Abstract
Objective
Nociplastic concept incorporates a broad continuum of pain phenotypes shared with clinical peculiarity. This study aimed to develop and validate a diagnostic tool, the preliminary Nociplastic‐based Fibromyalgia Features (NFF), to detect fibromyalgia (FM) in patients with chronic pain.
Methods
Items requiring yes or no responses and relating to the most relevant clinical nociplastic pain (NP) features of FM were compiled by a group of expert rheumatologists. The provisional list was tested in a prospective study on 185 consecutive patients with chronic pain (126 patients with FM and 59 patients with non‐FM non‐inflammatory chronic pain) diagnosed based on expert decision. Identification of the most discriminant combinations of items for FM and the calculation of their sensitivity and specificity were based on both univariate and multivariate (stepwise logistic regression) analyses. All participants were investigated through the final NFF, the 2011 American College of Rheumatology (ACR) criteria, and the 2016 ACR criteria. NFF performance was assessed with receiver operating characteristic curve analysis.
Results
Based on multivariate analyses, we retained only seven items in the final version of the NFF. A cut‐off score of 4 (corresponding to the number of positive items) gave the highest rate of correct identification of patients (85%), with a sensitivity of 82% and a specificity of 91%. The NFF showed the highest concordance rate with expert diagnosis (85%) and the lowest value (77%) with the ACR 2016 criteria.
Conclusion
The preliminary NFF with respect to the various aspects of NP showed good performance for detection of the FM in the clinical setting. This tool may provide a more pragmatic approach to the timely diagnosis of FM.
SIGNIFICANCE & INNOVATIONS.
This is the first study to introduce and validate a preliminary diagnostic tool, the Nociplastic‐based Fibromyalgia Features (NFF), which is based on the nociplastic pain (NP) features of fibromyalgia (FM).
The NFF differs from the traditional symptoms‐driven FM criteria in that it focuses on the attributes of NP instead of counting symptoms and pain locations.
The high concordance rates of the NFF with expert diagnosis, the 2011 American College of Rheumatology (ACR) criteria, and the 2016 ACR criteria indicate good performance of the NFF.
The NFF may promise to help clinicians detect FM in its early course, when it is mild, and before generalization or worsening of pain or other symptoms.
INTRODUCTION
Over recent decades, there have been efforts to develop classification or diagnostic criteria for fibromyalgia (FM), a condition that could easily have escaped capture by diagnostic tools used by the clinicians and researchers (1, 2, 3, 4, 5). The difficulty in FM identification is related to the multiplicity and subjectivity of FM symptoms, the presence of concurrent comorbidities, and the prominent effects of social and cultural factors (5, 6, 7). The vast heterogeneity in “clinico‐social” presentations of FM seems to originate from multiplex genetico‐biological pathways coded in the context of environmental, physical, and emotional stressors (8, 9). However, despite the complex clinical and etiopathogenic domains, there are remarkable basic similarities in pain attributes in FM, which could be classified as a new pain taxonomy called nociplastic pain (NP) (10). The nociplastic concept denotes the pain arising from altered nociception, with no evidence of tissue or somatosensory damage causing the pain. This condition is prototypically acknowledged in FM in which pain originates from the central nervous system and systemic factors rather than from ongoing inflammation (nociceptive pain) or nerve damage (neuropathic pain) (11, 12).
Apart from nomenclature, the NP concept could propose a real gateway to recognizing FM in the clinical setting. From the perspective of clinical features, NP could be inferred indirectly from some clinical phenomena (11, 12, 13). This pain state is thought to be a single lifelong condition that merely tends to manifest in multiple different bodily regions and to various extents over time (4, 5, 14). Individuals with NP may have a history of migratory chronic pain and also several nonpainful manifestations such as fatigue, sleep, and mood disorders (8, 13, 14). Furthermore, amplification or modification of sensory inputs leads to generalized sensory hyperresponsiveness to physical and emotional stimuli. This was originally identified in FM by diffuse tenderness to palpation, hyperalgesia, and pain intensification following physical/emotional distress (1, 8, 13). Indeed, NP cannot be applied to patients reporting pain without hypersensitivity (11).
To date, all introduced criteria to define FM have been symptom driven (1, 2, 3, 4, 15). Accordingly, each set of existing criteria has focused on some clinical aspects of FM and identifies different subsets of patients with FM, thus resulting in modest agreement among them (16). Along with a growing need for early screening/diagnosis of patients with FM, a pragmatic conceptualized‐based diagnosis built upon the core NP concept instead of just symptoms‐based scoring criteria seems necessary. The nociplastic concept may provide a new opportunity for early diagnosis of FM through identification of key NP features and hence can preclude overemphaizing inconsistent individual patients’ complaints or symptoms. This study aimed to propose core clinical attributes of NP into a new preliminary diagnostic tool for FM diagnosis in clinical practice. We also sought to explore the performance of this new diagnostic set—called the Nociplastic‐based Fibromyalgia Features (NFF)—in the clinical setting and to compare the concordance of the NFF with expert diagnosis (ED) and internationally accepted sets of diagnostic criteria (the 2011 and 2016 American College of Rheumatology [ACR] criteria).
MATERIALS AND METHODS
Development of the preliminary NFF tool
In order to compile the initial list of core features that are representative of NP, the study advisory committee, composed of six rheumatologists experienced in FM recognition and clinical research, was formed. Based on their clinical experience and a search of the literature on nociplastic characteristics, the advisory committee reached a consensus on potential discriminatory items that may differentiate NP from other pain types, such as peripheral or nociceptive pain. The experts were asked to present their own set of nociplastic features. Each feature or question was discussed and finally voted on by the expert group. The 11 binary items with “Yes/No” responses were finalized to identify the nociplastic core features of FM. All items were related to problems within the last 3 months. The first two items of the NFF evaluated pain extent. These items were “Do you have pain all over the body?” and “Does the patient have any dominant localized pain?” Item 1 provided the pain extent from the patient perspective. Item 2 captured dominant localized pain from the physician's viewpoint, based on the body pain sites definition (4). We defined the specific noncontiguous anatomical body sites including left and right arm and leg, chest, abdomen, upper back, and lower back. The pain that is maximally focused over one or two regional noncontiguous pain sites was considered as the dominant localized pain. Item 2 ascertained the fact that patients with FM may complain of unexplained dominant localized pain despite having more diffuse pain (12). Furthermore, considering dominant localized pain will help incorporate the more limited pain areas in the NP classification, particularly in the early FM course before pain generalization. Item 3 evaluated the presence of migratory pain pattern: “Is your pain migratory or nonconsistent?” Regarding the importance of pain history in particular sites in the NP category, items 4 and 5 focused on axial pain (neck and lumbar) and headache as the common sites of experienced pain in the course of the disease. Item 6 assessed aggravation of pain with physical or emotional stress: “Is your pain intensified with physical stress (such as excessive physical activity or cold exposure) or emotional stress?” Item 7 was related to pain response to non‐opioid analgesia. This item refers to the prevailing idea that NP responds poorly to non‐opioid analgesics and anti‐inflammatory drugs (13, 17, 18). Item 8 assessed the affective component of pain perception: “Do you experience the pain with excruciating or suffering quality?” This question is based on the fact that the excruciating/unpleasant quality of pain is prevalent in FM patients and is a crucial part of somatic complaints in the majority of cases (9). Item 9 was related to the presence of disabling fatigue, especially in the morning. Item 10 assessed the perceived invalidation by patients stemming from social environments: “Do you experience the invalidation or misunderstanding about yourself or your condition by others?” “Invalidation”—which has been recently proposed as a differentiating feature in FM symptomatology—seems an embedded social part of NP (9, 19, 20). Finally, the last item focused on pain hypersensitivity: “Are there tender points (TPs) in the manual examination?” The advisory committee suggested the fewer but more discriminatory TPs (only two instead of nine pairs of TPs) as a semi‐objective marker in order to reintegrate hyperalgesia as an intuitional nociplastic feature in NFF. Assessment of TPs for the detection of hyperalgesia was confined to the four easily accessed areas, which are known as the most discriminative TPs for FM, including paired trapezius and supraspinatus areas (21, 22). For the sake of feasibility in clinical evaluation, we opted for manual examination as opposed to dolorimetric assessment to detect TPs, based on clinical research that has shown the comparable value of manual TP examination (23). According to advisory committee consensus, the presence of two or more TPs (out of 4 points) was considered a positive response.
Finally, the preliminary 11‐item NFF was considered for testing to identify the combination of items with the best discriminating value between nociplastic‐based FM pain and other non‐FM common pain disorders seen in everyday clinical practice.
Patient recruitment and diagnosis
The study was conducted at the academic rheumatology clinic of Razi General Hospital, affiliated with Guilan University of Medical Sciences (GUMS), from March 2019 to October 2019. Study participants were recruited consecutively by two of the research rheumatologists (BG‐H and AB); both had long‐term experience in the diagnosis and management of FM and chronic pain disorders. All‐adult patients between 18 and 65 years of age with common chronic pain disorders of at least 3 months’ pain duration were enrolled in this study. To avoid the gender confounding factor, only female individuals (composing the vast majority of patients) were recruited. The diagnoses of patients were made by the recruiting rheumatologists based on their clinical experience. Satisfying the ACR classification criteria was not a requirement for diagnosis, and like previous studies, the rheumatologists’ diagnosis of FM was considered the gold standard (reference). Furthermore, all participants were assessed according to the ACR 2011 (3) and ACR 2016 (15) criteria by two medical assistants jointly trained for this task.
Non‐FM control subjects were female patients with common non‐inflammatory painful disorders including hip or hand osteoarthritis, mechanical neck and back syndromes, tendonitis, and other painful periarticular conditions (such as lateral or medial epicondylitis, adhesive capsulitis, etc) who had no concurrent diagnosis of FM at the time of enrollment. Patients were excluded if they were under age 18 years or over age 65 years or if they had a known systemic inflammatory rheumatic disease (eg, rheumatoid arthritis), any cultural or educational barriers to cooperation, organic nonrheumatic causes of pain (such as malignancy, neuropathy, or other painful medical conditions).
Written informed consent was obtained from all the participants. The study was approved by the GUMS ethical committee (IR.GUMS.REC.1396.511) and was in compliance with the Helsinki Declaration.
Study questionnaires
We collected data from all patients on demographic features, disease duration, and also the widespread pain index and symptom severity score to determine whether patients met the ACR 2011 and 2016 criteria (3, 15).
We applied the Revised Fibromyalgia Impact Questionnaire (FIQR). The FIQR evaluated disease impact and severity and entailed 21 numeric rating individual questions, organized into three sets of domains: function, overall impact, and symptoms. The total FIQR score was the sum of the three modified domain scores (24).
Furthermore, all participants completed the Short‐Form Health Survey (SF‐12). This questionnaire included eight dimensions: physical functioning, physical role, social role, emotional role, bodily pain, general health, vitality, and mental health. Scores ranged from 0 to 100, and the lower scores indicated worse health status (25).
We also administered the Pain Visual Analog Scale to all participants. This scale ranged from 0, indicating no pain, to 10 denoting the worst pain possible.
Finally, participants were requested to complete the 11‐item NFF questionnaire.
The main questionnaires applied in this study, including the SF‐12, the FIQR, and the ACR 2011 criteria, had been translated and validated in the Persian language before (25, 26, 27).
Two medical assistants received training to check and record the TPs. They also supported participants by helping them complete study questionnaires.
Processing and validation of preliminary NFF
The 11‐item NFF was tested in our study population to assess validity and reliability as follows:
Face validity was evaluated based on rating the quality of patient‐related questions (item 1, items 3‐10) in terms of word clarity, concept, and clinical relevance as bad, moderate, or good. Content validity was assessed independently by four experts (who were not members of the study advisory committee) to rate (bad, moderate, good, or very good) each item of the NFF, evaluating items’ relevancy and clarity to the nociplastic concept.
Proportions of positive responses for each item were compared between the FM and non‐FM groups based on the method of FM diagnosis: either the entry (ED) or diagnostic criteria (ACR 2011 and 2016 criteria).
Based on the gold standard (the ED of FM), we determined the odds ratio (OR) and the significance of individual independent variables with logistic regression. Determining the best discriminatory subset of items, we used the stepwise logistic regression model. Each item of the NFF was given a score of 0 or 1 for each negative or positive response, and the total score was calculated as the sum of scores for the responses to all the items.
The sensitivity, specificity, positive predictive value, and negative predictive value were calculated for various cut‐off points of the total score of the NFF final version. The corresponding receiver operating characteristics (ROC) curve was plotted and the area under the curve (AUC) was calculated.
In addition, we determined the discriminative accuracy of the final NFF for distinguishing the nociplastic FM pain from non‐FM as compared with the different diagnostic methods. Thereby, the diagnostic performance of the final NFF was assessed according to ED, the ACR 2011, and the ACR 2016 criteria.
Statistical methods
Quantitative data were described in terms of their mean, standard deviation, and range. Qualitative variables were reported as the frequency and percentages. All statistical tests were two‐tailed, and P < 0.05 was considered significant.
To find the best subset of items for FM discrimination, we used the stepwise logistic regression analysis. The Akaike Information Criterion was used to select the “final best model.” The ROC curve analysis was used to find the best cut‐off point for the final NFF and also to explore the discriminative accuracy of the NFF when compared with ED, the ACR 2011, and the ACR 2016 criteria as the external references.
The internal consistency of the NFF was assessed by calculating Cronbach's alpha coefficient. Data were analyzed using Stata software version 12 (StataCorp, College Station, Texas).
RESULTS
In total, 185 patients were recruited for the study: 126 patients with FM and 59 patients with common chronic pain disorders (31 patients with osteoarthritis, 13 patients with tendonitis or painful periarticular conditions, 9 patients with mechanical low back pain, and 6 patients with mechanical neck pain). The baseline demographic characteristics are shown in Table 1.
TABLE 1.
Demographic and baseline characteristics of patients with FM and those without FM
| Non‐FM (n:59) | FM (n = 126) | P Value | |
|---|---|---|---|
| Age, mean ± SD | 47.5 ± 11.5 | 44.8 ± 9.9 | 0.1 |
| Marital status | |||
| Single | 4 (6.8%) | 24 (19%) | 0.14 |
| Married | 54 (91.5%) | 98 (77.8%) | |
| Divorced | 0 (0) | 1 (0.8%) | |
| Widow | 1 (1.7%) | 3 (2.4%) | |
| Literacy | |||
| Elementary | 3 (5.1%) | 4 (3.2%) | 0.73 |
| High school | 12 (20.3%) | 30 (23.8%) | |
| Diploma | 29 (49.2%) | 54 (42.9%) | |
| Academic | 15 (25.4%) | 38 (30.2%) | |
| Work status | |||
| Employed | 8 (13.5%) | 14 (11.1%) |
0.53 |
| Unemployed | 51 (86.5%) | 112 (88.9%) | |
| Time to diagnosis | |||
| Month | 16.93 ± 21.6 | 45.7 ± 62.2 | 0.001* |
| Year | 1.4 ± 1.7 | 3.7 ± 5.2 | 0.001* |
| Widespread pain index | 1.6 ± 1.5 | 6.9 ± 4 | 0.001* |
| Symptom severity score | 3.2 ± 2.1 | 6.3 ± 2.2 | 0.001* |
| Mental component summary | 53.2 ± 12.7 | 45.7 ± 15.2 | 0.001* |
| Physical component summary | 43.1 ± 11.2 | 36.4 ± 10.3 | 0.001* |
| Total score of FIQR | 21.5 ± 15.2 | 51.9 ± 20.3 | 0.001* |
| Pain VAS | 4.7 ± 2.9 | 7.6 ± 1.8 | 0.001* |
P < 0.01.
Abbreviations: FIQR, Revised Fibromyalgia Impact Questionnaire; FM, fibromyalgia; SD, standard deviation; VAS, Visual Analogue Scale.
The word clarity and clinical relevance of patient‐related questions in the NFF were reported to be of “good” or “very good” quality by 95% of the patients.
In the assessment of content validity of the NFF items, the 10th item (invalidation) was considered to be less relevant to the NP by two experts. One expert considered the 7th item (pain response to nonopioid analgesics) was less relevant to discriminate between NP and other types of pain. The experts concluded independently that other NFF items were representative of the nociplastic features of FM.
Proportions of positive responses in each item in patients with FM and those without FM were compared with different diagnostic external criteria (ED, the ACR 2011, and the ACR 2016 criteria) (Table 2).
TABLE 2.
Proportions of positive and negative responses in the studied items between patients with FM and those without FM based on the three diagnostic external criterions (expert diagnosis, ACR 2011 criteria, and ACR 2016 criteria)
| Expert Diagnosis | 2011 | 2016 | ||||||
|---|---|---|---|---|---|---|---|---|
| Non‐FM | FM | Non‐FM | FM | Non‐FM | FM | |||
| Item 1 | Pain all over the body | No | 57 (96.6) | 17 (13.5) | 54 (68.4) | 20 (18.9) | 63 (66.3) | 11 (12.33) |
| Yes | 2 (3.4) | 109 (86.5) | 25 (31.6) | 86 (81.1) | 32 (33.7) | 79 (87.8) | ||
| P value | 0.0001* | 0.001* | 0.001* | |||||
| Item 2 | Dominant localized pain | No | 3 (5.1) | 32 (25.4) | 9 (11.4) | 26 (24.5) | 12 (12.6) | 23 (25.6) |
| Yes | 56 (94.9) | 94 (74.6) | 70 (88.6) | 80 (75.5) | 83 (87.4) | 67 (74.4) | ||
| P value | 0.001* | 0.002* | 0.02 | |||||
| Item 3 | Migratory pattern of pain | No | 50 (84.7) | 61 (48.4) | 58 (73.4) | 53 (50) | 64 (67.4) | 47 (53.2) |
| Yes | 9 (15.3) | 65 (51.6) | 21 (26.6) | 52 (50) | 31 (32.7) | 43 (47.8) | ||
| P value | 0.0001* | 0.001* | 0.03 | |||||
| Item 4 | Axial pain | No | 44 (74.6) | 36 (38.6) | 58 (73.4) | 22 (20.8) | 63 (66.3) | 17 (18.9) |
| Yes | 15 (25.4) | 90 (71.4) | 21 (26.6) | 84 (79.2) | 32 (33.7) | 73 (81.1) | ||
| P value | 0.001* | 0.001* | 0.001* | |||||
| Item 5 | Headache | No | 48 (81.4) | 75 (59.5) | 65 (82.3) | 58 (54.7) | 76 (80) | 47 (52.2) |
| Yes | 11 (18.6) | 51 (40.5) | 14 (17.7) | 48 (45.3) | 19 (20) | 43 (47.8) | ||
| P value | 0.003* | 0.001* | 0.001* | |||||
| Item 6 | Pain aggravation with the physical or emotional stress | No | 42 (71.2) | 27 (21.4) | 48 (60.8) | 21 (19.8) | 53 (55.8) | 16 (17.8) |
| Yes | 17 (28.8) | 99 (78.6) | 31 (39.2) | 85 (80.2) | 42 (44.22) | 74 (82.3) | ||
| P value | 0.001* | 0.001* | 0.001* | |||||
| Item 7 | Pain respond to analgesia | No | 55 (93.2) | 99 (78.6) | 73 (92.4) | 81 (73.4) | 82 (90.5) | 68 (75.5) |
| Yes | 4 (6.8) | 27 (26.4) | 6 (7.6) | 25 (23.6) | 9 (9.5) | 22 (24.4) | ||
| P value | 0.01 | 0.004* | 0.006* | |||||
| Item 8 | Excruciated pain quality | No | 43 (72.9) | 30 (23.7) | 54 (68.4) | 19 (17.9) | 57 (60) | 16 (17.8) |
| Yes | 16 (27.1) | 96 (76.1) | 25 (31.6) | 87 (83.1) | 38 (40) | 74 (82.2) | ||
| P value | 0.001* | 0.001* | 0.001* | |||||
| Item 9 | Fatigue (especially in morning) | No | 47 (79.7) | 32 (25.4) | 57 (74.4) | 22 (30.7) | 62 (65.3) | 17 (18.9) |
| Yes | 12 (20.3) | 94 (24.6) | 23 (27.8) | 84 (79.2) | 33 (34.7) | 73 (81.1) | ||
| P value | 0.001* | 0.001* | 0.001* | |||||
| Item 10 | Invalidation | No | 52 (88) | 94 (74.9) | 71 (89.9) | 75 (70.8) | 85 (89.5) | 61 (67.8) |
| Yes | 7 (11.5) | 32 (25.4) | 8 (10.1) | 31 (29.2) | 10 (10.5) | 29 (32.2) | ||
| P value | 0.03 | 0.002* | 0.001* | |||||
| Item 11 | TPs | No | 42 (71.3) | 32 (25.4) | 45 (57) | 29 (28.4) | 51 (53.7) | 23 (25.6) |
| Yes | 17 (28.8) | 94 (74.6) | 34 (43) | 77 (72.6) | 44 (46.3) | 67 (74.4) | ||
| P value | 0.001* | 0.001* | 0.001* | |||||
P < 0.01.
Chi‐squared test.
Abbreviations: ACR, American College of Rheumatology; FM, fibromyalgia; TP, tender point.
A significant OR was detected for all items of the NFF in logistic regression analysis, comparing patients with FM with those without FM. Item 1 showed the most powerful discriminative power among other variables. Using the stepwise logistic regression analysis, only seven items were retained in the final NFF model as the best discriminative set of nociplastic FM pain. Those included items 1, 2, 3, 6, 8, 9, and 11. The ORs of independent variables that were retained in the final model compared with ED as the gold standard were 9.2 (4.0‐16.2), 0.1 (0.02‐0.5), 4.2 (1.4‐12.4), 3.4 (1.4‐8.4), 3.5 (1.4‐8.8), 4.6 (1.8‐11.9), and 3.8 (1.5‐9.5), respectively.
Based on the ROC analysis and AUC of the final NFF, we determined a cut‐off point of 4 as the point with the highest proportion of correctly identified patients (85%), and a sensitivity of 82.5% and specificity of 91.5% (Table 3). The plotted ROC for the total NFF score is depicted in Figure 1. The AUC was obtained as 0.87.
TABLE 3.
Multiple diagnostic cut‐off points of the total NFF score resulting from ROC curve analysis
| Cut‐off Score | Sensitivity (%) | Specificity (%) | Correctly Classified (%) | LR+ | LR− |
|---|---|---|---|---|---|
| (≥0) | 100.00 | 0.00 | 68.11 | 1.0000 | |
| (≥1) | 99.21 | 1.69 | 68.11 | 1.0092 | 0.4683 |
| (≥2) | 96.83 | 35.59 | 77.30 | 1.5033 | 0.0892 |
| (≥3) | 90.48 | 64.41 | 82.16 | 2.5419 | 0.1479 |
| (≥4) | 82.50 | 91.50 | 85.00 | 7.4140 | 0.2739 |
| (≥5) | 42.24 | 94.92 | 61.08 | 8.8968 | 0.5770 |
| (≥6) | 23.02 | 98.31 | 47.03 | 13.5794 | 0.7831 |
| (≥6) | 0.00 | 100.00 | 31.89 | 1.0000 |
Abbreviations: LR+, positive likelihood ratio; LR−, negative likelihood ratio; NFF, Nociplastic‐based Fibromyalgia Features; ROC, receiver operating characteristics.
Figure 1.
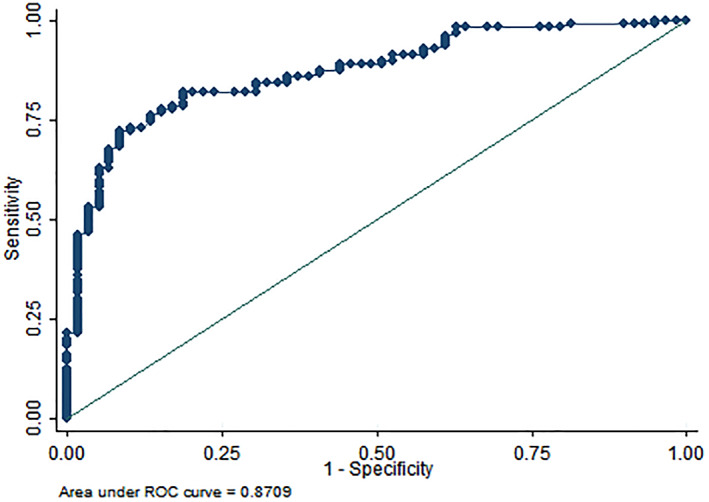
ROC curve and AUC for the total NFF score. AUC, area under the curve; NFF, Nociplastic‐based Fibromyalgia Features; ROC, receiver operating characteristics.
The diagnostic performance of the preliminary NFF was compared with the ED as the gold standard, and the ACR 2011 and 2016 criteria (Table 4). The preliminary NFF (Table 5) had the highest concordance rate with ED (85%) and the lowest rate (77%) with the ACR 2016 criteria.
TABLE 4.
The diagnostic performance of the NFF model as compared with expert diagnosis, ACR 2011 criteria, and ACR 2016 criteria
| Expert Diagnosis | 2011 | 2016 | ||||
|---|---|---|---|---|---|---|
| Non‐FM | FM | Non‐FM | FM | Non‐FM | FM | |
| The NFF Model | ||||||
| Non‐FM | 54 (91.5) | 22 (7.5) | 59 (74.7) | 17 (16) | 63 (66.3) | 13 (14.4) |
| FM | 5 (8.5) | 104 (92.5) | 20 (25.3) | 89 (84) | 32 (33.7) | 77 (83.6) |
| P value | 0.001 | 0.001 | 0.001 | |||
| Concordance (%) | 0.85 | 0.8 | 0.77 | |||
| Kappa (95% CI) | 0.68 (0.59‐0.76) | 0.58 (0.48‐0.67) | 0.51 (0.4‐0.61) | |||
| Sensitivity (%) (95% CI) | 82.5 (74.8‐88.7) | 84 (75.6‐90.4) | 85.6 (76.6‐92.1) | |||
| Specificity (%) (95% CI) | 91.5 (81.3‐97.2) | 74.4 (63.6‐83.8) | 66.3 (55.9‐75.7) | |||
| AUC | 0.87 (0.82‐0.92) | 0.79 (0.73‐0.85) | 0.76 (0.69‐0.82) | |||
| PPV (%) (95% CI) | 95.4 (89.6‐98.5) | 81.7 (73.1‐88.4) | 70.6 (61.2‐79) | |||
| NPV (%) (95% CI) | 71.1 (59.5‐80.9) | 77.6 (66.6‐86.4) | 82.9 (72.5‐90.6) | |||
| LR+ (95% CI) | 9.74 (4.2‐22.6) | 3.32 (2.25‐4.89) | 2.54 (1.89‐3.41) | |||
| LR− (95% CI) | 0.19 (0.13‐0.28) | 0.215 (0.14‐0.34) | 0.218 (0.13‐0.36) | |||
Abbreviations: ACR, American College of Rheumatology; AUC, area under the curve; CI, confidence interval; LR+, positive likelihood ratio; LR−, negative likelihood ratio; NFF, Nociplastic‐based Fibromyalgia Features Criteria; NPV, negative predictive value; PPV, positive predictive value.
TABLE 5.
Nociplastic‐based Fibromyalgia Features (NFF)
| Items | Yes | No |
|---|---|---|
| 1. Do you have pain all over the body? | ||
| 2. Does the patient have any dominant localized pain? a | ||
| 3. Is your pain migratory or nonconsistent? | ||
| 4. Is your pain intensified with physical stress (such as excessive physical activity or cold exposure) or emotional stress? | ||
| 5. Do you experience the pain with excruciated or suffering quality? | ||
| 6. Do you have disabling fatigue, especially in the morning? | ||
| 7. Are there TPs in the manual examination? b |
Note: All items were related to problems within the last 3 months.
The dominant localized pain is defined as the pain maximally focused over one or two the noncontiguous anatomical body sites including left and right arm and leg, chest, abdomen, upper back, and lower back.
The presence of two or more TPs (out of paired trapezius and supraspinatus areas) is considered a positive response.
Abbreviation: TP, tender point.
Cronbach's alpha coefficient was 0.72, reflecting the good internal consistency but lack of redundancy between the items.
DISCUSSION
In this study, we developed and validated the preliminary NFF as an alternative tool, constructed on the key nociplastic features, to discriminate the FM among non‐inflammatory painful disorders in the clinical setting. We also found NFF to have a satisfactory performance and high agreement compared with the ED and the 2011 and 2016 ACR criteria.
The nociplastic concept, proposed recently as a third mechanistic descriptor in addition to nociceptive and neuropathic pain, could introduce a broader aspect in the clinical and neurobiological research than just pain description (11, 13, 28, 29). Recently, the diagnosis of patients according to different pain phenotypes is gaining attention (12, 28). To our best knowledge, this study is the first effort to develop an NP‐based diagnostic tool for FM. The current FM criteria are symptom driven and rely on the presence of a constellation of somatic complaints. In contrast, NFF is based on attributes of NP that are more consistent across time and across different somatic presentations. It contains some core FM symptoms, including generalized pain, unexplained non‐neuroanatomical dominant localized pain, and morning fatigue. In addition, NFF encompasses pain attributes such as migratory pattern, lack of response to analgesia, affective and social aspects (excruciating quality of pain and invalidation), emotional and physical stressors, and hyperalgesia represented as TPs. Therefore, the NFF differs from the currently used FM criteria in that it takes localized pain, peculiar aspects of NP, and a modified TP examination into consideration. Thereby using NFF, physicians would be able to prospect beyond considering only the FM symptoms and shift their attention toward the essential features of NP for early detection of FM.
Of note, in response to NFF item 2, 74% of our FM population had dominant localized pain. It is conceivable that having generalized pain does not negate the reality that patients may complain of localized symptoms due to the out‐of‐proportionate regional discomfort. Indeed, NP represents a nonanatomical bizarre sensation that is expressed differently by patients. The complaint of localized pain in FM is potentially misleading to physicians by suggesting that the patient is affected by a low‐impact and less severe painful state (30). Furthermore, when a physician misses the nociplastic features of pain, the dominant localized pain may shift the clinical impression toward a nociceptive origin of pain (31).
The other items retained in the final NFF model were a migratory pattern of pain, pain aggravation with emotional or physical stress, an excruciating quality of pain, morning fatigue, and TPs. These items showed a significant discriminatory value in differentiating FM from other chronic pain disorders. Whereas pain generalization and polysymptomatology are known as the core features of FM and are employed in FM criteria (2, 3, 15), the significance of shifting patterns of pain and symptoms has not gained proportional attention. Our findings substantiate that anatomical inconsistency of pain across time would be further support for FM.
Pain aggravation with emotional or physical stress differentiated FM from other non‐inflammatory pain disorders in our study. Although this feature has already been described in central sensitization syndrome (CSS) (32) and was included in the initial draft of representative symptoms of the Fibromyalgia Rapid Screening Tool (FIRST) (33), it was not used for the description of FM or CSS. The FIRST study demonstrated no discriminatory value of this item, probably because of selecting a control group of inflammatory rheumatic diseases, inherently prone to stress‐induced disease exacerbation or having a concomitant NP component. This feature is only used in the 2014 Bennett et al criteria and was ranked second in importance as a diagnostic question (34).
Item 8, which probes the affective component of NP, was retained in the final NFF. It is a crucial ingredient in NP syndromes (9, 31) and is still an undervalued part of clinical assessment. Indeed, the FIRST study showed that pain descriptions such as burns, electric shock, and cramps, which suggest an affective component, can discriminate FM (33). These descriptions, however, were not clearly intended to define the affective component of pain. In contrast, the NFF asks about suffering or excruciating pain, which is a more straightforward question.
We found that item 10, which asks about morning fatigue, was a predictor of FM in logistic regression analysis. In the Outcome Measures in Rheumatology study, 94% of expert participants agreed that fatigue was an essential domain in FM assessment (35). Fatigue has various patterns and dimensions, but morning fatigue is more specific for FM and refers to fatigue that does not improve with sleep, indirectly pointing out unrefreshing sleep in FM.
Hyperalgesia is an essential feature of NP and was assessed by examining 18 defined TPs in the ACR 1990 criteria for FM. However, because the task was clinically unfeasible, it was later abandoned (30). That led to overlooking a guide for assessing hyperalgesia. In the NFF, we restated a modified 4‐point exam, consisting of points with the most discriminatory value. We found that this exam discriminated patients with FM from those without FM. Our data are consistent with several studies, including the 2010 Wolfe et al study, which introduced the TP‐free pillars’ criteria. The latter study found that the widespread pain index and TPs were the most remarkable variables in differentiating FM, although TPs were not used in the final formulation of the criteria (2).
Although axial pain, headache, non‐response to analgesia, and invalidation were not retained in the final NFF, our data should not preclude the use and examination of these worthy variables in future research. Our results may have been affected by the comparison of the FM group with the non‐FM group who had localized pain such as mechanical axial pain, and also due to the suboptimal sample size of the study.
The NFF detected FM with good sensitivity (82%) and specificity (91%) when compared with the gold standard (ED). The ROC AUC indicated a good ability to discriminate between FM and non‐FM with a score of 4 as the best cut‐off. The NFF showed high concordance rates with all diagnostic references, especially the ED reference (85%, k = 0.68). The lowest agreement of the NFF with the 2016 ACR criteria was probably due to the more rigid criterion of pain generalization in the 2016 ACR criteria (15), which might be non‐concordant with eligible more localized pain in the NFF.
Our study had some limitations. Only female patients aged between 18 and 65 years were recruited, and therefore the study findings cannot be extrapolated to the general population of FM. Also, we recruited our patients from two tertiary care centers in Iran with a relatively small sample size, making the extrapolation of data difficult. Furthermore, we compared patients with FM only with patients with non‐inflammatory chronic pain disorders who had more localized pain. Future studies are needed to validate the NFF against other generalized pain conditions such as systemic inflammatory rheumatic diseases with the probability of having mixed type pain. Of note, neuropathic pain could have overlapping features with the NP. Small fiber neuropathy has been reported in up to 50% of patients with FM (36). Although we did not specifically evaluate neuropathic pain in our patients, our cases did not manifest overt clinical findings in favor of neuropathy at the time of presentation.
It appears that we stand at the starting point of a long path toward the nociplastic approach to FM diagnosis. The next larger population studies across the different conditions and cultures need to validate the preliminary NFF for FM diagnosis.
In conclusion, the NFF showed good performance for discriminating FM from other common chronic pain disorders. It differs from the traditional symptom‐driven FM criteria in that it focuses on the attributes of NP instead of counting symptoms and pain locations. The presence of item 1, as the strongest discriminatory variable in the model, besides other NFF items, clearly points out the FM diagnosis. However, we demonstrated that dominant localized pain is common in FM. Hence, the expression of dominant pain in association with the attributes of NP, such as the presence of TPs, could be suggestive of FM. Therefore, the NFF can help clinicians detect FM in the early course and when it is mild, before generalization or deepening of pain or other symptoms. It must be kept in mind that classic FM is an easy‐to‐detect but also hard‐to‐control condition. The preliminary NFF tool as a kind of NP conceptualization‐based approach may prevent the wasting of inappropriate diagnostic testing and treatments and would shift clinicians’ attitude to a more effective, and less expensive, intervention.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Professor Bidari had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Ghavidel‐Parsa, Bidari, Atrkarroushan.
Acquisition of data
Ghavidel‐Parsa, Khososi.
Analysis and interpretation of data
Ghavidel‐Parsa, Bidari, Atrkarroushan, Khososi.
Supporting information
Disclosureform
ACKNOWLEDGMENTS
The authors thank Jafar Forghanizadeh, Asghar Hajiabbasi, Irandokht Shenavar, Habib Zayeni, and the members of the study advisory committee, which participated in the preparation and finalization of the initial draft of the tool in this work. The authors also thank Sajjad Hosseini and Amir Hassankhani for data gathering and facilitating recruitment. The authors are grateful to the participants.
This study was supported by Guilan University of Medical Sciences (GUMS).
No potential conflicts of interest relevant to this article were reported.
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Facr2.11390&file=acr211390-sup-0001-Disclosureform.pdf.
REFERENCES
- 1. Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Arthritis Rheum 1990;33:160‐72. [DOI] [PubMed] [Google Scholar]
- 2. Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62:600‐10. [DOI] [PubMed] [Google Scholar]
- 3. Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz RS, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol 2011;38:1113‐22. [DOI] [PubMed] [Google Scholar]
- 4. Arnold LM, Bennett RM, Crofford LJ, Dean LE, Clauw DJ, Goldenberg DL, et al. AAPT Diagnostic Criteria for Fibromyalgia. J Pain 2019;20:611‐28. [DOI] [PubMed] [Google Scholar]
- 5. Bidari A, Ghavidel Parsa B, Ghalehbaghi B. Challenges in fibromyalgia diagnosis: from meaning of symptoms to fibromyalgia labeling. Korean J Pain 2018;31:147‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hadker N, Garg S, Chandran AB, Crean SM, McNett MM, Silverman SL. Efficient practices associated with diagnosis, treatment and management of fibromyalgia among primary care physicians. Pain Res Manag 2011;16:440‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Häuser W, Ablin J, Fitzcharles MA, Littlejohn G, Luciano JV, Usui C, et al. Fibromyalgia. Nat Rev Dis Primers 2015;1:15022. [DOI] [PubMed] [Google Scholar]
- 8. Sluka KA, Clauw DJ. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience 2016;338:114‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ghavidel‐Parsa B, Bidari A. Two sides on the fibromyalgia coin: physical pain and social pain (invalidation). Clinical Rheumatol 2021;40:841‐8. [DOI] [PubMed] [Google Scholar]
- 10. Kosek E, Cohen M, Baron R, Gebhart GF, Mico JA, Rice ASC, et al. Do we need a third mechanistic descriptor for chronic pain states? Pain 2016;157:1382‐6. [DOI] [PubMed] [Google Scholar]
- 11. Trouvin AP, Perrot S. New concepts of pain. Best Pract Res Clin Rheumatol 2019;33:101415. [DOI] [PubMed] [Google Scholar]
- 12. Kosek E, Clauw D, Nijs J, Baron R, Gilron I, Harris RE, et al. Chronic nociplastic pain affecting the musculoskeletal system: clinical criteria and grading system. Pain 2021;162:2629‐34. [DOI] [PubMed] [Google Scholar]
- 13. Sarzi‐Puttini P, Giorgi V, Marotto D, Atzeni F. Fibromyalgia: an update on clinical characteristics, aetiopathogenesis and treatment. Nat Rev Rheumatol 2020;16:645‐60. [DOI] [PubMed] [Google Scholar]
- 14. Williams DA, Clauw DJ. Understanding fibromyalgia: lessons from the broader pain research community. J Pain 2009;10:777‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz RL, et al. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum 2016;46:319‐29. [DOI] [PubMed] [Google Scholar]
- 16. Clauw D. Time to stop the fibromyalgia criteria wars and refocus on identifying and treating individuals with this type of pain earlier in their illness. Arthritis Care Res (Hoboken) 2021;73:613‐6. [DOI] [PubMed] [Google Scholar]
- 17. Diers M. Neuroimaging the pain network—implications for treatment. Best Pract Res Clin Rheumatol 2019;33:101418. [DOI] [PubMed] [Google Scholar]
- 18. Kia S, Choy E. Update on treatment guideline in fibromyalgia syndrome with focus on pharmacology. Biomedicines 2017;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Santiago MG, Marques A, Kool M, Geenen R, da Silva JAP. Invalidation in patients with rheumatic diseases: clinical and psychological framework. J Rheumatol 2017;44:512‐8. [DOI] [PubMed] [Google Scholar]
- 20. Ghavidel‐Parsa B, Bidari A, Tohidi S, Shenavar I, Kazemnezhad Leyli E, Hosseini K, et al. Implication of invalidation concept in fibromyalgia diagnosis. Clin Rheumatol 2021;40:2369‐76. [DOI] [PubMed] [Google Scholar]
- 21. Tastekin N, Uzunca K, Sut N, Birtane M, Mercimek OB. Discriminative value of tender points in fibromyalgia syndrome. Pain Med 2010;11:466‐71. [DOI] [PubMed] [Google Scholar]
- 22. Segura‐Jiménez V, Álvarez‐Gallardo IC, Carbonell‐Baeza A, Aparicio VA, Ortega FB, Casimiro AJ, et al. Fibromyalgia has a larger impact on physical health than on psychological health, yet both are markedly affected: the al‐Ándalus project. Semin Arthritis Rheum 2015;44:563‐70. [DOI] [PubMed] [Google Scholar]
- 23. Tastekin N, Birtane M, Uzunca K. Which of the three different tender points assessment methods is more useful for predicting the severity of fibromyalgia syndrome? Rheumatology International 2007;27:447‐51. [DOI] [PubMed] [Google Scholar]
- 24. Bennett RM, Friend R, Jones KD, Ward R, Han BK, Ross RL. The Revised Fibromyalgia Impact Questionnaire (FIQR): validation and psychometric properties. Arthritis Res Ther 2009;11:R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Montazeri A, Vahdaninia M, Mousavi SJ, Omidvari S. The Iranian version of 12‐item Short Form Health Survey (SF‐12): factor structure, internal consistency and construct validity. BMC Public Health 2009;9:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bidari A, Ghavidel‐Parsa B, Amir Maafi A, Montazeri A, Ghalehbaghi B, Hassankhani A, et al. Validation of fibromyalgia survey questionnaire and polysymptomatic distress scale in a Persian population. Rheumatol Int 2015;35:2013‐9. [DOI] [PubMed] [Google Scholar]
- 27. Ghavidel Parsa B, Amir Maafi A, Haghdoost A, Arabi Y, Khojamli M, Chatrnour G, et al. The validity and reliability of the Persian version of the Revised Fibromyalgia Impact Questionnaire. Rheumatol Int 2014;34:175‐80. [DOI] [PubMed] [Google Scholar]
- 28. Shraim MA, Massé‐Alarie H, Hodges PW. Methods to discriminate between mechanism‐based categories of pain experienced in the musculoskeletal system: a systematic review. Pain 2021;162:1007‐37. [DOI] [PubMed] [Google Scholar]
- 29. López‐Solà M, Woo C‐W, Pujol J, Deus J, Harrison BJ, Monfort J, et al. Towards a neurophysiological signature for fibromyalgia. Pain 2017;158:34‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Egloff N, von Känel R, Müller V, Egle UT, Kokinogenis G, Lederbogen S, et al. Implications of proposed fibromyalgia criteria across other functional pain syndromes. Scand J Rheumatol 2015;44:416‐24. [DOI] [PubMed] [Google Scholar]
- 31. Nijs J, George SZ, Clauw DJ, Fernández‐de‐las‐Peñas C, Kosek E, Ickmans K, et al. Central sensitisation in chronic pain conditions: latest discoveries and their potential for precision medicine. Lancet Rheumatol 2021;3:e383‐e92. [DOI] [PubMed] [Google Scholar]
- 32. Mayer TG, Neblett R, Cohen H, Howard KJ, Choi YH, Williams MJ, et al. The development and psychometric validation of the central sensitization inventory. Pain Practice 2012;12:276‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Perrot S, Bouhassira D, Fermanian J. Development and validation of the Fibromyalgia Rapid Screening Tool (FiRST). Pain 2010;150:250‐6. [DOI] [PubMed] [Google Scholar]
- 34. Bennett RM, Friend R, Marcus D, Bernstein C, Han BK, Yachoui R, et al. Criteria for the diagnosis of fibromyalgia: validation of the modified 2010 preliminary American College of Rheumatology criteria and the development of alternative criteria. Arthritis Care Res (Hoboken) 2014;66:1364‐73. [DOI] [PubMed] [Google Scholar]
- 35. Vincent A, Hoskin TL, Whipple MO, Clauw DJ, Barton DL, Benzo RP, et al. OMERACT‐based fibromyalgia symptom subgroups: an exploratory cluster analysis. Arthritis Res Ther 2014;16:463‐. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grayston R, Czanner G, Elhadd K, Goebel A, Frank B, Üçeyler N, et al. A systematic review and meta‐analysis of the prevalence of small fiber pathology in fibromyalgia: implications for a new paradigm in fibromyalgia etiopathogenesis. Semin Arthritis Rheum 2019;48:933‐40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosureform


