the study? At study entry? At the time of outcome assessment?
For each included study, we described the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We assessed the methods as: adequate, inadequate or unclear for participants; adequate, inadequate or unclear for study personnel; and adequate, inadequate or unclear for outcome assessors and specific outcomes assessed.
4. Incomplete outcome data: Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. We assessed methods as: adequate (< 20% missing data); inadequate (≥20% missing data) or unclear.
5. Selective outcome reporting: Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we assessed the possibility of selective outcome reporting bias as: adequate (where it is clear that all of the study's pre‐specified outcomes and all expected outcomes of interest to the review have been reported); inadequate (where not all the study's pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or unclear.
6. Other sources of bias: Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns regarding other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as: yes; no; or unclear.
Measures of treatment effect
For dichotomous outcomes, relative risk (RR) and its associated confidence interval were calculated. For continuous outcomes, treatment effect was expressed as mean difference and its calculated standard deviation.
Assessment of heterogeneity
Heterogeneity was examined using the I‐2 test. Potential sources of heterogeneity might include differences in the timing of surgical ligation or use of cyclooxygenase inhibitors in the second arm of the study, the population under study, and study quality.
Data synthesis
If appropriate, meta‐analysis of pooled data was performed assuming a fixed effect model. Review Manager 4.2.8 software was used for statistical analysis.
Subgroup analysis and investigation of heterogeneity
The review includes two comparisons: 1. prophylactic surgical ligation vs. no prophylactic treatment; 2. prophylactic surgical ligation vs. prophylactic cyclooxygenase inhibitors.
If possible, a sensitivity analysis was planned to assess the effect of trials methodological quality on results of the meta‐analysis.
In addition, subgroup analyses were planned to investigate the effect of prophylactic surgical ligation of the ductus in high risk infants < 750 grams, to investigate the effect of timing of surgical prophylaxis (i.e. < 24 hr vs. > 24 hr after birth).
Results
Description of studies
Initial electronic search using OVID MEDLINE and EMBASE yielded 106 MEDLINE and 86 EMBASE potentially relevant citations. After reading abstracts, two articles were identified as potentially relevant. Review of full text articles identified one study (Cassady 1989) investigating the use of prophylactic PDA surgical ligation in preterm infants. Only one study (Cotton 1978) was excluded for reasons outlined in the table "Characteristics of Excluded Studies". There was total agreement among both review authors with regard to the search process. Details of the included study is shown in the table "Characteristics of Included Studies".
Cassady 1989 was a single center randomised controlled trial. Eighty‐four preterm infants less than 1000 g were enrolled in both study arms. The surgical intervention group (n = 40) had a PDA ligation within 24 hours of life following a pre‐specified protocol while the control group (n = 44) received standard care defined as: 1) nasal CPAP initially for hypoxemia due to RDS or pulmonary edema; 2) endotracheal intubation and ventilation for respiratory failure (PCo2 > 70) or refractory apnea; 3) bedside Echo and radionuclide studies routinely performed to detect "silent" ducts.
Ligation was performed for hemodynamically significant ducts based on predefined criteria. Neither group received any cyclooxygenase inhibitor during or before the study period. Infants with echo defined criteria or hemodynamically significant PDA underwent subsequent surgical ligation. The main outcome measures were survival to one year after birth, NEC (Bell's stage III, IV), IVH defined by cranial ultrasound, BPD and ROP. Long‐term neurosensory outcomes were not assessed. The study was suspended after 14 months since only few physicians were available to conduct the study properly.
Risk of bias in included studies
Details of the included study are presented in the table "Characteristics of Included Studies". The methodologic details of the study were extracted from the published data.
Cassady et al (Cassady 1989) was a single center study. Two groups of 84 infants were randomised to receive either early prophylactic surgical PDA ligation (within 24 hours of birth) or control (no intervention). Randomization was possibly adequate and allocation was adequately concealed. The intervention was not blinded. Measurement of outcomes was blinded for NEC and BPD. Blinding of other outcomes was not clear. All randomised infants were accounted for in the analysis. Of note, the sample size of this study was calculated to detect a large difference of 50%, which rendered the study underpowered to detect smaller meaningful clinical differences among the study groups.
Effects of interventions
PROPHYLACTIC SURGICAL DUCTAL LIGATION VS. CONTROL (Comparison 1):
Results of outcomes presented in Cassady 1989 study are summarized in the analysis part of this review.
Death The prespecified outcome of all cause neonatal mortality at 36 weeks postmenstrual age was not presented in the study. The author presented data with regard to death within 28 days of birth and within one year of life. These results are described below.
Death within 28 days of birth (Outcome 1.1): 16/40 infants died within 28 days of life in the treatment group as compared 20/44 in the control group [RR 0.88, 95% CI (0.53, 1.45), p value 0.62].
Death to one year of age (Outcome 1.2): A total of 25/40 infants died within one year of age in the treatment group as compared to 26/44 in the control group [RR 1.06, 95% CI 0.75, 1.49), p value 0.75].
Severe grade III, IV Intraventricular Hemorrhage (Outcome 1.3): 16/35 infants in the prophylactic ductal ligation group developed grade III or IVH as compared to 23/41 in the control group [RR 0.81, 95% CI (0.52, 1.28), p value 0.37]. Data with regard to all grades of IVH were not presented in the published manuscript.
Severe NEC (Outcome 1.4): The assessment of this outcome was blinded. Only 3/40 infants in the prophylactic ductal ligation group developed severe stage III or IV NEC as compared to 13/44 in the control group [RR 0.25, 95% CI (0.08,0.83), p value 0.02, NNT 5].
Bronchopulmonary Dysplasia (Outcome 1.5): The definition used for BPD was different from the pre‐specified definition (i.e. oxygen requirement at 36 weeks postmenstrual age). In Cassady 1989, BPD was defined using the criteria of Bancalari et al (histologic finding or radiographic criteria). 15/24 infants in the prophylactic ligation group developed BPD as compared to 14/24 in the control group [RR 1.07, 95% CI (0.68, 1.69), p value 0.77].
Retinopathy of Prematurity (Outcome 1.6): The criteria and method of measurement of this outcome was not specified in the manuscript. 7/22 infants in the prophylactic group developed ROP as compared to 10/21 in the control group [RR 0.67, 95% CI (0.31, 1.43), p value 0.30]. Out of these, only one infant had severe stage III or IV ROP in the prophylactic ligation group as compared to 3 in the control group [RR 0.32, 95% CI (0.04, 2.8), p value 0.30].
Subgroup analysis: The secondary objective of this review was to investigate the role of prophylactic surgical ligation in a subgroup of high risk infants <750 g at birth. No data was found in the included trial that relates specifically to this high‐risk group.
Data with regard to nosocomial sepsis, hospital stay, pneumothorax, vocal cord palsy, periventricular leucomalacia, and long term neurosensory impairment were not presented in this study.
Discussion
Only one study (Cassady 1989) that compared the effect of early prophylactic PDA ligation in extreme low birth weight infants versus later or no intervention was eligible for inclusion in this review. Prophylactic surgical ligation of the duct resulted in no significant differences in mortality, IVH, BPD and ROP between the two study groups. Interestingly, the study was able to show a significant reduction of severe NEC in the prophylactic surgical group as compared to infants who received selective treatment for hemodynamically significant PDA (20% vs. 79%). While it is possible this is a result of a true effect, it is important to note that feeds were introduced earlier in the control group. Strong conclusions can not be made since the study is underpowered to detect minimally important clinical differences in outcomes other than NEC.
Ligation of the ductus arteriosus is not without risks and it is often associated with significant hypotension and postoperative cardiorespiratory instability secondary to cardiovascular physiologic adaptation. There is limited information on the ability of preterm infants to compensate for the dramatic changes in left ventricular afterload following ligation (Teixeira 2006). Surgical ligation also poses risks of potential morbidity from infection, pneumothorax, chylothorax and vocal cord paralysis. Surgical ligation may not be easily accessible to all centers. In addition, evidence is accumulating regarding PDA ligation and its association with a higher incidence of BPD, ROP and unfavorable neurodevelopmental outcomes (Kabra 2007).
Since the publication of the included trial, the practice of neonatal medicine has advanced significantly. Over the last 16 years, many trials were published addressing the efficacy of prophylactic cyclooxygenase inhibitors (i.e. indomethacin and ibuprofen) in reducing the incidence of PDA and other short and long‐term morbidities in ELBW infants. The use of prophylactic indomethacin reduces the incidence of PDA by more than 50%, surgical ligation of the duct by 50% and severe grade III and IV IVH by 35% with no apparent adverse effect in long‐term neurosensory outcomes (Fowlie 2002). With the high rate of spontaneous closure, availability of effective safe medical therapies, potential short and long‐term complications of prophylactic surgical ligation (Kabra 2007), and the recent doubts raised concerning the need to close all significant PDA's in preterm infants (Laughon 2004, Laughon 2007), there is no obvious role for prophylactic surgical ligation in the management of preterm infants.
Current evidence demonstrates that prophylactic indomethacin given to preterm infants reduces the incidence of symptomatic PDA, severe IVH, and serious pulmonary hemorrhage (Schmidt 2001; Fowlie 2002; AlFaleh 2004) . Despite the reduction of the aforementioned morbidities (all of which are associated with poor long‐term outcome), no improvements in long‐term neurosensory outcome was noted. Giving indomethacin to unselected group of infants, irrespective of the ductal size, is a possible explanation (Osborn DA 2003). It has been suggested that future studies aimed at preventing IVH and improving neurodevelopmental outcome using medical agents to close the PDA should be directed at infants with large, hemodynamically significant PDA in the first hours of life (Osborn DA 2003).
The conclusions of our review are weakened by the inclusion of only one, small trial that addressed the intervention under study.
Authors' conclusions
Implications for practice.
Prophylactic surgical ligation of the PDA did not decrease mortality or the incidence of BPD in ELBW infants. A significant reduction in the incidence of stage II or III NEC was noted. Based on the current evidence, the high rate of spontaneous closure, availability of effective and safe medical therapies, and the potential short and long‐term complications of surgical ligation, the use such prophylactic surgical therapy is not indicated in the management of the preterm infants.
Implications for research.
A well‐powered large randomised controlled trial to investigate early targeted prophylactic use of cyclooxygenase inhibitors (within first 24 hours of life and based on echocardiographic criteria) in reducing neonatal morbidities and long‐term neurosensory outcomes in ELBW infants is indicated.
What's new
| Date | Event | Description |
|---|---|---|
| 23 April 2010 | New search has been performed | This review updates the existing review "Prophylactic surgical ligation of patent ductus arteriosus for prevention of mortality and morbidity in extremely low birth weight infants" published in the Cochrane Database of Systematic Reviews (Mosalli 2007). Updated search found no new trials. No changes to conclusions. |
History
Protocol first published: Issue 4, 2006 Review first published: Issue 1, 2008
| Date | Event | Description |
|---|---|---|
| 17 February 2009 | Amended | Plain language summary added. |
| 11 September 2008 | Amended | Converted to new review format. |
| 31 August 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank Dr Roger Soll and Dr Bosco Paes for their thoughtful advise throughout the process of our review preparation.
The Cochrane Neonatal Review Group has been funded in part with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN267200603418C.
Data and analyses
Comparison 1. Prophylactic ductal ligation vs. control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death within 28 days of birth | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.53, 1.45] |
| 2 Death till one year of age | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.75, 1.49] |
| 3 Severe grade III and IV IVH | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.52, 1.28] |
| 4 Severe NEC | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.08, 0.83] |
| 5 Bronchopulmonary dysplasia | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.68, 1.69] |
| 6 Retinopathy of prematurity | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 All ROP | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.31, 1.43] |
| 6.2 ROP stage III or IV | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.04, 2.82] |
1.1. Analysis.
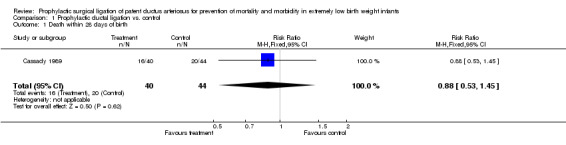
Comparison 1 Prophylactic ductal ligation vs. control, Outcome 1 Death within 28 days of birth.
1.2. Analysis.
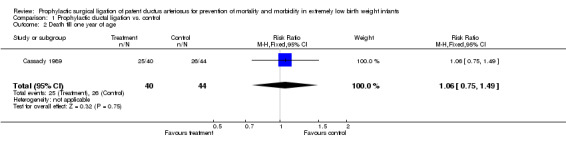
Comparison 1 Prophylactic ductal ligation vs. control, Outcome 2 Death till one year of age.
1.3. Analysis.
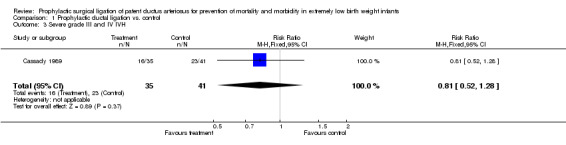
Comparison 1 Prophylactic ductal ligation vs. control, Outcome 3 Severe grade III and IV IVH.
1.4. Analysis.
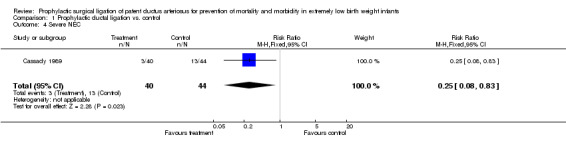
Comparison 1 Prophylactic ductal ligation vs. control, Outcome 4 Severe NEC.
1.5. Analysis.
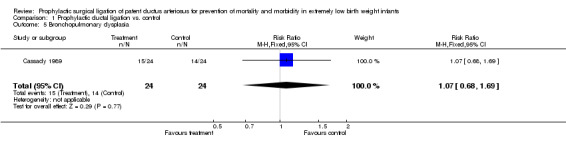
Comparison 1 Prophylactic ductal ligation vs. control, Outcome 5 Bronchopulmonary dysplasia.
1.6. Analysis.
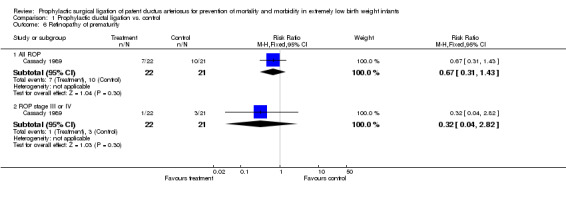
Comparison 1 Prophylactic ductal ligation vs. control, Outcome 6 Retinopathy of prematurity.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cassady 1989.
| Methods | Randomized controlled un‐blinded study Method of generating randomization sequence: Modified, balanced randomization technique Allocation concealment: Adequate Blinding of Intervention: No Blinding of outcome measurement: Yes for NEC and BPD Complete Follow‐up: Yes | |
| Participants | 84 infants, weighed 1000 g or less at birth Exclusion criteria: Lethal anomalies, failure to be randomised within 24 hours of birth and judged to be moribund by medical staff. Demographic data: Intervention group : n=40, gestational age (weeks) 27(1.8), birth weight (g) 800 (126.3) Control group n=44, gestational age (weeks) 28(1.5), birth weight (g) 795 (97.7) | |
| Interventions | Surgical group (n=40) had ligation within 24 hours of birth following designed protocol Control group (n=44) received standard care defined as 1) Initial NCPAP for hypoxemia due to RDS or pulmonary edema (2) Endotracheal intubation and ventilation for respiratory failure or refractory apnea (3) Echo and radionuclide studies routinely performed to detect silent ducts. Ligation was performed hemodynamically significant ducts based on a predefined criteria; Both groups received no indomethacin during or before the study period. Infants with echo defined criteria or hemodynamically significant PDA underwent subsequent surgical ligation. |
|
| Outcomes | Survival to one year after birth NEC, stage III, IV IVH defined by HUS BPD ROP | |
| Notes | USA Period of study: Oct 1980‐ Oct 1981 suspended for 14 months Jan 1983‐ May 1986 (four years and four months) Published: 1989 Source of Funding: not specified in paper | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Randomized controlled unblinded study Method of generating randomization sequence: Modified, balanced randomization technique |
| Blinding? All outcomes | High risk | Blinding of Intervention: No Blinding of outcome measurement: Yes for NEC and BPD |
| Incomplete outcome data addressed? All outcomes | Low risk | Complete Follow‐up: Yes |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cotton 1978 | Surgery was done after one week of birth for infants needing ventilation or Oxygen supplementation. Therefore, the intervention was not prophylactic. |
Contributions of authors
In joint meetings, both review authors, Rafat Mosalli (RM) and Khalid AlFaleh (KA), searched the literature, stated the objectives of the review and wrote the text of the protocol and review.
The April 2010 update was conducted centrally by the Cochrane Neonatal Review Group staff (Yolanda Montagne, Diane Haughton, and Roger Soll). This update was reviewed and approved by RM.
Sources of support
Internal sources
McMaster University Medical Center, Canada.
External sources
No sources of support supplied
Declarations of interest
None
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Cassady 1989 {published data only}
- Cassady G, Crouse DT, Kirklin JW, Strange MJ, Joiner CH, Godoy G, et al. A randomized, controlled trial of very early prophylactic ligation of the ductus arteriosus in babies who weighed 1000 g or less at birth. New England Journal of Medicine 1989;320:1511‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Cotton 1978 {published data only}
- Cotton RB, Stahlman MT, Bender HW, Graham TP, Catterton WZ, Kovar I. Randomized trial of early closure of symptomatic patent ductus arteriosus in small preterm infants. Journal of Pediatrics 1978;93:647‐51. [DOI] [PubMed] [Google Scholar]
Additional references
AlFaleh 2004
- Al‐Faleh K, Smyth JA, Roberts RA, Solimano A, Asztalos EV, Schmidt B. Prevention and 18‐month outcome of serious pulmonary hemorrhage in extremely‐low‐birth‐weight (ELBW) infants: results from the trial of indomethacin prophylaxis in preterms (TIPP). PAS2004:2589. [DOI] [PubMed] [Google Scholar]
Bancalari 2003
- Bancalari E, Claure N, Sosenko IR. Bronchopulmonary dysplasia: changes in pathogenesis, epidemiology and definition. Seminars in Neonatology 2003;1:63‐71. [DOI] [PubMed] [Google Scholar]
Bayley 1993
- Bayley N. Manual for the Bayley Scales of Infant Development. 2nd Edition. San Antonio, Tex.: Psychological Corporation, 1993. [Google Scholar]
Bell 1978
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, Brotherton T. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brook 1995
- Brook M, Heymann M. Patent ductus arteriosus. In: Emmanouilides GC, Riemenschneider TA, Allen HD, Gutgesell HP editor(s). Heart Disease in Infants, Children and Adolescents Including the Fetus and Young Adult. Williams & Wilkins, 1995:746‐64. [Google Scholar]
Brown 1979
- Brown ER. Increased risk of bronchopulmonary dysplasia in infants with patent ductus arteriosus. The Journal of Pediatrics 1979;95:865‐6. [DOI] [PubMed] [Google Scholar]
Chen 1999
- Chen KB, Tu KT, Cheng HC, Wu YL, Chang JS. The anesthetic management of a preterm infant weighing 500 gms undergoing ligation of patent ductus arteriosus. Acta Anaesthesiologica Sinica 1999;37:89‐92. [PubMed] [Google Scholar]
Clyman 1990
- Clyman R. Developmental physiology of the ductus arteriosus. In: Long W editor(s). Fetal and Neonatal Cardiology. Philadelphia: WB Saunders, 1990:64‐75. [Google Scholar]
Clyman 1996
- Clyman RI. Recommendations for the postnatal use of indomethacin: an analysis of four separate treatment strategies. Journal of Pediatrics 1996;128:601‐7. [DOI] [PubMed] [Google Scholar]
Clyman 1998
- Clyman RI, Waleh N, Black SM. Regulation of ductus arteriosus patency by nitric oxide in fetal lambs: the role of gestation, oxygen tension, and vasa vasorum. Pediatric Research 1998;43:633‐44. [DOI] [PubMed] [Google Scholar]
Clyman 2000
- Clyman R, Narayanan M. Patent ductus arteriosus: a physiologic basis for current treatment practices. Current Topics in Neonatology. Philadelphia: WB Saunders, 2000:71‐91. [Google Scholar]
Cotton 1979
- Cotton RB, Stahlman MT, Kovar I, Catterton WZ. Medical management of small preterm infants with symptomatic patent ductus arteriosus. The Journal of Pediatrics 1979;2:467‐73. [DOI] [PubMed] [Google Scholar]
Duddell 1984
- Duddell GG, Gersony M. Patent ductus arteriosus in neonates with severe respiratory disease. Journal of Pediatrics 1984;104:915‐20. [DOI] [PubMed] [Google Scholar]
Fowlie 2002
- Fowlie PW, Davis PG. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD000174.] [DOI] [PubMed] [Google Scholar]
Hammerman 1995
- Hammerman C. Patent ductus arteriosus. Clinical relevance of prostaglandins and prostaglandin inhibitors in PDA pathophysiology and treatment. Clinics of Perinatology 1995;22:457‐79. [PubMed] [Google Scholar]
Kabra 2007
- Kabra NS, Schmidt B, Roberts RS, Doyle LW, Papile L, Fanaroff A. Neurosensory impairment after surgical closure of patent ductus arteriosus in extremely low birth weight infants: results from the Trial of Indomethacin Prophylaxis in Preterms. Journal of Pediatrics 2007;150:229‐34. [DOI] [PubMed] [Google Scholar]
Kitterman 1972
- Kitterman JA, Edmunds LH, Gregory GA, Heymann MA, Tooley H, Rudolph AM. Patent ductus arteriosus in premature infants: incidence and relation to pulmonary disease and management. New England Journal of Medicine 1972;287:473‐7. [DOI] [PubMed] [Google Scholar]
Kluckow 2000
- Kluckow M, Evans N. Ductal shunting, high pulmonary blood flow and pulmonary hemorrhage. Journal of Pediatrics 2000;137:68‐72. [DOI] [PubMed] [Google Scholar]
Laughon 2007
- Laughon M, Bose C, Clark R. Treatment strategies to prevent or close a patent ductus arteriosus in preterm infants and outcomes. Journal of Perinatology 2007;27:164‐70. [DOI] [PubMed] [Google Scholar]
Laughon 2004
- Laughon MM, Simmons MA, Bose CL. Patency of the ductus arteriosus in the premature infant: is it pathologic? Should it be treated?. Current Opinion in Pediatrics 2004;16:146‐51. [DOI] [PubMed] [Google Scholar]
Malviya 2003
- Malviya M, Ohlsson A, Shah S. Surgical versus medical treatment with cyclooxygenase inhibitors for symptomatic patent ductus arteriosus in preterm infants. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD003951.pub2] [DOI] [PubMed] [Google Scholar]
Martin 1982
- Martin CG, Snider AR, Katz SM, Peabody JL, Brady JP. Abnormal cerebral flow patterns in preterm infants with a large patent ductus arteriosus. Journal of Pediatrics 1982;101:587‐93. [DOI] [PubMed] [Google Scholar]
Mouzinho 1991
- Mouzinho AI, Rosenfeld CR, Risser R. Symptomatic patent ductus arteriosus in very low birth weight infants. Early Human Development 1991;27:65‐77. [DOI] [PubMed] [Google Scholar]
Nestrud 1980
- Nestrud RM, Hill DE, Arrington RW, Beard AG, Dungan WT, Norton JB, Radinger RI. Indomethacin treatment in patent ductus arteriosus: a double blind study utilizing indomethacin plasma levels. Developmental Pharmacology and Therapeutics 1980;1:125‐36. [PubMed] [Google Scholar]
Osborn 2003
- Osborn DA, Evans N, Kluckow M. Hemodynamic and antecedent risk factors of early and late periventricular/intraventricular hemorrhage in premature infants. Pediatrics 2003;112:33‐9. [DOI] [PubMed] [Google Scholar]
Osborn DA 2003
- Osborn DA, Evans N, Kluckow M. Effect of early targeted indomethacin on the ductus arteriosus and blood flow to the upper body and brain in the preterm infant. Archives of Disease in Childhood 2003;88:F477‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Palder 1987
- Palder SB, Schwartz M, Tyson K, Marr C. Management of patent ductus arteriosus: comparison of operative vs pharmacologic treatment. Journal of Pediatric Surgery 1987;22:1171‐4. [DOI] [PubMed] [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Kofer H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92:529‐34. [DOI] [PubMed] [Google Scholar]
Pokharel 1998
- Pokharel R, Hisano K, Yasufuku M, Ataka K, Okada M, Yoshimoto S. Ligation of medically refracted patent ductus arteriosus (PDA) in an extremely low body weight premature infant. Surgery Today 1998;28:1290‐4. [DOI] [PubMed] [Google Scholar]
Reller 1993
- Reller MD, Rice MJ, McDonald RW. Review of studies evaluating ductal patency in the premature infant. Journal of Pediatrics 1993;122:S59‐62. [DOI] [PubMed] [Google Scholar]
Rojas 1995
- Rojas MA, Gonzalez A, Bancalari E, Claure N, Poole C, Silva‐Neto G, et al. Changing trends in the epidemiology and pathogenesis of neonatal chronic lung disease. Journal of Pediatrics 1995;126:605‐10. [DOI] [PubMed] [Google Scholar]
Satur 1991
- Satur CR, Walker DR, Dickinson DF. Day case ligation of patent ductus arteriosus in preterm infants. A ten year review. Archives of Disease in Childhood 1991;66:477‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmidt 2001
- Schmidt B, Davis P, Moddemann D, Ohlsson A, Roberts RS, SaigalS, et al. Long‐term effects of indomethacin prophylaxis in extremely low‐birth‐weight infants. New England Journal of Medicine 2001;344:1966‐72. [DOI] [PubMed] [Google Scholar]
Shimada 1994
- Shimada S, Kasai T, Konishi M, Fujiwara T. Effects of patent ductus arteriosus on left ventricular output and oxygen blood flow in preterm infants with respiratory distress syndrome treated with surfactant. Journal of Pediatrics 1994;125:270‐7. [DOI] [PubMed] [Google Scholar]
Teixeira 2006
- Teixeira L, McNamara P. Enhanced intensive care for the neonatal ductus arteriosus. Acta Paediatrica 2006;95:394‐403. [DOI] [PubMed] [Google Scholar]
Trus 1993
- Trus T, Winthrop AL, Pipe S, Shah J, Langer JC, Lau GY. Optimal management of patent ductus arteriosus in the neonate weighing less than 800 g. Journal of Pediatric Surgery 1993;28:1137‐9. [DOI] [PubMed] [Google Scholar]
Wagner 1984
- Wagner HR, Ellison C, Zierler S, Lang P, Purohit DM, Behrendt D, et al. Surgical closure of patent ductus arteriosus in 268 preterm infants. Journal of Thoracic and Cardiovascular Surgery 1984;87:870‐5. [PubMed] [Google Scholar]
Weir 1999
- Weir FJ, Ohlsson A, Myhr TL, Fong K, Rayan ML. A patent ductus arteriosus is associated with reduced middle cerebral artery blood flow velocity. European Journal of Pediatrics 1999;158:484‐7. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Mosalli 2007
- Mosalli R, AlFaleh KM. Prophylactic surgical ligation of patent ductus arteriosus for prevention of mortality and morbidity in extremely low birth weight infants. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006181.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


