Abstract
Objective
Pain reduction with baricitinib was assessed in patients with rheumatoid arthritis (RA) who either used opioids or did not use opioids during three randomized, double‐blind phase 3 trials.
Methods
Analysis populations were as follows: i) baricitinib 4 mg once daily versus placebo groups integrated from RA‐BEAM (NCT01710358) for patients with inadequate response (IR) to methotrexate, RA‐BUILD (NCT01721057) with IR to conventional disease‐modifying antirheumatic drugs, and RA‐BEACON (NCT01721044) with IR to at least one tumor necrosis factor inhibitors; ii) baricitinib 2 mg versus placebo from RA‐BUILD and RA‐BEACON; and iii) adalimumab 40 mg every other week versus placebo from RA‐BEAM. Pain was measured by the Patient Assessment of Pain Visual Analog Scale. Analysis of covariance modeling assessed differences in pain reduction between treatments at each time point through Week 24, with an interaction term to test heterogeneous treatment effects across opioid users and nonusers.
Results
Baricitinib 4 mg had greater pain reduction versus placebo in opioid users and nonusers (P < 0.05) at all time points starting from Week 1; the pain reduction was similar between opioid users and nonusers. Baricitinib 2 mg had greater pain reduction versus placebo in opioid users and nonusers starting at Week 4. A significant difference in pain reduction was not observed for adalimumab versus placebo in the opioid users but was observed in nonusers at all time points.
Conclusion
Pain reduction was observed and was similar between opioid users and nonusers with baricitinib 2 mg and 4 mg but not adalimumab in this post hoc analysis.
Introduction
Pain control is an important clinical management target for patients with rheumatoid arthritis (RA) (1). Current research indicates that multiple cytokines are involved in the pathogenesis of pain (2), many of which signal through the Janus kinase (JAK) signal transducer and activator of transcription (STAT) pathway. Therapies for RA, such as JAK inhibitors, have been developed to inhibit a subgroup of cytokines implicated in the pathogenesis of inflammation but may also affect pain by other pathways. Important pain reductions have been observed in randomized clinical trials (RCTs) of baricitinib, an oral, selective, JAK1/2 inhibitor, as have been observed in other effective treatments for RA.
Meanwhile, use of opioids to treat RA‐related pain has increased; in one assessment, up to 40% of patients with RA reported using prescription opioids (3). Current research indicates that short‐term opioid use is modestly effective for pain relief; however, long‐term opioid use is associated with reduced efficacy and more safety concerns (4). These safety concerns with opioids include psychological and neurological effects, increased risk of serious infections and nonvertebral fractures, misuse, and addiction (4, 5, 6). The misuse of opioids has resulted in a public health crisis in the United States and highlights the need for safe, effective nonaddictive alternatives for pain management (7). Furthermore, despite the use of long‐term opioids, patients with RA often have significant residual pain (8).
A prior multiple mediation analysis assessed the relative contribution of surrogates of inflammation to the change in pain observed in the context of a double‐blind, randomized, controlled clinical trial which compared the efficacy of baricitinib with the tumor necrosis factor (TNF) inhibitor adalimumab in RA patients on background methotrexate (MTX) (1). In that analysis, JAK inhibition and TNF inhibition gave rise to a similar improvement in markers of inflammation, but that overall pain relief was greater with JAK inhibition, raising the possibility that JAK inhibition may ameliorate pain of both inflammatory and non‐inflammatory origin. Although the precise mechanism accounting for an experiential pain of noninflammatory origin with JAK inhibition is not understood, it is thought unlikely to be mediated via the opiate pathway. Therefore, we reasoned that, in RA patients on MTX and receiving opiate treatment, baricitinib might provide additional pain improvement over adalimumab.
To understand whether pain relief by baricitinib is influenced by opioid use across different patient populations, we conducted hypothesis‐generating analyses to assess pain reduction in patients who used opioids and those who did not with data from three randomized, double‐blind phase 3 studies of baricitinib (RA‐BEAM, RA‐BUILD, and RA‐BEACON) (9, 10, 11, 12, 13, 14).
Patients and methods
Data sources and study designs
Data from randomized, double‐blind, parallel‐group, multicenter, international phase 3 RCTs were included in this analysis; detailed methods for the RCTs have been published previously (9, 10, 11, 12, 13, 14). In brief, across all RCTs, patients were 18 years of age or older with active RA (a tender joint count of at least 6 of 68, a swollen joint count of at least 6 of 66). Patients were randomly allocated to treatment, as described later, added to existing background therapy, including MTX. In RA‐BEAM (NCT01710358), RA patients with an inadequate response to MTX were randomly allocated to placebo, baricitinib 4 mg once daily, or biweekly subcutaneous adalimumab 40 mg (12, 14). In RA‐BUILD (NCT01721057), patients with an inadequate response to conventional synthetic disease‐modifying antirheumatic drugs (DMARDs) were randomly allocated to once‐daily placebo, baricitinib 2 mg, or baricitinib 4 mg (9, 10). In RA‐BEACON (NCT01721044), patients with an inadequate response to at least one TNF inhibitors (TNFis) were randomly allocated to once‐daily placebo, baricitinib 2 mg, or baricitinib 4 mg (11, 13).
Study population
We identified opioid users as those patients who reported any opioid use during the RCTs, assuming that opioid use would likely be stable in the analysis because additional medication use was discouraged during the RCTs, unless needed for an adverse event or for treatment of an ongoing medical condition.
We evaluated three analysis populations. In the first analysis population, data from baricitinib 4 mg and placebo were pooled from all three trials (RA‐BEAM, RA‐BUILD, and RA‐BEACON). In the second analysis population, we evaluated baricitinib 2 mg and placebo with data pooled from RA‐BUILD and RA‐BEACON. In the third analysis population, we evaluated subcutaneous adalimumab 40 mg every other week and placebo with data from RA‐BEAM.
Pain assessment
Pain was measured by the Patient Assessment of Pain Visual Analog Scale (VAS; 0‐100 mm). Change from baseline in pain was assessed through Week 24.
Statistical analysis
Analysis of covariance models assessed the differences in pain reduction for opioid users and nonusers at each time point between i) baricitinib 4 mg and placebo, ii) baricitinib 2 mg and placebo, and iii) adalimumab and placebo. The covariates in the model included baseline pain VAS, age, body mass index, and RCT (for analyses in which multiple trials are combined). Therapeutic effects of active treatment versus placebo within opioid users and nonusers were compared with an interaction term. Patients who discontinued treatment or were rescued had their last observation before discontinuation or rescue carried forward through Week 24. These post hoc analyses were not adjusted for multiplicity. All analyses were conducted with SAS (SAS Institute Inc.).
Results
Characteristics of the study populations
The number of opioid users in the RCTs was 143 (11%) in RA‐BEAM, 133 (19%) in RA‐BUILD, and 176 (33%) in RA‐BEACON. Opioid use by class and by RCT is presented in Table 1. The number of opioid users of the total number of patients randomized to treatment was 171 of 891 (19%) for baricitinib 4 mg and 153 of 892 (17%) for placebo in the pooled 4‐mg analysis population, 94 of 403 (23%) for baricitinib 2 mg and 103 of 404 (25%) for placebo in the pooled 2‐mg analysis population, and 34 of 330 (10%) for adalimumab and 50 of 488 (10%) for placebo in RA‐BEAM. Among the opioid users, we observed that 42 of 153 (28%) placebo‐treated patients, 42 of 265 (16%) baricitinib‐treated patients, and 10 of 34 (29%) adalimumab‐treated patients initiated opioid use during the RCTs.
Table 1.
Opioid use by class and by randomized clinical trial
| Opioid Class, N (%) | RA‐BEAM (N = 1,305) | RA‐BUILD (N = 684) | RA‐BEACON (N = 527) |
|---|---|---|---|
| Tramadol | 95 (7.3) | 71 (10.4) | 68 (12.9) |
| Oxycodone | 12 (0.9) | 19 (2.8) | 38 (7.2) |
| Hydrocodone | 5 (0.4) | 11 (1.6) | 28 (5.3) |
| Fentanyl | 4 (0.3) | 6 (0.9) | 7 (1.3) |
| Morphine | 4 (0.3) | 7 (1.0) | 9 (1.7) |
| Other | 44 (3.4) | 34 (5.0) | 61 (11.6) |
| Total | 143 | 133 | 176 |
The opioid classes are not mutually exclusive. The “Other” category included aporex, buprenorphine, butorphanol, co‐dafalgan, cyclimorph, dextropropoxyphene, dihydrocodeine, hydromorphone, klosidol, lamaline, lenoltec with codeine, mersyndol, mersyndol, myprodol, new dickinin, new lulu a, nolotil, omunin, oxymorphone, panadeine co, pethidine, procet, solpadeine, stilpane, supragesic, tapentadol, tilidine, valoron, and Vicodin. Number of patients receiving each treatment and % of total number of patients per study.
The average age across the different study populations spanned from 52 to 59 years. Most patients (68% to 85%) were women, and the mean baseline pain ranged from 56 to 64 based on 100‐mm VAS scores. Baseline characteristics were comparable between treatment groups and between opioid users and nonusers in general (Table 2).
Table 2.
Patient baseline characteristics by analysis population
| Pooled 4‐Mg Data Set | Pooled 2‐Mg Data Set | RA‐BEAM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Opioid User | Opioid Nonuser | Opioid User | Opioid Nonuser | Opioid User | Opioid Nonuser | |||||||
| Characteristic | Placebo(N = 153) | Baricitinib 4 mg(N = 171) | Placebo(N = 739) | Baricitinib 4 mg (N = 720) | Placebo(N = 103) | Baricitinib 2 mg (N = 94) | Placebo (N = 301) | Baricitinib 2 mg (N = 309) | Placebo (N = 50) | Adalimumab 40 mg (N = 34) | Placebo (N = 438) | Adalimumab 40 mg (N = 296) |
| Age, years, mean (SD) | 57 (11) | 56 (12) | 53 (12) | 53 (12) | 55 (10) | 57 (11) | 53 (13) | 53 (12) | 59 (11) | 52 (14) | 53 (12) | 53 (12) |
| Female, n(%) | 128 (84) | 134 (78) | 588 (80) | 577 (80) | 88 (85) | 79 (84) | 246 (82) | 242 (78) | 40 (80) | 23 (68) | 342 (78) | 228 (77) |
| Pain, 0‐100 mm, mean (SD) | 61 (23) | 64 (22) | 60 (22) | 61 (23) | 63 (22) | 60 (21) | 60 (22) | 61 (22) | 56 (24) | 64 (25) | 60 (22) | 61 (23) |
| DAS28‐CRP, mean (SD) | 5.7 (1.0) | 6.0 (0.9) | 5.7 (0.9) | 5.7 (0.9) | 5.9 (1.0) | 5.9 (1.0) | 5.6 (0.9) | 5.7 (0.9) | 5.5 (1.1) | 5.9 (1.1) | 5.7 (0.9) | 5.7 (0.9) |
Abbreviations: DAS28‐CRP, Disease Activity Score with 28 joint count using the CRP (c‐reactive protein); SD, standard deviation.
Baricitinib 4 mg versus placebo (pooled analysis population: RA‐BEAM, RA‐BUILD, and RA‐BEACON)
Greater pain reduction was observed with baricitinib 4 mg compared with placebo for both opioid users and nonusers across all time points (P < 0.05; Figure 1A). Furthermore, the treatment difference in the amount of pain reduction was similar between opioid users and nonusers at all time points (interaction P > 0.1). At Week 24, the difference in pain VAS reduction between baricitinib 4 mg and placebo was −13.4 (95% confidence interval CI: −19.0 to −7.8) in opioid users and −14.3 (−16.7 to −11.9) in nonusers (interaction P = 0.8).
Figure 1.
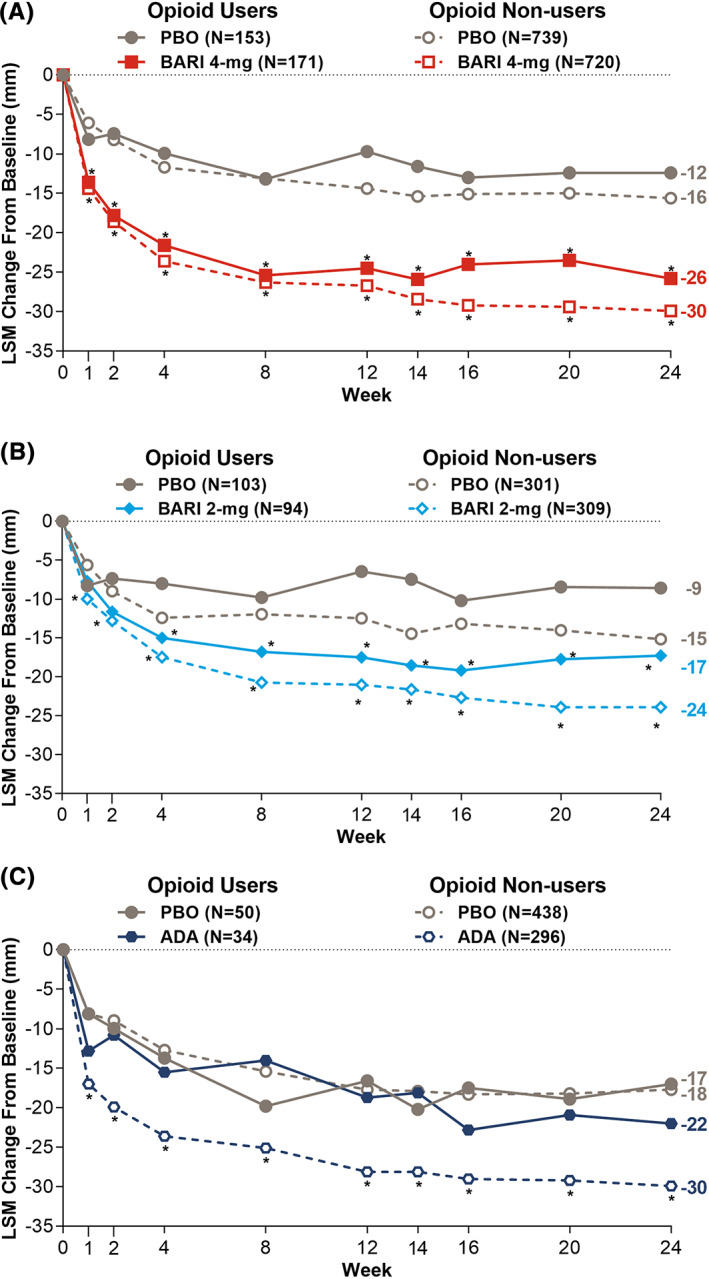
Pain reduction between opioid users and nonusers by analysis population. A, Baricitinib 4 mg versus placebo with data from the pooled analysis population (RA‐BEAM, RA‐BUILD, and RA‐BEACON). B, Baricitinib 2 mg versus placebo with data from pooled analysis population (RA‐BUILD and RA‐BEACON). C, Adalimumab 40 mg versus placebo with data from RA‐BEAM.
Baricitinib 2 mg versus placebo (pooled analysis population: RA‐BUILD and RA‐BEACON)
Greater pain reduction was observed with baricitinib 2 mg compared with placebo for both opioid users and nonusers beginning at Week 4 (P < 0.05; Figure 1B). Prior to Week 4, statistical significance compared with placebo was observed only for baricitinib 2‐mg patients who did not use opioids. The treatment difference in the amount of pain reduction was similar between opioid users and nonusers at all time points, except for Week 1 (interaction P > 0.1). At Week 24, the difference in pain VAS reduction between baricitinib 2 mg compared with placebo was −8.7 (−15.4 to −2.0) in opioid users and −8.7 (−12.6 to −4.9) in nonusers (interaction P = 0.9).
Because only a dose level of baricitinib 2 mg, once daily, was approved by the United States Food and Drug Administration for RA patients with an incomplete response to TNFis, sensitivity analysis on baricitinib 2 mg was conducted in RA‐BEACON alone. Similar results were observed, yet greater pain reduction was observed with baricitinib 2 mg compared with placebo starting at Week 12 for both opioid users and nonusers. At Week 24, the difference in pain VAS reduction between baricitinib 2 mg and placebo was −10.7 (−19.5 to −1.9) in opioid users and −8.3 (−15.0 to −1.6) in nonusers (interaction P = 0.8).
Adalimumab 40 mg versus placebo (RA‐BEAM)
In the 84 patients using opioids in the adalimumab (n = 34) and placebo arms (n = 50), no significant difference in pain reduction was observed between adalimumab and placebo, whereas for nonusers, a difference in pain reduction was observed for adalimumab compared with placebo at all time points (P < 0.05; Figure 1C). At Week 24, the difference in pain VAS reduction between adalimumab and placebo was −4.9 (−16.4 to 6.6) in opioid users and −12.2 (−15.6 to −8.9) in nonusers (interaction P = 0.2).
Discussion
In this analysis, baricitinib 4 mg had greater pain reduction compared with placebo in opioid users and nonusers (P < 0.05) at all time points starting from Week 1; the treatment difference in the pain reduction was similar between opioid users and nonusers. Baricitinib 2 mg had greater pain reduction compared with placebo in opioid users (P < 0.05) at all time points and in opioid nonusers (P < 0.05) starting at Week 4. The sample size for adalimumab was small, relative to the baricitinib data, yet trends in the data suggest that there was no difference in pain reduction between adalimumab and placebo among opioid users. Differences, however, were observed between adalimumab and placebo for nonusers at all time points.
The mechanisms underlying pain in RA are not fully understood (15). The JAK–STAT pathway may potentially mediate the signaling of multiple cytokines known to have a role in nociception, acting at a variety of neuroanatomical locations from the periphery to the brain. The present data suggest that at least some of these nociception checkpoints are nonconvergent with opioid signaling pathways. Additionally, prior analyses have implied that JAK1 and JAK2 inhibition may have antinociceptive effects that may be independent of some aspects of the inflammatory process (1). Furthermore, the pain reduction possibly related to the biologic effects of baricitinib may subsequently lead to subjective improvement in pain by cortical pathways linked to overall well‐being and pain perception at the central nervous system level (16). We do not know why pain reduction with adalimumab may be attenuated by opioid use. One hypothesis is that inhibiting JAK 1/2 may impact more afferent pain signaling than inhibiting TNF alone. The results with adalimumab from the current analysis, however, may be affected by the play of chance because of the small sample size. Additionally, the lack of statistically significant interaction of treatment and opioid use in adalimumab‐treated patients likely was due to the study being underpowered.
There are several limitations to this post hoc hypothesis‐generating analysis. The data are from RCTs, which may not be generalizable to a real‐world, clinical setting. The trial databases captured limited detail about the opioids, and given the lack of dosing information, we could not stratify by morphine equivalent dose. We also could not stratify or adjust for the extent of inflammation, central pain sensitization, or by comorbidities, such as osteoarthritis, degenerative disc disease, fibromyalgia, or depression. Randomization of the large number of patients in these RCTs would on expectation balance these factors between baricitinib‐treated patients and placebo‐treated patients in the subgroups of opioid users and nonusers, but the play of chance may yield covariate imbalances in small subgroups, such as the adalimumab‐treated patients (n = 34) and placebo‐treated patients (n = 50) who used opioids (Table 2). Subsequently, it is not possible to draw a conclusion on the attenuation of pain reduction with adalimumab by opioid use. We also defined opioid users as those patients who reported any opioid use during the RCTs and assumed opioid use was likely stable throughout the course of the RCTs. Among the opioid users, however, approximately, 16% of baricitinib‐treated patients and 28% of placebo‐treated patients initiated opioid treatment during the RCTs. Because there is a greater percentage of placebo‐treated patients starting opioids during the RCTs, we believe the greater pain reduction observed in the baricitinib‐treated group compared with the placebo‐treated group among opioid users is not likely biased by later opioid use. Lastly, these analyses were not adjusted for multiplicity.
In conclusion, this novel, post hoc, hypothesis‐generating analysis assessed the differential effect of advanced DMARDs on pain reduction in patients with RA who were opioid users. The current results suggest that pain reduction with baricitinib is robust both for nonusers and users of opioids. This observation may be particularly meaningful for patients with RA who use opioids because prior research indicates that opioid use for chronic pain is associated with less benefit and more safety concerns (16, 17). Further research, however, is needed to replicate these results with other JAK inhibitors and with more robust TNFi data. Analyses such as these would confirm whether these observations are reproducible, whether they apply to other JAK inhibitors, and whether the results are a consequence of JAK1/2 selective inhibition. These additional lines of research will address the unmet need of pain control for patients with RA.
Author Contributions
All authors made substantial contributions to study conception and design and to analysis and interpretation of data, and all authors drafted the article or revised it critically for important intellectual content and approved the final version to be published.
Supporting information
Disclosure Form
Acknowledgments
The authors would like to thank Molly E. Tomlin, MS, MEd, of Eli Lilly and Company for her assistance with manuscript preparation and process support and Amanda Quebe for her review and feedback on the manuscript. Lilly provides access to relevant anonymized patient‐level data from studies on approved medicines and indications as defined by the sponsor‐specific information on www.clinicalstudydatarequest.com. For details on submitting a request, see the instructions provided at www.clinicalstudydatarequest.com. Dr. Taylor would like to thank the National Institute of Health Research for their funding of The National Institute for Health Research Biomedical Research Centre in Musculoskeletal Disease at Oxford University Hospitals National Health System Trust and the University of Oxford.
This analysis and report were sponsored by Eli Lilly and Company, under license from Incyte Corporation.
No potential conflicts of interest relevant to this article were reported.
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Facr2.11380&file=acr211380-sup-0001-Disclosureform.pdf.
References
- 1. Taylor PC, Lee YC, Fleischmann R, Takeuchi T, Perkins EL, Fautrel B, et al. Achieving pain control in rheumatoid arthritis with baricitinib or adalimumab plus methotrexate: Results from the RA‐BEAM trial. J Clin Med 2019; 8:831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang A, Lee YC. Mechanisms for joint pain in rheumatoid arthritis (RA): From cytokines to central sensitization. Curr Osteoporos Rep 2018;16:603‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Curtis JR, Xie F, Smith C, Saag KG, Chen L, Beukelman T, et al. Changing trends in opioid use among patients with rheumatoid arthritis in the United States. Arthritis Rheumatol 2017;69:1733–40. [DOI] [PubMed] [Google Scholar]
- 4. Day AL, Curtis JR. Opioid use in rheumatoid arthritis: trends, efficacy, safety, and best practices. Curr Opin Rheumatol 2019;31:264–70. [DOI] [PubMed] [Google Scholar]
- 5. Black RJ, Richards B, Lester S, Buchbinder R, Barrett C, Lassere M, et al. Factors associated with commencing and ceasing opioid therapy in patients with rheumatoid arthritis. Semin Arthritis Rheum 2019;49:351–7. [DOI] [PubMed] [Google Scholar]
- 6. Wiese AD, Griffin MR, Stein CM, Mitchel EF, Jr. , Grijalva CG. Opioid analgesics and the risk of serious infections among patients with rheumatoid arthritis: A self‐controlled case series study. Arthritis Rheumatol 2016;68:323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pope JE. Prescribing opioids for severe hip and knee osteoarthritis varies widely in the United States: The devil is in the details. Arthritis Rheumatol 2019;71:659–61. [DOI] [PubMed] [Google Scholar]
- 8. Michaud K, Pope J, van M , de L , Curtis JR, Kannowski C, et al. A systematic literature review of residual symptoms and unmet need in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). Published online July 3, 2020. doi: 10.1002/acr.24369 [DOI] [PMC free article] [PubMed]
- 9. Dougados M, van der Heijde D, Chen YC, Greenwald M, Drescher E, Liu J, et al. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: Results from the RA‐BUILD study. Ann Rheum Dis 2017;76:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Emery P, Blanco R, Maldonado Cocco J, Chen YC, Gaich CL, DeLozier AM, et al. Patient‐reported outcomes from a phase III study of baricitinib in patients with conventional synthetic DMARD‐refractory rheumatoid arthritis. RMD Open 2017;3:e000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Genovese MC, Kremer J, Zamani O, Ludivico C, Krogulec M, Xie L, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med 2016;374:1243–52. [DOI] [PubMed] [Google Scholar]
- 12. Keystone EC, Taylor PC, Tanaka Y, Gaich C, DeLozier AM, Dudek A, et al. Patient‐reported outcomes from a phase 3 study of baricitinib versus placebo or adalimumab in rheumatoid arthritis: secondary analyses from the RA‐BEAM study. Ann Rheum Dis 2017;76:1853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smolen JS, Kremer JM, Gaich CL, DeLozier AM, Schlichting DE, Xie L, et al. Patient‐reported outcomes from a randomised phase III study of baricitinib in patients with rheumatoid arthritis and an inadequate response to biological agents (RA‐BEACON). Ann Rheum Dis 2017;76:694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taylor PC, Keystone EC, van der Heijde D, Weinblatt ME, Del Carmen Morales L, Reyes Gonzaga J, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med 2017;376652–62. [DOI] [PubMed] [Google Scholar]
- 15. Simon LS, Taylor PC, Choy EH, Sebba A, Quebe A, Knopp KL, et al. The Jak/STAT pathway: A focus on pain in rheumatoid arthritis. Semin Arthritis Rheum 2021;51:278–84. [DOI] [PubMed] [Google Scholar]
- 16. Yang S, Chang MC. Chronic pain: Structural and functional changes in brain structures and associated negative affective states. Int J Mol Sci 2019;20:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rivat C, Ballantyne J. The dark side of opioids in pain management: Basic science explains clinical observation. Pain Rep 2016;12:e570. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form


