Abstract
Background
Noninvasive electrocardiographic markers (NIEMs) are promising arrhythmic risk stratification tools for assessing the risk of sudden cardiac death. However, little is known about their utility in patients with chronic kidney disease (CKD) and organic heart disease. This study aimed to determine whether NIEMs can predict cardiac events in patients with CKD and structural heart disease (CKD‐SHD).
Methods
We prospectively analyzed 183 CKD‐SHD patients (median age, 69 years [interquartile range, 61−77 years]) who underwent 24‐h ambulatory electrocardiographic monitoring and assessed the worst values for ambulatory‐based late potentials (w‐LPs), heart rate turbulence, and nonsustained ventricular tachycardia (NSVT). The primary endpoint was the occurrence of documented lethal ventricular tachyarrhythmias (ventricular fibrillation or sustained ventricular tachycardia) or cardiac death. The secondary endpoint was admission for cardiovascular causes.
Results
Thirteen patients reached the primary endpoint during a follow‐up period of 24 ± 11 months. Cox univariate regression analysis showed that existence of w‐LPs (hazard ratio [HR] = 6.04, 95% confidence interval [CI]: 1.4−22.3, p = .007) and NSVT [HR = 8.72, 95% CI: 2.8−26.5: p < .001] was significantly associated with the primary endpoint. Kaplan–Meier analysis demonstrated that the combination of w‐LPs and NSVT resulted in a lower event‐free survival rate than did other NIEMs (p < .0001). No NIEM was useful in predicting the secondary endpoint, although the left ventricular mass index was correlated with the secondary endpoint.
Conclusion
The combination of w‐LPs and NSVT was a significant risk factor for lethal ventricular tachyarrhythmias and cardiac death in CKD‐SHD patients.
Keywords: ambulatory electrocardiography, chronic kidney disease, late potentials, nonsustained ventricular tachycardia, sudden cardiac death, ventricular tachycardia
1. INTRODUCTION
Patients with chronic kidney disease (CKD), which number more than 20 million in the United States (Pun, 2014), have a 4‐ to 20‐fold higher risk of sudden cardiac death (SCD) than does the general population. Cardiovascular disease (CVD) and CKD are closely related, with the former having a significant impact on CKD mortality (Go et al., 2004; Ronco et al., 2008). A large cohort study has shown that while only 3.1% of stage 2–4 CKD patients progress to renal replacement therapy, 24.9% die (Keith et al., 2004), and that a higher cumulative cardiovascular comorbidity was associated with the risk of death regardless of CKD severity (Jesky et al., 2013). Therefore, in CKD management, clinicians should pay attention not only to renal function deterioration but also to CVD. Identifying CKD patients at high risk, particularly those with structural heart disease (SHD), is of great importance. However, few studies have addressed this issue to date.
It has been reported that noninvasive electrocardiographic markers (NIEMs), including late potentials (LPs) (Gatzoulis et al., 2018), heart rate turbulence (HRT) (Bauer et al., 2008), and nonsustained ventricular tachycardia (NSVT) (Kinoshita et al., 2020), are useful predictors of lethal arrhythmic events and SCD in patients with SHD. We have recently demonstrated that a combined assessment of NIEMs obtained from high‐resolution digital electrocardiogram (ECG) systems accurately predicts the occurrence of lethal arrhythmias and SCD in patients with SHD (Hashimoto et al., 2020; Kinoshita et al., 2020). Although LPs have been associated with mortality and SCD in patients with end‐stage renal disease (Morales et al., 1998), little is known about the usefulness of other NIEMs in predicting lethal arrhythmias or cardiac mortality in patients with CKD, regardless of disease severity or the presence of concurrent SHD.
This study aimed to investigate whether noninvasive ECG parameters assessed using new ambulatory ECG systems could predict cardiac death, lethal arrhythmic events, and nonfatal cardiovascular events in CKD patients with SHD.
2. METHODS
2.1. Study design and population
This study was a sub‐study of the Japanese Noninvasive Electrocardiographic Risk Stratification for Prediction of Sudden Cardiac Death (JANIES) study (Kinoshita et al., 2020). The JANIES study was a multicenter, observational, prospective cohort study. It assessed the role of noninvasive ECG markers, obtained simultaneously using a 24‐h high‐resolution digital ambulatory ECG system, in predicting severe cardiac events, such as lethal arrhythmias and cardiac mortality in high‐risk patients. Recruitment was performed between April 2012 and March 2015. The inclusion and exclusion criteria used have been reported previously (Kinoshita et al., 2020). Briefly, the JANIES study included patients with structural or idiopathic cardiac disorders who underwent 24‐h high‐resolution digital ambulatory monitoring (Figure 1). The exclusion criteria were as follows: hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, persistent atrial fibrillation or flutter, right or left bundle branch block and intraventricular conduction delay, permanent pacing, second‐ or third‐degree atrioventricular block, nonsimultaneous measurement of ECG markers, and patient dropout (missing follow‐up data or unknown cause).
FIGURE 1.
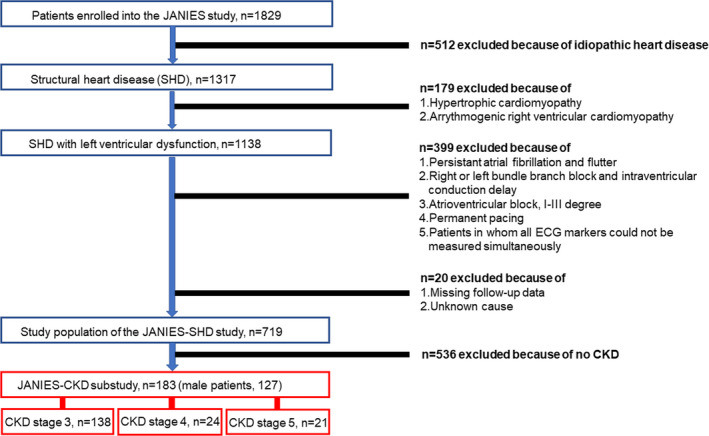
Study population. Patients were enrolled from April 2012 to March 2015. Follow‐up data collection was performed every 6 months until September 2015. CKD, chronic kidney disease; JANIES‐CKD, The Japanese Noninvasive Electrocardiographic Risk Stratification of Sudden Cardiac Death in Chronic Kidney Disease; SHD, structural heart disease
In this study, we exclusively selected patients with CKD for analysis. CKD was defined according to the Kidney Disease: Improving Global Outcomes (KDIGO) 2012 classification (Levey et al., 2005). Overall, 183 patients (mean age 70 years, 127 men) were eligible for analysis. Patient characteristics are presented in Table 1. The mean left ventricular mass index (LVMI) was 107 ± 37.1 g/m2 (reference values were <90 g/m2 for men and <84 g/m2 for women) (Daimon et al., 2008). Approximately 75% of patients in this study had left ventricular hypertrophy (LVH). Renal replacement therapy (e.g., hemodialysis) was required in 19 patients (10.3%). The major causes of heart disease were ischemic heart disease and chronic heart failure. Other clinical characteristics, including the prevalence of hypertension, dyslipidemia, diabetes mellitus, serum brain natriuretic peptide levels, New York Heart Association (NYHA) functional class, and medication use, are detailed in Table 1.
TABLE 1.
Baseline characteristics of the study patients (n = 183)
| Characteristics | |
|---|---|
| Age, years | 70 (61.0, 77.0) |
| Male sex | 127 (69) |
| Hypertension | 133 (73) |
| Dyslipidemia | 109 (60) |
| Diabetes mellitus | 82 (45) |
| Estimated GFR, ml/min/1.73 m2 | |
| Mean | 42.4 ± 18.9 |
| 45–59 (stage 3a) | 94 (51) |
| 30–44 (stage 3b) | 44 (24) |
| 15–29 (stage 4) | 24 (13) |
| <15 (stage 5) | 21 (11) |
| LVEF, % | 55.1 (39.8, 68.0) |
| LVDd, mm | 51.2 ± 9.6 |
| LVM, g | 181.4 (146.2, 224.3) |
| LVMI, g/m2 | 107.5 ± 37.1 |
| BNP, pg/dl | 199.3 (65.9, 397.6) |
| NYHA functional class | |
| I | 123 (67) |
| II | 38 (21) |
| III | 15 (8) |
| IV | 8 (4) |
| Heart disease | |
| Ischemic heart disease | 82 (45) |
| Chronic heart failure | 71 (39) |
| Dilated cardiomyopathy | 9 (5) |
| Hypertensive heart disease | 11 (6) |
| Unknown cardiomyopathy | 10 (5) |
| ICD implantation | 16/183 (9) |
| RRT | 19/183 (10) |
| Medications | |
| β‐blocker | 134 (73) |
| RAS inhibitor | 124 (68) |
| Ca‐channel blocker | 56 (31) |
| Diuretic | 58 (32) |
| Amiodarone | 27 (15) |
| Class Ia or Ic antiarrhythmic | 5 (3) |
Values are expressed as n (%), mean ± standard deviation or median (interquartile range).
Abbreviations: BNP, brain natriuretic peptide; GFR, glomerular filtration rate; HHD, hypertensive heart disease; ICD, implantable cardioverter defibrillator; LVDd, left ventricular diastolic diameter; LVEF, left ventricular ejection fraction; LVM, left ventricular mass; LVMI, left ventricular mass index; NYHA, New York Heart Association; RAS, renin–angiotensin–aldosterone system; RRT, renal replacement therapy.
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Competent Authorities and Ethics Committees of the participating centers. Written informed consent was obtained from all patients. The JANIES study was approved by the Ethics Committee of Toho University Omori Medical Center (approval number 23‐135) and registered in the UMIN Clinical Trials Registry (UMIN000007683).
2.2. Ambulatory ECG recordings and echocardiography assessments
All ECGs were recorded using a FM‐180 Digital Holter ECG Recorder (Fukuda Denshi Co., Ltd.) and analyzed using a high‐resolution digital ambulatory 24‐h ECG system (SCM 8000, Fukuda Denshi Co., Ltd.). Routine ambulatory ECG parameters, including NSVT, ambulatory‐based LPs (a‐LPs), and HRT, were also analyzed. The presence of NSVT was defined as more than three consecutive ventricular premature contractions (VPCs) at >100 beats/min, as previously reported (Lin et al., 2016). All participants underwent 24‐h ECG assessment during their ordinary daily activities. Additionally, ECGs were obtained to assess cardiac structure and function; the left ventricular ejection fraction (LVEF), left ventricular end‐diastolic diameter, left ventricular mass (LVM), and LVMI were measured.
2.3. Measurement of ambulatory‐based LPs
Electrocardiogram data for measurement of ambulatory‐based LPs were obtained at a sampling rate of 1000 Hz and amplitude resolution using a 20‐bit analog to digital converter. A three‐pole (18 dB/octave) corrected bidirectional filter (infinite pulse response method) was used, with a bandpass ranging from 40 to 200 Hz. ECG data were filtered and ranged from 40 to 250 Hz. Orthogonal X, Y, and Z bipolar leads with silver–silver chloride electrodes (Magnerode®; Fukuda Denshi Co., Ltd.) were used for all recordings. Ambulatory‐based LPs were automatically measured every 30 min over 24 h. Values were presented on a trend graph and evaluated according to three parameters: filtered QRS duration (fQRS), duration of low‐amplitude signals <40 μV in the terminal filtered QRS complex (LAS40), and root mean square voltage of the terminal 40 ms in the filtered QRS complex (RMS40). Ambulatory‐based LPs were considered positive on fulfillment of two of the following three criteria: fQRS > 135 ms, RMS40 < 15 μV, and LAS40 > 39 ms (Abe et al., 2012). The worst values for a‐LPs (w‐LPs) were calculated and used for analysis in this study according to a previously published method (Hashimoto et al., 2020; Kinoshita et al., 2020). W‐LPs were defined as measurements detected when the RMS40 was the smallest over 24 h.
2.4. HRT measurements
Heart rate turbulence was measured according to a previously established protocol (Bauer et al., 2008) and recorded when more than one VPC occurred. HRT is characterized by two parameters, turbulence onset (TO) and turbulence slope (TS). TO captures the early‐phase sinus rhythm acceleration after VPC, followed by TS, which captures the compensatory deceleration phase. TO was calculated as the shortening ratio of RR intervals immediately after the compensatory pause of the VPC. TS was calculated as the steepest regression over any five consecutive sinus rhythm RR intervals after VPC and within 15 sinus rhythm beats (Bauer et al., 2008). TO ≥ 0% and TS ≤ 2.5 ms/RR intervals were considered abnormal. HRT was identified when both TO and TS were abnormal. Isolated TO and TS abnormalities were not regarded as HRT, nor was the inability to calculate HRT due to the absence of VPC (Bauer et al., 2008).
2.5. Study endpoints and follow‐up
The primary endpoint was the occurrence of lethal ventricular tachyarrhythmias, such as ventricular fibrillation (VF) or sustained ventricular tachycardia (SVT) and cardiac death. Shock delivery using an implantable cardioverter–defibrillator (ICD) and anti‐tachycardia pacing for sustained VT were included as lethal ventricular tachyarrhythmias. The secondary endpoint was a composite of hospital admission due to heart failure, percutaneous coronary intervention (PCI), coronary artery bypass surgery (CABG), peripheral artery disease intervention, or aortic dissection. Causes of death were retrieved from medical or autopsy records and testimonies of the primary doctors or witnesses. The occurrence of lethal ventricular tachyarrhythmias was verified through ECG monitoring performed in the hospital or by 24‐h Holter ECG recorded during hospitalization or inferred using an ICD. Follow‐up data were collected at 6‐month intervals until September 2015.
2.6. Statistical analyses
Data are presented as mean ± standard deviation for normally distributed continuous variables and as medians (interquartile range: 25th–75th percentile) for nonnormally distributed variables. The normality of the distribution was tested using the Shapiro–Wilk method. Patient characteristics were compared using the χ2 test for categorical variables, the Student's t‐test for continuous and parametric data, and the Mann–Whitney test for nonparametric data. Cox univariate and multivariate regression analyses were performed to investigate associations between the endpoints and clinical parameters. Cardiac event‐free survival rates were calculated for each NIEM using the Kaplan–Meier method, and differences in cardiac event‐free survival rates were calculated using the log‐rank test. Statistical analyses were conducted using SPSS software version 25 (IBM Corp). All tests were two‐sided, and a p‐value of .05 was considered significant.
3. RESULTS
3.1. Cardiac events during follow‐up
During the mean follow‐up period of 20.7 ± 11.1 months, 13 patients reached the primary endpoint of the study. Their characteristics are shown in Tables 2 and 3.
TABLE 2.
Comparison of risk factors between the primary endpoint group and the event‐free group
| Characteristics | Primary endpoint (n = 13) | Event free (n = 170) | p‐value | Univariate analysis HR (95% CI) | p‐value | Multivariate analysis HR (95% CI) | p‐value |
|---|---|---|---|---|---|---|---|
| Age, years | 70 (66.5, 81.0) | 69.0 (61.0,77.0) | .41 | 1.02 (0.98–1.08) | .31 | 1.02 (0.98–1.08) | .27 |
| Male | 8 (84.6) | 118 (69.4) | .25 | 1.06 (1.03–1.10) | .001 | 1.07 (1.03–1.11) | .001 |
| Hypertension | 8 (61.5) | 125 (73.5) | .35 | 0.56 (0.18–1.72) | .31 | ||
| Dyslipidemia | 8 (61.5) | 101 (59.4) | .88 | 1.04 (0.34–3.19) | .94 | ||
| Diabetes mellitus | 7 (53.8) | 75 (44.1) | .69 | 1.43 (0.48–4.26) | .52 | ||
| ICD | 4 (30.8) | 12 (7.1) | .022 | 6.79 (1.98–23.26) | .002 | ||
| RRT | 2 (15.3) | 17 (10) | .289 | 2.53 (0.53–11.95) | .24 | ||
| eGFR, (ml/min/1.73 m2) | 32.5 ± 24.3 | 42.8 ± 18 | .028 | 0.97 (0.94–0.99) | .014 | 0.96 (0.93–1.00) | .055 |
| LVEF, % | 38.0 (29.1, 52.9) | 56.9 (41.0, 69.4) | .023 | 0.95 (0.92–0.99) | .004 | 0.98 (0.94–1.03) | .44 |
| LVM, g | 205.0 (135.5, 236.4) | 180.5 (147.8, 224.4) | .81 | 1.00 (0.99–1.02) | .98 | ||
| LVMI, g/m2 | 108.7 ± 35.9 | 106.5 ± 37.6 | .88 | 1.003 (0.98–1.029) | .79 | ||
| BNP, pg/dl | 393.9 (220.0, 853.1) | 174.3 (55.7, 335.1) | .005 | ||||
| NYHA I, II | 8 (61.5) | 152 (89.4) | Ref | Ref | |||
| NYHA III, IV | 5 (38.4) | 18 (10.5) | .003 | 3.94 (1.29–12.06) | .016 | ||
| β‐blocker | 11 (92.3) | 123 (72.3) | .34 | ||||
| RAS inhibitor | 8 (61.5) | 116 (68.2) | .62 | ||||
| Amiodarone | 4 (23.4) | 23 (13.5) | .096 | ||||
| w‐LPs | 10 (76.9) | 69 (40.6) | .011 | 6.04 (1.40–22.3) | .007 | 3.18 (0.72–13.99) | .13 |
| HRT | 8 (61.5) | 68 (40.5) | .13 | 3.01 (0.98–9.25) | .54 | ||
| NSVT | 7 (53.8) | 20 (11.8) | <.001 | 8.72 (2.80–26.5) | <.001 | 10.41 (2.81–38.51) | <.0001 |
Values are expressed as n (%), mean ± standard deviation, median (interquartile range), or hazard ratio (95% confidence interval).
Abbreviations: BNP, brain natriuretic peptide; CHF, chronic heart failure; CI, confidence intervals; DCM, dilated cardiomyopathy; GFR, glomerular filtration rate; HHD, hypertensive heart disease; HRT, heart rate turbulence; ICD, implantable cardioverter defibrillator; IHD, ischemic heart disease; LVDd, left ventricular dimension diameter; LVEF, left ventricular ejection fraction; LVM, left ventricular mass; LVMI, left ventricular mass index; NSVT, nonsustained ventricular tachycardia; RAS, renin–angiotensin–aldosterone system; ref, reference; RRT, renal replacement therapy; w‐LP, worst value of ambulatory‐based late potentials.
TABLE 3.
The characteristics of patients who developed the primary endpoint
| No | Age/Sex | eGFR (ml/min/m2) | CKD stage | Heart disease | NYHA class | EF (%) | LVDD (mm) | NIEP positive | ICD | RRT | Time to event (days) | SVT/VF | Death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 76/F | 54.3 | 3a | IHD | 4 | 53.0 | 44.5 | HRT | 304 | SVT/VF | |||
| 2 | 49/M | 53.9 | 3a | DCM | 4 | 33.7 | 63.2 | w‐LPs, NSVT | + | 126 | SVT | ||
| 3 | 56/F | 48.9 | 3a | DCM | 3 | 27.9 | 70.1 | w‐LPs, HRT, NSVT | + | 495 | SVT | ||
| 4 | 86/M | 44.8 | 3b | HF | 2 | 52.8 | 55.6 | w‐LPs, NSVT | 978 | Death (Heart failure) | |||
| 5 | 86/M | 34 | 3b | IHD | 2 | 37.0 | 49.0 | w‐LPs, HRT | + | 101 | SVT | ||
| 6 | 68/M | 33 | 3b | DCM | 2 | 38.0 | 65.0 | w‐LPs, NSVT | 630 | Death (Heart failure) | |||
| 7 | 68/M | 27.2 | 4 | DCM | 3 | 30.3 | 64.9 | w‐LPs, NSVT | 29 | Death (Heart failure) | |||
| 8 | 68/M | 22 | 4 | IHD | 2 | 22.0 | 72.0 | w‐LPs, HRT, NSVT | + | 33 | Death (Heart failure) | ||
| 9 | 71/M | 20.3 | 4 | IHD | 3 | 48.7 | 47.9 | HRT, NSVT | 264 | Death (Heart failure) | |||
| 10 | 70/M | 14.6 | 5 | IHD | 2 | 74.7 | 37.2 | w‐LPs, HRT, NSVT | 481 | Death (Heart failure) | |||
| 11 | 89/M | 14.1 | 5 | IHD | 2 | 26.0 | 57.2 | w‐LPs, HRT | 490 | Death (Heart failure) | |||
| 12 | 74/M | 9.8 | 5 | HF | 2 | 47.1 | 56.8 | none | + | 950 | Death (Heart failure) | ||
| 13 | 65/M | 4.4 | 5 | IHD | 1 | 67.9 | 54.7 | w‐LPs, HRT | + | 384 | Death (Heart failure) |
Abbreviations: CKD, chronic heart failure; DCM, dilated cardiomyopathy; EF, ejection fraction; eGFR, estimated glomerular filtration rate; F, female; HF, heart failure; HRT, heart rate turbulence; ICD, implantable cardioverter defibrillator; IHD, ischemic heart disease; M, male; NIEPs, noninvasive electrocardiographic parameters; NSVT, nonsustained ventricular tachycardia; NYHA, New York Heart Association; RRT, renal replacement therapy; SVT, sustained ventricular tachycardia; VF, ventricular fibrillation; w‐LPs, worst value of ambulatory‐based late potentials.
3.2. Association between ECG markers and endpoints
3.2.1. Primary endpoint
Univariate analysis (Table 2) revealed that sex, ICD implantation, estimated glomerular filtration rate (eGFR), LVEF, NYHA cardiac functional classification of III or IV, w‐LPs, and NSVT were significantly associated with the primary endpoint (p = .001, .002, .014, .004, .016, .007, and <.001, respectively). However, NSVT was the factor that correlated most significantly with the primary endpoint in multivariate analysis (hazard ratio [HR], 10.41; 95% confidence interval [CI], 2.81−38.51). Table 3 demonstrates the individual characteristics of patients who reached the primary endpoint. Six of 13 patients were categorized as having CKD stage 3 (patient No. 1–6); the remaining seven patients were categorized as having CKD stage 4 or 5 (patient No. 7–13). Severe arrhythmic events such as SVT/VF were documented in four of the six patients with CKD stage 3 (No. 1–3, 5). Moreover, two patients of these four were positive for both w‐LP and NSVT. These two patients (No. 2 and 3) survived due to anti‐tachycardia pacing or appropriate ICD shock delivery. However, all seven patients with CKD stage 4 or 5 died of heart failure. None of the patients developed serious arrhythmic events. The predictive ability of single and combined ECG risk markers is presented in Table 4. The highest positive predictive value (PPV) (47%), positive likelihood ratios (P‐LRs) (11.4), predictive accuracy (PA) (92%), and lowest negative likelihood ratios (N‐LRs) were obtained when both w‐LPs and NSVT were present (HR, 18.22; 95% CI, 5.64−58.81; p < .0001). This combination remained significantly associated with the primary endpoint after adjusting for age, sex, eGFR, and LVEF (HR, 19.18; 95% CI, 4.97−74.08; p < .0001) (Table S1). Kaplan–Meier analysis revealed that the concurrence of w‐LPs and NSVT positivity was associated with a significantly lower event‐free survival rate than was the presence of either variable alone (log‐rank, p < .0001) (Figure 2).
TABLE 4.
Association and prognostic ability of ambulatory electrocardiographic parameters with respect to the primary endpoint
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | P‐LRs | N‐LRs | PA (%) | RH (95% CI) | p‐value | |
|---|---|---|---|---|---|---|---|---|---|
| w‐LPs | 77 | 59 | 13 | 97 | 1.9 | 0.39 | 61 | 6.04 (1.63–22.27) | .007 |
| HRT | 62 | 60 | 11 | 95 | 1.5 | 0.64 | 60 | 3.01 (0.98–9.25) | .054 |
| NSVT | 54 | 89 | 27 | 96 | 4.8 | 0.51 | 86 | 8.72 (2.87–26.47) | <.001 |
| w‐LPs + HRT | 46 | 82 | 17 | 95 | 2.61 | 0.65 | 80 | 5.07 (1.69–15.27) | .004 |
| NSVT + HRT | 31 | 94 | 27 | 95 | 4.76 | 0.74 | 89 | 6.55 (1.99–21.59) | .02 |
| w‐LPs + NSVT | 54 | 95 | 47 | 96 | 11.4 | 0.48 | 92 | 18.22 (5.64–58.81) | <.001 |
Abbreviations: CI, confidence interval; HRT, heart rate turbulence; N‐LRs, negative likelihood ratios; NPV, negative predictive value; NSVT, nonsustained ventricular tachycardia; PA, predictive accuracy; P‐LRs, positive likelihood ratios; PPV, positive predictive value; RH, relative hazard; w‐LPs, worst value of ambulatory‐based late potentials.
FIGURE 2.
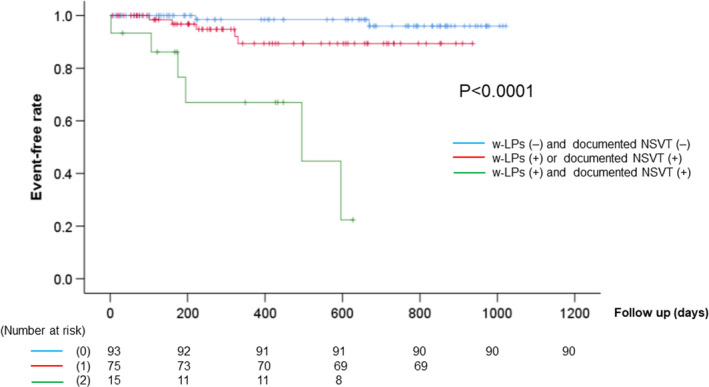
Kaplan–Meier event‐free survival curves of NIEMs for cardiac death and lethal arrhythmias (primary endpoint). The combination of a‐LP and NSVT was associated with a significantly lower event‐free survival rate than was any other combination. NIEMs, noninvasive electrocardiographic markers; NSVT, nonsustained ventricular tachycardia; w‐LPs, worst values of ambulatory‐based late potentials
3.2.2. Secondary endpoint
Univariate analysis (Table S2) revealed that sex, LVEF, LVM, LVMI, NYHA cardiac functional classification of III or IV, w‐LPs, and HRT were significantly associated with the secondary endpoint (p = .006, .029, .003, .004, and .049, respectively). However, multivariate analysis showed that none of these variables could predict the secondary endpoint. Cox multivariate regression analysis showed that left ventricle (LV) mass and LVMI were significantly associated with the secondary endpoint (HR, 1.007; 95% CI, 1.001−1.013; p < .034) (Table S2).
4. DISCUSSION
The main findings of this study were as follows. In patients with CKD and SHD, the combination of w‐LPs and NSVT was a strong predictor of lethal ventricular tachyarrhythmia or cardiac death, even after adjusting for factors, such as age, sex, eGFR, and LVEF. The combined positivity of w‐LP and NSVT was associated with a significantly lower event‐free survival rate than was the positivity of either variable alone, and no independent factor was useful for predicting the secondary endpoint.
4.1. Combined ECG markers for primary endpoint prediction
To the best of our knowledge, this is the first study to evaluate the usefulness of w‐LPs, HRT, and NSVT in patients with CKD. Furthermore, we evaluated the utility of w‐LPs recorded using a new and highly versatile high‐resolution ambulatory ECG system. Individually, w‐LPs, HRT, and NSVT had high negative predictive values for predicting lethal ventricular tachyarrhythmias and cardiac death (90%−95%), with PPVs of 10%–20%. We believe that the strong point of our study is the finding that LPs and NSVT combined have a superior PPV than does either variable alone. A meta‐analysis by Fu et al. (2017) reported that ICD implantation improved the survival rate in patients with CKD stage 3 but was not beneficial in patients with stage 4 or 5 disease. They suggested that this may be because as CKD progresses, sympathetic nervous system activity increases and is modified by heart failure, making the ICD less effective. Therefore, ICD implantation should be considered, especially in patients with CKD stage 3 who are NSVT and w‐LP positive and have not had ICD implantation. In our study, all four patients who developed SVT/VF had stage 3 CKD, and the presence of NSVT and w‐LP was a useful risk marker in 50% (2/4) of these patients (Table 3). These two patients may have survived due to ICD implantation. In addition, only one of the 183 patients in our study resulted in death without any NIEM (Table 3, no 12). Therefore, if a CKD patient with cardiac disease has no NIEMs, it is highly improbable that a fatal event such as SVT/VF or heart failure death will occur in the near future.
4.2. Pathophysiology of lethal ventricular tachyarrhythmias and heart failure death in CKD patients and its relationship with ECG markers
The pathophysiology of lethal ventricular arrhythmias in CKD patients has not been fully elucidated. In the general population, electrophysiological abnormalities due to SHD and modifying factors, such as electrolyte imbalance or heart failure, facilitate the progression of transient arrhythmic triggers (e.g., extrasystoles) into lethal arrhythmias (Ikeda et al., 2007). It is speculated that the interaction between CKD and SHD accelerates these root causes, increasing the risk of sudden arrhythmic death. It is well known that LPs reflect an abnormal electrophysiological condition in patients with SHD (Ikeda et al., 2007; Zimmermann et al., 1997). Additionally, NSVT could be a trigger of lethal arrhythmias, such as VT and VF (Ikeda et al., 2007). Because w‐LPs and NSVT are expressions of underlying arrhythmic mechanisms, they were predictive of the primary endpoint in this study.
Nine of the 13 patients who reached the primary endpoint died of heart failure, and more than four developed fatal arrhythmias. CKD patients are likely to suffer from ischemic heart disease and LVH, which lead to myocardial electrophysiological abnormalities, especially ventricular conduction delay (Morales et al., 1998). Morales et al (Morales et al., 1998) found that in patients with CKD, LVH could prolong the fQRS duration in LPs due to a longer depolarization time. Additionally, pump failure is common in CKD patients due to neurohormonal abnormalities, left diastolic dysfunction, inflammation due to decreased coronary perfusion, and coronary tissue calcification (Ronco et al., 2008). In CKD patients, complex ventricular arrhythmias such as NSVT were significantly associated with a lower LVEF, a higher LVMI, and an increased calcium score (Bonato et al., 2016). Additionally, it has been reported that documented NSVT is associated with increased mortality in CKD patients with SHD (Bonato et al., 2016). Because w‐LPs and NSVT reflect the severity of heart failure due to LVH, they were predictive of death due to heart failure in this study. However, there were no significant differences in LVMI between the primary endpoint and event‐free groups (Table 2). Cox univariate analysis showed that neither LVM nor LVMI could predict the primary endpoint. Therefore, the combination of w‐LPs and NSVT was a superior surrogate marker to predict the progression of heart failure leading to death.
4.3. Noninvasive ECG markers and the secondary endpoint
In this study, NIEMs were not useful for predicting cardiac events requiring hospital admission (secondary endpoint). Expectations in this regard were low because these markers have ordinarily been associated with severe events, such as SCD or lethal arrhythmias (Hashimoto et al., 2020; Kinoshita et al., 2020; Morales et al., 1998). In our study, there was a significant association between LVMI and the secondary endpoint (Table 4), which is consistent with the results from several other studies that showed a positive correlation between an increased LVMI and complications, such as heart failure, myocardial infarction, angina requiring PCI or CABG, and stroke (Dubin et al., 2017; Lee et al., 2020; Sundström et al., 2001).
4.4. Limitations
Our study has some limitations. First, although the JANIES study included a large population (n = 1829), our sample size was small because we focused solely on CKD patients. Additionally, since all subjects had SHD, our results cannot be extrapolated to CKD patients without cardiac disease. Finally, because of the limited scope of our study, T‐wave alternans were not included for analysis. This recognized repolarization abnormality increases the risk for lethal ventricular tachyarrhythmias or SCD and may be a useful predictive parameter in CKD patients.
5. CONCLUSION
In patients with CKD and SHD, the combined assessment of w‐LPs and NSVT was a strong predictor of lethal ventricular tachyarrhythmia and cardiac death. Additionally, the presence of combined w‐LPs and NSVT was associated with a significantly lower event‐free survival rate. However, NIEMs were not useful in predicting cardiovascular events requiring hospital admission. Our results suggest that these parameters may be clinically useful for risk assessment in patients with CKD and SHD. Patients with positive w‐LP and documented NSVT should be considered for ICD implantation, particularly those with stage 3 CKD. Future studies, including larger populations of CVD and CKD patients, are needed to confirm our results and explore further applications of these robust risk markers.
CONFLICT OF INTEREST
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
ETHICAL APPROVAL
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Competent Authorities and Ethics Committees of the participating centers. Written informed consent was obtained from all patients. The JANIES study was approved by the Ethics Committee of Toho University Omori Medical Center (approval number 23‐135) and registered in the UMIN Clinical Trials Registry (UMIN000007683).
Supporting information
Table S1‐S2
ACKNOWLEDGMENTS
The first author (K.H.) would like to express his gratitude and appreciation for his advisor, Hiroaki Shimabukuro, who has been an exceptional mentor, encouraging the author throughout this study. The JANIES study was supported, in part, by a Grant‐in‐aid for Scientific Research (JSPS KAKENHI) [grant numbers 21590909, 24591074, and 15K09103] from the Japanese Society of Promotion of Science.
Hashimoto, K. , Kinoshita, T. , Miwa, Y. , Amino, M. , Yoshioka, K. , Yodogawa, K. , Nakagawa, M. , Nakamura, K. , Watanabe, E. , Nakamura, K. , Watanabe, T. , Kasamaki, Y. , & Ikeda, T. (2022). Ambulatory electrocardiographic markers predict serious cardiac events in patients with chronic kidney disease: The Japanese Noninvasive Electrocardiographic Risk Stratification of Sudden Cardiac Death in Chronic Kidney Disease (JANIES‐CKD) study. Annals of Noninvasive Electrocardiology, 27, e12923. 10.1111/anec.12923
DATA AVAILABILITY STATEMENT
The data used in this report are available from the corresponding author on reasonable request.
REFERENCES
- Abe, A. , Kobayashi, K. , Yuzawa, H. , Sato, H. , Fukunaga, S. , Fujino, T. , Okano, Y. , Yamazaki, J. , Miwa, Y. , Yoshino, H. , & Ikeda, T. (2012). Comparison of late potentials for 24 hours between Brugada syndrome and arrhythmogenic right ventricular cardiomyopathy using a novel signal‐averaging system based on Holter ECG. Circulation: Arrhythmia and Electrophysiology, 5, 789–795. 10.1161/CIRCEP.111.969865 [DOI] [PubMed] [Google Scholar]
- Bauer, A. , Malik, M. , Schmidt, G. , Barthel, P. , Bonnemeier, H. , Cygankiewicz, I. , Guzik, P. , Lombardi, F. , Müller, A. , Oto, A. , Schneider, R. , Watanabe, M. , Wichterle, D. , & Zareba, W. (2008). Heart rate turbulence: Standards of measurement, physiological interpretation, and clinical use: International Society for Holter and Noninvasive Electrophysiology Consensus. Journal of the American College of Cardiology, 52, 1353–1365. 10.1016/j.jacc.2008.07.041 [DOI] [PubMed] [Google Scholar]
- Bonato, F. O. , Watanabe, R. , Lemos, M. M. , Cassiolato, J. L. , Wolf, M. , & Canziani, M. E. (2016). Asymptomatic ventricular arrhythmia and clinical outcomes in chronic kidney disease: A pilot study. Cardiorenal Medicine, 7, 66–73. 10.1159/000449260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daimon, M. , Watanabe, H. , Abe, Y. , Hirata, K. , Hozumi, T. , Ishii, K. , Ito, H. , Iwakura, K. , Izumi, C. , Matsuzaki, M. , Minagoe, S. , Abe, H. , Murata, K. , Nakatani, S. , Negishi, K. , Yoshida, K. , Tanabe, K. , Tanaka, N. , Tokai, K. , … The JAMP Study Investigators . (2008). Normal values of echocardiographic parameters in relation to age in a healthy Japanese population: The JAMP study. Circulation Journal, 72, 1859–1866. 10.1253/circj.cj-08-0171 [DOI] [PubMed] [Google Scholar]
- Dubin, R. F. , Deo, R. , Bansal, N. , Anderson, A. H. , Yang, P. , Go, A. S. , Keane, M. , Townsend, R. , Porter, A. , Budoff, M. , Malik, S. , He, J. , Rahman, M. , Wright, J. , Cappola, T. , Kallem, R. , Roy, J. , Sha, D. , & Shlipak, M. G. (2017). Associations of conventional echocardiographic measures with incident heart failure and mortality: The chronic renal insufficiency cohort. Clinical Journal of the American Society of Nephrology, 12, 60–68. 10.2215/CJN.02700316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, L. , Zhou, Q. , Zhu, W. , Lin, H. , Ding, Y. , Shen, Y. , Hu, J. , & Hong, K. (2017). Do implantable cardioverter defibrillators reduce mortality in patients with chronic kidney disease at all stages? International Heart Journal, 58, 371–377. 10.1536/ihj.16-357 [DOI] [PubMed] [Google Scholar]
- Gatzoulis, K. A. , Arsenos, P. , Trachanas, K. , Dilaveris, P. , Antoniou, C. , Tsiachris, D. , Sideris, S. , Kolettis, T. M. , & Tousoulis, D. (2018). Signal‐averaged electrocardiography: Past, present, and future. Journal of Arrhythmia, 34, 222–229. 10.1002/joa3.12062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go, A. S. , Chertow, G. M. , Fan, D. , McCulloch, C. E. , & Hsu, C. Y. (2004). Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. New England Journal of Medicine, 351, 1296–1305. 10.1056/NEJMoa041031 [DOI] [PubMed] [Google Scholar]
- Hashimoto, K. , Amino, M. , Yoshioka, K. , Kasamaki, Y. , Kinoshita, T. , & Ikeda, T. (2020). Combined evaluation of ambulatory‐based late potentials and nonsustained ventricular tachycardia to predict arrhythmic events in patients with previous myocardial infarction: A Japanese noninvasive electrocardiographic risk stratification of sudden cardiac death (JANIES) substudy. Annals of Noninvasive Electrocardiology, 26, e12803. 10.1111/anec.12803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda, T. , Yusu, S. , Nakamura, K. , & Yoshino, H. (2007). Risk stratification for sudden cardiac death. Circulation Journal, 71(Suppl A), 106–114. 10.1253/circj.71.a106 [DOI] [PubMed] [Google Scholar]
- Jesky, M. , Lambert, A. , Burden, A. C. , & Cockwell, P. (2013). The impact of chronic kidney disease and cardiovascular comorbidity on mortality in a multiethnic population: A retrospective cohort study. British Medical Journal Open, 3, e003458. 10.1136/bmjopen-2013-003458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith, D. S. , Nichols, G. A. , Gullion, C. M. , Brown, J. B. , & Smith, D. H. (2004). Longitudinal follow‐up and outcomes among a population with chronic kidney disease in a large managed care organization. Archives of Internal Medicine, 164, 659–663. 10.1001/archinte [DOI] [PubMed] [Google Scholar]
- Kinoshita, T. , Hashimoto, K. , Yoshioka, K. , Miwa, Y. , Yodogawa, K. , Watanabe, E. , Nakamura, K. , Nakagawa, M. , Nakamura, K. , Watanabe, T. , Yusu, S. , Tachibana, M. , Nakahara, S. , Mizumaki, K. , & Ikeda, T. (2020). Risk stratification for cardiac mortality using electrocardiographic markers based on 24‐hour Holter recordings: The JANIES‐SHD study. Journal of Cardiology, 75, 155–163. 10.1016/j.jjcc.2019.07.012 [DOI] [PubMed] [Google Scholar]
- Lee, S. W. , Min, H. K. , Chae, D.‐W. , Oh, K.‐H. , Ahn, C. , Chung, W. , Lee, J. , Kim, Y.‐S. , & Sung, S. A. (2020). Representing the KNOW‐CKD Study Group. Indexation of left ventricular mass to predict adverse clinical outcomes in pre‐dialysis patients with chronic kidney disease: Korean cohort study of the outcome in patients with chronic kidney disease. PLoS One, 15, e0233310. 10.1371/journal.pone.0233310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey, A. S. , Eckardt, K.‐U. , Tsukamoto, Y. , Levin, A. , Coresh, J. , Rossert, J. , Zeeuw, D. D. E. , Hostetter, T. H. , Lameire, N. , & Eknoyan, G. (2005). Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney International, 67, 2089–2100. 10.1111/j.1523-1755.2005.00365.x [DOI] [PubMed] [Google Scholar]
- Lin, C.‐Y. , Chang, S.‐L. , Chung, F.‐P. , Chen, Y.‐Y. , Lin, Y.‐J. , Lo, L.‐W. , Hu, Y.‐F. , Tuan, T.‐C. , Chao, T.‐F. , Liao, J.‐N. , Chang, Y.‐T. , Lin, C.‐H. , Allamsetty, S. , Walia, R. , Te, A. L. D. , Yamada, S. , Chiang, S.‐J. , Tsao, H.‐M. , & Chen, S.‐A. (2016). Long‐term outcome of non‐sustained ventricular tachycardia in structurally normal hearts. PLoS One, 11, e0160181. 10.1371/journal.pone.0160181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales, M. , Gremigni, C. , Dattolo, P. , Piacenti, M. , Cerrai, T. , Fazi, A. , Pelosi, G. , Vergassola, R. , & Maggiore, Q. (1998). Signal‐averaged ECG abnormalities in haemodialysis patients. Role of dialysis. Nephrology Dialysis Transplantation, 13, 668–673. 10.1093/ndt/13.3.668 [DOI] [PubMed] [Google Scholar]
- Pun, P. H. (2014). The interplay between CKD, sudden cardiac death, and ventricular arrhythmias. Advances in Chronic Kidney Disease, 21, 480–488. 10.1053/j.ackd.2014.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronco, C. , Haapio, M. , House, A. A. , Anavekar, N. , & Bellomo, R. (2008). Cardiorenal syndrome. Journal of the American College of Cardiology, 52, 1527–1539. 10.1016/j.jacc.2008.07.051 [DOI] [PubMed] [Google Scholar]
- Sundström, J. , Lind, L. , Arnlöv, J. , Zethelius, B. , Andrén, B. , & Lithell, H. O. (2001). Echocardiographic and electrocardiographic diagnoses of left ventricular hypertrophy predict mortality independently of each other in a population of elderly men. Circulation, 103, 2346–2351. 10.1161/01.cir.103.19.2346 [DOI] [PubMed] [Google Scholar]
- Zimmermann, M. , Sentici, A. , Adamec, R. , Metzger, J. , Mermillod, B. , & Rutishauser, W. (1997). Long‐term prognostic significance of ventricular late potentials after a first acute myocardial infarction. American Heart Journal, 134, 1019–1028. 10.1016/s0002-8703(97)70021-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S2
Data Availability Statement
The data used in this report are available from the corresponding author on reasonable request.


