Abstract
Neuroimmunometabolism is an emerging field that examines the intersection of immunologic and metabolic cascades in the brain. Neuroinflammatory conditions often involve differential metabolic reprogramming in neuronal and glial cells through their immunometabolic sensors. The impact of such bioenergetic adaptation on general brain function is poorly understood, but this cross-talk becomes increasingly important in neurodegenerative disorders that exhibit reshaping of neuroimmunometabolic pathways. Here we summarize the intrinsic balance of neuroimmunometabolic substrates and sensors in the healthy brain and how their dysregulation can contribute to the pathophysiology of various neurodegenerative disorders. This review also proposes possible avenues for disease management through neuroimmunometabolic profiling and therapeutics to bridge translational gaps and guide future treatment strategies.
SIGNIFICANCE STATEMENT Neuroimmunometabolism intersects with neuroinflammation and immunometabolic regulation of neurons and glial cells in the CNS. There is emerging evidence that neuroimmunometabolism plays an essential role in the manifestation of CNS degeneration. This review highlights how neuroimmunometabolic homeostasis is disrupted in various neurodegenerative conditions and could be a target for new therapeutic strategies.
Keywords: neuroimmunometabolism, neuroinflammation, neurodegeneration, immunometabolic sensors
Introduction
Neuroimmunometabolism is a broad umbrella term for an emerging area of research that involves understanding how reprogramming of cellular metabolism can alter immune responses in the CNS (Larabee et al., 2020). The interface of immune regulation and metabolic states is essential for maintaining dynamic cellular balance in an organism (Watts et al., 2018; Larabee et al., 2020).
Metabolic control (also conventionally referred to as neuroenergetics) is essential in the brain, where there is a high metabolic demand. Despite comprising ∼2% of the body mass, the brain consumes ∼20% of energy substrates at rest, mainly to reverse ion fluxes that mediate synaptic and action potentials; this demand is elevated during activity-dependent processes (Mink et al., 1981; Attwell and Laughlin, 2001; Harris et al., 2012). Neurons are responsible for most of these energy demands, while glial cells serve as energy suppliers (Jha and Morrison, 2018). Efficient energy shuttling requires metabolic flexibility in microglia, astrocytes, and oligodendrocytes (Philips and Rothstein, 2017; Morita et al., 2019; Bernier et al., 2020). This is achieved through robust regulation by metabolic sensors, such as receptors, transporters, and enzymes that allow glial cells to expend energy in response to elevated neuronal demands. These regulators also modulate glial inflammatory responses through crosstalk between metabolic and immune signaling pathways (Robb et al., 2020a).
Neuroinflammation is an important cellular defense mechanism that involves an immune response to noxious and harmful stimuli (Mitra et al., 2020). The triggering, activation, and persistence of inflammation, mediated by glial cells, are influenced by environmental factors and genetic predispositions (Lucas et al., 2006). Circadian rhythms, age, and lifestyle choices (diet, exercise, drug abuse, etc.) also affect the glial metabolic and inflammatory profile and thus influence glial function (Marpegan et al., 2011; Garcia-Caceres et al., 2012; Camandola and Mattson, 2017; Lacagnina et al., 2017; Chi-Castaneda and Ortega, 2018; Jin et al., 2020; X. Wang et al., 2020). Neuroinflammatory responses entail reprogramming of several transcriptional and translational pathways in glial cells, resulting in the production of inflammatory cytokines and reactive oxygen species (ROS) and reactive nitrogen species (RNS) (Mitra et al., 2020). These cellular adaptations involving concerted action of metabolic and immune functions are essential in balancing immunometabolic changes in a metabolically active organ, such as the brain.
It is unclear whether alterations in neuroimmunometabolism in neurodegenerative conditions drive or oppose disease progression. Neurodegenerative diseases are debilitating disorders of the CNS that are characterized by progressive loss of neurons leading to abnormalities in behavioral domains associated with cognition, movement, and affective functions (Granholm et al., 2008; Bowman et al., 2011; Levenson et al., 2014; Dugger and Dickson, 2017; Gitler et al., 2017). With the lack of effective disease-modifying therapeutic interventions and the limited translational success of potential drug molecules, it is imperative to revisit these diseases with a renewed perspective to identify novel and effective therapeutic targets. Degenerative disorders are typically characterized by dysregulation in many cellular processes, which include imbalances in intracellular mechanisms, such as cellular stress, aberrant clearance of cellular debris by the proteasome and lysosomes, and abnormal immunometabolic activity (Gan et al., 2018).
In this review, we focus on how immunometabolic imbalances across CNS neurons and glia can be investigated to better understand the pathophysiology of neurodegenerative diseases. We discuss the regulatory roles of immunometabolic substrates and sensors in the brain and detail the immunometabolic aberrations occurring in particular neurodegenerative conditions. Finally, we introduce an integrative approach to devise effective therapeutic measures.
Neuroimmunometabolic sensors
The relationship between metabolism and immune functions in the CNS is tightly regulated by several sensors (“immunometabolic sensors”) that form a critical regulatory hub for maintaining homeostasis in the metabolic and immune pathways. Sensors predominantly comprise energy substrates, such as sugars, amino acids, and lipids, along with transporters/receptors that sense fluctuations in these substrates and trigger metabolic and biosynthetic cascades that use the substrates. These sensors, expressed in neurons and glia, provide necessary signals to alter the metabolic and immune status based on the overall energy demand (Argente-Arizon et al., 2017; Saravia et al., 2020). Each cell type in the brain exhibits a unique metabolic profile based on its functions. Switching between metabolic states can have detrimental consequences in cells' structural and functional properties. In this section, we describe regulation of some of these sensors across CNS cell types.
Sugar sensors
Glucose is the most important sugar for generating energy in the brain, and it acts as an essential precursor for neurotransmitter synthesis (Mergenthaler et al., 2013). Glucose homeostasis in the brain is tightly regulated through neuro-glia metabolic coupling (Afridi et al., 2020). Glucose is sensed and transported across the cell membrane by a saturable transport system composed of various glucose transporters (GLUTs) (Navale and Paranjape, 2016). These transporters, based on sequence polymorphisms, exhibit differential affinity and distribution across cell types in the CNS (Mueckler and Thorens, 2013). Neurons primarily express high-affinity GLUT3 transporters, while oligodendrocytes, microglia, and astrocytes use GLUT1 and GLUT5. GLUT1 is also expressed in brain vasculature to enable glucose transport across the blood–brain barrier (BBB); but unlike in glial cells, the form expressed in the vasculature is highly glycosylated (Jurcovicova, 2014). Additionally, sodium/glucose cotransporters and the Kir6.2 subunit of an ATP-sensitive potassium channel sense and enable glucose transport in neurons and astrocytes (M. Liu et al., 1999; Vega et al., 2006; Yu et al., 2013; Koepsell, 2020).
Although neurons express a high-affinity glucose sensor (GLUT3), they have a low level of glycolysis because of the rapid degradation of the rate-limiting glycolytic enzyme 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatase, isoform 3 (PFKFB3) (Herrero-Mendez et al., 2009; Bolaños et al., 2010). Therefore, it has been suggested that glial cells in the brain uptake and metabolize more glucose than neurons, and neurons depend on glucose metabolites, such as lactate released by glial cells (Chuquet et al., 2010; Fünfschilling et al., 2012; S. Lee et al., 2012). There are conflicting reports indicating that neurons rely on direct glycolysis rather than lactate from astrocytes (Díaz-García et al., 2017; Díaz-García and Yellen, 2019). These reports suggest that the mechanism underlying metabolic interchange between neurons and astrocytes is still unsettled.
Astrocytes provide metabolic sustenance to neurons by detecting circulating glucose and transporting it to neurons (Fig. 1) (Jurcovicova, 2014). Both GLUT1 and GLUT2 are critical for this astrocytic function. Astrocytes have low levels of the malate-aspartate shuttle, which is generally responsible for the reduction of NADH to NAD+ during glycolysis. Instead, in astrocytes, NADH reduces pyruvate to lactate, leading to high levels of lactate production. The lactate is then shuttled into neurons by the monocarboxylate transporter MCT4 where it supports oxidative metabolism before being imported back to astrocytes by MCT1 (Roosterman and Cottrell, 2020).
Figure 1.
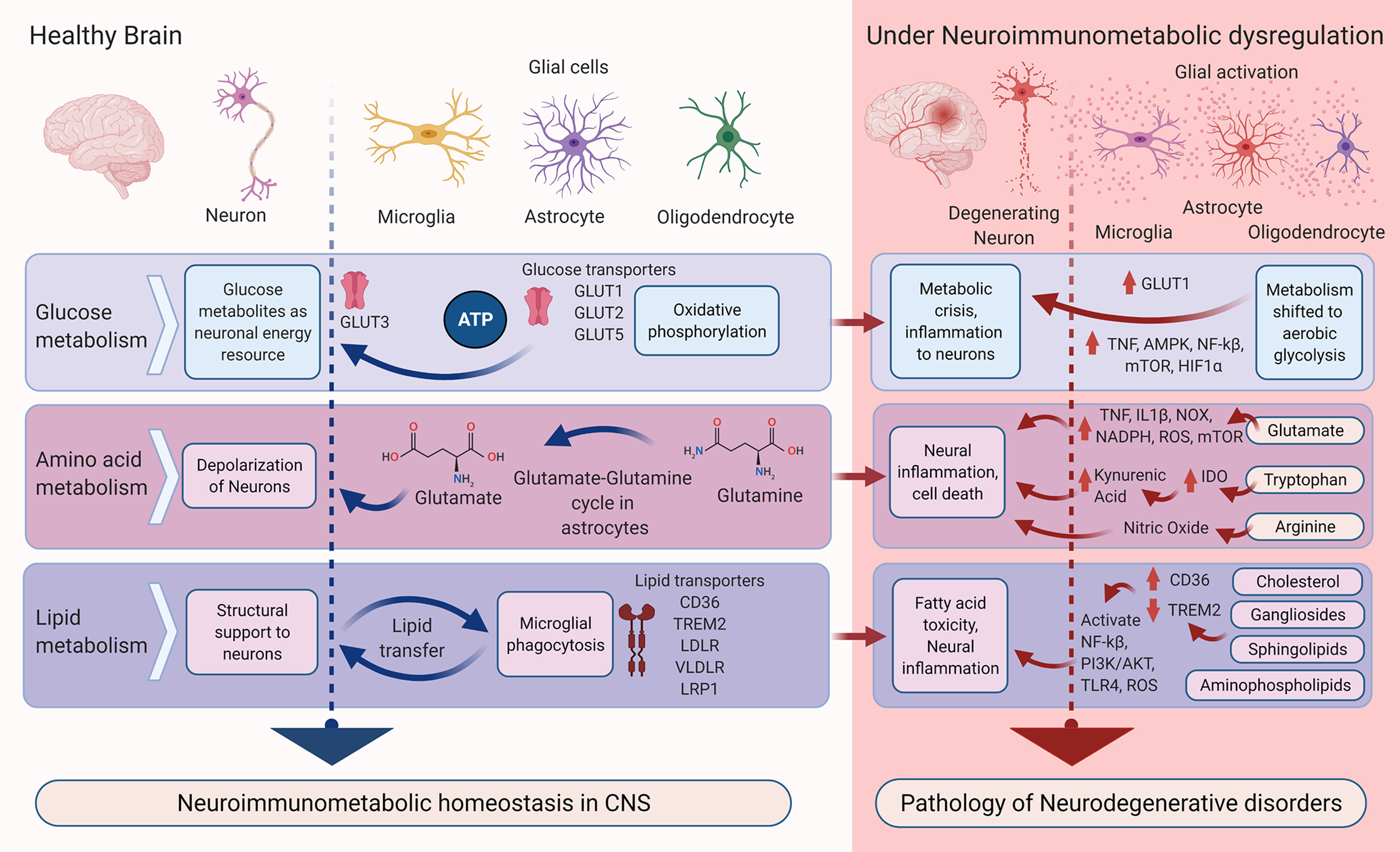
Predominant neuroimmunometabolic homeostasis in the healthy brain and its disruption in neurodegenerative conditions. In the healthy state (left, blue arrows), glucose, amino acid, and lipid metabolism occur in various cell types. Neuroimmunometabolism disrupts this homeostasis (right, red arrows) contributing to degenerative conditions. IDO, Indoleamine-2,3 dioxygenase; PI3K/AKT, phosphoinositide 3-kinases.
There is emerging evidence that oligodendrocytes also shuttle lactate to neurons. Lactate produced in the mitochondria of oligodendrocytes is essential for local axonal support, and disrupting this function leads to elevated extracellular lactate. Lactate shuttling to neurons is blocked on inhibition of the lactate transporters MCT1/2 and GLUT1 (Fünfschilling et al., 2012; Meyer et al., 2018).
Sugar metabolism in microglia depends on their activation state. Microglia normally survey their environment for indications of damage or infection. This surveillance is powered by oxidative phosphorylation (OXPHOS). When damage is detected, however, it triggers microglial activation, which involves conversion to pro-inflammatory or phagocytic states. Like other immune cells (Warburg, 1956; Palsson-McDermott and O'Neill, 2013), activated microglia upregulate glycolysis and move away from oxidative phosphorylation to meet the inflammatory demand (Moss and Bates, 2001; Chénais et al., 2002; Gimeno-Bayón et al., 2014; L. Wang et al., 2019; Lauro and Limatola, 2020). This shift from OXPHOS to glycolysis in microglia is because of upregulation of GLUT1, which is concomitant with increased synthesis of hexokinase and PFKFB3 (the rate-limiting enzymes of the glycolytic pathway) and activation of mammalian target of rapamycin (mTOR), which regulates transcriptional control of glycolysis in a process involving hypoxia-inducible factor-1α (HIF-1α) (Yecies and Manning, 2011; Saxton and Sabatini, 2017; Li et al., 2018; Rubio-Araiz et al., 2018). In addition, activated microglia upregulate GLUT1 expression to promote glycolysis, which can be limited by inhibition of GLUT1 (L. Wang et al., 2019).
Although microglia are the primary inflammatory mediators of the brain, astrocytes also play an essential role. With their copious numbers and proximity to neurons, astrocytes can amplify inflammatory signals by releasing several proinflammatory chemokines and cytokines (Szepesi et al., 2018). This metabolically expensive inflammatory response requires astrocytes to rely on mitochondrial oxidation (Chao et al., 2019). Several in vitro studies have demonstrated that inflammatory challenges activate regulators of aerobic glycolysis, such as HIF-1α and AMP-activated protein kinase (AMPK) in astrocytes (Almeida et al., 2004; Brix et al., 2012). A similar metabolic switch occurs in the aging brain, where astrocytes decrease their trophic support to neurons and use energy substrates for their own metabolism (Jiang and Cadenas, 2014). An in vivo study in rats has shown that the switch to aerobic metabolism during aging is correlated with the activation of nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB), a regulator of innate and adaptive immunity (Jiang and Cadenas, 2014).
Outside the CNS, inflammation leads to uninterrupted glycolysis, which supplements the production of ATP to sustain the inflammatory mechanisms of macrophages and other immune cells (e.g., T cells), resulting in the amplification of pro-inflammatory chemokines and cytokines to fight the infection (Kaushik and Yong, 2020). This metabolic alteration in activated macrophages and T cells is linked to two major break points in the tricarboxylic acid (TCA) cycle, causing accumulation of citrate and succinate. The accumulation of succinate leads to the production of IL-6, IL-1β, and nitric oxide (NO), largely through a glycolytic flux from OXPHOS by activation of HIF-1α, while citrate accumulation reroutes the metabolism toward prostaglandin synthesis, the major metabolic pathway inducing inflammation in both the PNS and the CNS (Tannahill et al., 2013; Infantino et al., 2013; Jha et al., 2015; Ryan and O'Neill, 2017).
Overall, the results discussed above demonstrate that neurons and glia synergistically depend on glycolytic and substitute pathways of sugar metabolism for energy production to sustain metabolic homeostasis. Various sugar sensors can act as neuroimmunometabolic regulators, suggesting that sugars play an essential role in modulating several aspects of neuroimmunometabolic machinery and can be putative factors in disease pathologies.
Amino acid sensors
Although amino acids are not a primary energy precursor, they still play a critical role in neuroimmunometabolic programming (Xie et al., 2020). For example, the amino acid tryptophan is an essential precursor for coenzyme NAD+ biosynthesis, which is critical for metabolic processes, such as glycolysis, the TCA cycle, and OXPHOS (Schröcksnadel et al., 2006). Tryptophan metabolism occurs through four different pathways, the most important being the kynurenine pathway (Kita et al., 2002; Gostner et al., 2020). Tryptophan is converted into kynurenine and downstream effector molecules by indoleamine-2,3 dioxygenase, a rate-limiting enzyme whose expression is elevated during inflammation, including the brain (Kwidzinski and Bechmann, 2007; O'Neill et al., 2016; Moffett et al., 2020). Tryptophan metabolic enzymes are expressed in both astrocytes and microglia. The key kynurenine metabolites quinolinic acid, 3-hydroxykynurenine, and kynurenic acid produce different inflammatory responses in these glial cells. In astrocytes, kynurenic acid protects neurons by removing excess glutamate spillover, whereas quinolinic acid and 3-hydroxykynurenine inflict neurotoxic effects by activating the NMDAR NR2B subunit, which leads to excessive calcium influx (Guillemin et al., 2005; Tao et al., 2020).
Glutamate is another quintessential amino acid required for maintaining optimum metabolic performance of neurons and glia. In the healthy CNS, glutamate is the predominant excitatory neurotransmitter. After taking up glutamate via excitatory amino acid transporters (EAAT1 and EAAT2), astrocytic glutaminase hydrolyzes glutamate to glutamine, which is then transported to neurons (Fig. 1) (Yudkoff, 2017; Mahmoud et al., 2019). Glutamate also binds to glutamatergic receptors on microglia and acts as a chemotactic molecule to recruit microglia to sites of injury, where excess glutamate release catalyzes neural excitotoxicity (Domercq et al., 2013). Microglia produce ROS in response to glutamate stimulation via increased activity of nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase (NOX) and NADPH. Microglia also release glutamate and exchange it with cystine through cystine/glutamate antiporter xCT (a membrane-bound Cl–-dependent antiporter) (Barger et al., 2007; Bridges et al., 2012; Mead et al., 2012; Domercq et al., 2013). Conversely, a neuroprotective role of glutamate has been reported in mixed cortical cultures, where estrogen receptor α and metabotropic glutamate receptor 1 interact to attenuate amyloid β (Aβ)-induced neurotoxicity (Spampinato et al., 2018). The precise downstream targets mediating these neuroprotective effects however remain to be investigated.
Glutamatergic homeostasis is disrupted under inflammatory conditions when the microglial inflammatory cytokines TNF and IL-1β impair the function of astrocytic EAATs (Domercq et al., 2007). In addition, conversion of glutamine to glutamate (glutaminolysis) in microglia activates mTOR signaling, halts autophagy, and creates a pro-inflammatory environment (Duran and Hall, 2012; Duran et al., 2012; G. Gao et al., 2020). Moreover, inhibition of glutamine synthetase, which catalyzes the reverse reaction of glutamate to glutamine, has been shown to enhance the inflammatory response of microglial cells (Palmieri et al., 2017). Based on these studies, one could speculate that astrocytes and microglia together play an essential role in maintaining the glutamine-glutamate balance, and any disturbance in this homeostasis can induce microglial neuroinflammation in the CNS.
The amino acid arginine has also been reported to influence immunometabolic mechanisms. Arginine is the starting substrate in the NO synthetic pathway, which involves the conversion of arginine to citrulline by the enzyme nitric oxide synthase 2 (Forstermann and Sessa, 2012). NO, the final product of this pathway, is an essential immunometabolite: it inhibits OXPHOS (Tengan and Moraes, 2017), thus shifting the balance toward the glycolytic pathway, a phenomenon that occurs in microglia exclusively during inflammation.
Lipid sensors
Brain lipid metabolism relies on both de novo local synthesis and peripheral lipid reserves. Lipids provide structural support to neurons, and intercellular exchange of lipids through microvesicles, lipoproteins, and nonesterified fatty acids (FAs) modulates energy and redox status (Tracey et al., 2018). Additionally, lipid sensing is essential for myelination by oligodendrocytes and debris clearance by microglia. After glucose metabolism, the brain draws a considerable amount of energy from lipid metabolism. Among many lipid sources, FA oxidation provides 20% of its total metabolic energy to the brain (Panov et al., 2014)). It is believed that FA oxidation occurs largely in astrocytes, but other glial and neuronal cells also use FAs, such as oleate and palmitate for oxidative metabolism (Ebert et al., 2003; Chausse et al., 2019). FA oxidation homeostasis also plays a major role in inflammatory responses in microglia. Polyunsaturated FAs can induce an anti-inflammatory phenotype in microglia, whereas saturated FAs can produce an inflammatory state (Namgaladze et al., 2014; X. Chen et al., 2018).
Microglia express several lipid-sensing receptors that remodel the microglial lipidome during cellular stress. Pattern recognition receptor cluster of differentiation 36 (CD36) is an essential lipid sensor that recognizes low-density lipoprotein (LDL) and Aβ (El Khoury et al., 2003; Kim et al., 2008; D. Gao et al., 2010). Upon activation, CD36 influences inflammatory remodeling through uptake of palmitate, which is a ligand of Toll-like receptors (TLRs). TLR activation results in phagocytosis, and production of ROS (Li et al., 2019; Tzeng et al., 2019). CD36-containing microglia also express LDL receptor (LDLR), very-LDL receptor (VLDR), and LDL-receptor-related protein 1 (LRP1). These receptors regulate the endocytosis of different isoforms of apolipoprotein (APOE2, APOE3, and APOE4). APOEs act as a hub for lipid transfer between neurons and microglia (Loving and Bruce, 2020). Although APOE is thought to be a negative regulator of inflammation (Rebeck, 2017), its isoforms (APOE2, APOE3, and APOE4) perform a dual role in inflammatory mechanisms. While APOE3 and APOE4 attenuate Aβ-induced inflammatory responses in glial culture, both isoforms exert an inflammatory response in the absence of Aβ (Guo et al., 2004). Further, the ε4 allele of APOE4 is the most common risk factor for persistent neuroinflammation underlying cardiovascular and neurodegenerative diseases (Sullivan et al., 1997; Tu et al., 2009; Mannix et al., 2011; Zhu et al., 2012; Rodriguez et al., 2014). Concomitantly, inflammatory cytokines can regulate the levels of APOE (H. Zhang et al., 2011).
Another lipid sensor expressed in microglia is triggering receptor expressed on myeloid cells 2 (TREM2), which recognizes APOE, Aβ, and aminophospholipids for phagocytosis and autophagy in microglial cells (Hsieh et al., 2009). There is a lack of consensus regarding the role of TREM2 during inflammatory responses. A soluble form of TREM2 has been found to stimulate the production of inflammatory cytokines by activating phosphoinositide 3 kinase/protein kinase B (Zhong et al., 2017). On the other hand, loss of TREM2 mitigates neuroinflammation in a mouse model of Tau-mediated neurodegeneration (Leyns et al., 2017).
Cholesterol is yet another important substrate in immune signaling involving lipid metabolism (Fig. 1). Astrocytes synthesize cholesterol in the endoplasmic reticulum, and it is shuttled to the membrane by ATP-binding cassette transporters (Nieweg et al., 2009). Membranes enriched in cholesterol act as platforms for effective dimerization of TLR4, which promotes inflammation (Varshney et al., 2016). Depletion of membrane cholesterol can therefore alter the neuroinflammatory status of astrocytes. An increase in brain glucose metabolism has been reported after astrocyte-selective knockdown of sterol-regulatory-element-binding protein 2, a receptor-bound transcription factor that regulates genes involved in cholesterol biosynthesis and uptake (Ferris et al., 2017).
Sphingolipids are also key components of astrocytic lipid reserves. Neurons, astrocytes, and microglia act in concert to sustain sphingolipid metabolism in the brain; and in the inflammatory state, this homeostasis is disturbed by production of inflammatory mediators through sphingolipid intermediate metabolites, such as ceramide and sphingosine-1-phosphate (J. Y. Lee et al., 2020). On the other hand, gangliosides, a class of glycosphingolipid, are produced by astrocytes in abundance and can regulate inflammatory status by triggering astrocytic autophagic pathways (Hwang et al., 2010).
Lipid metabolic coupling with neurons is tightly regulated by astrocytes. This is essential to avoid FA toxicity in neurons (Ioannou et al., 2018). Neurons typically have a low capacity to house the lipid droplets that store FAs to thwart toxicity and loss of mitochondrial membrane integrity. The lipid droplets act as an alternative energy source during stress and nutrient depletion (Jarc and Petan, 2019). Under constant stimulation, neurons produce ROS that catalyze the oxidation of FAs and thus build up lipid-induced toxicity. Astrocytes host lipid droplets and are able to clear FAs expelled from neurons. Additionally, astrocytes regulate transcription of putative targets that participate in energy metabolism and neutralization of oxidative species (Reynolds and Hastings, 1995; Unger et al., 2010; Rambold et al., 2015; Nguyen et al., 2017). Overall, this indicates that lipid biosynthetic and metabolic sensors have an essential role in regulating immunometabolic status of the CNS.
Neuroimmunometabolism and neurodegenerative disorders
Neurodegenerative diseases differ in multiple attributes, such as the brain regions affected, underlying molecular pathways, associated genetic and immunologic aberrations, and specific symptoms (Dugger and Dickson, 2017). The molecular mechanisms implicated in these diseases often include protein aggregation and associated neurotoxicity in the brain, for example, tau neurofibrillary tangles and amyloid plaques in Alzheimer's disease (AD), α-synuclein-containing Lewy bodies in Parkinson's disease (PD), and polyQ protein aggregates in Huntington's disease (HD) (Dugger and Dickson, 2017). Advances in brain imaging and systematic recording of clinical symptoms have contributed to better understanding of different stages of disease progression. But because of the complex and multifactorial etiologies of these diseases, the precise neurobiological underpinnings remain elusive. Although studies have elucidated the role of genetic and environmental factors that trigger neuroinflammatory conditions in these diseases, the contribution of neuroimmunometabolic factors to neurodegenerative pathophysiology is not well examined. Perhaps, that is why translational success in these diseases is limited. In this section, we look at different neurodegenerative disorders from the neuroimmunometabolic angle to highlight potential targets for better disease management (Fig. 2).
Figure 2.
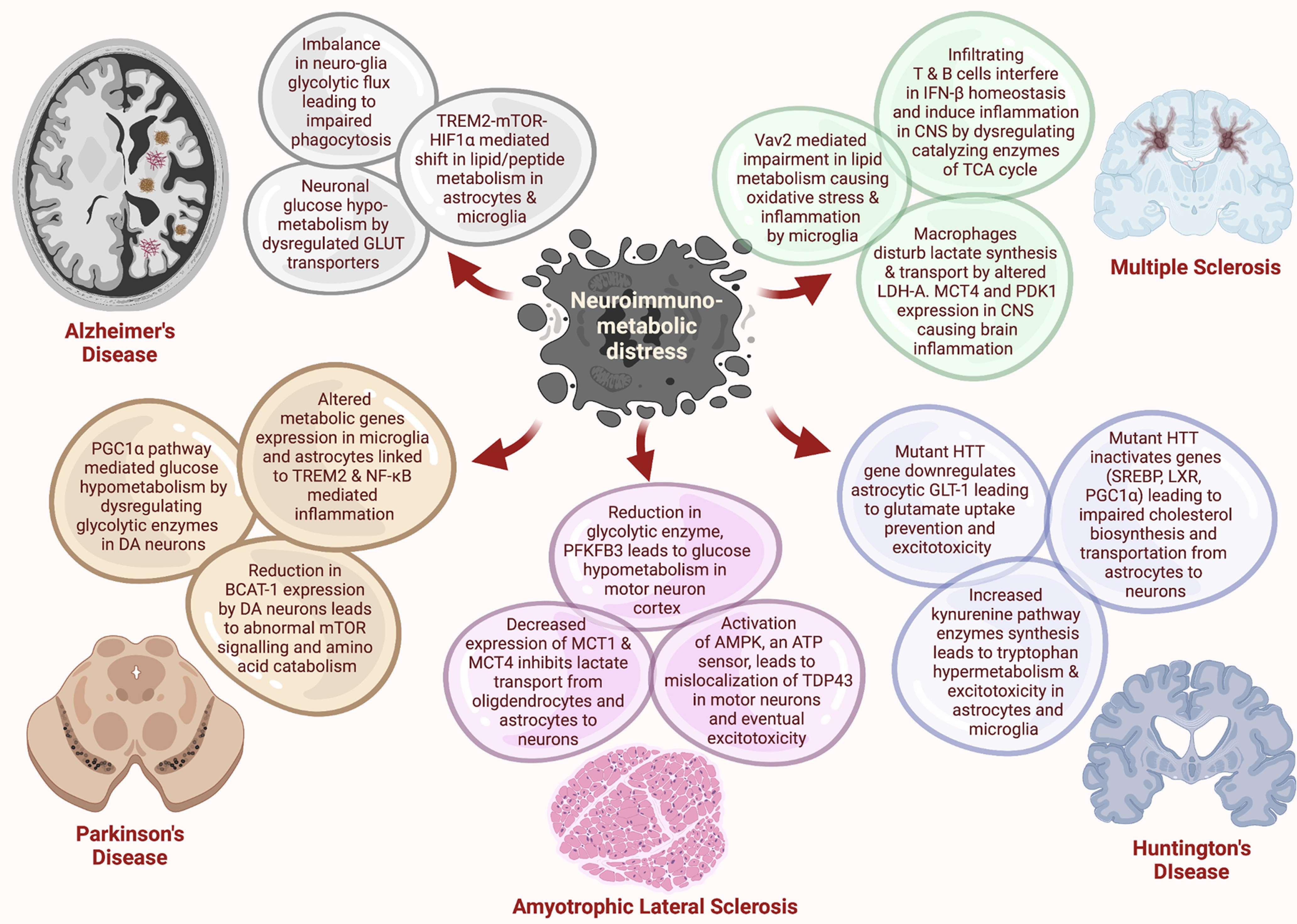
Major neuroimmunometabolic imbalances in neurodegenerative disorders. Several glucose, peptide, and lipid metabolic pathways are hindered in neurons and glia in these neurodegenerative diseases. These perturbations potentiate altered synthesis and transportation of different metabolites, leading to excitotoxicity, impaired phagocytosis, and inflammation in the CNS.
AD: a neuroimmunometabolic disorder?
Extracellular amyloid plaques and intracellular neurofibrillary tau tangles are the central pathologic hallmarks of AD. But therapies targeting amyloid and tau pathology have generally failed to exert any positive outcomes in clinical trials, suggesting that multiple associated cofactors play roles in disease manifestation (Banik et al., 2015). Recent reports show a correlation between inflammatory and immunometabolic pathways and amyloid and tau pathologies in AD models (Holland et al., 2018; McIntosh et al., 2019). There is accumulating evidence that metabolic disturbance plays an important role in the underlying microglial mediated neuroinflammation (Devanney et al., 2020). Furthermore, age-related decline in cerebral glucose metabolism (hypometabolism) is associated with cognitive loss in mild cognitive impairment and AD patients (Mosconi et al., 2008; Lin and Rothman, 2014), along with amyloid deposition and white matter disruption (Schilling et al., 2019). Glucose hypometabolism in AD brains is largely linked to mitochondrial calcium dysregulation in neurons, which may subsequently lead to cell death (Moreira et al., 2010). The presence of Aβ peptides in mitochondria may be another causative factor in neuronal death (Cha et al., 2012). Notably, some evidence, albeit limited, suggests that hypometabolism in AD is linked with disturbed homeostasis of GLUT1 and GLUT2, which is mostly expressed in glial cells, and GLUT3, which is predominantly expressed in neurons. While GLUT1 and GLUT3 levels are decreased in the cerebral cortex, the levels of GLUT2 are increased to compensate for the reduction in ATP production (Szablewski, 2016).
A role for astrocytes and microglia in disruptive metabolism has also been identified in the experimental models of AD. In cultured astrocytes derived from transgenic AD mice, exogenous Aβ can reduce glycolytic capacity by decreasing GLUT1 levels. This impairs glucose uptake and lactate production by astrocytes. Further, decreased levels of MCT1 reduce lactate transport to neurons (Merlini et al., 2011). In addition, calcium dysregulation in astrocytes may activate several pro-inflammatory regulators through NF-κB and HIF-1 pathways, responsible for ROS and NO production (Abramov et al., 2004; Hsiao et al., 2007; Schubert et al., 2009; Mesquita Dá et al., 2016; Shigetomi et al., 2019; Robb et al., 2020b). These studies strongly suggest a central role of glucose metabolism coupled with inflammation in inducing pathophysiological states in AD.
Exogenous introduction of Aβ or inflammatory stimuli, such as LPS, reduce oxidative metabolism of macrophages and microglia through activation of inflammatory cascades, and this leads to metabolically inefficient glycolysis (Rubio-Araiz et al., 2018; Finucane et al., 2019). A change in neuro-glial glycolytic flux was also found in a 76-year-old individual: magnetic resonance spectroscopy data revealed that there was a 28% decrease in TCA-cycle activity in neurons and a 30% increase in TCA-cycle activity in astrocytes compared with a 26-year-old individual (Boumezbeur et al., 2010). Finally, cultured microglia isolated from APP/PS1 mice, a transgenic model of AD, exhibit disrupted phagocytosis and chemotactic activity in tandem with an increased rate of glycolysis (Holland et al., 2018; McIntosh et al., 2019). This suggests that microglia adopt an altered neuroimmunomodulatory phenotype in AD, which may influence disease pathology.
Genome-wide association studies (GWASs) have revealed several predisposing genetic risk factors for AD, including variants of APOE4, TREM2, and TLR4, which are linked to metabolic functions and are highly expressed in microglia. It is demonstrated that APOE can trigger the formation of Aβ peptides in the brain of transgenic AD mice by using astrocytic cholesterol. APOE promotes two-way traffic of neuronal amyloid precursor protein from intracellular lipid clusters to increase the insoluble Aβ burden in the brain (H. Wang et al., 2021). The APOEε4 allele is one of the most investigated genetic risk factors for AD with a 15-fold increased risk for AD in people homozygous for this allele (Mosconi, 2013; Lumsden et al., 2020). Positron emission tomography (PET) studies of young adults (20-39 years of age) carrying APOEε4 alleles show reduced glucose metabolism in the cerebral cortex, similar to the pattern seen in the cortex of AD patients at progressive stages of the disease (Reiman et al., 2004; Mosconi, 2013). Hexokinase, one of the key cytosolic enzymes in glucose metabolism, was reported to be downregulated in the brains carrying an APOEε4 allele (compared with APOEε2 and APOEε3) leading to a deficiency in glucose metabolism (Ding et al., 2013). This glucose hypometabolism is linked to the inhibition of PPAR-γ/PGC-1α (peroxisome proliferator-activated receptor γ/ coactivator-1α), implicating this signaling pathway in AD pathophysiology (L. Wu et al., 2018).
TREM2, an immune receptor expressed on microglia, actively binds to Aβ oligomers and maintains phagocytic clearance of amyloid peptides from the healthy brain (Zhao et al., 2018). A deficiency or functional mutation in TREM2 increases the risk of AD (Jonsson et al., 2013). Recently, TREM2 deficiency was found to increase levels of cholesterol ester, which exacerbates inflammation and increases clearance burden on microglial phagocytic machinery (Nugent et al., 2020). Microglia from TREM2−/−/5XFAD mice also exhibit a reduced rate of glycolysis and decreased production of ATP that is linked to defective mTOR signaling and leads to increased autophagy. The impairment in TREM2-mTOR signaling is associated with fewer activated microglial cells migrating to surround amyloid plaques in AD patients and TREM2-deficient AD-model mice. RNA-seq data reveal downregulation of genes related to metabolic and bioenergetic pathways in TREM2-deficient mice, which likely causes autophagic abnormalities (Ulland et al., 2017). Additionally, genetic variants of TREM2 disrupt autophagy by impairing mTOR signaling in AD brains (Ulland et al., 2017; Zhou et al., 2018). Interestingly, TREM2 KO results in elevated tau phosphorylation leading to microglial activation in AD mice (Audrain et al., 2019). Surprisingly, TREM2 haploinsufficiency increases tau pathology and inflammatory responses more than complete knockdown of TREM2 in a mouse model of tauopathy, suggesting a dynamic role of TREM2 in regulating neuroinflammation by a probable compensatory mechanism in microglia (Sayed et al., 2018).
Hypoxia signaling pathways are also linked to metabolic distress and pro-inflammatory induction in the AD brain (Bazan et al., 2002; Baik et al., 2019) A recent report highlights the role of TLR4 in the immunometabolism of Aβ-treated microglial cells through the mTOR-HIF-1α hypoxia signaling pathway. Aβ induces phosphorylation of mTOR and higher expression of HIF-1α in primary microglia, activating proinflammatory cascades (Baik et al., 2019). A higher rate of mTOR-HIF-1α signaling is associated with decreased oxygen consumption rate and increased extracellular acidification rate, critically shifting the glycolytic balance in these cells (Ulland et al., 2017). Overall, these findings suggest a strong neuroimmunometabolic dysregulation pertaining to lipid and sugar metabolism that may mediate pathologic conditions associated with AD (Fig. 2).
PD: a neuroimmunometabolic anomaly?
PD impacts ∼7-10 million people around the world, making it the second most common neurodegenerative disease (Beitz, 2014). The pathologic features of PD include the loss of dopaminergic neurons in the substantia nigra pars compacta and accumulation of α-synuclein in intracellular inclusions known as Lewy bodies (Kalia and Lang, 2015; Maulik et al., 2017). Although genetic alterations and environmental exposures are thought to trigger PD, the exact molecular mechanisms linking the risk factors to PD pathology are unclear (Trinh and Farrer, 2013). Postmortem analyses of human PD samples and animal studies have demonstrated the presence of activated astrocytes and microglia in the brain (McGeer et al., 1988; Yamada et al, 1992; Q. Wang et al., 2015). The pro-inflammatory cytokines released by these cells can exacerbate degeneration of midbrain dopaminergic neurons (Hirsch et al, 2003). It can be predicted that neuroimmunometabolic modulators play certain passive roles in the neuroinflammatory characteristics of PD by altering the metabolic status of neurons and associated glial cells.
Glucose hypometabolism has been implicated in PD pathogenesis using MRI and fluorodeoxyglucose (18F) PET (Borghammer, 2012; González-Redondo et al., 2014). Moreover, reduced glucose metabolism in the cortex of PD patients is linked to the development of PD dementia (Firbank et al., 2017). Associations between glucose metabolism, changes in cell bioenergetics, and neurodegenerative pathology in PD may be mediated in part by PD-related risk factors linked to glucose and amino acid transporters, metabolic enzymes, and transcription factors that regulate metabolic pathways (Dunn et al., 2014; Y. Zhang et al., 2016; Anandhan et al., 2017). Nuclear receptors, such as peroxisome proliferator-activated receptor (PPAR), which is known to regulate inflammation and energy metabolism in the brain, are linked to several neurodegenerative disorders, including PD (Chaturvedi and Beal, 2008). Upon activation, PPARγ/PGC-1α promotes transcription of genes encoding mitochondrial respiratory subunits in the substantia nigra, thus regulating glucose metabolism in dopaminergic neurons (Zheng et al., 2010). Branched-chain amino acid transferase (BCAT1), which catalyzes degradation of branched-chain amino acids, is downregulated in the substantia nigra of sporadic PD patients (Yao et al., 2018). Knockdown of BCAT1 increases mitochondrial respiration and results in oxidative damage in neurons through mTOR-independent pathways (Mor et al., 2020). Finally, dysregulation of several glycolytic enzymes, including GLUT, MCT1, MCT4, pyruvate dehydrogenase kinase 1 (PDK1), LDH-A, and pyruvate kinase M2, has been reported to produce a hypometabolic state in the PD brain (Vallée et al., 2019). Knockdown of at least one of these genes MCT4 (SLC16A3) also results in impaired motor function in experimental mice (Lundquist et al., 2021).
Many PD-associated genes are expressed in glial cells, indicating that dysfunction of the encoded proteins in microglia and/or astrocytes could contribute to PD etiology (Joe et al., 2018). For example, the microglial receptors TREM2 and sialic acid-binding Ig-like lectin 3, which are associated with AD risk, are also related to PD risk (Rayaprolu, 2013; Chan et al., 2016). The recycling of microglial TREM2 at the plasma membrane is suggested to be regulated by the vacuolar protein sorting 35, which is often implicated in late-onset autosomal-dominant familial PD (J. Yin et al., 2016). Activation of microglia via TREM2 signaling also results in neuroinflammation in the environmental-toxin-induced model of PD (Belloli et al., 2017). However, there are conflicting reports regarding the association between TREM2 and PD pathology (Mengel et al., 2016; Ren et al., 2018); therefore, further investigation is warranted.
PD-related genes that are highly expressed in astrocyte include cytosolic ubiquitin-E3-ligase PARK2, PARK7, PTEN induced kinase 1, leucine-rich repeat kinase 2, α-synuclein, and glucocerebrosidase. These proteins play vital roles in astrocytic functions, such as inflammatory responses, uptake of glutamate, oxidative stress responses, and neuroprotection (Sonninen et al., 2020). Studies using astrocytes derived from induced pluripotent stem cells obtained from PD patients have shown dysfunction in α-synuclein clearance and downregulation of matrix metallopeptidase 2 and TGF genes. Furthermore, leucine-rich repeat kinase 2- and glucocerebrosidase-deficient astrocytes exhibit elevated levels of α-synuclein, increased reactivity to inflammatory stimulation, greater Ca2+ release from the endoplasmic reticulum, and altered polyamine metabolism: crucial hallmarks of PD pathophysiology (Sonninen et al., 2020).
Loci related to NF-κB signaling, such as methylcrotonyl-CoA carboxylase 1, DDRGK domain-containing protein 1, ras like without CAAX 2, and scavenger receptor Class B member 2 (Xi et al., 2013; Cao et al., 2016; van der Poel et al., 2019) have been identified in GWAS meta-analysis from PD patients (Jimenez-Ferrer and Swanberg, 2018). Similarly, metabolic genes, such as transmembrane glycoprotein NMB, sterol regulatory element-binding protein 1, and aminocarboxymuconate semialdehyde decarboxylase, that induce inflammatory signaling have been associated with PD pathology (Ivatt et al., 2014; Murthy et al., 2017; Vilas et al., 2017). Overall, these findings show the potential contribution of neuroimmunometabolic genes in PD pathogenesis (Fig. 2).
Multiple sclerosis (MS): intersecting peripheral and central immunometabolism
MS is an autoimmune disorder affecting sensory, motor, and autonomic functions in patients. It is a progressive neurodegenerative condition in which autoreactive immune cells (T and B lymphocytes) infiltrate the CNS, triggering a cascade of neuroinflammatory responses that cause demyelination, gliotic scarring, and axonal loss (Doshi and Chataway, 2017). In the disease state, the pro-inflammatory subsets of T and B cells (T helper cells, monocytes/macrophages, and dendritic cells) are activated in the peripheral immune system. The regular metabolic resources for energy production in these cells, on activation, are found to be shifted from the TCA cycle to glycolysis once they cross the BBB and reach the CNS.
The treatment for MS so far is heavily dependent on variants of IFN-β to reduce inflammation in the brain by shifting the balance of T and B cells toward an anti-inflammatory state. Although INF-β treatment significantly improves the medical care for MS patients, the mechanism of action is still unclear, raising doubts about its long-term responsiveness and associated risks versus benefits (Jakimovski et al., 2018). The involvement of IFN-β in targeting catalyzing enzymes of the TCA cycle links its effects to immunometabolic mechanisms of immune cells (Kaushik and Yong, 2020). Consistent with this, IFN-β treatment restores the metabolic enzymes related to glycolysis and mitochondrial respiration in relapsing-remitting MS patients (La Rocca et al., 2017). Hence, a renewed angle of investigation is warranted to identify the immunometabolic components of MS progression.
The interplay between the peripheral immune system and neuroinflammatory cascades in the pathophysiology of MS makes MS a unique degenerative disorder of the CNS. Immune-reprogramming moves from blood to brain, integrating peripheral and central immunometabolic mechanisms. Postmortem brain tissues from MS patients and mouse models have revealed the presence of brain-infiltrating macrophages that exhibit an elevated glycolytic state, suggesting a peripheral immune role in regulating metabolic homeostasis and neuroinflammation in the MS brain. Such compromised metabolic and neuroinflammatory states are most likely because of a leaky BBB that enables infiltration of the macrophages into the CNS (Popescu et al., 2013). These infiltrating macrophages express high levels of LDH-A and MCT4, which are responsible for lactate synthesis and transport. When LDH-A and MCT4 are knocked down in cultured macrophages from MS-model mice, their migration is restricted (Kaushik et al., 2019). Another study reports accumulation of lactate in infiltrating macrophages in the parenchyma of the mouse brain, and this lactate accumulation is correlated with high levels of PDK1. Increased PDK1 leads to production of lactate that triggers the inflammatory M1 phenotype in macrophages (Tan et al., 2015. This confirms an immunometabolic switch in the CNS toward the activation of proinflammatory cascades (Guglielmetti et al., 2017).
Several gene array studies have identified potential metabolic targets in the pathogenesis of MS. One potential candidate identified in GWAS of MS is the guanine nucleotide exchange factor Vav2, which, in microglia stimulated with fibrillar Aβ, activates NADPH oxidase by activating Rho-family GTPases, which in turn upregulate the NLRP3 inflammasome leading to oxidative stress (Wilkinson et al., 2006; Conley et al., 2017). Interestingly, Vav2 functions downstream of TREM2 (Y. Wang et al., 2015), indicating a possible involvement of Vav2 in lipid metabolism underlying MS pathology.
An independent transcription profiling study in microglial cells isolated from human MS postmortem brains reported higher transcription of genes related to lipid metabolism, iron metabolism, and regulation of the NF-κB pathway (van der Poel et al., 2019). These findings demonstrate the parallel immunometabolic changes occurring in neuroimmune cells along with peripheral immune cells in the MS brain (Fig. 2). How the resident immune cells of CNS metabolically react to the infiltrating peripheral immune cells is still not clearly understood and warrants further investigation.
Amyotrophic lateral sclerosis (ALS): neuroimmunometabolic swing between spinal cord and motor cortex
ALS is a fatal and aggressive neurodegenerative disorder affecting motor neurons. It is characterized by rapid progression of neuronal loss, brain and spinal cord atrophy, active astrogliosis, and a self-perpetuating inflammatory cycle. Motor neuron degeneration leads to muscle degeneration, eventual respiratory failure, and death (Brown and Al-Chalabi, 2017). Treatments for the disorder remain elusive.
There is abundant evidence indicating a role for immunometabolic dysregulation in ALS. Metabolic and energy perturbations have been observed in both clinical populations and preclinical models of ALS (Vandoorne et al., 2018; Kirk et al., 2019). PET scanning of ALS brains has shown hypometabolism of glucose in the motor cortex and frontal lobe, while the midbrain, occipital cortex, hippocampus, and spinal cord exhibit a hypermetabolic profile (Ludolph et al., 1992; Claassen et al., 2010; Pagani et al., 2014). Hypermetabolism in the spinal cord is congruent with elevated glucose levels in the cerebrospinal fluid of patients (Toczylowska et al., 2013). Conversely, postmortem analysis from ALS brains has demonstrated twofold attenuation in cortical mRNA content of the glycolytic enzyme PFKFB3 (X. S. Wang et al., 2006). Given that glia are more glycolytic than neurons, it is plausible that the increased glycolysis in the CNS of individuals with ALS could be largely because of glial cell activation (Vandoorne et al., 2018). This is corroborated by clinical studies showing downregulation of MCT1 transporters in oligodendrocytes of ALS patients (S. Lee et al., 2012; S. H. Kang et al., 2013; Philips et al., 2013), whereas expression of glutamate transporter EAAT2 is reduced in astrocytes of postmortem samples (Rothstein et al., 1995). MCT1 expression is also reduced in oligodendrocytes of ALS-model mice, and this occurs alongside cell degeneration, leading to reduced trophic support of lactate to neurons (S. Lee et al., 2012; S. H. Kang et al., 2013; Philips et al., 2013). Meanwhile, increases in synaptic glutamate resulting from reduced expression of glutamate transporter 1 (GLT-1) in astrocytes might lead to excitotoxic cell death (Lasiene and Yamanaka, 2011).
Certain kinases regulate energy metabolism through downstream immunometabolic mediators (Salminen et al., 2011). AMPK, a crucial energy regulatory kinase, is activated in motor neurons, and this causes mislocalization of TAR DNA-binding protein 43 (TDP43) in ALS patients and in an ALS mouse model expressing mutant SOD1 (Lim et al., 2012; Y. J. Liu et al., 2015). Reducing AMPK activity reverses these pathologic features (Lim et al., 2012). Interestingly, metabolic imbalances are often concomitant with neuroinflammatory aberrations. Microglial upregulation of NOX and NO synthase leads to exacerbated production of ROS and RNS in a process involving the prostanoid synthesis pathway (Almer et al., 2001; Beers et al., 2006; D. C. Wu et al., 2006; Boillée and Cleveland, 2008). Microglia also amplify NF-κB signaling in the mutant-SOD1 mouse model of ALS (Frakes et al., 2014). On the other hand, astrocytes from ALS patients, when cocultured with neurons, exhibit neurotoxicity by an unknown mechanism (Haidet-Phillips et al., 2011) that might involve the upregulation of chemokines and cytokines (Haidet-Phillips et al., 2011) or insufficient astrocytic metabolic support to neurons (Ferraiuolo et al., 2011; Philips and Rothstein, 2014). Overall, these reports suggest a contribution of neuroimmunometabolic pathways to ALS (Fig. 2), but further scrutiny will be required to establish any causative links to the disease pathology.
HD: susceptible genes linked to neuroimmunometabolism
HD is an autosomal dominant disease characterized by progressive deficits in motor function. It is caused by expanded repeats of glutamate residues at the N-terminal of the huntingtin gene (HTT). Inflammation is common in the brains of HD patients, having been identified in both presymptomatic and postmortem HD patients (Sapp et al., 2001; Tai et al., 2007; Vonsattel et al., 2008, 2011). Cell-autonomous expression of dysfunctional mHTT in the microglia results in its activation (Crotti et al., 2014; H. M. Yang et al., 2017), whereas selective expression of mHTT in astrocytes causes motor and transcriptional dysfunction (Bradford et al., 2009; Wood et al., 2019). The HTT gene is also reported to dysregulate several genes involved in cholesterol metabolism. For example, inhibition in SREBP (SREBF1), LXR (NR1H3), and PGC1α (PPARGC1A) genes results in impaired synthesis and transport of cholesterol from astrocytes to neurons in HD (Leoni and Caccia, 2015). These findings indicate immunometabolic imbalance induced by several genetic risk factors in HD (Fig. 2)
Molecules related to amino acid metabolism are also involved in the inflammatory cascade in HD brains. For example, studies have indicated that mHTT can downregulate GLT-1 in astrocytes, preventing uptake of glutamate and causing excitotoxicity (Lievens et al., 2001; Shin et al., 2005; Khakh et al., 2017). Another notable example is kynurenine-pathway metabolites, which induce neuroinflammation and neuroexcitotoxicity by activating microglia and astrocytes (Satyasaikumar et al., 2010; Campesan et al., 2011; Palpagama et al., 2019). Multiple lines of evidence point toward reduced kynurenine levels and increased quinolinic acid, an intermediate of the kynurenine pathway, in the early stages of HD (Beal et al., 1992; Guidetti et al., 2004; Giorgini et al., 2008; Crotti et al., 2014). Indeed, genetic ablation of kynurenine 3-monooxygenase, an enzyme that converts kynurenine to toxic 3-hydroxykynurenine, suppresses huntingtin-mediated excitotoxicity in a yeast model system (Giorgini et al., 2005). Further, both in vitro and in vivo studies have shown that mHTT can cause activation of NF-κB signaling, an important immunometabolic pathway in the CNS, via the IκB kinase of the IκB kinase complex. (Khoshnan et al., 2004; Träger et al., 2014). These studies also strengthen the plausibility that NF-κB-mediated increases in kynurenine-pathway activity play a role in HD (Ligam et al., 2010).
GWASs have also implicated many neuroimmunometabolic genes in relationship to HD. One of these is Autophagy related 7 (ATG7), a gene associated with defective autophagy (Squitieri et al., 2003; Metzger et al., 2010). Atg7 has been linked to (CRTC1) activity and glycolysis (Wei et al., 2016), suggesting a neuroimmunometabolic role in driving autophagy. Mechanistically, ATG7 deletion results in disrupted autophagy and activation of (CRTC1) and the glycolytic pathway. Inhibiting mTORC1 reverses the metabolic dysregulation caused by Atg7-mediated autophagic deficits (Wei et al., 2016).
Cofactors triggering neuroimmunometabolic switching
Aging and lifestyle choices, such as stress, diet, exercise, and substance abuse are some of the critical modulators of immunometabolic processes. In this section we aim to highlight how the process of aging and lifestyle factors may lead to an imbalance in immunometabolic homeostasis in the CNS (Fig. 3).
Figure 3.
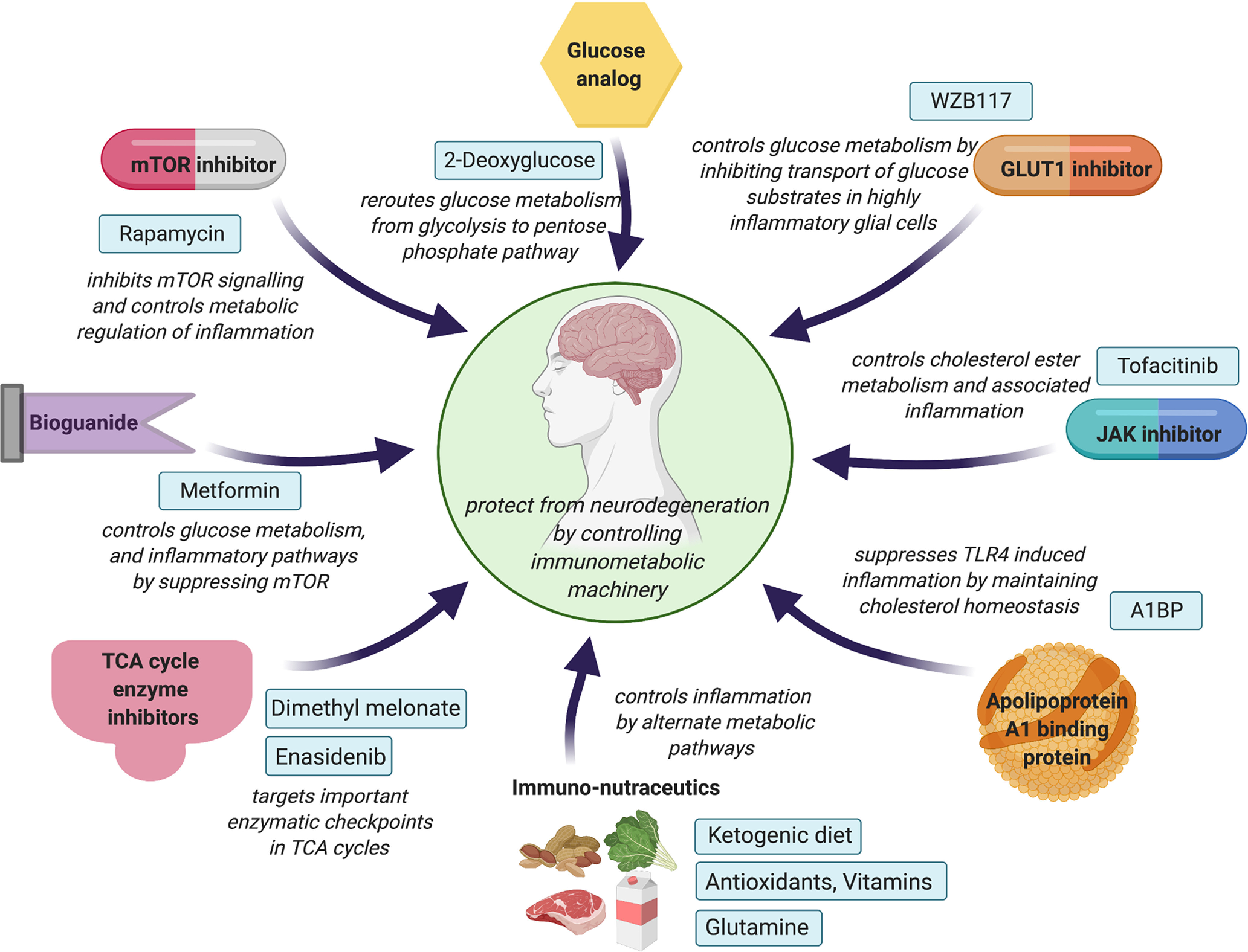
Novel therapeutic strategies to control immunometabolic machinery in neurodegenerative disorders. Glucose analogs, inhibitors of GLUT1 and TCA-cycle enzymes, act on essential regulators to sustain or control glycolytic homeostasis in the brain. While bioguanides and mTOR inhibitors control glucose metabolism, JAK inhibitors and A1BP maintain cholesterol homeostasis and associated neuroinflammation. Immuno-nutraceutics, such as ketogenic diet, antioxidants, vitamins, and glutamine supplements, can also influence neuroinflammatory events in neurodegenerative conditions by fulfilling the brain's energy requirements through alternative metabolic pathways.
Aging
Aging is a major risk factor for neurodegenerative diseases. There is a gradual decrease in neuronal viability in brain regions related to memory, motivation, and locomotion with age (Fearnley and Lees, 1991; Umegaki et al., 2008; Kusindarta et al., 2018; Maxwell et al., 2018). Aging may contribute to neurodegeneration in multiple, complex ways. Aging is associated with hyperactivation or hypoactivation in certain brain regions; this has been supported by PET scanning showing increased or decreased glucose metabolism in cortical regions and hippocampus of aged compared with young brains (Prvulovic et al., 2005; Kaup et al., 2014; F. Yin et al., 2016; Nyberg et al., 2019). Hypometabolism is manifested by metabolic shifts involving decreased neuronal glucose uptake and alterations in mitochondrial TCA cycle (Boumezbeur et al., 2010; Winklhofer and Haass, 2010; Jiang and Cadenas, 2014; J. Yin et al., 2016). Hyperactivated states are thought to be associated with drastic immunometabolic shifts, as excessive excitation can induce glutamate spillover leading to neurotoxicity (Esposito et al., 2013; Assefa et al., 2018). Further, aging is associated with increases in reactive astrocytes and microglia in the frontal cortex and the hippocampus (Rodríguez et al., 2016; Muddapu et al., 2020). In the aging brain, increased signaling by prostaglandin E2 in microglia promotes glucose sequestration into glycogen, reducing glucose flux and mitochondrial respiration. This hypometabolic state is compounded by the dependence of myeloid cells on glucose. Inhibiting prostaglandin E2 signaling in myeloid cells of aged mice reverses these deficits, improving synaptic plasticity and spatial memory (Minhas et al., 2021).
Diet
A century of industrialization and mass urbanization have led to a steady decline in the intake of fruits, vegetables, and fibers and increased consumption of animal products, saturated fats, and refined sugars (Popkin and Gordon-Larsen, 2004; Popkin et al., 2012). This, coupled with a modern sedentary lifestyle, has increased the incidence of metabolic syndromes that have gained a pandemic status (Bray et al., 2004; Bray and Popkin, 2014). There is compelling evidence that diets rich in fat and sugars adversely influence the metabolic and inflammatory profile of brain cells (Beilharz et al., 2015; Chianese et al., 2018). Apart from FAs synthesized in the brain, dietary FAs can cross the BBB (Freund Levi et al., 2014) and modulate cellular processes in the brain. It is unknown how central versus peripheral lipid pools distinctly modulate neuroimmunometabolic mechanisms, necessitating further investigation.
Disturbances in immunometabolic signaling and day/night rhythmicity because of hypercaloric diet have been demonstrated in microglia (Milanova et al., 2019). Dietary habits influence microglial phenotypic polarization, a morphologic adaptation driven by cell surface receptors. These receptors recognize harmful stimuli that lead to transcriptional changes necessary for the phenotypic switch. High-fat diet induces region-specific inflammatory states and metabolic imbalances in microglia, astrocytes, and oligodendrocytes (Valdearcos et al., 2014; Guillemot-Legris et al., 2016; Guillemot-Legris and Muccioli, 2017; Jin et al., 2020), mainly as a result of saturated FAs activating putative immunometabolic mediators, such as myelin disruption, and pathways linked to NF-κB, TLR receptors, IFN-γ, TNF, and IL-33 (Buckman et al., 2015; Guillemot-Legris et al., 2016; Ng and Say, 2018; H. T. Huang et al., 2019). Additionally, high-fat diets attenuate the positive effects of nutraceuticals, such as Alaskan bog blueberries, on neurodegenerative pathophysiology by microglial activation (Maulik et al., 2019). In contrast, unsaturated and ω3 FAs, abundantly present in foods, such as fish oils, attenuate anti-inflammatory phenotypes in microglia (Inoue et al., 2017). Omega-3 FAs also help in eliminating myelin debris and extracellular Aβ peptides by glial phagocytosis (Oksman et al., 2006; S. Chen et al., 2014; Dong et al., 2018).
Exercise
There is accumulating evidence for a role of exercise in regulating neuroinflammation and glial activation. Exercise releases anti-inflammatory myokines, such as IL-6, from skeletal muscles, and this increases the production of IL-10, an anti-inflammatory cytokine. Myokines can travel across the BBB, bind to microglial receptors, and promote a quiescent phenotype as opposed to a more active inflammatory phenotype (Cianciulli et al., 2015; Kelly, 2018; Vecchio et al., 2018; Pederson, 2019). Long-term treadmill exercise elevates neuronal expression of CD200, a Type 1 membrane glycoprotein. CD200 binds to the CD200R receptor expressed on the microglia and leads to glycosylation of CD200R (N-glycosylated at asparagine 44). This CD200-CD200R interaction checks on the microglial activation in PD mice, sustaining metabolic homeostasis (Sung et al., 2012; C. Liu et al., 2018).
BDNF, a ubiquitous modulator of neurogenesis, synaptic plasticity, and inflammation, is produced by neurons, microglia, and astrocytes and is upregulated by exercise (Wrann et al., 2013; Sleiman et al., 2016). The precise mechanisms by which BDNF reduces inflammation is unknown. The most likely target is the cholinergic system where an imbalance in neuroimmune communication may lead to inflammation through phosphoinositide 3-kinases/GSK-3β-mediated pathways (Papathanassoglou et al., 2015; Halder and Lal, 2021). Notably, acute bouts of exercise have positive impact on BDNF levels and inflammatory status in both healthy individuals and PD patients (Małczyńska-Sims et al., 2020).
Finally, exercise produces antioxidants, such as GSH and SOD, which play a vital role in maintaining redox balance and anti-inflammatory status in astrocytes and microglia (Radak et al., 2001), as well as attenuating TLR activation on microglial cells by high-fat diets (E. B. Kang et al., 2016).
Substance abuse
Substance abuse and stress can also significantly influence the immunometabolic status of the brain. Longitudinal studies have shown progressive changes in brain metabolic activity in cocaine abusers (Volkow et al., 2011) and in animals after abstinence from cocaine self-administration (Nicolas et al., 2017). Similarly, alcohol decreases brain glucose metabolism in heavy drinkers (Volkow et al., 2015), and methamphetamine and 3,4-methylenedioxymethamphetamine abuse causes oxidative stress, metabolic compromise, and inflammation (Yamamoto and Raudensky, 2008). Both 3,4-methylenedioxymethamphetamine and methamphetamine elicit acute decreases in glucose utilization, and this is linked to long-term impairment in energy metabolism and increased inflammation in different brain regions. These neurotoxic effects were found to be selective and long-lasting or irreversible (Pontieri et al., 1990; Y. H. Huang et al., 1999; Quate et al., 2004).
Neuroimmunometabolic therapeutics: time to change the course of disease management?
Designing treatment strategies centered around optimizing neuroimmunometabolic aberration could be challenging but may lead to effective therapeutic intervention for these neurodegenerative disorders (Fig. 3). Most current clinical treatments serve to cope with symptoms rather than addressing the underlying pathophysiology (X. Chen and Pan, 2015). Further, most of these drugs (e.g., Levodopa, amantadine, galantamine, memantine, etc.) carry severe side effects, such as gait disturbances, tremors, hives, headache, drowsiness, etc. (Duraes et al., 2018). There remains a huge opportunity in using drugs that target the metabolic and immune machinery to halt the progression of these diseases.
Metformin
Metformin is a biguanide that controls glucose metabolism and inhibits mitochondrial electron transport and ROS production. Metformin activates the AMPK pathway by activating serine–threonine liver kinase B1, inhibiting mTOR and its coupling to downstream mediators (Kalender et al., 2010; Y. Wang et al., 2018), which are disrupted in degenerative conditions. Moreover, metformin significantly reduces mitochondrial distress caused by BCAT-1 deficiency, thus reversing neurotoxicity, improving motor function, and enhancing neuronal viability in Caenorhabditis elegans models of PD (Mor et al., 2020). Furthermore, clinical trials are currently recruiting ALS and AD patients to test the efficacy of metformin in altering the pathophysiology in these disorders (www.clinicaltrials.gov, NCT04098666, 2020; NCT04220021, 2020).
Rapamycin and inhibitors of glucose metabolism
Given the plethora of evidence implicating mTOR in the metabolic regulation of inflammation, the mTOR inhibitor rapamycin may be an effective therapeutic to combat immunometabolic imbalances. Rapamycin, sold under the trade name Sirolimus, exhibited efficacy in clinical trials of inflammatory disorders, such as systemic lupus erythematosus (Lai et al., 2018).
The glucose metabolism inhibitor, 2-deoxyglucose, which is used as a noninvasive diagnostic tool in PET scanning, can inhibit excessive glycolysis alone or in combination by rerouting glucose metabolism into the pentose phosphate pathway involving hexokinase (Maher et al., 2004; Pajak et al., 2019), and this might be able to attenuate the excessive glycolytic surge observed in several pathologic conditions. Another drug, WZB117, inhibits the glucose transporter GLUT1 and thus reduces inflammation by suppressing metabolism (Y. Liu et al., 2012). Furthermore, drugs, such as dimethyl malonate (a succinate dehydrogenase inhibitor) and enasidenib (an isocitrate dehydrogenase inhibitor) that target the TCA cycle at various enzymatic steps, may ameliorate immunometabolic dysregulation by regulating energy metabolism (Stein et al., 2019).
Tofacinib
Like drugs targeting glucose metabolism, drugs targeting lipid substrates are under clinical investigation for immune and metabolic disorders. Dysregulation of the JAK/STAT signaling pathway and the resulting disruption of lipid metabolism is evident in several metabolic disorders (Xu et al., 2013; Gurzov et al., 2016; Bharadwaj et al., 2020). Tofacitinib, an FDA-approved drug for rheumatoid arthritis (RA) and other immune disorders, is a JAK inhibitor, and it ameliorates macrophage-induced inflammation in RA patients by controlling the metabolism of cholesterol esters (Fleischmann et al., 2012; Kremer et al., 2012). In a rabbit model mimicking the lipid paradox in RA (a high cardiovascular risk despite low levels of LDL), tofacitinib treatment reverses inflammation-induced inhibition of reverse cholesterol transport (Perez-Baos et al., 2017). Although the precise mechanism is unknown, tofacitinib is believed to influence reverse cholesterol transport by upregulating ATP-binding cassette A1 transporters that promote phospholipid and cholesterol transport on pre-high-density lipoprotein (Perez-Baos et al., 2017). This drug could be further investigated for treating neurodegenerative disorders, such as AD and PD, where pathways related to lipid metabolism are disrupted. Notably, a recent nonrandomized clinical trial is registered to test the efficacy of tofacitinib in AD and other dementias. Further, a tofacitinib trial is currently underway in RA patients (www.clinicaltrials.gov, NCT04529876, 2020).
Apolipoprotein A-I binding protein (AIBP)
AIBP helps to maintain cholesterol homeostasis in lipid rafts in immune cells by removing excess cholesterol. AIBP is reported to promote cholesterol efflux from macrophages by binding to ATP-binding cassette A1, the key transporter involved in cholesterol metabolism (M. Zhang et al., 2016). Further, AIBP can inhibit inflammatory responses in macrophages by inhibiting the formation of foam cells in the circulation through a process involving the activation of MAPK and NF-κB signaling pathways (M. Zhang et al., 2018). Furthermore, AIBP inhibits LPS-induced TLR4 dimerization and inflammatory cytokine production in microglial lipid rafts (Woller et al., 2018).
A role for AIBP has also been tested in other degenerative models, such as glaucoma. Glaucoma involves apoptosis in retinal ganglion cells, and glia-driven neuroinflammation. In a mouse model of glaucoma, the level of AIBP in retinal ganglion cells was significantly reduced, and exogenous administration of recombinant AIBP protected retinal ganglion cells from glaucomatous neurodegeneration and associated inflammatory responses (Choi et al., 2020).
Immuno-nutraceutics
Another new line of research has been the development of immuno-nutraceutics. This field explores the possibility of using nutritional intervention and physical activity to improve neuroimmunometabolic balance. For example, carbohydrates influence immune responses to chronic intense exercise. Further, data from exercise-immune studies have revealed positive benefits of using antioxidants, vitamins, amino acids, such as glutamine, and other nutraceuticals in exercise-induced immunometabolic restoration (Gould and Pazdro, 2019).
In the 1920s, the ketogenic diet was formulated for the treatment of drug-resistant epilepsy (Wheless, 2008). This carbohydrate-depleted and fat-enriched diet shifts metabolic pathways from glycolysis to the TCA cycle, pushing the body into a state of ketosis that involves burning fat for energy production. The ketogenic diet has also been shown to exert anti-inflammatory effect in several experimental models and clinical cohorts of AD, PD, HD, and other disorders (Ruskin et al., 2011; J. Y. Chen et al., 2016; Phillips et al., 2018; Brenton et al., 2019; Rusek et al., 2019; Bahr et al., 2020; Koh et al., 2020). There is growing evidence that the ketogenic diet suppresses potential pro-inflammatory pathways, such as the NRLP3, PPARγ, and mTOR cascades (Huttenlocher, 1976; Koh et al., 2020).
While such dietary interventions could hold potential in treating neuroimmunometabolic pathologies, it is also important to understand that there are confounding factors that influence success of such therapeutic strategies. This includes the stage of diagnosis, potential side effects, specificity of action, and individual responses to these therapies or interventions. Moreover, adjuvant or combinational treatment involving immunonutrition and multiple drugs could be an alternative intervention. This hypothesis, however, remains to be tested in different models of CNS neurodegenerative disorders and thus warrants further investigation.
Neuroimmunometabolic profiling to bridge the translational gap
As described above, neurodegenerative diseases are affected by multiple genetic and nongenetic factors, including neuroimmunometabolic sensors. They differ in their disease manifestation but share numerous features, including scarce availability of efficient tools for early diagnosis and managing disease progression. Preclinical evaluation of pathways related to genes implicated in these diseases has usually failed to produce drugs that prove effective in clinical trial. The complexity of the diseases lies in the involvement of multiple risk genes, each of which imposes only modest risk on its own. Moreover, the encoded genes may have roles in complex pathways, the disruption of which may affect numerous other pathways in various cell types. For example, metabolic aberration in glial cells may induce a recalibration of immune responses, which might lead to reconfiguration of neural connectivity, thus resulting in an array of neuronal abnormalities. Such a network of events can initiate at any node, resulting in a unidirectional or bidirectional effect. Thus, functional studies are essential to evaluate the role of risk genes and verify their involvement in discrete biological cascades. The gap between preclinical and clinical findings suggests that a more holistic preclinical approach is necessary.
Understanding disease mechanisms is contingent on identifying all the implicated genes, verifying their roles, and testing for therapeutic potential. This can be achieved by approaches, such as genomics (genotype arrays, GWAS, whole genome, and exome sequencing), epigenomics, transcriptomics, proteomics, and metabolomics. GWASs are unbiased, as they have often shown association of completely unrelated genes previously considered to be remotely associated with the disease.
For example, screening of AD-associated genes expressed in CNS cell types has led to the discovery of many hits in microglia, such as TREM2 and APOE, as potential risk factors for AD (Andreasson et al., 2016; Jansen et al., 2019). Comparing the results of studies of one disease with those from another disease can reveal risk factors that affect more than one disease. Cross evaluation of multiple conditions will not only expand our understanding of diseases, but will also aid in designing informed therapeutic interventions.
Epigenomics, involving epigenome wide association studies, addresses reversible or irreversible modification of DNA and histones, including methylation and acetylation (Hasin et al., 2017). Differentially methylated immunometabolic factors can serve as indicators of disease status (Piunti and Shilatifard, 2016). Similarly, transcriptomics measures genome-wide RNA levels using various advanced RNA sequencing techniques (Z. Wang et al., 2009; Lowe et al., 2017), whereas proteomics quantifies peptide expression, interaction, and clearance using mass spectroscopy-based and mass spectroscopy-independent methods (Hasin et al., 2017; Timp and Timp, 2020). Metabolomics, on the other hand, quantifies small molecules and metabolites, which can be used to determine neuroimmunometabolic reprogramming (Johnson et al., 2016).
Seldom are all risk genes produced by any single one of these approaches translatable. Further, evaluating therapeutic potential of each risk gene in a global framework might be time- and resource-intensive. Thus, a combinatorial approach to verify risk genes may help bridge therapeutic gaps. After omics evaluation, risk genes can be studied in vivo or in vitro to examine in detail how they contribute to various cascading effects that disturb neuroimmunometabolism. This will aid in unraveling more therapeutic hits. Some of the common techniques for studying the onset of neuroimmunometabolic shifts include examination of metabolic changes through colorimetric techniques, specialized biosensor imaging, mass spectroscopy, PET and imaging, as reviewed previously (Bernier et al., 2020). Given the flood of data available in individual fields, such as immunological, neurodegenerative, and metabolic diseases, the neuroimmunometabolic gene profiles in animal models identified by omics have great potential to increase translational success. The omics databases of risk genes can be used by the health care system to track individual health for personalized treatment. At this point, the cost of generating individual omics data is too expensive for the payers to appreciate, but as the technology advances, these problems can be overcome.
Conclusion
In conclusion, we have provided an overview of the immunometabolic mediators and mechanisms that play critical roles in regulating energy balance in the CNS. Abnormalities in neuroimmunometabolic functioning linking cellular intermediates and pathways can lead to neurodegenerative conditions. Unraveling the cellular mediators underlying such pathways in these disorders can provide a basis for future therapeutic intervention. Further, translating these preclinical findings may help us in synergizing personalized treatment for many currently untreatable neurodegenerative disorders.
Footnotes
We thank Dr. Raymond Dingledine (Emory University School of Medicine) for help in overall insight and edits to improve this manuscript. Figures were created with www.BioRender.com.
The authors declare no competing financial interests.
References
- Abramov AY, Canevari L, Duchen MR (2004) Calcium signals induced by amyloid beta peptide and their consequences in neurons and astrocytes in culture. Biochim Biophys Acta 1742:81–87. 10.1016/j.bbamcr.2004.09.006 [DOI] [PubMed] [Google Scholar]
- Afridi R, Kim JH, Rahman MH, Suk K (2020) Metabolic regulation of glial phenotypes: implications in neuron-glia interactions and neurological disorders. Front Cell Neurosci 14:20. 10.3389/fncel.2020.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida A, Moncada S, Bolaños JP (2004) Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat Cell Biol 6:45–51. 10.1038/ncb1080 [DOI] [PubMed] [Google Scholar]
- Almer G, Guégan C, Teismann P, Naini A, Rosoklija G, Hays AP, Chen C, Przedborski S (2001) Increased expression of the pro-inflammatory enzyme cyclooxygenase-2 in amyotrophic lateral sclerosis. Ann Neurol 49:176–185. [PubMed] [Google Scholar]
- Anandhan A, Jacome MS, Lei S, Hernandez-Franco P, Pappa A, Panayiotidis MI, Powers R, Franco R (2017) Metabolic dysfunction in Parkinson's disease: bioenergetics, redox homeostasis and central carbon metabolism. Brain Res Bull 133:12–30. 10.1016/j.brainresbull.2017.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasson KI, Bachstetter AD, Colonna M, Ginhoux F, Holmes C, Lamb B, Landreth G, Lee DC, Low D, Lynch MA, Monsonego A, O'Banion MK, Pekny M, Puschmann T, Russek-Blum N, Sandusky LA, Selenica ML, Takata K, Teeling J, Town T, et al. (2016) Targeting innate immunity for neurodegenerative disorders of the central nervous system. J Neurochem 138:653–693. 10.1111/jnc.13667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argente-Arizón P, Guerra-Cantera S, Garcia-Segura LM, Argente J, Chowen JA (2017) Glial cells and energy balance. J Mol Endocrinol 58:R59–R71. 10.1530/JME-16-0182 [DOI] [PubMed] [Google Scholar]
- Assefa BT, Gebre AK, Altaye BM (2018) Reactive astrocytes as drug target in Alzheimer's disease. Biomed Res Int 2018:4160247. 10.1155/2018/4160247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB (2001) An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab 21:1133–1145. 10.1097/00004647-200110000-00001 [DOI] [PubMed] [Google Scholar]
- Audrain M, Haure-Mirande JV, Wang M, Kim SH, Fanutza T, Chakrabarty P, Fraser P, St George-Hyslop PH, Golde TE, Blitzer RD, Schadt EE, Zhang B, Ehrlich ME, Gandy S (2019) Integrative approach to sporadic Alzheimer's disease: deficiency of TYROBP in a tauopathy mouse model reduces C1q and normalizes clinical phenotype while increasing spread and state of phosphorylation of tau. Mol Psychiatry 24:1383–1397. 10.1038/s41380-018-0258-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr LS, Bock M, Liebscher D, Bellmann-Strobl J, Franz L, Prüß A, Schumann D, Piper SK, Kessler CS, Steckhan N, Michalsen A, Paul F, Mähler A (2020) Ketogenic diet and fasting diet as Nutritional Approaches in Multiple Sclerosis (NAMS): protocol of a randomized controlled study. Trials 21:3. 10.1186/s13063-019-3928-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baik SH, Kang S, Lee W, Choi H, Chung S, Kim JI, Mook-Jung I (2019) A breakdown in metabolic reprogramming causes microglia dysfunction in Alzheimer's disease. Cell Metab 30:493–507.e496. 10.1016/j.cmet.2019.06.005 [DOI] [PubMed] [Google Scholar]
- Banik A, Brown RE, Bamburg J, Lahiri DK, Khurana D, Friedland RP, Chen W, Ding Y, Mudher A, Padjen AL, Mukaetova-Ladinska E, Ihara M, Srivastava S, Padma Srivastava MV, Masters CL, Kalaria RN, Anand A (2015) Translation of pre-clinical studies into successful clinical trials for Alzheimer's disease: what are the roadblocks and how can they be overcome? J Alzheimers Dis 47:815–843. 10.3233/JAD-150136 [DOI] [PubMed] [Google Scholar]
- Barger SW, Goodwin ME, Porter MM, Beggs ML (2007) Glutamate release from activated microglia requires the oxidative burst and lipid peroxidation. J Neurochem 101:1205–1213. 10.1111/j.1471-4159.2007.04487.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG, Palacios-Pelaez R, Lukiw WJ (2002) Hypoxia signaling to genes: significance in Alzheimer's disease. Mol Neurobiol 26:283–298. 10.1385/MN:26:2-3:283 [DOI] [PubMed] [Google Scholar]
- Beal MF, Matson WR, Storey E, Milbury P, Ryan EA, Ogawa T, Bird ED (1992) Kynurenic acid concentrations are reduced in Huntington's disease cerebral cortex. J Neurol Sci 108:80–87. 10.1016/0022-510X(92)90191-M [DOI] [PubMed] [Google Scholar]
- Beers DR, Henkel JS, Xiao Q, Zhao W, Wang J, Yen AA, Siklos L, McKercher SR, Appel SH (2006) Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc Natl Acad Sci USA 103:16021–16026. 10.1073/pnas.0607423103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitz JM (2014) Parkinson's disease: a review. Front Biosci (Schol Ed) 6:65–74. 10.2741/s415 [DOI] [PubMed] [Google Scholar]
- Beilharz JE, Maniam J, Morris MJ (2015) Diet-induced cognitive deficits: the role of fat and sugar, potential mechanisms and nutritional interventions. Nutrients 7:6719–6738. 10.3390/nu7085307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belloli S, Pannese M, Buonsanti C, Maiorino C, Di Grigoli G, Carpinelli A, Monterisi C, Moresco RM, Panina-Bordignon P (2017) Early upregulation of 18-kDa translocator protein in response to acute neurodegenerative damage in TREM2-deficient mice. Neurobiol Aging 53:159–168. 10.1016/j.neurobiolaging.2017.01.010 [DOI] [PubMed] [Google Scholar]
- Bernier LP, York EM, MacVicar BA (2020) Immunometabolism in the brain: how metabolism shapes microglial function. Trends Neurosci 43:854–869. 10.1016/j.tins.2020.08.008 [DOI] [PubMed] [Google Scholar]
- Bharadwaj U, Kasembeli MM, Robinson P, Tweardy DJ (2020) Targeting janus kinases and signal transducer and activator of transcription 3 to treat inflammation, fibrosis, and cancer: rationale, progress, and caution. Pharmacol Rev 72:486–526. 10.1124/pr.119.018440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boillée S, Cleveland DW (2008) Revisiting oxidative damage in ALS: microglia, Nox, and mutant SOD1. J Clin Invest 118:474–478. 10.1172/JCI34613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolaños JP, Almeida A, Moncada S (2010) Glycolysis: a bioenergetic or a survival pathway? Trends Biochem Sci 35:145–149. 10.1016/j.tibs.2009.10.006 [DOI] [PubMed] [Google Scholar]
- Borghammer P (2012) Perfusion and metabolism imaging studies in Parkinson's disease. Dan Med J 59:B4466. [PubMed] [Google Scholar]
- Boumezbeur F, Mason GF, de Graaf RA, Behar KL, Cline GW, Shulman GI, Rothman DL, Petersen KF (2010) Altered brain mitochondrial metabolism in healthy aging as assessed by in vivo magnetic resonance spectroscopy. J Cereb Blood Flow Metab 30:211–221. 10.1038/jcbfm.2009.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman AB, Kwakye GF, Herrero Hernández E, Aschner M (2011) Role of manganese in neurodegenerative diseases. J Trace Elem Med Biol 25:191–203. 10.1016/j.jtemb.2011.08.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford J, Shin JY, Roberts M, Wang CE, Li XJ, Li S (2009) Expression of mutant huntingtin in mouse brain astrocytes causes age-dependent neurological symptoms. Proc Natl Acad Sci USA 106:22480–22485. 10.1073/pnas.0911503106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray GA, Popkin BM (2014) Dietary sugar and body weight: have we reached a crisis in the epidemic of obesity and diabetes? Health be damned! Pour on the sugar. Diabetes Care 37:950–956. 10.2337/dc13-2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray GA, Nielsen SJ, Popkin BM (2004) Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr 79:537–543. 10.1093/ajcn/79.4.537 [DOI] [PubMed] [Google Scholar]
- Brenton JN, Banwell B, Bergqvist AG, Lehner-Gulotta D, Gampper L, Leytham E, Coleman R, Goldman MD (2019) Pilot study of a ketogenic diet in relapsing-remitting MS. Neurol Neuroimmunol Neuroinflamm 6:e565. 10.1212/NXI.0000000000000565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges R, Lutgen V, Lobner D, Baker DA (2012) Thinking outside the cleft to understand synaptic activity: contribution of the cystine-glutamate antiporter (System xc-) to normal and pathological glutamatergic signaling. Pharmacol Rev 64:780–802. 10.1124/pr.110.003889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brix B, Mesters JR, Pellerin L, Jöhren O (2012) Endothelial cell-derived nitric oxide enhances aerobic glycolysis in astrocytes via HIF-1α-mediated target gene activation. J Neurosci 32:9727–9735. 10.1523/JNEUROSCI.0879-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RH, Al-Chalabi A (2017) Amyotrophic lateral sclerosis. N Engl J Med 377:162–172. 10.1056/NEJMra1603471 [DOI] [PubMed] [Google Scholar]
- Buckman LB, Thompson MM, Lippert RN, Blackwell TS, Yull FE, Ellacott KL (2015) Evidence for a novel functional role of astrocytes in the acute homeostatic response to high-fat diet intake in mice. Mol Metab 4:58–63. 10.1016/j.molmet.2014.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camandola S, Mattson MP (2017) Brain metabolism in health, aging, and neurodegeneration. EMBO J 36:1474–1492. 10.15252/embj.201695810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campesan S, Green EW, Breda C, Sathyasaikumar KV, Muchowski PJ, Schwarcz R, Kyriacou CP, Giorgini F (2011) The kynurenine pathway modulates neurodegeneration in a Drosophila model of Huntington's disease. Curr Biol 21:961–966. 10.1016/j.cub.2011.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Xia Z, Zhou Y, Yang X, Hao H, Peng N, Liu S, Zhu Y (2016) Methylcrotonoyl-CoA carboxylase 1 potentiates RLR-induced NF-κB signaling by targeting. Sci Rep 6:33557. 10.1038/srep33557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha MY, Han SH, Son SM, Hong HS, Choi YJ, Byun J, Mook-Jung I (2012) Mitochondria-specific accumulation of amyloid β induces mitochondrial dysfunction leading to apoptotic cell death. PLoS One 7:e34929. 10.1371/journal.pone.0034929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G, White CC, Winn PA, Cimpean M, Replogle JM, Glick LR, Cuerdon NE, Ryan KJ, Johnson KA, Schneider JA, Bennett DA, Chibnik LB, Sperling RA, De Jager PL, Bradshaw EM (2016) Trans-pQTL study identifies immune crosstalk between Parkinson and Alzheimer loci. Neurol Genet 2:e90. 10.1212/NXG.0000000000000090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CC, Gutiérrez-Vázquez C, Rothhammer V, Mayo L, Wheeler MA, Tjon EC, Zandee SE, Blain M, de Lima KA, Takenaka MC, Avila-Pacheco J, Hewson P, Liu L, Sanmarco LM, Borucki DM, Lipof GZ, Trauger SA, Clish CB, Antel JP, Prat A, et al. (2019) Metabolic control of astrocyte pathogenic activity via cPLA2-MAVS. Cell 179:1483–1498.e1422. 10.1016/j.cell.2019.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi RK, Beal MF (2008) PPAR: a therapeutic target in Parkinson's disease. J Neurochem 106:506–518. 10.1111/j.1471-4159.2008.05388.x [DOI] [PubMed] [Google Scholar]
- Chausse B, Kakimoto PA, Caldeira-da-Silva CC, Chaves-Filho AB, Yoshinaga MY, da Silva RP, Miyamoto S, Kowaltowski AJ (2019) Distinct metabolic patterns during microglial remodeling by oleate and palmitate. Biosci Rep 39:BSR20190072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JY, Tran C, Hwang L, Deng G, Jung ME, Faull KF, Levine MS, Cepeda C (2016) Partial amelioration of peripheral and central symptoms of Huntington's disease via modulation of lipid metabolism. J Huntingtons Dis 5:65–81. 10.3233/JHD-150181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Zhang H, Pu H, Wang G, Li W, Leak RK, Chen J, Liou AK, Hu X (2014) n-3 PUFA supplementation benefits microglial responses to myelin pathology. Sci Rep 4:7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Pan W (2015) The treatment strategies for neurodegenerative diseases by integrative medicine. Integr Med Int 1:223–225. 10.1159/000381546 [DOI] [Google Scholar]
- Chen X, Chen C, Fan S, Wu S, Yang F, Fang Z, Fu H, Li Y (2018) Omega-3 polyunsaturated fatty acid attenuates the inflammatory response by modulating microglia polarization through SIRT1-mediated deacetylation of the HMGB1/NF-κB pathway following experimental traumatic brain injury. J Neuroinflammation 15:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chénais B, Morjani H, Drapier JC (2002) Impact of endogenous nitric oxide on microglial cell energy metabolism and labile iron pool. J Neurochem 81:615–623. 10.1046/j.1471-4159.2002.00864.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi-Castaneda D, Ortega A (2018) Glial cells in the genesis and regulation of circadian rhythms. Front Physiol 9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chianese R, Coccurello R, Viggiano A, Scafuro M, Fiore M, Coppola G, Operto FF, Fasano S, Laye S, Pierantoni R, Meccariello R (2018) Impact of dietary fats on brain functions. Curr Neuropharmacol 16:1059–1085. 10.2174/1570159X15666171017102547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Kim KY, Perkins GA, Phan S, Edwards G, Xia Y, Kim J, Skowronska-Krawczyk D, Weinreb RN, Ellisman MH, Miller YI, Ju WK (2020) AIBP protects retinal ganglion cells against neuroinflammation and mitochondrial dysfunction in glaucomatous neurodegeneration. Redox Biol 37:101703. 10.1016/j.redox.2020.101703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuquet J, Quilichini P, Nimchinsky EA, Buzsáki G (2010) Predominant enhancement of glucose uptake in astrocytes versus neurons during activation of the somatosensory cortex. J Neurosci 30:15298–15303. 10.1523/JNEUROSCI.0762-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianciulli A, Dragone T, Calvello R, Porro C, Trotta T, Lofrumento DD, Panaro MA (2015) IL-10 plays a pivotal role in anti-inflammatory effects of resveratrol in activated microglia cells. Int Immunopharmacol 24:369–376. 10.1016/j.intimp.2014.12.035 [DOI] [PubMed] [Google Scholar]
- Claassen DO, Josephs KA, Peller PJ (2010) The stripe of primary lateral sclerosis: focal primary motor cortex hypometabolism seen on fluorodeoxyglucose F18 positron emission tomography. Arch Neurol 67:122–125. 10.1001/archneurol.2009.298 [DOI] [PubMed] [Google Scholar]
- Conley SM, Abais-Battad JM, Yuan X, Zhang Q, Boini KM, Li PL (2017) Contribution of guanine nucleotide exchange factor Vav2 to NLRP3 inflammasome activation in mouse podocytes during hyperhomocysteinemia. Free Radic Biol Med 106:236–244. 10.1016/j.freeradbiomed.2017.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotti A, Benner C, Kerman BE, Gosselin D, Lagier-Tourenne C, Zuccato C, Cattaneo E, Gage FH, Cleveland DW, Glass CK (2014) Mutant Huntingtin promotes autonomous microglia activation via myeloid lineage-determining factors. Nat Neurosci 17:513–521. 10.1038/nn.3668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanney NA, Stewart AN, Gensel JC (2020) Microglia and macrophage metabolism in CNS injury and disease: the role of immunometabolism in neurodegeneration and neurotrauma. Exp Neurol 329:113310. 10.1016/j.expneurol.2020.113310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-García CM, Yellen G (2019) Neurons rely on glucose rather than astrocytic lactate during stimulation. J Neurosci Res 97:883–889. 10.1002/jnr.24374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-García CM, Mongeon R, Lahmann C, Koveal D, Zucker H, Yellen G (2017) Neuronal stimulation triggers neuronal glycolysis and not lactate uptake. Cell Metab 26:361–374.e364. 10.1016/j.cmet.2017.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding F, Yao J, Rettberg JR, Chen S, Brinton RD (2013) Early decline in glucose transport and metabolism precedes shift to ketogenic system in female aging and Alzheimer's mouse brain: implication for bioenergetic intervention. PLoS One 8:e79977. 10.1371/journal.pone.0079977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domercq M, Sánchez-Gómez MV, Sherwin C, Etxebarria E, Fern R, Matute C (2007) System xc- and glutamate transporter inhibition mediates microglial toxicity to oligodendrocytes. J Immunol 178:6549–6556. 10.4049/jimmunol.178.10.6549 [DOI] [PubMed] [Google Scholar]
- Domercq M, Vazquez-Villoldo N, Matute C (2013) Neurotransmitter signaling in the pathophysiology of microglia. Front Cell Neurosci 7:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Xu M, Kalueff AV, Song C (2018) Dietary eicosapentaenoic acid normalizes hippocampal omega-3 and 6 polyunsaturated fatty acid profile, attenuates glial activation and regulates BDNF function in a rodent model of neuroinflammation induced by central interleukin-1beta administration. Eur J Nutr 57:1781–1791. 10.1007/s00394-017-1462-7 [DOI] [PubMed] [Google Scholar]
- Doshi A, Chataway J (2017) Multiple sclerosis, a treatable disease. Clin Med (Lond) 17:530–536. 10.7861/clinmedicine.17-6-530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugger BN, Dickson DW (2017) Pathology of neurodegenerative diseases. Cold Spring Harb Perspect Biol 9:a028035. 10.1101/cshperspect.a028035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn L, Allen GF, Mamais A, Ling H, Li A, Duberley KE, Hargreaves IP, Pope S, Holton JL, Lees A, Heales SJ, Bandopadhyay R (2014) Dysregulation of glucose metabolism is an early event in sporadic Parkinson's disease. Neurobiol Aging 35:1111–1115. 10.1016/j.neurobiolaging.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duraes F, Pinto M, Sousa E (2018) Old drugs as new treatments for neurodegenerative diseases. Pharmaceuticals (Basel) 11:44. 10.3390/ph11020044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran RV, Hall MN (2012) Glutaminolysis feeds mTORC1. Cell Cycle 11:4107–4108. 10.4161/cc.22632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran RV, Oppliger W, Robitaille AM, Heiserich L, Skendaj R, Gottlieb E, Hall MN (2012) Glutaminolysis activates Rag-mTORC1 signaling. Mol Cell 47:349–358. 10.1016/j.molcel.2012.05.043 [DOI] [PubMed] [Google Scholar]
- Ebert D, Haller RG, Walton ME (2003) Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy. J Neurosci 23:5928–5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khoury JB, Moore KJ, Means TK, Leung J, Terada K, Toft M, Freeman MW, Luster AD (2003) CD36 mediates the innate host response to beta-amyloid. J Exp Med 197:1657–1666. 10.1084/jem.20021546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito Z, Belli L, Toniolo S, Sancesario G, Bianconi C, Martorana A (2013) Amyloid beta, glutamate, excitotoxicity in Alzheimer's disease: are we on the right track? CNS Neurosci Ther 19:549–555. 10.1111/cns.12095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ (1991) Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain 114: 2283–2301. 10.1093/brain/114.5.2283 [DOI] [PubMed] [Google Scholar]
- Ferraiuolo L, Higginbottom A, Heath PR, Barber S, Greenald D, Kirby J, Shaw PJ (2011) Dysregulation of astrocyte-motoneuron cross-talk in mutant superoxide dismutase 1-related amyotrophic lateral sclerosis. Brain 134:2627–2641. 10.1093/brain/awr193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris HA, Perry RJ, Moreira GV, Shulman GI, Horton JD, Kahn CR (2017) Loss of astrocyte cholesterol synthesis disrupts neuronal function and alters whole-body metabolism. Proc Natl Acad Sci USA 114:1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finucane OM, Sugrue J, Rubio-Araiz A, Guillot-Sestier MV, Lynch MA (2019) The NLRP3 inflammasome modulates glycolysis by increasing PFKFB3 in an IL-1β-dependent manner in macrophages. Sci Rep 9:4034. 10.1038/s41598-019-40619-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firbank MJ, Yarnall AJ, Lawson RA, Duncan GW, Khoo TK, Petrides GS, O'Brien JT, Barker RA, Maxwell RJ, Brooks DJ, Burn DJ (2017) Cerebral glucose metabolism and cognition in newly diagnosed Parkinson's disease: ICICLE-PD study. J Neurol Neurosurg Psychiatry 88:310–316. 10.1136/jnnp-2016-313918 [DOI] [PubMed] [Google Scholar]
- Fleischmann R, Kremer J, Cush J, Schulze-Koops H, Connell CA, Bradley JD, Gruben D, Wallenstein GV, Zwillich SH, Kanik KS (2012) Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med 367:495–507. 10.1056/NEJMoa1109071 [DOI] [PubMed] [Google Scholar]
- Forstermann U, Sessa WC (2012) Nitric oxide synthases: regulation and function. Eur Heart J 33:829–837. 10.1093/eurheartj/ehr304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frakes AE, Ferraiuolo L, Haidet-Phillips AM, Schmelzer L, Braun L, Miranda CJ, Ladner KJ, Bevan AK, Foust KD, Godbout JP, Popovich PG, Guttridge DC, Kaspar BK (2014) Microglia induce motor neuron death via the classical NF-κB pathway in amyotrophic lateral sclerosis. Neuron 81:1009–1023. 10.1016/j.neuron.2014.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund Levi Y, Vedin I, Cederholm T, Basun H, Irving G, Eriksdotter M, Hjorth E, Schultzberg M, Vessby B, Wahlund LO, Salem N Jr, Palmblad J (2014) Transfer of omega-3 fatty acids across the blood-brain barrier after dietary supplementation with a docosahexaenoic acid-rich omega-3 fatty acid preparation in patients with Alzheimer's disease: the OmegAD study. J Intern Med 275:428–436. 10.1111/joim.12166 [DOI] [PubMed] [Google Scholar]
- Fünfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Möbius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave KA (2012) Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485:517–521. 10.1038/nature11007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan L, Cookson MR, Petrucelli L, Spada AR (2018) Converging pathways in neurodegeneration, from genetics to mechanisms. Nat Neurosci 21:1300–1309. 10.1038/s41593-018-0237-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D, Ashraf MZ, Kar NS, Lin D, Sayre LM, Podrez EA (2010) Structural basis for the recognition of oxidized phospholipids in oxidized low density lipoproteins by class B scavenger receptors CD36 and SR-BI. J Biol Chem 285:4447–4454. 10.1074/jbc.M109.082800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Li C, Zhu J, Wang Y, Huang Y, Zhao S, Sheng S, Song Y, Li C, Yang X, Ye L, Qi X, Zhang Y, Xia X, Zheng JC (2020) Glutaminase 1 regulates neuroinflammation after cerebral ischemia through enhancing microglial activation and pro-inflammatory exosome release. Front Immunol 11:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Cáceres C, Fuente-Martín E, Argente J, Chowen JA (2012) Emerging role of glial cells in the control of body weight. Mol Metab 1:37–46. 10.1016/j.molmet.2012.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno-Bayón J, López-López A, Rodríguez MJ, Mahy N (2014) Glucose pathways adaptation supports acquisition of activated microglia phenotype. J Neurosci Res 92:723–731. 10.1002/jnr.23356 [DOI] [PubMed] [Google Scholar]
- Giorgini F, Guidetti P, Nguyen Q, Bennett SC, Muchowski PJ (2005) A genomic screen in yeast implicates kynurenine 3-monooxygenase as a therapeutic target for Huntington disease. Nat Genet 37:526–531. 10.1038/ng1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgini F, Moller T, Kwan W, Zwilling D, Wacker JL, Hong S, Tsai LC, Cheah CS, Schwarcz R, Guidetti P, Muchowski PJ (2008) Histone deacetylase inhibition modulates kynurenine pathway activation in yeast, microglia, and mice expressing a mutant huntingtin fragment. J Biol Chem 283:7390–7400. 10.1074/jbc.M708192200 [DOI] [PubMed] [Google Scholar]
- Gitler AD, Dhillon P, Shorter J (2017) Neurodegenerative disease: models, mechanisms, and a new hope. Dis Model Mech 10:499–502. 10.1242/dmm.030205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Redondo R, García-García D, Clavero P, Gasca-Salas C, García-Eulate R, Zubieta JL, Arbizu J, Obeso JA, Rodríguez-Oroz MC (2014) Grey matter hypometabolism and atrophy in Parkinson's disease with cognitive impairment: a two-step process. Brain 137:2356–2367. 10.1093/brain/awu159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gostner JM, Geisler S, Stonig M, Mair L, Sperner-Unterweger B, Fuchs D (2020) Tryptophan metabolism and related pathways in psychoneuroimmunology: the impact of nutrition and lifestyle. Neuropsychobiology 79:89–99. 10.1159/000496293 [DOI] [PubMed] [Google Scholar]
- Gould RL, Pazdro R (2019) Impact of supplementary amino acids, micronutrients, and overall diet on glutathione homeostasis. Nutrients 11:1056. 10.3390/nu11051056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm AC, Boger H, Emborg ME (2008) Mood, memory and movement: an age-related neurodegenerative complex? Curr Aging Sci 1:133–139. 10.2174/1874609810801020133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmetti C, Najac C, Didonna A, Van der Linden A, Ronen SM, Chaumeil MM (2017) Hyperpolarized (13)C MR metabolic imaging can detect neuroinflammation in vivo in a multiple sclerosis murine model. Proc Natl Acad Sci USA 114:E6982–E6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidetti P, Luthi-Carter RE, Augood SJ, Schwarcz R (2004) Neostriatal and cortical quinolinate levels are increased in early grade Huntington's disease. Neurobiol Dis 17:455–461. 10.1016/j.nbd.2004.07.006 [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Smythe G, Takikawa O, Brew BJ (2005) Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia 49:15–23. 10.1002/glia.20090 [DOI] [PubMed] [Google Scholar]
- Guillemot-Legris O, Muccioli GG (2017) Obesity-induced neuroinflammation: beyond the hypothalamus. Trends Neurosci 40:237–253. 10.1016/j.tins.2017.02.005 [DOI] [PubMed] [Google Scholar]
- Guillemot-Legris O, Masquelier J, Everard A, Cani PD, Alhouayek M, Muccioli GG (2016) High-fat diet feeding differentially affects the development of inflammation in the central nervous system. J Neuroinflammation 13:206. 10.1186/s12974-016-0666-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, LaDu MJ, Van Eldik LJ (2004) A dual role for apolipoprotein E in neuroinflammation. J Mol Neurosci 23:205–212. 10.1385/JMN:23:3:205 [DOI] [PubMed] [Google Scholar]
- Gurzov EN, Stanley WJ, Pappas EG, Thomas HE, Gough DJ (2016) The JAK/STAT pathway in obesity and diabetes. FEBS J 283:3002–3015. 10.1111/febs.13709 [DOI] [PubMed] [Google Scholar]
- Haidet-Phillips AM, Hester ME, Miranda CJ, Meyer K, Braun L, Frakes A, Song S, Likhite S, Murtha MJ, Foust KD, Rao M, Eagle A, Kammesheidt A, Christensen A, Mendell JR, Burghes AH, Kaspar BK (2011) Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat Biotechnol 29:824–828. 10.1038/nbt.1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder N, Lal G (2021) Cholinergic system and its therapeutic importance in inflammation and autoimmunity. Front Immunol 12:660342. 10.3389/fimmu.2021.660342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JJ, Jolivet R, Attwell D (2012) Synaptic energy use and supply. Neuron 75:762–777. 10.1016/j.neuron.2012.08.019 [DOI] [PubMed] [Google Scholar]
- Hasin Y, Seldin M, Lusis A (2017) Multi-omics approaches to disease. Genome Biol 18:83. 10.1186/s13059-017-1215-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero-Mendez A, Almeida A, Fernández E, Maestre C, Moncada S, Bolaños JP (2009) The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C–Cdh1. Nat Cell Biol 11:747–752. 10.1038/ncb1881 [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Breidert T, Rousselet E, Hunot S, Hartmann A, Michel PP (2003) The role of glial reaction and inflammation in Parkinson's disease. Ann NY Acad Sci 991:214–228. 10.1111/j.1749-6632.2003.tb07478.x [DOI] [PubMed] [Google Scholar]
- Holland R, McIntosh AL, Finucane OM, Mela V, Rubio-Araiz A, Timmons G, McCarthy SA, Gun'ko YK, Lynch MA (2018) Inflammatory microglia are glycolytic and iron retentive and typify the microglia in APP/PS1 mice. Brain Behav Immun 68:183–196. 10.1016/j.bbi.2017.10.017 [DOI] [PubMed] [Google Scholar]
- Hsiao HY, Mak OT, Yang CS, Liu YP, Fang KM, Tzeng SF (2007) TNF-alpha/IFN-gamma-induced iNOS expression increased by prostaglandin E2 in rat primary astrocytes via EP2-evoked cAMP/PKA and intracellular calcium signaling. Glia 55:214–223. 10.1002/glia.20453 [DOI] [PubMed] [Google Scholar]
- Hsieh CL, Koike M, Spusta SC, Niemi EC, Yenari M, Nakamura MC, Seaman WE (2009) A role for TREM2 ligands in the phagocytosis of apoptotic neuronal cells by microglia. J Neurochem 109:1144–1156. 10.1111/j.1471-4159.2009.06042.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HT, Tsai SF, Wu HT, Huang HY, Hsieh HH, Kuo YM, Chen PS, Yang CS, Tzeng SF (2019) Chronic exposure to high fat diet triggers myelin disruption and interleukin-33 upregulation in hypothalamus. BMC Neurosci 20:33. 10.1186/s12868-019-0516-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Tsai SJ, Su TW, Sim CB (1999) Effects of repeated high-dose methamphetamine on local cerebral glucose utilization in rats. Neuropsychopharmacology 21:427–434. 10.1016/S0893-133X(99)00029-9 [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR (1976) Ketonemia and seizures: metabolic and anticonvulsant effects of two ketogenic diets in childhood epilepsy. Pediatr Res 10:536–540. 10.1203/00006450-197605000-00006 [DOI] [PubMed] [Google Scholar]
- Hwang J, Lee S, Lee JT, Kwon TK, Kim DR, Kim H, Park HC, Suk K (2010) Gangliosides induce autophagic cell death in astrocytes. Br J Pharmacol 159:586–603. 10.1111/j.1476-5381.2009.00563.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infantino V, Iacobazzi V, Palmieri F, Menga A (2013) ATP-citrate lyase is essential for macrophage inflammatory response. Biochem Biophys Res Commun 440:105–111. 10.1016/j.bbrc.2013.09.037 [DOI] [PubMed] [Google Scholar]
- Inoue T, Tanaka M, Masuda S, Ohue-Kitano R, Yamakage H, Muranaka K, Wada H, Kusakabe T, Shimatsu A, Hasegawa K, Satoh-Asahara N (2017) Omega-3 polyunsaturated fatty acids suppress the inflammatory responses of lipopolysaccharide-stimulated mouse microglia by activating SIRT1 pathways. Biochim Biophys Acta Mol Cell Biol Lipids 1862:552–560. 10.1016/j.bbalip.2017.02.010 [DOI] [PubMed] [Google Scholar]
- Ioannou MS, Jackson J, Sheu SH, Chang CL, Weigel AV, Liu H, Pasolli HA, Xu CS, Pang S, Hess HF, Lippincott-Schwartz J, Liu Z (2018) Neuron-astrocyte metabolic coupling during neuronal stimulation protects against fatty acid toxicity. bioRxiv 465237. [DOI] [PubMed] [Google Scholar]
- Ivatt RM, Sanchez-Martinez A, Godena VK, Brown S, Ziviani E, Whitworth AJ (2014) Genome-wide RNAi screen identifies the Parkinson disease GWAS risk locus SREBF1 as a regulator of mitophagy. Proc Natl Acad Sci USA 111:8494–8499. 10.1073/pnas.1321207111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakimovski D, Kolb C, Ramanathan M, Zivadinov R, Weinstock-Guttman B (2018) Interferon β for multiple sclerosis. Cold Spring Harb Perspect Med 8:a032003. 10.1101/cshperspect.a032003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen IE, Savage JE, Watanabe K, Bryois J, Williams DM, Steinberg S, Sealock J, Karlsson IK, Hägg S, Athanasiu L, Voyle N, Proitsi P, Witoelar A, Stringer S, Aarsland D, Almdahl IS, Andersen F, Bergh S, Bettella F, Bjornsson S, et al. (2019) Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer's disease risk. Nat Genet 51:404–413. 10.1038/s41588-018-0311-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarc E, Petan T (2019) Lipid droplets and the management of cellular stress. Yale J Biol Med 92:435–452. [PMC free article] [PubMed] [Google Scholar]
- Jha AK, Huang SC, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, Chmielewski K, Stewart KM, Ashall J, Everts B, Pearce EJ, Driggers EM, Artyomov MN (2015) Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 42:419–430. 10.1016/j.immuni.2015.02.005 [DOI] [PubMed] [Google Scholar]
- Jha MK, Morrison BM (2018) Glia-neuron energy metabolism in health and diseases: new insights into the role of nervous system metabolic transporters. Exp Neurol 309:23–31. 10.1016/j.expneurol.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Cadenas E (2014) Astrocytic metabolic and inflammatory changes as a function of age. Aging Cell 13:1059–1067. 10.1111/acel.12268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Ferrer I, Swanberg M (2018) Immunogenetics of Parkinson's disease. In: Parkinson's disease: pathogenesis and clinical aspects (Stoker TB, Greenland JC, eds). Brisbane (AU): Codon Publications. [Google Scholar]
- Jin S, Kim KK, Park BS, Kim DH, Jeong B, Kang D, Lee TH, Park JW, Kim JG, Lee BJ (2020) Function of astrocyte MyD88 in high-fat-diet-induced hypothalamic inflammation. J Neuroinflammation 17:195. 10.1186/s12974-020-01846-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe EH, Choi DJ, An J, Eun JH, Jou I, Park S (2018) Astrocytes, microglia, and Parkinson's disease. Exp Neurobiol 27:77–87. 10.5607/en.2018.27.2.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CH, Ivanisevic J, Siuzdak G (2016) Metabolomics: beyond biomarkers and towards mechanisms. Nat Rev Mol Cell Biol 17:451–459. 10.1038/nrm.2016.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, Rujescu D, Hampel H, Giegling I, Andreassen OA, Engedal K, Ulstein I, Djurovic S, Ibrahim-Verbaas C, Hofman A, Ikram MA, et al. (2013) Variant of TREM2 associated with the risk of Alzheimer's disease. N Engl J Med 368:107–116. 10.1056/NEJMoa1211103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurcovicova J (2014) Glucose transport in brain: effect of inflammation. Endocr Regul 48:35–48. 10.4149/endo_2014_01_35 [DOI] [PubMed] [Google Scholar]
- Kalender A, Selvaraj A, Kim SY, Gulati P, Brule S, Viollet B, Kemp BE, Bardeesy N, Dennis P, Schlager JJ, Marette A, Kozma SC, Thomas G (2010) Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab 11:390–401. 10.1016/j.cmet.2010.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia LV, Lang AE (2015) Parkinson's disease. Lancet 386:896–912. 10.1016/S0140-6736(14)61393-3 [DOI] [PubMed] [Google Scholar]
- Kang EB, Koo JH, Jang YC, Yang CH, Lee Y, Cosio-Lima LM, Cho JY (2016) Neuroprotective effects of endurance exercise against high-fat diet-induced hippocampal neuroinflammation. J Neuroendocrinol 28:5. 10.1111/jne.12385 [DOI] [PubMed] [Google Scholar]
- Kang SH, Li Y, Fukaya M, Lorenzini I, Cleveland DW, Ostrow LW, Rothstein JD, Bergles DE (2013) Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat Neurosci 16:571–579. 10.1038/nn.3357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaup AR, Drummond SP, Eyler LT (2014) Brain functional correlates of working memory: reduced load-modulated activation and deactivation in aging without hyperactivation or functional reorganization. J Int Neuropsychol Soc 20:945–950. 10.1017/S1355617714000824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik DK, Yong VW (2020) Metabolic needs of brain-infiltrating leukocytes and microglia in multiple sclerosis. J Neurochem 158:14–24. [DOI] [PubMed] [Google Scholar]
- Kaushik DK, Bhattacharya A, Mirzaei R, Rawji KS, Ahn Y, Rho JM, Yong VW (2019) Enhanced glycolytic metabolism supports transmigration of brain-infiltrating macrophages in multiple sclerosis. J Clin Invest 129:3277–3292. 10.1172/JCI124012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM (2018) Exercise-induced modulation of neuroinflammation in models of Alzheimer's disease. Brain Plast 4:81–94. 10.3233/BPL-180074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Beaumont V, Cachope R, Munoz-Sanjuan I, Goldman SA, Grantyn R (2017) Unravelling and exploiting astrocyte dysfunction in Huntington's disease. Trends Neurosci 40:422–437. 10.1016/j.tins.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshnan A, Ko J, Watkin EE, Paige LA, Reinhart PH, Patterson PH (2004) Activation of the IkappaB kinase complex and nuclear factor-kappaB contributes to mutant huntingtin neurotoxicity. J Neurosci 24:7999–8008. 10.1523/JNEUROSCI.2675-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Tolhurst AT, Qin LY, Chen XY, Febbraio M, Cho S (2008) CD36/fatty acid translocase, an inflammatory mediator, is involved in hyperlipidemia-induced exacerbation in ischemic brain injury. J Neurosci 28:4661–4670. 10.1523/JNEUROSCI.0982-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk SE, Tracey TJ, Steyn FJ, Ngo ST (2019) Biomarkers of metabolism in amyotrophic lateral sclerosis. Front Neurol 10:191–191. 10.3389/fneur.2019.00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita T, Morrison PF, Heyes MP, Markey SP (2002) Effects of systemic and central nervous system localized inflammation on the contributions of metabolic precursors to the L-kynurenine and quinolinic acid pools in brain. J Neurochem 82:258–268. 10.1046/j.1471-4159.2002.00955.x [DOI] [PubMed] [Google Scholar]
- Koepsell H (2020) Glucose transporters in brain in health and disease. Pflugers Arch 472:1299–1343. 10.1007/s00424-020-02441-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh S, Dupuis N, Auvin S (2020) Ketogenic diet and neuroinflammation. Epilepsy Res 167:106454. 10.1016/j.eplepsyres.2020.106454 [DOI] [PubMed] [Google Scholar]
- Kremer JM, Cohen S, Wilkinson BE, Connell CA, French JL, Gomez-Reino J, Gruben D, Kanik KS, Krishnaswami S, Pascual-Ramos V, Wallenstein G, Zwillich SH (2012) A phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum 64:970–981. 10.1002/art.33419 [DOI] [PubMed] [Google Scholar]
- Kusindarta DL, Wihadmadyatami H, Haryanto A (2018) The analysis of hippocampus neuronal density (CA1 and CA3) after Ocimum sanctum ethanolic extract treatment on the young adulthood and middle age rat model. Vet World 11:135–140. 10.14202/vetworld.2018.135-140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwidzinski E, Bechmann I (2007) IDO expression in the brain: a double-edged sword. J Mol Med (Berl) 85:1351–1359. 10.1007/s00109-007-0229-7 [DOI] [PubMed] [Google Scholar]
- La Rocca C, Carbone F, Rosa V, Colamatteo A, Galgani M, Perna F, Lanzillo R, Morra V, Orefice G, Cerillo I, Florio C, Maniscalco GT, Salvetti M, Centonze D, Uccelli A, Longobardi S, Visconti A, Matarese G (2017) Immunometabolic profiling of T cells from patients with relapsing-remitting multiple sclerosis reveals an impairment in glycolysis and mitochondrial respiration. Metabolism 77:39–46. 10.1016/j.metabol.2017.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacagnina MJ, Rivera PD, Bilbo SD (2017) Glial and neuroimmune mechanisms as critical modulators of drug use and abuse. Neuropsychopharmacology 42:156–177. 10.1038/npp.2016.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai ZW, Kelly R, Winans T, Marchena I, Shadakshari A, Yu J, Dawood M, Garcia R, Tily H, Francis L, Faraone SV, Phillips PE, Perl A (2018) Sirolimus in patients with clinically active systemic lupus erythematosus resistant to, or intolerant of, conventional medications: a single-arm, open-label, phase 1/2 trial. Lancet 391:1186–1196. 10.1016/S0140-6736(18)30485-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larabee CM, Neely OC, Domingos AI (2020) Obesity: a neuroimmunometabolic perspective. Nat Rev Endocrinol 16:30–43. 10.1038/s41574-019-0283-6 [DOI] [PubMed] [Google Scholar]
- Lasiene J, Yamanaka K (2011) Glial cells in amyotrophic lateral sclerosis. Neurol Res Int 2011:1–7. 10.1155/2011/718987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauro C, Limatola C (2020) Metabolic reprogramming of microglia in the regulation of the innate inflammatory response. Front Immunol 11:493. 10.3389/fimmu.2020.00493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Leach MK, Redmond SA, Chong SY, Mellon SH, Tuck SJ, Feng ZQ, Corey JM, Chan JR (2012) A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat Methods 9:917–922. 10.1038/nmeth.2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Jin HK, Bae JS (2020) Sphingolipids in neuroinflammation: a potential target for diagnosis and therapy. BMB Rep 53:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoni V, Caccia C (2015) The impairment of cholesterol metabolism in Huntington disease. Biochim Biophys Acta 1851:1095–1105. 10.1016/j.bbalip.2014.12.018 [DOI] [PubMed] [Google Scholar]
- Levenson RW, Sturm VE, Haase CM (2014) Emotional and behavioral symptoms in neurodegenerative disease: a model for studying the neural bases of psychopathology. Annu Rev Clin Psychol 10:581–606. 10.1146/annurev-clinpsy-032813-153653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyns CE, Ulrich JD, Finn MB, Stewart FR, Koscal LJ, Remolina Serrano J, Robinson GO, Anderson E, Colonna M, Holtzman DM (2017) TREM2 deficiency attenuates neuroinflammation and protects against neurodegeneration in a mouse model of tauopathy. Proc Natl Acad Sci USA 114:11524–11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lu B, Sheng L, Zhu Z, Sun H, Zhou Y, Yang Y, Xue D, Chen W, Tian X, Du Y, Yan M, Zhu W, Xing F, Li K, Lin S, Qiu P, Su X, Huang Y, Yan G, et al. (2018) Hexokinase 2-dependent hyperglycolysis driving microglial activation contributes to ischemic brain injury. J Neurochem 144:186–200. 10.1111/jnc.14267 [DOI] [PubMed] [Google Scholar]
- Li Y, Deng SL, Lian ZX, Yu K (2019) Roles of toll-like receptors in nitroxidative stress in mammals. Cells 8:576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lievens JC, Woodman B, Mahal A, Spasic-Boscovic O, Samuel D, Kerkerian-Le Goff L, Bates GP (2001) Impaired glutamate uptake in the R6 Huntington's disease transgenic mice. Neurobiol Dis 8:807–821. 10.1006/nbdi.2001.0430 [DOI] [PubMed] [Google Scholar]
- Ligam PDN, Manuelpillai U, Wallace E, Walker DW (2010) NFκB-dependent increase of kynurenine pathway activity in human placenta: inhibition by sulfasalazine. Placenta 31:997–1002. 10.1016/j.placenta.2010.09.002 [DOI] [PubMed] [Google Scholar]
- Lim MA, Selak MA, Xiang Z, Krainc D, Neve RL, Kraemer BC, Watts JL, Kalb RG (2012) Reduced activity of AMP-activated protein kinase protects against genetic models of motor neuron disease. J Neurosci 32:1123–1141. 10.1523/JNEUROSCI.6554-10.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AL, Rothman DL (2014) What have novel imaging techniques revealed about metabolism in the aging brain? Future Neurol 9:341–354. 10.2217/fnl.14.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Shen Y, Tang Y, Gu Y (2018) The role of N-glycosylation of CD200-CD200R1 interaction in classical microglial activation. J Inflamm (Lond) 15:28. 10.1186/s12950-018-0205-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Seino S, Kirchgessner AL (1999) Identification and characterization of glucoresponsive neurons in the enteric nervous system. J Neurosci 19:10305–10317. 10.1523/JNEUROSCI.19-23-10305.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Cao Y, Zhang W, Bergmeier S, Qian Y, Akbar H, Colvin R, Ding J, Tong L, Wu S, Hines J, Chen X (2012) A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell-cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol Cancer Ther 11:1672–1682. 10.1158/1535-7163.MCT-12-0131 [DOI] [PubMed] [Google Scholar]
- Liu YJ, Ju TC, Chen HM, Jang YS, Lee LM, Lai HL, Tai HC, Fang JM, Lin YL, Tu PH, Chern Y (2015) Activation of AMP-activated protein kinase α1 mediates mislocalization of TDP-43 in amyotrophic lateral sclerosis. Hum Mol Genet 24:787–801. 10.1093/hmg/ddu497 [DOI] [PubMed] [Google Scholar]
- Loving BA, Bruce KD (2020) Lipid and lipoprotein metabolism in microglia. Front Physiol 11:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe R, Shirley N, Bleackley M, Dolan S, Shafee T (2017) Transcriptomics technologies. PLoS Comput Biol 13:e1005457. 10.1371/journal.pcbi.1005457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas SM, Rothwell NJ, Gibson RM (2006) The role of inflammation in CNS injury and disease. Br J Pharmacol 147: S232–S240. 10.1038/sj.bjp.0706400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludolph AC, Langen KJ, Regard M, Herzog H, Kemper B, Kuwert T, Böttger IG, Feinendegen L (1992) Frontal lobe function in amyotrophic lateral sclerosis: a neuropsychologic and positron emission tomography study. Acta Neurol Scand 85:81–89. 10.1111/j.1600-0404.1992.tb04003.x [DOI] [PubMed] [Google Scholar]
- Lumsden AL, Mulugeta A, Zhou A, Hyppönen E (2020) Apolipoprotein E (APOE) genotype-associated disease risks: a phenome-wide, registry-based, case-control study utilising the UK Biobank. EBioMedicine 59:102954. 10.1016/j.ebiom.2020.102954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist AJ, Llewellyn GN, Kishi SH, Jakowec NA, Cannon PM, Petzinger GM, Jakowec MW (2021) Knockdown of astrocytic monocarboxylate transporter 4 (MCT4) in the motor cortex leads to loss of dendritic spines and a deficit in motor learning. bioRxiv 2021.2007.2001.450797. [DOI] [PubMed] [Google Scholar]
- Maher JC, Krishan A, Lampidis TJ (2004) Greater cell cycle inhibition and cytotoxicity induced by 2-deoxy-D-glucose in tumor cells treated under hypoxic vs aerobic conditions. Cancer Chemother Pharmacol 53:116–122. 10.1007/s00280-003-0724-7 [DOI] [PubMed] [Google Scholar]
- Mahmoud S, Gharagozloo M, Simard C, Gris D (2019) Astrocytes maintain glutamate homeostasis in the CNS by controlling the balance between glutamate uptake and release. Cells 8:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Małczyńska-Sims P, Chalimoniuk M, Sułek A (2020) The effect of endurance training on brain-derived neurotrophic factor and inflammatory markers in healthy people and Parkinson's disease: a narrative review. Front Physiol 11:578981. 10.3389/fphys.2020.578981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannix RC, Zhang J, Park J, Zhang X, Bilal K, Walker K, Tanzi RE, Tesco G, Whalen MJ (2011) Age-dependent effect of apolipoprotein E4 on functional outcome after controlled cortical impact in mice. J Cereb Blood Flow Metab 31:351–361. 10.1038/jcbfm.2010.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marpegan L, Swanstrom AE, Chung K, Simon T, Haydon PG, Khan SK, Liu AC, Herzog ED, Beaule C (2011) Circadian regulation of ATP release in astrocytes. J Neurosci 31:8342–8350. 10.1523/JNEUROSCI.6537-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maulik M, Mitra S, Bult-Ito A, Taylor BE, Vayndorf EM (2017) Behavioral phenotyping and pathological indicators of Parkinson's disease in C. elegans models. Front Genet 8:77. 10.3389/fgene.2017.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maulik M, Mitra S, Sweeney M, Lu B, Taylor BE, Bult-Ito A (2019) Complex interaction of dietary fat and Alaskan bog blueberry supplementation influences manganese mediated neurotoxicity and behavioral impairments. J Funct Foods 53:306–317. 10.1016/j.jff.2018.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell N, Castro RW, Sutherland NM, Vaughan KL, Szarowicz MD, de Cabo R, Mattison JA, Valdez G (2018) α-Motor neurons are spared from aging while their synaptic inputs degenerate in monkeys and mice. Aging Cell 17:e12726. 10.1111/acel.12726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG (1988) Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology 38:1285–1291. 10.1212/wnl.38.8.1285 [DOI] [PubMed] [Google Scholar]
- McIntosh A, Mela V, Harty C, Minogue AM, Costello DA, Kerskens C, Lynch MA (2019) Iron accumulation in microglia triggers a cascade of events that leads to altered metabolism and compromised function in APP/PS1 mice. Brain Pathol 29:606–621. 10.1111/bpa.12704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead EL, Mosley A, Eaton S, Dobson L, Heales SJ, Pocock JM (2012) Microglial neurotransmitter receptors trigger superoxide production in microglia: consequences for microglial-neuronal interactions. J Neurochem 121:287–301. 10.1111/j.1471-4159.2012.07659.x [DOI] [PubMed] [Google Scholar]
- Mengel D, Thelen M, Balzer-Geldsetzer M, Söling C, Bach JP, Schaeffer E, Herold C, Becker T, Liepelt I, Becker J, Riedel-Heller S, Scherer M, Jessen F, Maier W, Dodel R, Ramirez A (2016) TREM2 rare variant p.R47H is not associated with Parkinson's disease. Parkinsonism Relat Disord 23:109–111. 10.1016/j.parkreldis.2015.11.026 [DOI] [PubMed] [Google Scholar]
- Mergenthaler P, Lindauer U, Dienel GA, Meisel A (2013) Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci 36:587–597. 10.1016/j.tins.2013.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlini M, Meyer EP, Ulmann-Schuler A, Nitsch RM (2011) Vascular β-amyloid and early astrocyte alterations impair cerebrovascular function and cerebral metabolism in transgenic arcAβ mice. Acta Neuropathol 122:293–311. 10.1007/s00401-011-0834-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita Dá S, Ferreira AC, Sousa JC, Correia-Neves M, Sousa N, Marques F (2016) Insights on the pathophysiology of Alzheimer's disease: the crosstalk between amyloid pathology, neuroinflammation and the peripheral immune system. Neurosci Biobehav Rev 68:547–562. 10.1016/j.neubiorev.2016.06.014 [DOI] [PubMed] [Google Scholar]
- Metzger S, Saukko M, Van Che H, Tong L, Puder Y, Riess O, Nguyen HP (2010) Age at onset in Huntington's disease is modified by the autophagy pathway: implication of the V471A polymorphism in Atg7. Hum Genet 128:453–459. 10.1007/s00439-010-0873-9 [DOI] [PubMed] [Google Scholar]
- Meyer N, Richter N, Fan Z, Siemonsmeier G, Pivneva T, Jordan P, Steinhäuser C, Semtner M, Nolte C, Kettenmann H (2018) Oligodendrocytes in the mouse corpus callosum maintain axonal function by delivery of glucose. Cell Rep 22:2383–2394. 10.1016/j.celrep.2018.02.022 [DOI] [PubMed] [Google Scholar]
- Milanova IV, Kalsbeek MJ, Wang XL, Korpel NL, Stenvers DJ, Wolff SE, de Goede P, Heijboer AC, Fliers E, la Fleur SE, Kalsbeek A, Yi CX (2019) Diet-induced obesity disturbs microglial immunometabolism in a time-of-day manner. Front Endocrinol (Lausanne) 10:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minhas PS, Latif-Hernandez A, McReynolds MR, Durairaj AS, Wang Q, Rubin A, Joshi AU, He JQ, Gauba E, Liu L, Wang C, Linde M, Sugiura Y, Moon PK, Majeti R, Suematsu M, Mochly-Rosen D, Weissman IL, Longo FM, Rabinowitz JD, et al. (2021) Restoring metabolism of myeloid cells reverses cognitive decline in ageing. Nature 590:122–128. 10.1038/s41586-020-03160-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink JW, Blumenschine RJ, Adams DB (1981) Ratio of central nervous system to body metabolism in vertebrates: its constancy and functional basis. Am J Physiol 241:R203–R212. 10.1152/ajpregu.1981.241.3.R203 [DOI] [PubMed] [Google Scholar]
- Mitra S, Khatri SN, Maulik M, Bult-Ito A, Schulte M (2020) Allosterism of nicotinic acetylcholine receptors: therapeutic potential for neuroinflammation underlying brain trauma and degenerative disorders. Int J Mol Sci 21:4918. 10.3390/ijms21144918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett JR, Arun P, Puthillathu N, Vengilote R, Ives JA, Badawy AA, Namboodiri AM (2020) Quinolinate as a marker for kynurenine metabolite formation and the unresolved question of NAD+ synthesis during inflammation and infection. Front Immunol 11:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor DE, Sohrabi S, Kaletsky R, Keyes W, Tartici A, Kalia V, Miller GW, Murphy CT (2020) Metformin rescues Parkinson's disease phenotypes caused by hyperactive mitochondria. Proc Natl Acad Sci USA 117:26438–26447. 10.1073/pnas.2009838117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira PI, Carvalho C, Zhu X, Smith MA, Perry G (2010) Mitochondrial dysfunction is a trigger of Alzheimer's disease pathophysiology. Biochim Biophys Acta 1802:2–10. 10.1016/j.bbadis.2009.10.006 [DOI] [PubMed] [Google Scholar]
- Morita M, Ikeshima-Kataoka H, Kreft M, Vardjan N, Zorec R, Noda M (2019) Metabolic plasticity of astrocytes and aging of the brain. Int J Mol Sci 20:941. 10.3390/ijms20040941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, De Santi S, Li J, Tsui WH, Li Y, Boppana M, Laska E, Rusinek H, de Leon MJ (2008) Hippocampal hypometabolism predicts cognitive decline from normal aging. Neurobiol Aging 29:676–692. 10.1016/j.neurobiolaging.2006.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L (2013) Glucose metabolism in normal aging and Alzheimer's disease: methodological and physiological considerations for PET studies. Clin Transl Imaging 1:217–233. 10.1007/s40336-013-0026-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss DW, Bates TE (2001) Activation of murine microglial cell lines by lipopolysaccharide and interferon-gamma causes NO-mediated decreases in mitochondrial and cellular function. Eur J Neurosci 13:529–538. 10.1046/j.1460-9568.2001.01418.x [DOI] [PubMed] [Google Scholar]
- Muddapu VR, Dharshini SA, Chakravarthy VS, Gromiha MM (2020) Neurodegenerative diseases: is metabolic deficiency the root cause? Front Neurosci 14:213. 10.3389/fnins.2020.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueckler M, Thorens B (2013) The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med 34:121–138. 10.1016/j.mam.2012.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy MN, Blauwendraat C, Guelfi S, Hardy J, Lewis PA, Trabzuni D, IPDGC (2017) Increased brain expression of GPNMB is associated with genome wide significant risk for Parkinson's disease on chromosome 7p15.3. Neurogenetics 18:121–133. 10.1007/s10048-017-0514-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namgaladze D, Lips S, Leiker TJ, Murphy RC, Ekroos K, Ferreiros N, Geisslinger G, Brüne B (2014) Inhibition of macrophage fatty acid β-oxidation exacerbates palmitate-induced inflammatory and endoplasmic reticulum stress responses. Diabetologia 57:1067–1077. 10.1007/s00125-014-3173-4 [DOI] [PubMed] [Google Scholar]
- NCT04098666 (2020) Safety and therapeutic potential of the FDA-approved drug metformin for C9orf72 ALS/FTD. https://clinicaltrials.gov/ct2/show/NCT04098666.
- NCT04220021 (2020) Safety and therapeutic potential of the FDA-approved drug metformin for C9orf72 ALS/FTD. https://ClinicalTrials.gov/show/NCT04220021.
- NCT04529876 (2020) Data analysis for drug repurposing for effective Alzheimer's medicines (DREAM): tofacitinib vs abatacept. https://ClinicalTrials.gov/show/NCT04529876.
- Navale AM, Paranjape AN (2016) Glucose transporters: physiological and pathological roles. Biophys Rev 8:5–9. 10.1007/s12551-015-0186-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng YW, Say YH (2018) Palmitic acid induces neurotoxicity and gliatoxicity in SH-SY5Y human neuroblastoma and T98G human glioblastoma cells. PeerJ 6:e4696. 10.7717/peerj.4696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TB, Louie SM, Daniele JR, Tran Q, Dillin A, Zoncu R, Nomura DK, Olzmann JA (2017) DGAT1-dependent lipid droplet biogenesis protects mitochondrial function during starvation-induced autophagy. Dev Cell 42:9–21.e25. 10.1016/j.devcel.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas C, Tauber C, Lepelletier FX, Chalon S, Belujon P, Galineau L, Solinas M (2017) Longitudinal changes in brain metabolic activity after withdrawal from escalation of cocaine self-administration. Neuropsychopharmacology 42:1981–1990. 10.1038/npp.2017.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieweg K, Schaller H, Pfrieger FW (2009) Marked differences in cholesterol synthesis between neurons and glial cells from postnatal rats. J Neurochem 109:125–134. 10.1111/j.1471-4159.2009.05917.x [DOI] [PubMed] [Google Scholar]
- Nugent AA, Lin K, van Lengerich B, Lianoglou S, Przybyla L, Davis SS, Llapashtica C, Wang J, Kim DJ, Xia D, Lucas A, Baskaran S, Haddick PC, Lenser M, Earr TK, Shi J, Dugas JC, Andreone BJ, Logan T, Solanoy HO, et al. (2020) TREM2 regulates microglial cholesterol metabolism upon chronic phagocytic challenge. Neuron 105:837–854.e839. 10.1016/j.neuron.2019.12.007 [DOI] [PubMed] [Google Scholar]
- Nyberg L, Andersson M, Lundquist A, Salami A, Wåhlin A (2019) Frontal contribution to hippocampal hyperactivity during memory encoding in aging. Front Mol Neurosci 12:229. 10.3389/fnmol.2019.00229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill LA, Kishton RJ, Rathmell J (2016) A guide to immunometabolism for immunologists. Nat Rev Immunol 16:553–565. 10.1038/nri.2016.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksman M, Iivonen H, Hogyes E, Amtul Z, Penke B, Leenders I, Broersen L, Lutjohann D, Hartmann T, Tanila H (2006) Impact of different saturated fatty acid, polyunsaturated fatty acid and cholesterol containing diets on beta-amyloid accumulation in APP/PS1 transgenic mice. Neurobiol Dis 23:563–572. 10.1016/j.nbd.2006.04.013 [DOI] [PubMed] [Google Scholar]
- Pagani M, Chiò A, Valentini MC, Öberg J, Nobili F, Calvo A, Moglia C, Bertuzzo D, Morbelli S, De Carli F, Fania P, Cistaro A (2014) Functional pattern of brain FDG-PET in amyotrophic lateral sclerosis. Neurology 83:1067–1074. 10.1212/WNL.0000000000000792 [DOI] [PubMed] [Google Scholar]
- Pajak B, Siwiak E, Soltyka M, Priebe A, Zielinski R, Fokt I, Ziemniak M, Jaskiewicz A, Borowski R, Domoradzki T, Priebe W (2019) Deoxy-d-glucose and its analogs: from diagnostic to therapeutic agents. Int J Mol Sci 21:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri EM, Menga A, Lebrun A, Hooper DC, Butterfield DA, Mazzone M, Castegna A (2017) Blockade of glutamine synthetase enhances inflammatory response in microglial cells. Antioxid Redox Signal 26:351–363. 10.1089/ars.2016.6715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palpagama TH, Waldvogel HJ, Faull RL, Kwakowsky A (2019) The role of microglia and astrocytes in Huntington's disease. Front Mol Neurosci 12:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palsson-McDermott EM, O'Neill LA (2013) The Warburg effect then and now: from cancer to inflammatory diseases. BioEssays 35:965–973. 10.1002/bies.201300084 [DOI] [PubMed] [Google Scholar]
- Papathanassoglou ED, Miltiadous P, Karanikola MN (2015) May BDNF be implicated in the exercise-mediated regulation of inflammation? Critical review and synthesis of evidence. Biol Res Nurs 17:521–539. 10.1177/1099800414555411 [DOI] [PubMed] [Google Scholar]
- Perez-Baos S, Barrasa JI, Gratal P, Larranaga-Vera A, Prieto-Potin I, Herrero-Beaumont G, Largo R (2017) Tofacitinib restores the inhibition of reverse cholesterol transport induced by inflammation: understanding the lipid paradox associated with rheumatoid arthritis. Br J Pharmacol 174:3018–3031. 10.1111/bph.13932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips T, Rothstein JD (2014) Glial cells in amyotrophic lateral sclerosis. Exp Neurol 262:111–120. 10.1016/j.expneurol.2014.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips T, Rothstein JD (2017) Oligodendroglia: metabolic supporters of neurons. J Clin Invest 127:3271–3280. 10.1172/JCI90610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips T, Bento-Abreu A, Nonneman A, Haeck W, Staats K, Geelen V, Hersmus N, Küsters B, Van Den Bosch L, Van Damme P, Richardson WD, Robberecht W (2013) Oligodendrocyte dysfunction in the pathogenesis of amyotrophic lateral sclerosis. Brain 136:471–482. 10.1093/brain/aws339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piunti A, Shilatifard A (2016) Epigenetic balance of gene expression by Polycomb and COMPASS families. Science 352:aad9780. 10.1126/science.aad9780 [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Crane AM, Seiden LS, Kleven MS, Porrino LJ (1990) Metabolic mapping of the effects of intravenous methamphetamine administration in freely moving rats. Psychopharmacology (Berl) 102:175–182. 10.1007/BF02245919 [DOI] [PubMed] [Google Scholar]
- Popescu BF, Pirko I, Lucchinetti CF (2013) Pathology of multiple sclerosis: where do we stand? Continuum (Minneap Minn) 19:901–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popkin BM, Gordon-Larsen P (2004) The nutrition transition: worldwide obesity dynamics and their determinants. Int J Obes 28: S2–S9. 10.1038/sj.ijo.0802804 [DOI] [PubMed] [Google Scholar]
- Popkin BM, Adair LS, Ng SW (2012) Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev 70:3–21. 10.1111/j.1753-4887.2011.00456.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prvulovic D, Van de Ven V, Sack AT, Maurer K, Linden DE (2005) Functional activation imaging in aging and dementia. Psychiatry Res 140:97–113. 10.1016/j.pscychresns.2005.06.006 [DOI] [PubMed] [Google Scholar]
- Quate L, McBean DE, Ritchie IM, Olverman HJ, Kelly PA (2004) Acute methylenedioxymethamphetamine administration: effects on local cerebral blood flow and glucose utilisation in the Dark Agouti rat. Psychopharmacology (Berl) 173:287–295. 10.1007/s00213-004-1784-z [DOI] [PubMed] [Google Scholar]
- Radak Z, Taylor AW, Ohno H, Goto S (2001) Adaptation to exercise-induced oxidative stress: from muscle to brain. Exerc Immunol Rev 7:90–107. [PubMed] [Google Scholar]
- Rambold AS, Cohen S, Lippincott-Schwartz J (2015) Fatty acid trafficking in starved cells: regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev Cell 32:678–692. 10.1016/j.devcel.2015.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayaprolu S (2013) TREM2 in neurodegeneration: evidence for association of the p.R47H variant with frontotemporal dementia and Parkinson's disease. Mol Neurodegener 8:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeck GW (2017) The role of APOE on lipid homeostasis and inflammation in normal brains. J Lipid Res 58:1493–1499. 10.1194/jlr.R075408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J (2004) Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proc Natl Acad Sci USA 101:284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M, Guo Y, Wei X, Yan S, Qin Y, Zhang X, Jiang F, Lou H (2018) TREM2 overexpression attenuates neuroinflammation and protects dopaminergic neurons in experimental models of Parkinson's disease. Exp Neurol 302:205–213. 10.1016/j.expneurol.2018.01.016 [DOI] [PubMed] [Google Scholar]
- Reynolds IJ, Hastings TG (1995) Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J Neurosci 15:3318–3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb JL, Morrissey NA, Weightman Potter PG, Smithers HE, Beall C, Ellacott KL (2020a) Immunometabolic changes in glia: a potential role in the pathophysiology of obesity and diabetes. Neuroscience 447:167–181. 10.1016/j.neuroscience.2019.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb JL, Hammad NA, Weightman Potter PG, Chilton JK, Beall C, Ellacott KL (2020b) The metabolic response to inflammation in astrocytes is regulated by nuclear factor-kappa B signaling. Glia 68:2246–2263. 10.1002/glia.23835 [DOI] [PubMed] [Google Scholar]
- Rodriguez GA, Tai LM, LaDu MJ, Rebeck GW (2014) Human APOE4 increases microglia reactivity at Aβ plaques in a mouse model of Aβ deposition. J Neuroinflammation 11:111. 10.1186/1742-2094-11-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez JJ, Butt AM, Gardenal E, Parpura V, Verkhratsky A (2016) Complex and differential glial responses in Alzheimer's disease and ageing. Curr Alzheimer Res 13:343–358. 10.2174/1567205013666160229112911 [DOI] [PubMed] [Google Scholar]
- Roosterman D, Cottrell GS (2020) Astrocytes and neurons communicate via a monocarboxylic acid shuttle. AIMS Neurosci 7:94–106. 10.3934/Neuroscience.2020007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW (1995) Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann Neurol 38:73–84. 10.1002/ana.410380114 [DOI] [PubMed] [Google Scholar]
- Rubio-Araiz A, Finucane OM, Keogh S, Lynch MA (2018) Anti-TLR2 antibody triggers oxidative phosphorylation in microglia and increases phagocytosis of β-amyloid. J Neuroinflammation 15:247. 10.1186/s12974-018-1281-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusek M, Pluta R, Ulamek-Koziol M, Czuczwar SJ (2019) Ketogenic diet in Alzheimer's disease. Int J Mol Sci 20:3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskin DN, Ross JL, Kawamura M Jr, Ruiz TL, Geiger JD, Masino SA (2011) A ketogenic diet delays weight loss and does not impair working memory or motor function in the R6/2 1J mouse model of Huntington's disease. Physiol Behav 103:501–507. 10.1016/j.physbeh.2011.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan DG, O'Neill LA (2017) Krebs cycle rewired for macrophage and dendritic cell effector functions. FEBS Lett 591:2992–3006. 10.1002/1873-3468.12744 [DOI] [PubMed] [Google Scholar]
- Salminen A, Hyttinen JM, Kaarniranta K (2011) AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on healthspan and lifespan. J Mol Med (Berl) 89:667–676. 10.1007/s00109-011-0748-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapp E, Kegel KB, Aronin N, Hashikawa T, Uchiyama Y, Tohyama K, Bhide PG, Vonsattel JP, DiFiglia M (2001) Early and progressive accumulation of reactive microglia in the Huntington disease brain. J Neuropathol Exp Neurol 60:161–172. 10.1093/jnen/60.2.161 [DOI] [PubMed] [Google Scholar]
- Saravia J, Raynor JL, Chapman NM, Lim SA, Chi H (2020) Signaling networks in immunometabolism. Cell Res 30:328–342. 10.1038/s41422-020-0301-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton RA, Sabatini DM (2017) mTOR signaling in growth, metabolism, and disease. Cell 168:960–976. 10.1016/j.cell.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayed FA, Telpoukhovskaia M, Kodama L, Li Y, Zhou Y, Le D, Hauduc A, Ludwig C, Gao F, Clelland C, Zhan L, Cooper YA, Davalos D, Akassoglou K, Coppola G, Gan L (2018) Differential effects of partial and complete loss of TREM2 on microglial injury response and tauopathy. Proc Natl Acad Sci USA 115:10172–10177. 10.1073/pnas.1811411115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling LP, Pascoal TA, Zimmer ER, Mathotaarachchi S, Shin M, de Mello Rieder CR, Gauthier S, Palmini A, Rosa-Neto P, Alzheimer's Disease Neuroimaging Initiative (2019) Regional amyloid-β load and white matter abnormalities contribute to hypometabolism in Alzheimer's dementia. Mol Neurobiol 56:4916–4924. 10.1007/s12035-018-1405-1 [DOI] [PubMed] [Google Scholar]
- Schröcksnadel K, Wirleitner B, Winkler C, Fuchs D (2006) Monitoring tryptophan metabolism in chronic immune activation. Clin Chim Acta 364:82–90. 10.1016/j.cca.2005.06.013 [DOI] [PubMed] [Google Scholar]
- Schubert D, Soucek T, Blouw B (2009) The induction of HIF-1 reduces astrocyte activation by amyloid beta peptide. Eur J Neurosci 29:1323–1334. 10.1111/j.1460-9568.2009.06712.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Saito K, Sano F, Koizumi S (2019) Aberrant calcium signals in reactive astrocytes: a key process in neurological disorders. Int J Mol Sci 20:996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JY, Fang ZH, Yu ZX, Wang CE, Li SH, Li XJ (2005) Expression of mutant huntingtin in glial cells contributes to neuronal excitotoxicity. J Cell Biol 171:1001–1012. 10.1083/jcb.200508072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleiman SF, Henry J, Al-Haddad R, El Hayek L, Abou Haidar E, Stringer T, Ulja D, Karuppagounder SS, Holson EB, Ratan RR, Ninan I, Chao MV (2016) Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body beta-hydroxybutyrate. Elife 5:e15092. 10.7554/eLife.15092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonninen TM, Hämäläinen RH, Koskuvi M, Oksanen M, Shakirzyanova A, Wojciechowski S, Puttonen K, Naumenko N, Goldsteins G, Laham-Karam N, Lehtonen M, Tavi P, Koistinaho J, Lehtonen Š (2020) Metabolic alterations in Parkinson's disease astrocytes. Sci Rep 10:14474. 10.1038/s41598-020-71329-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampinato SF, Copani A, Nicoletti F, Sortino MA, Caraci F (2018) Metabotropic glutamate receptors in glial cells: a new potential target for neuroprotection? Front Mol Neurosci 11:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squitieri F, Cannella M, Gaudio L, Martino T, Maglione V, Giallonardo P, Simonelli M, Simonelli G, Mangeruga D, Ciarmiello A, Pierelli F (2003) Italian Huntington disease patients: data and tissue bank. Neurol Sci 24:215–216. 10.1007/s10072-003-0137-8 [DOI] [PubMed] [Google Scholar]
- Stein EM, DiNardo CD, Fathi AT, Pollyea DA, Stone RM, Altman JK, Roboz GJ, Patel MR, Collins R, Flinn IW, Sekeres MA, Stein AS, Kantarjian HM, Levine RL, Vyas P, MacBeth KJ, Tosolini A, VanOostendorp J, Xu Q, Gupta I, et al. (2019) Molecular remission and response patterns in patients with mutant-IDH2 acute myeloid leukemia treated with enasidenib. Blood 133:676–687. 10.1182/blood-2018-08-869008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PM, Mezdour H, Aratani Y, Knouff C, Najib J, Reddick RL, Quarfordt SH, Maeda N (1997) Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem 272:17972–17980. 10.1074/jbc.272.29.17972 [DOI] [PubMed] [Google Scholar]
- Sung YH, Kim SC, Hong HP, Park CY, Shin MS, Kim CJ, Seo JH, Kim DY, Kim DJ, Cho HJ (2012) Treadmill exercise ameliorates dopaminergic neuronal loss through suppressing microglial activation in Parkinson's disease mice. Life Sci 91:1309–1316. 10.1016/j.lfs.2012.10.003 [DOI] [PubMed] [Google Scholar]
- Szablewski L (2016) Glucose transporters in brain: in health and in Alzheimer's disease. J Alzheimers Dis 55:1307–1320. 10.3233/JAD-160841 [DOI] [PubMed] [Google Scholar]
- Szepesi Z, Manouchehrian O, Bachiller S, Deierborg T (2018) Bidirectional microglia-neuron communication in health and disease. Front Cell Neurosci 12:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai YF, Pavese N, Gerhard A, Tabrizi SJ, Barker RA, Brooks DJ, Piccini P (2007) Microglial activation in presymptomatic Huntington's disease gene carriers. Brain 130:1759–1766. 10.1093/brain/awm044 [DOI] [PubMed] [Google Scholar]
- Tan Z, Xie N, Cui H, Moellering DR, Abraham E, Thannickal VJ, Liu G (2015) Pyruvate dehydrogenase kinase 1 participates in macrophage polarization via regulating glucose metabolism. J Immunol 194:6082–6089. 10.4049/jimmunol.1402469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, Zheng L, Gardet A, Tong Z, Jany SS, Corr SC, Haneklaus M, Caffrey BE, Pierce K, Walmsley S, Beasley FC, et al. (2013) Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496:238–242. 10.1038/nature11986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Yan M, Wang L, Zhou Y, Wang Z, Xia T, Liu X, Pan R, Chang Q (2020) Homeostasis imbalance of microglia and astrocytes leads to alteration in the metabolites of the kynurenine pathway in LPS-induced depressive-like mice. Int J Mol Sci 21:1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tengan CH, Moraes CT (2017) NO control of mitochondrial function in normal and transformed cells. Biochim Biophys Acta Bioenerg 1858:573–581. 10.1016/j.bbabio.2017.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timp W, Timp G (2020) Beyond mass spectrometry, the next step in proteomics. Sci Adv 6:eaax8978. 10.1126/sciadv.aax8978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toczylowska B, Jamrozik Z, Liebert A, Kwiecinski H (2013) NMR-based metabonomics of cerebrospinal fluid applied to amyotrophic lateral sclerosis. Biocybernet Biomed Eng 33:21–32. 10.1016/S0208-5216(13)70053-6 [DOI] [Google Scholar]
- Tracey TJ, Steyn FJ, Wolvetang EJ, Ngo ST (2018) Neuronal lipid metabolism: multiple pathways driving functional outcomes in health and disease. Front Mol Neurosci 11:10. 10.3389/fnmol.2018.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Träger U, Andre R, Lahiri N, Magnusson-Lind A, Weiss A, Grueninger S, McKinnon C, Sirinathsinghji E, Kahlon S, Pfister EL, Moser R, Hummerich H, Antoniou M, Bates GP, Luthi-Carter R, Lowdell MW, Björkqvist M, Ostroff GR, Aronin N, Tabrizi SJ (2014) HTT-lowering reverses Huntington's disease immune dysfunction caused by NFkappaB pathway dysregulation. Brain 137:819–833. 10.1093/brain/awt355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh J, Farrer M (2013) Advances in the genetics of Parkinson disease. Nat Rev Neurol 9:445–454. 10.1038/nrneurol.2013.132 [DOI] [PubMed] [Google Scholar]
- Tu JL, Zhao CB, Vollmer T, Coons S, Lin HJ, Marsh S, Treiman DM, Shi J (2009) APOE 4 polymorphism results in early cognitive deficits in an EAE model. Biochem Biophys Res Commun 384:466–470. 10.1016/j.bbrc.2009.04.153 [DOI] [PubMed] [Google Scholar]
- Tzeng HT, Chyuan IT, Chen WY (2019) Shaping of innate immune response by fatty acid metabolite palmitate. Cells 8:1633. 10.3390/cells8121633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulland TK, Song WM, Huang SC, Ulrich JD, Sergushichev A, Beatty WL, Loboda AA, Zhou Y, Cairns NJ, Kambal A, Loginicheva E, Gilfillan S, Cella M, Virgin HW, Unanue ER, Wang Y, Artyomov MN, Holtzman DM, Colonna M (2017) TREM2 maintains microglial metabolic fitness in Alzheimer's disease. Cell 170:649–663.e613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umegaki H, Roth GS, Ingram DK (2008) Aging of the striatum: mechanisms and interventions. Age (Dordr) 30:251–261. 10.1007/s11357-008-9066-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger RH, Clark GO, Scherer PE, Orci L (2010) Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta 1801:209–214. 10.1016/j.bbalip.2009.10.006 [DOI] [PubMed] [Google Scholar]
- Valdearcos M, Robblee MM, Benjamin DI, Nomura DK, Xu AW, Koliwad SK (2014) Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep 9:2124–2138. 10.1016/j.celrep.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallée A, Lecarpentier Y, Vallée JN (2019) Circadian rhythms and energy metabolism reprogramming in Parkinson's disease. Curr Issues Mol Biol 31:21–44. 10.21775/cimb.031.021 [DOI] [PubMed] [Google Scholar]
- van der Poel M, Ulas T, Mizee MR, Hsiao CC, Miedema SS, Schuurman KG, Helder B, Tas SW, Schultze JL, Hamann J, Huitinga I (2019) Transcriptional profiling of human microglia reveals grey–white matter heterogeneity and multiple sclerosis-associated changes. Nat Commun 10:1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandoorne T, De Bock K, Van Den Bosch L (2018) Energy metabolism in ALS: an underappreciated opportunity? Acta Neuropathol 135:489–509. 10.1007/s00401-018-1835-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney P, Yadav V, Saini N (2016) Lipid rafts in immune signalling: current progress and future perspective. Immunology 149:13–24. 10.1111/imm.12617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchio LM, Meng Y, Xhima K, Lipsman N, Hamani C, Aubert I (2018) The neuroprotective effects of exercise: maintaining a healthy brain throughout aging. Brain Plast 4:17–52. 10.3233/BPL-180069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega C, Sachleben LJ, Gozal D, Gozal E (2006) Differential metabolic adaptation to acute and long-term hypoxia in rat primary cortical astrocytes. J Neurochem 97:872–883. 10.1111/j.1471-4159.2006.03790.x [DOI] [PubMed] [Google Scholar]
- Vilas D, Fernández-Santiago R, Sanchez E, Azcona LJ, Santos-Montes M, Casquero P, Argandoña L, Tolosa E, Paisán-Ruiz C (2017) A novel p.Glu298Lys mutation in the ACMSD gene in sporadic Parkinson's disease. J Parkinsons Dis 7:459–463. 10.3233/JPD-171146 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Tomasi D, Wang GJ, Fowler JS, Telang F, Goldstein RZ, Alia-Klein N, Wong C (2011) Reduced metabolism in brain 'control networks' following cocaine-cues exposure in female cocaine abusers. PLoS One 6:e16573. 10.1371/journal.pone.0016573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G, Kojori E, Fowler JS, Benveniste H, Tomasi D (2015) Alcohol decreases baseline brain glucose metabolism more in heavy drinkers than controls but has no effect on stimulation-induced metabolic increases. J Neurosci 35:3248–3255. 10.1523/JNEUROSCI.4877-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonsattel JP, Keller C, Amaya M (2008) Neuropathology of Huntington's disease. Handb Clin Neurol 89:599–618. [DOI] [PubMed] [Google Scholar]
- Vonsattel JP, Keller C, Ramirez EP (2011) Huntington's disease: neuropathology. Handb Clin Neurol 100:83–100. [DOI] [PubMed] [Google Scholar]
- Wang H, Kulas JA, Wang C, Holtzman DM, Ferris HA, Hansen SB (2021) Regulation of beta-amyloid production in neurons by astrocyte-derived cholesterol. Proc Natl Acad Sci USA 118:e2102191118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Pavlou S, Du X, Bhuckory M, Xu H, Chen M (2019) Glucose transporter 1 critically controls microglial activation through facilitating glycolysis. Mol Neurodegener 14:2. 10.1186/s13024-019-0305-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Liu Y, Zhou J (2015) Neuroinflammation in Parkinson's disease and its potential as therapeutic target. Transl Neurodegener 4:19–19. 10.1186/s40035-015-0042-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Yang J, Lu T, Zhan Z, Wei W, Lyu X, Jiang Y, Xue X (2020) The effect of swimming exercise and diet on the hypothalamic inflammation of ApoE-/- mice based on SIRT1-NF-kappaB-GnRH expression. Aging (Albany NY) 12:11085–11099. 10.18632/aging.103323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XS, Simmons Z, Liu W, Boyer PJ, Connor JR (2006) Differential expression of genes in amyotrophic lateral sclerosis revealed by profiling the post mortem cortex. Amyotroph Lateral Scler 7:201–210. 10.1080/17482960600947689 [DOI] [PubMed] [Google Scholar]
- Wang Y, Cella M, Mallinson K, Ulrich JD, Young KL, Robinette ML, Gilfillan S, Krishnan GM, Sudhakar S, Zinselmeyer BH, Holtzman DM, Cirrito JR, Colonna M (2015) TREM2 lipid sensing sustains the microglial response in an Alzheimer's disease model. Cell 160:1061–1071. 10.1016/j.cell.2015.01.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xu W, Yan Z, Zhao W, Mi J, Li J, Yan H (2018) Metformin induces autophagy and G0/G1 phase cell cycle arrest in myeloma by targeting the AMPK/mTORC1 and mTORC2 pathways. J Exp Clin Cancer Res 37:63. 10.1186/s13046-018-0731-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Gerstein M, Snyder M (2009) RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 10:57–63. 10.1038/nrg2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O (1956) On the origin of cancer cells. Science 123:309–314. 10.1126/science.123.3191.309 [DOI] [PubMed] [Google Scholar]
- Watts ME, Pocock R, Claudianos C (2018) Brain energy and oxygen metabolism: emerging role in normal function and disease. Front Mol Neurosci 11:216. 10.3389/fnmol.2018.00216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheless JW (2008) History of the ketogenic diet. Epilepsia 49 Suppl 8:3–5. [DOI] [PubMed] [Google Scholar]
- Wei J, Long L, Yang K, Guy C, Shrestha S, Chen Z, Wu C, Vogel P, Neale G, Green DR, Chi H (2016) Autophagy enforces functional integrity of regulatory T cells by coupling environmental cues and metabolic homeostasis. Nat Immunol 17:277–285. 10.1038/ni.3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B, Koenigsknecht-Talboo J, Grommes C, Lee CY, Landreth G (2006) Fibrillar beta-amyloid-stimulated intracellular signaling cascades require Vav for induction of respiratory burst and phagocytosis in monocytes and microglia. J Biol Chem 281:20842–20850. 10.1074/jbc.M600627200 [DOI] [PubMed] [Google Scholar]
- Winklhofer KF, Haass C (2010) Mitochondrial dysfunction in Parkinson's disease. Biochim Biophys Acta 1802:29–44. 10.1016/j.bbadis.2009.08.013 [DOI] [PubMed] [Google Scholar]
- Woller SA, Choi SH, An EJ, Low H, Schneider DA, Ramachandran R, Kim J, Bae YS, Sviridov D, Corr M, Yaksh TL, Miller YI (2018) Inhibition of neuroinflammation by AIBP: spinal effects upon facilitated pain states. Cell Rep 23:2667–2677. 10.1016/j.celrep.2018.04.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood TE, Barry J, Yang Z, Cepeda C, Levine MS, Gray M (2019) Mutant huntingtin reduction in astrocytes slows disease progression in the BACHD conditional Huntington's disease mouse model. Hum Mol Genet 28:487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM (2013) Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metab 18:649–659. 10.1016/j.cmet.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DC, Ré DB, Nagai M, Ischiropoulos H, Przedborski S (2006) The inflammatory NADPH oxidase enzyme modulates motor neuron degeneration in amyotrophic lateral sclerosis mice. Proc Natl Acad Sci USA 103:12132–12137. 10.1073/pnas.0603670103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Zhang X, Zhao L (2018) Human ApoE isoforms differentially modulate brain glucose and ketone body metabolism: implications for Alzheimer's disease risk reduction and early intervention. J Neurosci 38:6665–6681. 10.1523/JNEUROSCI.2262-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi P, Ding D, Zhou J, Wang M, Cong YS (2013) DDRGK1 regulates NF-κB activity by modulating IκBα stability. PLoS One 8:e64231. 10.1371/journal.pone.0064231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie N, Zhang L, Gao W, Huang C, Huber PE, Zhou X, Li C, Shen G, Zou B (2020) NAD+ metabolism: pathophysiologic mechanisms and therapeutic potential. Sig Transduct Target Ther 5:227. 10.1038/s41392-020-00311-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Yin C, Wang S, Xiao Y (2013) JAK-STAT in lipid metabolism of adipocytes. JAKSTAT 2:e27203. 10.4161/jkst.27203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Kawamata T, Walker DG, McGeer PL (1992) Vimentin immunoreactivity in normal and pathological human brain tissue. Acta Neuropathol 84:157–162. 10.1007/BF00311389 [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Raudensky J (2008) The role of oxidative stress, metabolic compromise, and inflammation in neuronal injury produced by amphetamine-related drugs of abuse. J Neuroimmune Pharmacol 3:203–217. 10.1007/s11481-008-9121-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HM, Yang S, Huang SS, Tang BS, Guo JF (2017) Microglial activation in the pathogenesis of Huntington's disease. Front Aging Neurosci 9:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao V, Kaletsky R, Keyes W, Mor DE, Wong AK, Sohrabi S, Murphy CT, Troyanskaya OG (2018) An integrative tissue-network approach to identify and test human disease genes. Nat Biotechnol 36:1091–1099. 10.1038/nbt.4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yecies JL, Manning BD (2011) Transcriptional control of cellular metabolism by mTOR signaling. Cancer Res 71:2815–2820. 10.1158/0008-5472.CAN-10-4158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, Sancheti H, Liu Z, Cadenas E (2016) Mitochondrial function in ageing: coordination with signalling and transcriptional pathways. J Physiol 594:2025–2042. 10.1113/JP270541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Liu X, He Q, Zhou L, Yuan Z, Zhao S (2016) Vps35-dependent recycling of Trem2 regulates microglial function. Traffic 17:1286–1296. 10.1111/tra.12451 [DOI] [PubMed] [Google Scholar]
- Yu AS, Hirayama BA, Timbol G, Liu J, Diez-Sampedro A, Kepe V, Satyamurthy N, Huang SC, Wright EM, Barrio JR (2013) Regional distribution of SGLT activity in rat brain in vivo. Am J Physiol Cell Physiol 304:C240–C247. 10.1152/ajpcell.00317.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkoff M (2017) Interactions in the metabolism of glutamate and the branched-chain amino acids and ketoacids in the CNS. Neurochem Res 42:10–18. 10.1007/s11064-016-2057-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wu LM, Wu J (2011) Cross-talk between apolipoprotein E and cytokines. Mediators Inflamm 2011:1–10. 10.1155/2011/949072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Li L, Xie W, Wu JF, Yao F, Tan YL, Xia XD, Liu XY, Liu D, Lan G, Zeng MY, Gong D, Cheng HP, Huang C, Zhao ZW, Zheng XL, Tang CK (2016) Apolipoprotein A-1 binding protein promotes macrophage cholesterol efflux by facilitating apolipoprotein A-1 binding to ABCA1 and preventing ABCA1 degradation. Atherosclerosis 248:149–159. 10.1016/j.atherosclerosis.2016.03.008 [DOI] [PubMed] [Google Scholar]
- Zhang M, Zhao GJ, Yin K, Xia XD, Gong D, Zhao ZW, Chen LY, Zheng XL, Tang XE, Tang CK (2018) Apolipoprotein A-1 binding protein inhibits inflammatory signaling pathways by binding to apolipoprotein A-1 in THP-1 macrophages. Circ J 82:1396–1404. 10.1253/circj.CJ-17-0877 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Tan F, Xu P, Qu S (2016) Recent advance in the relationship between excitatory amino acid transporters and Parkinson's disease. Neural Plast 2016:1–8. 10.1155/2016/8941327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Wu X, Li X, Jiang LL, Gui X, Liu Y, Sun Y, Zhu B, Piña-Crespo JC, Zhang M, Zhang N, Chen X, Bu G, An Z, Huang TY, Xu H (2018) TREM2 is a receptor for β-amyloid that mediates microglial function. Neuron 97:1023–1031.e7. 10.1016/j.neuron.2018.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Liao Z, Locascio JJ, Lesniak KA, Roderick SS, Watt ML, Eklund AC, Zhang-James Y, Kim PD, Hauser MA, Grünblatt E, Moran LB, Mandel SA, Riederer P, Miller RM, Federoff HJ, Wüllner U, Papapetropoulos S, Youdim MB, Cantuti-Castelvetri I, et al. (2010) PGC-1α, a potential therapeutic target for early intervention in Parkinson's disease. Sci Transl Med 2:52ra73. 10.1126/scitranslmed.3001059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Chen XF, Wang T, Wang Z, Liao C, Wang Z, Huang R, Wang D, Li X, Wu L, Jia L, Zheng H, Painter M, Atagi Y, Liu CC, Zhang YW, Fryer JD, Xu H, Bu G (2017) Soluble TREM2 induces inflammatory responses and enhances microglial survival. J Exp Med 214:597–607. 10.1084/jem.20160844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Ulland TK, Colonna M (2018) TREM2-dependent effects on microglia in Alzheimer's disease. Front Aging Neurosci 10:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Nwabuisi-Heath E, Dumanis SB, Tai LM, Yu C, Rebeck GW, LaDu MJ (2012) APOE genotype alters glial activation and loss of synaptic markers in mice. Glia 60:559–569. 10.1002/glia.22289 [DOI] [PMC free article] [PubMed] [Google Scholar]


