Abstract
While individual neurons are the basic unit of the nervous system, they process information by working together in neuronal circuits with specific patterns of synaptic connectivity. Here we review common circuit motifs and architectural plans used in diverse brain regions and animal species. We also consider how these circuit architectures assemble during development and might have evolved. Understanding how specific patterns of synaptic connectivity can implement specific neural computations will help bridge the huge gap between the biology of the individual neuron and the function of the entire brain, will allow us to better understand the neural basis of behavior, and may inspire new advances in artificial intelligence.
One-sentence summary:
Neuronal circuit architectures and their function, evolution, and development are reviewed here.
Over a century ago, Santiago Ramón y Cajal and his contemporaries proposed that individual neurons are the basic unit of the nervous system. Cajal further proposed that information flows from dendrites to cell bodies to axons within individual neurons (Fig. 1) (1). Given that dendrites and axons of most vertebrate neurons are readily distinguishable morphologically, systematic studies of isolated neurons labeled by Golgi stains (2) provided the first overview of how information flows within vertebrate nervous systems (1).
Fig. 1. Information flow within a vertebrate neuron.
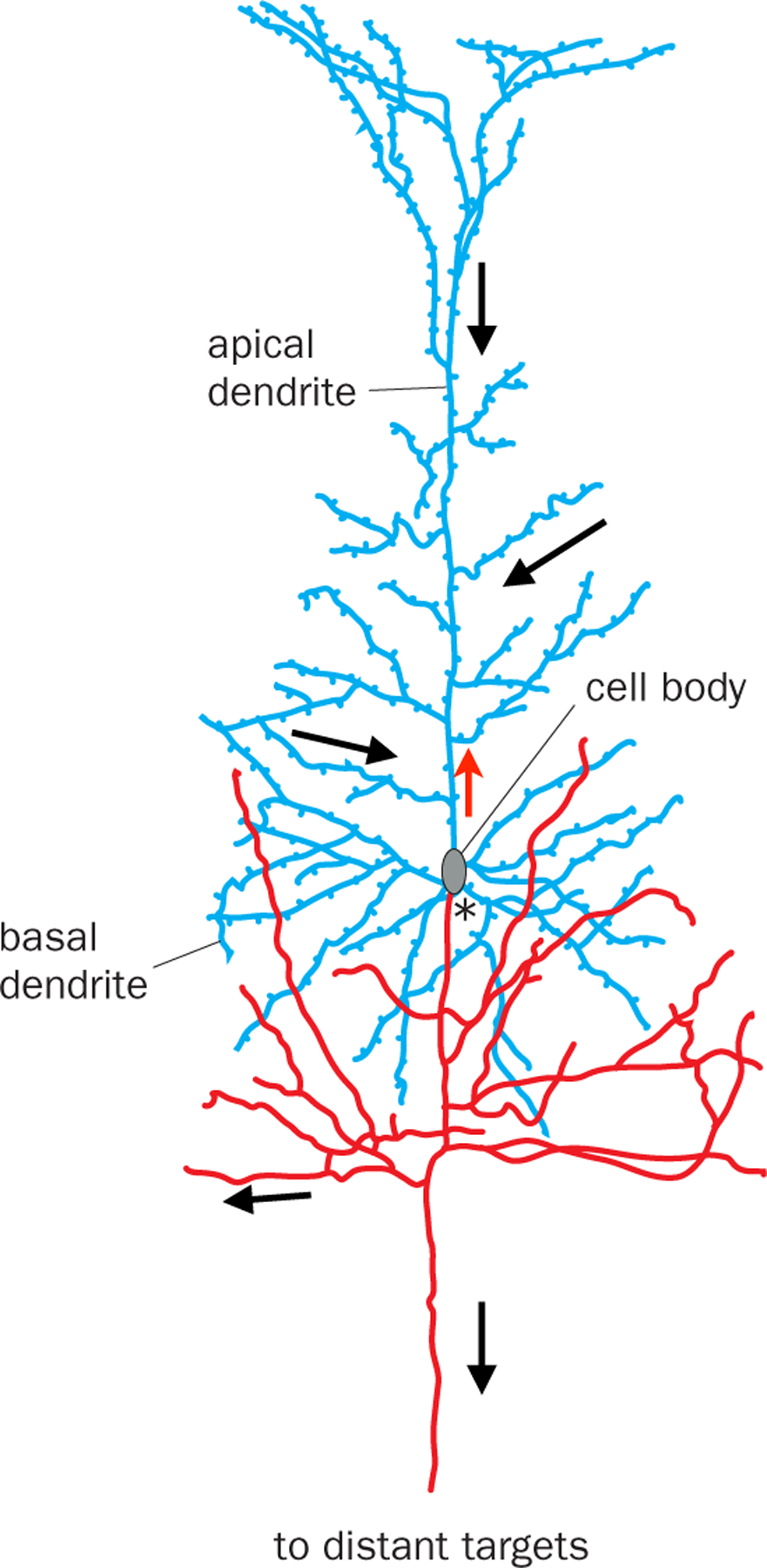
A pyramidal neuron from rabbit cerebral cortex. Neurons generally use dendrites (blue) to receive information from their presynaptic partners and the axon (red) to send information to their postsynaptic partners. Thus, information flows from dendrites to cell bodies to the axon (black arrows). *, axon initial segment, where action potentials are generated. Red arrow indicates back-propagating action potentials that can interact with synaptic inputs in complex ways for dendritic computation (115). While axons and dendrites are readily distinguishable by morphological criteria in most vertebrate neurons, most invertebrate CNS neurons extend a single process from the cell body that gives rise to both dendrites and the axon. Thus, in invertebrate neurons, information flow is less easily deciphered from morphological criteria alone. Modified after (1, 5).
With the advent of modern technologies (Box 1), we have accumulated vast amounts of knowledge of the anatomical, physiological, and functional properties of individual neurons. However, individual neurons do not work in isolation: they work together in neuronal circuits to process information. What is less clear is whether there are generalizable principles about the structural organization of neuronal circuits across different brain regions and animal species. Here I discuss principles underlying how neurons communicate with each other through specific patterns of synaptic connectivity. While the importance of activity dynamics in neuronal populations has been increasingly recognized in information processing in diverse systems from invertebrates to mammals (3, 4), synaptic connectivity patterns provide the physical bases on which neuronal dynamics execute their functions. Understanding how these connectivity patterns implement specific computations will allow us to decipher information processing principles in the nervous system and should inspire new advances in artificial intelligence.
Box 1: Tools for mapping neuronal circuit architecture.
Diverse tools have been used to study the structural organization of the nervous system (94, 95).
Single neuron tracing.
In this approach, a dense library of single neurons within a neural region is created by sparse labeling in individual animals, using the Golgi stain (2), intracellular dye filling (8), or genetic methods (96–100), such that dendritic morphology and axonal projections are clearly resolved using light microscopy. One can infer that neuron A synapses onto neuron B when A’s axon overlaps with B’s dendrites. Cajal used this approach to chart the coarse organization of the vertebrate nervous system (1). Genetic methods for sparse labeling now allow researchers to infer connectivity between neurons of specific types. A key limitation of this method is that spatial overlap visualized with light microscopy between dendrites and axons is necessary but insufficient to confidently classify two neurons as synaptic partners. Thus, it is only useful for inferring possible connectivity at a coarse level.
Serial electron microscopic (EM) reconstruction.
This is the most comprehensive way of deciphering synaptic wiring diagrams, as EM is the only method able to unambiguously visualize synapses. All synapses can be visualized in the same specimen, with the potential of producing a complete wiring diagram. Serial EM reconstruction has been used to decipher the synaptic wiring diagram of the entire C. elegans nervous system (101). Recent years have seen rapid progress in the acquisition and partial reconstruction of EM volumes of neural regions from multiple organisms (89, 102–105). A densely reconstructed Drosophila hemi-brain has been achieved (106), and the entire mouse brain has been proposed as the next ambitious target (107). Limitations include the extensive labor needed to accurately reconstruct connections from EM volumes, especially across large distances, and the difficulty of deciphering cell types or connection signs (excitatory vs. inhibitory) unless the region is also well characterized by other means.
Trans-synaptic tracing.
This approach relies on an event such as gene expression or viral transduction occurring in one neuron to trigger the labeling of its presynaptic partners (retrograde trans-synaptic labeling) or postsynaptic partners (anterograde trans-synaptic labeling). The most widely used methods in mammals utilize viruses that naturally transduce neurons across synapses, in particular rabies virus for retrograde trans-synaptic tracing from a defined neuron type in a specific location (108, 109). Axon terminal-initiated rabies tracing can reveal inputs to neuronal populations that project to specific targets, allowing inference of input–output architecture (Fig. 4). Anterograde methods have also been reported (110–113). Limitations include poor understanding of trans-synaptic transmission mechanisms, potential biases due to cell type and subcellular locations of synapses, and incomplete characterization of false negatives (synaptic partners not labeled) and false positives (labeling of non-synaptic partners) for most methods.
Electrophysiological and optical methods.
Simultaneous intracellular recordings can reveal synaptic connections between multiple neurons, as well as their sign and strength. This method is mostly limited to in vitro preparations and is therefore mostly used to map local connectivity. However, channelrhodopsin (ChR2)-assisted circuit mapping (114) can map long-range connections between a specific input population (expressing ChR2) and its target neurons in a brain slice, as photostimulating ChR2+ axon terminals can often elicit responses in postsynaptic neurons. Due to their low throughput, these electrophysiological and optical methods are mostly used to validate connections suggested by other methods and for detailed analysis of synaptic properties, rather than to reveal connectivity within or between neural regions de novo.
Commonly Used Circuit Motifs
If individual neurons are ‘letters’ in an alphabet used to write an ‘article’ that is a brain, then what are the intermediates? In this section, we focus on circuit motifs used across diverse brain regions and animal species (5) (Fig. 2), which can be considered ‘words.’ In the next section, we explore circuit architectures that might operate at the level of ‘sentences.’ Here, we discuss the most commonly used circuit motifs involving excitatory and inhibitory neurons. Some of these motifs apply not only to neuronal circuits but also to gene regulatory circuits (6). Architectures based on some of these motifs have also been used in artificial intelligence to great effect (7).
Fig. 2. Commonly used circuit motifs.
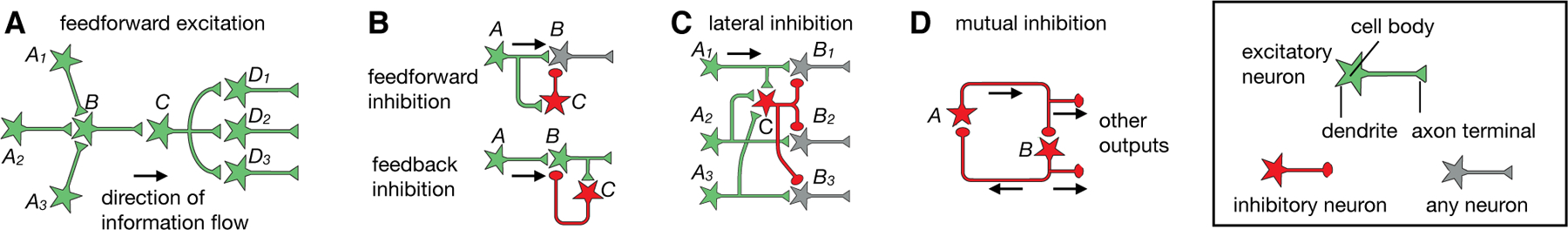
See box on the right for notations. (A) Feedforward excitation. Information flows through a series of excitatory neurons, A to D. Three different A neurons synapse onto B, exemplifying convergent excitation. C synapses onto three distinct D neurons, exemplifying divergent excitation. (B) In feedforward inhibition (top), inhibitory neuron C receives input from presynaptic excitatory neuron A and sends inhibitory output to postsynaptic neuron B; in feedback inhibition (bottom), inhibitory neuron C receives input from and sends inhibitory output to postsynaptic excitatory neuron B. (C) In lateral inhibition, parallel pathways (An → Bn; 3 are shown) each excite inhibitory neuron C, which in turn sends inhibitory output to all pathways. (D) In mutual inhibition, two inhibitory neurons form reciprocal connections and also provide outputs through branched axons to broadcast their activity states. The inhibitory neurons can also act through intermediary excitatory neurons to inhibit each other (not shown). A–C, modified after (5).
Feedforward excitation.
The primary means by which signals flow from one region to another is through feedforward excitation, a series of connections between excitatory neurons (Fig. 2A). At each stage, a neuron often receives input from multiple presynaptic partners (convergent excitation) and sends output via branched axons to multiple postsynaptic partners (divergent excitation). Convergent excitation can enable postsynaptic neurons to respond selectively to features not solely or explicitly present in any of the presynaptic neurons. It can also increase signal-to-noise ratio if multiple input neurons carry the same signal but uncorrelated noise. Divergent excitation allows the same signal to be processed by multiple downstream pathways.
One of the best characterized examples of feedforward excitation is the mammalian visual system, where signals flow from photoreceptors → bipolar cells → retinal ganglion cells → lateral geniculate nucleus (LGN) relay neurons → layer 4 primary visual cortical (V1) neurons → V1 neurons in other layers → neurons in higher cortical areas (1, 8, 9). (Note that while the discussion here focuses on individual neurons and their synaptic connections, feedforward excitation can also be applied to neural regions in broad strokes, such as retina → LGN → V1.) Along these feedforward pathways, representations of visual information are transformed from light intensity to contrasts, edges, objects, and motion. The feedforward architecture of the mammalian visual system inspired the development of the ‘perceptron’ (10) and ‘deep neural network’ (11) for image recognition and categorization; deep neural networks have also been used in artificial intelligence to solve problems far beyond image analysis (7).
Feedforward/feedback inhibition.
While long-range signals in the nervous system are mostly delivered by excitatory neurons (notable exceptions include basal ganglia and cerebellum circuits), inhibitory interneurons play key roles in sculpting such signals locally (12–14). Two widely used motifs are feedforward and feedback inhibition (Fig. 2B). In feedforward inhibition, an inhibitory neuron receives input from a presynaptic excitatory neuron, and both inhibitory and presynaptic excitatory inputs converge onto a postsynaptic neuron. In feedback inhibition, an inhibitory neuron receives input from and projects back onto an excitatory neuron, often at its presynaptic terminals. Almost every excitatory connection in the visual pathway described above is accompanied by feedforward inhibition, feedback inhibition, or both. For example, LGN neurons directly excite V1 GABAergic neurons to provide feedforward inhibition to layer 4 excitatory neurons, and layer 4 excitatory neurons also activate V1 GABAergic neurons to provide feedback inhibition onto themselves (15, 16).
Feedforward inhibition acts more rapidly than feedback inhibition, as it reaches the postsynaptic target cell with only one synaptic delay after excitatory signals, whereas feedback inhibition has two synaptic delays (Fig. 2B). Feedforward inhibition is proportional to the strength of the input, whereas feedback inhibition is proportional to the strength of the output. Both are used to regulate the duration and magnitude of incoming excitatory signals. For example, limiting the duration of activation in response to sensory input allows circuits to quickly return to their baseline activity levels, so as to maximize their sensitivity to future inputs that signal changes in the environment. Networks of feedforward and feedback inhibitory neurons often act in concert and can perform many interesting functions, such as regulating the gain and dynamic range of input signals and facilitating synchronous or oscillatory firing (14, 17). Feedforward and feedback inhibition also play an essential role in maintaining a ‘balance’ between excitation and inhibition (e.g., strong excitation is accompanied by strong inhibition) to prevent overly excited or inhibited states. Such ‘balanced’ networks can enhance the speed and signal-to-noise ratio of information processing (18, 19).
Lateral inhibition.
Lateral inhibition (Fig. 2C) is a widely occurring circuit motif. It selects information to be propagated to downstream circuits by amplifying differences in activity between parallel pathways. For example, photoreceptor neurons in the vertebrate retina activate horizontal cells, which provide feedback inhibition to many photoreceptor neurons nearby. This action is a major contributor to the classic center–surround receptive field in downstream ganglion cells (20, 21), conferring on these neurons the ability to extract information about spatial or color contrast. Lateral inhibition is also used in other sensory systems (3, 22, 23), with the general purpose of sharpening representations of ethologically relevant information to be processed by downstream circuits.
Mutual inhibition.
Communication between inhibitory neurons can confer circuits interesting properties. For example, if inhibitory neuron A directly inhibits inhibitory neuron B, then activation of A would disinhibit target neurons of B. If B also inhibits A, then they form the mutual (reciprocal) inhibition motif (Fig. 2D). Mutual inhibition is widely used in circuits that exhibit rhythmic activity, such as those involved in locomotion (24). A classic example is the crustacean stomatogastric ganglion (25). Operating on a longer timescale, mutual inhibition can also be used to regulate brain states, such as the sleep–wake cycle (26, 27).
So far, our discussion has involved an alphabet comprising just two letters: excitatory and inhibitory neurons. In reality, the neuronal alphabet is far richer. Both excitatory and inhibitory neurons have many variations, thanks to the heterogeneity in their dendrite morphology, ion channel composition, spiking properties, and the subcellular distribution and strength of their input and output synapses. For example, in the mammalian neocortex, three classes of inhibitory neurons, the Martinotti, basket, and chandelier cells, target their presynaptic terminals to distal dendrites, cell bodies, and axon initial segments of excitatory pyramidal neurons, respectively, and thus control different aspects of how pyramidal neurons integrate synaptic inputs and produce spikes (28, 29). In the stomatogastric ganglion, mutually inhibiting neurons have distinct ion channel compositions and input/output synaptic strengths, which underlie their sequential firing patterns within each rhythmic cycle (30). Finally, the neuronal ‘alphabet’ also includes many modulatory neuron types to be discussed later.
At the level of core motifs, there are also many variations. For example, the mutual inhibition motif often includes intermediary neurons (e.g., inhibitory neuron A inhibits an excitatory neuron that excites inhibitory neuron B). It is important to note that the core motifs discussed above are almost always used in concert. Indeed, the large-scale architectural patterns discussed in the next section always contain these motifs.
In summary, a rich alphabet of neurons with diverse intrinsic properties can be used to compose words using a set of core motifs and their variations. These words are often used in concert to produce phrases, which together form the basis for sentences, as we discuss next.
Specialized Architectures for Specific Functions
The next level of organization is more heterogeneous in scale and configuration and less readily generalizable. Nevertheless, I attempt here to extract some high-order circuit architectural patterns that have been found in multiple neural regions and diverse species.
Continuous topographic mapping:
This is a common organizational scheme for representing information in the nervous system. Neighboring input neurons connect to neighboring target neurons through orderly axonal projections (Fig. 3A). A prime example is retinotopy: neighboring retinal ganglion cells synapse onto neighboring LGN neurons, which then connect to neighboring V1 neurons, which in turn connect to neighboring higher-order visual cortical neurons. Retinotopy enables spatial relationships in the outside world captured by the retina to be recapitulated in V1 and higher visual cortical areas. Continuous topographic mapping is also used elsewhere. In the sensory and motor homunculi, somatosensory stimuli from neighboring body parts are coarsely represented in neighboring areas of the primary somatosensory cortex, and motor outputs to neighboring body parts are coarsely controlled by neighboring areas of the motor cortex (31).
Fig. 3. Specialized architectures for specific functions.
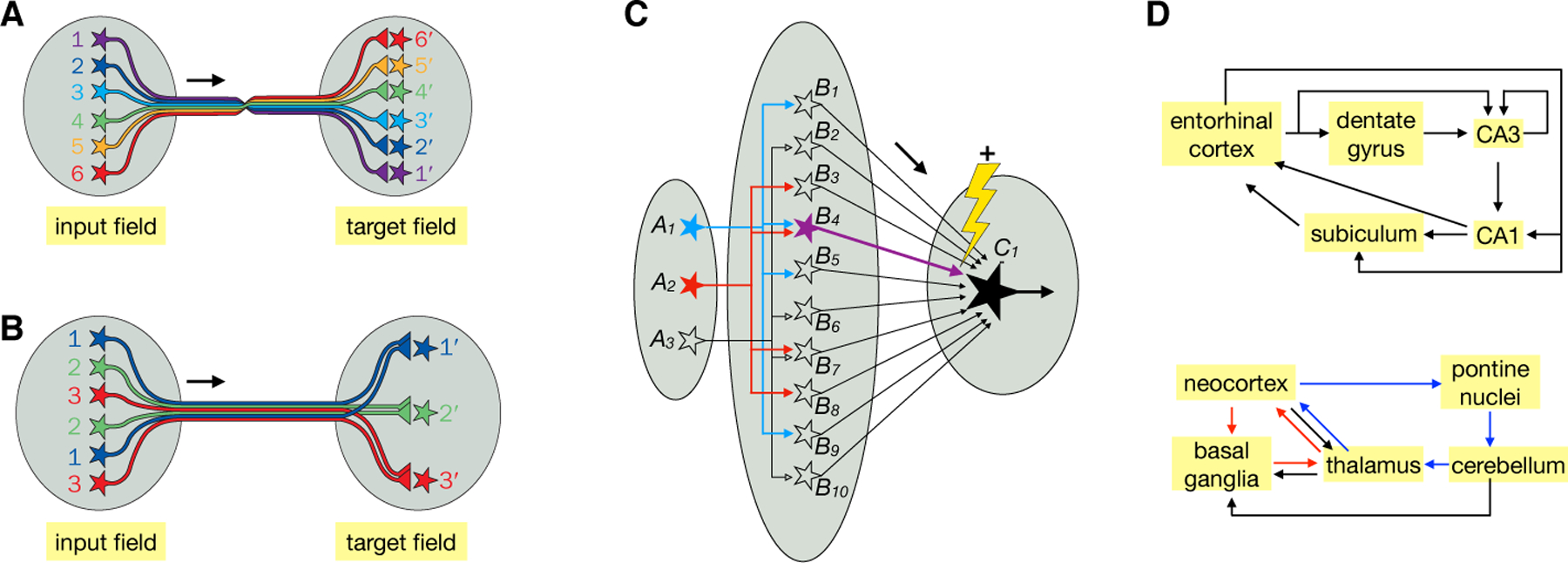
(A) Continuous topographic mapping. Neighboring neurons in the input field project their axons in an orderly fashion to connect to neighboring neurons in the target field, preserving their spatial relationships. A prime example is the retinotopic map. (B) Discrete parallel processing. Neurons of a specific type (same color) in the input field, regardless of their spatial locations, connect to the corresponding neuron type in the target field. A prime example is the olfactory glomerular map. Target neurons do not need to be spatially ordered as shown; they could extend their dendrites to connect specifically with the axons of specific input neuron types. (C) Dimensionality expansion. Signals represented by a small number of neurons in field A are represented by a much larger number of neurons in field B, such that activation of B neurons can represent specific combinations of A neurons (e.g., B4 represents co-activation of A1 and A2). Furthermore, the synaptic connections between B and C can be altered by coactivation of a teaching signal (the lightening and + sign signals strengthening) and a specific B neuron to modify the synaptic strength between that particular B and C. Thus, only after training would coactivation of A1 and A2 reaches the threshold (thick arrow for B4) for activating C1. Likewise, a C2 neuron (not shown) can be trained to respond to the coactivation of A2 and A3 by modifying the strength of B7 → C2. Filled and open symbols represent active and inactive neurons, respectively. (D) Two examples of recurrent loops. In the entorhinal–hippocampal loop (top), arrows indicate direct connections between neurons within the indicated regions. Many connections within these regions are topographically organized (116). In the neocortex–basal ganglia–thalamus (red) and neocortex–pons–cerebellum–thalamus loops (blue), arrows represent connections between these brain regions but not necessarily direct synaptic connections between specific neuron types. Within the basal ganglia and cerebellum, for example, inputs are transformed at intermediary stages to produce outputs. A and B, modified after (117).
Topographic maps provide a convenient way to organize information at successive stages of processing and can be constructed via robust developmental mechanisms (Fig. 5A). They have a variety of computational advantages. For example, retinotopy facilitates extraction of local contrast through lateral inhibition for object recognition. Furthermore, by placing circuit elements that are more often functionally connected nearby each other, topographic maps save energy by minimizing wiring length (32). The design of ‘convolutional neural networks’ (7) takes a page from topographic mapping to greatly reduce the number of variables needed to tune an artificial neural network and thus speed up computation.
Fig. 5. Wiring up neuronal circuits.
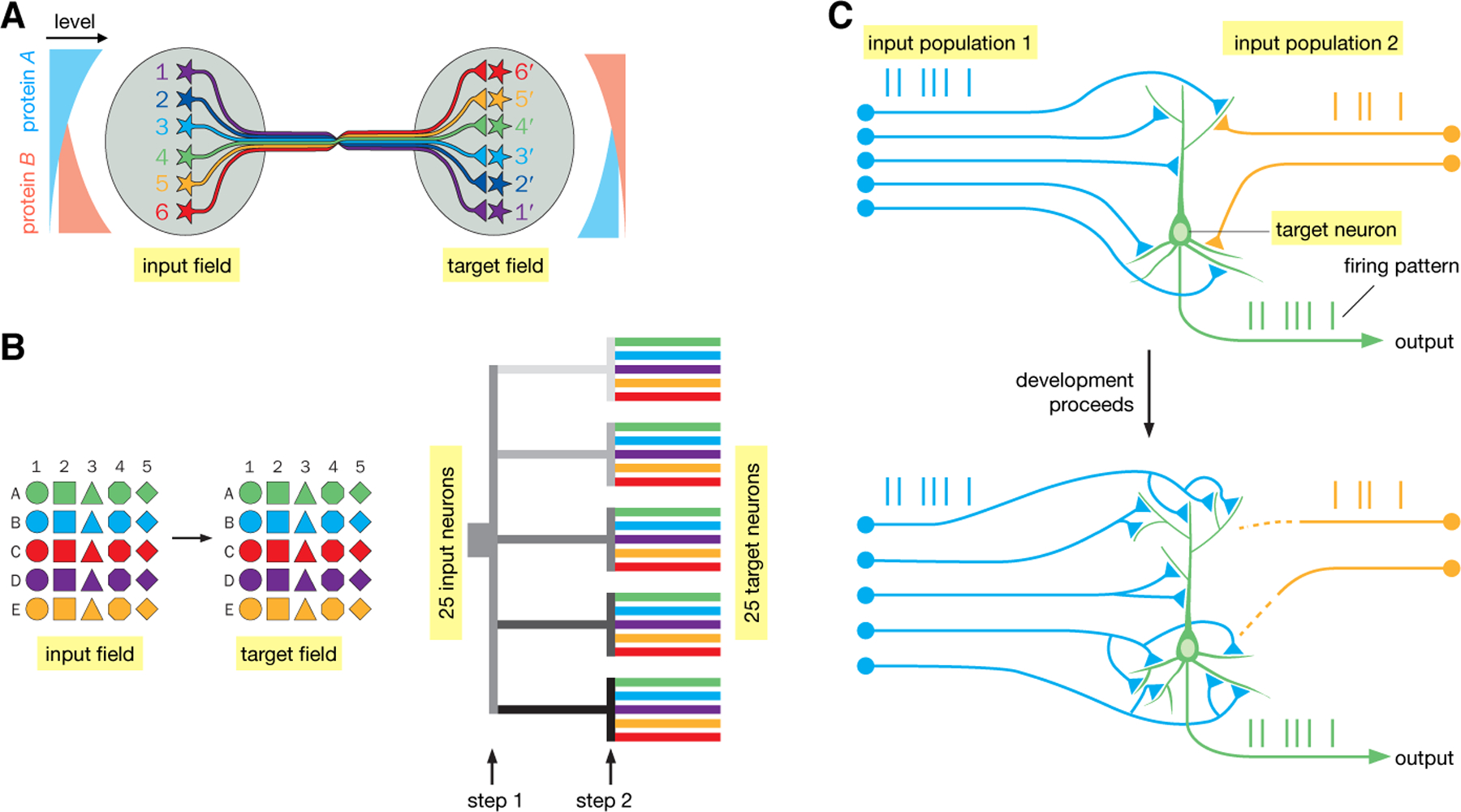
(A) Protein gradients can be used to construct continuous topographic maps. In this example, both input and target fields are patterned by opposing gradients of cell-surface proteins A and B. Suppose that neuronal processes expressing protein A and protein B mutually repel each other. Because Neuron 1 has the highest level of protein A, it seeks a target field with the lowest level of protein B; likewise, Neuron 6 seeks a target field with the lowest level of protein A. (B) Illustration of combinatorial strategies to specify connections between 25 discrete cell types in the input and target fields. (Left) Suppose that connection specificities between the input and target fields are mediated by homophilic attraction molecules. If each connection is specified by a single molecule, 25 molecules are needed to specify 25 connections. If each connection is specified by a combination of two molecules (a letter and a number), only 10 molecules are needed. (Right) The combinatorial strategy is realized by dividing the wiring process into 2 steps. At step 1, 5 molecules (represented by different shades of gray) separate 5 input axons into 5 groups; at step 2, 5 more molecules (represented by different colors) are used in each of the 5 groups to specify the final connections. (C) Schematic illustration of Hebb’s rule in instructing wiring. At an early developmental stage, the target neuron is connected with two groups of input neurons with distinct coincident firing patterns (blue and yellow vertical lines). Because stronger connections to the group 1 input drive the target neuron to fire in a pattern (green vertical lines) similar to that of group 1, their connections are strengthened. Synaptic connections between group 2 input and the target neuron weaken due to their dissimilar firing patterns. Eventually, the target neuron is only connected with group 1 input. Modified after (5).
Discrete parallel processing:
Discrete parallel processing (Fig. 3B) allows signals to be represented and processed in parallel by discrete information channels. A prime example is the glomerular organization of the vertebrate olfactory bulb and insect antennal lobe: olfactory receptor neurons (ORNs) expressing the same odorant receptors send their axons to the same glomerulus to synapse onto the dendrites of their corresponding second-order projection neurons, forming discrete olfactory processing channels (33, 34). Tens to thousands of individual ORNs expressing the same odorant receptor converge their axons onto the same glomerulus, thus enhancing the signal-to-noise ratio. Rather than representing continuous values, different glomeruli represent signals from discrete ORN types, and thus the nature of the chemicals that activate those odorant receptors. Discrete parallel processing also characterizes the mammalian taste system (35).
Discrete parallel processing is often used in conjunction with continuous topographic mapping. In the retina, for example, superimposed on the continuous retinotopy are discrete layers where different bipolar and ganglion cell types form specific connections to process different types of visual signals such as luminance, color, and motion in parallel. Compared to serial processing, parallel processing reduces computational depth, hence decreasing error rate and increasing processing speed. Indeed, massively parallel processing is a salient feature of complex nervous systems with large numbers of neurons and large numbers of connections per neuron (5). This architecture is increasingly being adopted in computer systems design (11).
Dimensionality expansion:
In this architecture, signals from a relatively small number of input neurons diverge onto a much larger number of output neurons (Fig. 3C), allowing output neurons to represent distinct combinations of inputs. Similar signals at the input level are more readily distinguished at the output level, facilitating pattern separation by downstream neurons (36–39). Two prime examples are the insect mushroom body (olfactory projection neurons → mushroom body Kenyon cells → mushroom body output neurons) and the vertebrate cerebellum (mossy fibers → cerebellar granule cells → Purkinje cells). In both cases, a relatively small number of inputs (projection neurons or mossy fibers, respectively) synapse onto a much larger number of output neurons (Kenyon cells or granule cells, respectively). Information at the level of the output neurons can thus be represented in a much higher dimensional space, with each dimension representing the firing rate of one cell. Small differences in input firing patterns (e.g., different projection neuron populations representing different odor combinations) can more readily be distinguished by the population firing patterns of their postsynaptic partners. This architecture allows for learning by adjusting the synaptic strengths of the output neurons via ‘teaching’ signals from dopamine neurons in the mushroom body (40, 41) and climbing fibers in the cerebellum (42). After training, the same input can produce different output patterns (Fig. 3C).
Another example of dimensionality expansion is the entorhinal cortex → dentate gyrus granule cell → CA3 pyramidal neuron circuit (Fig. 3D, top). The large number of dentate gyrus granule cells can perform pattern separation for information from the entorhinal cortex regarding space and objects for further processing by the downstream hippocampal circuit (43, 44). Unlike in the mushroom body and cerebellar cortex, ‘teaching neurons’ have not been identified here. This may be because the hippocampal circuit uses unsupervised learning, whereas the cerebellar and mushroom body circuits implement algorithms akin to supervised and reinforcement learning.
Recurrent loops:
Nervous systems are full of recurrent loops: neurons connect back onto themselves, often through intermediary neurons. These recurrent loops are heterogeneous in scale, ranging from within a particular neural region (e.g., mutual inhibition employed in the crustacean stomatogastric circuit) to spanning large parts of the brain. In the mammalian visual system, for example, in addition to ‘bottom-up’ projections from LGN → V1 → higher cortical areas, ‘top-down’ projections from higher cortical areas → V1 → LGN serve several functions such as attentional control. Long-range recurrent loops may incorporate continuous topographic mapping or discrete parallel processing architectures. Fig. 3D illustrates two examples in the mammalian brain at the level of neuronal populations (top) and brain regions (bottom). Recurrent loops generally support rich neural activity dynamics, but their exact computational roles are not clear in most cases and are likely to differ on a case-by-case basis. Understanding the general principles of information processing in recurrent loops is a major challenge in modern neuroscience.
Biased input–segregated output:
The above discussions have focused on circuits comprising excitatory and inhibitory neurons. Nervous systems also employ modulatory neurons for important functions. Modulatory neurons use neurotransmitters such as monoamines and neuropeptides that primarily engage G-protein-coupled receptors; hence their actions on postsynaptic neurons are slower and last between tens of milliseconds to seconds, compared to fast excitatory and inhibitory neurotransmitters, which engage ionotropic receptors and act within a few milliseconds. Besides acting across the synaptic cleft, modulatory neurotransmitters can also be released at sites without postsynaptic specializations—so-called ‘volume release’—and can thus influence targets at distances greater than that of a typical synaptic cleft.
Some modulatory neurons in the mammalian brain have cell bodies clustered in small regions but project axons broadly and receive diverse inputs. Viral-genetic tracing in the mouse (Box 1) revealed that midbrain dopamine, dorsal raphe serotonin, and hypothalamic neuropeptide galanin systems all adopt a ‘biased input–segregated output’ architecture at the population level (45–48) (Fig. 4A). Each system can be divided into parallel subsystems defined based on their segregated output projections to distinct target regions that serve different behavioral functions. Each output subsystem receives inputs from similar regions with quantitative biases, allowing these subsystems to be differentially regulated by external and internal stimuli. One exception is the locus coeruleus (LC) norepinephrine system: at the population level, LC norepinephrine axons projecting to one brain region also project broadly to other regions, even though branching patterns of individual neurons can be idiosyncratic (49, 50). These observations suggest that the LC norepinephrine system adopts an integration-and-broadcast architecture (Fig. 4B), which may suit its role in regulating global brain states such as arousal.
Fig. 4. Input–output organization of neuromodulatory circuits with broad projections.
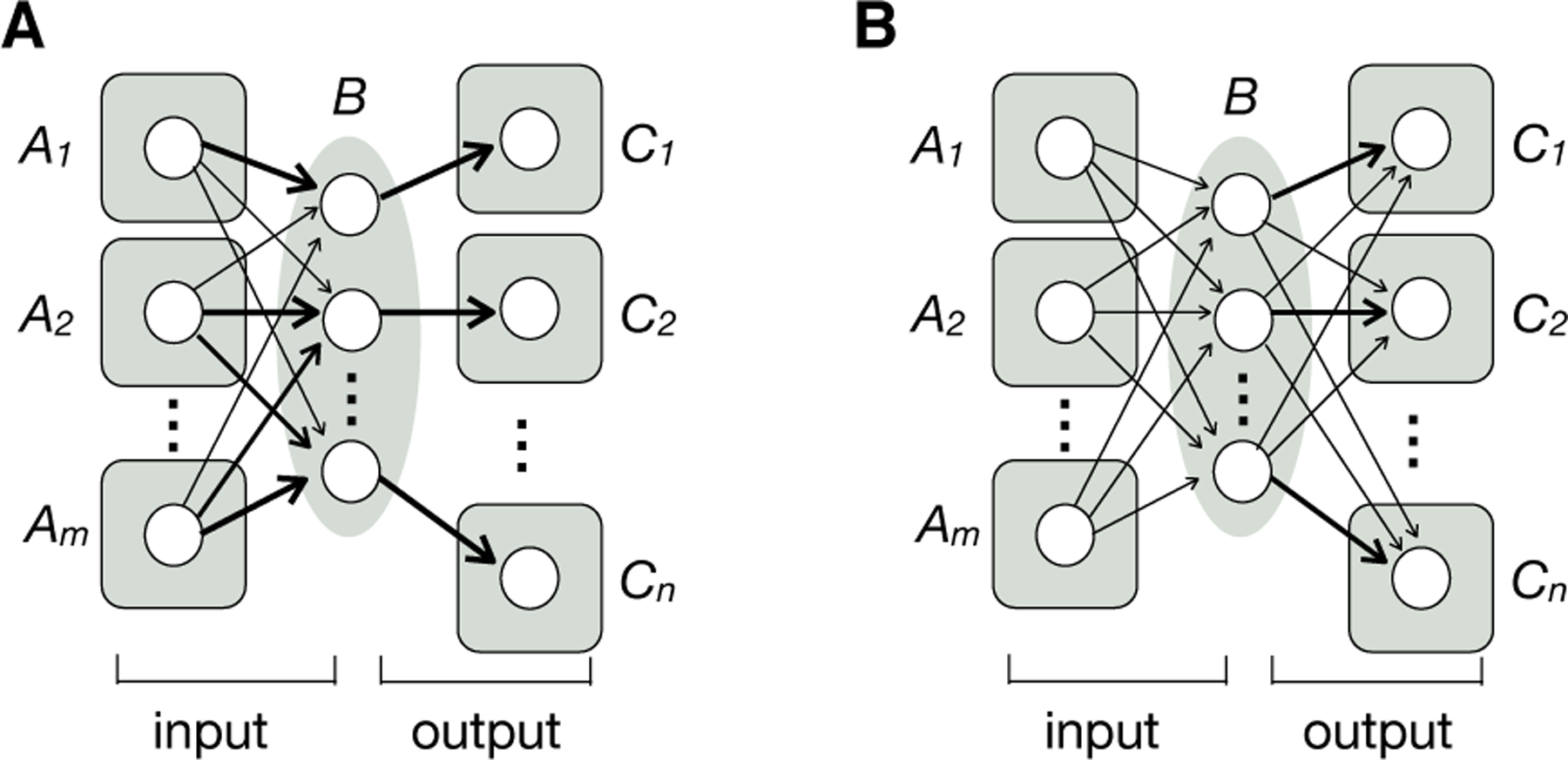
Modulatory neurons in region B collectively receive inputs from regions A1–Am and send broad output to regions C1–Cn. (A) Biased input–segregated output architecture. This architecture applies to several neuromodulatory systems, including midbrain dopamine neurons, dorsal raphe serotonin neurons, and preoptic area galanin neurons. Arrows of different thickness represent different input strengths. (B) Integration-and-broadcast architecture. Neuronal populations in region B that project to a specific output region also send output to other output regions, with the possibility of a quantitative bias; these populations also receive similar inputs. Locus coeruleus norepinephrine neurons approximate this architecture. Each circle symbolizes a neuronal population rather than an individual neuron, as the input–output organization summarized here is based on studies at the population level rather than at the level of individual neurons. Modified after (48, 49).
Nervous systems also employ architectures not discussed above. A prominent architecture in bilaterians is interconnected bilateral symmetry (51); formal network analysis identified bilateral symmetry as the top-level organization in forebrain connectivity maps (52). The architectures of many neuronal circuits, such as those of the canonical mammalian neocortex (53) and basal ganglia (54) circuits, do not fit neatly into the categories described above, even though they utilize the aforementioned core circuit motifs, and can participate as parts of other architectures, such as topographic maps (55) and recurrent loops (Fig. 3D bottom). This may be because we have not dug deep enough into these specific circuits to decipher their computational principles or because our understanding of the nervous system is not broad enough to identify shared architectures. We expect ample future opportunities to explore both the depth and breadth of neural circuit architectures by collecting greater amounts of data with increasingly sophisticated tools (Box 1). Only when we know more about these ‘sentences’ and their numerous variations and complex interactions will we have a deeper understanding of how they constitute ‘paragraphs’ (e.g., brain regions) and eventually the ‘article’—the overall organization of an entire nervous system.
Evolutionary and Developmental Perspectives
Whereas computer circuits are products of top-down design, complex neuronal circuits have evolved over hundreds of millions of years. Neuronal circuits also self-assemble during development using evolutionarily selected genetic instructions and are fine-tuned by experience. Thus, existing neuronal circuit architectures are likely a selection of those that can be readily evolved and assembled during development. Looking at a neuronal circuit in isolation may not tell us what elements are functionally important. Seeing what has been evolutionarily selected, expanded, shrunken, eliminated, or repeatedly produced through convergent evolution can, however, suggest what elements to focus on in functional studies.
Evolution of neuronal circuits.
Extant bilaterian nervous systems (including all vertebrate and most invertebrate phyla) likely derived from ancestors via progressive sophistication: those with only myocytes, followed by the sequential evolution of sensorimotor neurons, separate sensory and motor neurons, interneurons, and centralized interneuron networks that gave rise to the central nervous system (CNS) and brain (51, 56). The ubiquity of some core motifs, such as feedforward excitation and feedforward/feedback inhibition, may have originated early in animals with interneurons and a CNS, and have since been conserved across diverse species and spread across neural regions within each species due to their utility. Other architectures have evolved independently. The glomerular organization of the insect and vertebrate olfactory systems is likely the result of convergent evolution, as many clades descended from their last common ancestor do not have this organization, and different types of molecules are used as odorant receptors. Visual systems provide striking examples of convergent evolution of many fundamental features from retinotopy to motion detection algorithms in invertebrate and vertebrate lineages (57, 58).
Progressive sophistication of the nervous system requires expansion of neuronal numbers (59), neuron types and their connections (60), and brain regions (61). All these processes must result from changes to DNA. A key mechanism of evolutionary innovation is the duplication and divergence of genes; for example, duplication and divergence of a cone opsin gene conferred trichromacy on some primates (62). Duplication-and-divergence is also used in the evolution of neuron types (63–65) and brain regions (66). Duplication-and-divergence for brain region evolution should in principle make neuronal circuits modular: rich connections within a duplicated unit and sparse connections between units (as opposed to all-to-all nonmodular architectures employed as the starting conditions in many artificial neural networks). In turn, the modular nature of neuronal circuits might speed up evolution (6), as different modules can evolve independently of each other.
Development of neuronal circuits.
Evolution exerts its influence on neuronal circuits primarily by modifying genes involved in circuit wiring during development. A key question is how a limited number of genes (~20,000 across many animal species) can construct nervous systems with much larger numbers of synaptic connections (~107 in fruit flies, ~1011 in mice, >1014 in humans) with specific motifs and architectures.
Extracellular cues and their cell-surface receptors enable recognition of specific targets by axonal and dendritic growth cones. These molecules are the predominant force for establishing a coarse organization of the nervous system and can also specify synaptic connectivity with great precision in certain circuits and organisms (67–69). One strategy to establish specificity of a large number of connections with a limited number of genes is to use different expression levels of the same protein to specify different connections. This strategy is readily used in constructing continuous topographic maps (70, 71) (Fig. 5A), perhaps contributing to the prevalence of this circuit architecture (Fig. 3A). Graded expression of cell-surface molecules is also used in the early steps of constructing discrete maps (72, 73). However, discrete parallel processing (Fig. 3B) requires distinguishing between discrete cell types, and often utilizes combinatorial cell-surface protein codes such that a small number of proteins can specify many more connections (Fig. 5B, left). An efficient way to implement combinatorial coding is to divide the wiring process into distinct spatiotemporal steps (Fig. 5B, right); in addition to conserving molecules, this strategy can also enhance robustness, as growth cones are faced with few simultaneous choices at each step. The same wiring molecules can be used at different times and places, sometimes in different parts of the same circuit, through elaborate spatiotemporal regulation of their expression patterns (74–76).
Neuronal activity, both spontaneous and experience-driven, refines synaptic wiring diagrams. Activity-dependent wiring, often via competition between neurons with different activity levels, has been well documented (77–81). A prominent mechanism by which neuronal activity influences wiring is by implementing Hebb’s rule: synapses at which firing of presynaptic neurons causes firing of postsynaptic neurons are strengthened—colloquially, ‘fire together, wire together’ (82, 83) (Fig. 5C). Non-Hebbian mechanisms, such as homeostatic synaptic plasticity, also contribute to activity-dependent circuit wiring (84). These activity-dependent mechanisms continue to operate in the adult nervous system, enabling animals to change their synaptic connectivity patterns as a consequence of experience throughout life.
Many synaptic connections are not completely specified. In vertebrate neuromuscular systems, for example, while the connections between motor neuron pools and muscles are precisely specified (85), the specific connection patterns between individual motor neurons and muscle fibers within a motor pool are highly variable (86). Likewise, in the fly olfactory circuit, synaptic connections between specific olfactory projection neuron types and mushroom body Kenyon cells (Fig. 3C) are mostly random (87–89). In both cases, it is not necessary, or even desirable, to have more stereotyped connectivity. As more synaptic connectomes are mapped (Box 1), more examples of wiring variability will surely emerge.
In summary, two broad kinds of mechanisms are used to establish wiring patterns of neuronal circuits: molecular cues hard-wire the nervous system, and neuronal activity and experience fine-tune connectivity. There is also interplay between neuronal activity and molecular cues; for example, neuronal activity can regulate expression of molecular cues or complement their action (90, 91). However, apart from the limited examples discussed above, most developmental studies have not focused on addressing how specific circuit motifs and architectures are established, while most investigations of circuit function have not considered developmental constraints. There is ample opportunity for cross-fertilization of developmental and functional studies of neuronal circuits.
Outlook
Applications of circuit mapping tools such as serial electron microscopy and trans-synaptic tracing (Box 1) to diverse neural regions and organisms will surely generate a wealth of data from which we can distill common principles of structural organization of neuronal circuits. Relating structures to the functions they implement will be an important next step. This can be done by leveraging powerful tools that have been developed and applied to functionally interrogate neuronal circuits in the context of animal behavior (92–94). Such interrogation is essential for identifying the functions of each circuit elements. In addition, a key challenge is to investigate how these motifs and architectures interact with each other across scales. Understanding how different architectures cooperate in an individual nervous system should also inspire new artificial neural networks that might someday achieve general-purpose artificial intelligence.
We are still only beginning to gain insights into the evolutionary and developmental processes that give rise to circuit architecture in complex nervous systems. We still do not know, for example, whether and to what degree algorithmic changes in the wiring process and operation of neuronal circuits can account for the increased complexity of the mammalian brain. A deliberate effort to investigate how letters are assembled into words and words into sentences in key circuit architectures across different species could yield valuable insights. Comparative study of neuron type composition of homologous brain regions using single-cell transcriptomics (63–66) is a useful first step. This can be followed by investigation of the mechanisms that establish their connectivity patterns and that underlie their functional operations. Integrating studies of the structure, function, development, and evolution of neuronal circuits will enable a deeper understanding of nervous system organization beyond the level of individual neurons.
Acknowledgements
I thank Will Allen, Tom Clandinin, Marla Feller, Justus Kebschull, Fei-Fei Li, Jan Lui, Jing Ren, Massimo Scanziani, Andrew Shuster, Lubert Stryer, and Mark Wagner for helpful critiques, and Taylors & Francis Group, LLC for the permission of adapting figures from Principles of Neurobiology (2020).
Funding:
National Institutes of Health, National Science Foundation, Howard Hughes Medical Institute, and Wu Tsai Neurosciences Institute at Stanford.
Footnotes
Competing interests: The author declares no competing interests.
References and Notes
- 1.Cajal SR, Histology of the Nervous System of Man and Vertebrates (Oxford University Press, Inc., translation by Swanson N & Swanson LW, Oxford, 1995). [Google Scholar]
- 2.Golgi C, Sulla struttura della sostanza grigia del cervello. Gazetta medica lombarda IV, (1873). [Google Scholar]
- 3.Laurent G et al. , Odor encoding as an active, dynamical process: Experiments, computation, and theory. Annu Rev Neurosci 24, 263–297 (2001). 11283312 [DOI] [PubMed] [Google Scholar]
- 4.Shenoy KV, Sahani M, Churchland MM, Cortical control of arm movements: A dynamical systems perspective. Annu Rev Neurosci 36, 337–359 (2013). 23725001 [DOI] [PubMed] [Google Scholar]
- 5.Luo L, Principles of Neurobiology, 2nd ed. (CRC Press/Garland Science, 2020). [Google Scholar]
- 6.Alon U, An Introduction to Systems Biology, 2nd ed. (CRC Press, 2020). [Google Scholar]
- 7.LeCun Y, Bengio Y, Hinton G, Deep learning. Nature 521, 436–444 (2015). 26017442 [DOI] [PubMed] [Google Scholar]
- 8.Gilbert CD, Wiesel TN, Morphology and intracortical projections of functionally characterised neurones in the cat visual cortex. Nature 280, 120–125 (1979). 552600 [DOI] [PubMed] [Google Scholar]
- 9.Felleman DJ, Van Essen DC, Distributed hierarchical processing in the primate cerebral cortex. Cerebral cortex 1, 1–47 (1991). 1822724 [DOI] [PubMed] [Google Scholar]
- 10.Rosenblatt F, The perceptron: A probabilistic model for information storage and organization in the brain. Psychol Rev 65, 386–408 (1958). 13602029 [DOI] [PubMed] [Google Scholar]
- 11.Krizhevsky A, Sutskever I, Hinton G, Imagenet classification with deep convolutional neural networks. In Proc. Advances in Neural Information Processing Systems 25, 1090–1098 (2012). [Google Scholar]
- 12.Sherrington CS, Remarks on some aspects of reflex inhibition. Proc R Sco Lond B 97, 519–545 (1925). [Google Scholar]
- 13.Coombs JS, Eccles JC, Fatt P, The inhibitory suppression of reflex discharges from motoneurones. The Journal of physiology 130, 396–413 (1955). 13278907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isaacson JS, Scanziani M, How inhibition shapes cortical activity. Neuron 72, 231–243 (2011). 22017986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin KA, Somogyi P, Whitteridge D, Physiological and morphological properties of identified basket cells in the cat’s visual cortex. Exp Brain Res 50, 193–200 (1983). 6641854 [DOI] [PubMed] [Google Scholar]
- 16.Ji XY et al. , Thalamocortical innervation pattern in mouse auditory and visual cortex: Laminar and cell-type specificity. Cerebral cortex 26, 2612–2625 (2016). 25979090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roux L, Buzsaki G, Tasks for inhibitory interneurons in intact brain circuits. Neuropharmacology 88, 10–23 (2015). 25239808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Vreeswijk C, Sompolinsky H, Chaos in neuronal networks with balanced excitatory and inhibitory activity. Science 274, 1724–1726 (1996). 8939866 [DOI] [PubMed] [Google Scholar]
- 19.Shadlen MN, Newsome WT, The variable discharge of cortical neurons: Implications for connectivity, computation, and information coding. J Neurosci 18, 3870–3896 (1998). 9570816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuffler SW, Discharge patterns and functional organization of mammalian retina. J Neurophysiol 16, 37–68 (1953). 13035466 [DOI] [PubMed] [Google Scholar]
- 21.Barlow HB, Summation and inhibition in the frog’s retina. The Journal of physiology 119, 69–88 (1953). 13035718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mountcastle VB, Modality and topographic properties of single neurons of cat’s somatic sensory cortex. J. Neurophysiol 20, 408–434 (1957). [DOI] [PubMed] [Google Scholar]
- 23.Shepherd GM, Chen WR, Greer CA, in The synaptic organization of the brain, Shepherd GM, Ed. (Oxford University Press, Oxford, 2004). [Google Scholar]
- 24.Grillner S, Biological pattern generation: The cellular and computational logic of networks in motion. Neuron 52, 751–766 (2006). 17145498 [DOI] [PubMed] [Google Scholar]
- 25.Marder E, Bucher D, Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annual review of physiology 69, 291–316 (2007). 17009928 [DOI] [PubMed] [Google Scholar]
- 26.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE, Sleep state switching. Neuron 68, 1023–1042 (2010). 21172606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weber F, Dan Y, Circuit-based interrogation of sleep control. Nature 538, 51–59 (2016). 27708309 [DOI] [PubMed] [Google Scholar]
- 28.Markram H et al. , Interneurons of the neocortical inhibitory system. Nat Rev Neurosci 5, 793–807 (2004). 15378039 [DOI] [PubMed] [Google Scholar]
- 29.Huang ZJ, Paul A, The diversity of gabaergic neurons and neural communication elements. Nat Rev Neurosci 20, 563–572 (2019). 31222186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prinz AA, Bucher D, Marder E, Similar network activity from disparate circuit parameters. Nat Neurosci 7, 1345–1352 (2004). 15558066 [DOI] [PubMed] [Google Scholar]
- 31.Penfield W, Rasmussen T, The cerebral cortex of man: A clinical study of localization of function (Macmillan, New York, 1950). [Google Scholar]
- 32.Wang IE, Clandinin TR, The influence of wiring economy on nervous system evolution. Curr Biol 26, R1101–R1108 (2016). 27780051 [DOI] [PubMed] [Google Scholar]
- 33.Axel R, The molecular logic of smell. Sci Am 273, 154–159 (1995). 7481719 [DOI] [PubMed] [Google Scholar]
- 34.Vosshall LB, Stocker RF, Molecular architecture of smell and taste in drosophila. Annu Rev Neurosci 30, 505–533 (2007). 17506643 [DOI] [PubMed] [Google Scholar]
- 35.Yarmolinsky DA, Zuker CS, Ryba NJ, Common sense about taste: From mammals to insects. Cell 139, 234–244 (2009). 19837029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marr D, A theory of cerebellar cortex. The Journal of physiology 202, 437–470 (1969). 5784296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Albus JS, A theory of cerebellar dunction. Math. Biosci 10, 25–61 (1971). [Google Scholar]
- 38.Litwin-Kumar A, Harris KD, Axel R, Sompolinsky H, Abbott LF, Optimal degrees of synaptic connectivity. Neuron 93, 1153–1164 e1157 (2017). 28215558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cayco-Gajic NA, Silver RA, Re-evaluating circuit mechanisms underlying pattern separation. Neuron 101, 584–602 (2019). 30790539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hige T, Aso Y, Modi MN, Rubin GM, Turner GC, Heterosynaptic plasticity underlies aversive olfactory learning in drosophila. Neuron 88, 985–998 (2015). 26637800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohn R, Morantte I, Ruta V, Coordinated and compartmentalized neuromodulation shapes sensory processing in drosophila. Cell 163, 1742–1755 (2015). 26687359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Zeeuw CI, Lisberger SG, Raymond JL, Diversity and dynamism in the cerebellum. Nat Neurosci 24, 160–167 (2021). 33288911 [DOI] [PubMed] [Google Scholar]
- 43.Marr D, Simple memory: A theory for archicortex. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 262, 23–81 (1971). 4399412 [DOI] [PubMed] [Google Scholar]
- 44.Neunuebel JP, Knierim JJ, Ca3 retrieves coherent representations from degraded input: Direct evidence for ca3 pattern completion and dentate gyrus pattern separation. Neuron 81, 416–427 (2014). 24462102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beier KT et al. , Circuit architecture of vta dopamine neurons revealed by systematic input-output mapping. Cell 162, 622–634 (2015). 26232228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lerner TN et al. , Intact-brain analyses reveal distinct information carried by snc dopamine subcircuits. Cell 162, 635–647 (2015). 26232229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ren J et al. , Anatomically defined and functionally distinct dorsal raphe serotonin sub-systems. Cell 175, 472–487 e420 (2018). 30146164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kohl J et al. , Functional circuit architecture underlying parental behaviour. Nature 556, 326–331 (2018). 29643503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwarz LA et al. , Viral-genetic tracing of the input-output organization of a central noradrenaline circuit. Nature 524, 88–92 (2015). 26131933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kebschull JM et al. , High-throughput mapping of single-neuron projections by sequencing of barcoded rna. Neuron 91, 975–987 (2016). 27545715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swanson LW, Brain Architecture, 2nd ed. (Oxford University Press, 2012). [Google Scholar]
- 52.Swanson LW, Hahn JD, Sporns O, Structure-function subsystem models of female and male forebrain networks integrating cognition, affect, behavior, and bodily functions. Proc Natl Acad Sci U S A 117, 31470–31481 (2020). 33229546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Douglas RJ, Martin KA, Neuronal circuits of the neocortex. Annu Rev Neurosci 27, 419–451 (2004). 15217339 [DOI] [PubMed] [Google Scholar]
- 54.Gerfen CR, Surmeier DJ, Modulation of striatal projection systems by dopamine. Annu Rev Neurosci 34, 441–466 (2011). 21469956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee J, Wang W, Sabatini BL, Anatomically segregated basal ganglia pathways allow parallel behavioral modulation. Nat Neurosci 23, 1388–1398 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaas JH, Evolutionary Nneuroscience, 2nd ed. (Elsevier, 2020). [Google Scholar]
- 57.Salvini-Plawen LV, Mayr E, On the evolution of photoreceptors and eyes. Evo Bio 10, 207–263 (1977). [Google Scholar]
- 58.Clark DA et al. , Flies and humans share a motion estimation strategy that exploits natural scene statistics. Nat Neurosci 17, 296–303 (2014). 24390225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lui JH, Hansen DV, Kriegstein AR, Development and evolution of the human neocortex. Cell 146, 18–36 (2011). 21729779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arendt D et al. , The origin and evolution of cell types. Nature reviews. Genetics 17, 744–757 (2016). 27818507 [DOI] [PubMed] [Google Scholar]
- 61.Chakraborty M, Jarvis ED, Brain evolution by brain pathway duplication. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 370, (2015). 26554045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jacobs GH, Nathans J, The evolution of primate color vision. Sci Am 300, 56–63 (2009). 19363921 [DOI] [PubMed] [Google Scholar]
- 63.Tosches MA et al. , Evolution of pallium, hippocampus, and cortical cell types revealed by single-cell transcriptomics in reptiles. Science 360, 881–888 (2018). 29724907 [DOI] [PubMed] [Google Scholar]
- 64.Peng YR et al. , Molecular classification and comparative taxonomics of foveal and peripheral cells in primate retina. Cell 176, 1222–1237 e1222 (2019). 30712875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hodge RD et al. , Conserved cell types with divergent features in human versus mouse cortex. Nature 573, 61–68 (2019). 31435019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kebschull JM et al. , Cerebellar nuclei evolved by repeatedly duplicating a conserved cell-type set. Science 370, eabd5059 (2020). 33335034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kolodkin AL, Tessier-Lavigne M, Mechanisms and molecules of neuronal wiring: A primer. Cold Spring Harb Perspect Biol 3, a001727 (2011). 21123392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hong W, Luo L, Genetic control of wiring specificity in the fly olfactory system. Genetics 196, 17–29 (2014). 24395823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sanes JR, Zipursky SL, Synaptic specificity, recognition molecules, and assembly of neural circuits. Cell 181, 536–556 (2020). 32359437 [DOI] [PubMed] [Google Scholar]
- 70.Drescher U et al. , In vitro guidance of a retinal ganglion cell axons by rags, a 25kda tectal protein related to ligands for eph receptor tyrosine kinase. Cell 82, 369–370 (1995). [DOI] [PubMed] [Google Scholar]
- 71.Cheng H-J, Nakamoto M, Bergemann AD, Flanagan JG, Complementary gradients in expression and binding of elf-1 and mek4 in development of the topographic retinotectal projection map. Cell 82, 371–381 (1995). [DOI] [PubMed] [Google Scholar]
- 72.Imai T, Suzuki M, Sakano H, Odorant receptor-derived camp signals direct axonal targeting. Science 314, 657–661 (2006). 16990513 [DOI] [PubMed] [Google Scholar]
- 73.Komiyama T, Sweeney LB, Schuldiner O, Garcia KC, Luo L, Graded expression of semaphorin-1a cell-autonomously directs dendritic targeting of olfactory projection neurons. Cell 128, 399–410 (2007). 17254975 [DOI] [PubMed] [Google Scholar]
- 74.Joo WJ, Sweeney LB, Liang L, Luo L, Linking cell fate, trajectory choice, and target selection: Genetic analysis of sema-2b in olfactory axon targeting. Neuron 78, 673–686 (2013). 23719164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cang J, Feldheim DA, Developmental mechanisms of topographic map formation and alignment. Annu Rev Neurosci 36, 51–77 (2013). 23642132 [DOI] [PubMed] [Google Scholar]
- 76.Pederick DT et al. , Reciprocal repulsions instruct the assembly of parallel hippocampal networks. Science 372, 1068–1073 (2021). 34083484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wiesel TN, Hubel DH, Single cell responses in striate cortex of kittens deprived of vision in one eye. Journal of Neurophysiology 26, 1003–1017 (1963). [DOI] [PubMed] [Google Scholar]
- 78.Constantine-Paton M, Law MI, Eye-specific termination bands in tecta of three-eyed frogs. Science 202, 639–641 (1978). 309179 [DOI] [PubMed] [Google Scholar]
- 79.Stryker MP, Harris WA, Binocular impulse blockade prevents the formation of ocular dominance columns in cat visual cortex. J Neurosci 6, 2117–2133 (1986). 3746403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Penn AA, Riquelme PA, Feller MB, Shatz CJ, Competition in retinogeniculate patterning driven by spontaneous activity. Science 279, 2108–2112 (1998). 9516112 [DOI] [PubMed] [Google Scholar]
- 81.Buffelli M et al. , Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature 424, 430–434 (2003). 12879071 [DOI] [PubMed] [Google Scholar]
- 82.Hebb DO, The Organization of Behavior (John Wiley and Sons, New York, 1949). [Google Scholar]
- 83.Stent GS, A physiological mechanism for hebb’s postulate of learning. Proc Natl Acad Sci U S A 70, 997–1001 (1973). 4352227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Turrigiano GG, The dialectic of Hebb and homeostasis. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 372, 20160258 (2017). 28093556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lance-Jones C, Landmesser L, Pathway selection by embryonic chick motoneurons in an experimentally altered environment. Proc R Soc Lond B Biol Sci 214, 19–52 (1981). 6121329 [DOI] [PubMed] [Google Scholar]
- 86.Lu J, Tapia JC, White OL, Lichtman JW, The interscutularis muscle connectome. PLoS biology 7, e32 (2009). 19209956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Caron SJ, Ruta V, Abbott LF, Axel R, Random convergence of olfactory inputs in the drosophila mushroom body. Nature 497, 113–117 (2013). 23615618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li F et al. , The connectome of the adult drosophila mushroom body provides insights into function. eLife 9, e62576 (2020). 33315010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zheng Z et al. , A complete electron microscopy volume of the brain of adult drosophila melanogaster. Cell 174, 730–743 e722 (2018). 30033368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Serizawa S et al. , A neuronal identity code for the odorant receptor-specific and activity-dependent axon sorting. Cell 127, 1057–1069 (2006). 17129788 [DOI] [PubMed] [Google Scholar]
- 91.McLaughlin T, Torborg CL, Feller MB, O’Leary DD, Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron 40, 1147–1160 (2003). 14687549 [DOI] [PubMed] [Google Scholar]
- 92.Lin MZ, Schnitzer MJ, Genetically encoded indicators of neuronal activity. Nat Neurosci 19, 1142–1153 (2016). 27571193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim CK, Adhikari A, Deisseroth K, Integration of optogenetics with complementary methodologies in systems neuroscience. Nat Rev Neurosci 18, 222–235 (2017). 28303019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Luo L, Callaway EM, Svoboda K, Genetic dissection of neural circuits: A decade of progress. Neuron 98, 256–281 (2018). 29673479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cowan WM, The emergence of modern neuroanatomy and developmental neurobiology. Neuron 20, 413–426 (1998). [DOI] [PubMed] [Google Scholar]
- 96.Lee T, Luo L, Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22, 451–461 (1999). S0896–6273(00)80701–1 [pii]; 10197526 [DOI] [PubMed] [Google Scholar]
- 97.Feng G et al. , Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of gfp. Neuron 28, 41–51 (2000). 11086982 [DOI] [PubMed] [Google Scholar]
- 98.Zong H, Espinosa JS, Su HH, Muzumdar MD, Luo L, Mosaic analysis with double markers in mice. Cell 121, 479–492 (2005). 15882628 [DOI] [PubMed] [Google Scholar]
- 99.Livet J et al. , Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 450, 56–62 (2007). nature06293 [pii] 17972876 [DOI] [PubMed] [Google Scholar]
- 100.Nern A, Pfeiffer BD, Rubin GM, Optimized tools for multicolor stochastic labeling reveal diverse stereotyped cell arrangements in the fly visual system. Proc Natl Acad Sci U S A 112, E2967–2976 (2015). 25964354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.White JG, Southgate E, Thomson JN, Brenner S, The structure of the nervous system of the nematode caenohabditis elegans. Philos. Trans. R. Soc. Lond. B 314, 1–340 (1986). [DOI] [PubMed] [Google Scholar]
- 102.Takemura SY et al. , A visual motion detection circuit suggested by drosophila connectomics. Nature 500, 175–181 (2013). 23925240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim JS et al. , Space-time wiring specificity supports direction selectivity in the retina. Nature 509, 331–336 (2014). 24805243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee WC et al. , Anatomy and function of an excitatory network in the visual cortex. Nature 532, 370–374 (2016). 27018655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hildebrand DGC et al. , Whole-brain serial-section electron microscopy in larval zebrafish. Nature 545, 345–349 (2017). 28489821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Scheffer LK et al. , A connectome and analysis of the adult drosophila central brain. eLife 9, e57443 (2020). 32880371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Abbott LF et al. , The mind of a mouse. Cell 182, 1372–1376 (2020). 32946777 [DOI] [PubMed] [Google Scholar]
- 108.Wickersham IR et al. , Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron 53, 639–647 (2007). 17329205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Callaway EM, Luo L, Monosynaptic circuit tracing with glycoprotein-deleted rabies viruses. J Neurosci 35, 8979–8985 (2015). 26085623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lo L, Anderson DJ, A cre-dependent, anterograde transsynaptic viral tracer for mapping output pathways of genetically marked neurons. Neuron 72, 938–950 (2011). 22196330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zingg B et al. , Aav-mediated anterograde transsynaptic tagging: Mapping corticocollicular input-defined neural pathways for defense behaviors. Neuron 93, 33–47 (2017). 27989459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Talay M et al. , Transsynaptic mapping of second-order taste neurons in flies by trans-tango. Neuron 96, 783–795 e784 (2017). 29107518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huang TH et al. , Tracing neuronal circuits in transgenic animals by transneuronal control of transcription (tract). eLife 6, e32027 (2017). 29231171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Petreanu L, Huber D, Sobczyk A, Svoboda K, Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat Neurosci 10, 663–668 (2007). 17435752 [DOI] [PubMed] [Google Scholar]
- 115.London M, Hausser M, Dendritic computation. Annu Rev Neurosci 28, 503–532 (2005). 16033324 [DOI] [PubMed] [Google Scholar]
- 116.van Strien NM, Cappaert NL, Witter MP, The anatomy of memory: An interactive overview of the parahippocampal-hippocampal network. Nat Rev Neurosci 10, 272–282 (2009). 19300446 [DOI] [PubMed] [Google Scholar]
- 117.Luo L, Flanagan JG, Development of continuous and discrete neural maps. Neuron 56, 284–300 (2007). 17964246 [DOI] [PubMed] [Google Scholar]


