Abstract
Background
Telerehabilitation is an alternative clinic‐based rehabilitation. A remote monitoring (RM) system attached to a cardiac rhythm device can collect physiological data and the device function. This study aimed to evaluate the safety and feasibility of telerehabilitation supervised by an RM in patients receiving cardiac resynchronization therapy (CRT).
Methods
A single group pre–post exercise program was implemented for 3 months in 18 CRT recipients. The exercise regimen consisted of walking a prescribed number of steps based on a 6‐min walk distance (6MWD) achieved at baseline. The patients were asked to exercise 3 to 5 times per week for up to 30 min per session, wearing an accelerometer to document the number of steps taken. The safety was assessed by the heart failure hospitalizations and all‐cause death. The feasibility was measured by the improvement in the quality of life (QOL) using the EuroQol 5 dimensions, and daily active time measured by the CRT, 6MWD, B‐type natriuretic peptide (BNP) level, and left ventricular ejection fraction (LVEF).
Results
No patients had heart failure hospitalizations or died. No patients had any ventricular tachyarrhythmias. One patient needed to suspend the exercise due to signs of exacerbated heart failure by the RM. Compared to baseline, there were significant improvements in the QOL (−0.037, p < .05), active time (1.12%/day, p < .05), and 6MWD (11 m, p < .001), but not the BNP (–32.4 pg/ml, p = .07) or LVEF (0.28%, p = .55).
Conclusions
Three months of RM‐guided walking exercise in patients with CRT significantly increased the QOL, active time, and exercise capacity without any adverse effects.
Keywords: arrhythmia, cardiac rehabilitation, heart failure, quality of life
![]()
1. INTRODUCTION
Heart failure is a major and growing public health problem, affecting more than 26 million people worldwide (Ponikowski et al., 2014). Cardiac resynchronization therapy (CRT) is effective for heart failure associated with severe left ventricular dysfunction and a wide QRS duration. Numerous trials have consistently shown significant improvements in the cardiac function and quality of life (QOL) and a reduction in heart failure‐related hospitalizations and mortality after CRT implementations (Tracy et al., 2012). The remote monitoring (RM) technology incorporated into cardiac electrical devices including CRT devices enables continuous monitoring of physiological parameters, arrhythmias, and the device integrity (Watanabe et al., 2013). RM transmits a monthly report in addition to real‐time alerts to medical personnel when pre‐specified adverse events occur. These early notifications enable a rapid clinical intervention and enhance the survival (Slotwiner et al., 2015; Varma et al., 2015).
Cardiac rehabilitation has also been shown to improve the QOL and reduce the risk of mortality in heart failure, but only a small number of eligible patients participate in clinical center‐based cardiac rehabilitation due to older age, comorbidities, and limited mobility both physically and in the means of transportation (Piepoli et al., 2010, 2016). Furthermore, there is often reluctance to prescribe cardiac rehabilitation in patients with a poor cardiac function because exacerbation of heart failure and arrhythmias can occur with rehabilitation (Lau et al., 2016). In light of these concerns, home‐based telerehabilitation with RM may facilitate the adoption of exercise programs in patients with CRT, while ensuring their safety (Pedretti et al., 2021; Piepoli et al., 2016; Thomas et al., 2019).
Many guidelines recommend conducting symptom‐limited cardiopulmonary exercise testing before prescribing an exercise dose and evaluating the effect of exercise training (Pedretti et al., 2021; Piepoli et al., 2010). In the real‐life setting, however, exercise testing is frequently impossible in patients with CRT because of a limited exercise capacity (Arena et al., 2007). We therefore devised a sub‐maximal walking program tailored to an individual's 6‐min walk distance (6MWD) based on our belief that walking is easy to fit into a patient's schedule and to build up gradually (Schwartz, 2004; Zubin Maslov et al., 2018). The purpose of this study was to evaluate the safety and feasibility of our telemonitoring‐guided walking exercise program in patients with CRT. This is a pilot study with 18 patients who were willing to participate and had device monitoring enabling collection of vital data.
2. METHODS
2.1. Patients
This study used a single‐center, single group pre–post design to assess the safety and feasibility of a 3‐month walking program conducted between December 2017 and November 2018. We recruited consecutive patients who visited our cardiac device clinic. The inclusion criteria were patients in sinus rhythm and who underwent either CRT with defibrillator (CRT‐D) or CRT with pacemaker (CRT‐P) implantation. The devices were manufactured by Biotronik (Biotronik SE & Co. KG, Berlin, Germany) or Boston Scientific and had been implanted for more than 1 year before participation in this study. No patient had previous experience of cardiac rehabilitation. All patients belonged to New York Heart Association (NYHA) functional class II or III, were receiving optimal medical therapy for heart failure, and had been in a stable condition for more than 6 months (defined as no hospitalizations for cardiovascular causes and no change in their cardiac medications). All had QRS width ≥120 ms and a left ventricular ejection fraction (LVEF) ≤35% at the time of the CRT implantation. A CRT responder was defined as a patient who achieved ≥15% reduction in the left ventricular end systolic volume (Leclercq et al., 2019). Exclusion criteria included NYHA functional class I or IV, arrhythmogenic right ventricular cardiomyopathy, hypertrophic cardiomyopathy, severe pulmonary hypertension, uncontrolled hypertension, persistent atrial fibrillation, peripheral artery disease, a history of myocardial infarction, coronary artery bypass grafting or percutaneous coronary intervention within the last 6 months, and significant untreated valvular disease. We also excluded lower limb disabilities, orthopedic pain, neurologic symptoms, and mental impairment leading to the inability to cooperate. We did not exclude patients with upgraded DDD pacemakers. The study protocol conformed to the 1975 Declaration of Helsinki and was approved by the Ethics Committee of the Fujita Health University School of Medicine (approval number HM17‐263). All patients gave their written informed consent prior to the study.
2.2. CRT device measurements of the physical activity and remote monitoring
The CRT devices measured the physical activity through an incorporated single‐axis accelerometer and stored the duration of the active period per day (ACT, %/day). This information could be retrieved using the RM system. The ACT was calculated from the number of minutes in a day the patient was active, where a minute was considered active if the threshold was exceeded that incorporated both the number and magnitude of the deflections of the accelerometer signal. According to the technical details provided by the manufacturer, the threshold was set to 0.05 g for the Biotronik and 0.025 g for Boston Scientific device, respectively. The acceleration of a free fall was 1.00 g . The acceleration of standing up from a chair was roughly 0.05 g . The CRT devices also captured the heart rate, rhythm statistics, mode switch episodes during atrial tachyarrhythmia, lead impedance, sensing and pacing thresholds, pacing rate, battery status, and intracardiac electrograms. The researchers reviewed all the transmitted data on a website once a day during the study period (Home Monitoring® for Biotronik and LATTITUDE™ for Boston Scientific). Furthermore, we received automatic alert notifications by email if the pre‐specified criteria were met. We used the weekday activity averaged over the 1 month before the study period as the baseline (Figure 1). In the primary prevention patients, we programmed the detection and therapy zones for ventricular tachyarrhythmias in the CRT‐Ds as follows: ventricular fibrillation: 261–280 ms, 8/12 or 30/40 intervals, and ventricular tachycardia: 351–353 ms, 28–48 intervals. For the secondary prevention patients, the programs were tailored to the individual. We activated the rate–response function.
FIGURE 1.
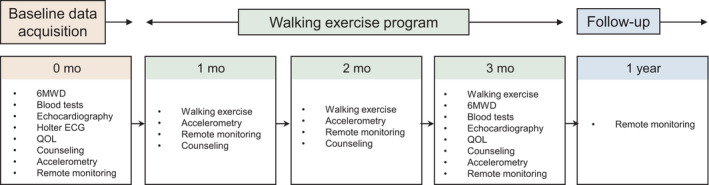
Study flow diagram. After termination of the walking exercise at 3 months, the patients were followed for 1 year. f/u: follow‐up
2.3. Accelerometer‐detected physical activity
The patients were asked to wear a single‐axis accelerometer (Lifecorder GS. Suzuken Co. Ltd, 71 × 40 × 19 mm /40 g) on their waist during the daytime. This device detected a physical activity of 0.06 g or more every 2 min and calculated an exercise intensity from 1.8 to 8.3 metabolic equivalents (METs) (Kumahara et al., 2004). The exercise intensity, which was classified as light (<2.9 METs), moderate (3–6 METs), or vigorous (>6.1 METs), was displayed by a bar graph on the device screen. The data can be downloaded offline in a summary report format via a personal computer. The internal real‐time clock also helped identify the activity patterns. In a preliminary study, we assessed the correlation between the total activity time determined by the accelerometer and that by the CRT device in 10 healthy adults. We found a significant linear correlation between the ACT measured by the accelerometer and both the Biotronik and Boston Scientific CRT devices (Supplementary File 1). These data validated our use of the Biotronik and Boston Scientific CRTs in this study and the monitoring of the daily ACT by the RM system. All patients visited the device clinic monthly for an interrogation of the CRT device and to download the accelerometer data.
2.4. Six‐minute walk distance and exercise dose prescription
A baseline 6MWD test was conducted between 2 and 4 p.m. In this test, the patients wore a pulse oximeter and completed as many laps on a 30 m walkway as they could within a 6‐min period, supervised by a laboratory technician. We counted the total steps and confirmed that their perceived exertion was less than 12–14 on the Borg scale, the minimum SpO2 was more than 95%, and the maximum heart rate was less than the upper tracking rate and programmed zones for defibrillation in each patient (Vanhees et al., 2004). Exercise dose was carefully personalized based on the 6MWD and stride length (Supplementary File 2) (Zubin Maslov et al., 2018). In brief, one target exercise session consisted of taking 5 times the number of steps achieved in 6 min on a level surface, within 30 min. The goal was to complete one session per day, 3–5 times per week, for 12 consecutive weeks. The patients were requested to wear an accelerometer to document the exercise intensity and number of steps. For example, a patient who was able to walk 400 m (4 km/h) with a stride of 66 cm in the 6MWD was asked to walk approximately 3,000 steps ([400 m × 5]/0.66) within a 30‐min session. We asked the patients to perform lower limb stretch exercises before and after walking to warm up and cool down. Patients were also instructed to increase their pace slowly over time during the session, to limit their perceived exertion to 12–14 on the Borg scale, and to avoid entering the vigorous activity zone (>6.1 METs) by monitoring the color displayed on the accelerometer. The study team also provided counseling on their medications, nutrition, risk factor management (smoking, alcohol, blood pressure, and weight), and lifestyle. Such counseling was conducted every month at the device clinic until the end of the 3‐month exercise program (Figure 1). Patients chose the time of day they completed their exercise, and patients were not monitored in real time by medical personnel during the exercise session.
2.5. Quality of life measurements
The health‐related QOL was assessed at baseline and 3 months after the start of the exercise program. We used a validated Japanese version of the EuroQol 5 dimensions (EQ‐5D; mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression), with three levels of severity (none 0, moderate 1, and severe 2). A higher score indicated better QOL (Shiroiwa et al., 2016; Tsuchiya et al., 2002).
2.6. Laboratory tests, echocardiography, and Holter electrocardiograms
The blood tests and echocardiography were performed at baseline and at the end of the 3‐month exercise program. The plasma B‐type natriuretic peptide (BNP) concentration was measured using a chemiluminescence enzyme immunoassay for human BNP (LLMIPULSE Presto, Fujirebio Inc.). A single echocardiographer who was blinded to the patients’ clinical information performed an offline echocardiographic analysis using Vivid 7 (GE Healthcare). We confirmed the absence of any significant tricuspid valve regurgitation due to the right ventricular lead. Echocardiographic atrioventricular and/or interventricular optimization were not performed during the study period. We recorded a 24 h Holter ECG (RAC‐2512, NIHON KOHDEN) equipped with single‐axis accelerometer to ensure the chronotropic incompetence before this study.
2.7. Outcome measures
The safety endpoint of our exercise program was hospitalizations due to worsening heart failure, and all‐cause mortality over the 3‐month period. We also examined the number of ventricular tachyarrhythmias, and therapies delivered for patients with CRT‐Ds (shocks and antitachycardia pacing). The data were collected by the RM and in‐office follow‐up. Absence of hospitalizations and emergency room visits were verified using the hospital records. The feasibility of the exercise program was determined by the QOL, 6MWD, and ACT measured by the CRT, BNP, LVEF, body weight, heart rate (HR) during nighttime hours (from 1 a.m. to 5 a.m.), average of a 5‐min standard deviation of the normal–normal R‐R period (SDANN), and right ventricular lead impedance between the baseline and 3 months after the exercise program. The patients were followed for 1 year after termination of the exercise program. The study members adjudicated all outcomes.
2.8. Statistical analysis
The baseline variables are presented as the number and frequency or mean ± standard deviation (SD) values, or median and interquartile range. The time‐dependent changes were tested with a paired sample Student's t‐test, and the mean differences were calculated with 95% confidence intervals (CI). The standardized mean differences (Hedges’ g) were also calculated to measure the effect size. A two‐tailed p‐value of <.05 was considered significant. The analyses were performed using JMP version 15 (SAS Institute, Inc.) and R project (Vienna, Austria) software.
3. RESULTS
During the recruitment period, 125 consecutive patients were assessed for their enrollment eligibility. We excluded 107 patients because of predetermined criteria. The baseline characteristics of the remaining 18 patients comprising the study cohort are shown in Table 1. The median age of the patients was 65 (range, 60–72) years, with two (11%) patients older than 80 years. The mean BNP and LVEF were 168 ± 111 pg/ml and 38 ± 12%, respectively. The CRTs were implanted at a median of 2.2 years before this study and 6 (33%) patients were CRT responders.
TABLE 1.
Baseline characteristics of the patients
| Overall (n = 18) | |
|---|---|
| Age, years | 66 ± 10 |
| 65 [60–72] | |
| Male, (n) % | 13 (72) |
| Body weight (kg) | 69 ± 14 |
| Systolic blood pressure (mmHg) | 122 ± 13 |
| Diastolic blood pressure (mmHg) | 75 ± 11 |
| Comorbidities, n (%) | |
| Hypertension | 9 (50) |
| Diabetes | 6 (33) |
| Dyslipidemia | 10 (56) |
| Ischemic cardiomyopathy | 6 (33) |
| Non‐ischemic cardiomyopathy | 12 (67) |
| BNP (pg/ml) | 168 ± 111 |
| 146 [87–228] | |
| LVEF at study enrollment (%) | 38 ± 12 |
| 6MWD (m) | 437 ± 67 |
| <400 m / ≥400 m, n (%) | 5 (28) / 13 (72) |
| Number of steps | 678 ± 88 |
| Stride (cm) | 66 ± 7 |
| Number of steps at baseline (/day) | 4210 ± 2312 |
| 4119 [2477–4408] | |
| CRT device | |
| Primary prevention/secondary prevention, n (%) | 12 (67) / 6 (33) |
| CRT‐P/CRT‐D, n (%) | 2 (11) / 16 (89) |
| Interval between the CRT implantation and rehabilitation (years) | 3.2 ± 1.4 |
| 2.2 [1.7–4.6] | |
| Responder, n (%) | 6 (33) |
| Patient activity (%/day) | 9.7 ± 4.9 |
| LV pacing rate (%) | 95 ± 3 |
| RV lead impedance (Ω) | 79 ± 10 |
| Night HR (/min) | 65 ± 5 |
| SDANN (ms) | 64 ± 16 |
| EQ−5D score a | |
| Utility score | 0.91 ± 0.10 |
| Mobility | 0.96 ± 0.03 |
| Self‐care | 0.98 ± 0.02 |
| Usual activities | 0.99 ± 0.01 |
| Pain/discomfort | 0.96 ± 0.04 |
| Anxiety/depression | 0.98 ± 0.02 |
| Medications, n (%) | |
| Beta‐blocker | 17 (94) |
| ACE inhibitor or ARB | 18 (100) |
| Loop diuretics | 16 (89) |
| Mineralocorticoid receptor antagonists | 12 (67) |
| Digitalis | 3 (17) |
| Amiodarone | 4 (22) |
| Antiplatelet agents | 8 (44) |
| Statin | 10 (56) |
Data represent number, frequency, means ± standard deviation (SD), or median and interquartile range.
Abbreviations: 6MWD, 6‐min walk distance; ACE, angiotensin converting enzyme; ARB, angiotensin II type 1 receptor blocker; BNP, B‐type natriuretic peptide; CRT, cardiac resynchronization therapy; CRT‐D, CRT with defibrillator; CRT‐P, CRT with pacemaker; EQ‐5D, EuroQol Research Foundation survey instrument for measuring the self‐reported health status in five dimensions; HR, heart rate; LV, left ventricular; LVEF, left ventricular ejection fraction; RV, right ventricular; SDANN, average of 5 min of the standard deviation of the normal–normal R‐R period (P‐P interval variability in Biotronik).
For the EQ‐5D Japan‐weighted index, a score of 1 indicates no problems for mobility, self‐care, usual activities, pain/discomfort, or depression/anxiety, whereas a score of 0 indicates a state as bad as death.
3.1. Safety
Death or the need for hospitalizations due to heart failure or arrhythmias did not occur during the 1‐year follow‐up. One patient showed signs of worsening heart failure, as indicated by a gradual decrease in the lead impedance, ACT, and SDANN associated with exceeding the prescribed exercise dose (Supplementary File 3). Heart failure hospitalizations were prevented by suspending the exercise and prescribing diuretics for 5 days. The patients recovered, resumed exercise, and completed the 3‐month program. At the 1‐year follow‐up after the end of the program, 5 (28%) patients were voluntarily continuing their walking exercise 3 times per week.
3.2. Feasibility
The median number of prescribed steps per session was 3,300 (range, 3,000–3,700). We found that 15 (83%) patients walked 3 times per week and 2 (11%) walked 5 times per week during the study period. The remaining one patient walked twice per week. Table 2 shows the changes in the parameters measured between baseline and 3 months after the exercise training. There was a significant improvement in the Δ EQ‐5D. Of the five domains in the EQ‐5D, the mobility domain had a significant improvement, but the remaining four domains did not. Significant changes were observed in the Δ ACT, suggesting that the activity time had increased by an average of 14 min per day. There were significant changes in the Δ 6MWD, Δ number of steps, Δ weight, and ΔSDANN. The standardized mean differences and Hedges’ g are shown in Figure 2. It shows that the Δ number of steps had a moderate difference. The utility score and mobility domain of the Δ EQ‐5D had a small‐to‐moderate difference, and the Δ ACT, Δ 6MWD, Δ Body weight, Δ Night HR, and Δ SDANN had a small difference.
TABLE 2.
Between‐group differences in parameters
| Difference | 95% CI | p‐value | |
|---|---|---|---|
| EQ−5D | |||
| Utility score | –0.037 | –0.071 to – 0.003 | <.05 |
| Mobility | –0.017 | –0.033 to – 0.001 | <.05 |
| Self‐care | –0.002 | –0.009 to 0.003 | .33 |
| Usual activities | –0.002 | –0.006 to 0.002 | .33 |
| Pain/discomfort | –0.009 | –0.022 to 0.004 | .16 |
| Anxiety/depression | 0.003 | –0.009 to 0.016 | .57 |
| ACT (%/day) | 1.12 | 0.07 to 2.18 | <.05 |
| 6MWD (m) | 11.0 | 6.51 to 15.5 | <.001 |
| Number of steps (/day) | 1663 | 710 to 2616 | <.01 |
| Body weight (kg) | –1.4 | –2.3 to – 0.5 | <.05 |
| BNP (pg/ml) | –32.4 | –67.9 to 3.0 | .07 |
| LVEF (%) | 0.28 | –1.23 to 0.68 | .55 |
| Night HR (beat/min) | –1.4 | –0.17 to – 2.36 | <.05 |
| SDANN (ms) | 3.5 | 0.52 to 6.12 | <.05 |
| RV lead impedance (Ω) | 5.9 | –15.6 to 3.6 | .20 |
The other abbreviations are as in Table 1.
Abbreviation: CI, confidence interval.
FIGURE 2.
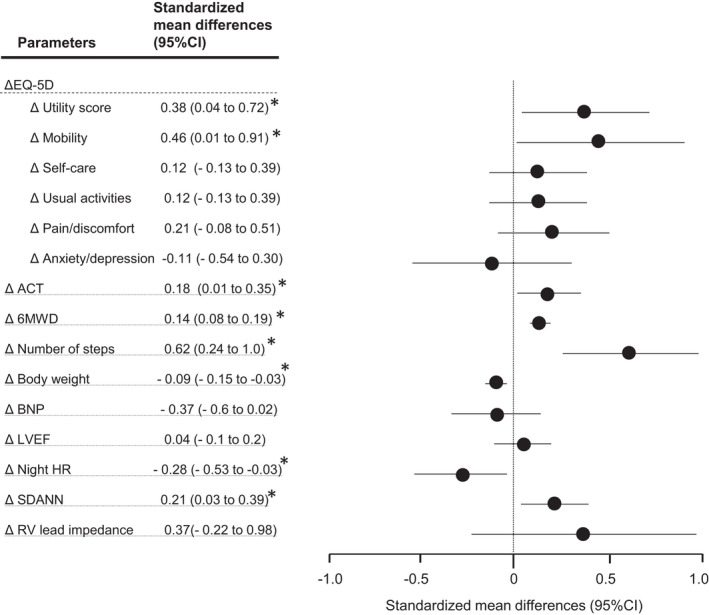
Standardized mean differences in the outcome parameters at the 3‐month follow‐up. The Hedges’ g and 95% confidence intervals are shown. A standardized mean difference of 0.20 was considered a small difference between the groups; 0.50, a moderate difference; and 0.80, a large difference. * p < .05. The abbreviations are as in Table 1
3.3. Arrhythmic events
No patients developed any ventricular tachyarrhythmias requiring defibrillator therapy. Three patients had a total of 10 asymptomatic paroxysmal atrial fibrillation episodes lasting <6 min.
4. DISCUSSION
In this pilot study, we showed that 3 months of telerehabilitation improved the QOL, ACT, and 6MWD, and resulted in an increased SDANN and weight loss. No patient developed any ventricular tachyarrhythmias. One patient had signs of worsening heart failure that were detected by RM and were promptly and successfully treated with suspension of exercise and diuretics. No adverse events occurred during 1 year of follow‐up.
Cardiac rehabilitation has been proven to improve the exercise capacity, cardiac function, QOL, and prognosis in patients with various cardiac diseases (Piepoli et al., 2010). An increasing number of heart failure patients referred to cardiac rehabilitation centers have CRT devices. However, doctors are often reluctant to prescribe exercise for CRT patients because of the potential for the exercise to cause ventricular arrhythmias and hospitalizations due to heart failure. In the current study, all 18 patients were able to complete the 3‐month walking exercise program. One patient demonstrated signs of worsening heart failure due to overtraining and had to stop exercising for 5 days.
To date, several studies have examined the safety and efficacy of exercise rehabilitation in patients with heart failure and CRT (Belardinelli et al., 2006; Conraads et al., 2007; Nobre et al., 2016; Patwala et al., 2009; Santa‐Clara et al., 2019; Smolis‐Bak et al., 2015; Zeitler et al., 2015). A recent meta‐analysis by Ye et al. (7 randomized controlled studies with 661 patients) showed that exercise rehabilitation in patients with CRT may improve the exercise capacity and cardiac function as well as the QOL (Ye et al., 2020). These results were consistent with our study. We further found a stabilization of the autonomic function evidenced by an increase in the SDANN. In the meta‐analysis by Ye et al., all seven cardiac rehabilitation programs were initiated shortly after the CRT implantation. Therefore, the positive impact on the cardiopulmonary fitness and health outcomes seen in those studies could be attributed to a combination of resynchronization and exercise therapy (Ye et al., 2020). In the current study, all patients had received CRTs at a median of 2.2 years prior, separating the beneficial effects that could result from the walking exercise and heart failure education beyond the improvements brought on by the CRT.
Cardiac telerehabilitation was devised in an attempt to increase the participation rates and adherence. A recent scientific statement comparing center‐based and home‐based cardiac rehabilitation studies in patients with a range of cardiac diseases showed that there was no statistically significant difference in the changes in the QOL and mortality for up to 12 months after the intervention (Thomas et al., 2019). However, most of those studies included low‐risk patients after a myocardial infarction without any complications and very few studies examined the safety and efficacy of a center‐based and home‐based cardiac rehabilitation in high‐risk patients such as transplant patients.
We did not determine the training heart rate using the Karvonen formula (Karvonen et al., 1957) since the Karvonen formula calculated that 11 of 16 (69%) patients had more than the upper tracking rate or programmed zones for defibrillation. To prevent cardioversion or shock therapies by the walking exercise, we confirmed that the maximum heart rate during the 6‐min walk test was less than the upper tracking rate and programmed zones for defibrillation in each patient. We did not monitor the heart rate during the walking exercise, but we monitored the maximum heart rate through the daily remote monitoring report.
In the current study, the patients self‐monitored their step count and exercise intensity with an accelerometer, and we could estimate it using the RM system. This cooperative monitoring by both the patient and physician may ensure the safety and be reassuring to both parties. The cardiac electronic devices continuously monitor various physiological parameters via built‐in sensors, which may result in early notification of abnormal events. However, a daily review of the RM is a burden for the medical staff. Boehmer et al. recently proposed a novel heart failure alert system (HeartLogic) that combines the heart sounds, respirations, thoracic impedance, heart rate, and activity, which predicts impending worsening heart failure events with a 70% sensitivity (Boehmer et al., 2017). Such efficient algorithms may help reduce heart failure hospitalizations in patients with CRT by proactively alerting them to signs of an exacerbation of their heart failure.
Previous home‐based cardiac rehabilitation programs involved walking programs supported by telephone calls by medical staff. Recent studies have adopted heart rate monitors and smartphones with special applications designed for research (O'Connor et al., 2009; Snoek et al., 2020). In a recent study, Kikuchi et al. reported a real‐time home‐based cardiac rehabilitation session monitored by a video conferencing program installed in a tablet. After assessing the patient's condition via oral history taking, a study coordinator observes the electrocardiogram and vital signs during ergometer exercise (Kikuchi et al., 2021). The current COVID‐19 pandemic has forced many center‐based cardiac rehabilitation programs to end or limit their regular offerings. IoT technology and cloud computing tools have the potential to transform cardiac rehabilitation from a center‐based to home‐based telerehabilitation.
4.1. Study limitations
The findings of this study were limited by the lack of a control group and short duration of follow‐up. The sample size was small because only two CRT vendors were suitable for this study and less than 15% of our CRT patients could be enrolled. Furthermore, this study was a pilot study to assess the safety and feasibility of the walking exercise and calculate the statistical power for planning a randomized control study. While some patients took drugs that may have affected the SDANN, we did not change the pharmacological therapy during the study period. In this study, we performed exercise training only and not endurance or resistance respiratory training. This study was conducted with patients of the Japanese race only. The RM systems of Biotronik and Boston Scientific cannot assess the ST‐T changes.
5. CONCLUSION
Our study showed that a 3‐month walking exercise program for CRT patients was safe and feasible, and walking exercise improved the QOL, physical activity, and exercise capacity without inducing any ventricular tachyarrhythmias or heart failure hospitalizations. This walking exercise‐based telerehabilitation program can be implemented in outpatients receiving CRTs.
CONFLICT OF INTEREST
Dr. Harada has received lecture fees from Daiichi‐Sankyo. Dr. Kiyono has received research funding from Kurabo Industries Ltd. Dr. Watanabe has received lecture fees from Daiichi‐Sankyo. Ms. Koike, Dr. Sobue, Dr. Kawai, Dr. Yamamoto, and Ms. Ban have no COI.
AUTHOR CONTRIBUTIONS
All authors contributed to the study conception and design. The material preparation and data collection and analysis were performed by Asami Koike, Yoshihiro Sobue, Mayumi Kawai, Masaru Yamamoto, Yukina Banno, Mashide Harada, Ken Kiyono, and Eiichi Watanabe. The first draft of the manuscript was written by Eiichi Watanabe and all authors commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
ETHICAL APPROVAL
The study was approved by the ethics committee of the Fujita Health University School of Medicine (approval number HM17‐263) and complied with the Declaration of Helsinki. Written informed consent was provided by all patients prior to the procedure.
Supporting information
Supplementary Material
ACKNOWLEDGMENT
The authors thank the representatives of Biotronik and Boston Scientific for supporting the pilot study.
Koike, A. , Sobue, Y. , Kawai, M. , Yamamoto, M. , Banno, Y. , Harada, M. , Kiyono, K. , & Watanabe, E. (2022). Safety and feasibility of a telemonitoring‐guided exercise program in patients receiving cardiac resynchronization therapy. Annals of Noninvasive Electrocardiology, 27, e12926. 10.1111/anec.12926
Funding information
This research was funded by Grants‐in‐Aid for Scientific Research from Japan Society for the Promotion of Science (Grant Number 21K08140) and Japan Agency for Medical Research and Development (Grant Number 20hk0102071h0001)
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- Arena, R. , Myers, J. , Williams, M. A. , Gulati, M. , Kligfield, P. , Balady, G. J. , Collins, E. , & Fletcher, G. (2007). Assessment of functional capacity in clinical and research settings: A scientific statement from the American heart association committee on exercise, rehabilitation, and prevention of the council on clinical cardiology and the council on cardiovascular nursing. Circulation, 116(3), 329–343. 10.1161/CIRCULATIONAHA.106.184461 [DOI] [PubMed] [Google Scholar]
- Belardinelli, R. , Capestro, F. , Misiani, A. , Scipione, P. , & Georgiou, D. (2006). Moderate exercise training improves functional capacity, quality of life, and endothelium‐dependent vasodilation in chronic heart failure patients with implantable cardioverter defibrillators and cardiac resynchronization therapy. European Journal of Cardiovascular Prevention and Rehabilitation, 13(5), 818–825. 10.1097/01.hjr.0000230104.93771.7d [DOI] [PubMed] [Google Scholar]
- Boehmer, J. P. , Hariharan, R. , Devecchi, F. G. , Smith, A. L. , Molon, G. , Capucci, A. , An, Q. , Averina, V. , Stolen, C. M. , Thakur, P. H. , Thompson, J. A. , Wariar, R. , Zhang, Y. , & Singh, J. P. (2017). A multisensor algorithm predicts heart failure events in patients with implanted devices: Results from the MultiSENSE Study. JACC Heart Fail, 5(3), 216–225. 10.1016/j.jchf.2016.12.011 [DOI] [PubMed] [Google Scholar]
- Conraads, V. M. , Vanderheyden, M. , Paelinck, B. , Verstreken, S. , Blankoff, I. , Miljoen, H. , De Sutter, J. , & Beckers, P. (2007). The effect of endurance training on exercise capacity following cardiac resynchronization therapy in chronic heart failure patients: A pilot trial. European Journal of Cardiovascular Prevention and Rehabilitation, 14(1), 99–106. 10.1097/HJR.0b013e32801164b3 [DOI] [PubMed] [Google Scholar]
- Karvonen, M. J. , Kentala, E. , & Mustala, O. (1957). The effects of training on heart rate; A longitudinal study. Annales Medicinae Experimentalis Et Biologiae Fenniae, 35(3), 307–315. https://www.ncbi.nlm.nih.gov/pubmed/13470504 [PubMed] [Google Scholar]
- Kikuchi, A. , Taniguchi, T. , Nakamoto, K. , Sera, F. , Ohtani, T. , Yamada, T. , & Sakata, Y. (2021). Feasibility of home‐based cardiac rehabilitation using an integrated telerehabilitation platform in elderly patients with heart failure: A pilot study. Journal of Cardiology, 78(1), 66–71. 10.1016/j.jjcc.2021.01.010 [DOI] [PubMed] [Google Scholar]
- Kumahara, H. , Schutz, Y. , Ayabe, M. , Yoshioka, M. , Yoshitake, Y. , Shindo, M. , Ishii, K. , & Tanaka, H. (2004). The use of uniaxial accelerometry for the assessment of physical‐activity‐related energy expenditure: A validation study against whole‐body indirect calorimetry. British Journal of Nutrition, 91(2), 235–243. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14756909 [DOI] [PubMed] [Google Scholar]
- Lau, E. T. , Thompson, E. A. , Burr, R. L. , & Dougherty, C. M. (2016). Safety and efficacy of an early home‐based walking program after receipt of an initial implantable cardioverter‐defibrillator. Archives of Physical Medicine and Rehabilitation, 97(8), 1228–1236. 10.1016/j.apmr.2016.02.007 [DOI] [PubMed] [Google Scholar]
- Leclercq, C. , Burri, H. , Curnis, A. , Delnoy, P. P. , Rinaldi, C. A. , Sperzel, J. , Lee, K. , Calo, L. , Vicentini, A. , Concha, J. F. , & Thibault, B. (2019). Cardiac resynchronization therapy non‐responder to responder conversion rate in the more response to cardiac resynchronization therapy with MultiPoint Pacing (MORE‐CRT MPP) study: Results from Phase I. European Heart Journal, 40(35), 2979–2987. 10.1093/eurheartj/ehz109 [DOI] [PubMed] [Google Scholar]
- Nobre, T. S. , Antunes‐Correa, L. M. , Groehs, R. V. , Alves, M. J. , Sarmento, A. O. , Bacurau, A. V. , Urias, U. , Alves, G. B. , Rondon, M. U. , Brum, P. C. , Martinelli, M. , Middlekauff, H. R. , & Negrao, C. E. (2016). Exercise training improves neurovascular control and calcium cycling gene expression in patients with heart failure with cardiac resynchronization therapy. American Journal of Physiology‐Heart and Circulatory Physiology, 311(5), H1180–H1188. 10.1152/ajpheart.00275.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor, C. M. , Whellan, D. J. , Lee, K. L. , Keteyian, S. J. , Cooper, L. S. , Ellis, S. J. , Leifer, E. S. , Kraus, W. E. , Kitzman, D. W. , Blumenthal, J. A. , Rendall, D. S. , Miller, N. H. , Fleg, J. L. , Schulman, K. A. , McKelvie, R. S. , Zannad, F. , Pina, I. L. , & HF‐ACTION Investigators . (2009). Efficacy and safety of exercise training in patients with chronic heart failure: HF‐ACTION randomized controlled trial. [Multicenter Study Randomized Controlled Trial Research Support, N.I.H., Extramural]. JAMA, 301(14), 1439. 10.1001/jama.2009.454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwala, A. Y. , Woods, P. R. , Sharp, L. , Goldspink, D. F. , Tan, L. B. , & Wright, D. J. (2009). Maximizing patient benefit from cardiac resynchronization therapy with the addition of structured exercise training: A randomized controlled study. Journal of the American College of Cardiology, 53(25), 2332–2339. 10.1016/j.jacc.2009.02.063 [DOI] [PubMed] [Google Scholar]
- Pedretti, R. F. E. , Iliou, M. C. , Israel, C. W. , Abreu, A. , Miljoen, H. , Corra, U. , Stellbrink, C. , Gevaert, A. B. , Theuns, D. A. , Piepoli, M. F. , Reibis, R. , Schmid, J. P. , Wilhelm, M. , Heidbuchel, H. , & Voller, H. (2021). Comprehensive multicomponent cardiac rehabilitation in cardiac implantable electronic devices recipients: A consensus document from the European Association of Preventive Cardiology (EAPC; Secondary prevention and rehabilitation section) and European Heart Rhythm Association (EHRA). Europace: European Pacing, Arrhythmias, and Cardiac Electrophysiology, 23(9), 1336–1337o. 10.1093/europace/euaa427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepoli, M. F. , Corra, U. , Benzer, W. , Bjarnason‐Wehrens, B. , Dendale, P. , Gaita, D. , McGee, H. , Mendes, M. , Niebauer, J. , Zwisler, A. D. , & Schmid, J. P. (2010). Secondary prevention through cardiac rehabilitation: From knowledge to implementation. A position paper from the cardiac rehabilitation section of the european association of cardiovascular prevention and rehabilitation. European Journal of Cardiovascular Prevention and Rehabilitation, 17(1), 1–17. 10.1097/HJR.0b013e3283313592 [DOI] [PubMed] [Google Scholar]
- Piepoli, M. F. , Hoes, A. W. , Agewall, S. , Albus, C. , Brotons, C. , Catapano, A. L. , Cooney, M. T. , Corra, U. , Cosyns, B. , Deaton, C. , Graham, I. , Hall, M. S. , Hobbs, F. D. R. , Lochen, M. L. , Lollgen, H. , Marques‐Vidal, P. , Perk, J. , Prescott, E. , Redon, J. , … Binno, S. (2016). 2016 European guidelines on cardiovascular disease prevention in clinical practice: The sixth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). European Heart Journal, 37(29), 2315‐2381. 10.1093/eurheartj/ehw106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponikowski, P. , Anker, S. D. , AlHabib, K. F. , Cowie, M. R. , Force, T. L. , Hu, S. , Jaarsma, T. , Krum, H. , Rastogi, V. , Rohde, L. E. , Samal, U. C. , Shimokawa, H. , Budi Siswanto, B. , Sliwa, K. , & Filippatos, G. E. S. C. (2014). Heart failure: Preventing disease and death worldwide. Heart Fail, 1(1), 4–25. 10.1002/ehf2.12005 [DOI] [PubMed] [Google Scholar]
- Santa‐Clara, H. , Abreu, A. , Melo, X. , Santos, V. , Cunha, P. , Oliveira, M. , Pinto, R. , Carmo, M. M. , & Fernhall, B. (2019). High‐intensity interval training in cardiac resynchronization therapy: A randomized control trial. European Journal of Applied Physiology, 119(8), 1757–1767. 10.1007/s00421-019-04165-y [DOI] [PubMed] [Google Scholar]
- Schwartz, A. L. (2004). Physical activity after a cancer diagnosis: Psychosocial outcomes. Cancer Investigation, 22(1), 82–92. 10.1081/CNV-120027582 [DOI] [PubMed] [Google Scholar]
- Shiroiwa, T. , Fukuda, T. , Ikeda, S. , Igarashi, A. , Noto, S. , Saito, S. , & Shimozuma, K. (2016). Japanese population norms for preference‐based measures: EQ‐5D‐3L, EQ‐5D‐5L, and SF‐6D. Quality of Life Research, 25(3), 707–719. 10.1007/s11136-015-1108-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotwiner, D. , Varma, N. , Akar, J. G. , Annas, G. , Beardsall, M. , Fogel, R. I. , Galizio, N. O. , Glotzer, T. V. , Leahy, R. A. , Love, C. J. , McLean, R. C. , Mittal, S. , Morichelli, L. , Patton, K. K. , Raitt, M. H. , Ricci, R. P. , Rickard, J. , Schoenfeld, M. H. , Serwer, G. A. , … Yu, C. M. (2015). HRS expert consensus statement on remote interrogation and monitoring for cardiovascular implantable electronic devices. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 12(7), e69‐e100. 10.1016/j.hrthm.2015.05.008 [DOI] [PubMed] [Google Scholar]
- Smolis‐Bak, E. , Dabrowski, R. , Piotrowicz, E. , Chwyczko, T. , Dobraszkiewicz‐Wasilewska, B. , Kowalik, I. , Kazimierska, B. , Jedrzejczyk, B. , Smolis, R. , Gepner, K. , Maciag, A. , Sterlinski, M. , & Szwed, H. (2015). Hospital‐based and telemonitoring guided home‐based training programs: Effects on exercise tolerance and quality of life in patients with heart failure (NYHA class III) and cardiac resynchronization therapy. A randomized, prospective observation. International Journal of Cardiology, 199, 442–447. 10.1016/j.ijcard.2015.07.041 [DOI] [PubMed] [Google Scholar]
- Snoek, J. A. , Prescott, E. I. , van der Velde, A. E. , Eijsvogels, T. M. H. , Mikkelsen, N. , Prins, L. F. , Bruins, W. , Meindersma, E. , Gonzalez‐Juanatey, J. R. , Pena‐Gil, C. , Gonzalez‐Salvado, V. , Moatemri, F. , Iliou, M. C. , Marcin, T. , Eser, P. , Wilhelm, M. , Van't Hof, A. W. J. , & de Kluiver, E. P. (2020). Effectiveness of home‐based Mobile guided cardiac rehabilitation as alternative strategy for nonparticipation in clinic‐based cardiac rehabilitation among elderly patients in Europe: A randomized clinical trial. JAMA Cardiology, 6(4), 463. 10.1001/jamacardio.2020.5218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, R. J. , Beatty, A. L. , Beckie, T. M. , Brewer, L. C. , Brown, T. M. , Forman, D. E. , Franklin, B. A. , Keteyian, S. J. , Kitzman, D. W. , Regensteiner, J. G. , Sanderson, B. K. , & Whooley, M. A. (2019). Home‐based cardiac rehabilitation: A scientific statement from the American association of cardiovascular and pulmonary rehabilitation, the American heart association, and the American College of cardiology. Journal of the American College of Cardiology, 74(1), 133–153. 10.1016/j.jacc.2019.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy, C. M. , Epstein, A. E. , Darbar, D. , DiMarco, J. P. , Dunbar, S. B. , Estes, N. A. 3rd , Ferguson, T. B. Jr , Hammill, S. C. , Karasik, P. E. , Link, M. S. , Marine, J. E. , Schoenfeld, M. H. , Shanker, A. J. , Silka, M. J. , Stevenson, L. W. , Stevenson, W. G. , Varosy, P. D. , Ellenbogen, K. A. , Freedman, R. A. , … Heart Rhythm Society . (2012). 2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device‐based therapy of cardiac rhythm abnormalities: A report of the American College of cardiology foundation/American Heart Association task force on practice guidelines and the heart rhythm society. [corrected]. Circulation, 126(14), 1784‐1800. 10.1161/CIR.0b013e3182618569 [DOI] [PubMed] [Google Scholar]
- Tsuchiya, A. , Ikeda, S. , Ikegami, N. , Nishimura, S. , Sakai, I. , Fukuda, T. , Hamashima, C. , Hisashige, A. , & Tamura, M. (2002). Estimating an EQ‐5D population value set: The case of Japan. Health Economics, 11(4), 341–353. 10.1002/hec.673 [DOI] [PubMed] [Google Scholar]
- Vanhees, L. , Kornaat, M. , Defoor, J. , Aufdemkampe, G. , Schepers, D. , Stevens, A. , Van Exel, H. , Van Den Beld, J. , Heidbuchel, H. , & Fagard, R. (2004). Effect of exercise training in patients with an implantable cardioverter defibrillator. European Heart Journal, 25(13), 1120‐1126. 10.1016/j.ehj.2004.04.034 [DOI] [PubMed] [Google Scholar]
- Varma, N. , Piccini, J. P. , Snell, J. , Fischer, A. , Dalal, N. , & Mittal, S. (2015). The relationship between level of adherence to automatic wireless remote monitoring and survival in pacemaker and defibrillator patients. Journal of the American College of Cardiology, 65(24), 2601–2610. 10.1016/j.jacc.2015.04.033 [DOI] [PubMed] [Google Scholar]
- Watanabe, E. , Kasai, A. , Fujii, E. , Yamashiro, K. , & Brugada, P. (2013). Reliability of implantable cardioverter defibrillator home monitoring in forecasting the need for regular office visits, and patient perspective. Japanese HOME‐ICD study. Circulation Journal, 77(11), 2704–2711. https://www.ncbi.nlm.nih.gov/pubmed/23903000 [DOI] [PubMed] [Google Scholar]
- Ye, L. F. , Wang, S. M. , & Wang, L. H. (2020). Efficacy and safety of exercise rehabilitation for heart failure patients with cardiac resynchronization therapy: A systematic review and meta‐analysis. Frontiers in Physiology, 11, 980. 10.3389/fphys.2020.00980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitler, E. P. , Piccini, J. P. , Hellkamp, A. S. , Whellan, D. J. , Jackson, K. P. , Ellis, S. J. , Kraus, W. E. , Keteyian, S. J. , Kitzman, D. W. , Ewald, G. A. , Fleg, J. L. , Piña, I. L. , & O'Connor, C. M. (2015). Exercise training and pacing status in patients with heart failure: Results from HF‐ACTION. Journal of Cardiac Failure, 21(1), 60–67. 10.1016/j.cardfail.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubin Maslov, P. , Schulman, A. , Lavie, C. J. , & Narula, J. (2018). Personalized exercise dose prescription. European Heart Journal, 39(25), 2346–2355. 10.1093/eurheartj/ehx686 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


