Abstract
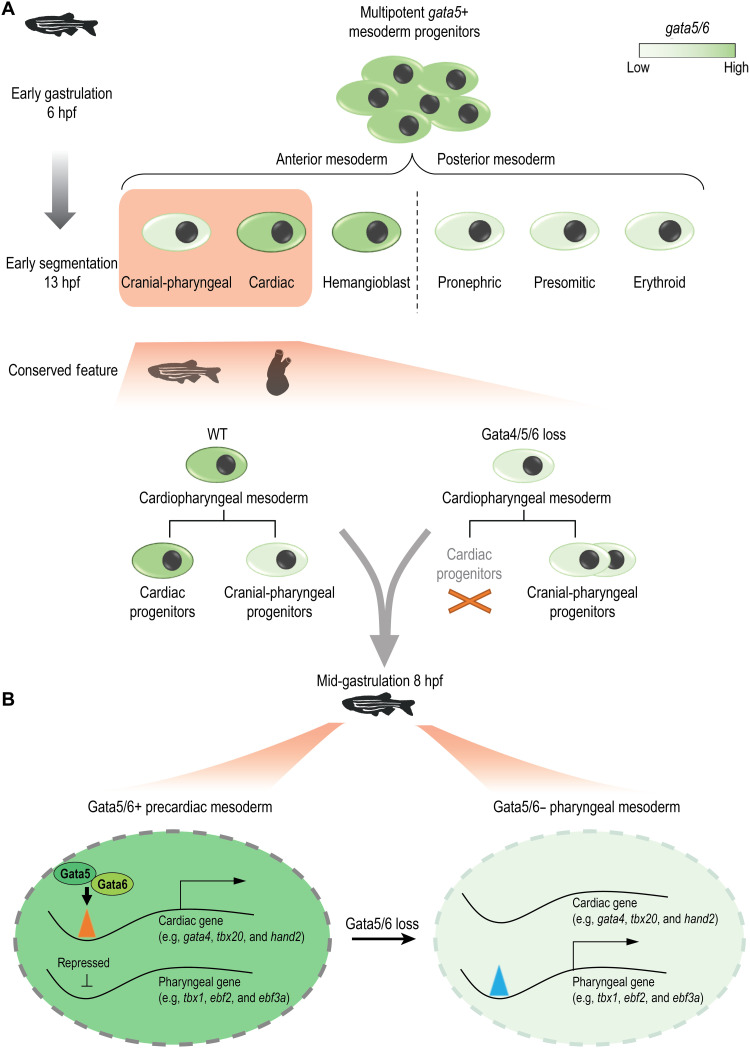
GATA4/5/6 transcription factors play essential, conserved roles in heart development. To understand how GATA4/5/6 modulates the mesoderm-to-cardiac fate transition, we labeled, isolated, and performed single-cell gene expression analysis on cells that express gata5 at precardiac time points spanning zebrafish gastrulation to somitogenesis. We found that most mesendoderm-derived lineages had dynamic gata5/6 expression. In the absence of Gata5/6, the population structure of mesendoderm-derived cells was substantially altered. In addition to the expected absence of cardiac mesoderm, we confirmed a concomitant expansion of cranial-pharyngeal mesoderm. Moreover, Gata5/6 loss led to extensive changes in chromatin accessibility near cardiac and pharyngeal genes. Functional analyses in zebrafish and the tunicate Ciona, which has a single GATA4/5/6 homolog, revealed that GATA4/5/6 acts upstream of tbx1 to exert essential and cell-autonomous roles in promoting cardiac and inhibiting pharyngeal mesoderm identity. Overall, cardiac and pharyngeal mesoderm fate choices are achieved through an evolutionarily conserved GATA4/5/6 regulatory network.
Cardiac and pharyngeal fate decisions are balanced by an ancient GATA4/5/6 regulatory network.
INTRODUCTION
Heart development is a complex process that requires tightly controlled gene regulatory networks, which direct the correct specification, differentiation, patterning, and morphogenesis of the heart and its constituents. Transcription factors (TFs) are the core components of this regulatory network as they play critical roles in initiating cardiac specification (1). Among the earliest TFs that are expressed in the cardiac mesoderm, GATA4, GATA5, and GATA6 paralogs (herein referred to as GATA4/5/6) function at or near the top of the cardiac regulatory network hierarchy in animals. For example, the loss of Gata4/6 in mice and gata5/6 in zebrafish both lead to a heartless phenotype (2–4); targeted expression of a dominant negative form of the sole GATA4/5/6 homolog in the chordate Ciona, Ci-Gata-a, results in a substantial reduction of cardiac gene expression (5); and the loss of the GATA4/5/6 homolog pannier in Drosophila leads to a heartless phenotype (6). Highlighting their essential and dosage-sensitive roles in heart development, individual heterozygous loss-of-function mutations in GATA4/5/6 in humans have been associated with congenital heart disease (7).
Cross-species comparisons suggest that zebrafish gata5 is the functional homolog of mammalian Gata4 (8, 9). The mutation of zebrafish gata5 results in greatly reduced expression of the canonical cardiac mesoderm marker nkx2.5, with improper migration of remaining cardiac progenitors (9), likely due to defects in endoderm development and morphogenesis. Loss of gata6 in zebrafish embryos leads to a reduction of the ventricular chamber and a dilation of the atrial chamber (3). gata4, however, is dispensable for early heart development in zebrafish (3). Its expression is first observed in the anterior lateral plate mesoderm (ALPM) at early segmentation stages and dependent on gata5/6 (10). Combined mutations or injection of morpholinos (MOs) targeting both gata5 and gata6 leads to a “triple” loss of the entire gata4/5/6 family and completely blocks cardiogenesis (2, 3, 10). Besides regulating heart and endoderm development, gata4/5/6 family members are also important for the development of zebrafish anterior hemangioblasts and fin bud progenitors (2, 11). Despite these insights, how GATA4/5/6 family members facilitate the early separation of cardiac and noncardiac lineages remains unclear.
Zebrafish gata5 and mouse Gata4 are among the first TFs expressed in precardiac mesoderm (9, 12). Lineage tracing, clonal analyses, and single-cell transcriptome profiling suggest that cardiac progenitors with distinct potential and transcriptional profiles are present as early as the onset of gastrulation (13–16). At least two distinct cardiac progenitor populations, the first heart field (FHF) and second heart field (SHF), arise during gastrulation, with the SHF being composed of late-differentiating progenitors that add to the developing heart tube derived from the FHF. Several lines of evidence from the chordate Ciona indicate that heart and pharyngeal skeletal muscles originate from a common developmental pool termed the cardiopharyngeal field (17, 18). The pharyngeal mesoderm found at the core of the developing pharyngeal arches is made up of the pharyngeal arch arteries (PAAs), the pharyngeal muscle progenitors that develop into the head and neck muscles, and the SHF progenitors (19). The early development of the cardiopharyngeal field is regulated by a conserved set of factors that are required for both cardiac and pharyngeal mesoderm development, including Isl1, Tbx1, and Nkx2.5 (20–23). Despite a later requirement of GATA4/6 to regulate outflow tract (OFT) development together with Hedgehog signaling (24), they are dispensable for early specification of the SHF (4). It therefore remains to be resolved at which stages and during which specific cardiogenesis processes the GATA4/5/6 family of TFs are essential and whether they are also involved in the development of the pharyngeal mesoderm.
To investigate the roles of gata5/6 in the early cardiac and pharyngeal lineage specification, we used a reporter line to isolate gata5–green fluorescent protein (GFP)–positive cells from zebrafish embryos at stages spanning early gastrulation to early segmentation and subjected them to single-cell mRNA sequencing (scRNA-seq). We found dynamic, lineage-specific regulation of gata5/6 transcripts, with gata5/6 expression being maintained in the cardiac lineage while being gradually turned off in the pharyngeal mesoderm. scRNA-seq analysis of wild-type (WT) and Gata5/6 knockdown (KD) embryos revealed both expected (loss of cardiac mesoderm and reduced endoderm) and unexpected (reduced pronephric mesoderm and expanded erythroid progenitors and pharyngeal mesoderm) changes in cell lineage composition. Zebrafish transplantation experiments showed that Gata5/6 KD promotes pharyngeal mesoderm fate in a cell-autonomous manner. Open chromatin profiling in WT and Gata5/6-deficient GFP+ cells at the mid-gastrula stage revealed a requirement for Gata5/6 in establishing the open chromatin state of cardiac enhancers while repressing that of pharyngeal enhancers. Enhancer reporter assays confirmed that cardiac and pharyngeal enhancers at the tbx1 locus responded differently to Gata5/6 loss, in accordance with their normal cardiac- or pharyngeal-specific activities. Last, targeted overexpression and CRISPR-mediated loss of the single GATA4/5/6 homolog in cardiopharyngeal progenitors of the tunicate Ciona indicated that sustained GATA4/5/6 expression is a conserved mechanism that promotes cardiac fate specification, in part by preventing ectopic activation of the pharyngeal muscle determinants Tbx1/10 and Ebf. Overall, we propose that GATA4/5/6 family members are central players in a conserved GATA-TBX1 regulatory axis that acts via modulating the open chromatin state of cardiac and pharyngeal determinants to promote the divergence of cardiac and pharyngeal fates.
RESULTS
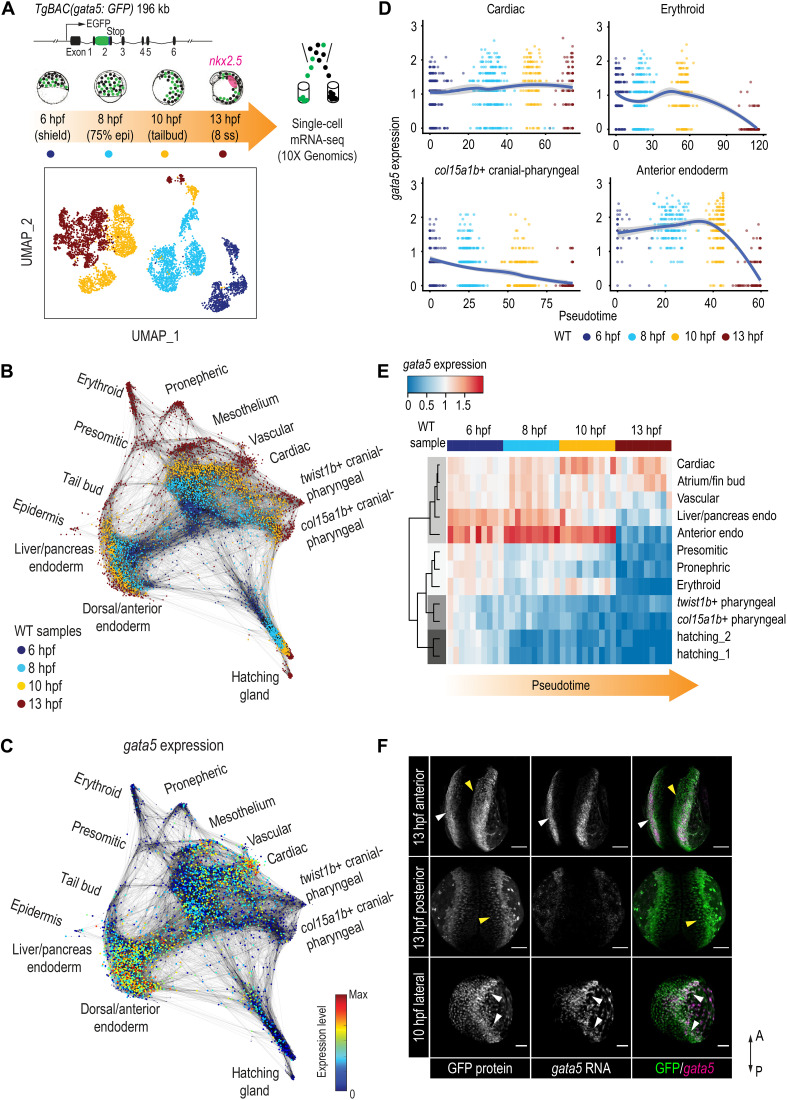
Dynamic expression of gata5 in mesoderm lineages
The heartless phenotype in zebrafish gata5/6 mutants and morphants (embryos injected with gata5/6 MO) can first be detected at the bilateral heart field stage, with loss of nkx2.5, the earliest apparent definitive marker of cardiac mesoderm. However, the expression of gata5 and gata6 can be detected before the onset of gastrulation, broadly within the mesendoderm and yolk syncytial layer (YSL), and become restricted to the ALPM by 13 hours postfertilization (hpf; fig. S1, A to D) (9, 25). This suggests that gata5 and gata6 may be required for the early specification of cardiac progenitor cells, either during or immediately after gastrulation. We therefore sought to profile how gata5/6 facilitates the early segregation of the cardiac lineage. We used the TgBAC (gata5:EGFP)pd25 line that faithfully recapitulated the endogenous expression of gata5 (fig. S1, A to D). Because of the perdurance of GFP and the rapid development of early zebrafish embryos, this approach allowed us to capture all cells that expressed gata5 before 13 hpf. We first conducted scRNA-seq on gata5:GFP+ cells at times spanning early to late gastrulation (shield: 6 hpf, 75% epiboly: 8 hpf, and tailbud: 10 hpf) and early somite stages [eight somite stage (ss): 13 hpf] (Fig. 1A). As gata5:GFP+ cells make up 6 to 15% of the embryo at the stages that we profiled, we obtained scRNA-seq data for 901 to 1985 cells for each stage using the 10X Genomics platform, in theory reaching two to three times more coverage of the gata5:GFP+ populations compared to a published whole-embryo single-cell dataset (26). After stringent quality control, 5358 cells were used for downstream analyses (data S1).
Fig. 1. Dynamic regulation of gata5 in various mesendoderm lineages.
(A) Schematic representation of the scRNA-seq experimental design and UMAP visualization of gata5:GFP+ cells collected at four developmental time points. TgBAC(gata5:GFP) contains a 196-kb genomic region spanning the gata5 locus, with the GFP sequence inserted after the first exon of gata5. (B) Force-directed graph showing the connection between all single cells from the four WT samples. Nodes were colored by developmental time points. Edges link nodes with their neighboring cells. For each cell, up to 20 mutual nearest neighbor edges within or between time points are retained. (C) Expression of gata5 projected on the force-directed graph. (D) Pseudotemporal expression profiles of gata5 along the developmental trajectories of representative lineages (cardiac, pharyngeal_2, erythroid, and anterior endoderm). x axis, pseudotime; y axis, normalized log expression level. The smoothed lines show conditional means after local polynomial regression fitting [LOESS (locally estimated scatterplot smoothing) method] and shaded areas indicate SEs. (E) Heatmap showing gata5 expression along the developmental trajectory of major lineages. Pseudotime was scaled across all lineages for each stage. Each column represents a quantile bin of the scaled pseudotime and each row represents a lineage. gata5 expression mean was calculated for each pseudotime bin for each lineage. The row dendrogram shows the unsupervised hierarchical clustering of each lineage based on gata5 expression dynamics. (F) FISH against gata5 transcripts and immunostaining against GFP at 10 and 13 hpf. White arrowheads show cells positive for both gata5 transcripts and GFP protein; yellow arrowheads indicate cells positive for GFP protein only. A, anterior; P, posterior. Scale bars, 100 μm.
We first performed graph-based clustering to examine the cellular heterogeneity of gata5:GFP+ cells at each stage (27). Consistent with previous reports, we observed an increasing complexity of mesodermal and endodermal lineages from 6 to 13 hpf (26, 28), with a divergence of cells evident as early as 6 hpf (fig. S1, E to P). At 10 hpf, expression of ttn.2, which encodes the cardiomyocyte-specific sarcomeric component Titin, was evident in an apparent cardiac mesoderm group that coexpressed other genes associated with cardiogenesis (nkx2.7, gata6, and foxh1) (cluster 7; fig. S1M), suggesting that expression signatures of cardiac commitment are evident by the end of gastrulation. Cellular heterogeneity was greatly increased at 13 hpf, with the emergence of 16 discernible clusters (fig. S1, N to P). Consistent with known roles of gata5/6 (2, 9), at 13 hpf, we detected cardiac progenitors (cluster 9: nkx2.5, mef2ca, and mef2cb) and two distinct endoderm populations (cluster 5: foxa2 and nkx2.7; cluster 12: onecut1 and angptl3). Using established marker genes, we further identified unexpected gata5-labeled cell types at 13 hpf: erythroid (cluster 1: gata1a, cldng, and gfi1aa), endothelial (cluster 11: etv2, fli1b, and kdrl), pronephric (cluster 2: acy3.2, hnf1bb, and foxj1a), presomitic (cluster 10: tbx16 and bambia), somitic (cluster 16: myod1, ttn.1, and myf5), and two cranial-pharyngeal (cluster 3: twist1b and thbs3b; cluster 7: pitx3 and col15a1b) populations. We also observed a likely mesothelium progenitor population (cluster 7: hand2, cxcl12a, and cdx4) (fig. S1, N to P) (29).
To further interpret the developmental progression of GFP+ cells from 6 to 13 hpf, we used a graph-based method to “stitch” cells with similar gene expression from consecutive time points and visualized them using a force-directed layout (26). We observed three major branches representing mesoderm, endoderm, and hatching gland starting from 6 hpf (fig. S1Q). At 13 hpf (red dots), branching tips representing distinct cell types are evident, including all major lineages that we observed in the clustering analysis (Fig. 1B, fig. S1R, and Supplementary Text). Together, this indicated that a broad spectrum of lineages arises from early gata5-expressing progenitors.
As the role of gata5 has not been linked to the specification of many of the lineages identified at 13 hpf (30), we next examined gata5 expression in our scRNA-seq data (Fig. 1C). In most lineages at 13 hpf (branching tips), including hatching gland, pharyngeal mesoderm, and ventral mesoderm derivatives (erythroid, presomitic, and pronephric progenitors), gata5 was no longer expressed, with high levels of gata5 only evident in cardiac, vascular, and mesothelium progenitors (Fig. 1C). To better delineate the temporal expression dynamics of gata5, we established pseudotime developmental trajectories for each distinct lineage (see Materials and Methods for details). The pseudotime trajectory corresponded well with actual developmental time points, with gata5 expression being regulated in distinctive manners in different trajectories (Fig. 1D and fig. S1T). We next aligned the pseudotime of all trajectories to better compare the regulation of gata5 in different differentiation paths. Although the expression of gata5 was relatively robust at the beginning of all lineage trajectories, its expression was only maintained in the cardiac, atrium/fin bud, and vascular progenitors while being down-regulated over 8 to 13 hpf in most other lineages. Although the expression of gata6 was relatively sparse, as compared to gata5, we observed that gata5 and gata6 shared similar expression dynamics in almost all trajectories (Fig. 1, D and E, and fig. S1T).
The observation of 13 hpf populations that lack detectable gata5 expression was consistent with the perdurance of GFP protein, which exceeds the 7 hours of developmental time we examined. To confirm this, we conducted fluorescence RNA in situ hybridization (FISH) against gata5 transcripts and immunofluorescent staining against GFP protein at 10 and 13 hpf. Consistent with our trajectory analysis, cells actively expressing gata5 were largely located at the ALPM and YSL at 13 hpf, whereas GFP+ cells that were located more medially or posteriorly did not express detectable gata5 transcripts (Fig. 1F). Together, our transgenic system captured cells that expressed gata5 before 13 hpf, with regulation of gata5 and gata6 being highly dynamic within these lineages. Both the maintenance and inhibition of gata5/6 expression may therefore be important events in the differentiation of these lineages.
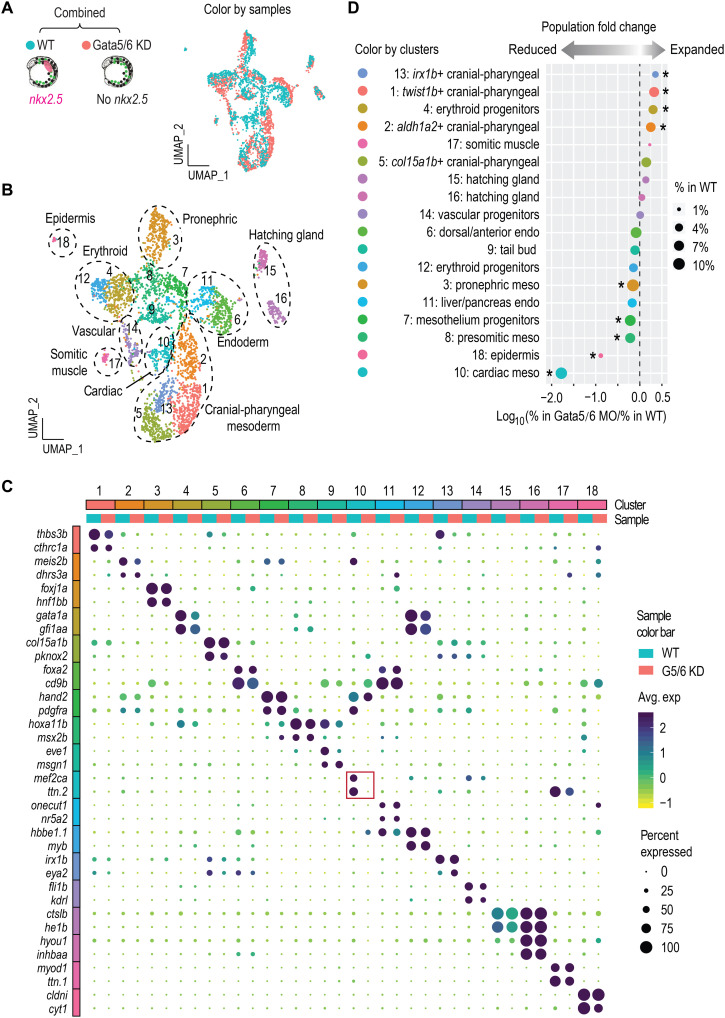
Loss of Gata5/6 leads to changes in mesodermal cell compositions
To directly investigate the roles of gata5/6 in early lineage commitment, we conducted scRNA-seq on 13 hpf gata5:EGFP+ cells collected from Gata5/6 morphants. We confirmed the repression of gata4 expression in these morphants (see fig. S5O), consistent with a previous study (10). We first combined the 13 hpf WT and morphant scRNA-seq data and followed a similar clustering analysis pipeline as above (Fig. 2, A and B, and fig. S2, A and B). We observed a slight increase in cluster numbers, likely due to the boost of analytic power obtained from doubling the number of cells (compare Fig. 2B and fig. S1, N to P). Overall, we detected very similar cell types in both datasets, with marker genes of most clusters shared between WT and Gata5/6 morphant cells (Fig. 2, A to C, and fig. S1, N to P). A notable exception was cardiac progenitors (cluster 10), which were largely absent in morphants (Fig. 2, C and D). While cell identities were highly comparable between WT and Gata5/6 morphants, we observed significant changes in the overall proportions of many lineages. Aside from the absence of the cardiac lineage, there was a significant expansion of cranial-pharyngeal mesoderm (clusters 1, 2, 5, and 13) and one erythroid progenitor population (cluster 4), with reduction of several posterior mesoderm clusters including those with pronephric and presomitic mesoderm signatures (Fig. 2D). These lineage proportion changes remained evident when the cardiac cluster was removed from the WT data (fig. S2C). Expansion and reduction of cell types not currently thought to be dependent on Gata5/6 function suggested that regulation of gata5/6 expression may play an unappreciated role in multiple, perhaps antagonistic, fate decisions.
Fig. 2. Loss of Gata5/6 leads to expansion and reduction of multiple mesoderm lineages.
(A and B) UMAP visualization of merged single-cell datasets from 13 hpf WT and Gata5/6 KD samples, colored by sample (A) or cluster IDs (B). (C) Dot plot showing the mean expression levels (color) of marker genes and the percentages of cells in which marker genes are expressed (size) in each cluster, with WT and Gata5/6 KD cells plotted separately. Red square indicates the marker gene expression in the cardiac lineage. (D) Cell composition changes of each cluster between Gata5/6 KD and WT samples. Asterisks indicate significant differences (Fisher’s exact test, Bonferroni correction, adjusted P < 0.05). Dot sizes show the percentage of each cluster within the whole WT population.
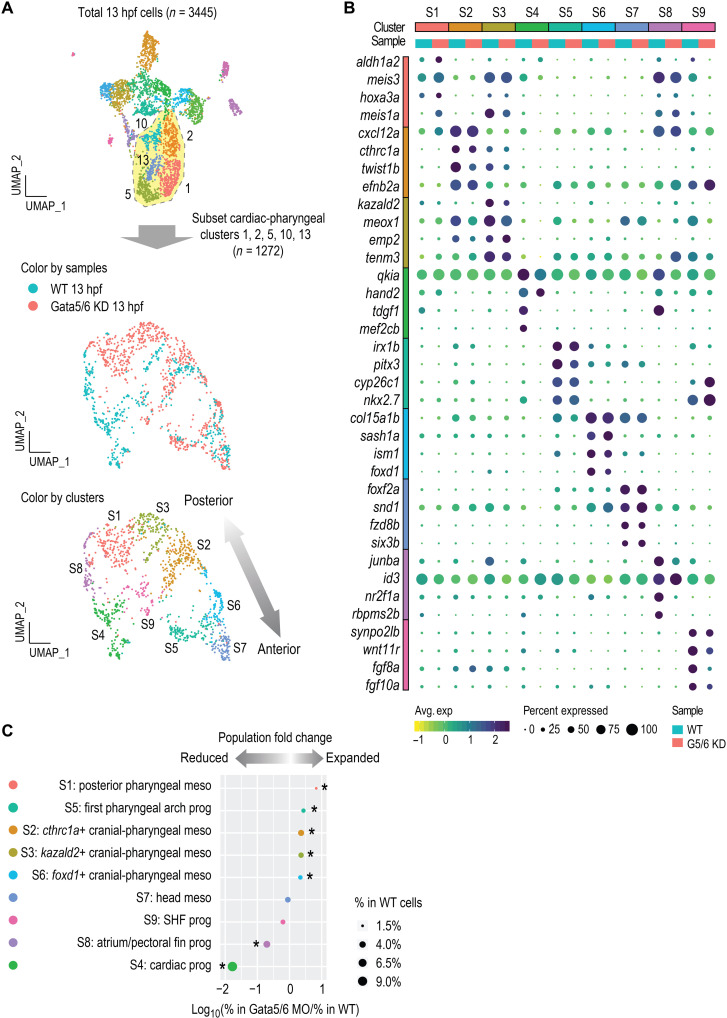
Characterization of molecularly distinct cardiac and pharyngeal subtypes
The most notable effect observed in the Gata5/6 morphant scRNA-seq data was the reciprocal decrease in cardiac and increase in pharyngeal mesoderm populations (Fig. 2D). This observation was highly reminiscent of a previously demonstrated common origin of cardiac and pharyngeal mesoderm: cardiopharyngeal field (17, 18, 31). To further investigate the heterogeneity of the cardiac and pharyngeal clusters, we subclustered these cell populations (clusters 1, 2, 5, 10, and 13). We identified nine molecularly distinct cardiac and pharyngeal clusters, including three cardiac (subclusters S4, S8, and S9), five cranial-pharyngeal (subclusters S1, S2, S3, S5, and S6), and one head (subcluster S7) mesoderm population (Fig. 3A and fig. S3, A and B). In addition to a clear cardiac population (subcluster S4: mef2cb and hand2), we also identified clusters expressing markers of putative atrium/pectoral fin bud (subcluster S8: nr2f1a and hoxb5b) (32) and SHF (subcluster S9: fgf10a and fgf8a) progenitors (Fig. 3C) (33, 34). By mining published gene expression data of the marker genes from each cluster at the pharyngula stage, we made speculations regarding the progenies of the five cranial-pharyngeal subclusters: S2, putative pharyngeal arch two (based on efn2b; table S5); S3, one of the posterior arches (based on tenm3; table S5); and S6, one of the anterior arches (based on foxd1; table S5). Together with the S1, aldh1a2+ posterior pharyngeal mesoderm, and S5, cyp26c1+ first pharyngeal arch progenitors (see table S5 for markers used to define these), the progenies of these pharyngeal subpopulations displayed an anterior-posterior organization that corresponded well with their relative positions on the subclustering UMAP (Uniform Manifold Approximation and Projection) (Fig. 3A). Canonical pharyngeal progenitor genes, such as tbx1 and ebf gene family members, showed distinct combinatorial expression in different pharyngeal subclusters (fig. S3D and Supplementary Text).
Fig. 3. High-resolution clustering analysis of the cardiac and pharyngeal mesoderm populations.
(A) Subclustering analysis of the cardiac and pharyngeal mesoderm lineages. Clusters 1, 2, 5, 13, and 14 were subset from the combined 13 hpf dataset shown in Fig. 2 and reanalyzed. New clustering results are visualized by UMAPs that are colored by samples (top) and by cluster IDs (bottom). (B) Dot plot showing the mean expression levels (color) of marker genes and the percentages of cells in which marker genes are expressed (size) in each cluster, with WT and Gata5/6 KD cells plotted separately. (C) Cell composition changes of each cluster (after subclustering) between Gata5/6 KD and WT samples. Asterisks indicate significant differences (Fisher’s exact test, Bonferroni correction, adjusted P < 0.05). Dot sizes show the percentage of each cluster within the whole WT population (total 13 hpf WT cells).
While SHF progenitors have been identified in several mouse single-cell studies at early stages [embryonic day 7.25 (E7.25)] (14, 15), potential molecular signatures of the SHF at 13 hpf are largely unknown in zebrafish. Supporting that we identified zebrafish SHF cell populations, the 62 zebrafish marker genes of the SHF-like subcluster (S9) were enriched for regulation of SHF cardioblast proliferation [GO:0003266, false discovery rate (FDR) = 1.45 × 10−2; data S2]. We next asked whether the mouse orthologs of the 62 zebrafish marker genes found in our putative SHF-like subcluster (S9) were also found in SHF progenitor cell populations identified by a mouse single-cell study at early stages (E7.75 and E8.25) (35). Among all our cardiac and pharyngeal subclusters (fig. S3C), marker genes of the SHF-like subcluster (S9) overlapped the most (12 of 62, 19.4%) with the genes defining the mouse anterior SHF (n = 212, corresponding to 221 orthologs in zebrafish). We confirmed the ALPM expression pattern of one of the SHF-like subcluster marker genes, wnt11r (wnt11 related; fig. S4E), suggesting that it may also be a zebrafish SHF progenitor marker.
To spatially map the five cranial-pharyngeal and the head mesoderm subclusters relative to the cardiac subclusters at early segmentation stages, we first conducted chromogenic RNA ISH for marker genes of each cardiac and pharyngeal subcluster (Fig. 4, A, B, D, and E, and fig. S4A). We observed that each subcluster occupied a spatially distinct domain at early somite stages. We next sought to characterize the relative spatial occupancy between the pan-cardiac and SHF populations at a single-cell resolution using RNAscope. The putative fgf8a+ SHF progenitor population occupied a more anterior and medial domain compared to the other cardiac progenitors at 13 hpf (fig. S4, B and C), as expected from previous fate-mapping studies (36). Moreover, FISH against nkx2.5 (S4, pan-cardiac) and cyp26c1 (S5, pharyngeal arch one) showed that the putative pharyngeal arch one progenitors occupied a more anterior and slightly more medial domain compared to the cardiac progenitors at 13 hpf (fig. S4F), in line with previous work showing a limited number of cells coexpressing these two genes at the anterior limit of the nkx2.5+ cardiac cells (37). As a subset of nkx2.5+ cells at the ALPM give rise to pharyngeal mesoderm, cells double positive for cyp26c1 and nkx2.5 likely represent the pharyngeal progenitors (38, 39). By further performing double FISH coupled with GFP immunostaining in gata5:EGFP embryos, we confirmed that the putative pharyngeal arch one progenitors had low GFP expression and were located medially to high-GFP cells that were likely to be cardiovascular progenitors (fig. S4D). This further supports that gata5/gfp transcription was selectively turned off in the cranial-pharyngeal populations. Overall, we were able to molecularly resolve distinct cardiac and cranial-pharyngeal populations and spatially mapped the most anterior subset of them (S4, S5, and S9) at early somite stages (fig. S4G), in agreement with previous fate-mapping work showing very early divergence of pharyngeal mesoderm subpopulations (38–40) and atrial/ventricular progenitors (41).
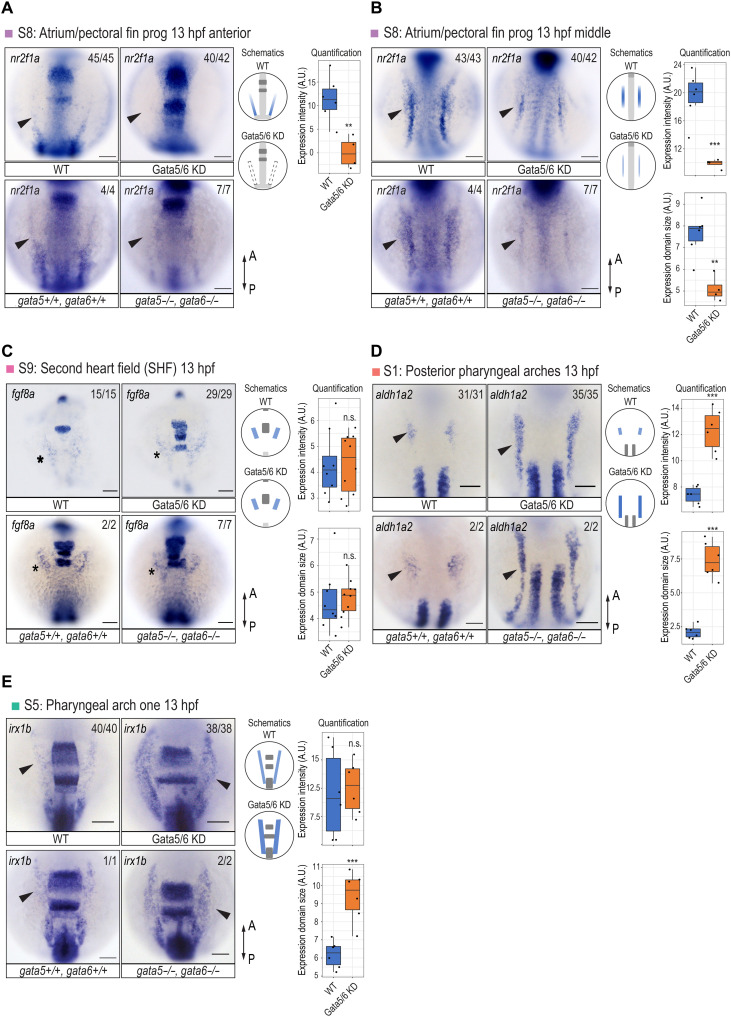
Fig. 4. Specification defects of the cardiac and pharyngeal mesoderm upon Gata5/6 loss.
RNA ISH against atrial/pectoral fin progenitor marker nr2f1a (A and B), SHF marker fgf8a (C), posterior pharyngeal arch progenitor marker aldh1a2 (D), and pharyngeal arch one progenitor marker irx1b (E) in WT, Gata5/6 KD embryos, and gata5/6 mutants at 13 hpf. For each gene, quantification and quantification schematics are shown on the right side. Only the lateral staining (colored blue in the schematics) was quantified. For all staining except the anterior expression of nr2f1a, both expression intensity and expression domain sizes were quantified. Since the anterior lateral expression of nr2f1a was not detectable in Gata5/6 KD embryos, we applied the same regions of interest (ROIs) that we used in WT to measure the intensity. Arrowheads in (A), (B), and (D) indicate the lateral domains that show differential expression between WT and Gata5/6-deficient embryos. Stars in (C) denote the unaffected anterior lateral expression of fgf8a in Gata5/6 KD embryos and gata5/6 mutants. Dorsal views are shown. t test was used to determine statistical significance. ***P < 0.001; **P < 0.01; not significant (n.s.), P > 0.05. Scale bars, 100 μm. A.U., arbitrary units.
Gata5/6 controls cardiac and pharyngeal lineage specification
To further examine the roles of gata5/6 in the cardiac and pharyngeal subclusters, we performed a cell composition analysis. This revealed distinct effects of Gata5/6 loss on different cardiac subpopulations, with the pan-cardiac (subcluster S4) and atrium/fin bud (subcluster S8) progenitors greatly reduced, while the SHF progenitors (subcluster S9) were not significantly affected (Fig. 3C). To validate these observations, we first used RNA ISH to examine the expression of tbx5a, a marker for cardiac and fin bud progenitors (42). The expression of tbx5a was no longer detected in cardiomyocytes while being reduced in the developing pectoral fin bud at 24 hpf upon Gata5/6 loss (fig. S4H). We next examined nr2f1a, which was expressed in both the ALPM and a more posterior domain at 13 hpf, likely representing atrial and pectoral fin bud progenitors, respectively (43). The ALPM expression of nr2f1a was completely depleted, while its expression in the more posterior domain was greatly reduced in Gata5/6-deficient embryos, which likely represented impaired specification of the atrial/pectoral fin bud progenitors (Fig. 4, A and B). Next, to confirm the existence of an SHF-like population upon loss of Gata5/6, we analyzed the expression of tbx1 and fgf8a and found that their ALPM expression remained largely unaltered in Gata5/6 morphants (Fig. 4C and fig. S4I). This suggests that SHF progenitors may still be specified upon loss of Gata5/6, indicating that cardiac progenitor subtypes have distinct requirements for gata5/6.
Cell composition analysis showed significant expansion of all five cranial-pharyngeal mesoderm subclusters upon loss of Gata5/6 (Fig. 3C). To characterize this further, we first used RNA ISH to analyze the expression of marker genes in WT and Gata5/6-deficient embryos. At 13 hpf, the aldh1a2-expressing posterior pharyngeal arches (subcluster S1) showed the strongest expansion in Gata5/6 morphants (Figs. 3C and 4D). The expansion of the putative pharyngeal arch one population (cluster S5), which was enriched for cyp26c1 and irx1b transcripts, was also confirmed in Gata5/6 morphants by RNA ISH (Figs. 3C and 4E). For other cranial-pharyngeal or head mesoderm populations, we observed either small increases in marker gene expression (subclusters S2 and S6) or expression domains (subcluster S3) or no obvious changes (subcluster S7), largely consistent with the cell composition changes in our single-cell experiments (Fig. 3C and fig. S4A).
To examine a direct, cell-autonomous role for Gata5/6 in regulating cardiac and pharyngeal cell fate, we performed transplantation experiments. In these experiments, we isolated WT or Gata5/6-depleted cells isolated from donor embryos and assessed their ability to contribute to cardiac and pharyngeal lineages. We first used the Tg(acta1:GFP)zf13 embryos either uninjected or injected with gata5/6 MO as donors, as this transgenic line labels not only facial muscles but also trunk muscles (40), which can serve as a control. Consistent with our single-cell analysis, we found that cells deficient in Gata5/6 had a higher propensity to contribute to facial muscle than WT cells (13.10% versus 3.87% of transplants, P < 0.005), while the contribution to trunk muscles remained similar (46.43% versus 41.94%, P > 0.4; Fig. 5, A and B). Next, we used the Tg (nkx2.5: zsYellow)fb7 transgenic line that marks both the heart and pharyngeal mesoderm derivatives, predominantly the PAAs (21, 44). We observed that Gata5/6-deficient donor Tg (nkx2.5: zsYellow)fb7 cells contributed more than WT to the pharyngeal (9.35% versus 2.45% of transplants, P < 0.004) but negligibly to the cardiac (0.36% versus 7.35%, P < 0.0002) mesoderm (Fig. 5, C and D). Together, this showed that loss of Gata5/6 biased cells to both pharyngeal muscle and pharyngeal vasculature fates in a cell-autonomous manner, possibly at the expense of the cardiac lineage.
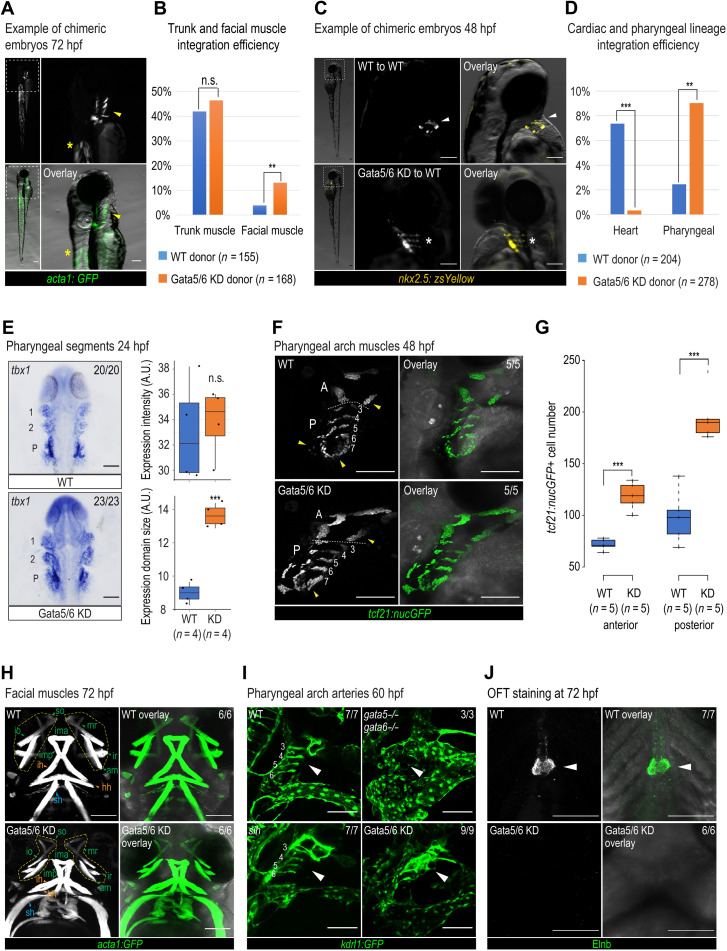
Fig. 5. Pharyngeal arch specification and patterning defects observed at later developmental stages.
Representative chimeric embryos from transplant experiments using Tg (acta1:GFP)zf13 (A) and Tg (nkx2.5:zsYellow)fb7 (C) donors. (A) Yellow arrowheads, pharyngeal muscle; asterisks, trunk muscle. (C) White arrowheads, cardiomyocytes; asterisks, pharyngeal cells. Quantification of integration efficiency in Tg (acta1:GFP)zf13 (B) and Tg (nkx2.5:zsYellow)fb7 (D) transplant experiments. Fisher’s exact test was used. (E) tbx1 RNA ISH and quantification in WT and Gata5/6 morphants at 24 hpf. 1, pharyngeal arch 1 segment; 2, pharyngeal arch 2 segment; P, posterior pharyngeal arch segment. (F) Confocal images of tcf21+ pharyngeal muscle cells in WT and Gata5/6 morphants at 48 hpf. Yellow arrowheads, expanded anterior and posterior pharyngeal arches upon Gata5/6 loss. A, anterior pharyngeal arches; P, posterior pharyngeal arches, separated by dotted lines. (G) Quantification of tcf21: nucGFP+ cell numbers within the anterior and posterior pharyngeal arches (arches 3 to 7) at 48 hpf. t test was used. (H) Confocal images of acta1:GFP+ facial muscle in WT and Gata5/6 morphants at 72 hpf. Green arrowheads, PA1-derived muscle; orange arrowheads, PA2-derived muscle; blue arrowheads, somite-derived muscle. Yellow boxes, muscle around eyes. am, adductor mandibulae; hh, hyohyal; ih, interhyal; ima, intermandibular anterior; imp, intermandibular posterior; io, inferior oblique; ir, inferior rectus; mr, medial rectus; sh, sternohyoideus; so, superior oblique. (I) Confocal images of kdrl:GFP+ vasculature in control, Gata5/6 morphants, gata5/6 mutants, and sih mutants at 60 hpf. Arrowheads, PAA areas. (J) Confocal images of Elnb antibody staining in WT and Gata5/6 morphants. Arrowheads, OFT smooth muscle cells. ***P < 0.001; **P < 0.01; n.s., P > 0.05. Scale bars, 100 μm.
Gata5/6 dosage regulates the balance of cardiac versus pharyngeal fates
At the time of this work being carried out, gata6 mutants were unavailable, and it was uncertain whether published gata5 mutant alleles were true nulls (s26 and tm236a) (9). While gata5 mutant and Gata5 morphant phenotypes are highly comparable (9), nonspecific side effects have been ascribed to MOs (45). We therefore aimed to validate the scRNA-seq results in mutant embryos by generating new null mutations in gata5 and gata6 using CRISPR-Cas9–mediated genome editing. Both a gata5 mutant with a 29–base pair (bp) deletion in the second exon and a gata6 mutant with an 11-bp deletion in the second exon were recovered (fig. S5A). Analysis of mutants revealed expected redundant functions for gata5 and gata6 in cardiac development, with double homozygous mutants lacking the expression of cardiac progenitor markers at 13 hpf and later heart formation (fig. S5, B and C), in full agreement with the cardiac defects reported in independently generated zebrafish gata5/6 mutants (3). In addition, mutant embryos formed much smaller pectoral fins with morphological defects (Fig. 4A and fig. S5D). Overall, the cardiac phenotypes of gata5/gata6 double homozygous mutants largely phenocopied those in Gata5/6 morphants (fig. S5, E and F), validating the MO usage for earlier stages of development when no obvious morphological phenotypes allowed for preselection of gata5/6 mutants.
Analysis of gata5/6 compound homozygous mutants confirmed the reduction of the cardiac and fin bud mesoderm and the expansion of the pharyngeal lineages observed in the morphant embryos (Fig. 4 and fig. S5, A to L). By analyzing all nine genotypes obtained from incrossing gata5+/−; gata6+/− double heterozygous mutants, we further observed that the cardiac and pharyngeal phenotypes can be well explained by the reduction of the gata5/6 gene dosage, with gata5 playing a more prominent role (fig. S5, G to L). The robust dosage effect of gata5/6 combined with the transplant experiments strongly indicated that the dosage of gata5/6 is a principal determinant underlying cardiac and pharyngeal divergence.
The progressive reduction of nkx2.5 expression in the ALPM following the loss of gata5/6 seems to contradict the observed expansion of the pharyngeal mesoderm, which has been previously shown to emerge from nkx2.5+ mesodermal cells at 11 to 12 hpf (21). To address this discrepancy, we examined the temporal expression of nkx2.5 by RNA ISH in WT and Gata5/6 morphants from 11 to 28 hpf (fig. S5M). As indicated by our scRNA-seq data, RNA ISH confirmed that nkx2.5 expression at the ALPM was absent following loss of gata5/6 at early segmentation stages (11.5, 12, and 14 hpf). However, at 24 and 28 hpf, although cardiac nkx2.5 expression was absent in Gata5/6 morphants, its expression was apparent in the developing pharyngeal arch segments (fig. S5M). At 11 to 12 hpf, ALPM expression of tbx1, a regulator upstream of nkx2.5 during pharyngeal fate determination (21), was apparent in Gata5/6 morphants (fig. S5N). As zebrafish embryos deficient for nkx2.5 display normal PAA specification and patterning (44), we therefore concluded that Gata5/6 is required for the expression of nkx2.5 in the pharyngeal progenitors at early somite stages but not at late segmentation stages.
Gata4 is not required for the early specification of cardiac and pharyngeal lineages
Next, we investigated whether Gata4 takes part in cardiac and pharyngeal mesoderm fate decisions together with Gata5 and Gata6. Embryos lacking gata4 alone initiate cardiac development normally, with later defects in heart development (3). We first confirmed that gata4 expression in the ALPM was absent in gata5/6 double mutants, consistent with what has been observed in Gata5/6 morphants (Fig. 5, O and P) (10). In addition, cardiac-related expression of gata4 was not detected until the ALPM stage and marked a subset of gata5:GFP+ cells (fig. S5, O and U). Therefore, we hypothesized that gata4 is unlikely to participate in the early cardiac versus pharyngeal mesoderm fate decisions. To test this, we examined the expression of aldh1a2 (the posterior pharyngeal arch marker) and irx1b (the pharyngeal arch marker) in offspring from gata4−/−; gata5+/− and gata4+/+; gata5+/− parents, respectively (fig. S5, Q to T). The absence of gata5 in a gata4-null background led to significant expansion of the aldh1a2+ and irx1b+ domains, similar to the effect of gata5 loss in the WT background. However, loss of gata4 alone did not affect the expression of these two markers (gata4−/−; gata5+/+ versus gata4+/+; gata5+/+). Similarly, in a gata5-null background, the expression of aldh1a2 and irx1b was unaffected in gata4-null animals (gata4−/−; gata5−/− versus gata4+/+; gata5−/−) (fig. S5, Q to T). Thus, in zebrafish, gata4 is likely dispensable for the early specification of the cardiac and pharyngeal lineages.
Gata5/6 regulates the development of pharyngeal mesoderm–derived muscles and vasculature
We next sought to determine how an early expansion of pharyngeal mesoderm progenitors at early stages following Gata5/6 loss affects the later development of pharyngeal structures. First, using tbx1 ISH, we observed significantly expanded but properly patterned pharyngeal arch segments in Gata5/6 morphants at 24 hpf, consistent with our observations of pharyngeal progenitors at 13 hpf (Fig. 5E). Next, we used two transgenic lines, TgBAC(tcf21:NLS-EGFP)pd41 and Tg(acta1:GFP)zf13, to characterize pharyngeal mesoderm–derived head and neck muscles. We observed an increase in tcf21+ muscle cells within both anterior and posterior pharyngeal arches in Gata5/6-deficient embryos at 48 hpf (anterior, P < 0.0001; posterior, P < 0.0004; Fig. 5, F and G). The facial muscle fibers marked by the Tg(acta1:GFP)zf13, which are primarily derived from pharyngeal mesoderm (40), were abnormally patterned in Gata5/6 morphants at 72 hpf, although all major muscles appeared to be specified (Fig. 5H). We next examined the pharyngeal vasculature of Gata5/6-deficient embryos using a Tg(kdrl:GFP)s843 transgenic line. At 60 hpf, the PAAs, which were well segmented in WT embryos, were severely mispatterned in Gata5/6 morphants and gata5/6 double mutants (Fig. 5I). This dysmorphia was not caused by the absence of blood flow as the PAAs were correctly patterned in silent heart mutants, which lack heart contraction and blood flow (Fig. 5I). Given that proper segmentation and patterning of pharyngeal primordia was evident at 24 hpf (Fig. 5E), it is likely that the proper dosage of Gata5/6 is required for the migration of the PAA endothelial cells after 24 hpf. Last, expression of the OFT smooth muscle marker Elastin was found to be absent in 72 hpf Gata5/6-deficient embryos (Fig. 5J), indicating a defect in the development of this pharyngeal mesoderm–derived lineage. Therefore, the major pharyngeal mesoderm–derived cell types are found mispatterned, often in expanded numbers in Gata5/6-deficient embryos, with the exception of absent OFT cells, which likely reflects the heartless phenotype.
The loss of Gata5/6 leads to extensive chromatin accessibility changes in the gata5:GFP-positive mesoderm
To identify mechanisms through which Gata5/6 might promote cardiac fate while repressing the pharyngeal program, we conducted assay for transposase-accessible chromatin using sequencing (ATAC-seq) on gata5:GFP+ and gata5:GFP− cells isolated from control and Gata5/6 KD embryos at 8 hpf, a stage when a committed cardiac lineage was not yet evident (Fig. 6A). We detected roughly 100,000 accessible chromatin regions (ATAC-seq peaks) in each of the four samples (WT GFP+, WT GFP−, Gata5/6 KD GFP+, and Gata5/6 KD GFP−) (data S1). By conducting pairwise comparisons, we identified 15,596 peaks showing significant differential accessibility between WT GFP+ and WT GFP− populations (FDR < 0.05, fold change > 1), which we termed GFP+-specific (n = 8323) or GFP−-specific (n = 7273) regions (Fig. 6, B and C; fig. S6, A and B; data S2; and Supplementary Text). Upon Gata5/6 KD, we detected 2597 differentially accessible regions (DARs) in the GFP+ populations (FDR < 0.05, fold change > 1), with 2225 regions losing accessibility (GFP+ closed DARs) and another 372 regions gaining accessibility (GFP+ open DARs) (Fig. 6, B and D, fig. S6C, and data S3). In contrast, we only detected one DAR in the GFP− populations upon Gata5/6 KD (GFP− DARs) using the same threshold (Fig. 6B and data S3). This suggests that Gata5/6 regulates chromatin accessibility mostly through a direct, cell-autonomous mechanism.
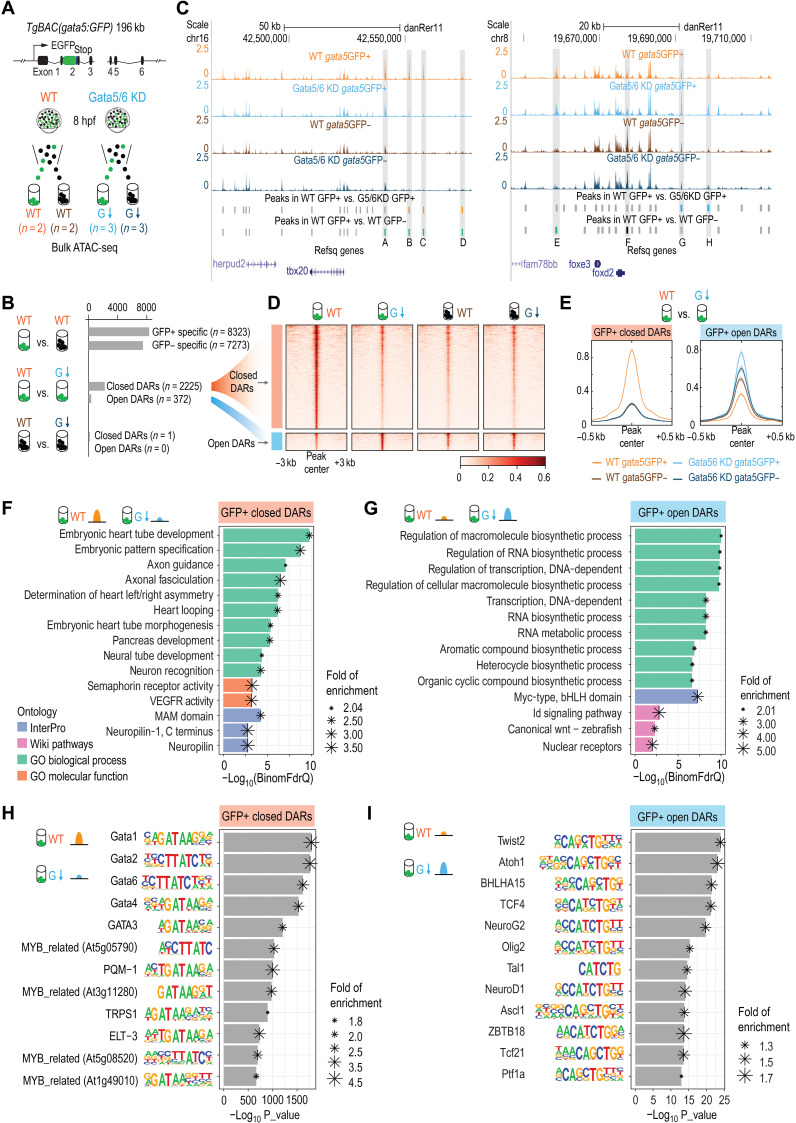
Fig. 6. Changes of open chromatin landscapes upon loss of Gata5/6.
(A) Schematic representation of the ATAC-seq experimental design. The same BAC transgenic lines used for the scRNA-seq were used for ATAC-seq to isolate gata5:GFP+ and gata5:GFP− cells. (B) Schematic representation of the three pairwise comparisons and the numbers of DARs identified in each comparison. (C) Genome browser view of the ATAC-seq signals and peaks identified in the four different conditions at the tbx20 and foxd2 loci. The sequence coverage tracks show replicate-merged, sequence depth-normalized (counts per million) read coverage for each sample. Gray sticks, shared peaks; orange sticks, closed DARs in GFP+ cells; blue sticks, open DARs in GFP+ cells; green sticks, GFP+-specific peaks in WT; black sticks, GFP−-specific peaks in WT. A to H denote several representative differential peaks. (D) Heatmap showing the closed and open DARs identified in GFP+ cells. The read intensity within 3 kb of the peak center was plotted for each peak. (E) Aggregate plots showing the ATAC-seq signals in closed and open DARs identified in GFP+ cells. (F and G) Bar plots showing the top 10 most enriched terms (or all enriched terms if the total number < 10) obtained from GFP+ closed DARs (F) and GFP+ open DARs (G) using GREAT analysis. Terms from four categories (GO biological process, GO molecular function, InterPro, and WikiPathways) were plotted. (H and I) Motif enrichments identified by Homer within closed DARs in GFP+ cells (H) and open DARs in GFP+ cells (I). The top 12 motifs are plotted.
Indicative of a direct role of Gata5/6 in establishing chromatin accessibility in zebrafish embryos, regions where chromatin accessibility was reduced after the KD of Gata5/6 (closed DARs) showed a strong enrichment for GATA motifs based on two independent motif enrichment programs and databases (Fig. 6H, fig. S6E, and data S4). Moreover, Gata5/6 KD reduced the accessibility signals within the GFP+ closed DARs to a basal level similar to that in the Gata5-negative populations (GFP− cells) (Fig. 6, D and E). Notably, the accessibility signals of these closed DARs were not sensitive to the loss of Gata5/6 in the Gata5-negative populations (GFP− cells) (Fig. 6, D and E). In contrast to the closed DARs, the accessibility signals within the GFP+ open DARs did not purely depend on the presence of Gata5/6 (e.g., the aggregated signals in Gata5/6 KD GFP+ and Gata5/6 KD GFP− populations were not the same; Fig. 6E), suggesting that other factors may work together with Gata5/6 to repress the chromatin accessibility, either directly or indirectly. Together, these results suggest that Gata5/6 themselves may directly promote high accessibility within these 2225 Gata5/6-dependent accessible chromatin regions, consistent with previous studies in mammalian cells demonstrating that GATA4 is a pioneer TF that can establish accessible chromatin (46).
Gata5/6 regulates chromatin accessibility at cardiac and pharyngeal regulatory genes
We next examined the function of DARs whose open chromatin status was dependent on Gata5/6. Closed DARs were highly enriched for proximity to genes related to many heart development–related processes (embryonic heart tube development: FDR = 1.82 × 10−10; heart looping: FDR = 7.33 × 10−7, binomial test; Fig. 6F and data S4), including known regulators of cardiac development such as gata4, hand2, tbx20, and mef2cb (fig. S5V and data S4). Two regions coinciding with closed DARs [one near hand2 (aCNE1) and the other one close to tbx20 (aCNE20)] were previously characterized using GFP reporter assays and RNA ISH against gfp, both of which displayed early cardiac activity at ALPM stages (13 hpf) (47). In contrast, open DARs were enriched for metabolic-, biosynthetic-, and transcription-related processes (regulation of cellular macromolecule biosynthetic process: FDR = 1.76 × 10−10; regulation of transcription, DNA-dependent: FDR = 1.48 × 10−10, binomial test; Fig. 6G and data S3). Notably, motifs of factors involved in pharyngeal and head muscle development—such as Twist, Tcf21, Myf5, Isl1, Lhx2, and MyoD—were highly enriched in the open DARs (Fig. 6I and data S3), suggesting the involvement of the open DARs in pharyngeal development. Although GFP+ Gata5/6 KD open DARs were not significantly associated with cranial-pharyngeal development processes (data S3), we observed a significant enrichment (fold enrichment = 4.47, P = 1.04 × 10−4, hypergeometric test) of the open DARs near pharyngeal mesoderm genes conserved between mouse and zebrafish (fig. S7, A to C), including tbx1, twist1a, irx5a, foxd2, ebf2, and ebf3a (table S6 and Supplementary Text).
As Tbx1 is required for both proper cardiogenesis and pharyngeal development (21, 34), Gata5/6-dependent DARs at the tbx1 locus were of particular interest for further analysis of Gata5/6 function. We detected three distal regions that, following Gata5/6 KD, showed a decrease in chromatin accessibility (tbx1 closed DARs; 110 to 599 kb upstream of the tbx1 TSS) and one proximal region that showed a gain in chromatin accessibility (tbx1 open DAR; 29 kb upstream of the tbx1 TSS) (Fig. 7A). We hypothesized that the tbx1 closed DARs are cardiac enhancers, whereas the tbx1 open DAR is a pharyngeal enhancer. We generated stable GFP reporter transgenic lines for all four regions. We found that the three tbx1 closed DARs, all of which contain one or more cardiac TF binding motifs (GATA, TBX5, NKX2.5, and MEF2C), consistently drove GFP activity in the ventricle and OFT of the developing heart at 50 to 60 hpf (Fig. 7, B to D; fig. S8, A to G; and data S5). These tbx1 closed DARs were also active at the end of gastrulation or early segmentation stages (fig. S8, I to N), suggesting a requirement for Gata5/6 in establishing the open chromatin states of early cardiac enhancers. In contrast, the tbx1 open DAR, which contained muscle-specific MYOD1 and MYF5 TF motifs (data S5), drove expression in the pharyngeal mesoderm–derived facial muscles marked by Tg(tcf21: dsRed)sd2 at 72 hpf (Fig. 7E, fig. S8H, and Supplementary Text). Together, our results suggest that Gata5/6 regulates cardiac and pharyngeal mesodermal fates by promoting the accessibility of cardiac enhancers while repressing that of the pharyngeal enhancers, in particular those that regulate essential cardiac/pharyngeal genes, such as tbx1.
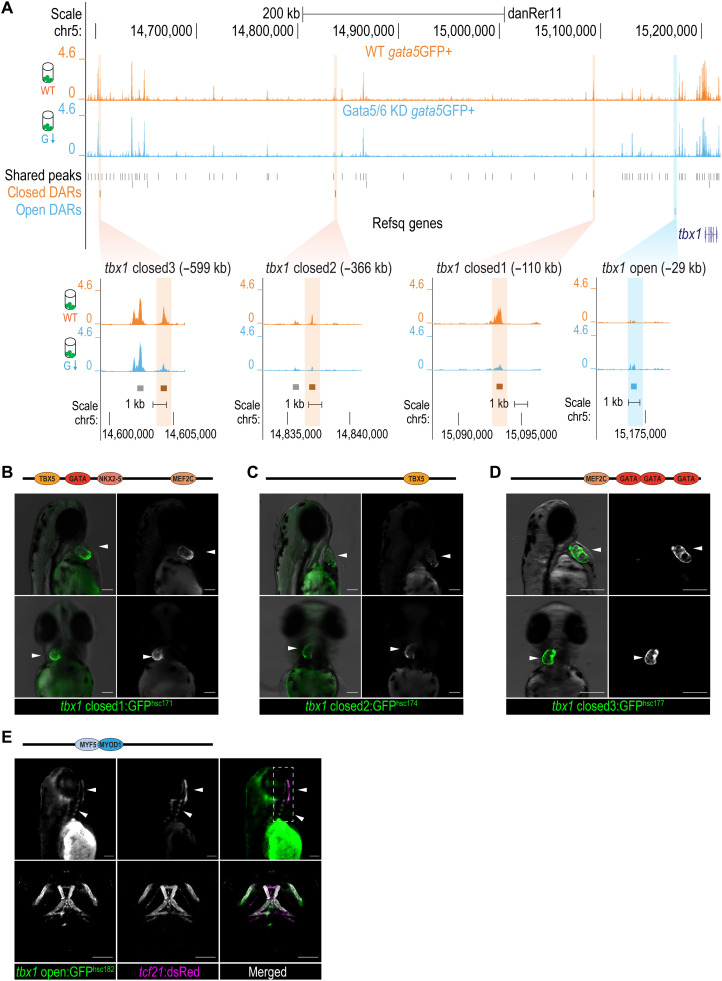
Fig. 7. Cardiac and pharyngeal enhancer activities of DARs at the tbx1 locus.
(A) Genome browser view of the ATAC-seq signals and zoomed-in views of the DARs at tbx1 locus. The sequence coverage tracks show replicate-merged, sequence depth-normalized (counts per million) read coverage for each sample. Gray sticks, shared peaks; orange sticks, closed DARs in GFP+ cells; blue sticks, open DARs in GFP+ cells. (B to D) Fluorescent images of F2 transgenic lines at 50 to 60 hpf, generated using tbx1 closed DAR sequences: closed_1hsc172(B), closed_2hsc174(C), and closed_3hsc180(D). White arrowheads show the GFP activity in the developing heart. Cardiac TF motifs within each sequence identified via motif scans were annotated in the schematics above the fluorescent images. (E) Fluorescent (top) and confocal (bottom) images of Tg(tbx1open:GFPhsc182, tcf21:dsRedpd37) at 72 hpf. White arrowheads show GFP and dsRed signal overlays in the pharyngeal mesoderm–derived facial muscles. Head muscle TF motifs via motif scans were annotated in the schematics above the fluorescent images. The full motif scan results are shown in data S5. Scale bars, 100 μm.
Evolutionarily conserved role of Gata4/5/6 in cardiopharyngeal development
The invertebrate chordate Ciona robusta has a single GATA4/5/6 homolog (Gata.a) (5), making it an ideal model to examine both the potential conservation and direct role of GATA4/5/6 in cardiopharyngeal lineage divergence. Moreover, Ciona displays a simplified and stereotyped cardiopharyngeal lineage, whereby two multipotent progenitors on either side of the embryo each undergoes two asymmetric and oriented cell divisions to produce one first heart progenitor, one second heart progenitor, and one pharyngeal muscle progenitor (Fig. 8, A and B) (17, 48). Similar to zebrafish gata5 and gata6, Ciona Gata4/5/6 is expressed in migrating cardiopharyngeal progenitors (5), transiently down-regulated after their first division, and sequentially reactivated in the first and second heart precursor cells (23), a dynamic that is captured by trajectory reconstruction from scRNA-seq data (49). Thus, we used Ciona as a model to directly test whether Gata4/5/6 regulates the heart versus pharyngeal muscle fate decision in the cardiopharyngeal lineage.
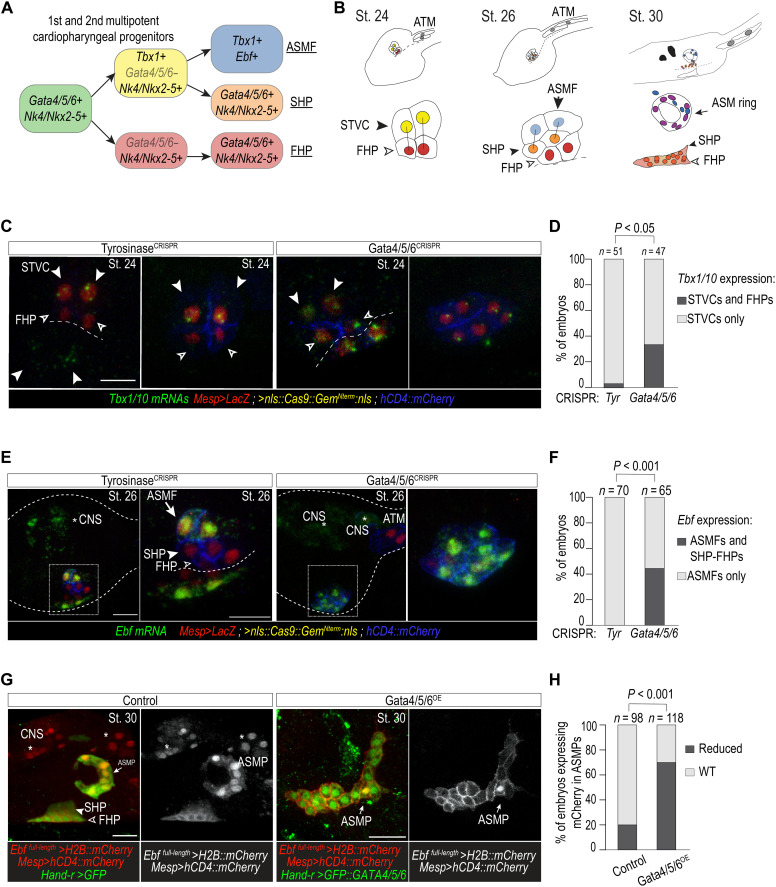
Fig. 8. Gata4/5/6 is required to promote a cardiac and antagonize a pharyngeal muscle fate in Ciona.
(A) Summarized Ciona cardiopharyngeal lineage and expression of markers. Rounded squares, cells; arrows, cell divisions. Note the transient down-regulation of Gata4/5/6 and subsequent reactivation in FHP and SHP (first and second heart progenitors). (B) Schematic representation of cardiopharyngeal lineages at stages 24, 26, and 30. ASM ring (arrows); ATM, anterior tail muscle; black bars link sister cells. Endogenous expression of Tbx1/10 at stage 24 (C) and Ebf at stage 26 (E) visualized by ISH (green) in control (TyrosinaseCRISPR) and Gata4/5/6CRISPR knockout embryos. Proportions of embryos expressing Tbx1/10 (D) and Ebf (F) transcripts in indicated cell-type progenitors. (D) STVC only (Tyr: 97%, n = 48; Gata4/5/6CRISPR: 66%, n = 31), both STVC and FHP (Tyr: 3%, n = 3; Gata4/5/6CRISPR: 34%, n = 16). (F) ASMF only (Tyr: 100%, n = 70), both ASMF and SHP-FHP (Tyr: 0%, Gata4/5/6CRISPR: 43% ± 3% SE; n = 65). Nuclei, red; cell membranes, blue. (G) Target overexpression of Gata4/5/6 in cardiopharyngeal progenitor cells. B7.5 lineage cells, red. ASMP, atrial siphon muscle precursor cells. (H) Proportions of embryos with normal or reduced numbers of Ebf-expressing ASMPs. Control: 20% (n = 98), Gata4/5/6OE: 68 ± 3% SE (n = 118) reduced Ebf-expressing ASMPs. Fisher’s exact test was used. White asterisks, central nervous system (CNS). Scale bars, 10 or 15 μm (zoomed-in views).
Using previously established reagents (50), we targeted CRISPR-Cas9–mediated mutagenesis of Gata4/5/6 to the cardiopharyngeal lineage and assayed the expression of pharyngeal muscle program markers. We first focused on Tbx1/10, a conserved essential regulator of pharyngeal mesoderm development expressed in the common progenitor [second trunk ventral cells (STVCs)] of the SHF and branchiomeric muscle cells (Fig. 8A). At stage 24, where the first heart lineage has segregated from the STVCs (Fig. 8, A and B), Tbx1/10 expression was restricted to the STVCs in the control embryos (TyrosinaseCRISPR) (Fig. 8, C and D). In contrast, >30% of Gata4/5/6CRISPR embryos exhibited conspicuous ectopic Tbx1/10 expression throughout the cardiopharyngeal lineage (Fig. 8, C and D). Similarly, at stage 26, when pharyngeal muscle progenitors have diverged from second heart progenitors (Fig. 8, A and B), expression of the pharyngeal muscle determinant Ebf, which is normally restricted to the lateral pharyngeal muscle precursor cells, was detected ectopically throughout the cardiopharyngeal lineage (Fig. 8, E and F). Conversely, cardiopharyngeal lineage–specific overexpression of a GFP::Gata4/5/6 fusion protein sufficed to inhibit the expression of an Ebf reporter construct and proper migration of pharyngeal muscle progenitors in ~70% of stage 30 swimming larvae, which normally exhibit a ring of pharyngeal muscles around the atrial siphon placode [Fig. 8, G and H; (17)]. As Ebf acts as a master regulator of pharyngeal muscle identity and is sufficient to inhibit the cardiac fate (17, 50, 51), this result indicates that Gata4/5/6 is necessary to both promote cardiac and prevent ectopic pharyngeal muscle fate in the common cardiopharyngeal progenitors. This further suggests that the function of GATA4/5/6 family members in the divergence of the cardiopharyngeal lineage is conserved between vertebrates and tunicates, with TBX1 acting as a conserved downstream target. This conserved GATA-TBX1 regulatory logic is likely to be an ancestral feature involved in chordate cardiopharyngeal development.
DISCUSSION
Our study highlights that, as opposed to being strictly a cardiac TF, zebrafish gata5/6 is broadly expressed in multipotent mesendodermal progenitors and subsequently regulated in distinct manners in different developmental lineages (Fig. 9A). Multiple TFs have been shown to share similar dynamics including key regulators of pluripotency/neural development (Sox2) (52) and hematopoiesis (Gata2) (53). In addition to the de novo expressed lineage-specific TFs, we show in the case of GATA4/5/6 that the selective silencing or maintenance of broadly expressed early TFs may serve as another key mechanism of cell fate determination. Given their role as potential pioneer factors that can initiate reprogramming of genes and cell fates (54), it is conceivable that a silencing program is required in certain lineages. This complex regulation of gene expression is likely characteristic of TFs that can both promote and repress related cell fates, directly or indirectly, and may represent a widely used strategy in early development.
Fig. 9. An evolutionarily conserved GATA4/5/6 regulatory axis regulates cardiac and pharyngeal mesoderm fate balance.
(A) Loss of Gata4/5/6 family TFs in zebrafish and Ciona results in a bias to a pharyngeal fate, which requires down-regulation of Gata4/5/6 expression in a WT-like context. (B) Gata5/6 promotes the accessibility of enhancers near cardiac genes (e.g., gata4, tbx20, and hand2) while repressing that of the pharyngeal enhancers (e.g., tbx1, ebf2, and ebf3a) in the precardiac mesoderm. In the pharyngeal mesoderm, where gata5/6 is down-regulated, the repression of the pharyngeal enhancers is removed.
Single-cell gene expression atlases of whole zebrafish embryos continue to provide novel insights into early zebrafish embryogenesis (26, 28). However, previous whole-embryo datasets have naturally been dominated by ectoderm-derived cells of the nervous system and epidermis, leaving mesoderm and endoderm lineages relatively underrepresented. For example, at a comparable stage (14 hpf), one study profiled 677 mesoderm (17.2% in total) and 57 endoderm (1.45% in total) cells, roughly five times less than the mesendoderm cells (n = 3445) included in our 13 hpf data (26). By specifically focusing on the gata5:GFP+ mesendoderm population, our study covers many of the lineages that are underrepresented in published whole-embryo datasets. By further subclustering and RNA ISH characterization, we provide a high-resolution map of the molecular signatures of many cardiac and pharyngeal lineages. Future analysis of the function and expression of genes that define these populations will be of great interest. More generally, this lineage-focused scRNA-seq approach provides a powerful means to broadly phenotype without the need or cost of sequencing very large numbers of cells.
Our study demonstrates that GATA-mediated cardiac-pharyngeal divergence is evolutionarily conserved between vertebrates and tunicates (Fig. 9A). Evidence suggests that the SHF shares a common progenitor pool with the cranial-pharyngeal mesoderm (17, 18, 31). Consistent with the previously described phenotype that SHF progenitors remained intact in Gata4/6 mutant mice, we observed that a putative SHF population still formed in a gata5/6 loss-of-function context in zebrafish despite the heartless phenotypes in both models (4). In mouse embryos, the early expression patterns of Gata4/6 follow a similar trend of broad expression in lateral plate mesoderm and later restricted expression in the developing heart (12, 55, 56). Note that the SHF population that remained in zebrafish lacking gata5/6 is in a more anterior position, and our SHF cluster showed greater similarity with mammalian aSHF cells. It is unclear why the anterior SHF progenitors, but not the retinoic acid (RA) target gene–enriched posterior ones, were less sensitive to gata5/6 loss. As RA has a well-documented role in restricting FHF and aspects of SHF populations in zebrafish (57), one model would be that RA signaling is heightened by gata5/6 loss. Further investigation will be needed to determine the genetic interactions between gata5/6 and RA signaling pathways in the context of early cardiac fate specification. Also related to the SHF, the ultimate fate of SHF cells that remain in gata5/6 mutants remains unclear. In the absence of an FHF-derived cardiac structure, it seems likely that these cells are unable to execute a cardiac differentiation program and either take on alternate fates or are depleted via a lack of proliferative and prosurvival signals.
In Ciona, Tbx1/10 activity is required to inhibit GATA4/5/6 while promoting the pharyngeal muscle program (Ebf, etc.) in atrial siphon and body wall muscle progenitors (23). Likewise, Tbx1 could be responsible for the down-regulation of gata5/6 in gastrulating zebrafish embryos. The cardiac-pharyngeal separation may therefore be controlled by a GATA4/5/6-TBX1 regulatory circuit. Future experiments that address how the expression of gata5/6 is maintained in cardiac and turned off in pharyngeal mesoderm and identification of Gata5/6 target genes will yield novel mechanistic insights into how these lineages segregate during embryogenesis. In this context, subtle alterations in the expression of key regulators such as gata5/6 may represent an important means to drive morphological changes via modulating cell-type proportions.
GATA4 has been shown to have pioneer activity that can engage condensed chromatin and promote an open chromatin state in mammalian cells in vitro (46). In the developing mouse heart, GATA4 also promotes the deposition of H3K27ac at heart enhancers (58). In Ciona, putative GATA binding sites are enriched in noncoding elements that open specifically in multipotent cardiopharyngeal progenitors in response to fibroblast growth factor (FGF)–mitogen-activated protein kinase signaling (59). Consistent with these previous studies, we observed GATA binding sites within the majority of regions losing accessibilities upon Gata5/6 KD (74.7 to 91.4%; data S4). Furthermore, these Gata5/6-dependent regions are highly associated with heart development processes, indicating that Gata5/6 contributes to the establishment of a substantial fraction of heart enhancers in early development, potentially establishing the template for early cardiac cell fate determination. The pioneering activity of the cardiac GATA factors is likely required to prepattern cardiac enhancers for cardiac fate inductive cues (e.g., signaling and binding of other TFs) at the early- and mid-gastrulation stage, presetting cardiac competence (60). In contrast, we further observed 372 regions gaining accessibilities upon Gata5/6 KD. Such ectopic activation of noncardiac regulatory elements and genes has also been observed in cardiomyocytes containing the disease-associated mutation GATA4-G296S (61). Together, it suggests that lineage-specific modulation of chromatin accessibility is likely an important and evolutionarily conserved mechanism through which GATA4/5/6 controls cardiac and pharyngeal gene expression and balances the early specification of these two lineages (Fig. 9B).
Besides the known role of GATA4/5/6 as transcriptional activators that promote cardiac specification (62, 63), our observation that GATA4/5/6 is crucial for preventing the ectopic expression of the pharyngeal program suggests a repressive role, which is at least partially achieved by repressing the activation of pharyngeal enhancers. It has been shown that mouse GATA4 can suppress the expression of noncardiac programs (64) and a distal intestinal program in the proximal intestine through the interaction with its cofactor FOG (65). It is likely that GATA4/5/6 inhibits the pharyngeal program through a similar mechanism, perhaps by engaging distinct cofactors. The enrichment of the variety of TF motifs within the regions that gain accessibility upon Gata5/6 KD concordantly suggests the participation of other TFs in repressing chromatin accessibility either in concert with or downstream of Gata5/6. Intriguingly, GATA family TF binding sites are frequently found in validated silencers, with a prevalence almost comparable to that of well-known chromatin repressors EZH2 and SUZ12 (66). Further studies that look into temporal changes in cobinding factors and target genes of GATA4/5/6 and how GATA4/5/6 may integrate key signaling pathways are needed to expand the gene regulatory network that governs the development of the cardiopharyngeal lineage. While our work focused on the divergence of cardiac and pharyngeal fate, it is likely that the proper regulation of zebrafish gata5/6 is also important for other lineages derived from gata5-expressing progenitors that require further characterization.
Our work uncovers the highly dynamic, lineage-specific transcriptional regulation of gata5/6 in multiple mesendoderm lineages during zebrafish gastrulation. We show that GATA4/5/6 family members play key, conserved, and early roles in cardiac versus pharyngeal fate separation. This implies that precisely controlled GATA4/5/6 expression during cardiac and pharyngeal specification is an ancestral mechanism that likely affects an unexpectedly large number of additional mesendodermal lineages. Together, this adds support to a model where repression of key lineage regulators, such as GATA4/5/6, is as essential a step in development as their activation.
MATERIALS AND METHODS
Zebrafish line husbandry
All the zebrafish lines were maintained under the guidance and approval of the Canadian Council on Animal Care and the Hospital for Sick Children Laboratory Animal Services. Embryos were maintained at 28.5°C in embryo medium and harvested at appropriate stages as previously described (67). To harvest samples at 13 hpf, embryos were first raised at 28.5°C to 10 hpf and transferred to 22°C overnight to 13 hpf. Staging was performed under the dissecting scope by embryonic morphology. All previously established transgenic and mutant lines used in this study are shown in table S1.
Zebrafish CRISPR-Cas9 mutagenesis
CRISPR-Cas9 genome editing technology was used to generate gata5 and gata6 mutant lines as previously described (68). A 29-bp deletion allele gata5hsc115 (starting at nucleotide 3403 within exon 2) was generated, which results in a premature stop codon at amino acid 233. An additional allele with a 7-bp deletion displayed an equivalent phenotype. The 11-bp deletion allele gata6hsc116 (starting at nucleotide 3522 within exon 2) was isolated with a premature stop codon formed at amino acid 332. Alternatively, another allele with a 10-bp deletion and 5-bp insertion displayed an equivalent phenotype. gata5 heterozygotes were crossed to gata6 heterozygotes to generate the compound heterozygous gata5hsc115; gata6hsc116 lines. Compound heterozygotes were then crossed to various transgenic backgrounds, including Tg(myl7:EGFP)twu34, Tg(gata1a: dsRed)sd2, Tg(nkx2.5:zsYellow)fb7, and Tg(kdrl:EGFP)s843, to perform downstream phenotypic characterization. Primers used for generating guide RNAs are provided in table S2.
Genotyping
Genomic DNA was extracted by incubating embryos or adult fin clips in 50 mM NaOH at 95°C for 20 min. Tris (1 M; pH 8.0) was added at 1/10 volume to neutralize the reaction before polymerase chain reaction (PCR). The gata5 or gata6 mutant allele was identified separately by amplifying a ~150-bp sequence containing the indel locus from the genomic DNA extracted from embryos or adult fin clips. Mutant, heterozygous, and WT embryos were distinguished through the sizes of the PCR products. The same PCR cycling condition was used for all genotyping: denaturing at 94°C for 5 min, thermocycling (35 cycles) at 94°C for 30 s, 60°C for 30 s and 72°C for 30 s, and final elongation at 72°C for 10 min. Primers used for genotyping gata5 mutants were as follows: forward, 5′-GACATGCTGGATGACCTGCCG-3′; reverse, 5′-GCGTTTCTGTGGTTTGATAAGAG-3′. Primers used for genotyping gata6 mutants were as follows: forward, 5′-CGCGATTTATTGACTGAGTG-3′; reverse, 5′-CAGAGAAAGTGTCCCGTGC-3′.
MO injections
MO oligonucleotides were purchased from Gene Tools. MOs targeting the splicing site of gata5 (ssGata5: 5′-TGTTAAGATTTTTACCTATACTGGA-3′) and the translation start site of gata6 (ATGGata6: 5′-AGCTGTTATCACCCAGGTCCATCCA-3′) were used as previously described (2). Injection of 9 ng of ssGata5 MO together with 1.5 ng of tbGata6 MO at the one-cell stage displayed a heartless phenotype in line with cardiac defects in gata5hsc115, gata6hsc116 compound homozygous mutants.
Colorimetric ISH
Digoxigenin (DIG)–labeled and fluorescein (Flu)–labeled in situ probes were synthesized using in vitro transcription with Flu/DIG RNA labeling kits, respectively (Roche, catalog nos. 11175025910 and 11685619910). RNA ISH was conducted according to the published protocol (69). Embryos synchronized to the right developmental stages were fixed in 4% paraformaldehyde (PFA) and then stored in 100% methanol at −20°C until used. After rehydration in phosphate-buffered saline (PBS) with 0.1% Tween 20 (PBSTw), embryos older than 48 hpf were permeabilized using proteinase K before being incubated with DIG-labeled antisense RNA probes at 65°C overnight. RNA-probe hybrids were detected by an alkaline phosphatase (AP)–conjugated antibody (anti–DIG-AP or anti–Flu-AP, Fab fragments; 1:5000; Roche, catalog nos. 11093274910 and 11426338910). Embryos were washed in MABT (Maleic acid buffer with 0.1% Tween 20) before being stained with nitro blue tetrazolium (NBT)/bromochloroindolyl phosphate (BCIP) (Roche, catalog no. 11681451001). Stained embryos were cleared in BBA solution (2:1 benzyl benzoate:benzyl alcohol) and imaged under a Zeiss Axio Zoom.V16 stereoscope. Each ISH experiment was performed at least twice to identify a certain gene expression pattern in both WT and Gata5/6 KD embryos (n > 15). Embryos incrossed from gata5/6 compound heterozygous mutants were dehydrated in 80% glycerol for imaging and genotyped after imaging.
All previously described probes used in this study are summarized in table S3. Other in situ probes were synthesized using the following primers: nr2f1a (5′-ATGGTAGTTAGCGTCTGGCG-3′ and 5′-AAAGCTTGCCAAAGCGACTG-3′), irx1b (5′-ACAGAGTTCCTCGGGAGT-3′ and 5′-CCGATGACGTATATGCTGTTGC-3′), cyp26c1 (5′-GATGCCGTCTTACCGACAGT-3′ and 5′-ATCGCAGTAATCGCTGGCTTG-3′), cthrc1a (5′-CTGCATTTCGGTAATGATGG-3′ and 5′-TGGTGATGCTCATTTTGGAAGC-3′), kazald2 (5′-ATGCTGGTTTCCGTATC-3′ and 5′-CACGGTGAGCTAATATTTCTGGTT-3′), foxd1 (5′-CATCGGGAAACCCGAGAG-3′ and 5′-ATTAGCAGCCGCTAGTGCCACTGA-3′), six3b (5′-TCCCCGTCGTTTTGTCTCTG-3′ and 5′-TGTCCGACGGTGCATCATAC-3′), tbx1 (5′-AAGGAGCGCAGTGGATGAAG-3′ and 5′-GTACATGTTGGCTGTGGTTG-3′), aldh1a2 (5′-TCGGCAGGGGAAAAAAACCC-3′ and 5′-TTTTTTTTTTTTTTTCAGAGGTAA-3′), and wnt11r (5′-ATGAAGCGAACCTTCCCTTCCCTCC-3′ and 5′-CCAGTAGCTCATTTGCAGACG-3′).
Quantification of colorimetric (NBT/BCIP) ISH was performed according to a previously published protocol (70). We first converted the raw images to the 8-bit grayscale format in Fiji and then drew the region of interest (ROI) around the relevant signals. Only the anterior lateral staining of each gene (highlighted in Fig. 4 schematics) was quantified. For all staining except the anterior expression of nr2f1a, both expression intensity and expression domain sizes were quantified. Since the anterior lateral expression of nr2f1a was not detectable in Gata5/6 KD embryos, we applied the same ROIs that we used in WT to measure the intensity only.
Transplantation and statistical test
Transplantation was performed as previously described (71). Transgenic lines incrossed from Tg (nkx2.5: zsYellow)fb7 or Tg (acta1: GFP)zf13 were used as donors to indicate cell fates for heart and pharyngeal arteries, trunk, and facial muscle, respectively. At 4 hpf, around 20 to 30 cells taken from the animal cap of these transgenic embryos either uninjected or injected with gata5/6 MOs were placed into the margin of WT host embryos. Host embryos were imaged and scored at 48 or 72 hpf for the contribution of donor cells to cell fates or ROIs using a Zeiss Axio Zoom.V16 stereoscope. Fisher’s exact tests were performed (**P < 0.01 and ***P < 0.001).
FISH with immunofluorescence
TgBAC (gata5: EGFP)pd25 embryos at the right developmental stages were fixed in 4% PFA at 4°C overnight. Prehybridization, hybridization, and the SSC washes were performed at 65°C (69). Detection of DIG-labeled probes was performed using an anti-DIG antibody, conjugated with horseradish peroxidase (HRP) (POD) (Roche, catalog no. 11207733910) 1:5000 followed by incubation with 1:50 to 1:100 TSA plus cyanine 3 solution (Akoya Biosciences, catalog no. NEL744001KT). To detect two genes at a time, embryos were incubated with a probe mix containing DIG- and Flu-labeled probes. Flu-labeled probes were detected using an anti–Flu-POD antibody (Roche, catalog no. 11426346910) and deposition of 1:50 to 1:100 TSA plus cyanine 3 solution. DIG-labeled probes were detected using an anti–DIG-AP antibody, developed with FastBlue (Sigma-Aldrich, F3378) and NAMP (naphtol-AS-MX-phosphate) (Sigma-Aldrich, N5000), and monitored under a dissecting scope. GFP was detected using primary antibody rabbit anti-GFP 1:500 (Torrey Pines Biolabs) and secondary antibody 488 goat anti-rabbit (Thermo Fisher Scientific, catalog no. A-11008) 1:1000. Embryos were stained for 4′,6-diamidino-2-phenylindole (DAPI) 1:2000 before mounting in low-melt agarose for imaging under a Nikon A1R Si point scanning confocal microscope at ×20 and ×40 magnification.
RNAscope and quantification
Embryos at 13 hpf were fixed in 4% PFA at 4°C overnight. RNAscope was performed following instructions from the RNAscope Assay on Whole Zebrafish Embryos (ACD) and RNAscope Multiplex Fluorescent Reagent Kit V2 Assay (ACD, catalog nos. 322381, 322000, 323110, 322809, and 310091). RNAscope target probes fgf8a-C1 and nxk2.5-C2 were hybridized at a ratio of 50:1 at 40°C for 2 hours. The HRP signals were developed using Opal 570 (Akoya Biosciences, catalog no. FP1488001KT) and Opal 690 (Akoya Biosciences, catalog no. FP1497001KT), respectively, with a dilution of 1:1000. Embryos were stained with DAPI 1:2000 before mounting in low-melt agarose for imaging under a Nikon A1R Si point scanning confocal microscope at ×20 and ×40 magnification. To quantify the spatial distribution of the signals, each image was divided into 10 bins horizontally. The number of foci and the number of cells with foci (positive cells) were counted separately within each bin. The number of foci or positive cells in a given bin was plotted against the total number of foci or positive cells with average moving trend lines (period = 2).
Immunostaining
WT and Gata5/6 KD embryos at 72 hpf were fixed in 4% PFA at 4°C overnight. After 3 × 10 min washes in PBSTw, embryos were bleached in bleach buffer (400 μl of 100% KOH, 150 μl of 30% H2O2, and 50 μl of Tween 20 to a final volume of 5 ml with H2O) in the dark until the pigment was fully eliminated. Embryos were then rinsed twice with PBSTw and permeabilized in PBS with 3% Triton (PBSTx) for 4 hours at room temperature (RT). Blocking was performed in PBS with 5% normal goat serum (Millipore, catalog no. S26-LITER) and 0.075% saponin for 1.5 to 2 hours at RT before incubation with the primary antibody [Elnb, 1:500 (72)] at 4°C overnight. The next day, embryos were washed several times for 4 to 6 hours with PBSTx at RT followed by incubation of the secondary antibody (α-rabbit immunoglobulin G, Alex Fluor 488, 1:1000; Thermo Fisher Scientific, catalog no. A-11008) at 4°C overnight. After 3 × 10 min washes in PBSTw, embryos were mounted in low-melt agarose for imaging under a Nikon A1R Si point scanning confocal microscope at ×20 and ×40 magnification.
Imaging and cell counting
Bright-field images were taken using a Zeiss Axio Zoom.V16. Confocal microscopy was performed under a Nikon A1R Si point scanning confocal microscope at ×40 magnification to determine the tcf21+ cell number in TgBAC(tcf21: NLS-EGFP)pd41 embryos and to image heart morphology in Tg(myl7:EGFP)twu34 embryos and FISH experiments. A magnification of ×20 was used to examine facial muscle patterns in Tg(acta1:GFP)zf13, PAA morphology in Tg(kdrl:GPF)s843 embryos, and Elnb antibody staining for OFT smooth muscle. The numbers of tcf21+ cells were counted using the Cell Counter plugin in Fiji (https://imagej.nih.gov/ij/index.html).
Embryo dissociation and cell isolation
WT and Gata5/6 KD TgBAC (gata5: GFP)pd25 embryos were synchronized and dechorionated with pronase at 1 mg/ml (Sigma-Aldrich, catalog no. 11459643001) at the desired stages. For embryos at gastrulation stages (6, 8, and 10 hpf), the dissociation was performed as previously described (47). Embryos at the early segmentation stage (13 hpf) were treated similarly but incubated with 500 μl of 0.25 trypsin (Gibco, catalog no. 15090046)/EDTA instead of TrypLE (Gibco, catalog no. 12604013) for dissociation. Fluorescence-activated cell sorting (FACS) was performed on a Sony SH800S cell sorter, MoFlo XDP, or MoFlo Astrios with a 100-μm nozzle by the SickKids-UHN Flow and Mass Cytometry Facility. Live GFP+ cells were isolated using a similar gating strategy as described previously (47). A total of 20,000 to 50,000 gata5:GFP+ cells and 100,000 GFP− cells were usually obtained in one FACS experiment. The sorted cells were used immediately for scRNA-seq or bulk ATAC-seq.
scRNA-seq on 10X Genomics platform
Single-cell complementary DNA libraries were prepared through the 10X Chromium Single Cell Gene Expression platform in the TCAG sequencing facility at SickKids. After FACS, GFP+ cells were counted twice with a Countess automated cell counter (Thermo Fisher Scientific) before adjusting to a suspension of 300 to 500 cells/μl in 10% fetal bovine serum/Dulbecco’s modified Eagle’s medium. A total of 3000 to 6000 cells per sample were loaded into the 10X Chromium machine, with roughly 30% of cells captured. After Gel Bead-In Emulsions generation and expression library construction as per the manufacturer’s instructions, final sequencing was performed on an Illumina HiSeq 2500 platform (rapid run mode). A total of 1000 to 2000 cells were collected for each condition with a sequencing depth of 116,497 ± 12,552 reads per cell, which were estimated to reach 78.6 ± 2.88% saturation of the whole libraries (data S1). The median of unique read (unique molecular identifier) counts per cell varied from 5845 to 10,366 in different libraries (data S1).
Assay for transposase-accessible chromatin using sequencing
ATAC-seq was performed with minor modifications to the published protocol (73). A total of 30,000 to 50,000 cells obtained from FACS were used for nuclei preparation. The isolated nuclei were incubated in the Tn5 transposase mix (Illumina, catalog no. FC-121-1030; 25 μl of 2× TD buffer, 2.5 μl of transposase, and 22.5 μl of nuclease-free water) for 30 min at 37°C to obtain genomic DNA enriched for accessible chromatin regions. Transposed DNA fragments were then amplified using the following PCR condition: 1 cycle of 72°C for 5 min and 98°C for 30 s, followed by 12 cycles of 98°C for 10 s, 63°C for 30 s, and 72°C for 1 min. Amplified libraries were purified twice using Agencourt Ampure XP beads (Beckman Coulter, catalog no. A63880) with a bead-to-sample ratio of 1.8:1. A Qubit fluorometer and Agilent Bioanalyzer were used to check library quality and concentration. All libraries were 68-bp single-end sequenced on an Illumina HiSeq 2500 platform in two batches to a final depth of 89 ± 11 million reads per library (data S1). Two biological replicates were collected for control GFP+ and control GFP− cells, while three biological replicates were collected for Gata5/6 KD GFP+ and Gata5/6 KD GFP− cells.
Analyses of scRNA-seq and ATAC-seq data
Detailed analysis pipelines of scRNA-seq and ATAC-seq data are described in the Supplementary Materials.
Characterization of tbx1 DARs
ROIs were cloned into pDONOR221 vector (Invitrogen, Gateway BP Clonase II Enzyme Mix, catalog no. 11789020). Entry vectors were then recombined into E1b-Tol2-GFP-gw vector (Invitrogen, Gateway LR Clonase II Enzyme Mix, catalog no. 11791020). E1b-Tol2-CRE-GFP-gw plasmid (22.5 ng) and 150 ng of Tol2 mRNA were injected in WT embryos at the one-cell stage. F0 founder embryos with GFP activity were raised. Two to six independent alleles have been isolated for each ROI: Tg(tbx1closed1:GFP)hsc171,hsc172, Tg(tbx1closed2:GFP)hsc173,hsc174, Tg(tbx1closed3:GFP)hsc175–180, and Tg(tbx1open:GFP)hss181–184. F1 lines were outcrossed to either WT or Tg(tcf21:dsRed)pd37 background and imaged at 48 to 60 hpf for cardiac activities and 72 hpf for facial muscle expressions. The genomic coordinates and primers for cloning are listed as follows: tbx1_open: chr5:15173728-15174226 (5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTTCACCCTCTCCCTAAGGTCCTA-3′ and 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTTTTCTGCAATTATGGCATTTATCCG-3′), tbx1_closed1: chr5:15093019-15093519 (5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTGGCACTAGAATATCGCTGGCA-3′ and 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTGGTGCCATTAATTCAGTCTTCGC-3′), tbx1_closed2: chr5: 14836719-14837219 (5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTGCAGGCACTAGAATATCGCTGGC-3′ and 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTTTAAGCTGAATTGTCAGGTGCC-3′), and tbx1_closed3: chr5:14604003-14604503 (5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTCGCTGAGGAGGCATCTATTCT-3′ and 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTTGGGTGAGATTGGGGTAGTG-3′).
Motif scan of each tbx1 DAR was performed individually using FIMO with the JASPAR 2020 CORE vertebrate nonredundant motif database. The full motif scan results are shown in data S5.
Ciona experiments
Gravid adults were obtained from M-Rep (San Diego, USA), and gametes were obtained surgically and used for in vitro fertilization and electroporation as previously described (74, 75). Lineage-specific loss of function by CRISPR-Cas9–mediated mutagenesis was performed as described in (50) with a Mesp>nls::Cas9::GemNter::nls, which contains the N terminus of human Geminin, and exhibited increased efficacy, and U6 promoter-driven single-guide RNAs targeting Gata4/5/6 or the control target Tyrosinase (a pigment cell-specific gene inactive in the cardiopharyngeal lineage) as described and validated previously (76). Overexpression was achieved by subcloning the coding sequence of GFP::Gata4/5/6 fusion protein downstream of the trunk ventral cell/cardiopharyngeal progenitor–specific minimal Hand-r enhancer (77). Cardiopharyngeal lineage cells were visualized with Mesp>hCD4::mCherry and Mesp>nls::LacZ reporters, which label the membranes and nuclei, respectively. Nuclei of B7.5 lineage cells are labeled by Mesp>nls::LacZ and revealed with an anti–β-galactosidase antibody (Promega). Mesp-driven hCD4::mCherry accumulates at the cell membrane as revealed by an anti-mCherry antibody (Bio Vision, catalog no. 5993-100). GFP was revealed with an anti-GFP (mouse monoclonal antibody, Roche) and H2B::mCherry was revealed with an anti-mCherry antibody (Bio Vision, catalog no. 5993-100). The atrial siphon muscle/pharyngeal muscle progenitors were identified with an Ebf>H2B::mCherry reporter (23, 59) or by FISH as previously described (59). Note that only one side of the embryo is imaged.
Acknowledgments
We would like to thank A. Salazar, E. Schimmens, M. Westaway, S. Knox, and N. Bendo for excellent animal care and zebrafish facility maintenance. Members of the Scott and Wilson laboratories provided invaluable suggestions and feedback during this project, in particular H. Hou (comments and discussions on computational analyses and the manuscript), A. Prentice (feedback on the manuscript), L. Wu (pilot experiments), and D. Wagner at USCF for help with the STITCH tool. Transgenic lines were provided by C. and G. Burns [Tg(nkx2.5: ZsYellow) fb7], K. Poss [TgBAC (gata5:GFP)pd25, TgBAC(tcf21:NSL-EGFP)pd41, and TgBAC(tcf21:dsRED)pd37], N. Chi [Tg(myl7:EGFP)twu34], and J. Dowling [Tg (acta1: GFP)zf13]. We thank J. Waxman for providing the Elnb antibody and T. Evans for providing embryos from gata4; gata5 incrosses. We would like to thank the Centre for Applied Genomics for DNA sequencing and assistance with scRNA-seq, in particular M. Coole, K. Ho, and S. Pereira, who were supported in part by a Genome Canada Genomics Technology Platform grant. Because of limits on the numbers of references that can be included, we apologize to researchers whose work could not be cited.
Funding: This work was supported in part by NSERC grant RGPIN-2019-07014 to M.D.W., NIH awards R01 HD096770 and R01 HL108643 to L.C., an Ontario Graduate Studentship to M.L., Ontario Trillium Scholarship and Ted Rogers Centre for Heart Research Education Fund to M.S., and Hospital for Sick Children Restracomp Studentship and Connaught International Scholarship to X.Y. M.D.W. is supported by the Canada Research Chairs Program and an Early Researcher Award from the Ontario Ministry of Research and Innovation. Key funding support was provided by NSERC (RGPIN-2017-06502 to I.C.S.), the Heart and Stroke Foundation of Canada (G-16-00013798 to I.C.S.), and the CIHR (FRN 86663 to I.C.S. and FRN 156318 to M.D.W. and I.C.S.).
Author contributions: Conception and study design: M.S., X.Y., C.R., L.C., M.D.W., and I.C.S. Acquisition of data: M.S., X.Y., C.R., M.L., N.S., and A.A. Analysis and interpretation of data: X.Y., M.S., C.R., L.C., M.D.W., and I.C.S. Supervision of work: M.D.W. and I.C.S.; M.S., X.Y., L.C., M.D.W., and I.C.S. wrote the manuscript, and all authors assisted with drafting and revision of the manuscript.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The raw single-cell sequencing data and count matrices can be accessed at ArrayExpress (E-MTAB-9193). The raw ATAC-seq data, aligned bam files, and the coverage bigwig files can be accessed at ArrayExpress (E-MTAB-10427). All scripts for downstream analysis are available at https://zenodo.org/record/5550772 and the GitHub repository (https://github.com/angleYuan/gata5_SC.git), and Rdata can be downloaded using the figShare link (https://figshare.com/s/72704b3218b17b071523).
Supplementary Materials
This PDF file includes:
Supplementary Text
Figs. S1 to S8
Tables S1 to S6
References
Other Supplementary Material for this manuscript includes the following:
Data S1 to S5
REFERENCES AND NOTES
- 1.Bruneau B. G., Signaling and transcriptional networks in heart development and regeneration. Cold Spring Harb. Perspect. Biol. 5, a008292 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holtzinger A., Evans T., Gata5 and Gata6 are functionally redundant in zebrafish for specification of cardiomyocytes. Dev. Biol. 312, 613–622 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sam J., Mercer E. J., Torregroza I., Banks K. M., Evans T., Specificity, redundancy and dosage thresholds among gata4/5/6 genes during zebrafish cardiogenesis. Biol. Open 9, 053611 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao R., Watt A. J., Battle M. A., Li J., Bondow B. J., Duncan S. A., Loss of both GATA4 and GATA6 blocks cardiac myocyte differentiation and results in acardia in mice. Dev. Biol. 317, 614–619 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ragkousi K., Beh J., Sweeney S., Starobinska E., Davidson B., A single GATA factor plays discrete, lineage specific roles in ascidian heart development. Dev. Biol. 352, 154–163 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gajewski K., Fossett N., Molkentin J. D., Schulz R. A., The zinc finger proteins Pannier and GATA4 function as cardiogenic factors in Drosophila. Development 126, 5679–5688 (1999). [DOI] [PubMed] [Google Scholar]
- 7.Morton S. U., Quiat D., Seidman J. G., Seidman C. E., Genomic frontiers in congenital heart disease. Nat. Rev. Cardiol. 19, 26–42 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molkentin J. D., Lin Q., Duncan S. A., Olson E. N., Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 11, 1061–1072 (1997). [DOI] [PubMed] [Google Scholar]
- 9.Reiter J. F., Alexander J., Rodaway A., Yelon D., Patient R., Holder N., Stainier D. Y., Gata5 is required for the development of the heart and endoderm in zebrafish. Genes Dev. 13, 2983–2995 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peterkin T., Gibson A., Patient R., Redundancy and evolution of GATA factor requirements in development of the myocardium. Dev. Biol. 311, 623–635 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peterkin T., Gibson A., Patient R., Common genetic control of haemangioblast and cardiac development in zebrafish. Development 136, 1465–1474 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heikinheimo M., Scandrett J. M., Wilson D. B., Localization of transcription factor GATA-4 to regions of the mouse embryo involved in cardiac development. Dev. Biol. 164, 361–373 (1994). [DOI] [PubMed] [Google Scholar]
- 13.Devine W. P., Wythe J. D., George M., Koshiba-Takeuchi K., Bruneau B. G., Early patterning and specification of cardiac progenitors in gastrulating mesoderm. eLife 3, e03848 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lescroart F., Chabab S., Lin X., Rulands S., Paulissen C., Rodolosse A., Auer H., Achouri Y., Dubois C., Bondue A., Simons B. D., Blanpain C., Early lineage restriction in temporally distinct populations of Mesp1 progenitors during mammalian heart development. Nat. Cell Biol. 16, 829–840 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lescroart F., Wang X., Lin X., Swedlund B., Gargouri S., Sànchez-Dànes A., Moignard V., Dubois C., Paulissen C., Kinston S., Göttgens B., Blanpain C., Defining the earliest step of cardiovascular lineage segregation by single-cell RNA-seq. Science 359, 1177–1181 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meilhac S. M., Esner M., Kelly R. G., Nicolas J.-F., Buckingham M. E., The clonal origin of myocardial cells in different regions of the embryonic mouse heart. Dev. Cell 6, 685–698 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Stolfi A., Gainous T. B., Young J. J., Mori A., Levine M., Christiaen L., Early chordate origins of the vertebrate second heart field. Science 329, 565–568 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diogo R., Kelly R. G., Christiaen L., Levine M., Ziermann J. M., Molnar J. L., Noden D. M., Tzahor E., A new heart for a new head in vertebrate cardiopharyngeal evolution. Nature 520, 466–473 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tzahor E., Evans S. M., Pharyngeal mesoderm development during embryogenesis: Implications for both heart and head myogenesis. Cardiovasc. Res. 91, 196–202 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harel I., Maezawa Y., Avraham R., Rinon A., Ma H.-Y., Cross J. W., Leviatan N., Hegesh J., Roy A., Jacob-Hirsch J., Rechavi G., Carvajal J., Tole S., Kioussi C., Quaggin S., Tzahor E., Pharyngeal mesoderm regulatory network controls cardiac and head muscle morphogenesis. Proc. Natl. Acad. Sci. U.S.A. 109, 18839–18844 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guner-Ataman B., González-Rosa J. M., Shah H. N., Butty V. L., Jeffrey S., Abrial M., Boyer L. A., Burns C. G., Burns C. E., Failed progenitor specification underlies the cardiopharyngeal phenotypes in a zebrafish model of 22q11.2 deletion syndrome. Cell Rep. 24, 1342–1354.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tirosh-Finkel L., Elhanany H., Rinon A., Tzahor E., Mesoderm progenitor cells of common origin contribute to the head musculature and the cardiac outflow tract. Development 133, 1943–1953 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Wang W., Razy-Krajka F., Siu E., Ketcham A., Christiaen L., NK4 antagonizes Tbx1/10 to promote cardiac versus pharyngeal muscle fate in the ascidian second heart field. PLoS Biol. 11, e1001725 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J., Cheng H., Xiang M., Zhou L., Wu B., Moskowitz I. P., Zhang K., Xie L., Gata4 regulates hedgehog signaling and Gata6 expression for outflow tract development. PLOS Genet. 15, e1007711 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tseng W.-F., Jang T.-H., Huang C.-B., Yuh C.-H., An evolutionarily conserved kernel of gata5, gata6, otx2 and prdm1a operates in the formation of endoderm in zebrafish. Dev. Biol. 357, 541–557 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Wagner D. E., Weinreb C., Collins Z. M., Briggs J. A., Megason S. G., Klein A. M., Single-cell mapping of gene expression landscapes and lineage in the zebrafish embryo. Science 360, 981–987 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blondel V. D., Guillaume J.-L., Lambiotte R., Lefebvre E., Fast unfolding of community hierarchies in large networks. J. Stat. Mech. Theory Exp. JSTAT , 1–6 (2008). [Google Scholar]
- 28.Farrell J. A., Wang Y., Riesenfeld S. J., Shekhar K., Regev A., Schier A. F., Single-cell reconstruction of developmental trajectories during zebrafish embryogenesis. Science 360, eaar3131 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prummel K. D., Crowell H. L., Nieuwenhuize S., Brombacher E. C., Daetwyler S., Soneson C., Kresoja-Rakic J., Ronner M., Kocere A., Ernst A., Labbaf Z., Clouthier D. E., Firulli A. B., Sanchez-Iranzo H., O’Rourke R., Raz E., Mercader N., Burger A., Felley-Bosco E., Huisken J., Robinson M. D., Mosimann C., Hand2 delineates mesothelium progenitors and is reactivated in mesothelioma. bioRxiv, 2020.11.11.355693 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tremblay M., Sanchez-Ferras O., Bouchard M., GATA transcription factors in development and disease. Development 145, dev164384 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Lescroart F., Kelly R. G., Garrec J.-F. L., Nicolas J.-F., Meilhac S. M., Buckingham M., Clonal analysis reveals common lineage relationships between head muscles and second heart field derivatives in the mouse embryo. Development 137, 3269–3279 (2010). [DOI] [PubMed] [Google Scholar]
- 32.Martin K. E., Waxman J. S., Atrial and sinoatrial node development in the zebrafish heart. J. Cardiovasc. Dev. Dis. 8, 15 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ilagan R., Abu-Issa R., Brown D., Yang Y.-P., Jiao K., Schwartz R. J., Klingensmith J., Meyers E. N., Fgf8 is required for anterior heart field development. Development 133, 2435–2445 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Nevis K., Obregon P., Walsh C., Guner-Ataman B., Burns C. G., Burns C. E., Tbx1 is required for second heart field proliferation in zebrafish. Dev. Dyn. 242, 550–559 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Soysa T. Y., Ranade S. S., Okawa S., Ravichandran S., Huang Y., Salunga H. T., Schricker A., Del Sol A., Gifford C. A., Srivastava D., Single-cell analysis of cardiogenesis reveals basis for organ-level developmental defects. Nature 572, 120–124 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dyer L. A., Kirby M. L., The role of secondary heart field in cardiac development. Dev. Biol. 336, 137–144 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rydeen A. B., Waxman J. S., Cyp26 enzymes are required to balance the cardiac and vascular lineages within the anterior lateral plate mesoderm. Development 141, 1638–1648 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paffett-Lugassy N., Singh R., Nevis K. R., Guner-Ataman B., O’Loughlin E., Jahangiri L., Harvey R. P., Burns C. G., Burns C. E., Heart field origin of great vessel precursors relies on nkx2.5-mediated vasculogenesis. Nat. Cell Biol. 15, 1362–1369 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paffett-Lugassy N., Novikov N., Jeffrey S., Abrial M., Guner-Ataman B., Sakthivel S., Burns C. E., Burns C. G., Unique developmental trajectories and genetic regulation of ventricular and outflow tract progenitors in the zebrafish second heart field. Development 144, 4616–4624 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schilling T. F., Kimmel C. B., Musculoskeletal patterning in the pharyngeal segments of the zebrafish embryo. Development 124, 2945–2960 (1997). [DOI] [PubMed] [Google Scholar]
- 41.Keegan B. R., Meyer D., Yelon D., Organization of cardiac chamber progenitors in the zebrafish blastula. Development 131, 3081–3091 (2004). [DOI] [PubMed] [Google Scholar]
- 42.Begemann G., Ingham P. W., Developmental regulation of Tbx5 in zebrafish embryogenesis. Mech. Dev. 90, 299–304 (2000). [DOI] [PubMed] [Google Scholar]
- 43.Duong T. B., Ravisankar P., Song Y. C., Gafranek J. T., Rydeen A. B., Dohn T. E., Barske L. A., Crump J. G., Waxman J. S., Nr2f1a balances atrial chamber and atrioventricular canal size via BMP signaling-independent and -dependent mechanisms. Dev. Biol. 434, 7–14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagelberg D., Wang J., Su R., Torres-Vázquez J., Targoff K. L., Poss K. D., Knaut H., Origin, specification, and plasticity of the great vessels of the heart. Curr. Biol. 25, 2099–2110 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kok F. O., Shin M., Ni C.-W., Gupta A., Grosse A. S., van Impel A., Kirchmaier B. C., Peterson-Maduro J., Kourkoulis G., Male I., DeSantis D. F., Sheppard-Tindell S., Ebarasi L., Betsholtz C., Schulte-Merker S., Wolfe S. A., Lawson N. D., Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Dev. Cell 32, 97–108 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cirillo L. A., Lin F. R., Cuesta I., Friedman D., Jarnik M., Zaret K. S., Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol. Cell 9, 279–289 (2002). [DOI] [PubMed] [Google Scholar]
- 47.Yuan X., Song M., Devine P., Bruneau B. G., Scott I. C., Wilson M. D., Heart enhancers with deeply conserved regulatory activity are established early in zebrafish development. Nat. Commun. 9, 4977 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaplan N., Razy-Krajka F., Christiaen L., Regulation and evolution of cardiopharyngeal cell identity and behavior: Insights from simple chordates. Curr. Opin. Genet. Dev. 32, 119–128 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang W., Niu X., Stuart T., Jullian E., Mauck W. M., Kelly R. G., Satija R., Christiaen L., A single-cell transcriptional roadmap for cardiopharyngeal fate diversification. Nat. Cell Biol. 21, 674–686 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stolfi A., Gandhi S., Salek F., Christiaen L., Tissue-specific genome editing in Ciona embryos by CRISPR/Cas9. Development 141, 4115–4120 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Razy-Krajka F., Lam K., Wang W., Stolfi A., Joly M., Bonneau R., Christiaen L., Collier/OLF/EBF-dependent transcriptional dynamics control pharyngeal muscle specification from primed cardiopharyngeal progenitors. Dev. Cell 29, 263–276 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang S., Cui W., Sox2, a key factor in the regulation of pluripotency and neural differentiation. World J. Stem Cells 6, 305–311 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vicente C., Conchillo A., García-Sánchez M. A., Odero M. D., The role of the GATA2 transcription factor in normal and malignant hematopoiesis. Crit. Rev. Oncol. Hematol. 82, 1–17 (2012). [DOI] [PubMed] [Google Scholar]
- 54.Iwafuchi-Doi M., Zaret K. S., Cell fate control by pioneer transcription factors. Development 143, 1833–1837 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koutsourakis M., Langeveld A., Patient R., Beddington R., Grosveld F., The transcription factor GATA6 is essential for early extraembryonic development. Development 126, 723–732 (1999). [PubMed] [Google Scholar]
- 56.Simon C. S., Zhang L., Wu T., Cai W., Saiz N., Nowotschin S., Cai C.-L., Hadjantonakis A.-K., A Gata4 nuclear GFP transcriptional reporter to study endoderm and cardiac development in the mouse. Biol. Open 7, bio036517 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duong T. B., Holowiecki A., Waxman J. S., Retinoic acid signaling restricts the size of the first heart field within the anterior lateral plate mesoderm. Dev. Biol. 473, 119–129 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.He A., Gu F., Hu Y., Ma Q., Yi Ye L., Akiyama J. A., Visel A., Pennacchio L. A., Pu W. T., Dynamic GATA4 enhancers shape the chromatin landscape central to heart development and disease. Nat. Commun. 5, 4907 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Racioppi C., Wiechecki K. A., Christiaen L., Combinatorial chromatin dynamics foster accurate cardiopharyngeal fate choices. eLife 8, e49921 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stutt N., Song M., Wilson M. D., Scott I. C., Cardiac specification during gastrulation—The yellow brick road leading to Tinman. Semin. Cell Dev. Biol. 10.1016/j.semcdb.2021.11.011 , (2021). [DOI] [PubMed] [Google Scholar]
- 61.Ang Y.-S., Rivas R. N., Ribeiro A. J. S., Srivas R., Rivera J., Stone N. R., Pratt K., Mohamed T. M. A., Fu J.-D., Spencer C. I., Tippens N. D., Li M., Narasimha A., Radzinsky E., Moon-Grady A. J., Yu H., Pruitt B. L., Snyder M. P., Srivastava D., Disease model of GATA4 mutation reveals transcription factor cooperativity in human cardiogenesis. Cell 167, 1734–1749.e22 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ieda M., Fu J.-D., Delgado-Olguin P., Vedantham V., Hayashi Y., Bruneau B. G., Srivastava D., Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142, 375–386 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takeuchi J. K., Bruneau B. G., Directed transdifferentiation of mouse mesoderm to heart tissue by defined factors. Nature 459, 708–711 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou L., Liu Y., Lu L., Lu X., Dixon R. A. F., Cardiac gene activation analysis in mammalian non-myoblasic cells by Nkx2-5, Tbx5, Gata4 and Myocd. PLOS ONE 7, e48028 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beuling E., Bosse T., aan de Kerk D. J., Piaseckyj C. M., Fujiwara Y., Katz S. G., Orkin S. H., Grand R. J., Krasinski S. D., GATA4 mediates gene repression in the mature mouse small intestine through interactions with friend of GATA (FOG) cofactors. Dev. Biol. 322, 179–189 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Doni Jayavelu N., Jajodia A., Mishra A., Hawkins R. D., Candidate silencer elements for the human and mouse genomes. Nat. Commun. 11, 1061 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.M. Westerfield, The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio) (University of Oregon Press, Eugene, Oregon, 2007). [Google Scholar]
- 68.Varshney G. K., Pei W., LaFave M. C., Idol J., Xu L., Gallardo V., Carrington B., Bishop K., Jones M., Li M., Harper U., Huang S. C., Prakash A., Chen W., Sood R., Ledin J., Burgess S. M., High-throughput gene targeting and phenotyping in zebrafish using CRISPR/Cas9. Genome Res. 25, 1030–1042 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thisse C., Thisse B., High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 3, 59–69 (2008). [DOI] [PubMed] [Google Scholar]
- 70.Dobrzycki T., Krecsmarik M., Monteiro R., Genotyping and quantification of in situ hybridization staining in zebrafish. J. Vis. Exp. e59956 (2020). [DOI] [PubMed] [Google Scholar]
- 71.Scott I. C., Masri B., D’Amico L. A., Jin S.-W., Jungblut B., Wehman A. M., Baier H., Audigier Y., Stainier D. Y. R., The g protein-coupled receptor agtrl1b regulates early development of myocardial progenitors. Dev. Cell 12, 403–413 (2007). [DOI] [PubMed] [Google Scholar]
- 72.Song Y. C., Dohn T. E., Rydeen A. B., Nechiporuk A. V., Waxman J. S., HDAC1-mediated repression of the retinoic acid-responsive gene ripply3 promotes second heart field development. PLOS Genet. 15, e1008165 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Buenrostro J. D., Giresi P. G., Zaba L. C., Chang H. Y., Greenleaf W. J., Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 10, 1213–1218 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Christiaen L., Wagner E., Shi W., Levine M., Electroporation of transgenic DNAs in the sea squirt Ciona. Cold Spring Harb. Protoc. 2009, pdb.prot5345 (2009). [DOI] [PubMed] [Google Scholar]
- 75.Christiaen L., Wagner E., Shi W., Levine M., Isolation of sea squirt (Ciona) gametes, fertilization, dechorionation, and development. Cold Spring Harb. Protoc. 2009, pdb.prot5344 (2009). [DOI] [PubMed] [Google Scholar]
- 76.Gandhi S., Haeussler M., Razy-Krajka F., Christiaen L., Stolfi A., Evaluation and rational design of guide RNAs for efficient CRISPR/Cas9-mediated mutagenesis in Ciona. Dev. Biol. 425, 8–20 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Woznica A., Haeussler M., Starobinska E., Jemmett J., Li Y., Mount D., Davidson B., Initial deployment of the cardiogenic gene regulatory network in the basal chordate, Ciona intestinalis. Dev. Biol. 368, 127–139 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gu X., Xu F., Wang X., Gao X., Zhao Q., Molecular cloning and expression of a novel CYP26 gene (cyp26d1) during zebrafish early development. Gene Expr. Patterns 5, 733–739 (2005). [DOI] [PubMed] [Google Scholar]
- 79.Takeuchi M., Yamaguchi S., Sakakibara Y., Hayashi T., Matsuda K., Hara Y., Tanegashima C., Shimizu T., Kuraku S., Hibi M., Gene expression profiling of granule cells and Purkinje cells in the zebrafish cerebellum. J. Comp. Neurol. 525, 1558–1585 (2017). [DOI] [PubMed] [Google Scholar]
- 80.Tambalo M., Mitter R., Wilkinson D. G., A single cell transcriptome atlas of the developing zebrafish hindbrain. Development 147, dev184143 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Inomata C., Yuikawa T., Nakayama-Sadakiyo Y., Kobayashi K., Ikeda M., Chiba M., Konishi C., Ishioka A., Tsuda S., Yamasu K., Involvement of an Oct4-related PouV gene, pou5f3/pou2, in neurogenesis in the early neural plate of zebrafish embryos. Dev. Biol. 457, 30–42 (2020). [DOI] [PubMed] [Google Scholar]
- 82.Hafemeister C., Satija R., Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 20, 296 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Finak G., McDavid A., Yajima M., Deng J., Gersuk V., Shalek A. K., Slichter C. K., Miller H. W., McElrath M. J., Prlic M., Linsley P. S., Gottardo R., MAST: A flexible statistical framework for assessing transcriptional changes and characterizing heterogeneity in single-cell RNA sequencing data. Genome Biol. 16, 278 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ashburner M., Ball C. A., Blake J. A., Botstein D., Butler H., Cherry J. M., Davis A. P., Dolinski K., Dwight S. S., Eppig J. T., Harris M. A., Hill D. P., Issel-Tarver L., Kasarskis A., Lewis S., Matese J. C., Richardson J. E., Ringwald M., Rubin G. M., Sherlock G., Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 25, 25–29 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.The Gene Ontology Consortium , The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 47, D330–D338 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saelens W., Cannoodt R., Todorov H., Saeys Y., A comparison of single-cell trajectory inference methods. Nat. Biotechnol. 37, 547–554 (2019). [DOI] [PubMed] [Google Scholar]
- 87.Street K., Risso D., Fletcher R. B., Das D., Ngai J., Yosef N., Purdom E., Dudoit S., Slingshot: Cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genomics 19, 477 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.S. Andrews, FastQC: A Quality Control Tool for High Throughput Sequence Data (2010); www.bioinformatics.babraham.ac.uk/projects/fastqc/.
- 89.Bolger A. M., Lohse M., Usadel B., Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li H., Durbin R., Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., Durbin R.; 1000 Genome Project Data Processing Subgroup , The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang Y., Liu T., Meyer C. A., Eeckhoute J., Johnson D. S., Bernstein B. E., Nussbaum C., Myers R. M., Brown M., Li W., Liu X. S., Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.R. Stark, G. Brown, DiffBind: Differential binding analysis of ChIP-Seq peak data (2011).
- 94.Ramírez F., Ryan D. P., Grüning B., Bhardwaj V., Kilpert F., Richter A. S., Heyne S., Dündar F., Manke T., deepTools2: A next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 44, W160–W165 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Heinz S., Benner C., Spann N., Bertolino E., Lin Y. C., Laslo P., Cheng J. X., Murre C., Singh H., Glass C. K., Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bailey T. L., Boden M., Buske F. A., Frith M., Grant C. E., Clementi L., Ren J., Li W. W., Noble W. S., MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 37, W202–W208 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fornes O., Castro-Mondragon J. A., Khan A., van der Lee R., Zhang X., Richmond P. A., Modi B. P., Correard S., Gheorghe M., Baranašić D., Santana-Garcia W., Tan G., Chèneby J., Ballester B., Parcy F., Sandelin A., Lenhard B., Wasserman W. W., Mathelier A., JASPAR 2020: Update of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 48, D87–D92 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hiller M., Agarwal S., Notwell J. H., Parikh R., Guturu H., Wenger A. M., Bejerano G., Computational methods to detect conserved non-genic elements in phylogenetically isolated genomes: Application to zebrafish. Nucleic Acids Res. 41, e151 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kuhn R. M., Haussler D., Kent W. J., The UCSC genome browser and associated tools. Brief. Bioinform. 14, 144–161 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lawrence M., Huber W., Pagès H., Aboyoun P., Carlson M., Gentleman R., Morgan M. T., Carey V. J., Software for computing and annotating genomic ranges. PLoS Comput. Biol. 9, e1003118 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Howe K. L., Achuthan P., Allen J., Allen J., Alvarez-Jarreta J., Amode M. R., Armean I. M., Azov A. G., Bennett R., Bhai J., Billis K., Boddu S., Charkhchi M., Cummins C., Da Rin Fioretto L., Davidson C., Dodiya K., El Houdaigui B., Fatima R., Gall A., Garcia Giron C., Grego T., Guijarro-Clarke C., Haggerty L., Hemrom A., Hourlier T., Izuogu O. G., Juettemann T., Kaikala V., Kay M., Lavidas I., Le T., Lemos D., Gonzalez Martinez J., Marugán J. C., Maurel T., McMahon A. C., Mohanan S., Moore B., Muffato M., Oheh D. N., Paraschas D., Parker A., Parton A., Prosovetskaia I., Sakthivel M. P., Salam A. I. A., Schmitt B. M., Schuilenburg H., Sheppard D., Steed E., Szpak M., Szuba M., Taylor K., Thormann A., Threadgold G., Walts B., Winterbottom A., Chakiachvili M., Chaubal A., De Silva N., Flint B., Frankish A., Hunt S. E., IIsley G. R., Langridge N., Loveland J. E., Martin F. J., Mudge J. M., Morales J., Perry E., Ruffier M., Tate J., Thybert D., Trevanion S. J., Cunningham F., Yates A. D., Zerbino D. R., Flicek P., Ensembl 2021. Nucleic Acids Res. 49, D884–D891 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kikuchi K., Holdway J. E., Major R. J., Blum N., Dahn R. D., Begemann G., Poss K. D., Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Dev. Cell 20, 397–404 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou Y., Cashman T. J., Nevis K. R., Obregon P., Carney S. A., Liu Y., Gu A., Mosimann C., Sondalle S., Peterson R. E., Heideman W., Burns C. E., Burns C. G., Latent TGF-β binding protein 3 identifies a second heart field in zebrafish. Nature 474, 645–648 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Traver D., Paw B. H., Poss K. D., Penberthy W. T., Lin S., Zon L. I., Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat. Immunol. 4, 1238–1246 (2003). [DOI] [PubMed] [Google Scholar]
- 105.Higashijima S., Okamoto H., Ueno N., Hotta Y., Eguchi G., High-frequency generation of transgenic zebrafish which reliably express GFP in whole muscles or the whole body by using promoters of zebrafish origin. Dev. Biol. 192, 289–299 (1997). [DOI] [PubMed] [Google Scholar]
- 106.Huang C.-J., Tu C.-T., Hsiao C.-D., Hsieh F.-J., Tsai H.-J., Germ-line transmission of a myocardium-specific GFP transgene reveals critical regulatory elements in the cardiac myosin light chain 2 promoter of zebrafish. Dev. Dyn. 228, 30–40 (2003). [DOI] [PubMed] [Google Scholar]
- 107.Wang J., Panáková D., Kikuchi K., Holdway J. E., Gemberling M., Burris J. S., Singh S. P., Dickson A. L., Lin Y.-F., Sabeh M. K., Werdich A. A., Yelon D., MacRae C. A., Poss K. D., The regenerative capacity of zebrafish reverses cardiac failure caused by genetic cardiomyocyte depletion. Development 138, 3421–3430 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jin S.-W., Beis D., Mitchell T., Chen J.-N., Stainier D. Y. R., Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development 132, 5199–5209 (2005). [DOI] [PubMed] [Google Scholar]
- 109.Sehnert A. J., Huq A., Weinstein B. M., Walker C., Fishman M., Stainier D. Y. R., Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nat. Genet. 31, 106–110 (2002). [DOI] [PubMed] [Google Scholar]
- 110.Yelon D., Horne S. A., Stainier D. Y. R., Restricted expression of cardiac myosin genes reveals regulated aspects of heart tube assembly in zebrafish. Dev. Biol. 214, 23–37 (1999). [DOI] [PubMed] [Google Scholar]
- 111.Chen J. N., Fishman M. C., Zebrafish tinman homolog demarcates the heart field and initiates myocardial differentiation. Development 122, 3809–3816 (1996). [DOI] [PubMed] [Google Scholar]
- 112.Szeto D. P., Griffin K. J. P., Kimelman D., HrT is required for cardiovascular development in zebrafish. Development 129, 5093–5101 (2002). [DOI] [PubMed] [Google Scholar]
- 113.Lazic S., Scott I. C., Mef2cb regulates late myocardial cell addition from a second heart field-like population of progenitors in zebrafish. Dev. Biol. 354, 123–133 (2011). [DOI] [PubMed] [Google Scholar]
- 114.Schoenebeck J. J., Keegan B. R., Yelon D., Vessel and blood specification override cardiac potential in anterior mesoderm. Dev. Cell 13, 254–267 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Reifers F., Böhli H., Walsh E. C., Crossley P. H., Stainier D. Y., Brand M., Fgf8 is mutated in zebrafish acerebellar (ace) mutants and is required for maintenance of midbrain-hindbrain boundary development and somitogenesis. Development 125, 2381–2395 (1998). [DOI] [PubMed] [Google Scholar]
- 116.Ruvinsky I., Oates A. C., Silver L. M., Ho R. K., The evolution of paired appendages in vertebrates: T-box genes in the zebrafish. Dev. Genes Evol. 210, 82–91 (2000). [DOI] [PubMed] [Google Scholar]
- 117.Grandel H., Lun K., Rauch G.-J., Rhinn M., Piotrowski T., Houart C., Sordino P., Küchler A. M., Schulte-Merker S., Geisler R., Holder N., Wilson S. W., Brand M., Retinoic acid signalling in the zebrafish embryo is necessary during pre-segmentation stages to pattern the anterior-posterior axis of the CNS and to induce a pectoral fin bud. Development 129, 2851–2865 (2002). [DOI] [PubMed] [Google Scholar]
- 118.Piotrowski T., Nüsslein-Volhard C., The endoderm plays an important role in patterning the segmented pharyngeal region in zebrafish (Danio rerio). Dev. Biol. 225, 339–356 (2000). [DOI] [PubMed] [Google Scholar]
- 119.Cheung A., Trevers K. E., Reyes-Corral M., Antinucci P., Hindges R., Expression and roles of teneurins in zebrafish. Front. Neurosci. 13, 158 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.B. Thisse, Fast Release Clones: A High Throughput Expression Analysis. ZFIN Direct Data Submission (2004);http://zfin.org.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text
Figs. S1 to S8
Tables S1 to S6
References
Data S1 to S5